Enhanced Skin Permeation and Controlled Release of β-Sitosterol Using Cubosomes Encrusted with Dissolving Microneedles for the Management of Alopecia
Abstract
1. Introduction
2. Results and Discussion
2.1. Inspection of the Prepared β-ST-CUBs
2.2. Particle Size, Polydispersity Index, and Zeta Potential of β-ST-CUBs
2.3. The Entrapment Efficiency Determination of β-ST-CUBs
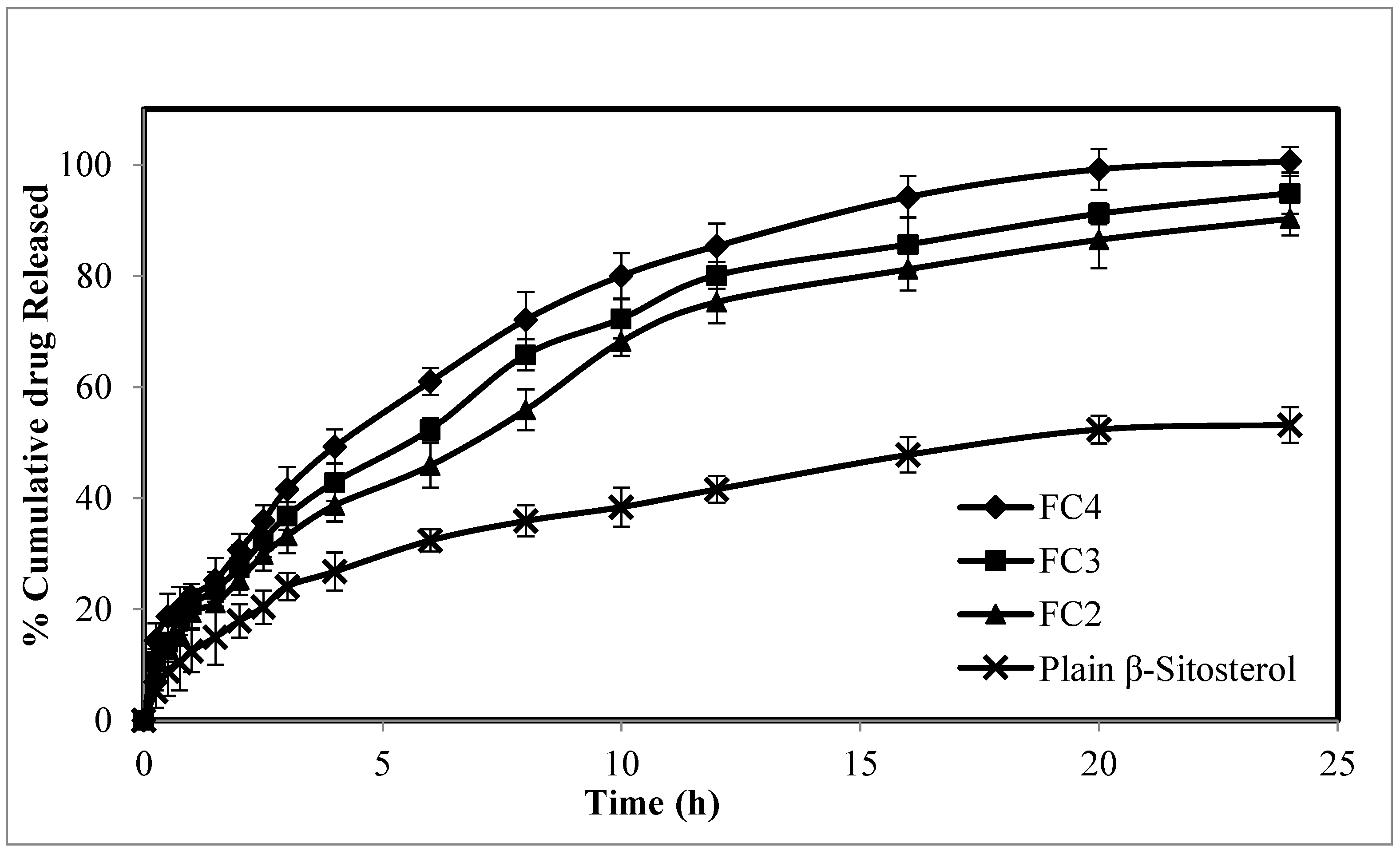
2.4. The In Vitro Release Study of β-ST-CUBs
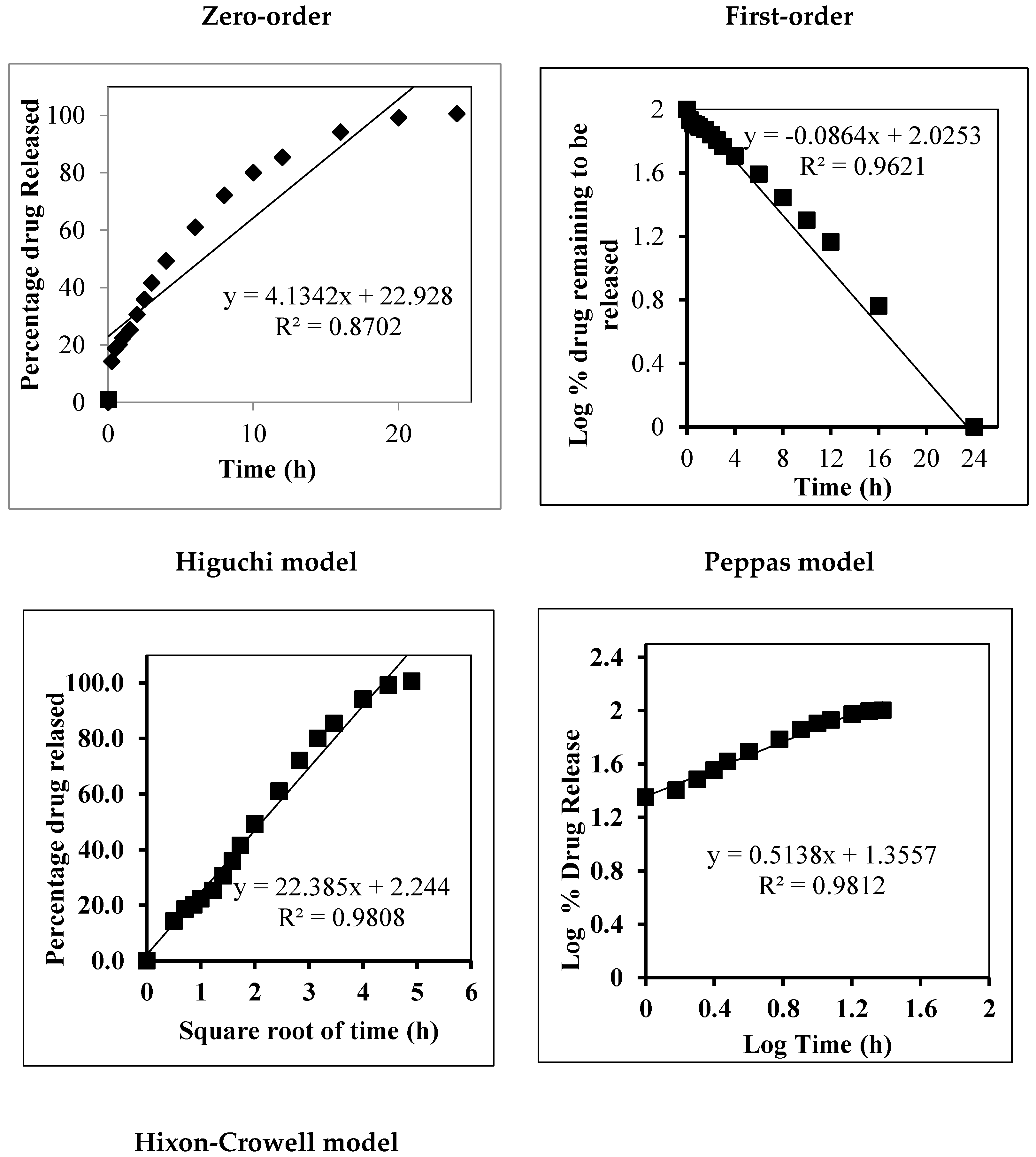
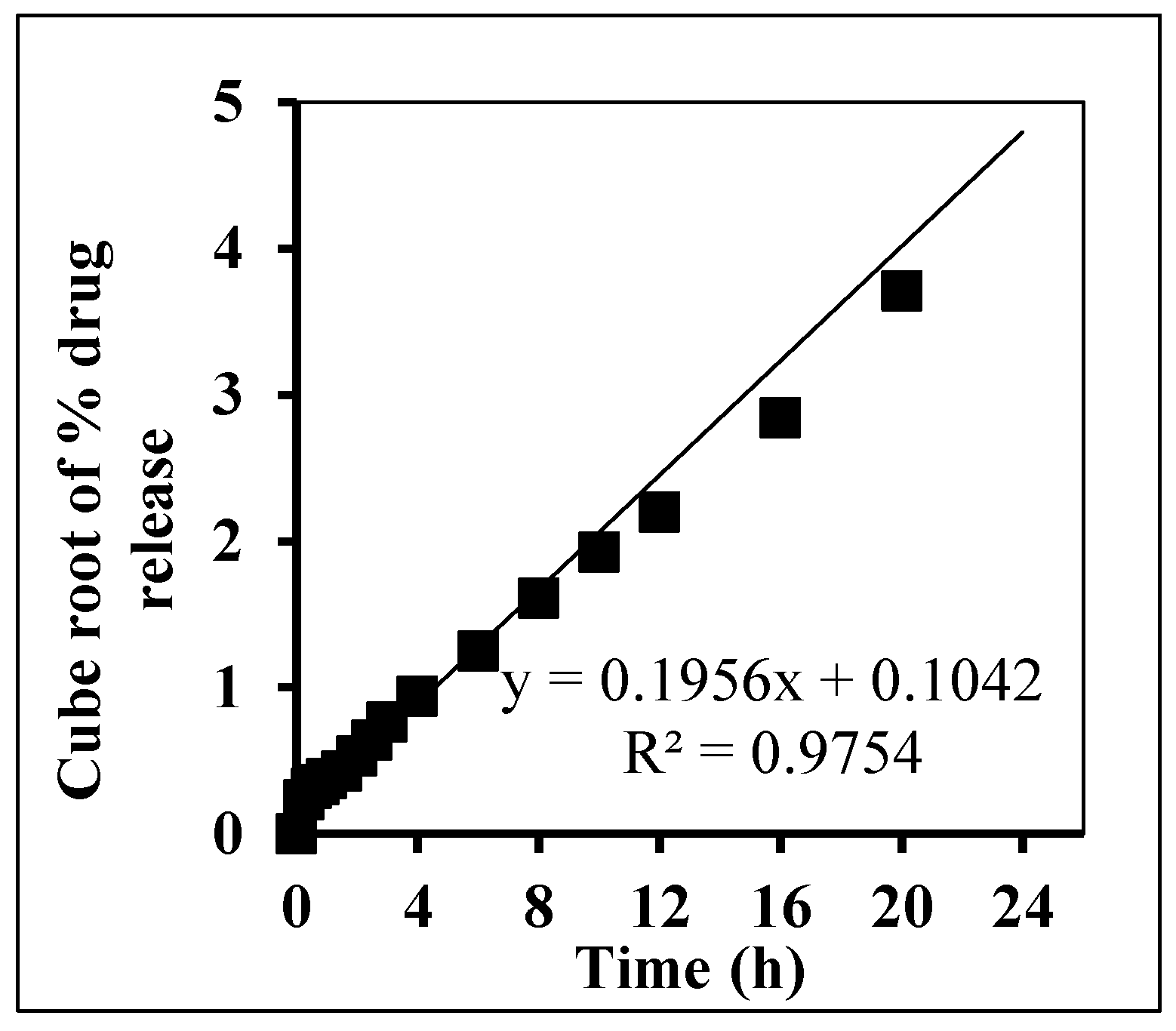
In Vitro Release Kinetic Analysis
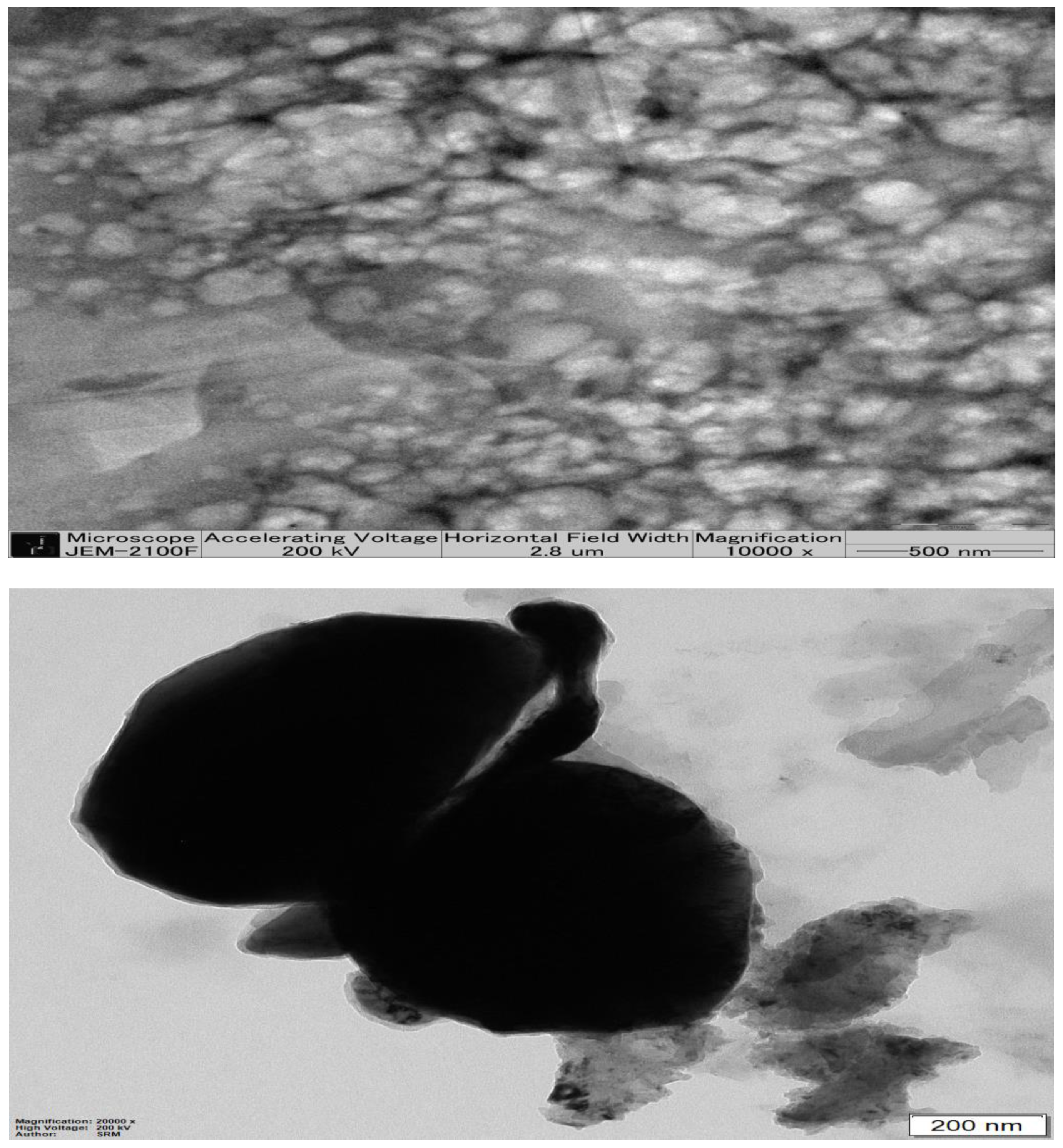
2.5. Transmission Electron Microscopy of Optimized β-ST-CUB
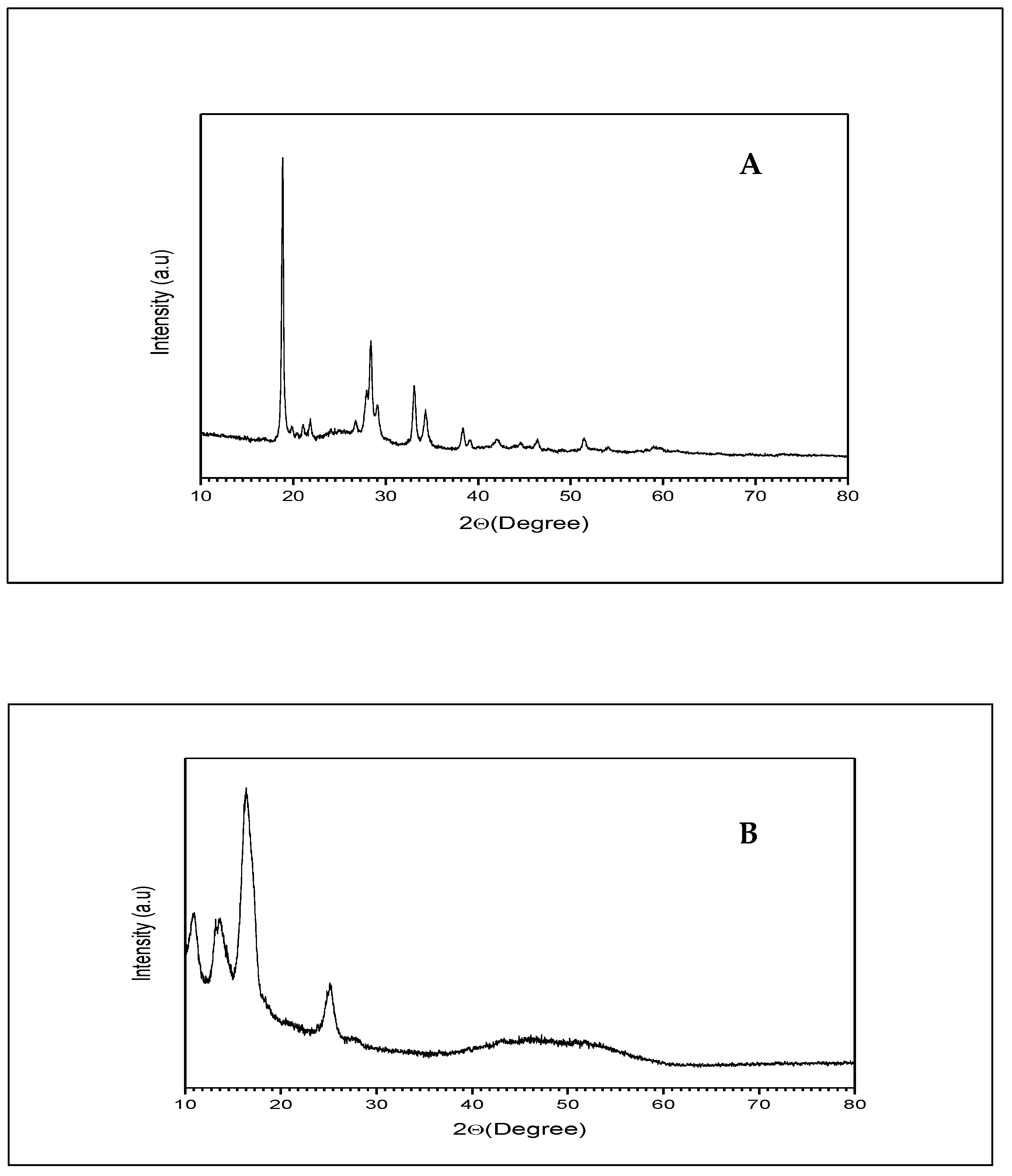
2.6. X-ray Diffraction of β-ST-CUBs
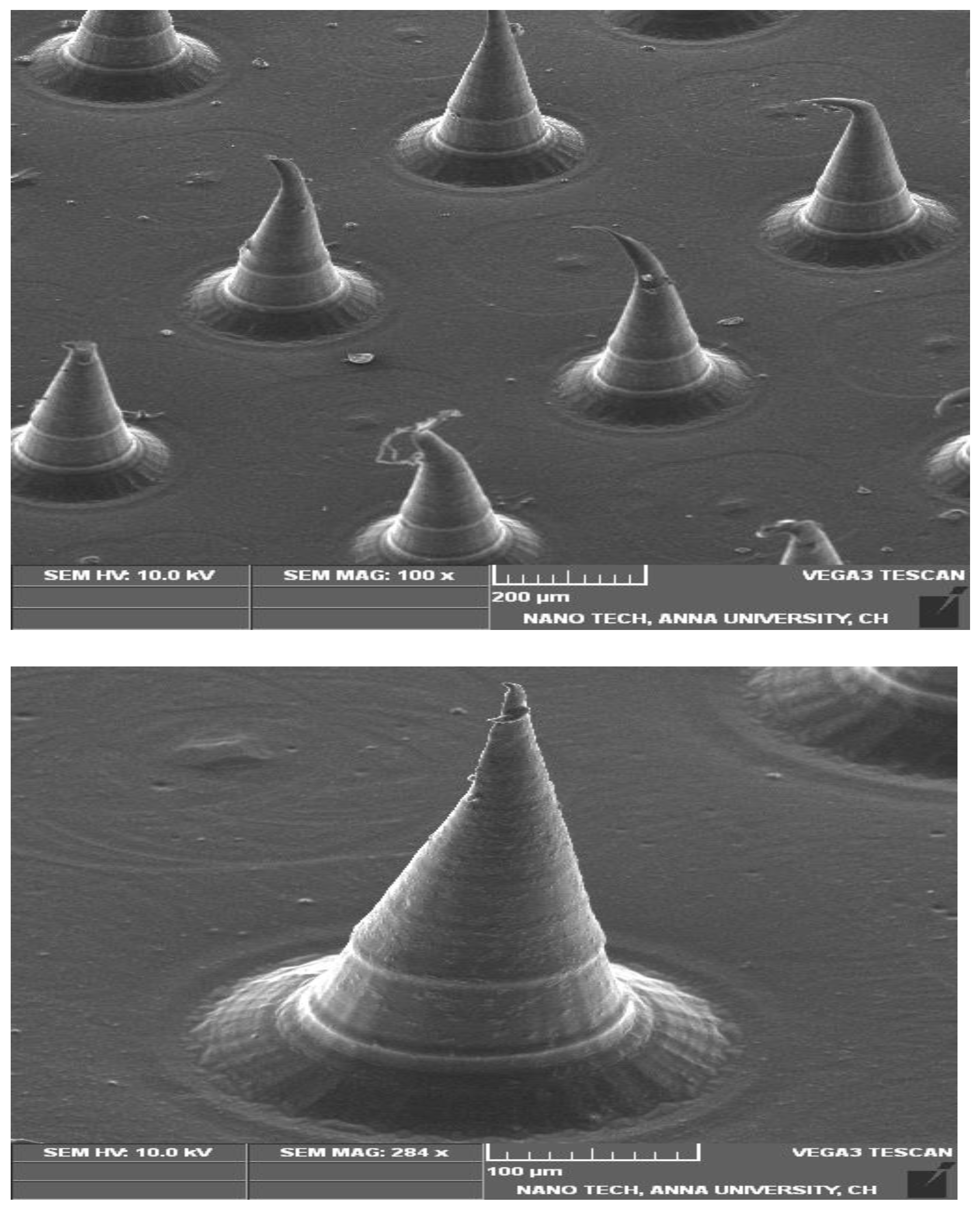
2.7. Characterization of β-ST-Loaded CUBs-MND
2.8. In Vitro Skin Permeation Study of β-ST from CUBs and CUBs-MND
2.9. The Stability Study of CUBs-MND
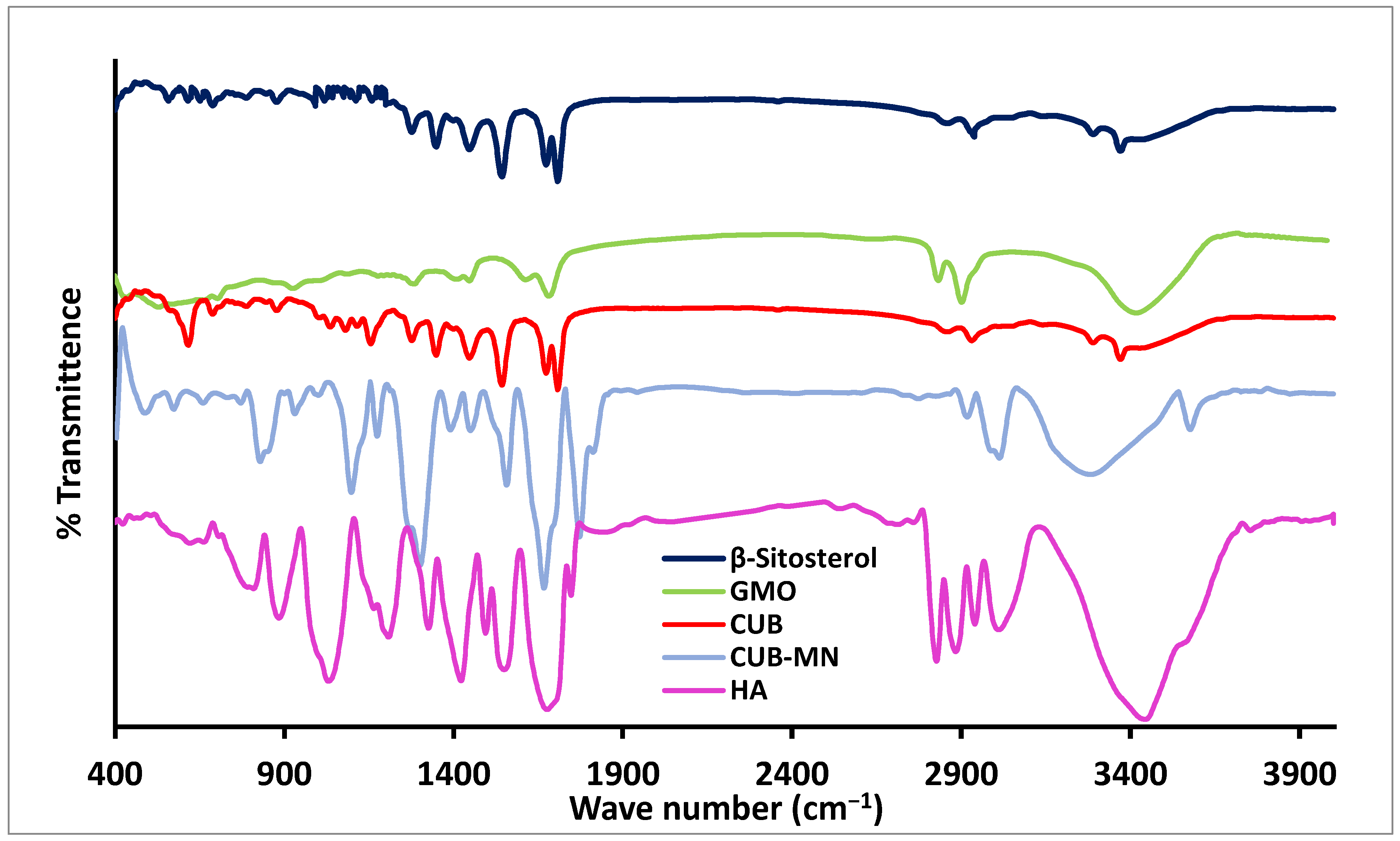
2.10. The IR Spectroscopy
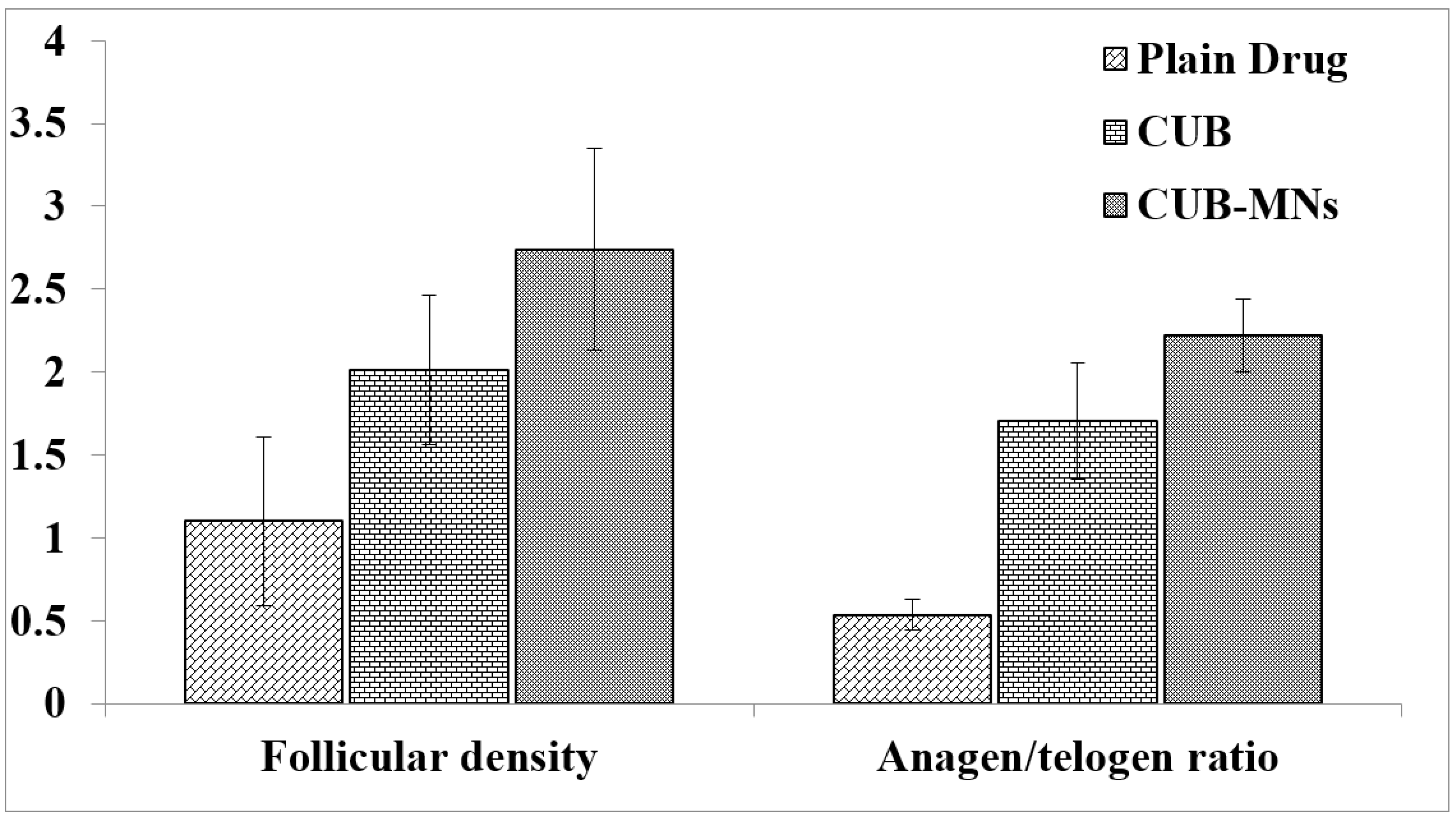
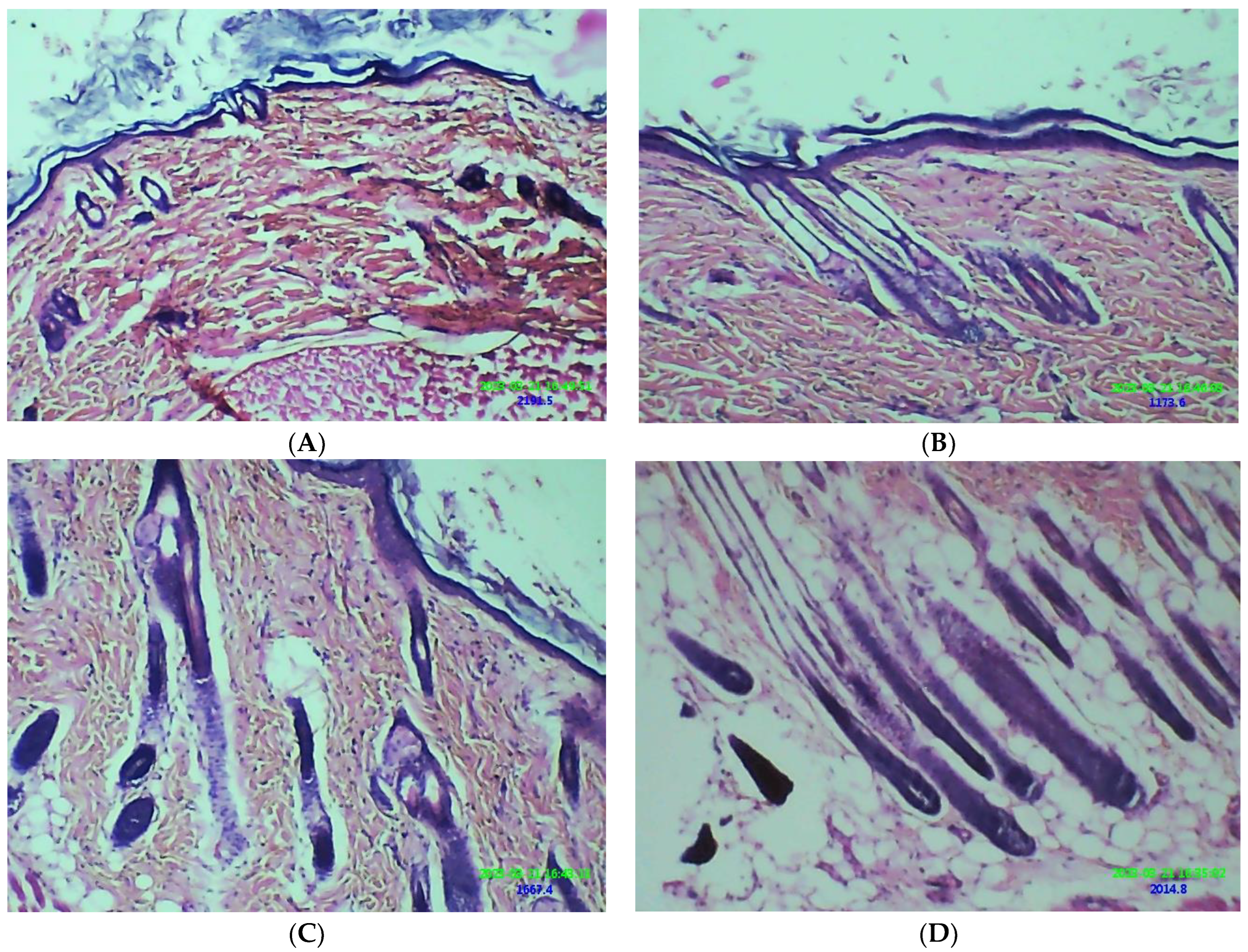
2.11. Hair Growth Effect of β-ST-Loaded CUBs-MND
3. Materials and Methods
3.1. Materials
3.2. Design and Preparation of Beta-Sitosterol Cubosomes (β-ST-CUBs)
3.3. Characterization of CUBs
3.3.1. Determination of the Particle Size, Polydispersity Index (PDI), and Zeta Potential (ZP) of β-ST-CUBs
3.3.2. Determination of the Entrapment Efficiency (EE%) of β-ST-CUBs
3.3.3. In Vitro Release Study of β-ST from the Prepared β-ST-CUBs
3.3.4. Transmission Electron Microscopy (TEM)
3.3.5. X-ray Diffraction Study
3.4. Preparation of β-ST-CUBs Loaded with Dissolving MND
3.4.1. Evaluation of CUB-MND
Morphological Studies
Drug Content
Scanning Electron Microscopy of CUBs-MND (SEM)
In Vitro Skin Permeation Study
Stability Testing of CUB-MND
Fourier-Transform Infrared (FTIR) Study
3.5. Animals
Animal Groups and Treatments
3.6. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sterkens, A.; Lambert, J.; Bervoets, A. Alopecia Areata: A Review on Diagnosis, Immunological Etiopathogenesis and Treatment Options. Clin. Exp. Med. 2021, 21, 215–230. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.; Chen, M.; Sun, Y.; Jin, Y.; Lu, C.; Pan, X.; Quan, G.; Wu, C. Microneedle-Mediated Transdermal Drug Delivery for Treating Diverse Skin Diseases. Acta Biomater. 2021, 121, 119–133. [Google Scholar] [CrossRef]
