Extra-Gonadal and Non-Canonical Effects of FSH in Males
Abstract
1. Introduction
2. Gonadal Effects of FSH
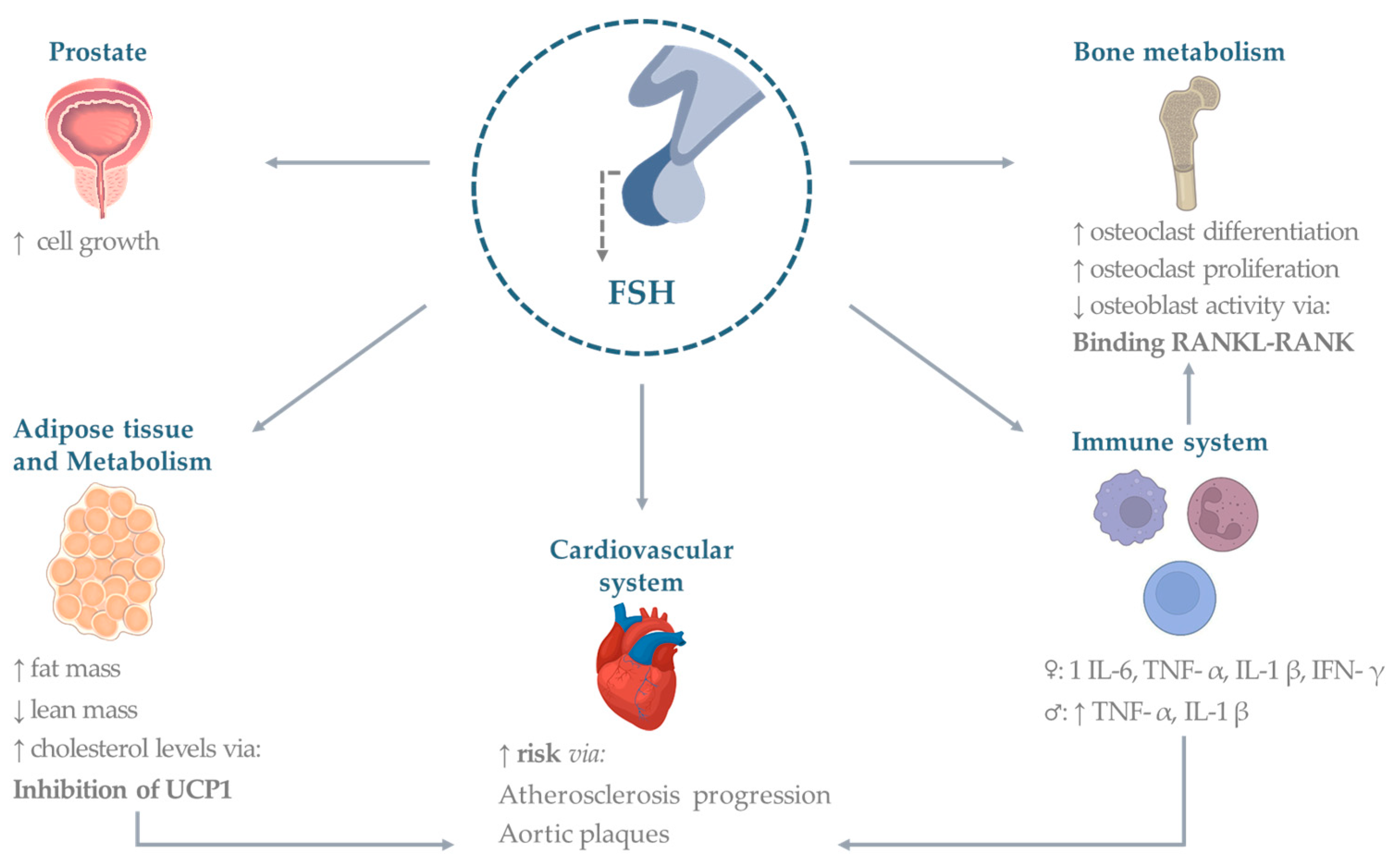
3. Extra-Gonadal Effects of FSH
3.1. Bone
3.2. Cardiovascular System
3.3. Adipose Tissue
3.4. Metabolism
3.5. Immune System
3.6. Prostate and Other Cancers
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Spaziani, M.; Tarantino, C.; Tahani, N.; Gianfrilli, D.; Sbardella, E.; Lenzi, A.; Radicioni, A.F. Hypothalamo-Pituitary axis and puberty. Mol. Cell. Endocrinol. 2021, 520, 111094. [Google Scholar] [CrossRef] [PubMed]
- Bhartiya, D.; Patel, H. An overview of FSH-FSHR biology and explaining the existing conundrums. J. Ovarian Res. 2021, 14, 144. [Google Scholar] [CrossRef]
- Recchia, K.; Jorge, A.S.; Pessoa, L.V.F.; Botigelli, R.C.; Zugaib, V.C.; de Souza, A.F.; Martins, D.D.S.; Ambrosio, C.E.; Bressan, F.F.; Pieri, N.C.G. Actions and Roles of FSH in Germinative Cells. Int. J. Mol. Sci. 2021, 22, 10110. [Google Scholar] [CrossRef]
- Simoni, M.; Gromoll, J.; Nieschlag, E. The follicle-stimulating hormone receptor: Biochemistry, molecular biology, physiology, and pathophysiology. Endocr. Rev. 1997, 18, 739–773. [Google Scholar] [PubMed]
- Bonfil, D.; Chuderland, D.; Kraus, S.; Shahbazian, D.; Friedberg, I.; Seger, R.; Naor, Z. Extracellular signal-regulated kinase, Jun N-terminal kinase, p38, and c-Src are involved in gonadotropin-releasing hormone-stimulated activity of the glycoprotein hormone follicle-stimulating hormone beta-subunit promoter. Endocrinology 2004, 145, 2228–2244. [Google Scholar] [CrossRef]
- Rannikko, A.; Penttila, T.L.; Zhang, F.P.; Toppari, J.; Parvinen, M.; Huhtaniemi, I. Stage-specific expression of the FSH receptor gene in the prepubertal and adult rat seminiferous epithelium. J. Endocrinol. 1996, 151, 29–35. [Google Scholar] [CrossRef]
- Colpi, G.M.; Francavilla, S.; Haidl, G.; Link, K.; Behre, H.M.; Goulis, D.G.; Krausz, C.; Giwercman, A. European Academy of Andrology guideline Management of oligo-astheno-teratozoospermia. Andrology 2018, 6, 513–524. [Google Scholar] [CrossRef] [PubMed]
- Barbonetti, A.; Calogero, A.E.; Balercia, G.; Garolla, A.; Krausz, C.; La Vignera, S.; Lombardo, F.; Jannini, E.A.; Maggi, M.; Lenzi, A.; et al. The use of follicle stimulating hormone (FSH) for the treatment of the infertile man: Position statement from the Italian Society of Andrology and Sexual Medicine (SIAMS). J. Endocrinol. Investig. 2018, 41, 1107–1122. [Google Scholar] [CrossRef]
- Chehab, M.; Madala, A.; Trussell, J.C. On-label and off-label drugs used in the treatment of male infertility. Fertil. Steril. 2015, 103, 595–604. [Google Scholar] [CrossRef]
- Casarini, L.; Crepieux, P.; Reiter, E.; Lazzaretti, C.; Paradiso, E.; Rochira, V.; Brigante, G.; Santi, D.; Simoni, M. FSH for the Treatment of Male Infertility. Int. J. Mol. Sci. 2020, 21, 2270. [Google Scholar] [CrossRef]
- Cannon, J.G.; Kraj, B.; Sloan, G. Follicle-stimulating hormone promotes RANK expression on human monocytes. Cytokine 2011, 53, 141–144. [Google Scholar] [CrossRef]
- Stilley, J.A.; Guan, R.; Duffy, D.M.; Segaloff, D.L. Signaling through FSH receptors on human umbilical vein endothelial cells promotes angiogenesis. J. Clin. Endocrinol. Metab. 2014, 99, E813–E820. [Google Scholar] [CrossRef] [PubMed]
- Oduwole, O.O.; Peltoketo, H.; Huhtaniemi, I.T. Role of Follicle-Stimulating Hormone in Spermatogenesis. Front. Endocrinol. 2018, 9, 763. [Google Scholar] [CrossRef]
- Santi, D.; Crepieux, P.; Reiter, E.; Spaggiari, G.; Brigante, G.; Casarini, L.; Rochira, V.; Simoni, M. Follicle-stimulating Hormone (FSH) Action on Spermatogenesis: A Focus on Physiological and Therapeutic Roles. J. Clin. Med. 2020, 9, 1014. [Google Scholar] [CrossRef] [PubMed]
