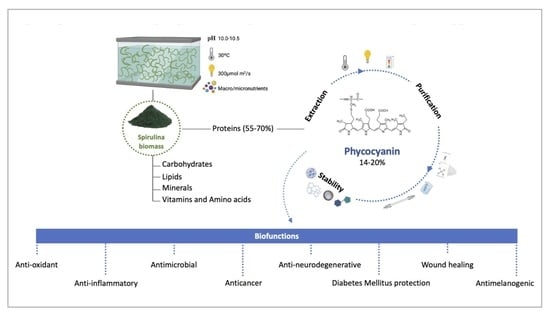
Exploring the Benefits of Phycocyanin: From Spirulina Cultivation to Its Widespread Applications
Abstract
1. Introduction
2. Overview of Spirulina
3. Phycocyanin
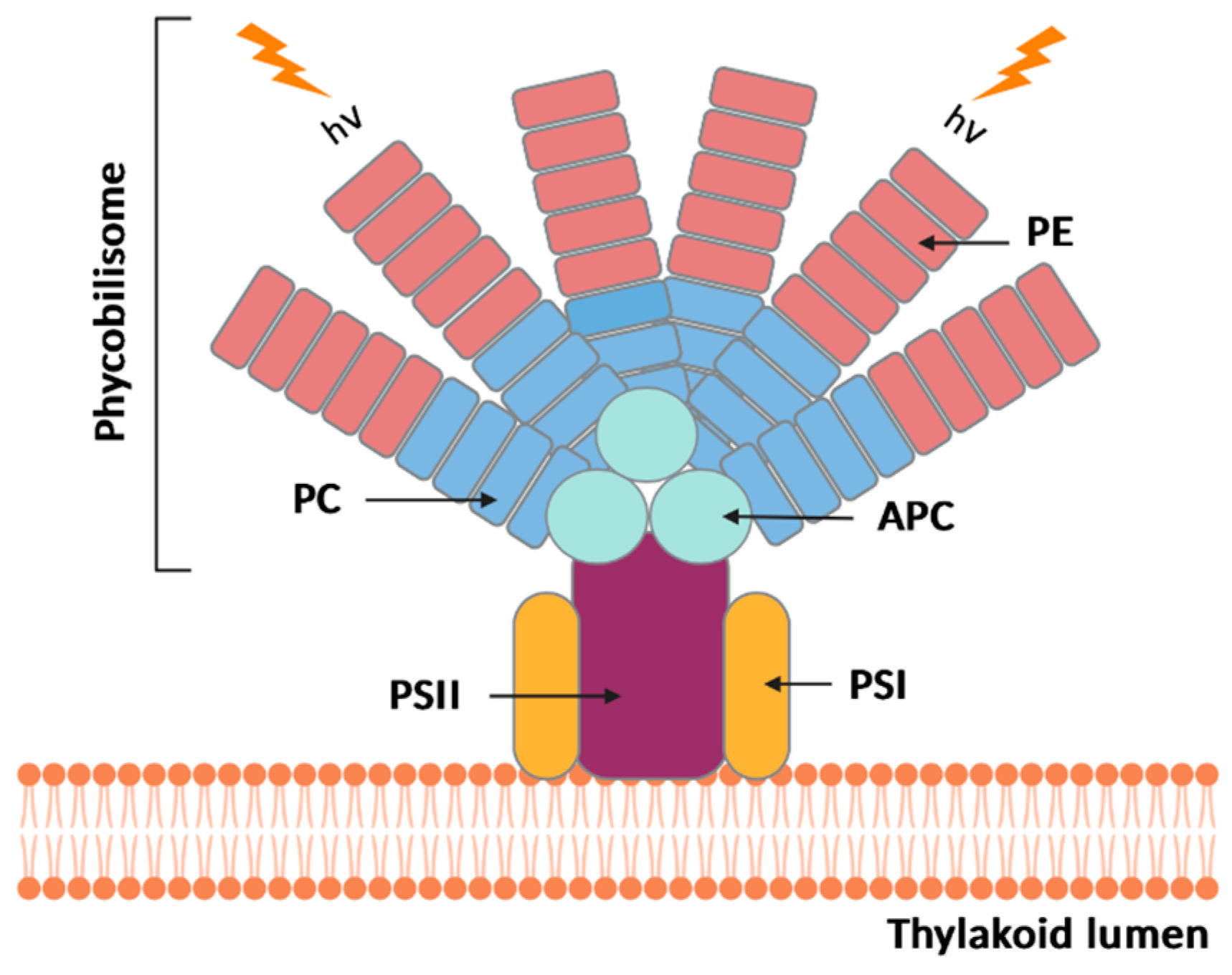
3.1. Classification and Structure
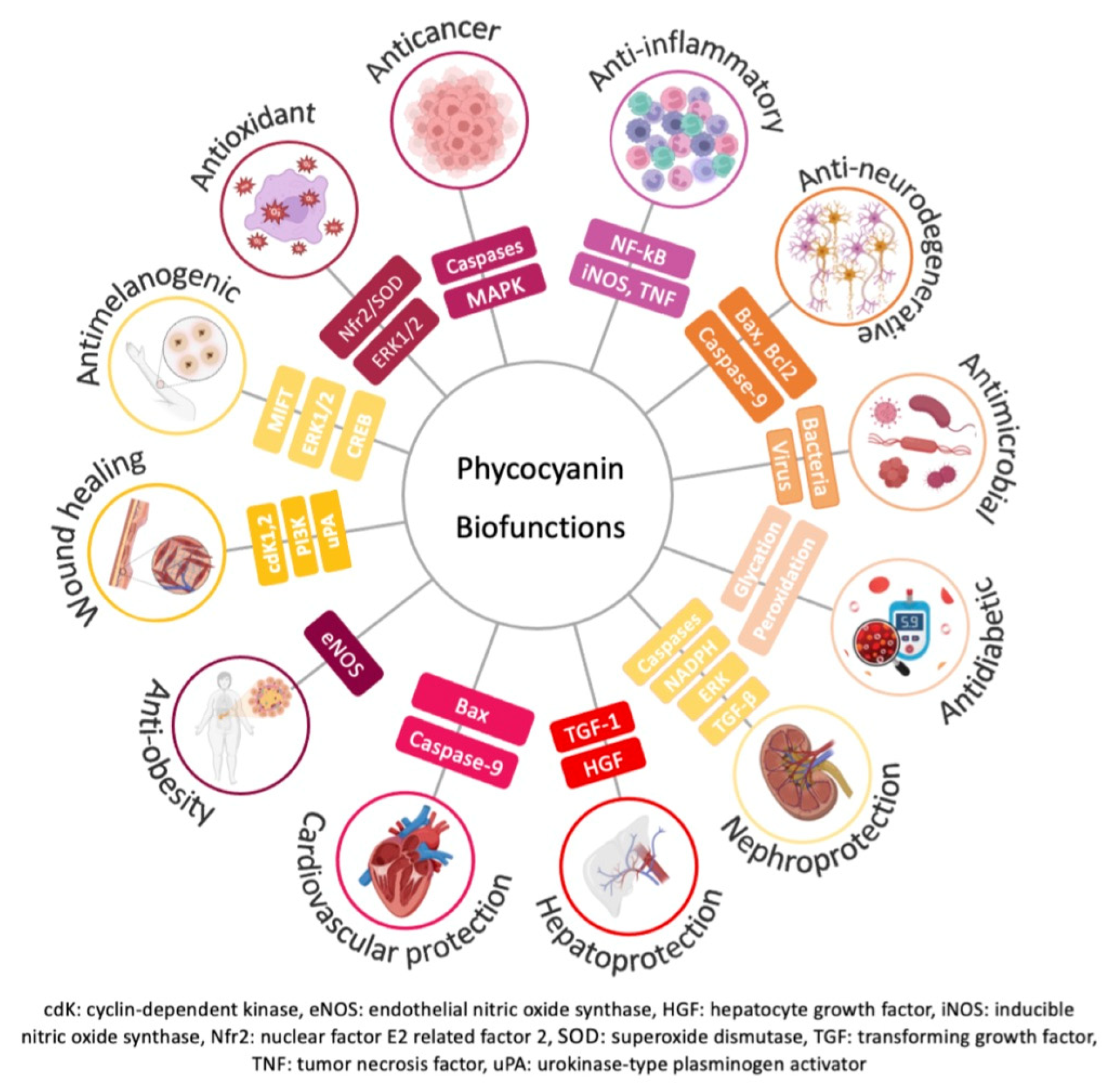
3.2. Biological Functions and Applicability
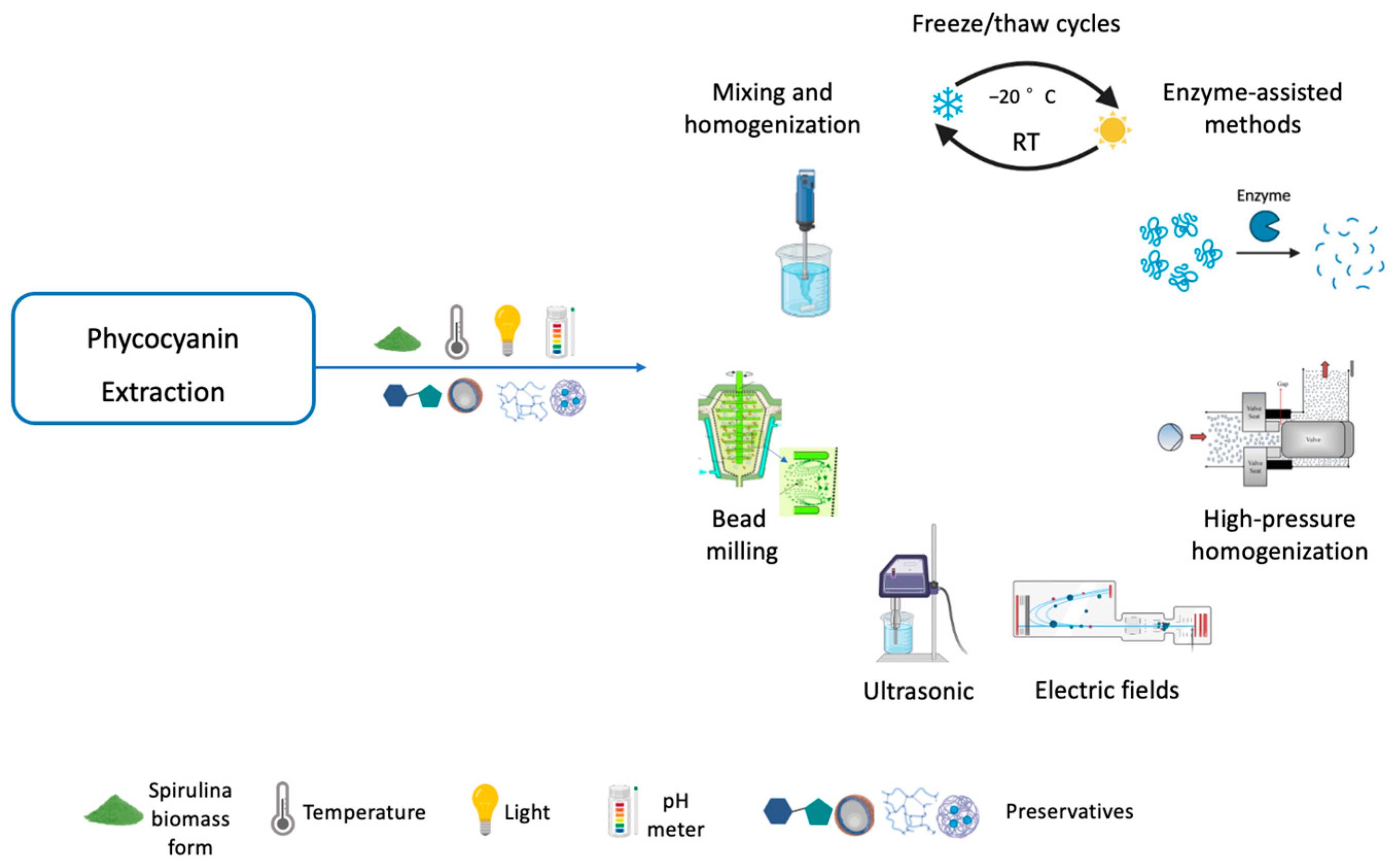
3.3. Phycocyanin Extraction Methods
3.3.1. Cell Disruption Methods
Freeze/Thaw Cycles
Mixing and Homogenization
Bead Milling
Ultrasonic
Electric Fields
High-Pressure Homogenization
Enzyme-Assisted Methods
3.4. Key Parameters for Phycocyanin Extraction and Stability
3.4.1. Biomass Form
3.4.2. Temperature
3.4.3. Light
3.4.4. pH
3.4.5. Type of Solvent
3.4.6. Biomass/Solvent Ratio
3.4.7. Preservatives
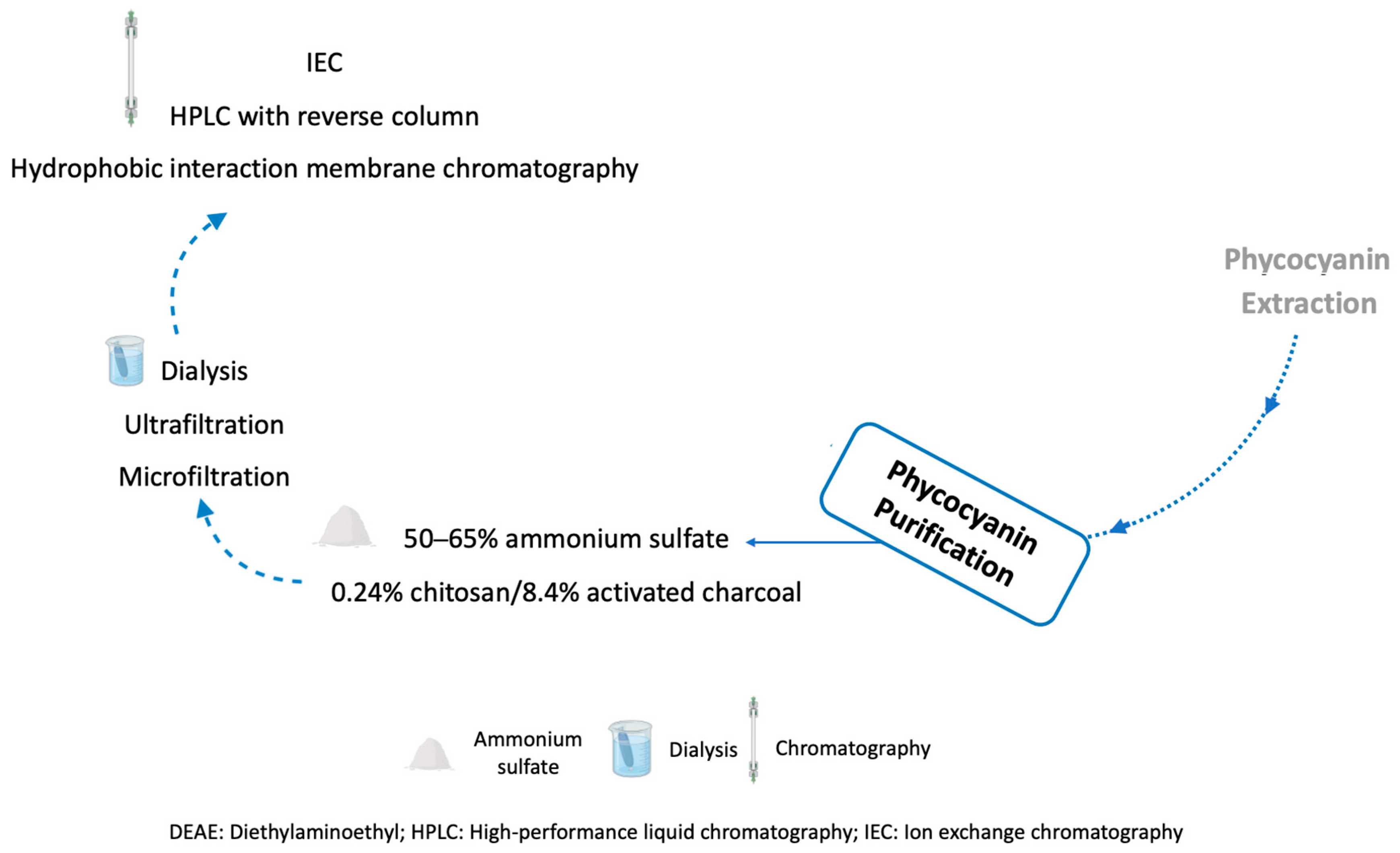
3.5. Phycocyanin Purification Methods
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- World Population Projected to Reach 9.8 Billion in 2050, and 11.2 Billion in 2100. Available online: https://www.un.org/en/desa/world-population-projected-reach-98-billion-2050-and-112-billion-2100 (accessed on 3 March 2023).
