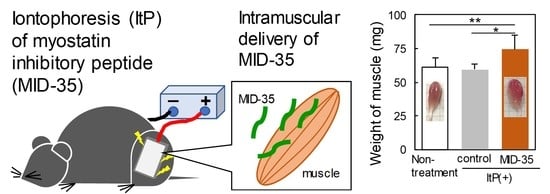
Increasing Skeletal Muscle Mass in Mice by Non-Invasive Intramuscular Delivery of Myostatin Inhibitory Peptide by Iontophoresis
Abstract
1. Introduction
2. Results
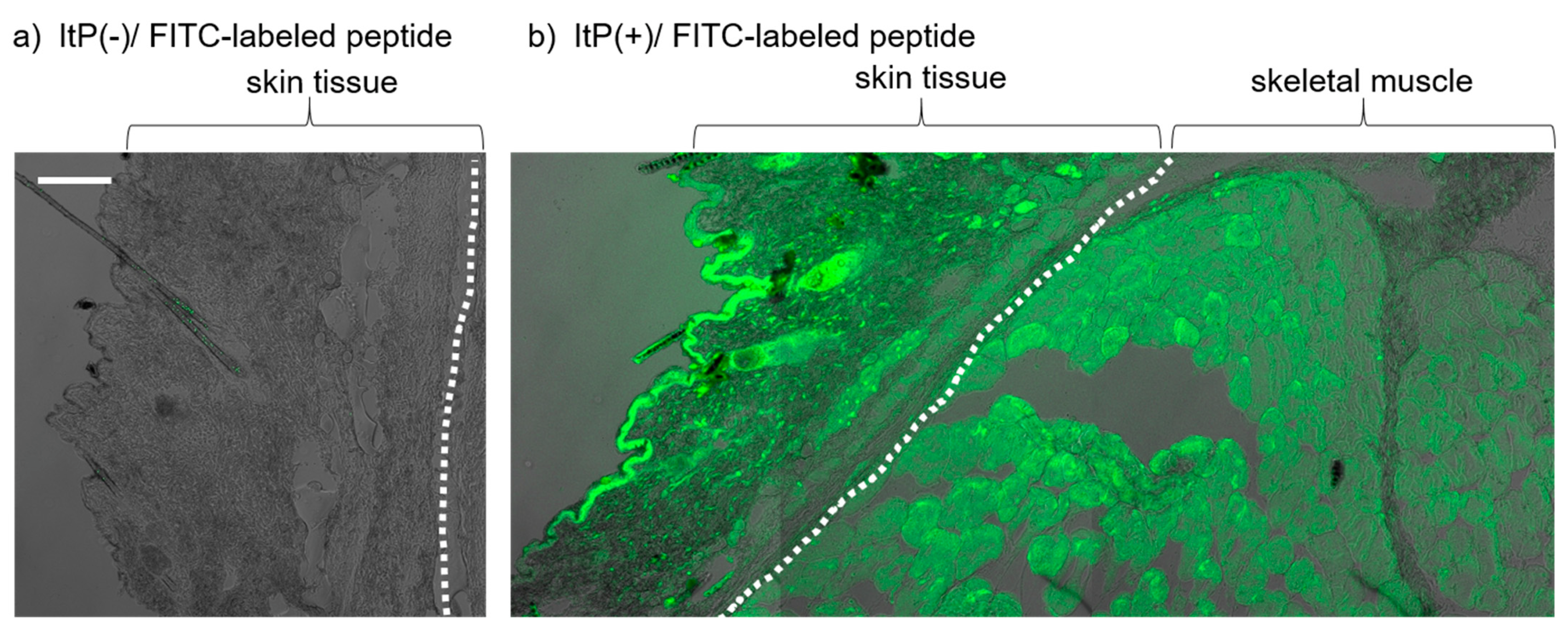
2.1. Delivery of a Peptide to the Skeletal Muscle from the Skin Using ItP
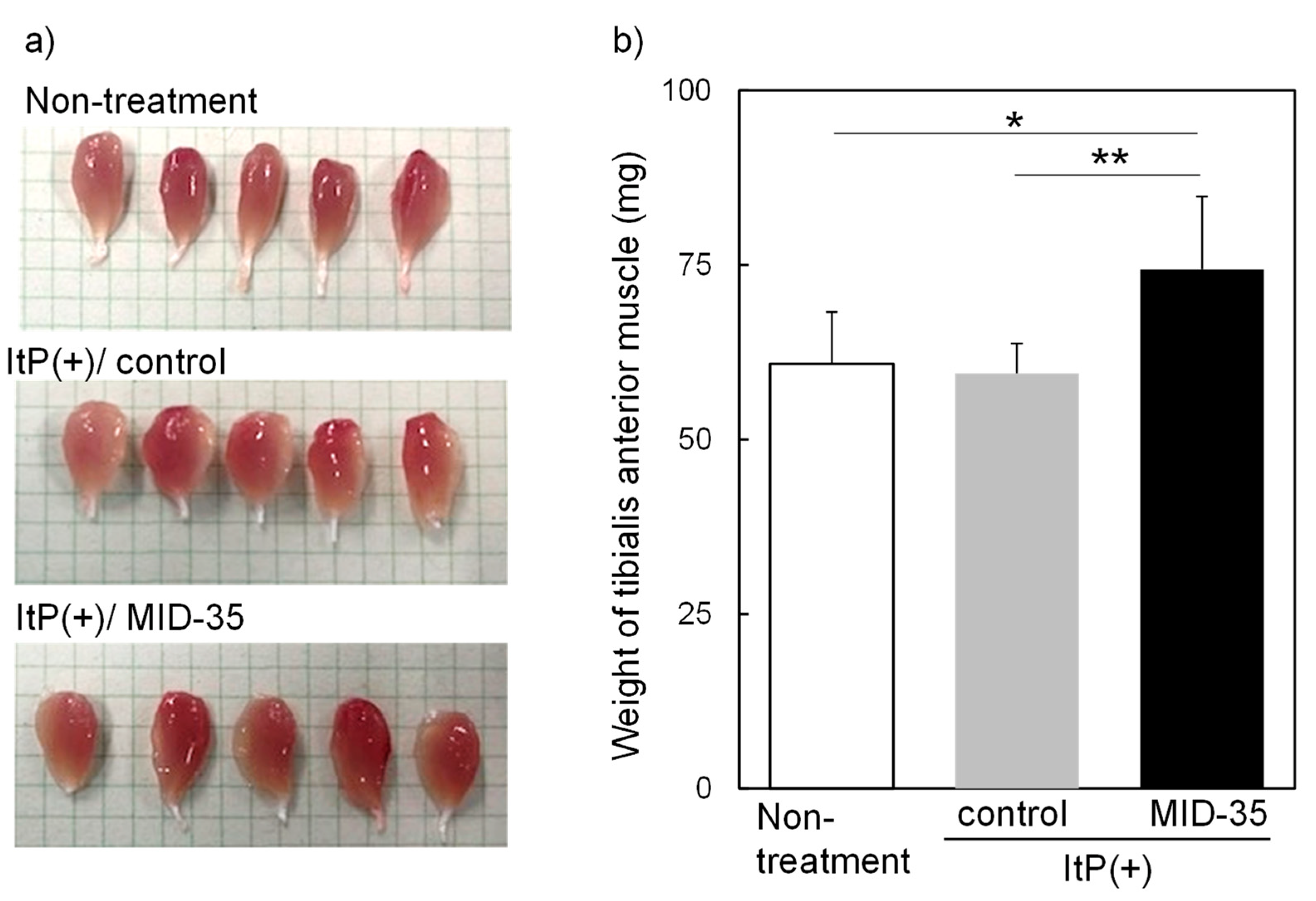
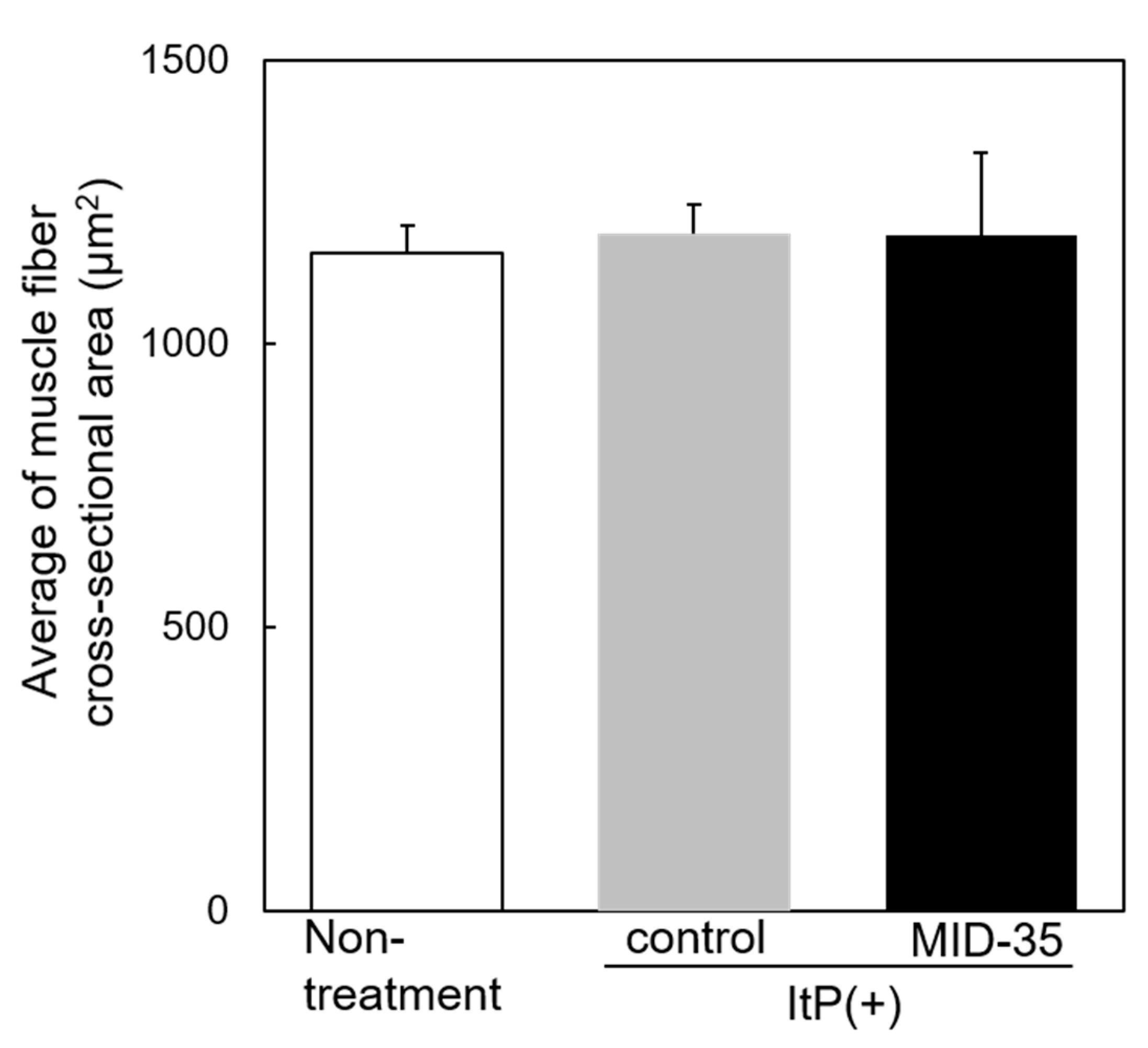
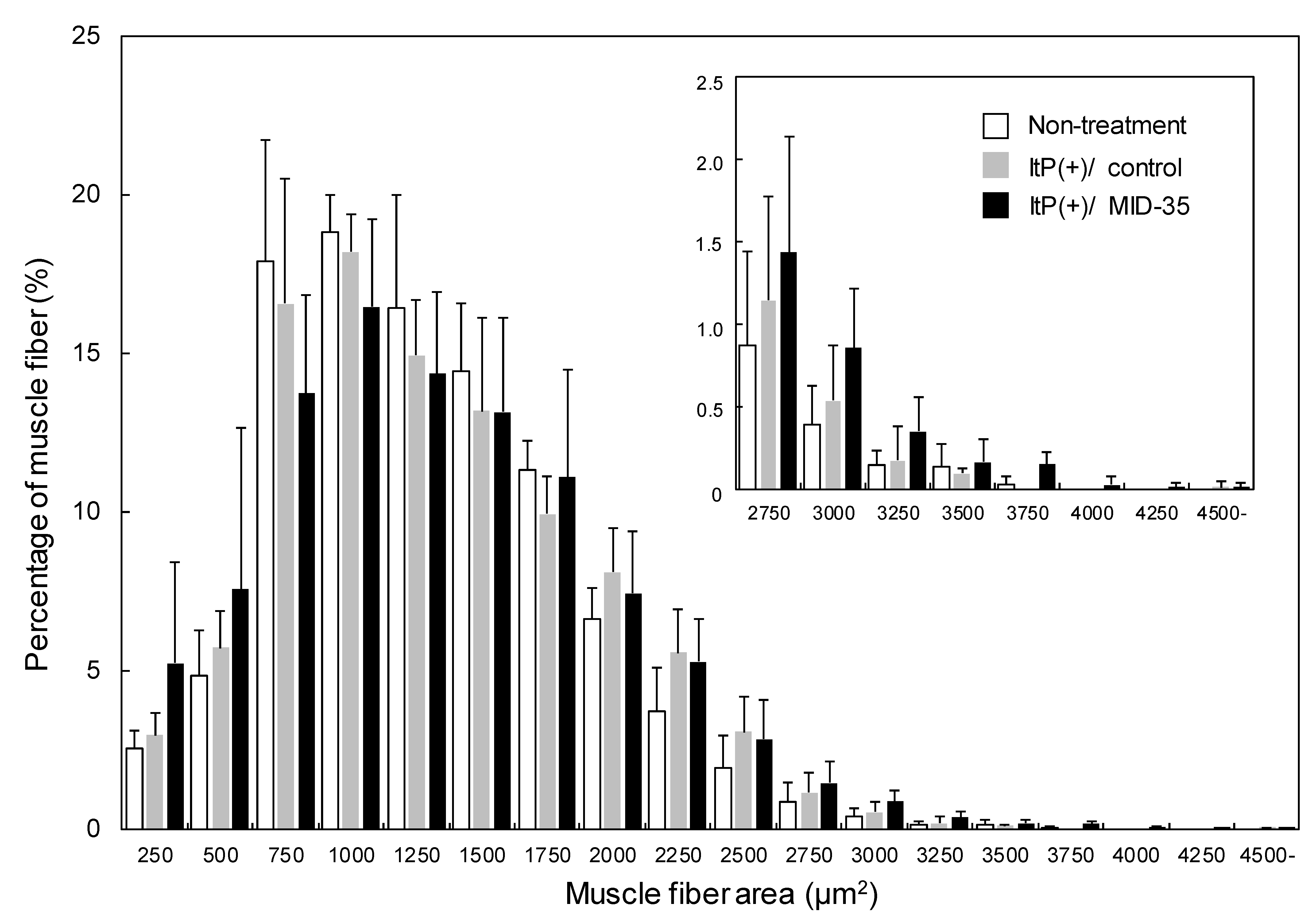
2.2. Effect of ItP of MID-35 on Skeletal Muscle
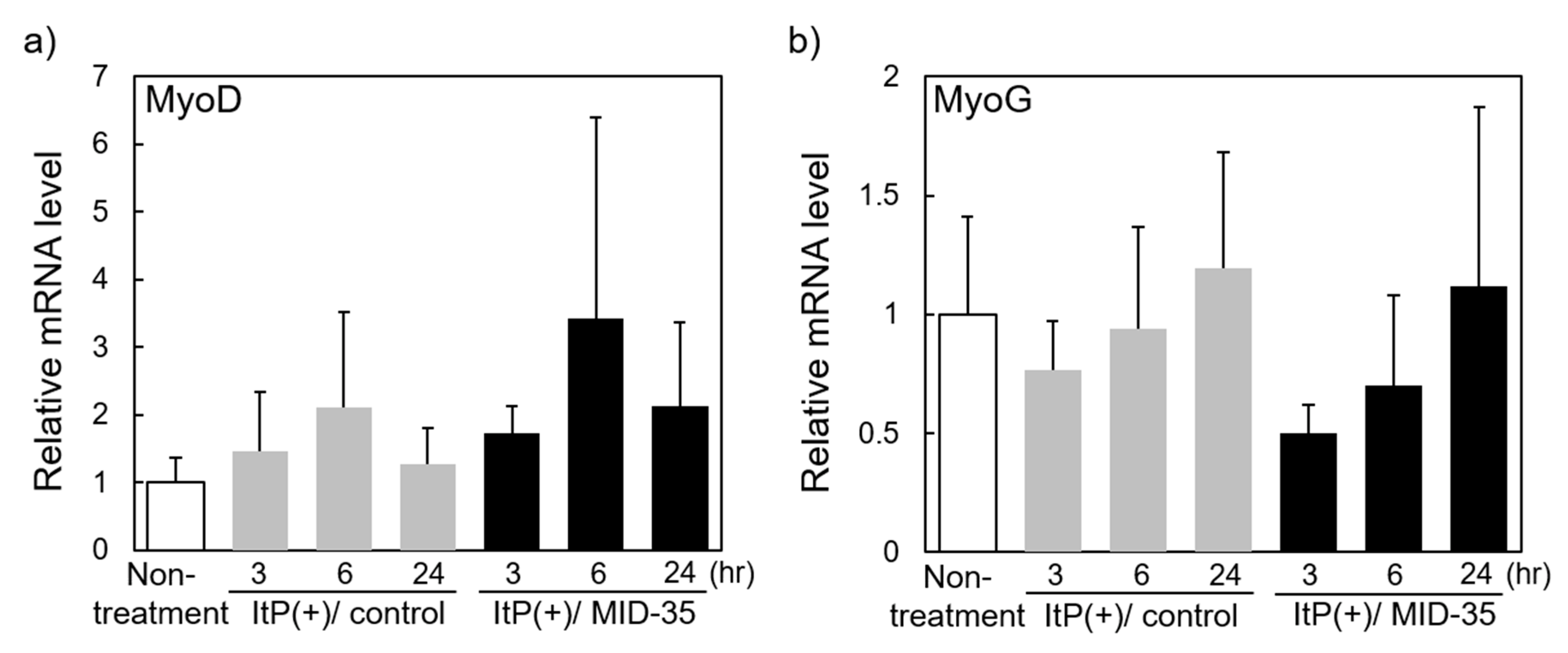
2.3. Effect of ItP of MID-35 on mRNA Expression of Genes Associated with Muscle Growth and Myostatin Signaling Pathways
3. Discussion
4. Materials and Methods
4.1. Materials and Animals
4.2. Synthesis of MID-35
4.3. Iontophoresis (ItP) Procedure
4.4. Analysis of Fluorescent Dye Distribution in the Tissue after ItP
4.5. Analysis of Muscle Tissue after ItP of MID-35
4.6. Quantitation of mRNA in the Muscle after ItP of MID-35
4.7. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bauer, J.; Morley, J.E.; Schols, A.M.W.J.; Ferrucci, L.; Cruz-Jentoft, A.J.; Dent, E.; Baracos, V.E.; Crawford, J.A.; Doehner, W.; Heymsfield, S.B.; et al. An SCWD Position Paper. J. Cachexia Sarcopenia Muscle 2019, 10, 956–961. [Google Scholar] [CrossRef]
