Exploring CD39 and CD73 Expression as Potential Biomarkers in Prostate Cancer
Abstract
1. Introduction
2. Results
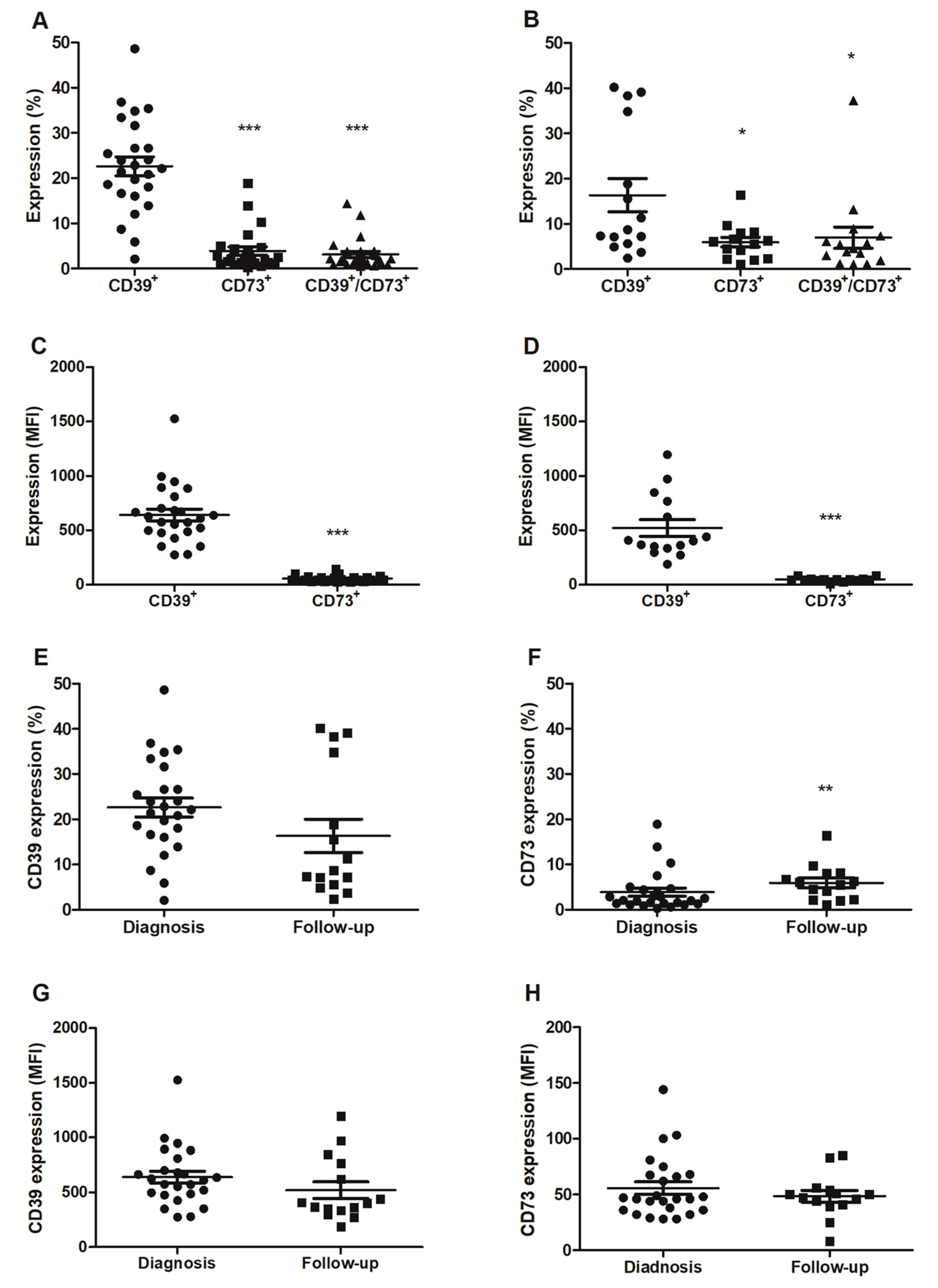
2.1. CD39 and CD73 Expression in Tissue Samples from Prostate Cancer Patients
2.2. Analysis of CD39 and CD73 Expression in Plasma Extracellular Vesicles
2.3. Analysis of ATP, ADP, and AMP Hydrolysis Profile in the Plasma
3. Discussion
4. Patients and Methods
4.1. Study Design
4.2. Sample Collection
4.3. Data Collection
4.4. Immunohistochemistry and Tissue Cytometry
4.5. Extracellular Vesicle Analysis
4.6. Analysis of ATP, ADP, and AMP Hydrolysis
4.7. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E. Cancer Statistics, 2022. CA Cancer J. Clin. 2022, 72, 7–33. [Google Scholar] [CrossRef] [PubMed]
- Sasse, A.D.; Wiermann, E.G.; Herchenhorn, D.; Bastos, D.A.; Schutz, F.A.; Maluf, F.C.; Filho, C.G.; Morbeck, I.A.P.; Cerci, J.J.; Smaletz, O.; et al. First Brazilian Consensus of Advanced Prostate Cancer: Recommendations for Clinical Practice. Int. Braz. J. Urol. 2017, 43, 407–415. [Google Scholar] [CrossRef] [PubMed]
- Parker, C.; Gillessen, S.; Heidenreich, A.; Horwich, A. ESMO Guidelines Committee. Cancer of the prostate: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2015, 26 (Suppl. S5), 69–77, Erratum in Ann. Oncol. 2016, 27 (Suppl. S5), 148. [Google Scholar] [CrossRef] [PubMed]
- Carter, H.B.; Albertsen, P.C.; Barry, M.J.; Etzioni, R.; Freedland, S.J.; Greene, K.L.; Holmberg, L.; Kantoff, P.; Konety, B.R.; Murad, M.H.; et al. Early Detection of Prostate Cancer: AUA Guideline. J. Urol. 2013, 190, 419–426. [Google Scholar] [CrossRef] [PubMed]
- Basch, E.; Oliver, T.K.; Vickers, A.; Thompson, I.; Kantoff, P.; Parnes, H.; Loblaw, D.A.; Roth, B.; Williams, J.; Nam, R.K. Screening for Prostate Cancer with Prostate-Specific Antigen Testing: American Society of Clinical Oncology Provisional Clinical Opinion. J. Clin. Oncol. 2012, 30, 3020–3025. [Google Scholar] [CrossRef]
- Burnstock, G.; Di Virgilio, F. Purinergic signalling and cancer. Purinergic Signal. 2013, 9, 491–540. [Google Scholar] [CrossRef] [PubMed]
- Vijayan, D.; Young, A.; Teng, M.W.L.; Smyth, M.J. Targeting immunosuppressive adenosine in cancer. Nat. Rev. Cancer 2017, 22, 765, Erratum in Nat. Rev. Cancer 2017, 17, 709–724. [Google Scholar] [CrossRef]
