Comparative Chemical Analysis and Bioactive Properties of Aqueous and Glucan-Rich Extracts of Three Widely Appreciated Mushrooms: Agaricus bisporus (J.E.Lange) Imbach, Laetiporus sulphureus (Bull.) Murill and Agrocybe aegerita (V. Brig.) Vizzini
Abstract
1. Introduction
2. Results and Discussion
2.1. Total α-Glucan and β-Glucan Content in AE and PE
2.2. Total Protein Content of AE and PE
2.3. Total Phenolic Content (TPC) of AE and PE
2.4. Protein Profile of Mushroom AE
2.5. UHPLC–QToF-MS Profiling of Mushroom Extracts
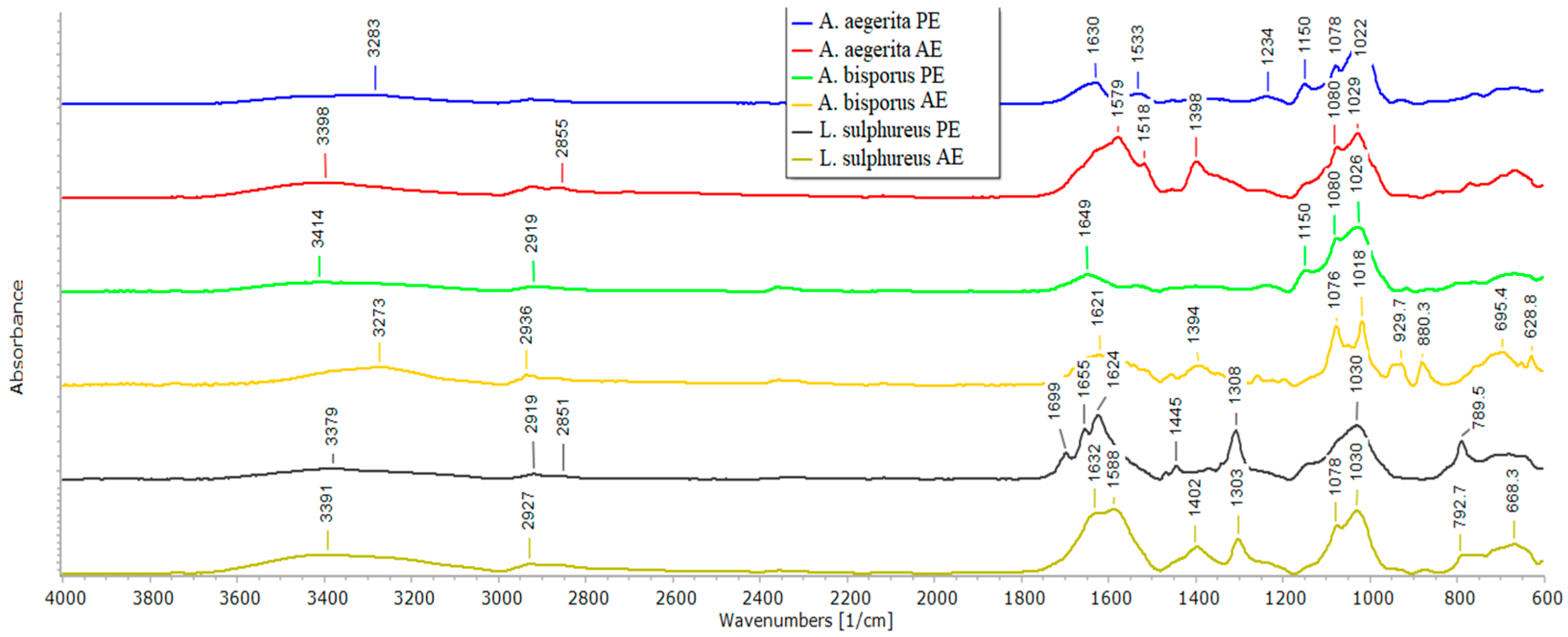
2.6. ATR-FTIR Spectroscopy of Mushroom AE and PE
2.7. Antioxidant Activity
2.8. Cytotoxicity of Extracts
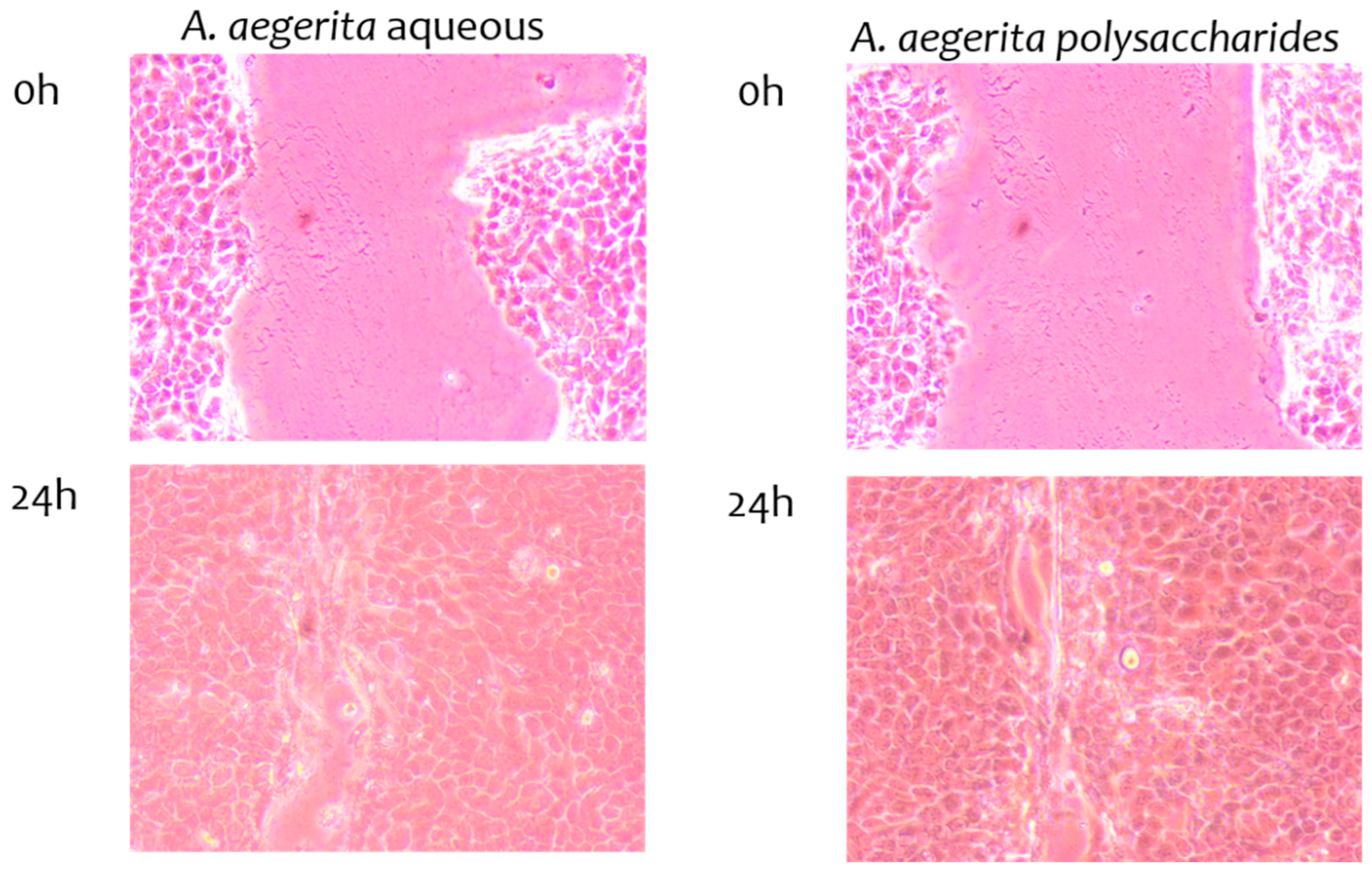
2.9. Wound-Healing Properties of Extracts
2.10. Anti-Inflammatory Properties of Extracts
2.11. Antimicrobial Activity Assays
2.11.1. Antibacterial Activity
| Microorganisms | A. aegerita AE | A. bisporus AE | L. sulphureus AE | A. aegerita PE | A. bisporus PE | L. sulphureus PE | E211 | E224 | |
|---|---|---|---|---|---|---|---|---|---|
| S. aureus (ATCC 11632) | MIC | 4.00 c | 4.00 c | 4.00 c | 4.00 c | 2.00 b | 4.00 c | 4.00 c | 1.00 a |
| MBC | 8.00 c | 8.00 c | 8.00 c | 8.00 c | 4.00 b | 8.00 c | 4.00 b | 1.00 a | |
| B. cereus (clinical isolate) | MIC | 1.00 b | 2.00 c | 2.00 c | 4.00 d | 2.00 b | 4.00 c | 0.50 a | 2.00 b |
| MBC | 2.00 b | 4.00 c | 4.00 c | 8.00 d | 4.00 c | 8.00 d | 0.50 a | 4.00 c | |
| L. monocytogenes (NCTC 7973) | MIC | 4.00 d | 4.00 d | 2.00 c | 4.00 d | 2.00 c | 4.00 d | 1.00 b | 0.50 a |
| MBC | 8.00 d | 8.00 d | 4.00 c | 8.00 d | 4.00 c | 8.00 d | 2.00 b | 1.00 a | |
| L. monocytogenes (ATCC 13932) | MIC | 1.00 b | 1.00 b | 1.00 b | 1.00 b | 1.00 b | 1.00 b | 0.50 a | 0.50 a |
| MBC | 2.00 b | 2.00 b | 2.00 b | 2.00 b | 2.00 b | 2.00 b | 1.00 a | 1.00 a | |
| L. monocytogenes (ATCC 15313) | MIC | 0.50 a | 0.50 a | 0.50 a | 0.50 a | 1.00 b | 0.50 a | 0.50 a | 1.00 b |
| MBC | 1.00 a | 1.00 a | 1.00 a | 1.00 a | 2.00 b | 1.00 a | 1.00 a | 2.00 b | |
| L. monocytogenes (ATCC 19111) | MIC | 1.00 a | 1.00 a | 1.00 a | 1.00 a | 1.00 a | 1.00 a | 1.00 a | 1.00 a |
| MBC | 2.00 a | 2.00 a | 2.00 a | 2.00 a | 2.00 a | 2.00 a | 2.00 a | 2.00 a | |
| L. monocytogenes (ATCC 35152) | MIC | 1.00 b | 1.00 b | 1.00 b | 1.00 b | 1.00 b | 1.00 b | 0.50 a | 1.00 b |
| MBC | 2.00 b | 2.00 b | 2.00 b | 2.00 b | 2.00 b | 2.00 b | 1.00 a | 2.00 b | |
| Y. enterocolitica (ATCC 23715) | MIC | 0.50 b | 0.25 a | 0.50 b | 0.50 b | 0.50 b | 0.50 b | 0.50 b | 0.50 b |
| MBC | 1.00 b | 0.50 a | 1.00 b | 1.00 b | 1.00 b | 1.00 b | 1.00 b | 1.00 b | |
| Y. enterocolitica (ATCC 9610) | MIC | 0.50 b | 0.50 b | 0.25 a | 0.50 b | 0.25 a | 0.25 a | 1.00 c | 0.50 b |
| MBC | 1.00 b | 1.00 b | 0.50 a | 1.00 b | 0.50 a | 1.00 b | 2.00 c | 1.00 b | |
| E. coli (ATCC 25922) | MIC | 4.00 d | 4.00 d | 2.00 c | 4.00 d | 2.00 c | 4.00 d | 1.00 b | 0.50 a |
| MBC | 8.00 d | 8.00 d | 4.00 c | 8.00 d | 4.00 c | 8.00 d | 2.00 b | 1.00 a | |
| E. coli (ATCC 11775) | MIC | 1.00 b | 1.00 b | 1.00 b | 1.00 b | 1.00 b | 1.00 b | 0.50 a | 0.50 a |
| MBC | 2.00 b | 2.00 b | 2.00 b | 2.00 b | 2.00 b | 2.00 b | 1.00 a | 1.00 a | |
| E. coli O157:H (ATCC 700728) | MIC | 1.00 b | 1.00 b | 1.00 b | 1.00 b | 1.00 b | 1.00 b | 1.00 b | 0.50 a |
| MBC | 2.00 b | 2.00 b | 2.00 b | 2.00 b | 2.00 b | 2.00 b | 2.00 b | 1.00 a | |
| E. coli O157:H (ATCC 43888) | MIC | 1.00 b | 1.00 b | 1.00 b | 1.00 b | 1.00 b | 1.00 b | 0.50 a | 1.00 b |
| MBC | 2.00 b | 2.00 b | 2.00 b | 2.00 b | 2.00 b | 2.00 b | 1.00 a | 2.00 b | |
