Asymmetric Synthesis of US-FDA Approved Drugs over Five Years (2016–2020): A Recapitulation of Chirality
Abstract
1. Introduction
1.1. Background
1.2. Chirality in FDA Drugs
2. Methodology
- Chiral pool approach: Synthesis from naturally occurring or chiral substrates;
- Chiral resolution: Resolving the racemic mixture at any stage of synthesis;
- Asymmetric synthesis: By adopting a chiral auxiliary, a chiral catalyst, or a chiral reagent.
3. Discussion
3.1. Drugs with One Chiral Center
3.1.1. Lifitegrast (2016)
3.1.2. Acalabrutinib (2017)
3.1.3. Pemafibrate (2017)
3.1.4. Letermovir (2017)
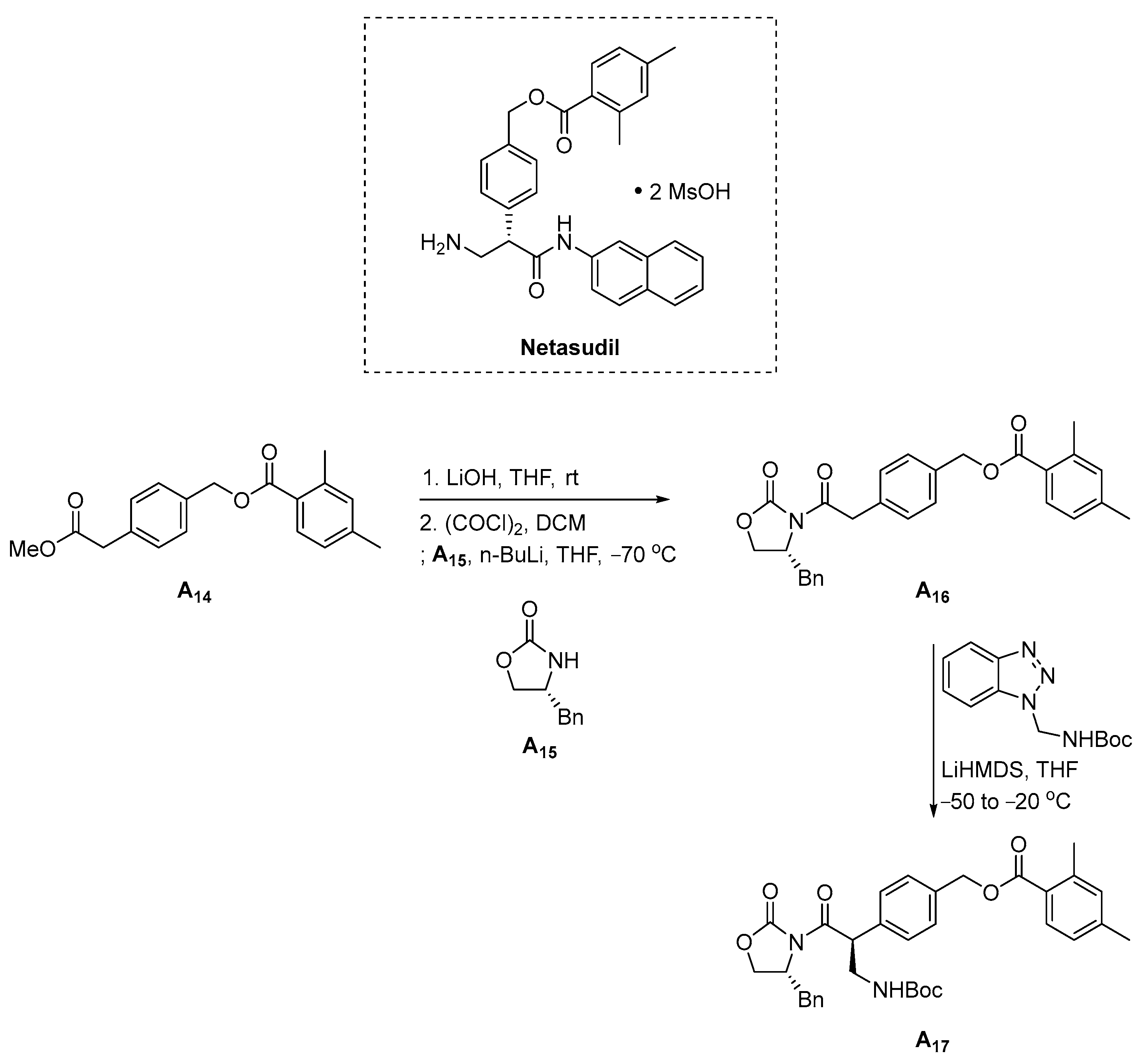
3.1.5. Netarsudil (2017)
3.1.6. Niraparib (2017)
3.1.7. Lorlatinib (2018)
3.1.8. Elobixibat Hydrate (2018)
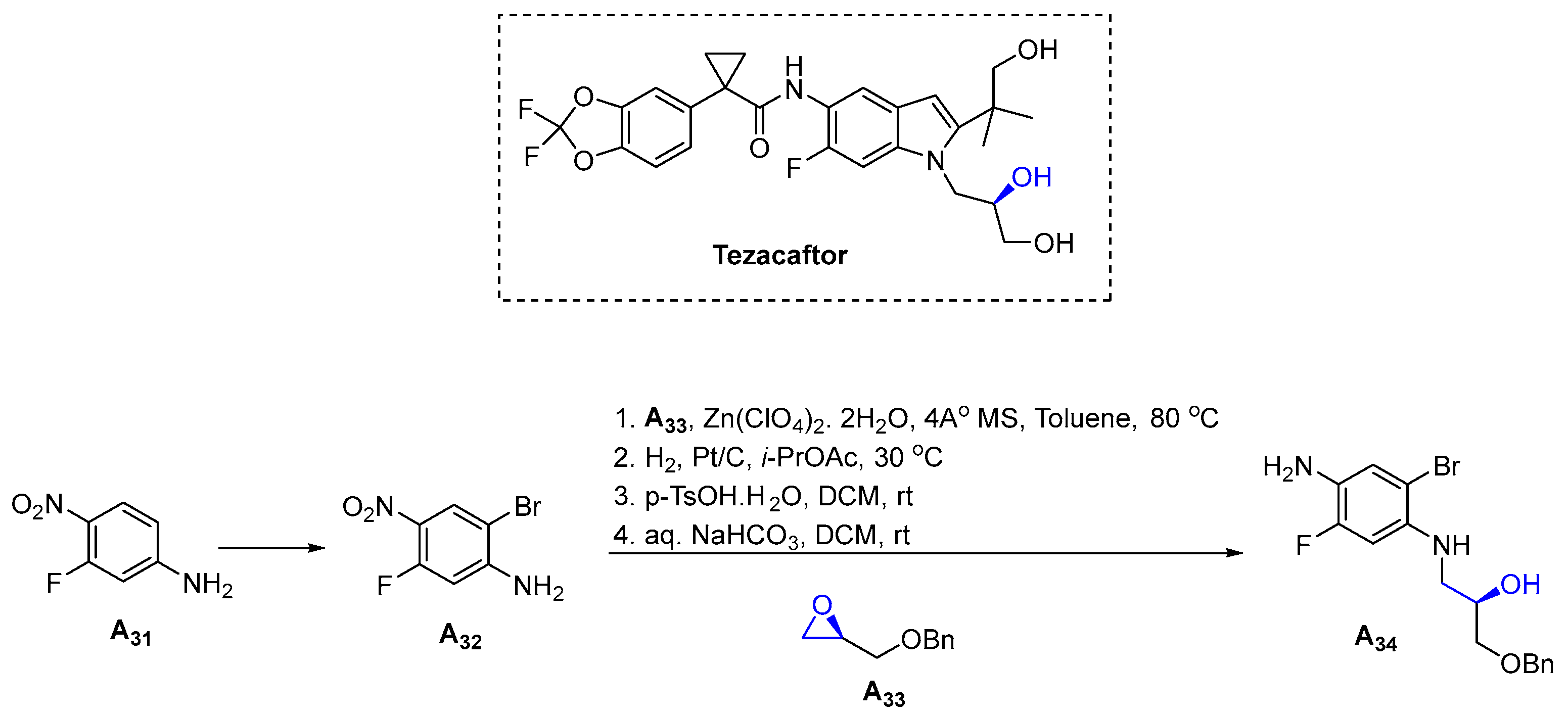
3.1.9. Tezacaftor (2018)
3.1.10. Pyrotinib Maleate (2018)
3.1.11. Encorafenib (2018)
3.1.12. Duvelisib Monohydrate (2018)
3.1.13. Elagolix Sodium (2018)
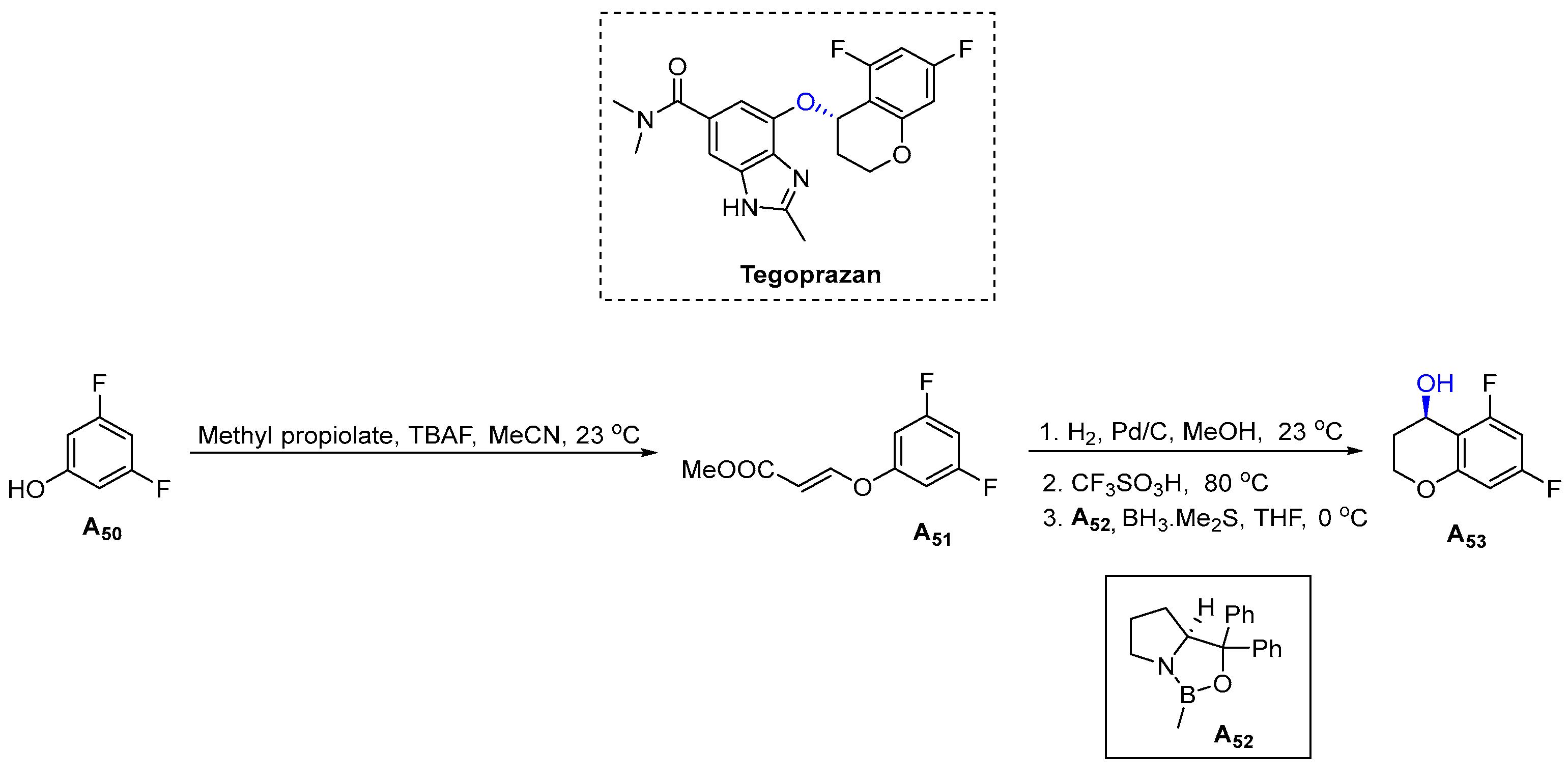
3.1.14. Tegoprazan (2018)
3.1.15. Alpelisib (2019)
3.1.16. Solriamfetol (2019)
3.1.17. Pretomanid (2019)
3.1.18. Zanubrutinib (2019)
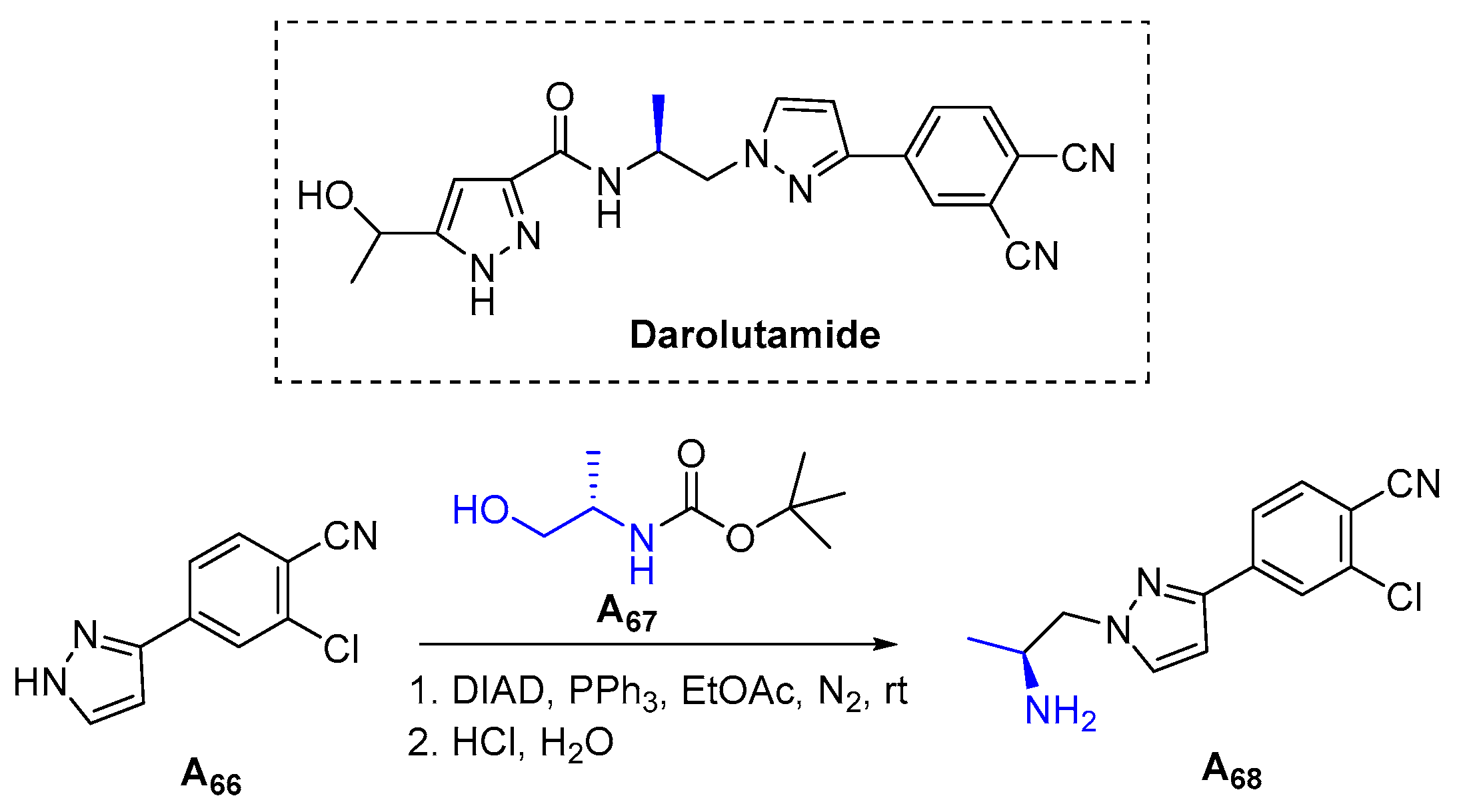
3.1.19. Darolutamide (2019)
3.1.20. Cenobamate (2019)
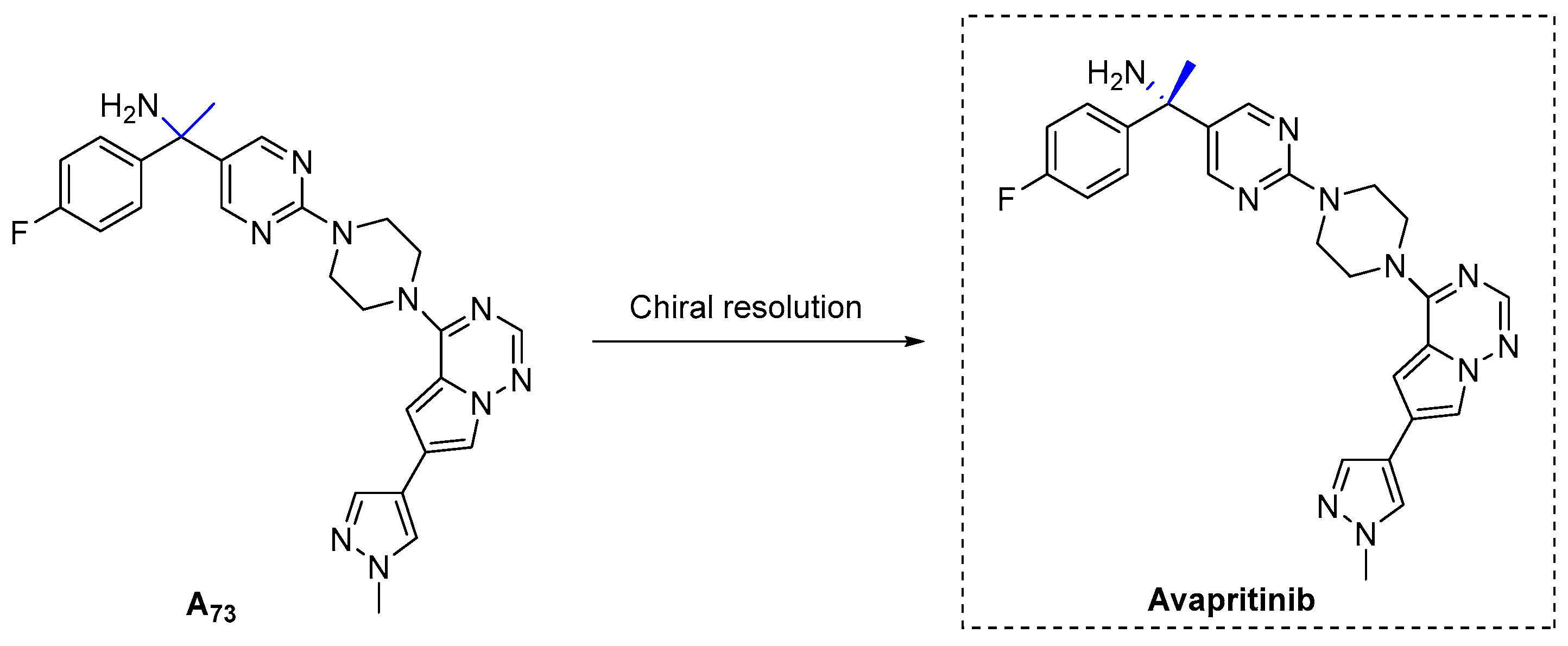
3.1.21. Avapritinib (2020)
3.1.22. Berotralstat (2020)
3.1.23. Lonafarnib (2020)
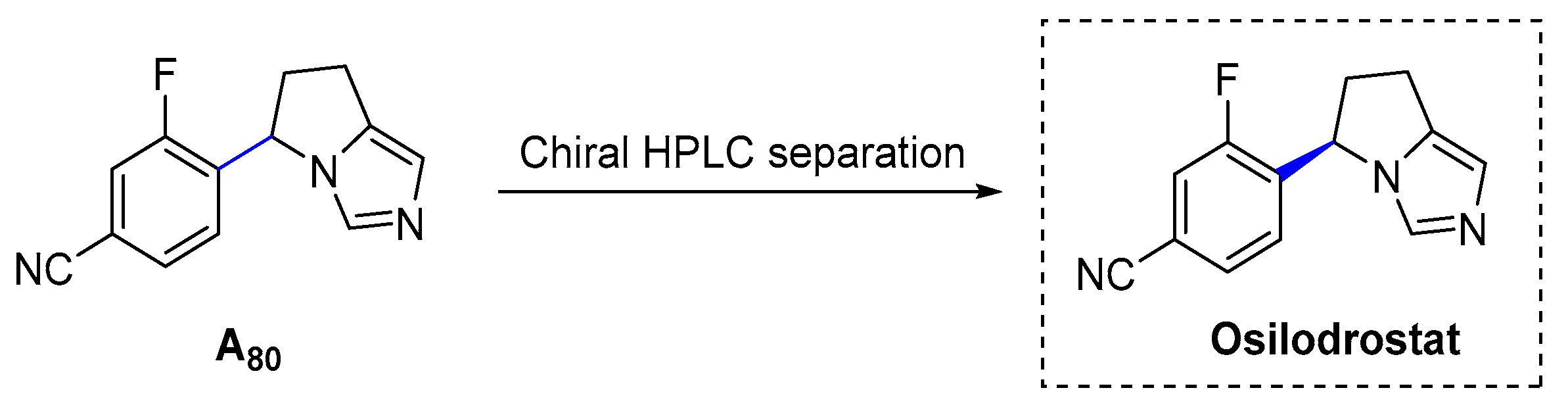
3.1.24. Osilodrostat (2020)
3.1.25. Oliceridine (2020)
3.1.26. Remimazolam (2020)
3.1.27. Ozanimod (2020)
3.2. Drugs with Two Chiral Centers
