Norcantharidin Nanoemulsion Development, Characterization, and In Vitro Antiproliferation Effect on B16F1 Melanoma Cells
Abstract
1. Introduction
2. Results and Discussion
2.1. Formulation Development
2.2. Drug Solubility Assays
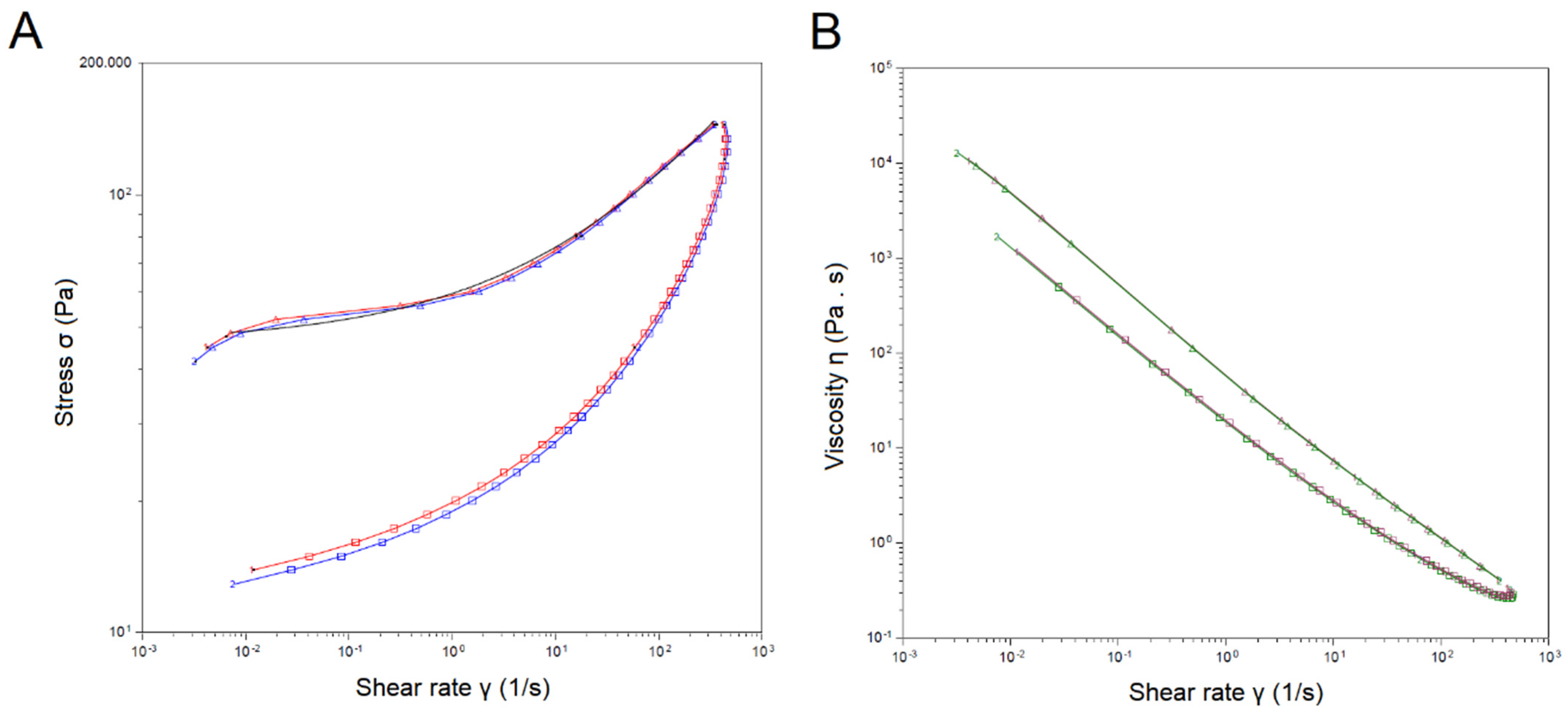
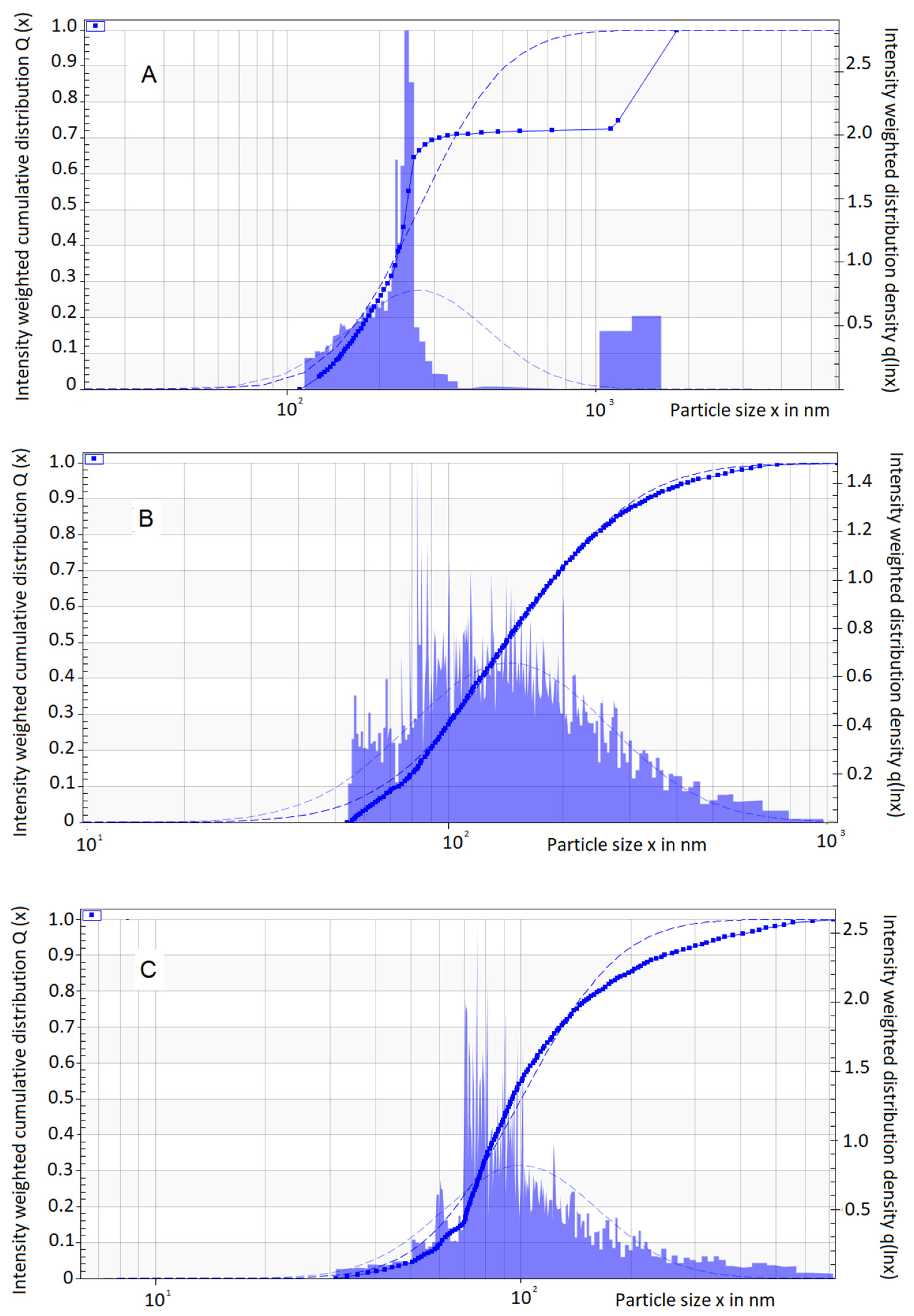
2.3. Droplet Size, Polydispersity Index, Zeta Potential, pH, and Viscosity
2.4. Accelerated Stability Assessment
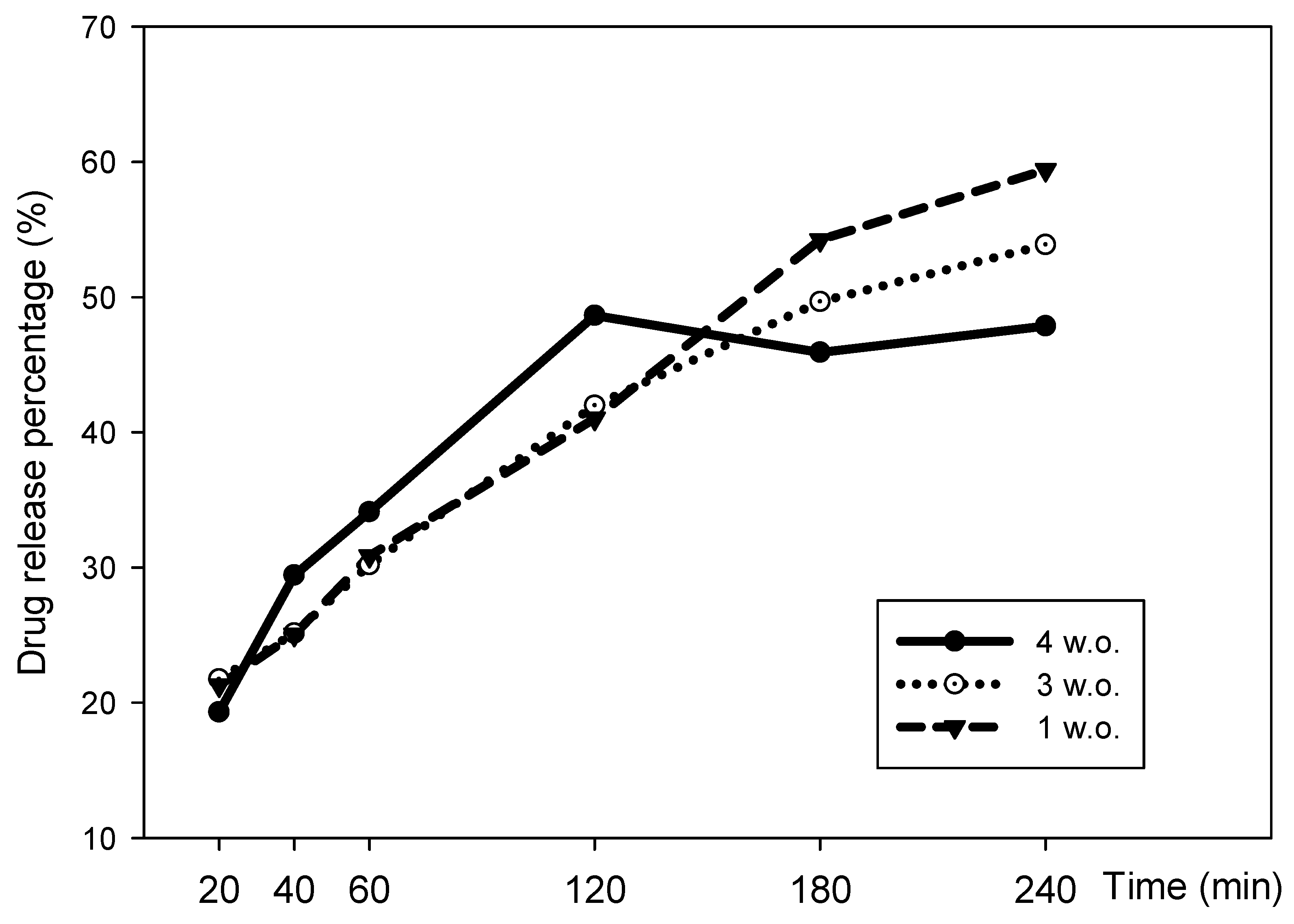
2.5. In Vitro Drug Release
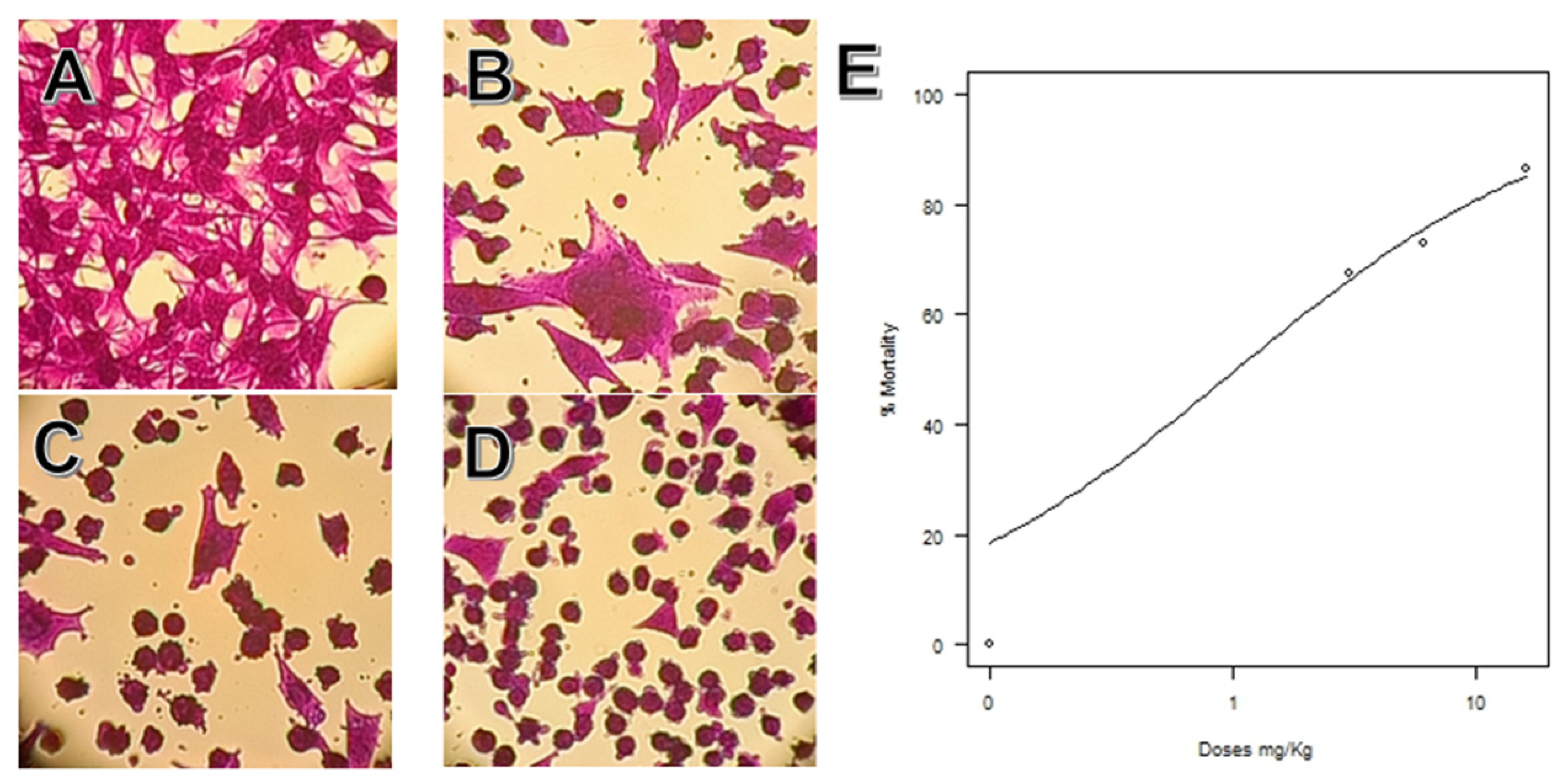
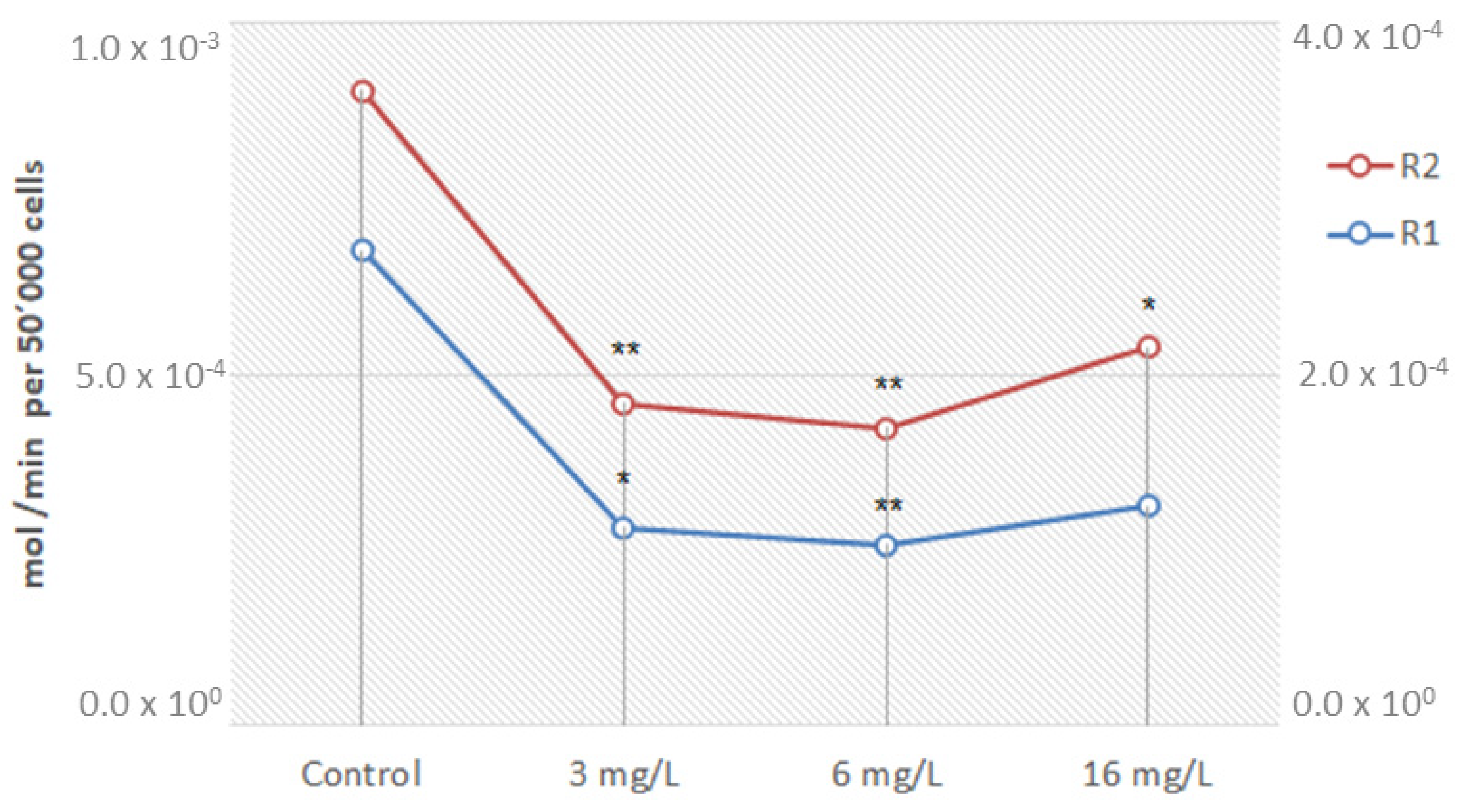
2.6. Melanoma Cells Nanoemulsion Exposure Assays
3. Materials and Methods
3.1. Materials
3.2. Formulation Development
3.3. Drug Solubility Assays
3.4. Droplet Size, Polydispersity Index, Zeta Potential, pH, and Viscosity
3.5. Accelerated Stability Assessment
3.6. In Vitro Drug Release Assay
3.7. Melanoma Cells Nanoemulsion Exposure Assays
3.8. Data Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ribero, S.; Glass, D.; Bataille, V. Genetic epidemiology of melanoma. Eur. J. Dermatol. 2016, 26, 335–339. [Google Scholar] [CrossRef] [PubMed]
