Fabrication of Nanoformulation Containing Carvedilol and Silk Protein Sericin against Doxorubicin Induced Cardiac Damage in Rats
Abstract
1. Introduction
2. Results
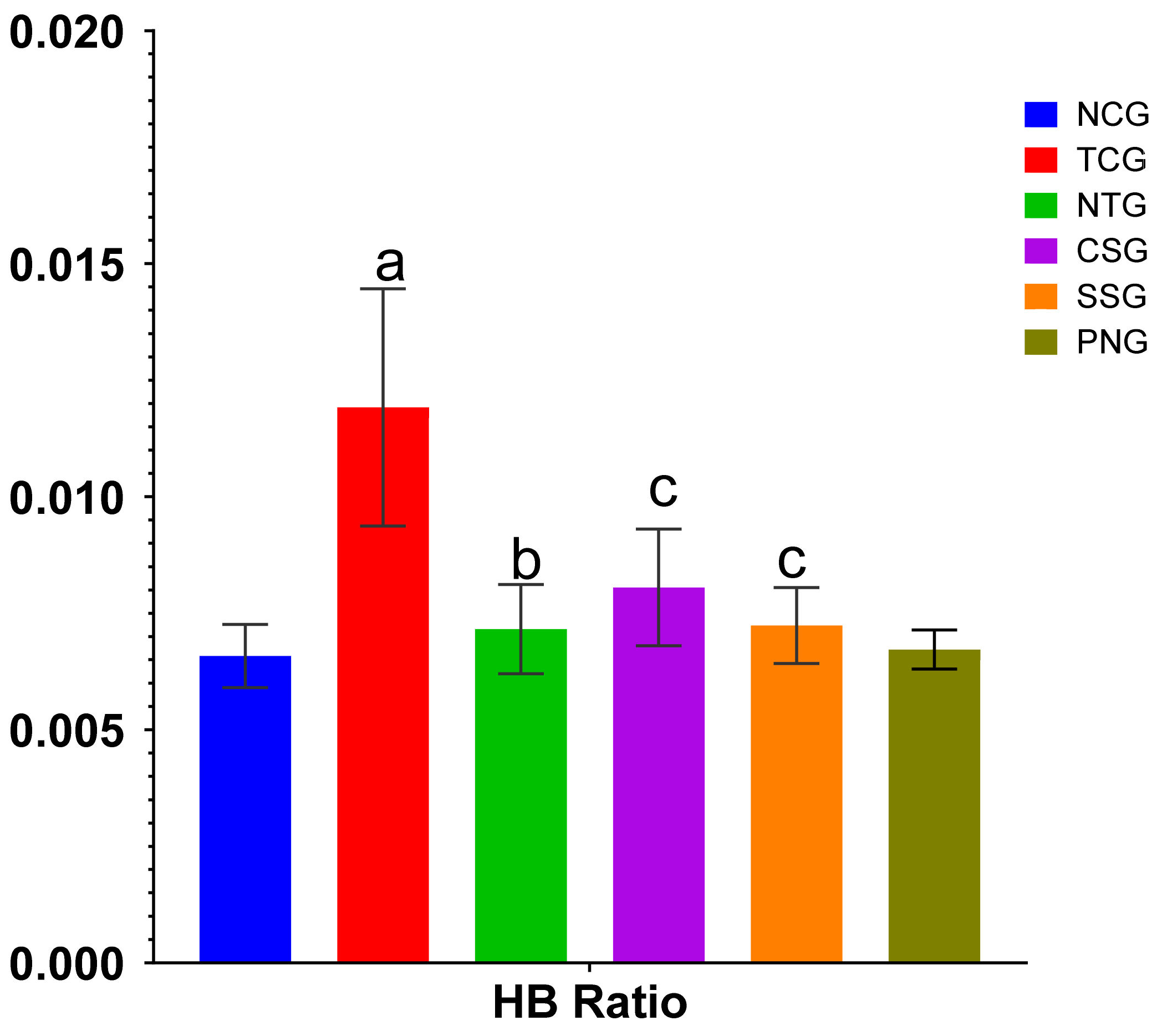
2.1. Heart Weight Body Weight Ratio
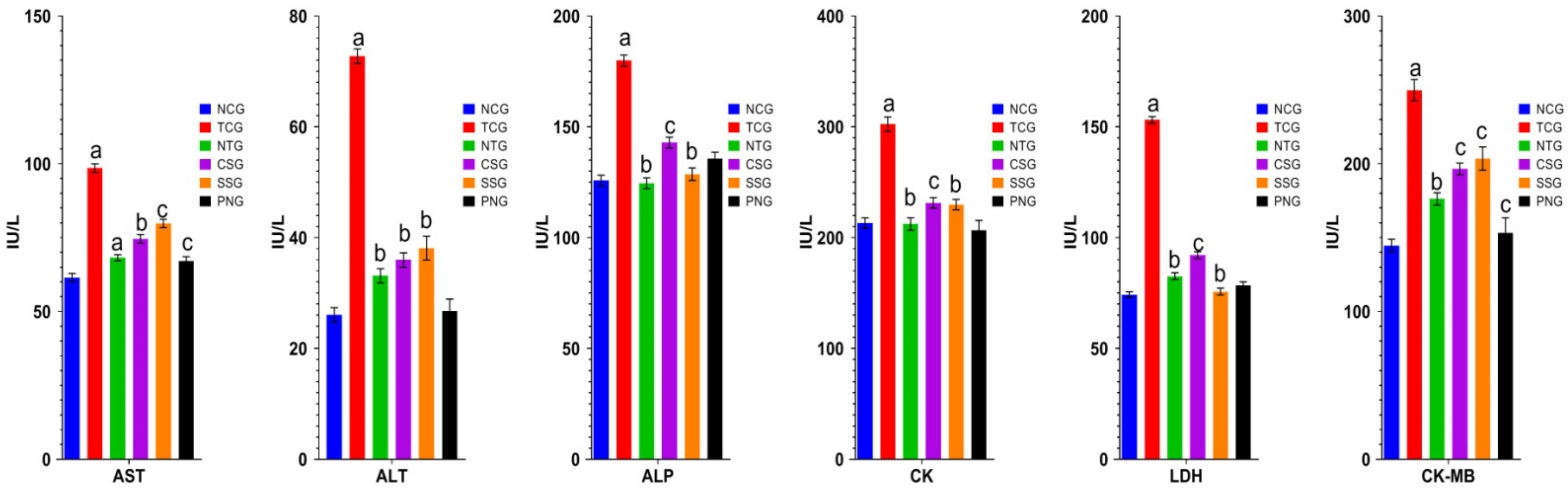
2.2. Biochemical Estimations (Serum)
2.3. Troponin T
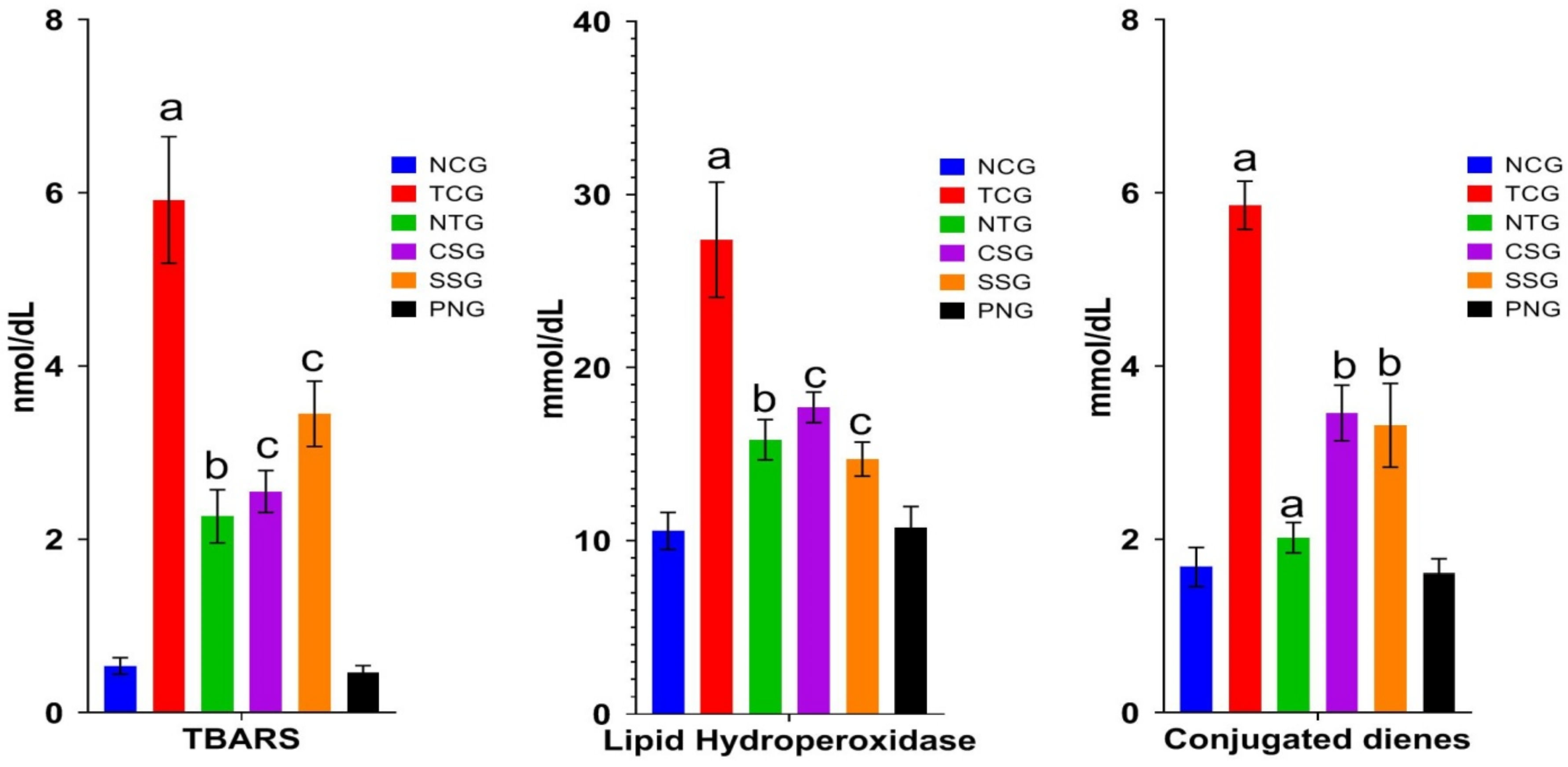
2.4. Lipid Peroxide Marker (Plasma)
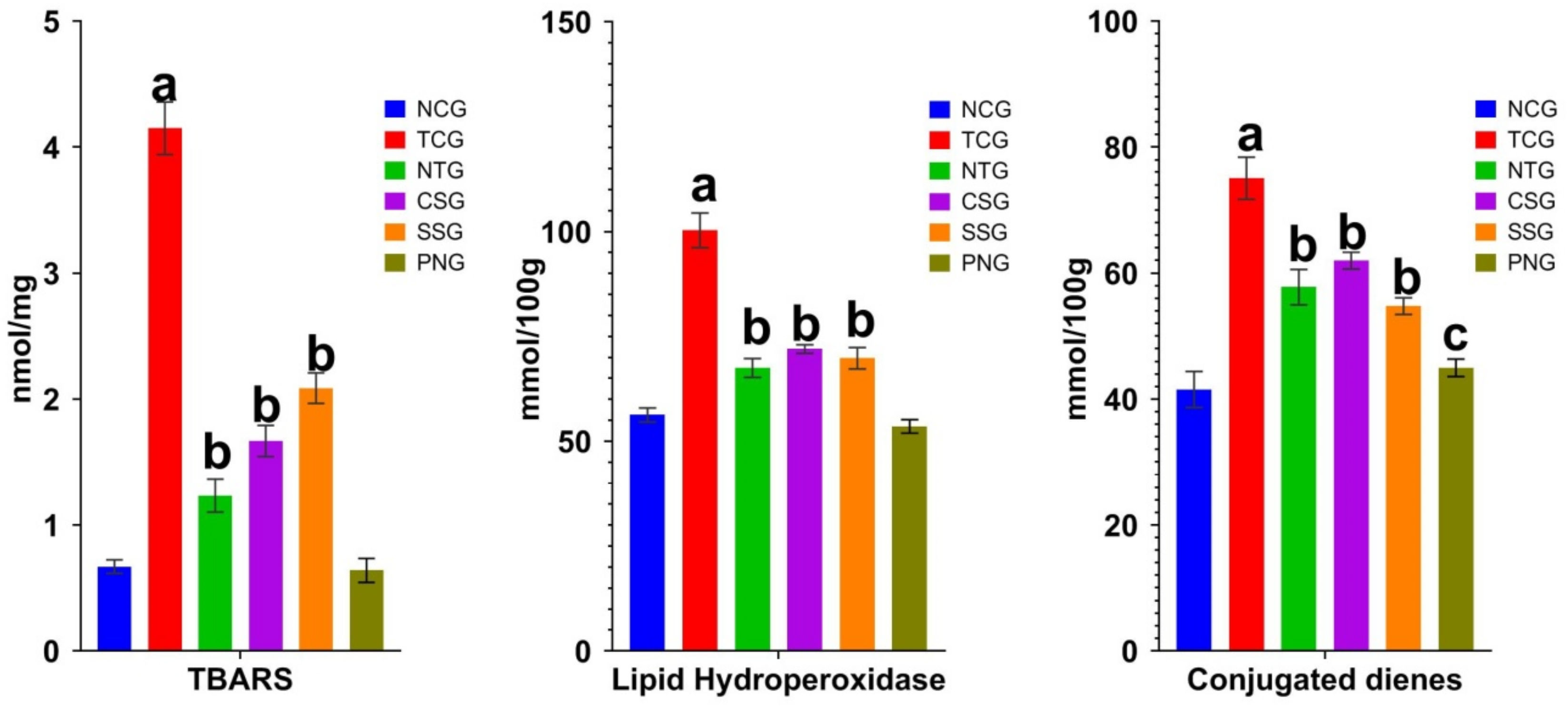
2.5. Lipid Peroxide Marker (Heart Tissue)
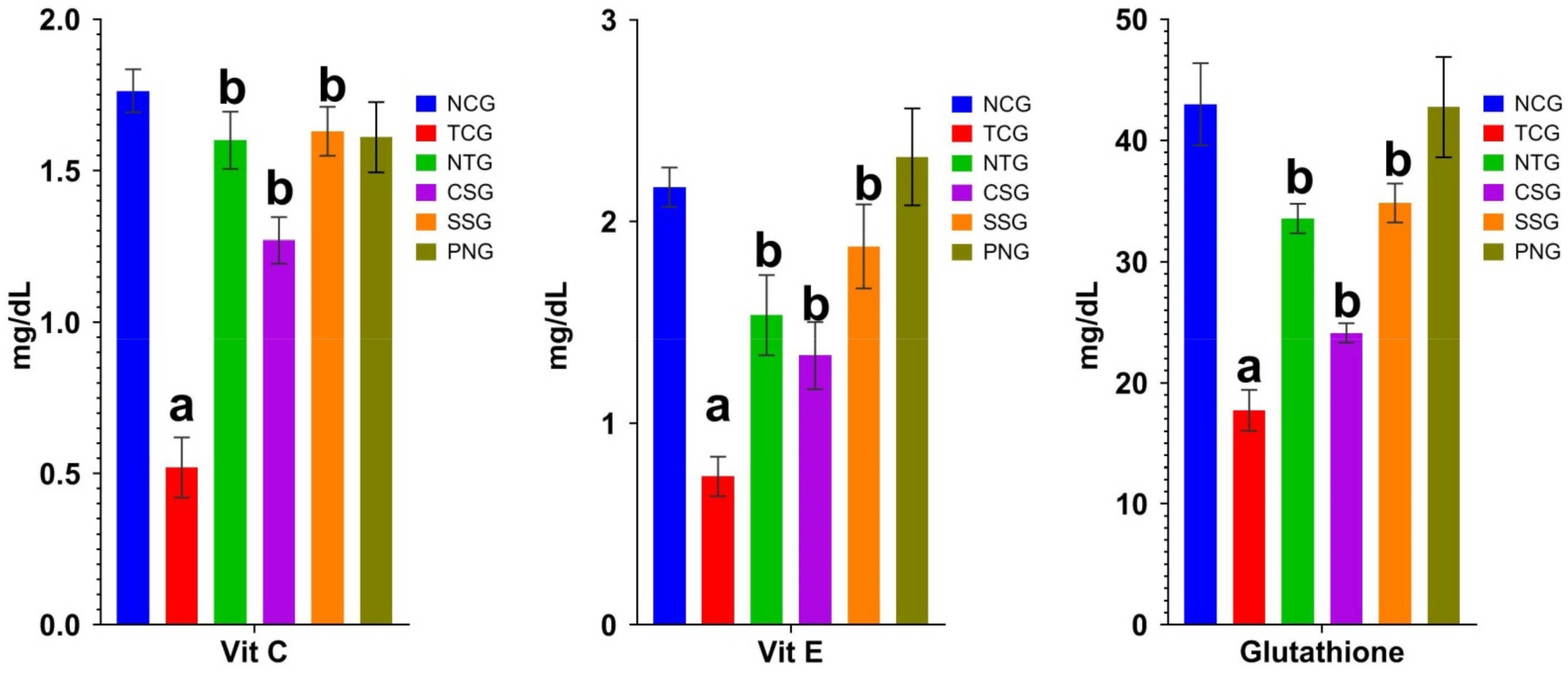
2.6. Non-Enzymatic Antioxidant Marker (Plasma)
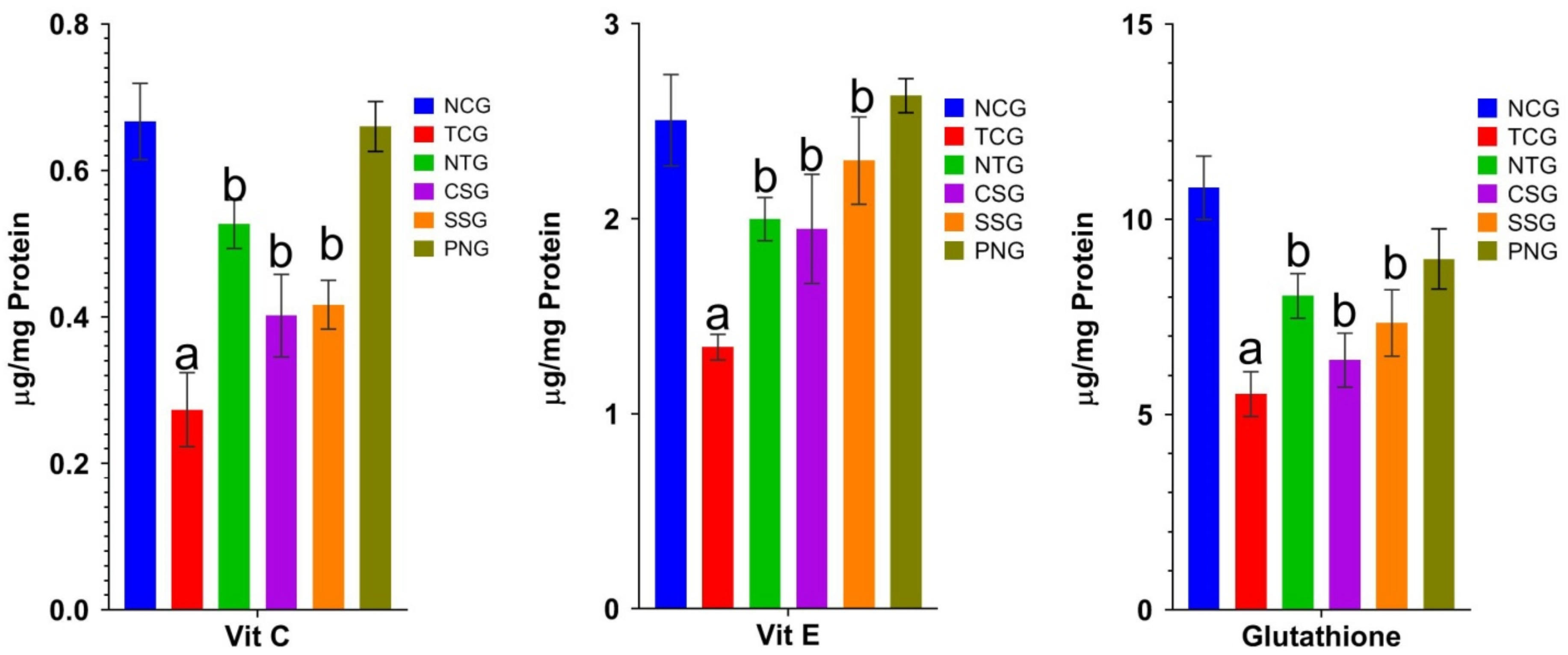
2.7. Non-Enzymatic Antioxidant Marker (Heart Tissue)
2.8. Enzymatic Antioxidant Marker (Heart Tissue)
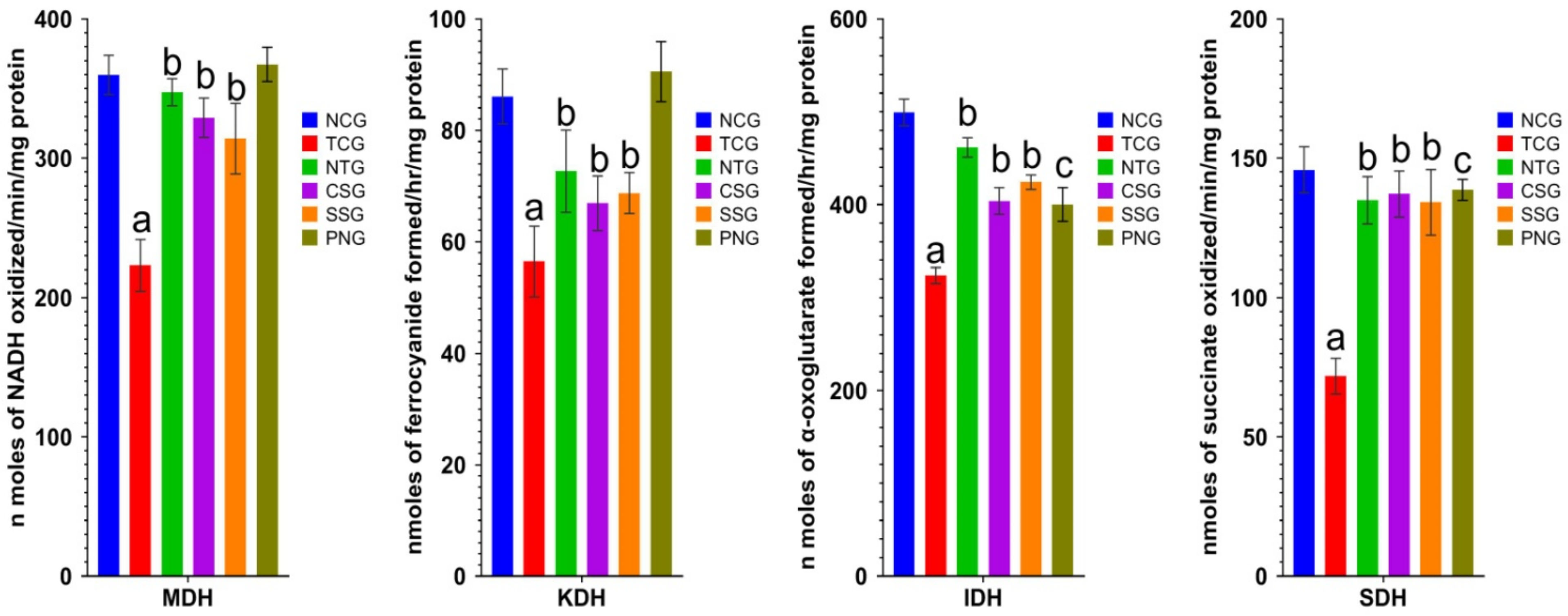
2.9. Mitochondrial Enzymes (Heart Tissue)
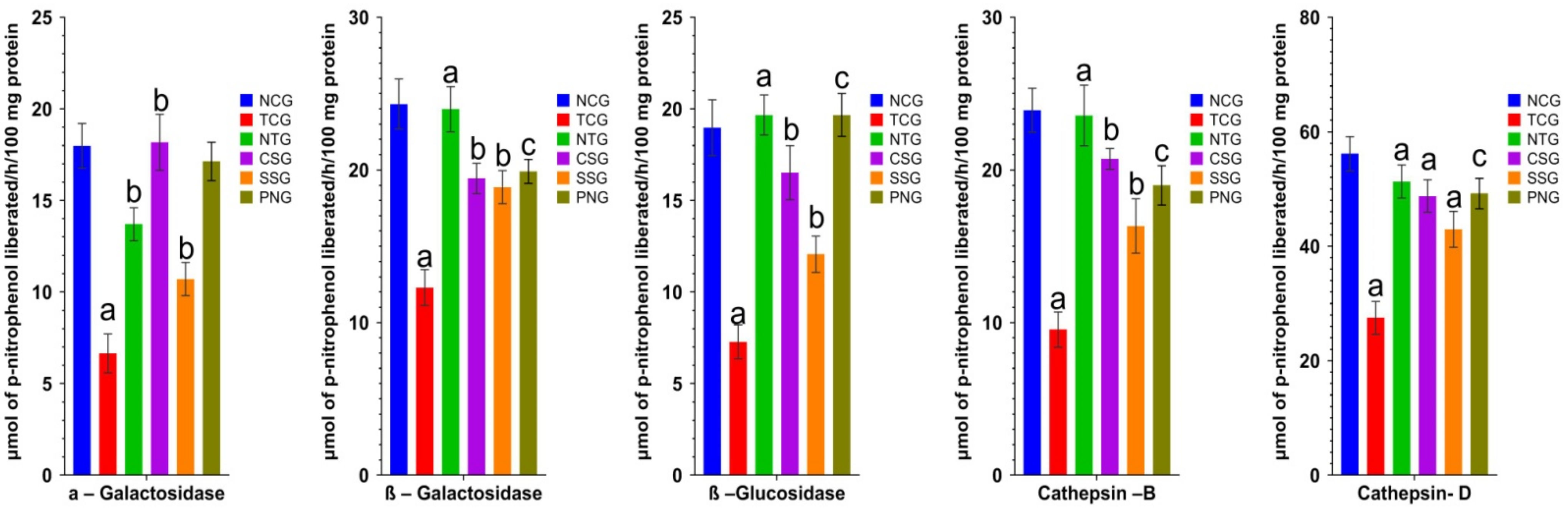
2.10. Lysosomal Hydrolases (Heart Tissue)
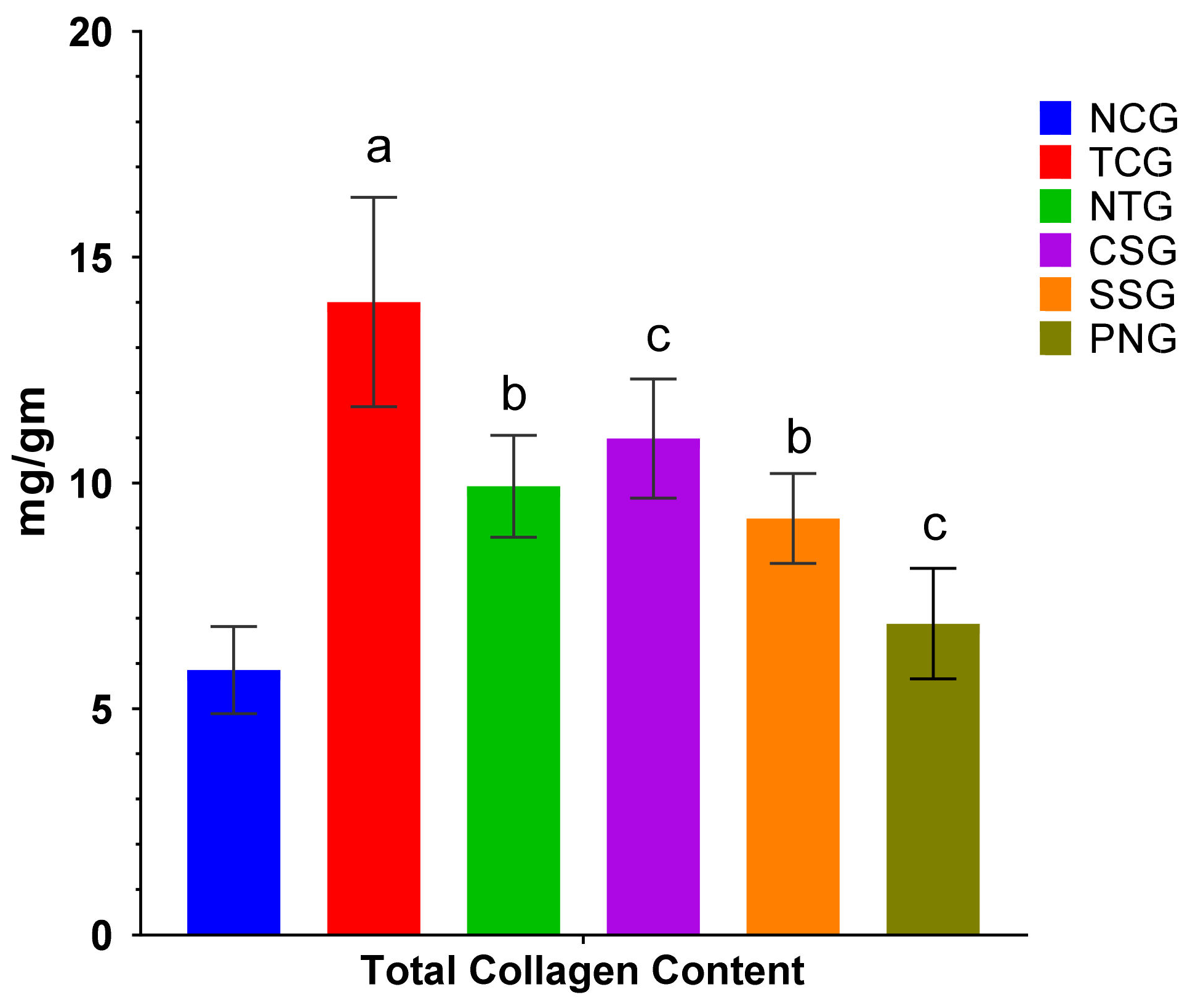
2.11. Total Collagen Content
2.12. Histopathology
3. Discussion
4. Material and Methods
4.1. Reagents
4.2. Preparation of Drug-Loaded Nanoparticles
4.3. Experimental Animals
4.4. Processing of Tissue and Blood Samples
4.5. Heart Weight Body Weight Ratio
4.6. Cardiac Markers
4.7. Oxidative Stress Parameter
4.8. Antioxidant Parameters
4.9. Lysosomal Hydrolases
4.10. Heart Mitochondrial Enzymes
4.11. Tissue Collagen Content
4.12. Histopathology
4.13. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Study Limitation
References
- Sahlgren, C.V.; Linden, M. Mesoporous silica nanoparticles in medicine-recent advances. Adv. Drug Deliv. Rev. 2013, 65, 689–702. [Google Scholar] [CrossRef]
- Nasiruddin, M.; Neyaz, M.K.; Das, S. Nanotechnology-based approach in tuberculosis treatment. Tuberc. Res. Treat. 2017, 2017, 4920209. [Google Scholar] [CrossRef]
- Heiligtag, F.J.; Niederberger, M. The fascinating world of Nanoparticle research. Mater. Today 2013, 16, 262–271. [Google Scholar] [CrossRef]
- Rastogi, T.; Reddy, K.S.; Vaz, M.; Spiegelman, D.; Prabhakaran, D.; Willett, W.C.; Stampfer, M.J.; Ascherio, A. Diet and risk of ischemic heart disease in India. Am. J. Clin. Nutr. 2004, 79, 582–592. [Google Scholar] [CrossRef] [PubMed]
