In Vitro and In Vivo Functional Viability, and Biocompatibility Evaluation of Bovine Serum Albumin-Ingrained Microemulsion: A Model Based on Sesame Oil as the Payload for Developing an Efficient Drug Delivery Platform
Abstract
1. Introduction
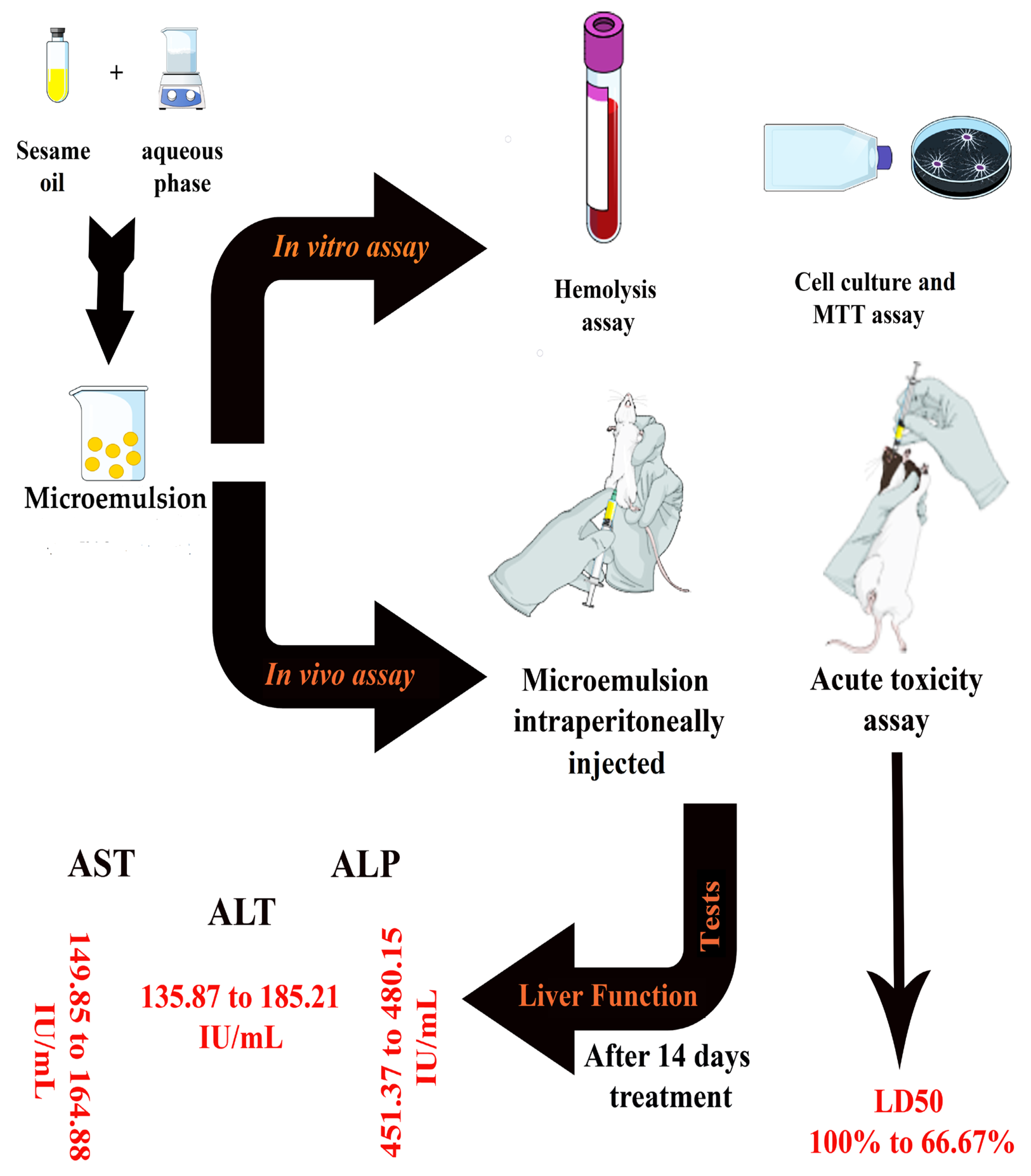
2. Results and Discussion
2.1. Formulation of Optimal Microemulsion Carrier
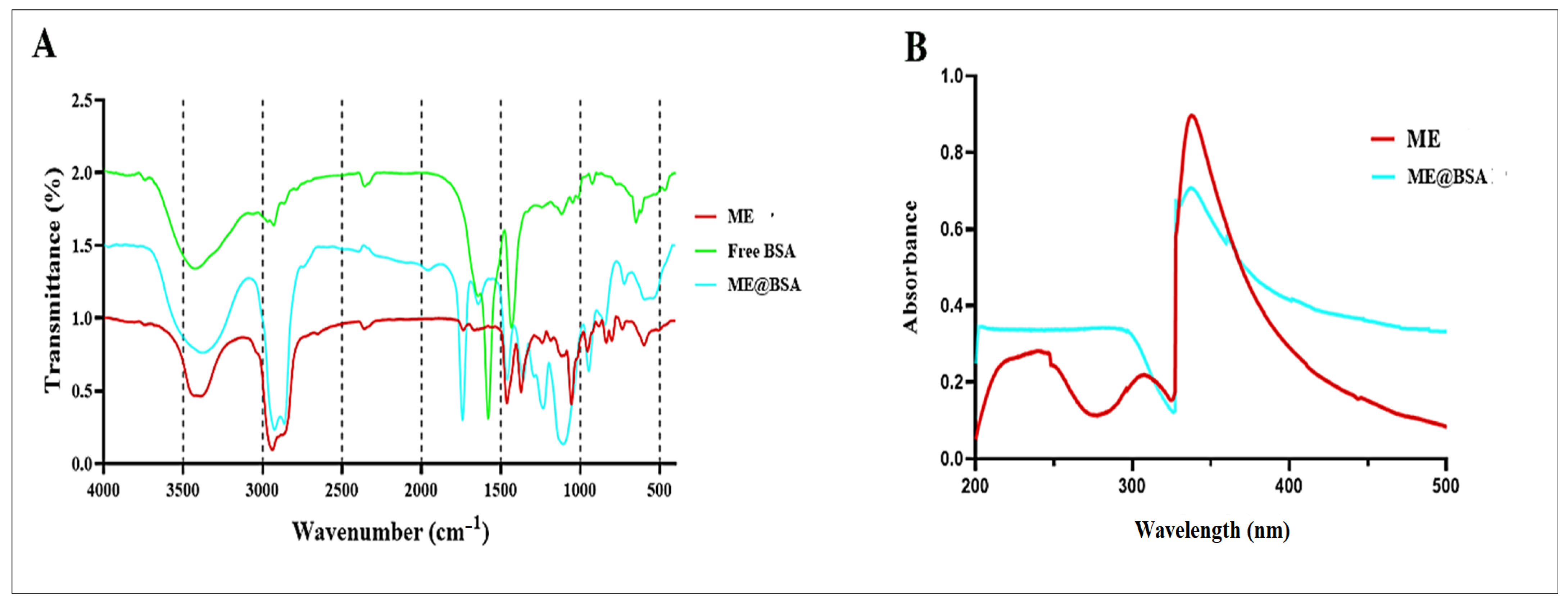
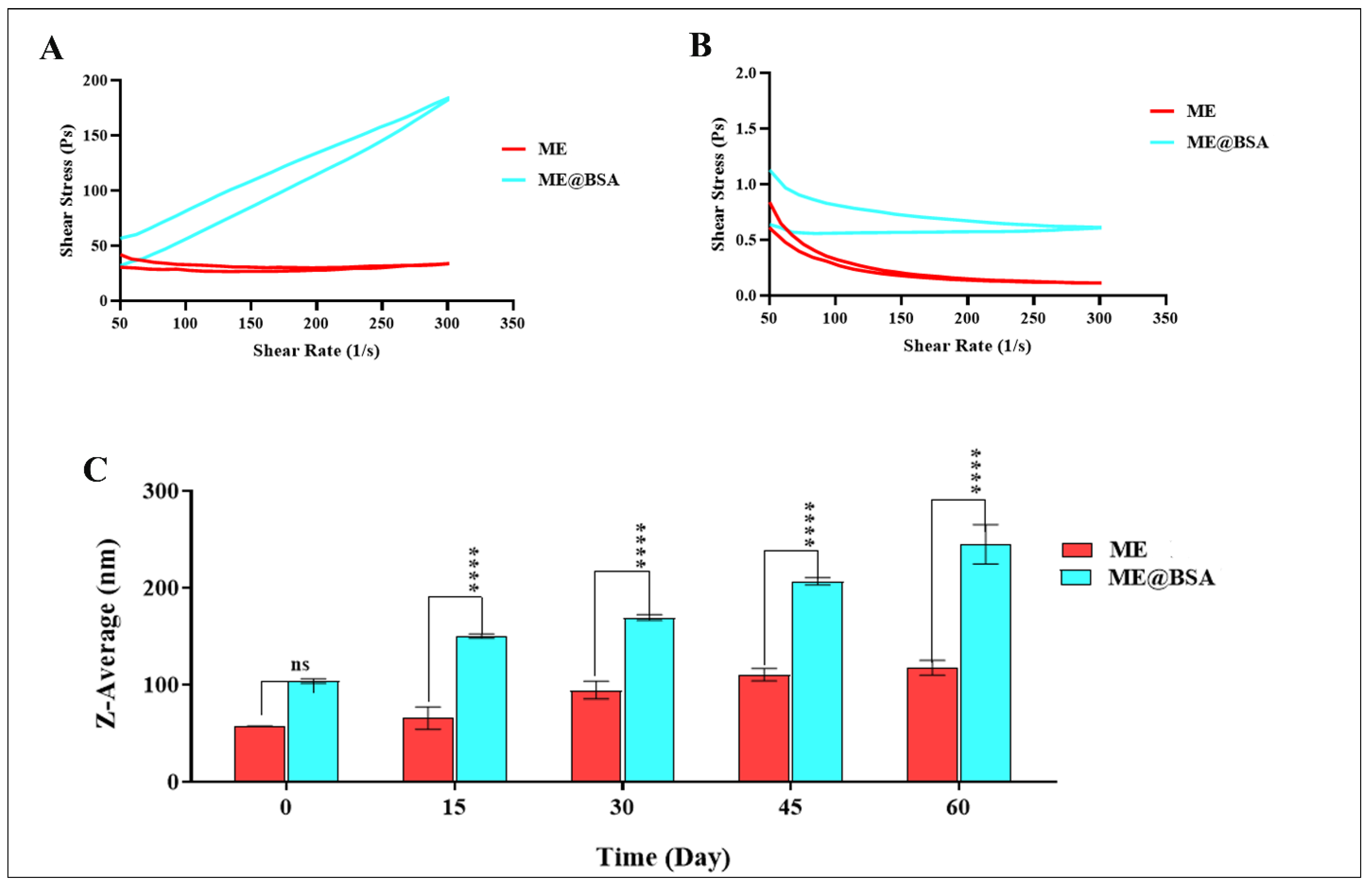
2.2. Physico-Chemical Characterizations
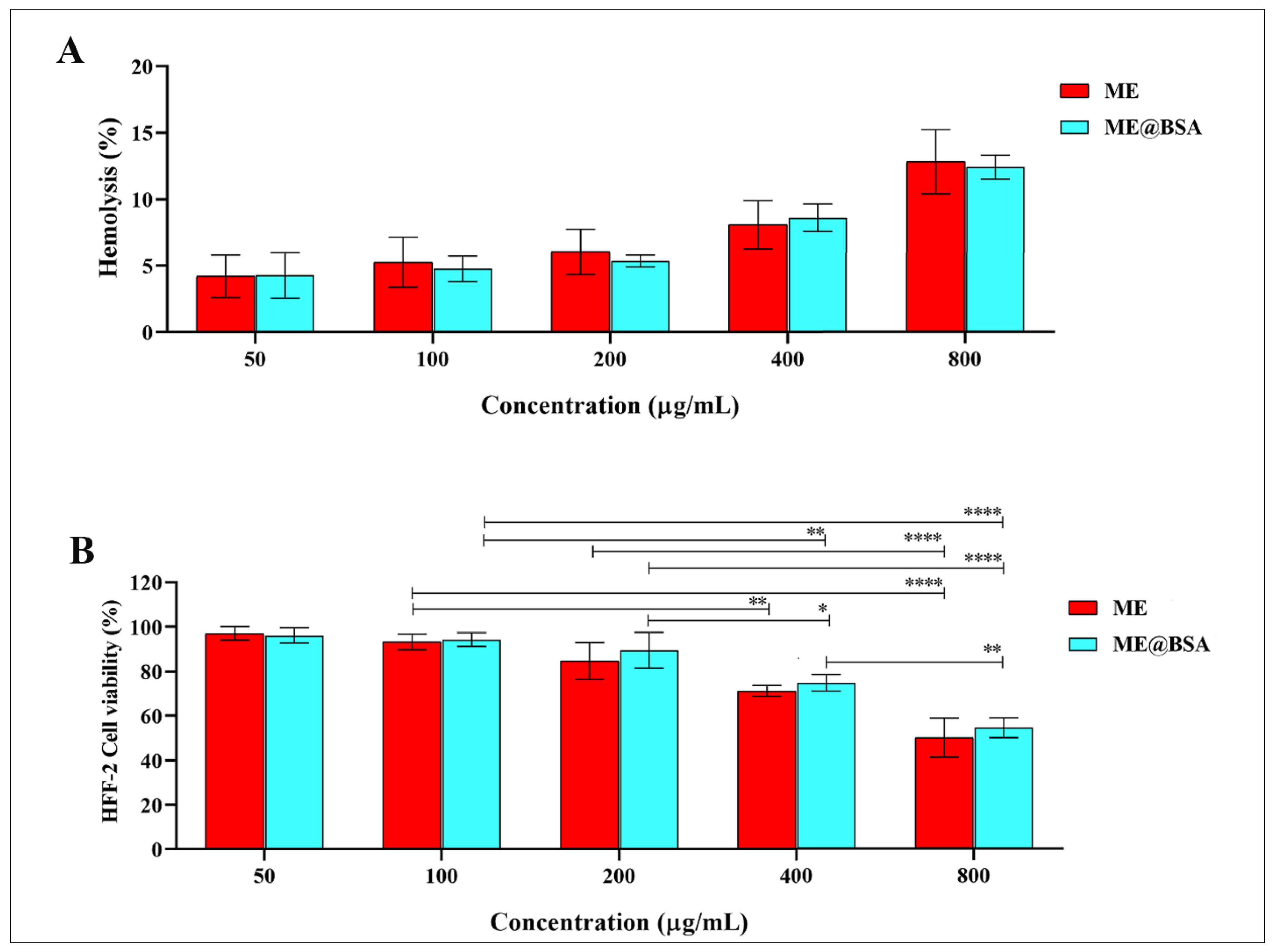
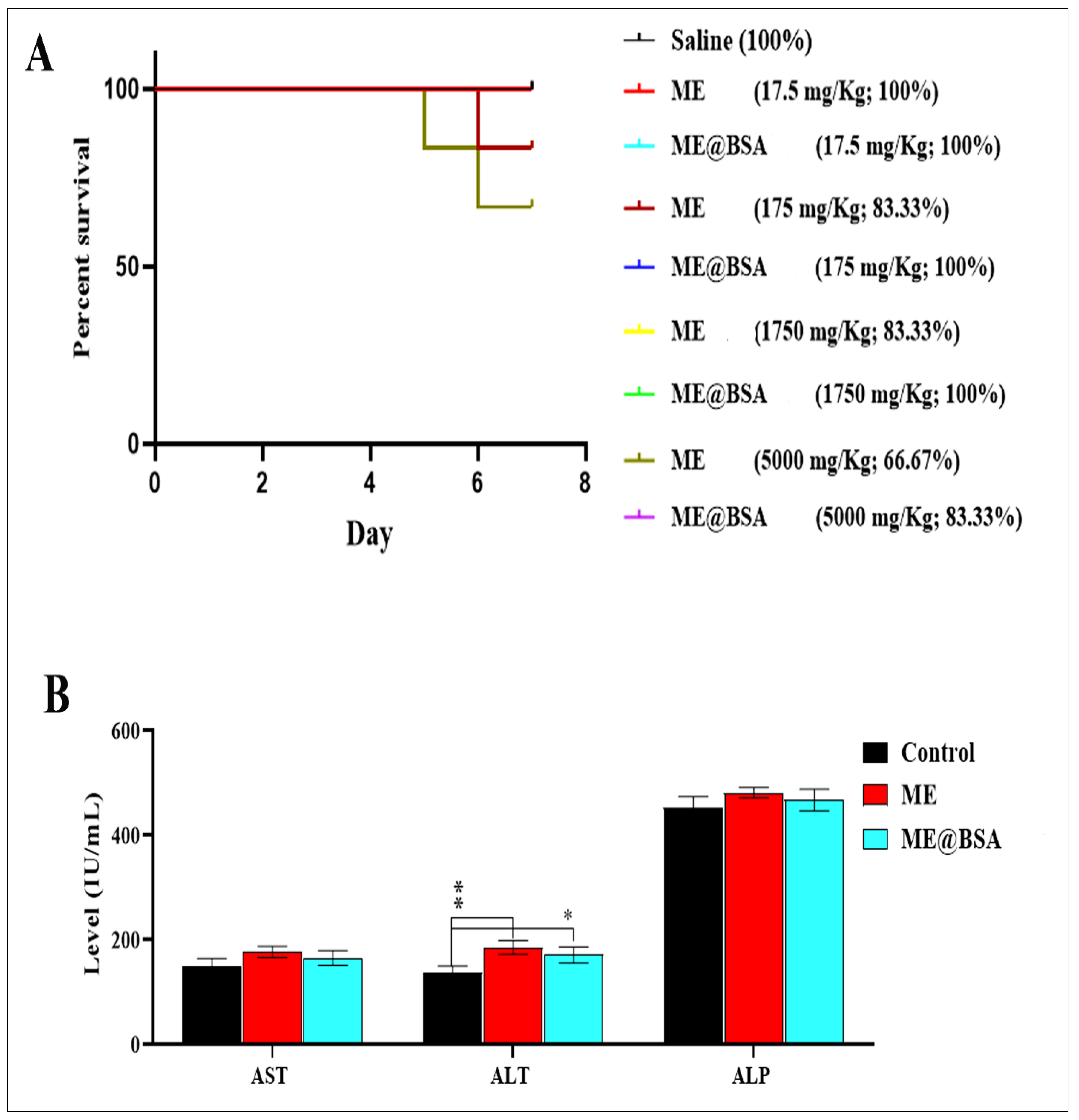
2.3. In Vitro and In Vivo Assays
3. Materials and Methods
3.1. Reagents and Materials
3.2. Preparation of O/W Microemulsion
3.3. Physicochemical Characterization
3.4. FT-IR Analysis
3.5. UV-VIS Spectroscopy
3.6. DLS and FE-SEM Analysis
3.7. Rheological Behavior
3.8. Hemo-Compatibility Assay
3.9. Cell Culture and Cell Viability Tests
3.10. In Vivo Experiments
3.11. LD50 Assay
3.12. Liver Function Tests
3.13. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Butt, U.; ElShaer, A.; Snyder, L.A.S.; Al-Kinani, A.A.; Le Gresley, A.; Alany, R.G. Fatty Acid Based Microemulsions to Combat Ophthalmia Neonatorum Caused by Neisseria gonorrhoeae and Staphylococcus aureus. Nanomaterials 2018, 8, 51. [Google Scholar] [CrossRef]
- Neto, A.O.W.; da Silva, V.L.; Rodrigues, D.V.; Ribeiro, L.S.; da Silva, D.N.N.; Freitas, J.C.D.O. A novel oil-in-water microemulsion as a cementation flushing fluid for removing non-aqueous filter cake. J. Pet. Sci. Eng. 2020, 184, 106536. [Google Scholar] [CrossRef]
- Dantas, T.N.C.; Santanna, V.C.; Souza, T.T.C.; Lucas, C.R.S.; Dantas Neto, A.A.; Aum, P.T.P. Microemulsios and Nanoemulsions Applied to Well Stimulation and Enhanced Oil Recovery. Brazil. J. Petrol. Gas 2018, 12, 251–265. [Google Scholar] [CrossRef]
- Mohammed, H.A.; Khan, R.A.; Singh, V.; Yusuf, M.; Akhtar, N.; Sulaiman, G.M.; Albukhaty, S.; Abdellatif, A.A.H.; Khan, M.; Mohammed, S.A.A.; et al. Solid lipid nanoparticles for targeted natural and synthetic drugs delivery in high-incidence cancers, and other diseases: Roles of preparation methods, lipid composition, transitional stability, and release profiles in nanocarriers’ development. Nanotechnol. Rev. 2023, 12, 20220517. [Google Scholar] [CrossRef]
