Jatropha Diterpenes: An Updated Review Concerning Their Structural Diversity, Therapeutic Performance, and Future Pharmaceutical Applications
Abstract
1. Introduction
2. Literature Search and Drug-Likeness Analysis
2.1. Literature Search Strategy
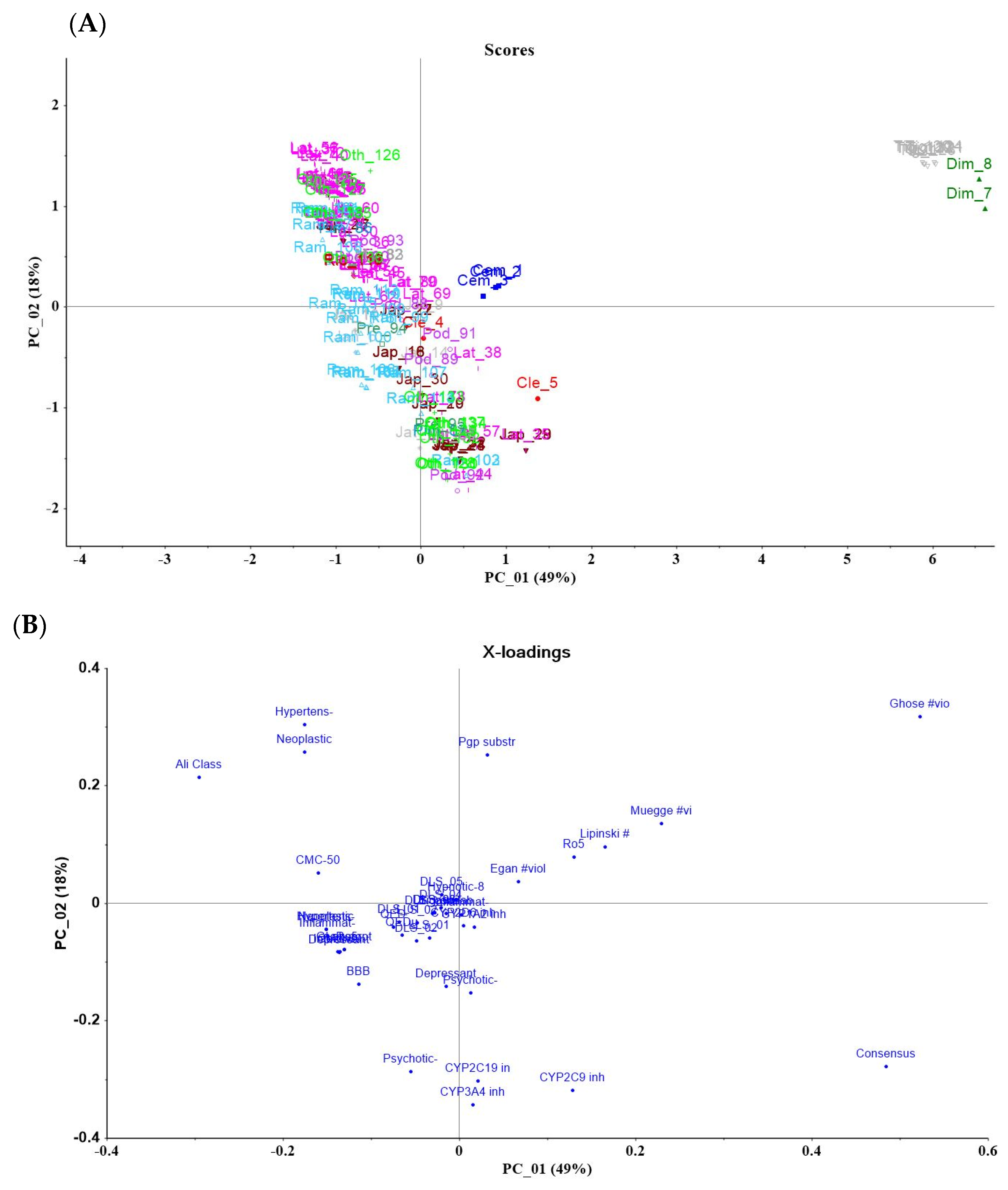
2.2. Jatropha Diterpenes Drug-Likeness
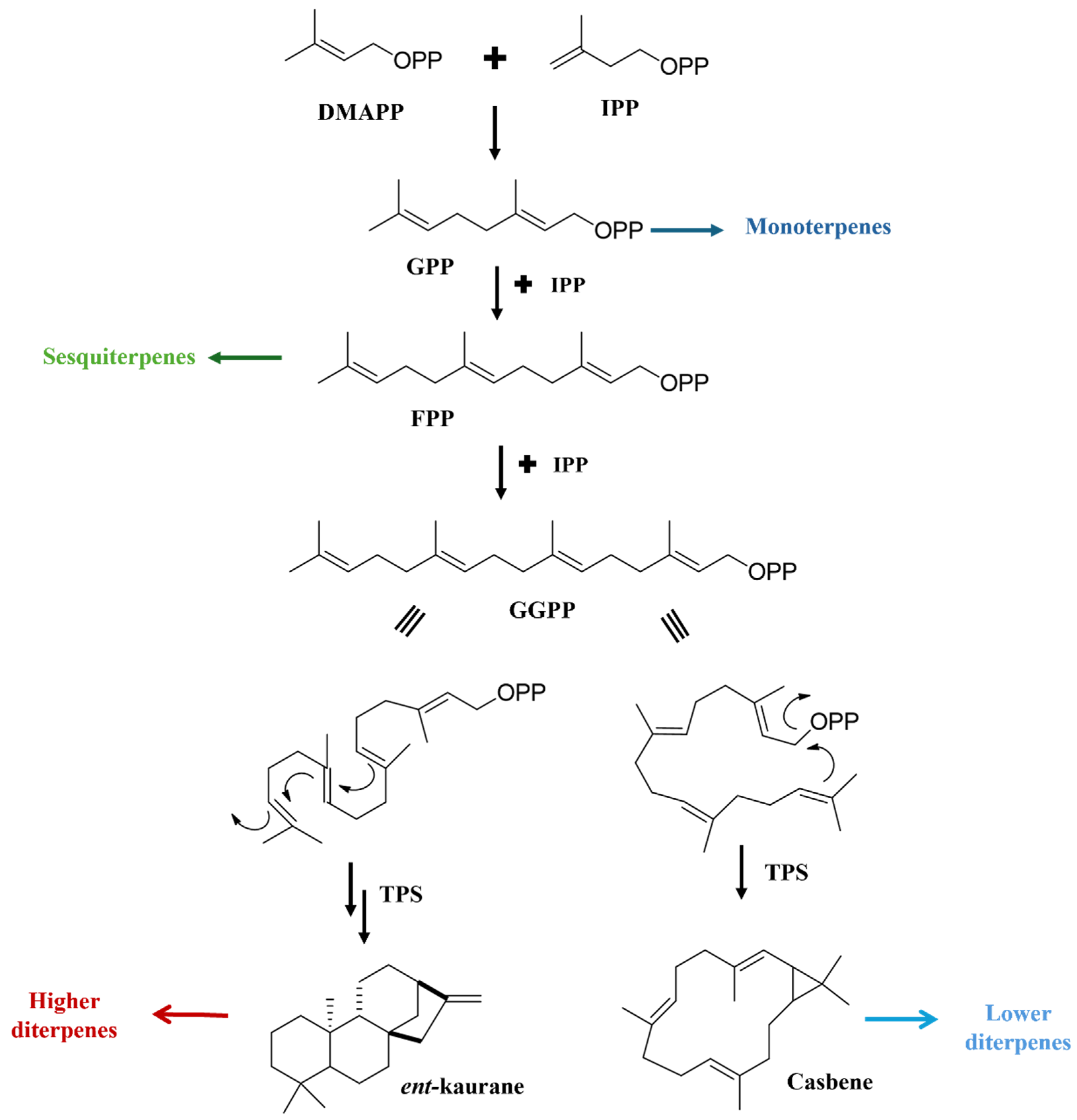
3. Biosynthesis
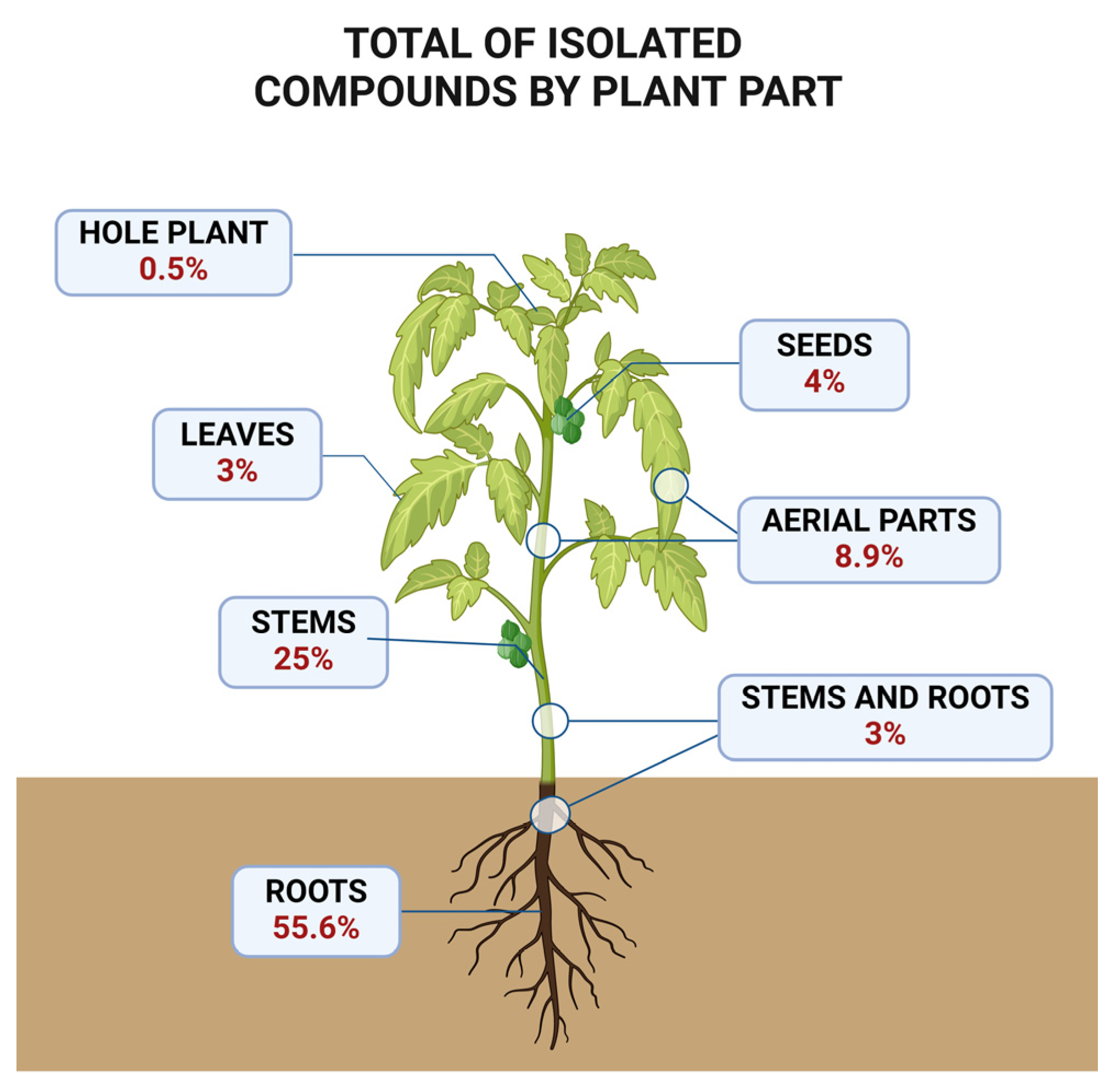
4. Extraction and Isolation
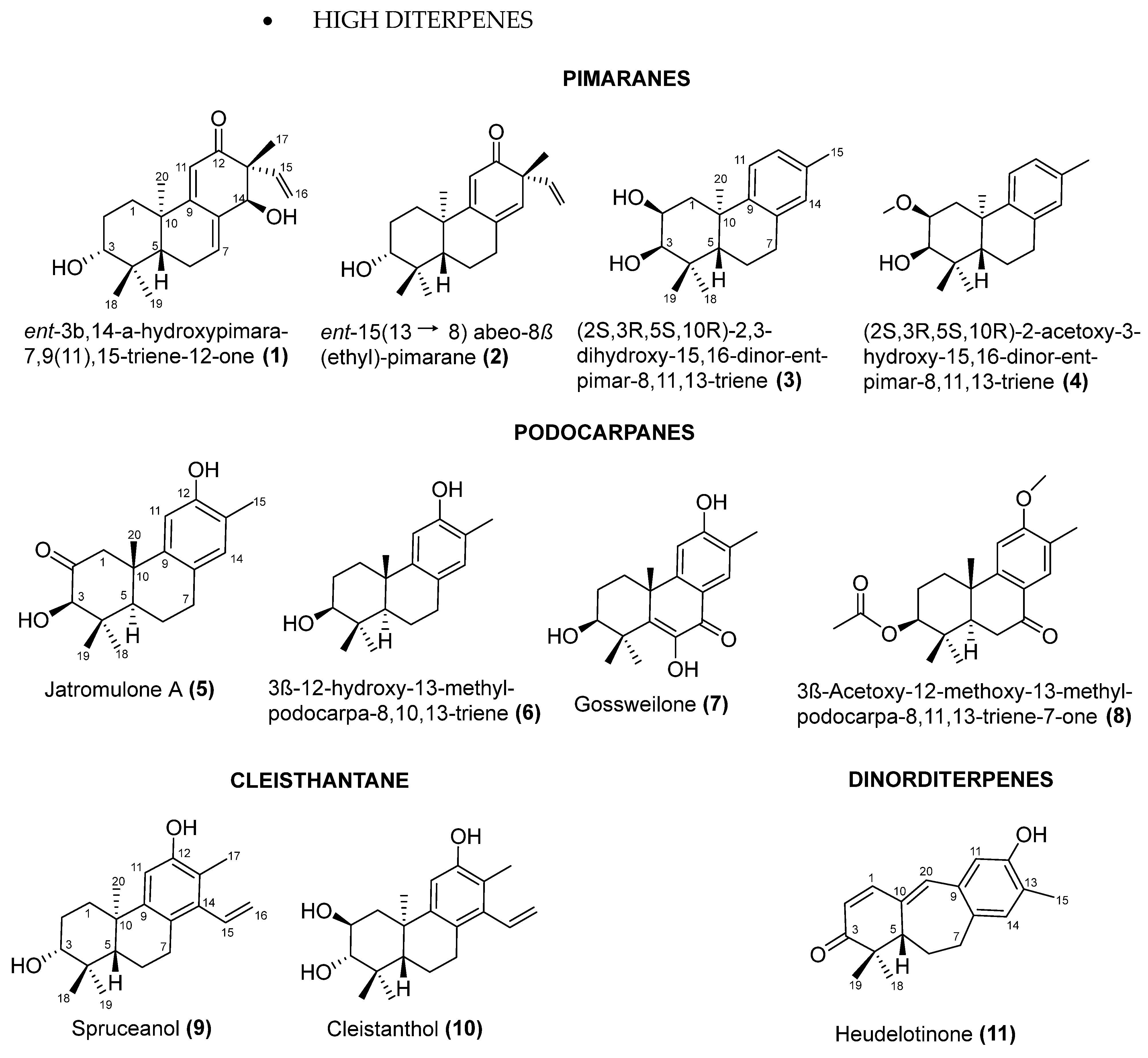
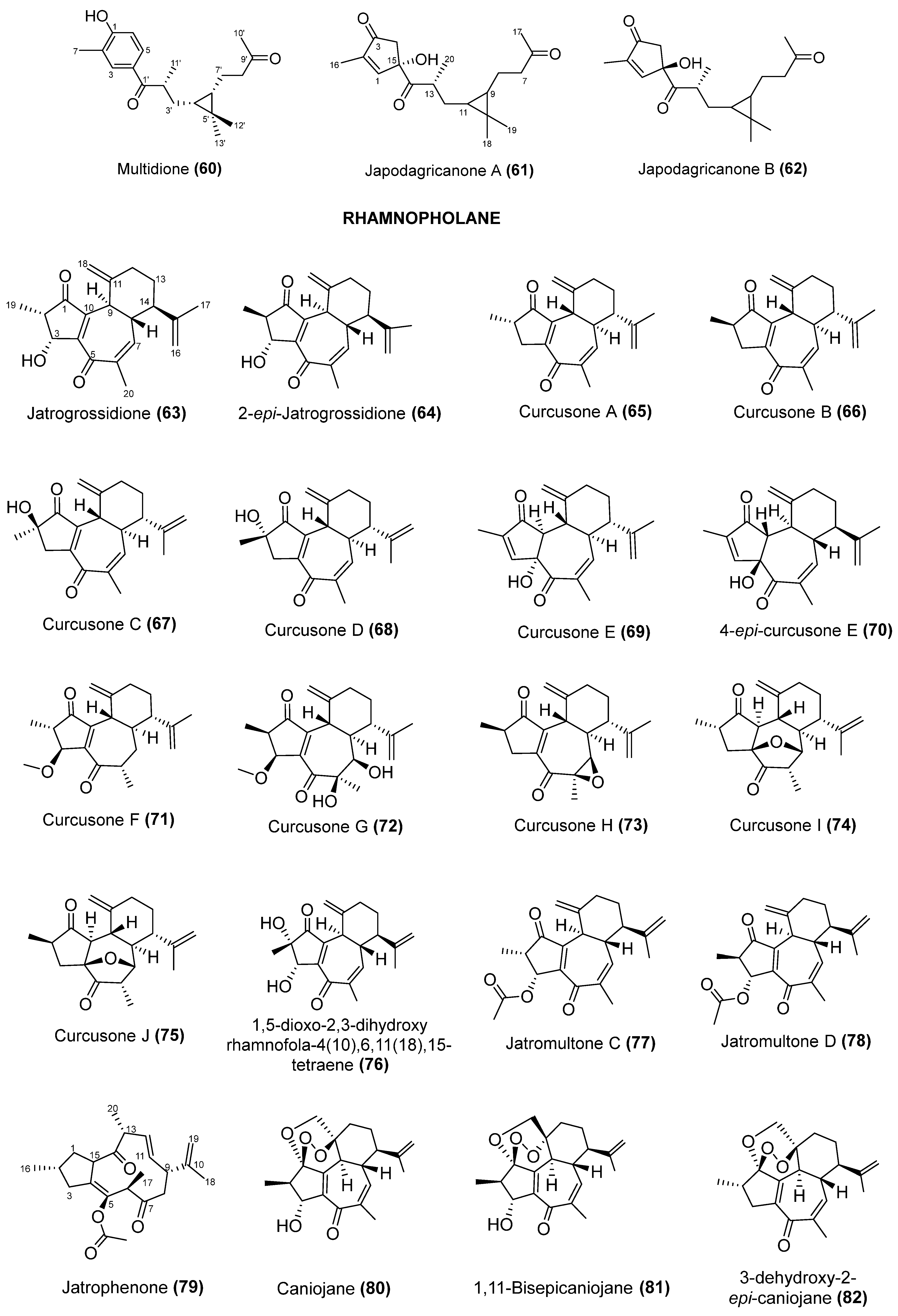
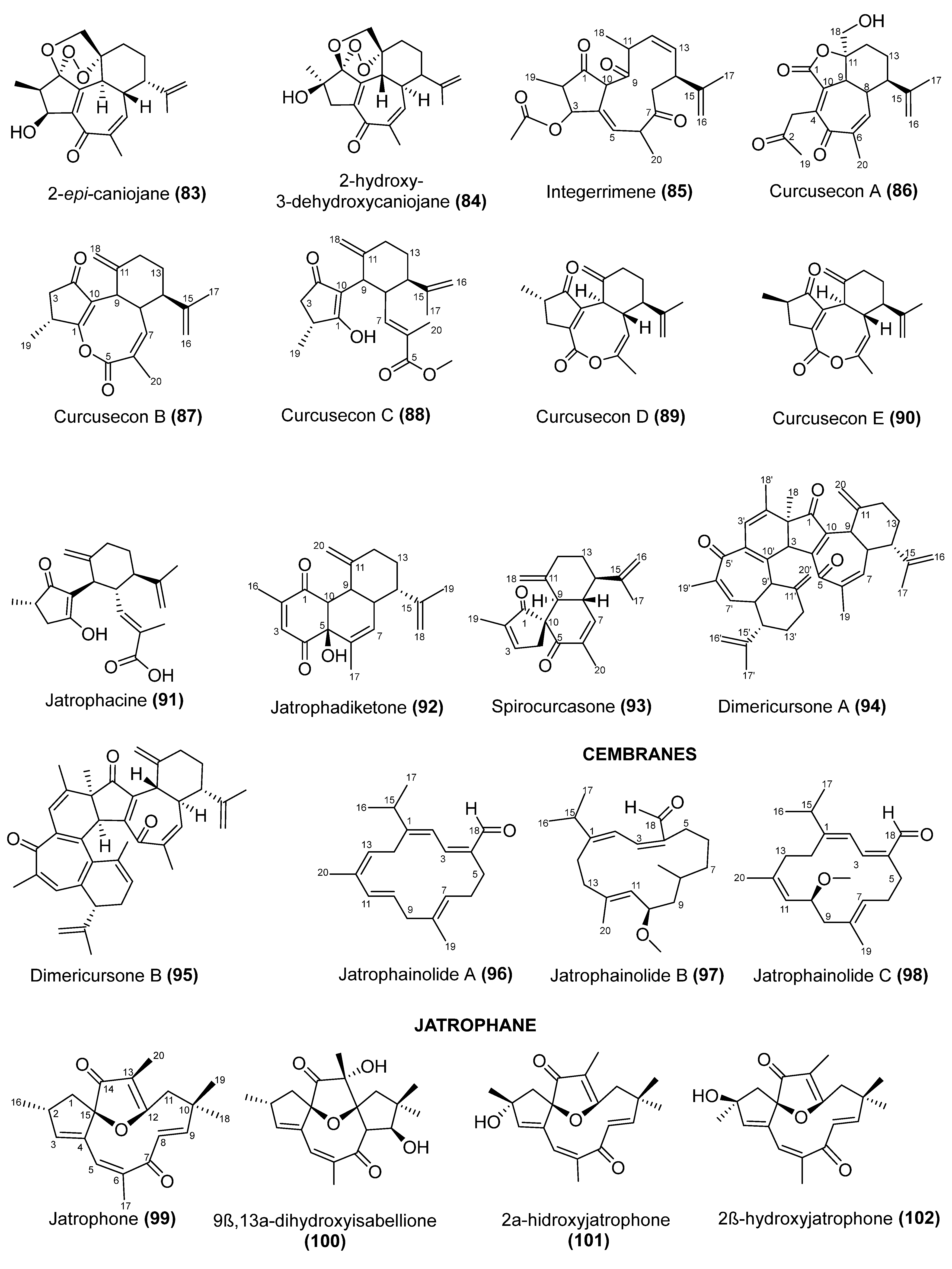
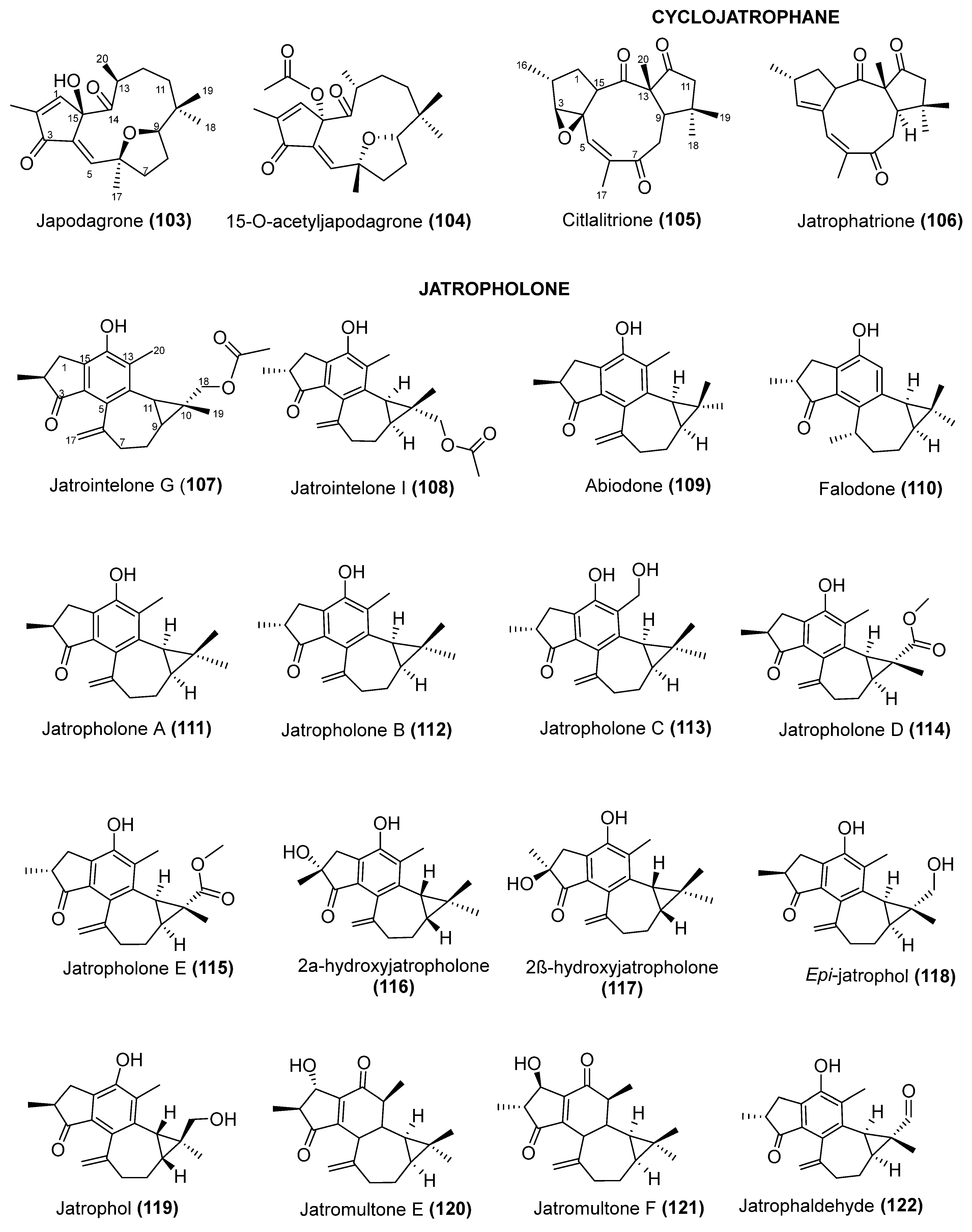
5. Chemical Diversify and Structural Characterization of Jatropha Diterpenes
6. Chemical Synthesis of Jatropha Diterpenes
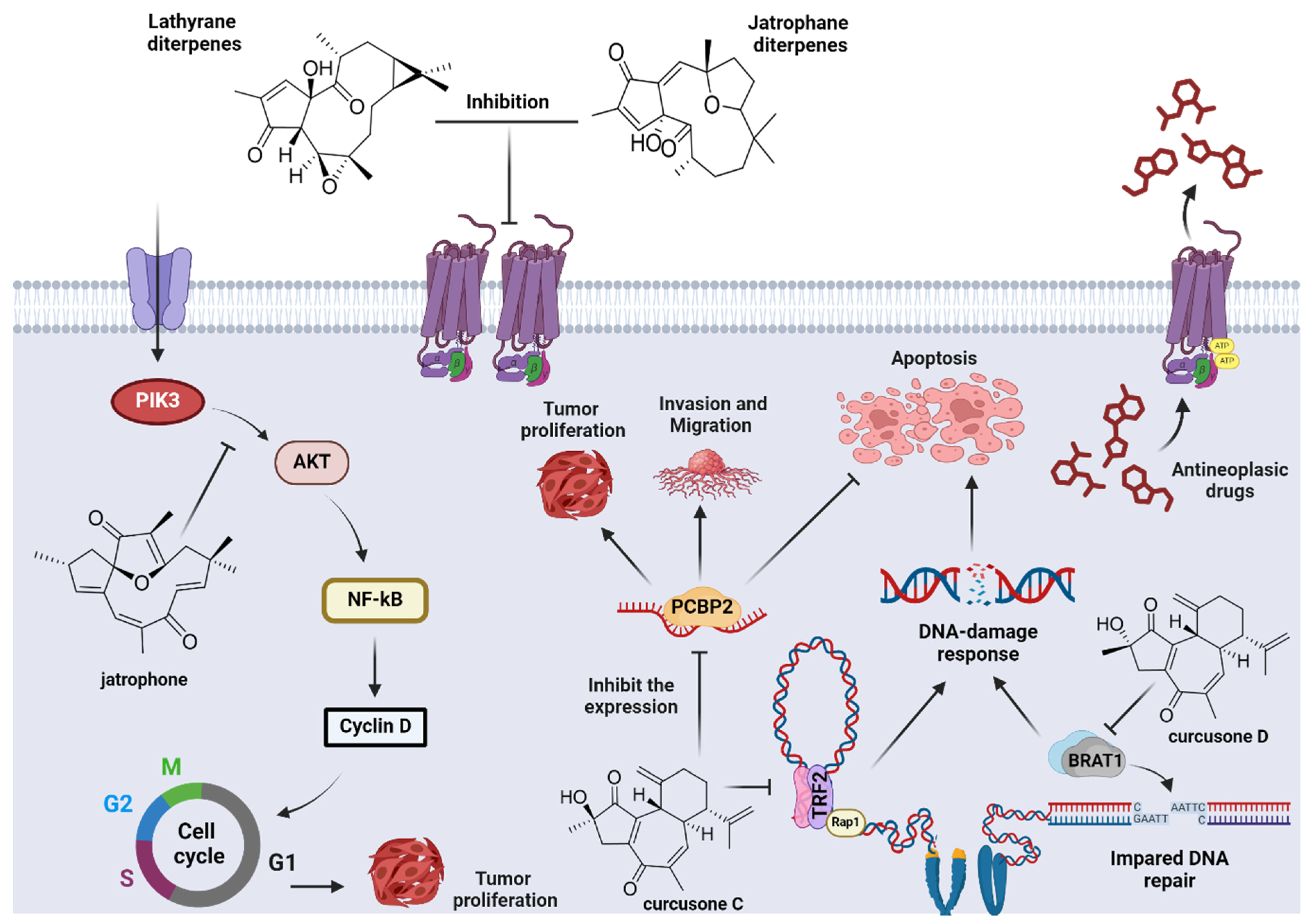
7. Biological Activity
8. Drug-Likeness Properties
9. Perspectives
10. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Brown, J.S.; Amend, S.R.; Austin, R.H.; Gatenby, R.A.; Hammarlund, E.U.; Pienta, K.J. Updating the Definition of Cancer. Mol. Cancer Res. 2023, 21, 1142–1147. [Google Scholar] [CrossRef] [PubMed]
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global Cancer Statistics 2022: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef]
- Huang, M.; Lu, J.-J.; Ding, J. Natural Products in Cancer Therapy: Past, Present and Future. Nat. Prod. Bioprospect. 2021, 11, 5–13. [Google Scholar] [CrossRef] [PubMed]
- Scott, E.C.; Baines, A.C.; Gong, Y.; Moore, R.; Pamuk, G.E.; Saber, H.; Subedee, A.; Thompson, M.D.; Xiao, W.; Pazdur, R.; et al. Trends in the Approval of Cancer Therapies by the FDA in the Twenty-First Century. Nat. Rev. Drug Discov. 2023, 22, 625–640. [Google Scholar] [CrossRef]
- Ghosh, S.; Das, S.K.; Sinha, K.; Ghosh, B.; Sen, K.; Ghosh, N.; Sil, P.C. The Emerging Role of Natural Products in Cancer Treatment. Arch. Toxicol. 2024, 98, 2353–2391. [Google Scholar] [CrossRef] [PubMed]
- Pavithra, R.; Khan, M.R.; Khan, M.S. Recent Advancements in Natural Compounds for Cancer Therapy and Prevention. Phytochem. Rev. 2024, 23, 1–25. [Google Scholar] [CrossRef]
- Shari, K.; Mohamed, O.G.; Meselhy, K.M.; Tripathi, A.; Khaleel, A.E.; Abdel-Sattar, E.; Gedaily, R.A. El Cytotoxic and Antiviral Activities of Jatropha Variegata and Jatropha Spinosa in Relation to Their Metabolite Profile. Sci. Rep. 2024, 14, 4846. [Google Scholar] [CrossRef] [PubMed]
- Paniagua-Zambrana, N.Y.; Bussmann, R.W.; Romero, C. Jatropha curcas L. Jatropha gossypifolia L. Jatropha multifida L. Euphorbiaceae. In Ethnobotany of the Andes; Narel Y. Paniagua-Zambrana, R.W.B., Ed.; Springer, Cham: Switzerland, 2020; pp. 1007–1015. ISBN 978-3-030-28933-1. [Google Scholar]
- Cavalcante, N.B.; Diego da Conceição Santos, A.; Guedes da Silva Almeida, J.R. The Genus Jatropha (Euphorbiaceae): A Review on Secondary Chemical Metabolites and Biological Aspects. Chem. Biol. Interact. 2020, 318, 108976. [Google Scholar] [CrossRef]
- Hanson, J.R. The Development of Strategies for Terpenoid Structure Determination. Nat. Prod. Rep. 2001, 18, 607–617. [Google Scholar] [CrossRef]
- Zeng, T.; Liu, Z.; Liu, H.; He, W.; Tang, X.; Xie, L.; Wu, R. Exploring Chemical and Biological Space of Terpenoids. J. Chem. Inf. Model. 2019, 59, 3667–3678. [Google Scholar] [CrossRef]
- Csizmadia, F. JChem: Java Applets and Modules Supporting Chemical Database Handling from Web Browsers. J. Chem. Inf. Comput. Sci. 2000, 40, 323–324. [Google Scholar] [CrossRef] [PubMed]
- Mauri, A. AlvaDesc: A Tool to Calculate and Analyze Molecular Descriptors and Fingerprints. In Ecotoxicological QSARs; Humana: New York, NY, USA, 2020; pp. 801–820. [Google Scholar]
- Daina, A.; Michielin, O.; Zoete, V. SwissADME: A Free Web Tool to Evaluate Pharmacokinetics, Drug-Likeness and Medicinal Chemistry Friendliness of Small Molecules. Sci. Rep. 2017, 7, 42717. [Google Scholar] [CrossRef] [PubMed]
- Opretzka, L.C.F.; Viana, M.D.M.; de Lima, A.A.; de Souza, T.A.; Scotti, M.T.; Tavares, J.F.; da Silva, M.S.; Soares, M.B.P.; Villarreal, C.F. Cleomin Exerts Acute Antinociceptive Effects in Mice via GABAB and Muscarinic Receptors. Pharmaceuticals 2023, 16, 1547. [Google Scholar] [CrossRef] [PubMed]
- Brown, S.D. The Unscrambler®, Version 5.5. Multivariate Analysis Software for MS-DOS. Available from CAMO A/S, Olav Tryggvasons Gate 24, N-7011 Trondheim, Norway (Telephone: (+47) 7351 4966, Fax: (+47) 7351 4257) or from CAMO USA, 435 Blake Street, Menlo Park, CA 94025, U.S.A. (Telephone/Fax: (+1 415) 324–4286, Internet: Teledyn@netcom.Com). Single-copy Price NOK 26 900 Commercial, NOK 18 800 Non-commercial, NOK 11 900 Academic. J. Chemom. 1995, 9, 527–529. [Google Scholar] [CrossRef]
- Muchlinski, A.; Chen, X.; Lovell, J.T.; Köllner, T.G.; Pelot, K.A.; Zerbe, P.; Ruggiero, M.; Callaway, L.; Laliberte, S.; Chen, F.; et al. Biosynthesis and Emission of Stress-Induced Volatile Terpenes in Roots and Leaves of Switchgrass (Panicum virgatum L.). Front. Plant Sci. 2019, 10, 1144. [Google Scholar] [CrossRef]
- Boncan, D.A.T.; Tsang, S.S.K.; Li, C.; Lee, I.H.T.; Lam, H.-M.; Chan, T.-F.; Hui, J.H.L. Terpenes and Terpenoids in Plants: Interactions with Environment and Insects. Int. J. Mol. Sci. 2020, 21, 7382. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, J.; Li, H.; Hua, J.; Luo, S. Esterification with a Long-Chain Fatty Acid Elevates the Exposure Toxicity of Tigliane Diterpenoids from Euphorbia fischeriana Roots against Nematodes. J. Agric. Food Chem. 2023, 71, 12730–12740. [Google Scholar] [CrossRef]
- Holstein, S.A. The Isoprenoid Biosynthetic Pathway and Statins. In The Enzymes; Elsevier: Amsterdam, The Netherlands, 2011; Volume 30, pp. 279–299. [Google Scholar]
- Schmidt, A.; Gershenzon, J. Cloning and Characterization of Two Different Types of Geranyl Diphosphate Synthases from Norway Spruce (Picea abies). Phytochemistry 2008, 69, 49–57. [Google Scholar] [CrossRef]
- Li, H.; Dickschat, J.S. Diterpene Biosynthesis from Geranylgeranyl Diphosphate Analogues with Changed Reactivities Expands Skeletal Diversity. Angew. Chem. Int. Ed. 2022, 61, 1–8. [Google Scholar] [CrossRef]
- Dewick, P.M. Medicinal Natural Products; John Wiley & Sons, Ltd.: Chichester, UK, 2009; Volume 1, ISBN 9780470742761. [Google Scholar]
- Xu, Y.; Tang, P.; Zhu, M.; Wang, Y.; Sun, D.; Li, H.; Chen, L. Diterpenoids from the Genus Euphorbia: Structure and Biological Activity (2013–2019). Phytochemistry 2021, 190, 112846. [Google Scholar] [CrossRef]
- Wang, H.-B.; Wang, X.-Y.; Liu, L.-P.; Qin, G.-W.; Kang, T.-G. Tigliane Diterpenoids from the Euphorbiaceae and Thymelaeaceae Families. Chem. Rev. 2015, 115, 2975–3011. [Google Scholar] [CrossRef] [PubMed]
- Jia, Q.; Brown, R.; Köllner, T.G.; Fu, J.; Chen, X.; Wong, G.K.-S.; Gershenzon, J.; Peters, R.J.; Chen, F. Origin and Early Evolution of the Plant Terpene Synthase Family. Proc. Natl. Acad. Sci. USA 2022, 119, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Lanier, E.R.; Andersen, T.B.; Hamberger, B. Plant Terpene Specialized Metabolism: Complex Networks or Simple Linear Pathways? Plant J. 2023, 114, 1178–1201. [Google Scholar] [CrossRef] [PubMed]
- Rudolf, J.D.; Chang, C.-Y. Terpene Synthases in Disguise: Enzymology, Structure, and Opportunities of Non-Canonical Terpene Synthases. Nat. Prod. Rep. 2020, 37, 425–463. [Google Scholar] [CrossRef]
- Whitehead, J.N.; Leferink, N.G.H.; Johannissen, L.O.; Hay, S.; Scrutton, N.S. Decoding Catalysis by Terpene Synthases. ACS Catal. 2023, 13, 12774–12802. [Google Scholar] [CrossRef]
- Zerbe, P.; Bohlmann, J. Plant Diterpene Synthases: Exploring Modularity and Metabolic Diversity for Bioengineering. Trends Biotechnol. 2015, 33, 419–428. [Google Scholar] [CrossRef]
- Christianson, D.W. Structural and Chemical Biology of Terpenoid Cyclases. Chem. Rev. 2017, 117, 11570–11648. [Google Scholar] [CrossRef]
- Zhang, Z.; Wu, Q.-Y.; Ge, Y.; Huang, Z.-Y.; Hong, R.; Li, A.; Xu, J.-H.; Yu, H.-L. Hydroxylases Involved in Terpenoid Biosynthesis: A Review. Bioresour. Bioprocess. 2023, 10, 39. [Google Scholar] [CrossRef]
- Bathe, U.; Tissier, A. Cytochrome P450 Enzymes: A Driving Force of Plant Diterpene Diversity. Phytochemistry 2019, 161, 149–162. [Google Scholar] [CrossRef]
- Kumari, M.; Checker, V.G.; Kathpalia, R.; Srivastava, V.; Singh, I.K.; Singh, A. Metabolic Engineering for Enhanced Terpenoid Production: Leveraging New Horizons with an Old Technique. Plant Physiol. Biochem. 2024, 210, 108511. [Google Scholar] [CrossRef]
- de Farias, A.R.B.; Almeida, N.P.; Domont, G.B.; Nogueira, F.C.S.; Campos, F.A.P. Quantitative Proteome Analysis of Jatropha curcas L. Genotypes with Contrasting Levels of Phorbol Esters. Proteomics 2020, 20, 1900273. [Google Scholar] [CrossRef] [PubMed]
- Al-Khayri, J.M.; Sudheer, W.N.; Preetha, T.R.; Nagella, P.; Rezk, A.A.; Shehata, W.F. Biotechnological Research Progress in Jatropha, a Biodiesel-Yielding Plant. Plants 2022, 11, 1292. [Google Scholar] [CrossRef] [PubMed]
- Tadano, S.; Chiyapo, G.; Ishimoto, Y.; Konaka, T.; Mazereku, C.; Tsujimoto, H.; Akashi, K. Multivariate Analysis of Seed Chemical Diversity among Jatropha curcas in Botswana. Agronomy 2021, 11, 1570. [Google Scholar] [CrossRef]
- Faria-Machado, A.F.; Licurgo, F.M.S.; Pires, J.M.F.; da Silveira Campos, R.; Wilhelm, A.E.; de Lourdes M. de Souza, M.; Antoniassi, R. Method Validation for Analysis of Phorbol Esters from Jatropha curcas. Ind. Crop. Prod. 2019, 140, 111627. [Google Scholar] [CrossRef]
- Chemat, F.; Vian, M.A.; Cravotto, G. Green Extraction of Natural Products: Concept and Principles. Int. J. Mol. Sci. 2012, 13, 8615–8627. [Google Scholar] [CrossRef]
- Majid, I.; Khan, S.; Aladel, A.; Dar, A.H.; Adnan, M.; Khan, M.I.; Mahgoub Awadelkareem, A.; Ashraf, S.A. Recent Insights into Green Extraction Techniques as Efficient Methods for the Extraction of Bioactive Components and Essential Oils from Foods. CyTA-J. Food 2023, 21, 101–114. [Google Scholar] [CrossRef]
- Canencia, C.D.; Ano, A.B.S.; Gayas, E.A.D.; Buntas, R.J.P.; Alvarez, P.V.; Ido, A.L.; Arazo, R.O.; Mabayo, V.I.F. Response Surface Optimization of the Bio-Oil Yield from Jatropha Gossypiifolia L. Seeds through Ultrasonic-Assisted Solvent Extraction. Biomass Convers. Biorefin. 2023, 13, 10971–10984. [Google Scholar] [CrossRef]
- Mouahid, A.; Bouanga, H.; Crampon, C.; Badens, E. Supercritical CO2 Extraction of Oil from Jatropha Curcas: An Experimental and Modelling Study. J. Supercrit. Fluids 2018, 141, 2–11. [Google Scholar] [CrossRef]
- Khalaf, M.; Abdel-Fadeel, W.; Hashish, H.M.A.; Wapet, D.E.M.; Mahmoud, M.M.; Elhady, S.A.; Esmail, M.F.C. Experimental Investigation of Different Extraction Methods for Producing Biofuel from Jatropha Seeds and Castor Seeds. Int. J. Energy Res. 2023, 2023, 1–15. [Google Scholar] [CrossRef]