- Ferlay, J.; Soerjomataram, I.; Ervik, M.; Dikshit, R.; Eser, S.; Mathers, C.; Rebelo, M.; Parkin, D.M.; Forman, D.; Bray, F. GLOBOCAN 2012 v1.0; Cancer Incidence and Mortality Worldwide: IARC Cancer Base No. 11; International Agency for Research on Cancer: Lyon, France, 2013; Available online: http://globocan.iarc.fr (accessed on 6 January 2022).
- Niewega, O.E.; Gallegos-Hernández, J.F. La cirugía en melanoma cutáneo maligno y las nuevas drogas. Cirugía y Cirujanos 2015, 83, 175–180. [Google Scholar] [CrossRef] [PubMed]
- Advances in neoadjuvant and adjuvant therapy for breast cancer. In Current Surgical Therapy; Cameron, J.L., Ed.; Elsevier: Philadelphia, PA, USA, 2017; Available online: Clinicalkey.com (accessed on 16 March 2018).
- Xie, M.-H.; Ge, M.; Peng, J.-B.; Jiang, X.-R.; Wang, D.-S.; Ji, L.-Q.; Ying, Y.; Wang, Z. In-vivo anti-tumor activity of a novel poloxamer-based thermosensitive in situ gel for sustained delivery of norcantharidin. Pharm. Dev. Technol. 2018, 24, 623–629. [Google Scholar] [CrossRef] [PubMed]
- Honkanen, R.E. Cantharidin, another natural toxin that inhibits the activity of serine/threonine protein phosphatases types 1 and 2A. FEBS Lett. 1993, 330, 283–286. [Google Scholar] [CrossRef]
- Pachuta-Stec, A.; Szuster-Ciesielska, A. New Norcantharidin Analogs: Synthesis and anticancer activity. Arch. Pharm. 2015, 348, 897–907. [Google Scholar] [CrossRef] [PubMed]