- Jabir, M.S.; Rashid, T.M.; Nayef, U.M.; Albukhaty, S.; AlMalki, F.A.; Albaqami, J.; AlYamani, A.A.; Taqi, Z.J.; Sulaiman, G.M. Inhibition of Staphylococcus aureus α-hemolysin production using nanocurcumin capped Au@ZnO nanocomposite. Bioinorg. Chem. Appl. 2022, 2022, 2663812. [Google Scholar] [CrossRef]
- Albukhaty, S.; Al-Karagoly, H.; Allafchian, A.R.; Jalali, S.A.H.; Al-Kelabi, T.; Muhannad, M. Production and characterization of biocompatible nanofibrous scaffolds made of β-sitosterol loaded polyvinyl alcohol/tragacanth gum composites. Nanotechnology 2021, 33, 085102. [Google Scholar] [CrossRef]
- Ghitman, J.; Stan, R.; Ghebaur, A.; Cecoltan, S.; Vasile, E.; Iovu, H. Novel PEG-Modified Hybrid PLGA-Vegetable Oils Nanostructured Carriers for Improving Performances of Indomethacin Delivery. Polymers 2018, 10, 579. [Google Scholar] [CrossRef]
- Al-Musawi, S.; Albukhaty, S.; Al-Karagoly, H.; Sulaiman, G.M.; Jabir, M.S.; Naderi-Manesh, H. Dextran-coated superparamagnetic nanoparticles modified with folate for targeted drug delivery of camptothecin. Adv. Nat. Sci. Nanosci. Nanotechnol. 2020, 11, 045009. [Google Scholar] [CrossRef]
- Kahdim, Q.S.; Abdelmoula, N.; Al-Karagoly, H.; Albukhaty, S.; Al-Saaidi, J. Fabrication of a Polycaprolactone/Chitosan Nanofibrous Scaffold Loaded with Nigella sativa Extract for Biomedical Applications. Biotech 2023, 12, 19. [Google Scholar] [CrossRef]
- Jihad, M.; Noori, F.; Jabir, M.; Albukhaty, S.; AlMalki, F.; Alyamani, A. Polyethylene Glycol Functionalized Graphene Oxide Nanoparticles Loaded with Nigella sativa Extract: A Smart Antibacterial Therapeutic Drug Delivery System. Molecules 2021, 26, 3067. [Google Scholar] [CrossRef]
- Minnelli, C.; Laudadio, E.; Galeazzi, R.; Barucca, G.; Notarstefano, V.; Cantarini, M.; Armeni, T.; Mobbili, G. Encapsulation of a Neutral Molecule into a Cationic Clay Material: Structural Insight and Cytotoxicity of Resveratrol/Layered Double Hydroxide/BSA Nanocomposites. Nanomaterials 2019, 10, 33. [Google Scholar] [CrossRef]
