An Insight into Advances in Developing Nanotechnology Based Therapeutics, Drug Delivery, Diagnostics and Vaccines: Multidimensional Applications in Tuberculosis Disease Management
Abstract
1. Introduction
2. Different forms of TB
2.1. Extra Pulmonary Tuberculosis (EPTB)
2.2. Multidrug-Resistant Tuberculosis (MDR TB)
2.3. Infection Stages
2.3.1. Primary Stage
2.3.2. Latent Stage
2.3.3. Active Stage
2.4. Pathogenesis and Immunology of TB
3. TB Diagnosis Method and Challenges with the Existing Methods
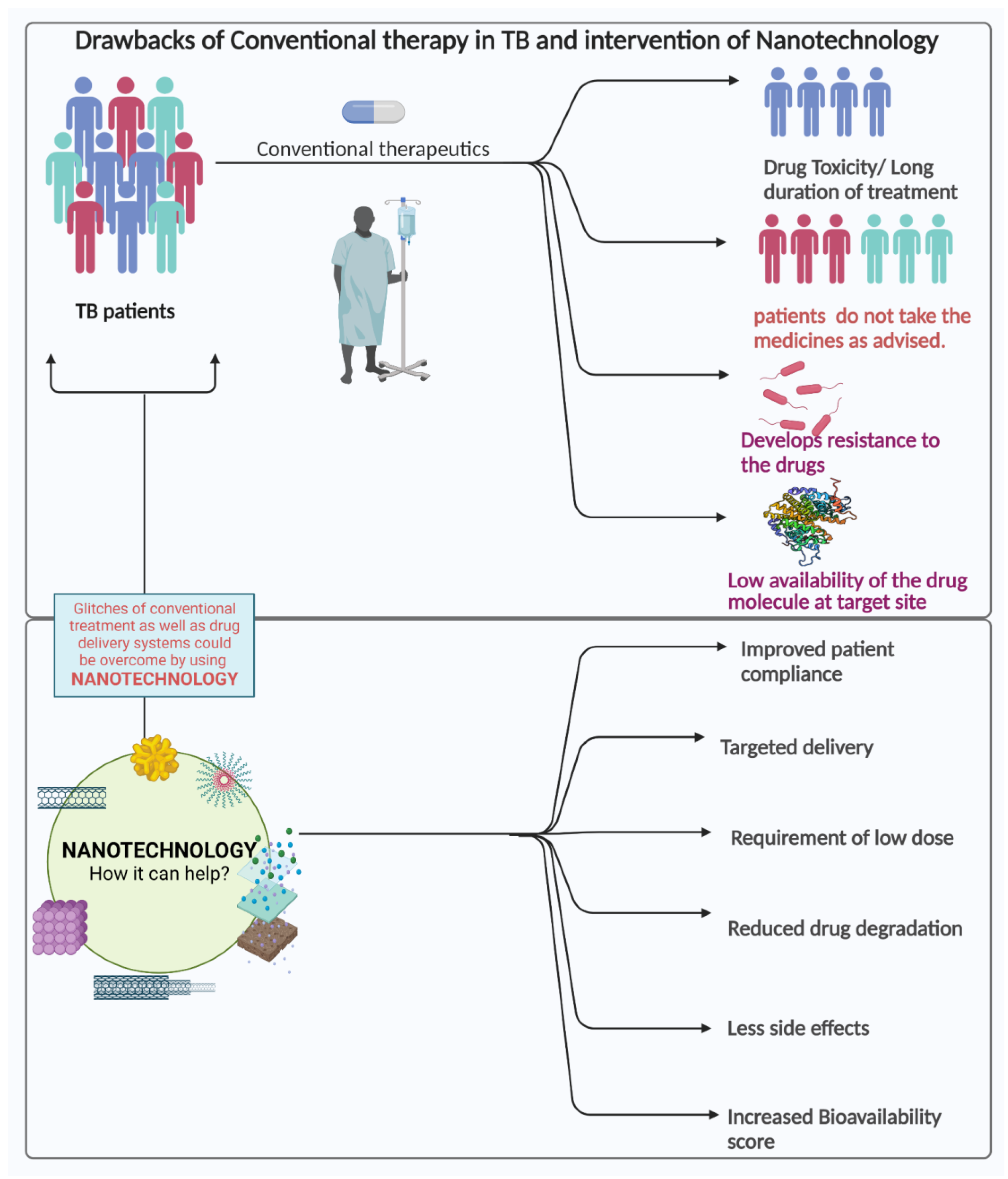
3.1. Current Therapy for Treatment of TB
3.2. Challenges in the Current Treatment Strategies
4. Nanotechnological Approach for Combating TB
4.1. Nanotechnology in the Treatment of Tuberculosis
4.2. Various Nanosystems
4.2.1. Dendrimers
4.2.2. Nanoparticle
4.2.3. Nanoemulsion
4.2.4. Liposomes
4.2.5. Miscellaneous
5. Nanotechnology in the Diagnosis of Tuberculosis
6. Nanotechnology in the Prevention of Tuberculosis
7. Future Perspectives and Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chakaya, J.; Khan, M.; Ntoumi, F.; Aklillu, E.; Fatima, R.; Mwaba, P.; Kapata, N.; Mfinanga, S.; Hasnain, S.E.; Katoto, P.D.M.C.; et al. Global Tuberculosis Report 2020—Reflections on the Global TB Burden, Treatment and Prevention Efforts. Int. J. Infect. Dis. 2021, 113, S7–S12. [Google Scholar] [CrossRef] [PubMed]
- ECDC. WHO 4% Annual Decrease Too Slow to End TB by 2030—Call for Europe’s Commitment to Increase Investment to End TB; ECDC: Solna, Sweden, 2018. [Google Scholar]
- Floyd, K.; Glaziou, P.; Houben, R.M.G.J.; Sumner, T.; White, R.G.; Raviglione, M. Global Tuberculosis Targets and Milestones Set for 2016–2035: Definition and Rationale. Int. J. Tuberc. Lung Dis. 2018, 22, 723–730. [Google Scholar] [CrossRef] [PubMed]
- Floyd, K.; Glaziou, P.; Zumla, A.; Raviglione, M. The Global Tuberculosis Epidemic and Progress in Care, Prevention, and Research: An Overview in Year 3 of the End TB Era. Lancet Respir. Med. 2018, 6, 299–314. [Google Scholar] [CrossRef] [PubMed]
- Churchyard, G.; Kim, P.; Shah, N.S.; Rustomjee, R.; Gandhi, N.; Mathema, B.; Dowdy, D.; Kasmar, A.; Cardenas, V. What We Know about Tuberculosis Transmission: An Overview. J. Infect. Dis. 2017, 216, S629–S635. [Google Scholar] [CrossRef]
- Wallenfels, J. Epidemiology of Tuberculosis. Kardiol. Rev. 2019, 21, 125–128. [Google Scholar] [CrossRef]
- World Health Organization. Global Tuberculosis Programme; WHO: Geneva, Switzerland, 2021. [Google Scholar]
- Fonseca, J.D.; Knight, G.M.; McHugh, T.D. The Complex Evolution of Antibiotic Resistance in Mycobacterium tuberculosis. Int. J. Infect. Dis. 2015, 32, 94–100. [Google Scholar] [CrossRef]
- Coll, F.; McNerney, R.; Preston, M.D.; Guerra-Assunção, J.A.; Warry, A.; Hill-Cawthorne, G.; Mallard, K.; Nair, M.; Miranda, A.; Alves, A.; et al. Rapid Determination of Anti-Tuberculosis Drug Resistance from Whole-Genome Sequences. Genome Med. 2015, 7, 51. [Google Scholar] [CrossRef]
- Esmail, H.; Barry, C.E.; Young, D.B.; Wilkinson, R.J. The Ongoing Challenge of Latent Tuberculosis. Philos. Trans. R. Soc. B Biol. Sci. 2014, 369, 0130437. [Google Scholar] [CrossRef]
- Turunc, T.; Habesoglu, M.A.; Demiroglu, Y.Z.; Karatasli, M.; Sen, N.; Ermis, H.; Aliskan, H.; Colakoglu, S.; Arslan, H. Comparative Evaluation of 113 Cases with Severe and Mild Forms of Extrapulmonary Tuberculosis. Mikrobiyoloji Bul. 2008, 42, 399–406. [Google Scholar]
- Sunnetcioglu, A.; Sunnetcioglu, M.; Binici, I.; Baran, A.I.; Karahocagil, M.K.; Saydan, M.R. Comparative Analysis of Pulmonary and Extrapulmonary Tuberculosis of 411 Cases. Ann. Clin. Microbiol. Antimicrob. 2015, 14, 1–5. [Google Scholar] [CrossRef]
- Ates Guler, S.; Bozkus, F.; Inci, M.F.; Kokoglu, O.F.; Ucmak, H.; Ozden, S.; Yuksel, M. Evaluation of Pulmonary and Extrapulmonary Tuberculosis in Immunocompetent Adults: A Retrospective Case Series Analysis. Med. Princ. Pract. 2015, 24, 75–79. [Google Scholar] [CrossRef] [PubMed]
- Sreeramareddy, C.T.; Panduru, K.V.; Verma, S.C.; Joshi, H.S.; Bates, M.N. Comparison of Pulmonary and Extrapulmonary Tuberculosis in Nepal—A Hospital-Based Retrospective Study. BMC Infect Dis. 2008, 8, 8. [Google Scholar] [CrossRef] [PubMed]
- Fader, T.; Parks, J.; Khan, N.U.; Manning, R.; Stokes, S.; Nasir, N.A. Extrapulmonary Tuberculosis in Kabul, Afghanistan: A Hospital-Based Retrospective Review. Int. J. Infect. Dis. 2010, 14, E102–E110. [Google Scholar] [CrossRef] [PubMed]
- Antony, S.J.; Harrell, V.; Christie, J.D.; Adams, H.G.; Rumley, R.L. Clinical Differences between Pulmonary and Extrapulmonary Tuberculosis: A 5-Year Retrospective Study. J. Natl. Med. Assoc. 1995, 87, 187–192. [Google Scholar] [PubMed]
- Musellim, B.; Erturan, S.; Sonmez Duman, E.; Ongen, G. Comparison of Extra-Pulmonary and Pulmonary Tuberculosis Cases: Factors Influencing the Site of Reactivation. Int. J. Tuberc. Lung Dis. 2005, 9, 1220–1223. [Google Scholar] [PubMed]
- Lin, J.N.; Lai, C.H.; Chen, Y.H.; Lee, S.S.J.; Tsai, S.S.; Huang, C.K.; Chung, H.C.; Liang, S.H.; Lin, H.H. Risk Factors for Extra-Pulmonary Tuberculosis Compared to Pulmonary Tuberculosis. Int. J. Tuberc. Lung Dis. 2009, 13, 620–625. [Google Scholar] [PubMed]
- Oren, S.; Jamal, J.; London, D.; Viskoper, J.R. Extrapulmonary Tuberculosis: Five Case Reports. Isr. J. Med. Sci. 1991, 27, 390–394. [Google Scholar]
- Pandit, V.R.; Shubha, S.; Rohit, V.; Vikas, M.; Vandana, K.E.; Ashwini, K.; Samarasinghe, C. Pott’s Spine with “bird Nest” Appearance. Int. J. Infect. Dis. 2010, 14, E390–E391. [Google Scholar] [CrossRef]
- Gupta, N.; Dass, A.; Goel, N.; Tiwari, S. Tuberculous Otitis Media Leading to Sequentialib Bilateral Facial Nerve Paralysis. Iran. J. Otorhinolaryngol. 2015, 27, 231–237. [Google Scholar]
- Lee, K.J.; Yoo, J.S.; Jeon, H.; Cho, S.K.; Lee, J.H.; Ha, S.S.; Cho, M.Y.; Kim, J.W. A Case of Splenic Tuberculosis Forming a Gastro-Splenic Fistula. Korean J. Gastroenterol. 2015, 66, 168–171. [Google Scholar] [CrossRef]
- Lombardi, R.; Pelusi, S.; Airaghi, L.; Fargion, S. Extrapulmonary Tuberculosis: An Unusual Presentation in an Immunocompetent Patient. BMJ Case Rep. 2015, 2015, bcr2014207146. [Google Scholar] [CrossRef] [PubMed]
- Sharma, V.; Chhabra, P.; Rana, S.S.; Bhasin, D.K. Pancreatic Tuberculosis: Look at the Kidney! Dig. Liver Dis. 2015, 47, e1. [Google Scholar] [CrossRef] [PubMed]
- Sherif, M.M.; Schauer, C.K.M.W. Multifocal Cutaneous and Osseous Tuberculosis. Int. J. Infect. Dis. 2015, 37, 11–13. [Google Scholar] [CrossRef] [PubMed]
- Gambhir, S.; Ravina, M.; Rangan, K.; Dixit, M.; Barai, S.; Bomanji, J. Imaging in Extrapulmonary Tuberculosis. Int. J. Infect. Dis. 2017, 56, 237–247. [Google Scholar] [CrossRef]
- Heye, T.; Stoijkovic, M.; Kauczor, H.U.; Junghanss, T.; Hosch, W. Extrapulmonary Tuberculosis: Radiological Imaging of an Almost Forgotten Transformation Artist. Rofo 2011, 183, 1019–1029. [Google Scholar] [CrossRef]
- Nachiappan, A.C.; Rahbar, K.; Shi, X.; Guy, E.S.; Mortani Barbosa, E.J.; Shroff, G.S.; Ocazionez, D.; Schlesinger, A.E.; Katz, S.I.; Hammer, M.M. Pulmonary Tuberculosis: Role of Radiology in Diagnosis and Management. Radiographics 2017, 37, 52–72. [Google Scholar] [CrossRef]
- Harisinghani, M.G.; McLoud, T.C.; Shepard, J.A.O.; Ko, J.P.; Shroff, M.M.; Mueller, P.R. Tuberculosis from Head to Toe. Radiographics 2000, 20, 449–470. [Google Scholar] [CrossRef]
- Akkerman, O.W.; ter Beek, L.; Centis, R.; Maeurer, M.; Visca, D.; Muñoz-Torrico, M.; Tiberi, S.; Migliori, G.B. Rehabilitation, Optimized Nutritional Care, and Boosting Host Internal Milieu to Improve Long-Term Treatment Outcomes in Tuberculosis Patients. Int. J. Infect. Dis. 2020, 92, S10–S14. [Google Scholar] [CrossRef]
- Seung, K.J.; Keshavjee, S.; Rich, M.L. Multidrug-Resistant Tuberculosis and Extensively Drug-Resistant Tuberculosis. Cold Spring Harb. Perspect. Med. 2015, 5, a017863. [Google Scholar] [CrossRef]
- World Health Organization. Meeting Report of the WHO Expert Consultation on Drug-Resistant Tuberculosis Treatment Outcome Definitions, 17–19 November 2020; World Health Organization: Geneva, Switzerland, 2020. [Google Scholar]
- Sasindran, S.J.; Torrelles, J.B. Mycobacterium tuberculosis Infection and Inflammation: What Is Beneficial for the Host and for the Bacterium? Front. Microbiol. 2011, 2, 1–16. [Google Scholar] [CrossRef]
- Bussi, C.; Gutierrez, M.G. Mycobacterium tuberculosis Infection of Host Cells in Space and Time. FEMS Microbiol. Rev. 2019, 43, 341–361. [Google Scholar] [CrossRef] [PubMed]
- Gengenbacher, M.; Kaufmann, S.H.E. Mycobacterium tuberculosis: Success through Dormancy. FEMS Microbiol. Rev. 2012, 36, 514–532. [Google Scholar] [CrossRef] [PubMed]
- Forrellad, M.A.; Klepp, L.I.; Gioffré, A.; García, J.S.; Morbidoni, H.R.; de la Paz Santangelo, M.; Cataldi, A.A.; Bigi, F. Virulence Factors of the Mycobacterium tuberculosis Complex. Virulence 2013, 4, 3–66. [Google Scholar] [CrossRef]