3.2.1. Nemonoxacin (2016)
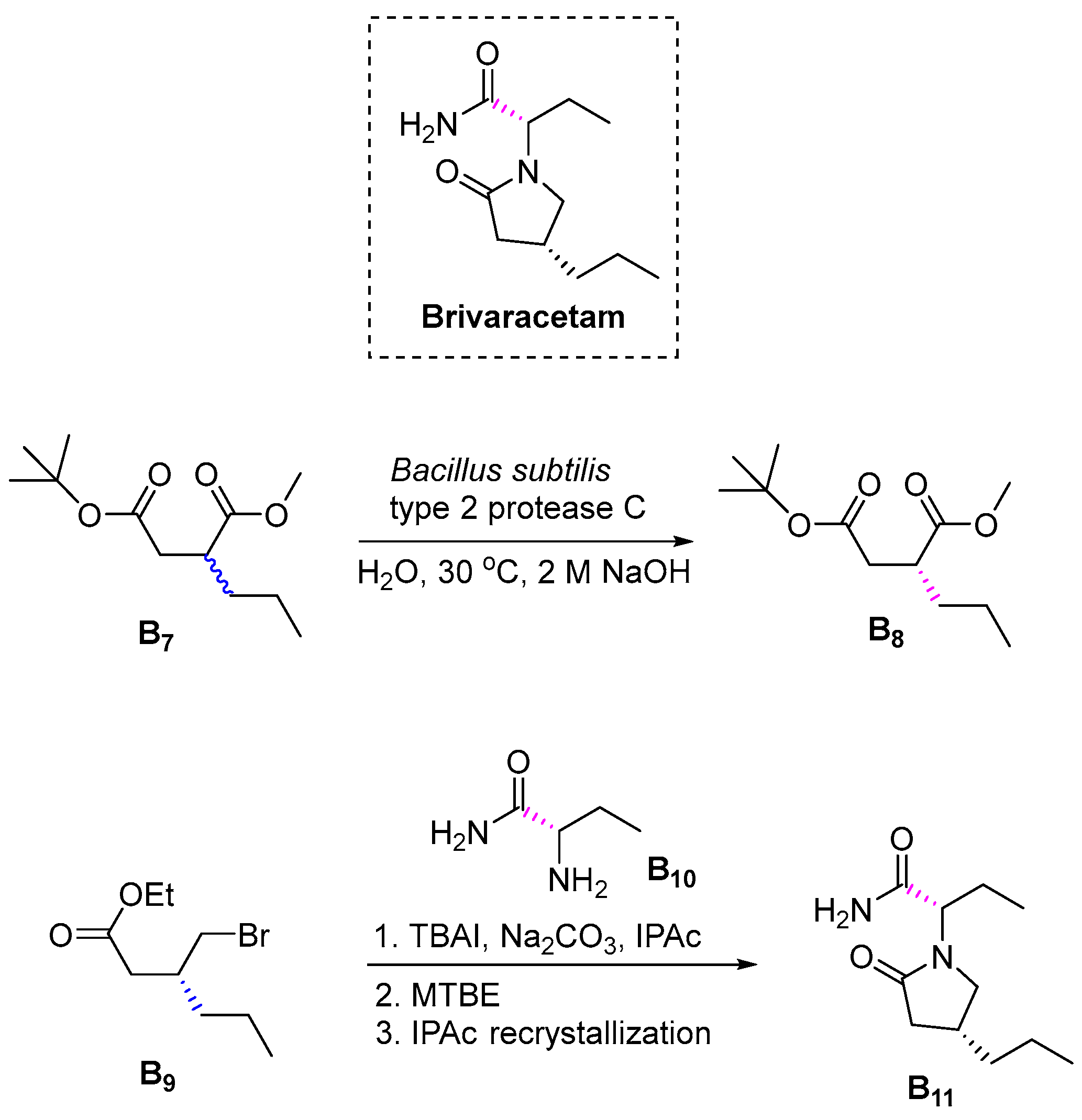
3.2.2. Brivaracetam (2016)
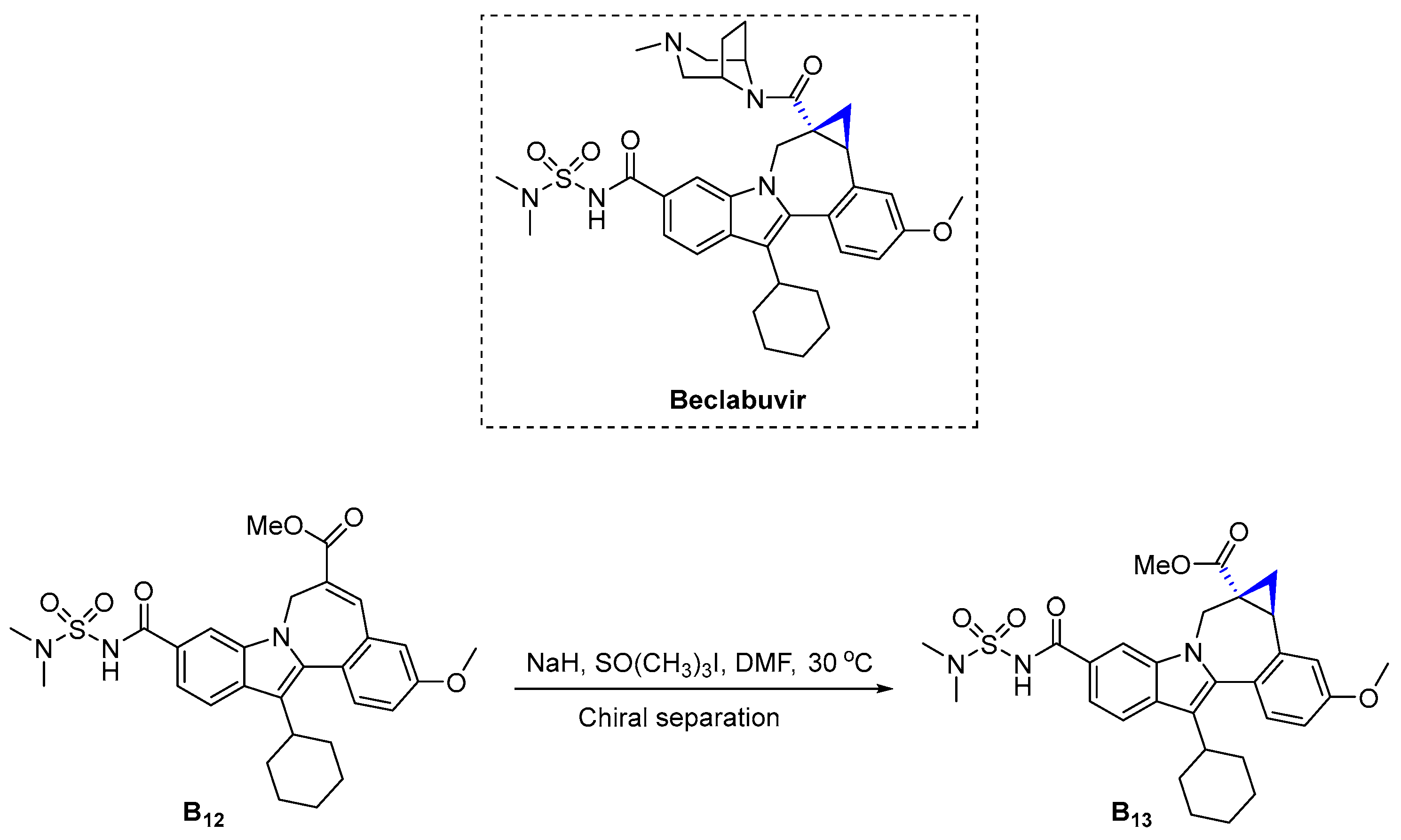
3.2.3. Beclabuvir (2016)
3.2.4. Inotuzumab Ozogamicin (2017)
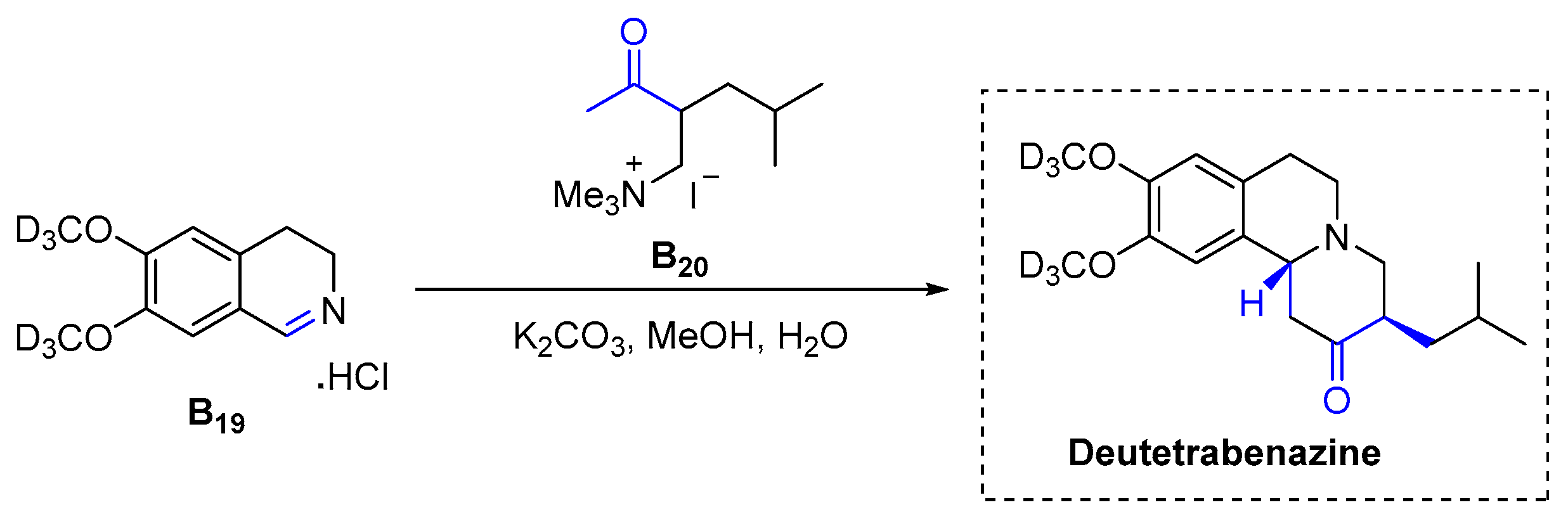
3.2.5. Deutetrabenazine (2017)
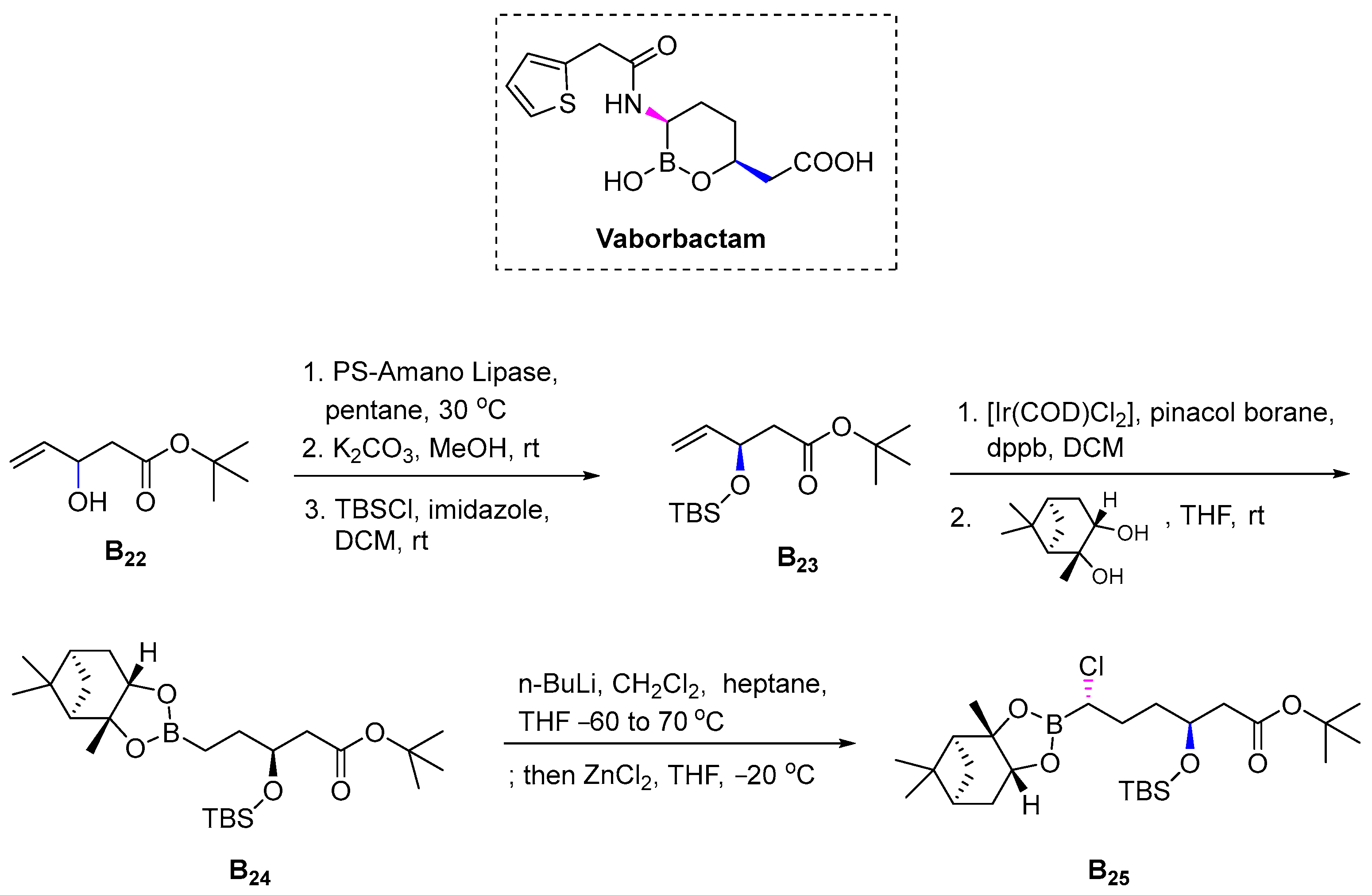
3.2.6. Vaborbactam (2017)
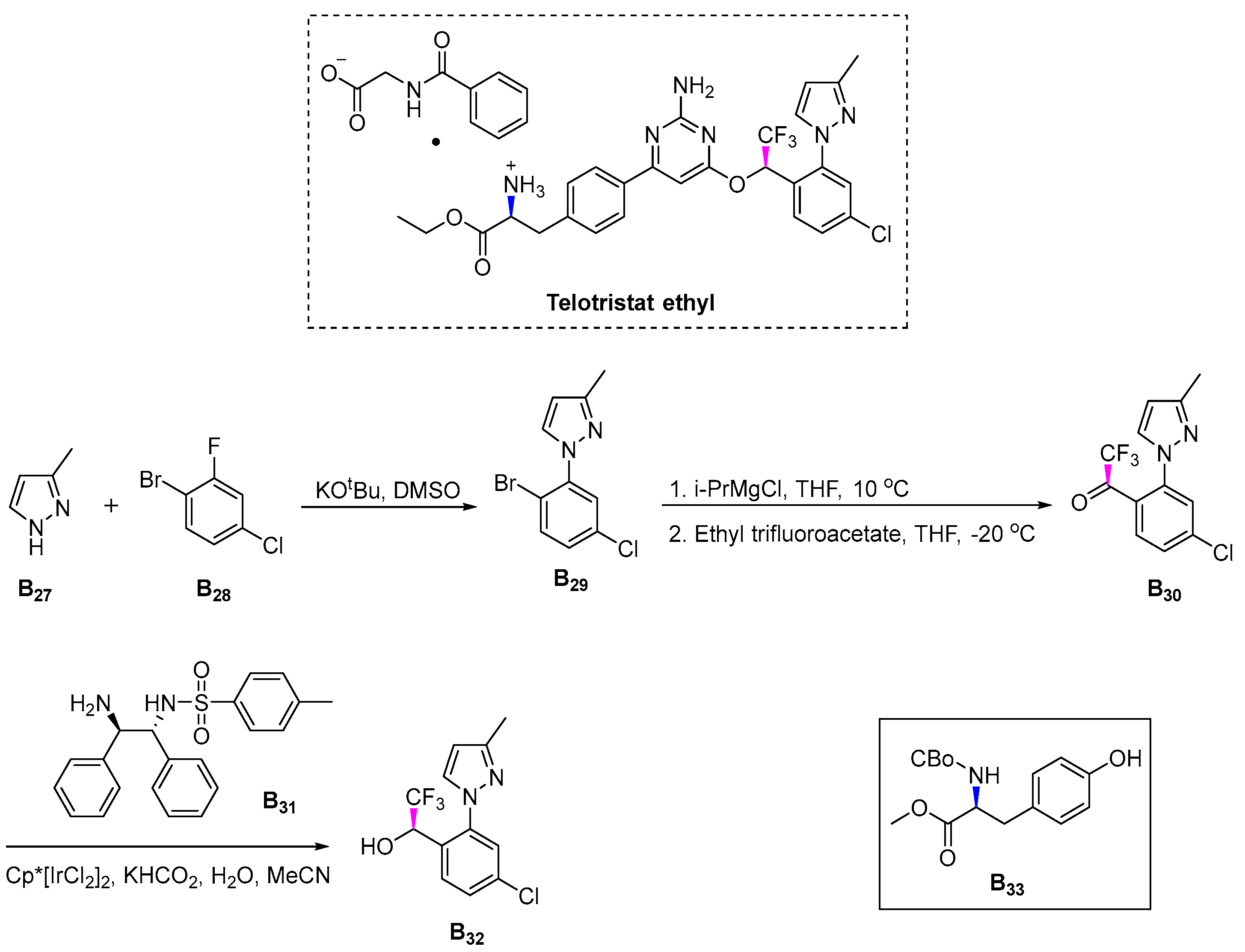
3.2.7. Telotristat Ethyl (2017)
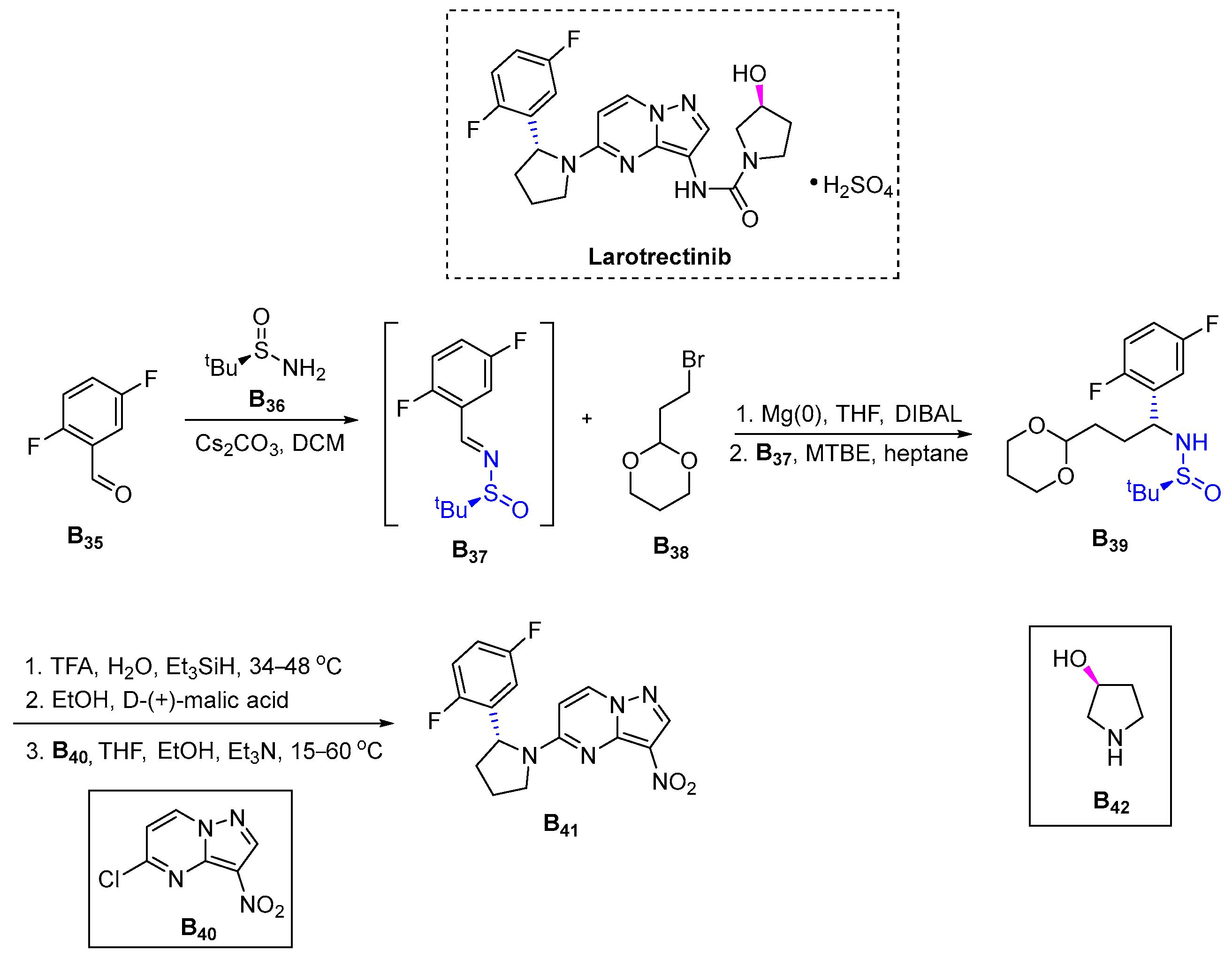
3.2.8. Larotrectinib (2018)
3.2.9. Glasdegib (2018)
3.2.10. Talazoparib (2018)
3.2.11. Ivosidenib (2018)
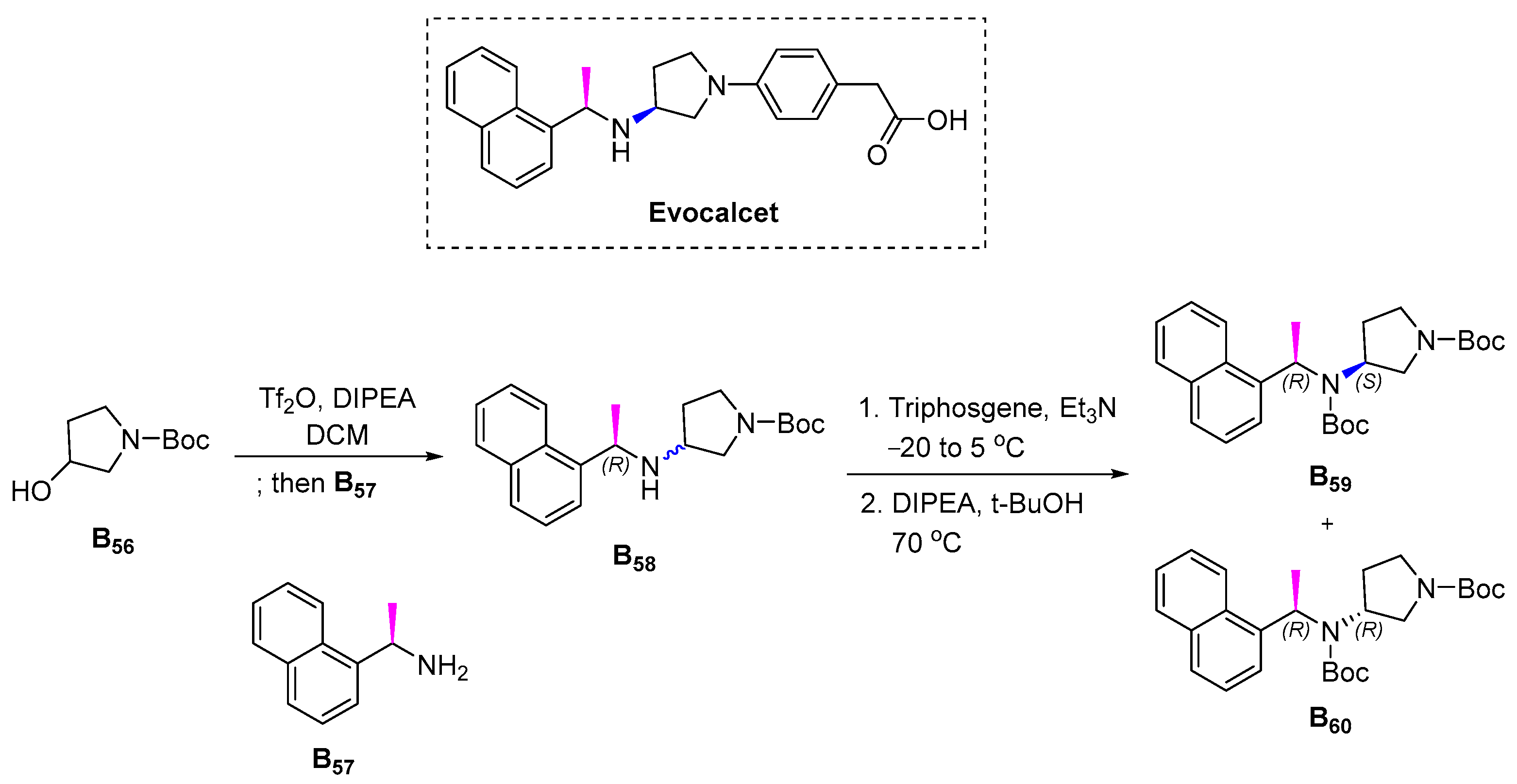
3.2.12. Evocalcet (2018)
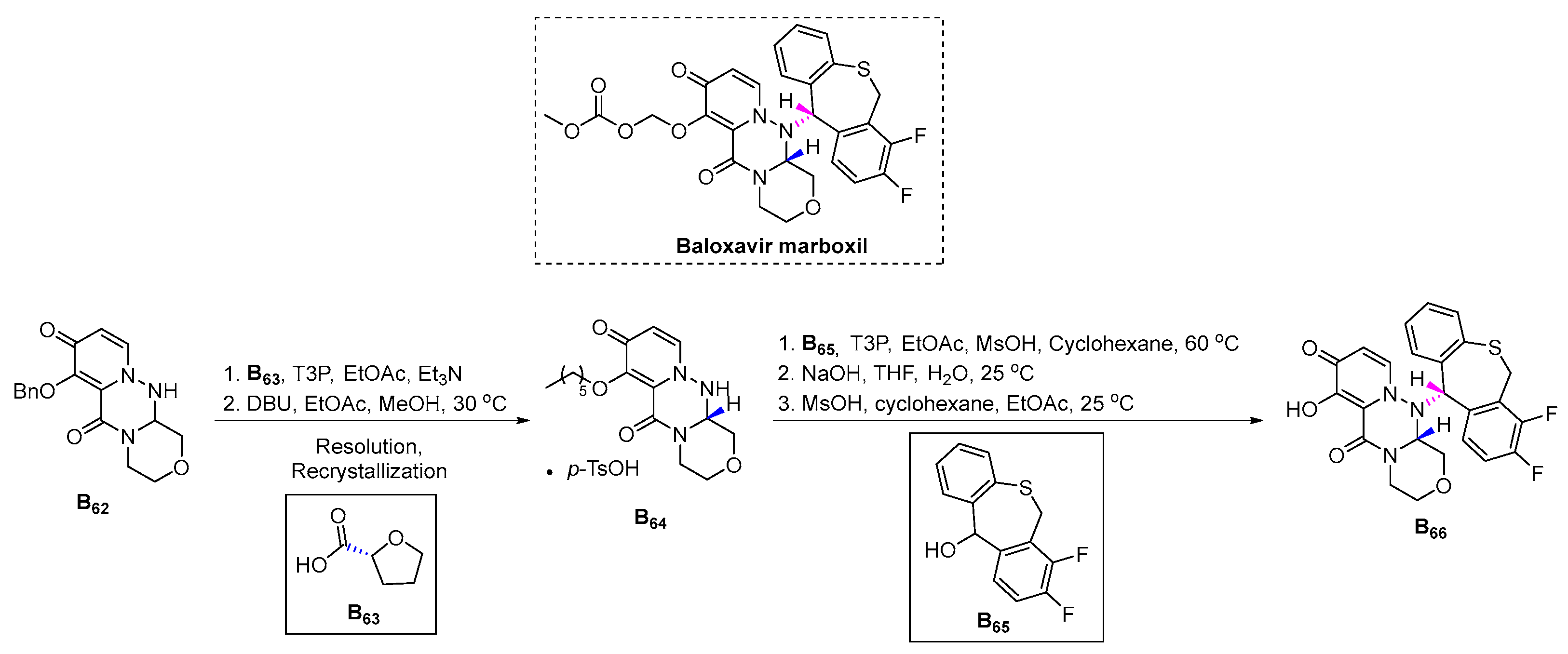
3.2.13. Baloxavir Marboxil (2018)
3.2.14. Fosravuconazole l-Lysine Ethanolate (2018)
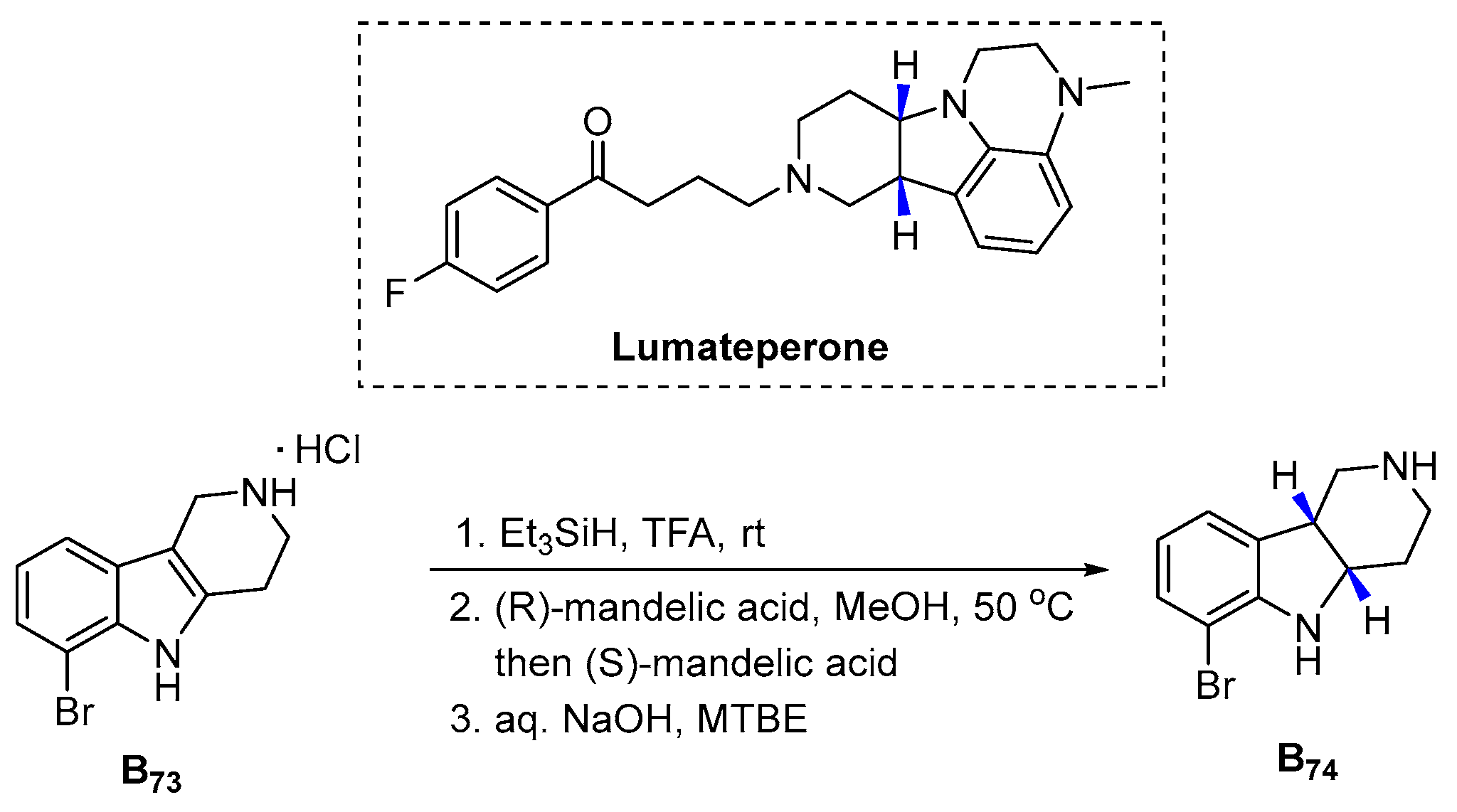
3.2.15. Lumateperone (2019)
3.2.16. Tenapanor (2019)
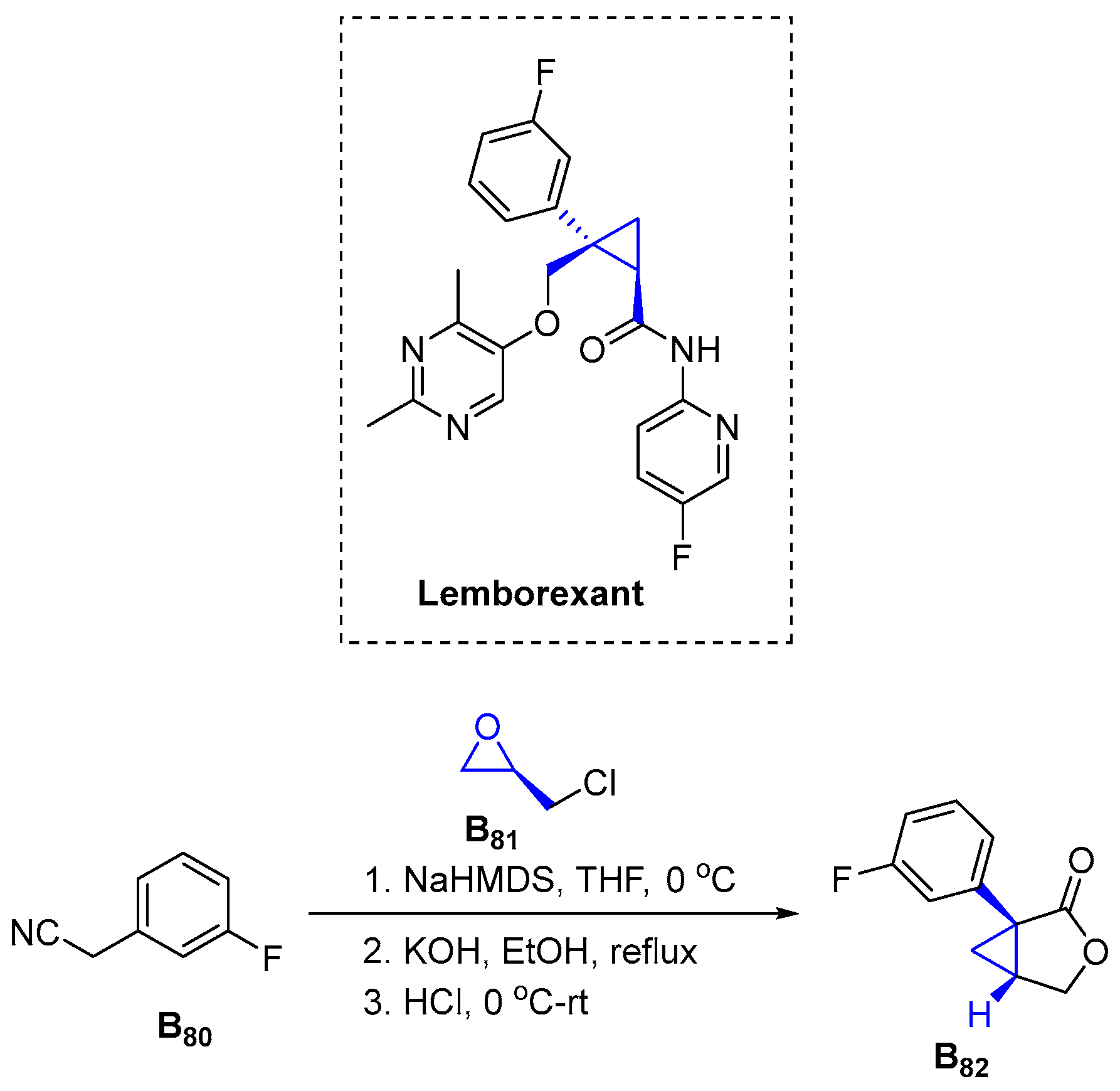
3.2.17. Lemborexant (2019)
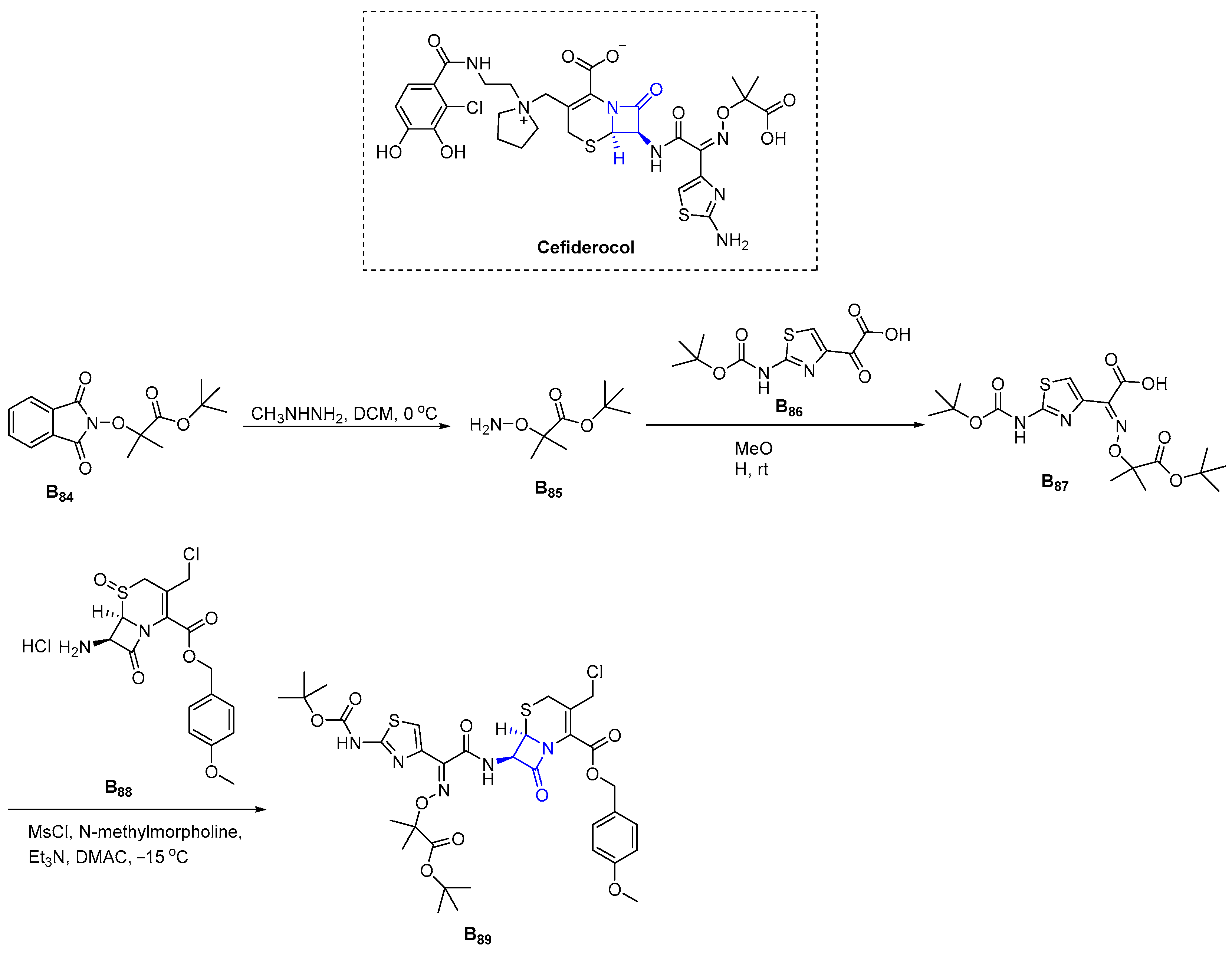
3.2.18. Cefiderocol (2019)
3.2.19. Upadacitinib (2019)
3.3. Drugs with Three Chiral Centers
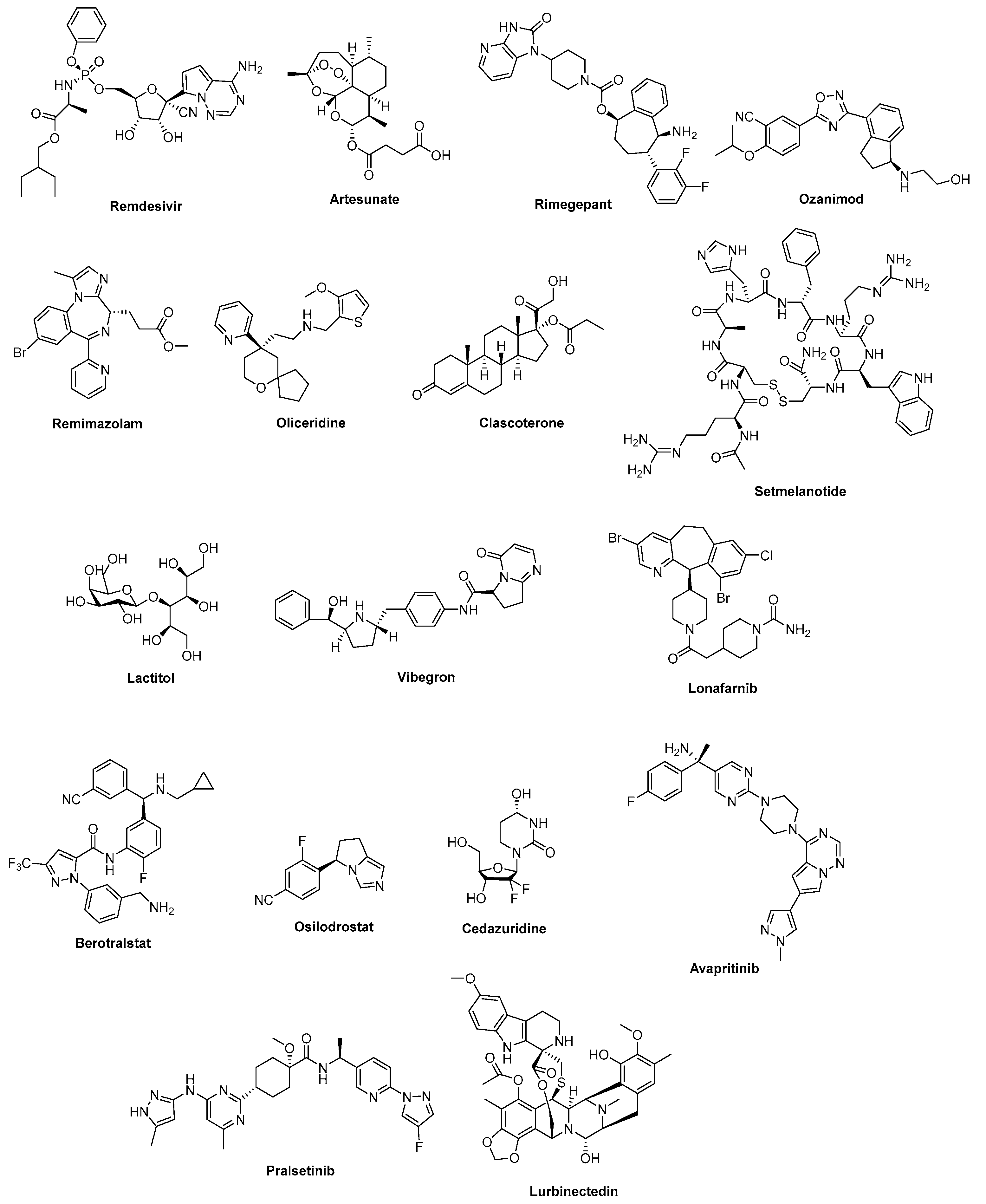
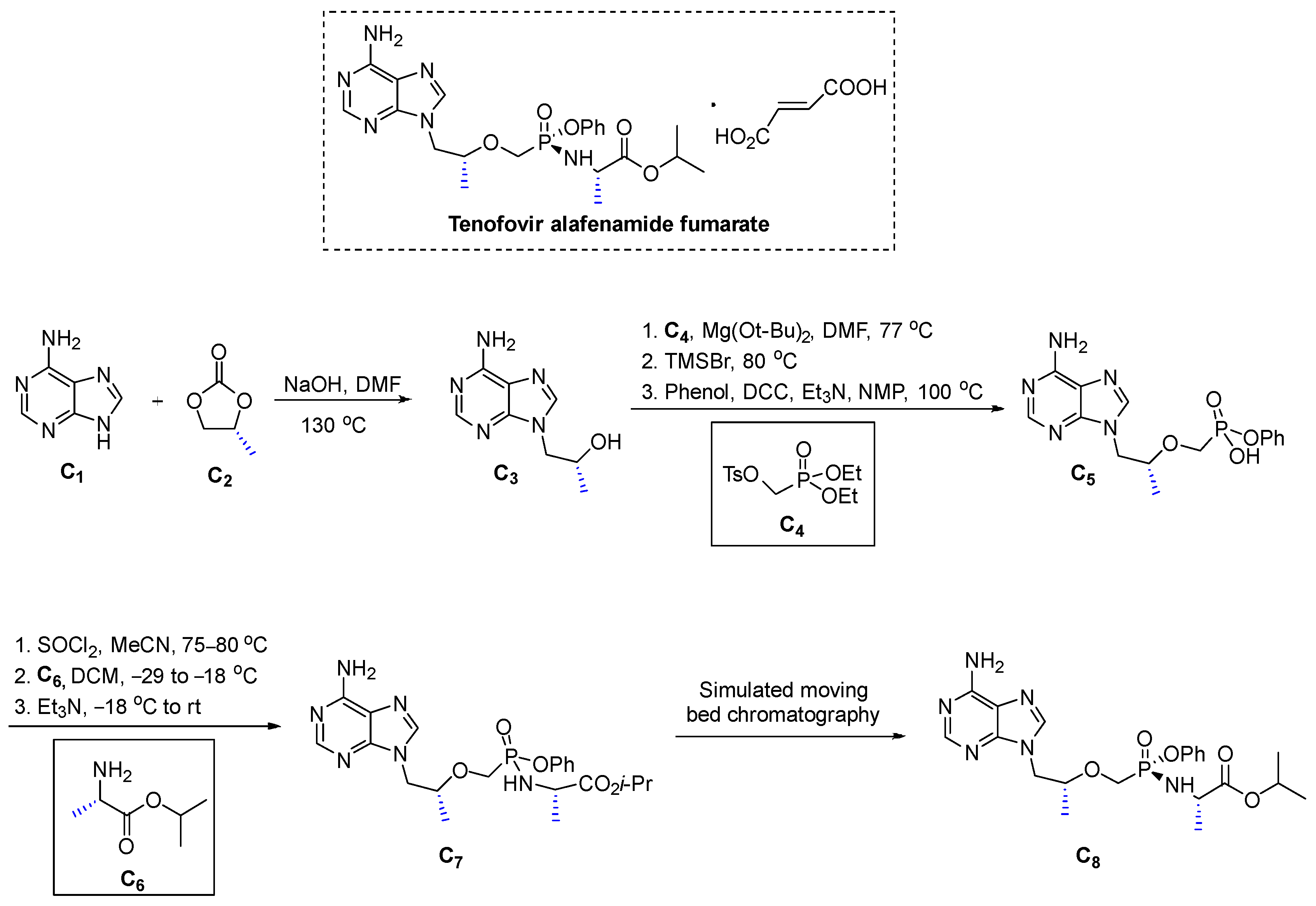
3.3.1. Tenofovir Alafenamide Fumarate (2016)
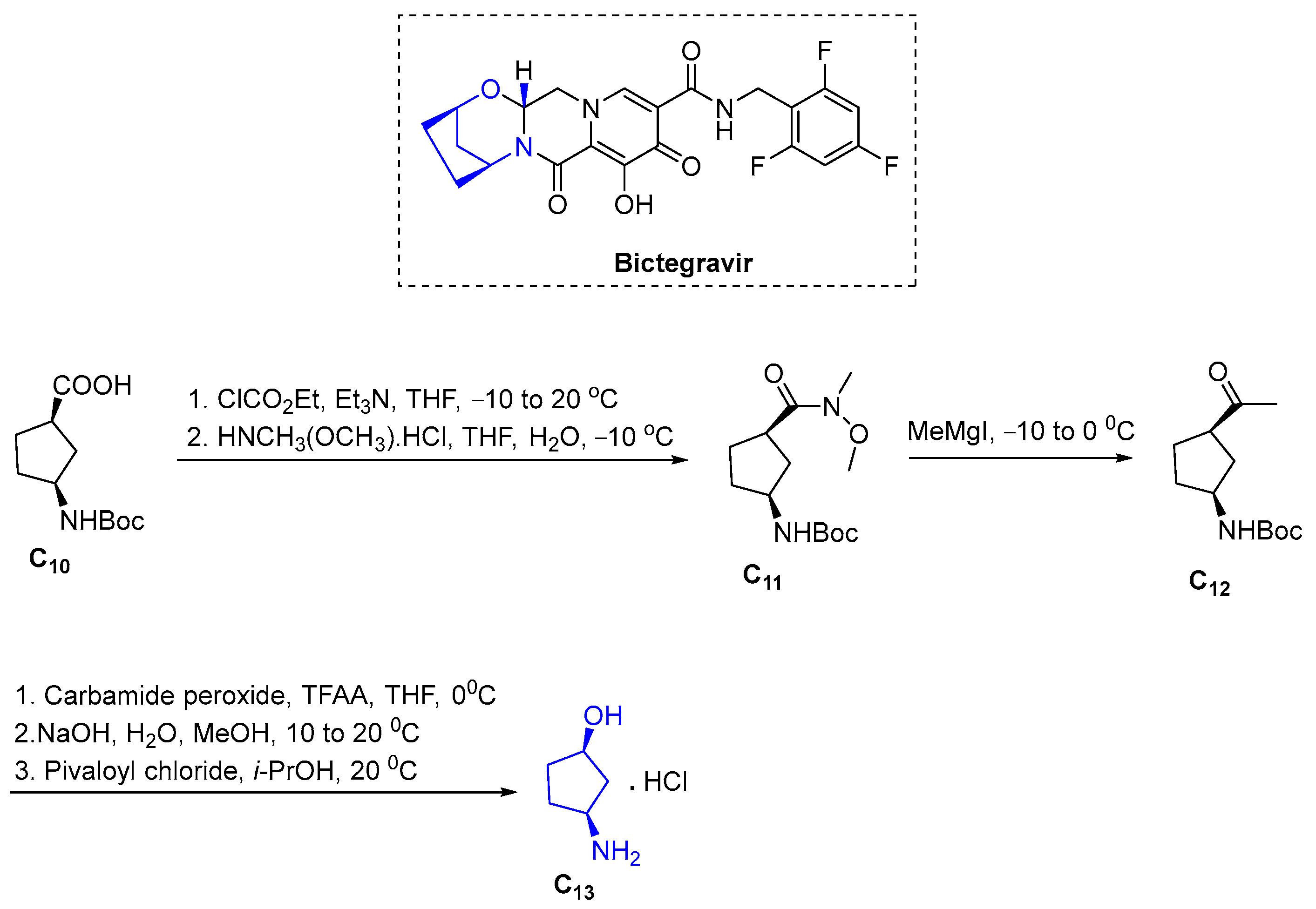
3.3.2. Bictegravir (2018)
3.3.3. Relebactam (2019)
3.3.4. Fam-Trastuzumab Deruxtecan-Nxki (2019)
- The linker C23 with one chiral center is obtained by a chiral pool approach (i.e., amino acid derivative C22 imparts its stereocenter to the drug);