- Savoji, H.; Mohammadi, M.H.; Rafatian, N.; Toroghi, M.K.; Wang, E.Y.; Zhao, Y.; Korolj, A.; Ahadian, S.; Radisic, M. Cardiovascular disease models: A game-changing paradigm in drug discovery and screening. Biomaterials 2019, 198, 3–26. [Google Scholar] [CrossRef] [PubMed]
- Erbaş, O.; Altuntaş, İ.; Çağlar, Ö.; Özyilmaz, E.; Sari, E.; Üzümcü, İ.; Erbakan, K. Experimental Model of Cardiotoxicity. Risk Factors Cardiovasc. Dis. 2022, 187. [Google Scholar]
- Kemp, C.D.; Conte, J.V. The pathophysiology of heart failure. Cardiovasc. Pathol. 2012, 21, 365–371. [Google Scholar] [CrossRef]
- Samak, M.; Fatullayev, J.; Sabashnikov, A.; Zeriouh, M.; Schmack, B.; Farag, M.; Popov, A.-F.; Dohmen, P.M.; Choi, Y.-H.; Wahlers, T.; et al. Cardiac hypertrophy: An introduction to molecular and cellular basis. Med. Sci. Monit. Basic Res. 2016, 22, 75–79. [Google Scholar] [CrossRef]
- Zhang, Y.W.; Shi, J.; Li, Y.J.; Wei, L. Cardiomyocyte death in doxorubicin-induced cardiotoxicity. Arch. Ther. 2009, 57, 435–445. [Google Scholar] [CrossRef]
- Rawat, P.S.; Jaiswal, A.; Khurana, A.; Bhatti, J.S.; Navik, U. Doxorubicin-induced cardiotoxicity: An update on the molecular mechanism and novel therapeutic strategies for effective management. Biomed. Pharmacother. 2021, 139, 111708. [Google Scholar] [CrossRef]
- Yeh, E.T.H.; Tong, A.T.; Lenihan, D.J.; Yusuf, S.W.; Swafford, J.; Champion, C.; Durand, J.-B.; Gibbs, H.; Zafarmand, A.A.; Ewer, M.S. Cardiovascular complications of cancer therapy: Diagnosis, pathogenesis, and management. Circulation 2004, 109, 3122–3131. [Google Scholar] [CrossRef]
- Ahsan, F.; Mahmood, T.; Wani, T.A.; Zargar, S.; Siddiqui, M.H.; Usmani, S.; Shamim, A.; Wahajuddin, M. Effectual endeavors of silk protein sericin against isoproterenol-induced cardiac toxicity and hypertrophy in wistar rats. Life 2022, 12, 1063. [Google Scholar] [CrossRef]
- Mizuochi, Y.; Okajima, K.; Harada, N.; Molor-Erdene, P.; Uchiba, M.; Komura, H.; Tsuda, T.; Katsuya, H. Carvedilol, a nonselective β-blocker, suppresses the production of tumor necrosis factor and tissue factor by inhibiting early growth response factor-1 expression in human monocytes in vitro. Transl. Res. 2007, 149, 223–230. [Google Scholar] [CrossRef]
- Veeresham, C. Natural products derived from plants as a source of drugs. J. Adv. Pharm. Technol. 2012, 3, 200–201. [Google Scholar] [CrossRef]