- Bhalla, A.S.; Goyal, A.; Guleria, R.; Gupta, A.K. Chest Tuberculosis: Radiological Review and Imaging Recommendations. Indian J. Radiol. Imaging 2015, 25, 213–225. [Google Scholar] [CrossRef] [PubMed]
- Hunter, R.L. The Pathogenesis of Tuberculosis–the Koch Phenomenon Reinstated. Pathogens 2020, 9, 813. [Google Scholar] [CrossRef] [PubMed]
- Peters, J.S.; Andrews, J.R.; Hatherill, M.; Hermans, S.; Martinez, L.; Schurr, E.; van der Heijden, Y.; Wood, R.; Rustomjee, R.; Kana, B.D. Advances in the Understanding of Mycobacterium tuberculosis Transmission in HIV-Endemic Settings. Lancet Infect Dis. 2019, 19, E65–E76. [Google Scholar] [CrossRef]
- Mathema, B.; Andrews, J.R.; Cohen, T.; Borgdorff, M.W.; Behr, M.; Glynn, J.R.; Rustomjee, R.; Silk, B.J.; Wood, R. Drivers of Tuberculosis Transmission. J. Infect. Dis. 2017, 216, S644–S653. [Google Scholar] [CrossRef]
- Perlman, D.C.; El-Helou, P.; Salomon, N. Tuberculosis in Patients with Human Immunodeficiency Virus Infection. Semin. Respir. Infect 1999, 14, 344–352. [Google Scholar]
- De Noronha, A.L.L.; Báfica, A.; Nogueira, L.; Barral, A.; Barral-Netto, M. Lung Granulomas from Mycobacterium tuberculosis/HIV-1 Co-Infected Patients Display Decreased in Situ TNF Production. Pathol. Res. Pract. 2008, 204, 155–161. [Google Scholar] [CrossRef]
- Saunders, B.M.; Frank, A.A.; Orme, I.M.; Cooper, A.M. CD4 Is Required for the Development of a Protective Granulomatous Response to Pulmonary Tuberculosis. Cell Immunol. 2002, 216, 65–72. [Google Scholar] [CrossRef]
- Lefford, M.J. Transfer of Adoptive Immunity to Tuberculosis in Mice. Infect Immun. 1975, 11, 1174–1181. [Google Scholar] [CrossRef] [PubMed]
- North, R.J. Importance of Thymus-Derived Lymphocytes in Cell-Mediated Immunity to Infection. Cell Immunol. 1973, 7, 166–176. [Google Scholar] [CrossRef] [PubMed]
- Orme, I.M. The Kinetics of Emergence and Loss of Mediator T Lymphocytes Acquired in Response to Infection with Mycobacterium tuberculosis. J. Immunol. 1987, 138, 293–298. [Google Scholar] [CrossRef]
- Mogues, T.; Goodrich, M.E.; Ryan, L.; LaCourse, R.; North, R.J. The Relative Importance of T Cell Subsets in Immunity and Immunopathology of Airborne Mycobacterium tuberculosis Infection in Mice. J. Exp. Med. 2001, 193, 271–280. [Google Scholar] [CrossRef]
- Flynn, J.A.L.; Chan, J.; Triebold, K.J.; Dalton, D.K.; Stewart, T.A.; Bloom, B.R. An Essential Role for Interferon γ in Resistance to Mycobacterium tuberculosis Infection. J. Exp. Med. 1993, 178, 2249–2254. [Google Scholar] [CrossRef] [PubMed]
- Cooper, A.M.; Dalton, D.K.; Stewart, T.A.; Griffin, J.P.; Russell, D.G.; Orme, I.M. Disseminated Tuberculosis in Interferon Gamma Gene-Distrupted Mice. J. Exp. Med. 1993, 178, 2243–2247. [Google Scholar] [CrossRef]
- Ottenhoff, T.H.M.; Kumararatne, D.; Casanova, J.L. Novel Human Immunodeficiencies Reveal the Essential Role of Type-I Cytokines in Immunity to Intracellular Bacteria. Immunol. Today 1998, 19, 491–494. [Google Scholar] [CrossRef]
- BañUls, A.L.; Sanou, A.; Van Anh, N.T.; Godreuil, S. Mycobacterium tuberculosis: Ecology and Evolution of a Human Bacterium. J. Med. Microbiol. 2015, 64, 1261–1269. [Google Scholar] [CrossRef]
- Caminero, J.A.; Matteelli, A.; Loddenkemper, R. Tuberculosis: Are We Making It Incurable? Eur. Respir. J. 2013, 42, 5–8. [Google Scholar] [CrossRef]
- Bai, Y.; Wang, Y.; Shao, C.; Hao, Y.; Jin, Y. GenoType MTBDRplus Assay for Rapid Detection of Multidrug Resistance in Mycobacterium tuberculosis: A Meta-Analysis. PLoS ONE 2016, 11, e0150321. [Google Scholar] [CrossRef]
- Raizada, N.; Sachdeva, K.S.; Chauhan, D.S.; Malhotra, B.; Reddy, K.; Dave, P.V.; Mundade, Y.; Patel, P.; Ramachandran, R.; Das, R.; et al. A Multi-Site Validation in India of the Line Probe Assay for the Rapid Diagnosis of Multi-Drug Resistant Tuberculosis Directly from Sputum Specimens. PLoS ONE 2014, 9, e088626. [Google Scholar] [CrossRef] [PubMed]
- Chakravorty, S.; Simmons, A.M.; Rowneki, M.; Parmar, H.; Cao, Y.; Ryan, J.; Banada, P.P.; Deshpande, S.; Shenai, S.; Gall, A.; et al. The New Xpert MTB/RIF Ultra: Improving Detection of Mycobacterium tuberculosis and Resistance to Rifampin in an Assay Suitable for Point-of-Care Testing. mBio 2017, 8, e00812–e00817. [Google Scholar] [CrossRef] [PubMed]
- Dorman, S.E.; Schumacher, S.G.; Alland, D.; Nabeta, P.; Armstrong, D.T.; King, B.; Hall, S.L.; Chakravorty, S.; Cirillo, D.M.; Tukvadze, N.; et al. Xpert MTB/RIF Ultra for Detection of Mycobacterium tuberculosis and Rifampicin Resistance: A Prospective Multicentre Diagnostic Accuracy Study. Lancet Infect Dis. 2018, 18, 76–84. [Google Scholar] [CrossRef] [PubMed]
- García-Basteiro, A.L.; DiNardo, A.; Saavedra, B.; Silva, D.R.; Palmero, D.; Gegia, M.; Migliori, G.B.; Duarte, R.; Mambuque, E.; Centis, R.; et al. Point of Care Diagnostics for Tuberculosis. Rev. Port. Pneumol. 2018, 24, 73–85. [Google Scholar] [CrossRef]
- Polu, G.P.; Mohammad Shaik, J.; Kota, N.M.K.; Karumanchi, D.; Allam, U.S. Analysis of Drug Resistance Mutations in Pulmonary Mycobacterium tuberculosis Isolates in the Southern Coastal Region of Andhra Pradesh, India. Braz. J. Infect. Dis. 2019, 23, 290–291. [Google Scholar] [CrossRef]
- Witney, A.A.; Cosgrove, C.A.; Arnold, A.; Hinds, J.; Stoker, N.G.; Butcher, P.D. Clinical Use of Whole Genome Sequencing for Mycobacterium tuberculosis. BMC Med. 2016, 14, 46. [Google Scholar] [CrossRef]
- WHO. WHO Consolidated Guidelines on Drug-Resistant Tuberculosis Treatment; World Health Organization: Geneva, Switzerland, 2019. [Google Scholar]
- Knight, G.M.; Colijn, C.; Shrestha, S.; Fofana, M.; Cobelens, F.; White, R.G.; Dowdy, D.W.; Cohen, T. The Distribution of Fitness Costs of Resistance-Conferring Mutations Is a Key Determinant for the Future Burden of Drug-Resistant Tuberculosis: A Model-Based Analysis. Clin. Infect. Dis. 2015, 61, S147–S154. [Google Scholar] [CrossRef]
- Jhun, B.W.; Koh, W.J. Treatment of Isoniazid-Resistant Pulmonary Tuberculosis. Tuberc. Respir. Dis. 2020, 83, 20–30. [Google Scholar] [CrossRef]
- Ramappa, V.; Aithal, G.P. Hepatotoxicity Related to Anti-Tuberculosis Drugs: Mechanisms and Management. J. Clin. Exp. Hepatol. 2013, 3, 37–49. [Google Scholar] [CrossRef]
- Metushi, I.; Uetrecht, J.; Phillips, E. Mechanism of Isoniazid-Induced Hepatotoxicity: Then and Now. Br. J. Clin. Pharmacol. 2016, 81, 1030–1036. [Google Scholar] [CrossRef]
- Bhargava, M.; Bhargava, A. Pyridoxine for Patients Suffering from Drug-Susceptible Tuberculosis in India. Public Health Action 2019, 8, 97. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Pradhan, K.; Zhong, X.B.; Ma, X. Isoniazid Metabolism and Hepatotoxicity. Acta Pharm. Sin. B 2016, 6, 384–392. [Google Scholar] [CrossRef] [PubMed]
- Sullins, A.K.; Abdel-Rahman, S.M. Pharmacokinetics of Antibacterial Agents in the CSF of Children and Adolescents. Pediatr. Drugs 2013, 15, 93–117. [Google Scholar] [CrossRef]
- Dhiman, R.K.; Saraswat, V.A.; Rajekar, H.; Reddy, C.; Chawla, Y.K. A Guide to the Management of Tuberculosis in Patients with Chronic Liver Disease. J. Clin. Exp. Hepatol. 2012, 2, 260–270. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.W.; Park, H.O.; Jang, H.N.; Yang, J.H.; Kim, S.H.; Moon, S.H.; Byun, J.H.; Lee, C.E.; Kim, J.W.; Kang, D.H. Side Effects Associated with the Treatment of Multidrug-Resistant Tuberculosis at a Tuberculosis Referral Hospital in South Korea. Medicine 2017, 96, e7482. [Google Scholar] [CrossRef]
- Chan, E.D.; Iseman, M.D. Multidrug-Resistant and Extensively Drug-Resistant Tuberculosis: A Review. Curr. Opin. Infect Dis. 2008, 21, 587–595. [Google Scholar] [CrossRef]
- Migliori, G.B.; Matteelli, A.; Cirillo, D.; Pai, M. Diagnosis of Multidrug-Resistant Tuberculosis and Extensively Drug-Resistant Tuberculosis: Current Standards and Challenges. Can. J. Infect. Dis. Med. Microbiol. 2008, 19, 169–172. [Google Scholar] [CrossRef]
- Griffiths, G.; Nyström, B.; Sable, S.B.; Khuller, G.K. Nanobead-Based Interventions for the Treatment and Prevention of Tuberculosis. Nat. Rev. Microbiol. 2010, 8, 827–834. [Google Scholar] [CrossRef]
- Nasiruddin, M.; Neyaz, K.; Das, S. Nanotechnology-Based Approach in Tuberculosis Treatment. Tuberc. Res. Treat. 2017, 2017, 1–12. [Google Scholar] [CrossRef]
- Wen, H.; Jung, H.; Li, X. Drug Delivery Approaches in Addressing Clinical Pharmacology-Related Issues: Opportunities and Challenges. AAPS J. 2015, 17, 1327–1340. [Google Scholar] [CrossRef]
- Griego, A.; Scarpa, E.; De Matteis, V.; Rizzello, L. Nanoparticle Delivery through the BBB in Central Nervous System Tuberculosis. Ibrain 2023, 9, 43–62. [Google Scholar] [CrossRef]
- Ifijen, I.H.; Atoe, B.; Ekun, R.O.; Ighodaro, A.; Odiachi, I.J. Treatments of Mycobacterium tuberculosis and Toxoplasma gondii with Selenium Nanoparticles. Bionanoscience 2023, 13, 249–277. [Google Scholar] [CrossRef] [PubMed]