- The polycyclic chiral fragment C27 is prepared by [4 + 2] cycloaddition, followed by chiral resolution adopting supercritical fluid chromatography of the racemic intermediate, C26.
3.3.5. Pralsetinib (2020)
3.3.6. Rimegepant (2020)
- Rhodium-catalyzed reduction of pyridine derivative C32 followed by hydroxyl protection with triisopropylsilyl trifluoromethanesulfonate (TIPSOTf) results in a chiral intermediate C33;
- Chiral fragment C33 then undergoes coupling with 1-bromo-2,3-difluorobenzene C34 resulting in an intermediate C35 with two stereocenters;
- The last chiral center in C36 is obtained by lithium-mediated reduction.
3.4. Drugs with Four Chiral Centers
3.4.1. Migalastat Hydrochloride (2016)
3.4.2. Midostaurin (2017)
3.4.3. Naldemedine (2017)
3.4.4. Valbenazine (2017)
3.4.5. Eravacycline (2018)
- Ellman sulfinamide auxiliary (D16) is used to convert aldehyde (D15) to sulfinimine (D17), which eventually leads to the formation of the chiral tartarate derivative, D18;
- Recrystallization with isopropanol provides pure chiral tricyclic fragment D20 from the corresponding enone derivative, D19.
3.4.6. Sarecycline Hydrochloride (2018)
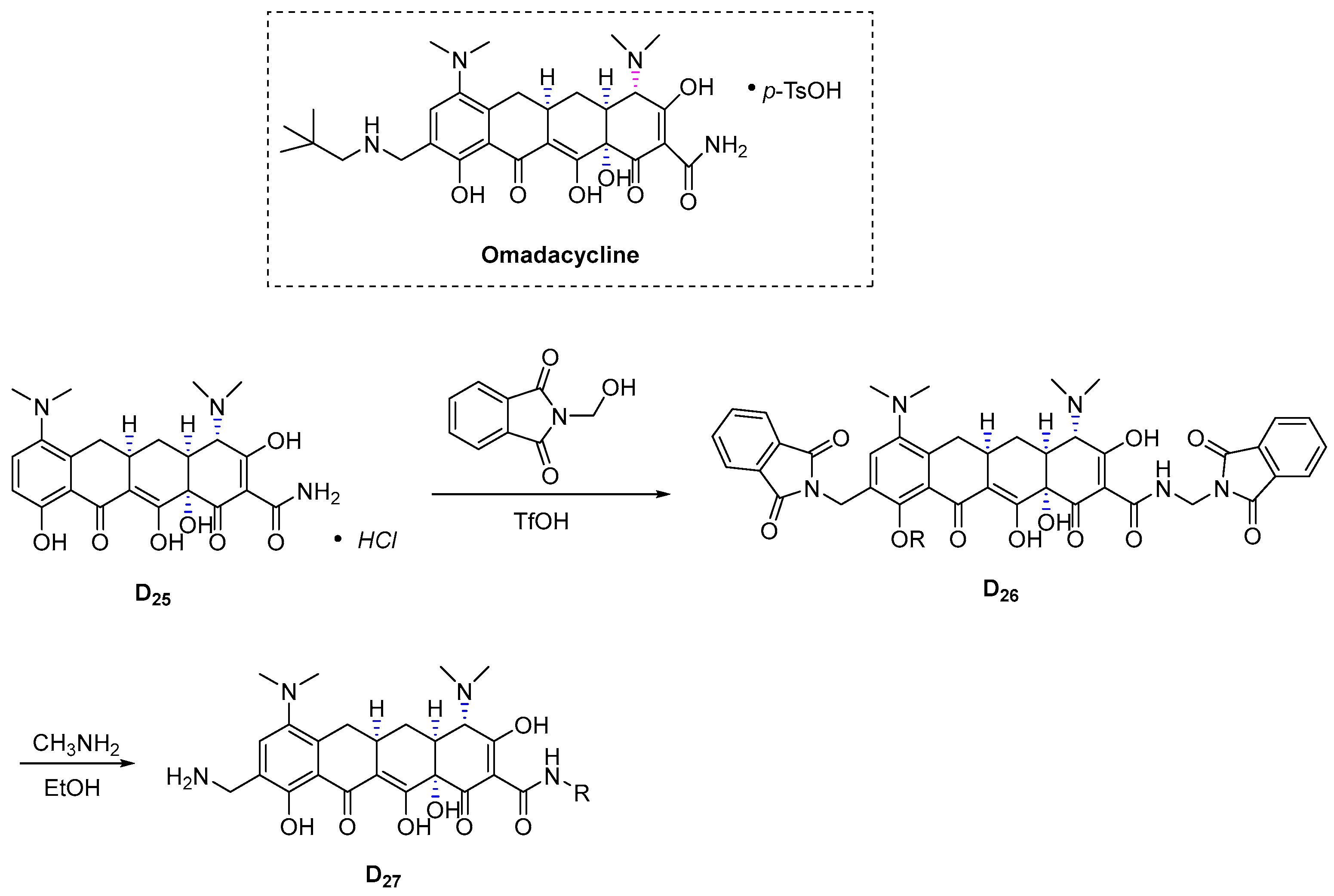
3.4.7. Omadacycline (2018)
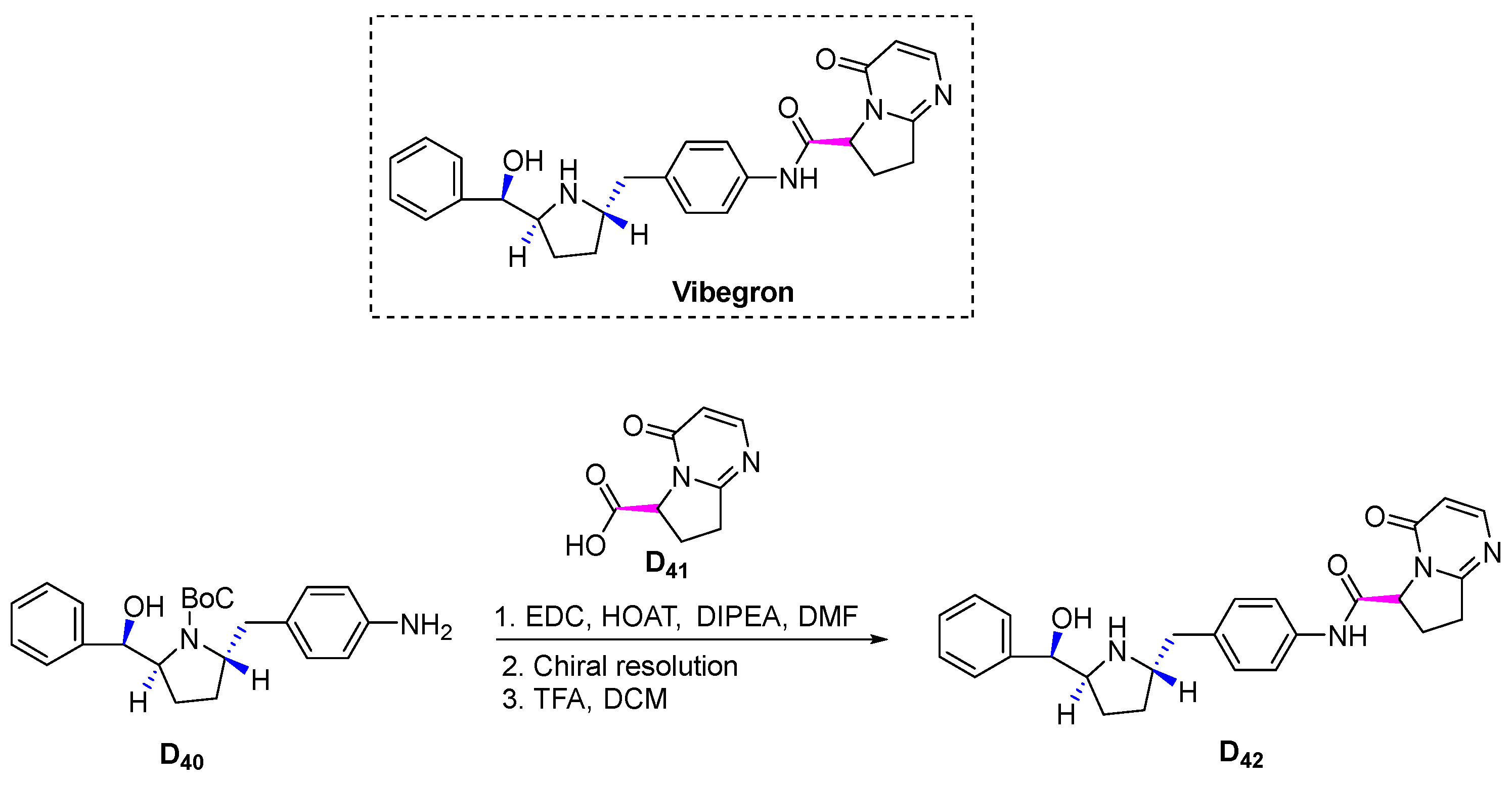
3.4.8. Vibegron (2018)
3.4.9. Ubrogepant (2019)
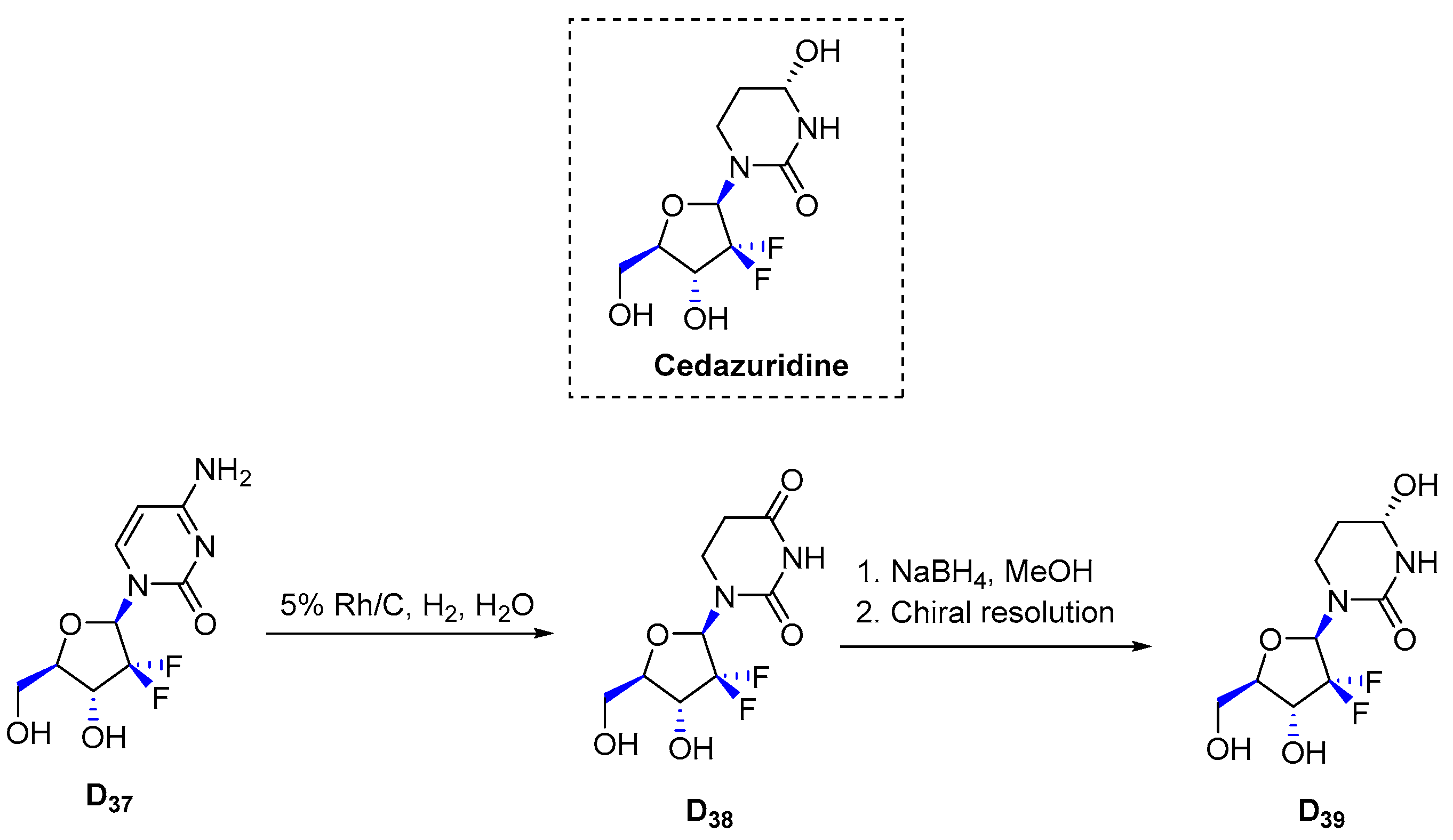
3.4.10. Cedazuridine (2020)
3.4.11. Vibegron (2020)
3.5. Drugs with Five Chiral Centers
3.5.1. Narlaprevir (2016)
3.5.2. Elbasvir (2016)
3.5.3. Latanoprostene Bunod (2017)