- Seabrooks, L.; Hu, L. Insects: An underrepresented resource for the discovery of biologically active natural products. Acta Pharm. Sin. B 2017, 7, 409–426. [Google Scholar] [CrossRef]
- Ahsan, F.; Ansari, T.M.; Usmani, S.; Bagga, P. An insight on silk protein sericin: From processing to biomedical application. Drug Res. 2018, 68, 317–327. [Google Scholar] [CrossRef]
- Mishra, R. Determinants of cardiovascular disease and sequential decision-making for treatment among women: AHeckman’s approach. SSM-Popul. Health 2019, 7, 100365. [Google Scholar] [CrossRef]
- Suchalatha, S.; Shyamala, C.D. Protective effect of Terminalia chebula against experimental myocardial injury induced by isoproterenol. Indian J. Exp. Biol. 2004, 42, 174–178. [Google Scholar]
- Antman, E.; Bassand, J.P.; Klein, W.; Ohman, M.; Sendon, J.L.; Rydén, L.; Tendera, M. Myocardial infarction redefined— A consensus document of the Joint European Society of Cardiology/American College of Cardiology committee for the redefinition of myocardial infarction: The Joint European Society of Cardiology/American College of Cardiology Committee. J. Am. Coll. Cardiol. 2000, 36, 959–969. [Google Scholar]
- Sushama Kumari, S.; Jayadeep, A.; Kumar, J.S.; Menon, V.P. Effect of carnitine on malon dialdehyde, taurine and glutathione levels in heart of rats subjected to myocardial stress by isoproterenol. Indian J. Exp. Biol. 1989, 27, 134–137. [Google Scholar]
- Sobel, B.E.; Mayer, S.E. Cyclic adenosine mono phosphate and cardiac contractility. Circ. Res. 1973, 32, 407–414. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, K.M.; Rana, A.C.; Dixit, V.K. Effect of Calotropis procera latex on isoproterenol induced myocardial infarction in albino rats. Phytomedicine 2004, 11, 327–330. [Google Scholar] [CrossRef] [PubMed]
- Mair, J.; Apple, F. Progress in myocardial damage detection: New biochemical markers for clinicians. Crit. Rev. Clin. Lab. Sci. 1997, 34, 1–66. [Google Scholar] [CrossRef]
- Bertinchant, J.P.; Polge, A.; Mohty, D.; Nguyen-Ngoc-Lam, R.; Estorc, J.; Cohendy, R.; Joubert, P.; Poupard, P.; Fabbro-Peray, P.; Monpeyroux, F.; et al. Evaluation of incidence, clinical significance, and prognostic value of circulating cardiac troponin Iand6 T elevation in hemodynamically stable patients with suspected myocardial contusion after blunt chest trauma. J. Trauma Acute Care Surg. 2000, 48, 924–931. [Google Scholar] [CrossRef] [PubMed]