- Lu, K.; Cao, M.; Mao, W.; Sun, X.; Tang, J.; Shen, Y.; Sui, M. Targeted acid-labile conjugates of NCTD for cancer chemotherapy. J. Mater. Chem. 2012, 22, 15804–15811. [Google Scholar] [CrossRef]
- Moed, L.; Shwayder, T.A.; Chang, M.W. Cantharidin revisited: A blistering defense of an ancient medicine. Arch. Dermatol. 2001, 137, 1357–1360. [Google Scholar] [CrossRef]
- Tsakovska, I.; Pajeva, I.; Al Sharif, M.; Alov, P.; Fioravanzo, E.; Kovarich, S.; Worth, A.P.; Richarz, A.-N.; Yang, C.; Mostrag-Szlichtyng, A.; et al. Quantitative structure-skin permeability relationships. Toxicology 2017, 387, 27–42. [Google Scholar] [CrossRef]
- Santos, A.C.; Pereira, I.; Pereira-Silva, M.; Ferreira, L.; Caldas, M.; Collado-González, M.; Magalhães, M.; Figueiras, A.; Ribeiro, A.J.; Veiga, F. Nanotechnology-based formulations for resveratrol delivery: Effects on resveratrol in vivo bioavailability and bioactivity. Colloids Surf. B Biointerfaces 2019, 180, 127–140. [Google Scholar] [CrossRef]
- Bernardo, J.; Santos, A.C.; Videira, R.A.; Valentão, P.; Veiga, F.; Andrade, P.B. Trichilia catigua and Turnera diffusa phyto-phospholipid nanostructures: Physicochemical characterization and bioactivity in cellular models of induced neuroinflammation and neurotoxicity. Int. J. Pharm. 2022, 620, 121774. [Google Scholar] [CrossRef]
