Anti-Atopic Dermatitis Effects of Abietic Acid Isolated from Rosin under Condition Optimized by Response Surface Methodology in DNCB-Spread BALB/c Mice
Abstract
1. Introduction
2. Results
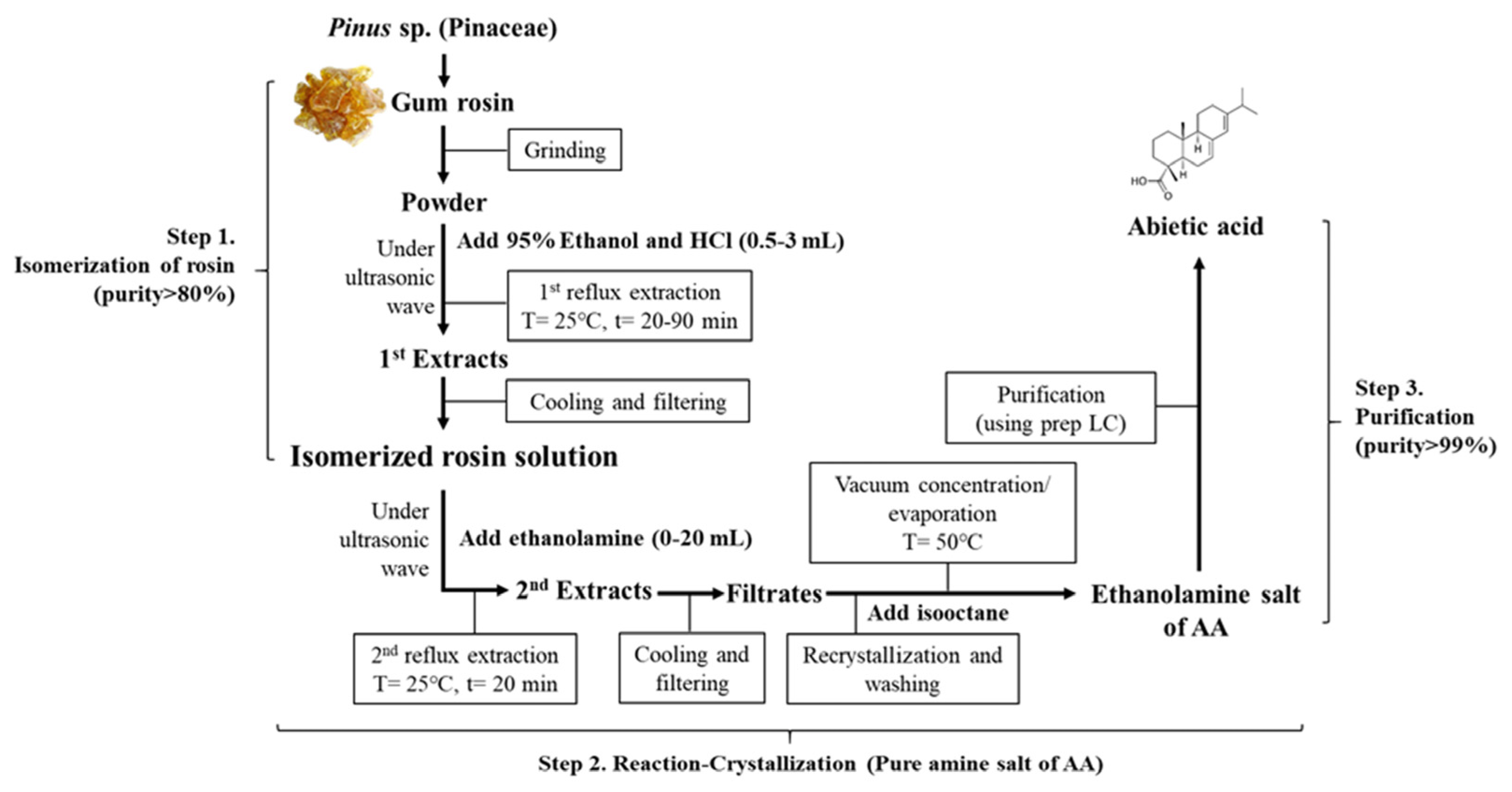
2.1. Isolation and Purification of AA under Optimal Condition Established by RSM, and Its Characterization
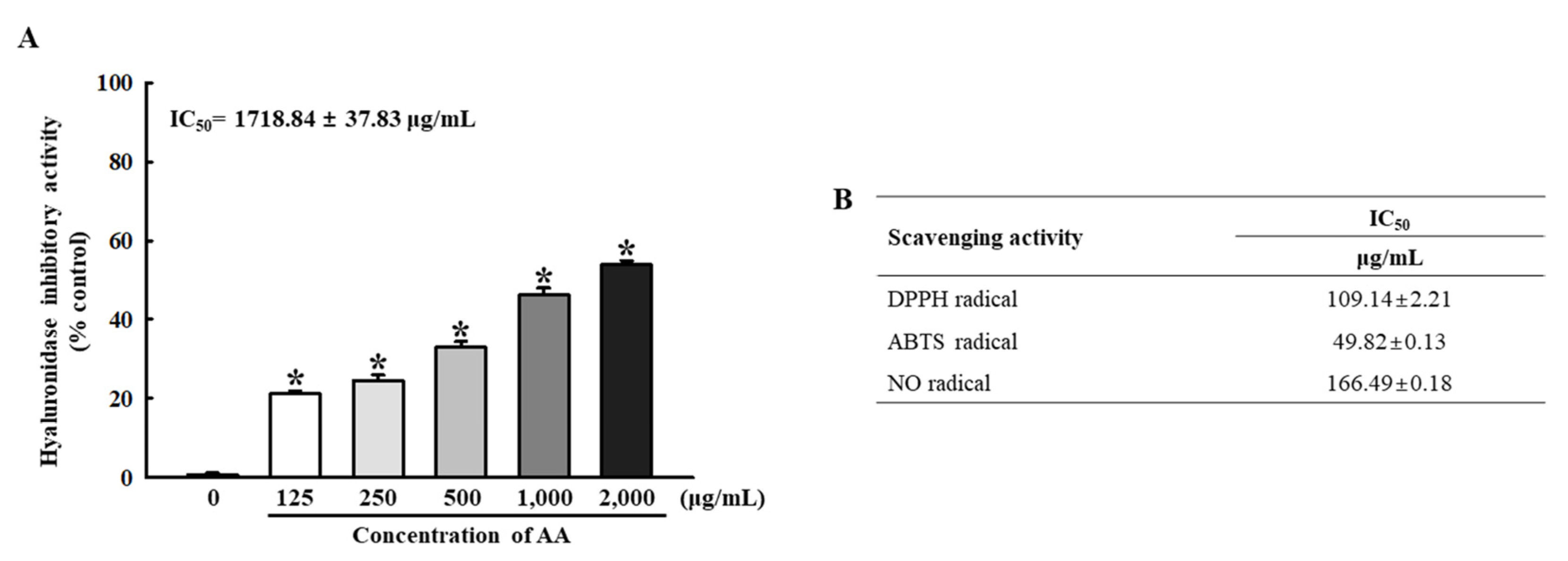
2.2. Biochemical Properties of AA against Inflammation and Oxidative Stress
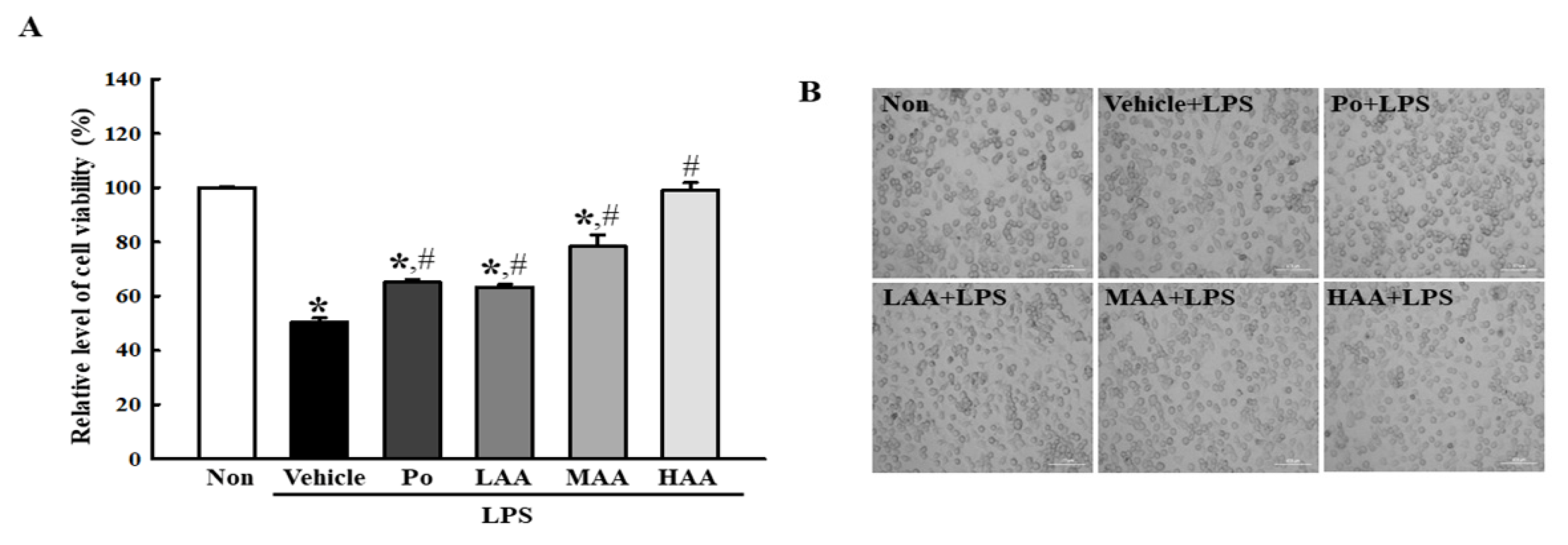
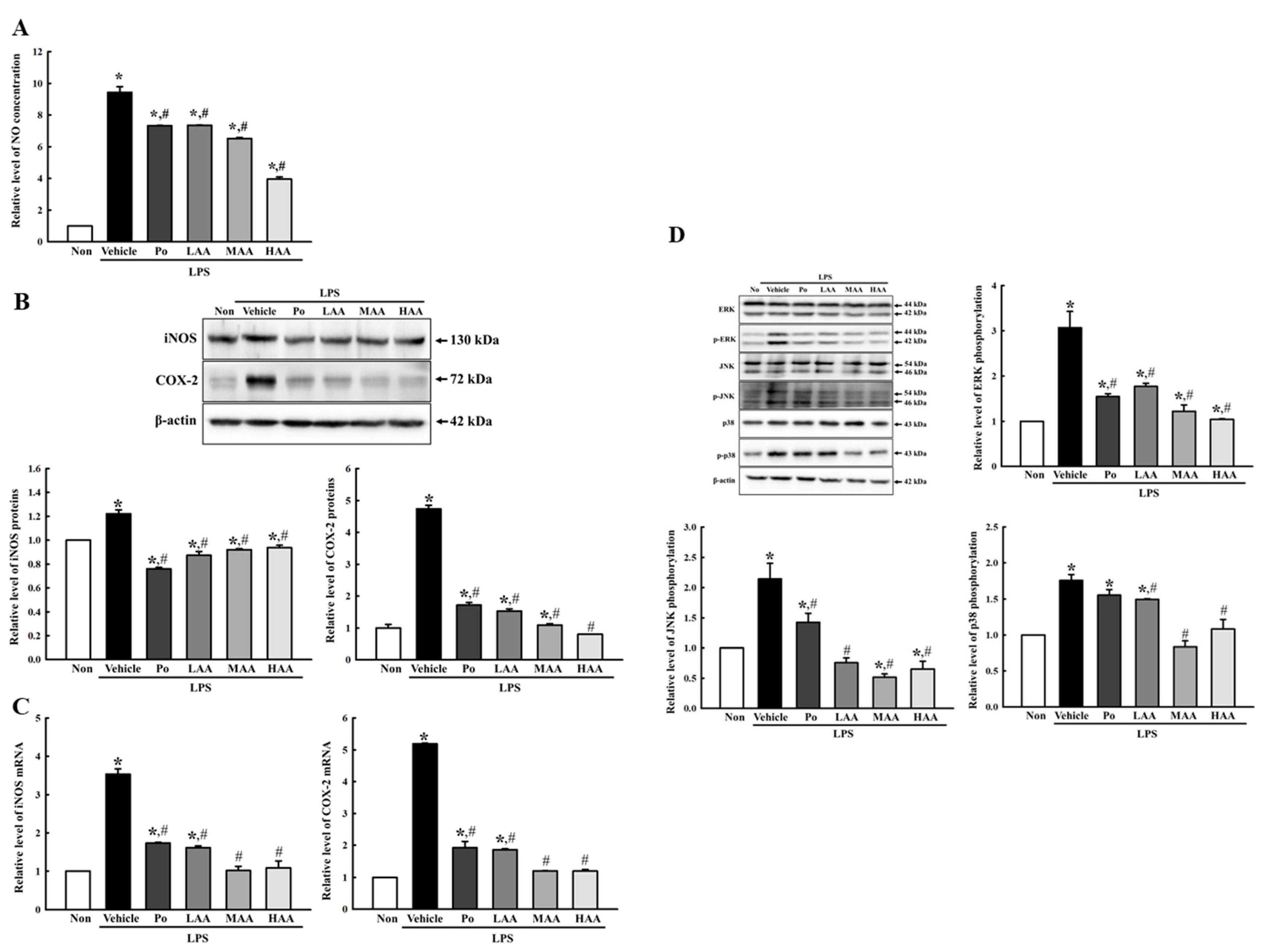
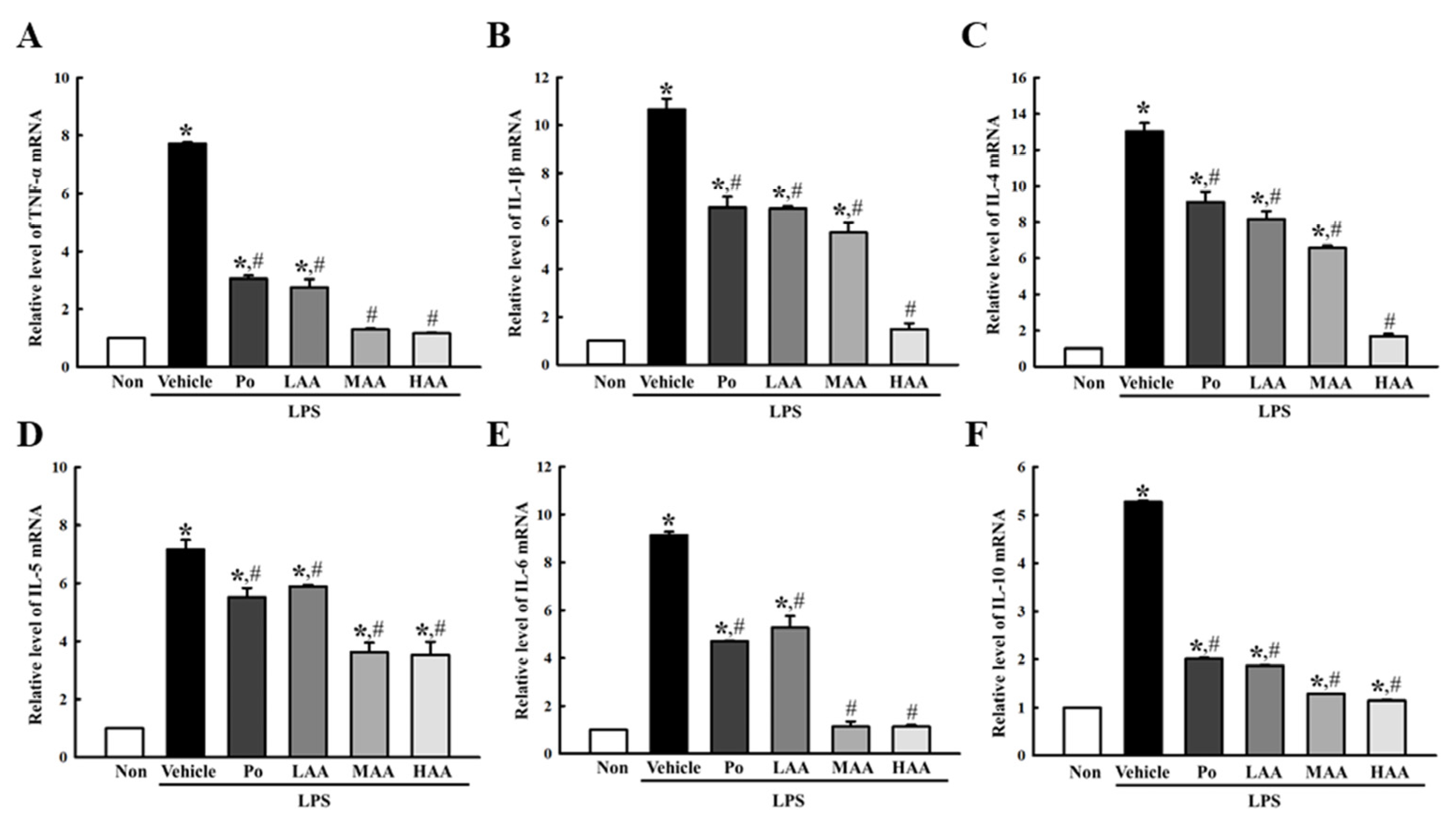
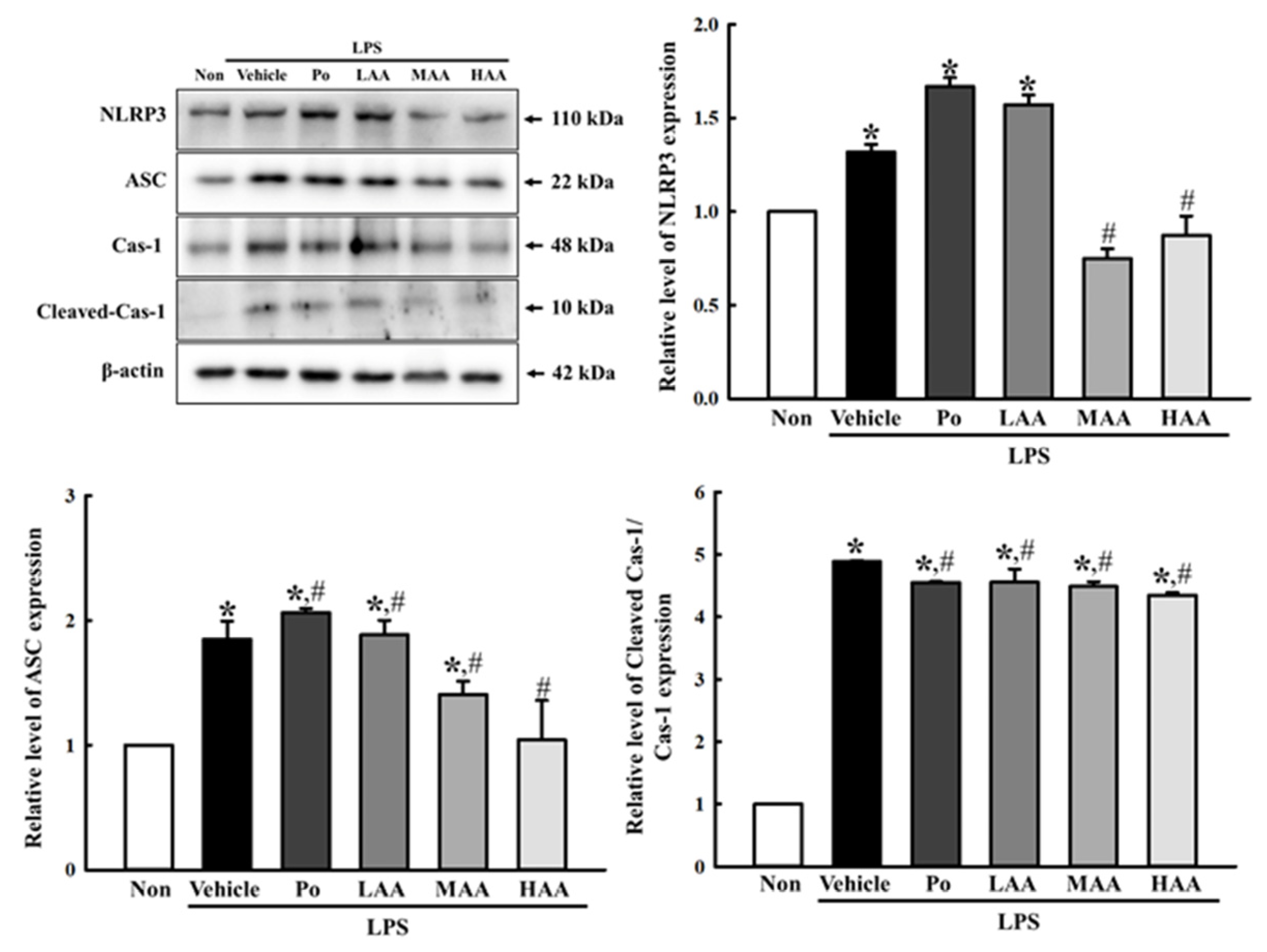
2.3. Verification of Anti-Inflammatory Activities of AA in LPS-Stimulated RAW264.7 Macrophages
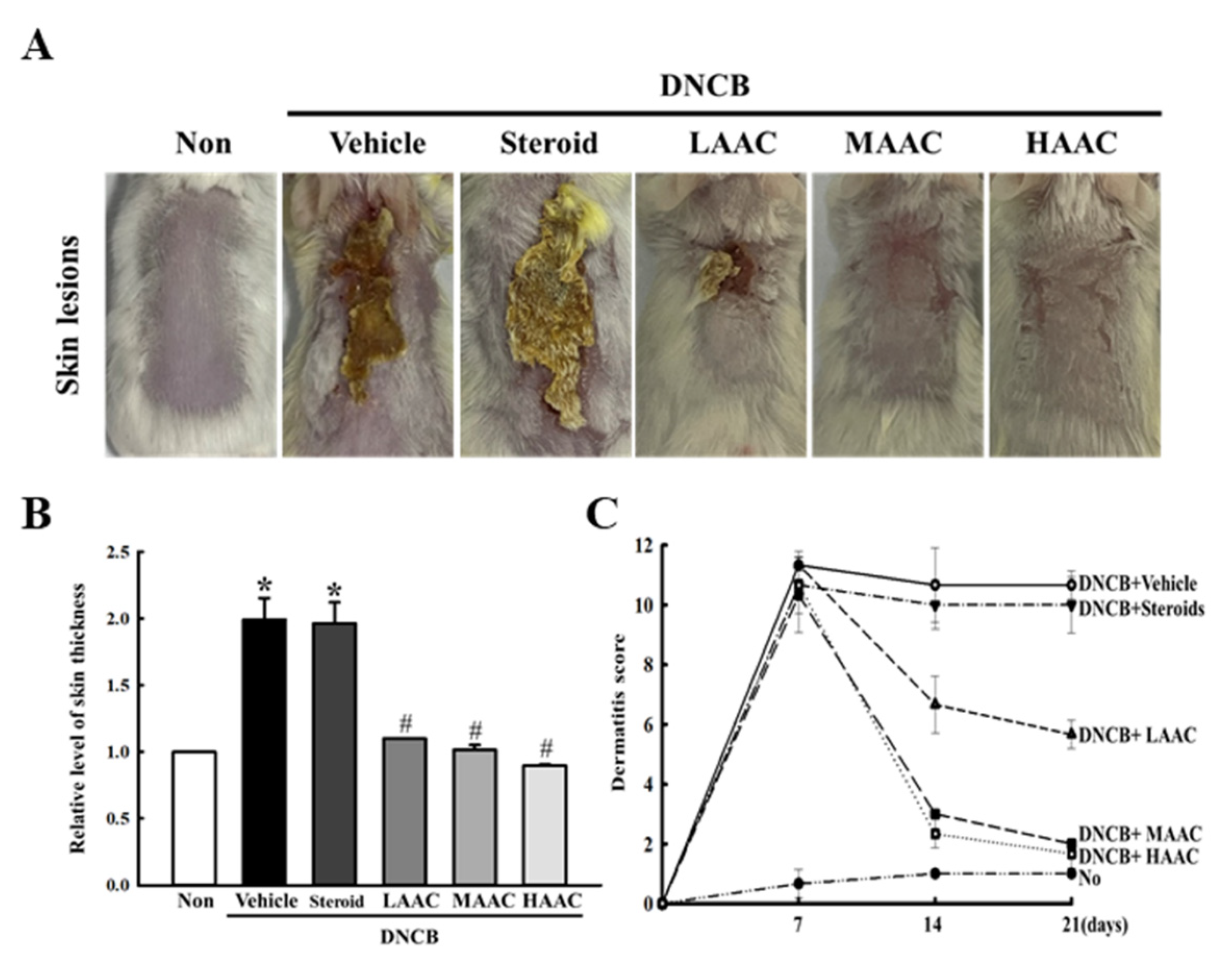
2.4. Amelioration of Skin Phenotypes in DNCB-Induced AD Mice by AA Spreading
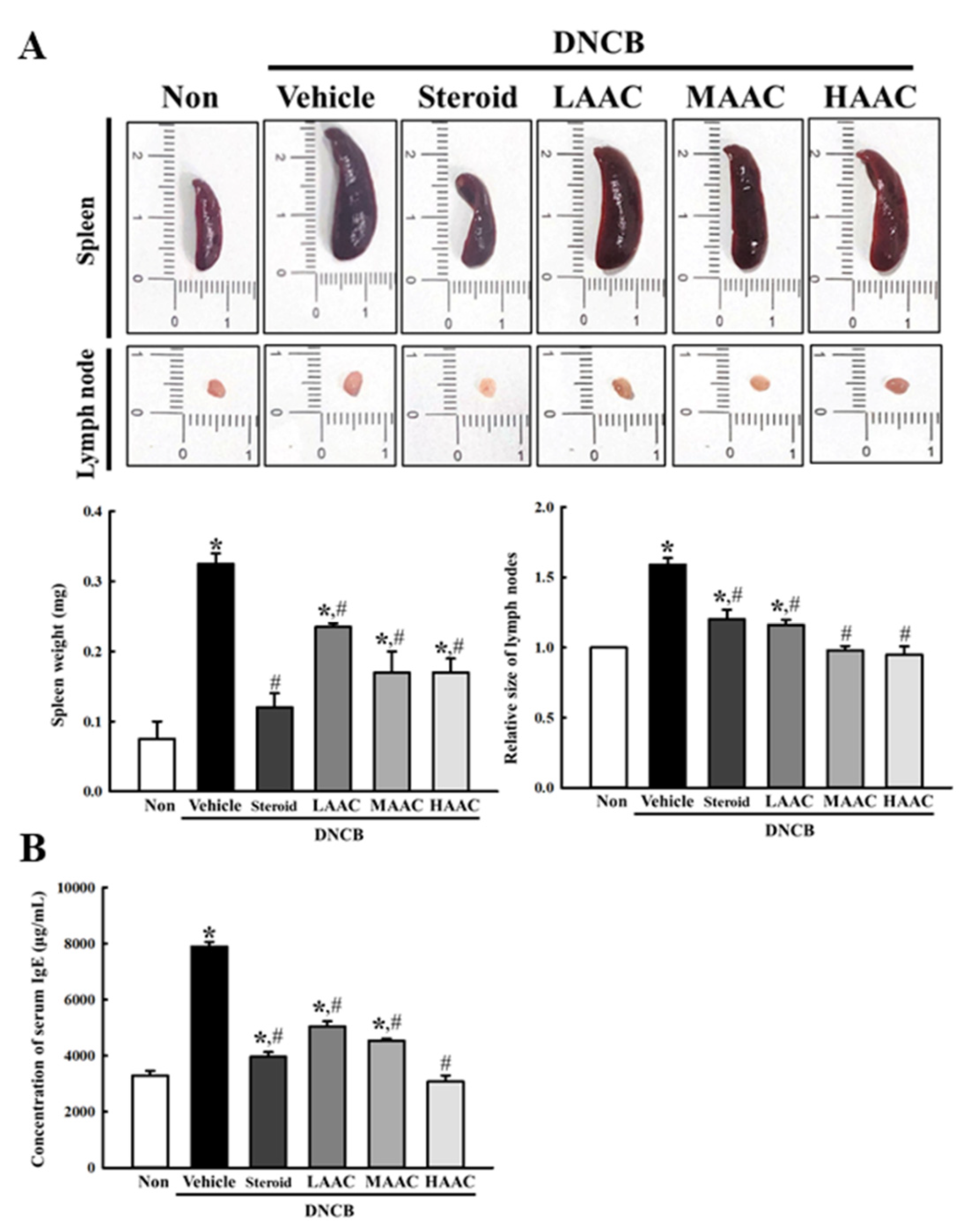
2.5. Amelioration of IgE-Mediated Symptoms in DNCB-Induced AD Mice by AA Spreading
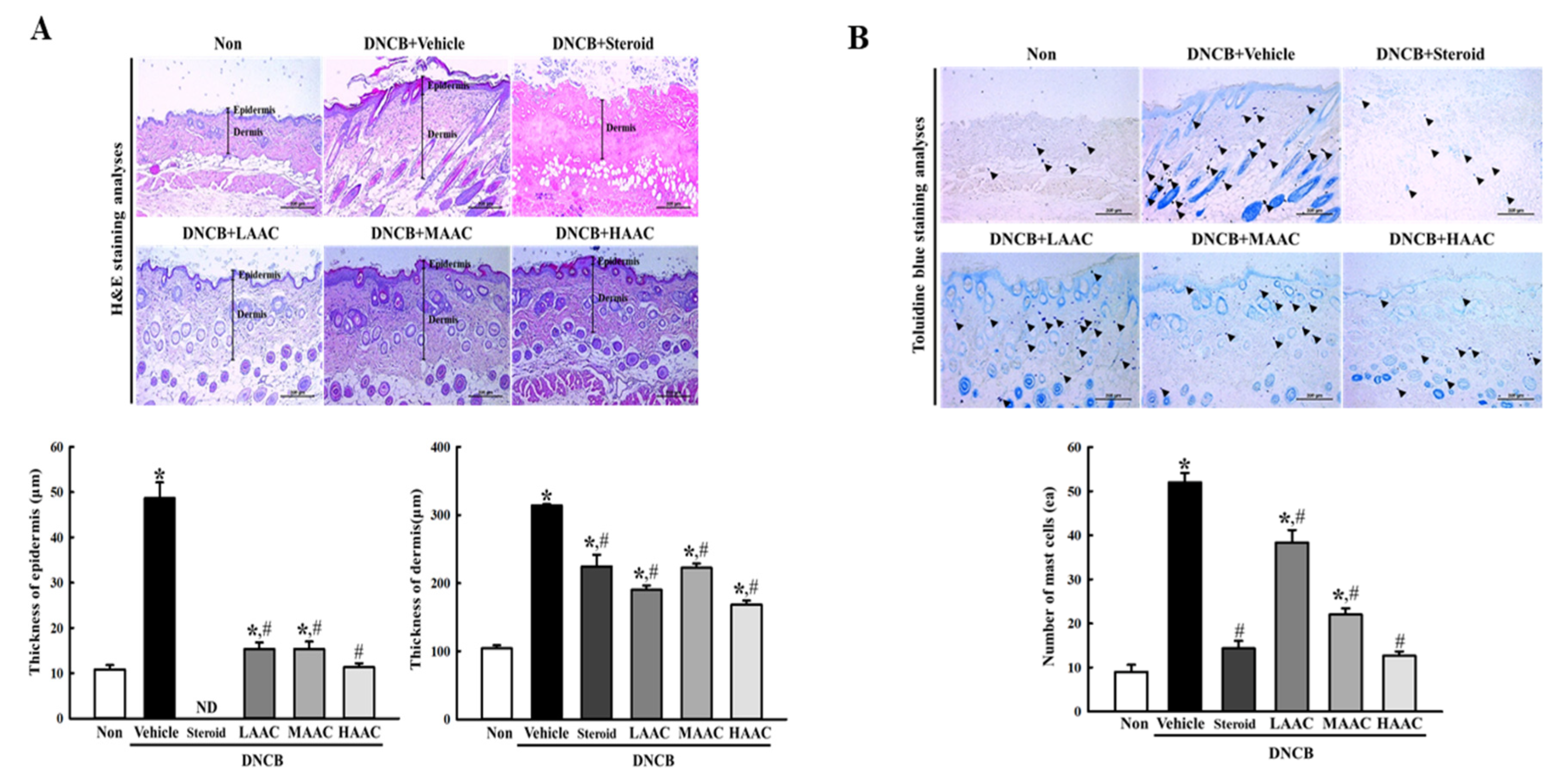
2.6. Amelioration of Skin Histopathological Structure in DNCB-Induced AD Mice by AA Spreading
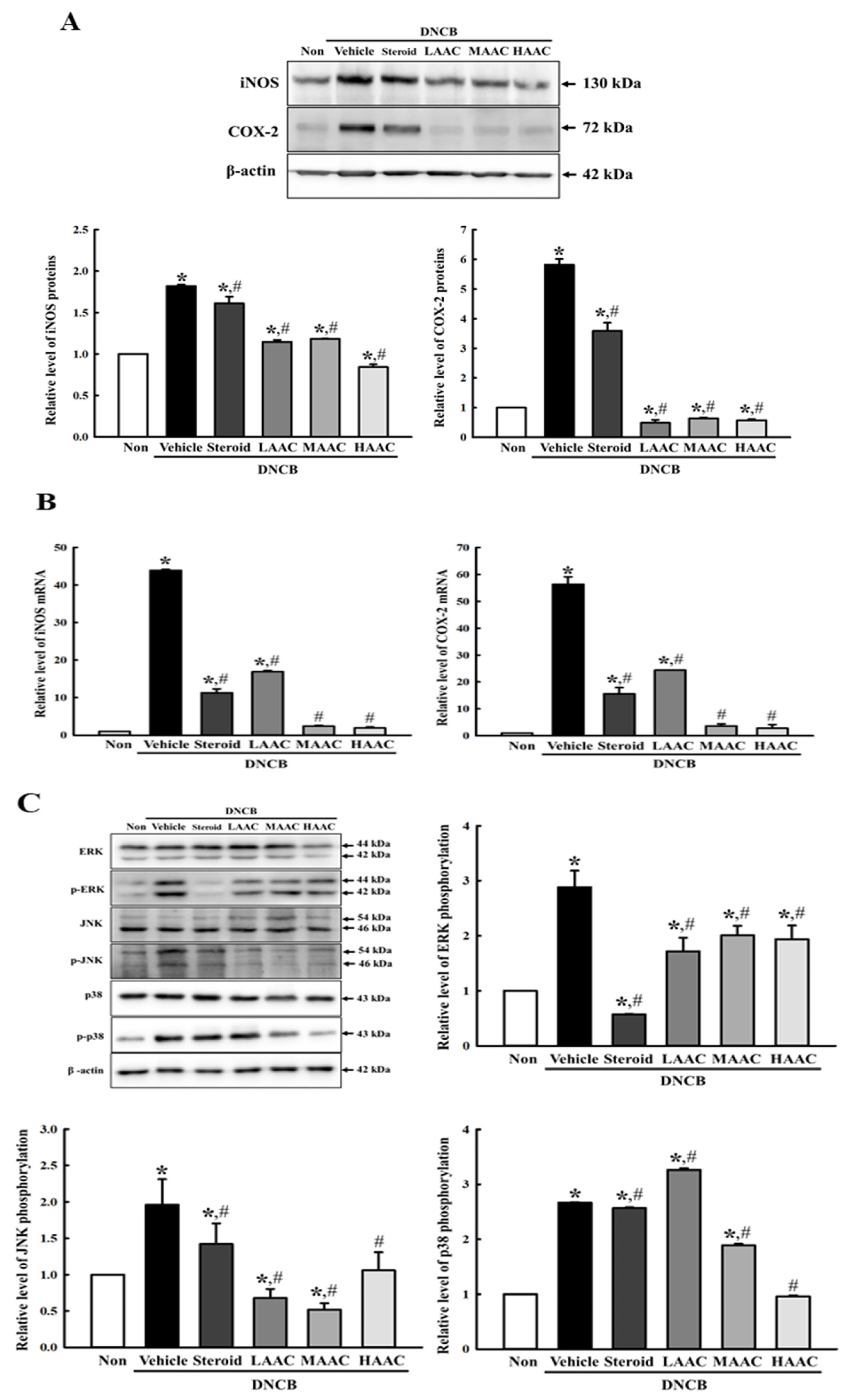
2.7. Amelioration of NO-Producing System in DNCB-Induced AD Mice by AA Spreading
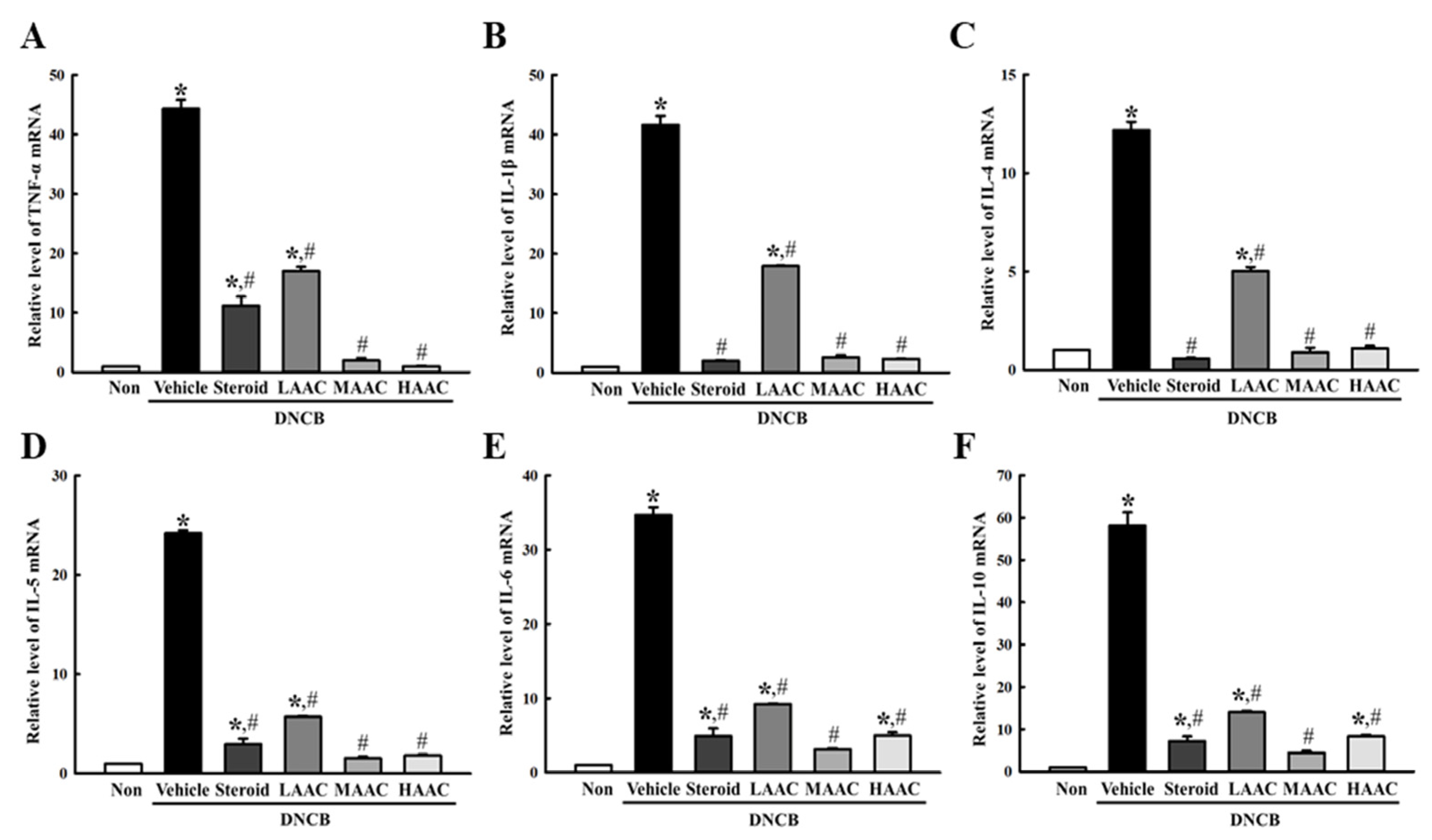
2.8. Amelioration of Inflammatory Cytokines Secretion in DNCB-Induced AD Mice by AA Spreading
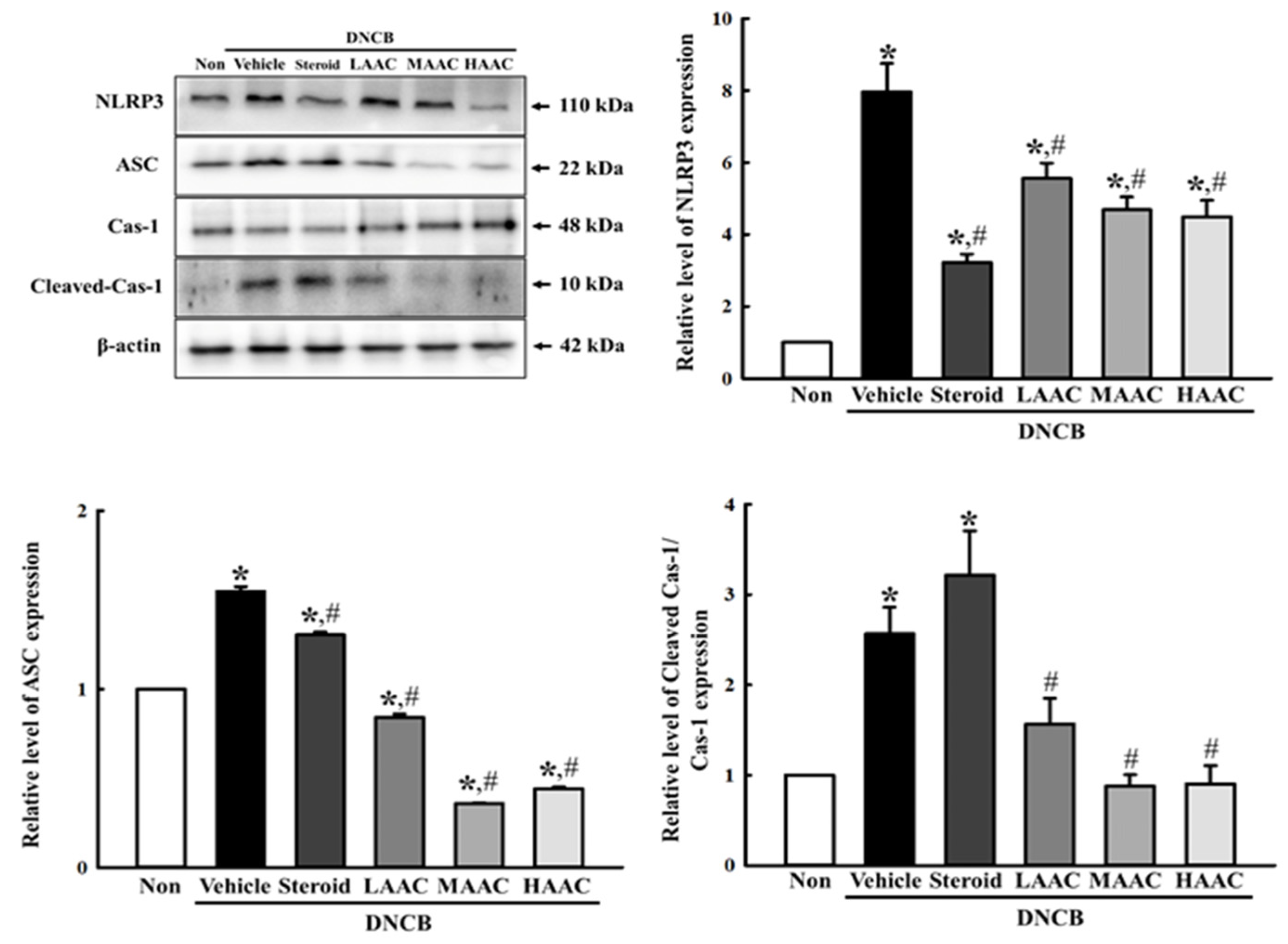
2.9. Amelioration of Inflammasome Activation in DNCB-Induced AD Mice by AA Spreading
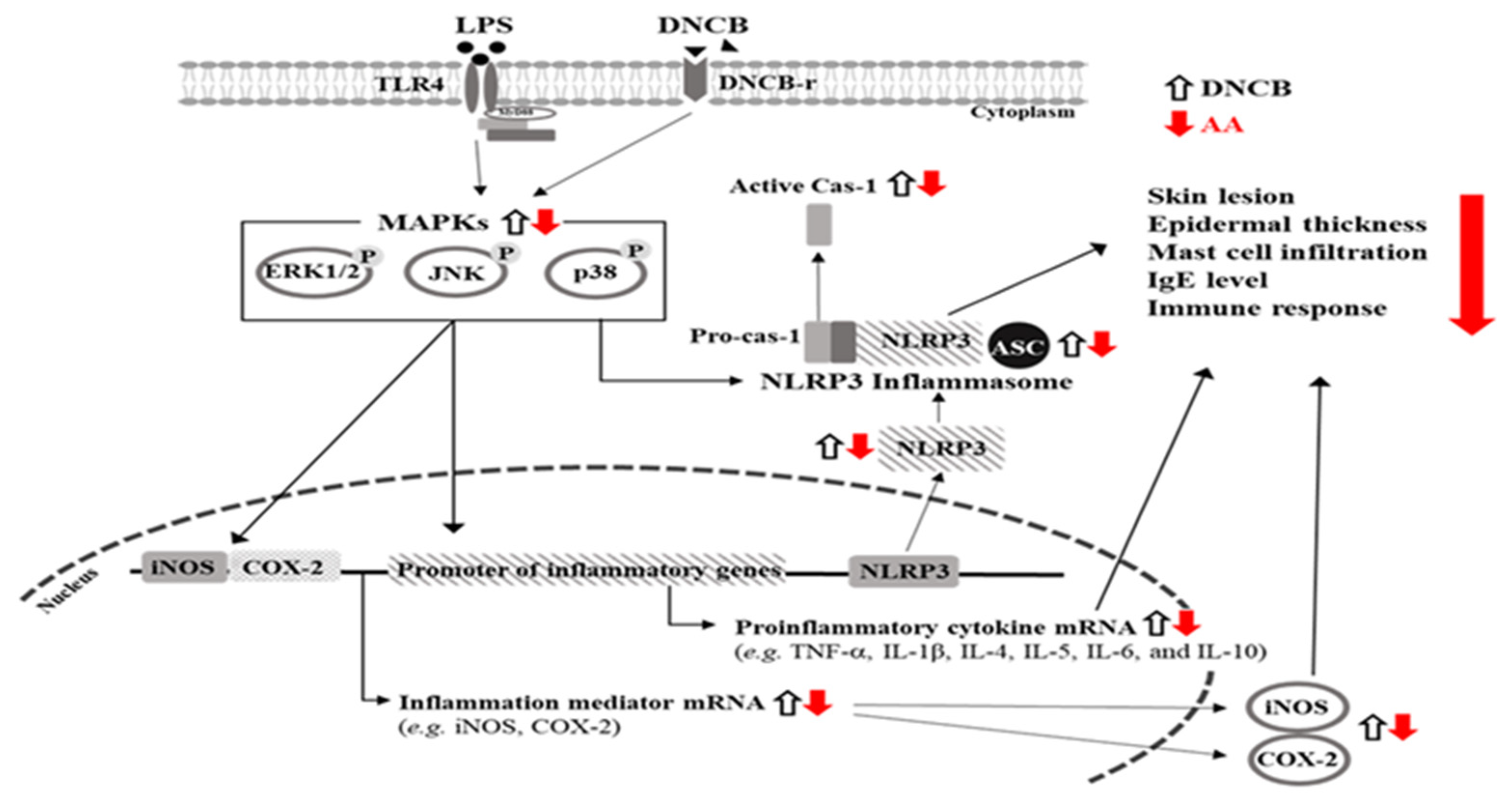
3. Discussion
4. Materials and Methods
4.1. RSM and Central Composite Design (CCD) Analysis
4.2. Isolation and Purification of AA from Rosin
4.3. HPLC, LC-MS, and FTIR Analysis
4.4. Assay for Hyaluronidase (HAase) Inhibition Activity
4.5. Free Radical Scavenging Activity Assay
4.6. Cell Culture and Viability
4.7. Measurement of NO Concentration
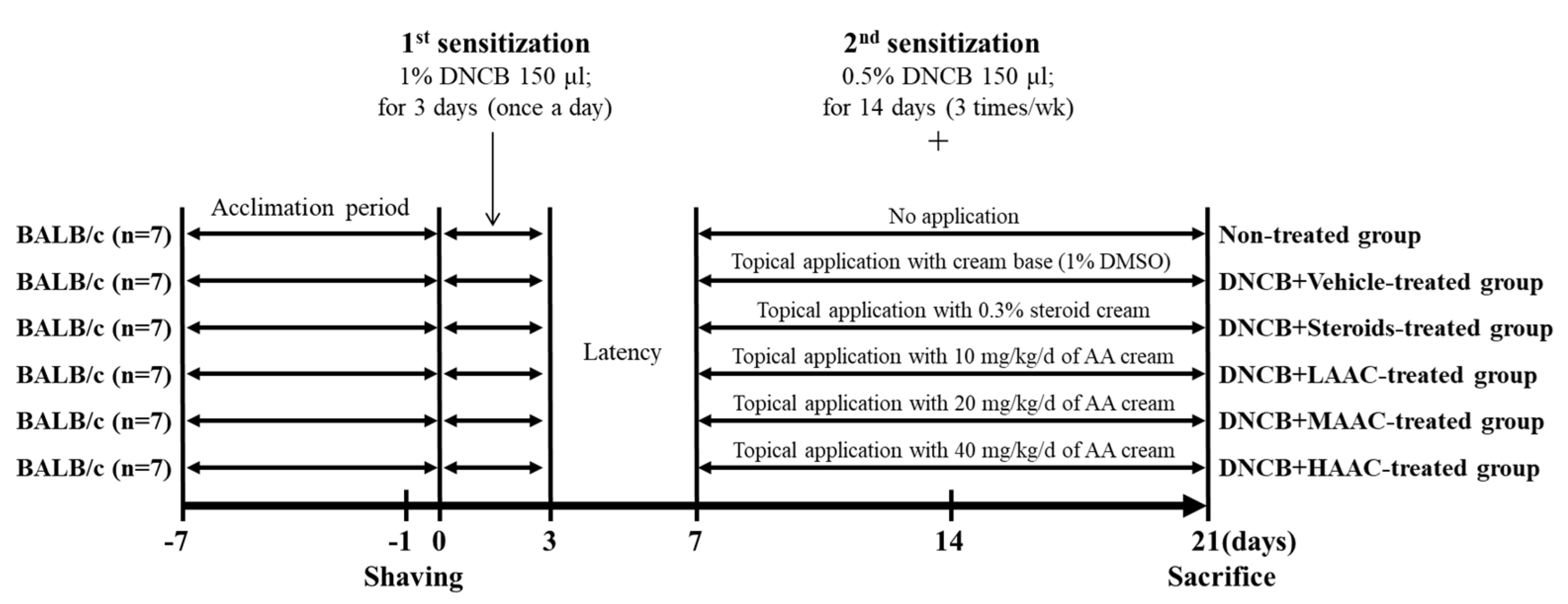
4.8. Experimental Design of the Animal Study
4.9. Measurement of Spleen Weight and Lymph Node Size
4.10. Determination of Dermatitis Score and Skin Thickness
