Protective Capacity of Helichrysum italicum Infusion Against Intestinal Barrier Disruption and Translocation of Salmonella Infantis
Abstract
1. Introduction
2. Results
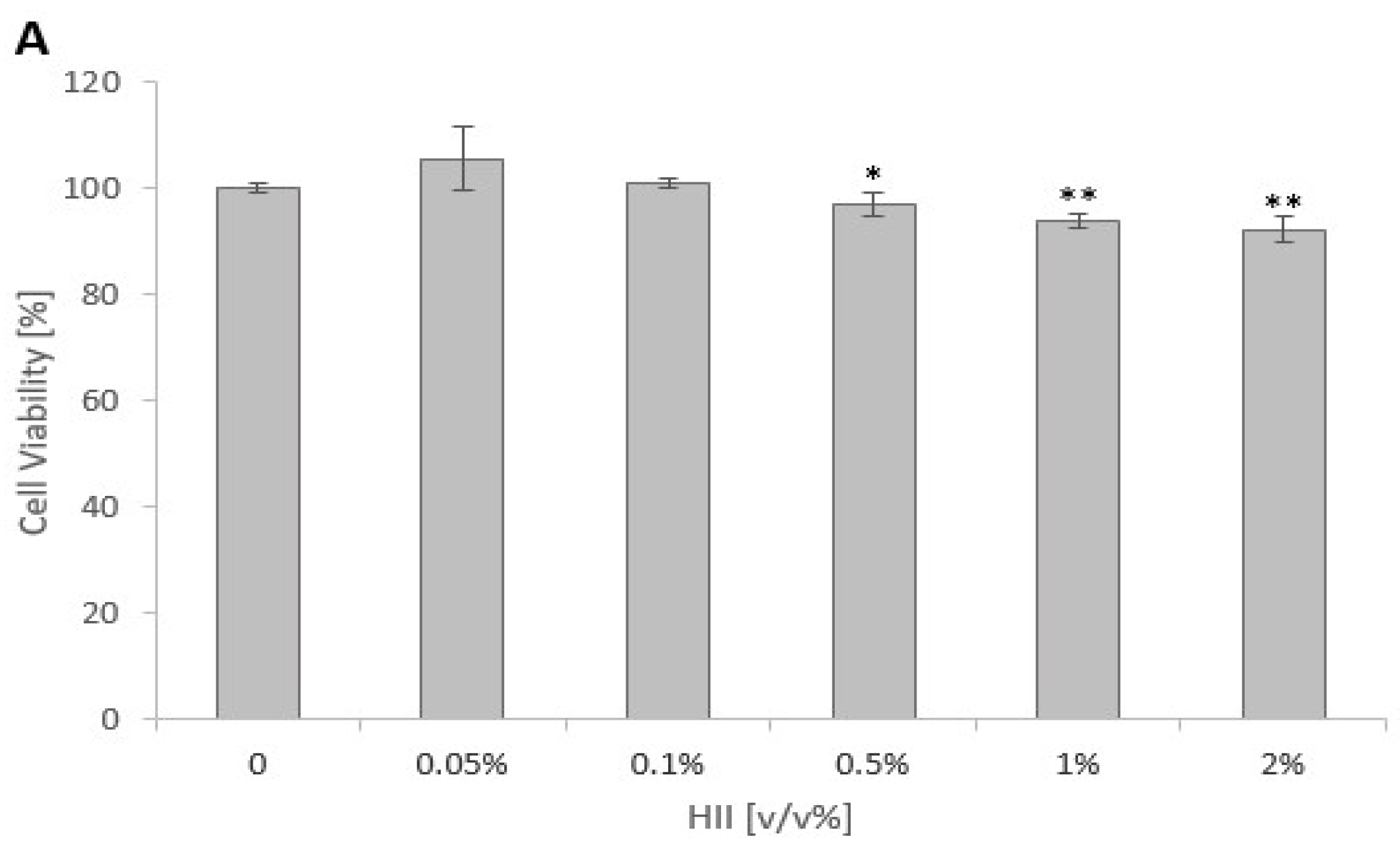
2.1. Cytotoxicity of HII and HKB
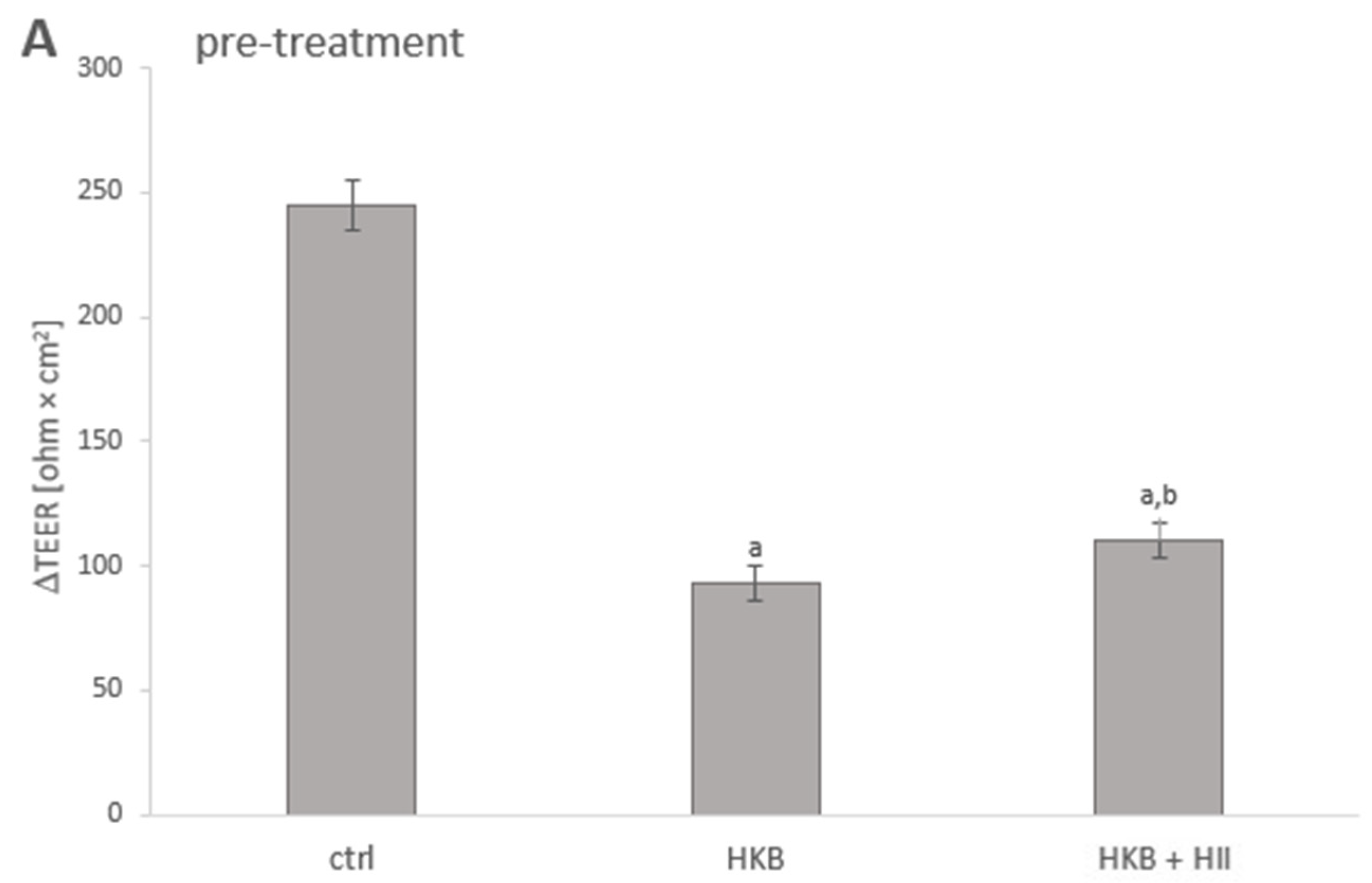
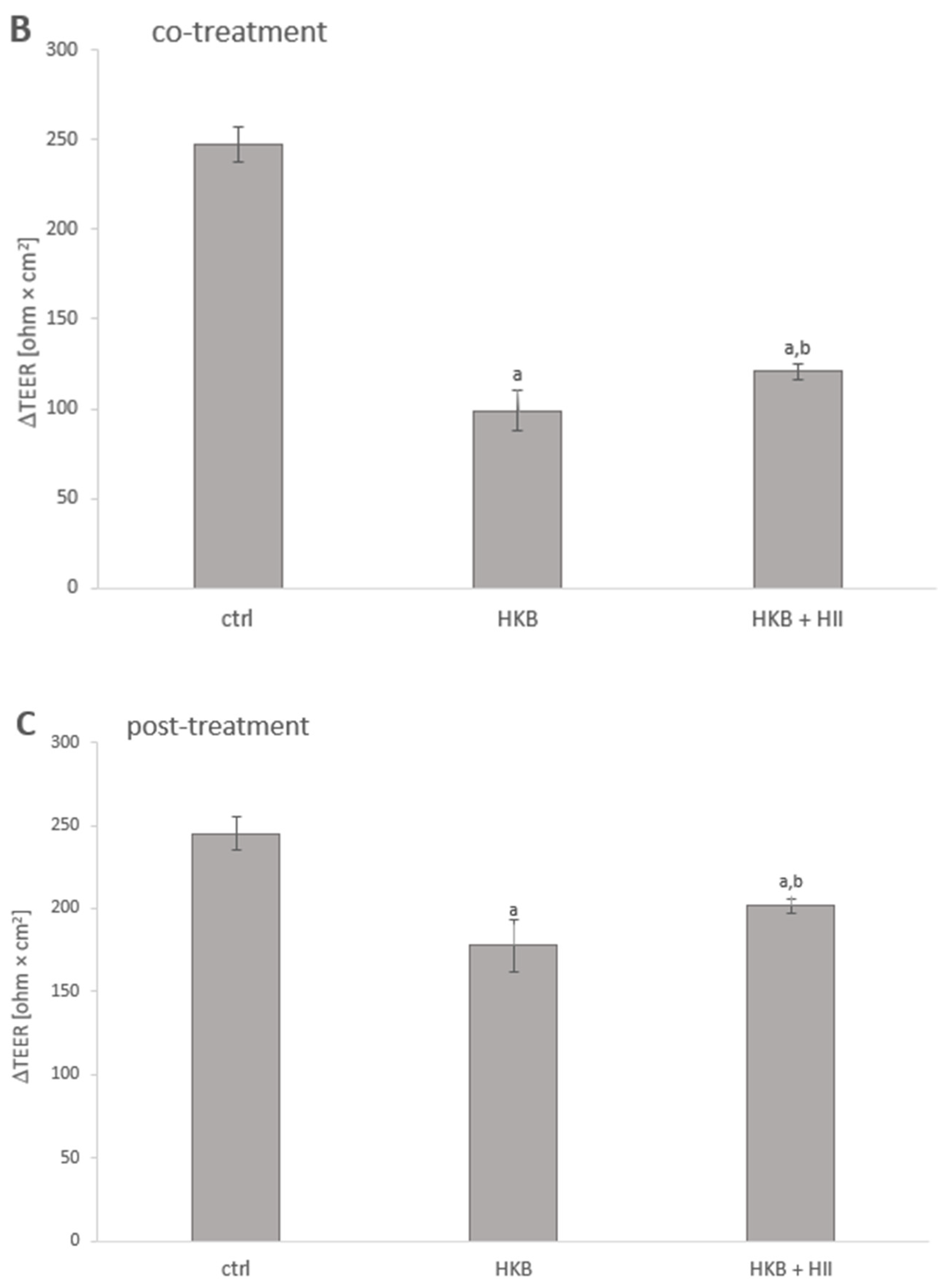
2.2. HII Improved HKB-Induced Integrity Disruption in Caco-2 Monolayer
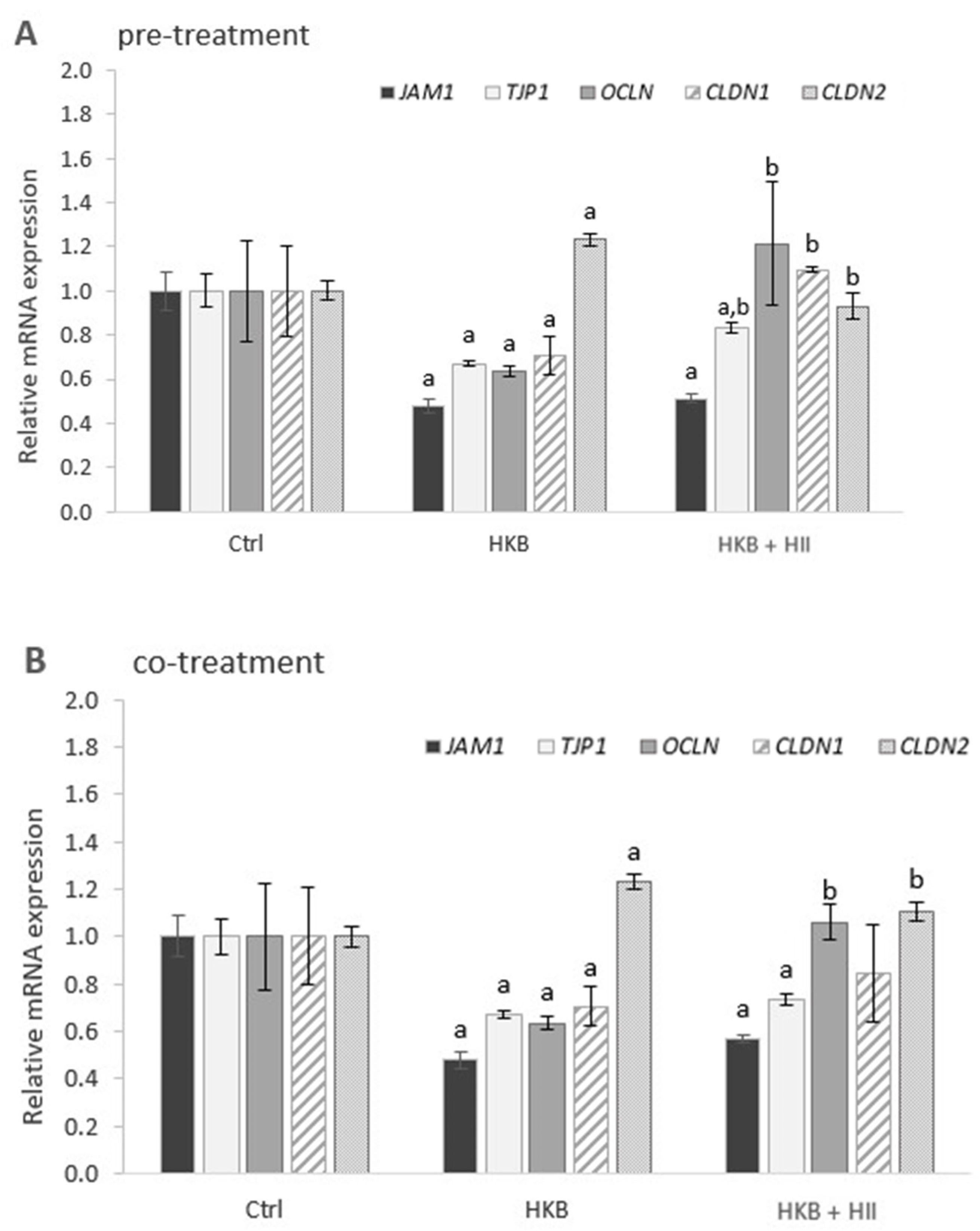
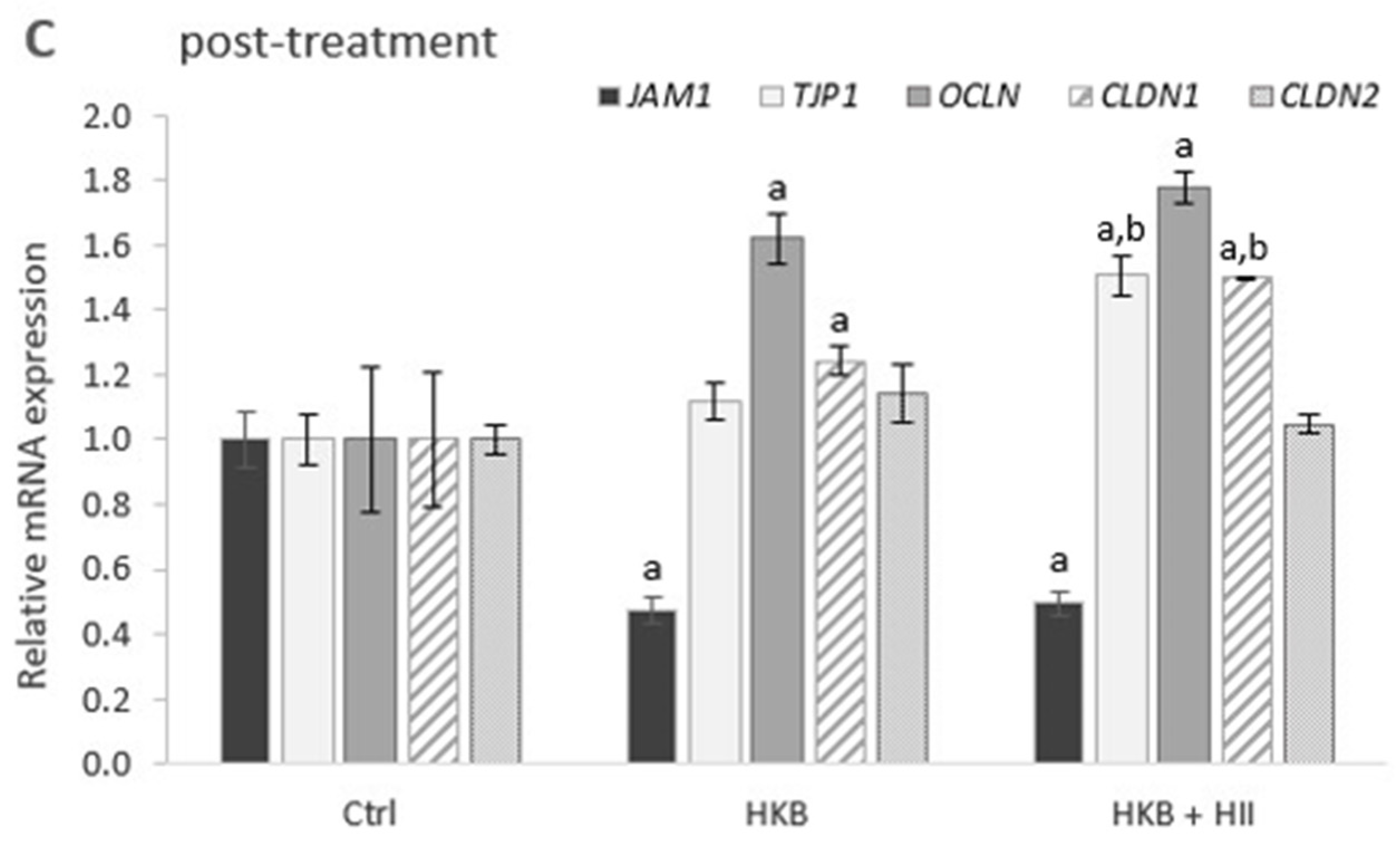
2.3. HII Improves HKB-Induced Down-Regulation of Genes Coding for Tight-Junction Proteins
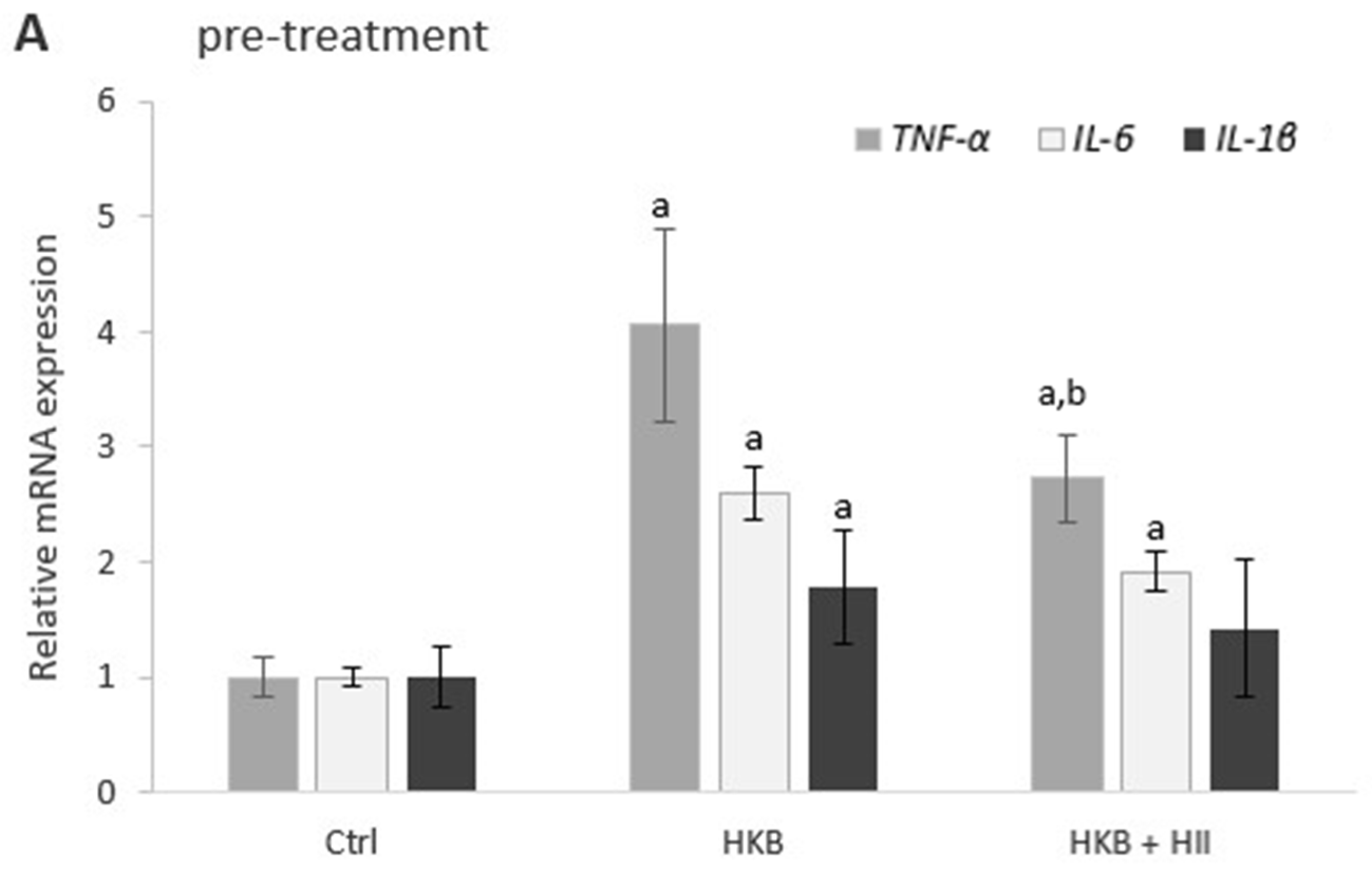
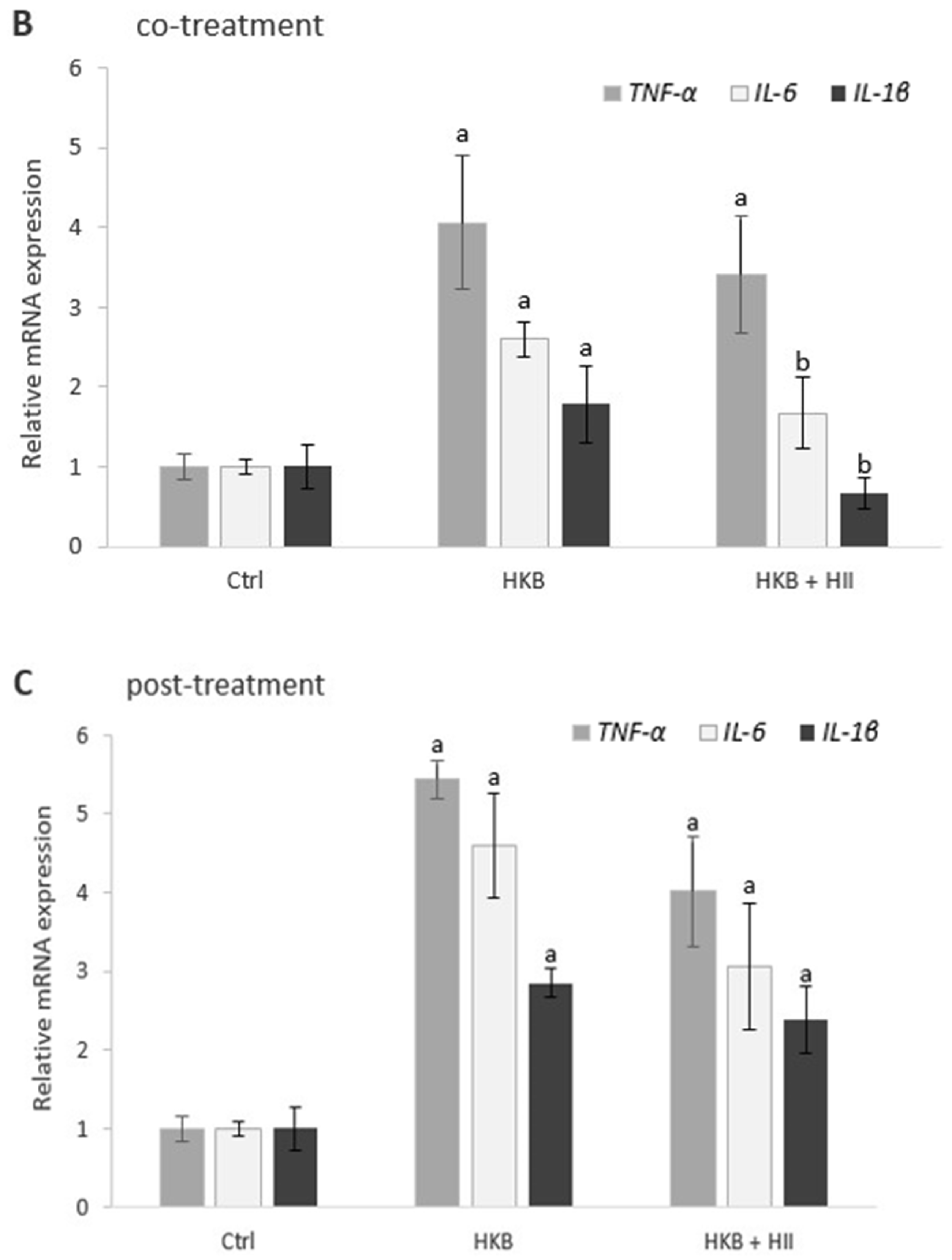
2.4. HII Reduces HKB-Induced Inflammation Response
2.5. HII Prevents Translocation of S. Infantis
3. Discussion
4. Materials and Methods
4.1. Plant Material and Infusion Preparation
4.2. Preparation of Heat-Killed Bacteria
4.3. Cell Culture
4.4. Cell Viability Assay
4.5. Intestinal Epithelial Barrier Function Measurements
4.6. RNA Isolation and RT-qPCR Analysis
4.7. Adhesion, Invasion, and Translocation of S. Infantis
4.7.1. Bacterial Culture
4.7.2. Infection Assay
4.8. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ji, J.; Qu, H.; Shu, D. Crosstalk Between Bioactive Peptide and Intestinal Barrier in Gut Homeostasis. Curr. Protein Pept. Sci. 2015, 16, 604–612. [Google Scholar] [CrossRef] [PubMed]
- König, J.; Wells, J.; Cani, P.D.; García-Ródenas, C.L.; MacDonald, T.; Mercenier, A.; Whyte, J.; Troost, F.; Brummer, R.-J. Human Intestinal Barrier Function in Health and Disease. Clin. Transl. Gastroenterol. 2016, 7, e196. [Google Scholar] [CrossRef] [PubMed]
- Chelakkot, C.; Ghim, J.; Ryu, S.H. Mechanisms Regulating Intestinal Barrier Integrity and Its Pathological Implications. Exp. Mol. Med. 2018, 50, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Wan, M.L.Y.; Forsythe, S.J.; El-Nezami, H. Probiotics Interaction with Foodborne Pathogens: A Potential Alternative to Antibiotics and Future Challenges. Crit. Rev. Food Sci. Nutr. 2019, 59, 3320–3333. [Google Scholar] [CrossRef]