- Paiva-Santos, A.C.; Gama, M.; Peixoto, D.; Sousa-Oliveira, I.; Ferreira-Faria, I.; Zeinali, M.; Abbaspour-Ravasjani, S.; Mascarenhas-Melo, F.; Hamishehkar, H.; Veiga, F. Nanocarrier-based dermopharmaceutical formulations for the topical management of atopic dermatitis. Int. J. Pharm. 2022, 618, 121656. [Google Scholar] [CrossRef] [PubMed]
- Nanoemulsions for Drug Delivery. Systems of Nanovesicular Drug Delivery; Chapter 2; Atanase, L.I., Nayak, A.K., Hasnain, S., Aminabhavi, T.M., Orchilin, V.P., Eds.; Academic Press: Cambridge, MA, USA, 2022. [Google Scholar]
- Wilson, R.J.; Li, Y.; Yang, G.; Zhao, C.-X. Nanoemulsions for drug delivery. Particuology 2021, 64, 85–97. [Google Scholar] [CrossRef]
- Pan, M.-S.; Cao, J.; Fan, Y.-Z. Insight into norcantharidin, a small-molecule synthetic compound with potential multi-target anticancer activities. Chin. Med. 2020, 15, 55. [Google Scholar] [CrossRef] [PubMed]
- Santos, A.C.; Morais, F.; Simões, A.; Pereira, I.; Sequeira, J.A.D.; Pereira-Silva, M.; Veiga, F.; Ribeiro, A. Nanotechnology for the development of new cosmetic formulations. Expert Opin. Drug Deliv. 2019, 16, 313–330. [Google Scholar] [CrossRef]