- Abel, M.H.; Baker, P.J.; Charlton, H.M.; Monteiro, A.; Verhoeven, G.; De Gendt, K.; Guillou, F.; O’Shaughnessy, P.J. Spermatogenesis and sertoli cell activity in mice lacking sertoli cell receptors for follicle-stimulating hormone and androgen. Endocrinology 2008, 149, 3279–3285. [Google Scholar] [CrossRef] [PubMed]
- Tenuta, M.; Carlomagno, F.; Cangiano, B.; Kanakis, G.; Pozza, C.; Sbardella, E.; Isidori, A.M.; Krausz, C.; Gianfrilli, D. Somatotropic-Testicular Axis: A crosstalk between GH/IGF-I and gonadal hormones during development, transition, and adult age. Andrology 2021, 9, 168–184. [Google Scholar] [CrossRef] [PubMed]
- Welsh, M.; Saunders, P.T.; Atanassova, N.; Sharpe, R.M.; Smith, L.B. Androgen action via testicular peritubular myoid cells is essential for male fertility. FASEB J. 2009, 23, 4218–4230. [Google Scholar] [CrossRef] [PubMed]
- Huhtaniemi, I. A hormonal contraceptive for men: How close are we? Prog. Brain Res. 2010, 181, 273–288. [Google Scholar]
- Davies, A.G. Role of FSH in the control of testicular function. Arch. Androl. 1981, 7, 97–108. [Google Scholar] [CrossRef]
- Hasenmajer, V.; Bonaventura, I.; Minnetti, M.; Sada, V.; Sbardella, E.; Isidori, A.M. Non-Canonical Effects of ACTH: Insights Into Adrenal Insufficiency. Front. Endocrinol. 2021, 12, 701263. [Google Scholar] [CrossRef]
- Khosla, S.; Oursler, M.J.; Monroe, D.G. Estrogen and the skeleton. Trends Endocrinol. Metab. 2012, 23, 576–581. [Google Scholar] [CrossRef] [PubMed]
- Khosla, S.; Monroe, D.G. Regulation of Bone Metabolism by Sex Steroids. Cold Spring Harb. Perspect. Med. 2018, 8, a031211. [Google Scholar] [CrossRef] [PubMed]
- Sowers, M.R.; Zheng, H.; McConnell, D.; Nan, B.; Harlow, S.; Randolph, J.F., Jr. Follicle stimulating hormone and its rate of change in defining menopause transition stages. J. Clin. Endocrinol. Metab. 2008, 93, 3958–3964. [Google Scholar] [CrossRef] [PubMed]
- Robinson, L.J.; Tourkova, I.; Wang, Y.; Sharrow, A.C.; Landau, M.S.; Yaroslavskiy, B.B.; Sun, L.; Zaidi, M.; Blair, H.C. FSH-receptor isoforms and FSH-dependent gene transcription in human monocytes and osteoclasts. Biochem. Biophys. Res. Commun. 2010, 394, 12–17. [Google Scholar] [CrossRef]
- Sun, L.; Peng, Y.; Sharrow, A.C.; Iqbal, J.; Zhang, Z.; Papachristou, D.J.; Zaidi, S.; Zhu, L.L.; Yaroslavskiy, B.B.; Zhou, H.; et al. FSH directly regulates bone mass. Cell 2006, 125, 247–260. [Google Scholar] [CrossRef]
- Gera, S.; Sant, D.; Haider, S.; Korkmaz, F.; Kuo, T.C.; Mathew, M.; Perez-Pena, H.; Xie, H.; Chen, H.; Batista, R.; et al. First-in-class humanized FSH blocking antibody targets bone and fat. Proc. Natl. Acad. Sci. USA 2020, 117, 28971–28979. [Google Scholar] [CrossRef]
- Zhu, L.L.; Blair, H.; Cao, J.; Yuen, T.; Latif, R.; Guo, L.; Tourkova, I.L.; Li, J.; Davies, T.F.; Sun, L.; et al. Blocking antibody to the beta-subunit of FSH prevents bone loss by inhibiting bone resorption and stimulating bone synthesis. Proc. Natl. Acad. Sci. USA 2012, 109, 14574–14579. [Google Scholar] [CrossRef]
- Ji, Y.; Liu, P.; Yuen, T.; Haider, S.; He, J.; Romero, R.; Chen, H.; Bloch, M.; Kim, S.M.; Lizneva, D.; et al. Epitope-specific monoclonal antibodies to FSHbeta increase bone mass. Proc. Natl. Acad. Sci. USA 2018, 115, 2192–2197. [Google Scholar] [CrossRef]
- Geng, W.; Yan, X.; Du, H.; Cui, J.; Li, L.; Chen, F. Immunization with FSHbeta fusion protein antigen prevents bone loss in a rat ovariectomy-induced osteoporosis model. Biochem. Biophys. Res. Commun. 2013, 434, 280–286. [Google Scholar] [CrossRef]
