Purinergic Activation of Store-Operated Calcium Entry (SOCE) Regulates Cell Migration in Metastatic Ovarian Cancer Cells
Abstract
1. Introduction
2. Results
2.1. UTP Induces Intracellular Ca2+ Mobilization Dependent on Extracellular Influx and Ca2+ Release from Internal Deposits in Metastatic Ovarian Carcinoma Cells
2.2. The Intracellular Ca2+ Mobilization Elicited by UTP in Non-Metastatic CAOV-3 Cells Is Similar in Presence or Absence of Extracellular Ca2+
2.3. Store-Operated Calcium Entry (SOCE) Is Mainly Present in Metastatic (SKOV-3) Cells
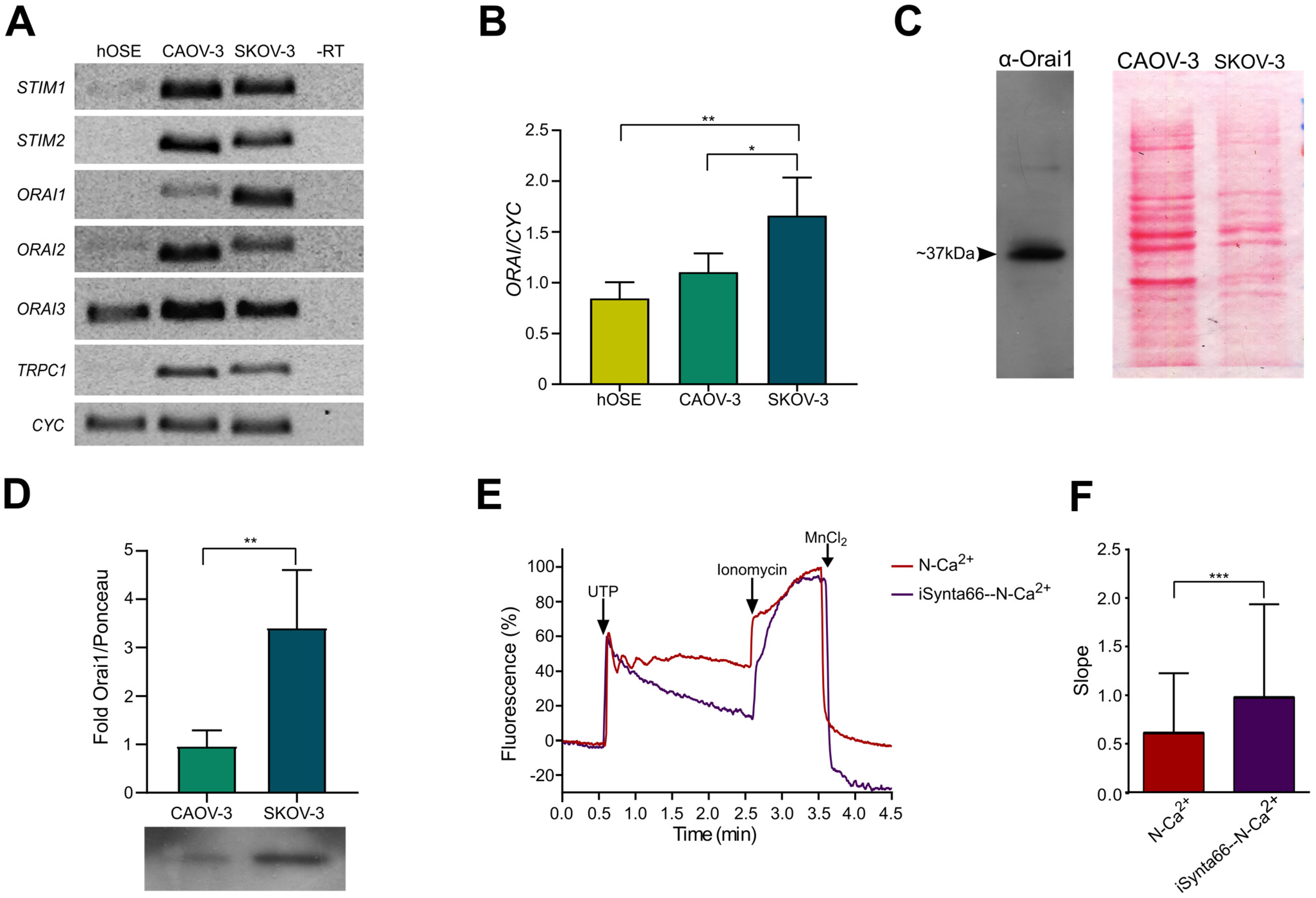
2.4. Molecular Components of SOCE Are Differentially Expressed in Healthy and Cancerous Cells
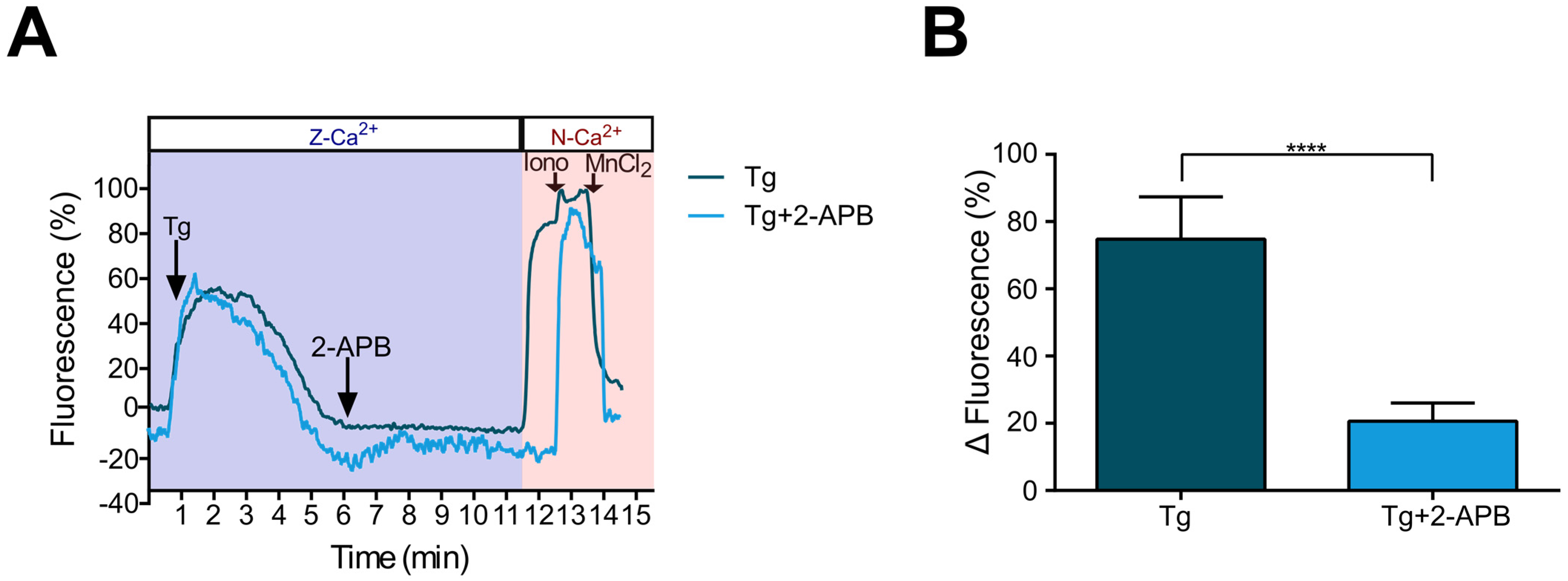
2.5. Ca2+ Influx Due to SOCE Activation by Thapsigargin Is Inhibited by 2-APB in Metastatic SKOV-3 Cells
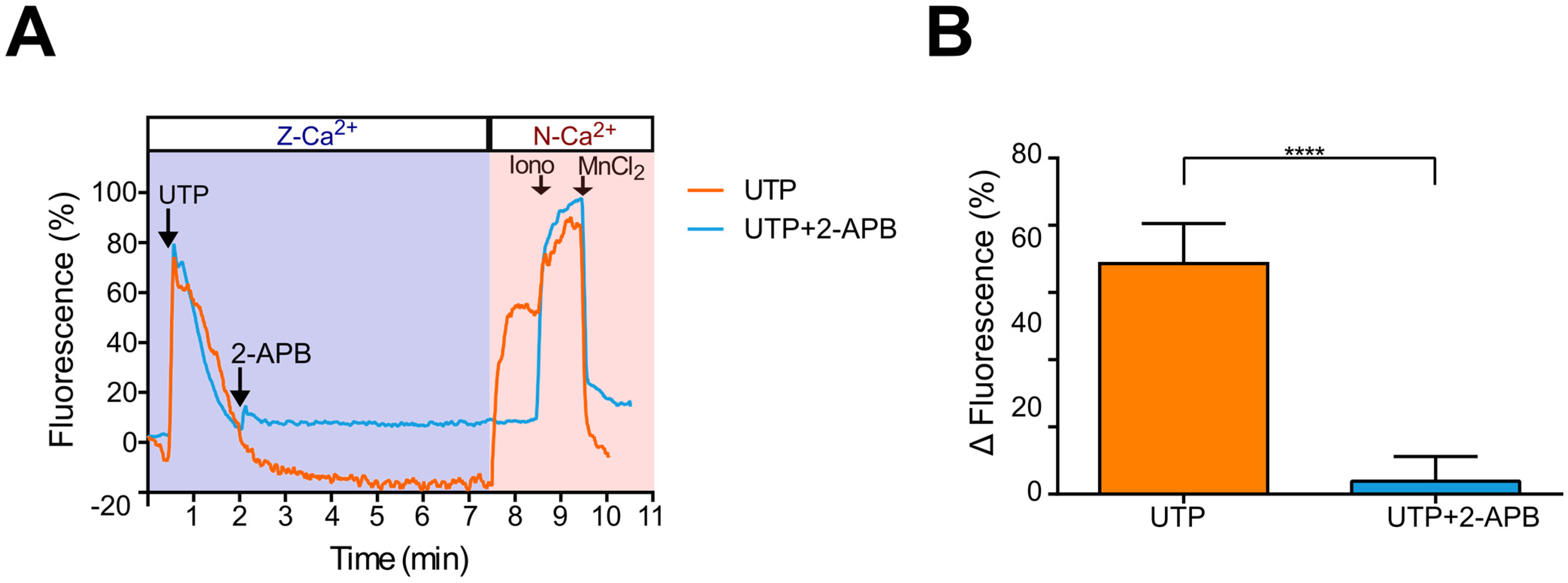
2.6. UTP-Induced Ca2+ Influx Is Inhibited by 2-APB in Metastatic SKOV-3 Cells, Indicating SOCE Participation
2.7. Blocking of SOCE with 2-APB Inhibits UTP-Induced Cell Migration
3. Discussion
4. Materials and Methods
4.1. Ovarian Carcinoma Cell Lines
4.2. Measurements of Intracellular Ca2+ in Single Cells
4.3. Wound Closure Assay
4.4. Cell Migration Assay in Boyden’s Chambers
4.5. Reverse Transcription, End-Point and Quantitative Polymerase Chain Reaction
4.6. Biotinylation of Plasma Membrane Proteins and Western Blot
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Global Cancer Observatory. Available online: https://gco.iarc.fr/ (accessed on 14 February 2023).