- Melhaoui, R.; Kodad, S.; Houmy, N.; Belhaj, K.; Mansouri, F.; Abid, M.; Addi, M.; Mihamou, A.; Sindic, M.; Serghini-Caid, H.; et al. Characterization of sweet almond oil content of four european cultivars (ferragnes, ferraduel, fournat, and marcona) recently introduced in Morocco. Scientifica 2021, 30, 9141695. [Google Scholar] [CrossRef]
- Gabriel, M.-R.; Azucena, M.-B.; Alejandra, S.-F.; Jorge, M.-H. Association between metabolic syndrome and erythrocyte fatty acid profile in Mexican adolescents: A trans fatty acid approach. Food Nutr. Sci. 2013, 4, 51–58. [Google Scholar] [CrossRef]
- Moghassemi, S.; Dadashzadeh, A.; Azevedo, R.B.; Amorim, C.A. Nanoemulsion applications in photodynamic therapy. J. Control. Release 2022, 351, 164–173. [Google Scholar] [CrossRef]
- Celleno, L. Topical urea in skincare: A review. Dermatol. Ther. 2018, 31, e12690. [Google Scholar] [CrossRef]
- Percutaneous Penetration Enhancers Drug Penetration into/through the Skin, 1st ed.; Dragicevic, N., Maibach, H.I., Eds.; Springer: Berlin/Heidelberg, Germany, 2017; pp. 77–93. ISBN 978-3-662-53270-6. [Google Scholar] [CrossRef]
- Gupta, A.; Eral, H.B.; Hatton, T.A.; Doyle, P.S. Nanoemulsions: Formation, properties and applications. Soft Matter 2016, 12, 2826–2841. [Google Scholar] [CrossRef]