- Iqbal, J.; Sun, L.; Kumar, T.R.; Blair, H.C.; Zaidi, M. Follicle-stimulating hormone stimulates TNF production from immune cells to enhance osteoblast and osteoclast formation. Proc. Natl. Acad. Sci. USA 2006, 103, 14925–14930. [Google Scholar] [CrossRef]
- Cannon, J.G.; Cortez-Cooper, M.; Meaders, E.; Stallings, J.; Haddow, S.; Kraj, B.; Sloan, G.; Mulloy, A. Follicle-stimulating hormone, interleukin-1, and bone density in adult women. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2010, 298, R790–R798. [Google Scholar] [CrossRef]
- Ritter, V.; Thuering, B.; Saint Mezard, P.; Luong-Nguyen, N.H.; Seltenmeyer, Y.; Junker, U.; Fournier, B.; Susa, M.; Morvan, F. Follicle-stimulating hormone does not impact male bone mass in vivo or human male osteoclasts in vitro. Calcif. Tissue Int. 2008, 82, 383–391. [Google Scholar] [CrossRef]
- Giovanelli, L.; Quinton, R.; Cangiano, B.; Colombo, S.; Persani, L.; Bonomi, M.; Chiodini, I. FSH and bone: Comparison between males with central versus primary hypogonadism. Front. Endocrinol. 2022, 13, 939897. [Google Scholar] [CrossRef] [PubMed]
- Ferlin, A.; Schipilliti, M.; Vinanzi, C.; Garolla, A.; Di Mambro, A.; Selice, R.; Lenzi, A.; Foresta, C. Bone mass in subjects with Klinefelter syndrome: Role of testosterone levels and androgen receptor gene CAG polymorphism. J. Clin. Endocrinol. Metab. 2011, 96, E739–E745. [Google Scholar] [CrossRef] [PubMed]
- Tahani, N.; Nieddu, L.; Prossomariti, G.; Spaziani, M.; Granato, S.; Carlomagno, F.; Anzuini, A.; Lenzi, A.; Radicioni, A.F.; Romagnoli, E. Long-term effect of testosterone replacement therapy on bone in hypogonadal men with Klinefelter Syndrome. Endocrine 2018, 61, 327–335. [Google Scholar] [CrossRef]
- Karim, N.; MacDonald, D.; Dolan, A.L.; Fogelman, I.; Wierzbicki, A.S.; Hampson, G. The relationship between gonadotrophins, gonadal hormones and bone mass in men. Clin. Endocrinol. 2008, 68, 94–101. [Google Scholar] [CrossRef]
- Sowers, M.; Zheng, H.; Tomey, K.; Karvonen-Gutierrez, C.; Jannausch, M.; Li, X.; Yosef, M.; Symons, J. Changes in body composition in women over six years at midlife: Ovarian and chronological aging. J. Clin. Endocrinol. Metab. 2007, 92, 895–901. [Google Scholar] [CrossRef] [PubMed]
- Sowers, M.R.; Finkelstein, J.S.; Ettinger, B.; Bondarenko, I.; Neer, R.M.; Cauley, J.A.; Sherman, S.; Greendale, G.A. Study of Women’s Health Across the N. The association of endogenous hormone concentrations and bone mineral density measures in pre- and perimenopausal women of four ethnic groups: SWAN. Osteoporos. Int. 2003, 14, 44–52. [Google Scholar] [CrossRef]
- Wang, N.; Li, Q.; Han, B.; Chen, Y.; Zhu, C.; Chen, Y.; Xia, F.; Lu, M.; Meng, Y.; Guo, Y.; et al. Follicle-stimulating hormone is associated with non-alcoholic fatty liver disease in Chinese women over 55 years old. J. Gastroenterol. Hepatol. 2016, 31, 1196–1202. [Google Scholar] [CrossRef]
- Antonio, L.; Priskorn, L.; Olesen, I.A.; Petersen, J.H.; Vanderschueren, D.; Jorgensen, N. High serum FSH is not a risk factor for low bone mineral density in infertile men. Bone 2020, 136, 115366. [Google Scholar] [CrossRef]
- Antonio, L.; Priskorn, L.; Nordkap, L.; Bang, A.K.; Jensen, T.K.; Skakkebaek, N.E.; Petersen, J.H.; Vanderschueren, D.; Jorgensen, N. Bone mineral density is preserved in men with idiopathic infertility. Andrology 2020, 8, 315–322. [Google Scholar] [CrossRef]
- Uihlein, A.V.; Finkelstein, J.S.; Lee, H.; Leder, B.Z. FSH suppression does not affect bone turnover in eugonadal men. J. Clin. Endocrinol. Metab. 2014, 99, 2510–2515. [Google Scholar] [CrossRef]