- Zwain, T.; Taneja, N.; Zwayen, S.; Shidhaye, A.; Palshetkar, A.; Singh, K.K. Albumin nanoparticles—A versatile and a safe platform for drug delivery applications. In Nanoparticle Therapeutics; Elsevier: Amsterdam, The Netherlands, 2022; pp. 327–358. [Google Scholar] [CrossRef]
- Solanki, R.; Rostamabadi, H.; Patel, S.; Jafari, S.M. Anticancer nano-delivery systems based on bovine serum albumin nanoparticles: A critical review. Int. J. Biol. Macromol. 2021, 193, 528–540. [Google Scholar] [CrossRef]
- Spada, A.; Emami, J.; Tuszynski, J.A.; Lavasanifar, A. The Uniqueness of Albumin as a Carrier in Nanodrug Delivery. Mol. Pharm. 2021, 18, 1862–1894. [Google Scholar] [CrossRef]
- Lombardo, D.; Kiselev, M.A.; Caccamo, M.T. Smart Nanoparticles for Drug Delivery Application: Development of Versatile Nanocarrier Platforms in Biotechnology and Nanomedicine. J. Nanomater. 2019, 2019, e3702518. [Google Scholar] [CrossRef]
- Skorb, E.V.; Andreeva, D.V. Surface Nanoarchitecture for Bio-Applications: Self-Regulating Intelligent Interfaces. Adv. Funct. Mater. 2013, 23, 4483–4506. [Google Scholar] [CrossRef]
- Gharbavi, M.; Danafar, H.; Sharafi, A. Microemulsion and bovine serum albumin nanoparticles as a novel hybrid nanocarrier system for efficient multifunctional drug delivery. J. Biomed. Mater. Res. Part A 2020, 108, 1688–1702. [Google Scholar] [CrossRef]
- Ji, J.; Liu, Y.; Shi, L.; Wang, N.; Wang, X. Effect of roasting treatment on the chemical composition of sesame oil. LWT 2019, 101, 191–200. [Google Scholar] [CrossRef]
- Kanu, P.; Bahsoon, J.; Kanu, J.; Kandeh, J. Nutraceutical Importance of Sesame Seed and Oil: A Review of the Contribution of their Lignans. Sierra Leone J. Biomed. Res. 2010, 2, 4–16. [Google Scholar] [CrossRef]
- Wu, X.; Zhao, Z.; Tian, R.; Shang, Z.; Liu, H. Identification and quantification of counterfeit sesame oil by 3D fluorescence spectroscopy and convolutional neural network. Food Chem. 2020, 311, 125882. [Google Scholar] [CrossRef]
