PUFAs and Their Derivatives as Emerging Players in Diagnostics and Treatment of Male Fertility Disorders
Abstract
1. Introduction
2. Role of Polyunsaturated Fatty Acids in Male Reproductive Health
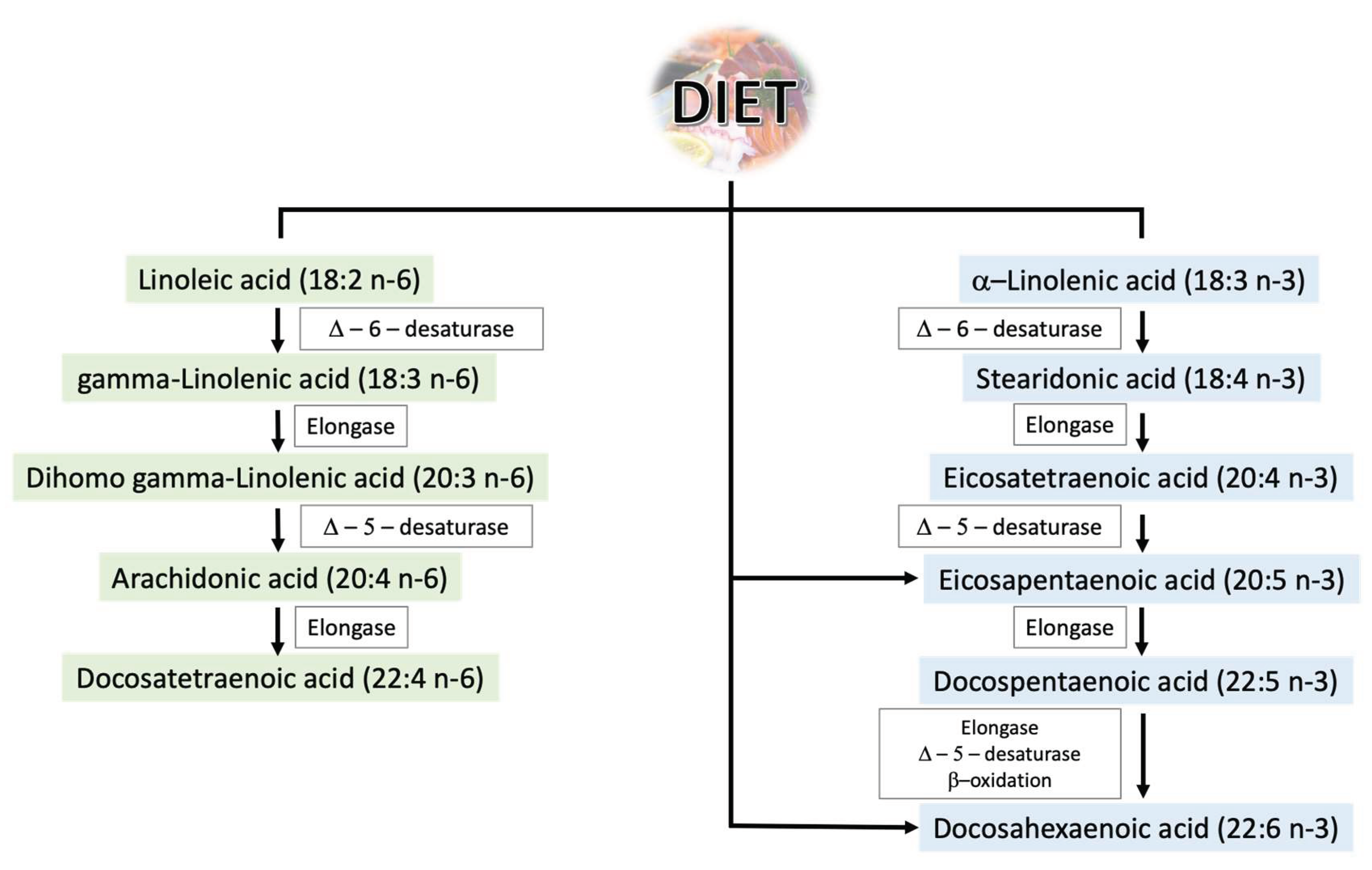
2.1. Polyunsaturated Fatty Acids—General Information
2.2. PUFAs in Male Infertility
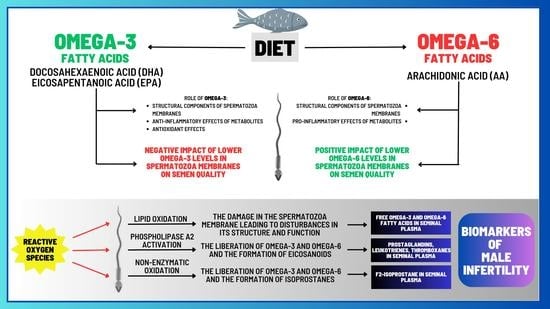
2.2.1. Omega-3 and Omega-6 PUFAs in the Ejaculate