- Chen, H.; Guo, J.H.; Lu, Y.C.; Ding, G.L.; Yu, M.K.; Tsang, L.L.; Fok, K.L.; Liu, X.M.; Zhang, X.H.; Chung, Y.W.; et al. Impaired CFTR-dependent amplification of FSH-stimulated estrogen production in cystic fibrosis and PCOS. J. Clin. Endocrinol. Metab. 2012, 97, 923–932. [Google Scholar] [CrossRef] [PubMed]
- Robinson, C.A.; Hofer, M.; Benden, C.; Schmid, C. Evaluation of bone disease in patients with cystic fibrosis and end-stage lung disease. J. Bras. Pneumol. 2019, 45, e20170280. [Google Scholar] [CrossRef] [PubMed]
- El Khoudary, S.R.; Wildman, R.P.; Matthews, K.; Thurston, R.C.; Bromberger, J.T.; Sutton-Tyrrell, K. Endogenous sex hormones impact the progression of subclinical atherosclerosis in women during the menopausal transition. Atherosclerosis 2012, 225, 180–186. [Google Scholar] [CrossRef] [PubMed]
- El Khoudary, S.R.; Santoro, N.; Chen, H.Y.; Tepper, P.G.; Brooks, M.M.; Thurston, R.C.; Janssen, I.; Harlow, S.D.; Barinas-Mitchell, E.; Selzer, F.; et al. Trajectories of estradiol and follicle-stimulating hormone over the menopause transition and early markers of atherosclerosis after menopause. Eur. J. Prev. Cardiol. 2016, 23, 694–703. [Google Scholar] [CrossRef]
- Munir, J.A.; Wu, H.; Bauer, K.; Bindeman, J.; Byrd, C.; Feuerstein, I.M.; Villines, T.C.; Taylor, A.J. The perimenopausal atherosclerosis transition: Relationships between calcified and noncalcified coronary, aortic, and carotid atherosclerosis and risk factors and hormone levels. Menopause 2012, 19, 10–15. [Google Scholar] [CrossRef]
- Hopmans, S.N.; Duivenvoorden, W.C.; Werstuck, G.H.; Klotz, L.; Pinthus, J.H. GnRH antagonist associates with less adiposity and reduced characteristics of metabolic syndrome and atherosclerosis compared with orchiectomy and GnRH agonist in a preclinical mouse model. Urol. Oncol. 2014, 32, 1126–1134. [Google Scholar] [CrossRef]
- Han, J.L.; Song, Y.X.; Yao, W.J.; Zhou, J.; Du, Y.; Xu, T. Follicle-Stimulating Hormone Provokes Macrophages to Secrete IL-1beta Contributing to Atherosclerosis Progression. J. Immunol. 2022, 210, 25–32. [Google Scholar] [CrossRef]
- Albertsen, P.C.; Klotz, L.; Tombal, B.; Grady, J.; Olesen, T.K.; Nilsson, J. Cardiovascular morbidity associated with gonadotropin releasing hormone agonists and an antagonist. Eur. Urol. 2014, 65, 565–573. [Google Scholar] [CrossRef]
- Crawford, E.D.; Schally, A.V.; Pinthus, J.H.; Block, N.L.; Rick, F.G.; Garnick, M.B.; Eckel, R.H.; Keane, T.E.; Shore, N.D.; Dahdal, D.N.; et al. The potential role of follicle-stimulating hormone in the cardiovascular, metabolic, skeletal, and cognitive effects associated with androgen deprivation therapy. Urol. Oncol. 2017, 35, 183–191. [Google Scholar] [CrossRef]
- Haring, R.; Teng, Z.; Xanthakis, V.; Coviello, A.; Sullivan, L.; Bhasin, S.; Murabito, J.M.; Wallaschofski, H.; Vasan, R.S. Association of sex steroids, gonadotrophins, and their trajectories with clinical cardiovascular disease and all-cause mortality in elderly men from the Framingham Heart Study. Clin. Endocrinol. 2013, 78, 629–634. [Google Scholar] [CrossRef]
- Kourbanhoussen, K.; Joncas, F.H.; Wallis, C.J.D.; Hovington, H.; Dagenais, F.; Fradet, Y.; Guillemette, C.; Lacombe, L.; Toren, P. Follicle-stimulating hormone (FSH) levels prior to prostatectomy are not related to long-term oncologic or cardiovascular outcomes for men with prostate cancer. Asian J. Androl. 2022, 24, 21–25. [Google Scholar] [PubMed]