- Lim, H.R.; Khoo, K.S.; Chew, K.W.; Chang, C.-K.; Munawaroh, H.S.H.; Kumar, P.S.; Huy, N.D.; Show, P.L. Perspective of Spirulina culture with wastewater into a sustainable circular bioeconomy. Environ. Pollut. 2021, 284, 117492. [Google Scholar] [CrossRef] [PubMed]
- Lafarga, T. Cultured Microalgae and Compounds Derived Thereof for Food Applications: Strain Selection and Cultivation, Drying, and Processing Strategies. Food Rev. Int. 2020, 36, 559–583. [Google Scholar] [CrossRef]
- Jaeschke, D.P.; Teixeira, I.R.; Marczak, L.D.F.; Mercali, G.D. Phycocyanin from Spirulina: A review of extraction methods and stability. Food Res. Int. 2021, 143, 110314. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. New Report Calls for Urgent Action to Avert Antimicrobial Resistance Crisis. International Organizations Unite on Critical Recommendations to Combat Drug-Resistant Infections and Prevent Staggering Number of Deaths Each Year. Available online: https://www.who.int/news/item/29-04-2019-new-report-calls-for-urgent-action-to-avert-antimicrobial-resistance-crisis (accessed on 3 March 2023).
- Nowruzi, B. Cyanobacteria Natural Products as Sources for Future Directions in Antibiotic Drug Discovery. In Cyanobacteria—Recent Advances and New Perspectives; IntechOpen: London, UK, 2022. [Google Scholar] [CrossRef]
- Saxena, R.; Rodríguez-Jasso, R.M.; Chávez-Gonzalez, M.L.; Aguilar, C.N.; Quijano, G.; Ruiz, H.A. Strategy Development for Microalgae Spirulina platensis Biomass Cultivation in a Bubble Photobioreactor to Promote High Carbohydrate Content. Fermentation 2022, 8, 374. [Google Scholar] [CrossRef]
- AlFadhly, N.K.Z.; Alhelfi, N.; Altemimi, A.B.; Verma, D.K.; Cacciola, F. Tendencies Affecting the Growth and Cultivation of Genus Spirulina: An Investigative Review on Current Trends. Plants 2022, 11, 3063. [Google Scholar] [CrossRef]
- Algae Products Market Size, Growth and Forecast 2028. Available online: https://www.credenceresearch.com/report/algae-products-market (accessed on 3 March 2023).
- Paniagua-Michel, J. Microalgal Nutraceuticals. In Handbook of Marine Microalgae: Biotechnology Advances; Academic Press: Cambridge, MA, USA, 2015; pp. 255–267. [Google Scholar] [CrossRef]
- Sorrenti, V.; Castagna, D.A.; Fortinguerra, S.; Buriani, A.; Scapagnini, G.; Willcox, D.C. Spirulina Microalgae and Brain Health: A Scoping Review of Experimental and Clinical Evidence. Mar. Drugs 2021, 19, 293. [Google Scholar] [CrossRef]
- de Morais, M.G.; da Fontoura Prates, D.; Moreira, J.B.; Duarte, J.H.; Costa, J.A.V. Phycocyanin from Microalgae: Properties, Extraction and Purification, with Some Recent Applications. Ind. Biotechnol. 2018, 14, 30–37. [Google Scholar] [CrossRef]
- Yuan, B.; Li, Z.; Shan, H.; Dashnyam, B.; Xu, X.; McClements, D.J.; Zhang, B.; Tan, M.; Wang, Z.; Cao, C. A review of recent strategies to improve the physical stability of phycocyanin. Curr. Res. Food Sci. 2022, 5, 2329–2337. [Google Scholar] [CrossRef]
- Jung, C.H.G.; Braune, S.; Waldeck, P.; Küpper, J.-H.; Petrick, I.; Jung, F. Morphology and Growth of Arthrospira platensis during Cultivation in a Flat-Type Bioreactor. Life 2021, 11, 536. [Google Scholar] [CrossRef]
- Trotta, T.; Porro, C.; Cianciulli, A.; Panaro, M.A. Beneficial Effects of Spirulina Consumption on Brain Health. Nutrients 2022, 14, 676. [Google Scholar] [CrossRef]
- A Review on Culture, Production and Use of Spirulina as Food for Humans and Feeds for Domestic Animals and Fish. Food and Agriculture Organization of the United Nations: Rome, Italy, 2008. Available online: https://www.fao.org/3/i0424e/i0424e00.pdf (accessed on 3 March 2023).
- Economic and Social Council Substantive Session For 2008 High-Level Segment. This Year’s Theme is “Achieving Sustainable Development”. Available online: https://www.un.org/en/ecosoc/docs/statement08/iimsam.pdf (accessed on 3 March 2023).
- OPINION of the French Agency for Food, Environmental and Occupational Health & Safety on the “Risks Associated with the Consumption of Food Supplements Containing Spirulina”. Available online: https://www.anses.fr/en/system/files/NUT2014SA0096EN.pdf (accessed on 3 March 2023).
- GRAS Notice (GRN) No. 742. Available online: https://www.fda.gov/media/113614/download (accessed on 3 March 2023).
- Code of Federal Regulations. Available online: https://www.ecfr.gov/ (accessed on 3 March 2023).
- Algae as Novel Food in Europe. Available online: https://www.algae-novel-food.com/output/algae-novel-food/download.pdf (accessed on 3 March 2023).
- Regulation (EU) 2015/2283 of the European Parliament and of the Council of 25 November 2015 on Novel Foods, Amending Regulation (EU) No 1169/2011 of the European Parliament and of the Council and Repealing Regulation (EC) No 258/97 of the European Parliament and of the Council and Commission Regulation (EC) No 1852/2001 (Text with EEA Relevance); 2015; Volume 327. Available online: http://data.europa.eu/eli/reg/2015/2283/oj/eng (accessed on 3 March 2023).
- Bortolini, D.G.; Maciel, G.M.; Fernandes, I.D.A.A.; Pedro, A.C.; Rubio, F.T.V.; Branco, I.G.; Haminiuk, C.W.I. Functional properties of bioactive compounds from Spirulina spp.: Current status and future trends. Food Chem. Mol. Sci. 2022, 5, 100134. [Google Scholar] [CrossRef] [PubMed]
- Grosshagauer, S.; Kraemer, K.; Somoza, V. The True Value of Spirulina. J. Agric. Food Chem. 2020, 68, 4109–4115. [Google Scholar] [CrossRef] [PubMed]
- Blue-Green Algae: MedlinePlus Supplements. Available online: https://medlineplus.gov/druginfo/natural/923.html (accessed on 3 March 2023).
- AlFadhly, N.K.Z.; Alhelfi, N.; Altemimi, A.B.; Verma, D.K.; Cacciola, F.; Narayanankutty, A. Trends and Technological Advancements in the Possible Food Applications of Spirulina and Their Health Benefits: A Review. Molecules 2022, 27, 5584. [Google Scholar] [CrossRef] [PubMed]
- Lafarga, T.; Fernández-Sevilla, J.M.; González-López, C.; Acién-Fernández, F.G. Spirulina for the food and functional food industries. Food Res. Int. 2020, 137, 109356. [Google Scholar] [CrossRef]
- Jung, F.; Krüger-Genge, A.; Waldeck, P.; Küpper, J.-H. Spirulina platensis, a super food? J. Cell. Biotechnol. 2019, 5, 43–54. [Google Scholar] [CrossRef]
- Manimekalan, A.; Parthiban, M.S. Reducing the Carbon Footprint by Cultivating and Consuming Spirulina: A Mini-review. Int. J. Environ. Clim. Chang. 2022, 12, 3069–3076. [Google Scholar] [CrossRef]
- de Medeiros, V.P.B.; da Costa, W.K.A.; da Silva, R.T.; Pimentel, T.C.; Magnani, M. Microalgae as source of functional ingredients in new-generation foods: Challenges, technological effects, biological activity, and regulatory issues. Crit. Rev. Food Sci. Nutr. 2022, 62, 4929–4950. [Google Scholar] [CrossRef]
- Ragusa, I.; Nardone, G.; Zanatta, S.; Bertin, W.; Amadio, E. Spirulina for Skin Care: A Bright Blue Future. Cosmetics 2021, 8, 7. [Google Scholar] [CrossRef]
- State of Technology Review—Algae Bioenergy. Available online: https://www.ieabioenergy.com/blog/publications/state-of-technology-review-algae-bioenergy (accessed on 3 March 2023).
- Spirulina Market by Distribution Channel (Consumer Channel, Business Channel), Product Type (Powder, Tablets, Capsules, Flakes, Phycocyanin Extract), Application (Nutraceuticals, Food and Beverages, Agriculture, Animal Feed)-Global Forecast to 2028. Available online: https://www.giiresearch.com/report/meti1018472-spirulina-market-by-distribution-channel-consumer.html (accessed on 3 March 2023).
