Nanostructured Lipid Carriers to Enhance the Bioavailability and Solubility of Ranolazine: Statistical Optimization and Pharmacological Evaluations
Abstract
1. Introduction
2. Results and Discussion
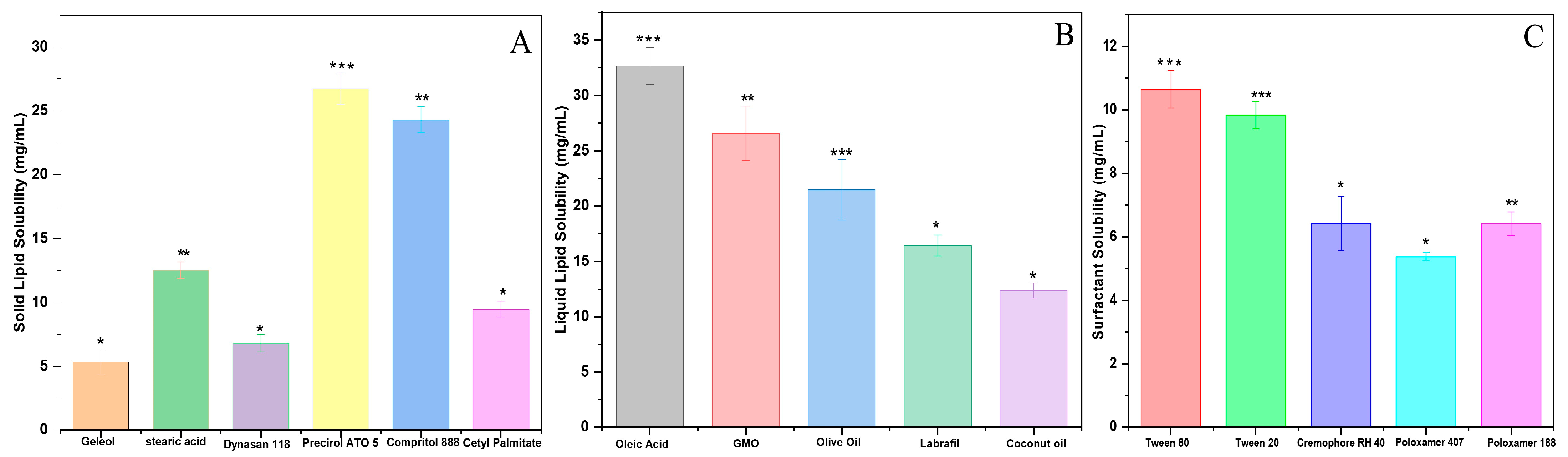
2.1. Selection of Solid and Liquid Lipids
2.2. Selection of Surfactants
2.3. Experimental Design Optimization
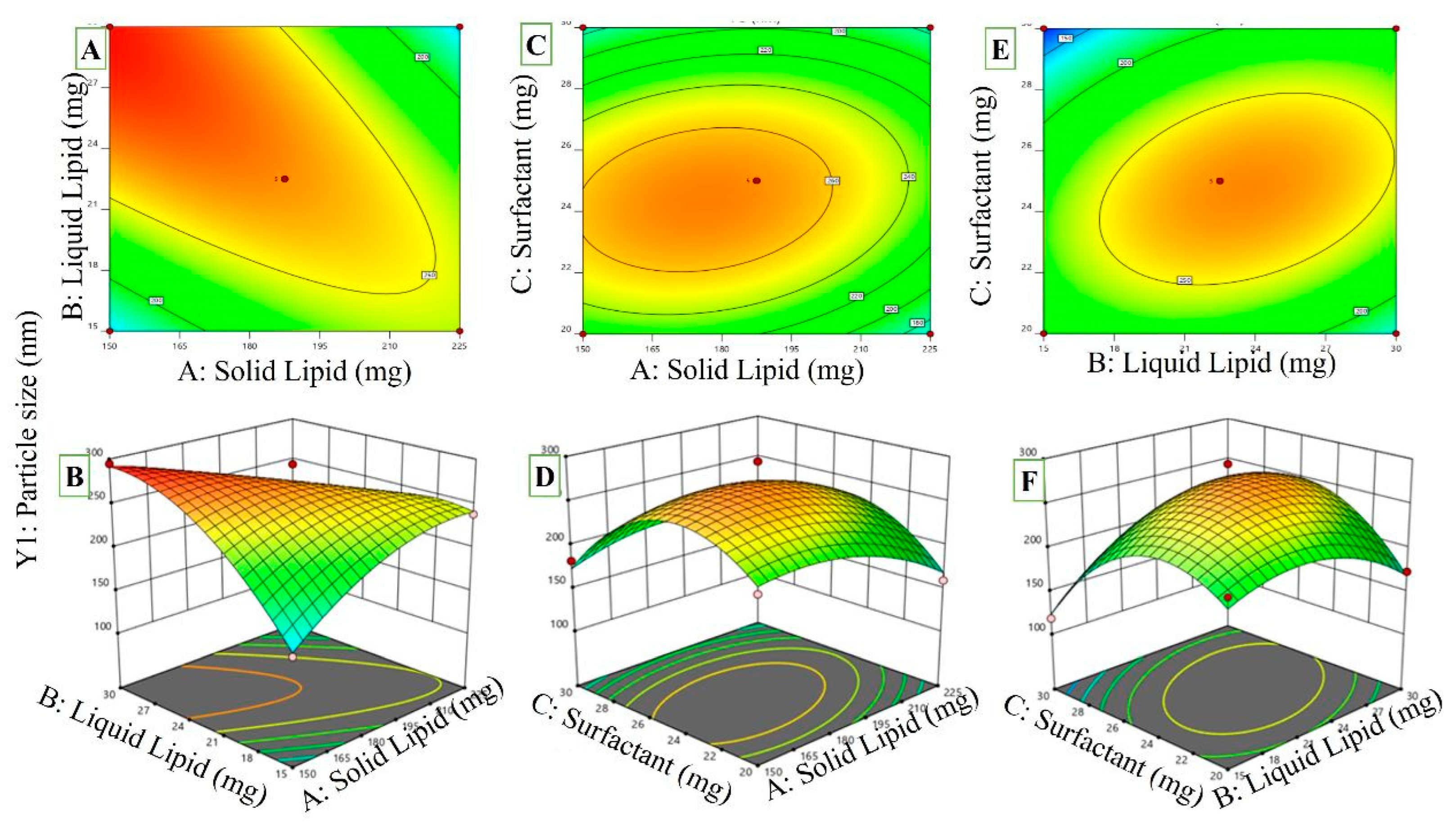
2.3.1. Effects of Independent Variables Different Types of Lipids (A): Solid Lipids (B): Liquid Lipids (C): Surfactant on Polystyrene
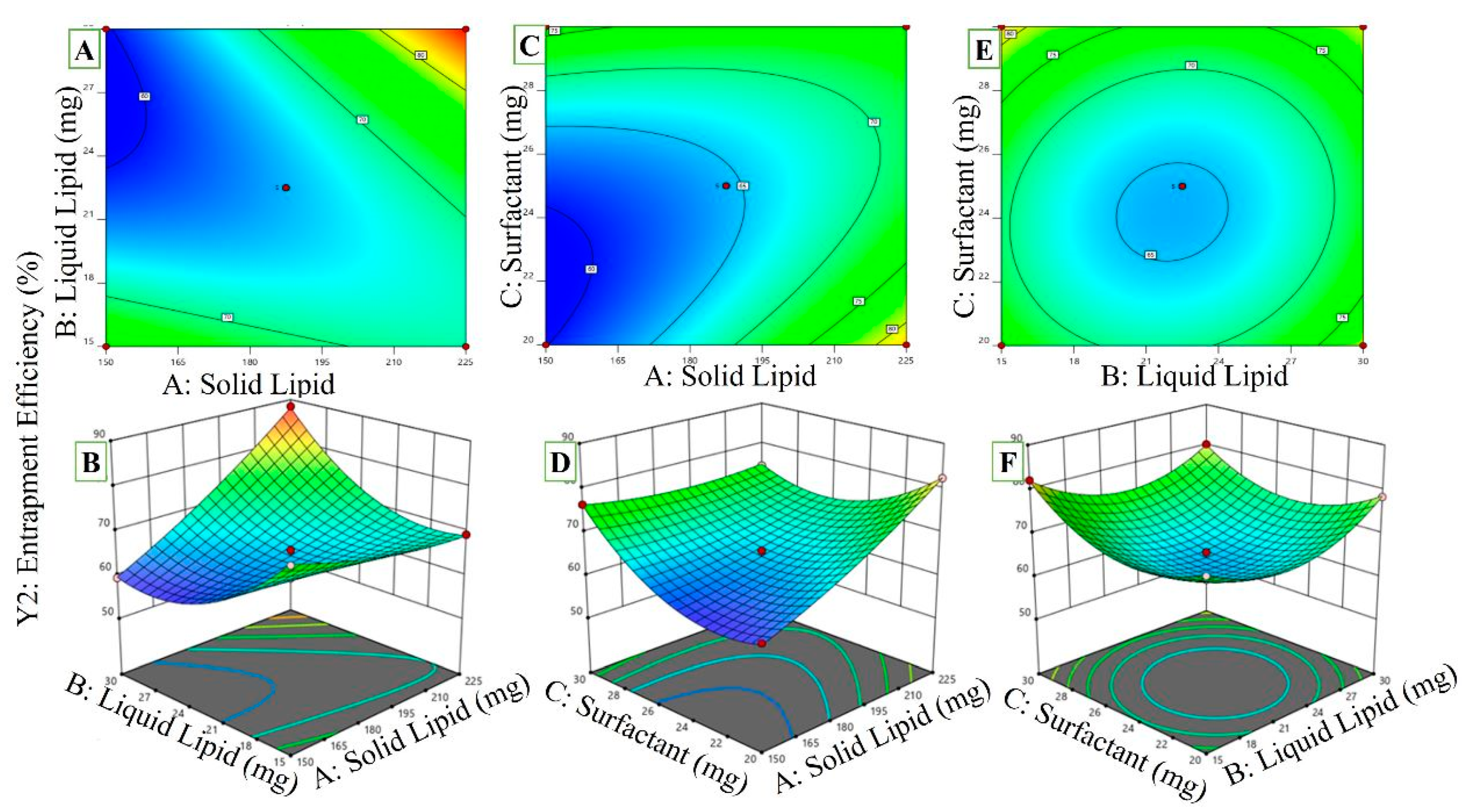
2.3.2. Effect of Independent Variables on EE
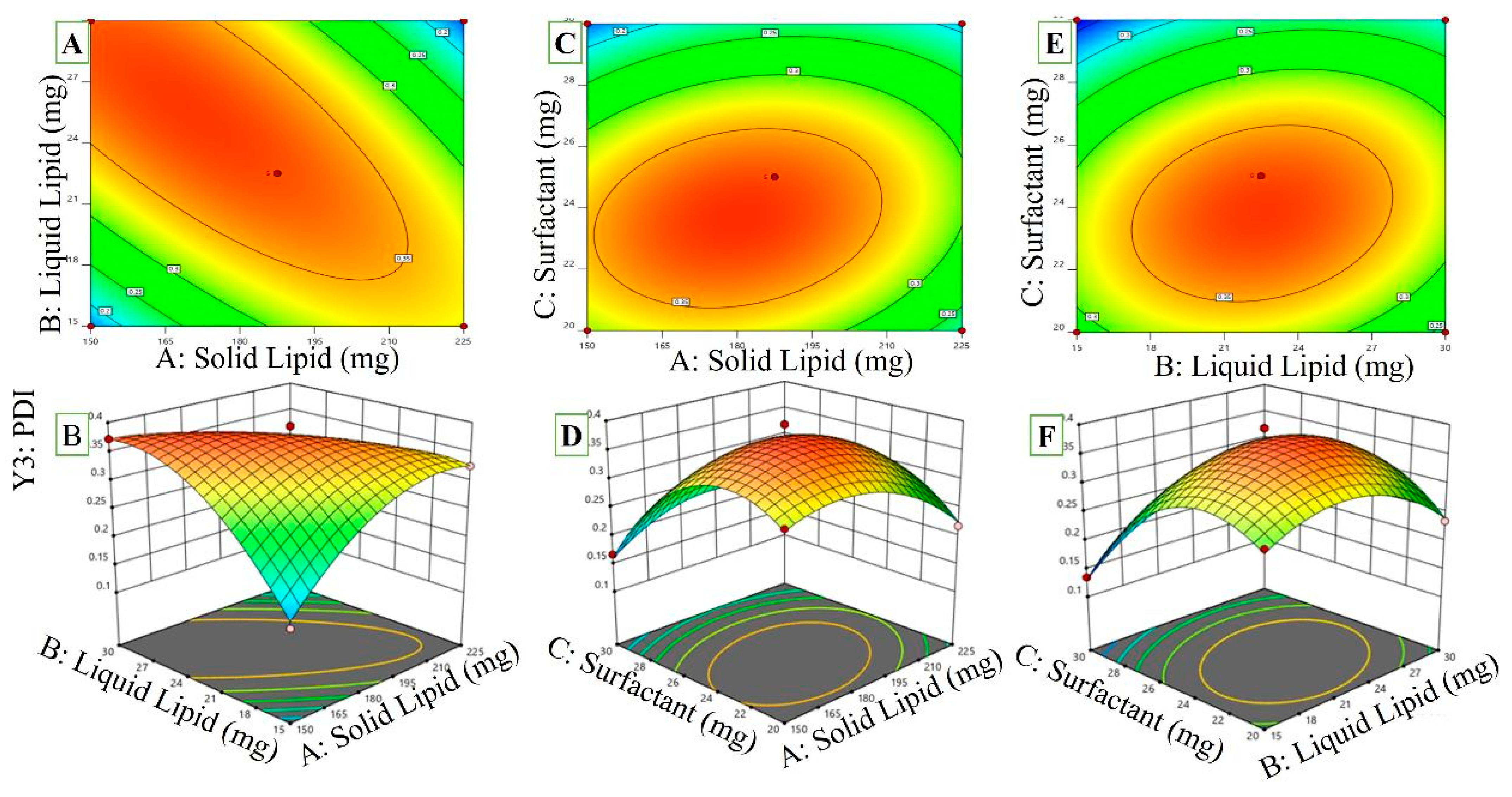
2.3.3. Effect of Independent Variables on PDI
2.3.4. Effects of Variables on ZP
2.4. Point Prediction