- Stefanska, A.; Sypniewska, G.; Ponikowska, I.; Cwiklinska-Jurkowska, M. Association of follicle-stimulating hormone and sex hormone binding globulin with the metabolic syndrome in postmenopausal women. Clin. Biochem. 2012, 45, 703–706. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Wang, E.S.; Xing, L.L.; Shi, S.; Qu, F.; Zhang, D.; Li, J.Y.; Shu, J.; Meng, Y.; Sheng, J.Z.; et al. Follicle-Stimulating Hormone Induces Postmenopausal Dyslipidemia Through Inhibiting Hepatic Cholesterol Metabolism. J. Clin. Endocrinol. Metab. 2016, 101, 254–263. [Google Scholar] [CrossRef] [PubMed]
- Cui, H.; Zhao, G.; Liu, R.; Zheng, M.; Chen, J.; Wen, J. FSH stimulates lipid biosynthesis in chicken adipose tissue by upregulating the expression of its receptor FSHR. J. Lipid Res. 2012, 53, 909–917. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.M.; Chan, H.C.; Ding, G.L.; Cai, J.; Song, Y.; Wang, T.T.; Zhang, D.; Chen, H.; Yu, M.K.; Wu, Y.T.; et al. FSH regulates fat accumulation and redistribution in aging through the Galphai/Ca(2+)/CREB pathway. Aging Cell. 2015, 14, 409–420. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Ji, Y.; Yuen, T.; Rendina-Ruedy, E.; DeMambro, V.E.; Dhawan, S.; Abu-Amer, W.; Izadmehr, S.; Zhou, B.; Shin, A.C.; et al. Blocking FSH induces thermogenic adipose tissue and reduces body fat. Nature 2017, 546, 107–112. [Google Scholar] [CrossRef]
- Han, X.; Guan, Z.; Xu, M.; Zhang, Y.; Yao, H.; Meng, F.; Zhuo, Y.; Yu, G.; Cao, X.; Du, X.; et al. A novel follicle-stimulating hormone vaccine for controlling fat accumulation. Theriogenology 2020, 148, 103–111. [Google Scholar] [CrossRef]
- Seth, B.; Arora, S.; Singh, R. Association of obesity with hormonal imbalance in infertility: A cross-sectional study in north Indian women. Indian. J. Clin. Biochem. 2013, 28, 342–347. [Google Scholar] [CrossRef]
- Bieniek, J.M.; Kashanian, J.A.; Deibert, C.M.; Grober, E.D.; Lo, K.C.; Brannigan, R.E.; Sandlow, J.I.; Jarvi, K.A. Influence of increasing body mass index on semen and reproductive hormonal parameters in a multi-institutional cohort of subfertile men. Fertil. Steril. 2016, 106, 1070–1075. [Google Scholar] [CrossRef]
- Yamacake, K.G.; Cocuzza, M.; Torricelli, F.C.; Tiseo, B.C.; Frati, R.; Freire, G.C.; Antunes, A.A.; Srougi, M. Impact of body mass index, age and varicocele on reproductive hormone profile from elderly men. Int. Braz. J. Urol. 2016, 42, 365–372. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Zhao, M.; Bo, T.; Ma, S.; Yuan, Z.; Chen, W.; He, Z.; Hou, X.; Liu, J.; Zhang, Z.; et al. Blocking FSH inhibits hepatic cholesterol biosynthesis and reduces serum cholesterol. Cell Res. 2019, 29, 151–166. [Google Scholar] [CrossRef] [PubMed]
- Keating, N.L.; O’Malley, A.J.; Freedland, S.J.; Smith, M.R. Does comorbidity influence the risk of myocardial infarction or diabetes during androgen-deprivation therapy for prostate cancer? Eur. Urol. 2013, 64, 159–166. [Google Scholar] [CrossRef] [PubMed]
- Basaria, S.; Muller, D.C.; Carducci, M.A.; Egan, J.; Dobs, A.S. Hyperglycemia and insulin resistance in men with prostate carcinoma who receive androgen-deprivation therapy. Cancer 2006, 106, 581–588. [Google Scholar] [CrossRef]
- Morote, J.; Gomez-Caamano, A.; Alvarez-Ossorio, J.L.; Pesqueira, D.; Tabernero, A.; Gomez Veiga, F.; Lorente, J.A.; Porras, M.; Lobato, J.J.; Ribal, M.J.; et al. The metabolic syndrome and its components in patients with prostate cancer on androgen deprivation therapy. J. Urol. 2015, 193, 1963–1969. [Google Scholar] [CrossRef]
- Ostergren, P.B.; Kistorp, C.; Fode, M.; Bennedbaek, F.N.; Faber, J.; Sonksen, J. Metabolic consequences of gonadotropin-releasing hormone agonists vs orchiectomy: A randomized clinical study. BJU Int. 2019, 123, 602–611. [Google Scholar] [CrossRef]
- Cheung, A.S.; Hoermann, R.; Dupuis, P.; Joon, D.L.; Zajac, J.D.; Grossmann, M. Relationships between insulin resistance and frailty with body composition and testosterone in men undergoing androgen deprivation therapy for prostate cancer. Eur. J. Endocrinol. 2016, 175, 229–237. [Google Scholar] [CrossRef]