- Larsson, L.; Degens, H.; Li, M.; Salviati, L.; Lee, Y.I.; Thompson, W.; Kirkland, J.L.; Sandri, M. Sarcopenia: Aging-related loss of muscle mass and function. Physiol. Rev. 2019, 99, 427–511. [Google Scholar] [CrossRef] [PubMed]
- Papadopoulou, S.K. Sarcopenia: A contemporary health problem among older adult populations. Nutrients 2020, 12, 1293. [Google Scholar] [CrossRef] [PubMed]
- McPherron, A.C.; Lawler, A.M.; Lee, S.-J. Regulation of skeletal muscle in mice by a new TGF-β superfamily member. Nature 1997, 387, 83–90. [Google Scholar] [CrossRef]
- Cho, M.R.; Lee, S.; Song, S.K. A Review of Sarcopenia Pathophysiology, Diagnosis, Treatment and Future Direction. J. Korean Med. Sci. 2022, 37, e146. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.-J.; McPherron, A.C. Regulation of myostatin activity and muscle growth. Proc. Natl. Acad. Sci. USA 2001, 98, 9306–9311. [Google Scholar] [CrossRef]
- Walker, R.G.; McCoy, J.C.; Czepnik, M.; Mills, M.J.; Hagg, A.; Walton, K.L.; Cotton, T.R.; Hyvönen, M.; Lee, R.T.; Gregorevic, P.; et al. Molecular characterization of latent GDF8 reveals mechanisms of activation. Proc. Natl. Acad. Sci. USA 2018, 115, E866–E875. [Google Scholar] [CrossRef]