| S. Typhimurium (ATCC 13311) | MIC | 4.00 c | 4.00 c | 2.00 b | 4.00 c | 2.00 b | 4.00 c | 1.00 a | 1.00 a |
| MBC | 8.00 d | 8.00 d | 4.00 c | 8.00 d | 4.00 c | 8.00 d | 2.00 b | 1.00 a | |
| E. cloacae (ATCC 35030) | MIC | 4.00 c | 8.00 d | 2.00 b | 4.00 c | 2.00 b | 4.00 c | 2.00 b | 0.50 a |
| MBC | 8.00 c | 8.00 c | 4.00 b | 8.00 c | 4.00 b | 8.00 c | 4.00 b | 0.50 a |
2.11.2. Antifungal Activity of Extracts
| A. fumigatus (ATCC 9197) | A. versicolor (ATCC 11730) | A. niger (ATCC 6275) | P. funiculosum (ATCC 36839) | P. verrucosum var. cyclopium (food isolate) | T. viride (IAM 5061) | ||
|---|---|---|---|---|---|---|---|
| A. aegerita AE | MIC | 2.00 c | 2.00 c | 1.00 a | 2.00 c | 2.00 b | 1.00 b |
| MFC | 4.00 | 4.00 c | 2.00 b | 4.00 c | 4.00 b | 2.00 b | |
| A. bisporus AE | MIC | 1.00 b | 2.00 c | 1.00 a | 2.00 c | 2.00 b | 1.00 b |
| MFC | 2.00 b | 4.00 c | 2.00 b | 4.00 c | 4.00 b | 2.00 b | |
| L. sulphureus AE | MIC | 0.50 a | 1.00 b | 1.00 a | >8.00 e | >8.00 c | 1.00 b |
| MFC | 1.00 a | 2.00 b | 2.00 b | >8.00 e | >8.00 c | 2.00 b | |
| A. aegerita PE | MIC | 0.50 a | 0.50 a | 1.00 a | 4.00 d | >8.00 c | 1.00 b |
| MFC | 1.00 a | 1.00 a | 2.00 b | 8.00 d | >8.00 c | 2.00 b | |
| A. bisporus PE | MIC | >8.00 c | 2.0 0c | >8.00 b | 4.00 d | >8.00 c | 1.00 b |
| MFC | >8.00 c | 2.00 b | >8.00 c | 8.00 d | >8.00 c | 2.00 b | |
| L. sulphureus PE | MIC | 0.50 a | 0.50 a | 1.00 a | 4.00 d | >8.00 c | 1.00 b |
| MFC | 1.00 a | 1.00 a | 2.00 b | 8.00 d | >8.00 c | 2.00 b | |
| E211 | MIC | 1.00 b | 2.00 c | 1.00 a | 1.00 b | 2.00 b | 1.00 b |
| MFC | 2.00 b | 4.00 c | 2.00 b | 2.00 b | 4.00 b | 2.00 b | |
| E224 | MIC | 1.00 b | 1.00 b | 1.00 a | 0.50 a | 1.00 a | 0.50 a |
| MFC | 1.00 a | 1.00 a | 1.00 a | 0.50 a | 1.00 a | 0.50 a |
2.11.3. Antibiofilm Activity
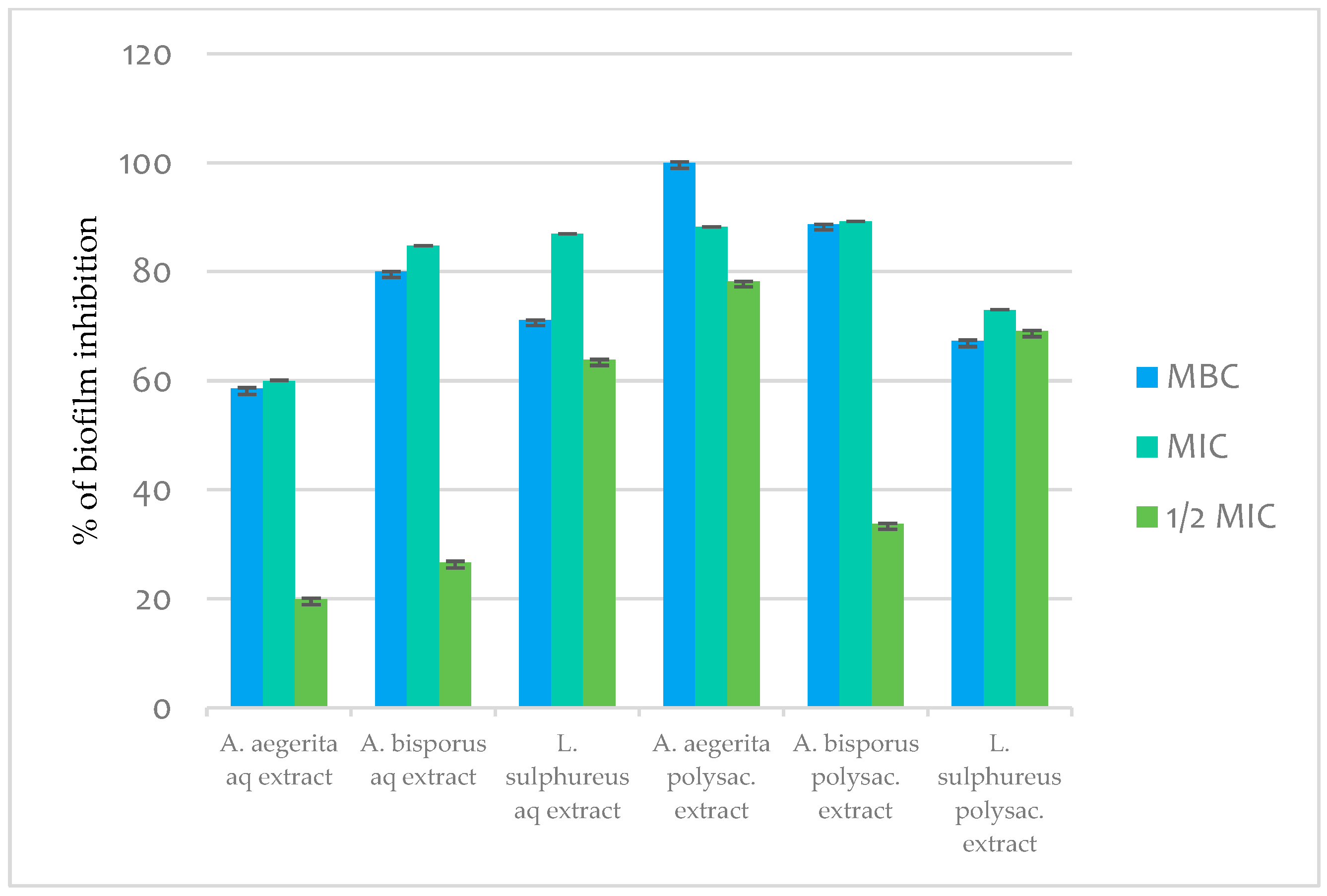
3. Material and Methods
3.1. Standards and Reagents
3.2. Mushroom Collection and Identification
3.3. Preparation of Mushroom Extracts
3.4. Total α-Glucan and β-Glucan Content
3.5. Total Protein Content
3.6. Total Phenolic Content
3.7. Protein Profile of Mushroom Aqueous Extracts
3.8. Phenolic Profile of Mushroom Aqueous Extracts
3.8.1. Preparation of Samples
3.8.2. UHPLC–QToF-MS Analysis
3.9. ATR-FTIR Spectroscopy
3.10. Antioxidant Assay
3.10.1. FRAP Assay
3.10.2. ABTS•+ Assay
3.10.3. DPPH• Assay
3.11. Cytotoxicity towards HaCaT Cells
3.12. Scratch Wound-Healing Assay
3.13. Anti-Inflammatory Properties
3.14. Antimicrobial Activity Assay
3.14.1. Microorganisms
3.14.2. Antibacterial Activity and Anticandidal Activity
3.14.3. Antifungal Activity
3.14.4. Inhibition of Biofilm Formation
3.15. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kostić, M.; Smiljković, M.; Petrović, J.; Glamočlija, J.; Barros, L.; Ferreira, I.C.F.R.; Ćirić, A.; Soković, M. Chemical, nutritive composition and a wide range of bioactive properties of honey mushroom: Armillaria mellea (Vahl: Fr.) Kummer. Food Funct. 2017, 8, 3239–3249. [Google Scholar] [CrossRef] [PubMed]
- Glamočlija, J.; Kostić, M.; Soković, M. Antimicrobial and hepatoprotective activities of edible mushrooms. In Biology of Macrofungi; Springer International Publishing AG: Cham, Switzerland, 2019; pp. 81–113. [Google Scholar]
- Kostić, M.; Ivanov, M.; Fernandes, A.; Pinela, J.; Calhelha, R.C.; Glamočlija, J.; Barros, L.; Ferreira, I.C.F.R.; Soković, M.; Ćirić, A. Antioxidant Extracts of Three Russula Genus Species Express Diverse Biological Activity. Molecules 2020, 25, 4336. [Google Scholar] [CrossRef]