- Gharbavi, M.; Manjili, H.K.; Amani, J.; Sharafi, A.; Danafar, H. In vivo and in vitro biocompatibility study of novel microemulsion hybridized with bovine serum albumin as nanocarrier for drug delivery. Heliyon 2019, 5, e01858. [Google Scholar] [CrossRef]
- Wang, G.; Li, H.; Yan, S.; Huang, Q.; Wang, S.; Fan, J. Effect of glycerol microemulsion on coal seam wetting and moisturizing performance. J. Mol. Liq. 2022, 367, 120405. [Google Scholar] [CrossRef]
- Zhang, H.; Feng, F.; Li, J.; Zhan, X.; Wei, H.; Li, H.; Wang, H.; Zheng, X. Formulation of food-grade microemulsions with glycerol monolaurate: Effects of short-chain alcohols, polyols, salts and nonionic surfactants. Eur. Food Res. Technol. 2008, 226, 613–619. [Google Scholar] [CrossRef]
- Fatehi, P.; Baba, A.S.; Suk, V.R.E.; Misran, M. Preparation and characterization of palm oil in water microemulsion for application in the food industry. Br. Food J. 2020, 122, 3077–3088. [Google Scholar] [CrossRef]
- Bichu, M.B.; Varsha, P. Microemulsion assisted transdermal delivery of a hydrophilic anti-osteoporotic drug: Formulation, in vivo pharmacokinetic studies, in vitro cell osteogenic activity. J. Appl. Pharm. Sci. 2020, 10, 8–19. [Google Scholar] [CrossRef]
- Swaidan, A.; Borthakur, P.; Boruah, P.K.; Das, M.R.; Barras, A.; Hamieh, S.; Toufaily, J.; Hamieh, T.; Szunerits, S.; Boukherroub, R. A facile preparation of CuS-BSA nanocomposite as enzyme mimics: Application for selective and sensitive sensing of Cr(VI) ions. Sens. Actuators B Chem. 2019, 294, 253–262. [Google Scholar] [CrossRef]
- Gharbavi, M.; Johari, B.; Eslami, S.S.; Mousazadeh, N.; Sharafi, A. Cholesterol-conjugated bovine serum albumin nanoparticles as a tamoxifen tumor-targeted delivery system. Cell Biol. Int. 2020, 44, 2485–2498. [Google Scholar] [CrossRef]
- Qi, X.; Xu, D.; Zhu, J.; Wang, S.; Peng, J.; Gao, W.; Cao, Y. Studying the interaction mechanism between bovine serum albumin and lutein dipalmitate: Multi-spectroscopic and molecular docking techniques. Food Hydrocoll. 2021, 113, 106513. [Google Scholar] [CrossRef]