- Bisht, N.; Patel, M.; Dwivedi, N.; Kumar, P.; Mondal, D.P.; Srivastava, A.K.; Dhand, C. Bio-Inspired Polynorepinephrine Based Nanocoatings for Reduced Graphene Oxide/Gold Nanoparticles Composite for High-Performance Biosensing of Mycobacterium tuberculosis. Environ. Res. 2023, 2023, 115684. [Google Scholar] [CrossRef] [PubMed]
- Parveen, S.; Sur, T.; Sarkar, S.; Roy, R. Antagonist Impact of Selenium-Based Nanoparticles Against Mycobacterium tuberculosis. Appl. Biochem. Biotechnol. 2023. [Google Scholar] [CrossRef]
- Bhattacharya, T.; Soares, G.A.B.E.; Chopra, H.; Rahman, M.; Hasan, Z.; Swain, S.S.; Cavalu, S. Applications of Phyto-Nanotechnology for the Treatment of Neurodegenerative Disorders. Materials 2022, 15, 804. [Google Scholar] [CrossRef]
- Chopra, H.; Dey, P.S.; Das, D.; Bhattacharya, T.; Shah, M.; Mubin, S.; Maishu, S.P.; Akter, R.; Rahman, H.; Karthika, C.; et al. Curcumin Nanoparticles as Promising Therapeutic Agents for Drug Targets. Molecules 2021, 26, 4998. [Google Scholar] [CrossRef]
- Chopra, H.; Bibi, S.; Mishra, A.K.; Tirth, V.; Yerramsetty, S.V.; Murali, S.V.; Ahmad, S.U.; Mohanta, Y.K.; Attia, M.S.; Algahtani, A.; et al. Nanomaterials: A Promising Therapeutic Approach for Cardiovascular Diseases. J. Nanomater. 2022, 2022, 4155729. [Google Scholar] [CrossRef]
- Chopra, H.; Bibi, S.; Islam, F.; Ahmad, S.U.; Olawale, O.A.; Alhumaydhi, F.A.; Marzouki, R.; Baig, A.A.; Emran, T. Bin Emerging Trends in the Delivery of Resveratrol by Nanostructures: Applications of Nanotechnology in Life Sciences. J. Nanomater. 2022, 2022, 3083728. [Google Scholar] [CrossRef]
- Singla, R.K.; Sai, C.S.; Chopra, H.; Behzad, S.; Bansal, H.; Goyal, R.; Gautam, R.K.; Tsagkaris, C.; Joon, S.; Singla, S.; et al. Natural Products for the Management of Castration-Resistant Prostate Cancer: Special Focus on Nanoparticles Based Studies. Front. Cell Dev. Biol. 2021, 9, 745177. [Google Scholar] [CrossRef]
- Elsadek, N.E.; Nagah, A.; Ibrahim, T.M.; Chopra, H.; Ghonaim, G.A.; Emam, S.E.; Cavalu, S.; Attia, M.S. Electrospun Nanofibers Revisited: An Update on the Emerging Applications in Nanomedicine. Materials 2022, 15, 1934. [Google Scholar] [CrossRef]
- Lin, W.; Fan, S.; Liao, K.; Huang, Y.; Cong, Y.; Zhang, J.; Jin, H.; Zhao, Y.; Ruan, Y.; Lu, H.; et al. Engineering Zinc Oxide Hybrid Selenium Nanoparticles for Synergetic Anti-Tuberculosis Treatment by Combining Mycobacterium tuberculosis Killings and Host Cell Immunological Inhibition. Front. Cell Infect Microbiol. 2023, 12, 1074533. [Google Scholar] [CrossRef] [PubMed]
- Sherje, A.P.; Jadhav, M.; Dravyakar, B.R.; Kadam, D. Dendrimers: A Versatile Nanocarrier for Drug Delivery and Targeting. Int. J. Pharm. 2018, 548, 707–720. [Google Scholar] [CrossRef] [PubMed]
- Esfand, R.; Tomalia, D.A. Poly(Amidoamine) (PAMAM) Dendrimers: From Biomimicry to Drug Delivery and Biomedical Applications. Drug Discov. Today 2001, 6, 427–436. [Google Scholar] [CrossRef] [PubMed]
- Sung, J.C.; Pulliam, B.L.; Edwards, D.A. Nanoparticles for Drug Delivery to the Lungs. Trends Biotechnol. 2007, 25, 563–570. [Google Scholar] [CrossRef] [PubMed]
- Mitchison, D.A.; Fourie, P.B. The near Future: Improving the Activity of Rifamycins and Pyrazinamide. Tuberculosis 2010, 90, 177–181. [Google Scholar] [CrossRef]
- Ahmed, R.; Aucamp, M.; Ebrahim, N.; Samsodien, H. Supramolecular Assembly of Rifampicin and PEGylated PAMAM Dendrimer as a Novel Conjugate for Tuberculosis. J. Drug Deliv. Sci. Technol. 2021, 66, 102773. [Google Scholar] [CrossRef]
- Pan, G.; Lemmouchi, Y.; Akala, E.O.; Bakare, O. Studies on PEGylated and Drug-Loaded PAMAM Dendrimers. J. Bioact. Compat. Polym. 2005, 20, 113–128. [Google Scholar] [CrossRef]
- Lee, H.; Larson, R.G. Effects of PEGylation on the Size and Internal Structure of Dendrimers: Self-Penetration of Long PEG Chains into the Dendrimer Core. Macromolecules 2011, 44, 2291–2298. [Google Scholar] [CrossRef]
- Dineshkumar, P.; Panneerselvam, T.; Brundavani, K.D.; Selvaraj, K.; Kumar, P.V. Formulation of Rifampicin Loaded PEGylated 5.0G EDA-PAMAM Dendrimers as Effective Long-Duration Release Drug Carriers. Curr. Drug Ther. 2016, 12, 115–126. [Google Scholar] [CrossRef]
- Bellini, R.G.; Guimarães, A.P.; Pacheco, M.A.C.; Dias, D.M.; Furtado, V.R.; De Alencastro, R.B.; Horta, B.A.C. Association of the Anti-Tuberculosis Drug Rifampicin with a PAMAM Dendrimer. J. Mol. Graph. Model 2015, 60, 34–42. [Google Scholar] [CrossRef]
- Mishra, V.; Gupta, U.; Jain, N.K. Surface-Engineered Dendrimers: A Solution for Toxicity Issues. J. Biomater. Sci. Polym. Ed. 2009, 20, 141–166. [Google Scholar] [CrossRef] [PubMed]
- Mignani, S.; Tripathi, V.D.; Soam, D.; Tripathi, R.P.; Das, S.; Singh, S.; Gandikota, R.; Laurent, R.; Karpus, A.; Caminade, A.M.; et al. Safe Polycationic Dendrimers as Potent Oral in Vivo Inhibitors of Mycobacterium tuberculosis: A New Therapy to Take down Tuberculosis. Biomacromolecules 2021, 22, 2659–2675. [Google Scholar] [CrossRef] [PubMed]
- Akbari, B.; Tavandashti, M.P.; Zandrahimi, M. Particle Size Characterization of Nanoparticles—A Practical Approach. Iran. J. Mater. Sci. Eng. 2011, 8, 48–56. [Google Scholar]
- Buzea, C.; Pacheco, I.I.; Robbie, K. Nanomaterials and Nanoparticles: Sources and Toxicity. Biointerphases 2007, 2, MR17–MR71. [Google Scholar] [CrossRef] [PubMed]
- Chopra, H.; Bibi, S.; Singh, I.; Hasan, M.M.; Khan, M.S.; Yousafi, Q.; Baig, A.A.; Rahman, M.; Islam, F.; Emran, T.B.; et al. Green Metallic Nanoparticles: Biosynthesis to Applications. Front. Bioeng. Biotechnol. 2022, 10, 874742. [Google Scholar] [CrossRef]
- Lee, B.K.; Yun, Y.H.; Park, K. Smart Nanoparticles for Drug Delivery: Boundaries and Opportunities. Chem. Eng. Sci. 2015, 125, 158–164. [Google Scholar] [CrossRef]
- Spirescu, V.A.; Chircov, C.; Grumezescu, A.M.; Vasile, B.Ș.; Andronescu, E. Inorganic Nanoparticles and Composite Films for Antimicrobial Therapies. Int. J. Mol. Sci. 2021, 22, 4595. [Google Scholar] [CrossRef]
- Sun, M.; Wang, T.; Li, L.; Li, X.; Zhai, Y.; Zhang, J.; Li, W. The Application of Inorganic Nanoparticles in Molecular Targeted Cancer Therapy: EGFR Targeting. Front. Pharmacol. 2021, 12, 702445. [Google Scholar] [CrossRef]
- Bayda, S.; Hadla, M.; Palazzolo, S.; Riello, P.; Corona, G.; Toffoli, G.; Rizzolio, F. Inorganic Nanoparticles for Cancer Therapy: A Transition from Lab to Clinic. Curr. Med. Chem. 2018, 25, 4269–4303. [Google Scholar] [CrossRef]
- Bhattacharya, T.; Das, D.; Borges e Soares, G.A.; Chakrabarti, P.; Ai, Z.; Chopra, H.; Hasan, M.A.; Cavalu, S. Novel Green Approaches for the Preparation of Gold Nanoparticles and Their Promising Potential in Oncology. Processes 2022, 10, 426. [Google Scholar] [CrossRef]
- Khalil, M.S.; Shakeel, M.; Gulfam, N.; Ahmad, S.U.; Aziz, A.; Ahmad, J.; Bibi, S.; Chopra, H.; Alhumaydhi, F.A.; Idris, A.M.; et al. Fabrication of Silver Nanoparticles from Ziziphus nummularia Fruit Extract: Effect on Hair Growth Rate and Activity against Selected Bacterial and Fungal Strains. J. Nanomater. 2022, 2022, 3164951. [Google Scholar] [CrossRef]
- Mehmood, Y.; Shahid, H.; Barkat, K.; Ibraheem, M.; Riaz, H.; Badshah, S.F.; Chopra, H.; Sharma, R.; Nepovimova, E.; Kuca, K.; et al. Designing of SiO2 Mesoporous Nanoparticles Loaded with Mometasone Furoate for Potential Nasal Drug Delivery: Ex Vivo Evaluation and Determination of pro-Inflammatory Interferon and Interleukin MRNA Expression. Front. Cell Dev. Biol. 2023, 10, 2411. [Google Scholar] [CrossRef] [PubMed]
- Heidary, M.; Bostanabad, S.Z.; Amini, S.M.; Jafari, A.; Nobar, M.G.; Ghodousi, A.; Kamalzadeh, M.; Darban-Sarokhalil, D. The Anti-Mycobacterial Activity of Ag, Zno, and Ag-Zno Nanoparticles against Mdr-and Xdr-Mycobacterium tuberculosis. Infect Drug Resist. 2019, 12, 3425–3435. [Google Scholar] [CrossRef]
- Ellis, T.; Chiappi, M.; García-Trenco, A.; Al-Ejji, M.; Sarkar, S.; Georgiou, T.K.; Shaffer, M.S.P.; Tetley, T.D.; Schwander, S.; Ryan, M.P.; et al. Multimetallic Microparticles Increase the Potency of Rifampicin against Intracellular Mycobacterium tuberculosis. ACS Nano 2018, 12, 5228–5240. [Google Scholar] [CrossRef] [PubMed]
- Jafari, A.; Mosavari, N.; Movahedzadeh, F.; Nodooshan, S.J.; Safarkar, R.; Moro, R.; Kamalzadeh, M.; Majidpour, A.; Boustanshenas, M.; Mosavi, T. Bactericidal Impact of Ag, ZnO and Mixed AgZnO Colloidal Nanoparticles on H37Rv Mycobacterium tuberculosis Phagocytized by THP-1 Cell Lines. Microb. Pathog. 2017, 110, 335–344. [Google Scholar] [CrossRef]
- Mohanty, S.; Jena, P.; Mehta, R.; Pati, R.; Banerjee, B.; Patil, S.; Sonawane, A. Cationic Antimicrobial Peptides and Biogenic Silver Nanoparticles Kill Mycobacteria without Eliciting Dna Damage and Cytotoxicity in Mouse Macrophages. Antimicrob. Agents Chemother. 2013, 57, 3688–3698. [Google Scholar] [CrossRef]
- Jena, P.; Mohanty, S.; Mallick, R.; Jacob, B.; Sonawane, A. Toxicity and Antibacterial Assessment of Chitosancoated Silver Nanoparticles on Human Pathogens and Macrophage Cells. Int. J. Nanomed. 2012, 7, 1805–1818. [Google Scholar] [CrossRef]