- Yadav, D.; Negi, P.S. Bioactive components of mushrooms: Processing effects and health benefits. Food Res. Int. 2021, 148, 110599. [Google Scholar] [CrossRef]
- Cateni, F.; Gargano, M.L.; Procida, G.; Venturella, G.; Cirlincione, F.; Ferarro, V. Mycochemicals in wild and cultivated mushrooms: Nutrition and health. Phytochem. Rev. 2022, 21, 339–383. [Google Scholar] [CrossRef]
- Diyabalanage, T.; Mulabagal, V.; Mills, G.; DeWitt, D.L.; Nair, M.G. Health-beneficial qualities of the edible mushroom, Agrocybe aegerita. Food Chem. 2008, 108, 97–102. [Google Scholar] [CrossRef]
- Petrović, J.; Stojković, D.; Reis, F.S.; Barros, L.; Glamočlija, J.; Ćirić, A.; Ferreira, C.F.R.I.; Soković, M. Study on chemical, bioactive and food preserving properties of Laetiporus sulphureus (Bull.: Fr.) Murr. Food Funct. 2014, 5, 1441–1451. [Google Scholar] [CrossRef]
- Petrović, J.; Glamočlija, J.; Stojković, D.; Ćirić, A.; Barros, L.; Ferreira, C.F.R.I.; Soković, M. Nutritional value, chemical composition, antioxidant activity and enrichment of cream cheese with chestnut mushroom Agrocybe aegerita (Brig.) Sing. JFST 2015, 52, 6711–6718. [Google Scholar] [CrossRef]
- Khatua, S.; Ghosh, S.; Acharya, K. Laetiporus sulphureus (Bull.: Fr.) Murr. as Food as Medicine. Pharmacogn. J. 2017, 9, s1–s15. [Google Scholar] [CrossRef]
- Cerletti, C.; Esposito, S.; Iacoviello, L. Edible Mushrooms and Beta-Glucans: Impact on Human Health. Nutrients 2021, 13, 2195. [Google Scholar] [CrossRef]
- Roncero-Ramos, I.; Mendiola-Lanao, M.; Pérez-Clavijo, M.; Delgado-Andrade, C. Effect of different cooking methods on nutritional value and antioxidant activity of cultivated mushrooms. Int. J. Food Sci. Nutr. 2017, 68, 287–297. [Google Scholar] [CrossRef]
- Sari, M.; Prange, A.; Lelley, J.I.; Hambitzer, R. Screening of beta-glucan contents in commercially cultivated and wild growing mushrooms. Food Chem. 2017, 216, 45–51. [Google Scholar] [CrossRef]
- Glamočlija, J.; Stojković, D.; Nikolić, M.; Ćirić, A.; Barros, L.; Ferreira, C.F.R.I.; Soković, M. Comparative study on edible Agaricus mushrooms as functional foods. Food Funct. 2015, 6, 1900–1910. [Google Scholar] [CrossRef] [PubMed]
- Banik, S.P.; Pal, S.; Ghorai, S.; Chowdhury, S.; Khowala, S. Interference of sugars in the Coomassie Blue G dye binding assay of proteins. Anal. Biochem. 2009, 386, 113–115. [Google Scholar] [CrossRef] [PubMed]
- Masoumi, B.; Tabibiazar, M.; Golchinfar, Z.; Mohammadifar, M.; Hamishehkar, H. A review of protein-phenolic acid interaction: Reaction mechanisms and applications. Crit. Rev. Food Sci. Nutr. 2024, 64, 3539–3555. [Google Scholar] [CrossRef] [PubMed]
- Alves, M.J.; Ferreira, I.C.; Froufe, H.J.; Abreu, R.M.; Martins, A.; Pintado, M. Antimicrobial activity of phenolic compounds identified in wild mushrooms, SAR analysis and docking studies. J. Appl. Microbiol. 2013, 115, 346–357. [Google Scholar] [CrossRef]
- Çayan, F.; Tel-Çayan, G.; Deveci, E.; Duru, M.E. A comprehensive study on phenolic compounds and bioactive properties of five mushroom species via chemometric approach. J. Food Process. 2021, 45, e15695. [Google Scholar] [CrossRef]
- Bauer Petrovska, B.; Panov, S.; Zafirovska, D.R.; Kulevanova, S. Electrophoretic study of mushroom proteins. J. Agric. Food Environ. 2004, 2, 148–153. [Google Scholar] [CrossRef]
- Prandi, B.; Cigognini, I.M.; Faccini, A.; Zurlini, C.; Rodríguez, Ó.; Tedeschi, T. Comparative Study of Different Protein Extraction Technologies Applied on Mushrooms By-products. Food Bioprocess. Technol. 2023, 16, 1570–1581. [Google Scholar] [CrossRef]