- Yoshida, T.; Delafontaine, P. Mechanisms of IGF-1-mediated regulation of skeletal muscle hypertrophy and atrophy. Cells 2020, 9, 1970. [Google Scholar] [CrossRef]
- Lee, S.J. Targeting the myostatin signaling pathway to treat muscle loss and metabolic dysfunction. J. Clin. Investig. 2021, 131, e148372. [Google Scholar] [CrossRef]
- Lee, S.-J.; Lehar, A.; Liu, Y.; Ly, C.H.; Pham, Q.-M.; Michaud, M.; Rydzik, R.; Youngstrom, D.W.; Shen, M.M.; Kaartinen, V.; et al. Functional redundancy of type I and type II receptors in the regulation of skeletal muscle growth by myostatin and activin A. Proc. Natl. Acad. Sci. USA 2020, 117, 30907–30917. [Google Scholar] [CrossRef]
- Iskenderian, A.; Liu, N.; Deng, Q.; Huang, Y.; Shen, C.; Palmieri, K.; Crooker, R.; Lundberg, D.; Kastrapeli, N.; Pescatore, B.; et al. Myostatin and activin blockade by engineered follistatin results in hypertrophy and improves dystrophic pathology in mdx mouse more than myostatin blockade alone. Skelet. Muscle 2018, 8, 34. [Google Scholar] [CrossRef] [PubMed]
- Ozawa, T.; Morikawa, M.; Morishita, Y.; Ogikubo, K.; Itoh, F.; Koinuma, D.; Nygren, P.; Miyazono, K. Systemic administration of monovalent follistatin-like 3-Fc-fusion protein increases muscle mass in mice. iScience 2021, 24, 102488. [Google Scholar] [CrossRef] [PubMed]