- Hatfield, S.M.; Kjaergaard, J.; Lukashev, D.; Schreiber, T.H.; Belikoff, B.; Abbott, R.; Sethumadhavan, S.; Philbrook, P.; Ko, K.; Cannici, R.; et al. Immunological mechanisms of the antitumor effects of supplemental oxygenation. Sci. Transl. Med. 2015, 7, 277ra30. [Google Scholar] [CrossRef]
- SSitkovsky, M.V.; Hatfield, S.; Abbott, R.; Belikoff, B.; Lukashev, D.; Ohta, A. Hostile, Hypoxia–A2-Adenosinergic Tumor Biology as the Next Barrier to Overcome for Tumor Immunologists. Cancer Immunol. Res. 2014, 2, 598–605. [Google Scholar] [CrossRef]
- Semenza, G.L. HIF-1 mediates metabolic responses to intratumoral hypoxia and oncogenic mutations. J. Clin. Investig. 2013, 123, 3664–3671. [Google Scholar] [CrossRef]
- Antonioli, L.; Pacher, P.; Vizi, E.S.; Haskó, G. CD39 and CD73 in immunity and inflammation. Trends Mol. Med. 2013, 19, 355–367. [Google Scholar] [CrossRef] [PubMed]
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef] [PubMed]
- Wilhelm, K.; Ganesan, J.; Müller, T.; Dürr, C.; Grimm, M.; Beilhack, A.; Krempl, C.D.; Sorichter, S.; Gerlach, U.V.; Jüttner, E.; et al. Graft-versus-host disease is enhanced by extracellular ATP activating P2X7R. Nat. Med. 2010, 16, 1434–1438. [Google Scholar] [CrossRef]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424, Erratum in CA Cancer J. Clin. 2020, 70, 313. [Google Scholar] [CrossRef]
- Scheffel, T.B.; Grave, N.; Vargas, P.; Diz, F.M.; Rockenbach, L.; Morrone, F.B. Immunosuppression in Gliomas via PD-1/PD-L1 Axis and Adenosine Pathway. Front. Oncol. 2021, 15, 617385. [Google Scholar] [CrossRef] [PubMed]
- Morrone, F.B.; Vargas, P.; Rockenbach, L.; Scheffel, T.B. P2Y12 Purinergic Receptor and Brain Tumors: Implications on Glioma Microenvironment. Molecules 2021, 26, 6146. [Google Scholar] [CrossRef] [PubMed]
- Burnstock, G. Purine and pyrimidine receptors. Cell. Mol. Life Sci. 2007, 64, 1471–1483. [Google Scholar] [CrossRef] [PubMed]
- Zimmermann, H.; Zebisch, M.; Sträter, N. Cellular function and molecular structure of ecto-nucleotidases. Purinergic Signal. 2012, 8, 437–502. [Google Scholar] [CrossRef]
- Gardani, C.F.F.; Cappellari, A.R.; de Souza, J.B.; da Silva, B.T.; Engroff, P.; Moritz, C.E.J.; Scholl, J.N.; Battastini, A.M.O.; Figueiró, F.; Morrone, F.B. Hydrolysis of ATP, ADP, and AMP is increased in blood plasma of prostate cancer patients. Purinergic Signal. 2019, 15, 95–105. [Google Scholar] [CrossRef]