- Kurien, B.T.; Scofield, R.H. Common artifacts and mistakes made in electrophoresis. Methods Mol. Biol. 2012, 869, 633–640. [Google Scholar] [CrossRef]
- Tehrani, M.H.H.; Fakhrehoseini, E.; Nejad, M.K.; Mehregan, H.; Hakemi-Vala, M. Search for Proteins in the Liquid Extract of Edible Mushroom, Agaricus bisporus, and Studying their Antibacterial Effects. Iran. J. Pharm. Res. 2012, 11, 145–150. [Google Scholar]
- Valentão, P.; Lopes, G.; Valente, M.; Barbosa, P.; Andrade, P.B.; Silva, B.M.; Baptista, P.; Seabra, R.M. Quantitation of Nine Organic Acids in Wild Mushrooms. J. Agric. Food Chem. 2005, 53, 3626–3630. [Google Scholar] [CrossRef]
- Valentão, P.; Andrade, P.B.; Rangel, J.; Ribeiro, B.; Silva, B.M.; Baptista, P.; Seabra, R.M. Effect of the Conservation Procedure on the Contents of Phenolic Compounds and Organic Acids in Chanterelle (Cantharellus cibarius) Mushroom. J. Agric. Food Chem. 2005, 53, 4925–4931. [Google Scholar] [CrossRef]
- Park, Y.J.; Jung, E.S.; Singh, D.; Lee, D.E.; Kim, S.; Lee, Y.W.; Kim, J.-G.; Lee, C.H. Spatial (cap & stipe) metabolomic variations affect functional components between brown and white beech mushrooms. Food Res. Int. 2017, 102, 544–552. [Google Scholar] [CrossRef] [PubMed]
- Gamboa-Becerra, R.; Montoya, L.; Bandala, V.M.; Monribot-Villanueva, J.L.; Guerrero-Analco, J.A.; Ramos, A. Metabolomic profiling, nutritional parameters and potential bioactive metabolites of the edible mushroom Tricholoma mesoamericanum. Int. J. Food Sci. 2024, 59, 4348–4358. [Google Scholar] [CrossRef]
- Ye, L.; Zhang, B.; Yang, X.; Li, X.; Tan, W.; Zhang, X. Ultra-performance liquid chromatography-tandem mass spectrometry revealed the significantly different metabolic profiles of Auricularia cornea growing on weakly acidic and weakly alkaline substrates. Can. J. Microbiol. 2023, 69, 262–278. [Google Scholar] [CrossRef]
- Palacios, I.; Lozano, M.; Moro, C.; D’Arrigo, M.; Rostagno, M.A.; Martínez, J.A.; García-Lafuente, A.; Guillamón, E.; Villares, A. Antioxidant properties of phenolic compounds occurring in edible mushrooms. Food Chem. 2011, 128, 674–678. [Google Scholar] [CrossRef]
- Reis, F.S.; Martins, A.; Barros, L.; Ferreira, I.C.F.R. Antioxidant properties and phenolic profile of the most widely appreciated cultivated mushrooms: A comparative study between in vivo and in vitro samples. Food Chem. Toxicol. 2012, 50, 1201–1207. [Google Scholar] [CrossRef]
- Quintero-Cabello, K.P.; Palafox-Rivera, P.; Lugo-Flores, M.A.; Gaitán-Hernández, R.; González-Aguilar, G.A.; Silva-Espinoza, B.A.; Tortoledo-Ortiz, O.; Ayala-Zavala, J.F.; Monribot-Villanueva, J.L.; Guerrero-Analco, J.A. Contribution of bioactive compounds to the antioxidant capacity of the edible mushroom Neolentinus lepideus. Chem. Biodivers. 2021, 18, e2100085. [Google Scholar] [CrossRef]
- Kim, M.-Y.; Seguin, P.; Ahn, J.-K.; Kim, J.-J.; Chun, S.-C.; Kim, E.-H.; Seo, S.-H.; Kang, E.-Y.; Kim, S.-L.; Park, Y.-J.; et al. Phenolic Compound Concentration and Antioxidant Activities of Edible and Medicinal Mushrooms from Korea. J. Agric. Food Chem. 2008, 56, 7265–7270. [Google Scholar] [CrossRef]
- Nowacka, N.; Nowak, R.; Drozd, M.; Olech, M.; Los, R.; Malm, A. Analysis of phenolic constituents, antiradical and antimicrobial activity of edible mushrooms growing wild in Poland. LWT Food Sci. Technol. 2014, 59, 689–694. [Google Scholar] [CrossRef]