- Nemane, S.T.; Bhusnure, O.G.; Gholve, S.B.; Mitakari, P.R.; Karwa, P.N. A Review on Finasteride: A 5-Alpha Reductase Inhibitors, Its Mechanism, Facts and Benefits. J. Drug Deliv. Ther. 2019, 9, 1132–1136. [Google Scholar]
- Coskuner, E.R.; Ozkan, B.; Culha, M.G. Sexual Problems of Men with Androgenic Alopecia Treated with 5-Alpha Reductase Inhibitors. Sex. Med. Rev. 2019, 7, 277–282. [Google Scholar] [CrossRef] [PubMed]
- Fu, D.; Huang, J.; Li, K.; Chen, Y.; He, Y.; Sun, Y.; Guo, Y.; Du, L.; Qu, Q.; Miao, Y. Dihydrotestosterone-Induced Hair Regrowth Inhibition by Activating Androgen Receptor in C57BL6 Mice Simulates Androgenetic Alopecia. Biomed. Pharmacother. 2021, 137, 111247. [Google Scholar] [CrossRef] [PubMed]
- Hatem, S.; Nasr, M.; Moftah, N.H.; Ragai, M.H.; Geneidi, A.S.; Elkheshen, S.A. Clinical Cosmeceutical Repurposing of Melatonin in Androgenic Alopecia Using Nanostructured Lipid Carriers Prepared with Antioxidant Oils. Expert Opin. Drug Deliv. 2018, 15, 927–935. [Google Scholar] [CrossRef]
- Avcil, M.; Çelik, A. Microneedles in Drug Delivery: Progress and Challenges. Micromachines 2021, 12, 1321. [Google Scholar] [CrossRef]
- Nagarkar, R.; Singh, M.; Nguyen, H.X.; Jonnalagadda, S. A Review of Recent Advances in Microneedle Technology for Transdermal Drug Delivery. J. Drug Deliv. Sci. Technol. 2020, 59, 101923. [Google Scholar] [CrossRef]
- Gowda, B.J.; Ahmed, M.G.; Sahebkar, A.; Riadi, Y.; Shukla, R.; Kesharwani, P. Stimuli-Responsive Microneedles as a Transdermal Drug Delivery System: A Demand-Supply Strategy. Biomacromolecules 2022, 23, 1519–1544. [Google Scholar] [CrossRef]
- Wang, M.; Hu, L.; Xu, C. Recent Advances in the Design of Polymeric Microneedles for Transdermal Drug Delivery and Biosensing. Lab A Chip 2017, 17, 1373–1387. [Google Scholar] [CrossRef]
- Yadav, P.R.; Han, T.; Olatunji, O.; Pattanayek, S.K.; Das, D.B. Mathematical Modelling, Simulation and Optimisation of Microneedles for Transdermal Drug Delivery: Trends and Progress. Pharmaceutics 2020, 12, 693. [Google Scholar] [CrossRef] [PubMed]
- Bin Sayeed, M.S.; Karim, S.M.R.; Sharmin, T.; Morshed, M.M. Critical Analysis on Characterization, Systemic Effect, and Therapeutic Potential of Beta-Sitosterol: A Plant-Derived Orphan Phytosterol. Medicines 2016, 3, 29. [Google Scholar] [CrossRef] [PubMed]