- Thevarajah, B.; Nishshanka, G.K.S.H.; Premaratne, M.; Nimarshana, P.; Nagarajan, D.; Chang, J.-S.; Ariyadasa, T.U. Large-scale production of Spirulina-based proteins and c-phycocyanin: A biorefinery approach. Biochem. Eng. J. 2022, 185, 108541. [Google Scholar] [CrossRef]
- Altmann, B.A.; Rosenau, S. Spirulina as Animal Feed: Opportunities and Challenges. Foods 2022, 11, 965. [Google Scholar] [CrossRef]
- Costa, J.A.V.; Freitas, B.C.B.; Rosa, G.M.; Moraes, L.; Morais, M.G.; Mitchell, B.G. Operational and economic aspects of Spirulina-based biorefinery. Bioresour. Technol. 2019, 292, 121946. [Google Scholar] [CrossRef]
- MacColl, R. Cyanobacterial Phycobilisomes. J. Struct. Biol. 1998, 124, 311–334. [Google Scholar] [CrossRef]
- Fabris, M.; Abbriano, R.; Pernice, M.; Sutherland, D.L.; Commault, A.S.; Hall, C.C.; Labeeuw, L.; McCauley, J.I.; Kuzhiumparambil, U.; Ray, P.; et al. Emerging Technologies in Algal Biotechnology: Toward the Establishment of a Sustainable, Algae-Based Bioeconomy. Front. Plant Sci. 2020, 11, 279. [Google Scholar] [CrossRef] [PubMed]
- Giwa, A.; Abuhantash, F.; Chalermthai, B.; Taher, H. Bio-Based Circular Economy and Polygeneration in Microalgal Produc-tion from Food Wastes: A Concise Review. Sustainability 2022, 14, 10759. [Google Scholar] [CrossRef]
- Bayramoglu, G.; Akbulut, A.; Arica, M.Y. Study of polyethyleneimine- and amidoxime-functionalized hybrid biomass of Spirulina (Arthrospira) platensis for adsorption of uranium (VI) ion. Environ. Sci. Pollut. Res. 2015, 22, 17998–18010. [Google Scholar] [CrossRef] [PubMed]
- Hsieh-Lo, M.; Castillo, G.; Ochoa-Becerra, M.A.; Mojica, L. Phycocyanin and phycoerythrin: Strategies to improve production yield and chemical stability. Algal Res. 2019, 42, 101600. [Google Scholar] [CrossRef]
- Wu, Q.; Liu, L.; Miron, A.; Klímová, B.; Wan, D.; Kuča, K. The antioxidant, immunomodulatory, and anti-inflammatory activities of Spirulina: An overview. Arch. Toxicol. 2016, 90, 1817–1840. [Google Scholar] [CrossRef]
- Pagels, F.; Guedes, A.C.; Amaro, H.M.; Kijjoa, A.; Vasconcelos, V. Phycobiliproteins from cyanobacteria: Chemistry and biotechnological applications. Biotechnol. Adv. 2019, 37, 422–443. [Google Scholar] [CrossRef]
- de Amarante, M.C.A.; Braga, A.R.C.; Sala, L.; Moraes, C.C.; Kalil, S.J. Design strategies for C-phycocyanin purification: Process influence on purity grade. Sep. Purif. Technol. 2020, 252, 117453. [Google Scholar] [CrossRef]
- C-Phycocyanin | Sigma-Aldrich. Available online: http://www.sigmaaldrich.com/ (accessed on 4 March 2023).
- Bennett, A.; Bogorad, L. Complementary chromatic adaptation in a filamentous blue-green alga. J. Cell Biol. 1973, 58, 419–435. [Google Scholar] [CrossRef]
- CFR-Code of Federal Regulations Title 21. Available online: https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/CFRSearch.cfm?fr=73.530 (accessed on 4 March 2023).
- Provision of Scientific and Technical Support with Respect to the Classification of Extracts/Concentrates with Colouring Properties Either as Food Colours (Food Additives Falling under Regulation (EC) No 1333/2008) or Colouring Foods. JRC Publications Repository. Available online: https://publications.jrc.ec.europa.eu/repository/handle/JRC96974 (accessed on 4 March 2023).
- GRAS Notice (GRN) No. 1000. Available online: https://www.fda.gov/media/155000/download (accessed on 4 March 2023).
- Enciso, P.; Cabrerizo, F.M.; Gancheff, J.S.; Denis, P.A.; Cerdá, M.F. Phycocyanin as Potential Natural Dye for its Use in Photovoltaic Cells. J. Appl. Sol. Chem. Model. 2013, 2, 225–233. Available online: https://www.semanticscholar.org/paper/Phycocyanin-as-Potential-Natural-Dye-for-its-Use-in-Enciso-Cabrerizo/677db30d4b30102e704d1f311f05e526fc277a7e (accessed on 4 March 2023).
- Kraseasintra, O.; Tragoolpua, Y.; Pandith, H.; Khonkarn, R.; Pathom-Aree, W.; Pekkoh, J.; Pumas, C. Application of phycocyanin from Arthrospira (Spirulina) platensis as a hair dye. Front. Mar. Sci. 2022, 9, 1024988. [Google Scholar] [CrossRef]
- Dewi, E.N.; Kurniasih, R.A.; Purnamayati, L. The Application of Microencapsulated Phycocyanin as a Blue Natural Colorant to the Quality of Jelly Candy. IOP Conf. Ser. Earth Environ. Sci. 2018, 116, 012047. [Google Scholar] [CrossRef]
- Vernes, L.; Granvillain, P.; Chemat, F.; Vian, M. Phycocyanin from Arthrospira platensis. Production, Extraction and Analysis. Curr. Biotechnol. 2015, 4, 481–491. [Google Scholar] [CrossRef]
- Romay, C.; Armesto, J.; Remirez, D.; González, R.; Ledon, N.; García, I. Antioxidant and anti-inflammatory properties of C-phycocyanin from blue-green algae. Inflamm. Res. 1998, 47, 36–41. [Google Scholar] [CrossRef] [PubMed]
- Safari, R.; Amiri, Z.R.; Kenari, R.E. Antioxidant and antibacterial activities of C-phycocyanin from common name Spirulina platensis. Iran. J. Fish. Sci. 2019, 19, 1911–1927. [Google Scholar] [CrossRef]
- Wu, H.-L.; Wang, G.-H.; Xiang, W.-Z.; Li, T.; He, H. Stability and Antioxidant Activity of Food-Grade Phycocyanin Isolated fromSpirulina platensis. Int. J. Food Prop. 2016, 19, 2349–2362. [Google Scholar] [CrossRef]
- Namasivayam, S.K.R.; Shivaramakrishnan, K.; Bharani, R.S.A. Potential antioxidative protein-pigment complex Spirulina platensis mediated food grade phycocyanin C -Extraction, purification, antioxidative activity and biocompatibility. Indian J. Biochem. Biophys. 2019, 56, 230–239. [Google Scholar]
- Grover, P.; Bhatnagar, A.; Kumari, N.; Bhatt, A.N.; Nishad, D.K.; Purkayastha, J. C-Phycocyanin-a novel protein from Spirulina platensis- In vivo toxicity, antioxidant and immunomodulatory studies. Saudi J. Biol. Sci. 2021, 28, 1853–1859. [Google Scholar] [CrossRef]
- Jang, Y.; Kim, B. Protective Effect of Spirulina-Derived C-Phycocyanin against Ultraviolet B-Induced Damage in HaCaT Cells. Medicina 2021, 57, 273. [Google Scholar] [CrossRef]
- Hsiao, G.; Chou, P.-H.; Shen, M.-Y.; Chou, D.-S.; Lin, C.-H.; Sheu, J.-R. C-Phycocyanin, a Very Potent and Novel Platelet Aggregation Inhibitor from Spirulina platensis. J. Agric. Food Chem. 2005, 53, 7734–7740. [Google Scholar] [CrossRef] [PubMed]
- Ivanova, K.G.; Stankova, K.G.; Nikolov, V.N.; Georgieva, R.T.; Minkova, K.M.; Gigova, L.G.; Rupova, I.T.; Boteva, R.N. The biliprotein C-phycocyanin modulates the early radiation response: A pilot study. Mutat. Res. Toxicol. Environ. Mutagen. 2010, 695, 40–45. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.; Varadharaj, S.; Ganesan, L.P.; Shobha, J.C.; Naidu, M.U.; Parinandi, N.L.; Tridandapani, S.; Kutala, V.K.; Kuppusamy, P. C-phycocyanin protects against ischemia-reperfusion injury of heart through involvement of p38 MAPK and ERK signaling. Am. J. Physiol. Heart Circ. Physiol. 2006, 290, H2136–H2145. [Google Scholar] [CrossRef] [PubMed]
- Ou, Y.; Zheng, S.; Lin, L.; Jiang, Q.; Yang, X. Protective effect of C-phycocyanin against carbon tetrachloride-induced hepatocyte damage in vitro and in vivo. Chem. Biol. Interact. 2010, 185, 94–100. [Google Scholar] [CrossRef] [PubMed]
- Farooq, S.M.; Boppana, N.B.; Asokan, D.; Sekaran, S.D.; Shankar, E.M.; Li, C.; Gopal, K.; Bakar, S.A.; Karthik, H.S.; Ebrahim, A.S. C-Phycocyanin Confers Protection against Oxalate-Mediated Oxidative Stress and Mitochondrial Dysfunctions in MDCK Cells. PLoS ONE 2014, 9, e93056. [Google Scholar] [CrossRef]
- Ravi, M.; Tentu, S.; Baskar, G.; Prasad, S.R.; Raghavan, S.; Jayaprakash, P.; Jeyakanthan, J.; Rayala, S.K.; Venkatraman, G. Molecular mechanism of anti-cancer activity of phycocyanin in triple-negative breast cancer cells. BMC Cancer 2015, 15, 768. [Google Scholar] [CrossRef]
- Subhashini, J.; Mahipal, S.V.; Reddy, M.C.; Reddy, M.M.; Rachamallu, A.; Reddanna, P. Molecular mechanisms in C-Phycocyanin induced apoptosis in human chronic myeloid leukemia cell line-K562. Biochem. Pharmacol. 2004, 68, 453–462. [Google Scholar] [CrossRef]
- Arunasree, K.M.; Roy, K.R.; Reddy, N.P.; Dheeraj, B.; Reddy, G.V.; Reddanna, P. Alteration of mitochondrial membrane potential by Spirulina platensis C-phycocyanin induces apoptosis in the doxorubicinresistant human hepatocellular-carcinoma cell line HepG2. Biotechnol. Appl. Biochem. 2007, 47, 159–167. [Google Scholar] [CrossRef]
- Kunte, M.; Desai, K. The Inhibitory Effect of C-phycocyanin Containing Protein Extract (C-PC Extract) on Human Matrix Metalloproteinases (MMP-2 and MMP-9) in Hepatocellular Cancer Cell Line (HepG2). Protein J. 2017, 36, 186–195. [Google Scholar] [CrossRef]
- Saini, M.K.; Sanyal, S.N. Targeting angiogenic pathway for chemoprevention of experimental colon cancer using C-phycocyanin as cyclooxygenase-2 inhibitor. Biochem. Cell Biol. 2014, 92, 206–218. [Google Scholar] [CrossRef]
- Chen, T.; Wong, Y.-S. In Vitro Antioxidant and Antiproliferative Activities of Selenium-Containing Phycocyanin from Selenium-Enriched Spirulina platensis. J. Agric. Food Chem. 2008, 56, 4352–4358. [Google Scholar] [CrossRef] [PubMed]
- Nishanth, R.P.; Ramakrishna, B.; Jyotsna, R.G.; Roy, K.R.; Reddy, G.V.; Reddy, P.K.; Reddanna, P. C-Phycocyanin inhibits MDR1 through reactive oxygen species and cyclooxygenase-2 mediated pathways in human hepatocellular carcinoma cell line. Eur. J. Pharmacol. 2010, 649, 74–83. [Google Scholar] [CrossRef] [PubMed]
- Shih, C.-M.; Cheng, S.-N.; Wong, C.-S.; Kuo, Y.-L.; Chou, T.-C. Antiinflammatory and Antihyperalgesic Activity of C-Phycocyanin. Anesth. Analg. 2009, 108, 1303–1310. [Google Scholar] [CrossRef]
- Cherng, S.-C.; Cheng, S.-N.; Tarn, A.; Chou, T.-C. Anti-inflammatory activity of c-phycocyanin in lipopolysaccharide-stimulated RAW 264.7 macrophages. Life Sci. 2007, 81, 1431–1435. [Google Scholar] [CrossRef] [PubMed]
- Leung, P.-O.; Lee, H.-H.; Kung, Y.-C.; Tsai, M.-F.; Chou, T.-C. Therapeutic Effect of C-Phycocyanin Extracted from Blue Green Algae in a Rat Model of Acute Lung Injury Induced by Lipopolysaccharide. Evid. Based Complement. Altern. Med. 2013, 2013, 916590. [Google Scholar] [CrossRef]
- Zhang, L.; Kong, D.; Huang, J.; Wang, Q.; Shao, L. The Therapeutic Effect and the Possible Mechanism of C-Phycocyanin in Lipopolysaccharide and Seawater-Induced Acute Lung Injury. Drug Des. Dev. Ther. 2022, 16, 1025–1040. [Google Scholar] [CrossRef]
- Pattarayan, D.; Rajarajan, D.; Ayyanar, S.; Palanichamy, R.; Subbiah, R. C-phycocyanin suppresses transforming growth factor-β1-induced epithelial mesenchymal transition in human epithelial cells. Pharmacol. Rep. 2017, 69, 426–431. [Google Scholar] [CrossRef]
- Liu, R.-Z.; Li, W.-J.; Zhang, J.-J.; Liu, Z.-Y.; Li, Y.; Liu, C.; Qin, S. The Inhibitory Effect of Phycocyanin Peptide on Pulmonary Fibrosis In Vitro. Mar. Drugs 2022, 20, 696. [Google Scholar] [CrossRef]
- Mohite, Y.; Shrivastava, N.; Sahu, D. Antimicrobial Activity of C-Phycocyanin from Arthrospira Platensis Isolated from Extreme Haloalkaline Environment of Lonar Lake. Semantic Scholar 2015. Available online: https://www.semanticscholar.org/paper/Antimicrobial-Activity-of-C-Phycocyanin-from-From-Mohite-Shrivastava/0ce72a6894d9670db805c76ac10247d48f3076a6 (accessed on 4 March 2023).