2.5. Effects on Particle Size, PDI, and ZP
2.6. Measurement of EE and Drug Concentration
2.7. Characterization of Optimized RZ-NLCG
pH, Viscosity, Spreadability, and Drug Content Estimation
2.8. Solid-State Characterization
2.8.1. Drug-Excipient Compatibility Studies by DSC
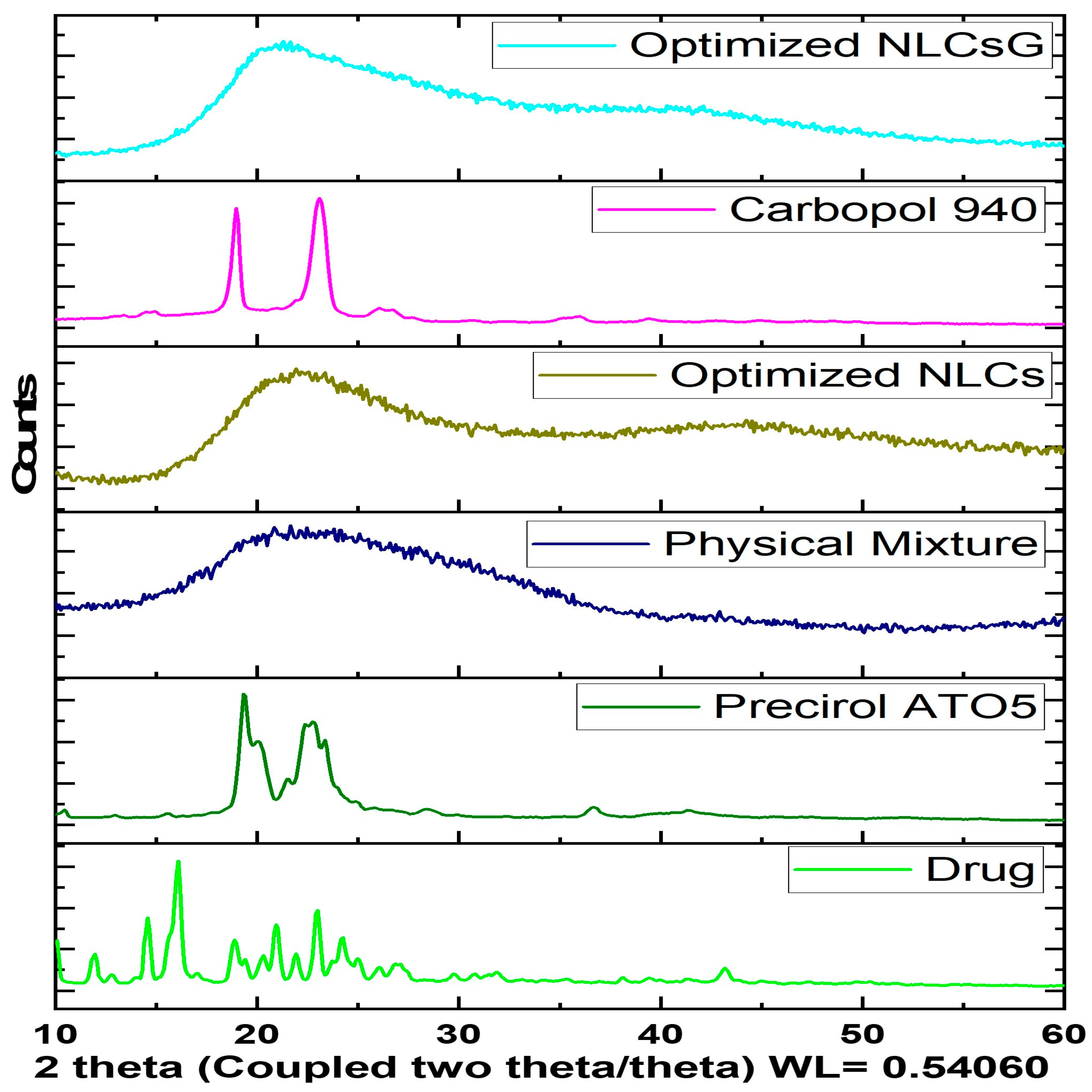
2.8.2. Powder X-ray Diffractometry
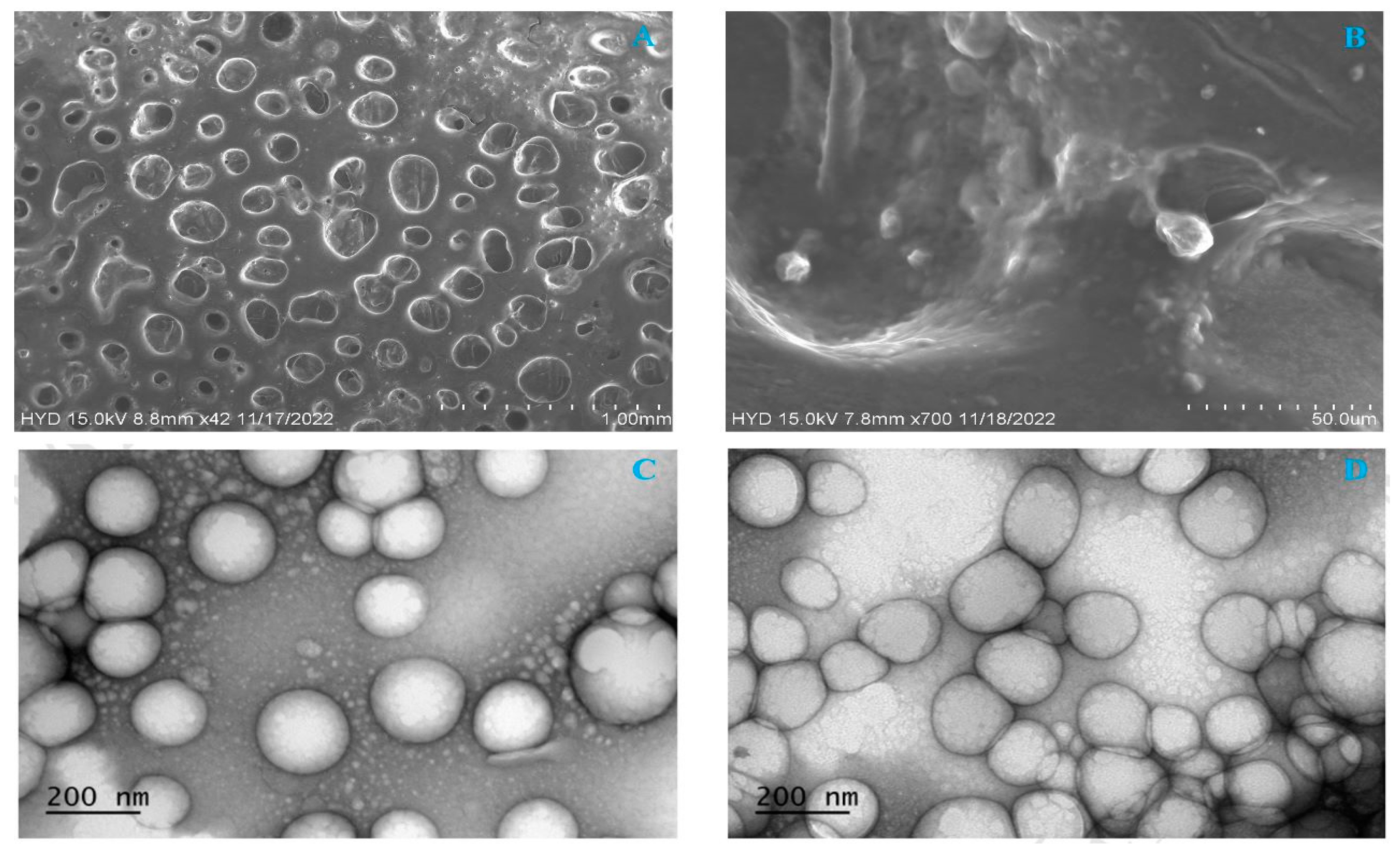
2.8.3. Morphology of NLCs Using Scanning Electron Microscopy
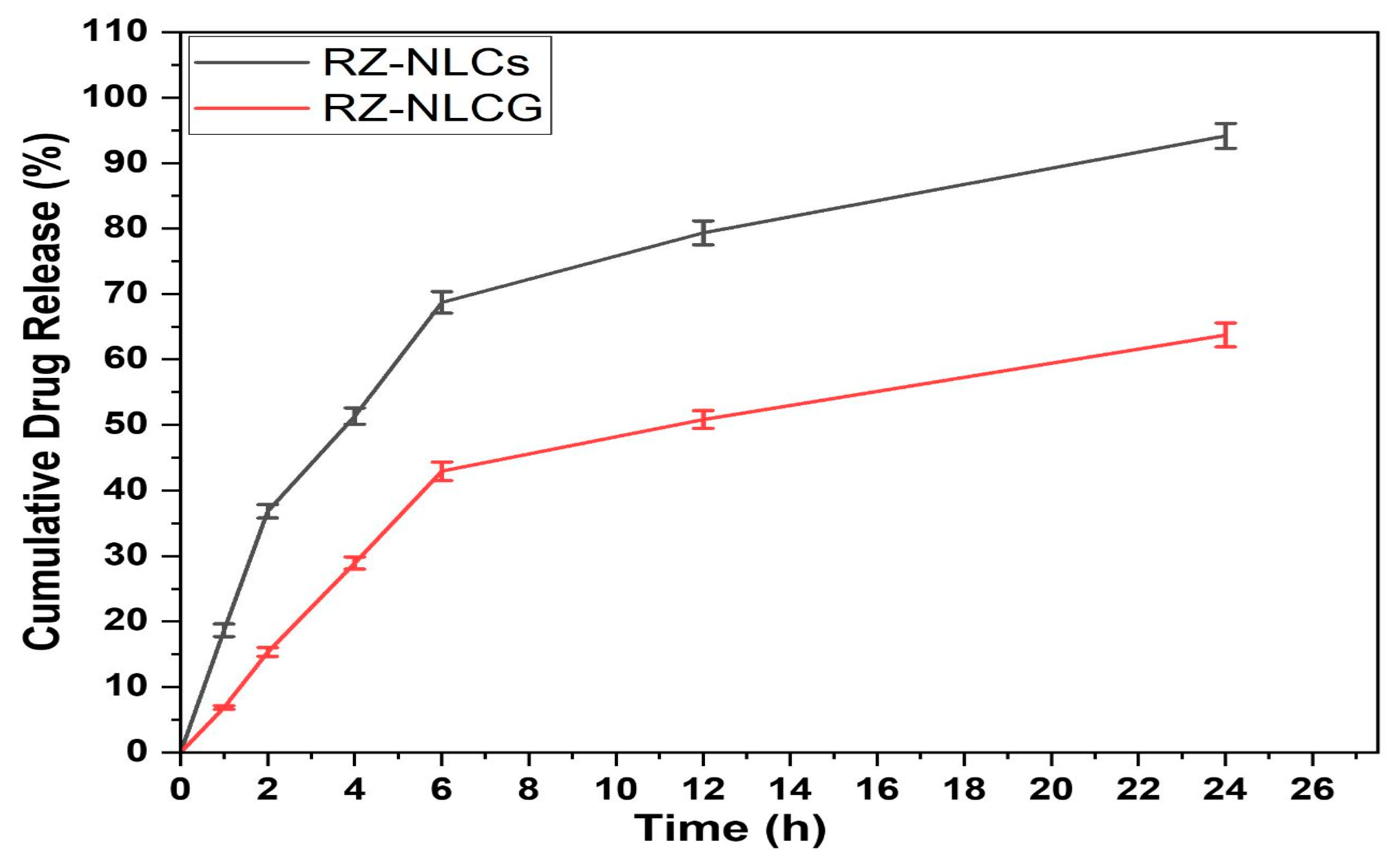
2.9. In Vitro Drug Release
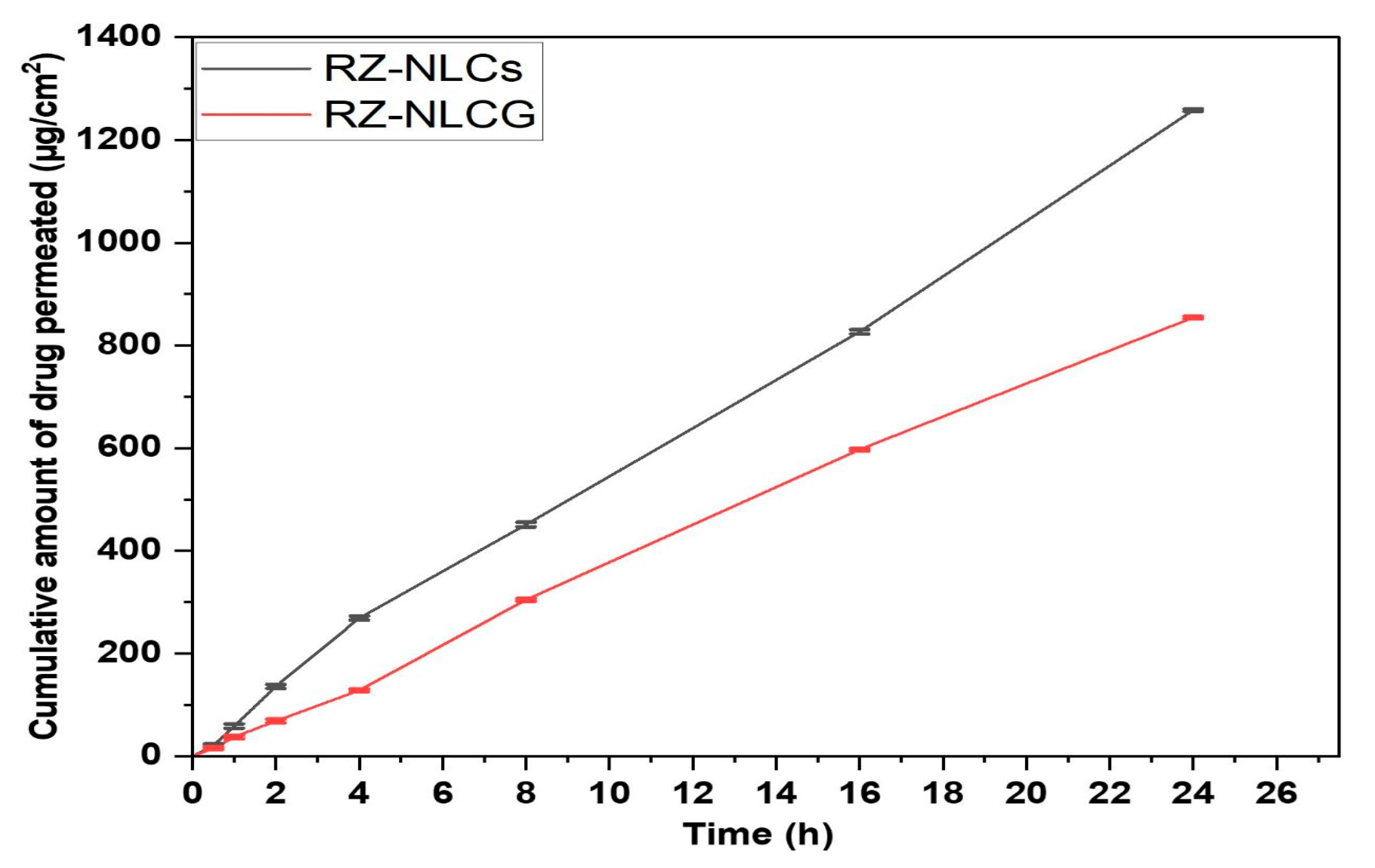
2.10. Ex Vivo Permeation Studies
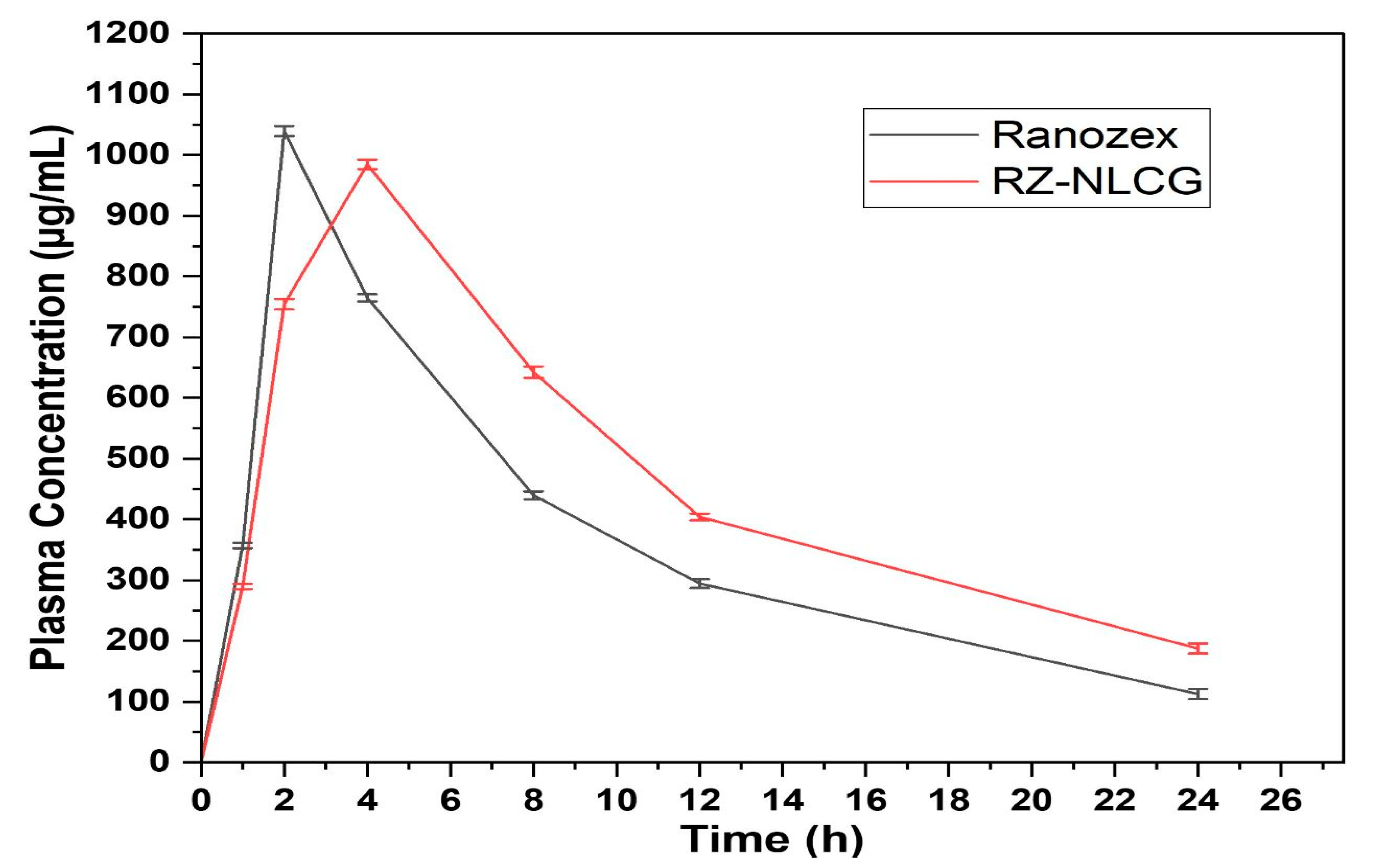
2.11. Pharmacokinetic Study
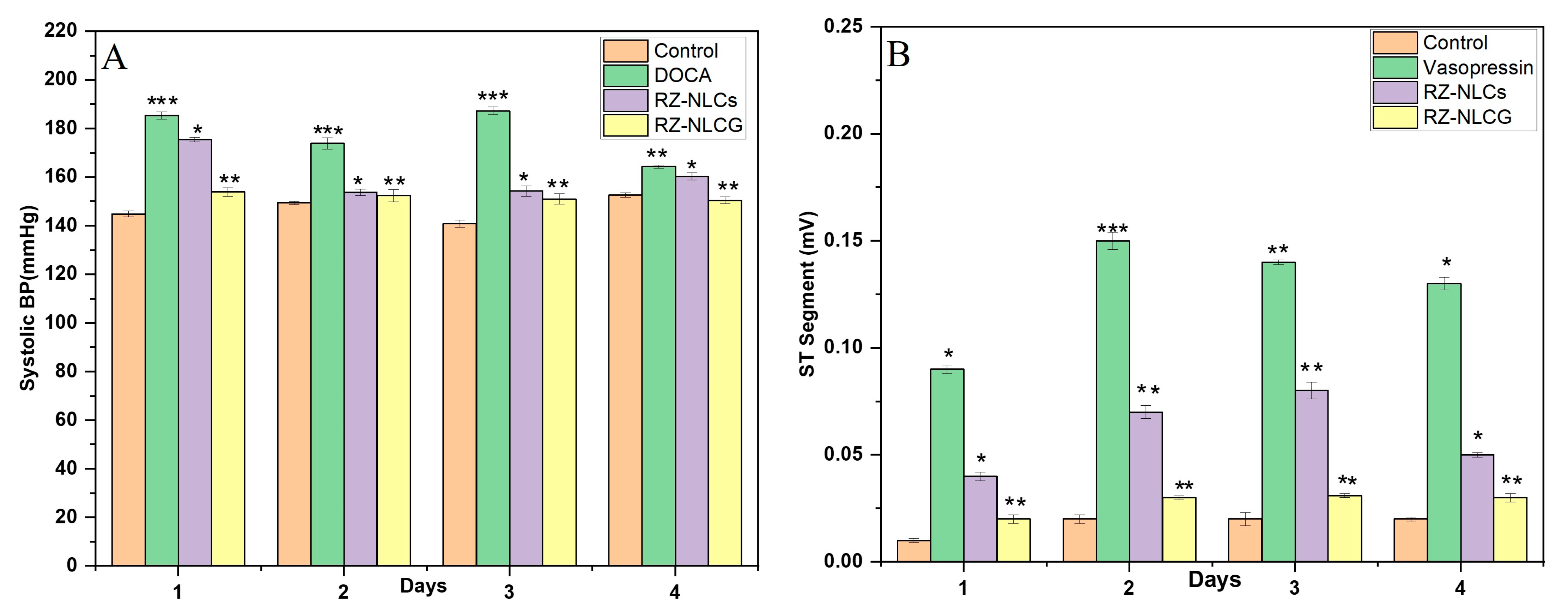
2.12. In Vivo Pharmacodynamic Studies