- Bharti, M.; Shrivastav, A.; Abid, M.; Khan, N.A. A Review on Hair Growth Regulator. J. Drug Deliv. Ther. 2020, 10, 368–375. [Google Scholar] [CrossRef]
- Prabahar, K.; Udhumansha, U.; Elsherbiny, N.; Qushawy, M. Microneedle Mediated Transdermal Delivery of β-Sitosterol Loaded Nanostructured Lipid Nanoparticles for Androgenic Alopecia. Drug Deliv. 2022, 29, 3022–3034. [Google Scholar] [CrossRef]
- Upadhyay, K.; Gupta, N.K.; Dixit, V.K. Development and Characterization of Phyto-Vesicles of β-Sitosterol for the Treatment of Androgenetic Alopecia. Arch. Dermatol. Res. 2012, 304, 511–519. [Google Scholar] [CrossRef] [PubMed]
- Alenzi, A.M.; Albalawi, S.A.; Alghamdi, S.G.; Albalawi, R.F.; Albalawi, H.S.; Qushawy, M. Review on Different Vesicular Drug Delivery Systems (VDDSs) and Their Applications. Recent Pat. Nanotechnol. 2023, 17, 18–32. [Google Scholar]
- Salim, S.; Kamalasanan, K. Controlled Drug Delivery for Alopecia: A Review. J. Control. Release 2020, 325, 84–99. [Google Scholar] [CrossRef]
- Hosny, K.M.; Rizg, W.Y.; Alfayez, E.; Elgebaly, S.S.; Alamoudi, A.J.; Felimban, R.I.; Tayeb, H.H.; Mushtaq, R.Y.; Safhi, A.Y.; Alharbi, M. Preparation and Optimization of Aloe Ferox Gel Loaded with Finasteride-Oregano Oil Nanocubosomes for Treatment of Alopecia. Drug Deliv. 2022, 29, 284–293. [Google Scholar] [CrossRef] [PubMed]
- Victorelli, F.D.; Manni, L.S.; Biffi, S.; Bortot, B.; Buzzá, H.H.; Lutz-Bueno, V.; Handschin, S.; Calixto, G.; Murgia, S.; Chorilli, M. Potential of Curcumin-Loaded Cubosomes for Topical Treatment of Cervical Cancer. J. Colloid Interface Sci. 2022, 620, 419–430. [Google Scholar] [CrossRef]
- Umar, H.; Wahab, H.A.; Gazzali, A.M.; Tahir, H.; Ahmad, W. Cubosomes: Design, Development, and Tumor-Targeted Drug Delivery Applications. Polymers 2022, 14, 3118. [Google Scholar] [CrossRef]
- Kwon, T.K.; Hong, S.K.; Kim, J.-C. In Vitro Skin Permeation of Cubosomes Containing Triclosan. J. Ind. Eng. Chem. 2012, 18, 563–567. [Google Scholar] [CrossRef]
- Peng, X.; Zhou, Y.; Han, K.; Qin, L.; Dian, L.; Li, G.; Pan, X.; Wu, C. Characterization of Cubosomes as a Targeted and Sustained Transdermal Delivery System for Capsaicin. Drug Des. Dev. Ther. 2015, 9, 4209. [Google Scholar] [CrossRef] [PubMed]