- Abdudeen, A.; Selim, M.Y.E.; Sekar, M.; Elgendi, M. Jatropha’s Rapid Developments and Future Opportunities as a Renewable Source of Biofuel—A Review. Energies 2023, 16, 828. [Google Scholar] [CrossRef]
- Bais, H.P.; Loyola-Vargas, V.M.; Flores, H.E.; Vivanco, J.M. Root-Specific Metabolism: The Biology and Biochemistry of Underground Organs. In Vitro Cell. Dev. Biol.-Plant 2001, 37, 730–741. [Google Scholar] [CrossRef]
- Touw, A.J.; van Dam, N.M. Optimal Chemical Defence Allocation in Roots: Where, Why and How? Phytochem. Rev. 2023, 1. [Google Scholar] [CrossRef]
- Zhao, W.; Peng, J.; Wang, F.; Tian, M.; Li, P.; Feng, B.; Yin, M.; Xu, Y.; Xue, J.-Y.; Xue, J.; et al. Integrating Metabolomics and Transcriptomics to Unveil the Spatiotemporal Distribution of Macrocyclic Diterpenoids and Candidate Genes Involved in Ingenol Biosynthesis in the Medicinal Plant Euphorbia lathyris L. Ind. Crop. Prod. 2022, 184, 115096. [Google Scholar] [CrossRef]
- Ricigliano, V.A.; Sica, V.P.; Knowles, S.L.; Diette, N.; Howarth, D.G.; Oberlies, N.H. Bioactive Diterpenoid Metabolism and Cytotoxic Activities of Genetically Transformed Euphorbia lathyris Roots. Phytochemistry 2020, 179, 112504. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Wei, L.; Li, S.; Chen, F.; Xu, Y. Tissue-Specific Accumulation Profiles of Phorbol Esters in Response to Abiotic and Biotic Stresses in Jatropha curcas. Seeds 2024, 3, 324–340. [Google Scholar] [CrossRef]
- de Almeida, N.P.; Neto, D.F.M.; Carneiro, G.R.A.; de Farias, A.R.B.; Domont, G.B.; de Assis de Paiva Campos, F.; Nogueira, F.C.S. Monitoring Casbene Synthase in Jatropha curcas Tissues Using Targeted Proteomics. Plant Methods 2021, 17, 1–13. [Google Scholar] [CrossRef]
- Wu, Q.; Patocka, J.; Nepovimova, E.; Kuca, K. Jatropha gossypiifolia L. and Its Biologically Active Metabolites: A Mini Review. J. Ethnopharmacol. 2019, 234, 197–203. [Google Scholar] [CrossRef]
- de Andrade, R.S.; Sales, K.A.; Abreu, L.S.; Campos, V.R.; dos Santos Junior, F.M.; Braz-Filho, R.; Scotti, M.T.; Tavares, J.F.; da Silva, M.S. Structure Revision of the Sesquiterpene Nordine Based on NMR Spectroscopic Analysis and X-Ray Crystallography. J. Nat. Prod. 2022, 85, 2480–2483. [Google Scholar] [CrossRef]
- Halabalaki, M.; Vougogiannopoulou, K.; Mikros, E.; Skaltsounis, A.L. Recent Advances and New Strategies in the NMR-Based Identification of Natural Products. Curr. Opin. Biotechnol. 2014, 25, 1–7. [Google Scholar] [CrossRef]
- Wolfender, J.-L.; Marti, G.; Thomas, A.; Bertrand, S. Current Approaches and Challenges for the Metabolite Profiling of Complex Natural Extracts. J. Chromatogr. A 2015, 1382, 136–164. [Google Scholar] [CrossRef]
- Borges, R.M.; de Assis Ferreira, G.; Campos, M.M.; Teixeira, A.M.; das Neves Costa, F.; das Chagas, F.O.; Colonna, M. NMR as a Tool for Compound Identification in Mixtures. Phytochem. Anal. 2023, 34, 385–392. [Google Scholar] [CrossRef] [PubMed]
- de Albuquerque, A.C.F.; Martorano, L.H.; dos Santos, F.M. Are We Still Chasing Molecules That Were Never There? The Role of Quantum Chemical Simulations of NMR Parameters in Structural Reassignment of Natural Products. Front. Nat. Prod. 2024, 2, 1321043. [Google Scholar] [CrossRef]
- Kuhn, S.; Nuzillard, J. Easy Structural Dereplication of Natural Products by Means of Predicted Carbon-13 Nuclear Magnetic Resonance Spectroscopy Data. Chemistry–Methods 2023, 3, e202200054. [Google Scholar] [CrossRef]
- Wolfender, J.-L.; Nuzillard, J.-M.; van der Hooft, J.J.J.; Renault, J.-H.; Bertrand, S. Accelerating Metabolite Identification in Natural Product Research: Toward an Ideal Combination of Liquid Chromatography–High-Resolution Tandem Mass Spectrometry and NMR Profiling, In Silico Databases, and Chemometrics. Anal. Chem. 2019, 91, 704–742. [Google Scholar] [CrossRef] [PubMed]
- Devappa, R.K.; Makkar, H.P.S.; Becker, K. Jatropha Diterpenes: A Review. J. Am. Oil Chem. Soc. 2011, 88, 301–322. [Google Scholar] [CrossRef]
- Costa, R.P.O.; Lucena, L.F.; Silva, L.M.A.; Zocolo, G.J.; Herrera-Acevedo, C.; Scotti, L.; Da-Costa, F.B.; Ionov, N.; Poroikov, V.; Muratov, E.N.; et al. The SistematX Web Portal of Natural Products: An Update. J. Chem. Inf. Model. 2021, 61, 2516–2522. [Google Scholar] [CrossRef]
- Denton, R.W.; Harding, W.W.; Anderson, C.I.; Jacobs, H.; McLean, S.; Reynolds, W.F. New Diterpenes from Jatropha divaricata. J. Nat. Prod. 2001, 64, 829–831. [Google Scholar] [CrossRef]
- Li, H.; Zhao, J.; Chen, J.; Zhu, L.; Wang, D.; Jiang, L.; Yang, D.; Zhao, Z. Diterpenoids from Aerial Parts of Flickingeria Fimbriata and Their Nuclear Factor-KappaB Inhibitory Activities. Phytochemistry 2015, 117, 400–409. [Google Scholar] [CrossRef]
- Zhu, J.-Y.; Zhang, C.-Y.; Dai, J.-J.; Rahman, K.; Zhang, H. Diterpenoids with Thioredoxin Reductase Inhibitory Activities from Jatropha multifida. Nat. Prod. Res. 2017, 31, 2753–2758. [Google Scholar] [CrossRef]
- Ravindranath, N.; Ravinder Reddy, M.; Ramesh, C.; Ramu, R.; Prabhakar, A.; Jagadeesh, B.; Das, B. New Lathyrane and Podocarpane Diterpenoids from Jatropha Curcas. Chem. Pharm. Bull. 2004, 52, 608–611. [Google Scholar] [CrossRef]
- Ngouela, S.; Ngoupayo, J.; Noungoue, D.T.; Tsamo, E.; Connolly, J.D. Gossweilone: A New Podocarpane Derivative from the Stem Bark of Drypetes gossweileri (Euphorbiaceae). Bull. Chem. Soc. Ethiop. 2004, 17. [Google Scholar] [CrossRef][Green Version]
- Kimbu, S.F.; Keumedjio, F.; Sondengam, L.B.; Connolly, J.D. Two Dinorditerpenoids from Ricinodendron heudelotii. Phytochemistry 1991, 30, 619–621. [Google Scholar] [CrossRef]
- Kanth, B.S.; Kumar, A.S.; Shinde, D.B.; Babu, K.H.; Raju, T.V.; Kumar, C.G.; Sujitha, P.; Das, B. New Bioactive Macrocyclic Diterpenoids from Jatropha multifida. Bioorg. Med. Chem. Lett. 2011, 21, 6808–6810. [Google Scholar] [CrossRef]
- Brum, R.L.; Honda, N.K.; Mazarin, S.M.; Hess, S.C.; Cavalheiro, A.J.; Monache, F.D. Jatrowedione, a Lathyrane Diterpene from Jatropha weddelliana. Phytochemistry 1998, 48, 1225–1227. [Google Scholar] [CrossRef]
- Brum, R.L.; Cavalheiro, A.J.; Monache, F.D.; Vencato, I. Jatrowediol, a Lathyrane Diterpene from Jatropha weddelliana. J. Braz. Chem. Soc. 2001, 12, 259–262. [Google Scholar] [CrossRef]
- Zhu, J.-Y.; Lou, L.-L.; Guo, Y.-Q.; Li, W.; Guo, Y.-H.; Bao, J.-M.; Tang, G.-H.; Bu, X.-Z.; Yin, S. Natural Thioredoxin Reductase Inhibitors from Jatropha integerrima. RSC Adv. 2015, 5, 47235–47243. [Google Scholar] [CrossRef]
- Das, B.; Ravikanth, B.; Reddy, K.R.; Thirupathi, P.; Raju, T.V.; Sridhar, B. Diterpenoids from Jatropha multifida. Phytochemistry 2008, 69, 2639–2641. [Google Scholar] [CrossRef]
- Aiyelaagbe, O.O.; Adesogan, K.; Ekundayo, O.; Gloer, J.B. Antibacterial Diterpenoids from Jatropha podagrica Hook. Phytochemistry 2007, 68, 2420–2425. [Google Scholar] [CrossRef] [PubMed]
- Schmeda-Hirschmann, G.; Tsichritzis, F.; Jakupovic, J. Diterpenes and a Lignan from Jatropha grossidentata. Phytochemistry 1992, 31, 1731–1735. [Google Scholar] [CrossRef]
- Falodun, A.; Imieje, V.; Erharuyi, O.; Joy, A.; Langer, P.; Jacob, M.; Khan, S.; Abaldry, M.; Hamann, M. Isolation of Antileishmanial, Antimalarial and Antimicrobial Metabolites from Jatropha multifida. Asian Pac. J. Trop. Biomed. 2014, 4, 374–378. [Google Scholar] [CrossRef]
- Liu, J.; Yang, Y.; Xia, J.; Li, X.; Li, Z.; Zhou, L.; Qiu, M. Cytotoxic Diterpenoids from Jatropha curcas Cv. nigroviensrugosus CY Yang Roots. Phytochemistry 2015, 117, 462–468. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.-D.; Zhang, C.; Xu, W.-J.; Lian, C.-L.; Liu, X.-M.; Wang, C.-F.; Liu, J.-Q. New Lathyrane Diterpenoids with Anti-Inflammatory Activity Isolated from the Roots of Jatropha curcas L. J. Ethnopharmacol. 2021, 268, 113673. [Google Scholar] [CrossRef]
- Zhang, J.-S.; Zhang, Y.; Li, S.; Ahmed, A.; Tang, G.-H.; Yin, S. Cytotoxic Macrocyclic Diterpenoids from Jatropha multifida. Bioorg. Chem. 2018, 80, 511–518. [Google Scholar] [CrossRef]
- Liu, J.-Q.; Yang, Y.-F.; Wang, C.-F.; Li, Y.; Qiu, M.-H. Three New Diterpenes from Jatropha curcas. Tetrahedron 2012, 68, 972–976. [Google Scholar] [CrossRef]
- Naengchomnong, W.; Thebtaranonth, Y.; Wiriyachitra, P.; Okamoto, K.T.; Clardy, J. Isolation and Structure Determination of Two Novel Lathyrenes from Jatropha curcus. Tetrahedron. Lett. 1986, 27, 5675–5678. [Google Scholar] [CrossRef]
- Wang, X.-C.; Zheng, Z.-P.; Gan, X.-W.; Hu, L.-H. Jatrophalactam, A Novel Diterpenoid Lactam Isolated from Jatropha curcas. Org. Lett. 2009, 11, 5522–5524. [Google Scholar] [CrossRef]
- Li, W.; Tang, Y.-Q.; Sang, J.; Fan, R.-Z.; Tang, G.-H.; Yin, S. Jatrofolianes A and B: Two Highly Modified Lathyrane Diterpenoids from Jatropha gossypiifolia. Org. Lett. 2020, 22, 106–109. [Google Scholar] [CrossRef]
- Das, B.; Reddy, K.R.; Ravikanth, B.; Raju, T.V.; Sridhar, B.; Khan, P.U.; Rao, J.V. Multifidone: A Novel Cytotoxic Lathyrane-Type Diterpene Having an Unusual Six-Membered A Ring from Jatropha multifida. Bioorg. Med. Chem. Lett. 2009, 19, 77–79. [Google Scholar] [CrossRef]
- Das, B.; Laxminarayana, K.; Krishnaiah, M.; Srinivas, Y.; Raju, T.V. Multidione, a Novel Diterpenoid from Jatropha multifida. Tetrahedron Lett. 2009, 50, 4885–4887. [Google Scholar] [CrossRef]
- Liu, W.-W.; Zhang, Y.; Yuan, C.-M.; Yu, C.; Ding, J.-Y.; Li, X.-X.; Hao, X.-J.; Wang, Q.; Li, S.-L. Japodagricanones A and B, Novel Diterpenoids from Jatropha podagrica. Fitoterapia 2014, 98, 156–159. [Google Scholar] [CrossRef]
- Can-Aké, R.; Erosa-Rejón, G.; May-Pat, F.; Peña-Rodríguez, L.M.; Peraza-Sánchez, S.R. Bioactive Terpenoids from Roots and Leaves of Jatropha gaumeri. Rev. De La Soc. Química De México 2004, 48, 11–14. [Google Scholar]
- Cui, C.; Dwyer, B.G.; Liu, C.; Abegg, D.; Cai, Z.-J.; Hoch, D.G.; Yin, X.; Qiu, N.; Liu, J.-Q.; Adibekian, A.; et al. Total Synthesis and Target Identification of the Curcusone Diterpenes. J. Am. Chem. Soc. 2021, 143, 4379–4386. [Google Scholar] [CrossRef] [PubMed]
- Chianese, G.; Fattorusso, E.; Aiyelaagbe, O.O.; Luciano, P.; Schröder, H.C.; Müller, W.E.G.; Taglialatela-Scafati, O. Spirocurcasone, a Diterpenoid with a Novel Carbon Skeleton from Jatropha curcas. Org. Lett. 2011, 13, 316–319. [Google Scholar] [CrossRef]
- Liu, J.-Q.; Yang, Y.-F.; Li, X.-Y.; Liu, E.-Q.; Li, Z.-R.; Zhou, L.; Li, Y.; Qiu, M.-H. Cytotoxicity of Naturally Occurring Rhamnofolane Diterpenes from Jatropha curcas. Phytochemistry 2013, 96, 265–272. [Google Scholar] [CrossRef]
- Sutthivaiyakit, S.; Mongkolvisut, W.; Prabpai, S.; Kongsaeree, P. Diterpenes, Sesquiterpenes, and a Sesquiterpene−Coumarin Conjugate from Jatropha integerrima. J. Nat. Prod. 2009, 72, 2024–2027. [Google Scholar] [CrossRef]