- Rose, E.C.; Odle, J.; Blikslager, A.T.; Ziegler, A.L. Probiotics, Prebiotics and Epithelial Tight Junctions: A Promising Approach to Modulate Intestinal Barrier Function. Int. J. Mol. Sci. 2021, 22, 6729. [Google Scholar] [CrossRef]
- Paradis, T.; Bègue, H.; Basmaciyan, L.; Dalle, F.; Bon, F. Tight Junctions as a Key for Pathogens Invasion in Intestinal Epithelial Cells. Int. J. Mol. Sci. 2021, 22, 2506. [Google Scholar] [CrossRef]
- Di Tommaso, N.; Gasbarrini, A.; Ponziani, F.R. Intestinal Barrier in Human Health and Disease. Int. J. Environ. Res. Public Health 2021, 18, 12836. [Google Scholar] [CrossRef]
- Gómez, J.M.R. Chapter 2—Early Colonization of the Human Gut. In Human-Gut Microbiome; Goel, G., Requena, T., Bansal, S., Eds.; Developments in Microbiology; Academic Press: Cambridge, MA, USA, 2022; pp. 15–36. ISBN 978-0-323-91313-3. [Google Scholar]
- Jin, S.; Wetzel, D.; Schirmer, M. Deciphering Mechanisms and Implications of Bacterial Translocation in Human Health and Disease. Curr. Opin. Microbiol. 2022, 67, 102147. [Google Scholar] [CrossRef]
- Alexander, C.; Rietschel, E.T. Bacterial Lipopolysaccharides and Innate Immunity. J. Endotoxin Res. 2001, 7, 167–202. [Google Scholar] [CrossRef]
- André, P.; Laugerette, F.; Féart, C. Metabolic Endotoxemia: A Potential Underlying Mechanism of the Relationship between Dietary Fat Intake and Risk for Cognitive Impairments in Humans? Nutrients 2019, 11, 1887. [Google Scholar] [CrossRef]
- Dmytriv, T.R.; Storey, K.B.; Lushchak, V.I. Intestinal Barrier Permeability: The Influence of Gut Microbiota, Nutrition, and Exercise. Front. Physiol. 2024, 15, 1380713. [Google Scholar] [CrossRef] [PubMed]
- Cani, P.D.; Amar, J.; Iglesias, M.A.; Poggi, M.; Knauf, C.; Bastelica, D.; Neyrinck, A.M.; Fava, F.; Tuohy, K.M.; Chabo, C.; et al. Metabolic Endotoxemia Initiates Obesity and Insulin Resistance. Diabetes 2007, 56, 1761–1772. [Google Scholar] [CrossRef] [PubMed]
- Brown, B.I. Nutritional Management of Metabolic Endotoxemia: A Clinical Review. Altern. Ther. Health Med. 2017, 23, 42–54. [Google Scholar] [PubMed]