- Al-Mahallawi, A.M.; Abdelbary, A.A.; El-Zahaby, S.A. Norfloxacin Loaded Nano-Cubosomes for Enhanced Management of Otitis Externa: In Vitro and in Vivo Evaluation. Int. J. Pharm. 2021, 600, 120490. [Google Scholar] [CrossRef] [PubMed]
- Dubey, A.; Shetty, A.; Ravi, G.S.; Kiritkumar, M.C.; Prabhu, P.; Hebbar, S.; El-Zahaby, S.A. Development and Investigation of Novel Solid Self-Nanoemulsifying System Loaded with Hydrochlorothiazide for the Treatment of Hypertension. Int. J. Pharm. Investig. 2018, 8, 83–91. [Google Scholar] [CrossRef]
- Sherif, S.; Bendas, E.R.; Badawy, S. The Clinical Efficacy of Cosmeceutical Application of Liquid Crystalline Nanostructured Dispersions of Alpha Lipoic Acid as Anti-Wrinkle. Eur. J. Pharm. Biopharm. 2014, 86, 251–259. [Google Scholar] [CrossRef] [PubMed]
- Han, S.; Shen, J.; Gan, Y.; Geng, H.; Zhang, X.; Zhu, C.; Gan, L. Novel Vehicle Based on Cubosomes for Ophthalmic Delivery of Flurbiprofen with Low Irritancy and High Bioavailability. Acta Pharmacol. Sin. 2010, 31, 990–998. [Google Scholar] [CrossRef]
- Vasanth, S.; Dubey, A.; GS, R.; Lewis, S.A.; Ghate, V.M.; El-Zahaby, S.A.; Hebbar, S. Development and Investigation of Vitamin C-Enriched Adapalene-Loaded Transfersome Gel: A Collegial Approach for the Treatment of Acne Vulgaris. AAPS PharmSciTech 2020, 21, 1–17. [Google Scholar] [CrossRef]
- Kurangi, B.; Jalalpure, S.; Jagwani, S. Formulation and Evaluation of Resveratrol Loaded Cubosomal Nanoformulation for Topical Delivery. Curr. Drug Deliv. 2021, 18, 607–619. [Google Scholar] [CrossRef]
- Zhang, H.; Cui, Y.; Zhang, X.; Yuan, X.; Xu, D.; Zhang, L. Sustained Delivery of Salbutamol from Cubosomal Gel for Management of Paediatric Asthma: In Vitro and in Vivo Evaluation. J. Microencapsul. 2022, 39, 252–260. [Google Scholar] [CrossRef]
- Zewail, M.; Gaafar, P.M.E.; Ali, M.M.; Abbas, H. Lipidic Cubic-Phase Leflunomide Nanoparticles (Cubosomes) as a Potential Tool for Breast Cancer Management. Drug Deliv. 2022, 29, 1663–1674. [Google Scholar] [CrossRef]
- Zakaria, F.; Ashari, S.E.; Azmi, I.D.M.; Rahman, M.B.A. Recent Advances in Encapsulation of Drug Delivery (Active Substance) in Cubosomes for Skin Diseases. J. Drug Deliv. Sci. Technol. 2022, 68, 103097. [Google Scholar] [CrossRef]