- Koutrotsios, G.; Kalogeropoulos, N.; Kaliora, A.C.; Zervakis, G.I. Toward an Increased Functionality in Oyster (Pleurotus) Mushrooms Produced on Grape Marc or Olive Mill Wastes Serving as Sources of Bioactive Compounds. J. Agric. Food Chem. 2018, 66, 5971–5983. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Hengchao, E.; Dong, H.; Zhang, Y.; Qiu, J.; Qian, Y.; Zhou, C. Combination of untargeted metabolomics approach and molecular networking analysis to identify unique natural components in wild Morchella sp. by UPLC-Q-TOF-MS. Food Chem. 2022, 366, 130642. [Google Scholar] [CrossRef]
- Gonzaga, M.L.C.; Menezes, T.M.F.; de Souza, J.R.R.; Ricardo, N.M.P.S.; Soares, S.d.A. Structural characterization of β glucans isolated from Agaricus blazei Murill using NMR and FTIR spectroscopy. Bioact. Carbohydr. Diet Fibre 2013, 2, 152–156. [Google Scholar] [CrossRef]
- Taofiq, O.; González-Paramás, A.M.; Martins, A.; Barreiro, M.F.; Ferreira, I.C. Mushrooms extracts and compounds in cosmetics, cosmeceuticals and nutricosmetics—A review. Ind. Crop. Prod. 2016, 90, 38–48. [Google Scholar] [CrossRef]
- Majtan, J.; Jesenak, M. β-Glucans: Multi-Functional Modulator of Wound Healing. Molecules 2018, 23, 806. [Google Scholar] [CrossRef]
- Kim, J.H.; Tam, C.C.; Chan, K.L.; Mahoney, N.; Cheng, L.W.; Friedman, M.; Land, K.M. Antimicrobial Efficacy of Edible Mushroom Extracts: Assessment of Fungal Resistance. Appl. Sci. 2022, 12, 4591. [Google Scholar] [CrossRef]
- Moussa, A.Y.; Fayez, S.; Xiao, H.; Xu, B. New insights into antimicrobial and antibiofilm effects of edible mushrooms. Int. Food Res. 2022, 162, 111982. [Google Scholar] [CrossRef]
- Vamanu, E.; Nita, S. Antioxidant capacity and the correlation with major phenolic compounds, anthocyanin, and tocopherol content in various extracts from the wild edible Boletus edulis mushroom. Biomed. Res. Int. 2013, 2013, 313905. [Google Scholar] [CrossRef]
- Cheng, J.J.; Lin, C.Y.; Lur, H.S.; Chen, H.P.; Lu, M.K. Properties and biological functions of polysaccharides and ethanolic extracts isolated from medicinal fungus, Fomitopsis pinicola. Process Biochem. 2008, 43, 829–834. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Milinčić, D.D.; Kostić, A.Ž.; Gašić, U.M.; Lević, S.; Stanojević, S.P.; Barać, M.B.; Tešić, Ž.L.; Nedović, V.; Pešić, M.B. Skimmed Goat’s Milk Powder Enriched with Grape Pomace Seed Extract: Phenolics and Protein Characterization and Antioxidant Properties. Biomolecules 2021, 11, 965. [Google Scholar] [CrossRef] [PubMed]
- Milinčić, D.D.; Stanisavljević, N.S.; Kostić, A.Ž.; Soković Bajić, S.; Kojić, M.O.; Gašić, U.M.; Barać, M.B.; Stanojević, S.P.; Tešić, Ž.; Pešić, M.B. Phenolic compounds and biopotential of grape pomace extracts from Prokupac red grape variety. LWT Food Sci. Technol. 2021, 138, 110739. [Google Scholar] [CrossRef]
- Pesic, M.B.; Barac, M.B.; Stanojevic, S.P.; Ristic, N.M.; Macej, O.D.; Vrvic, M.M. Heat induced casein-whey protein interactions at natural pH of milk: A comparison between caprine and bovine milk. Small Ruminant Res. 2012, 108, 77–86. [Google Scholar] [CrossRef]
- Milinčić, D.D.; Vidović, B.B.; Gašić, U.M.; Milenković, M.; Kostić, A.Ž.; Stanojević, S.P.; Ilić, T.; Pešić, M.B. A systematic UHPLC Q-ToF MS approach for the characterization of bioactive compounds from freeze-dried red goji berries (L. barbarum L.) grown in Serbia: Phenolic compounds and phenylamides. Food Chem. 2024, 456, 140044. [Google Scholar] [CrossRef] [PubMed]
- Pešić, M.B.; Milinčić, D.D.; Kostić, A.Ž.; Stanisavljević, N.S.; Vukotić, G.N.; Kojić, M.O.; Gašić, U.M.; Barać, M.B.; Stanojević, S.P.; Popović, D.A.; et al. In vitro digestion of meat- and cereal-based food matrix enriched with grape extracts: How are polyphenol composition, bioaccessibility and antioxidant activity affected? Food Chem. 2019, 284, 28–44. [Google Scholar] [CrossRef]