- Levy, Y.; Zaltzberg, H.; Ben-Amotz, A.; Kanter, Y.; Aviram, M. β-Carotene affects antioxidants tatusinnon-insulin-dependent diabetes mellitus. Pathophysiology 1999, 6, 157–161. [Google Scholar] [CrossRef]
- Senthil, S.; Chandramohan, G.; Pugalendi, K.V. Isomers (oleanolic and ursolic acids) differ in their protective effect against isoproterenol-induced myocardial ischemia in rats. Int. J. Card. 2007, 119, 131–133. [Google Scholar] [CrossRef]
- Halliwell, B.; Gutteridge, J.M.C. Free Radicals in Biology and Medicine; Oxford University Press: Oxford, UK; New York, NY, USA, 1997. [Google Scholar]
- Dutta-Roy, A.K. Insulin mediated processes in platelets, erythrocytes and monocytes/macrophages: Effects of essential fatty acid metabolism. Prostaglandins Leukot. Essent. Fat. Acids 1994, 51, 385–399. [Google Scholar] [CrossRef]
- Bendich, A.; Machlin, L.J.; Scandurra, O.; Burton, G.W.; Wayner, D.D. The antioxidant role of vitamin C. Adv. Free Radic. Biol. Med. 1986, 2, 419–444. [Google Scholar] [CrossRef]
- Vennila, L.; Pugalendi, K.V. Protective effect of sesame against myocardial infarction caused by isoproterenol in Wistar rats. Redox Rep. 2010, 15, 36–42. [Google Scholar] [CrossRef]
- Karthikeyan, J.; Rani, P. Enzymatic and non-enzymatic antioxidants in selected Piper species. Indian J. Exp.Biol. 2003, 41, 135–140. [Google Scholar]
- Uhlig, S.; Wendel, A. Thephysiologicalconsequencesofglutathionevariations. Life Sci. 1992, 51, 1083–1094. [Google Scholar] [CrossRef]
- Sasikumar, C.S.; Devi, C.S. Protective effect of Abana®, a poly-herbal formulation, on isoproterenol-induced myocardial infarction in rats. Indian J. Pharmacol. 2000, 32, 198–201. [Google Scholar]
- Deori, M.; Devi, D.; Kumari, S.; Hazarika, A.; Kalita, H.; Sarma, R.; Devi, R. Antioxidant effect of sericin in brain in peripheral tissues of oxidative stress induced hyper cholesterolemic rats. Front. Pharmacol. 2016, 7, 319. [Google Scholar] [CrossRef]