- Yu, J.; Liu, J.-Y.; Xiong, W.-M.; Zhang, X.-Y.; Zheng, Y. Binding interaction of sodium benzoate food additive with bovine serum albumin: Multi-spectroscopy and molecular docking studies. BMC Chem. 2019, 13, 95. [Google Scholar] [CrossRef]
- Gharbavi, M.; Johari, B.; Rismani, E.; Mousazadeh, N.; Taromchi, A.H.; Sharafi, A. NANOG Decoy Oligodeoxynucleotide–Encapsulated Niosomes Nanocarriers: A Promising Approach to Suppress the Metastatic Properties of U87 Human Glioblastoma Multiforme Cells. ACS Chem. Neurosci. 2020, 11, 4499–4515. [Google Scholar] [CrossRef]
- Chen, X.; Zhang, Y.; Lin, H.; Xia, P.; Cai, X.; Li, X.; Li, X.; Li, W. Porous ZnMn2O4 nanospheres: Facile synthesis through microemulsion method and excellent performance as anode of lithium ion battery. J. Power Sources 2016, 312, 137–145. [Google Scholar] [CrossRef]
- Wang, H.; Niu, Y.; Fei, G.; Shen, Y.; Lan, J. In-situ polymerization, rheology, morphology and properties of stable alkoxysilane-functionalized poly (urethane-acrylate) microemulsion. Prog. Org. Coat. 2016, 99, 400–411. [Google Scholar] [CrossRef]
- Zhou, L.; Yang, H.; Wu, H.; Wang, M.; Cheng, D. Catalytic pyrolysis of rice husk by mixing with zinc oxide: Characterization of bio-oil and its rheological behavior. Fuel Process. Technol. 2013, 106, 385–391. [Google Scholar] [CrossRef]
- Chen, X.; Xu, X.; Liu, D.; Zhou, G.; Han, M.; Wang, P. Rheological behavior, conformational changes and interactions of water-soluble myofibrillar protein during heating. Food Hydrocoll. 2018, 77, 524–533. [Google Scholar] [CrossRef]
- Boonme, P.; Kaewbanjong, J.; Amnuaikit, T.; Andreani, T.; Silva, A.; Souto, E.B. Microemulsion and Microemulsion-Based Gels for Topical Antifungal Therapy with Phytochemicals. Curr. Pharm. Des. 2016, 22, 4257–4263. [Google Scholar] [CrossRef]
- Feng, J.; Zhang, C.-P. Preparation of Cu–Ni alloy nanocrystallites in water-in-oil microemulsions. J. Colloid Interface Sci. 2006, 293, 414–420. [Google Scholar] [CrossRef] [PubMed]