- Ritch, S.J.; Telleria, C.M. The Transcoelomic Ecosystem and Epithelial Ovarian Cancer Dissemination. Front. Endocrinol. 2022, 13, 886533. [Google Scholar] [CrossRef]
- Campos-Contreras, A.D.R.; Díaz-Muñoz, M.; Vázquez-Cuevas, F.G. Purinergic Signaling in the Hallmarks of Cancer. Cells 2020, 9, 1612. [Google Scholar] [CrossRef] [PubMed]
- Schiliro, C.; Firestein, B.L. Mechanisms of Metabolic Reprogramming in Cancer Cells Supporting Enhanced Growth and Proliferation. Cells 2021, 10, 1056. [Google Scholar] [CrossRef]
- Pellegatti, P.; Raffaghello, L.; Bianchi, G.; Piccardi, F.; Pistoia, V.; Di Virgilio, F. Increased level of extracellular ATP at tumor sites: In vivo imaging with plasma membrane luciferase. PLoS ONE 2008, 3, e2599. [Google Scholar] [CrossRef]
- Reyna-Jeldes, M.; Díaz-Muñoz, M.; Madariaga, J.A.; Coddou, C.; Vázquez-Cuevas, F.G. Autocrine and paracrine purinergic signaling in the most lethal types of cancer. Purinergic Signal. 2021, 17, 345–370. [Google Scholar] [CrossRef]
- Ralevic, V.; Burnstock, G. Receptors for purines and pyrimidines. Pharmacol. Rev. 1998, 50, 413–492. [Google Scholar] [CrossRef]
- Schäfer, R.; Sedehizade, F.; Welte, T.; Reiser, G. ATP- and UTP-activated P2Y receptors differently regulate proliferation of human lung epithelial tumor cells. Am. J. Physiol. Lung Cell. Mol. Physiol. 2003, 285, L376–L385. [Google Scholar] [CrossRef] [PubMed]
- Choi, K.C.; Tai, C.J.; Tzeng, C.R.; Auersperg, N.; Leung, P.C.K. Adenosine triphosphate activates mitogen-activated protein kinase in pre-neoplastic and neoplastic ovarian surface epithelial cells. Biol. Reprod. 2003, 68, 309–315. [Google Scholar] [CrossRef] [PubMed]
- Li, H.J.; Wang, L.Y.; Qu, H.N.; Yu, L.H.; Burnstock, G.; Ni, X.; Xu, M.; Ma, B. P2Y2 receptor-mediated modulation of estrogen-induced proliferation of breast cancer cells. Mol. Cell. Endocrinol. 2011, 338, 28–37. [Google Scholar] [CrossRef]
- Li, W.H.; Qiu, Y.; Zhang, H.Q.; Tian, X.X.; Fang, W.G. P2Y2 Receptor and EGFR Cooperate to Promote Prostate Cancer Cell Invasion via ERK1/2 Pathway. PLoS ONE 2015, 10, e0133165. [Google Scholar] [CrossRef]
- Li, W.H.; Qiu, Y.; Zhang, H.Q.; Liu, Y.; You, J.F.; Tian, X.X.; Fang, W.G. P2Y2 receptor promotes cell invasion and metastasis in prostate cancer cells. Br. J. Cancer 2013, 109, 1666–1675. [Google Scholar] [CrossRef] [PubMed]
- Muscella, A.; Elia, M.G.; Greco, S.; Storelli, C.; Marsigliante, S. Activation of P2Y2 receptor induces c-FOS protein through a pathway involving mitogen-activated protein kinases and phosphoinositide 3-kinases in HeLa cells. J. Cell. Physiol. 2003, 195, 234–240. [Google Scholar] [CrossRef] [PubMed]
- Jin, H.; Eun, S.Y.; Lee, J.S.; Park, S.W.; Lee, J.H.; Chang, K.C.; Kim, H.J. P2Y2 receptor activation by nucleotides released from highly metastatic breast cancer cells increases tumor growth and invasion via crosstalk with endothelial cells. Breast Cancer Res. 2014, 16, R77. [Google Scholar] [CrossRef]