- Farvin, K.S.; Anandan, R.; Kumar SH, S.; Shiny, K.S.; Sankar, T.V.; Thankappan, T.K. Effect of squalene on tissue defense system in isoproterenol-induced myocardial infarction in rats. Pharmacol. Res. 2004, 50, 231–236. [Google Scholar]
- Sushama, S.K.; Varghese, A.; Muraleedharan, D.; Menon, V.P. Protective action of aspirin in experimental myocardial infarction induced by isoproterenol in rats and its effect on lipid peroxidation. Indian J. Exp. Biol. 1990, 28, 480–485. [Google Scholar]
- Ghosal, S. Active constituents of Emblica officinalis: Part I. The chemistry and antioxidative effects of two new hydrolyzable tannins, Emblicanin A and B. Indian J. Chem. 1996, 35, 941–948. [Google Scholar]
- Ebenezar, K.K.; Sathish, V.; Devaki, T. Effect of L-arginine and L-lysine on lysosomal hydrolases and membrane bound phosphatises in experimentally induced myocardial infarction in rats. Mol. Cell. Biochem. 2003, 247, 163–169. [Google Scholar] [CrossRef]
- Ravichandran, L.V.; Puvanakrishnan, R.; Joseph, K.T. Influence of isoproterenol-induced myocardial infarction on certain glycohydrolases and cathepsins in rats. Biochem. Med. Metab. Biol. 1991, 45, 6–15. [Google Scholar] [CrossRef]
- Akila, P.; Asaikumar, L.; Vennila, L. Chlorogenic acid ameliorates isoproterenol-induced myocardial injury in rats by stabilizing mitochondrial and lysosomal enzymes. Biomed. Pharmacother. 2017, 85, 582–591. [Google Scholar] [CrossRef]
- Capetanaki, Y. Desmincyto skeleton: A potential regulator of muscle mitochondrial behavior and function. Trends Cardiovasc. Med. 2002, 12, 339–348. [Google Scholar] [CrossRef]
- Ricciutti, M.A. Myocardial lysosome stability in the early stages of acute ischemic injury. Am. J. Card. 1972, 30, 492–497. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.W.; Yu, X.F.; Xu, H.L.; Zhao, X.Z.; Sui, D.Y. Ginsenoside Reimproves isoproterenol-induced myocardial fibrosis and heart failure in rats. Evid.-Based Complement. Altern. Med. 2019, 2019, 3714508. [Google Scholar]
- Chatterjee, S. Artefacts in histopathology. J. Oral Maxillofac. Pathol. 2014, 18 (Suppl. S1), S111. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.I.; Kim, W.S.; Kim, D.H.; Kang, J.S. Histopathological evaluation of heart toxicity of a novel selective PPAR-γagonistsCKD-501indb/dbmice. Biomol. Ther. 2013, 21, 84. [Google Scholar] [CrossRef]