4.11. Histopathological Analysis
4.12. Enzyme-Linked Immunosorbent Assay (ELISA) of IgE
4.13. Western Blot Analysis
4.14. Quantitative Real-Time-PCR (RT-qPCR) Analysis
4.15. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Abd Wahab, N.A.; Giribabu, N.; Kilari, E.K.; Salleh, N. Abietic acid ameliorates nephropathy progression via mitigating renal oxidative stress, inflammation, fibrosis and apoptosis in high fat diet and low dose streptozotocin-induced diabetic rats. Phytomedicine 2022, 107, 154464. [Google Scholar] [CrossRef] [PubMed]
- Aminimoghadamfarouj, N.; Nematollahi, A. Propolis diterpenes as a remarkable bio-source for drug discovery development: A review. Int. J. Mol. Sci. 2017, 18, 1290. [Google Scholar] [CrossRef] [PubMed]
- Ulusu, N.N.; Ercil, D.; Sakar, M.K.; Tezcan, E.F. Abietic acid inhibits lipoxygenase activity. Phytother. Res. 2002, 16, 88–90. [Google Scholar] [CrossRef]
- Zeng, H.H.; Tu, P.F.; Zhou, K.; Wang, H.; Wang, B.H.; Lu, J.F. Antioxidant properties of phenolic diterpenes from Rosmarinus officinalis. Acta Pharmacol. Sin. 2001, 22, 1094–1098. [Google Scholar] [PubMed]
- Politi, M.; Braca, A.; De Tommasi, N.; Morelli, I.; Manunta, A.; Battinelli, L.; Mazzanti, G. Antimicrobial diterpenes from the seeds of Cephalotaxus harringtonia var. drupacea. Planta Med. 2003, 69, 468–470. [Google Scholar] [CrossRef] [PubMed]
- Rasoamiaranjanahary, L.; Marston, A.; Guilet, D.; Schenk, K.; Randimbivololona, F.; Hostettmann, K. Antifungal diterpenes from Hypoestes serpens (Acanthaceae). Phytochemistry 2003, 62, 333–337. [Google Scholar] [CrossRef]
- Devappa, R.K.; Makkar, H.P.; Becker, K. Jatropha diterpenes: A review. J. Am. Oil Chem. Soc. 2011, 88, 301–322. [Google Scholar] [CrossRef]
- Costa, J.P.; Ferreira, P.B.; De Sousa, D.P.; Jordan, J.; Freitas, R.M. Anticonvulsant effect of phytol in a pilocarpine model in mice. Neurosci. Lett. 2012, 523, 115–118. [Google Scholar] [CrossRef]
- Kesler, C.C.; Lowy, A.; Faragher, W.F. Purification of abietic acid from rosin and preparation of some of its derivatives. Amer. Chem. Soc. J. 1927, 49, 2898–2903. [Google Scholar] [CrossRef]