- Zhang, J.L.; Liu, Y.; Yang, H.; Zhang, H.Q.; Tian, X.X.; Fang, W.G. ATP-P2Y2-β-catenin axis promotes cell invasion in breast cancer cells. Cancer Sci. 2017, 108, 1318–1327. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Ramírez, A.S.; Garay, E.; García-Carrancá, A.; Vázquez-Cuevas, F.G. The P2RY2 Receptor Induces Carcinoma Cell Migration and EMT Through Cross-Talk with Epidermal Growth Factor Receptor. J. Cell. Biochem. 2016, 117, 1016–1026. [Google Scholar] [CrossRef]
- Reyna-Jeldes, M.; De la Fuente-Ortega, E.; Cerda, D.; Velázquez-Miranda, E.; Pinto, K.; Vázquez-Cuevas, F.G.; Coddou, C. Purinergic P2Y2 and P2X4 Receptors Are Involved in the Epithelial-Mesenchymal Transition and Metastatic Potential of Gastric Cancer Derived Cell Lines. Pharmaceutics 2021, 13, 1234. [Google Scholar] [CrossRef]
- El Hachmane, M.F.; Ermund, A.; Brännmark, C.; Olofsson, C.S. Extracellular ATP activates store-operated Ca2+ entry in white adipocytes: Functional evidence for STIM1 and ORAI1. Biochem. J. 2018, 475, 691–704. [Google Scholar] [CrossRef]
- Prakriya, M.; Lewis, R.S. Store-Operated Calcium Channels. Physiol. Rev. 2015, 95, 1383–1436. [Google Scholar] [CrossRef]
- Yang, S.; Zhang, J.J.; Huang, X.Y. Orai1 and STIM1 are critical for breast tumor cell migration and metastasis. Cancer Cell 2009, 15, 124–134. [Google Scholar] [CrossRef]
- Saleem, K.; Wani, W.A.; Haque, A.; Lone, M.N.; Hsieh, M.F.; Jairajpuri, M.A.; Ali, I. Synthesis, DNA binding, hemolysis assays and anticancer studies of copper(II), nickel(II) and iron(III) complexes of a pyrazoline-based ligand. Future Med. Chem. 2013, 5, 135–146. [Google Scholar] [CrossRef]
- Ali, I.; Wani, W.A.; Haque, A.; Saleem, K. Glutamic acid and its derivatives: Candidates for rational design of anticancer drugs. Future Med. Chem. 2013, 5, 961–978. [Google Scholar] [CrossRef] [PubMed]
- Lazarowski, E.R.; Watt, W.C.; Stutts, M.J.; Boucher, R.C.; Harden, T.K. Pharmacological selectivity of the cloned human P2U-purinoceptor: Potent activation by diadenosine tetraphosphate. Br. J. Pharmacol. 1995, 116, 1619–1627. [Google Scholar] [CrossRef] [PubMed]
- Caov-3 [Caov3]—HTB-75|ATCC. Available online: https://www.atcc.org/products/htb-75 (accessed on 14 February 2023).
- Maus, M.; Cuk, M.; Patel, B.; Lian, J.; Ouimet, M.; Kaufmann, U.; Yang, J.; Horvath, R.; Hornig-Do, H.T.; Chrzanowska-Lightowlers, Z.M.; et al. Store-Operated Ca2+ Entry Controls Induction of Lipolysis and the Transcriptional Reprogramming to Lipid Metabolism. Cell Metab. 2017, 25, 698–712. [Google Scholar] [CrossRef]
- Secondo, A.; Frischauf, I.; Kepler, J.; Bailey, M.A.; Shawer, H.; Norman, K.; Cheng, C.W.; Foster, R.; Beech, D.J. ORAI1 Ca2+ Channel as a Therapeutic Target in Pathological Vascular Remodelling. Front. Cell Dev. Biol. 2021, 9, 653812. [Google Scholar] [CrossRef]
