Pharmacological Therapy in Inflammatory Bowel Diseases: A Narrative Review of the Past 90 Years
Abstract
:1. Introduction
2. Methodology
3. Therapies and Therapeutic Goals
4. Amino Salicylates
5. Glucocorticoids
6. Immunomodulators: Thiopurines and Methotrexate
7. Biological Therapy
8. Anti-TNF
9. Anti-Integrin
10. Anti-Interleukin
11. Small Molecules
12. JAK Inhibitors
13. S1p Modulators
14. Advances in the Safety of Immunosuppressive Therapies
15. Future Perspectives
16. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Celebi Sozener, Z.; Ozdel Ozturk, B.; Cerci, P.; Turk, M.; Gorgulu Akin, B.; Akdis, M.; Altiner, S.; Ozbey, U.; Ogulur, I.; Mitamura, Y.; et al. Epithelial Barrier Hypothesis: Effect of the External Exposome on the Microbiome and Epithelial Barriers in Allergic Disease. Allergy 2022, 77, 1418–1449. [Google Scholar] [CrossRef]
- Barrett, B.; Charles, J.W.; Temte, J.L. Climate Change, Human Health, and Epidemiological Transition. Prev. Med. 2015, 70, 69–75. [Google Scholar] [CrossRef]
- Colella, M.; Charitos, I.A.; Ballini, A.; Cafiero, C.; Topi, S.; Palmirotta, R.; Santacroce, L. Microbiota Revolution: How Gut Microbes Regulate Our Lives. World J. Gastroenterol. 2023, 29, 4368–4383. [Google Scholar] [CrossRef]
- Ananthakrishnan, A.N. Epidemiology and Risk Factors for IBD. Nat. Rev. Gastroenterol. Hepatol. 2015, 12, 205–217. [Google Scholar] [CrossRef]
- Gearry, R.B. IBD and Environment: Are There Differences between East and West. Dig. Dis. 2016, 34, 84–89. [Google Scholar] [CrossRef]
- Actis, G.C.; Pellicano, R.; Fagoonee, S.; Ribaldone, D.G. History of Inflammatory Bowel Diseases. J. Clin. Med. 2019, 8, 1970. [Google Scholar] [CrossRef]
- Agrawal, M.; Jess, T. Implications of the Changing Epidemiology of Inflammatory Bowel Disease in a Changing World. United Eur. Gastroenterol. J. 2022, 10, 1113–1120. [Google Scholar] [CrossRef]
- Padoan, A.; Musso, G.; Contran, N.; Basso, D. Inflammation, Autoinflammation and Autoimmunity in Inflammatory Bowel Diseases. Curr. Issues Mol. Biol. 2023, 45, 5534–5557. [Google Scholar] [CrossRef]
- Jefremow, A.; Neurath, M.F. Novel Small Molecules in IBD: Current State and Future Perspectives. Cells 2023, 12, 1730. [Google Scholar] [CrossRef]
- Ashton, J.J.; Beattie, R.M. Inflammatory Bowel Disease: Recent Developments. Arch. Dis. Child. 2023, 325668. [Google Scholar] [CrossRef]
- Lomazi, E.A.; Oba, J.; Rodrigues, M.; Marmo, M.C.D.R.; Sandy, N.S.; Sdepanian, V.L.; Imbrizi, M.; Baima, J.P.; Magro, D.O.; Albuquerque, I.C.D.; et al. Brazilian Consensus on the Management of Inflammatory Bowel Diseases in Pediatric Patients: A Consensus of the Brazilian Organization for Crohn’s Disease and Colitis (GEDIIB). Arq. Gastroenterol. 2023, 59, 85–124. [Google Scholar] [CrossRef]
- Van Rheenen, P.F.; Aloi, M.; Assa, A.; Bronsky, J.; Escher, J.C.; Fagerberg, U.L.; Gasparetto, M.; Gerasimidis, K.; Griffiths, A.; Henderson, P.; et al. The Medical Management of Paediatric Crohn’s Disease: An ECCO-ESPGHAN Guideline Update. J. Crohns Colitis 2021, 15, 171–194. [Google Scholar] [CrossRef]
- Gkikas, K.; Gerasimidis, K.; Milling, S.; Ijaz, U.Z.; Hansen, R.; Russell, R.K. Dietary Strategies for Maintenance of Clinical Remission in Inflammatory Bowel Diseases: Are We There Yet? Nutrients 2020, 12, 2018. [Google Scholar] [CrossRef]
- Albenberg, L. The Role of Diet in Pediatric Inflammatory Bowel Disease. Gastroenterol. Clin. N. Am. 2023, 52, 565–577. [Google Scholar] [CrossRef]
- Raine, T.; Danese, S. Breaking Through the Therapeutic Ceiling: What Will It Take? Gastroenterology 2022, 162, 1507–1511. [Google Scholar] [CrossRef] [PubMed]
- Turner, D.; Ricciuto, A.; Lewis, A.; D’Amico, F.; Dhaliwal, J.; Griffiths, A.M.; Bettenworth, D.; Sandborn, W.J.; Sands, B.E.; Reinisch, W.; et al. STRIDE-II: An Update on the Selecting Therapeutic Targets in Inflammatory Bowel Disease (STRIDE) Initiative of the International Organization for the Study of IBD (IOIBD): Determining Therapeutic Goals for Treat-to-Target Strategies in IBD. Gastroenterology 2021, 160, 1570–1583. [Google Scholar] [CrossRef]
- Liu, J.; Di, B.; Xu, L. Recent Advances in the Treatment of IBD: Targets, Mechanisms and Related Therapies. Cytokine Growth Factor Rev. 2023, 71–72, 1–12. [Google Scholar] [CrossRef]
- Parigi, T.L.; D’Amico, F.; Abreu, M.T.; Dignass, A.; Dotan, I.; Magro, F.; Griffiths, A.M.; Jairath, V.; Iacucci, M.; Mantzaris, G.J.; et al. Difficult-to-Treat Inflammatory Bowel Disease: Results from an International Consensus Meeting. Lancet Gastroenterol. Hepatol. 2023, 8, 853–859. [Google Scholar] [CrossRef]