- Harris, G.C.; Sanderson, T.F. Abietic acid. Org. Synth. 1952, 32, 1. [Google Scholar] [CrossRef]
- Nong, W.J.; Chen, X.P.; Liang, J.Z.; Wang, L.L.; Tong, Z.F.; Huang, K.; Wu, R.; Xie, Q.; Jia, Y.; Li, K.X. Isolation and characterization of abietic acid. Adv. Mater. Res. 2014, 887, 551–556. [Google Scholar] [CrossRef]
- Hsieh, Y.S.; Yang, S.F.; Hsieh, Y.H.; Hung, C.H.; Chu, S.C.; Yang, S.H.; Chen, P.N. The inhibitory effect of abietic acid on melanoma cancer metastasis and invasiveness In Vitro and In Vivo. Am. J. Chin. Med. 2015, 43, 1697–1714. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Chen, W.; Liu, Q.; Dai, J. Abietic acid suppresses non-small-cell lung cancer cell growth via blocking IKKβ/NF-κB signaling. OncoTargets Ther. 2019, 12, 4825–4837. [Google Scholar] [CrossRef] [PubMed]
- Haffez, H.; Osman, S.; Ebrahim, H.Y.; Hassan, Z.A. Growth inhibition and apoptotic effect of pine extract and abietic acid on MCF-7 breast cancer cells via alteration of multiple gene expressions using in vitro approach. Molecules 2022, 27, 293. [Google Scholar] [CrossRef]
- Park, J.Y.; Lee, Y.K.; Lee, D.S.; Yoo, J.E.; Shin, M.S.; Kim, S.N.; Lee, S.A.; Kim, K.H.; Lee, H.J.; Roh, S.S.; et al. Abietic acid isolated from pine resin (Resina Pini) enhances angiogenesis in HUVECs and accelerates cutaneous wound healing in mice. J. Ethnopharmacol. 2017, 203, 279–287. [Google Scholar] [CrossRef]
- Fang, H.; Chen, J.; Luo, J.; Hu, J.; Wang, D.; Lv, L.; Zhang, W. Abietic acid attenuates sepsis-induced lung injury by inhibiting NF-kB pathway to inhibit M1 macrophage polarization. Exp. Anim. 2022, 71, 481–490. [Google Scholar] [CrossRef]
- Jung, T.W.; Jeong, J.-C.; Park, S.Y.; Cho, W.; Oh, H.; Lee, H.J.; Hacimuftuoglu, A.; Abd El-Aty, A.A.; Bang, J.S.; Jeong, J.H. Abietic acid alleviates endoplasmic reticulum stress and lipid accumulation in human primary hepatocytes through the AMPK/ORP150 signaling. Biochem. Biophys. Res. Commun. 2022, 608, 142–148. [Google Scholar] [CrossRef]