- Wu, X.-X.; Huang, X.-L.; Chen, R.-R.; Li, T.; Ye, H.-J.; Xie, W.; Huang, Z.-M.; Cao, G.-Z. Paeoniflorin Prevents Intestinal Barrier Disruption and Inhibits Lipopolysaccharide (LPS)-Induced Inflammation in Caco-2 Cell Monolayers. Inflammation 2019, 42, 2215–2225. [Google Scholar] [CrossRef]
- Cassotta, M.; Cianciosi, D.; De Giuseppe, R.; Navarro-Hortal, M.D.; Armas Diaz, Y.; Forbes-Hernández, T.Y.; Pifarre, K.T.; Pascual Barrera, A.E.; Grosso, G.; Xiao, J.; et al. Possible Role of Nutrition in the Prevention of Inflammatory Bowel Disease–Related Colorectal Cancer: A Focus on Human Studies. Nutrition 2023, 110, 111980. [Google Scholar] [CrossRef]
- Kramberger, K.; Barlič-Maganja, D.; Bandelj, D.; Baruca Arbeiter, A.; Peeters, K.; Miklavčič Višnjevec, A.; Jenko Pražnikar, Z. HPLC-DAD-ESI-QTOF-MS Determination of Bioactive Compounds and Antioxidant Activity Comparison of the Hydroalcoholic and Water Extracts from Two Helichrysum italicum Species. Metabolites 2020, 10, 403. [Google Scholar] [CrossRef]
- Appendino, G.; Ottino, M.; Marquez, N.; Bianchi, F.; Giana, A.; Ballero, M.; Sterner, O.; Fiebich, B.L.; Munoz, E. Arzanol, an Anti-Inflammatory and Anti-HIV-1 Phloroglucinol Alpha-Pyrone from Helichrysum italicum ssp. Microphyllum. J. Nat. Prod. 2007, 70, 608–612. [Google Scholar] [CrossRef]
- Kramberger, K.; Kenig, S.; Jenko Pražnikar, Z.; Kočevar Glavač, N.; Barlič-Maganja, D. A Review and Evaluation of the Data Supporting Internal Use of Helichrysum italicum. Plants 2021, 10, 1738. [Google Scholar] [CrossRef]
- Plamada, D.; Vodnar, D.C. Polyphenols—Gut Microbiota Interrelationship: A Transition to a New Generation of Prebiotics. Nutrients 2022, 14, 137. [Google Scholar] [CrossRef]
- Pereira, C.G.; Barreira, L.; Bijttebier, S.; Pieters, L.; Neves, V.; Rodrigues, M.; Rivas, R.; Varela, J.; Custodio, L. Chemical Profiling of Infusions and Decoctions of Helichrysum italicum subsp. Picardii by UHPLC-PDA-MS and in Vitro Biological Activities Comparatively with Green Tea (Camellia sinensis) and Rooibos Tisane (Aspalathus linearis). J. Pharm. Biomed. Anal. 2017, 145, 593–603. [Google Scholar] [CrossRef]