- Ravindranath, N.; Venkataiah, B.; Ramesh, C.; Jayaprakash, P.; Das, B. Jatrophenone, a Novel Macrocyclic Bioactive Diterpene from Jatropha gossypifolia. Chem. Pharm. Bull. 2003, 51, 870–871. [Google Scholar] [CrossRef] [PubMed]
- Jakupovic, J.; Grenz, M.; Schmeda-Hirschmann, G. Rhamnofolane Derivatives from Jatropha grossidentata. Phytochemistry 1988, 27, 2997–2998. [Google Scholar] [CrossRef]
- Sutthivaiyakit, S.; Mongkolvisut, W.; Ponsitipiboon, P.; Prabpai, S.; Kongsaeree, P.; Ruchirawat, S.; Mahidol, C. A Novel 8,9-Seco-Rhamnofolane and a New Rhamnofolane Endoperoxide from Jatropha integerrima Roots. Tetrahedron Lett. 2003, 44, 3637–3640. [Google Scholar] [CrossRef]
- Yang, Y.; Liu, J.; Li, X.; Liu, E.; Li, Z.; Qiu, M. New Terpenoids from the Roots of Jatropha curcas. Chin. Sci. Bull. 2013, 58, 1115–1119. [Google Scholar] [CrossRef]
- Hu, Y.; Zhao, H.; Yang, A.; Lv, Q.; Ding, N.; Lu, T.-L.; Hu, L.; Wang, X. Jatrophacine, a 4,5-Seco-Rhamnofolane Diterpenoid with Potent Anti-Inflammatory Activity from Jatropha curcas. Nat. Prod. Res. 2021, 35, 2748–2752. [Google Scholar] [CrossRef]
- Liu, J.-Q.; Xu, Y.; Xiao, Q.; Huang, J.-D.; Ma, J.-J.; Lian, C.-L.; Huang, M.-Y.; Du, Z.; Wang, C.-F. Dimericursones A and B: Two Unprecedented Hexacyclic Dimeric Diterpenoids from the Root Barks of Jatropha curcas. Org. Biomol. Chem. 2018, 16, 8305–8310. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.-B.; Wang, Z.; Liang, Y.-N.; Yu, J.-G.; Zhang, Z.; Liu, S.-J.; Zhang, Z.; Song, Z.-X.; Tang, Z.-S.; Duan, D.-Z. Jatrophainolides A–C, New Cembrane-Type Diterpenoids with PTP1B Inhibitory Activity from the Root Bark of Jatropha integerrima. Phytochem. Lett. 2020, 36, 166–170. [Google Scholar] [CrossRef]
- Taylor, M.D.; Smith, A.B.; Furst, G.T.; Gunasekara, S.P.; Bevelle, C.A.; Cordell, G.A.; Farnsworth, N.R.; Kupchan, S.M.; Uchida, H. Plant Anticancer Agents. 28. New Antileukemic Jatrophone Derivatives from Jatropha gossypiifolia: Structural and Stereochemical Assignment through Nuclear Magnetic Resonance Spectroscopy. J. Am. Chem. Soc. 1983, 105, 3177–3183. [Google Scholar] [CrossRef]
- Pertino, M.; Schmeda-Hirschmann, G.; Rodríguez, J.A.; Theoduloz, C. Gastroprotective Effect and Cytotoxicity of Terpenes from the Paraguayan Crude Drug “Yagua Rova” (Jatropha isabelli). J. Ethnopharmacol. 2007, 111, 553–559. [Google Scholar] [CrossRef]
- Batista, P.H.J.; de Andrade, J.R.M.; Matos, T.S.; da Silva Sousa, T.; das Chagas L. Pinto, F.; Silveira, E.R.; Loiola, M.I.B.; Pessoa, O.D.L. Terpenoids and Coumarins from Jatropha ribifolia (Pohl) Baill. Quim. Nova 2014. [Google Scholar] [CrossRef]
- Das, B.; Ravikanth, B.; Laxminarayana, K.; Ramarao, B.; Raju, T.V. New Macrocyclic Diterpenoids from Jatropha multifida. Chem. Pharm. Bull. 2009, 57, 318–320. [Google Scholar] [CrossRef]
- Yang, J.; Long, Y.O.; Paquette, L.A. Concise Total Syntheses of the Bioactive Mesotricyclic Diterpenoids Jatrophatrione and Citlalitrione. J. Am. Chem. Soc. 2003, 125, 1567–1574. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.-F.; Liu, J.-Q.; Shi, L.; Li, Z.-R.; Qiu, M.-H. New Jatropholane-Type Diterpenes from Jatropha curcas Cv. Multiflorum CY Yang. Nat. Prod. Bioprospect. 2013, 3, 99–102. [Google Scholar] [CrossRef][Green Version]
- Zhang, X.-Q.; Li, F.; Zhao, Z.-G.; Liu, X.-L.; Tang, Y.-X.; Wang, M.-K. Diterpenoids from the Root Bark of Jatropha Curcas and Their Cytotoxic Activities. Phytochem. Lett. 2012, 5, 721–724. [Google Scholar] [CrossRef]
- Naengchomnong, W.; Tarnchompoo, B.; Thebtaranonth, Y. (+)-JATROPHOL, (+)-MARMESIN, PROPACIN AND JATROPHIN FROM THE ROOTS OF JATROPHA CURCAS (EUPHORBIACEAE). ScienceAsia 1994, 20, 073–083. [Google Scholar] [CrossRef]
- Melchor-Martínez, E.M.; Silva-Mares, D.A.; Torres-López, E.; Waksman-Minsky, N.; Pauli, G.F.; Chen, S.-N.; Niemitz, M.; Sánchez-Castellanos, M.; Toscano, A.; Cuevas, G.; et al. Stereochemistry of a Second Riolozane and Other Diterpenoids from Jatropha dioica. J. Nat. Prod. 2017, 80, 2252–2262. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Fan, J.; Zeng, G.; Tan, N. A New Tetracyclic Diterpene from Jatropha curcas. Helv. Chim. Acta 2011, 94, 842–846. [Google Scholar] [CrossRef]
- Bao, J.-M.; Su, Z.-Y.; Lou, L.-L.; Zhu, J.-Y.; Tang, G.-H.; Gan, L.-S.; Bu, X.-Z.; Yin, S. Jatrocurcadiones A and B: Two Novel Diterpenoids with an Unusual 10,11-Seco-Premyrsinane Skeleton from Jatropha curcas. RSC Adv. 2015, 5, 62921–62925. [Google Scholar] [CrossRef]
- Chen, L.; Huang, J.-L.; Zhang, L.; Tian, H.-Y.; Yin, S. Jatrogricaine A: A New Diterpenoid with a 5/6/6/4 Carbon Ring System from the Stems of Jatropha podagrica. Chin. J. Nat. Med. 2019, 17, 298–302. [Google Scholar] [CrossRef] [PubMed]
- Haas, W.; Sterk, H.; Mittelbach, M. Novel 12-Deoxy-16-Hydroxyphorbol Diesters Isolated from the Seed Oil of Jatropha curcas. J. Nat. Prod. 2002, 65, 1434–1440. [Google Scholar] [CrossRef]
- Shenvi, R.A. Natural Product Synthesis in the 21st Century: Beyond the Mountain Top. ACS Cent. Sci. 2024, 10, 519–528. [Google Scholar] [CrossRef]
- Smith, A.B. Evolution of a Synthetic Strategy: Total Synthesis of Jatrophone. In Strategies and Tactics in Organic Synthesis; Academic Press: Cambridge, MA, USA, 1984; pp. 223–274. [Google Scholar]
- Smith, A.B.; Lupo, A.T.; Ohba, M.; Chen, K. Total Synthesis of (+)-Hydroxyjatrophone A and (+)-Hydroxyjatrophone B. J. Am. Chem. Soc. 1989, 111, 6648–6656. [Google Scholar] [CrossRef]
- Smith, A.B.; Malamas, M.S. Jatrophone Analogs: Synthesis of Cis- and Trans-Normethyljatropholactones. J. Org. Chem. 1982, 47, 3442–3447. [Google Scholar] [CrossRef]
- Han, Q.; Wiemer, D.F. Total Synthesis of (+)-Jatrophone. J. Am. Chem. Soc. 1992, 114, 7692–7697. [Google Scholar] [CrossRef]
- Gyorkos, A.C.; Stille, J.K.; Hegedus, L.S. The Total Synthesis of (.+-.)-Epi-Jatrophone and (.+-.)-Jatrophone Using Palladium-Catalyzed Carbonylative Coupling of Vinyl Triflates with Vinyl Stannanes as the Macrocycle-Forming Step. J. Am. Chem. Soc. 1990, 112, 8465–8472. [Google Scholar] [CrossRef]
- Rinner, U. Progress in the Preparation of Jatrophane Diterpenes. Eur. J. Org. Chem. 2015, 2015, 3197–3219. [Google Scholar] [CrossRef]
- Mohan, P.; Koushik, K.; Fuertes, M.J. Synthetic Studies Directed toward the Total Synthesis of a Jatrophane Diterpene. Tetrahedron Lett 2015, 56, 61–65. [Google Scholar] [CrossRef]
- Lentsch, C.; Fürst, R.; Mulzer, J.; Rinner, U. Jatrophane Diterpenes: Preparation of the Western Fragment of Pl-3. Eur. J. Org. Chem. 2014, 2014, 919–923. [Google Scholar] [CrossRef]
- Smith, A.B.; Liverton, N.J.; Hrib, N.J.; Sivaramakrishnan, H.; Winzenberg, K. Total Synthesis of (+)-Jatropholone A and B. J. Org. Chem. 1985, 50, 3239–3241. [Google Scholar] [CrossRef]
- Cai, W.; Huang, S.; Wu, J.; Song, Z.; Xin, Z.; Li, J.; Xue, X. Synthesis of Ent -Cleistanthane Diterpenoid Spruceanol: Construction of an Aromatic C Ring via Lewis Acid-Controlled Regioselective Diels–Alder Cycloaddition. J. Org. Chem. 2020, 85, 6709–6718. [Google Scholar] [CrossRef]
- Wright, A.C.; Lee, C.W.; Stoltz, B.M. Progress toward the Enantioselective Synthesis of Curcusones A–D via a Divinylcyclopropane Rearrangement Strategy. Org. Lett. 2019, 21, 9658–9662. [Google Scholar] [CrossRef]
- Wright, A.C.; Stoltz, B.M. Enantioselective Construction of the Tricyclic Core of Curcusones A–D via a Cross-Electrophile Coupling Approach. Chem. Sci. 2019, 10, 10562–10565. [Google Scholar] [CrossRef]
- Li, Y.; Dai, M. Total Syntheses of the Reported Structures of Curcusones I and J through Tandem Gold Catalysis. Angew. Chem. Int. Ed. 2017, 56, 11624–11627. [Google Scholar] [CrossRef]
- Löffler, L.E.; Wirtz, C.; Fürstner, A. Collective Total Synthesis of Casbane Diterpenes: One Strategy, Multiple Targets. Angew. Chem. Int. Ed. 2021, 60, 5316–5322. [Google Scholar] [CrossRef]
- Watanabe, A.; Hikone, Y.; Nagatomo, M.; Inoue, M. Conversion of Phorbol into Des-D-Ring Tricycle and Crotonianoid B via Peroxidation Reaction. Org. Lett. 2024, 26, 4335–4339. [Google Scholar] [CrossRef]
- Watanabe, A.; Nagatomo, M.; Hirose, A.; Hikone, Y.; Kishimoto, N.; Miura, S.; Yasutake, T.; Abe, T.; Misumi, S.; Inoue, M. Total Syntheses of Phorbol and 11 Tigliane Diterpenoids and Their Evaluation as HIV Latency-Reversing Agents. J. Am. Chem. Soc. 2024, 146, 8746–8756. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.; Wang, M.; Keasling, J.D.; Hu, T.; Yin, X. Expanding the Structural Diversity of Terpenes by Synthetic Biology Approaches. Trends Biotechnol. 2024, 42, 699–713. [Google Scholar] [CrossRef]
- Sun, L.; Wang, X.-M.; Tang, Q.; Xiao, Y.; Xu, J.-B.; Zhang, T.-T.; Liu, Y.-J.; Li, X.; Gao, F. Lathyrane and Premyrsinane Euphorbia Diterpenes against Alzheimer’s Disease: Bioinspired Synthesis, Anti-Cholinesterase and Neuroprotection Bioactivity. Bioorg. Chem. 2024, 147, 107377. [Google Scholar] [CrossRef]
- Schneider, A.; Lystbæk, T.B.; Markthaler, D.; Hansen, N.; Hauer, B. Biocatalytic Stereocontrolled Head-to-Tail Cyclizations of Unbiased Terpenes as a Tool in Chemoenzymatic Synthesis. Nat. Commun. 2024, 15, 4925. [Google Scholar] [CrossRef]
- de Souza, T.A.; Lins, F.S.V.; da Silva Lins, J.; Alves, A.F.; Cibulski, S.P.; de Araújo Medeiros, B.T.; Abreu, L.S.; Scotti, L.; Scotti, M.T.; da Silva, M.S.; et al. Asclepiadoideae Subfamily (Apocynaceae): Ethnopharmacology, Biological Activities and Chemophenetics Based on Pregnane Glycosides. Phytochem. Rev. 2024. [Google Scholar] [CrossRef]
- Rojas-Jiménez, S.; Valladares-Cisneros, M.G.; Salinas-Sánchez, D.O.; Pérez-Ramos, J.; Sánchez-Pérez, L.; Pérez-Gutiérrez, S.; Campos-Xolalpa, N. Anti-Inflammatory and Cytotoxic Compounds Isolated from Plants of Euphorbia Genus. Molecules 2024, 29, 1083. [Google Scholar] [CrossRef]
- Moremi, M.P.; Makolo, F.; Viljoen, A.M.; Kamatou, G.P. A Review of Biological Activities and Phytochemistry of Six Ethnomedicinally Important South African Croton Species. J. Ethnopharmacol. 2021, 280, 114416. [Google Scholar] [CrossRef] [PubMed]
- Aiyelaagbe, O.O.; Hamid, A.A.; Fattorusso, E.; Taglialatela-Scafati, O.; Schröder, H.C.; Müller, W.E.G. Cytotoxic Activity of Crude Extracts as Well as of Pure Components from Jatropha Species, Plants Used Extensively in African Traditional Medicine. Evid.-Based Complement. Altern. Med. 2011, 2011, 134954. [Google Scholar] [CrossRef] [PubMed]
- de S. Fernandes, E.; Rodrigues, F.A.; Tófoli, D.; Imamura, P.M.; de Carvalho, J.E.; Ruiz, A.L.T.G.; Foglio, M.A.; Minguzzi, S.; Silva, R.C.L. Isolation, Structural Identification and Cytotoxic Activity of Hexanic Extract, Cyperenoic Acid, and Jatrophone Terpenes from Jatropha Ribifolia Roots. Rev. Bras. De Farmacogn. 2013, 23, 441–446. [Google Scholar] [CrossRef]