- Young, J.J.; Cheng, K.M.; Young, Y.A.; Chen, X.A.; Chen, Y.H.; Chang, T.Y.; Yen, H.J.; Chen, C.C. Chondroitin Sulfate-Stabilized Silver Nanoparticles: Improved Synthesis and Their Catalytic, Antimicrobial, and Biocompatible Activities. Carbohydr. Res. 2018, 457, 14–24. [Google Scholar] [CrossRef]
- Chang, T.Y.; Chen, C.C.; Cheng, K.M.; Chin, C.Y.; Chen, Y.H.; Chen, X.A.; Sun, J.R.; Young, J.J.; Chiueh, T.S. Trimethyl Chitosan-Capped Silver Nanoparticles with Positive Surface Charge: Their Catalytic Activity and Antibacterial Spectrum Including Multidrug-Resistant Strains of Acinetobacter baumannii. Colloids Surf. B Biointerfaces 2017, 155, 61–70. [Google Scholar] [CrossRef]
- Wang, S.H.; Chen, C.C.; Lee, C.H.; Chen, X.A.; Chang, T.Y.; Cheng, Y.C.; Young, J.J.; Lu, J.J. Fungicidal and Anti-Biofilm Activities of Trimethylchitosan-Stabilized Silver Nanoparticles against Candida Species in Zebrafish Embryos. Int. J. Biol. Macromol. 2020, 143, 724–731. [Google Scholar] [CrossRef]
- Chen, C.C.; Chen, Y.Y.; Yeh, C.C.; Hsu, C.W.; Yu, S.J.; Hsu, C.H.; Wei, T.C.; Ho, S.N.; Tsai, P.C.; Song, Y.D.; et al. Alginate-Capped Silver Nanoparticles as a Potent Anti-Mycobacterial Agent Against Mycobacterium tuberculosis. Front. Pharmacol. 2021, 12, 746496. [Google Scholar] [CrossRef] [PubMed]
- Yin, I.X.; Zhang, J.; Zhao, I.S.; Mei, M.L.; Li, Q.; Chu, C.H. The Antibacterial Mechanism of Silver Nanoparticles and Its Application in Dentistry. Int. J. Nanomed. 2020, 15, 2555–2562. [Google Scholar] [CrossRef]
- Zhang, X.F.; Liu, Z.G.; Shen, W.; Gurunathan, S. Silver Nanoparticles: Synthesis, Characterization, Properties, Applications, and Therapeutic Approaches. Int. J. Mol. Sci. 2016, 17, 1534. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, P.; Mehta, A.; Kachhwaha, S.; Kothari, S.L. Green Synthesis of Silver Nanoparticles and Their Activity against Mycobacterium tuberculosis. Adv. Sci. Eng. Med. 2013, 5, 709–714. [Google Scholar] [CrossRef]
- Baulard, A.R.; Betts, J.C.; Engohang-Ndong, J.; Quan, S.; McAdam, R.A.; Brennan, P.J.; Locht, C.; Besra, G.S. Activation of the Pro-Drug Ethionamide Is Regulated in Mycobacteria. J. Biol. Chem. 2000, 275, 28326–28331. [Google Scholar] [CrossRef] [PubMed]
- Vannelli, T.A.; Dykman, A.; Ortiz De Montellano, P.R. The Antituberculosis Drug Ethionamide Is Activated by a Flavoprotein Monooxygenase. J. Biol. Chem. 2002, 277, 12824–12829. [Google Scholar] [CrossRef]
- Willand, N.; Dirié, B.; Carette, X.; Bifani, P.; Singhal, A.; Desroses, M.; Leroux, F.; Willery, E.; Mathys, V.; Déprez-Poulain, R.; et al. Synthetic EthR Inhibitors Boost Antituberculous Activity of Ethionamide. Nat. Med. 2009, 15, 537–544. [Google Scholar] [CrossRef]
- Costa-Gouveia, J.; Pancani, E.; Jouny, S.; Machelart, A.; Delorme, V.; Salzano, G.; Iantomasi, R.; Piveteau, C.; Queval, C.J.; Song, O.R.; et al. Combination Therapy for Tuberculosis Treatment: Pulmonary Administration of Ethionamide and Booster Co-Loaded Nanoparticles. Sci. Rep. 2017, 7, 5390. [Google Scholar] [CrossRef]
- Gelperina, S.; Kisich, K.; Iseman, M.D.; Heifets, L. The Potential Advantages of Nanoparticle Drug Delivery Systems in Chemotherapy of Tuberculosis. Am. J. Respir. Crit. Care Med. 2005, 172, 1487–1490. [Google Scholar] [CrossRef]
- Sosnik, A.; Carcaboso, Á.M.; Glisoni, R.J.; Moretton, M.A.; Chiappetta, D.A. New Old Challenges in Tuberculosis: Potentially Effective Nanotechnologies in Drug Delivery. Adv. Drug Deliv. Rev. 2010, 62, 547–559. [Google Scholar] [CrossRef]
- Barbé, C.; Bartlett, J.; Kong, L.; Finnie, K.; Lin, H.Q.; Larkin, M.; Calleja, S.; Bush, A.; Calleja, G. Silica Particles: A Novel Drug-Delivery System. Adv. Mater. 2004, 16, 1959–1966. [Google Scholar] [CrossRef]
- Anisimova, Y.V.; Gelperina, S.I.; Peloquin, C.A.; Heifets, L.B. Nanoparticles as Antituberculosis Drugs Carriers: Effect on Activity against Mycobacterium tuberculosis in Human Monocyte-Derived Macrophages. J. Nanoparticle Res. 2000, 2, 165–171. [Google Scholar] [CrossRef]
- Kisich, K.O.; Gelperina, S.; Higgins, M.P.; Wilson, S.; Shipulo, E.; Oganesyan, E.; Heifets, L. Encapsulation of Moxifloxacin within Poly(Butyl Cyanoacrylate) Nanoparticles Enhances Efficacy against Intracellular Mycobacterium tuberculosis. Int. J. Pharm. 2007, 345, 154–162. [Google Scholar] [CrossRef] [PubMed]
- Qurrat-ul-Ain, A.; Sharma, S.; Khuller, G.K.; Garg, S.K. Alginate-Based Oral Drug Delivery System for Tuberculosis: Pharmacokinetics and Therapeutic Effects. J. Antimicrob. Chemother. 2003, 51, 931–938. [Google Scholar] [CrossRef] [PubMed]
- Saraogi, G.K.; Gupta, P.; Gupta, U.D.; Jain, N.K.; Agrawal, G.P. Gelatin Nanocarriers as Potential Vectors for Effective Management of Tuberculosis. Int. J. Pharm. 2010, 385, 143–149. [Google Scholar] [CrossRef]
- Sharma, A.; Sharma, S.; Khuller, G.K. Lectin-Functionalized Poly (Lactide-Co-Glycolide) Nanoparticles as Oral/Aerosolized Antitubercular Drug Carriers for Treatment of Tuberculosis. J. Antimicrob. Chemother. 2004, 54, 761–766. [Google Scholar] [CrossRef]
- Muttil, P.; Kaur, J.; Kumar, K.; Yadav, A.B.; Sharma, R.; Misra, A. Inhalable Microparticles Containing Large Payload of Anti-Tuberculosis Drugs. Eur. J. Pharm. Sci. 2007, 32, 140–150. [Google Scholar] [CrossRef]
- Pandey, R.; Khuller, G.K. Solid Lipid Particle-Based Inhalable Sustained Drug Delivery System against Experimental Tuberculosis. Tuberculosis 2005, 85, 227–234. [Google Scholar] [CrossRef]
- Hwang, A.A.; Lee, B.Y.; Clemens, D.L.; Dillon, B.J.; Zink, J.I.; Horwitz, M.A. PH-Responsive Isoniazid-Loaded Nanoparticles Markedly Improve Tuberculosis Treatment in Mice. Small 2015, 11, 5066–5078. [Google Scholar] [CrossRef]
- Li, Z.Y.; Liu, Y.; Wang, X.Q.; Liu, L.H.; Hu, J.J.; Luo, G.F.; Chen, W.H.; Rong, L.; Zhang, X.Z. One-Pot Construction of Functional Mesoporous Silica Nanoparticles for the Tumor-Acidity-Activated Synergistic Chemotherapy of Glioblastoma. ACS Appl. Mater. Interfaces 2013, 5, 7995–8001. [Google Scholar] [CrossRef]
- Fan, J.; Fang, G.; Wang, X.; Zeng, F.; Xiang, Y.; Wu, S. Targeted Anticancer Prodrug with Mesoporous Silica Nanoparticles as Vehicles. Nanotechnology 2011, 22, 455102. [Google Scholar] [CrossRef] [PubMed]
- Clemens, D.L.; Lee, B.Y.; Xue, M.; Thomas, C.R.; Meng, H.; Ferris, D.; Nel, A.E.; Zink, J.I.; Horwitz, M.A. Targeted Intracellular Delivery of Antituberculosis Drugs to Mycobacterium tuberculosis-Infected Macrophages via Functionalized Mesoporous Silica Nanoparticles. Antimicrob. Agents Chemother. 2012, 56, 2535–2545. [Google Scholar] [CrossRef] [PubMed]
- Duan, H.; Nie, S. Cell-Penetrating Quantum Dots Based on Multivalent and Endosome-Disrupting Surface Coatings. J. Am. Chem. Soc. 2007, 129, 3333–3336. [Google Scholar] [CrossRef] [PubMed]
- Xia, T.; Kovochich, M.; Liong, M.; Meng, H.; Kabehie, S.; George, S.; Zink, J.I.; Nel, A.E. Polyethyleneimine Coating Enhances the Cellular Uptake of Mesoporous Silica Nanoparticles and Allows Safe Delivery of SiRNA and DNA Constructs. ACS Nano 2009, 3, 3273–3286. [Google Scholar] [CrossRef]
- Cao, Z.; Sun, Y. Chitosan-Based Rechargeable Long-Term Antimicrobial and Biofilm-Controlling Systems. J. Biomed. Mater. Res. A 2009, 89, 960–967. [Google Scholar] [CrossRef]
- Chokshi, N.V.; Khatri, H.N.; Patel, M.M. Formulation, Optimization, and Characterization of Rifampicin-Loaded Solid Lipid Nanoparticles for the Treatment of Tuberculosis. Drug Dev. Ind. Pharm. 2018, 44, 1975–1989. [Google Scholar] [CrossRef]
- Kora, A.J.; Beedu, S.R.; Jayaraman, A. Size-Controlled Green Synthesis of Silver Nanoparticles Mediated by Gum Ghatti (Anogeissus latifolia) and Its Biological Activity. Org. Med. Chem. Lett. 2012, 2, 1–10. [Google Scholar] [CrossRef]
- Singh, R.; Nawale, L.U.; Arkile, M.; Shedbalkar, U.U.; Wadhwani, S.A.; Sarkar, D.; Chopade, B.A. Chemical and Biological Metal Nanoparticles as Antimycobacterial Agents: A Comparative Study. Int. J. Antimicrob. Agents 2015, 46, 183–188. [Google Scholar] [CrossRef]
- Singh, R.; Nawale, L.; Arkile, M.; Wadhwani, S.; Shedbalkar, U.; Chopade, S.; Sarkar, D.; Chopade, B.A. Phytogenic Silver, Gold, and Bimetallic Nanoparticles as Novel Antitubercular Agents. Int. J. Nanomed. 2016, 11, 1889–1897. [Google Scholar] [CrossRef]
- Larimer, C.; Islam, M.S.; Ojha, A.; Nettleship, I. Mutation of Environmental Mycobacteria to Resist Silver Nanoparticles Also Confers Resistance to a Common Antibiotic. BioMetals 2014, 27, 695–702. [Google Scholar] [CrossRef]
- Sarkar, S.; Leo, B.F.; Carranza, C.; Chen, S.; Rivas-Santiago, C.; Porter, A.E.; Ryan, M.P.; Gow, A.; Chung, K.F.; Tetley, T.D.; et al. Modulation of Human Macrophage Responses to Mycobacterium tuberculosis by Silver Nanoparticles of Different Size and Surface Modification. PLoS ONE 2015, 10, e0143077. [Google Scholar] [CrossRef] [PubMed]