- Gheler, F.V.; Cappellari, A.R.; Renck, D.; de Souza, J.B.; de Melo, R.O.; Moehlecke, B.Z.; Moriguchi, C.A.; Engroff, P.; Lambert, A.P.F.; Rockenbach, L.; et al. AMP hydrolysis reduction in blood plasma of breast cancer elderly patients after different treatments. Mol. Cell. Biochem. 2021, 476, 3719–3727. [Google Scholar] [CrossRef]
- Bastid, J.; Bonnefoy, N.; Eliaou, J.-F.; Bensussan, A. Lymphocyte-derived interleukin-17A adds another brick in the wall of inflammation-induced breast carcinogenesis. OncoImmunology 2014, 3, e28273. [Google Scholar] [CrossRef] [PubMed]
- Jin, D.; Fan, J.; Wang, L.; Thompson, L.F.; Liu, A.; Daniel, B.J.; Shin, T.; Curiel, T.J.; Zhang, B. CD73 on Tumor Cells Impairs Antitumor T-Cell Responses: A Novel Mechanism of Tumor-Induced Immune Suppression. Cancer Res 2010, 70, 2245–2255. [Google Scholar] [CrossRef] [PubMed]
- Loi, S.; Pommey, S.; Haibe-Kains, B.; Beavis, P.A.; Darcy, P.K.; Smyth, M.J.; Stagg, J. CD73 promotes anthracycline resistance and poor prognosis in triple negative breast cancer. Proc. Natl. Acad. Sci. USA 2013, 110, 11091–11096. [Google Scholar] [CrossRef] [PubMed]
- Cappellari, A.R.; Rockenbach, L.; Dietrich, F.; Clarimundo, V.; Glaser, T.; Braganhol, E.; Abujamra, A.L.; Roesler, R.; Ulrich, H.; Battastini, A.M. Characterization of ectonucleotidases in human medulloblastoma cell lines: Ecto-5’NT/CD73 in metastasis as potential prognostic factor. PLoS ONE 2012, 7, e47468, Erratum in PLoS ONE 2012, 7. [Google Scholar] [CrossRef]
- Yang, Q.; Du, J.; Zu, L. Overexpression of CD73 in Prostate Cancer is Associated with Lymph Node Metastasis. Pathol. Oncol. Res. 2013, 19, 811–814. [Google Scholar] [CrossRef]
- Leclerc, B.G.; Charlebois, R.; Chouinard, G.; Allard, B.; Pommey, S.; Saad, F.; Stagg, J. CD73 Expression Is an Independent Prognostic Factor in Prostate Cancer. Clin. Cancer Res. 2016, 22, 158–166. [Google Scholar] [CrossRef]
- Bastid, J.; Regairaz, A.; Bonnefoy, N.; Déjou, C.; Giustiniani, J.; Laheurte, C.; Cochaud, S.; Laprevotte, E.; Funck-Brentano, E.; Hemon, P.; et al. Inhibition of CD39 Enzymatic Function at the Surface of Tumor Cells Alleviates Their Immunosuppressive Activity. Cancer Immunol. Res. 2015, 3, 254–265. [Google Scholar] [CrossRef]
- Pulte, E.D.; Broekman, M.J.; Olson, K.E.; Drosopoulos, J.H.; Kizer, J.R.; Islam, N.; Marcus, A.J. CD39/NTPDase-1 activity and expression in normal leukocytes. Thromb. Res. 2007, 121, 309–317. [Google Scholar] [CrossRef]