- Shari, K.; El Gedaily, R.A.; Allam, R.M.; Meselhy, K.M.; Khaleel, A.E.; Abdel-Sattar, E. Jatrophone: A Cytotoxic Macrocylic Diterpene Targeting PI3K/AKT/NF-ΚB Pathway, Inducing Apoptosis and Autophagy in Resistant Breast Cancer Cells. BMC Complement. Med. Ther. 2023, 23, 293. [Google Scholar] [CrossRef]
- Zhang, C.-Y.; Zhang, L.-J.; Lu, Z.-C.; Ma, C.-Y.; Ye, Y.; Rahman, K.; Zhang, H.; Zhu, J.-Y. Antitumor Activity of Diterpenoids from Jatropha gossypiifolia: Cell Cycle Arrest and Apoptosis-Inducing Activity in RKO Colon Cancer Cells. J. Nat. Prod. 2018, 81, 1701–1710. [Google Scholar] [CrossRef] [PubMed]
- Sanjai, C.; Hakkimane, S.S.; Guru, B.R.; Gaonkar, S.L. A Comprehensive Review on Anticancer Evaluation Techniques. Bioorg. Chem. 2024, 142, 106973. [Google Scholar] [CrossRef] [PubMed]
- Atanasov, A.G.; Zotchev, S.B.; Dirsch, V.M.; Supuran, C.T. Natural Products in Drug Discovery: Advances and Opportunities. Nat. Rev. Drug Discov. 2021, 20, 200–216. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Izzo, A.A.; Papapetropoulos, A.; Alexander, S.P.H.; Cortese-Krott, M.; Kendall, D.A.; Martemyanov, K.A.; Mauro, C.; Panettieri, R.A.; Patel, H.H.; et al. Natural Product Pharmacology: The British Journal of Pharmacology Perspective. Br. J. Pharmacol. 2024, 181, 3547–3555. [Google Scholar] [CrossRef]
- Wang, M.; Cao, J.; Zhu, J.-Y.; Qiu, J.; Zhang, Y.; Shu, B.; Ou, T.-M.; Tan, J.-H.; Gu, L.-Q.; Huang, Z.-S.; et al. Curcusone C Induces Telomeric DNA-Damage Response in Cancer Cells through Inhibition of Telomeric Repeat Factor 2. Biochim. Et Biophys. Acta (BBA)-Proteins Proteom. 2017, 1865, 1372–1382. [Google Scholar] [CrossRef] [PubMed]
- An, J.; Li, L.; Zhang, X. Curcusone C Induces Apoptosis in Endometrial Cancer Cells via Mitochondria-Dependent Apoptotic and ERK Pathway. Biotechnol. Lett. 2021, 43, 329–338. [Google Scholar] [CrossRef]
- Huang, L.; Ma, B.; Zhang, C.; Shi, J.; Shen, R.; Zhang, E.; Lian, C.; Wang, C.; Liu, J. Unveiling Poly(RC)-Binding Protein 2 as the Target Protein for Curcusone C against Prostate Cancer: Mechanism Validation through Click Chemistry-Activity Based Proteomics Profiling Approach. BMC Cancer 2023, 23, 957. [Google Scholar] [CrossRef]
- Yuan, C.; Chen, M.; Cai, X. Advances in Poly(RC)-Binding Protein 2: Structure, Molecular Function, and Roles in Cancer. Biomed. Pharmacother. 2021, 139, 111719. [Google Scholar] [CrossRef]
- Cao, M.-N.; Zhou, Y.-B.; Gao, A.-H.; Cao, J.-Y.; Gao, L.-X.; Sheng, L.; Xu, L.; Su, M.-B.; Cao, X.-C.; Han, M.; et al. Curcusone D, a Novel Ubiquitin–Proteasome Pathway Inhibitor via ROS-Induced DUB Inhibition, Is Synergistic with Bortezomib against Multiple Myeloma Cell Growth. Biochim. Et Biophys. Acta (BBA)-Gen. Subj. 2014, 1840, 2004–2013. [Google Scholar] [CrossRef]
- Liu, R.; Chen, Y.; Liu, G.; Li, C.; Song, Y.; Cao, Z.; Li, W.; Hu, J.; Lu, C.; Liu, Y. PI3K/AKT Pathway as a Key Link Modulates the Multidrug Resistance of Cancers. Cell Death Dis. 2020, 11, 797. [Google Scholar] [CrossRef]
- Kopecka, J.; Godel, M.; Dei, S.; Giampietro, R.; Belisario, D.C.; Akman, M.; Contino, M.; Teodori, E.; Riganti, C. Insights into P-Glycoprotein Inhibitors: New Inducers of Immunogenic Cell Death. Cells 2020, 9, 1033. [Google Scholar] [CrossRef] [PubMed]
- Vela, F.; Ezzanad, A.; Hunter, A.C.; Macías-Sánchez, A.J.; Hernández-Galán, R. Pharmacological Potential of Lathyrane-Type Diterpenoids from Phytochemical Sources. Pharmaceuticals 2022, 15, 780. [Google Scholar] [CrossRef] [PubMed]
- Fattahian, M.; Ghanadian, M.; Ali, Z.; Khan, I.A. Jatrophane and Rearranged Jatrophane-Type Diterpenes: Biogenesis, Structure, Isolation, Biological Activity and SARs (1984–2019). Phytochem. Rev. 2020, 19, 265–336. [Google Scholar] [CrossRef] [PubMed]
- Sousa, I.J.; Ferreira, M.-J.U.; Molnár, J.; Fernandes, M.X. QSAR Studies of Macrocyclic Diterpenes with P-Glycoprotein Inhibitory Activity. Eur. J. Pharm. Sci. 2013, 48, 542–553. [Google Scholar] [CrossRef]
- Kar, A.; Agarwal, S.; Singh, A.; Bajaj, A.; Dasgupta, U. Insights into Molecular Mechanisms of Chemotherapy Resistance in Cancer. Transl. Oncol. 2024, 42, 101901. [Google Scholar] [CrossRef]
- Nanayakkara, A.K.; Follit, C.A.; Chen, G.; Williams, N.S.; Vogel, P.D.; Wise, J.G. Targeted Inhibitors of P-Glycoprotein Increase Chemotherapeutic-Induced Mortality of Multidrug Resistant Tumor Cells. Sci. Rep. 2018, 8, 967. [Google Scholar] [CrossRef]
- Soto-Sánchez, J. Could Natural Terpenes Be an Alternative for the Treatment of Neglected Tropical Diseases? Chem. Biol. Drug Des. 2024, 103, e14470. [Google Scholar] [CrossRef]
- Barros de Alencar, M.V.O.; de Castro e Sousa, J.M.; Rolim, H.M.L.; das Graças Freire de Medeiros, M.; Cerqueira, G.S.; de Castro Almeida, F.R.; das Graças Lopes Citó, A.M.; Ferreira, P.M.P.; Lopes, J.A.D.; de Carvalho Melo-Cavalcante, A.A.; et al. Diterpenes as Lead Molecules against Neglected Tropical Diseases. Phytother. Res. 2017, 31, 175–201. [Google Scholar] [CrossRef]
- Valli, M.; Döring, T.H.; Marx, E.; Ferreira, L.L.G.; Medina-Franco, J.L.; Andricopulo, A.D. Neglected Tropical Diseases: A Chemoinformatics Approach for the Use of Biodiversity in Anti-Trypanosomatid Drug Discovery. Biomolecules 2024, 14, 1033. [Google Scholar] [CrossRef]
- Luna, I.S.; de Souza, T.A.; da Silva, M.S.; da Franca Rodrigues, K.A.; Scotti, L.; Scotti, M.T.; Mendonça-Junior, F.J.B. Computer-Aided Drug Design of New 2-Amino-Thiophene Derivatives as Anti-Leishmanial Agents. Eur. J. Med. Chem. 2023, 250, 115223. [Google Scholar] [CrossRef]
- Sülsen, V.P. Sesquiterpene Lactones and Diterpenes: Promising Therapeutic Candidates for Infectious Diseases, Neoplasms and Other Chronic Disorders. Molecules 2021, 26, 1251. [Google Scholar] [CrossRef] [PubMed]
- Calixto, J.B.; Sant’Ana, A.E.G. Pharmacological Analysis of the Inhibitory Effect of Jatrophone, a Diterpene Isolated from Jatropha elliptica, on Smooth and Cardiac Muscles. Phytother. Res. 1987, 1, 122–126. [Google Scholar] [CrossRef]
- Silva, A.M.; Brum, R.L.; Calixto, J.B. The Relaxant Action of Jatrophone in Rat Portal Vein. A Comparison with Protein Kinase C Inhibitors. Life Sci. 1995, 57, 863–871. [Google Scholar] [CrossRef] [PubMed]
- Calixto, J.B.; Sant’ana, A.E.G. Evidence for the Mechanism of the Inhibitory Action of Jatrophone in the Isolated Rat Uterine Muscle. Gen. Pharmacol. Vasc. Syst. 1990, 21, 117–122. [Google Scholar] [CrossRef]
- Bizzarri, M.; Giuliani, A.; Monti, N.; Verna, R.; Pensotti, A.; Cucina, A. Rediscovery of Natural Compounds Acting via Multitarget Recognition and Noncanonical Pharmacodynamical Actions. Drug Discov. Today 2020, 25, 920–927. [Google Scholar] [CrossRef]
- Zhang, W.; Pei, J.; Lai, L. Computational Multitarget Drug Design. J. Chem. Inf. Model. 2017, 57, 403–412. [Google Scholar] [CrossRef]
- Abdelgadir, H.A.; Van Staden, J. Ethnobotany, Ethnopharmacology and Toxicity of Jatropha curcas L. (Euphorbiaceae): A Review. S. Afr. J. Bot. 2013, 88, 204–218. [Google Scholar] [CrossRef]
- Sabandar, C.W.; Ahmat, N.; Jaafar, F.M.; Sahidin, I. Medicinal Property, Phytochemistry and Pharmacology of Several Jatropha Species (Euphorbiaceae): A Review. Phytochemistry 2013, 85, 7–29. [Google Scholar] [CrossRef]
- Patridge, E.; Gareiss, P.; Kinch, M.S.; Hoyer, D. An Analysis of FDA-Approved Drugs: Natural Products and Their Derivatives. Drug Discov. Today 2016, 21, 204–207. [Google Scholar] [CrossRef]
- Newman, D.J.; Cragg, G.M. Natural Products as Sources of New Drugs over the Nearly Four Decades from 01/1981 to 09/2019. J. Nat. Prod. 2020, 83, 770–803. [Google Scholar] [CrossRef]
- Lipinski, C.A.; Lombardo, F.; Dominy, B.W.; Feeney, P.J. Experimental and Computational Approaches to Estimate Solubility and Permeability in Drug Discovery and Development Settings 1PII of Original Article: S0169-409X(96)00423-1. The Article Was Originally Published in Advanced Drug Delivery Reviews 23 (1997) 3–25. 1. Adv. Drug Deliv. Rev. 2001, 46, 3–26. [Google Scholar] [CrossRef] [PubMed]
- Bickerton, G.R.; Paolini, G.V.; Besnard, J.; Muresan, S.; Hopkins, A.L. Quantifying the Chemical Beauty of Drugs. Nat. Chem. 2012, 4, 90–98. [Google Scholar] [CrossRef] [PubMed]
- Camp, D.; Davis, R.A.; Campitelli, M.; Ebdon, J.; Quinn, R.J. Drug-like Properties: Guiding Principles for the Design of Natural Product Libraries. J. Nat. Prod. 2012, 75, 72–81. [Google Scholar] [CrossRef]
- Shultz, M.D. Two Decades under the Influence of the Rule of Five and the Changing Properties of Approved Oral Drugs. J. Med. Chem. 2019, 62, 1701–1714. [Google Scholar] [CrossRef] [PubMed]
- Domingo-Fernández, D.; Gadiya, Y.; Mubeen, S.; Healey, D.; Norman, B.H.; Colluru, V. Exploring the Known Chemical Space of the Plant Kingdom: Insights into Taxonomic Patterns, Knowledge Gaps, and Bioactive Regions. J. Cheminform. 2023, 15, 107. [Google Scholar] [CrossRef]
- Talevi, A.; Bellera, C.L. Clustering of Small Molecules: New Perspectives and Their Impact on Natural Product Lead Discovery. Front. Nat. Prod. 2024, 3, 1367537. [Google Scholar] [CrossRef]
- Obende, S.O.; Ochieng, C.O.; Shikanga, E.A.; Cruz, J.N.; Santos, C.B.R.; Kimani, N.M. Croton’s Therapeutic Promise: A Review of Its Phytochemistry and Critical Computational ADME/Tox Analysis. S. Afr. J. Bot. 2024, 171, 648–672. [Google Scholar] [CrossRef]
- Parker, P.J.; Brown, S.J.; Calleja, V.; Chakravarty, P.; Cobbaut, M.; Linch, M.; Marshall, J.J.T.; Martini, S.; McDonald, N.Q.; Soliman, T.; et al. Equivocal, Explicit and Emergent Actions of PKC Isoforms in Cancer. Nat. Rev. Cancer 2021, 21, 51–63. [Google Scholar] [CrossRef]
- Otsuki, K.; Li, W. Tigliane and Daphnane Diterpenoids from Thymelaeaceae Family: Chemistry, Biological Activity, and Potential in Drug Discovery. J. Nat. Med. 2023, 77, 625–643. [Google Scholar] [CrossRef]
- Schmeda-Hirschrnann, G.; Razmilic, I.; Sauvain, M.; Moretti, C.; Muiioz, V.; Ruiz, E.; Balanza, E.; Fournet, A. Antiprotozoal Activity of Jatrogrossidione From Jatropha grossidentata and Jatrophone from Jatropha isabellii. Phytother. Res. 1996, 10, 375–378. [Google Scholar] [CrossRef]
- Wang, Y.; Zhou, D.; Meng, Q.; Xu, L.; Yao, X.; Ni, X.; Xie, H.; Wu, G.; Chen, G.; Hou, Y.; et al. Anti-Neuroinflammatory Effects in Vitro and in Vivo, and Chemical Profile of Jatropha curcas L. Bioorg. Chem. 2022, 122, 105720. [Google Scholar] [CrossRef] [PubMed]
- Chokchaisiri, R.; Srijun, J.; Chaichompoo, W.; Cheenpracha, S.; Ganranoo, L.; Suksamrarn, A. Anti-Herpes Simplex Type-1 (HSV-1) Activity from the Roots of Jatropha multifida L. Med. Chem. Res. 2020, 29, 328–333. [Google Scholar] [CrossRef]
- Gao, J.; Chen, Q.-B.; Liu, Y.-Q.; Xin, X.-L.; Yili, A.; Aisa, H.A. Diterpenoid Constituents of Euphorbia macrorrhiza. Phytochemistry 2016, 122, 246–253. [Google Scholar] [CrossRef] [PubMed]