2.12.1. DOCA Salt Model for Hypertension
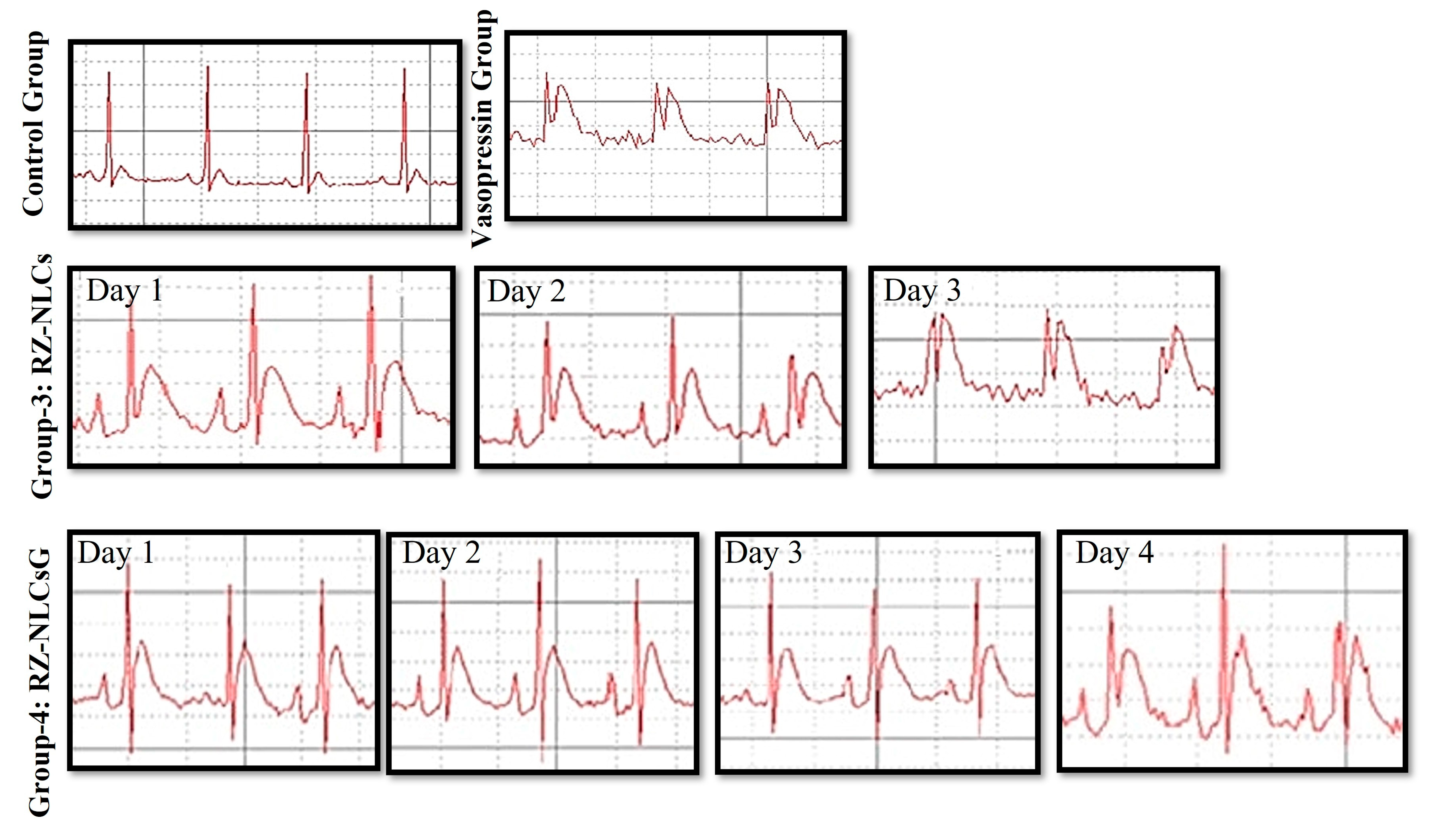
2.12.2. Vasopressin Model for Angina
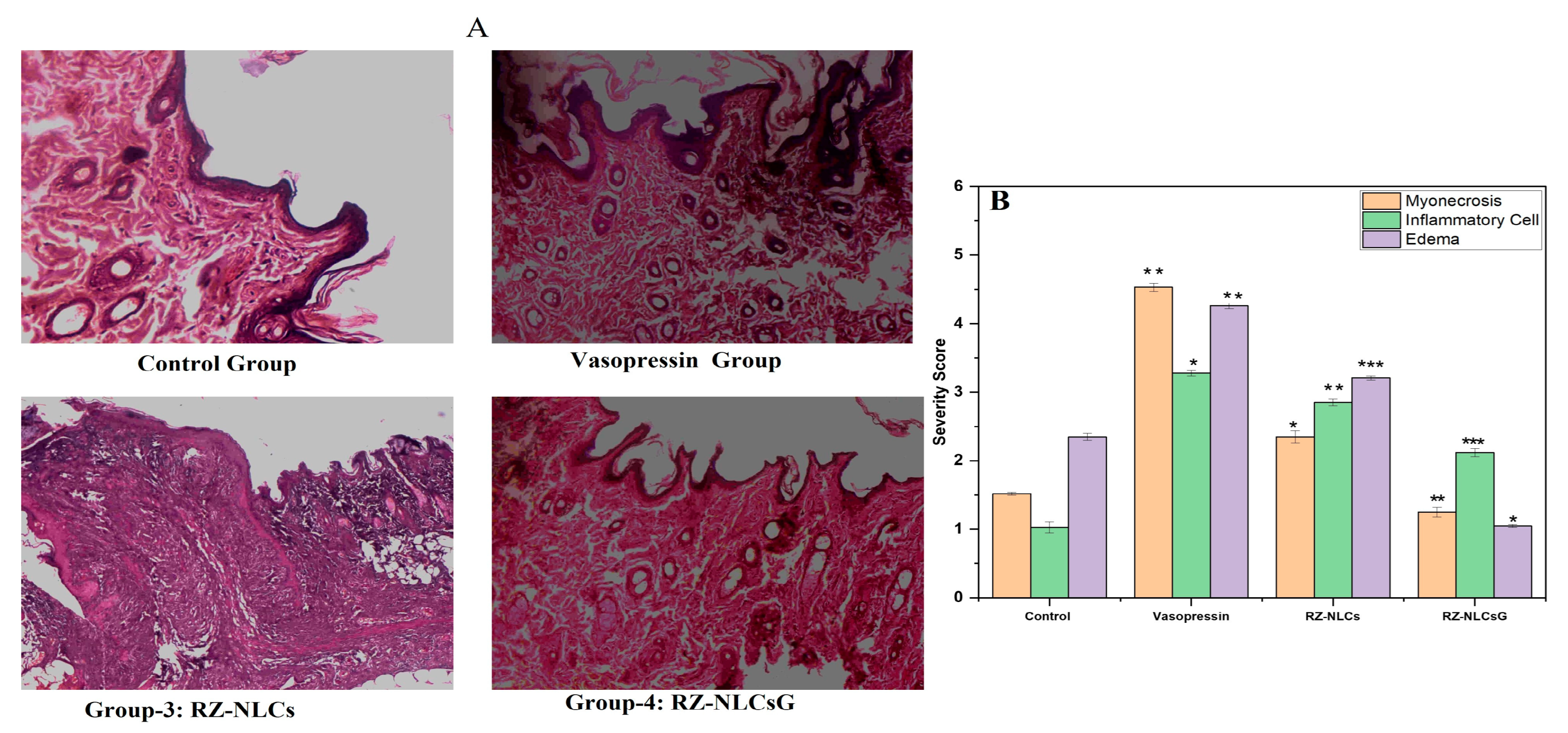
2.12.3. Histopathological Analysis
2.13. Stability Study
3. Materials and Methods
3.1. Selection of Lipids (Solid and Liquid)
3.2. Collection of Solid-Liquid Lipid Ratio/Miscibility Study
3.3. Development of RZ-NLCs
3.4. Statistical Optimization of the RZ-NLCs
3.5. Characterization of RZ-NLCs
3.5.1. Determination of PS, PDI, and ZP
3.5.2. Measurement of EE and RZ Content
3.6. Formulation of RZ-NLCG
3.6.1. Evaluation of RZ-NLCG
3.6.2. Viscosity and Spreadability
3.6.3. Texture Analysis
3.7. Solid-State Characterization
3.7.1. Drug-Excipient Compatibility Studies by DSC
3.7.2. Powder X-ray Diffraction Characterization of Crystallinity (PXRD)
3.7.3. Morphology by SEM
3.7.4. Transmission Electron Microscopy (TEM) Studies
3.8. In Vitro Drug Release
3.9. Skin Permeation Studies
3.10. Pharmacokinetics Study
3.11. In Vivo Pharmacodynamic Studies
3.11.1. DOCA Salt Model
3.11.2. Uni-Nephrectomy
3.11.3. Induction of Hypertension
3.11.4. Experimental Design
3.11.5. Measurement of Blood Pressure
3.11.6. Vasopressin-Induced Angina Model
3.11.7. Histopathology
3.12. Short-Term Stability Study
3.13. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Maton, A. Human Biology and Health; Prentice Hall: Hoboken, NY, USA, 2022. Available online: https://my.clevelandclinic.org/health/diseases/21489-angina (accessed on 1 May 2023).