- Kansas, G.S.; Wood, G.S.; Tedder, T.F. Expression, distribution, and biochemistry of human CD39. Role in activation-associated homotypic adhesion of lymphocytes. J. Immunol. 1991, 146, 2235–2244. [Google Scholar] [CrossRef]
- Campos, J.H.; Soares, R.P.; Ribeiro, K.; Andrade, A.C.; Batista, W.L.; Torrecilhas, A.C. Extracellular Vesicles: Role in Inflammatory Responses and Potential Uses in Vaccination in Cancer and Infectious Diseases. J. Immunol. Res. 2015, 2015, 832057. [Google Scholar] [CrossRef]
- Clayton, A.; Al-Taei, S.; Webber, J.; Mason, M.D.; Tabi, Z. Cancer Exosomes Express CD39 and CD73, Which Suppress T Cells through Adenosine Production. J. Immunol. 2011, 187, 676–683. [Google Scholar] [CrossRef] [PubMed]
- Panagopoulos, K.; Cross-Knorr, S.; Dillard, C.; Pantazatos, D.; Del Tatto, M.; Mills, D.; Goldstein, L.; Renzulli, J.; Quesenberry, P.; Chatterjee, D. Reversal of chemosensitivity and induction of cell malignancy of a non-malignant prostate cancer cell line upon extracellular vesicle exposure. Mol. Cancer 2013, 12, 118. [Google Scholar] [CrossRef] [PubMed]
- Raposo, G.; Stoorvogel, W. Extracellular vesicles: Exosomes, microvesicles, and friends. J. Cell Biol. 2013, 200, 373–383. [Google Scholar] [CrossRef] [PubMed]
- Lässer, C.; Théry, C.; Buzás, E.I.; Mathivanan, S.; Zhao, W.; Gho, Y.S.; Lötvall, J. The International Society for Extracellular Vesicles launches the first massive open online course on extracellular vesicles. J. Extracell. Vesicles 2016, 5, 34299. [Google Scholar] [CrossRef]
- Vlaeminck-Guillem, V. Extracellular Vesicles in Prostate Cancer Carcinogenesis, Diagnosis, and Management. Front. Oncol. 2018, 8, 222. [Google Scholar] [CrossRef]
- Gleason, D.F.; Mellinger, G.T. Prediction of prognosis for prostatic adenocarcinoma by combined histological grading and clinical staging. J. Urol. 1974, 111, 58–64. [Google Scholar] [CrossRef]
- Damber, J.E.; Aus, G. Prostate cancer. Lancet 2008, 371, 1710–1721. [Google Scholar] [CrossRef]
- Siegel, R.L.; Miller, K.D.; Wagle, N.S.; Jemal, A. Cancer statistics, 2023. CA A Cancer J. Clin. 2023, 73, 17–48. [Google Scholar] [CrossRef]
- Di Virgilio, F.; Dal Ben, D.; Sarti, A.C.; Giuliani, A.L.; Falzoni, S. The P2X7 Receptor in Infection and Inflammation. Immunity 2017, 47, 15–31. [Google Scholar] [CrossRef]