- Golan, T.; Geva, R.; Richards, D.; Madhusudan, S.; Lin, B.K.; Wang, H.T.; Walgren, R.A.; Stemmer, S.M. LY2495655, an antimyostatin antibody, in pancreatic cancer: A randomized, phase 2 trial. J. Cachexia Sarcopenia Muscle 2018, 9, 871–879. [Google Scholar] [CrossRef]
- Zhou, X.; Wang, J.L.; Lu, J.; Song, Y.; Kwak, K.S.; Jiao, Q.; Rosenfeld, R.; Chen, Q.; Boone, T.; Scott Simonet, W.; et al. Reversal of cancer cachexia and muscle wasting by ActRIIB antagonism leads to prolonged survival. Cell 2010, 142, 531–543. [Google Scholar] [CrossRef]
- Statland, J.M.; Campbell, C.; Desai, U.; Karam, C.; Díaz-Manera, J.; Guptill, J.T.; Korngut, L.; Genge, A.; Tawil, R.N.; Elman, L.; et al. Randomized phase 2 study of ACE-083, a muscle-promoting agent, in facioscapulohumeral muscular dystrophy. Muscle Nerve 2022, 66, 50–62. [Google Scholar] [CrossRef] [PubMed]
- Hanada, K.; Fukasawa, K.; Hinata, H.; Imai, S.; Takayama, K.; Hirai, H.; Ohfusa, R.; Hayashi, Y.; Itoh, F. Combination therapy with anamorelin and a myostatin inhibitor is advantageous for cancer cachexia in a mouse model. Cancer Sci. 2022, 113, 3547–3557. [Google Scholar] [CrossRef]