- Mohamed, S.A.; Osman, A.; Abo Eita, A.; Sitohy, M.Z. Estimation of antibacterial and antioxidant activities of phycocyanin isolated from Spirulina. Zagazig J. Agric. Res. 2018, 45, 657–666. [Google Scholar] [CrossRef]
- Sarada, D.V.L.; Kumar, C.S.; Rengasamy, R. Purified C-phycocyanin from Spirulina platensis (Nordstedt) Geitler: A novel and potent agent against drug resistant bacteria. World J. Microbiol. Biotechnol. 2011, 27, 779–783. [Google Scholar] [CrossRef]
- Nihal, B.; Gupta, N.V.; Gowda, D.V.; Manohar, M. Formulation and development of topical anti acne formulation of spirulina extract. Int. J. Appl. Pharm. 2018, 10, 229–233. [Google Scholar] [CrossRef]
- Murugan, T.; Radhamadhavan. Screening for Antifungal and Antiviral Activity of C-Phycocyanin from Spirulina Platensis. Association of Pharmaceutical Innovators 2011. Available online: https://scholar.google.com/scholar_lookup?title=Screening+for+Antifungal+and+Antiviral+activity+of+C-phycocyanin+from+Spirulina+platensis&author=T.Murugan+1+&publication_year=2011 (accessed on 4 March 2023).
- Pentón-Rol, G.; Martínez-Sánchez, G.; Cervantes-Llanos, M.; Lagumersindez-Denis, N.; Acosta-Medina, E.F.; Falcón-Cama, V.; Alonso-Ramírez, R.; Valenzuela-Silva, C.; Rodríguez-Jiménez, E.; Llópiz-Arzuaga, A.; et al. C-Phycocyanin ameliorates experimental autoimmune encephalomyelitis and induces regulatory T cells. Int. Immunopharmacol. 2011, 11, 29–38. [Google Scholar] [CrossRef] [PubMed]
- Koh, E.-J.; Kim, K.-J.; Choi, J.; Kang, D.-H.; Lee, B.-Y. Spirulina maxima extract prevents cell death through BDNF activation against amyloid beta 1-42 (Aβ 1-42) induced neurotoxicity in PC12 cells. Neurosci. Lett. 2018, 673, 33–38. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, M.; Perumal, Y.; Bansal, S.; Arora, S.; Chopra, K. Phycocyanin alleviates ICV-STZ induced cognitive and molecular deficits via PI3-Kinase dependent pathway. Food Chem. Toxicol. 2020, 145, 111684. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Gan, L.; Yan, S.; Yan, Y.; Huang, W. Effect of C-phycocyanin on HDAC3 and miRNA-335 in Alzheimer’s disease. Transl. Neurosci. 2020, 11, 161–172. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Jovcevski, B.; Pukala, T.L. C-Phycocyanin from Spirulina Inhibits α-Synuclein and Amyloid-β Fibril Formation but Not Amorphous Aggregation. J. Nat. Prod. 2019, 82, 66–73. [Google Scholar] [CrossRef] [PubMed]
- Prabakaran, G.; Sampathkumar, P.; Kavisri, M.; Moovendhan, M. Extraction and characterization of phycocyanin from Spirulina platensis and evaluation of its anticancer, antidiabetic and antiinflammatory effect. Int. J. Biol. Macromol. 2020, 153, 256–263. [Google Scholar] [CrossRef]
- Husain, A.; Alouffi, S.; Khanam, A.; Akasha, R.; Farooqui, A.; Ahmad, S. Therapeutic Efficacy of Natural Product ‘C-Phycocyanin’ in Alleviating Streptozotocin-Induced Diabetes via the Inhibition of Glycation Reaction in Rats. Int. J. Mol. Sci. 2022, 23, 14235. [Google Scholar] [CrossRef]
- Ou, Y.; Lin, L.; Yang, X.; Pan, Q.; Cheng, X. Antidiabetic potential of phycocyanin: Effects on KKAy mice. Pharm. Biol. 2013, 51, 539–544. [Google Scholar] [CrossRef]
- Hao, S.; Li, F.; Li, Q.; Yang, Q.; Zhang, W. Phycocyanin Protects against High Glucose High Fat Diet Induced Diabetes in Mice and Participates in AKT and AMPK Signaling. Foods 2022, 11, 3183. [Google Scholar] [CrossRef]
- El-Sayed, E.-S.M.; Hikal, M.S.; Abo El-Khair, B.E.; El-Ghobashy, R.E.; El-Assar, A.M. Hypoglycemic and hypolipidemic effects of spirulina platensis, phycocyanin, phycocyanopeptide and phycocyanobilin on male diabetic rats. Arab. Univ. J. Agric. Sci. 2018, 26, 1121–1134. [Google Scholar] [CrossRef]
- Khan, M.; Shobha, J.C.; Mohan, I.K.; Naidu, M.U.R.; Prayag, A.; Kutala, V.K. Spirulina attenuates cyclosporine-induced nephrotoxicity in rats. J. Appl. Toxicol. 2006, 26, 444–451. [Google Scholar] [CrossRef] [PubMed]
- Bhat, V.B.; Madyastha, K. C-Phycocyanin: A Potent Peroxyl Radical Scavenger in Vivo and in Vitro. Biochem. Biophys. Res. Commun. 2000, 275, 20–25. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Rojas, B.; Medina-Campos, O.N.; Hernández-Pando, R.; Negrette-Guzmán, M.; Huerta-Yepez, S.; Pedraza-Chaverri, J. C-Phycocyanin prevents cisplatin-induced nephrotoxicity through inhibition of oxidative stress. Food Funct. 2014, 5, 480–490. [Google Scholar] [CrossRef]
- Zheng, J.; Inoguchi, T.; Sasaki, S.; Maeda, Y.; McCarty, M.F.; Fujii, M.; Ikeda, N.; Kobayashi, K.; Sonoda, N.; Takayanagi, R. Phycocyanin and phycocyanobilin from Spirulina platensis protect against diabetic nephropathy by inhibiting oxidative stress. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2013, 304, R110–R120. [Google Scholar] [CrossRef]
- Lim, B.J.; Jeong, J.Y.; Chang, Y.-K.; Na, K.R.; Lee, K.W.; Shin, Y.-T.; Choi, D.E. C-Phycocyanin Attenuates Cisplatin-Induced Nephrotoxicity in Mice. Ren. Fail. 2012, 34, 892–900. [Google Scholar] [CrossRef]
- Strasky, Z.; Zemankova, L.; Nemeckova, I.; Rathouska, J.; Wong, R.J.; Muchova, L.; Subhanova, I.; Vanikova, J.; Vanova, K.; Vitek, L.; et al. Spirulina platensis and phycocyanobilin activate atheroprotective heme oxygenase-1: A possible implication for atherogenesis. Food Funct. 2013, 4, 1586–1594. [Google Scholar] [CrossRef]
- Blas-Valdivia, V.; Moran-Dorantes, D.N.; Rojas-Franco, P.; Franco-Colin, M.; Mirhosseini, N.; Davarnejad, R.; Halajisani, A.; Tavakoli, O.; Cano-Europa, E. C-Phycocyanin prevents acute myocardial infarction-induced oxidative stress, inflammation and cardiac damage. Pharm. Biol. 2022, 60, 755–763. [Google Scholar] [CrossRef]
- Sheu, M.-J.; Hsieh, Y.-Y.; Lai, C.-H.; Chang, C.-C.; Wu, C.-H. Antihyperlipidemic and Antioxidant Effects of C-phycocyanin in Golden Syrian Hamsters Fed with a Hypercholesterolemic Diet. J. Tradit. Complement. Med. 2013, 3, 41–47. [Google Scholar] [CrossRef]
- Ichimura, M.; Kato, S.; Tsuneyama, K.; Matsutake, S.; Kamogawa, M.; Hirao, E.; Miyata, A.; Mori, S.; Yamaguchi, N.; Suruga, K.; et al. Phycocyanin prevents hypertension and low serum adiponectin level in a rat model of metabolic syndrome. Nutr. Res. 2013, 33, 397–405. [Google Scholar] [CrossRef]
- Seo, Y.-J.; Kim, K.-J.; Choi, J.; Koh, E.-J.; Lee, B.-Y. Spirulina maxima Extract Reduces Obesity through Suppression of Adipogenesis and Activation of Browning in 3T3-L1 Cells and High-Fat Diet-Induced Obese Mice. Nutrients 2018, 10, 712. [Google Scholar] [CrossRef] [PubMed]
- Madhyastha, H.K.; Radha, K.S.; Nakajima, Y.; Omura, S.; Maruyama, M. uPA dependent and independent mechanisms of wound healing by C-phycocyanin. J. Cell. Mol. Med. 2008, 12, 2691–2703. [Google Scholar] [CrossRef]
- Madhyastha, H.; Radha, K.; Sugiki, M.; Omura, S.; Maruyama, M. C-phycocyanin transcriptionally regulates uPA mRNA through cAMP mediated PKA pathway in human fibroblast WI-38 cells. Biochim. Biophys. Acta Gen. Subj. 2006, 1760, 1624–1630. [Google Scholar] [CrossRef]
- Kose, A.; Oncel, S.S. Design of melanogenesis regulatory peptides derived from phycocyanin of the microalgae Spirulina platensis. Peptides 2022, 152, 170783. [Google Scholar] [CrossRef]
- Wu, L.-C.; Lin, Y.-Y.; Yang, S.-Y.; Weng, Y.-T.; Tsai, Y.-T. Antimelanogenic effect of c-phycocyanin through modulation of tyrosinase expression by upregulation of ERK and downregulation of p38 MAPK signaling pathways. J. Biomed. Sci. 2011, 18, 74. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Rojas, B.; Hernández-Juárez, J.; Pedraza-Chaverri, J. Nutraceutical properties of phycocyanin. J. Funct. Foods 2014, 11, 375–392. [Google Scholar] [CrossRef]
- Romay, C.; Gonzalez, R.; Pizarro, M.; Lissi, E. Kinetics of c-Phycocyanin Reaction with Hypochlorite. J. Protein Chem. 2000, 19, 151–155. [Google Scholar] [CrossRef] [PubMed]
- Bhat, V.B.; Madyastha, K. Scavenging of Peroxynitrite by Phycocyanin and Phycocyanobilin from Spirulina platensis: Protection against Oxidative Damage to DNA. Biochem. Biophys. Res. Commun. 2001, 285, 262–266. [Google Scholar] [CrossRef]
- Pleonsil, P.; Soogarun, S.; Suwanwong, Y. Anti-oxidant activity of holo- and apo-c-phycocyanin and their protective effects on human erythrocytes. Int. J. Biol. Macromol. 2013, 60, 393–398. [Google Scholar] [CrossRef]
- Patel, A.; Mishra, S.; Pawar, R.; Ghosh, P. Purification and characterization of C-Phycocyanin from cyanobacterial species of marine and freshwater habitat. Protein Expr. Purif. 2005, 40, 248–255. [Google Scholar] [CrossRef]
- Xu, F.; Zhang, Y.; Qiu, Y.; Yang, F.; Liu, G.; Dong, X.; Chen, G.; Cao, C.; Zhang, Q.; Zhang, S.; et al. Three novel antioxidant peptides isolated from C-phycocyanin against H2O2-induced oxidative stress in zebrafish via Nrf2 signaling pathway. Front. Mar. Sci. 2022, 9, 1098091. [Google Scholar] [CrossRef]
- Liu, Q.; Li, W.; Qin, S. Therapeutic effect of phycocyanin on acute liver oxidative damage caused by X-ray. Biomed. Pharmacother. 2020, 130, 110553. [Google Scholar] [CrossRef] [PubMed]
- Dranseikienė, D.; Balčiūnaitė-Murzienė, G.; Karosienė, J.; Morudov, D.; Juodžiukynienė, N.; Hudz, N.; Gerbutavičienė, R.J.; Savickienė, N. Cyano-Phycocyanin: Mechanisms of Action on Human Skin and Future Perspectives in Medicine. Plants 2022, 11, 1249. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Wang, Y.; Yin, Q.; Liu, G.; Liu, H.; Huang, Y.; Li, B. Phycocyanin: A Potential Drug for Cancer Treatment. J. Cancer 2017, 8, 3416–3429. [Google Scholar] [CrossRef] [PubMed]
- Morcos, N.C.; Berns, M.; Henry, W.L. Phycocyanin: Laser activation, cytotoxic effects, and uptake in human atherosclerotic plaque. Lasers Surg. Med. 1988, 8, 10–17. [Google Scholar] [CrossRef]
- Thangam, R.; Suresh, V.; Princy, W.A.; Rajkumar, M.; SenthilKumar, N.; Gunasekaran, P.; Rengasamy, R.; Anbazhagan, C.; Kaveri, K.; Kannan, S. C-Phycocyanin from Oscillatoria tenuis exhibited an antioxidant and in vitro antiproliferative activity through induction of apoptosis and G0/G1 cell cycle arrest. Food Chem. 2013, 140, 262–272. [Google Scholar] [CrossRef]
- Wynn, T.A. Cellular and molecular mechanisms of fibrosis. J. Pathol. 2008, 214, 199–210. [Google Scholar] [CrossRef]
- Byrd, A.L.; Belkaid, Y.; Segre, J.A. The human skin microbiome. Nat. Rev. Microbiol. 2018, 16, 143–155. [Google Scholar] [CrossRef]
- AMR Review Paper—Tackling a Crisis for the Health and Wealth of Nations_1.Pdf. Available online: https://amr-review.org/sites/default/files/AMR%20Review%20Paper%20-%20Tackling%20a%20crisis%20for%20the%20health%20and%20wealth%20of%20nations_1.pdf (accessed on 4 March 2023).