- Kenig, S.; Kramberger, K.; Novak, K.Š.; Karnjuš, I.; Bandelj, D.; Petelin, A.; Pražnikar, Z.J. Helichrysum italicum (Roth) G. Don and Helichrysum arenarium (L.) Moench Infusions in Reversing the Traits of Metabolic Syndrome: A Double-Blind Randomized Comparative Trial. Food Funct. 2022, 13, 7697–7706. [Google Scholar] [CrossRef] [PubMed]
- Petelin, A.; Šik Novak, K.; Hladnik, M.; Bandelj, D.; Baruca Arbeiter, A.; Kramberger, K.; Kenig, S.; Jenko Pražnikar, Z. Helichrysum italicum (Roth) G. Don and Helichrysum arenarium (L.) Moench Infusion Consumption Affects the Inflammatory Status and the Composition of Human Gut Microbiota in Patients with Traits of Metabolic Syndrome: A Randomized Comparative Study. Foods 2022, 11, 3277. [Google Scholar] [CrossRef] [PubMed]
- Kramberger, K.; Barlič-Maganja, D.; Pražnikar, Z.J.; Režen, T.; Rozman, D.; Pražnikar, J.; Kenig, S. Whole Transcriptome Expression Array Analysis of Human Colon Fibroblasts Culture Treated with Helichrysum italicum Supports Its Use in Traditional Medicine. J. Ethnopharmacol. 2022, 296, 115505. [Google Scholar] [CrossRef] [PubMed]
- Putt, K.K.; Pei, R.; White, H.M.; Bolling, B.W. Yogurt Inhibits Intestinal Barrier Dysfunction in Caco-2 Cells by Increasing Tight Junctions. Food Funct. 2017, 8, 406–414. [Google Scholar] [CrossRef]
- Valdez, J.C.; Cho, J.; Bolling, B.W. Aronia Berry Inhibits Disruption of Caco-2 Intestinal Barrier Function. Arch. Biochem. Biophys. 2020, 688, 108409. [Google Scholar] [CrossRef]
- Feldman, G.J.; Mullin, J.M.; Ryan, M.P. Occludin: Structure, Function and Regulation. Adv. Drug Deliv. Rev. 2005, 57, 883–917. [Google Scholar] [CrossRef]
- Kuo, W.-T.; Shen, L.; Zuo, L.; Shashikanth, N.; Ong, M.L.D.M.; Wu, L.; Zha, J.; Edelblum, K.L.; Wang, Y.; Wang, Y.; et al. Inflammation-Induced Occludin Downregulation Limits Epithelial Apoptosis by Suppressing Caspase 3 Expression. Gastroenterology 2019, 157, 1323–1337. [Google Scholar] [CrossRef]
- Yu, S.; He, J.; Xie, K. Zonula Occludens Proteins Signaling in Inflammation and Tumorigenesis. Int. J. Biol. Sci. 2023, 19, 3804–3815. [Google Scholar] [CrossRef]