- Hwang, K.-H.; Ahn, J.-Y.; Kim, S.; Park, J.-H.; Ha, T.-Y. Abietic acid has an anti-obesity effect in mice fed a high-fat diet. J. Med. Food 2011, 14, 1052–1056. [Google Scholar] [CrossRef]
- Thummuri, D.; Guntuku, L.; Challa, V.S.; Ramavat, R.N.; Naidu, V.G.M. Abietic acid attenuates RANKL induced osteoclastogenesis and inflammation associated osteolysis by inhibiting the NF-KB and MAPK signaling. J. Cell. Physiol. 2019, 234, 443–453. [Google Scholar] [CrossRef]
- Li, X.Q.; Chen, Y.; Dai, G.C.; Zhou, B.B.; Yan, X.N.; Tan, R.X. Abietic acid ameliorates psoriasis-like inflammation and modulates gut microbiota in mice. J. Ethnopharmacol. 2021, 272, 113934. [Google Scholar] [CrossRef]
- Kang, S.; Zhang, J.; Yuan, Y. Abietic acid attenuates IL-1β-induced inflammation in human osteoarthritis chondrocytes. Int. Immunopharmacol. 2018, 64, 110–115. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Zhaoyu, L.; Xiangming, F.; Chunyi, L.; Jiayu, P.; Lu, S.; Jitao, C.; Liangcai, C.; Jifang, L. Abietic acid attenuates allergic airway inflammation in a mouse allergic asthma model. Int. Immunopharmacol. 2016, 38, 261–266. [Google Scholar] [CrossRef] [PubMed]
- Beltran, V.; Salvadó, N.; Butí, S.; Pradell, T. Ageing of resin from Pinus species assessed by infrared spectroscopy. Anal. Bioanal. Chem. 2016, 408, 4073–4082. [Google Scholar] [CrossRef] [PubMed]
- Crucitti, V.C.; Migneco, L.M.; Piozzi, A.; Taresco, V.; Garnett, M.; Argent, R.H.; Francolini, I. Intermolecular interaction and solid state characterization of abietic acid/chitosan solid dispersions possessing antimicrobial and antioxidant properties. Eur. J. Pharm. Biopharm. 2018, 125, 114–123. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Deng, H.; Cui, H.; Fang, J.; Zuo, Z.; Deng, J.; Li, Y.; Wang, X.; Zhao, L. Inflammatory responses and inflammation-associated diseases in organs. Oncotarget 2018, 9, 7204. [Google Scholar] [CrossRef] [PubMed]
- Ferrero-Miliani, L.; Nielsen, O.H.; Andersen, P.S.; Girardin, S. Chronic inflammation: Importance of NOD2 and NALP3 in interleukin-1β generation. J. Clin. Exp. Immunol. 2007, 147, 227–235. [Google Scholar] [CrossRef]
