In Vitro Hepatic Models to Assess Herb–Drug Interactions: Approaches and Challenges
Abstract
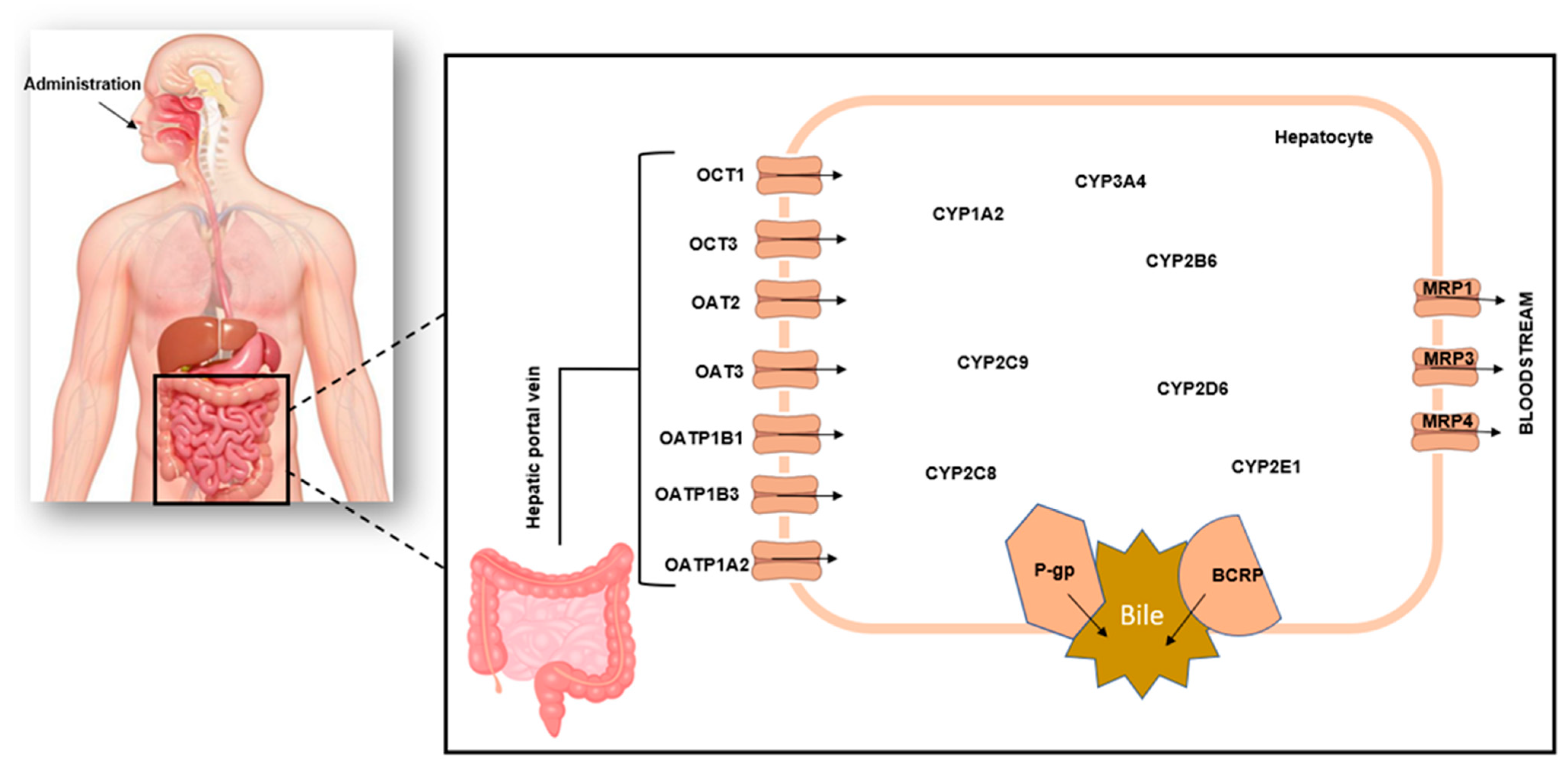
1. Introduction
2. Research Methodology and Search Strategy
3. Overview of Liver Models Used in Herb–Drug Interaction Studies
4. Recombinant Cytochrome P450 Enzymes
5. Human Liver Microsomes (HLM), S9 Fractions, and Cytosolic Liver Fractions
| Type of Liver Model | Advantages | Disadvantages/Limitations | Advancements/What Is New in the Field? | References |
|---|---|---|---|---|
| PHH | Maintain original structure and liver-specific functions in vivo, “gold standard.” | Significant batch-to-batch variation, limited availability, short lifespan. | Stem cell-derived hepatocytes. | [24,25] |
| PCLS | Contains all the cells of liver tissue in their natural environment. | Fierce competition for organ donors in research, short lifespan. | Long-term PCLS as “pre-clinical test.” | [26,27] |
| S9 Fractions | High throughput in vitro screening model, more readily available than hepatocytes. | Lack of major phase II metabolizing enzymes. | Organoids. | [21] |
| HLM | Model for high-throughput in vitro screening, greater availability than hepatocytes. | Lack of stability for long-term culture. | Organoids. | [21] |
| LFC | In vitro high-throughput screening model, greater availability than hepatocytes. | Lack of major phase I and phase II metabolizing enzymes. | Organoids. | [23,27] |
| HEPG2/C3A cells | Easily attainable, cost effective. | 2D monocultures show low expression of major CYPs. | 3D culture HEPG2/C3A liver spheroids. | [28] |
| 3D culture liver spheroids | Reproducibility of results from long-term drug/herbal treatment. | 3D spheroids expert skill. | 3D bioprinting technology/ liver organ-on-chip. | [9,24,29] |
| Liver organ-on-chip models | Real-time monitoring, high-throughput screening, cost-effective when compared to animals. | Limited functionality, short lifespan, lack of standardization. | 3D bioprinting technology. | [30,31,32] |
6. Precision-Cut Liver Slices
7. Primary Hepatocytes
7.1. HepG2/C3A Cells
7.2. 3D Cultured Liver Spheroids
8. Liver Organ-on-Chip Models
9. Discussion
10. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Williamson, E.M.; Liu, X.; Izzo, A.A. Trends in use, pharmacology, and clinical applications of emerging herbal nutraceuticals. Br. J. Pharmacol. 2020, 177, 1227–1240. [Google Scholar] [CrossRef]