3.5.4. Danoprevir (2018)
3.6. Drugs with More Than Five Chiral Centers
3.6.1. Velpatasvir (2016)
3.6.2. Obeticholic Acid (2016)
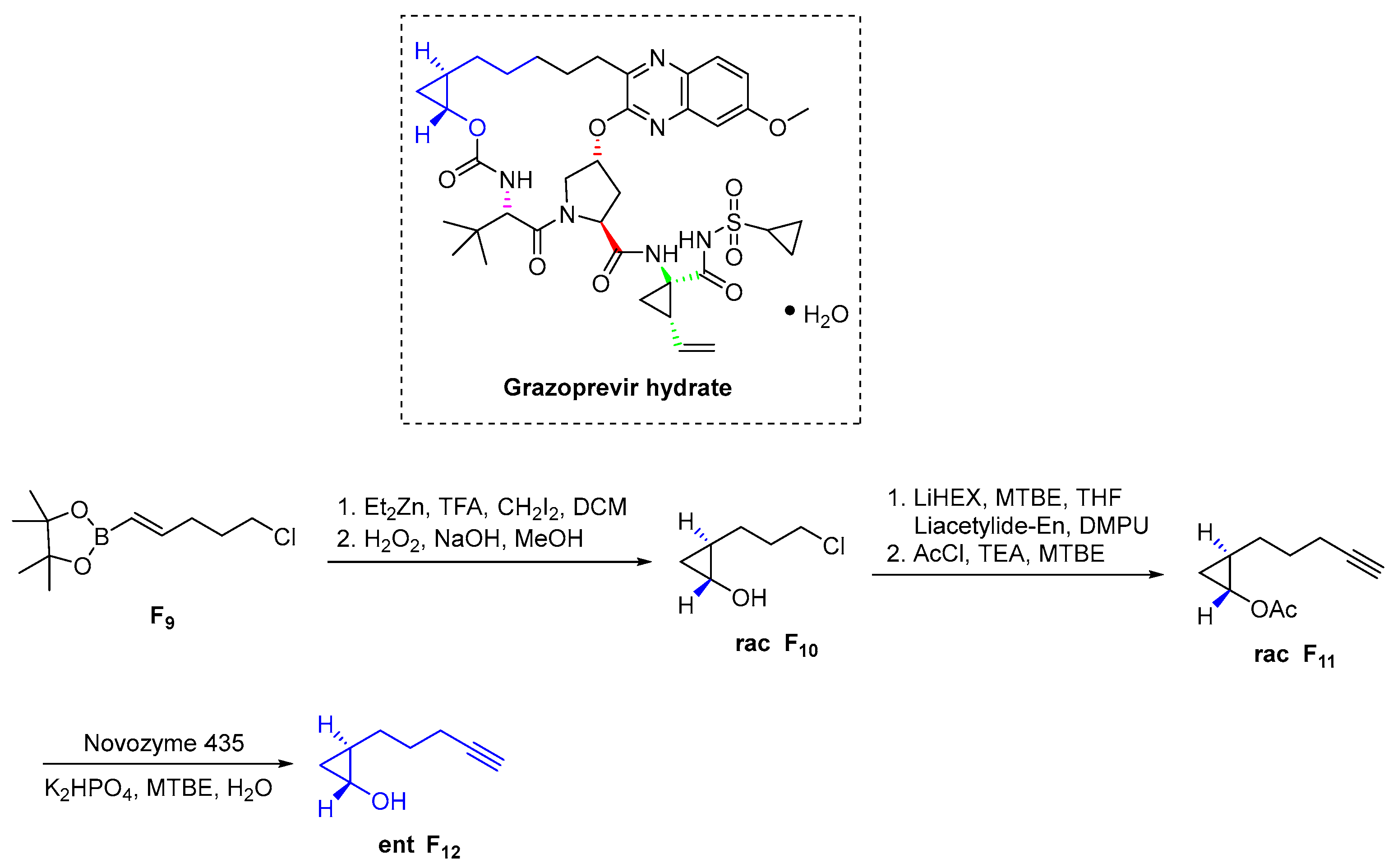
3.6.3. Grazoprevir Hydrate (2016)
3.6.4. Ertugliflozin-l-pyroglutamic Acid (2017)
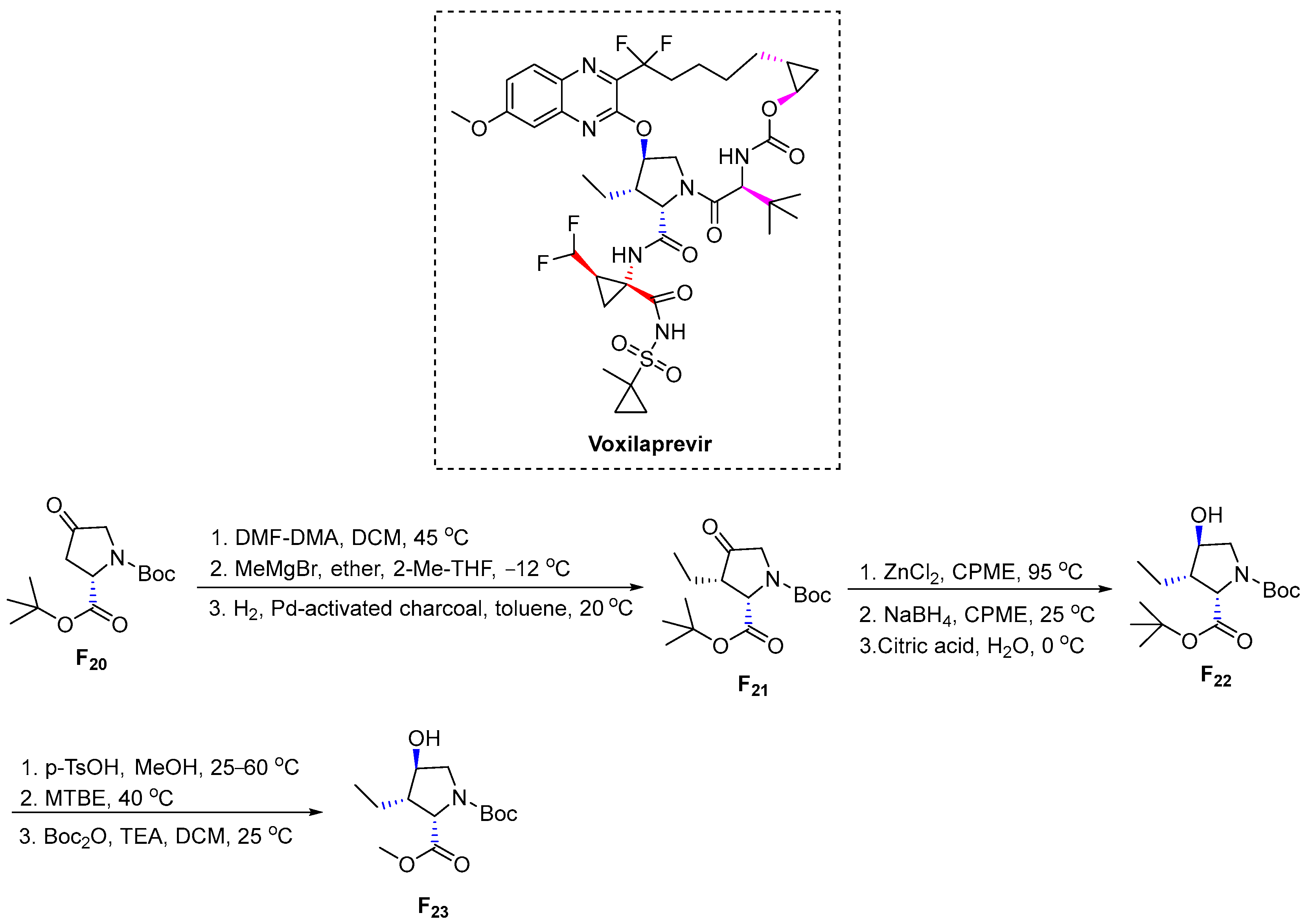
3.6.5. Voxilaprevir (2017)
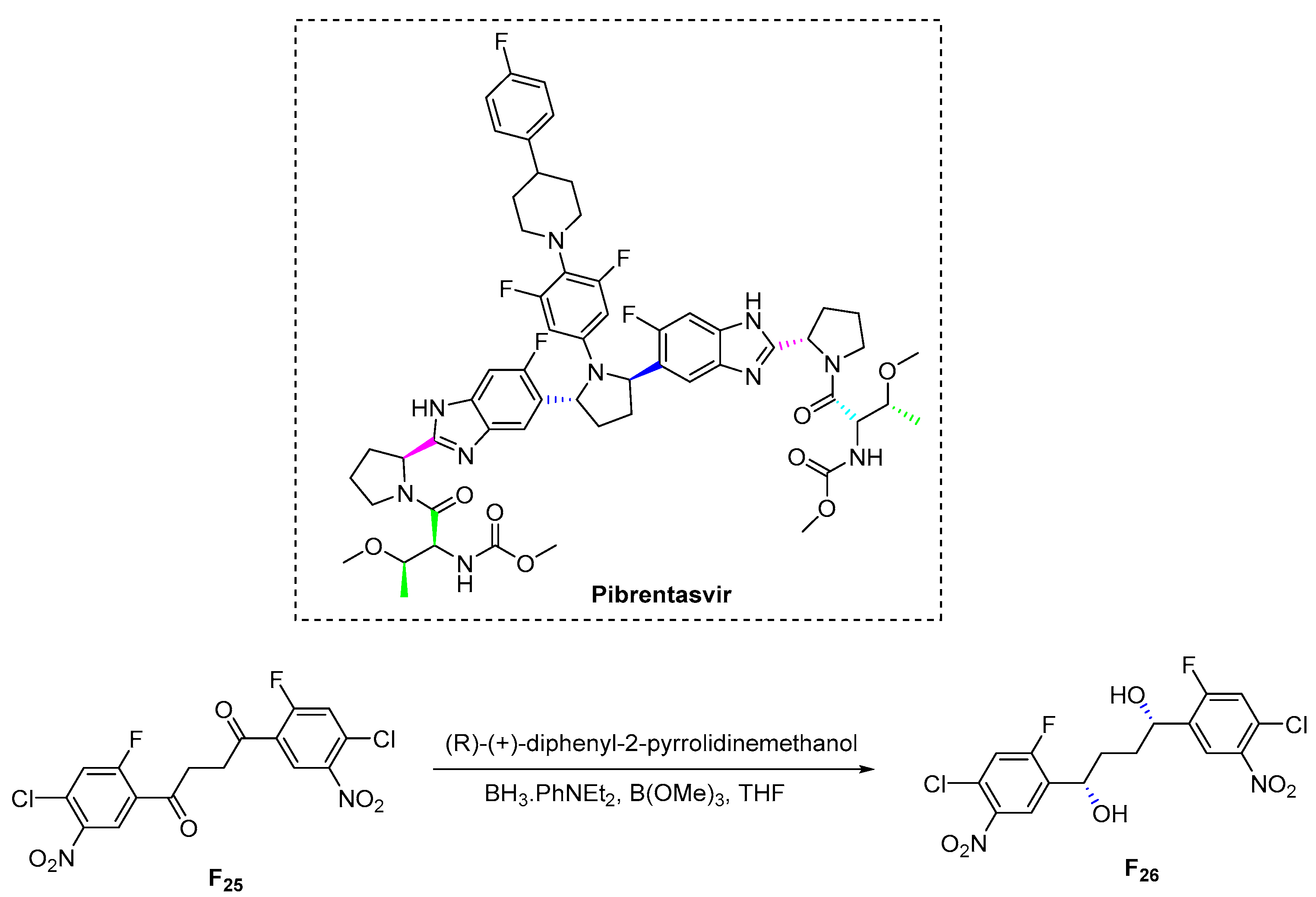
3.6.6. Pibrentasvir (2017)
3.6.7. Glecaprevir (2017)
3.6.8. Segesterone Acetate (2018)
3.6.9. Plitidepsin (2018)
3.6.10. Moxidectin (2018)
3.6.11. Plazomicin (2018)
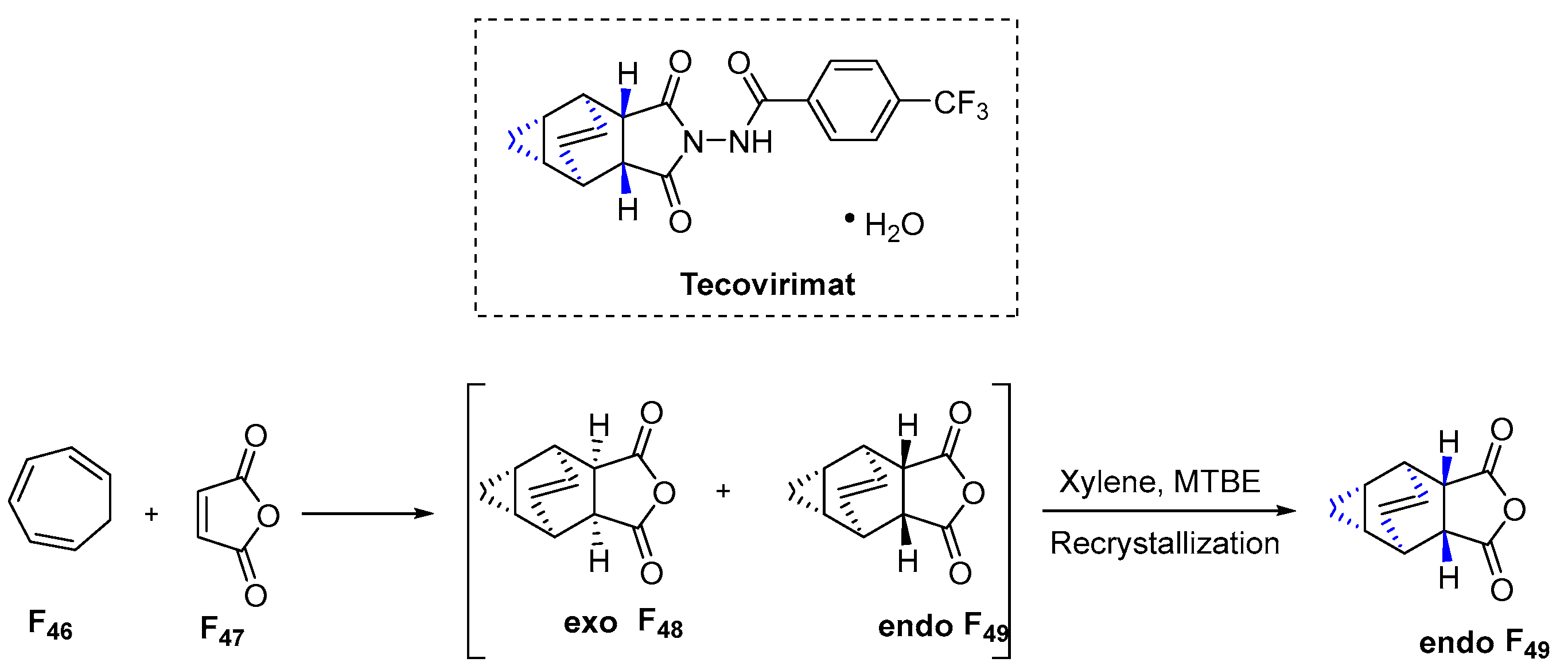
3.6.12. Tecovirimat (2018)
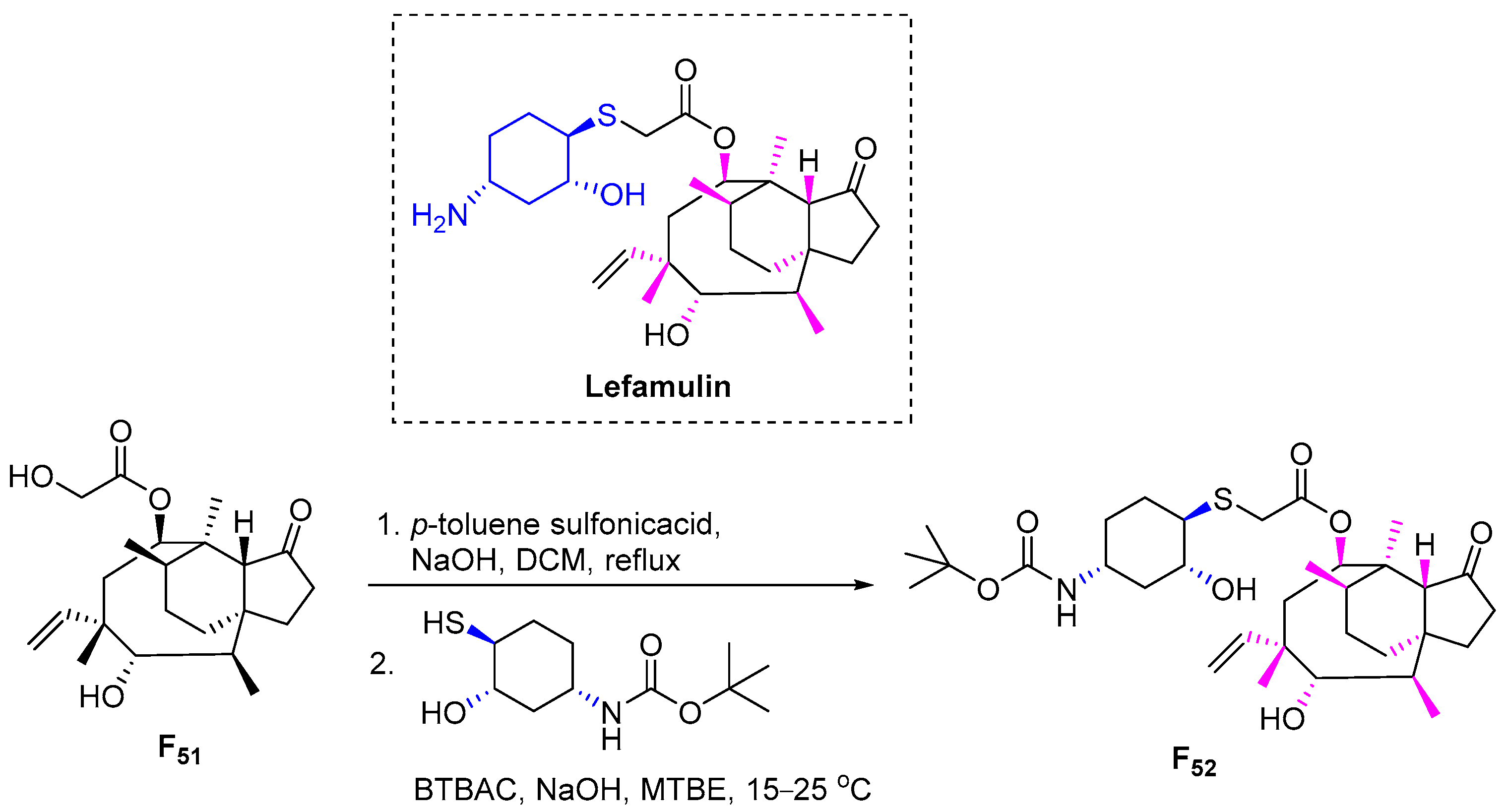
3.6.13. Lefamulin (2019)
3.6.14. Afamelanotide (2019)
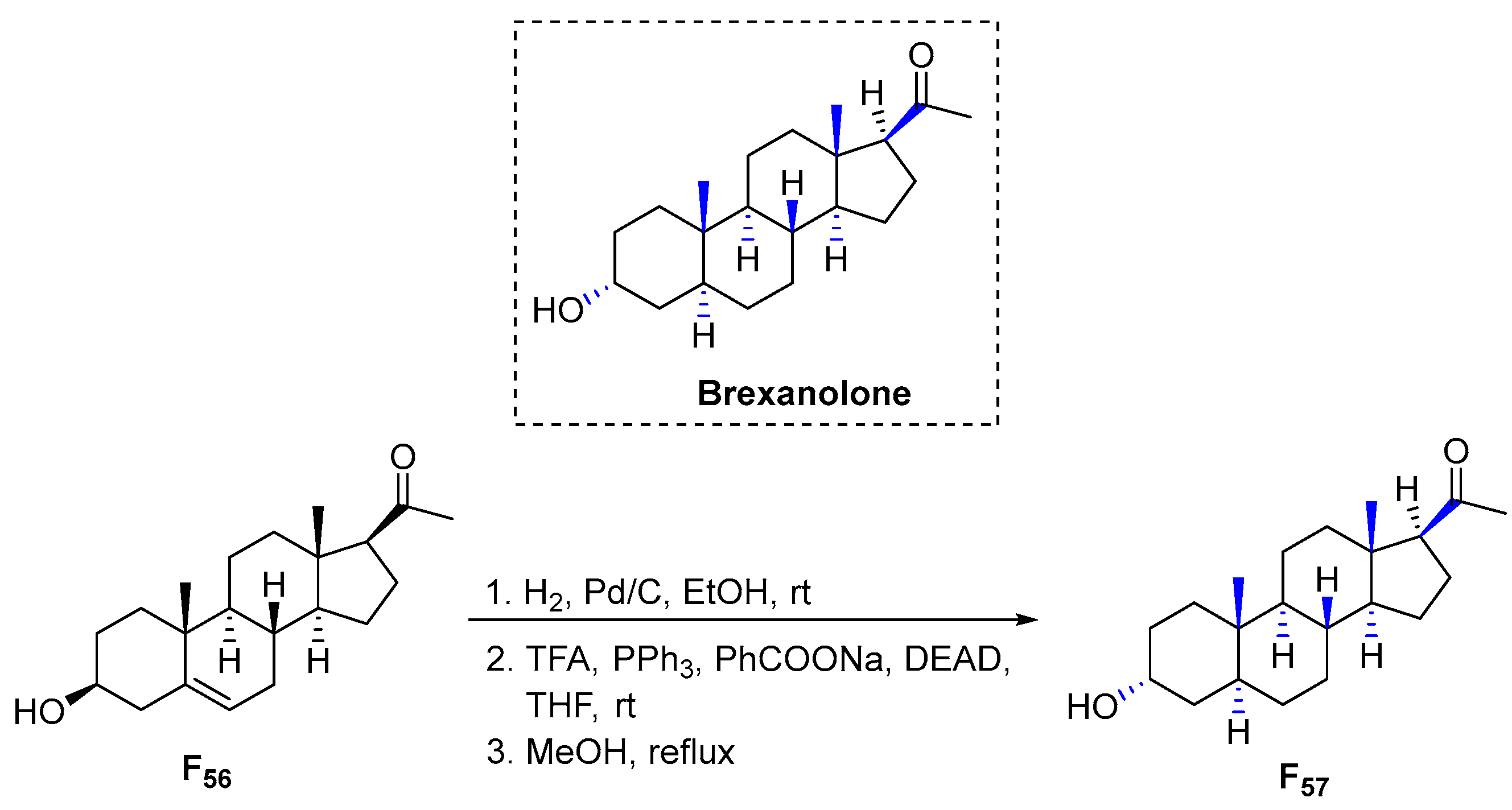
3.6.15. Brexanolone (2019)
3.6.16. Bremelanotide (2019)
3.6.17. Lurbinectedin (2020)
3.6.18. Lactitol (2020)
3.6.19. Setmelanotide (2020)
3.6.20. Clascoterone (2020)
3.6.21. Artesunate (2020)
3.6.22. Remdesivir (2020)
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Blaser, H.U. Industrial asymmetric hydrogenation. In Applications of Transition Metal Catalysis in Drug Discovery and Development; Crawley, M.L., Trost, B.M., Eds.; Wiley: New York, NY, USA, 2012; pp. 315–342. [Google Scholar]
- Biot, J.B. Phénomènes de polarisation successive, observés dans des fluides homogénes. Bull. Soc. Philomatique 1815, 190–192. [Google Scholar]
- Pasteur, L. Recherches sur les propriétés spécifiques des deux acides qui composent pacide racémique. Ann. Chim. Phys. 1850, 28, 56–99. [Google Scholar]