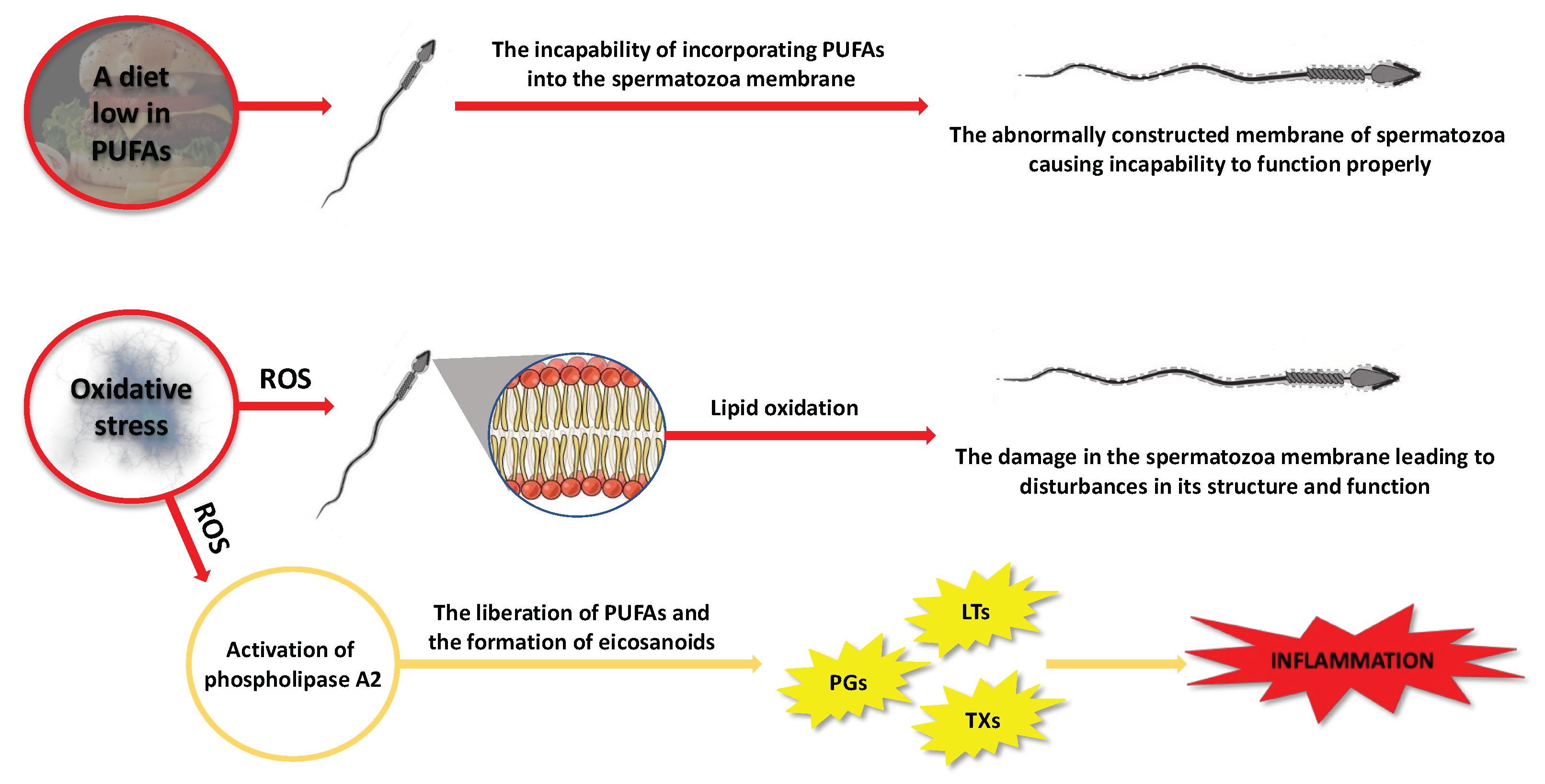
2.2.2. Factors That May Contribute to Alterations in the Composition of PUFAs
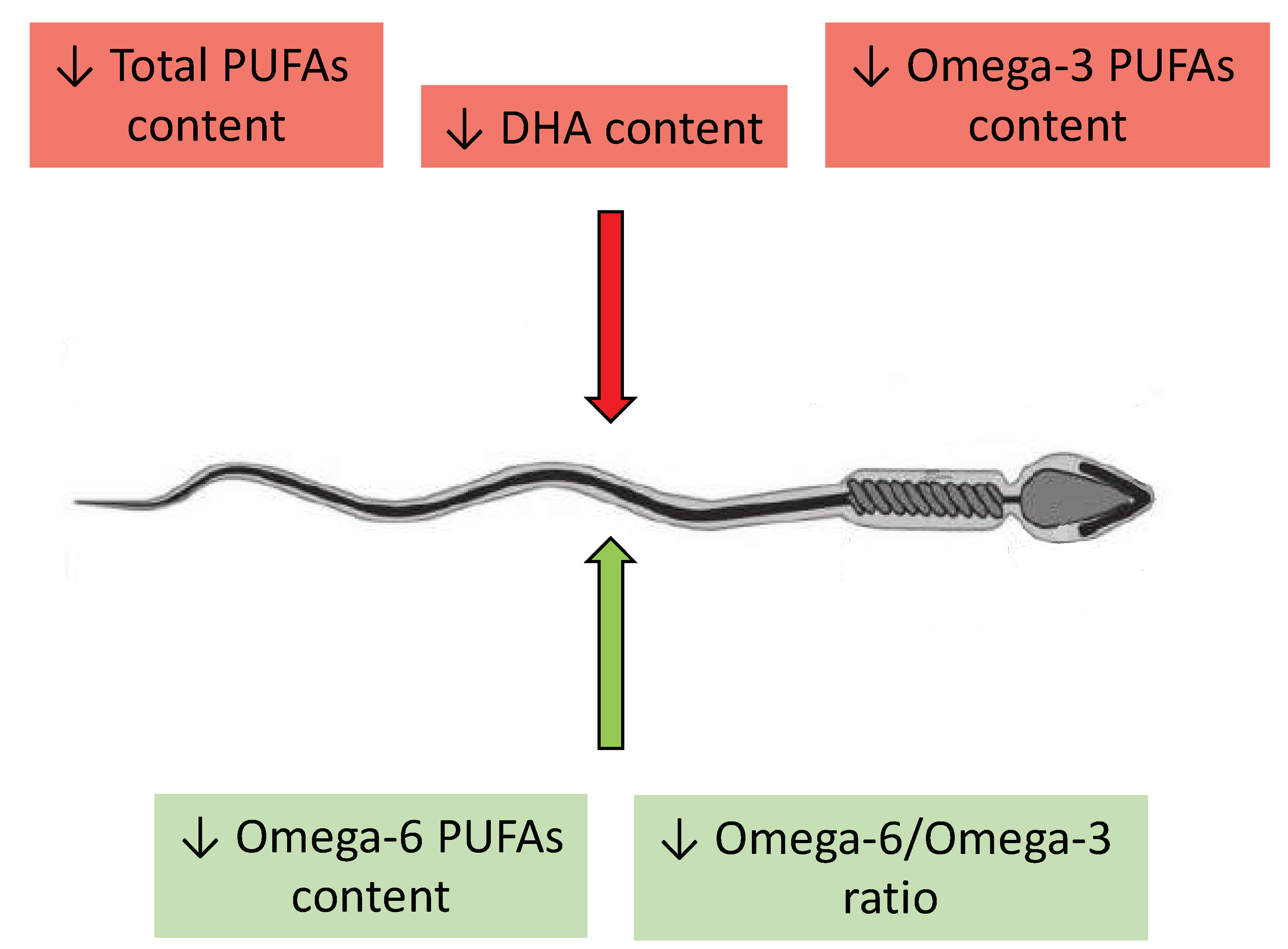
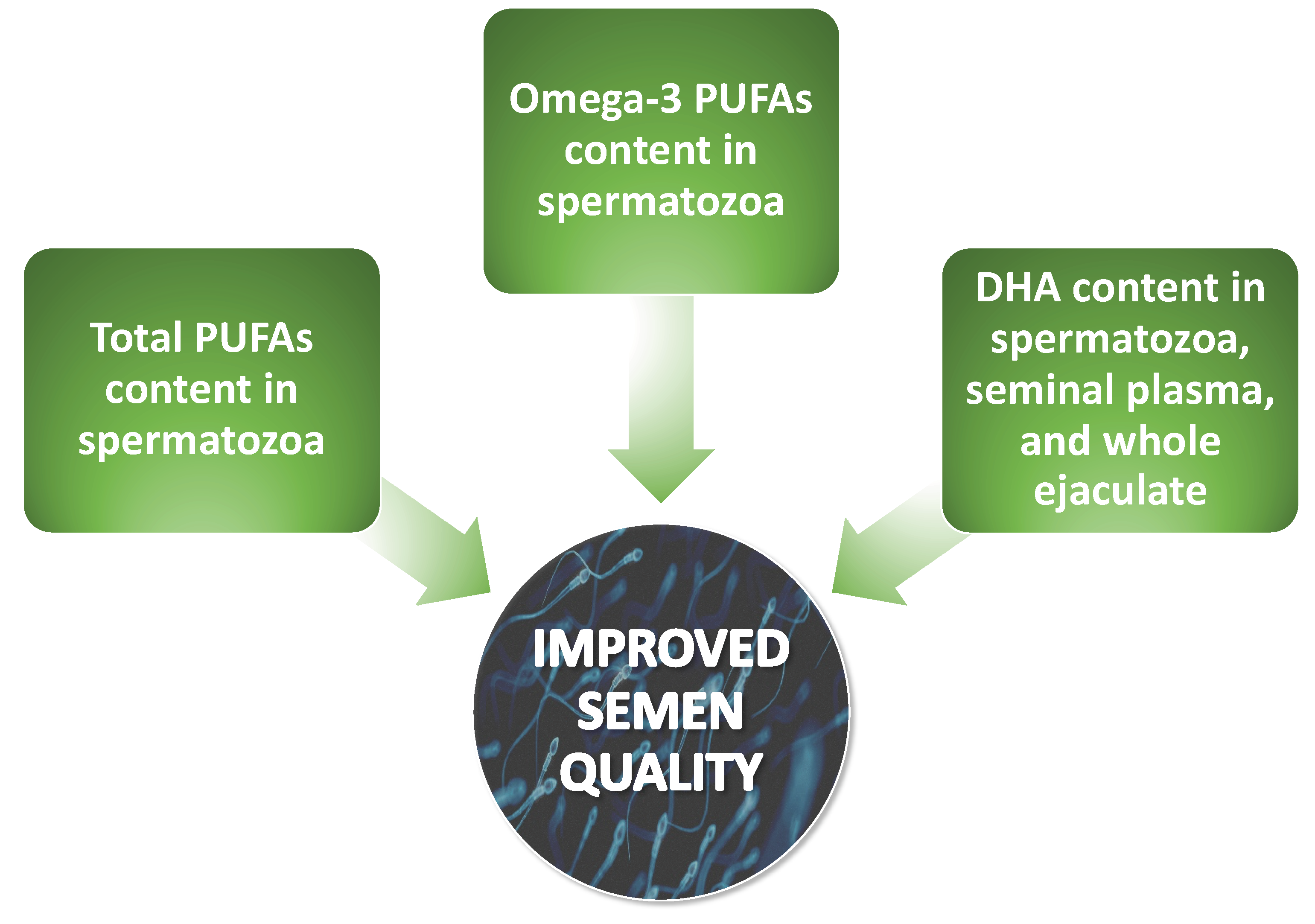
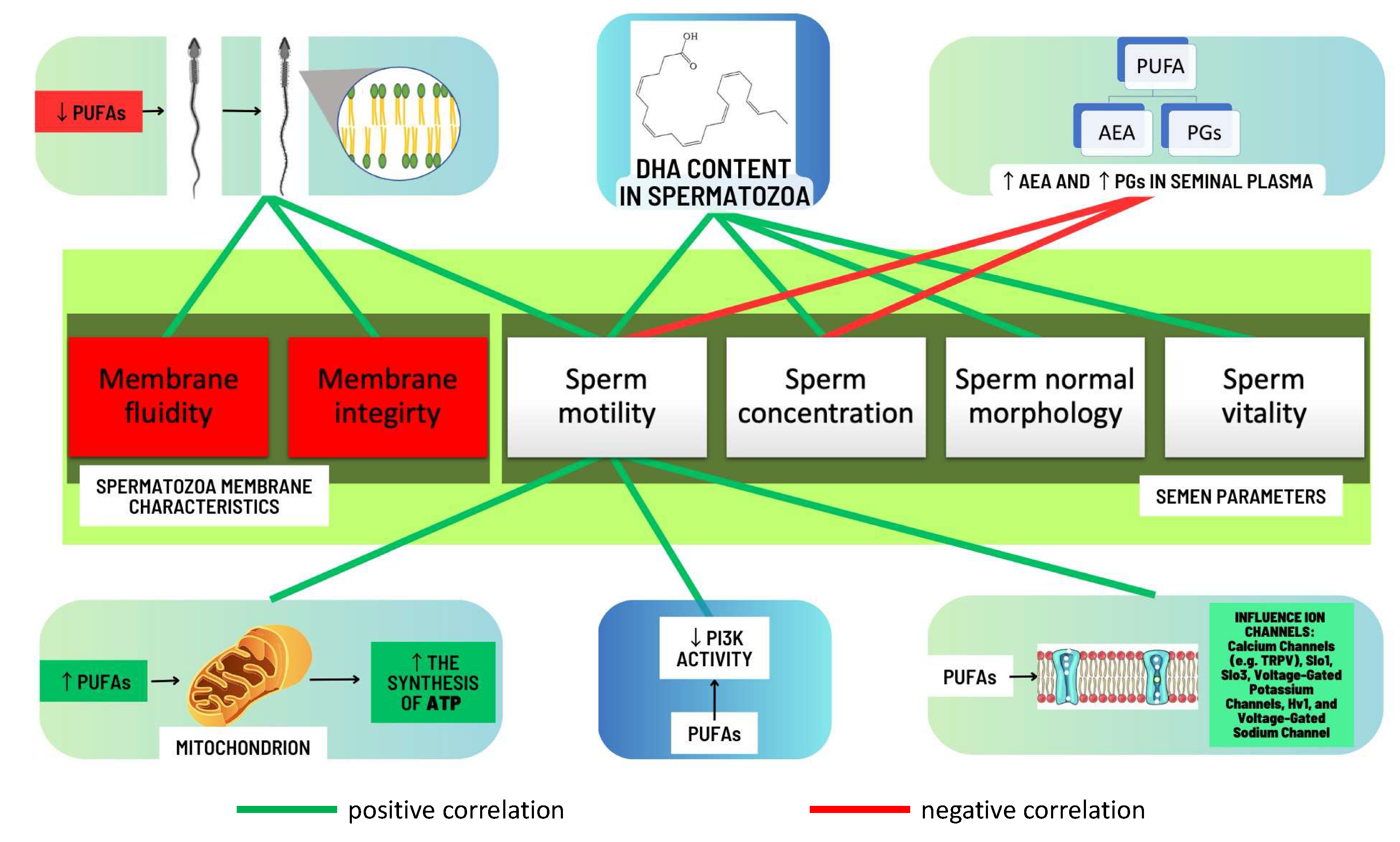
2.2.3. Correlations between Levels of Omega-3 and Omega-6 PUFAs and Semen Quality
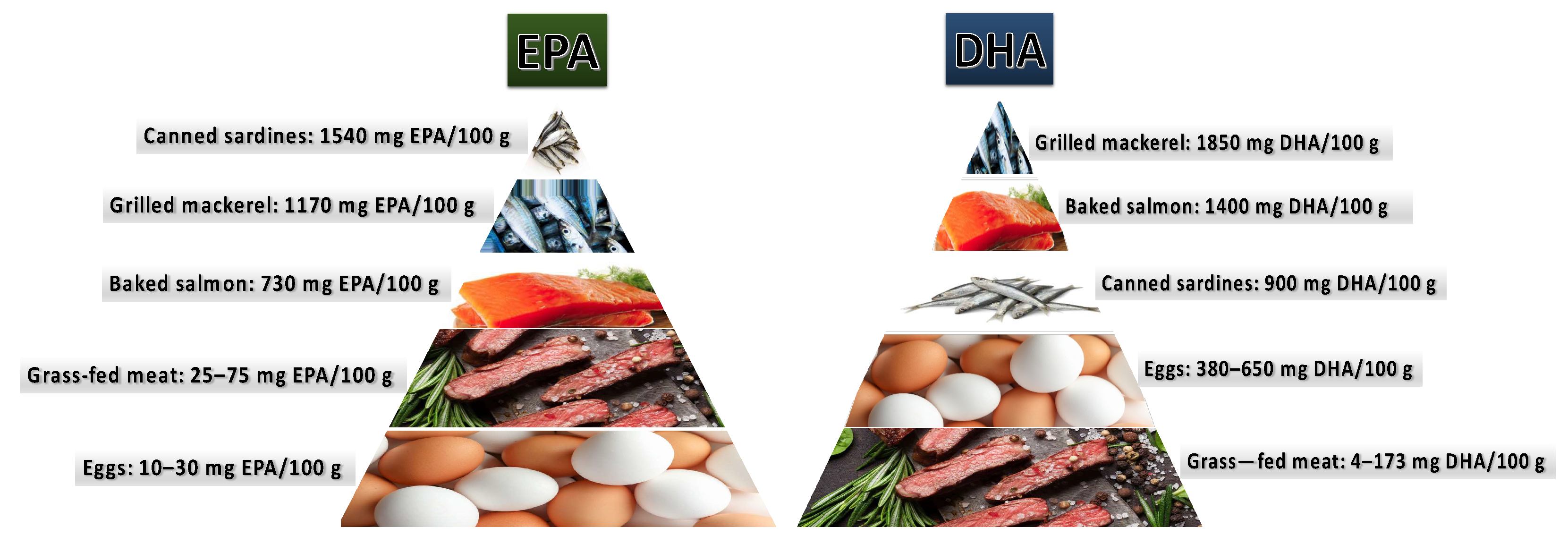
2.2.4. A Diet Rich in Omega-3 PUFAs as a Factor Enhancing Male Fertility