- Olakanmi, O.; Kesavalu, B.; Pasula, R.; Abdalla, M.Y.; Schlesinger, L.S.; Britigan, B.E. Gallium Nitrate Is Efficacious in Murine Models of Tuberculosis and Inhibits Key Bacterial Fe-Dependent Enzymes. Antimicrob. Agents Chemother. 2013, 57, 6074–6080. [Google Scholar] [CrossRef] [PubMed]
- Gifford, J.C.; Bresee, J.; Carter, C.J.; Wang, G.; Melander, R.J.; Melander, C.; Feldheim, D.L. Thiol-Modified Gold Nanoparticles for the Inhibition of Mycobacterium smegmatis. Chem. Commun. 2014, 50, 15860–15863. [Google Scholar] [CrossRef] [PubMed]
- El-Zowalaty, M.E.; Al-Ali, S.H.H.; Husseiny, M.I.; Geilich, B.M.; Webster, T.J.; Hussein, M.Z. The Ability of Streptomycin-Loaded Chitosan-Coated Magnetic Nanocomposites to Possess Antimicrobial and Antituberculosis Activities. Int. J. Nanomed. 2015, 10, 3269–3274. [Google Scholar] [CrossRef]
- Padwal, P.; Bandyopadhyaya, R.; Mehra, S. Polyacrylic Acid-Coated Iron Oxide Nanoparticles for Targeting Drug Resistance in Mycobacteria. Langmuir 2014, 30, 15266–15276. [Google Scholar] [CrossRef]
- Paranjpe, M.; Müller-Goymann, C.C. Nanoparticle-Mediated Pulmonary Drug Delivery: A Review. Int. J. Mol. Sci. 2014, 15, 5852–5873. [Google Scholar] [CrossRef]
- Rostami, E.; Kashanian, S.; Azandaryani, A.H.; Faramarzi, H.; Dolatabadi, J.E.N.; Omidfar, K. Drug Targeting Using Solid Lipid Nanoparticles. Chem. Phys. Lipids 2014, 181, 56–61. [Google Scholar] [CrossRef]
- Sharma, R.; Muttil, P.; Yadav, A.B.; Rath, S.K.; Bajpai, V.K.; Mani, U.; Misra, A. Uptake of Inhalable Microparticles Affects Defence Responses of Macrophages Infected with Mycobacterium tuberculosis H37Ra. J. Antimicrob. Chemother. 2007, 59, 499–506. [Google Scholar] [CrossRef]
- Lee, W.H.; Loo, C.Y.; Traini, D.; Young, P.M. Nano- and Micro-Based Inhaled Drug Delivery Systems for Targeting Alveolar Macrophages. Expert Opin. Drug Deliv. 2015, 12, 1009–1026. [Google Scholar] [CrossRef]
- Maretti, E.; Rossi, T.; Bondi, M.; Croce, M.A.; Hanuskova, M.; Leo, E.; Sacchetti, F.; Iannuccelli, V. Inhaled Solid Lipid Microparticles to Target Alveolar Macrophages for Tuberculosis. Int. J. Pharm. 2014, 462, 74–82. [Google Scholar] [CrossRef]
- Truzzi, E.; Nascimento, T.L.; Iannuccelli, V.; Costantino, L.; Lima, E.M.; Leo, E.; Siligardi, C.; Gualtieri, M.L.; Maretti, E. In Vivo Biodistribution of Respirable Solid Lipid Nanoparticles Surface-Decorated with a Mannose-Based Surfactant: A Promising Tool for Pulmonary Tuberculosis Treatment? Nanomaterials 2020, 10, 568. [Google Scholar] [CrossRef] [PubMed]
- Porter, C.J.H.; Trevaskis, N.L.; Charman, W.N. Lipids and Lipid-Based Formulations: Optimizing the Oral Delivery of Lipophilic Drugs. Nat. Rev. Drug Discov. 2007, 6, 231–248. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Shen, Q.; Katsumi, H.; Okada, N.; Fujita, T.; Jiang, X.; Yamamoto, A. Effects of Labrasol and Other Pharmaceutical Excipients on the Intestinal Transport and Absorption of Rhodamine123, a P-Glycoprotein Substrate, in Rats. Biol. Pharm. Bull. 2007, 30, 1301–1307. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Yao, M.; Morrison, R.A.; Chong, S. Commonly Used Surfactant, Tween 80, Improves Absorption of P-Glycoprotein Substrate, Digoxin, in Rats. Arch Pharm. Res. 2003, 26, 768–772. [Google Scholar] [CrossRef]
- Desjardins, M.; Griffiths, G. Phagocytosis: Latex Leads the Way. Curr. Opin. Cell Biol. 2003, 15, 498–503. [Google Scholar] [CrossRef]
- Ul-Ain, Q.; Sharma, S.; Khuller, G.K. Chemotherapeutic Potential of Orally Administered Poly(Lactide-Co-Glycolide) Microparticles Containing Isoniazid, Rifampin, and Pyrazinamide against Experimental Tuberculosis. Antimicrob. Agents Chemother. 2003, 47, 3005–3007. [Google Scholar] [CrossRef]
- Pandey, R.; Khuller, G.K. Chemotherapeutic Potential of Alginate-Chitosan Microspheres as Anti-Tubercular Drug Carriers. J. Antimicrob. Chemother. 2004, 53, 635–640. [Google Scholar] [CrossRef]
- Pandey, R.; Sharma, A.; Zahoor, A.; Sharma, S.; Khuller, G.K.; Prasad, B. Poly (DL-Lactide-Co-Glycolide) Nanoparticle-Based Inhalable Sustained Drug Delivery System for Experimental Tuberculosis. J. Antimicrob. Chemother. 2003, 52, 981–986. [Google Scholar] [CrossRef]
- Mahendra, C.; Chandra, M.N.; Murali, M.; Abhilash, M.R.; Singh, S.B.; Satish, S.; Sudarshana, M.S. Phyto-Fabricated ZnO Nanoparticles from Canthium dicoccum (L.) for Antimicrobial, Anti-Tuberculosis and Antioxidant Activity. Process Biochem. 2020, 89, 220–226. [Google Scholar] [CrossRef]
- Abdel-Aziz, M.M.; Elella, M.H.A.; Mohamed, R.R. Green Synthesis of Quaternized Chitosan/Silver Nanocomposites for Targeting Mycobacterium tuberculosis and Lung Carcinoma Cells (A-549). Int. J. Biol. Macromol. 2020, 142, 244–253. [Google Scholar] [CrossRef]
- Sivaraj, A.; Kumar, V.; Sunder, R.; Parthasarathy, K.; Kasivelu, G. Commercial Yeast Extracts Mediated Green Synthesis of Silver Chloride Nanoparticles and Their Anti-Mycobacterial Activity. J. Clust. Sci. 2020, 31, 287–291. [Google Scholar] [CrossRef]
- Pi, J.; Shen, L.; Yang, E.; Shen, H.; Huang, D.; Wang, R.; Hu, C.; Jin, H.; Cai, H.; Cai, J.; et al. Macrophage-Targeted Isoniazid–Selenium Nanoparticles Promote Antimicrobial Immunity and Synergize Bactericidal Destruction of Tuberculosis Bacilli. Angew. Chem. 2020, 59, 3226–3234. [Google Scholar] [CrossRef] [PubMed]
- Viswanathan, V.; Mehta, H.; Pharande, R.; Bannalikar, A.; Gupta, P.; Gupta, U.; Mukne, A. Mannosylated Gelatin Nanoparticles of Licorice for Use in Tuberculosis: Formulation, in Vitro Evaluation, in Vitro Cell Uptake, in Vivo Pharmacokinetics and in Vivo Anti-Tubercular Efficacy. J. Drug Deliv. Sci. Technol. 2018, 45, 255–263. [Google Scholar] [CrossRef]
- Hwang, J.; Son, J.; Seo, Y.; Jo, Y.; Lee, K.; Lee, D.; Khan, M.S.; Chavan, S.; Park, C.; Sharma, A.; et al. Functional Silica Nanoparticles Conjugated with Beta-Glucan to Deliver Anti-Tuberculosis Drug Molecules. J. Ind. Eng. Chem. 2018, 58, 376–385. [Google Scholar] [CrossRef]
- Kesavan, M.P.; Ayyanaar, S.; Vijayakumar, V.; Dhaveethu Raja, J.; Annaraj, J.; Sakthipandi, K.; Rajesh, J. Magnetic Iron Oxide Nanoparticles (MIONs) Cross-Linked Natural Polymer-Based Hybrid Gel Beads: Controlled Nano Anti-TB Drug Delivery Application. J. Biomed. Mater. Res. A 2018, 106, 1039–1050. [Google Scholar] [CrossRef]
- Amarnath Praphakar, R.; Jeyaraj, M.; Ahmed, M.; Suresh Kumar, S.; Rajan, M. Silver Nanoparticle Functionalized CS-g-(CA-MA-PZA) Carrier for Sustainable Anti-Tuberculosis Drug Delivery. Int. J. Biol. Macromol. 2018, 118, 1627–1638. [Google Scholar] [CrossRef]
- Abdelghany, S.; Parumasivam, T.; Pang, A.; Roediger, B.; Tang, P.; Jahn, K.; Britton, W.J.; Chan, H.K. Alginate Modified-PLGA Nanoparticles Entrapping Amikacin and Moxifloxacin as a Novel Host-Directed Therapy for Multidrug-Resistant Tuberculosis. J. Drug Deliv. Sci. Technol. 2019, 52, 642–651. [Google Scholar] [CrossRef]
- Ramalingam, V.; Sundaramahalingam, S.; Rajaram, R. Size-Dependent Antimycobacterial Activity of Titanium Oxide Nanoparticles against: Mycobacterium tuberculosis. J. Mater. Chem. B 2019, 7, 4338–4346. [Google Scholar] [CrossRef]
- Batalha, I.L.; Bernut, A.; Schiebler, M.; Ouberai, M.M.; Passemar, C.; Klapholz, C.; Kinna, S.; Michel, S.; Sader, K.; Castro-Hartmann, P.; et al. Polymeric Nanobiotics as a Novel Treatment for Mycobacterial Infections. J. Control. Release 2019, 314, 116–124. [Google Scholar] [CrossRef]
- Ahmad, Z.; Pandey, R.; Sharma, S.; Khuller, G.K. Alginate Nanoparticles as Antituberculosis Drug Carriers: Formulation Development, Pharmacokinetics and Therapeutic Potential. Indian J. Chest Dis. Allied Sci. 2006, 48, 171–176. [Google Scholar]
- Rajan, M.; Raj, V. Encapsulation, Characterisation and in-Vitro Release of Anti-Tuberculosis Drug Using Chitosan—Poly Ethylene Glycol Nanoparticles. Int. J. Pharm. Pharm. Sci. 2012, 4, 255–259. [Google Scholar]
- Banu, A.; Rathod, V. Biosynthesis of Monodispersed Silver Nanoparticles and Their Activity against Mycobacterium tuberculosis. Int. J. Biomed. Nanosci. Nanotechnol. 2013, 3, 1000110. [Google Scholar] [CrossRef]
- Patil, B.N.; Taranath, T.C. Limonia acidissima L. Leaf Mediated Synthesis of Zinc Oxide Nanoparticles: A Potent Tool against Mycobacterium tuberculosis. Int. J. Mycobacteriol. 2016, 5, 197–204. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Pandey, A.K.; Singh, S.S.; Shanker, R.; Dhawan, A. Engineered ZnO and TiO2 Nanoparticles Induce Oxidative Stress and DNA Damage Leading to Reduced Viability of Escherichia coli. Free Radic. Biol. Med. 2011, 51, 1872–1881. [Google Scholar] [CrossRef] [PubMed]
- Feng, Q.L.; Wu, J.; Chen, G.Q.; Cui, F.Z.; Kim, T.N.; Kim, J.O. A Mechanistic Study of the Antibacterial Effect of Silver Ions on Escherichia coli and Staphylococcus aureus. J. Biomed. Mater. Res. 2000, 52, 662–668. [Google Scholar] [CrossRef]
- Baruwati, B.; Kumar, D.K.; Manorama, S.V. Hydrothermal Synthesis of Highly Crystalline ZnO Nanoparticles: A Competitive Sensor for LPG and EtOH. Sens. Actuators B Chem. 2006, 119, 676–682. [Google Scholar] [CrossRef]