- Stojković, D.; Dias, M.I.; Drakulić, D.; Barros, L.; Stevanović, M.; Ferreira, I.C.F.R.; Soković, M. Methanolic Extract of the Herb Ononis spinosa L. Is an Antifungal Agent with no Cytotoxicity to Primary Human Cells. Pharmaceuticals 2020, 13, 78. [Google Scholar] [CrossRef]
- Stojković, D.; Kovačević-Grujičić, N.; Reis, F.; Davidović, S.; Barros, L.; Popović, J.; Petrović, I.; Pavić, A.; Glamočlija, J.; Ćirić, A.; et al. Chemical composition of the mushroom Meripilus giganteus Karst. and bioactive properties of its methanolic extract. LWT Food Sci. Technol. 2017, 79, 454–462. [Google Scholar] [CrossRef]
- CLSI. Clinical and Laboratory Standards Institute Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria that Grow Aerobically. Approved Standard, 8th ed.; CLSI Publication M07-A8; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2009. [Google Scholar]
- Kostić, M.; Ivanov, M.; Babić, S.S.; Petrović, J.; Soković, M.; Ćirić, A. An up-to-date review on bio-resource therapeutics effective against bacterial species frequently associated with chronic sinusitis and tonsillitis. Curr. Med. Chem. 2020, 27, 6892–6909. [Google Scholar] [CrossRef]

| Total Glucan Content (%) | α-Glucans (%) | β-Glucans (%) | |
|---|---|---|---|
| Polysaccharide extracts | |||
| A. aegerita | 44.28 ± 0.06 | 9.34 ± 0.86 | 34.95 ± 0.80 |
| A. bisporus | 33.37 ± 0.31 | 12.64 ± 1.82 | 20.73 ± 2.13 |
| L. sulphureus | 11.47 ± 0.38 | 0.74 ± 0.03 | 10.73 ± 0.35 |
| Aqueous extracts | |||
| A. aegerita | 25.47 ± 2.22 | 14.36 ± 0.57 | 11.10 ± 2.79 |
| A. bisporus | 7.32 ± 0.07 | 2.54 ± 0.02 | 4.77 ± 0.09 |
| L. sulphureus | 6.92 ± 1.45 | 2.27 ± 0.03 | 4.65 ± 0.05 |
| Total Protein Content (g BSA/100 g dw) | ||
|---|---|---|
| Aqueous Extracts | Polysaccharide Extracts | |
| A. aegerita | 1.84 ± 0.02 | n.d. * |
| A. bisporus | 2.70 ± 0.07 | n.d. * |
| L. sulphureus | 5.62 ± 0.91 | n.d. * |
| Total Phenolic Content (g GAE/100 g dw) | ||
|---|---|---|
| Aqueous Extracts | Polysaccharide Extracts | |
| A. aegerita | 23.72 ± 0.02 | n.d. * |
| A. bisporus | 7.9 ± 0.07 | n.d. * |
| L. sulphureus | 0.93 ± 0.91 | 0.19 ± 0.01 |
| RT, min | Compounds Name | Formula | Calculated Mass | m/z Exact Mass | mDa | MS2 Fragments (% Base Peak) | Samples | Refs | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ABP | ABA | AAP | AAA | LSP | LSA | ||||||||
| Organic acids and derivatives | |||||||||||||
| 0.73 | Fumaric acid | C4H3O4– | 115.00368 | 115.00491 | −1.23 | / | + | + | + | + | − | + | [22,23] |
| 0.73 | Malic acid | C4H5O5– | 133.01425 | 133.01571 | −1.46 | 115.0050 (100) | + | + | + | + | + | + | [22,23] |
| 0.83 | Citric acid | C6H7O7– | 191.01973 | 191.02299 | −3.26 | 111.0093 (100), 112.01131 | + | + | + | + | + | + | [22,23] |
| 0.94 | Succinic acid | C4H5O4– | 117.01933 | 117.02020 | −0.87 | / | + | + | − | + | + | + | [22] |
| 6.27 | Pimelic acid (6-Carboxyhexanoate) | C7H11O4– | 159.06628 | 159.06686 | −0.58 | 115.07504 (100), 116.08119, 118.04377 | − | + | − | + | + | + | / |
| 7.88 | 3-hydroxy sebacic acid | C10H17O5– | 217.10815 | 217.10986 | −1.72 | 171.1020 (100), 127.1115, 132.04799, 153.06758 | − | + | − | − | + | + | [33] |
| 8.49 | Azelaic acid | C9H15O4– | 187.09758 | 187.09846 | −0.88 | 123.0813 (100), 125.0971 | + | + | + | + | + | + | [24] |