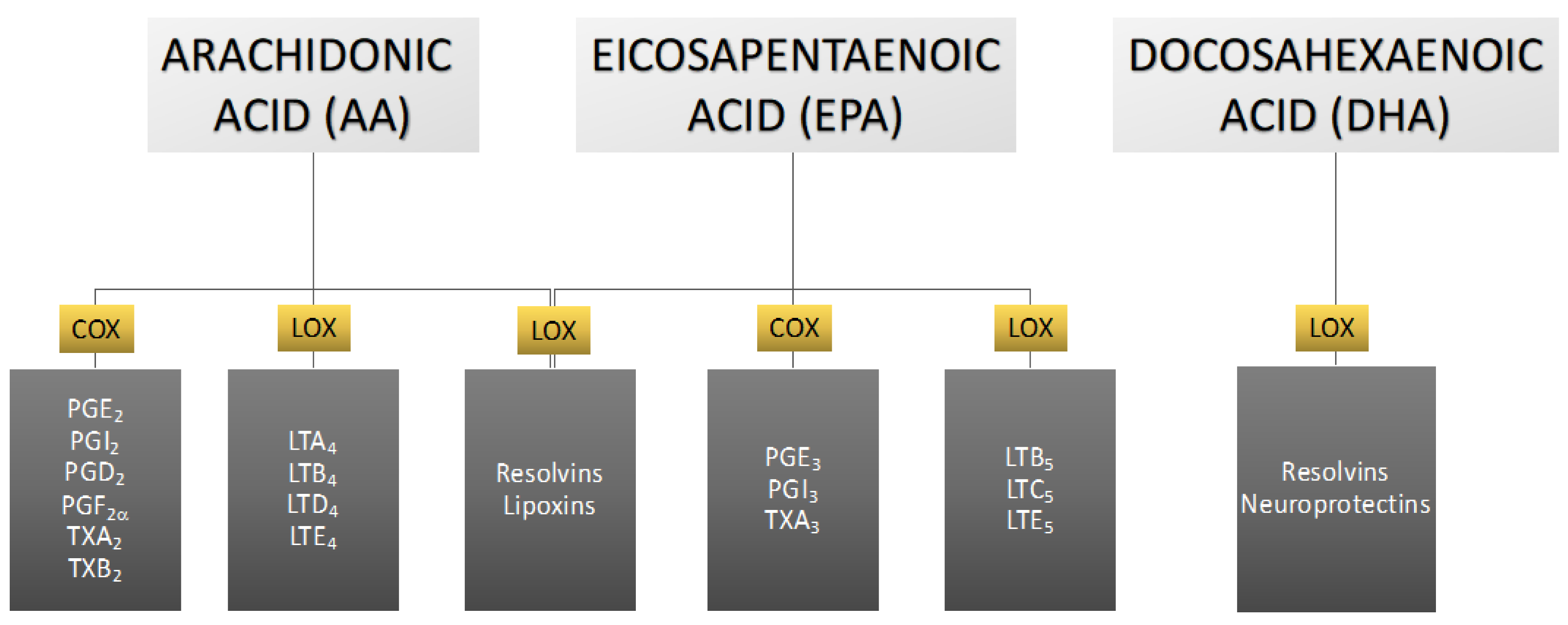
3. The Role of Eicosanoids in Male Reproductive Health
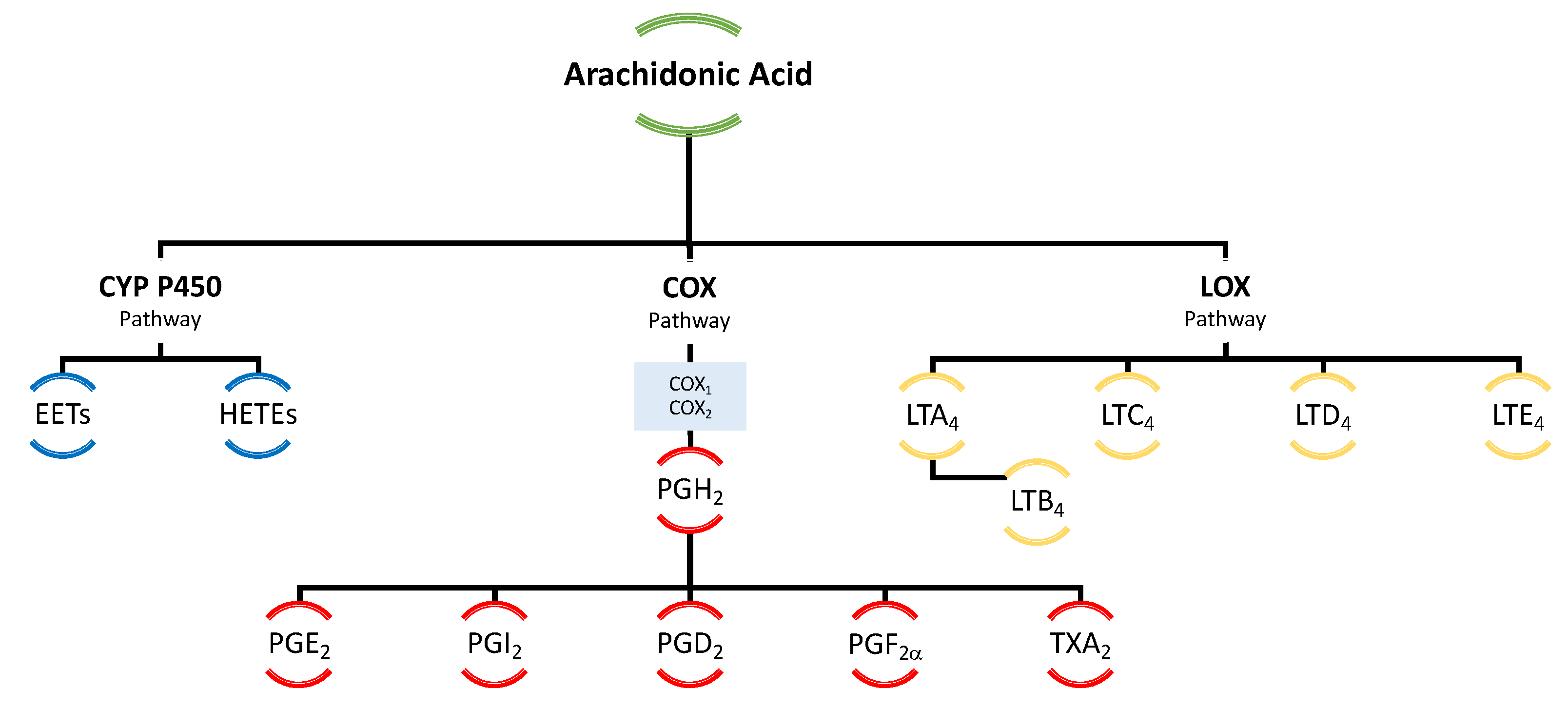
3.1. Derivatives of Arachidonic Acid
3.2. Arachidonic Acid Derivatives and Male Reproductive Potential
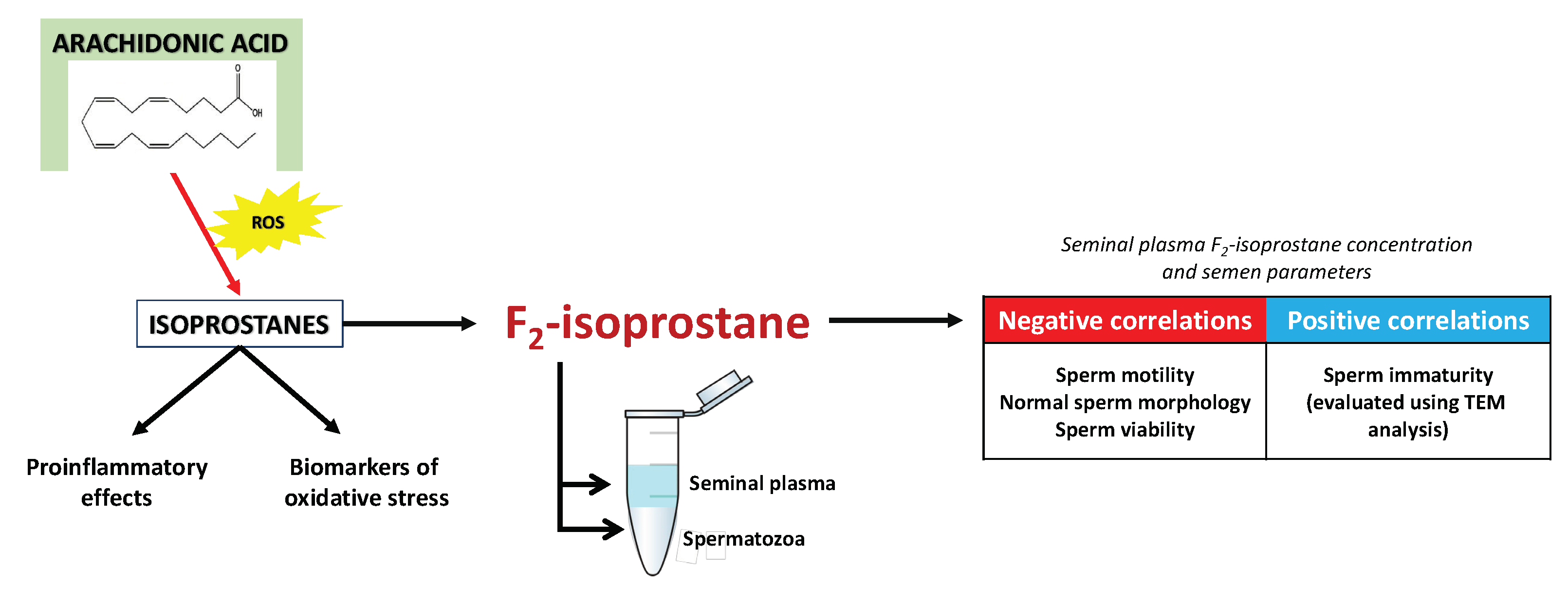
4. Isoprostanes—A Pioneering Approach in Diagnostics and Treatment of Male Infertility?
5. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Koperwas, M.; Głowacka, M. Problem niepłodności wśród kobiet i mężczyzn-epidemiologia, czynniki ryzyka i świadomość społeczna. Asp. Zdrowia Chor. 2017, 2, 31–49. [Google Scholar]
- Choy, J.T.; Eisenberg, M.L. Male infertility as a window to health. Fertil. Steril. 2018, 110, 810–814. [Google Scholar] [CrossRef] [PubMed]
- Infertility. Available online: https://www.who.int/news-room/fact-sheets/detail/infertility (accessed on 16 January 2023).