- Dubois-Camacho, K.; Ottum, P.A.; Franco-Muñoz, D.; De la Fuente, M.; Torres-Riquelme, A.; Díaz-Jiménez, D.; Olivares-Morales, M.; Astudillo, G.; Quera, R.; Hermoso, M.A. Glucocorticosteroid Therapy in Inflammatory Bowel Diseases: From Clinical Practice to Molecular Biology. World J. Gastroenterol. 2017, 23, 6628–6638. [Google Scholar] [CrossRef] [PubMed]
- Grevenitis, P.; Thomas, A.; Lodhia, N. Medical Therapy for Inflammatory Bowel Disease. Surg. Clin. N. Am. 2015, 95, 1159–1182. [Google Scholar] [CrossRef]
- Bonovas, S.; Nikolopoulos, G.K.; Lytras, T.; Fiorino, G.; Peyrin-Biroulet, L.; Danese, S. Comparative Safety of Systemic and Low-Bioavailability Steroids in Inflammatory Bowel Disease: Systematic Review and Network Meta-Analysis: Comparative Safety of Steroids in IBD. Br. J. Clin. Pharmacol. 2018, 84, 239–251. [Google Scholar] [CrossRef] [PubMed]
- Dignass, A.; Rath, S.; Kleindienst, T.; Stallmach, A. Review Article: Translating STRIDE-II into Clinical Reality—Opportunities and Challenges. Aliment. Pharmacol. Ther. 2023, 58, 492–502. [Google Scholar] [CrossRef] [PubMed]
- Le Berre, C.; Peyrin-Biroulet, L.; Sandborn, W.J.; Colombel, J.-F.; Rubin, D.; Chowers, Y.; Reinisch, W.; Schreiber, S.; Allez, M.; D’Haens, G.; et al. Selecting End Points for Disease-Modification Trials in Inflammatory Bowel Disease: The SPIRIT Consensus From the IOIBD. Gastroenterology 2021, 160, 1452–1460.e21. [Google Scholar] [CrossRef] [PubMed]
- Feuerstein, J.D.; Ho, E.Y.; Shmidt, E.; Singh, H.; Falck-Ytter, Y.; Sultan, S.; Terdiman, J.P.; Sultan, S.; Cohen, B.L.; Chachu, K.; et al. AGA Clinical Practice Guidelines on the Medical Management of Moderate to Severe Luminal and Perianal Fistulizing Crohn’s Disease. Gastroenterology 2021, 160, 2496–2508. [Google Scholar] [CrossRef]
- Feuerstein, J.D.; Isaacs, K.L.; Schneider, Y.; Siddique, S.M.; Falck-Ytter, Y.; Singh, S.; Chachu, K.; Day, L.; Lebwohl, B.; Muniraj, T.; et al. AGA Clinical Practice Guidelines on the Management of Moderate to Severe Ulcerative Colitis. Gastroenterology 2020, 158, 1450–1461. [Google Scholar] [CrossRef] [PubMed]
- Raine, T.; Bonovas, S.; Burisch, J.; Kucharzik, T.; Adamina, M.; Annese, V.; Bachmann, O.; Bettenworth, D.; Chaparro, M.; Czuber-Dochan, W.; et al. ECCO Guidelines on Therapeutics in Ulcerative Colitis: Medical Treatment. J. Crohn’s Colitis 2022, 16, 2–17. [Google Scholar] [CrossRef]
- Torres, J.; Bonovas, S.; Doherty, G.; Kucharzik, T.; Gisbert, J.P.; Raine, T.; Adamina, M.; Armuzzi, A.; Bachmann, O.; Bager, P.; et al. ECCO Guidelines on Therapeutics in Crohn’s Disease: Medical Treatment. J. Crohn’s Colitis 2020, 14, 4–22. [Google Scholar] [CrossRef]
- Baima, J.P.; Imbrizi, M.; Ribas, A.; Andrade, L.; Costa, M.; Freitas, N.S.; Azevedo, M.F.C.; de Magalhães, M.H.; Brito, R.d.S. Second Brazilian Consensus on the Management of Ulcerative Colitis in Adults: A Consensus of the Brazilian Organization for Crohn’s Disease and Colitis (GEDIIB). Arq. Gastroenterol. 2023, 59, 51–84. [Google Scholar] [CrossRef]
- Imbrizi, M.; Baima, J.P.; Azevedo, M.F.C.; Ribas, A.; Freitas, N.S.; Andrade, L.; Costa, M.; Yukie, L.; Serafim, R.; Botelho, A.; et al. Second Brazilian Consensus on the Management of Crohn’s Disease in Adults: A Consensus of the Brazilian Organization for Crohn’s Disease and Colitis (GEDIIB). Arq. Gastroenterol. 2023, 59, 20–50. [Google Scholar] [CrossRef]
- Juliao-Baños, F.; Grillo-Ardila, C.F.; Alfaro, I.; Andara-Ramírez, M.T.; Avelar-Escobar, O.; Barahona-Garrido, J.; Bautista-Martínez, S.; Bosques-Padilla, F.J.; De Paula, J.A.; Ernest-Suárez, K.; et al. Update of the PANCCO Clinical Practice Guidelines for the Treatment of Ulcerative Colitis in the Adult Population. Rev. Gastroenterol. M. Engl. Ed. 2022, 87, 342–361. [Google Scholar] [CrossRef]
- Travis, S.P.L.; Danese, S.; Kupcinskas, L.; Alexeeva, O.; D’Haens, G.; Gibson, P.R.; Moro, L.; Jones, R.; Ballard, E.D.; Masure, J.; et al. Once-Daily Budesonide MMX in Active, Mild-to-Moderate Ulcerative Colitis: Results from the Randomised CORE II Study. Gut 2014, 63, 433–441. [Google Scholar] [CrossRef] [PubMed]
- Edsbacker, S.; Andersson, T. Pharmacokinetics of Budesonide (Entocort EC) Capsules for Crohn’s Disease. Clin. Pharmacokinet. 2004, 43, 803–821. [Google Scholar] [CrossRef] [PubMed]
- Löwenberg, M.; Volkers, A.; Van Gennep, S.; Mookhoek, A.; Montazeri, N.; Clasquin, E.; Duijvestein, M.; Van Bodegraven, A.; Rietdijk, S.; Jansen, J.; et al. Mercaptopurine for the Treatment of Ulcerative Colitis: A Randomized Placebo-Controlled Trial. J. Crohn’s Colitis 2023, 17, 1055–1065. [Google Scholar] [CrossRef] [PubMed]
- Hanauer, S.B.; Feagan, B.G.; Lichtenstein, G.R.; Mayer, L.F.; Schreiber, S.; Colombel, J.F.; Rachmilewitz, D.; Wolf, D.C.; Olson, A.; Bao, W.; et al. Maintenance Infliximab for Crohn’s Disease: The ACCENT I Randomised Trial. Lancet 2002, 359, 1541–1549. [Google Scholar] [CrossRef] [PubMed]