- Salah, S.; Mahmoud, A.A.; Kamel, A.O. Etodolac Transdermal Cubosomes for the Treatment of Rheumatoid Arthritis: Ex Vivo Permeation and in Vivo Pharmacokinetic Studies. Drug Deliv. 2017, 24, 846–856. [Google Scholar] [CrossRef]
- Nasr, M.; Ghorab, M.K.; Abdelazem, A. In Vitro and in Vivo Evaluation of Cubosomes Containing 5-Fluorouracil for Liver Targeting. Acta Pharm. Sin. B 2015, 5, 79–88. [Google Scholar] [CrossRef]
- He, J.; Zhang, Z.; Zheng, X.; Li, L.; Qi, J.; Wu, W.; Lu, Y. Design and Evaluation of Dissolving Microneedles for Enhanced Dermal Delivery of Propranolol Hydrochloride. Pharmaceutics 2021, 13, 579. [Google Scholar] [CrossRef] [PubMed]
- Azeez, R.A.; Abaas, I.S.; Kadhim, E.J. Isolation and Characterization of β-Sitosterol from Elaeagnus Angustifolia Cultivated in Iraq. Asian J Pharm Clin Res 2018, 11, 442–446. [Google Scholar] [CrossRef]
- Mohammed, A.A.; Niamah, A.K. Identification and Antioxidant Activity of Hyaluronic Acid Extracted from Local Isolates of Streptococcus Thermophilus. Mater. Today: Proc. 2022, 60, 1523–1529. [Google Scholar] [CrossRef]
- Lee, S.G.; Jeong, J.H.; Lee, K.M.; Jeong, K.H.; Yang, H.; Kim, M.; Jung, H.; Lee, S.; Choi, Y.W. Nanostructured Lipid Carrier-Loaded Hyaluronic Acid Microneedles for Controlled Dermal Delivery of a Lipophilic Molecule. Int. J. Nanomed. 2014, 9, 289. [Google Scholar]
- Lai, J.; Chen, J.; Lu, Y.; Sun, J.; Hu, F.; Yin, Z.; Wu, W. Glyceryl Monooleate/Poloxamer 407 Cubic Nanoparticles as Oral Drug Delivery Systems: I. In Vitro Evaluation and Enhanced Oral Bioavailability of the Poorly Water-Soluble Drug Simvastatin. Aaps Pharmscitech 2009, 10, 960–966. [Google Scholar] [CrossRef]
- Dian, L.; Yang, Z.; Li, F.; Wang, Z.; Pan, X.; Peng, X.; Huang, X.; Guo, Z.; Quan, G.; Shi, X. Cubic Phase Nanoparticles for Sustained Release of Ibuprofen: Formulation, Characterization, and Enhanced Bioavailability Study. Int. J. Nanomed. 2013, 8, 845. [Google Scholar]
- Kim, T.H.; Yoo, D.S.; Kim, J.-C. In Vitro Dermal Delivery of Epidermal Growth Factor Using Redox-Responsive Cubosomes. Biotechnol. Bioprocess Eng. 2019, 24, 273–281. [Google Scholar] [CrossRef]
- Fornasier, M.; Biffi, S.; Bortot, B.; Macor, P.; Manhart, A.; Wurm, F.R.; Murgia, S. Cubosomes Stabilized by a Polyphosphoester-Analog of Pluronic F127 with Reduced Cytotoxicity. J. Colloid Interface Sci. 2020, 580, 286–297. [Google Scholar] [CrossRef]