| 8.56 | Diethyl 3-hydroxyglutarate | C9H15O5– | 203.09250 | 203.09342 | −0.92 | 113.0977 (100), 129.0912, 141.0931, 147.0154 | − | − | − | + | + | + | / |
| 9.31 | Sebacic acid | C10H17O4– | 201.11323 | 201.11399 | −0.76 | 139.1132 (100), 111.0815, 137.0974, 183.1025, 184.1039 | − | + | + | + | + | + | [24] |
| 10.58 | Pinellic acid | C18H33O5– | 329.23335 | 329.23534 | −2.00 | 171.1047 (100), 139.1139, 183.1382, 201.1145, 211.1345, 229.1449 | + | + | + | + | + | + | [25] |
| 13.41 | 9-Hydroxy-13-oxo-10-octadecenoic acid | C18H31O4– | 311.22278 | 311.22509 | −2.31 | 201.1138 (100), 127.1115, 139.1125, 171.1032, 183.1325, 197.1195 | − | + | − | + | + | + | [26] |
| 15.84 | 13-Hydroxy-9,11-octadecadienoic acid | C18H31O3– | 295.22787 | 295.22894 | −1.07 | 127.1132 (100), 111.0818, 171.1024, 183.1032 | + | + | + | + | + | + | [26] |
| Phenolic acids and derivatives | |||||||||||||
| 1.28 | Gallic acid | C7H5O5– | 169.01425 | 169.01871 | −4.46 | 125.0240 (100), 124.0164, 107.0145, 108.0226 | − | − | − | − | + | − | [27] |
| 2.49 | 3,4-Dihydroxybenzoic acid (like Protocatehuic acid) | C7H5O4– | 153.01933 | 153.02228 | −2.95 | 108.0215 (100), 109.0295 | − | + | − | − | + | + | [27,28] |
| 4.30 | p-Hydroxybenzoic acid | C7H5O3– | 137.02390 | 137.02649 | −2.59 | / | − | + | − | + | + | + | [27,28] |
| 4.31 | 4-Methoxybenzoic acid (like p-Anisic acid) | C8H7O3– | 151.04007 | 151.04025 | −0.18 | 108.0219 (100), 107.0389, 109.0327 | − | − | − | + | − | + | [29] |
| 4.44 | 3,4-Dimethoxybenzoate (like veratric acid) | C9H9O4– | 181.05063 | 181.05262 | −1.98 | 134.0381 (100), 107.0569, 135.0427, 137.0216, 119.0531 | − | − | − | + | + | + | [30] |
| 6.19 | Coumaric acid is. I | C9H7O3– | 163.04007 | 163.04142 | −1.35 | 117.0338 (100), 118.0352, 119.0237 | − | − | − | + | − | + | [27,28] |
| 7.28 | p-hydroxy-hydrocinnamic acid (or Dihydrocoumaric acid) | C9H9O3– | 165.05572 | 165.05574 | −0.02 | 119.0504 (100), 117.0343, 147.0475 | − | + | − | + | + | + | / |
| 7.58 | Vanillic acid | C8H7O4– | 167.03498 | 167.03916 | −4.18 | 105.0345 (100), 123.0457 | − | − | − | − | + | + | [30,31] |
| 7.81 | Sinapic acid | C11H11O5– | 223.06120 | 223.06138 | −0.18 | 164.0408 (100), 117.0675, 133.0661, 163.0411, 179.0703, 193.0171 | − | + | − | + | − | − | [31] |
| 9.77 | Coumaric acid is. II | C9H7O3– | 163.04007 | 163.04023 | −0.17 | 117.0357 (100), 119.0454, 145.9088 | − | − | − | + | − | + | [28,30] |
| 11.80 | Ethyl-homovanillate | C11H13O4– | 209.08193 | 209.08451 | −2.58 | 122.0374 (100), 135.0455, 150.0693 | − | − | − | − | + | − | / |
| Other compounds | |||||||||||||
| 13.82 | Triterpenoid (like maslinic acid) | C30H47O4– | 471.34798 | 471.34992 | −1.94 | 471.3453 (100) | − | − | − | − | + | + | [32] |
| Mushroom Extracts | ABTS (g Trolox/100 g) | FRP (µg AA/mL) | FCC (µg EDTA/mL) |
|---|---|---|---|
| A. aegerita, aqueous | 21.97 | 43.56 | 48.25 |
| A. bisporus, aqueous | 9.07 | 7.26 | 47.9 |
| L. sulphureus, aqueous | 4.92 | 219.52 | 0.19 |
| L. sulphureus, polysaccharides | 3.37 | 42.05 | 2.49 |
| Mushroom Extracts | IC50 Value (µg/mL) |
|---|---|
| A. bisporus, aqueous | 246.24 ± 5.46 |
| L. sulphureus, aqueous | 274.72 ± 2.54 |
| A. aegerita, aqueous | >400 |
| A. bisporus, polysaccharides | >400 |
| L. sulphureus, polysaccharides | >400 |
| A. aegerita, polysaccharides | >400 |
| Mushroom Extracts | Wound Closure % |
|---|---|
| A. bisporus, aqueous | 65.96 ± 6.98 |
| L. sulphureus, aqueous | 62.56 ± 9.43 |
| A. bisporus, polysaccharides | 84.27 ± 4.54 |
| L. sulphureus, polysaccharides | 77.06 ± 7.65 |
| Mushroom Extracts | IL-6 (pg/mL) |
|---|---|