- Kamada, N.; Seo, S.-U.; Chen, G.Y.; Núñez, G. Role of the gut microbiota in immunity and inflammatory disease. Nat. Rev. Immunol. 2013, 13, 321–335. [Google Scholar] [CrossRef]
- Hamulic, D.; Stadler, M.; Hering, S.; Padrón, J.M.; Bassett, R.; Rivas, F.; Loza-Mejia, M.A.; Dea-Ayuela, M.A.; González-Cardenete, M.A. Synthesis and biological studies of (+)-liquiditerpenoic acid A (abietopinoic acid) and representative analogues: SAR studies. J. Nat. Prod. 2019, 82, 823–831. [Google Scholar] [CrossRef]
- Laroche, B.; Nay, B. Harnessing the potential diversity of resinic diterpenes through visible light-induced sensitized oxygenation coupled to Kornblum–DeLaMare and Hock reactions. Org. Chem. Front. 2017, 4, 2412–2416. [Google Scholar] [CrossRef]
- San Feliciano, A.; Gordaliza, M.; Salinero, M.A.; del Corral, J.M.M. Abietane acids: Sources, biological activities, and therapeutic uses. Planta. Med. 1993, 59, 485–490. [Google Scholar] [CrossRef]
- Ruzicka, L.; Ankersmit, P.J.; Frank, B. Polyterpene und polyterpenoide LXXIII. Anlagerung von maleinsäure-anhydrid an abietinsäure und dextro-pimarsäure. Helv. Chim. Acta 1932, 15, 1289–1294. [Google Scholar] [CrossRef]
- Carman, R.M.; Marty, R.A.; Diterpenoids, X.X.I.V. A survey of the Agathis species of north Queensland. Two new resin acids. Aust. J. Chem. 1970, 23, 1457–1464. [Google Scholar] [CrossRef]
- Zinkel, D.F.; Critchfield, W.B. Diterpene resin acids in Pinus massoniana needles and cortex. Phytochemistry 1974, 13, 2876–2877. [Google Scholar] [CrossRef]
- Mantzaridis, C.; Brocas, A.L.; Llevot, A.; Cendejas, G.; Auvergne, R.; Caillol, S.; Carlotti, S.; Cramail, H. Rosin acid oligomers as precursors of DGEBA-free epoxy resins. Green Chem. 2013, 15, 3091–3098. [Google Scholar] [CrossRef]
- Wang, J.; Yao, K.; Wang, C.; Tang, C.; Jiang, X. Synthesis and drug delivery of novel amphiphilic block copolymers containing hydrophobic dehydroabietic moiety. J. Mater. Chem. B 2013, 1, 2324–2332. [Google Scholar] [CrossRef]