- Mohsen, A.M.; Younis, M.M.; Salama, A.; Darwish, A.B. Cubosomes as a Potential Oral Drug Delivery System for Enhancing the Hepatoprotective Effect of Coenzyme Q10. J. Pharm. Sci. 2021, 110, 2677–2686. [Google Scholar] [CrossRef] [PubMed]
- Ali, Z.; Sharma, P.K.; Warsi, M.H. Fabrication and Evaluation of Ketorolac Loaded Cubosome for Ocular Drug Delivery. J. Appl. Pharm. Sci. 2016, 6, 204–208. [Google Scholar] [CrossRef]
- Nithya, R.; Jerold, P.; Siram, K. Cubosomes of Dapsone Enhanced Permeation across the Skin. J. Drug Deliv. Sci. Technol. 2018, 48, 75–81. [Google Scholar] [CrossRef]
- Rapalli, V.K.; Banerjee, S.; Khan, S.; Jha, P.N.; Gupta, G.; Dua, K.; Hasnain, M.S.; Nayak, A.K.; Dubey, S.K.; Singhvi, G. QbD-Driven Formulation Development and Evaluation of Topical Hydrogel Containing Ketoconazole Loaded Cubosomes. Mater. Sci. Eng. C 2021, 119, 111548. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.; O’Brien, D.F.; Marder, S.R. Polymerized Bicontinuous Cubic Nanoparticles (Cubosomes) from a Reactive Monoacylglycerol. J. Am. Chem. Soc. 2002, 124, 13388–13389. [Google Scholar] [CrossRef]
- Kapoor, K.; Pandit, V.; Nagaich, U. Development and Characterization of Sustained Release Methotrexate Loaded Cubosomes for Topical Delivery in Rheumatoid Arthritis. Int J Appl Pharm 2020, 12, 33–39. [Google Scholar] [CrossRef]
- Prabahar, K.; Udhumansha, U.; Qushawy, M. Optimization of Thiolated Chitosan Nanoparticles for the Enhancement of in Vivo Hypoglycemic Efficacy of Sitagliptin in Streptozotocin-Induced Diabetic Rats. Pharmaceutics 2020, 12, 300. [Google Scholar] [CrossRef] [PubMed]
- Ganesh, M.; Ubaidulla, U.; Rathnam, G.; Jang, H.T. Chitosan-Telmisartan Polymeric Cocrystals for Improving Oral Absorption: In Vitro and in Vivo Evaluation. Int. J. Biol. Macromol. 2019, 131, 879–885. [Google Scholar] [CrossRef]
- Wei, S.; Quan, G.; Lu, C.; Pan, X.; Wu, C. Dissolving Microneedles Integrated with PH-Responsive Micelles Containing AIEgen with Ultra-Photostability for Enhancing Melanoma Photothermal Therapy. Biomater. Sci. 2020, 8, 5739–5750. [Google Scholar] [CrossRef]
- Nasiri, M.I.; Vora, L.K.; Ershaid, J.A.; Peng, K.; Tekko, I.A.; Donnelly, R.F. Nanoemulsion-Based Dissolving Microneedle Arrays for Enhanced Intradermal and Transdermal Delivery. Drug Deliv. Transl. Res. 2022, 12, 881–896. [Google Scholar] [CrossRef] [PubMed]