- Zhang, X.-N.; Guan, M.; Zhu, Q.-L.; Liu, Y.; Bei, Y.-Y.; Gu, Z.-L.; Zhang, Q. Uptake and transport of a novel anticancer drug-delivery system: Lactosyl-norcantharidin-associated N-trimethyl chitosan nanoparticles across intestinal Caco-2 cell monolayers. Int. J. Nanomed. 2012, 7, 1921–1930. [Google Scholar] [CrossRef]
- Jiang, Z.; Chi, J.; Han, B.; Liu, W. Preparation and pharmacological evaluation of norcantharidin-conjugated carboxymethyl chitosan in mice bearing hepatocellular carcinoma. Carbohydr. Polym. 2017, 174, 282–290. [Google Scholar] [CrossRef] [PubMed]
- Zeng, L.; Liu, Y.; Pan, J.; Liu, X. Formulation and evaluation of norcanthridin nanoemulsions against the Plutella xylostella (Lepidotera: Plutellidae). BMC Biotechnol. 2019, 19, 16. [Google Scholar] [CrossRef] [PubMed]
- Zou, Y.; Li, D.; Shen, M.; Shi, X. Polyethylenimine-Based nanogels for biomedical applications. Macromol. Biosci. 2019, 19, e1900272. [Google Scholar] [CrossRef] [PubMed]
- Lixin, W.; Haibing, H.; Xing, T.; Ruiying, S.; Dawei, C. A less irritant norcantharidin lipid microspheres: Formulation and drug distribution. Int. J. Pharm. 2006, 323, 161–167. [Google Scholar] [CrossRef]
- Measuring Zeta Potential Using Phase Analysis Light Scattering (PALS). Technical Note. 2022. Available online: www.malvern.com (accessed on 15 January 2023).