- Vandewalle, S.; Taes, Y.; Fiers, T.; Van Helvoirt, M.; Debode, P.; Herregods, N.; Ernst, C.; Van Caenegem, E.; Roggen, I.; Verhelle, F.; et al. Sex steroids in relation to sexual and skeletal maturation in obese male adolescents. J. Clin. Endocrinol. Metab. 2014, 99, 2977–2985. [Google Scholar] [CrossRef]
- Aydin, B.K.; Stenlid, R.; Ciba, I.; Cerenius, S.Y.; Dahlbom, M.; Bergsten, P.; Nergardh, R.; Forslund, A. High levels of FSH before puberty are associated with increased risk of metabolic syndrome during pubertal transition. Pediatr. Obes. 2022, 17, e12906. [Google Scholar] [CrossRef]
- Zhu, Y.; Xu, J.; Zhang, X.; Ke, Y.; Fu, G.; Guo, Q. A low follicle-stimulating hormone level is a protective factor for non-alcoholic fatty liver disease in older men aged over 80. BMC Geriatr. 2021, 21, 544. [Google Scholar] [CrossRef] [PubMed]
- Musabak, U.; Bolu, E.; Ozata, M.; Oktenli, C.; Sengul, A.; Inal, A.; Yesilova, Z.; Kilciler, G.; Ozdemir, I.C.; Kocar, I.H. Gonadotropin treatment restores in vitro interleukin-1beta and tumour necrosis factor-alpha production by stimulated peripheral blood mononuclear cells from patients with idiopathic hypogonadotropic hypogonadism. Clin. Exp. Immunol. 2003, 132, 265–270. [Google Scholar] [CrossRef] [PubMed]
- Yousefi, S.; Karamlou, K.; Vaziri, N.; Carandang, G.; Ocariz, J.; Cesario, T. The effect of gonadotropins on the production of human interferon-gamma by mononuclear cells. J. Interferon Res. 1993, 13, 213–220. [Google Scholar] [CrossRef] [PubMed]
- Komorowski, J.; Stepien, H. FSH and LH induce interleukin-6 (IL-6) release from human peripheral blood monocytes cultures in vitro. A dose-response study. Horm. Metab. Res. 1994, 26, 438–439. [Google Scholar] [CrossRef] [PubMed]
- Syed, V.; Gerard, N.; Kaipia, A.; Bardin, C.W.; Parvinen, M.; Jegou, B. Identification, ontogeny, and regulation of an interleukin-6-like factor in the rat seminiferous tubule. Endocrinology 1993, 132, 293–299. [Google Scholar] [CrossRef]
- Carbone, F.; Procaccini, C.; De Rosa, V.; Alviggi, C.; De Placido, G.; Kramer, D.; Longobardi, S.; Matarese, G. Divergent immunomodulatory effects of recombinant and urinary-derived FSH, LH, and hCG on human CD4+ T cells. J. Reprod. Immunol. 2010, 85, 172–179. [Google Scholar] [CrossRef]
- Biffoni, M.; Marcucci, I.; Ythier, A.; Eshkol, A. Effects of urinary gonadotrophin preparations on human in-vitro immune function. Hum. Reprod. 1998, 13, 2430–2434. [Google Scholar] [CrossRef]
- Cenci, S.; Toraldo, G.; Weitzmann, M.N.; Roggia, C.; Gao, Y.; Qian, W.P.; Sierra, O.; Pacifici, R. Estrogen deficiency induces bone loss by increasing T cell proliferation and lifespan through IFN-gamma-induced class II transactivator. Proc. Natl. Acad. Sci. USA 2003, 100, 10405–10410. [Google Scholar] [CrossRef]
- Stubelius, A.; Andersson, A.; Islander, U.; Carlsten, H. Ovarian hormones in innate inflammation. Immunobiology 2017, 222, 878–883. [Google Scholar] [CrossRef]
- Tyagi, A.M.; Srivastava, K.; Kureel, J.; Kumar, A.; Raghuvanshi, A.; Yadav, D.; Maurya, R.; Goel, A.; Singh, D. Premature T cell senescence in Ovx mice is inhibited by repletion of estrogen and medicarpin: A possible mechanism for alleviating bone loss. Osteoporos. Int. 2012, 23, 1151–1161. [Google Scholar] [CrossRef]
- Atsma, F.; Bartelink, M.L.; Grobbee, D.E.; van der Schouw, Y.T. Postmenopausal status and early menopause as independent risk factors for cardiovascular disease: A meta-analysis. Menopause 2006, 13, 265–279. [Google Scholar] [CrossRef] [PubMed]