- Raghupathi, K.R.; Koodali, R.T.; Manna, A.C. Size-Dependent Bacterial Growth Inhibition and Mechanism of Antibacterial Activity of Zinc Oxide Nanoparticles. Langmuir 2011, 27, 4020–4028. [Google Scholar] [CrossRef]
- Periasamy, S.; Joo, H.S.; Duong, A.C.; Bach, T.H.L.; Tan, V.Y.; Chatterjee, S.S.; Cheung, G.Y.C.; Otto, M. How Staphylococcus aureus Biofilms Develop Their Characteristic Structure. Proc. Natl. Acad. Sci. USA 2012, 109, 1281–1286. [Google Scholar] [CrossRef]
- Lazar, V. Quorum Sensing in Biofilms—How to Destroy the Bacterial Citadels or Their Cohesion/Power? Anaerobe 2011, 17, 280–285. [Google Scholar] [CrossRef]
- Choi, O.; Deng, K.K.; Kim, N.J.; Ross, L.; Surampalli, R.Y.; Hu, Z. The Inhibitory Effects of Silver Nanoparticles, Silver Ions, and Silver Chloride Colloids on Microbial Growth. Water Res. 2008, 42, 3066–3074. [Google Scholar] [CrossRef]
- McQuillan, J.S.; Groenaga Infante, H.; Stokes, E.; Shaw, A.M. Silver Nanoparticle Enhanced Silver Ion Stress Response in Escherichia coli K12. Nanotoxicology 2012, 6, 857–866. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, S.; Roy, S.; Bhaumik, K.N.; Pillai, J. Mechanisms of the Effectiveness of Lipid Nanoparticle Formulations Loaded with Anti-Tubercular Drugs Combinations toward Overcoming Drug Bioavailability in Tuberculosis. J. Drug Target 2020, 28, 55–69. [Google Scholar] [CrossRef] [PubMed]
- Hussain, A.; Altamimi, M.A.; Alshehri, S.; Imam, S.S.; Shakeel, F.; Singh, S.K. Novel Approach for Transdermal Delivery of Rifampicin to Induce Synergistic Antimycobacterial Effects against Cutaneous and Systemic Tuberculosis Using a Cationic Nanoemulsion Gel. Int. J. Nanomed. 2020, 15, 1073–1094. [Google Scholar] [CrossRef] [PubMed]
- Caon, T.; Campos, C.E.M.; Simões, C.M.O.; Silva, M.A.S. Novel Perspectives in the Tuberculosis Treatment: Administration of Isoniazid through the Skin. Int. J. Pharm. 2015, 494, 463–470. [Google Scholar] [CrossRef] [PubMed]
- Godwin, D.A.; Michniak, B.B.; Creek, K.E. Evaluation of Transdermal Penetration Enhancers Using a Novel Skin Alternative. J. Pharm. Sci. 1997, 86, 1001–1005. [Google Scholar] [CrossRef]
- Kang, K.C.; Lee, C.I.; Pyo, H.B.; Jeong, N.H. Preparation and Characterization of Nano-Liposomes Using Phosphatidylcholine. J. Ind. Eng. Chem. 2005, 11, 847–851. [Google Scholar]
- Kaur, I.P.; Bhandari, R.; Bhandari, S.; Kakkar, V. Potential of Solid Lipid Nanoparticles in Brain Targeting. J. Control. Release 2008, 127, 97–109. [Google Scholar] [CrossRef]
- Lee, G.-S.; Lee, D.-H.; Kang, K.-C.; Lee, C.-I.; Pyo, H.-B.; Choi, T.-B. Preparation and Characterization of Bis-Ethylhexyloxyphenolmethoxy-Phenyltriazine (BEMT) Loaded Solid Lipid Nano-Particles (SLN). J. Ind. Eng. Chem. 2007, 13, 1180–1187. [Google Scholar]
- Hu, L.; Tang, X.; Cui, F. Solid Lipid Nanoparticles (SLNs) to Improve Oral Bioavailability of Poorly Soluble Drugs. J. Pharm. Pharmacol. 2010, 56, 1527–1535. [Google Scholar] [CrossRef]
- Lim, S.J.; Lee, M.K.; Kim, C.K. Altered Chemical and Biological Activities of All-Trans Retinoic Acid Incorporated in Solid Lipid Nanoparticle Powders. J. Control. Release 2004, 100, 53–61. [Google Scholar] [CrossRef]
- Cortesi, R.; Esposito, E.; Luca, G.; Nastruzzi, C. Production of Lipospheres as Carriers for Bioactive Compounds. Biomaterials 2002, 23, 2283–2294. [Google Scholar] [CrossRef] [PubMed]
- De Matteis, L.; Jary, D.; Lucía, A.; García-Embid, S.; Serrano-Sevilla, I.; Pérez, D.; Ainsa, J.A.; Navarro, F.P.; de la Fuente, J.M. New Active Formulations against M. Tuberculosis: Bedaquiline Encapsulation in Lipid Nanoparticles and Chitosan Nanocapsules. Chem. Eng. J. 2018, 340, 181–191. [Google Scholar] [CrossRef]
- De Matteis, V.; Rinaldi, R. Toxicity Assessment in the Nanoparticle Era. In Cellular and Molecular Toxicology of Nanoparticles; Saquib, Q., Faisal, M., Al-Khedhairy, A.A., Alatar, A.A., Eds.; Springer International Publishing: Cham, Switzerland, 2018; pp. 1–19. ISBN 978-3-319-72041-8. [Google Scholar]
- Santos, N.C.; Castanho, M.A.R.B. Liposomes: Has the Magic Bullet Hit the Target? Quim. Nova 2002, 25, 1181–1185. [Google Scholar] [CrossRef]
- Li, J.; Wang, X.; Zhang, T.; Wang, C.; Huang, Z.; Luo, X.; Deng, Y. A Review on Phospholipids and Their Main Applications in Drug Delivery Systems. Asian J. Pharm. Sci. 2015, 10, 81–98. [Google Scholar] [CrossRef]
- Yokota, D.; Moraes, M.; Pinho, S.C. Characterization of Lyophilized Liposomes Produced with Non-Purified Soy Lecithin: A Case Study of Casein Hydrolysate Microencapsulation. Braz. J. Chem. Eng. 2012, 29, 325–335. [Google Scholar] [CrossRef]
- Adriana, R.M.; Leticia, M.d.A.; Maria, I.R.M.; Leonor, A.d.S.-S. Importance of Lecithin for Encapsulation Processes. Afr. J. Food Sci. 2014, 8, 176–183. [Google Scholar] [CrossRef]
- Nkanga, C.I.; Krause, R.W.; Noundou, X.S.; Walker, R.B. Preparation and Characterization of Isoniazid-Loaded Crude Soybean Lecithin Liposomes. Int. J. Pharm. 2017, 526, 466–473. [Google Scholar] [CrossRef]
- Payne, N.I.; Timmins, P.; Ambrose, C.V.; Ward, M.D.; Ridgway, F. Proliposomes: A Novel Solution to an Old Problem. J. Pharm. Sci. 1986, 75, 325–329. [Google Scholar] [CrossRef]
- Elhissi, A.; Phoenix, D.; Ahmed, W. Some Approaches to Large-Scale Manufacturing of Liposomes. In Emerging Nanotechnologies for Manufacturing; Elsevier: Amsterdam, The Netherlands, 2014; pp. 402–417. ISBN 9780323289900. [Google Scholar]
- Khan, I.; Elhissi, A.; Shah, M.; Alhnan, M.A.; Ahmed, W. Liposome-Based Carrier Systems and Devices Used for Pulmonary Drug Delivery. In Biomaterials and Medical Tribology: Research and Development; Elsevier Inc.: Amsterdam, The Netherlands, 2013; pp. 395–443. ISBN 9780857090171. [Google Scholar]
- Patil-Gadhe, A.; Pokharkar, V. Single Step Spray Drying Method to Develop Proliposomes for Inhalation: A Systematic Study Based on Quality by Design Approach. Pulm. Pharmacol. Ther. 2014, 27, 197–207. [Google Scholar] [CrossRef]
- Rojanarat, W.; Changsan, N.; Tawithong, E.; Pinsuwan, S.; Chan, H.K.; Srichana, T. Isoniazid Proliposome Powders for Inhalation-Preparation, Characterization and Cell Culture Studies. Int. J. Mol. Sci. 2011, 12, 4414–4434. [Google Scholar] [CrossRef]
- Viswanathan, V.; Pharande, R.; Bannalikar, A.; Gupta, P.; Gupta, U.; Mukne, A. Inhalable Liposomes of Glycyrrhiza glabra Extract for Use in Tuberculosis: Formulation, in Vitro Characterization, in Vivo Lung Deposition, and in Vivo Pharmacodynamic Studies. Drug Dev. Ind. Pharm. 2019, 45, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Sangboonruang, S.; Semakul, N.; Suriyaprom, S.; Kitidee, K.; Khantipongse, J.; Intorasoot, S.; Tharinjaroen, C.S.; Wattananandkul, U.; Butr-Indr, B.; Phunpae, P.; et al. Nano-Delivery System of Ethanolic Extract of Propolis Targeting Mycobacterium tuberculosis via Aptamer-Modified-Niosomes. Nanomaterials 2023, 13, 269. [Google Scholar] [CrossRef] [PubMed]
- Karbalaei Zadeh Babaki, M.; Soleimanpour, S.; Rezaee, S.A. Antigen 85 Complex as a Powerful Mycobacterium tuberculosis Immunogene: Biology, Immune-Pathogenicity, Applications in Diagnosis, and Vaccine Design. Microb. Pathog. 2017, 112, 20–29. [Google Scholar] [CrossRef] [PubMed]
- Barry, C.E.; Boshoff, H.I.; Dartois, V.; Dick, T.; Ehrt, S.; Flynn, J.A.; Schnappinger, D.; Wilkinson, R.J.; Young, D. The Spectrum of Latent Tuberculosis: Rethinking the Biology and Intervention Strategies. Nat. Rev. Microbiol. 2009, 7, 845–855. [Google Scholar] [CrossRef]
- Pai, N.P.; Pai, M. Point-of-Care Diagnostics for HIV and Tuberculosis: Landscape, Pipeline, and Unmet Needs. Discov. Med. 2013, 13, 35–45. [Google Scholar]
- Wang, S.Q.; Xu, F.; Demirci, U. Advances in Developing HIV-1 Viral Load Assays for Resource-Limited Settings. Biotechnol. Adv. 2010, 28, 770–781. [Google Scholar] [CrossRef]
- Sonawane, M.D.; Nimse, S.B. Surface Modification Chemistries of Materials Used in Diagnostic Platforms with Biomolecules. J. Chem. 2016, 2016, 9241378. [Google Scholar] [CrossRef]
- Kim, J.; Oh, S.Y.; Shukla, S.; Hong, S.B.; Heo, N.S.; Bajpai, V.K.; Chun, H.S.; Jo, C.H.; Choi, B.G.; Huh, Y.S.; et al. Heteroassembled Gold Nanoparticles with Sandwich-Immunoassay LSPR Chip Format for Rapid and Sensitive Detection of Hepatitis B Virus Surface Antigen (HBsAg). Biosens. Bioelectron. 2018, 107, 118–122. [Google Scholar] [CrossRef]
- Chandra, P.; Das, D.; Abdelwahab, A.A. Gold Nanoparticles in Molecular Diagnostics and Therapeutics. Dig. J. Nanomater. Biostruct. 2010, 5, 363–367. [Google Scholar]
- Baptista, P.; Pereira, E.; Eaton, P.; Doria, G.; Miranda, A.; Gomes, I.; Quaresma, P.; Franco, R. Gold Nanoparticles for the Development of Clinical Diagnosis Methods. Anal. Bioanal. Chem. 2008, 391, 943–950. [Google Scholar] [CrossRef]
- Cordeiro, M.; Carlos, F.F.; Pedrosa, P.; Lopez, A.; Baptista, P.V. Gold Nanoparticles for Diagnostics: Advances towards Points of Care. Diagnostics 2016, 6, 43. [Google Scholar] [CrossRef] [PubMed]