- Morrow, S.M.; Bissette, A.J.; Fletcher, S.P. Transmission of Chirality Through Space and Across Length Scales. Nat. Nanotechnol. 2017, 12, 410–419. [Google Scholar] [CrossRef]
- Nguyen, L.A.; He, H.; Pham-Huy, C. Chiral drugs. An Overview. Int. J. Biomed. Sci. 2006, 2, 85–100. [Google Scholar]
- Drayer, D.E. Pharmacodynamic and Pharmacokinetic Differences Between Drug Enantiomers in Human: An Overview. Clin. Pharmacol. Ther. 1986, 40, 125–133. [Google Scholar] [CrossRef]
- James, H.K.; Anthony, R.S. Thalidomide: The Tragedy of Birth Defects and the Effective Treatment of Disease. Toxicol. Sci. 2011, 122, 1–6. [Google Scholar]
- Yu, T.L.; Chan, Y.; Yan, W.; Jun, J.L.; Xiao, F.; Xiaofei, T.; Zhengzheng, Z.; Xiaoyan, P.; Shuwen, L.; Li, W.T. Axial Chiral Binaphthoquinone and Perylenequinones from the Stromata of Hypocrella bambusae Are SARS-CoV-2 Entry Inhibitors. J. Nat. Prod. 2021, 84, 436–443. [Google Scholar]
- Zhonglei, W. Advances in the Asymmetric Total Synthesis of Natural Products Using Chiral Secondary Amine Catalyzed Reactions of α,β-Unsaturated Aldehydes. Molecules 2019, 24, 3412–3449. [Google Scholar]
- Reilly, E.S.; Donald, M.C.; Justin, L.N. Chiral Analysis of Linalool, an Important Natural Fragrance and Flavor Compound, by Molecular Rotational Resonance Spectroscopy. Symmetry 2022, 14, 917–926. [Google Scholar]