- Acedhars Unilag COVID-19 Response Team; Akindele, A.J.; Agunbiade, F.O.; Sofidiya, M.O.; Awodele, O.; Sowemimo, A.; Ade-Ademilua, O.; Akinleye, M.O.; Ishola, I.O.; Orabueze, I.; et al. COVID-19 pandemic: A case for phytomedicines. Nat. Prod. Commun. 2020, 15, 1934578X20945086. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y. Use of herbal drugs to treat COVID-19 should be with caution. Lancet 2020, 395, 1689–1690. [Google Scholar] [CrossRef] [PubMed]
- Lazarou, J.; Pomeranz, B.H.; Corey, P.N. Incidence of adverse drug reactions in hospitalized patients: A meta-analysis of prospective studies. J. Am. Med. Assoc. 1998, 279, 1200–1205. [Google Scholar] [CrossRef] [PubMed]
- Gardiner, P.; Phillips, R.; Shaughnessy, A.F. Herbal and dietary supplement–drug interactions: A systematic review and meta-analysis. J. Clin. Pharmacol. 2008, 48, 768–776. [Google Scholar] [CrossRef]
- Shi, S.; Klotz, U. Drug interactions with herbal medicines. Clin. Pharmacokinet. 2012, 51, 77–104. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.Y.; Lee, J.Y.; Kang, W.; Kwon, K.I.; Park, S.K.; Oh, S.J.; Ma, J.Y.; Kim, S.K. Cytochrome P450-mediated herb–drug interaction potential of Galgeun-tang. Food Chem. Toxicol. 2013, 51, 343–349. [Google Scholar] [CrossRef]
- Kang, H.K.; Sarsenova, M.; Kim, D.H.; Kim, M.S.; Lee, J.Y.; Sung, E.A.; Kook, M.G.; Kim, N.G.; Choi, S.W.; Ogay, V.; et al. Establishing a 3D in vitro hepatic model mimicking physiologically relevant to in vivo state. Cells 2021, 10, 1268. [Google Scholar] [CrossRef]
- Bhise, N.S.; Manoharan, V.; Massa, S.; Tamayol, A.; Ghaderi, M.; Miscuglio, M.; Lang, Q.; Zhang, Y.S.; Shin, S.R.; Calzone, G.; et al. A liver-on-a-chip platform with bioprinted hepatic spheroids. Biofabrication 2016, 8, 014101. [Google Scholar] [CrossRef]
- Ehrlich, A.; Duche, D.; Ouedraogo, G.; Nahmias, Y. Challenges and opportunities in the design of liver-on-chip microdevices. Annu. Rev. Biomed. Eng. 2019, 21, 219–239. [Google Scholar] [CrossRef]
- Donato, M.T.; Castell, J.V. Strategies and molecular probes to investigate the role of cytochrome P450 in drug metabolism. Clin. Pharmacokinet. 2003, 42, 153–178. [Google Scholar] [CrossRef] [PubMed]
- Kishida, T.; Muto, S.I.; Hayashi, M.; Tsutsui, M.; Tanaka, S.; Murakami, M.; Kuroda, J. Strain differences in hepatic cytochrome P450 1A and 3A expression between Sprague-Dawley and Wistar rats. J. Toxicol. Sci. 2008, 33, 447–457. [Google Scholar] [CrossRef] [PubMed]
- Clarke, S.E. In vitro assessment of human cytochrome P450. Xenobiotica 1998, 28, 1167–1202. [Google Scholar] [CrossRef]
- Choi, Y.H. Interpretation of drug interaction using systemic and local tissue exposure changes. Pharmaceutics 2020, 12, 417. [Google Scholar] [CrossRef] [PubMed]
- Crespi, C.L. Xenobiotic-metabolizing human cells as tools for pharmacological and toxicological research. In Advances in Drug Research; Academic Press: Cambridge, MA, USA, 1995; Volume 26, pp. 179–235. [Google Scholar] [CrossRef]