- Tsai, T.T.; Huang, C.Y.; Chen, C.A.; Shen, S.W.; Wang, M.C.; Cheng, C.M.; Chen, C.F. Diagnosis of Tuberculosis Using Colorimetric Gold Nanoparticles on a Paper-Based Analytical Device. ACS Sens. 2017, 2, 1345–1354. [Google Scholar] [CrossRef] [PubMed]
- IUPAC. Biosensor. In The IUPAC Compendium of Chemical Terminology; International Union of Pure and Applied Chemistry (IUPAC): Research Triangle Park, NC, USA, 2014. [Google Scholar]
- Ferreira, G.N.M.; da-Silva, A.C.; Tomé, B. Acoustic Wave Biosensors: Physical Models and Biological Applications of Quartz Crystal Microbalance. Trends Biotechnol. 2009, 27, 689–697. [Google Scholar] [CrossRef]
- Nayak, M.; Kotian, A.; Marathe, S.; Chakravortty, D. Detection of Microorganisms Using Biosensors—A Smarter Way towards Detection Techniques. Biosens. Bioelectron. 2009, 25, 661–667. [Google Scholar] [CrossRef]
- Homola, J. Present and Future of Surface Plasmon Resonance Biosensors. Anal. Bioanal. Chem. 2003, 377, 528–539. [Google Scholar] [CrossRef]
- Fan, X.; White, I.M.; Shopova, S.I.; Zhu, H.; Suter, J.D.; Sun, Y. Sensitive Optical Biosensors for Unlabeled Targets: A Review. Anal. Chim. Acta 2008, 620, 8–26. [Google Scholar] [CrossRef] [PubMed]
- Zanchetta, G.; Lanfranco, R.; Giavazzi, F.; Bellini, T.; Buscaglia, M. Emerging Applications of Label-Free Optical Biosensors. Nanophotonics 2017, 6, 627–645. [Google Scholar] [CrossRef]
- Chuang, P.-C.; Liao, P.-C.; Chen, Y.-F. Enhancing the Sensitivity of Localized Surface Plasmon Resonance (LSPR) Biosensors Using Nanorods and DNA Aptamers. In Proceedings of the Plasmonics in Biology and Medicine XII; SPIE: Bellingham, MA, USA, 2015; Volume 9340, p. 93400T. [Google Scholar]
- Sun, W.; Yuan, S.; Huang, H.; Liu, N.; Tan, Y. A Label-Free Biosensor Based on Localized Surface Plasmon Resonance for Diagnosis of Tuberculosis. J. Microbiol. Methods 2017, 142, 41–45. [Google Scholar] [CrossRef]
- Silva, L.B.; Veigas, B.; Doria, G.; Costa, P.; Inácio, J.; Martins, R.; Fortunato, E.; Baptista, P.V. Portable Optoelectronic Biosensing Platform for Identification of Mycobacteria from the Mycobacterium tuberculosis Complex. Biosens. Bioelectron. 2011, 26, 2012–2017. [Google Scholar] [CrossRef]
- Kim, J.; Lee, J.; Lee, K.-I.; Park, T.J.; Kim, H.-J.; Lee, J. Rapid Monitoring of CFP-10 during Culture of Mycobacterium tuberculosis by Using a Magnetophoretic Immunoassay. Sens. Actuators B Chem. 2013, 177, 327–333. [Google Scholar] [CrossRef]
- Veigas, B.; Jacob, J.M.; Costa, M.N.; Santos, D.S.; Viveiros, M.; Inácio, J.; Martins, R.; Barquinha, P.; Fortunato, E.; Baptista, P.V. Gold on Paper-Paper Platform for Au-Nanoprobe TB Detection. Lab. Chip. 2012, 12, 4802–4808. [Google Scholar] [CrossRef] [PubMed]
- Bernacka-Wojcik, I.; Lopes, P.; Catarina Vaz, A.; Veigas, B.; Jerzy Wojcik, P.; Simoes, P.; Barata, D.; Fortunato, E.; Viana Baptista, P.; Águas, H.; et al. Bio-Microfluidic Platform for Gold Nanoprobe Based DNA Detection-Application to Mycobacterium tuberculosis. Biosens. Bioelectron. 2013, 48, 87–93. [Google Scholar] [CrossRef] [PubMed]
- Farré, M.; Kantiani, L.; Petrovic, M.; Pérez, S.; Barceló, D. Achievements and Future Trends in the Analysis of Emerging Organic Contaminants in Environmental Samples by Mass Spectrometry and Bioanalytical Techniques. J. Chromatogr. A 2012, 1259, 86–99. [Google Scholar] [CrossRef] [PubMed]
- Liandris, E.; Gazouli, M.; Andreadou, M.; Čomor, M.; Abazovic, N.; Sechi, L.A.; Ikonomopoulos, J. Direct Detection of Unamplified DNA from Pathogenic Mycobacteria Using DNA-Derivatized Gold Nanoparticles. J. Microbiol. Methods 2009, 78, 260–264. [Google Scholar] [CrossRef]
- Engström, A.; Zardán Gómez de la Torre, T.; Strømme, M.; Nilsson, M.; Herthnek, D. Detection of Rifampicin Resistance in Mycobacterium tuberculosis by Padlock Probes and Magnetic Nanobead-Based Readout. PLoS ONE 2013, 8, e62015. [Google Scholar] [CrossRef] [PubMed]
- Sabale, S.; Kandesar, P.; Jadhav, V.; Komorek, R.; Motkuri, R.K.; Yu, X.Y. Recent Developments in the Synthesis, Properties, and Biomedical Applications of Core/Shell Superparamagnetic Iron Oxide Nanoparticles with Gold. Biomater. Sci. 2017, 5, 2212–2225. [Google Scholar] [CrossRef] [PubMed]
- Bomanji, J.B.; Gupta, N.; Gulati, P.; Das, C.J. Imaging in Tuberculosis. Cold Spring Harb. Perspect. Med. 2015, 5, a017814. [Google Scholar] [CrossRef]
- Liong, M.; Hoang, A.N.; Chung, J.; Gural, N.; Ford, C.B.; Min, C.; Shah, R.R.; Ahmad, R.; Fernandez-Suarez, M.; Fortune, S.M.; et al. Magnetic Barcode Assay for Genetic Detection of Pathogens. Nat. Commun. 2013, 4, 1752. [Google Scholar] [CrossRef]
- Chen, Y.-T.; Kolhatkar, A.G.; Zenasni, O.; Xu, S.; Lee, T.R. Biosensing Using Magnetic Particle Detection Techniques. Sensors 2017, 17, 2300. [Google Scholar] [CrossRef]
- Yu, F.; Wang, J.; Dou, J.; Yang, H.; He, X.; Xu, W.; Zhang, Y.; Hu, K.; Gu, N. Nanoparticle-Based Adjuvant for Enhanced Protective Efficacy of DNA Vaccine Ag85A-ESAT-6-IL-21 against Mycobacterium tuberculosis Infection. Nanomedicine 2012, 8, 1337–1344. [Google Scholar] [CrossRef]
- Henriksen-Lacey, M.; Christensen, D.; Bramwell, V.W.; Lindenstrøm, T.; Agger, E.M.; Andersen, P.; Perrie, Y. Comparison of the Depot Effect and Immunogenicity of Liposomes Based on Dimethyldioctadecylammonium (DDA), 3β-[N-(N′,N′- Dimethylaminoethane)Carbomyl] Cholesterol (DC-Chol), and 1,2-Dioleoyl-3- Trimethylammonium Propane (DOTAP): Prolonged Liposome Retention Mediates Stronger Th1 Responses. Mol. Pharm. 2011, 8, 153–161. [Google Scholar] [CrossRef] [PubMed]
- Moingeon, P.; Haensler, J.; Lindberg, A. Towards the Rational Design of Th1 Adjuvants. Vaccine 2001, 19, 4363–4372. [Google Scholar] [CrossRef]
- Krishnan, L.; Sad, S.; Patel, G.B.; Sprott, G.D. Archaeosomes Induce Long-Term CD8+ Cytotoxic T Cell Response to Entrapped Soluble Protein by the Exogenous Cytosolic Pathway, in the Absence of CD4+ T Cell Help. J. Immunol. 2000, 165, 5177–5185. [Google Scholar] [CrossRef] [PubMed]
- Perrie, Y.; Frederik, P.M.; Gregoriadis, G. Liposome-Mediated DNA Vaccination: The Effect of Vesicle Composition. Vaccine 2001, 19, 3301–3310. [Google Scholar] [CrossRef] [PubMed]
- McDonald, R.J.; Liggitt, H.D.; Roche, L.; Nguyen, H.T.; Pearlman, R.; Raabe, O.G.; Bussey, L.B.; Gorman, C.M. Aerosol Delivery of Lipid: DNA Complexes to Lungs of Rhesus Monkeys. Pharm. Res. 1998, 15, 671–679. [Google Scholar] [CrossRef]
- Tyagi, R.K.; Garg, N.K.; Sahu, T. Vaccination Strategies against Malaria: Novel Carrier(s) More than a Tour de Force. J. Control. Release 2012, 162, 242–254. [Google Scholar] [CrossRef]
- Gram, G.J.; Karlsson, I.; Agger, E.M.; Andersen, P.; Fomsgaard, A. A Novel Liposome-Based Adjuvant CAF01 for Induction of CD8+ Cytotoxic T-Lymphocytes (CTL) to HIV-1 Minimal CTL Peptides in HLA-A*0201 Transgenic Mice. PLoS ONE 2009, 4, e6950. [Google Scholar] [CrossRef]
- Elvang, T.; Christensen, J.P.; Billeskov, R.; Hoang, T.T.K.T.; Holst, P.; Thomsen, A.R.; Andersen, P.; Dietrich, J. CD4 and CD8 T Cell Responses to the M. Tuberculosis Ag85B-TB10.4 Promoted by Adjuvanted Subunit, Adenovector or Heterologous Prime Boost Vaccination. PLoS ONE 2009, 4, e5139. [Google Scholar] [CrossRef]
- Agger, E.M.; Rosenkrands, I.; Hansen, J.; Brahimi, K.; Vandahl, B.S.; Aagaard, C.; Werninghaus, K.; Kirschning, C.; Lang, R.; Christensen, D.; et al. Cationic Liposomes Formulated with Synthetic Mycobacterial Cordfactor (CAF01): A Versatile Adjuvant for Vaccines with Different Immunological Requirements. PLoS ONE 2008, 3, e3116. [Google Scholar] [CrossRef]
- Derrick, S.C.; Dao, D.; Yang, A.; Kolibab, K.; Jacobs, W.R.; Morris, S.L. Formulation of a MmaA4 Gene Deletion Mutant of Mycobacterium Bovis BCG in Cationic Liposomes Significantly Enhances Protection against Tuberculosis. PLoS ONE 2012, 7, e32959. [Google Scholar] [CrossRef]
- Bivas-Benita, M.; Lin, M.Y.; Bal, S.M.; van Meijgaarden, K.E.; Franken, K.L.M.C.; Friggen, A.H.; Junginger, H.E.; Borchard, G.; Klein, M.R.; Ottenhoff, T.H.M. Pulmonary Delivery of DNA Encoding Mycobacterium tuberculosis Latency Antigen Rv1733c Associated to PLGA-PEI Nanoparticles Enhances T Cell Responses in a DNA Prime/Protein Boost Vaccination Regimen in Mice. Vaccine 2009, 27, 4010–4017. [Google Scholar] [CrossRef] [PubMed]
- Meerak, J.; Wanichwecharungruang, S.P.; Palaga, T. Enhancement of Immune Response to a DNA Vaccine against Mycobacterium tuberculosis Ag85B by Incorporation of an Autophagy Inducing System. Vaccine 2013, 31, 784–790. [Google Scholar] [CrossRef] [PubMed]
- Ai, W.; Yue, Y.; Xiong, S.; Xu, W. Enhanced Protection against Pulmonary Mycobacterial Challenge by Chitosan-Formulated Polyepitope Gene Vaccine Is Associated with Increased Pulmonary Secretory IgA and Gamma-Interferon+ T Cell Responses. Microbiol. Immunol. 2013, 57, 224–235. [Google Scholar] [CrossRef] [PubMed]