- Waldherr, L.; Tiffner, A.; Mishra, D.; Sallinger, M.; Schober, R.; Frischauf, I.; Schmidt, T.; Handl, V.; Sagmeister, P.; Köckinger, M.; et al. Blockage of Store-Operated Ca2+ Influx by Synta66 is Mediated by Direct Inhibition of the Ca2+ Selective Orai1 Pore. Cancers 2020, 12, 2876. [Google Scholar] [CrossRef]
- DeHaven, W.I.; Smyth, J.T.; Boyles, R.R.; Bird, G.S.; Putney, J.W. Complex actions of 2-aminoethyldiphenyl borate on store-operated calcium entry. J. Biol. Chem. 2008, 283, 19265–19273. [Google Scholar] [CrossRef]
- Alvarez, C.L.; Troncoso, M.F.; Espelt, M.V. Extracellular ATP and adenosine in tumor microenvironment: Roles in epithelial-mesenchymal transition, cell migration, and invasion. J. Cell. Physiol. 2022, 237, 389–400. [Google Scholar] [CrossRef]
- Vultaggio-Poma, V.; Sarti, A.C.; Di Virgilio, F. Extracellular ATP: A Feasible Target for Cancer Therapy. Cells 2020, 9, 2496. [Google Scholar] [CrossRef]
- Kepp, O.; Bezu, L.; Yamazaki, T.; Di Virgilio, F.; Smyth, M.J.; Kroemer, G.; Galluzzi, L. ATP and cancer immunosurveillance. EMBO J. 2021, 40, e108130. [Google Scholar] [CrossRef]
- Joo, Y.N.; Jin, H.; Eun, S.Y.; Park, S.W.; Chang, K.C.; Kim, H.J. P2Y2R activation by nucleotides released from the highly metastatic breast cancer cell contributes to pre-metastatic niche formation by mediating lysyl oxidase secretion, collagen crosslinking, and monocyte recruitment. Oncotarget 2014, 5, 9322–9334. [Google Scholar] [CrossRef]
- Chadet, S.; Jelassi, B.; Wannous, R.; Angoulvant, D.; Chevalier, S.; Besson, P.; Roger, S. The activation of P2Y2 receptors increases MCF-7 breast cancer cells migration through the MEK-ERK1/2 signalling pathway. Carcinogenesis 2014, 35, 1238–1247. [Google Scholar] [CrossRef] [PubMed]
- Eun, S.Y.; Ko, Y.S.; Park, S.W.; Chang, K.C.; Kim, H.J. P2Y2 nucleotide receptor-mediated extracellular signal-regulated kinases and protein kinase C activation induces the invasion of highly metastatic breast cancer cells. Oncol. Rep. 2015, 34, 195–202. [Google Scholar] [CrossRef]
- Xie, R.; Xu, J.; Wen, G.; Jin, H.; Liu, X.; Yang, Y.; Ji, B.; Jiang, Y.; Song, P.; Dong, H.; et al. The P2Y2 nucleotide receptor mediates the proliferation and migration of human hepatocellular carcinoma cells induced by ATP. J. Biol. Chem. 2014, 289, 19137–19149. [Google Scholar] [CrossRef]
- Schulien, I.; Hockenjos, B.; Van Marck, V.; Ayata, C.K.; Follo, M.; Thimme, R.; Hasselblatt, P. Extracellular ATP and Purinergic P2Y2 Receptor Signaling Promote Liver Tumorigenesis in Mice by Exacerbating DNA Damage. Cancer Res. 2020, 80, 699–708. [Google Scholar] [CrossRef] [PubMed]
- Hevia, M.J.; Castro, P.; Pinto, K.; Reyna-Jeldes, M.; Rodríguez-Tirado, F.; Robles-Planells, C.; Ramírez-Rivera, S.; Madariaga, J.A.; Gutierrez, F.; López, J.; et al. Differential Effects of Purinergic Signaling in Gastric Cancer-Derived Cells Through P2Y and P2X Receptors. Front. Pharmacol. 2019, 10, 612. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.H.; Ji, Y.G.; Lee, D.H. Uridine triphosphate increases proliferation of human cancerous pancreatic duct epithelial cells by activating P2Y2 receptor. Pancreas 2013, 42, 680–686. [Google Scholar] [CrossRef]