- Fuster, V.; Kelly, B.B. (Eds.) Committee on Preventing the Global Epidemic of Cardiovascular Disease: Meeting the Challenges in Developing Countries Board on Global Health. In Promoting Cardiovascular Health in the Developing World: A Critical Challenge to Achieve Global Health; National Academies Press: Washington, DC, USA, 2010. [Google Scholar]
- Pizzorno, J.E.; Murray, M.T.; Joiner-Bey, H. The Clinician’s Handbook of Natural Medicine, 3rd ed.; Elsevier: Amsterdam, The Netherlands, 2016. [Google Scholar]
- Jolicoeur, E.M.; Henry, T.D. Chronic Coronary Artery Disease; Elsevier: Amsterdam, The Netherlands, 2018. [Google Scholar]
- Rouhana, S.; Virsolvy, A.; Fares, N.; Richard, S.; Thireau, J. Ranolazine: An Old Drug with Emerging Potential; Lessons from Pre-Clinical and Clinical Investigations for Possible Repositioning. Pharmaceuticals 2022, 15, 31. [Google Scholar] [CrossRef] [PubMed]
- Balla, C.; Pavasini, R.; Ferrari, R. Treatment of Angina: Where Are We? Cardiology 2018, 140, 52–67. [Google Scholar] [CrossRef]
- Jitendra, G.; Govind, M.; Prabakaran, L.; Reena, G. Formulation Development and Characterization of Modified Release Microspheres of Antianginal Drug. Int. J. Drug Dev. Res 2014, 6, 252–265. [Google Scholar]
- Goodwill, A.G.; Dick, G.M.; Kiel, A.M.; Tune, J.D. Regulation of Coronary Blood Flow. Compr. Physiol. 2017, 7, 321–382. [Google Scholar]
- Tardif, J.-C.; Ford, I.; Tendera, M.; Bourassa, M.G.; Fox, K. Efficacy of Ivabradine, a New Selective I f Inhibitor, Compared with Atenolol in Patients with Chronic Stable Angina. Eur. Heart J. 2005, 26, 2529–2536. [Google Scholar] [CrossRef] [PubMed]
- Chauhan, I.; Yasir, M.; Verma, M.; Singh, A.P. Nanostructured Lipid Carriers: A Groundbreaking Approach for Transdermal Drug Delivery. Adv Pharm. Bull. 2020, 10, 150–165. [Google Scholar] [CrossRef] [PubMed]
- Peña-Juárez, M.C.; Guadarrama-Escobar, O.R.; Escobar-Chávez, J.J. Transdermal Delivery Systems for Biomolecules. J. Pharm. Innov. 2022, 17, 319–332. [Google Scholar] [CrossRef]
- Qadri, G.R.; Ahad, A.; Aqil, M.; Imam, S.S.; Ali, A. Invasomes of Isradipine for Enhanced Transdermal Delivery against Hypertension: Formulation, Characterization, and in Vivo Pharmacodynamic Study. Artif. Cells Nanomed. Biotechnol. 2017, 45, 139–145. [Google Scholar] [CrossRef]
- Imam, S.S.; Aqil, M.; Akhtar, M.; Sultana, Y.; Ali, A. Formulation by Design-Based Proniosome for Accentuated Transdermal Delivery of Risperidone: In Vitro Characterization and in Vivo Pharmacokinetic Study. Drug Deliv. 2015, 22, 1059–1070. [Google Scholar] [CrossRef]
- Kamran, M.; Ahad, A.; Aqil, M.; Imam, S.S.; Sultana, Y.; Ali, A. Design, Formulation and Optimization of Novel Soft Nano-Carriers for Transdermal Olmesartan Medoxomil Delivery: In Vitro Characterization and in Vivo Pharmacokinetic Assessment. Int. J. Pharm. 2016, 505, 147–158. [Google Scholar] [CrossRef]
- Olusanya, T.O.B.; Haj Ahmad, R.R.; Ibegbu, D.M.; Smith, J.R.; Elkordy, A.A. Liposomal Drug Delivery Systems and Anticancer Drugs. Molecules 2018, 23, 907. [Google Scholar] [CrossRef] [PubMed]
- Garg, T.; Bhandari, S.; Rath, G.; Goyal, A.K. Current Strategies for Targeted Delivery of Bio-Active Drug Molecules in the Treatment of Brain Tumor. J. Drug Target. 2015, 23, 865–887. [Google Scholar] [CrossRef] [PubMed]
- Garg, T.; Kumar Goyal, A. Iontophoresis: Drug Delivery System by Applying an Electrical Potential across the Skin. Drug Deliv. Lett. 2012, 2, 270–280. [Google Scholar] [CrossRef]
- Chauhan, A.S. Dendrimers for Drug Delivery. Molecules 2018, 23, 938. [Google Scholar] [CrossRef]
- Garg, T.; Goyal, A.K. Liposomes: Targeted and Controlled Delivery System. Drug Deliv. Lett. 2014, 4, 62–71. [Google Scholar] [CrossRef]
- Huang, C.-C.; Liao, Z.-X.; Chen, D.-Y.; Hsiao, C.-W.; Chang, Y.; Sung, H.-W. Injectable Cell Constructs Fabricated via Culture on a Thermoresponsive Methylcellulose Hydrogel System for the Treatment of Ischemic Diseases. Adv. Healthc. Mater. 2014, 3, 1133–1148. [Google Scholar] [CrossRef]
- Chourasia, M.K.; Kang, L.; Chan, S.Y. Nanosized Ethosomes Bearing Ketoprofen for Improved Transdermal Delivery. Results Pharma. Sci. 2011, 1, 60–67. [Google Scholar] [CrossRef]
- Pradhan, M.; Singh, D.; Singh, M.R. Influence of Selected Variables on Fabrication of Triamcinolone Acetonide Loaded Solid Lipid Nanoparticles for Topical Treatment of Dermal Disorders. Artif. Cells Nanomed. Biotechnol. 2016, 44, 392–400. [Google Scholar] [CrossRef]
- Jaiswal, P.; Gidwani, B.; Vyas, A. Nanostructured Lipid Carriers and Their Current Application in Targeted Drug Delivery. Artif. Cells Nanomed. Biotechnol. 2016, 44, 27–40. [Google Scholar] [CrossRef]
- Ghasemiyeh, P.; Mohammadi-Samani, S. Potential of Nanoparticles as Permeation Enhancers and Targeted Delivery Options for Skin: Advantages and Disadvantages. Drug Des. Devel. Ther. 2020, 14, 3271–3289. [Google Scholar] [CrossRef]