- Van Dullemen, H.M.; van Deventer, S.J.H.; Hommes, D.W.; Bijl, H.A.; Jansen, J.; Tytgat, G.N.J.; Woody, J. Treatment of Crohn’s Disease with Anti-Tumor Necrosis Factor Chimeric Monoclonal Antibody (CA2). Gastroenterology 1995, 109, 129–135. [Google Scholar] [CrossRef]
- Sands, B.E.; Anderson, F.H.; Bernstein, C.N.; Chey, W.Y.; Feagan, B.G.; Fedorak, R.N.; Kamm, M.A.; Korzenik, J.R.; Lashner, B.A.; Onken, J.E.; et al. Infliximab Maintenance Therapy for Fistulizing Crohn’s Disease. N. Engl. J. Med. 2004, 350, 876–885. [Google Scholar] [CrossRef]
- Buisson, A.; Nachury, M.; Reymond, M.; Yzet, C.; Wils, P.; Payen, L.; Laugie, M.; Manlay, L.; Mathieu, N.; Pereira, B.; et al. Effectiveness of Switching from Intravenous to Subcutaneous Infliximab in Patients with Inflammatory Bowel Diseases: The REMSWITCH Study. Clin. Gastroenterol. Hepatol. 2022, 21, 2338–2346.e3. [Google Scholar] [CrossRef]
- Hanauer, S.B.; Sandborn, W.J.; Rutgeerts, P.; Fedorak, R.N.; Lukas, M.; MacIntosh, D.; Panaccione, R.; Wolf, D.; Pollack, P. Human Anti–Tumor Necrosis Factor Monoclonal Antibody (Adalimumab) in Crohn’s Disease: The CLASSIC-I Trial. Gastroenterology 2006, 130, 323–333. [Google Scholar] [CrossRef]
- Sandborn, W.J.; Hanauer, S.B.; Rutgeerts, P.; Fedorak, R.N.; Lukas, M.; MacIntosh, D.G.; Panaccione, R.; Wolf, D.; Kent, J.D.; Bittle, B.; et al. Adalimumab for Maintenance Treatment of Crohn’s Disease: Results of the CLASSIC II Trial. Gut 2007, 56, 1232–1239. [Google Scholar] [CrossRef]
- Reinisch, W.; Sandborn, W.J.; Hommes, D.W.; D’Haens, G.; Hanauer, S.; Schreiber, S.; Panaccione, R.; Fedorak, R.N.; Tighe, M.B.; Huang, B.; et al. Adalimumab for Induction of Clinical Remission in Moderately to Severely Active Ulcerative Colitis: Results of a Randomised Controlled Trial. Gut 2011, 60, 780–787. [Google Scholar] [CrossRef]
- Sandborn, W.J.; Honiball, P.J.; Bloomfield, R.; Schreiber, S. Certolizumab Pegol for the Treatment of Crohn’s Disease. N. Engl. J. Med. 2007, 357, 228–238. [Google Scholar] [CrossRef] [PubMed]
- Schreiber, S.; Thomsen, O.Ø.; McColm, J. Maintenance Therapy with Certolizumab Pegol for Crohn’s Disease. N. Engl. J. Med. 2007, 357, 239–250. [Google Scholar] [CrossRef] [PubMed]
- Sandborn, W.J.; Feagan, B.G.; Marano, C.; Zhang, H.; Strauss, R.; Johanns, J.; Adedokun, O.J.; Guzzo, C.; Colombel, J.-F.; Reinisch, W.; et al. Subcutaneous Golimumab Induces Clinical Response and Remission in Patients with Moderate-to-Severe Ulcerative Colitis. Gastroenterology 2014, 146, 85–95. [Google Scholar] [CrossRef]
- Sandborn, W.J.; Feagan, B.G.; Marano, C.; Zhang, H.; Strauss, R.; Johanns, J.; Adedokun, O.J.; Guzzo, C.; Colombel, J.; Reinisch, W.; et al. Subcutaneous Golimumab Maintains Clinical Response in Patients with Moderate-to-Severe Ulcerative Colitis. Gastroenterology 2014, 146, 96–109.e1. [Google Scholar] [CrossRef] [PubMed]
- Targan, S.R.; Feagan, B.G.; Fedorak, R.N.; Lashner, B.A.; Panaccione, R.; Present, D.H.; Spehlmann, M.E.; Rutgeerts, P.J.; Tulassay, Z.; Volfova, M.; et al. Natalizumab for the Treatment of Active Crohn’s Disease: Results of the ENCORE Trial. Gastroenterology 2007, 132, 1672–1683. [Google Scholar] [CrossRef]
- Sandborn, W.J.; Feagan, B.G.; Rutgeerts, P.; Hanauer, S.; Colombel, J.-F.; Sands, B.E.; Lukas, M.; Fedorak, R.N.; Lee, S.; Bressler, B.; et al. Vedolizumab as Induction and Maintenance Therapy for Crohn’s Disease. N. Engl. J. Med. 2013, 369, 711–721. [Google Scholar] [CrossRef] [PubMed]
- Feagan, B.G.; Rutgeerts, P.; Sands, B.E.; Hanauer, S.; Colombel, J.-F.; Sandborn, W.J.; Van Assche, G.; Axler, J.; Kim, H.-J.; Danese, S.; et al. Vedolizumab as Induction and Maintenance Therapy for Ulcerative Colitis. N. Engl. J. Med. 2013, 369, 699–710. [Google Scholar] [CrossRef]
- Vermeire, S.; D’Haens, G.; Baert, F.; Danese, S.; Kobayashi, T.; Loftus, E.V.; Bhatia, S.; Agboton, C.; Rosario, M.; Chen, C.; et al. Efficacy and Safety of Subcutaneous Vedolizumab in Patients with Moderately to Severely Active Crohn’s Disease: Results from the VISIBLE 2 Randomised Trial. J. Crohn’s Colitis 2022, 16, 27–38. [Google Scholar] [CrossRef]
- Feagan, B.G.; Sandborn, W.J.; Gasink, C.; Jacobstein, D.; Lang, Y.; Friedman, J.R.; Blank, M.A.; Johanns, J.; Gao, L.-L.; Miao, Y.; et al. Ustekinumab as Induction and Maintenance Therapy for Crohn’s Disease. N. Engl. J. Med. 2016, 375, 1946–1960. [Google Scholar] [CrossRef]
- Sands, B.E.; Sandborn, W.J.; Panaccione, R.; O’Brien, C.D.; Zhang, H.; Johanns, J.; Adedokun, O.J.; Li, K.; Peyrin-Biroulet, L.; Van Assche, G.; et al. Ustekinumab as Induction and Maintenance Therapy for Ulcerative Colitis. N. Engl. J. Med. 2019, 381, 1201–1214. [Google Scholar] [CrossRef]