- Ilhan, G.; Atmaca, F.F.V.; Altan, E.; Zebitay, A.G.; Sozen, H.; Akyol, H.; Kurek Eken, M. Evaluation of Neutrophil-Lymphocyte Ratio, Platelet-Lymphocyte Ratio and Red Blood Cell Distribution Width-Platelet Ratio for Diagnosis of Premature Ovarian Insufficiency. J. Fam. Reprod. Health 2016, 10, 211–216. [Google Scholar] [PubMed]
- Tani, A.; Yasui, T.; Matsui, S.; Kato, T.; Kunimi, K.; Tsuchiya, N.; Yuzurihara, M.; Kase, Y.; Irahara, M. Different circulating levels of monocyte chemoattractant protein-1 and interleukin-8 during the menopausal transition. Cytokine 2013, 62, 86–90. [Google Scholar] [CrossRef] [PubMed]
- Abildgaard, J.; Tingstedt, J.; Zhao, Y.; Hartling, H.J.; Pedersen, A.T.; Lindegaard, B.; Dam Nielsen, S. Increased systemic inflammation and altered distribution of T-cell subsets in postmenopausal women. PLoS ONE 2020, 15, e0235174. [Google Scholar] [CrossRef]
- Giglio, T.; Imro, M.A.; Filaci, G.; Scudeletti, M.; Puppo, F.; De Cecco, L.; Indiveri, F.; Costantini, S. Immune cell circulating subsets are affected by gonadal function. Life Sci. 1994, 54, 1305–1312. [Google Scholar] [CrossRef]
- Kass, A.S.; Lea, T.E.; Torjesen, P.A.; Gulseth, H.C.; Forre, O.T. The association of luteinizing hormone and follicle-stimulating hormone with cytokines and markers of disease activity in rheumatoid arthritis: A case-control study. Scand. J. Rheumatol. 2010, 39, 109–117. [Google Scholar] [CrossRef]
- Sawalha, A.H.; Harley, J.B.; Scofield, R.H. Autoimmunity and Klinefelter’s syndrome: When men have two X chromosomes. J. Autoimmun. 2009, 33, 31–34. [Google Scholar] [CrossRef]
- Seminog, O.O.; Seminog, A.B.; Yeates, D.; Goldacre, M.J. Associations between Klinefelter’s syndrome and autoimmune diseases: English national record linkage studies. Autoimmunity 2015, 48, 125–128. [Google Scholar] [CrossRef]
- Rovensky, J.; Imrich, R.; Lazurova, I.; Payer, J. Rheumatic diseases and Klinefelter’s syndrome. Ann. NY. Acad. Sci. 2010, 1193, 1–9. [Google Scholar] [CrossRef]
- Karaoglan, M.; Nacarkahya, G. Immunological interpretation of minipuberty: Minipuberty as the driving force of sexual dimorphism in the immune response. Clin. Endocrinol. 2021, 94, 575–582. [Google Scholar] [CrossRef]
- Corrales, J.J.; Almeida, M.; Cordero, M.; Martin-Martin, L.; Mendez, C.; Miralles, J.M.; Orfao, A. Enhanced immunological response by dendritic cells in male hypogonadism. Eur. J. Clin. Investig. 2012, 42, 1205–1212. [Google Scholar] [CrossRef] [PubMed]
- Deiktakis, E.E.; Ieronymaki, E.; Zaren, P.; Hagsund, A.; Wirestrand, E.; Malm, J.; Tsatsanis, C.; Huhtaniemi, I.T.; Giwercman, A.; Giwercman, Y.L. Impact of add-back FSH on human and mouse prostate following gonadotropin ablation by GnRH antagonist treatment. Endocr. Connect. 2022, 11, e210639. [Google Scholar] [CrossRef] [PubMed]
- Dirnhofer, S.; Berger, C.; Hermann, M.; Steiner, G.; Madersbacher, S.; Berger, P. Coexpression of gonadotropic hormones and their corresponding FSH- and LH/CG-receptors in the human prostate. Prostate 1998, 35, 212–220. [Google Scholar] [CrossRef]
- Ben-Josef, E.; Yang, S.Y.; Ji, T.H.; Bidart, J.M.; Garde, S.V.; Chopra, D.P.; Porter, A.T.; Tang, D.G. Hormone-refractory prostate cancer cells express functional follicle-stimulating hormone receptor (FSHR). J. Urol. 1999, 161, 970–976. [Google Scholar] [CrossRef]
- Mariani, S.; Salvatori, L.; Basciani, S.; Arizzi, M.; Franco, G.; Petrangeli, E.; Spera, G.; Gnessi, L. Expression and cellular localization of follicle-stimulating hormone receptor in normal human prostate, benign prostatic hyperplasia and prostate cancer. J. Urol. 2006, 175, 2072–2077; discussion 2077. [Google Scholar] [CrossRef] [PubMed]