- White, T.A.; LeBrasseur, N.K. Myostatin and sarcopenia: Opportunities and challenges—A mini-review. Gerontology 2014, 60, 289–293. [Google Scholar] [CrossRef]
- Wagner, K.R. The elusive promise of myostatin inhibition for muscular dystrophy. Curr. Opin. Neurol. 2020, 33, 621–628. [Google Scholar] [CrossRef]
- Abati, E.; Manini, A.; Comi, G.P.; Corti, S. Inhibition of myostatin and related signaling pathways for the treatment of muscle atrophy in motor neuron diseases. Cell Mol. Life Sci. 2022, 79, 374. [Google Scholar] [CrossRef]
- Baig, M.H.; Ahmad, K.; Moon, J.S.; Park, S.Y.; Ho, L.J.; Chun, H.J.; Qadri, A.F.; Hwang, Y.C.; Jan, A.T.; Ahmad, S.S.; et al. Myostatin and its Regulation: A Comprehensive Review of Myostatin Inhibiting Strategies. Front. Physiol. 2022, 13, 876078. [Google Scholar] [CrossRef]
- Takayama, K.; Hitachi, K.; Okamoto, H.; Saitoh, M.; Odagiri, M.; Ohfusa, R.; Shimada, T.; Taguchi, A.; Taniguchi, A.; Tsuchida, K.; et al. Development of myostatin inhibitory D-peptides to enhance the potency, increasing skeletal muscle mass in mice. ACS Med. Chem. Lett. 2022, 13, 492–498. [Google Scholar] [CrossRef]