- Lee, Y.B.; Byun, E.J.; Kim, H.S. Potential Role of the Microbiome in Acne: A Comprehensive Review. J. Clin. Med. 2019, 8, 987. [Google Scholar] [CrossRef]
- Habib, R.; Noureen, N.; Nadeem, N. Decoding Common Features of Neurodegenerative Disorders: From Differentially Expressed Genes to Pathways. Curr. Genom. 2018, 19, 300–312. [Google Scholar] [CrossRef]
- Pentón-Rol, G.; Marín-Prida, J.; McCarty, M.F. C-Phycocyanin-derived Phycocyanobilin as a Potential Nutraceutical Approach for Major Neurodegenerative Disorders and COVID-19-induced Damage to the Nervous System. Curr. Neuropharmacol. 2021, 19, 2250–2275. [Google Scholar] [CrossRef] [PubMed]
- Hirata, T.; Tanaka, M.; Ooike, M.; Tsunomura, T.; Sakaguchi, M. Antioxidant activities of phycocyanobilin prepared from Spirulina platensis. J. Appl. Phycol. 2000, 12, 435–439. [Google Scholar] [CrossRef]
- Pentón-Rol, G.; Marín-Prida, J.; Falcón-Cama, V. C-Phycocyanin and Phycocyanobilin as Remyelination Therapies for Enhancing Recovery in Multiple Sclerosis and Ischemic Stroke: A Preclinical Perspective. Behav. Sci. 2018, 8, 15. [Google Scholar] [CrossRef]
- Hua, P.; Yu, Z.; Xiong, Y.; Liu, B.; Zhao, L. Regulatory Efficacy of Spirulina platensis Protease Hydrolyzate on Lipid Metabolism and Gut Microbiota in High-Fat Diet-Fed Rats. Int. J. Mol. Sci. 2018, 19, 4023. [Google Scholar] [CrossRef]
- Xie, Y.; Li, W.; Zhu, L.; Zhai, S.; Qin, S.; Du, Z. Effects of phycocyanin in modulating the intestinal microbiota of mice. Microbiologyopen 2019, 8, e00825. [Google Scholar] [CrossRef] [PubMed]
- Elbialy, Z.I.; Assar, D.H.; Abdelnaby, A.; Abu Asa, S.; Abdelhiee, E.Y.; Ibrahim, S.S.; Abdel-Daim, M.M.; Almeer, R.; Atiba, A. Healing potential of Spirulina platensis for skin wounds by modulating bFGF, VEGF, TGF-ß1 and α-SMA genes expression targeting angiogenesis and scar tissue formation in the rat model. Biomed. Pharmacother. 2021, 137, 111349. [Google Scholar] [CrossRef]
- Munawaroh, H.S.H.; Gumilar, G.G.; Nurjanah, F.; Yuliani, G.; Aisyah, S.; Kurnia, D.; Wulandari, A.P.; Kurniawan, I.; Ningrum, A.; Koyandev, A.K.; et al. In-vitro molecular docking analysis of microalgae extracted phycocyanin as an anti-diabetic candidate. Biochem. Eng. J. 2020, 161, 107666. [Google Scholar] [CrossRef]
- Ashaolu, T.J.; Samborska, K.; Lee, C.C.; Tomas, M.; Capanoglu, E.; Tarhan, Ö.; Taze, B.; Jafari, S.M. Phycocyanin, a super functional ingredient from algae; properties, purification characterization, and applications. Int. J. Biol. Macromol. 2021, 193 Pt B, 2320–2331. [Google Scholar] [CrossRef]
- Wen, X.; Han, Z.; Liu, S.-J.; Hao, X.; Zhang, X.-J.; Wang, X.-Y.; Zhou, C.-J.; Ma, Y.-Z.; Liang, C.-G. Phycocyanin Improves Reproductive Ability in Obese Female Mice by Restoring Ovary and Oocyte Quality. Front. Cell Dev. Biol. 2020, 8, 595373. [Google Scholar] [CrossRef]
- Syarina, P.N.A.; Karthivashan, G.; Abas, F.; Arulselvan, P.; Fakurazi, S. Wound healing potential of Spirulina platensis extracts on human dermal fibroblast cells. EXCLI J. 2015, 14, 385–393. [Google Scholar] [CrossRef]
- Liu, P.; Choi, J.; Lee, M.; Choi, Y.H.; Nam, T. Spirulina protein promotes skin wound repair in a mouse model of full-thickness dermal excisional wound. Int. J. Mol. Med. 2020, 46, 351–359. [Google Scholar] [CrossRef] [PubMed]
- Fu, C.; Chen, J.; Lu, J.; Yi, L.; Tong, X.; Kang, L.; Pei, S.; Ouyang, Y.; Jiang, L.; Ding, Y.; et al. Roles of inflammation factors in melanogenesis. Mol. Med. Rep. 2020, 21, 1421–1430. [Google Scholar] [CrossRef] [PubMed]
- Favas, R.; Morone, J.; Martins, R.; Vasconcelos, V.; Lopes, G. Cyanobacteria and microalgae bioactive compounds in skin-ageing: Potential to restore extracellular matrix filling and overcome hyperpigmentation. J. Enzym. Inhib. Med. Chem. 2021, 36, 1829–1838. [Google Scholar] [CrossRef] [PubMed]
- Nicolaidou, E.; Katsambas, A.D. Pigmentation disorders: Hyperpigmentation and hypopigmentation. Clin. Dermatol. 2014, 32, 66–72. [Google Scholar] [CrossRef] [PubMed]
- Sulzer, D.; Cassidy, C.; Horga, G.; Kang, U.J.; Fahn, S.; Casella, L.; Pezzoli, G.; Langley, J.; Hu, X.P.; Zucca, F.A.; et al. Neuromelanin detection by magnetic resonance imaging (MRI) and its promise as a biomarker for Parkinson’s disease. npj Park. Dis. 2018, 4, 11. [Google Scholar] [CrossRef]
- Mahendra, J.; Mahendra, L.; Muthu, J.; John, L.; Romanos, G.E. Clinical Effects of Subgingivally Delivered Spirulina Gel in Chronic Periodontitis Cases: A Placebo Controlled Clinical Trial. J. Clin. Diagn. Res. 2013, 7, 2330–2333. [Google Scholar] [CrossRef]
- Patil, S.; Al-Zarea, B.K.; Maheshwari, S.; Sahu, R. Comparative evaluation of natural antioxidants spirulina and aloe vera for the treatment of oral submucous fibrosis. J. Oral Biol. Craniofacial Res. 2015, 5, 11–15. [Google Scholar] [CrossRef]
- Ge, Y.; Kang, Y.-K.; Dong, L.; Liu, L.-H.; An, G.-Y. The efficacy of dietary Spirulina as an adjunct to chemotherapy to improve immune function and reduce myelosuppression in patients with malignant tumors. Transl. Cancer Res. 2019, 8, 1065–1073. [Google Scholar] [CrossRef]
- Tavanandi, H.A.; Mittal, R.; Chandrasekhar, J.; Raghavarao, K. Simple and efficient method for extraction of C-Phycocyanin from dry biomass of Arthospira platensis. Algal Res. 2018, 31, 239–251. [Google Scholar] [CrossRef]
- Silveira, S.T.; Burkert, J.F.D.M.; Costa, J.A.V.; Burkert, C.A.V.; Kalil, S.J. Optimization of phycocyanin extraction from Spirulina platensis using factorial design. Bioresour. Technol. 2007, 98, 1629–1634. [Google Scholar] [CrossRef]
- Chentir, I.; Hamdi, M.; Li, S.; Doumandji, A.; Markou, G.; Nasri, M. Stability, bio-functionality and bio-activity of crude phycocyanin from a two-phase cultured Saharian Arthrospira sp. strain. Algal Res. 2018, 35, 395–406. [Google Scholar] [CrossRef]
- Ferreira-Santos, P.; Nunes, R.; De Biasio, F.; Spigno, G.; Gorgoglione, D.; Teixeira, J.A.; Rocha, C.M. Influence of thermal and electrical effects of ohmic heating on C-phycocyanin properties and biocompounds recovery from Spirulina platensis. LWT 2020, 128, 109491. [Google Scholar] [CrossRef]
- Ilter, I.; Akyıl, S.; Demirel, Z.; Koç, M.; Conk-Dalay, M.; Kaymak-Ertekin, F. Optimization of phycocyanin extraction from Spirulina platensis using different techniques. J. Food Compos. Anal. 2018, 70, 78–88. [Google Scholar] [CrossRef]
- Pan-Utai, W.; Iamtham, S. Extraction, purification and antioxidant activity of phycobiliprotein from Arthrospira platensis. Process. Biochem. 2019, 82, 189–198. [Google Scholar] [CrossRef]
- Käferböck, A.; Smetana, S.; de Vos, R.; Schwarz, C.; Toepfl, S.; Parniakov, O. Sustainable extraction of valuable components from Spirulina assisted by pulsed electric fields technology. Algal Res. 2020, 48, 101914. [Google Scholar] [CrossRef]
- Pott, R.W.M. The release of the blue biological pigment C-phycocyanin through calcium-aided cytolysis of live Spirulina sp. Color. Technol. 2019, 135, 17–21. [Google Scholar] [CrossRef]
- Martínez, J.M.; Luengo, E.; Saldaña, G.; Álvarez, I.; Raso, J. C-phycocyanin extraction assisted by pulsed electric field from Artrosphira platensis. Food Res. Int. 2017, 99 Pt 3, 1042–1047. [Google Scholar] [CrossRef]
- Jaeschke, D.P.; Mercali, G.D.; Marczak, L.D.F.; Müller, G.; Frey, W.; Gusbeth, C. Extraction of valuable compounds from Arthrospira platensis using pulsed electric field treatment. Bioresour. Technol. 2019, 283, 207–212. [Google Scholar] [CrossRef]
- Liao, X.; Zhang, B.; Wang, X.; Yan, H.; Zhang, X. Purification of C-Phycocyanin from Spirulina platensis by Single-Step Ion-Exchange Chromatography. Chromatographia 2011, 73, 291–296. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, Z.; Paciulli, M.; Abbaspourrad, A. Extraction of phycocyanin—A natural blue colorant from dried spirulina biomass: Influence of processing parameters and extraction techniques. J. Food Sci. 2020, 85, 727–735. [Google Scholar] [CrossRef]
- Ruiz-Domínguez, M.C.; Jáuregui, M.; Medina, E.; Jaime, C.; Cerezal, P. Rapid Green Extractions of C-Phycocyanin from Arthrospira maxima for Functional Applications. Appl. Sci. 2019, 9, 1987. [Google Scholar] [CrossRef]
- Giannoglou, M.; Andreou, V.; Thanou, I.; Markou, G.; Katsaros, G. High pressure assisted extraction of proteins from wet biomass of Arthrospira platensis (spirulina)—A kinetic approach. Innov. Food Sci. Emerg. Technol. 2022, 81, 103138. [Google Scholar] [CrossRef]
- Tavanandi, H.A.; Raghavarao, K. Ultrasound-assisted enzymatic extraction of natural food colorant C-Phycocyanin from dry biomass of Arthrospira platensis. LWT 2020, 118, 108802. [Google Scholar] [CrossRef]
- Acker, J.P.; McGann, L.E. Protective effect of intracellular ice during freezing? Cryobiology 2003, 46, 197–202. [Google Scholar] [CrossRef] [PubMed]
- Ores, J.D.C.; de Amarante, M.C.A.; Kalil, S.J. Co-production of carbonic anhydrase and phycobiliproteins by Spirulina sp. and Synechococcus nidulans. Bioresour. Technol. 2016, 219, 219–227. [Google Scholar] [CrossRef] [PubMed]
- Garcia, E.S.; Lo, C.; Eppink, M.; Wijffels, R.; van den Berg, C. Understanding mild cell disintegration of microalgae in bead mills for the release of biomolecules. Chem. Eng. Sci. 2019, 203, 380–390. [Google Scholar] [CrossRef]
- Günerken, E.; D’Hondt, E.; Eppink, M.; Garcia-Gonzalez, L.; Elst, K.; Wijffels, R. Cell disruption for microalgae biorefineries. Biotechnol. Adv. 2015, 33, 243–260. [Google Scholar] [CrossRef]
- Montalescot, V.; Rinaldi, T.; Touchard, R.; Jubeau, S.; Frappart, M.; Jaouen, P.; Bourseau, P.; Marchal, L. Optimization of bead milling parameters for the cell disruption of microalgae: Process modeling and application to Porphyridium cruentum and Nannochloropsis oculata. Bioresour. Technol. 2015, 196, 339–346. [Google Scholar] [CrossRef]
- Soto-Sierra, L.; Stoykova, P.; Nikolov, Z.L. Extraction and fractionation of microalgae-based protein products. Algal Res. 2018, 36, 175–192. [Google Scholar] [CrossRef]
- Tiwari, B.K. Ultrasound: A clean, green extraction technology. TrAC Trends Anal. Chem. 2015, 71, 100–109. [Google Scholar] [CrossRef]
- Vernès, L.; Abert-Vian, M.; El Maâtaoui, M.; Tao, Y.; Bornard, I.; Chemat, F. Application of ultrasound for green extraction of proteins from spirulina. Mechanism, optimization, modeling, and industrial prospects. Ultrason. Sonochem. 2019, 54, 48–60. [Google Scholar] [CrossRef] [PubMed]
- Vorobiev, E.; Lebovka, N. 2–Extraction from Foods and Biomaterials Enhanced by Pulsed Electric Energy. In Innovative Food Processing Technologies; Knoerzer, K., Juliano, P., Smithers, G., Eds.; Woodhead Publishing Series in Food Science, Technology and Nutrition; Woodhead Publishing: Sawston, UK, 2016; pp. 31–56. [Google Scholar] [CrossRef]
- Lebovka, N.; Vorobiev, E. (Eds.) Electrotechnologies for Extraction from Food Plants and Biomaterials; Food Engineering Series; Springer: New York, NY, USA, 2009. [Google Scholar] [CrossRef]
- Song, W.; Zhao, C.; Wang, S. A Large-Scale Preparation Method of High Purity C-Phycocyanin. Int. J. Biosci. Biochem. Bioinform. 2013, 3, 293–297. Available online: http://www.ijbbb.org/papers/216-S007.pdf. (accessed on 4 March 2023).