- Gartrell, B.A.; Tsao, C.K.; Galsky, M.D. The follicle-stimulating hormone receptor: A novel target in genitourinary malignancies. Urol. Oncol. 2013, 31, 1403–1407. [Google Scholar] [CrossRef] [PubMed]
- Beer, T.M.; Garzotto, M.; Eilers, K.M.; Lemmon, D.; Wersinger, E.M. Targeting FSH in androgen-independent prostate cancer: Abarelix for prostate cancer progressing after orchiectomy. Urology 2004, 63, 342–347. [Google Scholar] [CrossRef]
- Radu, A.; Pichon, C.; Camparo, P.; Antoine, M.; Allory, Y.; Couvelard, A.; Fromont, G.; Hai, M.T.; Ghinea, N. Expression of follicle-stimulating hormone receptor in tumor blood vessels. N. Engl. J. Med. 2010, 363, 1621–1630. [Google Scholar] [CrossRef]
- Chrusciel, M.; Ponikwicka-Tyszko, D.; Wolczynski, S.; Huhtaniemi, I.; Rahman, N.A. Extragonadal FSHR Expression and Function-Is It Real? Front. Endocrinol. 2019, 10, 32. [Google Scholar] [CrossRef]
- Siraj, A.; Desestret, V.; Antoine, M.; Fromont, G.; Huerre, M.; Sanson, M.; Camparo, P.; Pichon, C.; Planeix, F.; Gonin, J.; et al. Expression of follicle-stimulating hormone receptor by the vascular endothelium in tumor metastases. BMC Cancer 2013, 13, 246. [Google Scholar] [CrossRef]
- Alam, H.; Weck, J.; Maizels, E.; Park, Y.; Lee, E.J.; Ashcroft, M.; Hunzicker-Dunn, M. Role of the phosphatidylinositol-3-kinase and extracellular regulated kinase pathways in the induction of hypoxia-inducible factor (HIF)-1 activity and the HIF-1 target vascular endothelial growth factor in ovarian granulosa cells in response to follicle-stimulating hormone. Endocrinology 2009, 150, 915–928. [Google Scholar] [PubMed]
- Castro-Fernandez, C.; Maya-Nunez, G.; Mendez, J.P. Regulation of follicle-stimulating and luteinizing hormone receptor signaling by. Endocrine 2004, 25, 49–54. [Google Scholar] [CrossRef] [PubMed]
- Zeng, H.; Zhao, D.; Yang, S.; Datta, K.; Mukhopadhyay, D. Heterotrimeric G alpha q/G alpha 11 proteins function upstream of vascular endothelial growth factor (VEGF) receptor-2 (KDR) phosphorylation in vascular permeability factor/VEGF signaling. J. Biol. Chem. 2003, 278, 20738–20745. [Google Scholar] [CrossRef] [PubMed]
- Panza, S.; Giordano, F.; De Rose, D.; Panno, M.L.; De Amicis, F.; Santoro, M.; Malivindi, R.; Rago, V.; Aquila, S. FSH-R Human Early Male Genital Tract, Testicular Tumors and Sperm: Its Involvement in Testicular Disorders. Life 2020, 10, 336. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Spaziani, M.; Carlomagno, F.; Tenuta, M.; Sesti, F.; Angelini, F.; Bonaventura, I.; Ferrari, D.; Tarantino, C.; Fiore, M.; Petrella, C.; et al. Extra-Gonadal and Non-Canonical Effects of FSH in Males. Pharmaceuticals 2023, 16, 813. https://doi.org/10.3390/ph16060813
Spaziani M, Carlomagno F, Tenuta M, Sesti F, Angelini F, Bonaventura I, Ferrari D, Tarantino C, Fiore M, Petrella C, et al. Extra-Gonadal and Non-Canonical Effects of FSH in Males. Pharmaceuticals. 2023; 16(6):813. https://doi.org/10.3390/ph16060813
Chicago/Turabian StyleSpaziani, Matteo, Francesco Carlomagno, Marta Tenuta, Franz Sesti, Francesco Angelini, Ilaria Bonaventura, Davide Ferrari, Chiara Tarantino, Marco Fiore, Carla Petrella, and et al. 2023. "Extra-Gonadal and Non-Canonical Effects of FSH in Males" Pharmaceuticals 16, no. 6: 813. https://doi.org/10.3390/ph16060813
APA StyleSpaziani, M., Carlomagno, F., Tenuta, M., Sesti, F., Angelini, F., Bonaventura, I., Ferrari, D., Tarantino, C., Fiore, M., Petrella, C., Tarani, L., Gianfrilli, D., & Pozza, C. (2023). Extra-Gonadal and Non-Canonical Effects of FSH in Males. Pharmaceuticals, 16(6), 813. https://doi.org/10.3390/ph16060813