- Li, Z.; Fang, X.; Yu, D. Transdermal Drug Delivery Systems and Their Use in Obesity Treatment. Int. J. Mol. Sci. 2021, 22, 12754. [Google Scholar] [CrossRef]
- Lim, D.J.; Kim, H.J. Microneedles in Action: Microneedling and Microneedles-Assisted Transdermal Delivery. Polymers 2022, 14, 1608. [Google Scholar] [CrossRef] [PubMed]
- Byrne, J.D.; Yeh, J.J.; DeSimone, J.M. Use of iontophoresis for the treatment of cancer. J. Control. Release 2018, 284, 144–151. [Google Scholar] [CrossRef] [PubMed]
- Karpiński, T.M. Selected Medicines Used in Iontophoresis. Pharmaceutics 2018, 10, 204. [Google Scholar] [CrossRef]
- Ragit, R.; Fulzele, P.; Rathi, N.V.; Thosar, N.R.; Khubchandani, M.; Malviya, N.S.; Das, S. Iontophoresis as an Effective Drug Delivery System in Dentistry: A Review. Cureus 2022, 14, e30658. [Google Scholar] [CrossRef] [PubMed]
- Hasan, M.; Khatun, A.; Kogure, K. Iontophoresis of Biological Macromolecular Drugs. Pharmaceutics 2022, 14, 525. [Google Scholar] [CrossRef]
- Colombini, M. Ceramide channels and mitochondrial outer membrane permeability. J. Bioenerg. Biomembr. 2017, 49, 57–64. [Google Scholar] [CrossRef] [PubMed]