- Iwatsubo, T.; Suzuki, H.; Shimada, N.; Chiba, K.; Ishizaki, T.; Green, C.E.; Tyson, C.A.; Yokoi, T.; Kamataki, T.; Sugiyama, Y. Prediction of in vivo hepatic metabolic clearance of YM796 from in vitro data by use of human liver microsomes and recombinant P-450 isozymes. J. Pharmacol. Exp. Ther. 1997, 282, 909–919. [Google Scholar] [PubMed]
- Yamamoto, T.; Itoga, H.; Kohno, Y.; Nagata, K.; Yamazoe, Y. Prediction of oral clearance from in vitro metabolic data using recombinant CYPs: Comparison among well-stirred, parallel-tube, distributed and dispersion models. Xenobiotica 2005, 35, 627–646. [Google Scholar] [CrossRef]
- Stringer, R.A.; Strain-Damerell, C.; Nicklin, P.; Houston, J.B. Evaluation of recombinant cytochrome P450 enzymes as an in vitro system for metabolic clearance predictions. Drug Metab. Dispos. 2009, 37, 1025–1034. [Google Scholar] [CrossRef] [PubMed]
- Knights, K.M.; Stresser, D.M.; Miners, J.O.; Crespi, C.L. In vitro drug metabolism using liver microsomes. Curr. Protoc. Pharmacol. 2016, 74, 7–8. [Google Scholar] [CrossRef]
- Jia, L.; Liu, X. The conduct of drug metabolism studies considered good practice (II): In vitro experiments. Curr. Drug Metab. 2007, 8, 822–829. [Google Scholar] [CrossRef]
- Richardson, S.J.; Bai, A.; Kulkarni, A.A.; Moghaddam, M.F. Efficiency in drug discovery: Liver S9 fraction assay as a screen for metabolic stability. Drug Metab. Lett. 2016, 10, 83–90. [Google Scholar] [CrossRef]
- Gajula, S.N.R.; Vora, S.A.; Dikundwar, A.G.; Sonti, R. In Vitro Drug Metabolism Studies Using Human Liver Microsomes; Intechopen: London, UK, 2022. [Google Scholar] [CrossRef]
- Esterbauer, H.; Zollner, H.; Lang, J. Metabolism of the lipid peroxidation product 4-hydroxynonenal by isolated hepatocytes and by liver cytosolic fractions. Biochem. J. 1985, 228, 363–373. [Google Scholar] [CrossRef] [PubMed]
- Basharat, A.; Rollison, H.E.; Williams, D.P.; Ivanov, D.P. HepG2 (C3A) spheroids show higher sensitivity compared to HepaRG spheroids for drug-induced liver injury (DILI). Toxicol. Appl. Pharmacol. 2020, 408, 115279. [Google Scholar] [CrossRef] [PubMed]
- Jin, M.; Yi, X.; Liao, W.; Chen, Q.; Yang, W.; Li, Y.; Li, S.; Gao, Y.; Peng, Q.; Zhou, S. Advancements in stem cell-derived hepatocyte-like cell models for hepatotoxicity testing. Stem Cell Res. Ther. 2021, 12, 84. [Google Scholar] [CrossRef] [PubMed]
- de Graaf, I.A.; Olinga, P.; De Jager, M.H.; Merema, M.T.; De Kanter, R.; Van De Kerkhof, E.G.; Groothuis, G.M. Preparation and incubation of precision-cut liver and intestinal slices for application in drug metabolism and toxicity studies. Nat. Protoc. 2010, 5, 1540–1551. [Google Scholar] [CrossRef]