- D’Haens, G.; Panaccione, R.; Baert, F.; Bossuyt, P.; Colombel, J.-F.; Danese, S.; Dubinsky, M.; Feagan, B.G.; Hisamatsu, T.; Lim, A.; et al. Risankizumab as Induction Therapy for Crohn’s Disease: Results from the Phase 3 ADVANCE and MOTIVATE Induction Trials. Lancet 2022, 399, 2015–2030. [Google Scholar] [CrossRef] [PubMed]
- Ferrante, M.; Panaccione, R.; Baert, F.; Bossuyt, P.; Colombel, J.-F.; Danese, S.; Dubinsky, M.; Feagan, B.G.; Hisamatsu, T.; Lim, A.; et al. Risankizumab as Maintenance Therapy for Moderately to Severely Active Crohn’s Disease: Results from the Multicentre, Randomised, Double-Blind, Placebo-Controlled, Withdrawal Phase 3 FORTIFY Maintenance Trial. Lancet 2022, 399, 2031–2046. [Google Scholar] [CrossRef] [PubMed]
- D’Haens, G.; Dubinsky, M.; Kobayashi, T.; Irving, P.M.; Howaldt, S.; Pokrotnieks, J.; Krueger, K.; Laskowski, J.; Li, X.; Lissoos, T.; et al. Mirikizumab as Induction and Maintenance Therapy for Ulcerative Colitis. N. Engl. J. Med. 2023, 388, 2444–2455. [Google Scholar] [CrossRef]
- Sandborn, W.J. Tofacitinib as Induction and Maintenance Therapy for Ulcerative Colitis. N. Engl. J. Med. 2017, 376, 1723–1736. [Google Scholar] [CrossRef]
- Feagan, B.G.; Danese, S.; Loftus, E.V.; Vermeire, S.; Schreiber, S.; Ritter, T.; Fogel, R.; Mehta, R.; Nijhawan, S.; Kempiński, R.; et al. Filgotinib as Induction and Maintenance Therapy for Ulcerative Colitis (SELECTION): A Phase 2b/3 Double-Blind, Randomised, Placebo-Controlled Trial. Lancet 2021, 397, 2372–2384. [Google Scholar] [CrossRef]
- Danese, S.; Vermeire, S.; Zhou, W.; Pangan, A.L.; Siffledeen, J.; Greenbloom, S.; Hébuterne, X.; D’Haens, G.; Nakase, H.; Panés, J.; et al. Upadacitinib as Induction and Maintenance Therapy for Moderately to Severely Active Ulcerative Colitis: Results from Three Phase 3, Multicentre, Double-Blind, Randomised Trials. Lancet 2022, 399, 2113–2128. [Google Scholar] [CrossRef] [PubMed]
- Loftus, E.V.; Panés, J.; Lacerda, A.P.; Peyrin-Biroulet, L.; D’Haens, G.; Panaccione, R.; Reinisch, W.; Louis, E.; Chen, M.; Nakase, H.; et al. Upadacitinib Induction and Maintenance Therapy for Crohn’s Disease. N. Engl. J. Med. 2023, 388, 1966–1980. [Google Scholar] [CrossRef]
- Sandborn, W.J.; Feagan, B.G.; Wolf, D.C.; D’Haens, G.; Vermeire, S.; Hanauer, S.B.; Ghosh, S.; Smith, H.; Cravets, M.; Frohna, P.A.; et al. Ozanimod Induction and Maintenance Treatment for Ulcerative Colitis. N. Engl. J. Med. 2016, 374, 1754–1762. [Google Scholar] [CrossRef]
- Sandborn, W.J.; Vermeire, S.; Peyrin-Biroulet, L.; Dubinsky, M.C.; Panes, J.; Yarur, A.; Ritter, T.; Baert, F.; Schreiber, S.; Sloan, S.; et al. Etrasimod as Induction and Maintenance Therapy for Ulcerative Colitis (ELEVATE): Two Randomised, Double-Blind, Placebo-Controlled, Phase 3 Studies. Lancet 2023, 401, 1159–1171. [Google Scholar] [CrossRef]
- Nikfar, S.; Rahimi, R.; Rezaie, A.; Abdollahi, M. A Meta-Analysis of the Efficacy of Sulfasalazine in Comparison with 5-Aminosalicylates in the Induction of Improvement and Maintenance of Remission in Patients with Ulcerative Colitis. Dig. Dis. Sci. 2009, 54, 1157–1170. [Google Scholar] [CrossRef]
- Magro, F.; Cordeiro, G.; Dias, A.M.; Estevinho, M.M. Inflammatory Bowel Disease—Non-Biological Treatment. Pharmacol. Res. 2020, 160, 105075. [Google Scholar] [CrossRef] [PubMed]
- Słoka, J.; Madej, M.; Strzalka-Mrozik, B. Molecular Mechanisms of the Antitumor Effects of Mesalazine and Its Preventive Potential in Colorectal Cancer. Molecules 2023, 28, 5081. [Google Scholar] [CrossRef] [PubMed]
- Le Berre, C.; Honap, S.; Peyrin-Biroulet, L. Ulcerative Colitis. Lancet 2023, 402, 571–584. [Google Scholar] [CrossRef] [PubMed]
- Barberio, B.; Segal, J.P.; Quraishi, M.N.; Black, C.J.; Savarino, E.V.; Ford, A.C. Efficacy of Oral, Topical, or Combined Oral and Topical 5-Aminosalicylates, in Ulcerative Colitis: Systematic Review and Network Meta-Analysis. J. Crohn’s Colitis 2021, 15, 1184–1196. [Google Scholar] [CrossRef]
- Chibbar, R.; Moss, A.C. Mesalamine in the Initial Therapy of Ulcerative Colitis. Gastroenterol. Clin. N. Am. 2020, 49, 689–704. [Google Scholar] [CrossRef]
- West, R.; Russel, M.; Bodelier, A.; Kuijvenhoven, J.; Bruin, K.; Jansen, J.; Van Der Meulen, A.; Keulen, E.; Wolters, L.; Ouwendijk, R.; et al. Lower Risk of Recurrence with a Higher Induction Dose of Mesalazine and Longer Duration of Treatment in Ulcerative Colitis: Results from the Dutch, Non-Interventional, IMPACT Study. J. Gastrointest. Liver Dis. 2022, 31, 18–24. [Google Scholar] [CrossRef]