- Lerche, D. Comprehensive characterization of nano-and microparticles by in-situ visualization of particle movement using advanced sedimentation techniques. KONA Powder Part. J. 2019, 36, 156–186. [Google Scholar] [CrossRef]
- Detloff, T.; Sobisch, T.; Lerche, D. Instability index. Dispers. Lett. Tech. 2014, T4, 1–4. [Google Scholar]
- Pires, P.C.; Peixoto, D.; Teixeira, I.; Rodrigues, M.; Alves, G.; Santos, A.O. Nanoemulsions and thermosensitive nanoemulgels of phenytoin and fosphenytoin for intranasal administration: Formulation development and in vitro characterization. Eur. J. Pharm. Sci. 2019, 141, 105099. [Google Scholar] [CrossRef]
- Batheja, P.; Sheihet, L.; Kohn, J.; Singer, A.J.; Michniak-Kohn, B. Topical drug delivery by a polymeric nanosphere gel: Formulation optimization and in vitro and in vivo skin distribution studies. J. Control. Release 2011, 149, 159–167. [Google Scholar] [CrossRef]
- Baboota, S.; Shakeel, F.; Ahuja, A.; Ali, J.; Shafiq, S. Design, development and evaluation of novel nanoemulsion formulations for transdermal potential of celecoxib. Acta Pharm. 2007, 57, 315–332. [Google Scholar] [CrossRef]
- Tambunlertchai, S.; Geary, S.M.; Salem, A.K. Skin penetration enhancement strategies used in the development of melanoma topical treatments. AAPS J. 2021, 23, 19. [Google Scholar] [CrossRef]
- Martínez-Razo, G.; Domínguez-López, M.L.; de la Rosa, J.M.; Fabila-Bustos, D.A.; Reyes-Maldonado, E.; Conde-Vázquez, E.; Vega-López, A. Norcantharidin toxicity profile: An in vivo murine study. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2022, 396, 99–108. [Google Scholar] [CrossRef] [PubMed]
- Mei, L.; Sang, W.; Cui, K.; Zhang, Y.; Chen, F.; Li, X. Norcantharidin inhibits proliferation and promotes apoptosis via c-Met/Akt/ mTOR pathway in human osteosarcoma cells. Cancer Sci. 2018, 110, 582–595. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; You, D.; Lu, M.; He, Y.; Yan, S. Inhibitory effect of norcantharidin on melanoma tumor growth and vasculogenic mimicry by suppressing MMP-2 expression. Oncol. Lett. 2017, 13, 1660–1664. [Google Scholar] [CrossRef]
- Kamiloglu, S.; Sari, G.; Ozdal, T.; Capanoglu, E. Guidelines for cell viability assays. Food Front. 2020, 1, 332–349. [Google Scholar] [CrossRef]
- Zhang, H.-X.; Du, G.-H.; Zhang, J.-T. Assay of mitochondrial functions by resazurin in vitro. Acta Pharmacol. Sin. 2004, 25, 385–389. [Google Scholar] [PubMed]
- Pasquali, S.; Hadjinicolaou, A.V.; Chiarion, S.V.; Rossi, C.R.; Mocellin, S. Systemic treatments for metastatic cutaneous melanoma. Cochrane Database Syst. Rev. 2018, 2, CD011123. [Google Scholar] [CrossRef]
- Testori, A.A.E.; Ribero, S.; Indini, A.; Mandalà, M. Adjuvant treatment of melanoma: Recent developments and future perspectives. Am. J. Clin. Dermatol. 2019, 20, 817–827. [Google Scholar] [CrossRef]
- Wang, L.; Otkur, W.; Wang, A.; Wang, W.; Lyu, Y.; Fang, L.; Shan, X.; Song, M.; Feng, Y.; Zhao, Y.; et al. Norcantharidin overcomes vemurafenib resistance in melanoma by inhibiting pentose phosphate pathway and lipogenesis via downregulating the mTOR pathway. Front. Pharmacol. 2022, 13, 906043. [Google Scholar] [CrossRef]
- Avdeef, A.; Berger, C.M.; Brownell, C. pH-Metric solubility. Correlation between the acid-base titration and the saturation shake-flask solubility- higher molecular weights and lipophilicity. Pharm. Res. 2000, 17, 85–89. [Google Scholar] [CrossRef]
- Medina-Bañuelos, E.F.; Marín-Santibáñez, B.M.; Pérez-González, J. Rheo-PIV analysis of the steady torsional parallel-plate flow of a viscoplastic microgel with wall slip. J. Rheol. 2022, 66, 31–48. [Google Scholar] [CrossRef]