- Hao, J.; Fang, X.; Zhou, Y.; Wang, J.; Guo, F.; Li, F.; Peng, X. Development and Optimization of Solid Lipid Nanoparticle Formulation for Ophthalmic Delivery of Chloramphenicol Using a Box-Behnken Design. Int. J. Nanomed. 2011, 6, 683. [Google Scholar]
- Thomareis, A.S.; Dimitreli, G. Processed Cheese Science and Technology; Elsevier: Amsterdam, The Netherlands, 2022. [Google Scholar]
- Chokshi, N.V.; Khatri, H.N.; Patel, M.M. Formulation, Optimization, and Characterization of Rifampicin-Loaded Solid Lipid Nanoparticles for the Treatment of Tuberculosis. Drug Dev. Ind. Pharm. 2018, 44, 1975–1989. [Google Scholar] [CrossRef] [PubMed]
- Amarachinta, P.R.; Sharma, G.; Samed, N.; Chettupalli, A.K.; Alle, M.; Kim, J.C. Central composite design for the development of carvedilol-loaded transdermal ethosomal hydrogel for extended and enhanced anti-hypertensive effect. J. Nanobiot. 2021, 19, 100. [Google Scholar] [CrossRef] [PubMed]
- Beg, S.; Al Robaian, M.; Rahman, M.; Imam, S.S.; Alruwaili, N.; Panda, S.K. (Eds.) Pharmaceutical Drug Product Development and Process Optimization: Effective Use of Quality by Design; CRC Press: Boca Raton, FL, USA, 2020. [Google Scholar]
- Jahangir, M.A.; Imam, S.S.; Muheem, A.; Chettupalli, A.; Al-Abbasi, F.A.; Nadeem, M.S.; Kazmi, I.; Afzal, M.; Alshehri, S. Nanocrystals: Characterization overview, applications in drug delivery, and their toxicity concerns. J. Pharm. Innov. 2020, 17, 237–248. [Google Scholar] [CrossRef]
- Das, S.; Ng, W.K.; Kanaujia, P.; Kim, S.; Tan, R.B.H. Formulation Design, Preparation and Physicochemical Characterizations of Solid Lipid Nanoparticles Containing a Hydrophobic Drug: Effects of Process Variables. Colloids Surf. B Biointerfaces 2017, 88, 483–489. [Google Scholar] [CrossRef]
- Zhuang, C.-Y.; Li, N.; Wang, M.; Zhang, X.-N.; Pan, W.-S.; Peng, J.-J.; Pan, Y.-S.; Tang, X. Preparation and Characterization of Vinpocetine Loaded Nanostructured Lipid Carriers (NLC) for Improved Oral Bioavailability. Int. J. Pharm. 2016, 394, 179–185. [Google Scholar] [CrossRef]
- Joshi, M.; Patravale, V. Formulation and evaluation of nanostructured lipid carrier (NLC)–based gel of Valdecoxib. Drug Dev. Ind. Pharm. 2006, 32, 911–918. [Google Scholar] [CrossRef]
- Shamma, R.N.; Aburahma, M.H. Follicular Delivery of Spironolactone via Nanostructured Lipid Carriers for Management of Alopecia. Int. J. Nanomed. 2014, 9, 5449. [Google Scholar] [CrossRef]
- Dandagi, P.M.; Dessai, G.A.; Gadad, A.P.; Desai, V.B. (2014). Formulation and evaluation of nanostructured lipid carrier (NLC) of lornoxicam. Int. J. Pharm. Pharm. Sci. 2014, 6, 73–77. [Google Scholar]
- Mitri, K.; Shegokar, R.; Gohla, S.; Anselmi, C.; Müller, R.H. Lipid Nanocarriers for Dermal Delivery of Lutein: Preparation, Characterization, Stability and Performance. Int. J. Pharm. 2011, 414, 267–275. [Google Scholar] [CrossRef]
- Madan, J.R.; Khobaragade, S.; Dua, K.; Awasthi, R. Formulation, optimization, and in vitro evaluation of nanostructured lipid carriers for topical delivery of Apremilast. Dermatol. Ther. 2020, 33, e13370. [Google Scholar] [CrossRef]
- Csóka, G.; Marton, S.; Zelko, R.; Otomo, N.; Antal, I. Application of Sucrose Fatty Acid Esters in Transdermal Therapeutic Systems. Eur. J. Pharm. Biopharm. 2007, 65, 233–237. [Google Scholar] [CrossRef]
- Shete, H.; Patravale, V. Long Chain Lipid Based Tamoxifen NLC. Part I: Preformulation Studies, Formulation Development and Physicochemical Characterization. Int. J. Pharm. 2013, 454, 573–583. [Google Scholar] [CrossRef]
- Sinhmar, G.K.; Shah, N.N.; Chokshi, N.V.; Khatri, H.N.; Patel, M.M. Process, Optimization, and Characterization of Budesonide-Loaded Nanostructured Lipid Carriers for the Treatment of Inflammatory Bowel Disease. Drug Dev. Ind. Pharm. 2018, 44, 1078–1089. [Google Scholar] [CrossRef] [PubMed]
- Kasongo, W.A.; Pardeike, J.; Müller, R.H.; Walker, R.B. Selection and Characterization of Suitable Lipid Excipients for Use in the Manufacture of Didanosine-Loaded Solid Lipid Nanoparticles and Nanostructured Lipid Carriers. J. Pharm. Sci. 2011, 100, 5185–5196. [Google Scholar] [CrossRef]
- Neupane, Y.R.; Srivastava, M.; Ahmad, N.; Kumar, N.; Bhatnagar, A.; Kohli, K. Lipid Based Nanocarrier System for the Potential Oral Delivery of Decitabine: Formulation Design, Characterization, Ex Vivo, and in Vivo Assessment. Int. J. Pharm. 2014, 477, 601–612. [Google Scholar] [CrossRef] [PubMed]
- Cunha, S.; Costa, C.P.; Loureiro, J.A.; Alves, J.; Peixoto, A.F.; Forbes, B.; Sousa Lobo, J.M.; Silva, A.C. Double Optimization of Rivastigmine-Loaded Nanostructured Lipid Carriers (NLC) for Nose-to-Brain Delivery Using the Quality by Design (QbD) Approach: Formulation Variables and Instrumental Parameters. Pharmaceutics 2020, 12, 599. [Google Scholar] [CrossRef] [PubMed]
- Gondrala, U.K.; Dudhipala, N.; Kishan, V. Preparation, Characterization and in Vivo Evaluation of Felodipine Solid-Lipid Nanoparticles for Improved Oral Bioavailability. Measurement 2015, 10, 2995–3002. [Google Scholar]