- US FDA. FDA’s Policy Statement for the Development of New Stereoisomeric Drugs. FDA Website [Online]. Available online: http://www.fda.gov/drugs/GuidanceComplianceRegulatoryInformation/Guidances/ucm122883.htm (accessed on 1 May 1992).
- Singh, M.; Sethi, S.; Bhushan, R. Liquid chromatographic methods for separation, determination, and bioassay of enantiomers of etodolac: A review. J. Sep. Sci. 2019, 43, 18–30. [Google Scholar] [CrossRef]
- Patocka, J.; Dvorak, A. Biomedical Aspects of Chiral Molecules. J. Appl. Med. 2004, 2, 95–100. [Google Scholar] [CrossRef]
- Coelho, M.M.; Fernandes, C.; Remião, F.; Tiritan, M.E. Enantioselectivity in drug pharmacokinetics and toxicity: Pharmacological relevance and analytical methods. Molecules 2021, 26, 3113. [Google Scholar] [CrossRef] [PubMed]
- Jarvis, L.M. FDA gives its nod to 53 new drugs in 2020. Chem. Eng. News 2021, 99. Available online: https://cen.acs.org/pharmaceuticals/drug-development/FDA-gives-nod-53-new/99/i2 (accessed on 5 August 2022).
- Available online: https://www.shire.com/newsroom/2016/july/9pks5v (accessed on 12 August 2017).
- Abidi, A.; Shukla, P.; Ahmad, A. Lifitegrast: A Novel Drug for Treatment of Dry Eye Disease. J. Pharmacol. Pharmacother. 2016, 7, 194–198. [Google Scholar] [CrossRef]
- Zeller, J.R.; Venkatraman, S.; Brot, E.C.A.; Iyer, S.; Hall, M. LFA-1 Inhibitor and Polymorph Thereof. WO 2014018748A1, 30 January 2014. [Google Scholar]
- Markham, A.; Dhillon, S. Acalabrutinib: First Global Approval. Drugs 2018, 78, 139–145. [Google Scholar] [CrossRef]
- Mo, G.; Zha, X. Preparation of Btk Inhibitor Acalabrutinib. CN 107522701A, 8 November 2019. [Google Scholar]