- Kisner, J.B.; Palmer, W.L. Ulcerative Colitis: Therapeutic Effects of Corticotropin (ACTH) and Cortisone in 120 Patients. J. Lab Clin. Med. 1950, 41, 232–250. [Google Scholar] [CrossRef]
- Bruscoli, S.; Febo, M.; Riccardi, C.; Migliorati, G. Glucocorticoid Therapy in Inflammatory Bowel Disease: Mechanisms and Clinical Practice. Front. Immunol. 2021, 12, 691480. [Google Scholar] [CrossRef]
- Lichtenstein, G.R. Budesonide Multi-Matrix for the Treatment of Patients with Ulcerative Colitis. Dig. Dis. Sci. 2016, 61, 358–370. [Google Scholar] [CrossRef]
- Hoy, S.M. Budesonide MMX®: A Review of Its Use in Patients with Mild to Moderate Ulcerative Colitis. Drugs 2015, 75, 879–886. [Google Scholar] [CrossRef]
- Campieri, M.; Ferguson, A.; Doe, W.; Persson, T.; Nilsson, L.-G. Oral Budesonide Is as Efective as Oral Prednisolone in Active Crohn’s Disease. Gut 1997, 41, 209–214. [Google Scholar] [CrossRef] [PubMed]
- Brooke, B.N.; Swarbrick, E.T. Azathioprine for Crohn’s Disease. Lancet 1969, 294, 612–614. [Google Scholar] [CrossRef] [PubMed]
- Teml, A.; Schaeffeler, E.; Herrlinger, K.R.; Klotz, U.; Schwab, M. Thiopurine Treatment in Inflammatory Bowel Disease. Clin. Pharmacokinet. 2007, 46, 187–208. [Google Scholar] [CrossRef]
- Estevinho, M.M.; Afonso, J.; Rosa, I.; Lago, P.; Trindade, E.; Correia, L.; Dias, C.C.; Magro, F.; On behalf GEDII [Portuguese IBD Group]. A Systematic Review and Meta-Analysis of 6-Thioguanine Nucleotide Levels and Clinical Remission in Inflammatory Bowel Disease. J. Crohn’s Colitis 2017, 11, 1381–1392. [Google Scholar] [CrossRef]
- Pariente, B.; Laharie, D. Review Article: Why, When and How to de-Escalate Therapy in Inflammatory Bowel Diseases. Aliment. Pharmacol. Ther. 2014, 40, 338–353. [Google Scholar] [CrossRef] [PubMed]
- Van Deventer, S.J. Tumour Necrosis Factor and Crohn’s Disease. Gut 1997, 40, 443–448. [Google Scholar] [CrossRef]
- Papadakis, K.A.; Targan, S.R. Role of Cytokines in the Pathogenesis of Inflammatory Bowel Disease. Annu. Rev. Med. 2000, 51, 289–298. [Google Scholar] [CrossRef]
- Neurath, M.F. Cytokines in Inflammatory Bowel Disease. Nat. Rev. Immunol. 2014, 14, 329–342. [Google Scholar] [CrossRef]
- Colombel, J.F.; Mantzaris, G.J.; Rachmilewitz, D.; Diamond, R.H. Infliximab, Azathioprine, or Combination Therapy for Crohn’s Disease. N. Engl. J. Med. 2010, 362, 1383–1395. [Google Scholar] [CrossRef]
- Hyams, J.; Crandall, W.; Kugathasan, S.; Griffiths, A.; Olson, A.; Johanns, J.; Liu, G.; Travers, S.; Heuschkel, R.; Markowitz, J.; et al. Induction and Maintenance Infliximab Therapy for the Treatment of Moderate-to-Severe Crohn’s Disease in Children. Gastroenterology 2007, 132, 863–873. [Google Scholar] [CrossRef]
- Rutgeerts, P.; Sandborn, W.J.; Feagan, B.G.; Reinisch, W.; Olson, A.; Johanns, J.; Suzanne, T.; Rachmilewitz, D.; Hanauer, S.B.; Lichtenstein, G.R.; et al. Infliximab for Induction and Maintenance Therapy for Ulcerative Colitis. N. Engl. J. Med. 2006, 353, 2462–2476. [Google Scholar] [CrossRef] [PubMed]
- Schreiber, S.; Ben-Horin, S.; Leszczyszyn, J.; Dudkowiak, R.; Lahat, A.; Gawdis-Wojnarska, B.; Pukitis, A.; Horynski, M.; Farkas, K.; Kierkus, J.; et al. Randomized Controlled Trial: Subcutaneous vs. Intravenous Infliximab CT-P13 Maintenance in Inflammatory Bowel Disease. Gastroenterology 2021, 160, 2340–2353. [Google Scholar] [CrossRef] [PubMed]
- Colombel, J.; Sandborn, W.J.; Rutgeerts, P.; Enns, R.; Hanauer, S.B.; Panaccione, R.; Schreiber, S.; Byczkowski, D.; Li, J.; Kent, J.D.; et al. Adalimumab for Maintenance of Clinical Response and Remission in Patients with Crohn’s Disease: The CHARM Trial. Gastroenterology 2007, 132, 52–65. [Google Scholar] [CrossRef] [PubMed]
- Panés, J.; Colombel, J.-F.; D’Haens, G.R.; Schreiber, S.; Panaccione, R.; Peyrin-Biroulet, L.; Loftus, E.V.; Danese, S.; Tanida, S.; Okuyama, Y.; et al. Higher vs. Standard Adalimumab Induction and Maintenance Dosing Regimens for Treatment of Ulcerative Colitis: SERENE UC Trial Results. Gastroenterology 2022, 162, 1891–1910. [Google Scholar] [CrossRef]
- Gubatan, J.; Keyashian, K.; Rubin, S.J.; Wang, J.; Buckman, C.; Sinha, S. Anti-Integrins for the Treatment of Inflammatory Bowel Disease: Current Evidence and Perspectives. Clin. Exp. Gastroenterol. 2021, 14, 333–342. [Google Scholar] [CrossRef]
- Ghosh, S.; Malchow, H.A.; Rutgeerts, P.; Palmer, T.; Donoghue, S. Natalizumab for Active Crohn’s Disease. N. Engl. J. Med. 2003, 348, 24–32. [Google Scholar] [CrossRef]
- Hellwig, K.; Gold, R. Progressive Multifocal Leukoencephalopathy and Natalizumab. J. Neurol. 2011, 258, 1920–1928. [Google Scholar] [CrossRef]
- McLean, L.P.; Shea-Donohue, T.; Cross, R.K. Vedolizumab for the Treatment of Ulcerative Colitis and Crohn’s Disease. Immunotherapy 2012, 4, 883–898. [Google Scholar] [CrossRef]