- Aziz, A.; Liu, Q.C.; Dilworth, F.J. Regulating a master regulator: Establishing tissue-specific gene expression in skeletal muscle. Epigenetics 2010, 5, 691–695. [Google Scholar] [CrossRef]
- De Esteves, L.J.; Relaix, F. Master regulators of skeletal muscle lineage development and pluripotent stem cells differentiation. Cell Regen. 2021, 10, 31. [Google Scholar] [CrossRef]
- Gumucio, J.P.; Mendias, C.L. Atrogin-1, MuRF-1, and sarcopenia. Endocrine 2013, 43, 12–21. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, A.; Aizawa, T.; Tomiya, A.; Matsubara, Y.; Kokubun, S.; Itoi, E. Effect of resting interval for muscle regeneration in mice. Upsala J. Med. Sci. 2007, 112, 175–181. [Google Scholar] [CrossRef] [PubMed]
- Hasan, M.; Fukuta, T.; Inoue, S.; Mori, H.; Kagawa, M.; Kogure, K. Iontophoresis-mediated direct delivery of nucleic acid therapeutics, without use of carriers, to internal organs via non-blood circulatory pathways. J. Control. Release 2022, 343, 392–399. [Google Scholar] [CrossRef] [PubMed]


| Gene Name | Forward(5′-3′) | Reverse(5′-3′) |
|---|---|---|
| MyoD | AGTGAATGAGGCCTTCGAGA | GCATCTGAGTCGCCACTGTA |
| MyoG | CCTTGCTCAGCTCCCTCA | TGGGAGTTGCATTCACTGG |
| Atrogin-1 | TAGTAAGGCTGTTGGAGCTGATAG | CTGCACCAGTGTGCATAAGG |
| MuRF-1 | CATCTTCCAGGCTGCGAATC | ACTGGAGCACTCCTGCTTGT |