- Lee, C.-W.; Bae, G.Y.; Bae, S.-H.; Suh, H.J.; Jo, K. Increased thermal stability of phycocyanin extracted from Spirulina platensis by cysteine addition during enzyme extraction. Food Sci. Technol. 2022, 42, e15021. [Google Scholar] [CrossRef]
- Patil, G.; Raghavarao, K.S.M.S. Aqueous two phase extraction for purification of C-phycocyanin. Biochem. Eng. J. 2007, 34, 156–164. [Google Scholar] [CrossRef]
- Chaiklahan, R.; Chirasuwan, N.; Bunnag, B. Stability of phycocyanin extracted from Spirulina sp.: Influence of temperature, pH and preservatives. Process. Biochem. 2012, 47, 659–664. [Google Scholar] [CrossRef]
- Antelo, F.S.; Costa, J.A.; Kalil, S.J. Thermal degradation kinetics of the phycocyanin from Spirulina platensis. Biochem. Eng. J. 2008, 41, 43–47. [Google Scholar] [CrossRef]
- Patel, A.; Pawar, R.; Mishra, S.; Sonawane, S.; Ghosh, P.K. Kinetic studies on thermal denaturation of C-phycocyanin. Indian J. Biochem. Biophys. 2004, 41, 254–257. [Google Scholar]
- Böcker, L.; Ortmann, S.; Surber, J.; Leeb, E.; Reineke, K.; Mathys, A. Biphasic short time heat degradation of the blue microalgae protein phycocyanin from Arthrospira platensis. Innov. Food Sci. Emerg. Technol. 2019, 52, 116–121. [Google Scholar] [CrossRef]
- Su, C.-H.; Liu, C.-S.; Yang, P.-C.; Syu, K.-S.; Chiuh, C.-C. Solid–liquid extraction of phycocyanin from Spirulina platensis: Kinetic modeling of influential factors. Sep. Purif. Technol. 2014, 123, 64–68. [Google Scholar] [CrossRef]
- Wicaksono, H.A.; Satyantini, W.H.; Masithah, E.D. The spectrum of light and nutrients required to increase the production of phycocyanin Spirulina platensis. IOP Conf. Ser. Earth Environ. Sci. 2019, 236, 012008. [Google Scholar] [CrossRef]
- Walter, A.; De Carvalho, J.C.; Soccol, V.T.; De Faria, A.B.B.; Ghiggi, V.; Soccol, C.R. Study of phycocyanin production from Spirulina platensis under different light spectra. Braz. Arch. Biol. Technol. 2011, 54, 675–682. [Google Scholar] [CrossRef]
- Escalante, F.M.E.; Pérez-Rico, D.A.; Alarcón-Jiménez, J.L.; González-Morales, E.; Guerra-Álvarez, L.F.; Ramírez-Vázquez, J.C.; Gutiérrez-Pulido, H. Phycocyanin Thermo-photostability: An Accelerated Life-test Analysis. J. Mex. Chem. Soc. 2020, 64, 218–229. [Google Scholar] [CrossRef]
- Choi, W.Y.; Lee, H.Y. Kinetic Analysis of Stabilizing C-Phycocyanin in the Spirulina platensis Extracts from Ultrasonic Process Associated with Effects of Light and Temperature. Appl. Sci. 2018, 8, 1662. [Google Scholar] [CrossRef]
- Aftari, R.V.; Rezaei, K.; Mortazavi, A.; Bandani, A.R. The Optimized Concentration and Purity of Spirulina platensis C-Phycocyanin: A Comparative Study on Microwave-Assisted and Ultrasound-Assisted Extraction Methods. J. Food Process. Preserv. 2015, 39, 3080–3091. [Google Scholar] [CrossRef]
- Wachda; Harjanto, G.D.; Huzain, M.L.; Hadiyanto, H.; Aji, R.W. Production of antioxidant C-phycocyanin using extraction process of Spirulina platensis in large scale industry. IOP Conf. Ser. Mater. Sci. Eng. 2019, 633, 012025. [Google Scholar] [CrossRef]
- Khandual, S.; Sanchez, E.O.L.; Andrews, H.E.; de la Rosa, J.D.P. Phycocyanin content and nutritional profile of Arthrospira platensis from Mexico: Efficient extraction process and stability evaluation of phycocyanin. BMC Chem. 2021, 15, 24. [Google Scholar] [CrossRef] [PubMed]
- Gorgich, M.; Passos, M.L.; Mata, T.M.; Martins, A.A.; Saraiva, M.L.M.; Caetano, N.S. Enhancing extraction and purification of phycocyanin from Arthrospira sp. with lower energy consumption. Energy Rep. 2020, 6, 312–318. [Google Scholar] [CrossRef]
- Adjali, A.; Clarot, I.; Chen, Z.; Marchioni, E.; Boudier, A. Physicochemical degradation of phycocyanin and means to improve its stability: A short review. J. Pharm. Anal. 2022, 12, 406–414. [Google Scholar] [CrossRef]
- Faieta, M.; Neri, L.; Sacchetti, G.; Di Michele, A.; Pittia, P. Role of saccharides on thermal stability of phycocyanin in aqueous solutions. Food Res. Int. 2020, 132, 109093. [Google Scholar] [CrossRef]
- Hadiyanto; Christwardana, M.; Sutanto, H.; Suzery, M.; Amelia, D.; Aritonang, R.F. Kinetic study on the effects of sugar addition on the thermal degradation of phycocyanin from Spirulina sp. Food Biosci. 2018, 22, 85–90. [Google Scholar] [CrossRef]
- Pan-utai, W.; Kahapana, W.; Iamtham, S. Extraction of C-phycocyanin from Arthrospira (Spirulina) and its thermal stability with citric acid. J. Appl. Phycol. 2018, 30, 231–242. [Google Scholar] [CrossRef]
- Mishra, S.K.; Shrivastav, A.; Mishra, S. Effect of preservatives for food grade C-PC from Spirulina platensis. Process. Biochem. 2008, 43, 339–345. [Google Scholar] [CrossRef]
- Sun, L.; Wang, S.; Qiao, Z. Chemical stabilization of the phycocyanin from cyanobacterium Spirulina platensis. J. Biotechnol. 2006, 121, 563–569. [Google Scholar] [CrossRef]
- Zhang, Z.; Li, Y.; Abbaspourrad, A. Improvement of the colloidal stability of phycocyanin in acidified conditions using whey protein-phycocyanin interactions. Food Hydrocoll. 2020, 105, 105747. [Google Scholar] [CrossRef]
- Zhang, Z.; Cho, S.; Dadmohammadi, Y.; Li, Y.; Abbaspourrad, A. Improvement of the storage stability of C-phycocyanin in beverages by high-pressure processing. Food Hydrocoll. 2020, 110, 106055. [Google Scholar] [CrossRef]
- Moreira, J.B.; Lim, L.-T.; Zavareze, E.D.R.; Dias, A.R.G.; Costa, J.A.V.; de Morais, M.G. Antioxidant ultrafine fibers developed with microalga compounds using a free surface electrospinning. Food Hydrocoll. 2019, 93, 131–136. [Google Scholar] [CrossRef]
- Hadiyanto, H.; Christwardana, M.; Suzery, M.; Sutanto, H.; Nilamsari, A.M.; Yunanda, A. Effects of Carrageenan and Chitosan as Coating Materials on the Thermal Degradation of Microencapsulated Phycocyanin from Spirulina sp. Int. J. Food Eng. 2019, 15, 20180290. [Google Scholar] [CrossRef]
- Pan-Utai, W.; Iamtham, S. Enhanced Microencapsulation of C-Phycocyanin from Arthrospira by Freeze-Drying with Different Wall Materials. Food Technol. Biotechnol. 2020, 58, 423–432. [Google Scholar] [CrossRef]
- Schmatz, D.A.; da Silveira Mastrantonio, D.J.; Costa, J.A.V.; de Morais, M.G. Encapsulation of phycocyanin by electrospraying: A promising approach for the protection of sensitive compounds. Food Bioprod. Process. 2019, 119, 206–215. [Google Scholar] [CrossRef]
- Pradeep, H.N.; Nayak, C.A. Enhanced stability of C-phycocyanin colorant by extrusion encapsulation. J. Food Sci. Technol. 2019, 56, 4526–4534. [Google Scholar] [CrossRef]
- Ilter, I.; Koç, M.; Demirel, Z.; Dalay, M.C.; Ertekin, F.K. Improving the stability of phycocyanin by spray dried microencapsulation. J. Food Process. Preserv. 2021, 45, e15646. [Google Scholar] [CrossRef]
- He, S.; Joseph, N.; Feng, S.; Jellicoe, M.; Raston, C.L. Application of microfluidic technology in food processing. Food Funct. 2020, 11, 5726–5737. [Google Scholar] [CrossRef]
- Yan, S.-G.; Zhu, L.-P.; Su, H.-N.; Zhang, X.-Y.; Chen, X.-L.; Zhou, B.-C.; Zhang, Y.-Z. Single-step chromatography for simultaneous purification of C-phycocyanin and allophycocyanin with high purity and recovery from Spirulina (Arthrospira) platensis. J. Appl. Phycol. 2011, 23, 1–6. [Google Scholar] [CrossRef]
- Minic, S.L.; Stanic-Vucinic, D.; Mihailovic, J.; Krstic, M.; Nikolic, M.R.; Velickovic, T.C. Digestion by pepsin releases biologically active chromopeptides from C-phycocyanin, a blue-colored biliprotein of microalga Spirulina. J. Proteom. 2016, 147, 132–139. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.-H.; Wang, S.S.-S.; Show, P.-L.; Hsu, S.-L.; Chang, Y.-K. Rapid and efficient recovery of C-phycocyanin from highly turbid Spirulina platensis algae using stirred fluidized bed ion exchange chromatography. Sep. Purif. Technol. 2019, 209, 636–645. [Google Scholar] [CrossRef]
- de Amarante, M.C.A.; Júnior, L.C.S.C.; Sala, L.; Kalil, S.J. Analytical grade C-phycocyanin obtained by a single-step purification process. Process. Biochem. 2020, 90, 215–222. [Google Scholar] [CrossRef]
- Lauceri, R.; Zittelli, G.C.; Maserti, B.; Torzillo, G. Purification of phycocyanin from Arthrospira platensis by hydrophobic interaction membrane chromatography. Algal Res. 2018, 35, 333–340. [Google Scholar] [CrossRef]
- Figueira, F.D.S.; Moraes, C.C.; Kalil, S.J. C-Phycocyanin purification: Multiple processes for different applications. Braz. J. Chem. Eng. 2018, 35, 1117–1128. [Google Scholar] [CrossRef]
- Moraes, C.C.; Kalil, S.J. Strategy for a protein purification design using C-phycocyanin extract. Bioresour. Technol. 2009, 100, 5312–5317. [Google Scholar] [CrossRef]
- Kumar, D.; Dhar, D.W.; Pabbi, S.; Kumar, N.; Walia, S. Extraction and purification of C-phycocyanin from Spirulina platensis (CCC540). Indian J. Plant Physiol. 2014, 19, 184–188. [Google Scholar] [CrossRef]