- Sands, B.E.; Feagan, B.G.; Rutgeerts, P.; Colombel, J.-F.; Sandborn, W.J.; Sy, R.; D’Haens, G.; Ben-Horin, S.; Xu, J.; Rosario, M.; et al. Effects of Vedolizumab Induction Therapy for Patients with Crohn’s Disease in Whom Tumor Necrosis Factor Antagonist Treatment Failed. Gastroenterology 2014, 147, 618–627.e3. [Google Scholar] [CrossRef]
- Travis, S.; Silverberg, M.S.; Danese, S.; Gionchetti, P.; Löwenberg, M.; Jairath, V.; Feagan, B.G.; Bressler, B.; Ferrante, M.; Hart, A.; et al. Vedolizumab for the Treatment of Chronic Pouchitis. N. Engl. J. Med. 2023, 388, 1191–1200. [Google Scholar] [CrossRef]
- Sandborn, W.J.; Baert, F.; Danese, S.; Krznarić, Ž.; Kobayashi, T.; Yao, X.; Chen, J.; Rosario, M.; Bhatia, S.; Kisfalvi, K.; et al. Efficacy and Safety of Vedolizumab Subcutaneous Formulation in a Randomized Trial of Patients with Ulcerative Colitis. Gastroenterology 2020, 158, 562–572.e12. [Google Scholar] [CrossRef] [PubMed]
- Moschen, A.R.; Tilg, H.; Raine, T. IL-12, IL-23 and IL-17 in IBD: Immunobiology and Therapeutic Targeting. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 185–196. [Google Scholar] [CrossRef] [PubMed]
- Al-Bawardy, B.; Shivashankar, R.; Proctor, D.D. Novel and Emerging Therapies for Inflammatory Bowel Disease. Front. Pharmacol. 2021, 12, 651415. [Google Scholar] [CrossRef] [PubMed]
- Imbrizi, M.; Coy, C.S.R. Tratado de Doença Inflamatória Intestinal. In Pequenas Moléculas, 1st ed.; Saad-Hosne, R., Sassaki, L.Y., Eds.; Atheneu: Rio de Janeiro, Brazil, 2023; Volume 1. [Google Scholar]
- Pippis, E.J.; Yacyshyn, B.R. Clinical and Mechanistic Characteristics of Current JAK Inhibitors in IBD. Inflamm. Bowel Dis. 2021, 27, 1674–1683. [Google Scholar] [CrossRef]
- Chaparro, M.; Ordás, I.; Cabré, E.; Garcia-Sanchez, V.; Bastida, G.; Peñalva, M.; Gomollón, F.; García-Planella, E.; Merino, O.; Gutiérrez, A.; et al. Safety of Thiopurine Therapy in Inflammatory Bowel Disease: Long-Term Follow-up Study of 3931 Patients. Inflamm. Bowel Dis. 2013, 19, 1404–1410. [Google Scholar] [CrossRef]
- Nielsen, O.H.; Steenholdt, C.; Juhl, C.B.; Rogler, G. Efficacy and Safety of Methotrexate in the Management of Inflammatory Bowel Disease: A Systematic Review and Meta-Analysis of Randomized, Controlled Trials. EClinicalMedicine 2020, 20, 100271. [Google Scholar] [CrossRef]
- Holmer, A.; Singh, S. Overall and Comparative Safety of Biologic and Immunosuppressive Therapy in Inflammatory Bowel Diseases. Expert Rev. Clin. Immunol. 2019, 15, 969–979. [Google Scholar] [CrossRef]
- Click, B.; Regueiro, M. Managing Risks with Biologics. Curr. Gastroenterol. Rep. 2019, 21, 1. [Google Scholar] [CrossRef]
- Peyrin-Biroulet, L.; Rahier, J.-F.; Kirchgesner, J.; Abitbol, V.; Shaji, S.; Armuzzi, A.; Karmiris, K.; Gisbert, J.P.; Bossuyt, P.; Helwig, U.; et al. I-CARE, a European Prospective Cohort Study Assessing Safety and Effectiveness of Biologics in Inflammatory Bowel Disease. Clin. Gastroenterol. Hepatol. 2023, 21, 771–788.e10. [Google Scholar] [CrossRef]
- Singh, S.; Kim, J.; Luo, J.; Paul, P.; Rudrapatna, V.; Park, S.; Zheng, K.; Syal, G.; Ha, C.; Fleshner, P.; et al. Comparative Safety and Effectiveness of Biologic Therapy for Crohn’s Disease: A CA-IBD Cohort Study. Clin. Gastroenterol. Hepatol. 2022, 21, 2359–2369.e5. [Google Scholar] [CrossRef]
- Pugliese, D.; Privitera, G.; Crispino, F.; Mezzina, N.; Castiglione, F.; Fiorino, G.; Laterza, L.; Viola, A.; Bertani, L.; Caprioli, F.; et al. Effectiveness and Safety of Vedolizumab in a Matched Cohort of Elderly and Nonelderly Patients with Inflammatory Bowel Disease: The IG-IBD LIVE Study. Aliment. Pharmacol. Ther. 2022, 56, 95–109. [Google Scholar] [CrossRef] [PubMed]
- Sandborn, W.J.; Rebuck, R.; Wang, Y.; Zou, B.; Adedokun, O.J.; Gasink, C.; Sands, B.E.; Hanauer, S.B.; Targan, S.; Ghosh, S.; et al. Five-Year Efficacy and Safety of Ustekinumab Treatment in Crohn’s Disease: The IM-UNITI Trial. Clin. Gastroenterol. Hepatol. 2022, 20, 578–590.e4. [Google Scholar] [CrossRef] [PubMed]
- Ferrante, M.; Feagan, B.G.; Panés, J.; Baert, F.; Louis, E.; Dewit, O.; Kaser, A.; Duan, W.R.; Pang, Y.; Lee, W.-J.; et al. Long-Term Safety and Efficacy of Risankizumab Treatment in Patients with Crohn’s Disease: Results from the Phase 2 Open-Label Extension Study. J. Crohn’s Colitis 2021, 15, 2001–2010. [Google Scholar] [CrossRef]
- Singh, S.; Murad, M.H.; Fumery, M.; Sedano, R.; Jairath, V.; Panaccione, R.; Sandborn, W.J.; Ma, C. Comparative Efficacy and Safety of Biologic Therapies for Moderate-to-Severe Crohn’s Disease: A Systematic Review and Network Meta-Analysis. Lancet Gastroenterol. Hepatol. 2021, 6, 1002–1014. [Google Scholar] [CrossRef] [PubMed]