- Kakegawa, H.; Matsumoto, H.; Satoh, T. Inhibitory effects of hydrangenol derivatives on the activation of hyaluronidase and their antiallergic activities. Planta Med. 1988, 54, 385–389. [Google Scholar] [CrossRef]
- González-Peña, D.; Colina-Coca, C.; Char, C.D.; Cano, M.P.; de Ancos, B.; Sánchez-Moreno, C. Hyaluronidase inhibiting activity and radical scavenging potential of flavonols in processed onion. J. Agric. Food Chem. 2013, 61, 4862–4872. [Google Scholar] [CrossRef]
- Noble, P.W. Hyaluronan and its catabolic products in tissue injury and repair. Matrix Biol. 2002, 21, 25–29. [Google Scholar] [CrossRef]
- Fernández, M.A.; Tornos, M.P.; Garcia, M.D.; De las Heras, B.; Villar, A.M.; Saenz, M.T. Anti-inflammatory activity of abietic acid, a diterpene isolated from Pimenta racemosa var. grissea. J. Pharm. Pharmacol. 2001, 53, 867–872. [Google Scholar] [CrossRef]
- Ramnath, M.G.; Thirugnanasampandan, R.; Sadasivam, M.; Mohan, P.S. Antioxidant, antibacterial and antiacetylcholinesterase activities of abietic acid from Isodon wightii (Bentham) H. Hara. Free Radic. Antioxid. 2015, 5, 1–5. [Google Scholar] [CrossRef]
- Miura, K.; Kikuzaki, H.; Nakatani, N. Antioxidant activity of chemical components from sage (Salvia officinalis L.) and thyme (Thymus vulgaris L.) measured by the oil stability index method. J. Agric. Food Chem. 2002, 50, 1845–1851. [Google Scholar] [CrossRef] [PubMed]
- Kabouche, A.; Kabouche, Z.; Öztürk, M.; Kolak, U.; Topçu, G. Antioxidant abietane diterpenoids from Salvia barrelieri. Food Chem. 2007, 102, 1281–1287. [Google Scholar] [CrossRef]
- Tam, N.M.; Thong, N.M.; Le Huyen, T.; Hoang, L.P.; Mechler, A.; Vo, Q.V. The radical scavenging activity of abietane diterpenoids: Theoretical insights. J. Mol. Graph. Model. 2021, 105, 107892. [Google Scholar] [CrossRef] [PubMed]
- Ngkelo, A.; Meja, K.; Yeadon, M.; Adcock, I.; Kirkham, P.A. LPS induced inflammatory responses in human peripheral blood mononuclear cells is mediated through NOX4 and Giα dependent PI-3kinase signaling. J. Inflamm. 2012, 9, 1. [Google Scholar] [CrossRef] [PubMed]