- Xu, X. Process for the Preparation of Acalabrutinib. CN 107056786A, 25 October 2018. [Google Scholar]
- Blair, H.A. Pemafibrate: First Global Approval. Drugs 2017, 77, 1805–1810. [Google Scholar] [CrossRef]
- Yamazaki, Y.; Araki, T.; Koura, M.; Shibuya, K. A Practical Synthesis of the PPARα Agonist, (R)-K-13675, Starting from (S)-2-Hydroxybutyrolactone. Tetrahedron 2008, 64, 8155–8158. [Google Scholar] [CrossRef]
- Kim, E.S. Letermovir: First Global Approval. Drugs 2018, 78, 147–152. [Google Scholar] [CrossRef]
- Humphrey, G.R.; Dalby, S.M.; Andreani, T.; Xiang, B.; Luzung, M.R.; Song, Z.J.; Shevlin, M.; Christensen, M.; Belyk, K.M.; Tschaen, D.M. Asymmetric Synthesis of Letermovir Using a Novel Phase-Transfer-Catalyzed Aza-Michael Reaction. Org. Process Res. Dev. 2016, 20, 1097–1103. [Google Scholar] [CrossRef]
- Hoy, S.M. Netarsudil Ophthalmic Solution 0.02%: First Global Approval. Drugs 2018, 78, 389–396. [Google Scholar] [CrossRef]
- Sturdivant, J.M.; Delong, M.A.; Chambournier, G.; Pamment, M.G.; Fedij, V. Process for the Preparation of Kinase Inhibitors and Intermediates Thereof. WO 2017086941A1, 26 May 2017. [Google Scholar]
- TESARO Announces, U.S. FDA Approval of ZEJULA (Niraparib) for Women with Recurrent Ovarian Cancer; TESARO, March 27, 2017. Available online: http://ir.tesarobio.com/news-releases/news-releasedetails/tesaro-announces-us-fda-approval-zejulatm-niraparib-women (accessed on 19 November 2018).