- Ytterberg, S.R.; Bhatt, D.L.; Mikuls, T.R.; Koch, G.G.; Fleischmann, R.; Rivas, J.L.; Germino, R.; Menon, S.; Sun, Y.; Wang, C.; et al. Cardiovascular and Cancer Risk with Tofacitinib in Rheumatoid Arthritis. N. Engl. J. Med. 2022, 386, 316–326. [Google Scholar] [CrossRef] [PubMed]
- McLornan, D.P.; Pope, J.E.; Gotlib, J.; Harrison, C.N. Current and Future Status of JAK Inhibitors. Lancet 2021, 398, 803–816. [Google Scholar] [CrossRef]
- Sivaraman, P.; Cohen, S.B. Malignancy and Janus Kinase Inhibition. Rheum. Dis. Clin. N. Am. 2017, 43, 79–93. [Google Scholar] [CrossRef]
- Queiroz, N.S.F.; Regueiro, M. Safety Considerations with Biologics and New Inflammatory Bowel Disease Therapies. Curr. Opin. Gastroenterol. 2020, 36, 257–264. [Google Scholar] [CrossRef]
- Sandborn, W.J.; Peyrin-Biroulet, L.; Quirk, D.; Wang, W.; Nduaka, C.I.; Mukherjee, A.; Su, C.; Sands, B.E. Efficacy and Safety of Extended Induction with Tofacitinib for the Treatment of Ulcerative Colitis. Clin. Gastroenterol. Hepatol. 2022, 20, 1821–1830.e3. [Google Scholar] [CrossRef]
- Kragstrup, T.W.; Glintborg, B.; Svensson, A.L.; McMaster, C.; Robinson, P.C.; Deleuran, B.; Liew, D.F. Waiting for JAK Inhibitor Safety Data. RMD Open 2022, 8, e002236. [Google Scholar] [CrossRef]
- Verstockt, B.; Vetrano, S.; Salas, A.; Nayeri, S.; Duijvestein, M.; Vande Casteele, N.; Alimentiv Translational Research Consortium (ATRC); Danese, S.; D’Haens, G.; Eckmann, L.; et al. Sphingosine 1-Phosphate Modulation and Immune Cell Trafficking in Inflammatory Bowel Disease. Nat. Rev. Gastroenterol. Hepatol. 2022, 19, 351–366. [Google Scholar] [CrossRef] [PubMed]
- Solitano, V.; Facciorusso, A.; Jess, T.; Ma, C.; Hassan, C.; Repici, A.; Jairath, V.; Armuzzi, A.; Singh, S. Comparative Risk of Serious Infections with Biologic Agents and Oral Small Molecules in Inflammatory Bowel Diseases: A Systematic Review and Meta-Analysis. Clin. Gastroenterol. Hepatol. 2023, 21, 907–921.e2. [Google Scholar] [CrossRef]
- Sattler, L.; Hanauer, S.B.; Malter, L. Immunomodulatory Agents for Treatment of Patients with Inflammatory Bowel Disease (Review Safety of Anti-TNF, Anti-Integrin, Anti IL-12/23, JAK Inhibition, Sphingosine 1-Phosphate Receptor Modulator, Azathioprine/6-MP and Methotrexate). Curr. Gastroenterol. Rep. 2021, 23, 30. [Google Scholar] [CrossRef] [PubMed]
- Andrew, B.; Srinivasan, A.; Zhou, A.; Vasudevan, A. Letter: Elderly Onset Inflammatory Bowel Disease—Treat to Target Approach Is Still Warranted. Aliment. Pharmacol. Ther. 2023, 58, 556–557. [Google Scholar] [CrossRef] [PubMed]
- Almario, C.V.; Keller, M.S.; Chen, M.; Lasch, K.; Ursos, L.; Shklovskaya, J.; Melmed, G.Y.; Spiegel, B.M.R. Optimizing Selection of Biologics in Inflammatory Bowel Disease: Development of an Online Patient Decision Aid Using Conjoint Analysis. Am. J. Gastroenterol. 2018, 113, 58–71. [Google Scholar] [CrossRef]
- Rubin, D.T.; Sninsky, C.; Siegmund, B.; Sans, M.; Hart, A.; Bressler, B.; Bouhnik, Y.; Armuzzi, A.; Afzali, A. International Perspectives on Management of Inflammatory Bowel Disease: Opinion Differences and Similarities Between Patients and Physicians from the IBD GAPPS Survey. Inflamm. Bowel Dis. 2021, 27, 1942–1953. [Google Scholar] [CrossRef]
- Bhat, S.; Click, B.; Regueiro, M. Safety and Monitoring of Inflammatory Bowel Disease Advanced Therapies. Inflamm. Bowel Dis. 2023, izad120. [Google Scholar] [CrossRef]
- Zurba, Y.; Gros, B.; Shehab, M. Exploring the Pipeline of Novel Therapies for Inflammatory Bowel Disease; State of the Art Review. Biomedicines 2023, 11, 747. [Google Scholar] [CrossRef]
- Dai, B.; Hackney, J.A.; Ichikawa, R.; Nguyen, A.; Elstrott, J.; Orozco, L.D.; Sun, K.-H.; Modrusan, Z.; Gogineni, A.; Scherl, A.; et al. Dual Targeting of Lymphocyte Homing and Retention through A4β7 and AEβ7 Inhibition in Inflammatory Bowel Disease. Cell Rep. Med. 2021, 2, 100381. [Google Scholar] [CrossRef]
- Sandborn, W.J.; D’Haens, G.R.; Reinisch, W.; Panés, J.; Chan, D.; Gonzalez, S.; Weisel, K.; Germinaro, M.; Frustaci, M.E.; Yang, Z.; et al. Guselkumab for the Treatment of Crohn’s Disease: Induction Results from the Phase 2 GALAXI-1 Study. Gastroenterology 2022, 162, 1650–1664.e8. [Google Scholar] [CrossRef]
- Danese, S.; Neurath, M.F.; Kopoń, A.; Zakko, S.F.; Simmons, T.C.; Fogel, R.; Siegel, C.A.; Panaccione, R.; Zhan, X.; Usiskin, K.; et al. Effects of Apremilast, an Oral Inhibitor of Phosphodiesterase 4, in a Randomized Trial of Patients with Active Ulcerative Colitis. Clin. Gastroenterol. Hepatol. 2020, 18, 2526–2534.e9. [Google Scholar] [CrossRef]