- Aati, H.Y.; El-Gamal, A.A.; Kayser, O.; Ahmed, A.F. The Phytochemical and Biological Investigation of Jatropha Pelargoniifolia Root Native to the Kingdom of Saudi Arabia. Molecules 2018, 23, 1892. [Google Scholar] [CrossRef]
- Muangman, S.; Thippornwong, M.; Tohtong, R. Anti-Metastatic Effects of Curcusone B, a Diterpene from Jatropha curcas. In Vivo 2005, 19, 265–268. [Google Scholar]
- Sahidin, N.; Sauba, T.; Muhammad, K.S.; Anil, A.I.; Solachuddin, J.A. Antiproliferative Activity of Curcusone B from Jatropha curcas on Human Cancer Cell Lines. Aust. J. Basic Appl. Sci. 2011, 5, 47–51. [Google Scholar]
- Sahidin, Y.; Ginting, S.; Manggau, M.A. Lukman Cytotoxic Potency of Diterpenes from Jatropha Plants. Int. J. Pharm. Pharm. Sci. 2013, 5, 417–420. [Google Scholar]
- Roach, J.S.; Devappa, R.K.; Makkar, H.P.S.; Becker, K. Isolation, Stability and Bioactivity of Jatropha curcas Phorbol Esters. Fitoterapia 2012, 83, 586–592. [Google Scholar] [CrossRef]
- Menezes, F.V.; Carneiro, E.M.; Delattre, E.; Boschero, A.C. Inhibition of Insulin Release by Jatrophone. Braz. J. Med. Biol. Res. 1992, 25, 305–307. [Google Scholar]
- dos Santos, A.F.; Sant’Ana, A.E. Molluscicidal Activity of the Diterpenoids Jatrophone and Jatropholones A and B Isolated from Jatropha elliptica (Pohl) Muell. Arg. Phytother. Res. 1999, 13, 660–664. [Google Scholar] [CrossRef]
- Duarte, D.F.P.; Sant’Ana, A.E.G.; Calixto, J.B. Analysis of the Vasorelaxant Action of Jatrophone in the Isolated Aorta of the Rat: Influence of Potassium Channel Blockers. Eur. J. Pharmacol. 1992, 215, 75–81. [Google Scholar] [CrossRef] [PubMed]
- de Moraes, V.L.G.; Rumjanek, V.M.; Calixto, J.B. Jatrophone and 12-O-Tetradecanoyl Phorbol-13-Acetate Antagonism of Stimulation of Natural Killer Activity and Lymphocyte Proliferation. Eur. J. Pharmacol. 1996, 312, 333–339. [Google Scholar] [CrossRef] [PubMed]
- Dutra, F.V.; Calixto, J.B.; Medeiros, Y.S.; Brum, R. Jatrophone Inhibits Platelet Rich Plasma Aggregation from Man, Rat and Guinea-Pig Induced by Different Agents. Phytother. Res. 1996, 10, 271–273. [Google Scholar] [CrossRef]
- Fatima, I.; El-Ayachi, I.; Taotao, L.; Lillo, M.A.; Krutilina, R.; Seagroves, T.N.; Radaszkiewicz, T.W.; Hutnan, M.; Bryja, V.; Krum, S.A.; et al. The Natural Compound Jatrophone Interferes with Wnt/β-Catenin Signaling and Inhibits Proliferation and EMT in Human Triple-Negative Breast Cancer. PLoS ONE 2017, 12, e0189864. [Google Scholar] [CrossRef] [PubMed]
- Martini, L.H.; Souza, C.R.; Marques, P.B.; Calixto, J.B.; Yunes, R.A.; Souza, D.O. Compounds Extracted from Phyllantus and Jatropha Elliptica Inhibit the Binding of [3H]Glutamate and [3H]GMP-PNP in Rat Cerebral Cortex Membrane. Neurochem. Res. 2000, 25, 211–215. [Google Scholar] [CrossRef]
- Li, K.; Xu, Y.; Yue, W. Anti-viral Activity of Jatrophone against RSV-induced Respiratory Infection via Increase in Interferon-γ Generating Dendritic Cells. Environ. Toxicol. 2020, 35, 888–894. [Google Scholar] [CrossRef]
- Theoduloz, C.; Rodríguez, J.; Pertino, M.; Schmeda-Hirschmann, G. Antiproliferative Activity of the Diterpenes Jatrophone and Jatropholone and Their Derivatives. Planta Med. 2009, 75, 1520–1522. [Google Scholar] [CrossRef]
- Coelho, I.P.; Dos Santos, L.B.B.; Kato Junior, W.H.; Corsino, J.; Cordeiro, K.W.; Boeing, T.; Coelho, J.M.; Garcez, F.R.; Garcez, W.S.; de Andrade, S.F.; et al. Chemical Profile and Gastroprotective Effect of Jatropha elliptica (Pohl) Oken Roots. Fitoterapia 2020, 146, 104707. [Google Scholar] [CrossRef]
- Huang, J.-D.; Wang, C.-F.; Lian, C.-L.; Huang, M.-Y.; Zhang, C.; Liu, J.-Q. Isolation and Identification of Five New Diterpenoids from Jatropha curcas. Phytochem. Lett. 2020, 40, 37–41. [Google Scholar] [CrossRef]
- Gunasekera, S.P.; Cordell, G.A.; Farnsworth, N.R. Potential Anticancer Agents. XIV. Isolation of Spruceanol and Montanin From Cunuria spruceana (Euphorbiaceae). J. Nat. Prod. 1979, 42, 658–662. [Google Scholar] [CrossRef]
- Maslovskaya, L.A.; Savchenko, A.I.; Gordon, V.A.; Reddell, P.W.; Pierce, C.J.; Parsons, P.G.; Williams, C.M. Isolation and Confirmation of the Proposed Cleistanthol Biogentic Link from Croton insularis. Org. Lett. 2011, 13, 1032–1035. [Google Scholar] [CrossRef] [PubMed]
- Su, D.; Yang, X.-Y.; Feng, X.; Shang, M.-Y.; Cai, S.-Q. The Diterpenes Ovoideal A–G from Tirpitzia Ovoidea. Molecules 2014, 19, 18966–18979. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Li, S.; Zhao, Y.; Ni, W.; Hao, X.; Li, J.; Hua, Y.; Xie, B.; Qing, C.; Chen, C. Four New Podocarpane-Type Trinorditerpenes from Aleurites moluccana. Helv. Chim. Acta 2007, 90, 2017–2023. [Google Scholar] [CrossRef]
- Sutthivaiyakit, S.; Nakorn, N.N.; Kraus, W.; Sutthivaiyakit, P. A Novel 29-nor-3,4-Seco-Friedelane Triterpene and a New Guaiane Sesquiterpene from the Roots of Phyllanthus oxyphyllus. Tetrahedron 2003, 59, 9991–9995. [Google Scholar] [CrossRef]
- Yuan, Y.-R.; Li, Y.-W.; Huang, Y.-Q.; Liu, Q.; Ren, Y.-H.; Yue, J.-M.; Zhou, B. Four New Diterpenoids from the Twigs and Leaves of Phyllanthus Acidus. Tetrahedron 2021, 91, 132224. [Google Scholar] [CrossRef]
- Sun, P.; Cao, D.-H.; Xiao, Y.-D.; Zhang, Z.-Y.; Wang, J.-N.; Shi, X.-C.; Xiao, C.-F.; Hu, H.-B.; Xu, Y.-K. Aspidoptoids A–D: Four New Diterpenoids from Aspidopterys Obcordata Vine. Molecules 2020, 25, 529. [Google Scholar] [CrossRef]
- Huang, D.; Luo, X.; Yin, Z.; Xu, J.; Gu, Q. Diterpenoids from the Aerial Parts of Flueggea Acicularis and Their Activity against RANKL-Induced Osteoclastogenesis. Bioorg. Chem. 2020, 94, 103453. [Google Scholar] [CrossRef]
- Kaemchantuek, P.; Chokchaisiri, R.; Prabpai, S.; Kongsaeree, P.; Chunglok, W.; Utaipan, T.; Chamulitrat, W.; Suksamrarn, A. Terpenoids with Potent Antimycobacterial Activity against Mycobacterium tuberculosis from Trigonostemon reidioides Roots. Tetrahedron 2017, 73, 1594–1601. [Google Scholar] [CrossRef]
- Chao, C.-H.; Cheng, J.-C.; Shen, D.-Y.; Wu, T.-S. Anti-Hepatitis C Virus Dinorditerpenes from the Roots of Flueggea virosa. J. Nat. Prod. 2014, 77, 22–28. [Google Scholar] [CrossRef]
- Zhu, G.; Wang, H.; Gan, K.; Gao, H.; Li, W. A New Purine Derivative from the Roots of Phyllanthus flexuosus. Nat. Prod. Commun. 2018, 13. [Google Scholar] [CrossRef]
- Yang, H.-Y.; Huang, P.-Z.; Ma, Q.; Sun, Y.; Feng, W.-J.; He, Y.-L.; Chen, J.-J.; Gao, K. Anti-Inflammatory Ent-Cleistanthane-Type Diterpenoids from Phyllanthus Rheophyticus. Phytochemistry 2023, 212, 113723. [Google Scholar] [CrossRef] [PubMed]
- Krebsa, H.C.; Duddeck, H.; Malik, S.; Beil, W.; Rasoanaivo, P.; Andrianarijaona, M. Chemical Composition and Antitumor Activities from Givotia madagascariensis. Z. Für Naturforschung B 2004, 59, 58–62. [Google Scholar] [CrossRef]
- Zhao, J.-Q.; Lv, J.-J.; Wang, Y.-M.; Xu, M.; Zhu, H.-T.; Wang, D.; Yang, C.-R.; Wang, Y.-F.; Zhang, Y.-J. Phyllanflexoid C: First Example of Phenylacetylene-Bearing 18-nor-Diterpenoid Glycoside from the Roots of Phyllanthus flexuosus. Tetrahedron Lett. 2013, 54, 4670–4674. [Google Scholar] [CrossRef]
- Wu, P.-Q.; Cui, Y.-S.; Han, X.-Y.; Wang, C.; An, P.-P.; Zhou, J.-S.; Ren, Y.-H.; Liu, Z.-L.; Lin, R.-T.; Zhou, B.; et al. Diterpenoids from Sauropus spatulifolius Leaves with Antimicrobial Activities. J. Nat. Prod. 2022, 85, 1304–1314. [Google Scholar] [CrossRef] [PubMed]
- Silva-Mares, D.; Torres-López, E.; Rivas-Estilla, A.M.; Cordero-Pérez, P.; Waksman-Minsky, N.; Rivas-Galindo, V.M. Plants from Northeast Mexico with Anti-HSV Activity. Nat. Prod. Commun. 2013, 8. [Google Scholar] [CrossRef]
- Torrance, S.J.; Wiedhopf, R.M.; Cole, J.R.; Arora, S.K.; Bates, R.B.; Beavers, W.A.; Cutler, R.S. Antitumor Agents from Jatropha macrorhiza (Euphorbiaceae). II. Isolation and Characterization of Jatrophatrione. J. Org. Chem. 1976, 41, 1855–1857. [Google Scholar] [CrossRef]
- Bautista, E.; Lozano-Gamboa, S.; Fragoso-Serrano, M.; Rivera-Chávez, J.; Salazar-Olivo, L.A. Jatrophenediol, a Pseudoguaiane Sesquiterpenoid from Jatropha dioica Rhizomes. Tetrahedron Lett. 2022, 104, 154040. [Google Scholar] [CrossRef]
- Falodun, A.; Sheng-Xiang, Q.; Parkinson, G.; Gibbons, S. Isolation and Characterization of a New Anticancer Diterpenoid from Jatropha gossypifolia. Pharm. Chem. J 2012, 45, 636–639. [Google Scholar] [CrossRef]
- Dekker, T.; Fourie, T.; Mathee E, S.; Snyckers, F. Jaherin, a New Daphthanane Diterpene with Antimicrobial Properties from Jatropha zeyheri. S. Afr. J. Chem 1987, 40, 74–76. [Google Scholar]




| Compound | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 |
| C1 | 34.4 | 36.0 | 40.4 | 36.5 | 51.7 | 37.0 | 34.5 | 35.9 | 37.3 | 44.4 | 149.6 | 42.0 | 128.6 | 156.0 |
| C2 | 27.3 | 27.4 | 67.2 | 71.6 | 210.6 | 27.9 | 34.1 | 24.0 | 27.9 | 72.5 | 138.1 | 40.2 | 148.5 | 140.5 |
| C3 | 78.2 | 78.3 | 79.0 | 76.8 | 82.7 | 78.8 | 217.8 | 79.8 | 78.8 | 79.4 | 207.1 | 82.9 | 80.0 | 211.9 |
| C4 | 39.3 | 39.6 | 38.5 | 38.7 | 44.9 | 38.9 | 49.0 | 37.7 | 38.9 | 39.3 | 45.4 | 145.1 | 145.2 | 47.6 |
| C5 | 47.9 | 53.1 | 43.1 | 43.1 | 49.2 | 49.8 | 138.7 | 49.0 | 49.3 | 51.1 | 49.2 | 134.5 | 137.5 | 79.2 |
| C6 | 24.0 | 17.9 | 18.6 | 18.5 | 19.1 | 18.9 | 144.5 | 35.3 | 19.0 | 20.0 | 28.5 | 74.5 | 73.6 | 73.6 |
| C7 | 131.7 | 33.7 | 30.2 | 30.1 | 29.4 | 29.7 | 181.4 | 197.6 | 29.2 | 30.4 | 32.5 | 41.8 | 42.1 | 41.3 |
| C8 | 131.0 | 47.0 | 134.8 | 134.9 | 126.7 | 126.8 | 121.7 | 125.3 | 125.0 | 124.4 | 124.7 | 19.3 | 19.4 | 18.9 |
| C9 | 164.2 | 172.7 | 146.4 | 146.2 | 145.8 | 121.2 | 152.9 | 123.6 | 147.6 | 149.5 | 132.3 | 27.1 | 27.3 | 28.6 |
| C10 | 37.3 | 41.4 | 38.7 | 38.8 | 43.4 | 37.3 | 41.0 | 38.0 | 37.5 | 38.3 | 136.3 | 17.5 | 17.2 | 16.0 |
| C11 | 116.7 | 122.0 | 124.2 | 124.1 | 110.3 | 110.6 | 112.5 | 104.0 | 109.7 | 110.8 | 117.5 | 19.0 | 19.2 | 19.9 |
| C12 | 202.2 | 88.7 | 126.8 | 126.8 | 152.2 | 148.3 | 162.7 | 162.6 | 152.1 | 154.3 | 153.0 | 28.1 | 28.5 | 28.3 |
| C13 | 73.1 | 131.4 | 135.1 | 135.2 | 122.2 | 151.9 | 126.8 | 155.6 | 119.3 | 119.3 | 132.6 | 38.9 | 38.1 | 43.8 |
| C14 | 55.5 | 150.9 | 129.8 | 129.9 | 131.5 | 131.1 | 130.5 | 129.8 | 139.1 | 140.0 | 130.5 | 212.8 | 212.3 | 210.5 |
| C15 | 138.9 | 140.0 | - | - | 15.2 | 15.3 | 27.1 | 16.1 | 135.1 | 137.0 | 22.6 | 83 | 87.8 | 51.5 |
| C16 | 117.9 | 113.7 | - | - | - | - | - | - | 119.5 | 120.4 | - | 16.9 | 13.9 | 10.1 |
| C17 | 14.0 | 15.2 | 21.0 | 21.0 | - | - | - | - | 12.9 | 13.3 | - | 29.7 | 29.3 | 26.0 |
| C18 | 27.4 | 28.3 | 28.5 | 28.4 | 29.2 | 28.1 | 21.8 | 27.5 | 28.1 | 30.2 | 19.8 | 28.5 | 28.8 | 28.9 |
| C19 | 15.5 | 15.6 | 21.8 | 21.9 | 16.2 | 15.3 | 25.2 | 15.6 | 15.4 | 17.3 | 15.4 | 14.9 | 14.9 | 15.0 |
| C20 | 20.7 | 21.4 | 25.8 | 25.7 | 25.9 | 24.8 | 16.3 | 23.4 | 24.9 | 27.4 | 122.5 | 16.9 | 16.6 | 13.4 |
| -OCH3 | - | - | - | - | - | - | - | 55.6 | - | - | - | - | - | - |