- Takashiba, S.; Van Dyke, T.E.; Amar, S.; Murayama, Y.; Soskolne, A.W.; Shapira, L. Differentiation of monocytes to macrophages primes cells for lipopolysaccharide stimulation via accumulation of cytoplasmic nuclear factor κB. Infect Immun. 1999, 67, 5573–5578. [Google Scholar] [CrossRef]
- Takahashi, N.; Kawada, T.; Goto, T.; Kim, C.S.; Taimatsu, A.; Egawa, K.; Yamamoto, T.; Jisaka, M.; Nishimura, K.; Yokota, K.; et al. Abietic acid activates peroxisome proliferator-activated receptor-γ (PPARγ) in RAW264. 7 macrophages and 3T3-L1 adipocytes to regulate gene expression involved in inflammation and lipid metabolism. FEBS Lett. 2003, 550, 190–194. [Google Scholar] [CrossRef]
- Kim, N.H.; Son, Y.; Jeong, S.O.; Hur, J.M.; Bang, H.S.; Lee, K.N.; Kim, E.C.; Chung, H.T.; Pae, H.O. Tetrahydroabietic acid, a reduced abietic acid, inhibits the production of inflammatory mediators in RAW264. 7 macrophages activated with lipopolysaccharide. J. Clin. Biochem. Nutr. 2010, 46, 119–125. [Google Scholar] [CrossRef]
- Elson, L.A.; Morgan, W.T.J. A colorimetric method for the determination of glucosamine and chondrosamine. Biochem. J. 1933, 27, 1824. [Google Scholar] [CrossRef]
- Blois, M.S. Antioxidant determinations by the use of a stable free radical. Nature 1958, 181, 1199–1200. [Google Scholar] [CrossRef]
- Lee, S.J.; Kim, J.E.; Choi, Y.J.; Gong, J.E.; Park, S.H.; Douangdeuane, B.; Souliya, O.; Park, J.M.; Lee, H.S.; Kim, B.H.; et al. Therapeutic effects of Dipterocarpus tuberculatus with high antioxidative activity against UV-induced photoaging of NHDF cells and nude mice. Antioxidants 2021, 10, 791. [Google Scholar] [CrossRef]
- Lee, N.R.; Lee, S.M.; Cho, K.S.; Jeong, S.Y.; Hwang, D.Y.; Kim, D.S.; Hong, C.O.; Son, H.J. Improved production of poly-γ-glutamic Acid by Bacillus subtilis D7 isolated from Doenjang, a Korean traditional fermented food, and its antioxidant activity. Appl. Biochem. Biotechnol. 2014, 173, 918–932. [Google Scholar] [CrossRef] [PubMed]