- Morello, S.; Capone, M.; Sorrentino, C.; Giannarelli, D.; Madonna, G.; Mallardo, D.; Grimaldi, A.M.; Pinto, A.; Ascierto, P.A. Soluble CD73 as biomarker in patients with metastatic melanoma patients treated with nivolumab. J. Transl. Med. 2017, 15, 244. [Google Scholar] [CrossRef]
- Moesta, A.K.; Li, X.-Y.; Smyth, M.J. Targeting CD39 in cancer. Nat. Rev. Immunol. 2020, 20, 739–755. [Google Scholar] [CrossRef] [PubMed]
- Biswas, S.K. Metabolic Reprogramming of Immune Cells in Cancer Progression. Immunity 2015, 43, 435–449. [Google Scholar] [CrossRef] [PubMed]
- Di Virgilio, F.; Sarti, A.C.; Falzoni, S.; De Marchi, E.; Adinolfi, E. Extracellular ATP and P2 purinergic signalling in the tumour microenvironment. Nat. Rev. Cancer 2018, 18, 601–618. [Google Scholar] [CrossRef] [PubMed]
- Tamajusuku, A.S.; Villodre, E.S.; Paulus, R.; Coutinho-Silva, R.; Battasstini, A.M.; Wink, M.R.; Lenz, G. Characterization of ATP-induced cell death in the GL261 mouse glioma. J. Cell. Biochem. 2010, 109, 983–991. [Google Scholar] [CrossRef]
- Battisti, V.; Maders, L.D.; Bagatini, M.D.; Battisti, I.E.; Bellé, L.P.; Santos, K.F.; Maldonado, P.A.; Thomé, G.R.; Schetinger, M.R.; Morsch, V.M. Ectonucleotide pyrophosphatase/phosphodiesterase (E-NPP) and adenosine deaminase (ADA) activities in prostate cancer patients: Influence of Gleason score, treatment and bone metastasis. BioMedicine 2013, 67, 203–208. [Google Scholar] [CrossRef] [PubMed]
- Pillat, M.M.; Lameu, C.; Trujillo, C.A.; Glaser, T.; Cappellari, A.R.; Negraes, P.D.; Battastini, A.M.; Schwindt, T.T.; Muotri, A.R.; Ulrich, H. Bradykinin promotes neuron-generating division of neural progenitor cells through ERK activation. J. Cell Sci. 2016, 129, 3437–3448. [Google Scholar] [CrossRef]
- Zaparte, A.; Cappellari, A.R.; Brandão, C.A.; de Souza, J.B.; Borges, T.J.; Kist, L.W.; Bogo, M.R.; Zerbini, L.F.; Ribeiro Pinto, L.F.; Glaser, T.; et al. P2Y2 receptor activation promotes esophageal cancer cells proliferation via ERK1/2 pathway. Eur. J. Pharmacol. 2021, 891, 173687. [Google Scholar] [CrossRef]
- Jiang, X.L.; Zhang, Y.; Tan, B.; Luo, C.L.; Wu, X.H. Renal tumor-derived exosomes inhibit hepaCAM expression of renal carcinoma cells in a p-AKT-dependent manner. Neoplasma 2014, 61, 416–423. [Google Scholar] [CrossRef][Green Version]
- Smith, P.K.; Krohn, R.I.; Hermanson, G.T.; Mallia, A.K.; Gartner, F.H.; Provenzano, M.D.; Fujimoto, E.K.; Goeke, N.M.; Olson, B.J.; Klenk, D.C. Measurement of protein using bicinchoninic acid. Anal. Biochem. 1985, 150, 76–85, Erratum in Anal. Biochem. 1987, 163, 279. [Google Scholar] [CrossRef]