- Hemlata, G.S.; Tejavath, K.K. ROS-Mediated Apoptosis Induced by BSA Nanospheres Encapsulated with Fruit Extract of Cucumis prophetarum in Various Human Cancer Cell Lines. ACS Omega 2021, 6, 10383–10395. [Google Scholar] [CrossRef] [PubMed]
- Shewaiter, M.A.; Hammady, T.M.; El-Gindy, A.; Hammadi, S.H.; Gad, S. Formulation and characterization of leflunomide/diclofenac sodium microemulsion base-gel for the transdermal treatment of inflammatory joint diseases. J. Drug Deliv. Sci. Technol. 2020, 61, 102110. [Google Scholar] [CrossRef]
- Schubert, M.; Müller-Goymann, C. Characterisation of surface-modified solid lipid nanoparticles (SLN): Influence of lecithin and nonionic emulsifier. Eur. J. Pharm. Biopharm. 2005, 61, 77–86. [Google Scholar] [CrossRef]
- Cantarovich, M.; Tzimas, G.N.; Barkun, J.; Deschenes, M.; Alpert, E.; Tchervenkov, J. Efficacy of mycophenolate mofetil combined with very low-dose cyclosporine microemulsion in long-term liver-transplant patients with renal dysfunction. Transplantation 2003, 76, 98–102. [Google Scholar] [CrossRef]
- Rahdar, A.; Hajinezhad, M.R.; Barani, M.; Sargazi, S.; Zaboli, M.; Ghazy, E.; Baino, F.; Cucchiarini, M.; Bilal, M.; Pandey, S. Pluronic F127/Doxorubicin microemulsions: Preparation, characterization, and toxicity evaluations. J. Mol. Liq. 2022, 345, 117028. [Google Scholar] [CrossRef]
- He, C.-X.; He, Z.-G.; Gao, J.-Q. Microemulsions as drug delivery systems to improve the solubility and the bioavailability of poorly water-soluble drugs. Expert Opin. Drug Deliv. 2010, 7, 445–460. [Google Scholar] [CrossRef]
- Chodari, L.; Aytemir, M.D.; Vahedi, P.; Alipour, M.; Vahed, S.Z.; Khatibi, S.M.H.; Ahmadian, E.; Ardalan, M.; Eftekhari, A. Targeting Mitochondrial Biogenesis with Polyphenol Compounds. Oxidative Med. Cell. Longev. 2021, 2021, 4946711. [Google Scholar] [CrossRef] [PubMed]
- Žigrayová, D.; Mikušová, V.; Mikuš, P. Advances in Antiviral Delivery Systems and Chitosan-Based Polymeric and Nanoparticulate Antivirals and Antiviral Carriers. Viruses 2023, 15, 647. [Google Scholar] [CrossRef] [PubMed]