- Atreya, R.; Peyrin-Biroulet, L.; Klymenko, A.; Augustyn, M.; Bakulin, I.; Slankamenac, D.; Miheller, P.; Gasbarrini, A.; Hébuterne, X.; Arnesson, K.; et al. Cobitolimod for Moderate-to-Severe, Left-Sided Ulcerative Colitis (CONDUCT): A Phase 2b Randomised, Double-Blind, Placebo-Controlled, Dose-Ranging Induction Trial. Lancet Gastroenterol. Hepatol. 2020, 5, 1063–1075. [Google Scholar] [CrossRef]
- Vuyyuru, S.K.; Kedia, S.; Ahuja, V. Considerations When Starting Patients on Multiple Biologics and Small Molecules. Curr. Opin. Gastroenterol. 2022, 38, 562–569. [Google Scholar] [CrossRef] [PubMed]
- Balderramo, D. Role of the Combination of Biologics and/or Small Molecules in the Treatment of Patients with Inflammatory Bowel Disease. World J. Gastroenterol. 2022, 28, 6743–6751. [Google Scholar] [CrossRef] [PubMed]
- Feagan, B.G.; Sands, B.E.; Sandborn, W.J.; Germinaro, M.; Vetter, M.; Shao, J.; Sheng, S.; Johanns, J.; Panés, J.; Tkachev, A.; et al. Guselkumab plus Golimumab Combination Therapy versus Guselkumab or Golimumab Monotherapy in Patients with Ulcerative Colitis (VEGA): A Randomised, Double-Blind, Controlled, Phase 2, Proof-of-Concept Trial. Lancet Gastroenterol. Hepatol. 2023, 8, 307–320. [Google Scholar] [CrossRef] [PubMed]
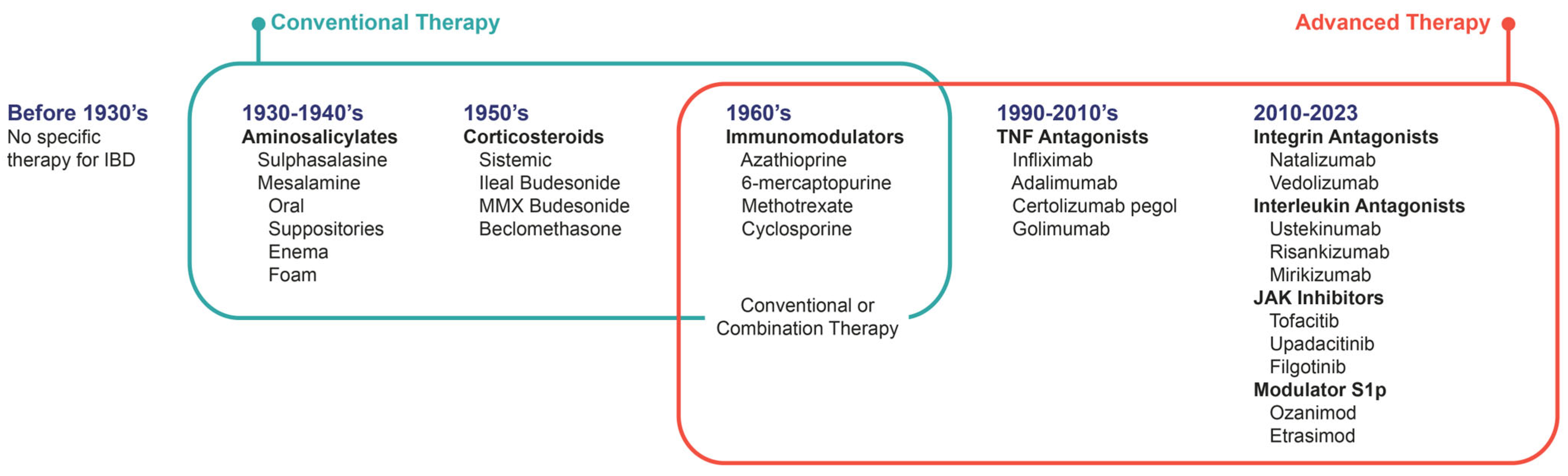

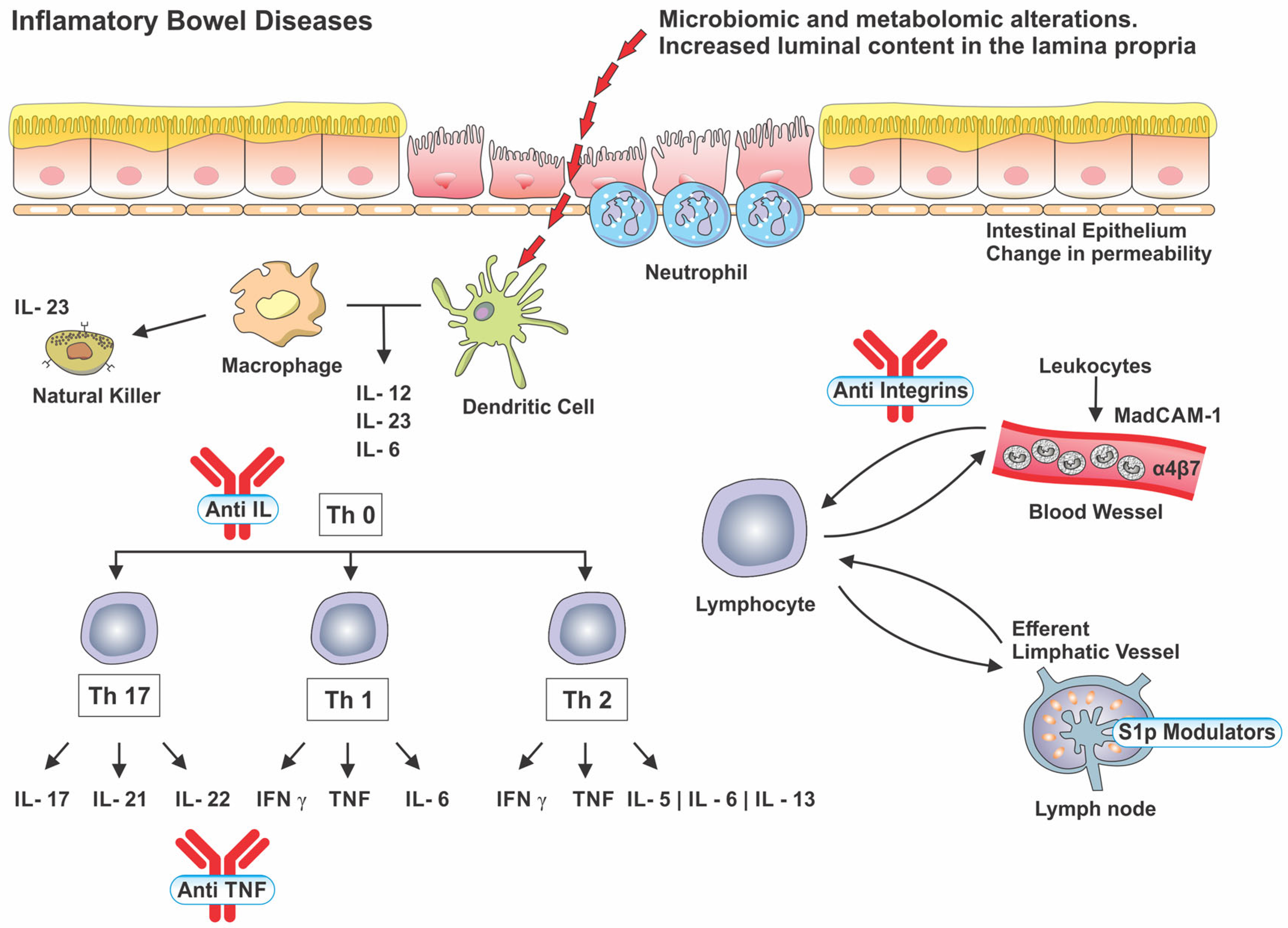
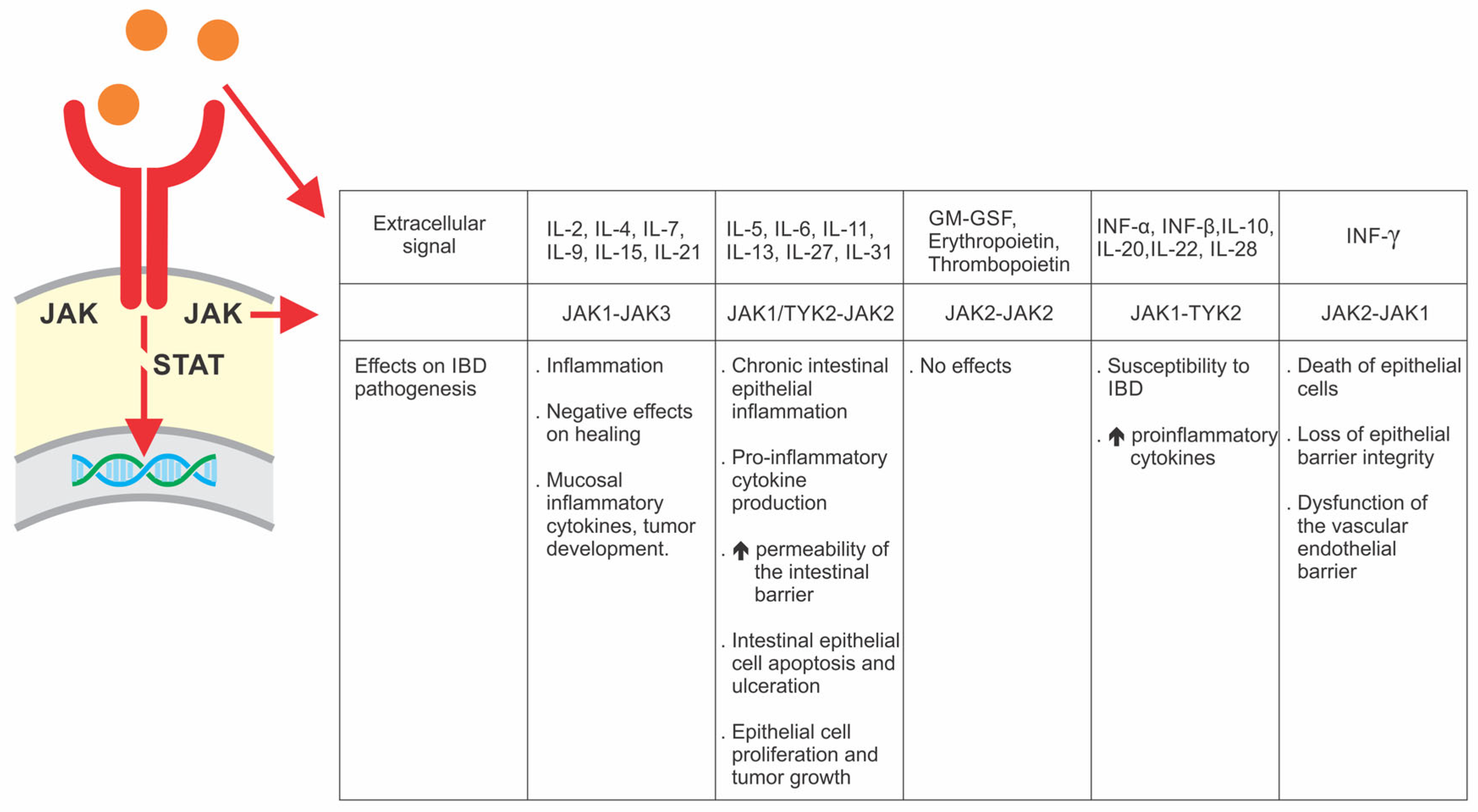
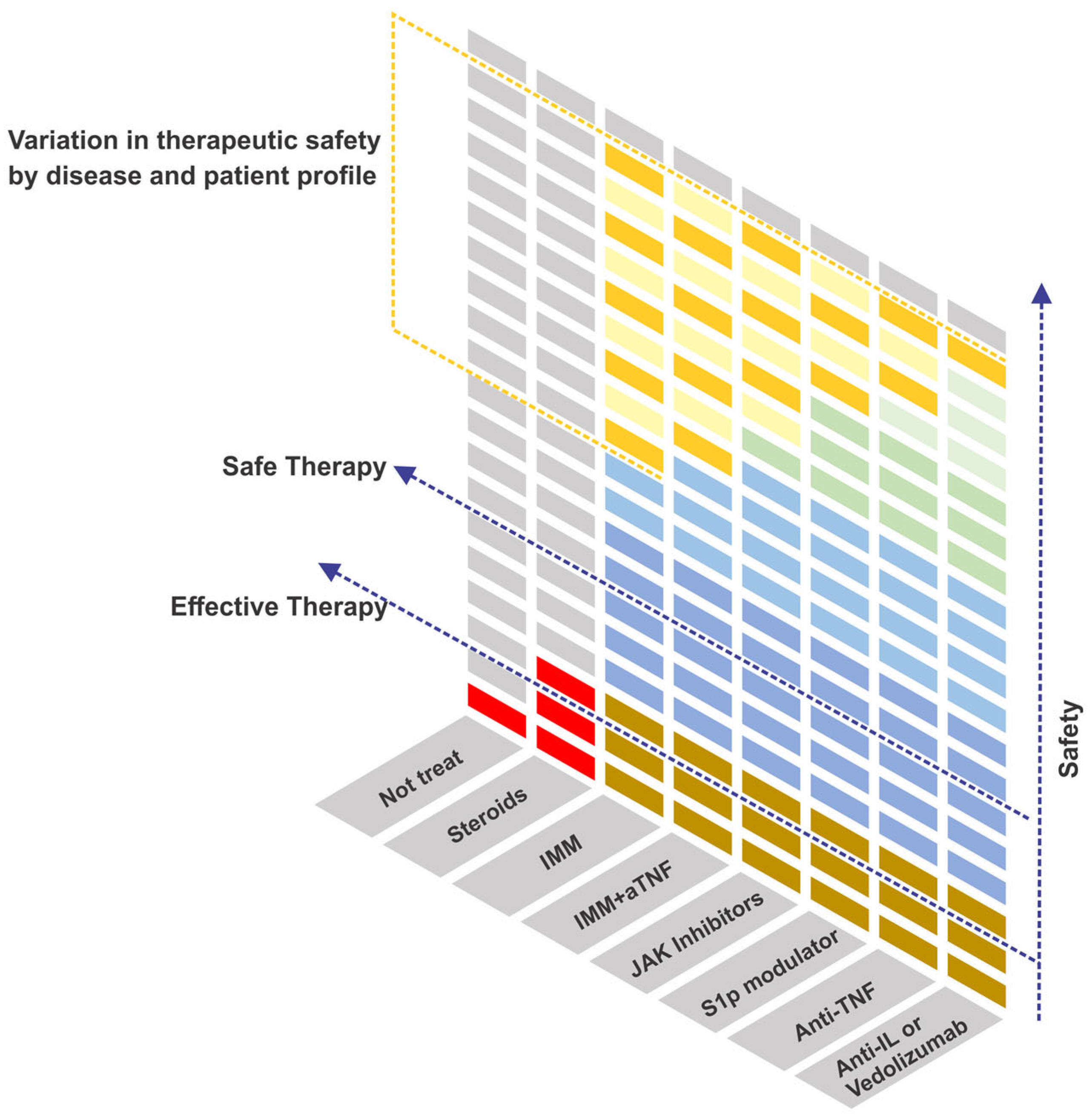
| Class | Drug | Disease | Dose | ||
|---|---|---|---|---|---|
| Induction | Maintenance | Dose Optimization | |||
| Aminosalicylates [26,28,30] | Sulfasalazine | UC | ≥3 g/d | ≥2 g/d | - |
| 5-ASA | UC | Distal colitis: rectal 5-ASA ≥ 1 g/d Extensive colitis: rectal 5-ASA ≥ 1 g/d + oral 5-ASA ≥ 2 g/d | ≥1 g/d >2 g/d | - >4 g/d | |
| Glucocorticoids [26,27,28,29,31,32] | Prednisolone | CD, UC | 0.5–0.75 mg/kg (maximum daily dose 60 mg) | - | - |
| Budesonide | CD | 9 mg/day 2–3 months | - | - | |
| Budesonide MMX | UC | 9 mg/day 2–3 months | - | - | |
| Immunomodulators [26,27,28,29,30,33] | Azathioprine | CD, UC | 1.5–2.5 mg/kg/d | - | |
| 6-Mercaptopurine | CD, UC | 1–1.5 mg/kg/d | - | ||
| Cyclosporine | UC | 2 mg/kg/d IV | 5 mg/kg/d (up to 3 months) | - | |
| Methotrexate | CD | 25 mg/w SC or IM (12w) | 15 mg/w SC or IM | - | |
| Anti-TNF | Infliximab [34,35,36,37] | CD, UC | 5 mg/kg IV at 0, 2 and 6 w | 5 mg/kg IV every 8w 120 mg SC every 2wfrom w6 | 10 mg/kg IV every 8w (label) or 5 mg/kg every 4w (off-label) |
| Adalimumab [38,39,40] | CD, UC | 160 mg, then 80 mg after 2w SC | 40 mg SC every 2w | 80 mg SC every 2w or 40 mg SC weekly | |
| Certolizumab pegol [41,42] | CD | 400 mg SC at weeks 0, 2 and 4 | 400 mg every 4w | - | |
| Golimumab [43,44] | UC | 200 mg, then 100 mg after 2w SC | 50–100 mg SC every 4w | <80 kg using 50 mg: 100 mg every 4w | |
| Anti-Integrin | Natalizumab [45] | CD | 300 mg IV every 4w | ||
| Vedolizumab [46,47,48] | CD, UC | 300 mg IV at weeks 0, 2 and 6. CD: an additional dose at w10 may be indicated | 300 mg IV every 8w 108 mg SC every 2w | 300 mg IV every 4w - | |
| Anti-Interleukin | Ustekinumab [49,50] | CD, UC | <55 kg: 260 mg, 55–85 kg: 390 mg, >85 kg: 520 mg IV single dose. | 90 mg SC every 12 or 8w | 90 mg SC every 4w (off-label) |
| Risankizumab [51,52] | CD | 600 mg IV at weeks 0, 4 and 8 | 360 mg SC at w12 and then every 8w | - | |
| Mirikizumab * [53] | UC | 300 mg IV every 4w for 12w | 200 mg IV every 4w | ||
| JAK-inhibitors | Tofacitinib [54] | UC | 10 mg BID PO 8–12w | 5 mg PO BID | In loss of response, consider new induction |
| Filgotinib [55] | UC | 200 mg PO OD 10w | 100–200 mg PO OD | - | |
| Upadacitinib [56,57] | CD, UC | UC: 45 mg PO OD 8w CD: 45 mg PO OD 12w | 15–30 mg PO OD | - | |
| S1p modulators | Ozanimod [58] | UC | Day 1–4: 0.92 mg PO Day 5–7: 0.46 mg PO | 0.92 mg PO | - |
| Etrasimod * [59] | UC | 2 mg PO OD | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Imbrizi, M.; Magro, F.; Coy, C.S.R. Pharmacological Therapy in Inflammatory Bowel Diseases: A Narrative Review of the Past 90 Years. Pharmaceuticals 2023, 16, 1272. https://doi.org/10.3390/ph16091272
Imbrizi M, Magro F, Coy CSR. Pharmacological Therapy in Inflammatory Bowel Diseases: A Narrative Review of the Past 90 Years. Pharmaceuticals. 2023; 16(9):1272. https://doi.org/10.3390/ph16091272
Chicago/Turabian StyleImbrizi, Marcello, Fernando Magro, and Claudio Saddy Rodrigues Coy. 2023. "Pharmacological Therapy in Inflammatory Bowel Diseases: A Narrative Review of the Past 90 Years" Pharmaceuticals 16, no. 9: 1272. https://doi.org/10.3390/ph16091272
APA StyleImbrizi, M., Magro, F., & Coy, C. S. R. (2023). Pharmacological Therapy in Inflammatory Bowel Diseases: A Narrative Review of the Past 90 Years. Pharmaceuticals, 16(9), 1272. https://doi.org/10.3390/ph16091272