- Shariq, M.; Ansari, T.M.; Kushwaha, P.; Parveen, S.; Shamim, A.; Ahsan, F.; Kazmi, M.T. Preparation, characterization and safety assessment of combinatorial nanoparticles of carvedilol and sericin. Int. J. App. Pharm. 2022, 14, 80–85. [Google Scholar] [CrossRef]
- Ahsan, F.; Siddiqui, H.H.; Mahmood, T.; Srivastav, R.K.; Nayeem, A. Evaluation of cardioprotective effect of Coleus forskohlii against Isoprenaline induced myocardial infarction in rats. Indian J. Pharm. Biol. Res. 2014, 2, 17. [Google Scholar] [CrossRef]
- Buege, J.A.; Aust, S.D. Microsomal lipid peroxidation. In Methodsin Enzymology; Academic Press: Cambridge, MA, USA, 1978; pp. 302–310. [Google Scholar]
- Ohkawa, H.; Ohishi, N.; Yagi, K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal. Biochem. 1979, 95, 351–358. [Google Scholar] [CrossRef]
- Jiang, Z.Y.; Hunt, J.V.; Wolff, S.P. Ferrous ion oxidation in the presence of xylenol orange for detection of lipid hydroperoxide in low-density lipoprotein. Anal. Biochem. 1992, 202, 384–389. [Google Scholar] [CrossRef]
- Rao, K.S.; Recknagel, R.O. Early onset of lipoperoxidation in rat liver after carbon tetrachloride administration. Exp. Mol. Pathol. 1968, 9, 271–278. [Google Scholar]
- Kyaw, A. A simple colorimetric method for ascorbic acid determination in blood plasma. Clin. Chim. Acta 1978, 86, 153–157. [Google Scholar] [CrossRef]
- Baker, H.; Frank, O. Clinical Vitaminology: Methods and Interpretation; John Wiley and Sons: New York, NY, USA, 1968. [Google Scholar]
- Ellman, G.L. Tissue sulfhydryl groups. Arch. Biochem. Biophys. 1959, 82, 70–77. [Google Scholar] [CrossRef]
- Sadasivam, S.; Manickam, A. Vitamins: Biochemical Methods; New Age International(P) Limited: New Delhi, India, 1997; pp. 185–186. [Google Scholar]
- Varley, H. Practical Clinical Biochemistry; William Heinemann Publishers Pvt Ltd.: New York, NY, USA, 1976. [Google Scholar]
- Boyne, A.F.; Ellman, G.L. A methodology for analysis of tissue sulfhydryl components. Anal. Biochem. 1972, 46, 639–653. [Google Scholar] [CrossRef]
- Marklund, S.; Marklund, G. Involvement of the superoxide anion radical in the autoxidation of pyrogallol and a convenient assay for superoxide dismutase. Eur. J. Biochem. 1974, 47, 469–474. [Google Scholar] [CrossRef]