| -OCOCH3 | - | - | - | - | - | - | - | 170.8 | - | - | - | - | - | - |
| -OCOCH3 | - | - | - | - | - | - | - | 21.2 | - | - | - | - | - | - |
| Solvent | CDCl3 125 MHz | CDCl3 125 MHz | CDCl3 100 MHz | CDCl3 100 MHz | CDCl3 100 MHz | CDCl3 125 MHz | CD3OD 125 MHZ | CDCl3 125 MHZ | CDCl3 125 MHz | CDCl3 125 MHz | CDCl3-CD3OD 50 MHz | CDCl3 100 MHz | CDCl3 100 MHz | Pyr-d5 N. I |
| Reference | [61] | [61] | [62] | [62] | [63] | [64] | [65] | [64] | [61] | [61] | [66] | [67] | [67] | [68] |
| Compound | 15 | 16 | 17 | 18 | 19 | 20 | 21 | 22 | 23 | 24 | 25 | 26 | 27 | 28 |
| C1 | 154.3 | 160.2 | 151.8 | 38.6 | 38.7 | 38.5 | 42.3 | 42.5 | 155.7 | 64.0 | 152.7 | 36.6 | 36.4 | 43.1 |
| C2 | 140.4 | 140.3 | 145.4 | 39.7 | 39.6 | 39.7 | 75.5 | 75.2 | 143.6 | 62.5 | 142.7 | 43.6 | 43.5 | 75.4 |
| C3 | 210.5 | 213.2 | 195.7 | 87.5 | 87.7 | 87.5 | 208.8 | 208.4 | 195.5 | 195.6 | 203.4 | 208.9 | 208.9 | 217.3 |
| C4 | 47.8 | 47.9 | 139.9 | 148.3 | 149.1 | 148.8 | 162.2 | 164.8 | 137.4 | 134.3 | 62.4 | 167.2 | 166.3 | 164.9 |
| C5 | 34.9 | 33.4 | 149.9 | 63.6 | 63.6 | 63.6 | 60.6 | 61.6 | 141.9 | 151.5 | 57.7 | 56.3 | 56.2 | 55.8 |
| C6 | 71.5 | 71.6 | 75.9 | 65.2 | 65.4 | 65.2 | 63.1 | 63.8 | 74.9 | 75.6 | 58.6 | 60.4 | 60.4 | 60.8 |
| C7 | 41.0 | 37.8 | 41.4 | 37.9 | 37.6 | 37.9 | 36.9 | 37.0 | 42.9 | 41.9 | 41.0 | 40.5 | 40.5 | 40.3 |
| C8 | 17.5 | 20.9 | 19.6 | 19.1 | 19.1 | 18.8 | 19.2 | 19.3 | 20.5 | 19.3 | 18.4 | 19.5 | 19.5 | 19.5 |
| C9 | 27.1 | 35.8 | 38.1 | 35.1 | 36.2 | 30.8 | 36.9 | 37.3 | 26.0 | 26.9 | 29.1 | 28.3 | 28.3 | 28.3 |
| C10 | 15.7 | 28.8 | 26.9 | 24.6 | 26.3 | 30.1 | 26.8 | 27.6 | 17.3 | 17.7 | 16.5 | 18.0 | 18.0 | 18.1 |
| C11 | 20.0 | 28.6 | 28.9 | 27.7 | 27.9 | 23.9 | 29.1 | 29.2 | 21.5 | 19.2 | 18.7 | 23.5 | 23.4 | 23.4 |
| C12 | 28.2 | 149.5 | 153.5 | 137.6 | 142.8 | 135.0 | 145.8 | 148.4 | 24.9 | 28.7 | 24.3 | 26.6 | 26.4 | 26.5 |
| C13 | 40.3 | 132.5 | 128.4 | 137.9 | 140.8 | 138.8 | 137.8 | 137.7 | 30.5 | 41.2 | 37.6 | 40.6 | 40.3 | 43.6 |
| C14 | 213.3 | 199.3 | 199.0 | 195.0 | 194.4 | 195.2 | 192.5 | 192.1 | 81.5 | 209.2 | 214.0 | 208.3 | 207.3 | 207.6 |
| C15 | 85.9 | 81.8 | 82.6 | 141.5 | 141.9 | 141.4 | 136.2 | 135.2 | 79.3 | 82.3 | 86.4 | 136.5 | 135.7 | 134.1 |
| C16 | 9.9 | 10.1 | 10.8 | 18.2 | 18.3 | 18.2 | 26.1 | 25.0 | 10.5 | 10.1 | 10.2 | 17.2 | 15.7 | 26.2 |
| C17 | 29.0 | 29.0 | 25.5 | 17.8 | 17.9 | 17.8 | 17.4 | 17.1 | 29.5 | 28.9 | 18.3 | 18.5 | 18.3 | 18.5 |
| C18 | 28.9 | 29.1 | 28.5 | 29.0 | 29.0 | 71.9 | 29.1 | 29.9 | 28.7 | 28.3 | 28.2 | 28.8 | 28.8 | 28.8 |
| C19 | 15.1 | 16.4 | 15.5 | 16.1 | 16.2 | 11.8 | 16.4 | 16.5 | 14.5 | 14.9 | 15.4 | 15.0 | 15.0 | 15.0 |
| C20 | 15.4 | 11.7 | 12.0 | 13.6 | 58.6 | 13.8 | 12.7 | 12.5 | 21.3 | 17.0 | 14.6 | 17.8 | 17.6 | 17.1 |
| Solvent | Pyr-d5 75 MHz | CDCl3 100 MHZ | CDCl3 100 MHz | CDCl3 100 MHz | CDCl3 100 MHz | CDCl3 100 MHz | CDCl3 100 MHz | CDCl3 100 MHz | CDCl3 100 MHz | CDCl3 100 MHz | CDCl3 100 MHz | CDCl3 100 MHz | CDCl3-DMSO-d6 (9:1) 100 MHz | CDCl3 100 MHz |
| Reference | [69] | [70] | [70] | [70] | [70] | [70] | [70] | [70] | [71] | [72] | [73] | [73] | [73] | [73] |
| Compound | 29 | 30 | 31 | 32 | 33 | 34 | 35 | 36 | 37 | 38 | 39 | 40 | 41 | 42 |
| C1 | 43.5 | 150.6 | 149.5 | 151.0 | 149.9 | 151.5 | 155.5 | 42.0 | 146.6 | 156.4 | 151.6 | 155.3 | 157.5 | 154.8 |
| C2 | 75.5 | 149.2 | 146.8 | 145.3 | 148.7 | 146.6 | 143.8 | 40.4 | 149.2 | 144.8 | 144.9 | 141.3 | 139.5 | 141.5 |
| C3 | 208.8 | 195.7 | 196.4 | 195.4 | 194.2 | 195.7 | 195.7 | 82.9 | 194.1 | 200.9 | 197.3 | 197.9 | 199.7 | 207.9 |
| C4 | 166.7 | 132.2 | 134.6 | 132.0 | 133.1 | 137.0 | 137.6 | 145.3 | 130.4 | 70.4 | 134.0 | 54.7 | 55.1 | 54.5 |
| C5 | 56.2 | 145.5 | 153.7 | 145.8 | 149.0 | 144.0 | 141.8 | 134.6 | 140.8 | 75.0 | 145.0 | 117.3 | 120.0 | 117.9 |
| C6 | 61.0 | 73.5 | 73.9 | 84.4 | 75.7 | 76.0 | 74.6 | 74.5 | 75.0 | 73.9 | 75.0 | 146.7 | 144.7 | 146.1 |
| C7 | 40.5 | 43.6 | 43.5 | 36.9 | 41.8 | 41.5 | 42.7 | 41.6 | 83.1 | 37.85 | 42.9 | 36.9 | 37.2 | 36.3 |
| C8 | 19.4 | 18.1 | 19.0 | 18.1 | 19.0 | 19.5 | 20.6 | 19.5 | 22.8 | 20.7 | 20.5 | 28.6 | 28.8 | 27.8 |
| C9 | 28.6 | 27.4 | 24.8 | 27.1 | 24.0 | 26.9 | 26.2 | 27.3 | 25.8 | 37.88 | 28.5 | 34.9 | 30.4 | 32.9 |
| C10 | 18.1 | 17.8 | 16.9 | 17.5 | 17.4 | 17.9 | 17.0 | 17.8 | 16.3 | 27.7 | 17.2 | 25.6 | 31.4 | 30.2 |
| C11 | 23.0 | 20.1 | 22.1 | 20.1 | 20.9 | 19.4 | 21.5 | 19.3 | 20.3 | 28.3 | 20.9 | 30.1 | 26.5 | 30.7 |
| C12 | 26.1 | 30.0 | 28.3 | 29.5 | 27.9 | 29.6 | 24.8 | 28.4 | 27.5 | 151.3 | 29.6 | 152.3 | 150.0 | 146.1 |
| C13 | 43.9 | 38.6 | 37.4 | 43.6 | 38.1 | 38.2 | 30.5 | 38.9 | 36.6 | 132.2 | 38.9 | 131.9 | 132.5 | 134.5 |
| C14 | 207.0 | 213.5 | 210.8 | 212.0 | 211.1 | 210.9 | 81.4 | 210.6 | 92.3 | 195.0 | 209.8 | 208.5 | 208.5 | 198.1 |
| C15 | 133.2 | 85.0 | 84.1 | 85.3 | 83.3 | 84.2 | 79.8 | 84.6 | 68.0 | 79.2 | 59.6 | 84.9 | 84.9 | 84.7 |
| C16 | 25.0 | 10.7 | 10.8 | 10.7 | 10.9 | 10.9 | 10.4 | 17.2 | 11.2 | 11.3 | 11.0 | 10.7 | 12.3 | 10.6 |
| C17 | 18.2 | 29.2 | 29.0 | 23.7 | 29.1 | 29.0 | 29.3 | 29.8 | 29.2 | 20.7 | 31.2 | 20.5 | 20.5 | 20.4 |
| C18 | 28.7 | 28.8 | 28.7 | 29.0 | 28.7 | 28.3 | 28.5 | 28.5 | 28.0 | 28.5 | 28.6 | 29.3 | 70.8 | - |
| C19 | 15.1 | 14.9 | 15.5 | 14.9 | 15.7 | 14.9 | 14.3 | 14.6 | 15.5 | 16.0 | 15.3 | 16.4 | 12.6 | 10.2 |
| C20 | 17.1 | 16.7 | 19.4 | 16.5 | 18.6 | 16.9 | 21.4 | 17.2 | 11.5 | 11.6 | 18.1 | 11.8 | 10.5 | 12.1 |
| -OCH3 | - | - | - | - | - | - | - | - | - | - | - | - | ||
| -OCOCH3 | - | - | - | 170.1 | - | - | - | - | - | - | - | - | - | 175.6 |
| -OCOCH3 | - | - | - | 22.1 | - | - | - | - | - | - | - | - | - | 52.5 |
| Solvent | CDCl3 100 MHz | CDCl3 100 MHz | CDCl3 100 MHz | CDCl3 100 MHz | CDCl3 100 MHz | CDCl3 100 MHz | CDCl3 100 MHz | CDCl3 100 MHz | Pyr-d5 150 MHz | CDCl3 150 MHz | Pyr-d5 150 MHz | CDCl3 150 MHz | Pyr-d5 150 MHz | CDCl3 125 Mhz |
| Reference | [73] | [73] | [73] | [73] | [73] | [73] | [74] | [74] | [75] | [75] | [75] | [75] | [75] | [76] |
| Compound | 43 | 44 | 45 | 46 | 47 | 48 | 49 | 50 | 51 | 52 | 53 | 54 | 55 | 56 |
| C1 | 154.2 | 152.2 | 147.6 | 35.6 | 159.8 | 159.8 | 39.0 | N.I | N.I | 39.1 | 39.0 | 171.9 | 38.6 | 171.0 |
| C2 | 140.2 | 149.4 | 129.3 | 40.4 | 143.8 | 143.8 | 75.4 | N.I | N.I | 76.5 | 76.4 | 131.0 | 203.5 | 170.4 |
| C3 | 211.1 | 199.0 | 194.9 | 208.2 | 210.2 | 210.2 | 208.5 | 146.7 | 164.9 | 212.9 | 213.1 | 60.3 | 168.1 | 82.0 |
| C4 | 45.6 | 61.2 | 136.1 | 130.7 | 53.9 | 53.9 | 151.0 | N.I | N.I | 155.5 | 155.3 | 123.8 | 145.2 | 121.3 |
| C5 | 89.2 | 63.5 | 135.7 | 57.7 | 71.8 | 71.8 | 44.8 | N.I | 55.7 | 70.3 | 70.7 | 138.1 | 55.0 | 137.6 |
| C6 | 74.0 | 70.9 | 74.2 | 60.4 | 75.1 | 75.1 | 152.7 | N.I | n.i | 50.2 | 50.1 | 73.7 | 88.8 | 73.7 |
| C7 | 40.0 | 84.7 | 84.8 | 40.5 | 41.2 | 41.2 | 40.4 | 41.6 | n.i | 32.5 | 32.4 | 42.2 | 38.8 | 45.7 |
| C8 | 17.9 | 23.5 | 22.8 | 19.5 | 16.1 | 16.1 | 26.8 | 29.7 | 26.5 | 19.6 | 19.9 | 20.0 | 20.1 | 20.1 |
| C9 | 27.7 | 25.9 | 27.1 | 28.6 | 28.7 | 28.7 | 27.1 | 195.8 | 207.6 | 27.6 | 27.6 | 27.0 | 24.9 | 26.77 |
| C10 | 16.1 | 16.5 | 17.6 | 18.2 | 17.1 | 17.1 | 20.0 | N.I | N.I | 20.5 | 20.5 | 18.4 | 19.7 | 18.9 |
| C11 | 19.3 | 20.1 | 21.8 | 23.6 | 19.4 | 19.4 | 27.2 | 38.2 | 43.6 | 22.2 | 22.2 | 21.8 | 27.7 | 21.8 |
| C12 | 28.1 | 27.1 | 35.1 | 26.8 | 24.3 | 24.3 | 48.6 | 19.6 | 19.5 | 25.1 | 25.2 | 28.4 | 41.1 | 30.1 |
| C13 | 44.0 | 37.2 | 34.2 | 36.5 | 38.7 | 38.7 | 77.0 | 27.0 | 28.2 | 55.7 | 55.6 | 47.7 | 75.3 | 44.8 |
| C14 | 209.6 | 90.9 | 158.0 | 69.6 | 213.5 | 213.5 | 203.6 | 19.5 | 23.4 | 203.6 | 203.4 | 205.8 | 202.6 | 202.2 |
| C15 | 51.1 | 66.9 | 116.6 | 180.5 | 84.0 | 84.0 | 154.9 | 18.0 | 18.1 | 144.7 | 143.4 | 157.4 | 133.5 | 126.7 |
| C16 | 10.4 | 11.3 | 10.8 | 17.7 | 10.0 | 10.0 | 24.6 | 17.0 | 18.5 | 25.4 | 25.3 | 14.7 | 30.3 | 13.8 |
| C17 | 25.3 | 26.8 | 29.8 | 29.1 | 23.9 | 23.9 | 111.7 | 15.0 | 17.7 | 13.7 | 13.8 | 22.1 | 20.6 | 26.80 |
| C18 | 28.9 | 28.9 | 29.4 | 15.3 | 15.6 | 15.6 | 16.5 | 10.9 | 15.0 | 29.0 | 29.0 | 29.4 | 16.4 | 29.2 |
| C19 | 15.1 | 15.4 | 15.8 | 17.3 | 28.3 | 28.3 | 28.7 | 29.1 | 28.8 | 15.6 | 15.6 | 15.6 | 28.7 | 15.8 |
| C20 | 13.3 | 11.9 | 12.8 | 13.9 | 14.9 | 14.9 | 21.7 | 28.4 | 26.2 | 17.7 | 18.0 | 13.7 | 21.3 | 11.8 |
| -OCH3 | 60.3 | - | - | - | - | - | - | - | - | - | - | - | - | - |
| -OCOCH3 | - | - | - | - | - | 171.0 | - | - | - | - | - | - | - | - |
| -OCOCH3 | - | - | - | - | - | 20.9 | - | - | - | - | - | - | - | - |
| Solvent | CDCl3 125 MHz | CDCl3 125 MHz | CDCl3 125 MHz | CDCl3 100 MHz | CDCl3 100 MHz | CDCl3 100 MHz | CDCl3 150 MHz | CDCl3 100 MHz | CDCl3 100 MHz | CDCl3 100 MHz | CDCl3 100 MHz | CDCl3 75 MHz | CDCl3 150 MHz | CDCl3 150 MHz |
| Reference | [76] | [76] | [76] | [77] | [77] | [77] | [78] | [79] | [79] | [77] | [77] | [80] | [78] | [75] |
| Compound | 57 | 58 | 59 | 60 | 61 | 62 | 64 | 65 | 66 | 67 | 68 | 69 | 70 | 71 |
| C1 | 34.4 | 35.9 | 152.7 | 158.8 | 154.5 | 154.7 | 209.6 | 212.7 | 212.1 | 212.3 | 209.3 | 204.1 | 203.3 | 204.7 |
| C2 | 36.2 | 37.5 | 142.7 | 124.2 | 146.3 | 146.2 | 48.3 | 39.2 | 39.8 | 74.7 | 73.0 | 145.7 | 144.8 | 49.2 |
| C3 | 74.1 | 81.8 | 203.4 | 131.8 | 205.5 | 205.7 | 76.8 | 36.3 | 36.4 | 43.7 | 43.1 | 152.1 | 152.6 | 80.3 |
| C4 | 148.0 | 137.8 | 62.4 | 129.4 | 45.8 | 46.3 | 148.4 | 160.5 | 158.6 | 158.1 | 158.4 | 88.9 | 86.3 | 160.0 |
| C5 | 122.4 | 135.0 | 57.7 | 128.3 | 209.2 | 209.0 | 198.4 | 199.1 | 198.5 | 198.4 | 197.5 | 206.3 | 208.0 | 209.6 |
| C6 | 79.2 | 80.7 | 58.6 | 114.7 | 44.2 | 44.1 | 141.3 | 141.2 | 141.0 | 141.2 | 140.9 | 131.2 | 136.3 | 37.3 |
| C7 | 110.1 | 213.7 | 41.0 | 15.8 | 18.8 | 18.9 | 137.2 | 136.4 | 136.7 | 136.9 | 137.5 | 139.0 | 134.4 | 33.8 |
| C8 | 29.8 | 37.8 | 18.4 | 203.8 | 26.0 | 26.1 | 43.7 | 43.9 | 43.8 | 43.5 | 43.8 | 45.7 | 45.5 | 45.0 |
| C9 | 21.8 | 46.6 | 29.1 | 40.3 | 17.8 | 17.7 | 45.4 | 45.6 | 46.0 | 45.4 | 45.6 | 42.8 | 43.8 | 40.6 |
| C10 | 17.7 | 145.7 | 16.5 | 29.0 | 24.8 | 24.1 | 160.5 | 149.0 | 149.0 | 148.7 | 148.3 | 57.4 | 59.1 | 142.7 |
| C11 | 21.9 | 138.8 | 18.7 | 24.5 | 29.1 | 29.5 | 148.9 | 147.7 | 148.8 | 146.8 | 146.9 | 148.7 | 146.7 | 147.7 |
| C12 | 25.6 | 129.8 | 24.3 | 17.3 | 39.8 | 39.2 | 36.4 | 36.7 | 36.7 | 36.7 | 36.4 | 36.9 | 36.4 | 34.2 |
| C13 | 37.9 | 72.4 | 37.6 | 25.9 | 213.3 | 213.6 | 34.3 | 34.6 | 34.6 | 34.6 | 34.3 | 34.4 | 33.7 | 32.3 |
| C14 | 107.0 | 176.2 | 214.0 | 19.1 | 82.6 | 82.7 | 51.4 | 51.9 | 51.9 | 52.0 | 51.5 | 53.7 | 51.9 | 48.4 |
| C15 | 47.7 | 46.7 | 86.4 | 44.1 | 10.5 | 10.5 | 146.6 | 147.0 | 147.0 | 145.4 | 146.0 | 146.8 | 150.1 | 145.4 |
| C16 | 14.2 | 12.9 | 10.2 | 209.8 | 30.3 | 30.3 | 113.4 | 113.4 | 113.4 | 113.6 | 113.5 | 112.5 | 112.5 | 111.7 |
| C17 | 15.9 | 27.6 | 18.3 | 29.9 | 29.3 | 29.4 | 18.7 | 18.8 | 18.8 | 18.9 | 18.8 | 20.2 | 18.7 | 17.6 |