- Julianti, E.; Susanti, S.; Singgih, M.; Mulyani, L.N. Optimization of Extraction Method and Characterization of Phycocyanin Pigment from Spirulina platensis. J. Math. Fundam. Sci. 2019, 51, 168–176. [Google Scholar] [CrossRef]
- Chew, K.W.; Chia, S.R.; Krishnamoorthy, R.; Tao, Y.; Chu, D.-T.; Show, P.L. Liquid biphasic flotation for the purification of C-phycocyanin from Spirulina platensis microalga. Bioresour. Technol. 2019, 288, 121519. [Google Scholar] [CrossRef] [PubMed]
- Patil, G.; Chethana, S.; Sridevi, A.S.; Raghavarao, K.S.M.S. Method to obtain C-phycocyanin of high purity. J. Chromatogr. A 2006, 1127, 76–81. [Google Scholar] [CrossRef]
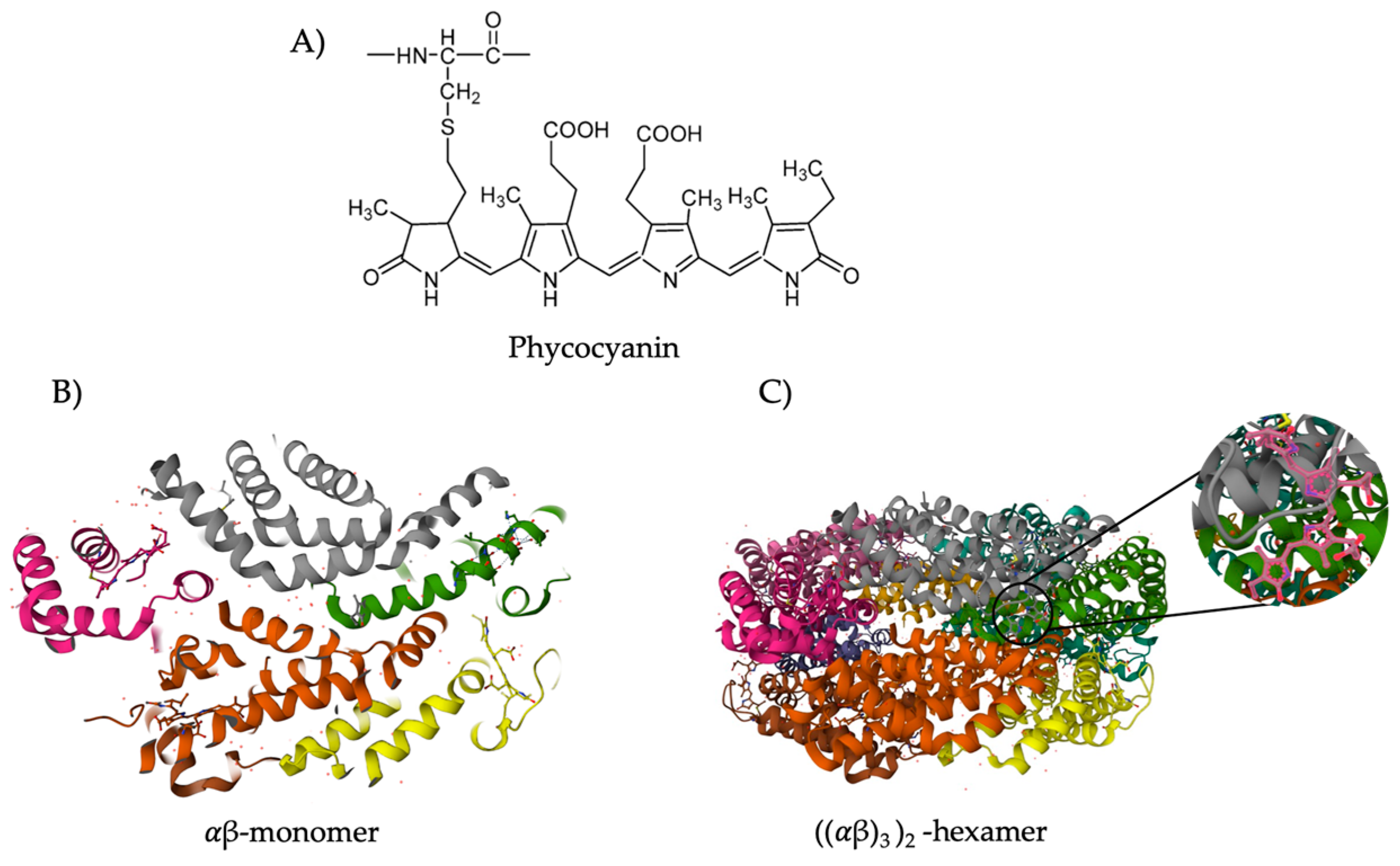
| Compound | Amount | Ref. | Compound | Amount | Ref. |
|---|---|---|---|---|---|
| General composition (in dry weight Spirulina) | Inositol | 6.4 mg | [28] | ||
| Proteins | Folic acid | [22,28] | Pantothenic acid | 10 μg | |
| Carbohydrates | Biotin | Folic acid | 1.0 μg | ||
| Total lipids | 5.0–6.0% | Biotin | 0.5 μg | ||
| Fibers | 3.6–6.0% | Minerals (per 10 g Spirulina) | |||
| Minerals | 7.0–13% | Potassium | 140 mg | [28] | |
| Vitamins (per 10 g Spirulina) | Sodium | 90 mg | |||
| Vitamin A | 23000 IU | [28] | Calcium | 70 mg | |
| Vitamin B1, B2, B3 | 0.4–1.4 mg | Phosphorus | 60 mg | ||
| Vitamin B12, B6 | 20–60 μg | Magnesium | 40 mg | ||
| Vitamin C | 0.8 mg | Iron | 15 mg | ||
| Vitamin D | 1200 IU | Manganese | 0.5 mg | ||
| Vitamin E | 1.0 mg | Zinc | 0.3 mg | ||
| Vitamin K1, K2 | 200 μg | Cooper | 120 μg | ||
| Germanium | 60 μg | [28] | Histidine | 100 mg | [29] |
| Iodine | 55 μg | Tryptophan | 90 mg | ||
| Chrome | 25 μg | Nonessential amino acid (per 10 g Spirulina) | |||
| Selenium | 10 μg | Glutamic acid | 910 mg | [29] | |
| Essential amino acid (per 10 g Spirulina) | Aspartic acid | 610 mg | |||
| Leucine | 540 mg | Alanine | 470 mg | ||
| Valine | 400 mg | Arginine | 430 mg | ||
| Isoleucine | 350 mg | Glycine | 320 mg | ||
| Threonine | 320 mg | Serine | 320 mg | ||
| Lysine | 290 mg | Tyrosine | 300 mg | ||
| Phenylalanine | 280 mg | Proline | 270 mg | ||
| Methionine | 140 mg | Cystine | 60 mg | ||
| Role | Effect | Dose or Concentration (Route of Administration) | Analysis Methodology | Ref. |
|---|---|---|---|---|
| Anti-oxidative | Scavenging of free radicals, lipid peroxidation inhibitor and metal chelator | 1–3 mg/mL | Luminol-enhanced chemiluminescence | [54] |
| 0–0.16 mM | Deoxyribose assay | |||
| 8–20 mg/mL | Inhibition of liver microsomal lipid peroxidation induced by Fe-ascorbic acid | |||
| 50–200 mg/kg (oral) | Glucose oxidase-induced inflammation in vivo | |||
| 62.34 mg/g | DPPH, FRAP and Fe2+—chelating activity | [55] | ||
| 0.125–2.00 mg/mL 0.3125–5.00 mg/mL | ABTS and DPPH | [56] | ||
| 10–100 μg/mL | DPPH | [57] | ||
| Serum antioxidant | 200–1000 mg/kg (oral) | SOD and catalase activity in vivo | [58] | |
| Attenuation of MMPs and ROS | 20–80 μg/mL | MMP-1 and MMP-9 and DCFDA staining | [59] | |
| Attenuation of platelet aggregation by decreasing hydroxyl radicals | 0.5–10 nM | Electron Spin Resonance Spectrometry | [60] | |
| Increase of antioxidant enzymes | 5 μM + 2 Gy radiation | RANDOX kit | [61] | |
| Attenuation of ROS | 10 μM (rat heart perfusion) | Electron paramagnetic resonance spectroscopy | [62] | |
| Attenuation of ROS, MDA and GSH, and maintenance of SOD activity | 100–400 mg/kg (intraperitoneal injection) 31–250 μg/mL | DCFDA staining and histopathologic analysis | [63] | |
| Free radical scavenger | 5–50 mM pre-treatment for 1 h and then co-treatment | DCFDA staining | [64] | |
| Anticancer | Cell cycle arrest in G0/G1, attenuation of proliferation and stimulation of apoptosis | 1–20 μM | Propidium iodide, annexin V-PE, 7-AAD, proliferative, and apoptotic markers | [65] |
| 10–100 μM | MTT assay, cytochrome c, ethidium bromide | [66] | ||
| Alteration of the mitochondrial membrane potential | 10–100 μM | Rhodamine 123 | [67] | |
| Attenuation of MMPs | 5–40 μg/mL | MMP-1, MMP-2, MMP-9, TIMP-1, TIMP-2 | [68] | |
| Attenuation of metastasis | 200 mg/kg (oral) | MMPs, VEGF-A and HIF-1α, activity of MMPs and HIF-1α | [69] | |
| Stimulation of mitochondria-mediated apoptosis | 5–40 μg | Depolarized mitochondria, apoptotic, and proliferative markers | [70] | |
| Drug resistance by preventing the induction of multidrug resistance protein | 1–100 μM | ROS production and COX-2 expression | [71] | |
| Anti-inflammatory | Attenuation of pro-inflammatory mediators and neutrophil infiltration | 30–50 mg/kg | TNF-α, IL-1β, IL-10, nitrite, nitrate, PGE2, COX-2, iNOS, MPO, and NF-kB activity | [72] |
| 0–250 μg/mL | [73] | |||
| Attenuation of lung injury | 50 mg/kg 100–400 mg/kg (intraperitoneal injection) | Lung injury, nitrate/nitrite, pro-inflammatory cytokines in BALF, MPO and NF-kB activity, iNOS, COX-2, lung edema, proapoptotic proteins | [74,75] | |
| Prevention of fibrosis | 10–50 μg/mL 0–200 μg/mL | Nrf2, NQO-1, EMT evaluated through the expression of vimentin, type-1-collagen, fibronectin, α-SMA, N-cadherin, and E-cadherin | [76,77] | |
| Antimicrobial | Decrease the growth of Escherichia coli, Bacillus sp., Staphylococcus aureus, and Salmonella Typhi | 35 μg/mL | Disc diffusion assay and determination of MIC. Comparation with Antibiotic Assay Medium (Himedia). | [78] |
| Attenuate the growth of Listeria monocytogenes, S. aureus, Yersinia ruckeri, E. coli, and Streptococcus iniae | 25 μg/mL | Agar well diffusion assay, MIC and MBC. Comparation with Tetracycline, Amikacin, and Doxycycline | [55] | |
| Impair the growth of S. aureus, Aeromonas hydrofila, and Salmonella Enteritidis. No effect in Enterococcus faecalis | 320 μg/mL | Agar well diffusion method and turbidity liquid media assay. MIC and turbidity at 600 nm | [79] | |
| Antibacterial activity against Pseudomonas aeruginosa MTCC 1034, Klebsiella pneumoniae (ESBL-KP) ATCC 700603, E. coli (ATCC 25922), and S. aureus ATCC 25,923 (MRSA). No effect on Acinetobacter baumanii, Enterococcus durans (P502). | 1000 μg/mL | Mueller–Hinton Agar plates and MIC using broth microdilution method | [80] | |
| Attenuation of acne symptoms and reduction of Propionibacterium acnes and Staphylococcus epidermidis | 10% extract | Disc diffusion method and MIC | [81] | |
| Inhibition of the growth of Candida albicans, Aspergillus niger, Aspergillus flavus, Penicillium sp., and Rhizopus sp. | 40–80 μg/mL | Agar block method and MIC | [82] | |
| Anti-neurodegenerative | Promotor of remyelination | 25 mg/kg (intraperitoneal injection) | Brain biopsies, pro-inflammatory mediators and populations, lipid peroxidation | [83] |
| Attenuation of Alzheimer’s disease markers | 0–20 μg/mL | Intracellular GSH, APP, BACE2, GSH-Px, SOD2, GR, BDNF, α-tubulin | [84] | |
| 50 or 100 mg/kg (intraperitoneal injection) | Morris water maze, novel object recognition and open field test, ChAT, inflammatory and apoptotic mediators, IRS-1, INS, PI3K/AKT, and PTEN gene expression | [85] | ||
| 200 mg/kg (intraperitoneal injection) | Eight-arm radial maze, HAC3, pro-inflammatory, and proapoptic mediators | [86] | ||
| Attenuation of Parkinson’s disease markers | 2.5–7.5 μM | Fibril formation of αS or Aβ40/42, ADH, catalase | [87] | |
| Antidiabetic | Antidiabetic and antiglycation | 100–500 μg/mL | Inhibitory effect of PPA and β-glucosidase | [88] |
| 100 and 200 mg/kg 100 mg/kg 200 mg/kg 50 mg/kg (oral) | Blood glucose, glycosylated hemoglobin HbA1c, BUN, urea, serum creatinine, SGOT/AST, SGPT/ALT, alkaline phosphatase, total bilirubin, TGs, LDL-C, TC, and HDL-C NBT assay, carbonyl content, reduced GSH | [89,90,91,92] | ||
| Hepatoprotection | Attenuation of nephrotoxicity | Not described | Plasma urea, creatinine, urinary N-acetyl-β-D-glucosaminidase, creatinine and lithium, histomorphology evaluation | [93] |
| Reduction of hepatocyte damage | 50–200 mg/kg (intraperitoneal injection) | Hepatic lipid peroxidation assayed by measuring malondialdehyde | [94] | |
| 1–100 μgM | ROS, MDA, GSH, GSH-Px, ALT, AST, SOD, TGF-β1, HGF | [71] | ||
| Nephroprotection | Prevention of cisplatin induced nephrotoxicity by reducing oxidative stress | 5–30 mg/kg (intraperitoneal injection) | Blood urea nitrogen, plasma glutathione peroxidase, plasma creatinine quantification, N-acetyl-β-D-glucosaminidase, apoptosis and histopathological changes; glutathione, malondialdehyde, 4-hydroxynonenal, and oxidized proteins quantification | [95] |
| Protection of Type 2 diabetes mice against oxidative stress and renal dysfunction | 300 mg/kg (oral) | Urinary 8-hydroxy-2-deoxyguanosine, 8-iso-prostaglandin F2α and albumin quantification; immunohistochemistry | [96] | |