- Laukoetter, M.G.; Nava, P.; Lee, W.Y.; Severson, E.A.; Capaldo, C.T.; Babbin, B.A.; Williams, I.R.; Koval, M.; Peatman, E.; Campbell, J.A.; et al. JAM-A Regulates Permeability and Inflammation in the Intestine in Vivo. J. Exp. Med. 2007, 204, 3067–3076. [Google Scholar] [CrossRef]
- Xia, Y.; Cao, H.; Zheng, J.; Chen, L. Claudin-1 Mediated Tight Junction Dysfunction as a Contributor to Atopic March. Front. Immunol. 2022, 13, 927465. [Google Scholar] [CrossRef]
- Ahmad, R.; Chaturvedi, R.; Olivares-Villagómez, D.; Habib, T.; Asim, M.; Shivesh, P.; Polk, D.B.; Wilson, K.T.; Washington, M.K.; Van Kaer, L.; et al. Targeted Colonic Claudin-2 Expression Renders Resistance to Epithelial Injury, Induces Immune Suppression, and Protects from Colitis. Mucosal Immunol. 2014, 7, 1340–1353. [Google Scholar] [CrossRef] [PubMed]
- Wan, M.L.Y.; Ling, K.H.; Wang, M.F.; El-Nezami, H. Green Tea Polyphenol Epigallocatechin-3-Gallate Improves Epithelial Barrier Function by Inducing the Production of Antimicrobial Peptide pBD-1 and pBD-2 in Monolayers of Porcine Intestinal Epithelial IPEC-J2 Cells. Mol. Nutr. Food Res. 2016, 60, 1048–1058. [Google Scholar] [CrossRef] [PubMed]
- Semple, F.; Dorin, J.R. β-Defensins: Multifunctional Modulators of Infection, Inflammation and More? J. Innate Immun. 2012, 4, 337–348. [Google Scholar] [CrossRef] [PubMed]
- Boyle, E.C.; Brown, N.F.; Finlay, B.B. Salmonella Enterica Serovar Typhimurium Effectors SopB, SopE, SopE2 and SipA Disrupt Tight Junction Structure and Function. Cell Microbiol. 2006, 8, 1946–1957. [Google Scholar] [CrossRef]
- Jepson, M.A.; Schlecht, H.B.; Collares-Buzato, C.B. Localization of Dysfunctional Tight Junctions in Salmonella Enterica Serovar Typhimurium-Infected Epithelial Layers. Infect. Immun. 2000, 68, 7202–7208. [Google Scholar] [CrossRef]
- Karacan Sever, N.; Akan, M. Molecular Analysis of Virulence Genes of Salmonella Infantis Isolated from Chickens and Turkeys. Microb. Pathog. 2019, 126, 199–204. [Google Scholar] [CrossRef]