- Bhadale, R.S.; Londhe, V.Y. Inclusion Complexed Iloperidone Loaded Dissolving Microneedles: Characterization, in-Vitro Study, and Dermatopharmacokinetics. J. Drug Deliv. Sci. Technol. 2022, 68, 103063. [Google Scholar] [CrossRef]
- Arshad, M.S.; Hassan, S.; Hussain, A.; Abbas, N.; Kucuk, I.; Nazari, K.; Ali, R.; Ramzan, S.; Alqahtani, A.; Andriotis, E.G. Improved Transdermal Delivery of Cetirizine Hydrochloride Using Polymeric Microneedles. DARU J. Pharm. Sci. 2019, 27, 673–681. [Google Scholar] [CrossRef] [PubMed]
- Guo, T.; Cheng, N.; Zhao, J.; Hou, X.; Zhang, Y.; Feng, N. Novel Nanostructured Lipid Carriers-Loaded Dissolving Microneedles for Controlled Local Administration of Aconitine. Int. J. Pharm. 2019, 572, 118741. [Google Scholar] [CrossRef]
- Ronnander, J.P.; Simon, L.; Koch, A. Transdermal Delivery of Sumatriptan Succinate Using Iontophoresis and Dissolving Microneedles. J. Pharm. Sci. 2019, 108, 3649–3656. [Google Scholar] [CrossRef]
- Sabri, A.H.; Kim, Y.; Marlow, M.; Scurr, D.J.; Segal, J.; Banga, A.K.; Kagan, L.; Lee, J.B. Intradermal and Transdermal Drug Delivery Using Microneedles–Fabrication, Performance Evaluation and Application to Lymphatic Delivery. Adv. Drug Deliv. Rev. 2020, 153, 195–215. [Google Scholar] [CrossRef]
- Prabhu, A.; Jose, J.; Kumar, L.; Salwa, S.; Vijay Kumar, M.; Nabavi, S.M. Transdermal Delivery of Curcumin-Loaded Solid Lipid Nanoparticles as Microneedle Patch: An In Vitro and In Vivo Study. AAPS PharmSciTech 2022, 23, 1–12. [Google Scholar] [CrossRef]
- Zaid Alkilani, A.; Nimrawi, S.; Al-Nemrawi, N.K.; Nasereddin, J. Microneedle-Assisted Transdermal Delivery of Amlodipine Besylate Loaded Nanoparticles. Drug Dev. Ind. Pharm. 2022, 48, 322–332. [Google Scholar] [CrossRef]
- Alafnan, A.; Seetharam, A.A.; Hussain, T.; Gupta, M.S.; Rizvi, S.M.D.; Moin, A.; Alamri, A.; Unnisa, A.; Awadelkareem, A.M.; Elkhalifa, A.O. Development and Characterization of PEGDA Microneedles for Localized Drug Delivery of Gemcitabine to Treat Inflammatory Breast Cancer. Materials 2022, 15, 7693. [Google Scholar] [CrossRef]

| Formulation Code | GMO (%) | β-ST (%) | Pluronic F-127 (%) | Water (mL) | Appearance |
|---|---|---|---|---|---|
| FC 1 | 100 | - | 10 | 10 | Transparent |