- Wan, H.; Xie, R.; Xu, J.; He, J.; Tang, B.; Liu, Q.; Wang, S.; Guo, Y.; Yang, X.; Dong, T.X.; et al. Anti-proliferative Effects of Nucleotides on Gastric Cancer via a Novel P2Y6/SOCE/Ca2+/β-catenin Pathway. Sci. Rep. 2017, 7, 1–13. [Google Scholar] [CrossRef]
- Prevarskaya, N.; Skryma, R.; Shuba, Y. Calcium in tumour metastasis: New roles for known actors. Nat. Rev. Cancer 2011, 11, 609–618. [Google Scholar] [CrossRef]
- Bong, A.H.L.; Monteith, G.R. Calcium signaling and the therapeutic targeting of cancer cells. Biochim. Biophys. Acta Mol. cell Res. 2018, 1865, 1786–1794. [Google Scholar] [CrossRef]
- Umemura, M.; Baljinnyam, E.; Feske, S.; De Lorenzo, M.S.; Xie, L.H.; Feng, X.; Oda, K.; Makino, A.; Fujita, T.; Yokoyama, U.; et al. Store-operated Ca2+ entry (SOCE) regulates melanoma proliferation and cell migration. PLoS ONE 2014, 9, e89292. [Google Scholar] [CrossRef]



| Transcript | Oligonucleotides | Amplicon Size |
|---|---|---|
| P2Y2 | Forward: CTG GTA GCG AGA ACA CTA AGG Reverse: GTC AAT ATC CTG AGC CCC TG | 189 bp |
| STIM-1 | Forward: CAC CAC AGC ACT TCC TAT TT Reverse: GGA AGT CAT GGC ATT GAG AG | 216 bp |
| STIM-2 | Forward: GAC ACT CTT CAG TGG TTG ATA Reverse: TAT GAG GTG GGC GTG TTA | 220 bp |
| ORAI-1 | Forward: CCT TCG GCC TGA TCT TTA TC Reverse: CCA AAG CAC TGG AAG GG | 206 bp |
| ORAI-2 | Forward: AAG GAG ATG GGA TGG AGA G Reverse: TAC CGA GTG GTG GTT AGA G | 196 bp |
| ORAI-3 | Forward: TGC CAC ACC CGA CTA AT Reverse: GAA ACA CCC AAA TCC CTC TAC | 220 bp |
| TRPC-1 | Forward: CTG TGG ATT ATT GGG ATG ATT TG Reverse: CAC CAG TGT AGG ATG GAA TG | 207 bp |
| CYC-1 | Forward: CTC CTG CCA CAG CAT GGA C Reverse: CAT GCC TAG CTC GCA CGA T | 251 bp |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mata-Martínez, E.; Gonzalez-Gallardo, A.; Díaz-Muñoz, M.; Vázquez-Cuevas, F.G. Purinergic Activation of Store-Operated Calcium Entry (SOCE) Regulates Cell Migration in Metastatic Ovarian Cancer Cells. Pharmaceuticals 2023, 16, 944. https://doi.org/10.3390/ph16070944
Mata-Martínez E, Gonzalez-Gallardo A, Díaz-Muñoz M, Vázquez-Cuevas FG. Purinergic Activation of Store-Operated Calcium Entry (SOCE) Regulates Cell Migration in Metastatic Ovarian Cancer Cells. Pharmaceuticals. 2023; 16(7):944. https://doi.org/10.3390/ph16070944
Chicago/Turabian StyleMata-Martínez, Esperanza, Adriana Gonzalez-Gallardo, Mauricio Díaz-Muñoz, and Francisco G. Vázquez-Cuevas. 2023. "Purinergic Activation of Store-Operated Calcium Entry (SOCE) Regulates Cell Migration in Metastatic Ovarian Cancer Cells" Pharmaceuticals 16, no. 7: 944. https://doi.org/10.3390/ph16070944
APA StyleMata-Martínez, E., Gonzalez-Gallardo, A., Díaz-Muñoz, M., & Vázquez-Cuevas, F. G. (2023). Purinergic Activation of Store-Operated Calcium Entry (SOCE) Regulates Cell Migration in Metastatic Ovarian Cancer Cells. Pharmaceuticals, 16(7), 944. https://doi.org/10.3390/ph16070944