- Green, L.C.; Wagner, D.A.; Glogowski, J.; Skipper, P.L.; Wishnok, J.S.; Tannenbaum, S.R. Analysis of nitrate, nitrite, and [15N] nitrate in biological fluids. Anal. Biochem. 1982, 126, 131–138. [Google Scholar] [CrossRef] [PubMed]
- Jaiswal, Y.S.; Tatke, P.A.; Gabhe, S.Y.; Ashok, V. Antioxidant activity of various extracts of leaves of Anacardium occidentale (cashew). Res. J. Pharm. Biol. Chem. Sci. 2010, 1, 112–119. [Google Scholar]
- Oranje, A.P.; Glazenburg, E.J.; Wolkerstorfer, A.; De Waard-van der Spek, F.B. Practical issues on interpretation of scoring atopic dermatitis: The SCORAD index, objective SCORAD and the three-item severity score. Br. J. Dermatol. 2007, 157, 645–648. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, J.; Kim, J.E.; Jin, Y.J.; Roh, Y.J.; Song, H.J.; Seol, A.; Park, S.H.; Seo, S.; Lee, H.; Hwang, D.Y. Anti-Atopic Dermatitis Effects of Abietic Acid Isolated from Rosin under Condition Optimized by Response Surface Methodology in DNCB-Spread BALB/c Mice. Pharmaceuticals 2023, 16, 407. https://doi.org/10.3390/ph16030407
Park J, Kim JE, Jin YJ, Roh YJ, Song HJ, Seol A, Park SH, Seo S, Lee H, Hwang DY. Anti-Atopic Dermatitis Effects of Abietic Acid Isolated from Rosin under Condition Optimized by Response Surface Methodology in DNCB-Spread BALB/c Mice. Pharmaceuticals. 2023; 16(3):407. https://doi.org/10.3390/ph16030407
Chicago/Turabian StylePark, Jumin, Ji Eun Kim, You Jeong Jin, Yu Jeong Roh, Hee Jin Song, Ayun Seol, So Hae Park, Sungbaek Seo, Heeseob Lee, and Dae Youn Hwang. 2023. "Anti-Atopic Dermatitis Effects of Abietic Acid Isolated from Rosin under Condition Optimized by Response Surface Methodology in DNCB-Spread BALB/c Mice" Pharmaceuticals 16, no. 3: 407. https://doi.org/10.3390/ph16030407
APA StylePark, J., Kim, J. E., Jin, Y. J., Roh, Y. J., Song, H. J., Seol, A., Park, S. H., Seo, S., Lee, H., & Hwang, D. Y. (2023). Anti-Atopic Dermatitis Effects of Abietic Acid Isolated from Rosin under Condition Optimized by Response Surface Methodology in DNCB-Spread BALB/c Mice. Pharmaceuticals, 16(3), 407. https://doi.org/10.3390/ph16030407