| C18 | 29.3 | 21.5 | 28.2 | 17.4 | 14.8 | 14.8 | 108.4 | 108.2 | 108.3 | 108.3 | 109.1 | 108.0 | 107.0 | 104.7 |
| C19 | 15.1 | 110.5 | 15.4 | 29.0 | 19.0 | 18.4 | 14.5 | 17.8 | 14.7 | 26.3 | 24.0 | 10.8 | 10.6 | 12.9 |
| C20 | 14.8 | 19.4 | 14.6 | 14.7 | - | - | 18.9 | 19.6 | 19.6 | 19.6 | 19.7 | 22.0 | 19.0 | 15.5 |
| -OCH3 | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| -OCOCH3 | 170.8 | 170.4 | - | - | - | - | - | - | - | - | - | - | - | - |
| -OCOCH3 | 21.0 | 21.2 | - | - | - | - | - | - | - | - | - | - | - | - |
| Solvent | CDCl3 125 MHz | CDCl3 125 MHz | CDCl3 100 MHz | CDCl3 100 MHz | CDCl3 150 MHz | CDCl3 125 MHz | CDCl3 125 MHz | CDCl3 125 MHz | CDCl3 125 MHz | CDCl3 125 MHz | CDCl3 125 MHz | CDCl3 125 MHz | CDCl3 150 MHz | CDCl3 150 MHz |
| Reference | [81] | [81] | [82] | [83] | [84] | [84] | [85] | [86] | [86] | [86] | [86] | [87] | [88] | [88] |
| Compound | 72 | 73 | 74 | 75 | 76 | 77 | 78 | 79 | 80 | 81 | 82 | 83 | 84 | 85 |
| C1 | 209.9 | 210.9 | 210.3 | 211.9 | 209.5 | 208.6 | 208.3 | 36.9 | 104.9 | 104.0 | 108.6 | 108.0 | 103.8 | 37.2 |
| C2 | 40.7 | 40.3 | 40.4 | 42.2 | 74.0 | 47.1 | 47.3 | 37.4 | 47.7 | 45.9 | 38.6 | 40.8 | 77.6 | 37.8 |
| C3 | 34.0 | 36.0 | 33.0 | 31.4 | N.I. | 72.2 | 77.8 | 91.0 | 80.4 | 80.3 | 37.3 | 74.9 | 43.9 | 81.4 |
| C4 | 167.9 | 153.8 | 84.1 | 86.6 | 157.9 | 150.7 | 149.5 | 140.0 | 138.3 | N.I. | 136.1 | 135.4 | 138.9 | 140.1 |
| C5 | 207.7 | 204.5 | 217.6 | 215.4 | 198.2 | 197.3 | 198.0 | 139.7 | 192.1 | 191.5 | 190.3 | 192.0 | 193.6 | 131.5 |
| C6 | 81.9 | 64.2 | 42.1 | 42.1 | 142.0 | 141.7 | 142.0 | 48.9 | 135.6 | 138.0 | 138.1 | 138.2 | 137.1 | 49.3 |
| C7 | 70.2 | 65.5 | 82.1 | 80.4 | 136.0 | 135.1 | 134.4 | 210.4 | 141.4 | 140.1 | 140.0 | 141.6 | 138.6 | 211.7 |
| C8 | 47.1 | 41.2 | 47.2 | 41.6 | 43.6 | 43.5 | 43.7 | 46.2 | 37.2 | 38.4 | 37.2 | 37.2 | 35.3 | 46.6 |
| C9 | 39.0 | 43.5 | 39.5 | 39.2 | 45.0 | 51.7 | 51.7 | 46.8 | 43.4 | 44.6 | 43.6 | 43.5 | 47.2 | 210.4 |
| C10 | 140.5 | 147.6 | 55.5 | 51.9 | 146.8 | 146.6 | 146.6 | 145.9 | 151.4 | 149.0 | 149.5 | 150.1 | 147.3 | 51.6 |
| C11 | 146.8 | 149.5 | 146.3 | 142.8 | 148.1 | 34.3 | 34.4 | 134.2 | 75.7 | 75.7 | 75.6 | 75.4 | 69.8 | 53.7 |
| C12 | 36.3 | 36.7 | 35.3 | 35.2 | 36.5 | 36.5 | 36.6 | 131.2 | 28.8 | 28.7 | 25.6 | 25.6 | 32.2 | 131.9 |
| C13 | 34.0 | 35.6 | 33.3 | 33.5 | 34.3 | 148.2 | 148.0 | 53.4 | 25.6 | 27.8 | 28.7 | 28.8 | 25.7 | 134.6 |
| C14 | 47.5 | 51.9 | 47.9 | 48.1 | 51.9 | 45.3 | 45.0 | 211.0 | 49.0 | 49.5 | 48.8 | 49.0 | 51.3 | 48.9 |
| C15 | 146.7 | 146.6 | 146.0 | 146.4 | 146.5 | 156.0 | 157.4 | 51.2 | 145.8 | 146.0 | 146.0 | 145.8 | 146.5 | 146.3 |
| C16 | 112.9 | 114.3 | 112.5 | 112.4 | 113.3 | 8.4 | 15.3 | 13.6 | 114.1 | 114.5 | 113.9 | 114.1 | 113.3 | 110.5 |
| C17 | 19.1 | 19.6 | 18.2 | 18.5 | 18.6 | 18.8 | 18.5 | 16.8 | 20.4 | 19.2 | 18.9 | 19.0 | 18.9 | 21.7 |
| C18 | 106.8 | 108.4 | 106.5 | 109.0 | 108.3 | 113.3 | 113.3 | 21.3 | 73.4 | 68.8 | 73.3 | 73.7 | 70.0 | 17.1 |
| C19 | 18.2 | 14.8 | 14.5 | 18.0 | 24.0 | 18.6 | 18.6 | 110.1 | 19.0 | 10.1 | 11.0 | 7.7 | 23.6 | 14.0 |
| C20 | 26.8 | 17.3 | 15.2 | 15.6 | 18.7 | 108.4 | 108.2 | 17.7 | 9.0 | 20.8 | 20.8 | 20.5 | 20.5 | 18.1 |
| -OCH3 | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| -OCOCH3 | - | - | - | - | - | 169.9 | 170.1 | 170.8 | - | - | - | - | - | 171.2 |
| -OCOCH3 | - | - | - | - | - | 20.4 | 20.6 | 21.3 | - | - | - | - | - | 21.6 |
| Solvent | CDCl3 150 MHz | CDCl3 150 MHz | CDCl3 150 MHz | CDCl3 150 MHz | CDCl3 100 MHz | CDCl3 100 MHz | CDCl3 100 MHz | CDCl3 125 MHz | CDCl3 100 MHz | CDCl3 100 MHz | CDCl3 150 MHz | CDCl3 100 MHz | CDCl3 150 MHz | CDCl3 100 MHz |
| Reference | [88] | [88] | [88] | [88] | [89] | [77] | [77] | [90] | [91] | [92] | [88] | [92] | [93] | [92] |
| Compound | 86 | 87 | 88 | 89 | 90 | 91 | 92 | 93 | 93 | 94 | 95 | 96 | 97 | 98 |
| C1 | 169.1 | 180.6 | 207.0 | 210.3 | 209.9 | 199.6 | 194.3 | 208.8 | 208.7 | 207.4 | 206.2 | 158.7 | 158.6 | 157.8 |
| C2 | 205.2 | 35.0 | 38.8 | 39.3 | 40.2 | 36.8 | 153.1 | 144.3 | 144.2 | 59.2 | 58.7 | 112.8 | 120.2 | 119.4 |
| C3 | 42.5 | 42.3 | 41.6 | 38.1 | 38.4 | 38.4 | 132.1 | 153.4 | 153.6 | 53.7 | 52.7 | 141.8 | 146.7 | 145.1 |
| C4 | 142.6 | 205.1 | 200.3 | 142.7 | 143.1 | 190.0 | 201.2 | 33.0 | 33.1 | 144.7 | 136.2 | 135.2 | 138.6 | 138.9 |
| C5 | 193.6 | 167.2 | 170.9 | 167.5 | 167.5 | 169.0 | 79.3 | 199.7 | 199.5 | 203.1 | 200.7 | 30.6 | 23.2 | 24.1 |
| C6 | 137.0 | 126.2 | 127.4 | 147.7 | 147.6 | 127.1 | 133.6 | 133.5 | 133.4 | 142.9 | 142.8 | 26.7 | 26.9 | 25.9 |
| C7 | 145.0 | 138.1 | 147.5 | 118.0 | 118.1 | 144.2 | 131.2 | 146.6 | 146.8 | 130.2 | 129.8 | 126.9 | 125.6 | 125.9 |
| C8 | 40.9 | 45.4 | 44.9 | 46.3 | 46.5 | 43.1 | 39.3 | 40.9 | 41.1 | 44.0 | 43.9 | 135.2 | 132.3 | 130.9 |
| C9 | 46.9 | 43.3 | 43.9 | 41.7 | 41.6 | 42.5 | 41.2 | 47.9 | 48.1 | 46.0 | 45.5 | 41.5 | 47.2 | 46.0 |
| C10 | 137.5 | 129.4 | 114.2 | 153.6 | 152.8 | 114.7 | 55.4 | 60.7 | 60.7 | 162.8 | 162.5 | 129.1 | 75.5 | 75.6 |
| C11 | 83.3 | 145.6 | 146.6 | 146.8 | 146.7 | 144.2 | 146.9 | 145.3 | 145.6 | 148.7 | 147.8 | 126.8 | 129.3 | 124.9 |
| C12 | 28.3 | 35.3 | 36.8 | 35.5 | 35.5 | 35.4 | 35.6 | 36.8 | 37.0 | 36.6 | 36.4 | 135.5 | 136.4 | 137.3 |
| C13 | 25.9 | 32.9 | 32.7 | 33.2 | 33.1 | 31.6 | 33.0 | 33.6 | 33.8 | 34.3 | 34.3 | 123.0 | 38.6 | 34.4 |
| C14 | 46.4 | 51.8 | 54.5 | 51.4 | 51.4 | 52.4 | 50.7 | 50.4 | 50.6 | 51.5 | 51.4 | 27.6 | 28.4 | 26.7 |
| C15 | 145.6 | 146.7 | 150.4 | 147.1 | 147.1 | 148.5 | 146.8 | 146.2 | 146.3 | 146.9 | 146.7 | 33.7 | 33.3 | 29.9 |
| C16 | 114.6 | 112.4 | 111.1 | 112.0 | 112.0 | 111.1 | 16.7 | 113.4 | 113.5 | 113.3 | 113.0 | 22.1 | 23.3 | 21.4 |
| C17 | 18.8 | 18.6 | 19.6 | 18.5 | 18.5 | 19.3 | 19.0 | 19.4 | 19.4 | 18.7 | 18.4 | 22.1 | 20.8 | 21.2 |
| C18 | 69.4 | 108.1 | 108.6 | 107.9 | 108.1 | 108.2 | 19.3 | 106.7 | 106.9 | 21.1 | 19.7 | 190.7 | 195.1 | 195.4 |
| C19 | 30.2 | 17.8 | 19.0 | 17.3 | 15.7 | 17.8 | 112.2 | 10.5 | 10.5 | 17.9 | 17.9 | 16.0 | 15.9 | 16.3 |
| C20 | 21.5 | 18.9 | 21.5 | 19.8 | 19.7 | 21.6 | 106.0 | 16.1 | 16.2 | 108.2 | 108.5 | 20.2 | 16.4 | 18.0 |
| -OCH3 | - | - | - | - | - | - | - | - | - | - | - | - | 55.3 | 55.5 |
| -OCOCH3 | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| -OCOCH3 | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| Solvent | CDCl3 150 MHz | CDCl3 150 MHz | MeOD-d4 150 MHz | CDCl3 150 MHz | CDCl3 150 MHZ | DMSO 1% TFA-d 125 MHz | CDCl3 150 MHz | CDCl3 125 MHz | CDCl3 125 MHz | CDCl3 150 MHz | CDCl3 150 MHz | CDCl3 125 MHz | CDCl3 125 MHz | CDCl3 125 MHz |
| Reference | [88] | [88] | [88] | [88] | [88] | [94] | [78] | [87] | [86] | [95] | [95] | [96] | [96] | [96] |
| Compound | 99 | 100 | 101 | 102 | 103 | 104 | 105 | 106 | 107 | 108 | 109 | 110 | 111 | 112 |
| C1 | 42.5 | 49.9 | 49.4 | 48.6 | 153.8 | 150.3 | 216.9 | 218.0 | 30.3 | 30.1 | 208.2 | 208.2 | 30.3 | 30.5 |
| C2 | 38.3 | 37.8 | 81.2 | 80.8 | 143.9 | 144.7 | 213.8 | 215.6 | 42.5 | 42.4 | 42.5 | 42.6 | 42.2 | 40.0 |
| C3 | 123.7 | 154.7 | 122.8 | 122.5 | 196.7 | 195.2 | 207.2 | 210.6 | 207.7 | 208.1 | 30.2 | 30.1 | 208.4 | 208.0 |
| C4 | 137.1 | 138.8 | 137.3 | 140.3 | 136.0 | 132.5 | 144.7 | 144.5 | 136.9 | 137.5 | 134.3 | 134.3 | 146.0 | 145.5 |
| C5 | 147.0 | 131.3 | 147.6 | 146.0 | 139.5 | 139.7 | 129.1 | 138.4 | 134.3 | 134.4 | 146.1 | 150.1 | 133.1 | 133.0 |
| C6 | 141.8 | 136.8 | 142.8 | 142.9 | 82.8 | 81.6 | 72.5 | 135.9 | 145.5 | 145.0 | 114.8 | 114.8 | 145.5 | 145.5 |
| C7 | 201.8 | 202.9 | 201.3 | 201.3 | 37.6 | 37.6 | 67.8 | 128.1 | 33.4 | 33.3 | 137.4 | 137.1 | 33.2 | 33.3 |
| C8 | 128.7 | 66.7 | 128.7 | 128.7 | 25.6 | 25.5 | 65.6 | 64.5 | 20.8 | 20.8 | 25.9 | 25.9 | 21.1 | 21.1 |
| C9 | 158.7 | 79.9 | 159.1 | 159.9 | 88.5 | 88.1 | 55.5 | 55.5 | 23.1 | 23.1 | 16.1 | 16.1 | 25.5 | 25.5 |
| C10 | 36.6 | 37.0 | 36.7 | 37.0 | 36.0 | 35.7 | 52.9 | 51.4 | 23.0 | 23.0 | 19.4 | 19.5 | 19.0 | 19.0 |
| C11 | 41.3 | 43.3 | 41.3 | 41.6 | 34.5 | 34.4 | 46.8 | 51.0 | 25.9 | 25.8 | 15.8 | 15.8 | 27.8 | 27.8 |
| C12 | 183.1 | 76.9 | 183.9 | 183.4 | 31.3 | 30.9 | 38.0 | 39.8 | 135.4 | 135.3 | 33.4 | 33.5 | 136.7 | 136.7 |
| C13 | 112.4 | 87.7 | 112.4 | 113.0 | 42.0 | 43.5 | 37.0 | 38.3 | 130.6 | 131.0 | 150.1 | 42.4 | 131.7 | 131.3 |
| C14 | 203.8 | 213.7 | 203.2 | 203.4 | 211.8 | 204.6 | 35.0 | 38.0 | 150.1 | 150.3 | 131.1 | 131.8 | 150.7 | 150.6 |
| C15 | 99.8 | 91.4 | 97.7 | 97.9 | 82.4 | 88.9 | 34.2 | 37.4 | 132.7 | 131.8 | 145.5 | 145.6 | 130.5 | 130.5 |
| C16 | 18.9 | 19.2 | 26.9 | 27.1 | 10.6 | 10.7 | 27.7 | 27.9 | 15.8 | 17.0 | 13.2 | 13.3 | 16.7 | 15.5 |
| C17 | 20.7 | 21.9 | 20.7 | 21.1 | 24.2 | 24.0 | 23.2 | 23.6 | 115.2 | 115.5 | 115.8 | 28.2 | 114.5 | 114.2 |
| C18 | 30.4 | 27.5 | 30.2 | 30.5 | 24.1 | 28.2 | 20.2 | 20.7 | 73.8 | 73.8 | 17.1 | 17.1 | 28.0 | 28.0 |
| C19 | 26.9 | 22.0 | 26.7 | 27.1 | 28.4 | 23.4 | 16.2 | 20.5 | 12.1 | 12.1 | 17.3 | 21.4 | 15.8 | 15.8 |
| C20 | 6.0 | 14.2 | 5.9 | 6.3 | 20.6 | 20.6 | 14.7 | 14.0 | 12.6 | 12.6 | 13.2 | 13.3 | 13.3 | |
| -OCH3 | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| -OCOCH3 | - | - | - | - | - | 169.3 | - | - | 171.3 | 171.3 | - | - | - | - |
| -OCOCH3 | - | - | - | - | - | 22.1 | - | - | 20.9 | 20.9 | - | - | - | - |
| Solvent | CDCl3 62,9 MHz | CDCl3 100 MHz | CDCl3 62,9 MHz | CDCl3 125 MHz | CDCl3 90/150 MHz | CDCl3 100 MHz | C6D6 150 MHz | CDCl3 125 MHz | CDCl3 100 MHz | CDCl3 100 MHz | CDCl3 75 MHz | CDCl3 100 MHz | CDCl3 50 MHz | CDCl3 50 MHz |
| Reference | [97] | [98] | [97] | [99] | [72] | [100] | [101] | [96] | [97] | [98] | [97] | [99] | [72] | [100] |
| Compound | 113 | 114 | 115 | 116 | 117 | 118 | 119 | 120 | 121 | 122 | 123 | 124 | 125 | 125 |
| C1 | 30.3 | 30.2 | 30.0 | 37.6 | 37.2 | 30.6 | 11.9 | 74.0 | 67.1 | 30.5 | 30.2 | 30.4 | 40.5 | 41.6 |
| C2 | 42.4 | 42.5 | 42.3 | 77.7 | 77.5 | 42.1 | 13.3 | 50.2 | 45.9 | 42.1 | 42.4 | 42.7 | 38.0 | 39.3 |
| C3 | 208.5 | 207.6 | 207.8 | 207.1 | 206.1 | 206.2 | 16.9 | 207.5 | 209.0 | 206.4 | 207.6 | 208.0 | 32.6 | 33.5 |
| C4 | 139.7 | 137.2 | 137.7 | 134.3 | 134.6 | 136.9 | 20.9 | 157.4 | 157.1 | 137.9 | 138.1 | 137.5 | 149.7 | 150.3 |
| C5 | 133.9 | 134.4 | 134.4 | 135.3 | 135.7 | 133.0 | 22.0 | 45.6 | 45.8 | 134.1 | 134.3 | 134.0 | 203.4 | 206.3 |
| C6 | 145.4 | 144.7 | 144.2 | 146.0 | 144.4 | 146.3 | 25.4 | 153.5 | 153.7 | 143.9 | 144.0 | 145.5 | 48.0 | 49.5 |
| C7 | 33.3 | 33.5 | 33.5 | 33.3 | 33.4 | 33.7 | 24.7 | 40.1 | 40.1 | 33.6 | 33.7 | 33.55 | 52.6 | 52.9 |
| C8 | 21.3 | 20.7 | 20.7 | 21.4 | 21.4 | 20.9 | 30.7 | 27.0 | 27.0 | 20.5 | 20.5 | N.I. | 181.3 | 184.8 |
| C9 | 26.2 | 28.0 | 28.0 | 26.0 | 25.9 | 22.1 | 33.5 | 26.5 | 26.7 | 25.9 | 26.0 | 26.2 | 65.5 | 67.3 |
| C10 | 19.6 | 26.7 | 26.7 | 19.6 | 19.6 | 25.0 | 41.9 | 19.5 | 19.5 | 35.8 | 35.8 | 23.3 | 206.8 | 208.8 |