- Othman, A.; Ehnert, S.; Dropmann, A.; Ruoß, M.; Nüssler, A.K.; Hammad, S. Precision-cut liver slices as an alternative method for long-term hepatotoxicity studies. Arch. Toxicol. 2020, 94, 2889–2891. [Google Scholar] [CrossRef] [PubMed]
- Godoy, P.; Hewitt, N.J.; Albrecht, U.; Andersen, M.E.; Ansari, N.; Bhattacharya, S.; Bode, J.G.; Bolleyn, J.; Borner, C.; Böttger, J.; et al. Recent advances in 2D and 3D in vitro systems using primary hepatocytes, alternative hepatocyte sources and non-parenchymal liver cells and their use in investigating mechanisms of hepatotoxicity, cell signaling and ADME. Arch. Toxicol. 2013, 87, 1315–1530. [Google Scholar] [CrossRef]
- Wrzesinski, K.; Magnone, M.C.; Hansen, L.V.; Kruse, M.E.; Bergauer, T.; Bobadilla, M.; Gubler, M.; Mizrahi, J.; Zhang, K.; Andreasen, C.M.; et al. HepG2/C3A 3D spheroids exhibit stable physiological functionality for at least 24 days after recovering from trypsinisation. Toxicol. Res. 2013, 2, 163–172. [Google Scholar] [CrossRef]
- Moradi, E.; Jalili-Firoozinezhad, S.; Solati-Hashjin, M. Microfluidic organ-on-a-chip models of human liver tissue. Acta Biomater. 2020, 116, 67–83. [Google Scholar] [CrossRef]
- Polidoro, M.A.; Ferrari, E.; Marzorati, S.; Lleo, A.; Rasponi, M. Experimental liver models: From cell culture techniques to microfluidic organs-on-chip. Liver Int. 2021, 41, 1744–1761. [Google Scholar] [CrossRef]
- Messelmani, T.; Morisseau, L.; Sakai, Y.; Legallais, C.; Le Goff, A.; Leclerc, E.; Jellali, R. Liver organ-on-chip models for toxicity studies and risk assessment. Lab Chip 2022, 22, 2423–2450. [Google Scholar] [CrossRef]
- Dewyse, L.; Reynaert, H.; van Grunsven, L.A. Best practices and progress in precision-cut liver slice cultures. Int. J. Mol. Sci. 2021, 22, 7137. [Google Scholar] [CrossRef] [PubMed]
- Starokozhko, V.; Abza, G.B.; Maessen, H.C.; Merema, M.T.; Kuper, F.; Groothuis, G.M. Viability, function and morphological integrity of precision-cut liver slices during prolonged incubation: Effects of culture medium. Toxicol. In Vitro 2015, 30, 288–299. [Google Scholar] [CrossRef] [PubMed]
- Dallas, S.; Sensenhauser, C.; Batheja, A.; Singer, M.; Markowska, M.; Zakszewski, C.; NVSMamidi, R.; McMillia, M.; Han, C.; Zhou, H.; et al. De-risking bio-therapeutics for possible drug interactions using cryopreserved human hepatocytes. Curr. Drug Metab. 2012, 13, 923–929. [Google Scholar] [CrossRef] [PubMed]