- Neutra, M.R.; Frey, A.; Kraehenbuhl, J.P. Epithelial M Cells: Gateways for Mucosal Infection and Immunization. Cell 1996, 86, 345–348. [Google Scholar] [CrossRef]
- Ebihara, C.; Kondoh, M.; Hasuike, N.; Harada, M.; Mizuguchi, H.; Horiguchi, Y.; Fujii, M.; Watanabe, Y. Preparation of a Claudin-Targeting Molecule Using a C-Terminal Fragment of Clostridium perfringens Enterotoxin. J. Pharmacol. Exp. Ther. 2006, 316, 255–260. [Google Scholar] [CrossRef]
- Rashidzadeh, H.; Danafar, H.; Rahimi, H.; Mozafari, F.; Salehiabar, M.; Rahmati, M.A.; Rahamooz-Haghighi, S.; Mousazadeh, N.; Mohammadi, A.; Ertas, Y.N.; et al. Nanotechnology against the Novel Coronavirus (Severe Acute Respiratory Syndrome Coronavirus 2): Diagnosis, Treatment, Therapy and Future Perspectives. Nanomedicine 2021, 16, 497–516. [Google Scholar] [CrossRef]
- Pandey, R.; Khuller, G.K. Nanoparticle-Based Oral Drug Delivery System for an Injectable Antibiotic—Streptomycin: Evaluation in a Murine Tuberculosis Model. Chemotherapy 2007, 53, 437–441. [Google Scholar] [CrossRef]
- Grotz, E.; Tateosian, N.; Amiano, N.; Cagel, M.; Bernabeu, E.; Chiappetta, D.A.; Moretton, M.A. Nanotechnology in Tuberculosis: State of the Art and the Challenges Ahead. Pharm. Res. 2018, 35, 213. [Google Scholar] [CrossRef]
- Wang, L.; Zhou, Y.; Wu, M.; Wu, M.; Li, X.; Gong, X.; Chang, J.; Zhang, X. Functional Nanocarrier for Drug and Gene Delivery via Local Administration in Mucosal Tissues. Nanomedicine 2018, 13, 69–88. [Google Scholar] [CrossRef]
- Manconi, M.; Manca, M.L.; Valenti, D.; Escribano, E.; Hillaireau, H.; Fadda, A.M.; Fattal, E. Chitosan and Hyaluronan Coated Liposomes for Pulmonary Administration of Curcumin. Int. J. Pharm. 2017, 525, 203–210. [Google Scholar] [CrossRef]
- Kumaresan, C.; Subramanian, N.; Gover Antoniraj, M.; Ruckmani, K. Dry Powder Inhaler—Formulation Aspects. Pharma Times 2012, 44, 14–18. [Google Scholar]
- Bourguignon, T.; Godinez-Leon, J.A.; Gref, R. Nanosized Drug Delivery Systems to Fight Tuberculosis. Pharmaceutics 2023, 15, 393. [Google Scholar] [CrossRef] [PubMed]
- He, S.; Gui, J.; Xiong, K.; Chen, M.; Gao, H.; Fu, Y. A Roadmap to Pulmonary Delivery Strategies for the Treatment of Infectious Lung Diseases. J. Nanobiotechnol. 2022, 20, 101. [Google Scholar] [CrossRef] [PubMed]
- Baranyai, Z.; Soria-Carrera, H.; Alleva, M.; Millán-Placer, A.C.; Lucía, A.; Martín-Rapún, R.; Aínsa, J.A.; la Fuente, J.M. Nanotechnology-Based Targeted Drug Delivery: An Emerging Tool to Overcome Tuberculosis. Adv. Ther. 2021, 4, 2000113. [Google Scholar] [CrossRef]
- Soni, A.; Bhandari, M.P.; Tripathi, G.K.; Bundela, P.; Khiriya, P.K.; Khare, P.S.; Kashyap, M.K.; Dey, A.; Vellingiri, B.; Sundaramurthy, S.; et al. Nano-biotechnology in Tumour and Cancerous Disease: A Perspective Review. J. Cell Mol. Med. 2023, 27, 737–762. [Google Scholar] [CrossRef] [PubMed]
- Gopinathan, A.; Naik, S.S.; Leela, K.V.; Ravi, S. Nano-Based Drug Delivery in Eliminating Tuberculosis. In Advances in Novel Formulations for Drug Delivery; Wiley: Hoboken, NJ, USA, 2023; pp. 207–218. [Google Scholar]
- Borah Slater, K.; Kim, D.; Chand, P.; Xu, Y.; Shaikh, H.; Undale, V. A Current Perspective on the Potential of Nanomedicine for Anti-Tuberculosis Therapy. Trop. Med. Infect. Dis. 2023, 8, 100. [Google Scholar] [CrossRef]



| Name of Active Nanoparticles | Size and Dimensions | Species of Mycobacterium Targeted | Outcome of Research | Reference |
|---|---|---|---|---|
| ZnO nanoparticles from Canthiumdicoccum | average size = 33 nm; zeta potential 7.3 mV | M. tuberculosis (ATCC No-27294) | Anti-TB activity by Alamar Blue Dye test revealed phytofabricated ZnO-NPs inhibited M. tuberculosis at 25 μg mL−1 | [163] |
| Quaternized chitosan/silver nanocomposites | Spherical 11 to 17.5 nm | M. tuberculosis (ATCC 25177) | Inhibition of growth. Disruption of the bacterial cell wall. | [164] |
| Silver chloride nanoparticles | Spherical 9 to 51 nm | M. tuberculosis (H37Ra, and MDR/XDR strains) | Inhibition of growth. | [165] |
| Isoniazid–Selenium Nanoparticles | Spherical 40 to 45 nm | M. tuberculosis strain H37Rv | Isoniazid-linked mannosylated selenium nanoparticles induced autophagy sequestration of Mtb, evolving into lysosome-associated Autophagosomal Mtb degradation linked to ROS-mitochondrial and PI3K/Akt/mTOR signaling pathways. | [166] |
| Mannosylated gelatin nanoparticles and licorice | 237.2 ± 5.11 to 289.6 ± 3.97 nm | M. tuberculosis strain H37Rv | Showed statistically significant reduction in bacterial counts in lungs and spleen of Mycobacterium tuberculosis H37Rv infected mice as compared to untreated animals. | [167] |
| Silica nanoparticles and isoniazid | 50 nm | Role in increasing the activation of immune cells through the attachment of particles, which will supplement the drug efficacy of INH. | [168] | |
| Magnetic iron oxide nanoparticles (MIONs) cross-linked polyethylene glycol hybrid chitosan and Rifampicin | 70.20 ± 3.50 nm | Magnetic gel beads show higher nano drug releasing efficacy at acidic medium (pH = 5.0) with a maximum efficiency of 71.00 ± 0.87%. This efficacy may also be tuned by altering the external magnetic field and the weight percentage (wt%) of PEG | [169] | |
| Amphiphilic chitosan–grafted- (cetyl alcohol-maleic anhydride-pyrazinamide), silver nanoparticles and rifampicin | 141.4 ± 1.61 nm and zeta sizer −8.44 | pH-dependent drug release, and this is of great potential for drug targets in a lysozyme environment. | [170] | |
| Alginate modified-PLGA nanoparticles entrapping amikacinand moxifloxacin | 640 ± 32 nm | M. tuberculosis H37Ra | Dual-loaded formulation revealed an enhanced inhibition of viable bacterial count compared to single drug-loaded nanoparticle formulations and untreated cells. | [171] |
| Ag, ZnO, and Ag-ZnO NPs | 5.4 ± 2.6 nm (Ag NPs) and 9.3 ± 3.9 nm (ZnO NPs) | MDR, XDR, and H37Rv (ATCC 27294) strains of M. tuberculosis | One microgram per milliliter of Ag and ZnO NPs can inhibit the growth of the XDR strains of M. tuberculosis. Moreover, 1–64 μg/mL of various dilutions of Ag-ZnO NPs can inhibit the MDR and H37Rv strains of M. tuberculosis. | [107] |
| Rifampicin-loaded solid lipid nanoparticles | 456 ± 11 nm | In vitro GI stability studies (at pH 1.2, pH 4.5, pH 6.8, and pH 7.4) revealed that the developed system could withstand various gastrointestinal tract media | [140] | |
| Titanium dioxide (TiO2) nanoparticles | M. tuberculosis, M. bovis and Mycobacterium sp. | The metabolic activity of mycobacteria was decreased up to 3–4-fold, with an increase in the concentration of the TiO2 nanoparticles hence affecting the biofilm formation. | [172] | |
| INH (conjugated), clofazimine (CFZ), coumarin-6, 1,8-Octanediol-dimethyl 2-oxoglutarate copolymer NPs | 284 ± 11 nm | M. marinum strain M carrying pTEC27 | Rapid, high-level accumulation in monocytes and neutrophils, and less efficient uptake by B and T cells in human PBMC; colocalization with Mtb H37Rv in phagosomes of dTHP-1; in a M. marinum-infected zebra fish model, NPs were taken up by macrophages; NPs had better activity in reducing bacterial burden and granuloma number. | [173] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chopra, H.; Mohanta, Y.K.; Rauta, P.R.; Ahmed, R.; Mahanta, S.; Mishra, P.K.; Panda, P.; Rabaan, A.A.; Alshehri, A.A.; Othman, B.; et al. An Insight into Advances in Developing Nanotechnology Based Therapeutics, Drug Delivery, Diagnostics and Vaccines: Multidimensional Applications in Tuberculosis Disease Management. Pharmaceuticals 2023, 16, 581. https://doi.org/10.3390/ph16040581
Chopra H, Mohanta YK, Rauta PR, Ahmed R, Mahanta S, Mishra PK, Panda P, Rabaan AA, Alshehri AA, Othman B, et al. An Insight into Advances in Developing Nanotechnology Based Therapeutics, Drug Delivery, Diagnostics and Vaccines: Multidimensional Applications in Tuberculosis Disease Management. Pharmaceuticals. 2023; 16(4):581. https://doi.org/10.3390/ph16040581
Chicago/Turabian StyleChopra, Hitesh, Yugal Kishore Mohanta, Pradipta Ranjan Rauta, Ramzan Ahmed, Saurov Mahanta, Piyush Kumar Mishra, Paramjot Panda, Ali A. Rabaan, Ahmad A. Alshehri, Basim Othman, and et al. 2023. "An Insight into Advances in Developing Nanotechnology Based Therapeutics, Drug Delivery, Diagnostics and Vaccines: Multidimensional Applications in Tuberculosis Disease Management" Pharmaceuticals 16, no. 4: 581. https://doi.org/10.3390/ph16040581
APA StyleChopra, H., Mohanta, Y. K., Rauta, P. R., Ahmed, R., Mahanta, S., Mishra, P. K., Panda, P., Rabaan, A. A., Alshehri, A. A., Othman, B., Alshahrani, M. A., Alqahtani, A. S., AL Basha, B. A., & Dhama, K. (2023). An Insight into Advances in Developing Nanotechnology Based Therapeutics, Drug Delivery, Diagnostics and Vaccines: Multidimensional Applications in Tuberculosis Disease Management. Pharmaceuticals, 16(4), 581. https://doi.org/10.3390/ph16040581