- Suárez, H.; Gámez-Valero, A.; Reyes, R.; López-Martín, S.; Rodríguez, M.J.; Carrascosa, J.L.; Cabañas, C.; Borràs, F.E.; Yáñez-Mó, M. A bead-assisted flow cytometry method for the semi-quantitative analysis of Extracellular Vesicles. Sci. Rep. 2017, 7, 11271. [Google Scholar] [CrossRef]
- Moritz, C.E.J.; Teixeira, B.C.; Rockenbach, L.; Reischak-Oliveira, A.; Casali, E.A.; Battastini, A.M.O. Altered extracellular ATP, ADP, and AMP hydrolysis in blood serum of sedentary individuals after an acute, aerobic, moderate exercise session. Mol. Cell. Biochem. 2016, 426, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Chan, K.-M.; Delfert, D.; Junger, K.D. A direct colorimetric assay for Ca2+-stimulated ATPase activity. Anal. Biochem. 1986, 157, 375–380. [Google Scholar] [CrossRef] [PubMed]



| Characteristics | N (23) | % |
|---|---|---|
| Age (Years) | ||
| Mean | 65.4 | |
| Range | 48–78 | |
| PSA | ||
| <10 | 15 | 65.5 |
| 10–20 | 6 | 26.1 |
| >20 | 2 | 8.7 |
| Gleason Grade * | ||
| Low grade | 7 | 30.5 |
| Intermediate grade | 12 | 52.2 |
| High grade | 4 | 17.3 |
| Histology | ||
| Adenocarcinoma | 23 | 100 |
| Clinical Stage (CS) ** | ||
| CS I/EC IIA | 7 | 30.5 |
| CS IIB/EC IIC | 11 | 47.9 |
| >CS IIIA | 5 | 21.6 |
| Transurethral Resection—TUR | ||
| Yes | 3 | 13.1 |
| No | 20 | 86.9 |
| Radical Prostatectomy | ||
| Yes | 12 | 52.2 |
| No | 11 | 47.8 |
| Radiotherapy | ||
| Yes | 18 | 78.3 |
| No | 5 | 21.7 |
| Hormonotherapy | ||
| Yes | 9 | 39.1 |
| No | 14 | 60.9 |
| CD39 Diagnosis | CD39 Follow-Up | CD73 Diagnosis | CD73 Follow-Up | |||||
|---|---|---|---|---|---|---|---|---|
| N | (%) | (MFI) | (%) | (MFI) | (%) | (MFI) | (%) | (MFI) |
| Age | ||||||||
| Mean (68.6) | −0.045 | 0.046 | −0.322 | −0.344 | −0.064 | −0.144 | −0.199 | −0.255 |
| Range (52–94) | ||||||||
| TUR | ||||||||
| Yes (17) | −0.107 | −0.048 | 0.327 | 0.196 | 0.631 ** | 0.197 | 0.491 | 0.344 |
| No (8) | ||||||||
| Prostatectomy | ||||||||
| Yes (8) | −0.83 | −0.131 | −0.098 | −0.131 | −0.553 ** | −0.197 | −0.229 | −0.115 |
| No (17) | ||||||||
| Gleason Score | ||||||||
| <6 (5) | ||||||||
| 7 (13) | −0.017 | 0.048 | 0.342 | 0.197 | 0.455 * | 0.170 | 0.594 * | 0.318 |
| 8–10 (7) | ||||||||
| CS | ||||||||