- Bachhav, Y.G.; Patravale, V.B. Microemulsion Based Vaginal Gel of Fluconazole: Formulation, in Vitro and in Vivo Evaluation. Int. J. Pharm. 2009, 365, 175–179. [Google Scholar] [CrossRef]
- Dudhipala, N.; Janga, K.Y.; Gorre, T. Comparative Study of Nisoldipine-Loaded Nanostructured Lipid Carriers and Solid Lipid Nanoparticles for Oral Delivery: Preparation, Characterization, Permeation and Pharmacokinetic Evaluation. Artif. Cells Nanomed. Biotechnol. 2018, 46, 616–625. [Google Scholar] [CrossRef]
- Korsmeyer, R.W.; Gurny, R.; Doelker, E.; Buri, P.; Peppas, N.A. Mechanisms of Solute Release from Porous Hydrophilic Polymers. Int. J. Pharm. 1983, 15, 25–35. [Google Scholar] [CrossRef]
- Peppas, N.A. Analysis of Fickian and Non-Fickian Drug Release from Polymers. Pharm. Acta Helv. 1985, 60, 110–111. [Google Scholar] [PubMed]
- Cirri, M.; Maestrini, L.; Maestrelli, F.; Mennini, N.; Mura, P.; Ghelardini, C.; Di Cesare Mannelli, L. Design, characterization and in vivo evaluation of nanostructured lipid carriers (NLC) as a new drug delivery system for hydrochlorothiazide oral administration in pediatric therapy. Drug Deliv. 2018, 25, 1910–1921. [Google Scholar] [CrossRef] [PubMed]
- Kapoor, H.; Aqil, M.; Imam, S.S.; Sultana, Y.; Ali, A. Formulation of Amlodipine Nano Lipid Carrier: Formulation Design, Physicochemical and Transdermal Absorption Investigation. J. Drug Deliv. Sci. Technol. 2019, 49, 209–218. [Google Scholar] [CrossRef]
- Jang, D.-J.; Sim, T.; Oh, E. Formulation and Optimization of Spray-Dried Amlodipine Solid Dispersion for Enhanced Oral Absorption. Drug Dev. Ind. Pharm. 2013, 39, 1133–1141. [Google Scholar] [CrossRef]
- Sharma, V.; Dewangan, H.K.; Maurya, L.; Vats, K.; Verma, H. Rational design and in-vivo estimation of Ivabradine Hydrochloride loaded nanoparticles for management of stable angina. J. Drug Deliv. Sci. Technol. 2019, 54, 101337. [Google Scholar] [CrossRef]
- Pliquett, R.U.; Brandes, R.P. Experimental uninephrectomy associates with less parasympathetic modulation of heart rate and facilitates sodium-dependent arterial hypertension. PLoS ONE 2022, 17, e0265086. [Google Scholar] [CrossRef]
- Van Vliet, B.N.; Chafe, L.L.; Antic, V.; Schnyder-Candrian, S.; Montani, J.-P. Direct and Indirect Methods Used to Study Arterial Blood Pressure. J. Pharmacol. Toxicol. Methods 2000, 44, 361–373. [Google Scholar] [CrossRef]
- Pacher, P.; Nagayama, T.; Mukhopadhyay, P.; Bátkai, S.; Kass, D.A. Measurement of cardiac function using pressure-volume conductance catheter technique in mice and rats. Nat. Protoc. 2008, 3, 1422–1434. [Google Scholar] [CrossRef]
- Ikeda, J.; Matsubara, M.; Yao, K. Effects of Benidipine in a Rat Model of Experimental Angina. Yakugaku Zasshi 2006, 126, 1377–1381. [Google Scholar] [CrossRef]
- Gürses, İ.; Özeren, M.; Serin, M.; Yücel, N.; Erkal, H.Ş. Histopathological Evaluation of Melatonin as a Protective Agent in Heart Injury Induced by Radiation in a Rat Model. Pathol. Pract. 2014, 210, 863–871. [Google Scholar] [CrossRef] [PubMed]
- Kruse, J.J.C.M.; Strootman, E.G.; Wondergem, J. Effects of Amifostine on Radiation-Induced Cardiac Damage. Acta Oncol. 2003, 42, 4–9. [Google Scholar] [CrossRef] [PubMed]
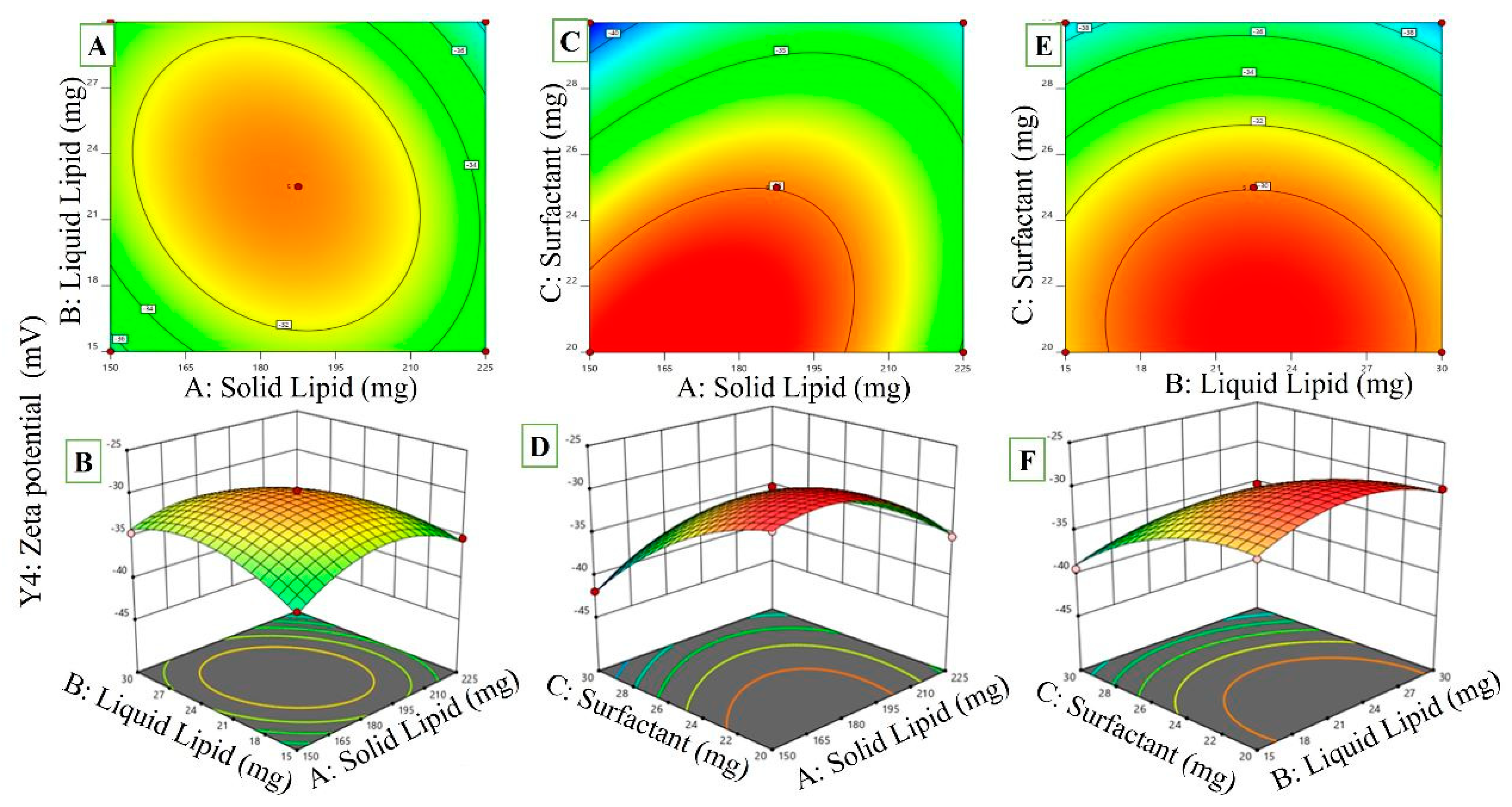
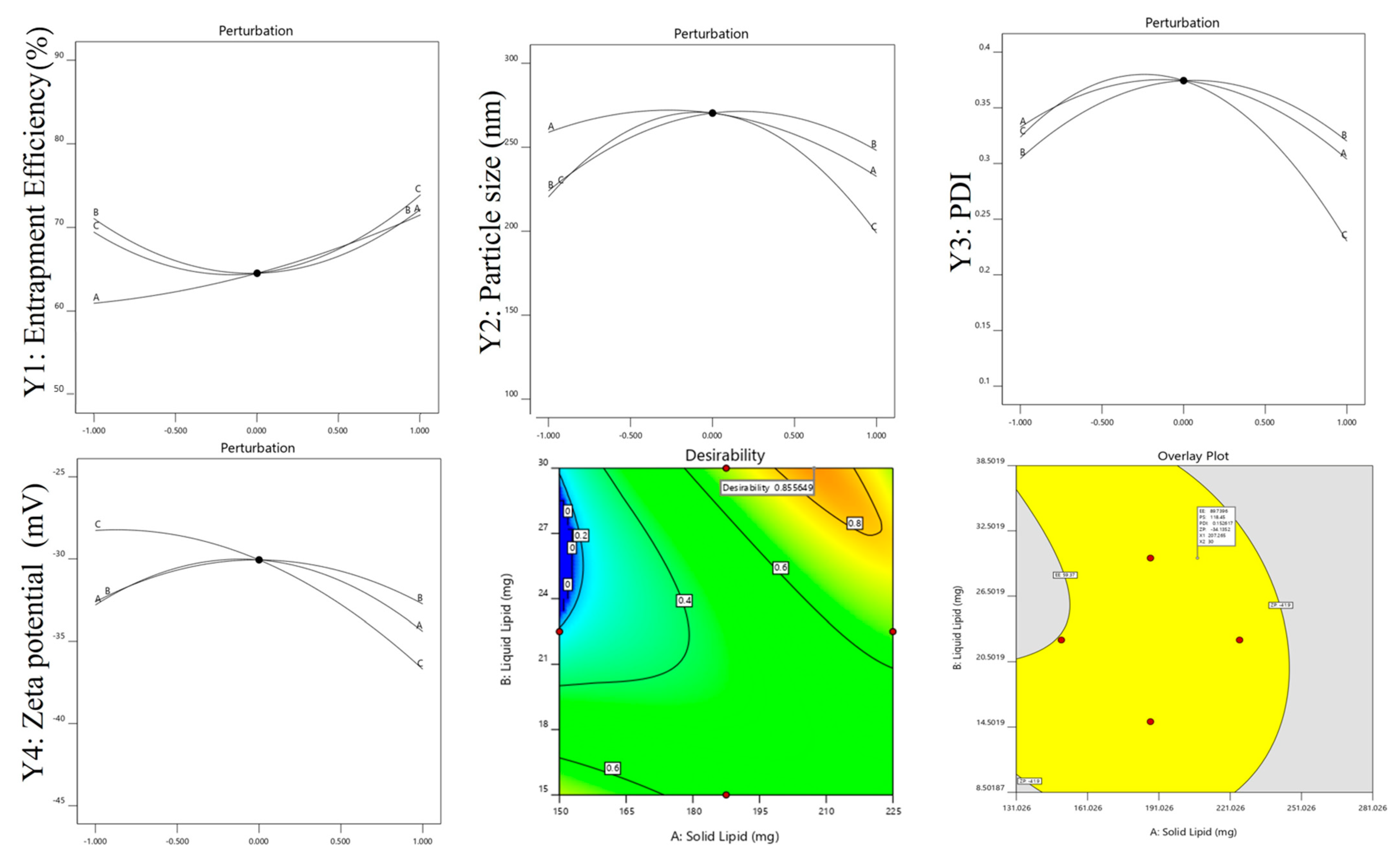
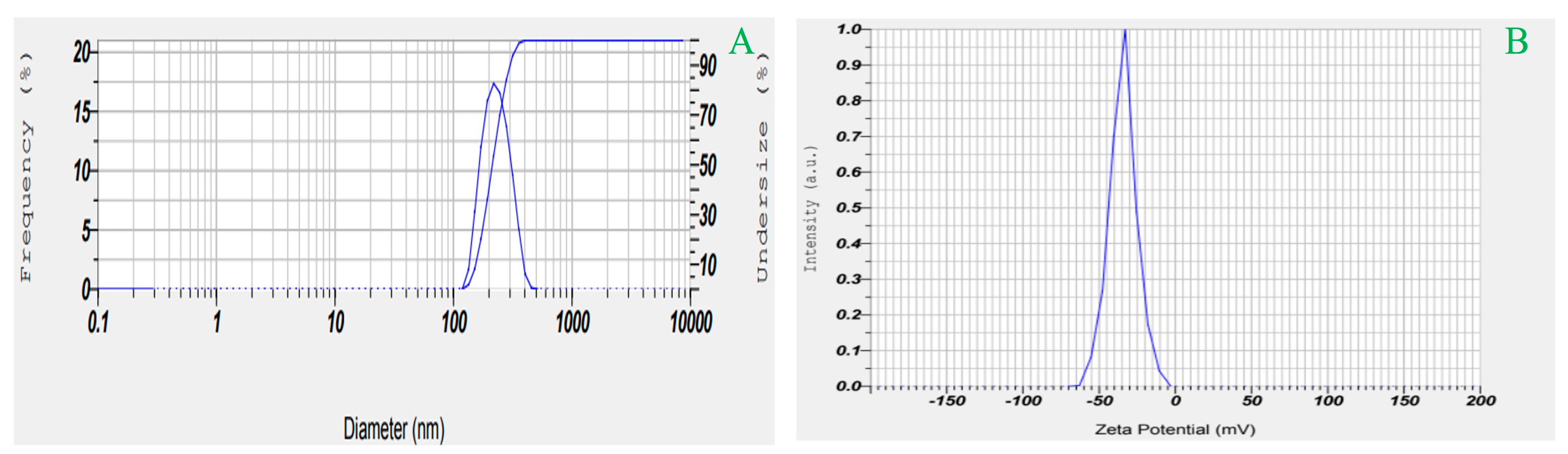
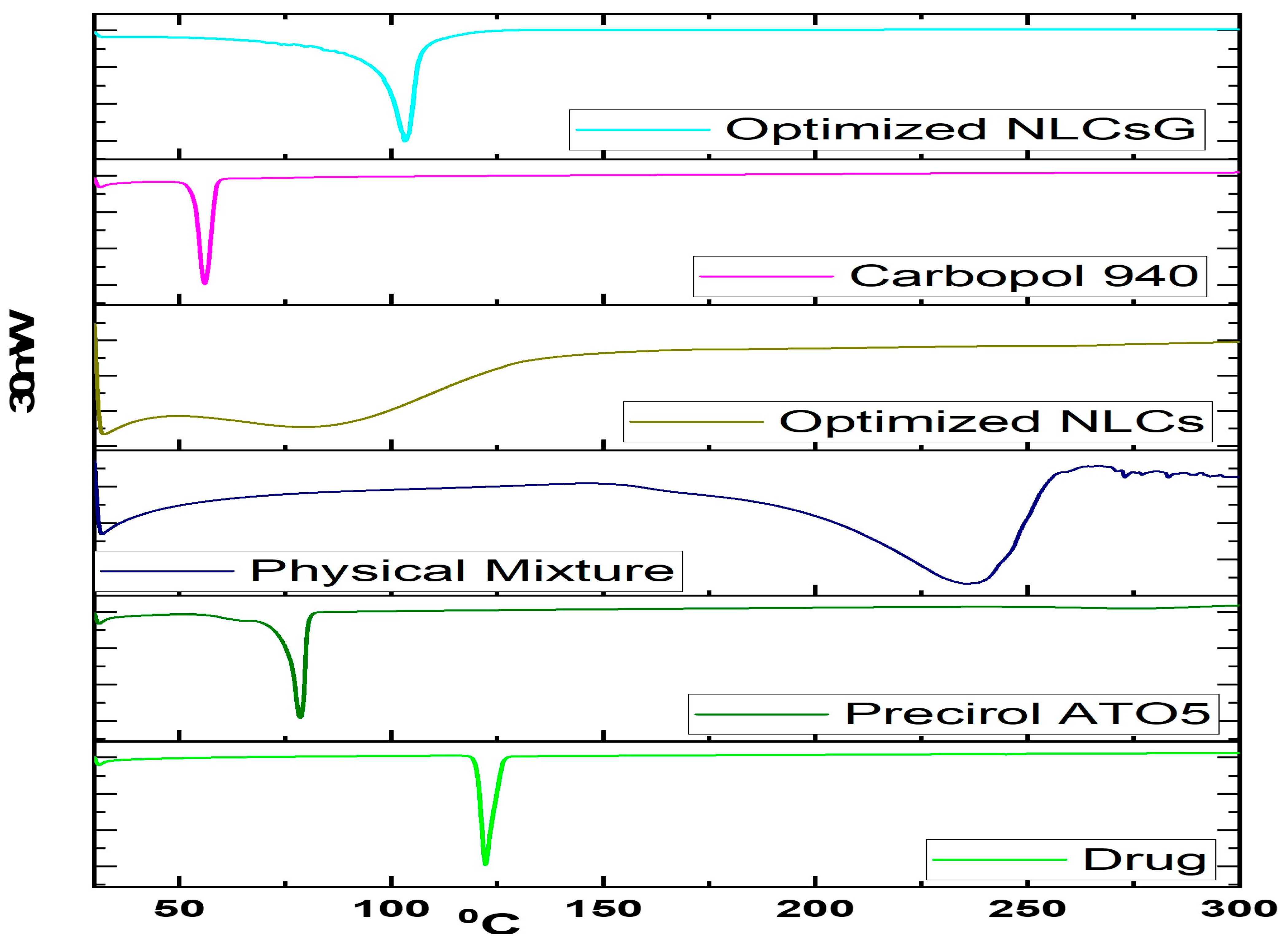
| Std | X1 | X2 | X3 | Y1 | Y2 | Y3 | Y4 |
|---|---|---|---|---|---|---|---|
| 1 | 187.5 | 22.5 | 25 | 65.81 ± 2.3 | 264.3 ± 5.39 | 0.394 ± 0.043 | −29.6 ± 1.26 |
| 2 | 187.5 | 22.5 | 25 | 65.81 ± 2.3 | 264.3 ± 5.39 | 0.394 ± 0.043 | −29.6 ± 1.26 |
| 3 | 187.5 | 15 | 20 | 74.39 ± 4.9 | 215.4 ± 6.5 | 0.291 ± 0.012 | −31.4 ± 1.05 |
| 4 | 187.5 | 22.5 | 25 | 65.81 ± 2.3 | 264.3 ± 5.39 | 0.394 ± 0.043 | −29.6 ± 1.26 |
| 5 | 187.5 | 22.5 | 25 | 65.81 ± 2.3 | 264.3 ± 5.39 | 0.394 ± 0.043 | −29.6 ± 1.26 |
| 6 | 225 | 22.5 | 20 | 82.34 ± 2.6 | 159.7 ± 6.4 | 0.218 ± 0.028 | −35.4 ± 2.15 |
| 7 | 187.5 | 22.5 | 25 | 59.37 ± 2.3 | 264.3 ± 5.39 | 0.394 ± 0.043 | −29.6 ± 1.26 |
| 8 | 225 | 30 | 25 | 88.39 ± 3.1 | 159.3 ± 3.1 | 0.168 ± 0.033 | −38.7 ± 0.61 |
| 9 | 187.5 | 30 | 20 | 78.43 ± 2.4 | 173.4 ± 8.6 | 0.235 ± 0.152 | −30.2 ± 0.98 |
| 10 | 187.5 | 30 | 30 | 80.19 ± 6.3 | 194.2 ± 3.33 | 0.198 ± 0.032 | −39.4 ± 2.15 |
| 11 | 150 | 22.5 | 30 | 76.38 ± 1.8 | 182.3 ± 2.84 | 0.167 ± 0.051 | −41.9 ± 1.38 |
| 12 | 187.5 | 15 | 30 | 82.16 ± 4.5 | 118.4 ± 5.94 | 0.135 ± 0.038 | −39.4 ± 1.52 |
| 13 | 225 | 22.5 | 30 | 74.52 ± 1.9 | 182.9 ± 3.51 | 0.184 ± 0.015 | −38.4 ± 0.68 |
| 14 | 225 | 15 | 25 | 69.31 ± 8.3 | 238.6 ± 6.25 | 0.326 ± 0.102 | −35.2 ± 1.53 |
| 15 | 150 | 22.5 | 20 | 60.28 ± 6.4 | 215.3 ± 3.38 | 0.314 ± 0.038 | −28.4 ± 0.75 |
| 16 | 150 | 15 | 25 | 76.18 ± 5.2 | 153.1 ± 5.13 | 0.158 ± 0.028 | −36.4 ± 2.38 |
| 17 | 150 | 30 | 25 | 59.43 ± 7.3 | 294.8 ± 2.49 | 0.372 ± 0.125 | −34.6 ± 3.24 |