| FC 2 | 90 | 10 | 05 | 10 | Transparent |
| FC 3 | 80 | 10 | 10 | 10 | Transparent |
| FC 4 | 70 | 10 | 15 | 10 | Transparent |
| FC 5 | 60 | 10 | 15 | 10 | Opaque white |
| Formulation Code | Particle Size (nm) | PDI | ZP (mV) |
|---|---|---|---|
| FC 2 | 265.03 ± 0.71 | 0.187 ± 0.01 | −28.67 ± 0.34 |
| FC 3 | 224.41 ± 0.97 | 0.267 ± 0.02 | −31.27 ± 1.48 |
| FC 4 | 173.67 ± 0.52 | 0.285 ± 0.02 | −35.88 ± 1.32 |
| Physical Characteristics (n = 20) | |
|---|---|
| Length (μm) | 621.34 ± 15.70 μm |
| Base diameter (μm) | 328.82 ± 18.39 μm |
| Tip diameter (μm) | 27.25 ± 4.17 μm |
| Aspect ratio | 0.86 ± 0.08 |
| Drug content (n = 3) | 93.79 ± 2.55% |
| Storage Condition | Initial Storage | 25 ± 2 °C (60 ± 5% RH) | 40 ± 2 °C (75 ± 5% RH) | 5 ± 2 °C |
|---|---|---|---|---|
| Time Point | 0 Month | 6 Month | 6 Month | 6 Month |
| Physical appearance | Microneedles | No Change | No Change | No Change |
| Drug content (%) | 93.79 ± 2.55 | 92.20 ± 1.42 | 91.10 ± 1.90 | 93.02 ± 1.57 |
| Drug Permeation (Jss) μg/cm2/h | 101.17 ± 2.32 | 100.65 ± 2.96 | 99.04 ± 2.17 | 100.3 ± 2.58 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Prabahar, K.; Uthumansha, U.; Elsherbiny, N.; Qushawy, M. Enhanced Skin Permeation and Controlled Release of β-Sitosterol Using Cubosomes Encrusted with Dissolving Microneedles for the Management of Alopecia. Pharmaceuticals 2023, 16, 563. https://doi.org/10.3390/ph16040563
Prabahar K, Uthumansha U, Elsherbiny N, Qushawy M. Enhanced Skin Permeation and Controlled Release of β-Sitosterol Using Cubosomes Encrusted with Dissolving Microneedles for the Management of Alopecia. Pharmaceuticals. 2023; 16(4):563. https://doi.org/10.3390/ph16040563
Chicago/Turabian StylePrabahar, Kousalya, Ubaidulla Uthumansha, Nehal Elsherbiny, and Mona Qushawy. 2023. "Enhanced Skin Permeation and Controlled Release of β-Sitosterol Using Cubosomes Encrusted with Dissolving Microneedles for the Management of Alopecia" Pharmaceuticals 16, no. 4: 563. https://doi.org/10.3390/ph16040563
APA StylePrabahar, K., Uthumansha, U., Elsherbiny, N., & Qushawy, M. (2023). Enhanced Skin Permeation and Controlled Release of β-Sitosterol Using Cubosomes Encrusted with Dissolving Microneedles for the Management of Alopecia. Pharmaceuticals, 16(4), 563. https://doi.org/10.3390/ph16040563