- Gharbavi, M.; Johari, B.; Ghorbani, R.; Madanchi, H.; Sharafi, A. Green synthesis of Zn nanoparticles and in situ hybridized with BSA nanoparticles for Baicalein targeted delivery mediated with glutamate receptors to U87-MG cancer cell lines. Appl. Organomet. Chem. 2022, 37, e6926. [Google Scholar] [CrossRef]
- Gharbavi, M.; Sharafi, A.; Fath, P.M.; Oruji, S.; Pakzad, H.; Manjili, H.K. Formulation and Biocompatibility of Microemulsion-Based PMBN as an Efficient System for Paclitaxel Delivery. J. Appl. Biotechnol. Rep. 2021, 8, 51–62. [Google Scholar] [CrossRef]
- Pundi, K.; Pundi, K.N.; Kamath, P.S.; Cetta, F.; Li, Z.; Poterucha, J.T.; Driscoll, D.J.; Johnson, J.N. Liver Disease in Patients After the Fontan Operation. Am. J. Cardiol. 2016, 117, 456–460. [Google Scholar] [CrossRef]


| Formulation Number | Sesame Oil | Tween-80 (%, w/w) | Span-60 (%, w/w) | Glycerol (%, w/w) | Ethanol (%, w/w) | Water (%, w/w) | State |
|---|---|---|---|---|---|---|---|
| 1. | 9.50 | 19.91 | - | 4.10 | - | 66.49 | Separation phase |
| 2. | 9.50 | 19.91 | - | - | 4.10 | 66.49 | Separation phase |
| 3. | 8.60 | 19.91 | - | 5.00 | - | 66.49 | Separation phase |
| 4. | 8.60 | 19.91 | - | - | 5.00 | 66.49 | Separation phase |
| 5. | 8.60 | 20.81 | - | 4.10 | - | 66.49 | Separation phase |
| 6. | 8.60 | 20.81 | - | - | 4.10 | 66.49 | Separation phase |
| 7. | 8.60 | 17.60 | 3.21 | 4.10 | - | 66.49 | Slightly cloudy, phases separation after 1 week |
| 8. | 8.60 | 17.60 | 3.21 | - | 4.10 | 66.49 | Slightly cloudy, phases separation after 10 days |
| 9. | 8.60 | 18.60 | 2.21 | 4.10 | - | 66.49 | Slightly cloudy, phases separation after 2 weeks |
| 10. | 8.60 | 18.60 | 2.21 | - | 4.10 | 66.49 | Slightly cloudy, phases separation after 2 weeks |
| 11. | 8.10 | 18.60 | 2.21 | 4.10 | - | 66.99 | Slightly cloudy, and stable |
| 12. | 8.10 | 18.60 | 2.21 | - | 4.10 | 66.99 | Slightly cloudy, and stable |
| 13. | 8.00 | 18.60 | 2.21 | 4.10 | - | 70.00 | Very slightly cloudy, and stable |
| 14. | 8.00 | 18.60 | 2.21 | - | 4.10 | 70.00 | Very slightly cloudy, and stable |
| 15. | 7.75 | 18.60 | 2.21 | 4.10 | - | 70.25 | Very slightly cloudy, and stable |
| 16. | 7.75 | 18.60 | 2.21 | - | 4.10 | 70.25 | Very slightly cloudy, and stable |