| Optimized Formula | Optimized Concentration | Responses | Predicted Value | Experimental Value |
|---|---|---|---|---|
| Solid Lipid (X1) | 187.5 | EE (%) | 89.749 | 88.39 ± 8.1 |
| Liquid Lipid (X2) | 15 | PS (nm) | 118.4 | 118.4 ± 5.94 |
| Surfactant (X3) | 30 | PDI | 0.1525 | 0.135 ± 0.038 |
| ZP (mV) | −34.13 | −29.6 ± 1.26 |
| Physicochemical Properties | Mechanical Properties. | |||||||
|---|---|---|---|---|---|---|---|---|
| Viscosity (cps) | Spreadability (g-cm/s) | pH | Swelling Index | Firmness (g) | Toughness | Consistency (g/s) | Cohesiveness (g) | Index of Viscosity (g.s) |
| 38513 ± 0.59 | 16.84 ± 0.32 | 6.35 ± 0.26 | 3.642 ± 0.005 | 0.158 ± 0.01 | 0.492 ± 0.15 | 1.486 ± 0.23 | −0.08 ± 0.58 | −1.068 ± 0.21 |
| Pharmacokinetics Parameter | Marketed | RZ-NLCG |
|---|---|---|
| Intercept | −0.033964629 | −0.020708832 |
| Slope | 2.891306469 | 2.845944685 |
| C0 (mcg/mL) | 778.585782 ± 25.36 | 701.3659608 ± 13.47 |
| K(h−1) | 0.07822054 ± 0.02 | 0.04769244 ± 0.002 |
| Dose (mg) | 100 | 100 |
| Vd (mL) | 128.437999 ± 8.34 | 142.5789183 ± 6.48 |
| Vd (L) | 0.128438 ± 0.24 | 0.142578918 ± 0.002 |
| t1/2 (h) | 8.85956546 ± 0.05 | 14.53060491 ± 0.59 |
| Clearance (L/h) | 0.01004649 ± 0.02 | 0.006799936 ± 0.004 |
| AUC0-t (µg.h/mL) | 779.585782 ± 13.24 | 702.3659608 ± 14.52 |
| AUC1-t (µg.h/mL) | 8820.125 ± 16.58 | 11,155.465 ± 20.46 |
| AUCt-inf (µg.h/mL) | 1437.72977 ± 10.34 | 3928.505246 ± 18.94 |
| AUCTotal (µg.h/mL) | 11,037.4406 ± 13.49 | 15,786.33621 ± 18.73 |
| Cmax | 1039.68 ± 5.48 | 986.52 ± 8.45 |
| Tmax | 2.05 ± 0.13 | 4.09 ± 0.48 |
| Relative Bioavailability | - | 1.64 ± 0.13 |
| Group | Myonecrosis | Inflammatory Cell | Edema |
|---|---|---|---|
| Control | - | - | + |
| Vasopressin | +++ | +++ | +++ |
| RZ-NLCs | ++ | + | + |
| RZ-NLCsG | - | - | - |
| Stability Parameters | 0 month | 1 month | 3 months | 6 months |
|---|---|---|---|---|
| Storage conditions (5 ± 3 °C) | ||||
| PS (nm) | 118.4 ± 5.94 | 125.28 ± 2.31 | 129.32 ± 3.52 | 135.67 ± 5.94 |
| EE (%) | 88.39 ± 8.1 | 85.37 ± 1.05 | 82.46 ± 1.29 | 76.49 ± 0.95 |
| PDI | 0.118 ± 0.028 | 0.135 ± 0.025 | 0.139 ± 0.038 | 0.153 ± 0.051 |
| ZP (mV) | −41.9 ± 1.38 | −43.68 ± 0.29 | −41.26 ± 0.83 | −48.92 ± 0.61 |
| Storage conditions (25 ± 2 °C/60 ± 5%RH) | ||||
| PS (nm) | 118.4 ± 5.94 | 294.35 ± 18.23 | 457.22 ± 0.00 | 958.34 ± 0.00 |
| EE (%) | 88.39 ± 8.1 | 69.38 ± 5.64 | NM | NM |
| PDI | 0.118 ± 0.028 | 0.85 ± 0.35 | NM | NM |
| ZP (mV) | −41.9 ± 1.38 | −49.35 ± 2.35 | NM | NM |
| Storage condition (40 ± 2 °C/75 ± 5% RH) | ||||
| PS (nm) | 118.4 ± 5.94 | 356.49 ± 25.34 | NM | NM |
| EE (%) | 88.39 ± 8.1 | 59.43 ± 6.49 | NM | NM |
| PDI | 0.118 ± 0.028 | 0.94 ± 1.23 | NM | NM |
| ZP (mV) | −41.9 ± 1.38 | −45.32 ± 2.11 | NM | NM |
| Parameter | Low (−1) | Medium (0) | High (+1) |
|---|---|---|---|
| Independent Variables | |||
| Solid Lipid (X1) | 4 | 6 | 8 |
| Liquid Lipid (X2) | 1 | 2.5 | 5 |
| surfactant (X3) | 1 | 5.5 | 10 |
| Dependent variables | |||
| Y1: Entrapment Efficiency (%) | 59.37 ± 2.3 | 88.39 ± 8.1 | |
| Y2: Vesicle Size (nm) | 118.4 ± 5.94 | 294.8 ± 2.49 | |
| Y3: polydispersity index | 0.118 ± 0.028 | 0.372 ± 0.125 | |
| Y4: zetapotential (mV) | −41.9 ± 1.38 | −29.6 ± 1.26 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Unnisa, A.; Chettupalli, A.K.; Alazragi, R.S.; Alelwani, W.; Bannunah, A.M.; Barnawi, J.; Amarachinta, P.R.; Jandrajupalli, S.B.; Elamine, B.A.; Mohamed, O.A.; et al. Nanostructured Lipid Carriers to Enhance the Bioavailability and Solubility of Ranolazine: Statistical Optimization and Pharmacological Evaluations. Pharmaceuticals 2023, 16, 1151. https://doi.org/10.3390/ph16081151
Unnisa A, Chettupalli AK, Alazragi RS, Alelwani W, Bannunah AM, Barnawi J, Amarachinta PR, Jandrajupalli SB, Elamine BA, Mohamed OA, et al. Nanostructured Lipid Carriers to Enhance the Bioavailability and Solubility of Ranolazine: Statistical Optimization and Pharmacological Evaluations. Pharmaceuticals. 2023; 16(8):1151. https://doi.org/10.3390/ph16081151
Chicago/Turabian StyleUnnisa, Aziz, Ananda K. Chettupalli, Reem S. Alazragi, Walla Alelwani, Azzah M. Bannunah, Jameel Barnawi, Padmanabha R. Amarachinta, Suresh B. Jandrajupalli, Badria A. Elamine, Omkalthoum A. Mohamed, and et al. 2023. "Nanostructured Lipid Carriers to Enhance the Bioavailability and Solubility of Ranolazine: Statistical Optimization and Pharmacological Evaluations" Pharmaceuticals 16, no. 8: 1151. https://doi.org/10.3390/ph16081151
APA StyleUnnisa, A., Chettupalli, A. K., Alazragi, R. S., Alelwani, W., Bannunah, A. M., Barnawi, J., Amarachinta, P. R., Jandrajupalli, S. B., Elamine, B. A., Mohamed, O. A., & Hussain, T. (2023). Nanostructured Lipid Carriers to Enhance the Bioavailability and Solubility of Ranolazine: Statistical Optimization and Pharmacological Evaluations. Pharmaceuticals, 16(8), 1151. https://doi.org/10.3390/ph16081151