| GAPDH | GAGGACCAGGTTGTCTCCTG | ATGTAGGCCATGAGGTCCAC |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Michiue, K.; Takayama, K.; Taniguchi, A.; Hayashi, Y.; Kogure, K. Increasing Skeletal Muscle Mass in Mice by Non-Invasive Intramuscular Delivery of Myostatin Inhibitory Peptide by Iontophoresis. Pharmaceuticals 2023, 16, 397. https://doi.org/10.3390/ph16030397
Michiue K, Takayama K, Taniguchi A, Hayashi Y, Kogure K. Increasing Skeletal Muscle Mass in Mice by Non-Invasive Intramuscular Delivery of Myostatin Inhibitory Peptide by Iontophoresis. Pharmaceuticals. 2023; 16(3):397. https://doi.org/10.3390/ph16030397
Chicago/Turabian StyleMichiue, Kohki, Kentaro Takayama, Atsuhiko Taniguchi, Yoshio Hayashi, and Kentaro Kogure. 2023. "Increasing Skeletal Muscle Mass in Mice by Non-Invasive Intramuscular Delivery of Myostatin Inhibitory Peptide by Iontophoresis" Pharmaceuticals 16, no. 3: 397. https://doi.org/10.3390/ph16030397
APA StyleMichiue, K., Takayama, K., Taniguchi, A., Hayashi, Y., & Kogure, K. (2023). Increasing Skeletal Muscle Mass in Mice by Non-Invasive Intramuscular Delivery of Myostatin Inhibitory Peptide by Iontophoresis. Pharmaceuticals, 16(3), 397. https://doi.org/10.3390/ph16030397