| Control | 2.96 ± 0.02 |
| S. aureus | 16.64 ± 0.06 |
| S. aureus + AE A. aegerita | 4.56 ± 0.08 |
| S. aureus + PE A. aegerita | 4.21 ± 0.02 |
| S. aureus + AE A. bisporus | 7.42 ± 0.02 |
| S. aureus + PE A. bisporus | 6.78 ± 0.04 |
| S. aureus + AE L. sulphureus | 15.12 ± 0.10 |
| S. aureus + PE L. sulphureus | 10.21 ± 0.09 |
| C. albicans 475/15 | C. albicans 13/15 | C. albicans 17/15 | C. parapsilosis ATCC 22019 | C. tropicalis ATCC 750 | C. krusei H1/16 | ||
|---|---|---|---|---|---|---|---|
| A. aegerita AE | MIC | 2.00 d | 2.00 d | 2.00 b | 1.00 b | 2.00 c | 4.00 c |
| MFC | 4.00 d | 4.00 d | 4.00 b | 2.00 b | 4.00 c | 8.00 c | |
| A. bisporus AE | MIC | 1.00 c | 2.00 d | 2.00 b | 1.00 b | 2.00 c | 4.00 c |
| MFC | 2.00 c | 4.00 d | 4.00 b | 2.00 b | 4.00 c | 8.00 c | |
| L. sulphureus AE | MIC | 0.50 b | 0.50 b | 4.00 c | 1.00 b | 1.00 b | 4.00 c |
| MFC | 1.00 b | 1.00 b | 8.00 c | 2.00 b | 2.00 b | 8.00 c | |
| A. aegerita PE | MIC | 0.50 b | 0.50 b | 2.00 b | 1.00 b | 2.00 c | 4.00 c |
| MFC | 1.00 b | 1.00 b | 4.00 b | 2.00 b | 4.00 c | 8.00 c | |
| A. bisporus PE | MIC | 1.00 c | 1.00 c | 2.00 b | 1.00 b | 2.00 c | 2.00 b |
| MFC | 2.00 c | 2.00 c | 4.00 b | 2.00 b | 4.00 c | 4.00 b | |
| L. sulphureus PE | MIC | 0.50 b | 1.00 c | 2.00 b | 1.00 b | 2.00 c | 2.00 b |
| MFC | 1.00 b | 2.00 c | 4.00 b | 2.00 b | 4.00 c | 4.00 b | |
| Ketoconazole (× 10−3) | MIC | 3.20 a | 1.60 a | 1.60 a | 3.20 a | 1.60 a | 1.60 a |
| MFC | 6.40 a | 51.20 a | 51.20 a | 6.40 a | 6.40 a | 3.20 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Petrović, J.; Glamočlija, J.; Milinčić, D.D.; Doroški, A.; Lević, S.; Stanojević, S.P.; Kostić, A.Ž.; Minić, D.A.P.; Vidović, B.B.; Plećić, A.; et al. Comparative Chemical Analysis and Bioactive Properties of Aqueous and Glucan-Rich Extracts of Three Widely Appreciated Mushrooms: Agaricus bisporus (J.E.Lange) Imbach, Laetiporus sulphureus (Bull.) Murill and Agrocybe aegerita (V. Brig.) Vizzini. Pharmaceuticals 2024, 17, 1153. https://doi.org/10.3390/ph17091153
Petrović J, Glamočlija J, Milinčić DD, Doroški A, Lević S, Stanojević SP, Kostić AŽ, Minić DAP, Vidović BB, Plećić A, et al. Comparative Chemical Analysis and Bioactive Properties of Aqueous and Glucan-Rich Extracts of Three Widely Appreciated Mushrooms: Agaricus bisporus (J.E.Lange) Imbach, Laetiporus sulphureus (Bull.) Murill and Agrocybe aegerita (V. Brig.) Vizzini. Pharmaceuticals. 2024; 17(9):1153. https://doi.org/10.3390/ph17091153
Chicago/Turabian StylePetrović, Jovana, Jasmina Glamočlija, Danijel D. Milinčić, Ana Doroški, Steva Lević, Slađana P. Stanojević, Aleksandar Ž. Kostić, Dušanka A. Popović Minić, Bojana B. Vidović, Ana Plećić, and et al. 2024. "Comparative Chemical Analysis and Bioactive Properties of Aqueous and Glucan-Rich Extracts of Three Widely Appreciated Mushrooms: Agaricus bisporus (J.E.Lange) Imbach, Laetiporus sulphureus (Bull.) Murill and Agrocybe aegerita (V. Brig.) Vizzini" Pharmaceuticals 17, no. 9: 1153. https://doi.org/10.3390/ph17091153
APA StylePetrović, J., Glamočlija, J., Milinčić, D. D., Doroški, A., Lević, S., Stanojević, S. P., Kostić, A. Ž., Minić, D. A. P., Vidović, B. B., Plećić, A., Nedović, V. A., Pešić, M. B., & Stojković, D. (2024). Comparative Chemical Analysis and Bioactive Properties of Aqueous and Glucan-Rich Extracts of Three Widely Appreciated Mushrooms: Agaricus bisporus (J.E.Lange) Imbach, Laetiporus sulphureus (Bull.) Murill and Agrocybe aegerita (V. Brig.) Vizzini. Pharmaceuticals, 17(9), 1153. https://doi.org/10.3390/ph17091153