- Aebi, H. Catalase in Vitro. Methods Enzymol. 1984, 105, 114–121. [Google Scholar]
- Rotruck, J.T.; Pope, A.L.; Ganther, H.E.; Swanson, A.B.; Hafeman, D.G.; Hoekstra, W.G. Selenium: Biochemical role as a component of glutathione peroxidase. Nutr. Rev. 1980, 38, 280–283. [Google Scholar] [CrossRef]
- Carlberg, I.; Mannervik, B. Glutathione reductase. Methods Enzymol. 1985, 113, 484–490. [Google Scholar]
- Habig, W.H.; Pabst, M.J.; Jakoby, W.B. Glutathione S-transferases the first enzymatic step in mercapturic acid formation. J. Biol. Chem. 1974, 249, 7130–7139. [Google Scholar] [CrossRef]
- Conchie, J.; Gelman, A.L.; Levvy, G.A. Inhibition of glycosidases by aldono lactones of corresponding configuration: The C-4- and C-6-specificity of β-glucosidase and β-galactosidase. Biochem. J. 1967, 103, 609–615. [Google Scholar] [CrossRef]
- Barrett, A.J. Fluorimetric assays for cathepsin B and cathepsin H with methyl coumaryl amide substrates. Biochem. J. 1980, 187, 909. [Google Scholar] [CrossRef]
- Sapolsky, A.I.; Altman, R.D.; Howell, D.S. Cathepsin D activity in normal and osteoarthritic human cartilage. Fed. Proc. 1973, 32, 1489–1493. [Google Scholar]
- Fontana-Ayoub, M.; Krumschnabel, G. Laboratory protocol: Isolation of mouse heart mitochondria. In Mitochondrial Physiology Network; Bioblast: Philadelphia, PA, USA, 2015; pp. 1–2. [Google Scholar]
- Bell, J.L.; Baron, D.N. A colorimetric method for determination of iso citric dehydrogenase. Clin. Acta 1960, 5, 740–747. [Google Scholar] [CrossRef]
- Reed, L.J.; Mukherjee, B.B. α-ketoglutarate dehydrogenase complex from Escherichia coli. Methods Enzymol. 1969, 13, 55–61. [Google Scholar]
- Slater, E.C.; Bonner, W.D. The effect of fluorideon succinic oxidase system. Biochem. J. 1952, 52, 185–196. [Google Scholar] [CrossRef]
- Mehler, A.; Kobnberg, A.; Gbisolia, S.; Ochoa, S. The enzymatic mechanism of oxidation-redactions between malateor isocitrate and pyravate. J. Biol. Chem. 1948, 174, 961–977. [Google Scholar] [CrossRef] [PubMed]
- Woessner, J.F., Jr. The determination of hydroxyproline in tissue and protein samples containing small proportions of this aminoacid. Arch. Biochem. Biophys. 1961, 93, 440–447. [Google Scholar] [CrossRef] [PubMed]