- Sun, L.; Yang, S.; Deng, Q.; Dong, K.; Li, Y.; Wu, S.; Huang, R. Salmonella Effector SpvB Disrupts Intestinal Epithelial Barrier Integrity for Bacterial Translocation. Front. Cell. Infect. Microbiol. 2020, 10, 606541. [Google Scholar] [CrossRef]
- Marino, M.; Venturi, S.; Rendine, M.; Porrini, M.; Gardana, C.; Klimis-Zacas, D.; Del Bo’, C.; Riso, P. Wild Blueberry (V. angustifolium) Improves TNFα-Induced Cell Barrier Permeability through Claudin-1 and Oxidative Stress Modulation in Caco-2 Cells. Food Funct. 2023, 14, 7387–7399. [Google Scholar] [CrossRef]
- Kramberger, K.; Jenko Pražnikar, Z.; Baruca Arbeiter, A.; Petelin, A.; Bandelj, D.; Kenig, S. A Comparative Study of the Antioxidative Effects of Helichrysum italicum and Helichrysum arenarium Infusions. Antioxidants 2021, 10, 380. [Google Scholar] [CrossRef]
- Wang, S.; Guo, L.; Gu, F.; Bao, J.; Guo, Y.; Zhang, Y.; Wang, Z.; Li, R.; Wu, Z.; Li, J. Quercetin Restores Respiratory Mucosal Barrier Dysfunction in Mycoplasma Gallisepticum-Infected Chicks by Enhancing Th2 Immune Response. Phytomedicine 2024, 133, 155953. [Google Scholar] [CrossRef]
- Cornelius, V.; Droessler, L.; Amasheh, S. Quercetin Improves Barrier Properties in Porcine Small Intestine but Not in Peyer’s Patches. Int. J. Mol. Sci. 2024, 25, 1530. [Google Scholar] [CrossRef] [PubMed]
- Fan, J.; Zhao, X.-H.; Zhao, J.-R.; Li, B.-R. Galangin and Kaempferol Alleviate the Indomethacin-Caused Cytotoxicity and Barrier Loss in Rat Intestinal Epithelial (IEC-6) Cells Via Mediating JNK/Src Activation. ACS Omega 2021, 6, 15046–15056. [Google Scholar] [CrossRef] [PubMed]
- Song, L.; Wu, T.; Zhang, L.; Wan, J.; Ruan, Z. Chlorogenic Acid Improves the Intestinal Barrier by Relieving Endoplasmic Reticulum Stress and Inhibiting ROCK/MLCK Signaling Pathways. Food Funct. 2022, 13, 4562–4575. [Google Scholar] [CrossRef] [PubMed]
- Leláková, V.; Béraud-Dufour, S.; Hošek, J.; Šmejkal, K.; Prachyawarakorn, V.; Pailee, P.; Widmann, C.; Václavík, J.; Coppola, T.; Mazella, J.; et al. Therapeutic Potential of Prenylated Stilbenoid Macasiamenene F through Its Anti-Inflammatory and Cytoprotective Effects on LPS-Challenged Monocytes and Microglia. J. Ethnopharmacol. 2020, 263, 113147. [Google Scholar] [CrossRef]