- Swan, S.H.; Elkin, E.P.; Fenster, L. The question of declining sperm density revisited: An analysis of 101 studies published 1934–1996. Environ. Health Perspect. 2000, 108, 961–966. [Google Scholar] [CrossRef]
- Jose-Miller, A.B.; Boyden, J.W.; Frey, K.A. Infertility. Am. Fam. Physician 2007, 75, 849–856. [Google Scholar]
- Naz, M.; Kamal, M. Classification, causes, diagnosis and treatment of male infertility: A review. Orient. Pharm. Exp. Med. 2017, 17, 89–109. [Google Scholar] [CrossRef]
- Klepinowski, T.; Klepinowska, M.; Sagan, L.; Syrenicz, A. Does SARS-CoV-2 Affect Human Semen? A Systematic Review and Meta-Analysis. Arch. Sex. Behav. 2023, 52, 669–677. [Google Scholar] [CrossRef]
- WHO World Health Organization. WHO Laboratory Manual for the Examination and Processing of Human Semen, 6th ed.; WHO Press: Geneva, Switzerland, 2021. [Google Scholar]
- Drabovich, A.P.; Saraon, P.; Jarvi, K.; Diamandis, E.P. Seminal plasma as a diagnostic fluid for male reproductive system disorders. Nat. Rev. Urol. 2014, 11, 278–288. [Google Scholar] [CrossRef]
- Cholewski, M.; Tomczykowa, M.; Tomczyk, M. A comprehensive review of chemistry, sources and bioavailability of omega-3 fatty acids. Nutrients 2018, 10, 1662. [Google Scholar] [CrossRef]
- Calder, P.C.; Grimble, R.F. Polyunsaturated fatty acids, inflammation and immunity. Eur. J. Clin. Nutr. 2002, 56 (Suppl. S3), S14–S19. [Google Scholar] [CrossRef] [PubMed]
- Kim, W.; McMurray, D.N.; Chapkin, R.S. N-3 Polyunsaturated fatty acids-Physiological relevance of dose. Prostaglandins Leukot. Essent. Fat. Acids 2010, 82, 155–158. [Google Scholar] [CrossRef]
- Dyall, S.C.; Balas, L.; Bazan, N.G.; Brenna, J.T.; Chiang, N.; da Costa Souza, F.; Dalli, J.; Durand, T.; Galano, J.M.; Lein, P.J.; et al. Polyunsaturated fatty acids and fatty acid-derived lipid mediators: Recent advances in the understanding of their biosynthesis, structures, and functions. Prog. Lipid Res. 2022, 86, 101165. [Google Scholar] [CrossRef] [PubMed]
- Dennis, E.A.; Norris, P.C. Eicosanoid storm in infection and inflammation. Nat. Rev. Immunol. 2015, 15, 511–523. [Google Scholar] [CrossRef]
- Hussein, J.S. Cell membrane fatty acids and health. Int. J. Pharm. Pharm. Sci. 2013, 5, 38–46. [Google Scholar]
- Lenzi, A.; Gandini, L.; Maresca, V.; Rago, R.; Sgrò, P.; Dondero, F.; Picardo, M. Fatty acid composition of spermatozoa and immature germ cells. Mol. Hum. Reprod. 2000, 6, 226–231. [Google Scholar] [CrossRef]
- Hou, T.Y.; McMurray, D.N.; Chapkin, R.S. Omega-3 fatty acids, lipid rafts, and T cell signaling. Eur. J. Pharmacol. 2016, 785, 2–9. [Google Scholar] [CrossRef] [PubMed]
- Mazza, M.; Pomponi, M.; Janiri, L.; Bria, P.; Mazza, S. Omega-3 fatty acids and antioxidants in neurological and psychiatric diseases: An overview. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2007, 31, 12–26. [Google Scholar] [CrossRef]
- Chatterjee, S.; Gagnon, C. Production of reactive oxygen species by spermatozoa undergoing cooling, freezing, and thawing. Mol. Reprod. Dev. 2001, 59, 451–458. [Google Scholar] [CrossRef] [PubMed]
- Zalata, A.A.; Christophe, A.B.; Depuydt, C.E.; Schoonjans, F.; Comhaire, F.H. The fatty acid composition of phospholipids of spermatozoa from infertile patients. Mol. Hum. Reprod. 1998, 4, 111–118. [Google Scholar] [CrossRef]
- Zerbinati, C.; Caponecchia, L.; Rago, R.; Leoncini, E.; Bottaccioli, A.G.; Ciacciarelli, M.; Pacelli, A.; Salacone, P.; Sebastianelli, A.; Pastore, A.; et al. Fatty acids profiling reveals potential candidate markers of semen quality. Andrology 2016, 4, 1094–1101. [Google Scholar] [CrossRef]
- Flesch, F.M.; Gadella, B.M. Dynamics of the mammalian sperm plasma membrane in the process of fertilization. Biochim. Biophys. Acta-Rev. Biomembr. 2000, 1469, 197–235. [Google Scholar] [CrossRef]
- Sturmey, R.G.; Reis, A.; Leese, H.J.; McEvoy, T.G. Role of fatty acids in energy provision during oocyte maturation and early embryo development. Reprod. Domest. Anim. 2009, 44, 50–58. [Google Scholar] [CrossRef] [PubMed]
- Mirabi, P.; Chaichi, M.J.; Esmaeilzadeh, S.; Ali Jorsaraei, S.G.; Bijani, A.; Ehsani, M.; Hashemi Karooee, S.F. The role of fatty acids on ICSI outcomes: A prospective cohort study. Lipids Health Dis. 2017, 16, 18. [Google Scholar] [CrossRef] [PubMed]
- Aitken, R.J. Reactive oxygen species as mediators of sperm capacitation and pathological damage. Mol. Reprod. Dev. 2017, 84, 1039–1052. [Google Scholar] [CrossRef]
- Breitbart, H.; Rotman, T.; Rubinstein, S.; Etkovitz, N. Role and regulation of PI3K in sperm capacitation and the acrosome reaction. Mol. Cell. Endocrinol. 2010, 314, 234–238. [Google Scholar] [CrossRef] [PubMed]
- Couplan, E.; Le Cann, M.; Le Foll, C.; Corporeau, C.; Blondell, M.; Delarue, J. Polyunsaturated fatty acids inhibit PI3K activity in a yeast-based model system. Biotechnol. J. 2009, 4, 1190–1197. [Google Scholar] [CrossRef]
- Conquer, J.A.; Martin, J.B.; Tummon, I.; Watson, L.; Tekpetey, F. Fatty acid analysis of blood serum, seminal plasma, and spermatozoa of normozoospermic vs. asthenozoospermic males. Lipids 1999, 34, 793–799. [Google Scholar] [CrossRef]
- Tavilani, H.; Doosti, M.; Abdi, K.; Vaisiraygani, A.; Joshaghani, H.R. Decreased polyunsaturated and increased saturated fatty acid concentration in spermatozoa from asthenozoospermic males as compared with normozoospermic males. Andrologia 2006, 38, 173–178. [Google Scholar] [CrossRef]
- Aksoy, Y.; Aksoy, H.; Altinkaynak, K.; Aydin, H.R.; Özkan, A. Sperm fatty acid composition in subfertile men. Prostaglandins Leukot. Essent. Fat. Acids 2006, 75, 75–79. [Google Scholar] [CrossRef]
- Martínez-Soto, J.C.; Landeras, J.; Gadea, J. Spermatozoa and seminal plasma fatty acids as predictors of cryopreservation success. Andrology 2013, 1, 365–375. [Google Scholar] [CrossRef]
- Tang, L.X.; Yuan, D.J.; Wang, Q.L.; Jiang, F.; Guo, J.; Tang, Y.G.; Zheng, L.X.; Kang, J.X. Association of decreased spermatozoa omega-3 fatty acid levels and increased oxidative DNA damage with varicocele in infertile men: A case control study. Reprod. Fertil. Dev. 2016, 28, 648–654. [Google Scholar] [CrossRef]
- Khosrowbeygi, A.; Zarghami, N. Fatty acid composition of human spermatozoa and seminal plasma levels of oxidative stress biomarkers in subfertile males. Prostaglandins Leukot. Essent. Fat. Acids 2007, 77, 117–121. [Google Scholar] [CrossRef]