| Recovery of cisplatin-induced renal injury in renal tissue and HK-2 cell and reduction of p-ERK, p-JNK, p-p38, Bax, caspase-9, and caspase-3 | 50 mg/kg (intraperitoneal injection) | Light microscopy examination, cell viability Assay, western blot, caspase-3 activity assay, and apoptosis detection by the terminal deoxynucleotidyl transferase-mediated dUTP-biotin nick end labeling method | [97] | |
| Cardiovascular protection | Reduction of atherosclerotic disease | 200 μM | HMOX-1, eNOS, P22, VCAM-1 | [98] |
| Prevention of AMI-induced oxidative stress, inflammation and heart damage | 50 mg/kg (subcutaneous injection) | CK, AST, ALT, ROS, nitrites, oxidized glutathione, pro-inflammatory and proapoptotic cytokines, lipid peroxidation | [99] | |
| Prevention of cardiovascular diseases and atherosclerotic formation | 0.25% and 1.25% (oral) | Cholesterol, MDA, GOT, GPT, catalase, SOD, GSH-Px, HMG CoA | [100] | |
| Anti-obesity | Prevention of endothelial dysfunction and attenuation of obesity | 2500, 5000, or 10,000 mg/kg (oral) | Serum triglyceride, total cholesterol, HDL-C, and glucose, insulin and leptin, immunohistochemistry analysis | [101] |
| Reduction of adipogenesis and lipogenesis | 0, 0.625, 1.25, 2.5, 5, 10, or 20 μg/mL (oral) | Western blots of adipogenic proteins (C/EBPα, PPARγ, and aP2) and lipogenic proteins (SREBP1, ACC, FAS, LPAATβ, Lipin1, and DGAT1) | [102] | |
| Wound healing | Proliferation of fibroblasts, synthesis of ECM components and regeneration | 10–200 μg/mL (superficial collagen films) 50 μg/mL | Cytotoxicity and proliferation/viability of fibroblasts, cdK1, cdK2, uPA, PI3K, and in vivo wound healing analysis | [103,104] |
| Antimelanogenic | Attenuation of melanin production | Not described | Cellular tyrosinase, production of melanin, DPPH | [105] |
| 0.05–2.00 mg/mL | Tyrosinase activity, melanin, intracellular cAMP, MITF, tyrosinase, ERK, pERK1/2, MEK1/2, p38, CREB | [106] |
| Cell Disruption Method | Purity * | Yield (mg/g) | Conditions | Advantages | Limitations | Ref. |
|---|---|---|---|---|---|---|
| Freeze/thaw cycles | 0.56–0.66- | 73.73–74.51 | 6 cycles, −40 °C/4 h + room temperature/1 h, 0.1 M phosphate buffer pH (6.8), 1:6, 1:8 and 1:10 S/L ratios | Continuous damage to the plasmatic membrane, easy to perform, availability | Time and energy consuming, and often require high solvent, leading to an increase in the production costs. Not suitable for industrial scale | [141] |
| 0.4 | ND | 25 °C/4 h, distilled water, 1:25 S/L ratio | [142] | |||
| 0.77 | 217.18 | 3 cycles, −20 °C + room temperature/24 h, 20 mM of sodium acetate and 50 mM of NaCl buffer (pH 5.1) 1:20 S/L ratio | [143] | |||
| 2.10 | 41.90 | 4 cycles, −20 °C/4 h + room temperature/1.5 h, sodium hydroxide, (pH 6.8), 1:20 S/L ratio | [144] | |||
| Mixing and homogenization | 0.6 | 52.11 | 25,200× g/10 min, 0.1 M phosphate buffer (pH 6.8), 1:6 S/L ratio | Simple, availability, reproducibility | Increased temperatures during the process, time consuming, not suitable for industrial scale, cell debris released | [141] |
| ND | ND | Rotary shaker at 30 °C, 10 mM sodium phosphate buffer (pH 7.0), 10 mM sodium acetate buffer (pH 5.0), NaCl 0.15 M and CaCl2 10 g/L, 1:25 S/L ratio | [142] | |||
| ND | 67.61 | 1.71% biomass/solvent ratio, 6237.66 homogenization rate, 15 min extraction time | [145] | |||
| 0.67 | 103.07 | Oven-dried biomass preparation, 70 °C/4 h, extraction at 25 °C/24 h by 0.01 M phosphate buffer using homogenization assisted method at 0.02 g/mL biomass concentration | [146] | |||
| Bead milling | 0.46 | 217.14 | Low-density beads for low viscosity media, 80–85% degree of bead filling | Low time, high biomass disruption, low energy | Time very dependent of the type of bead, low purities, cell debris, additional purification steps needed | [144] |
| ND | 119.48 mg/g | Bead diameter 0.3 mm, glass beads at a speed of 3580 rpm. 4 cycles of milling each 25 s and subsequent cooling at 4 °C | [147] | |||
| ND | 90% recovery | Dakot Zirconia beads (0.5–1.4 mm), 330 rpm agitation, 8 h under 0.5 M Ca (II) in a 0.35 M acetate buffer (pH 6.8) | [148] | |||
| 0.21 | ND | Bead Beater, diameter 0.1 mm, agitation of 4800 rpm, 10 cycles of 10 s. Following each cycle, samples cool down in water at 0 °C | [149] | |||
| ND | 94.90 | Glass beads 0.25–0.5 mm of diameter) in 2 mL flasks, 4 cycles of 25 s at 30 Hz of vibrational frequency | [150] | |||
| Ultrasound | 0.62 | 51.51 | Pre-soaked for 120 min, ultrasonication amplitude of 50%, 2.5 min, 1:6 S/L | High purities, reproducibility, suitable for industrial scale, temperature could be controlled | Increased temperatures during the process, complex process, expensive, specific equipment | [141] |
| ND | 98.84 | 1% biomass/solvent ratio, 60% amplitude, 16.23 min extraction time | [145] | |||
| 0.67–0.93 | 90.00 | Frequencies of 20–100 kHz, power intensities >1 W/cm2, PBS soaking | [146] | |||
| 0.65 | 18.20 | Power 60 W, extraction for 10 s, 30 cycles in total, in ice bath | [151] | |||
| Electric field | 2.45 | 143.33 | Freeze/thaw and pulsed electric field maximum charging voltage of 30 kV, square bipolar pulses with a variable pulse width of 4–32 μs and a pulse frequency up to 300 Hz. | Increased permeability of the membrane | Long time to optimization, complex equipment, intracellular compounds might not be completely released | [141] |
| 0.51 | 151.94 | 40 °C, 25 kV/cm, 150 μs | [149] | |||
| ND | ND | 50 to 200 pulses at 20 kV | [152] | |||
| High-pressure homogenization | ND | ND | 3.5 min with pressures between 50 and 600 MPa, distilled water ratio of 6% (wt%) | Simpler, scalable for industrial application, environmentally friendly, high recovery | Expensive, not useful in extracting dry biomass, can lead to protein denaturation | [152] |
| ND | 291.90 | 100 mM Na-phosphate solvent (pH 7), 1400 bar | [153] | |||
| 1.2–1.4 | 90% recovery | 300 MPa for 10 min, deionized water or phosphate buffer (pH 6.8), 1/20 (w/v) ratio | [154] | |||
| Enzyme-assisted | 0.80–0.90 | 20–25 | 1 mg/mL lysozyme, high pressure homogenizer D-15M at 10–12,000 p.s.i., 4–8 °C | Stable, efficient, eco-friendly | More efficient when combined with other methods | [153] |
| 1.19 | 82.07 | 1.0% enzyme concentration, 16 h incubation time, 1:6 S/L ratio | [155] | |||
| 1.09 | 92.73 | 2.5 min Ultrasonication at 50% Amplitude, 0.6% enzyme concentration, 16 h incubation, 1:6 S/L ratio |
| Purification Method | Conditions | Purity * | Recovery (%) | Ref. |
|---|---|---|---|---|
| Ammonium sulfate (AS) precipitation | 50–65% AS | 2.11 | 86 | [197] |
| Chitosan and activated charcoal | 0.24% chitosan/8.4% activated charcoal | 3.14 | 79 | [198] |
| Stirred fluidized bed IEC | 1%, dw/v in STREAMLINE DEAE 10%, dw/v in STREAMLINE DEAE | 2.70 3.00 | 90 64 | [199] |
| IEC | IEC with pH gradient using an anion-exchanger Q-Sepharose Fast Flow column | 4.20 | 49 | [200] |
| Combined methods | 50–65% AS, dialysis in sodium acetate buffer, IEC on a DEAE-Sepharose Fast Flow column | 5.59 | 67 | [197] |
| 50% AS, dialysis, ultrafiltration with MWCO of 50 kDa and IEC (anion-exchanger resin Q-Sepharose Fast Flow column) with pH gradient | 4.00 | 80 | [44] | |
| 65% AS, 65% dialysis with 12–14 kDa membranes, microfiltration, IEC in a Sephadex-G-100 column, and HPLC with a reverse column | 92% | 53 | [88] | |
| 1.113 M AS, filtration with a PVDF membrane and two hydrophobic interaction membrane chromatography steps | 4.20 | 67 | [201] | |
| 2% w/v chitosan solution, 80 g/L activated charcoal, ultrafiltration, and IEC on DEAE Sephadex A-25 | 4.30 | 42 | [151] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fernandes, R.; Campos, J.; Serra, M.; Fidalgo, J.; Almeida, H.; Casas, A.; Toubarro, D.; Barros, A.I.R.N.A. Exploring the Benefits of Phycocyanin: From Spirulina Cultivation to Its Widespread Applications. Pharmaceuticals 2023, 16, 592. https://doi.org/10.3390/ph16040592
Fernandes R, Campos J, Serra M, Fidalgo J, Almeida H, Casas A, Toubarro D, Barros AIRNA. Exploring the Benefits of Phycocyanin: From Spirulina Cultivation to Its Widespread Applications. Pharmaceuticals. 2023; 16(4):592. https://doi.org/10.3390/ph16040592
Chicago/Turabian StyleFernandes, Raquel, Joana Campos, Mónica Serra, Javier Fidalgo, Hugo Almeida, Ana Casas, Duarte Toubarro, and Ana I. R. N. A. Barros. 2023. "Exploring the Benefits of Phycocyanin: From Spirulina Cultivation to Its Widespread Applications" Pharmaceuticals 16, no. 4: 592. https://doi.org/10.3390/ph16040592
APA StyleFernandes, R., Campos, J., Serra, M., Fidalgo, J., Almeida, H., Casas, A., Toubarro, D., & Barros, A. I. R. N. A. (2023). Exploring the Benefits of Phycocyanin: From Spirulina Cultivation to Its Widespread Applications. Pharmaceuticals, 16(4), 592. https://doi.org/10.3390/ph16040592