- Karimi Ardestani, S.; Tafvizi, F.; Tajabadi Ebrahimi, M. Heat-Killed Probiotic Bacteria Induce Apoptosis of HT-29 Human Colon Adenocarcinoma Cell Line via the Regulation of Bax/Bcl2 and Caspases Pathway. Hum. Exp. Toxicol. 2019, 38, 1069–1081. [Google Scholar] [CrossRef]
- Srinivasan, B.; Kolli, A.R.; Esch, M.B.; Abaci, H.E.; Shuler, M.L.; Hickman, J.J. TEER Measurement Techniques for in Vitro Barrier Model Systems. J. Lab. Autom. 2015, 20, 107–126. [Google Scholar] [CrossRef]
- Spandidos, A.; Wang, X.; Wang, H.; Seed, B. PrimerBank: A Resource of Human and Mouse PCR Primer Pairs for Gene Expression Detection and Quantification. Nucleic Acids Res. 2010, 38, D792–D799. [Google Scholar] [CrossRef]
- Vreeburg, R.A.M.; Bastiaan-Net, S.; Mes, J.J. Normalization Genes for Quantitative RT-PCR in Differentiated Caco-2 Cells Used for Food Exposure Studies. Food Funct. 2011, 2, 124–129. [Google Scholar] [CrossRef]
- Papić, B.; Kušar, D.; Mićunović, J.; Pirš, M.; Ocepek, M.; Avberšek, J. Clonal Spread of pESI-Positive Multidrug-Resistant ST32 Salmonella Enterica Serovar Infantis Isolates among Broilers and Humans in Slovenia. Microbiol. Spectr. 2022, 10, e0248122. [Google Scholar] [CrossRef]
- Bezek, K.; Kramberger, K.; Barlič-Maganja, D. Antioxidant and Antimicrobial Properties of Helichrysum italicum (Roth) G. Don Hydrosol. Antibiotics 2022, 11, 1017. [Google Scholar] [CrossRef]
- Šikić Pogačar, M.; Langerholc, T.; Mičetić-Turk, D.; Možina, S.S.; Klančnik, A. Effect of Lactobacillus Spp. on Adhesion, Invasion, and Translocation of Campylobacter Jejuni in Chicken and Pig Small-Intestinal Epithelial Cell Lines. BMC Vet. Res. 2020, 16, 34. [Google Scholar] [CrossRef]

| Positive Control (S. Inf) | (a) S. Inf and HII | (b) 24 h HII + S. Inf | (c) 24 h HII + S. Inf and HII | |
|---|---|---|---|---|
| Adhesion | 1.67 ± 0.30 | 1.74 ± 0.19 | 1.65 ± 0.30 | 1.63 ± 0.30 |
| Invasion | 5.99 ± 0.18 | 6.07 ± 0.27 | 6.31 ± 0.30 | 6.02 ± 0.17 |