- Safarinejad, M.R. Effect of omega-3 polyunsaturated fatty acid supplementation on semen profile and enzymatic anti-oxidant capacity of seminal plasma in infertile men with idiopathic oligoasthenoteratospermia: A double-blind, placebo-controlled, randomised study. Andrologia 2011, 43, 38–47. [Google Scholar] [CrossRef] [PubMed]
- Gulaya, N.M.; Margitich, V.M.; Govseeva, N.M.; Klimashevsky, V.M.; Gorpynchenko, I.I.; Boyko, M.I. Phospholipid composition of human sperm and seminal plasma in relation to sperm fertility. Arch. Androl. 2001, 46, 169–175. [Google Scholar] [CrossRef]
- Rato, L.; Alves, M.G.; Cavaco, J.E.; Oliveira, P.F. High-energy diets: A threat for male fertility? Obes. Rev. 2014, 15, 996–1007. [Google Scholar] [CrossRef] [PubMed]
- Sæther, T.; Tran, T.N.; Rootwelt, H.; Grav, H.J.; Christophersen, B.O.; Haugen, T.B. Essential fatty acid deficiency induces fatty acid desaturase expression in rat epididymis, but not in testis. Reproduction 2007, 133, 467–477. [Google Scholar] [CrossRef]
- Conquer, J.A.; Martin, J.B.; Tummon, I.; Watson, L.; Tekpetey, F. Effect of DHA supplementation on DHA status and sperm motility in asthenozoospermic males. Lipids 2000, 35, 149–154. [Google Scholar] [CrossRef] [PubMed]
- González-Ravina, C.; Aguirre-Lipperheide, M.; Pinto, F.; Martín-Lozano, D.; Fernández-Sánchez, M.; Blasco, V.; Santamaría-López, E.; Candenas, L. Effect of dietary supplementation with a highly pure and concentrated docosahexaenoic acid (DHA) supplement on human sperm function. Reprod. Biol. 2018, 18, 282–288. [Google Scholar] [CrossRef]
- Ferramosca, A.; Moscatelli, N.; Di Giacomo, M.; Zara, V. Dietary fatty acids influence sperm quality and function. Andrology 2017, 5, 423–430. [Google Scholar] [CrossRef]
- Eslamian, G.; Amirjannati, N.; Rashidkhani, B.; Sadeghi, M.R.; Baghestani, A.R.; Hekmatdoost, A. Dietary fatty acid intakes and asthenozoospermia: A case-control study. Fertil. Steril. 2015, 103, 190–198. [Google Scholar] [CrossRef] [PubMed]
- Ayala, A.; Muñoz, M.F.; Argüelles, S. Lipid peroxidation: Production, metabolism, and signaling mechanisms of malondialdehyde and 4-hydroxy-2-nonenal. Oxid. Med. Cell. Longev. 2014, 2014. [Google Scholar] [CrossRef]
- Collodel, G.; Moretti, E.; Micheli, L.; Menchiari, A.; Moltoni, L.; Cerretani, D. Semen characteristics and malondialdehyde levels in men with different reproductive problems. Andrology 2015, 3, 280–286. [Google Scholar] [CrossRef]
- Roberts, L.J.; Morrow, J.D. Products of the isoprostane pathway: Unique bioactive compounds and markers of lipid peroxidation. Cell. Mol. Life Sci. 2002, 59, 808–820. [Google Scholar] [CrossRef] [PubMed]
- Aksu, D.S.; Saglam, Y.S.; Yildirim, S.; Aksu, T. Effect of pomegranate (Punica granatum L.) juice on kidney, liver, heart and testis histopathological changes, and the tissues lipid peroxidation and antioxidant status in lead acetate-treated rats. Cell. Mol. Biol. 2017, 63, 33–43. [Google Scholar] [CrossRef]
- Jannatifar, R.; Parivar, K.; Roodbari, N.H.; Nasr-Esfahani, M.H. Effects of N-acetyl-cysteine supplementation on sperm quality, chromatin integrity and level of oxidative stress in infertile men. Reprod. Biol. Endocrinol. 2019, 17, 24. [Google Scholar] [CrossRef]
- Martínez-Soto, J.C.; Domingo, J.C.; Cordobilla, B.; Nicolás, M.; Fernández, L.; Albero, P.; Gadea, J.; Landeras, J. Dietary supplementation with docosahexaenoic acid (DHA) improves seminal antioxidant status and decreases sperm DNA fragmentation. Syst. Biol. Reprod. Med. 2016, 62, 387–395. [Google Scholar] [CrossRef] [PubMed]
- Retterstøl, K.; Tran, T.N.; Haugen, T.B.; Christophersen, B.O. Metabolism of very long chain polyunsaturated fatty acids in isolated rat germ cells. Lipids 2001, 36, 601–606. [Google Scholar] [CrossRef]
- Collodel, G.; Moretti, E.; Noto, D.; Iacoponi, F.; Signorini, C. Fatty Acid Profile and Metabolism Are Related to Human Sperm Parameters and Are Relevant in Idiopathic Infertility and Varicocele. Mediat. Inflamm. 2020, 2020, 3640450. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.X.; Wu, Y.; Chen, H.G.; Duan, P.; Wang, L.; Shen, H.Q.; Lu, W.Q.; Sun, B.; Wang, Q.; Zhang, B.; et al. Seminal plasma metabolome in relation to semen quality and urinary phthalate metabolites among Chinese adult men. Environ. Int. 2019, 129, 354–363. [Google Scholar] [CrossRef] [PubMed]
- Gilany, K.; Moazeni-Pourasil, R.S.; Jafarzadeh, N.; Savadi-Shiraz, E. Metabolomics fingerprinting of the human seminal plasma of asthenozoospermic patients. Mol. Reprod. Dev. 2014, 81, 84–86. [Google Scholar] [CrossRef]
- Mehrparvar, B.; Chashmniam, S.; Nobakht, F.; Amini, M.; Javidi, A.; Minai-Tehrani, A.; Arjmand, B.; Gilany, K. Metabolic profiling of seminal plasma from teratozoospermia patients. J. Pharm. Biomed. Anal. 2020, 178, 112903. [Google Scholar] [CrossRef] [PubMed]
- Qiao, S.; Wu, W.; Chen, M.; Tang, Q.; Xia, Y.; Jia, W.; Wang, X. Seminal plasma metabolomics approach for the diagnosis of unexplained male infertility. PLoS ONE 2017, 12, e0181115. [Google Scholar] [CrossRef]
- Zhang, Y.; Jiang, L.; Hu, W.; Zheng, Q.; Xiang, W. Mitochondrial dysfunction during in vitro hepatocyte steatosis is reversed by omega-3 fatty acid-induced up-regulation of mitofusin 2. Metabolism. 2011, 60, 767–775. [Google Scholar] [CrossRef] [PubMed]
- Cooray, A.; Kim, J.H.; Chae, M.R.; Lee, S.; Lee, K.P. Perspectives on Potential Fatty Acid Modulations of Motility Associated Human Sperm Ion Channels. Int. J. Mol. Sci. 2022, 23, 3718. [Google Scholar] [CrossRef]
- Elgar, K. Curcumin: A Review of Clinical Use and Efficacy. Nutr. Med. J. 2022, 1, 10–31. [Google Scholar]
- Afeiche, M.C.; Gaskins, A.J.; Williams, P.L.; Toth, T.L.; Wright, D.L.; Tanrikut, C.; Hauser, R.; Chavarro, J.E. Processed meat intake is unfavorably and fish intake favorably associated with semen quality indicators among men attending a fertility clinic. J. Nutr. 2014, 144, 1091–1098. [Google Scholar] [CrossRef] [PubMed]
- Gaskins, A.J.; Sundaram, R.; Buck Louis, G.M.; Chavarro, J.E. Seafood intake, sexual activity, and time to pregnancy. J. Clin. Endocrinol. Metab. 2018, 103, 2680–2688. [Google Scholar] [CrossRef]