- Tucker, G.T.; Houston, J.B.; Huang, S.M. Optimizing drug development: Strategies to assess drug metabolism/transporter interaction potential–towards a consensus. Pharm. Res. 2001, 18, 1071–1080. [Google Scholar] [CrossRef] [PubMed]
- Knowles, B.B.; Howe, C.C.; Aden, D.P. Human hepatocellular carcinoma cell lines secrete the major plasma proteins and hepatitis B surface antigen. Science 1980, 209, 497–499. [Google Scholar] [CrossRef] [PubMed]
- Elaut, G.; Henkens, T.; Papeleu, P.; Snykers, S.; Vinken, M.; Vanhaecke, T.; Rogiers, V. Molecular mechanisms underlying the dedifferentiation process of isolated hepatocytes and their cultures. Curr. Drug Metab. 2006, 7, 629–660. [Google Scholar] [CrossRef]
- Dixit, R.; David, F.S. Market watch: Trends in pharmaceutical company R&D spending: 2005–2015. Nat. Rev. Drug Discov. 2017, 16, 376–377. [Google Scholar] [CrossRef]
- Enosawa, S.; Miyashita, T.; Fujita, Y.; Suzuki, S.; Amemiya, H.; Omasa, T.; Hiramatsu, S.; Suga, K.; Matsumura, T. In vivo estimation of bioartificial liver with recombinant HepG2 cells using pigs with ischemic liver failure. Cell Transplant. 2001, 10, 429–433. [Google Scholar] [CrossRef]
- Fey, S.J.; Wrzesinski, K. Determination of drug toxicity using 3D spheroids constructed from an immortal human hepatocyte cell line. Toxicol. Sci. 2012, 127, 403–411. [Google Scholar] [CrossRef]
- Štampar, M.; Frandsen, H.S.; Rogowska-Wrzesinska, A.; Wrzesinski, K.; Filipič, M.; Žegura, B. Hepatocellular carcinoma (HepG2/C3A) cell-based 3D model for genotoxicity testing of chemicals. Sci. Total Environ. 2021, 755, 143255. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
N., H.; C., M.; T. R., M.; S., S.; S., N.; K. E., M.; S. C., S.; Y., N.; P. V., D.; R. N., M. In Vitro Hepatic Models to Assess Herb–Drug Interactions: Approaches and Challenges. Pharmaceuticals 2023, 16, 409. https://doi.org/10.3390/ph16030409
N. H, C. M, T. R. M, S. S, S. N, K. E. M, S. C. S, Y. N, P. V. D, R. N. M. In Vitro Hepatic Models to Assess Herb–Drug Interactions: Approaches and Challenges. Pharmaceuticals. 2023; 16(3):409. https://doi.org/10.3390/ph16030409
Chicago/Turabian StyleN., Hlengwa, Masilela C., Mtambo T. R., Sithole S., Naidoo S., Machaba K. E., Shabalala S. C., Ntamo Y., Dludla P. V., and Milase R. N. 2023. "In Vitro Hepatic Models to Assess Herb–Drug Interactions: Approaches and Challenges" Pharmaceuticals 16, no. 3: 409. https://doi.org/10.3390/ph16030409
APA StyleN., H., C., M., T. R., M., S., S., S., N., K. E., M., S. C., S., Y., N., P. V., D., & R. N., M. (2023). In Vitro Hepatic Models to Assess Herb–Drug Interactions: Approaches and Challenges. Pharmaceuticals, 16(3), 409. https://doi.org/10.3390/ph16030409