| 17. | 7.60 | 18.60 | 2.21 | 4.10 | - | 70.40 | Transparent |
| 18. | 7.60 | 18.60 | 2.21 | - | 4.10 | 70.40 | Very slightly cloudy, and stable |
| 19. | 7.40 | 18.80 | 2.21 | 4.10 | - | 70.40 | Transparent |
| 20. | 7.40 | 18.80 | 2.21 | - | 4.10 | 70.40 | Transparent |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rhyaf, A.; Naji, H.; Al-Karagoly, H.; Albukhaty, S.; Sulaiman, G.M.; Alshammari, A.A.A.; Mohammed, H.A.; Jabir, M.; Khan, R.A. In Vitro and In Vivo Functional Viability, and Biocompatibility Evaluation of Bovine Serum Albumin-Ingrained Microemulsion: A Model Based on Sesame Oil as the Payload for Developing an Efficient Drug Delivery Platform. Pharmaceuticals 2023, 16, 582. https://doi.org/10.3390/ph16040582
Rhyaf A, Naji H, Al-Karagoly H, Albukhaty S, Sulaiman GM, Alshammari AAA, Mohammed HA, Jabir M, Khan RA. In Vitro and In Vivo Functional Viability, and Biocompatibility Evaluation of Bovine Serum Albumin-Ingrained Microemulsion: A Model Based on Sesame Oil as the Payload for Developing an Efficient Drug Delivery Platform. Pharmaceuticals. 2023; 16(4):582. https://doi.org/10.3390/ph16040582
Chicago/Turabian StyleRhyaf, Atiaf, Hala Naji, Hassan Al-Karagoly, Salim Albukhaty, Ghassan M. Sulaiman, Abdulaziz Arif A. Alshammari, Hamdoon A. Mohammed, Majid Jabir, and Riaz A. Khan. 2023. "In Vitro and In Vivo Functional Viability, and Biocompatibility Evaluation of Bovine Serum Albumin-Ingrained Microemulsion: A Model Based on Sesame Oil as the Payload for Developing an Efficient Drug Delivery Platform" Pharmaceuticals 16, no. 4: 582. https://doi.org/10.3390/ph16040582
APA StyleRhyaf, A., Naji, H., Al-Karagoly, H., Albukhaty, S., Sulaiman, G. M., Alshammari, A. A. A., Mohammed, H. A., Jabir, M., & Khan, R. A. (2023). In Vitro and In Vivo Functional Viability, and Biocompatibility Evaluation of Bovine Serum Albumin-Ingrained Microemulsion: A Model Based on Sesame Oil as the Payload for Developing an Efficient Drug Delivery Platform. Pharmaceuticals, 16(4), 582. https://doi.org/10.3390/ph16040582