- Jensen, T.K.; Priskorn, L.; Holmboe, S.A.; Nassan, F.L.; Andersson, A.M.; Dalgård, C.; Petersen, J.H.; Chavarro, J.E.; Jørgensen, N. Associations of Fish Oil Supplement Use with Testicular Function in Young Men. JAMA Netw. Open 2020, 3, e1919462. [Google Scholar] [CrossRef]
- Attaman, J.A.; Toth, T.L.; Furtado, J.; Campos, H.; Hauser, R.; Chavarro, J.E. Dietary fat and semen quality among men attending a fertility clinic. Hum. Reprod. 2012, 27, 1466–1474. [Google Scholar] [CrossRef] [PubMed]
- Falsig, A.M.L.; Gleerup, C.S.; Knudsen, U.B. The influence of omega-3 fatty acids on semen quality markers: A systematic PRISMA review. Andrology 2019, 7, 794–803. [Google Scholar] [CrossRef]
- Wathne, A.M.; Devle, H.; Naess-Andresen, C.F.; Ekeberg, D. Identification and Quantification of Fatty Acids in T. viridissima, C. biguttulus, and C. brunneus by GC-MS. J. Lipids 2018, 2018, 3679247. [Google Scholar] [CrossRef]
- Bosviel, R.; Joumard-Cubizolles, L.; Chinetti-Gbaguidi, G.; Bayle, D.; Copin, C.; Hennuyer, N.; Duplan, I.; Staels, B.; Zanoni, G.; Porta, A.; et al. DHA-derived oxylipins, neuroprostanes and protectins, differentially and dose-dependently modulate the inflammatory response in human macrophages: Putative mechanisms through PPAR activation. Free Radic. Biol. Med. 2017, 103, 146–154. [Google Scholar] [CrossRef] [PubMed]
- Rapino, C.; Battista, N.; Bari, M.; Maccarrone, M. Endocannabinoids as biomarkers of human reproduction. Hum. Reprod. Update 2014, 20, 501–516. [Google Scholar] [CrossRef]
- Burke, J.E.; Dennis, E.A. Phospholipase A 2 structure/function, mechanism, and signaling. J. Lipid Res. 2009, 50, S237–S242. [Google Scholar] [CrossRef] [PubMed]
- Templeton, A.A.; Cooper, I.; Kelly, R.W. Prostaglandin concentrations in the semen of fertile men. J. Reprod. Fertil. 1978, 52, 147–150. [Google Scholar] [CrossRef]
- Samuelsson, B. Isolation and identification of prostaglandins from human seminal. J. Biol. Chem. 1963, 238, 3229–3234. [Google Scholar] [CrossRef] [PubMed]
- Bygdeman, M.; Fredricsson, B.; Svanborg, K.; Samuelsson, B. The relation between fertility and prostaglandin content of seminal fluid in man. Fertil. Steril. 1970, 21, 622–629. [Google Scholar] [CrossRef]
- Collier, J.G.; Flower, R.J.; Stanton, S.L. Seminal prostaglandins in infertile men. Fertil. Steril. 1975, 26, 868–871. [Google Scholar] [CrossRef]
- Jonsson, H.T.; Middleditch, B.S.; Desiderio, D.M. Prostaglandins in human seminal fluid: Two novel compounds. Science 1975, 187, 1093–1094. [Google Scholar] [CrossRef]
- Cosentino, M.J.; Emilson, L.B.V.; Cockett, A.T.K. Prostaglandins in semen and their relationship to male fertility: A study of 145 men. Fertil. Steril. 1984, 41, 88–94. [Google Scholar] [CrossRef]
- Bendvold, E.; Svanborg, K.; Eneroth, P.; Gottlieb, C.; Bygdeman, M. The natural variations in prostaglandin concentration in human seminal fluid and its relation to sperm quality. Fertil. Steril. 1984, 41, 743–747. [Google Scholar] [CrossRef]
- Kelly, R.W.; Cooper, I.; Templeton, A.A. Reduced prostaglandin levels in the semen of men with very high sperm concentrations. J. Reprod. Fertil. 1979, 56, 195–199. [Google Scholar] [CrossRef] [PubMed]
- Isidori, A.; Conte, D.; Laguzzi, G.; Giovenco, P.; Dondero, F. Role of seminal prostaglandins in male fertility. I. Relationship of prostaglandin E and 19-OH prostaglandin E with seminal parameters. J. Endocrinol. Investig. Off. J. Ital. Soc. Endocrinol. 1980, 3, 1–4. [Google Scholar] [CrossRef]
- Rios, M.; Carreño, D.V.; Oses, C.; Barrera, N.; Kerr, B.; Villalón, M. Low physiological levels of prostaglandins E2 and F2α improve human sperm functions. Reprod. Fertil. Dev. 2016, 28, 434–439. [Google Scholar] [CrossRef] [PubMed]
- Schaefer, M.; Hofmann, T.; Schultz, G.; Gudermann, T. A new prostaglandin E receptor mediates calcium influx and acrosome reaction in human spermatozoa. Proc. Natl. Acad. Sci. USA 1998, 95, 3008–3013. [Google Scholar] [CrossRef] [PubMed]
- Joseph, T.; Zalenskaya, I.A.; Sawyer, L.C.; Chandra, N.; Doncel, G.F. Seminal plasma induces prostaglandin-endoperoxide synthase (PTGS) 2 expression in immortalized human vaginal cells: Involvement of semen prostaglandin E2 in PTGS2 upregulation. Biol. Reprod. 2013, 88, 1–10. [Google Scholar] [CrossRef]
- Iliev, I.D.; Spadoni, I.; Mileti, E.; Matteoli, G.; Sonzogni, A.; Sampietro, G.M.; Foschi, D.; Caprioli, F.; Viale, G.; Rescigno, M. Human intestinal epithelial cells promote the differentiation of tolerogenic dendritic cells. Gut 2009, 58, 1481–1489. [Google Scholar] [CrossRef]
- Aluvihare, V.R.; Kallikourdis, M.; Betz, A.G. Regulatory T cells mediate maternal tolerance to the fetus. Nat. Immunol. 2004, 5, 266–271. [Google Scholar] [CrossRef]
- Holtel, M.; Chosed, R.J.; Zimmerman, S.; Wynia, B.; Roudebush, W.E. Relationship between Human Seminal Prostaglandins on Intrauterine Insemination Pregnancy Outcomes. Gynecol. Women’s Health Res. 2021, 3, e367. [Google Scholar] [CrossRef]
- Saad, M.H.; Burka, J.F. Isolation of leukotriene C4 from human seminal fluid. Prostaglandins 1983, 26, 943–954. [Google Scholar] [CrossRef]
- Reddy, G.P.; Prasad, M.; Sailesh, S.; Kumar, Y.V.K.; Reddanna, P. The production of arachidonic acid metabolites in rat testis. Prostaglandins 1992, 44, 497–507. [Google Scholar] [CrossRef]
- Mai, J.; Goswami, S.K.; Bruckner, G.; Kinsella, J.E. Determination of prostaglandins and thromboxane as their pentafluorobenzyl-trimethylsilyl derivatives by electron-capture gas chromatography. J. Chromatogr. B Biomed. Sci. Appl. 1982, 230, 15–28. [Google Scholar] [CrossRef] [PubMed]
- Cascio, M.G. PUFA-derived endocannabinoids: An overview. In Proceedings of the Nutrition Society; Cambridge University Press: Cambridge, UK, 2013; Volume 72, pp. 451–459. [Google Scholar]
- Francavilla, F.; Battista, N.; Barbonetti, A.; Vassallo, M.R.C.; Rapino, C.; Antonangelo, C.; Pasquariello, N.; Catanzaro, G.; Barboni, B.; Maccarrone, M. Characterization of the endocannabinoid system in human spermatozoa and involvement of transient receptor potential vanilloid 1 receptor in their fertilizing ability. Endocrinology 2009, 150, 4692–4700. [Google Scholar] [CrossRef] [PubMed]
- Rossato, M. Endocannabinoids, sperm functions and energy metabolism. Mol. Cell. Endocrinol. 2008, 286 (Suppl. S1), S31–S35. [Google Scholar] [CrossRef]