| I/IIA (9) | 0.133 | 0.188 | 0.015 | −0.165 | 0.392 | 0.173 | 0.559 * | 0.065 |
| IIB/IIC (14) | ||||||||
| III (3) | ||||||||
| Perineural Invasion | ||||||||
| Yes (18) | −0.334 | −0.383 | −0.182 | 0.045 | −0.192 | −0.445 * | 0.091 | −0.023 |
| No (7) | ||||||||
| PSA | ||||||||
| <10 (18) | 0.166 | 0.174 | 0.354 | 0.343 | 0.083 | 0.171 | −0.221 | 0.204 |
| 10–20 (4) | ||||||||
| >20 (3) | ||||||||
| Diagnosis | Follow-Up | |||||
|---|---|---|---|---|---|---|
| ATP Hydrolysis | ADP Hydrolysis | AMP Hydrolysis | ATP Hydrolysis | ADP Hydrolysis | AMP Hydrolysis | |
| Age | 0.202 | 0.300 | −0.037 | −0.245 | −0.141 | 0.048 |
| TUR | 0.217 | 0.549 ** | 0.013 | −0.393 | 0.033 | −0.229 |
| Prostatectomy | −0.013 | −0.421 * | 0.051 | 0.360 | −0.065 | −0.065 |
| Gleason Score | 0.254 | 0.427 * | 0.397 | −0.097 | 0.220 | 0.147 |
| CS | 0.246 | 0.179 | 0.241 | 0.094 | 0.320 | 0.335 |
| Perineural Invasion | −0.049 | 0.125 | 0.431 * | 0.454 | 0.409 | 0.136 |
| PSA | 0.010 | −0.023 | −0.171 | −0.182 | −0.357 | 0.207 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gardani, C.F.F.; Pedrazza, E.L.; Paz, V.S.; Zanirati, G.G.; da Costa, J.C.; Andrejew, R.; Ulrich, H.; Scholl, J.N.; Figueiró, F.; Rockenbach, L.; et al. Exploring CD39 and CD73 Expression as Potential Biomarkers in Prostate Cancer. Pharmaceuticals 2023, 16, 1619. https://doi.org/10.3390/ph16111619
Gardani CFF, Pedrazza EL, Paz VS, Zanirati GG, da Costa JC, Andrejew R, Ulrich H, Scholl JN, Figueiró F, Rockenbach L, et al. Exploring CD39 and CD73 Expression as Potential Biomarkers in Prostate Cancer. Pharmaceuticals. 2023; 16(11):1619. https://doi.org/10.3390/ph16111619
Chicago/Turabian StyleGardani, Carla Fernanda Furtado, Eduardo Luiz Pedrazza, Victória Santos Paz, Gabriele Goulart Zanirati, Jaderson Costa da Costa, Roberta Andrejew, Henning Ulrich, Juliete Nathali Scholl, Fabrício Figueiró, Liliana Rockenbach, and et al. 2023. "Exploring CD39 and CD73 Expression as Potential Biomarkers in Prostate Cancer" Pharmaceuticals 16, no. 11: 1619. https://doi.org/10.3390/ph16111619
APA StyleGardani, C. F. F., Pedrazza, E. L., Paz, V. S., Zanirati, G. G., da Costa, J. C., Andrejew, R., Ulrich, H., Scholl, J. N., Figueiró, F., Rockenbach, L., & Morrone, F. B. (2023). Exploring CD39 and CD73 Expression as Potential Biomarkers in Prostate Cancer. Pharmaceuticals, 16(11), 1619. https://doi.org/10.3390/ph16111619