| Groups | Treatment | Dose, Route, and Duration |
|---|---|---|
| Normal Control Group (NCG) | Normal Saline (NS) | 2 mL/kg/p.o/day for 7 days |
| Toxic Control Group (TCG) | Dox + NS | Dox 20 mg/kg i.p. once on 1st day + DW 2 mL/kg/p.o/day for next 6 days. |
| Nanoparticle Treated Group (NTG) | Doxorubicin + Nanoparticle | 200 mg/kg p.o. from day 1 to day 7 + Dox 20 mg/kg i.p. once on 1st day after 2 h of Nanoparticle administration |
| Carvedilol Standard Group (CSG) | Doxorubicin + Carvedilol | 10 mg/kg p.o. from day 1 to day 7 + Dox 20 mg/kg i.p. once on 1st day after 2 h of Carvedilol administration |
| Sericin Standard Group (SSG) | Doxorubicin + Sericin | 100 mg/kg p.o. from day 1 to day 7 + Dox 20 mg/kg i.p. once on 1st day after 2 h of Sericin administration |
| Per Se Group (PNG) | Nanoparticle | 200 mg/kg p.o. for 7 days |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shariq, M.; Mahmood, T.; Kushwaha, P.; Parveen, S.; Shamim, A.; Ahsan, F.; Wani, T.A.; Zargar, S.; Wasim, R.; Wahajuddin, M. Fabrication of Nanoformulation Containing Carvedilol and Silk Protein Sericin against Doxorubicin Induced Cardiac Damage in Rats. Pharmaceuticals 2023, 16, 561. https://doi.org/10.3390/ph16040561
Shariq M, Mahmood T, Kushwaha P, Parveen S, Shamim A, Ahsan F, Wani TA, Zargar S, Wasim R, Wahajuddin M. Fabrication of Nanoformulation Containing Carvedilol and Silk Protein Sericin against Doxorubicin Induced Cardiac Damage in Rats. Pharmaceuticals. 2023; 16(4):561. https://doi.org/10.3390/ph16040561
Chicago/Turabian StyleShariq, Mohammad, Tarique Mahmood, Poonam Kushwaha, Saba Parveen, Arshiya Shamim, Farogh Ahsan, Tanveer A. Wani, Seema Zargar, Rufaida Wasim, and Muhammad Wahajuddin. 2023. "Fabrication of Nanoformulation Containing Carvedilol and Silk Protein Sericin against Doxorubicin Induced Cardiac Damage in Rats" Pharmaceuticals 16, no. 4: 561. https://doi.org/10.3390/ph16040561
APA StyleShariq, M., Mahmood, T., Kushwaha, P., Parveen, S., Shamim, A., Ahsan, F., Wani, T. A., Zargar, S., Wasim, R., & Wahajuddin, M. (2023). Fabrication of Nanoformulation Containing Carvedilol and Silk Protein Sericin against Doxorubicin Induced Cardiac Damage in Rats. Pharmaceuticals, 16(4), 561. https://doi.org/10.3390/ph16040561