- Barbonetti, A.; Vassallo, M.R.C.; Fortunato, D.; Francavilla, S.; Maccarrone, M.; Francavilla, F. Energetic metabolism and human sperm motility: Impact of CB1 receptor activation. Endocrinology 2010, 151, 5882–5892. [Google Scholar] [CrossRef]
- Lewis, S.E.M.; Rapino, C.; Di Tommaso, M.; Pucci, M.; Battista, N.; Paro, R.; Simon, L.; Lutton, D.; Maccarrone, M. Differences in the Endocannabinoid System of Sperm from Fertile and Infertile Men. PLoS ONE 2012, 7, e47704. [Google Scholar] [CrossRef]
- Amoako, A.A.; Marczylo, T.H.; Marczylo, E.L.; Elson, J.; Willets, J.M.; Taylor, A.H.; Konje, J.C. Anandamide modulates human sperm motility: Implications for men with asthenozoospermia and oligoasthenoteratozoospermia. Hum. Reprod. 2013, 28, 2058–2066. [Google Scholar] [CrossRef] [PubMed]
- Vigor, C.; Bertrand-Michel, J.; Pinot, E.; Oger, C.; Vercauteren, J.; Le Faouder, P.; Galano, J.M.; Lee, J.C.Y.; Durand, T. Non-enzymatic lipid oxidation products in biological systems: ASSESSMENT of the metabolites from polyunsaturated fatty acids. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2014, 964, 65–78. [Google Scholar] [CrossRef]
- Morrow, J.D.; Harris, T.M.; Jackson Roberts, L. Noncyclooxygenase oxidative formation of a series of novel prostaglandins: Analytical ramifications for measurement of eicosanoids. Anal. Biochem. 1990, 184, 1–10. [Google Scholar] [CrossRef]
- Morrow, J.D.; Awad, J.A.; Boss, H.J.; Blair, I.A.; Roberts, L.J. Non-cyclooxygenase-derived prostanoids (F2-isoprostanes) are formed in situ on phospholipids. Proc. Natl. Acad. Sci. USA 1992, 89, 10721–10725. [Google Scholar] [CrossRef]
- Montuschi, P.; Barnes, P.J.; Roberts, L.J. Isoprostanes: Markers and mediators of oxidative stress. FASEB J. 2004, 18, 1791–1800. [Google Scholar] [CrossRef]
- Milne, G.L.; Dai, Q.; Roberts, L.J. The isoprostanes-25 years later. Biochim. Biophys. Acta-Mol. Cell Biol. Lipids 2015, 1851, 433–445. [Google Scholar] [CrossRef] [PubMed]
- Collodel, G.; Castellini, C.; Iacoponi, F.; Noto, D.; Signorini, C. Cytosolic phospholipase A2 and F2 isoprostanes are involved in semen quality and human infertility—A study on leucocytospermia, varicocele and idiopathic infertility. Andrologia 2020, 52, e13465. [Google Scholar] [CrossRef] [PubMed]
- Collodel, G.; Moretti, E.; Longini, M.; Pascarelli, N.A.; Signorini, C. Increased F2-isoprostane levels in semen and immunolocalization of the 8-iso prostaglandin F2α in spermatozoa from infertile patients with varicocele. Oxid. Med. Cell. Longev. 2018, 2018. [Google Scholar] [CrossRef]
- Moretti, E.; Collodel, G.; Salvatici, M.C.; Belmonte, G.; Signorini, C. New insights into sperm with total globozoospermia: Increased fatty acid oxidation and centrin1 alteration. Syst. Biol. Reprod. Med. 2019, 65, 390–399. [Google Scholar] [CrossRef] [PubMed]
- Khosrowbeygi, A.; Zarghami, N. Levels of oxidative stress biomarkers in seminal plasma and their relationship with seminal parameters. BMC Clin. Pathol. 2007, 7, 6. [Google Scholar] [CrossRef]
- Longini, M.; Moretti, E.; Signorini, C.; Noto, D.; Iacoponi, F.; Collodel, G. Relevance of seminal F2-dihomo-IsoPs, F2-IsoPs and F4-NeuroPs in idiopathic infertility and varicocele. Prostaglandins Other Lipid Mediat. 2020, 149, 106448. [Google Scholar] [CrossRef]
- Moretti, E.; Signorini, C.; Ferretti, F.; Noto, D.; Collodel, G. A Study to Validate the Relevance of Semen F2-Isoprostanes on Human Male Infertility. Int. J. Environ. Res. Public Health 2022, 19, 1642. [Google Scholar] [CrossRef]
- Fukunaga, M.; Makita, N.; Roberts, L.J.; Morrow, J.D.; Takahashi, K.; Badr, K.F. Evidence for the existence of F2-isoprostane receptors on rat vascular smooth muscle cells. Am. J. Physiol.-Cell Physiol. 1993, 264, C1619–C1624. [Google Scholar] [CrossRef]

| Disorder | Description |
|---|---|
| Oligozoospermia | The number of spermatozoa in the semen is below the lower reference limit (<16 mln./mL of ejaculate) |
| Teratozoospermia | >96% of spermatozoa have morphological malformations such as double head, double tail, or abnormal midpiece |
| Asthenozoospermia | The spermatozoa have poor movement and are unable to move effectively (<42% of motile spermatozoa) |
| Azoospermia | There are no spermatozoa present in the semen |
| Mixed: Oligoteratozoospermia, Oligoasthenozoospermia, Asthenoteratozoospermia, Oligoasthenoteratozoospermia | Two or three disorders occurring simultaneously |
| Study | Examined Group | Observed Changes in Omega-3, Omega-6, and Total PUFA Levels in Comparison to Normozoospermic Men | |
|---|---|---|---|
| ↑ | ↓ | ||
| Zalata et al. [20] | Asthenozoospermic | Total omega-6 PUFAs, omega-6/omega-3 | Total PUFAs, total omega-3 PUFAs, linoleic acid, DHA |
| Oligozoospermic | Total omega-6 PUFAs, omega-6/omega-3, linoleic acid | Total omega-3 PUFAs, DHA | |
| Conquer et al. [28] | Asthenozoospermic | – | Total PUFAs, total omega-3 PUFAs, DHA |
| Tavilani et al. [29] | Asthenozoospermic | – | Linoleic acid, DHA |
| Aksoy et al. [30] | Asthenozoospermic | omega-6/omega-3 | Total PUFAs, DHA |
| Oligozoospermic | – | Total PUFAs, DHA | |
| Oligoasthenozoospermic | – | Total PUFAs, DHA | |
| Martínez-Soto et al. [31] | Asthenozoospermic | – | Total PUFAs, DHA |
| Oligozoospermic | – | Total PUFAs, DHA | |
| Oligoasthenozoospermic | – | Total PUFAs, DHA | |
| Tang et al. [32] | Infertile men without varicocele | Total omega-6 PUFAs | Total omega-3 PUFAs, DHA, EPA |
| Khosrowbeygi et al. [33] | Asthenoteratozoospermic | Linoleic acid, AA, DHA | – |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rodak, K.; Kratz, E.M. PUFAs and Their Derivatives as Emerging Players in Diagnostics and Treatment of Male Fertility Disorders. Pharmaceuticals 2023, 16, 723. https://doi.org/10.3390/ph16050723
Rodak K, Kratz EM. PUFAs and Their Derivatives as Emerging Players in Diagnostics and Treatment of Male Fertility Disorders. Pharmaceuticals. 2023; 16(5):723. https://doi.org/10.3390/ph16050723
Chicago/Turabian StyleRodak, Kamil, and Ewa Maria Kratz. 2023. "PUFAs and Their Derivatives as Emerging Players in Diagnostics and Treatment of Male Fertility Disorders" Pharmaceuticals 16, no. 5: 723. https://doi.org/10.3390/ph16050723
APA StyleRodak, K., & Kratz, E. M. (2023). PUFAs and Their Derivatives as Emerging Players in Diagnostics and Treatment of Male Fertility Disorders. Pharmaceuticals, 16(5), 723. https://doi.org/10.3390/ph16050723