| C11 | 27.3 | 30.4 | 30.4 | 28.3 | 28.3 | 25.5 | 70.5 | 35.3 | 35.3 | 28.5 | 28.6 | 23.1 | 33.2 | 34.1 |
| C12 | 134.7 | 134.2 | 134.3 | 138.1 | 137.9 | 136.0 | 114.5 | 47.1 | 46.9 | 132.9 | 133.0 | 136.0 | 22.73 | 23.7 |
| C13 | 130.0 | 130.1 | 130.3 | 132.1 | 132.4 | 132.6 | 131.1 | 49.2 | 49.5 | 130.1 | 130.2 | 131.0 | 35.9 | 36.8 |
| C14 | 153.2 | 150.1 | 150.1 | 150.3 | 150.1 | 151.3 | 132.3 | 202.3 | 201.8 | 150.4 | 150.4 | 150.6 | 207.1 | 209.2 |
| C15 | 133.3 | 132.9 | 132.1 | 129.7 | 128.7 | 131.4 | 132.8 | 152.8 | 153.7 | 132.2 | 132.4 | 131.8 | 26.2 | 27.1 |
| C16 | 17.0 | 15.8 | 17.0 | 26.1 | 25.7 | 15.0 | 135.7 | 14.2 | 9.0 | 15.0 | 16.4 | 17.3 | 28.3 | 28.4 |
| C17 | 115.3 | 116.0 | 116.3 | 115.1 | 116.2 | 113.9 | 138.0 | 110.3 | 110.3 | 115.8 | 116.5 | 115.6 | 16.7 | 17.2 |
| C18 | 28.0 | 176.2 | 176.2 | 28.1 | 28.1 | 70.7 | 145.5 | 16.8 | 16.8 | 202.3 | 202.4 | 12.5 | 12.4 | 11.9 |
| C19 | 16.5 | 10.3 | 10.2 | 16.1 | 16.2 | 11.4 | 151.3 | 28.4 | 28.4 | 7.8 | 7.9 | 74.1 | 21.5 | 22.0 |
| C20 | 62.1 | 13.0 | 13.0 | 13.3 | 13.4 | 12.7 | 207.6 | 11.4 | 11.1 | 12.8 | 12.8 | 13.2 | 17.6 | 17.8 |
| -OCH3 | - | 52.3 | 52.3 | - | - | - | - | - | - | - | - | - | - | - |
| -OCOCH3 | - | - | - | - | - | - | - | - | - | - | - | 170.5 | - | - |
| -OCOCH3 | - | - | - | - | - | - | - | - | - | - | - | 10.9 | - | - |
| Solvent | CDCl3 150 MHz | CDCl3 150 MHz | CDCl3 150 MHz | CDCl3 100 MHz | CDCl3 100 MHz | CDCl3 150 MHz | DMSO-d6 100 MHz | CDCl3 100 MHz | CDCl3 150 MHz | CDCl3 150 MHz | CDCl3 150 MHz | CDCl3 125 MHz | CDCl3 100 MHz | MeOD-d4 100 MHz |
| Reference | [102] | [102] | [102] | [89] | [89] | [103] | [104] | [77] | [77] | [103] | [103] | [87] | [105] | [105] |
| Compound | 126 | 126 | 127 | 128 | 129 | 130 | 131 | 132 | 133 | 134 | ||||
| C1 | 40.4 | 41.5 | 150.9 | 27.3 | 27.8 | 34.3 | 34.3 | 75.7 | 77.0 | 31.6 | ||||
| C2 | 37.8 | 39.1 | 144.6 | 41.6 | 42.8 | 39.7 | 39.5 | 51.0 | 48.3 | 41.5 | ||||
| C3 | 32.7 | 33.6 | 195.2 | 207.3 | 205.3 | 213.1 | 213.1 | 209.2 | 207.9 | 214.8 | ||||
| C4 | 149.3 | 150.0 | 129.8 | 128.7 | 129.5 | 142.0 | 142.0 | 142.3 | 145.3 | 157.6 | ||||
| C5 | 203.3 | 206.6 | 149.3 | 148.5 | 147.9 | 33.9 | 34.0 | 35.5 | 35.3 | 71.0 | ||||
| C6 | 50.3 | 51.8 | 39.4 | 38.4 | 38.1 | 38.5 | 38.4 | 39.8 | 39.3 | 48.0 | ||||
| C7 | 45.6 | 46.9 | 37.9 | 38.0 | 38.4 | 38.1 | 38.0 | 39.3 | 39.0 | 30.4 | ||||
| C8 | 180.6 | 184.1 | 16.0 | 16.0 | 16.1 | 16.4 | 16.4 | 24.3 | 24.0 | 21.2 | ||||
| C9 | 68.6 | 70.2 | 19.2 | 19.3 | 19.4 | 24.0 | 23.8 | 157.7 | 158.0 | 37.1 | ||||
| C10 | 205.3 | 207.5 | 17.4 | 17.3 | 17.1 | 21.7 | 21.7 | 36.7 | 36.5 | 44.0 | ||||
| C11 | 33.2 | 34.5 | 20.9 | 20.4 | 20.0 | 29.9 | 29.5 | 121.8 | 121.3 | 73.7 | ||||
| C12 | 21.8 | 23.3 | 40.9 | 42.1 | 42.1 | 157.7 | 157.7 | 155.8 | 155.7 | 45.3 | ||||
| C13 | 37.8 | 38.0 | 81.7 | 81.8 | 81.7 | 137.2 | 137.2 | 132.1 | 131.6 | 54.0 | ||||
| C14 | 206.8 | 209.5 | 208.3 | 208.5 | 209.1 | 192.3 | 192.5 | 191.3 | 188.6 | 204.9 | ||||
| C15 | 26.3 | 27.4 | 81.3 | 43.1 | 44.1 | 161.3 | 161.3 | 163.4 | 158.4 | 147.6 | ||||
| C16 | 28.4 | 28.6 | 10.8 | 16.4 | 14.2 | 16.4 | 16.3 | 14.3 | 14.7 | 16.1 | ||||
| C17 | 16.5 | 16.9 | 19.4 | 19.4 | 18.9 | 24.6 | 24.4 | 20.7 | 20.7 | 14.3 | ||||
| C18 | 10.7 | 11.0 | 15.7 | 28.6 | 28.8 | 28.6 | 28.7 | 21.9 | 21.2 | 28.3 | ||||
| C19 | 21.5 | 22.1 | 28.5 | 15.7 | 15.7 | 16.1 | 16.1 | 21.6 | 21.4 | 15.1 | ||||
| C20 | 11.8 | 12.1 | 27.7 | 26.9 | 27.0 | 17.0 | 16.9 | 16.2 | 16.0 | 14.0 | ||||
| -OCH3 | - | - | - | - | - | - | - | - | - | - | ||||
| -OCOCH3 | - | - | - | - | - | - | - | - | 170.8 | - | ||||
| -OCOCH3 | - | - | - | - | - | - | - | - | 20.8 | - | ||||
| Solvent | CDCl3 100 MHz | MeOD-d4 100 MHz | CDCl3 125 MHz | CDCl3 150 MHz | CDCl3 150 MHz | CDCl3 125 MHz | CDCl3 125 MHz | Pyr-d5 100 MHz | Pyr-d5 150 MHz | CDCl3 125 MHz | ||||
| Reference | [105] | [105] | [75] | [106] | [106] | [106] | [106] | [107] | [107] | [108] | ||||
| Compound | 135 | 136 | 137 | |||||||||||
| C1 | 161.2 | C1’ | 173.3 | C1 | 161.2 | C1’ | 173.7 | C1 | 160.8 | C1’ | 173.8 | |||
| C2 | 133.4 | C2’ | 38.1 | C2 | 133.0 | C2’ | 38.1 | C2 | 133.1 | C2’ | 38.6 | |||
| C3 | 208.7 | C3’ | 125.0 | C3 | 208.8 | C3’ | 121.8 | C3 | 208.7 | C3’ | 123.3 | |||
| C4 | 74.0 | C4’ | 136.8 | C4 | 73.6 | C4’ | 135.9 | C4 | 74.0 | C4’ | 133.9 | |||
| C5 | 39.1 | C5’ | 51.7 | C5 | 38.8 | C5’ | 132.6 | C5 | 38.9 | C5’ | 129.8 | |||
| C6 | 140.9 | C6’ | 45.0 | C6 | 140.4 | C6’ | 128.9 | C6 | 140.7 | C6’ | 131.6 | |||
| C7 | 129.5 | C7’ | 133.0 | C7 | 129.4 | C7’ | 47.0 | C7 | 129.1 | C7’ | 48.9 | |||
| C8 | 38.9 | C8’ | 131.0 | C8 | 38.3 | C8’ | 49.2 | C8 | 38.5 | C8’ | 47.1 | |||
| C9 | 76.4 | C9’ | 133.1 | C9 | 75.9 | C9’ | 139.4 | C9 | 76.0 | C9’ | 137.7 | |||
| C10 | 56.1 | C10’ | 118.1 | C10 | 55.7 | C10’ | 115.8 | C10 | 55.9 | C10’ | 115.9 | |||
| C11 | 36.7 | C11’ | 175.2 | C11 | 36.3 | C11’ | 174.8 | C11 | 36.5 | C11’ | 166.6 | |||
| C12 | 32.0 | C12’ | 51.1 | C12 | 31.7 | C12’ | 53.6 | C12 | 31.9 | C12’ | 119.0 | |||
| C13 | 63.4 | C13’ | 26.8 | C13 | 64.3 | C13’ | 30.0 | C13 | 64.4 | C13’ | 145.9 | |||
| C14 | 33.4 | C14’ | 32.3 | C14 | 30.0 | C14’ | 35.3 | C14 | 30.2 | C14’ | 42.1 | |||
| C15 | 25.9 | C15’ | 23.3 | C15 | 26.1 | C15’ | 24.4 | C15 | 26.3 | C15’ | 44.1 | |||
| C16 | 74.0 | C16’ | 130.0 | C16 | 68.7 | C16’ | 134.1 | C16 | 69.4 | C16’ | 132.0 | |||
| C17 | 12.0 | C17’ | 134.9 | C17 | 10.6 | C17’ | 128.8 | C17 | 10.9 | C17’ | 130.4 or 132.7 | |||
| C18 | 18.7 | C18’ | 130.8 | C18 | 18.4 | C18’ | 130.7 | C18 | 18.6 | C18’ | 130.4 or 132.8 | |||
| C19 | 10.1 | C19’ | 131.8 | C19 | 10.2 | C19’ | 130.7 | C19 | 10.2 | C19’ | 130.4 or 132.9 | |||
| C20 | 68.4 | C20’ | 131.0 | C20 | 68.2 | C20’ | 130.7 | C20 | 68.2 | C20’ | 130.7 | |||
| C21’ | 135.8 | C21’ | 134.6 | C21’ | 135.6 | |||||||||
| C22’ | 35.3 | C22’ | 35.0 | C22’ | 35.3 | |||||||||
| C23’ | 22.9 | C23’ | 22.6 | C23’ | 23.0 | |||||||||
| C24’ | 13.9 | C24’ | 14.0 | C24’ | 14.0 | |||||||||
| Solvent | CDCl3/125 MHz | CDCl3 125 MHz | CDCl3 125 MHz | 35.3 | C22’ | 35.3 | C22’ | 35.3 | ||||||
| 22.8 | C23’ | 22.8 | C23’ | 23.0 | ||||||||||
| C24’ | 13.9 | C24’ | 13.9 | C24’ | 14.0 | |||||||||
| Reference | [109] | [109] | [109] | |||||||||||
| Compound | 138 | 139 | 140 | |||||||||||
| C1 | 161.0 | C1’ | 173.5 | C1 | 161.0 | C1’ | 173.5 | C1 | 161.0 | C1’ | 173.9 | |||
| C2 | 133.3 | C2’ | 39.0 | C2 | 133.3 | C2’ | 39.0 | C2 | 133.2 | C2’ | 39.0 | |||
| C3 | 208.9 | C3’ | 122.0 | C3 | 208.9 | C3’ | 122.3 | C3 | 208.7 | C3’ | 122.1 | |||
| C4 | 74.2 | C4’ | 136.6 | C4 | 74.2 | C4’ | 136.4 | C4 | 74.2 | C4’ | 136.7 | |||
| C5 | 38.8 | C5’ | 49.9 | C5 | 38.8 | C5’ | 51.7 | C5 | 38.9 | C5’ | 129.9–132.3 | |||
| C6 | 140.8 | C6’ | 28.6 | C6 | 140.8 | C6’ | 26.8 | C6 | 140.9 | C6’ | 130.6 | |||
| C7 | 129.3 | C7’ | 31.7 | C7 | 129.3 | C7’ | 29.6 | C7 | 129.4 | C7’ | 129.9–132.3 | |||
| C8 | 38.8 | C8’ | 22.1 | C8 | 38.8 | C8’ | 24.1 | C8 | 38.6 | C8’ | 135.1 | |||
| C9 | 76.2 | C9’ | 139.8 | C9 | 76.2 | C9’ | 135.1 | C9 | 76.0 | C9’ | 35.7 | |||
| C10 | 55.9 | C10’ | 112.4 | C10 | 55.9 | C10’ | 118.7 | C10 | 56.1 | C10’ | 31.9 | |||
| C11 | 36.5 | C11’ | 173.9 | C11 | 36.5 | C11’ | 174.6 | C11 | 36.6 | C11’ | 166.3 | |||
| C12 | 31.9 | C12’ | 52.0 | C12 | 31.9 | C12’ | 54.7 | C12 | 31.9 | C12’ | 119.6 | |||
| C13 | 63.4 | C13’ | 45.4 | C13 | 63.4 | C13’ | 44.3 | C13 | 64.6 | C13’ | 153.3 | |||
| C14 | 33.2 | C14’ | 134.8 | C14 | 33.2 | C14’ | 135.1 | C14 | 30.3 | C14’ | 46.1 | |||
| C15 | 25.5 | C15’ | 130.3 | C15 | 25.5 | C15’ | 130.3 | C15 | 26.6 | C15’ | 43.4 | |||
| C16 | 73.9 | C16’ | 129–134 | C16 | 73.9 | C16’ | 129–134 | C16 | 69.3 | C16’ | 136.4 | |||
| C17 | 12.1 | C17’ | 129–134 | C17 | 12.1 | C17’ | 129–134 | C17 | 11.0 | C17’ | 129.9–132.3 | |||
| C18 | 18.5 | C18’ | 129–134 | C18 | 18.5 | C18’ | 129–134 | C18 | 18.6 | C18’ | 129.9–132.3 | |||
| C19 | 10.2 | C19’ | 129–134 | C19 | 10.2 | C19’ | 129–134 | C19 | 10.4 | C19’ | 129.9–132.3 | |||
| C20 | 68.2 | C20’ | 131.1 | C20 | 68.2 | C20’ | 131.1 | C20 | 68.2 | C20’ | 130.9 | |||
| C21’ | 135.9 | C21’ | 135.9 | C21’ | 135.3 | |||||||||
| C22’ | 35.3 | C22’ | 35.3 | C22’ | 35.3 | |||||||||
| C23’ | 22.8 | C23’ | 22.8 | C23’ | 23.0 | |||||||||
| C24’ | 13.9 | C24’ | 13.9 | C24’ | 14.0 | |||||||||
| Solvent | CDCl3 / 125 MHz | CDCl3 125 MHz | CDCl3 125 MHz | |||||||||||
| Reference | [109] | [109] | [109] | |||||||||||
| Compound | IC50 (Cell Lineage) * | Assay ** | Reference |
|---|---|---|---|
| Curcusone C | 0.08 µM (HEPG2) 0.25 µM (L5178y) | MTT | [88,133] |
| Curcusone D | 0.15 µM (HEPG2) 0.51 µM (L5178y) | MTT | [88,133] |
| Curcusone B | 0.70 µM (L5178y) 2.64 µM (HL-60) | MTT | |
| Curcusone A | 0.91 µM (L5178y) 1.63 µM (HL-60) | MTT | |
| Jatrophone | 1.80 µM (MCF-7) 1.82 µM (U-251) | SRB | [134,135] |
| Jatromultone D | 2.69 µM (A549) | MTT | [77] |
| Jatrogrossidione | 2.6 µM (RKO) | CKK-8 | [136] |
| 15-epi-4Z- Jatrogrossidentadion | 2.61 µM (HEPG2) | MTT | [77] |
| 2-epi-hydroxyiso Jatrogrossidion | 2.67 µM (HEPG2) 3.20 µM (HL-60) 3.20 µM (MCF-7) | MTS | [77] |
| 3-dehydroxy-2-epi-caniojane | 2.86 µM (HL-60) | MTT | [88] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Souza, T.A.d.; Pereira, L.H.A.; Alves, A.F.; Dourado, D.; Lins, J.d.S.; Scotti, M.T.; Scotti, L.; Abreu, L.S.; Tavares, J.F.; Silva, M.S. Jatropha Diterpenes: An Updated Review Concerning Their Structural Diversity, Therapeutic Performance, and Future Pharmaceutical Applications. Pharmaceuticals 2024, 17, 1399. https://doi.org/10.3390/ph17101399
Souza TAd, Pereira LHA, Alves AF, Dourado D, Lins JdS, Scotti MT, Scotti L, Abreu LS, Tavares JF, Silva MS. Jatropha Diterpenes: An Updated Review Concerning Their Structural Diversity, Therapeutic Performance, and Future Pharmaceutical Applications. Pharmaceuticals. 2024; 17(10):1399. https://doi.org/10.3390/ph17101399
Chicago/Turabian StyleSouza, Thalisson A. de, Luiz H. A. Pereira, Alan F. Alves, Douglas Dourado, Jociano da S. Lins, Marcus T. Scotti, Luciana Scotti, Lucas S. Abreu, Josean F. Tavares, and Marcelo S. Silva. 2024. "Jatropha Diterpenes: An Updated Review Concerning Their Structural Diversity, Therapeutic Performance, and Future Pharmaceutical Applications" Pharmaceuticals 17, no. 10: 1399. https://doi.org/10.3390/ph17101399
APA StyleSouza, T. A. d., Pereira, L. H. A., Alves, A. F., Dourado, D., Lins, J. d. S., Scotti, M. T., Scotti, L., Abreu, L. S., Tavares, J. F., & Silva, M. S. (2024). Jatropha Diterpenes: An Updated Review Concerning Their Structural Diversity, Therapeutic Performance, and Future Pharmaceutical Applications. Pharmaceuticals, 17(10), 1399. https://doi.org/10.3390/ph17101399