- Po-Shen, L. Simple proof of the quadratic formula. arXiv 2019, arXiv:1910.06709. [Google Scholar]
- Doornaert, B.; Leblond, V.; Galiacy, S.; Gras, G.; Planus, E.; Laurent, V.; Isabey, D.; Lafuma, C. Negative impact of DEP exposure on human airway epithelial cell adhesion, stiffness, and repair. Am. J. Physiol. Cell. Mol. Physiol. 2003, 284, L119–L132. [Google Scholar] [CrossRef]
- Nájera-Martínez, M.; Landon-Hernández, G.G.; Romero-López, J.P.; Domínguez-López, M.L.; Vega-López, A. Disruption of neurotransmission, membrane potential, and mitochondrial calcium in the brain and spinal cord of nile tilapia elicited by microcystis aeruginosa extract: An uncommon consequence of the eutrophication process. Water Air Soil Pollut. 2021, 233, 6. [Google Scholar] [CrossRef]
- Präbst, K.; Engelhardt, H.; Ringgeler, S.; Hübner, H. Basic Colorimetric Proliferation Assays: MTT, WST, and Resazurin. In Cell Viability Assays; Gilbert, D.F., Friedrich, O., Eds.; Springer: New York, NY, USA, 2017; Volume 1601, pp. 1–17. [Google Scholar]
- Hammer, Ø.; Harper, D.A.T.; Ryan, P.D. PAST: Paleontological Statistics software package for education and data analysis. Palaeontol. Electron. 2001, 4, 9. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2022; Available online: www.R-project.org/ (accessed on 3 January 2022).
- Ritz, C.; Baty, F.; Streibig, J.C.; Gerhard, D. Dose-response analysis using r. PLoS ONE 2015, 10, e0146021. [Google Scholar] [CrossRef]



| Batch | Droplet Size (nm) | Creaming Velocity (µm/s) | Instability Index | |||||
|---|---|---|---|---|---|---|---|---|
| Id | Median | S.D. | sig | Median | S.D | sig | Mean | sig |
| 4 w.o. | 243.6 | 575.6 | - | 8.42 | 59.63 | - | 0.321 | - |
| 3 w.o. | 142.1 | 59.63 | a ** | 14.5 | 82.28 | a ** | 0.568 | - |
| 1 w.o. | 94.7 | 104 | a ** b ** | 53.6 | 77.9 | a ** b ** | 0.658 | - |
| Mean | S.D. | Coeff. | Std. Err. | p | R2 | |
|---|---|---|---|---|---|---|
| DRR (%·cm2·t1/2) | ||||||
| 4.w.o. | 48.14 | 13.94 | 0.070 | 0.055 | 0.336 | 0.845 |
| 3 w.o. | 47.56 | 15.61 | −0.079 | 0.218 | 0.753 | 0.992 |
| 1 w.o. | 49.53 | 18.33 | 0.232 | 0.153 | 0.269 | 0.989 |
| CDRR (mg·cm2·t1/2) | ||||||
| 4.w.o. | 24.34 | 13.31 | 0.417 | 0.300 | 0.299 | 0.994 |
| 3 w.o. | 23.66 | 13.57 | −0.296 | 1.173 | 0.824 | 0.979 |
| 1 w.o. | 24.22 | 14.45 | 0.163 | 0.857 | 0.866 | 0.971 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martínez-Razo, G.; Pires, P.C.; Domínguez-López, M.L.; Veiga, F.; Vega-López, A.; Paiva-Santos, A.C. Norcantharidin Nanoemulsion Development, Characterization, and In Vitro Antiproliferation Effect on B16F1 Melanoma Cells. Pharmaceuticals 2023, 16, 501. https://doi.org/10.3390/ph16040501
Martínez-Razo G, Pires PC, Domínguez-López ML, Veiga F, Vega-López A, Paiva-Santos AC. Norcantharidin Nanoemulsion Development, Characterization, and In Vitro Antiproliferation Effect on B16F1 Melanoma Cells. Pharmaceuticals. 2023; 16(4):501. https://doi.org/10.3390/ph16040501
Chicago/Turabian StyleMartínez-Razo, Gabriel, Patrícia C. Pires, María Lilia Domínguez-López, Francisco Veiga, Armando Vega-López, and Ana Cláudia Paiva-Santos. 2023. "Norcantharidin Nanoemulsion Development, Characterization, and In Vitro Antiproliferation Effect on B16F1 Melanoma Cells" Pharmaceuticals 16, no. 4: 501. https://doi.org/10.3390/ph16040501
APA StyleMartínez-Razo, G., Pires, P. C., Domínguez-López, M. L., Veiga, F., Vega-López, A., & Paiva-Santos, A. C. (2023). Norcantharidin Nanoemulsion Development, Characterization, and In Vitro Antiproliferation Effect on B16F1 Melanoma Cells. Pharmaceuticals, 16(4), 501. https://doi.org/10.3390/ph16040501