| Translocation | 3.10 ± 0.52 | 3.49 ± 0.19 | 0.44 ± 0.88 * | 0 * |
| Gene Name | Primer Sequence |
|---|---|
| TNF-α | F: 5′-CCTCTCTCTAATCAGCCCTCTG-3′ |
| R: 5′-GAGGACCTGGGAGTAGATGAG-3′ | |
| IL-6 | F: 5′- ACTCACCTCTTCAGAACGAATTG-3′ |
| R: 5′-CCATCTTTGGAAGGTTCAGGTTG-3′ | |
| IL-1β | F: 5′-ATGATGGCTTATTACAGTGGCAA-3′ |
| R: 5′-GTCGGAGATTCGTAGCTGGA-3′ | |
| TJP1 | F: 5′-CAACATACAGTGACGCTTCACA-3′ R: 5′-CACTATTGACGTTTCCCCACTC-3′ |
| CLDN1 | F: 5′-GGCAACTAAAATAGCCAGACC-3′ R: 5′-CCTCCTGGGAGTGATAGCAAT-3′ |
| CLDN2 | F: 5′-CGGGACTTCTACTCACCACTG-3′ R: 5′-GGATGATTCCAGCTATCAGGGA-3′ |
| OCLN | F: 5′-ACAAGCGGTTTTATCCAGAGTC-3′ R: 5′-GTCATCCACAGGCGAAGTTAAT-3′ |
| JAM1 | F: 5′-ACCTTCTTGCCAACTGGTATCA-3′ R: 5′-AGCACGATGAGCTTGACCTTG-3′ |
| GAPDH | F: 5′-GGAGCGAGATCCCTCCAAAAT-3′ R: 5′-GGCTGTTGTCATACTTCTCATGG-3′ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kramberger, K.; Bezek Kranjc, K.; Jenko Pražnikar, Z.; Barlič-Maganja, D.; Kenig, S. Protective Capacity of Helichrysum italicum Infusion Against Intestinal Barrier Disruption and Translocation of Salmonella Infantis. Pharmaceuticals 2024, 17, 1398. https://doi.org/10.3390/ph17101398
Kramberger K, Bezek Kranjc K, Jenko Pražnikar Z, Barlič-Maganja D, Kenig S. Protective Capacity of Helichrysum italicum Infusion Against Intestinal Barrier Disruption and Translocation of Salmonella Infantis. Pharmaceuticals. 2024; 17(10):1398. https://doi.org/10.3390/ph17101398
Chicago/Turabian StyleKramberger, Katja, Katja Bezek Kranjc, Zala Jenko Pražnikar, Darja Barlič-Maganja, and Saša Kenig. 2024. "Protective Capacity of Helichrysum italicum Infusion Against Intestinal Barrier Disruption and Translocation of Salmonella Infantis" Pharmaceuticals 17, no. 10: 1398. https://doi.org/10.3390/ph17101398
APA StyleKramberger, K., Bezek Kranjc, K., Jenko Pražnikar, Z., Barlič-Maganja, D., & Kenig, S. (2024). Protective Capacity of Helichrysum italicum Infusion Against Intestinal Barrier Disruption and Translocation of Salmonella Infantis. Pharmaceuticals, 17(10), 1398. https://doi.org/10.3390/ph17101398