- Chung, C.K.; Bulger, P.G.; Kosjek, B.; Belyk, K.M.; Rivera, N.; Scott, M.E.; Humphrey, G.R.; Limanto, J.; Bachert, D.C.; Emerson, K.M. Process Development of C−N Cross-Coupling and Enantioselective Biocatalytic Reactions for the Asymmetric Synthesis of Niraparib. Org. Process Res. Dev. 2014, 18, 215–227. [Google Scholar] [CrossRef]
- Hughes, D.L. Patent Review of Manufacturing Routes to Recently Approved PARP. Inhibitors: Olaparib, Rucaparib and Niraparib. Org. Process Res. Dev. 2017, 21, 1227–1244. [Google Scholar] [CrossRef]
- Chung, C.K.; Scott, M.E.; Bulger, P.G.; Belyk, K.M.; Limanto, J.; Humphrey, G.R. Regioselective N-2 Arylation of Indazoles. WO 2014088983A1, 12 June 2014. [Google Scholar]
- Bulger, P.G.; Kosjek, B.; Rivera, N. Biocatalytic Transamination Process. US. 20160040201A1, 22 August 2017. [Google Scholar]
- Syed, Y.Y. Lorlatinib: First Global Approval. Drugs 2019, 79, 93–98. [Google Scholar] [CrossRef]
- Duan, S.; Li, B.; Dugger, R.W.; Conway, B.; Kumar, R.; Martinez, C.; Makowski, T.; Pearson, R.; Olivier, M.; Colon-Cruz, R. Developing an Asymmetric Transfer Hydrogenation Process for (S)-5-Fluoro-3-Methylisobenzofuran-1(3h)-One, a Key Intermediate to Lorlatinib. Org. Process Res. Dev. 2017, 21, 1340–1348. [Google Scholar] [CrossRef]
- Chedid, V.; Vijayvargiya, P.; Camilleri, M. Elobixibat for the Treatment of Constipation. Expert Rev. Gastroenterol. Hepatol. 2018, 12, 951–960. [Google Scholar] [CrossRef]
- Starke, I.; Dahlstrom, M.U.J.; Blomberg, D.; Alenfalk, S.; Nordberg, P.; Wallberg, A.C.; Bostrom, S.J. Preparation of Benzothiazepine Derivatives for Potential Use as Ileal Bile Acid Transport Inhibitors for the Treatment of Hyperlipidemia. WO2003020710A1, 13 March 2003. [Google Scholar]
- Donaldson, S.H.; Pilewski, J.M.; Griese, M.; Cooke, J.; Viswanathan, L.; Tullis, E.; Davies, J.C.; Lekstrom-Himes, J.A.; Wang, L.T. Tezacaftor/Ivacaftor in Subjects with Cystic Fibrosis and F508del/F508del-CFTR or F508del/G551D-CFTR. Am. J. Respir. Crit. Care Med. 2018, 197, 214–224. [Google Scholar] [CrossRef]
- Taylor-Cousar, J.L.; Munck, A.; McKone, E.F.; van der Ent, C.K.; Moeller, A.; Simard, C.; Wang, L.T.; Ingenito, E.P.; McKee, C.; Lu, Y.; et al. Tezacaftor-Ivacaftor in Patients with Cystic Fibrosis Homozygous for Phe508del. N. Engl. J. Med. 2017, 377, 2013–2023. [Google Scholar] [CrossRef]
- Phenix, B.D.; Bagnol, L.J.-C.; Brodeur, G.G.; Chandran, S.; Dokou, E.; Ferris, L.A.; Knezic, D.; McCarty, K.L.; Medek, A.; Waggener, S.A. Method for Preparation of Quinolinone Carboxamides, Indole Carboxamides and Pharmaceutical Compositions Containing Them for the Treatment of Cystic Fibrosis Transmembrane Conductance Regulator Mediated Diseases. WO2015160787A1, 22 October 2015. [Google Scholar]
- Blair, H.A. Pyrotinib: First Global Approval. Drugs 2018, 78, 1751–1755. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.; Zhang, Q.; Cao, Y. Method for Preparing Tyrosine Kinase Inhibitor and Derivative Thereof. WO2017186140A1, 15 December 2021. [Google Scholar]
- Shirley, M. Encorafenib and Binimetinib: First Global Approvals. Drugs 2018, 78, 1277–1284. [Google Scholar] [CrossRef]
- Huang, S.; Jin, X.; Liu, Z.; Poon, D.; Tellew, J.; Wan, Y.; Wang, X.; Xie, Y. Compounds and Compositions as Protein Kinase Inhibitors. WO2011025927A1, 15 December 2021. [Google Scholar]
- Anastasia, A.; Rossi, G. Novel Drugs in Follicular Lymphoma. Mediterr. J. Hematol. Infect. Dis. 2015, 8, e2016061. [Google Scholar]
- Rodrigues, D.A.; Sagrillo, F.S.; Fraga, C.A.M. Duvelisib: A 2018 Novel FDA-Approved Small Molecule Inhibiting Phosphoinositide 3-Kinases. Pharmaceuticals 2019, 12, 69. [Google Scholar] [CrossRef]
- Vangapandu, H.V.; Jain, N.; Gandhi, V. Duvelisib: A Phosphoinositide-3 Kinase Delta/Gamma Inhibitor for Chronic Lymphocytic Leukemia. Expert Opin. Investig. Drugs 2017, 26, 625–632. [Google Scholar] [CrossRef]
- Ren, P.; Martin, M.; Isbester, P.; Lane, B.; Kropp, J. Process for Preparing Isoquinolines and Solid Forms of Isoquinolines. WO2012097000A1, 15 December 2021. [Google Scholar]
- Lamb, Y.N. Elagolix: First Global Approval. Drugs 2018, 78, 1501–1508. [Google Scholar] [CrossRef]
- Chen, C.; Wu, D.; Guo, Z.; Xie, Q.; Reinhart, G.J.; Madan, A.; Wen, J.; Chen, T.; Huang, C.Q.; Chen, M.; et al. Discovery of Sodium R-(+)-4-{2-[5-(2- Fluoro-3-Methoxyphenyl)-3-(2-Fluoro-6-[Trifluoromethyl]Benzyl)-4- Methyl-2,6-Dioxo-3,6-Dihyd ro-2h-Pyrimidin-1-Yl]-1- Phenylethylamino}Butyrate (Elagolix), a Potent and Orally Available Nonpeptide Antagonist of the Human Gonadotropin-Releasing Hormone Receptor. J. Med. Chem. 2008, 51, 7478–7485. [Google Scholar]
- Takahashi, N.; Take, Y. Tegoprazan, a Novel PotassiumCompetitive Acid Blocker to Control Gastric Acid Secretion and Motility. J. Pharmacol. Exp. Ther. 2018, 364, 275–286. [Google Scholar] [CrossRef] [PubMed]
- Xiaolong, Q.; Lin, H.; Wenbo, L.; Ping, Z.; Lingling, C.; Xingang, Z.; Ping, W.; Donghui, W.; Lei, C.; Jun, C. Method for Synthetizing Tegoprazan Chiral Alcohol. CN109320485, 28 May 2021. [Google Scholar]
- Stirrups, R. Alpelisib Plus Fulvestrant for PIK3CA-Mutated Breast Cancer. Lancet Oncol. 2019, 20, 347. [Google Scholar] [CrossRef] [PubMed]
- Andre, F.; Ciruelos, E.; Rubovszky, G.; Campone, M.; Loibl, S.; Rugo, H.S.; Iwata, H.; Conte, P.; Mayer, I.A.; Kaufman, B.; et al. Alpelisib for PIK3CA-Mutated, Hormone Receptor-Positive Advanced Breast Cancer. N. Engl. J. Med. 2019, 380, 1929–1940. [Google Scholar] [CrossRef]
- Caravatti, R.A.; Fairhurst, P.; Furet, V.; Guagnano, P. Imbach, Thiazole Derivatives Used as PI3 Kinase Inhibitors. WO2009080694, 2 July 2009. [Google Scholar]
- Strollo, P.J.; Hedner, J.; Collop, N.; Lorch, D.G.; Chen, D.; Carter, L.P.; Lu, Y.; Lee, L.; Black, J.; Pepin, J.L.; et al. Solriamfetol for the Treatment of Excessive Sleepiness in OSA: A Placebo-Controlled Randomized Withdrawal Study. Chest 2019, 155, 364–374. [Google Scholar] [CrossRef]
- Markham, A. Solriamfetol: First Global Approval. Drugs 2019, 79, 785–790. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.M.; Kim, M.W. Process of Preparing O-Carbamoyl Compounds in the Presence of Active Amine Group. WO2005033064, 14 April 2005. [Google Scholar]
- Wang, X.; Inoyama, D.; Russo, R.; Li, S.G.; Jadhav, R.; Stratton, T.P.; Mittal, N.; Bilotta, J.A.; Singleton, E.; Kim, T.; et al. Antitubercular Triazines: Optimization and Intrabacterial Metabolism. Cell Chem. Biol. 2020, 27, 172–185. [Google Scholar] [CrossRef]
- Miao, D.; Hu, Q.; Li, Y.; Yu, Z.; Wang, H.; Zhu, X. Synthesis Method of PA-824 (Pretomanid) for Treating Tuberculosis. CN107915747, 10 November 2020. [Google Scholar]
- Tam, C.S.; Trotman, J.; Opat, S.; Burger, J.A.; Cull, G.; Gottlieb, D.; Harrup, R.; Johnston, P.B.; Marlton, P.; Munoz, J.; et al. Phase 1 Study of the Selective BTK Inhibitor Zanubrutinib in Bcell Malignancies and Safety and Efficacy Evaluation in CLL. Blood 2019, 134, 851–859. [Google Scholar] [CrossRef]
- Guo, Y.; Wang, Z. Fused Heterocyclic Compounds as Protein Kinase Inhibitors. WO2014173289, 30 October 2014. [Google Scholar]
- Markham, A.; Duggan, S. Darolutamide: First Approval. Drugs 2019, 79, 1813–1818. [Google Scholar] [CrossRef]
- Fizazi, K.; Shore, N.; Tammela, T.L.; Ulys, A.; Vjaters, E.; Polyakov, S.; Jievaltas, M.; Luz, M.; Alekseev, B.; Kuss, I.; et al. Darolutamide in Nonmetastatic, Castration-Resistant Prostate Cancer. N. Engl. J. Med. 2019, 380, 1235–1246. [Google Scholar] [CrossRef]
- Laitinen, I.; Karjalainen, O. Process for the Preparation of Androgen Receptor Antagonists and Intermediates Thereof. WO2016162604, 13 October 2016. [Google Scholar]
- Pan, T.; Xia, C.; Yang, Y.; Zhang, A. Process and Intermediates for Preparation of Androgen Receptor Antagonist. CN108218908, 29 June 2018. [Google Scholar]
- Tormakangas, O.; Wohlfahrt, G.; Salo, H.; Ramasubramanian, R.D.; Patra, P.K.; Martin, A.E.; Heikkinen, T.; Vesalainen, A.; Moilanen, A.; Karjalainen, A. Androgen Receptor Modulating Carboxamides. WO2012143599, 26 October 2012. [Google Scholar]
- Kasteleijn-Nolst Trenite, D.G.A.; DiVentura, B.D.; Pollard, J.R.; Krauss, G.L.; Mizne, S.; French, J.A. Suppression of the Photoparoxysmal Response in Photosensitive Epilepsy with Cenobamate (YKP3089). Neurology 2019, 93, 559–567. [Google Scholar] [CrossRef]
- Choi, Y.M.; Kim, C.G.; Kang, Y.S.; Yi, H.J.; Lee, H.S.; Ku, B.C.; Lee, E.H.; Im, D.J.; Shin, Y.J. Preparation of Triazoles and Tetrazoles Containing Carbamoyl Group as Anticonvulsants. WO2006112685, 26 October 2006. [Google Scholar]
- Dhillon, S. Avapritinib: First Approval. Drugs 2020, 80, 433–439. [Google Scholar] [CrossRef]
- Zhang, Y.; Hodous, B.L.; Kim, J.L.; Wilson, K.J.; Wilson, D. Compositions Useful for Treating Disorders Related to KIT. WO2015057873, 23 April 2015. [Google Scholar]
- Ohsawa, I.; Honda, D.; Suzuki, Y.; Fukuda, T.; Kohga, K.; Morita, E.; Moriwaki, S.; Ishikawa, O.; Sasaki, Y.; Tago, M.; et al. Oral Berotralstat for the Prophylaxis of Hereditary Angioedema Attacks in Patients in Japan: A Phase 3 Randomized Trial. Allergy 2021, 76, 1789–1799. [Google Scholar] [CrossRef] [PubMed]
- El-Kattan, Y.; Babu, Y.S. Crystalline Salts of a Plasma Kallikrein Inhibitor. US20200140389, 26 May 2020. [Google Scholar]
- Gordon, L.B.; Kleinman, M.E.; Massaro, J.; D’Agostino, R.B.; Shappell, S.H.; Gerhard-Herman, M.; Smoot, L.B.; Gordon, C.M.; Cleveland, R.H.; Nazarian, A.; et al. Clinical Trial of the Protein Farnesylation Inhibitors Lonafarnib, Pravastatin, and Zoledronic Acid in Children with Hutchinson-Gilford Progeria Syndrome. Circulation 2016, 134, 114–125. [Google Scholar] [CrossRef]
- Njoroge, F.G.; Vibulbhan, B.; Rane, D.F.; Bishop, W.R.; Petrin, J.; Patton, R.; Bryant, M.S.; Chen, K.J.; Nomeir, A.A.; Lin, C.C.; et al. Structure-Activity Relationship of 3- Substituted N-(Pyridinylacetyl)-4-(8-Chloro-5,6-dihydro-11H-benzo[5,6] Cyclohepta[1,2-b]Pyridin-11-ylidene)Piperidine Inhibitors of Farnesylprotein Transferase: Design and Synthesis of in vivo Active Antitumor Compounds. J. Med. Chem. 1997, 40, 4290–4301. [Google Scholar]
- Duggan, S. Osilodrostat: First Approval. Drugs 2020, 80, 495–500. [Google Scholar] [CrossRef]
- Zhang, C.; Chakma, J. 3-Fluoro-Benzonitrile Inhibitors of 11-Beta-Hydroxylase. WO2016109361, 7 July 2016. [Google Scholar]
- Markham, A. Oliceridine: First Approval. Drugs 2020, 80, 1739–1744. [Google Scholar] [CrossRef]
- Yamashita, D.; Gotchev, D.; Pitis, P.; Chen, X.T.; Liu, G.; Yuan, C.C.K. Opioid Receptor Ligands and Methods of Using and Making Same. WO2012129495, 27 September 2012. [Google Scholar]
- Sneyd, J.R.; Rigby-Jones, A.E. Remimazolam for Anaesthesia or Sedation. Curr. Opin. Anaesthesiol. 2020, 33, 506–511. [Google Scholar] [CrossRef]
- Feldman, P.L.; Jung, D.K.; Kaldor, I.; Pacofsky, G.J.; Stafford, J.A.; Tidwell, J.H. Short-Acting Benzodiazepines. WO2000069836, 23 November 2000. [Google Scholar]
- Lamb, Y.N. Ozanimod: First Approval. Drugs 2020, 80, 841–848. [Google Scholar] [CrossRef]
- Martinborough, E.; Boehm, M.F.; Yeager, A.R.; Tamiya, J.; Huang, L.; Brahmachary, E.; Moorjani, M.; Timony, G.A.; Brooks, J.L.; Peach, R.; et al. Selective Sphingosine 1 Phosphate Receptor Modulators and Combination Therapy Therewith. WO2015066515, 7 May 2015. [Google Scholar]
- Poole, R.M. Nemonoxacin: First Global Approval. Drugs 2014, 74, 1445–1453. [Google Scholar] [CrossRef]
- Redman-Furey, N.L.; Godlewski, J.E.; Dicks, M.L. Malate Salts, and Polymorphs of (3S,5S)-7-[3-Amino-5-Methyl-Piperidinyl]-1-Cyclopropyl-1,4-Dihydro-8-Methoxy-4-oxo-3-Quinolinecarboxylic Acid. US 8039485B2, 18 October 2011. [Google Scholar]
- Markham, A. Brivaracetam: First Global Approval. Drugs 2016, 76, 517–522. [Google Scholar] [CrossRef]
- Strzelczyk, A.; Klein, K.M.; Willems, L.M.; Rosenow, F.; Bauer, S. Brivaracetam in the Treatment of Focal and Idiopathic Generalized Epilepsies and of Status Epilepticus. Expert Rev. Clin. Pharmacol. 2016, 9, 637–645. [Google Scholar] [CrossRef]
- Schüle, A.; Merschaert, A.; Szczepaniak, C.; Marechal, C.; Carly, N.; O’Rourke, J.; Ates, C. A Biocatalytic Route to the Novel Antiepileptic Drug Brivaracetam. Org. Process Res. Dev. 2016, 20, 1566–1575. [Google Scholar] [CrossRef]
- Gentles, R.G.; Ding, M.; Bender, J.A.; Bergstrom, C.P.; Grant-Young, K.; Hewawasam, P.; Hudyma, T.; Martin, S.; Nickel, A.; Regueiro-Ren, A.; et al. Discovery and Preclinical Characterization of the Cyclopropylindolobenzazepine BMS-791325, A Potent Allosteric Inhibitor of the Hepatitis C Virus NS5B Polymerase. J. Med. Chem. 2014, 57, 1855–1879. [Google Scholar] [CrossRef] [PubMed]
- Bender, J.A.; Ding, M.; Gentles, R.G.; Hewawasam, P. Preparation of Cyclopropyl Fused Indolobenzazepine Derivatives as Hepatitis C Virus (HCV) NS5B Polymerase Inhibitors. WO 20070270405, 25 November 2008. [Google Scholar]
- Jabbour, E.J.; DeAngelo, D.J.; Stelljes, M.; Stock, W.; Liedtke, M.; Goekbuget, N.; O’Brien, S.; Wang, T.; Paccagnella, M.L.; Sleight, B.; et al. Efficacy and Safety Analysis by Age Cohort of Inotuzumab Ozogamicin in Patients with Relapsed or Refractory Acute Lymphoblastic Leukemia Enrolled in Ino-Vate. Cancer 2018, 124, 1722–1732. [Google Scholar] [CrossRef]
- Maiese, W.M.; Lechevalier, M.P.; Lechevalier, H.A.; Korshalla, J.; Kuck, N.; Fantini, A.; Wildey, M.J.; Thomas, J.; Greenstein, M. Calicheamicins, a Novel Family of Antitumor Antibiotics: Taxonomy, Fermentation and Biological Properties. J. Antibiot. 1989, 42, 558–563. [Google Scholar] [CrossRef]
- Lee, M.D.; Greenstein, M.; Labeda, D.P.; Fantini, A.A. Fermentative Manufacture of Antitumor Antibiotics (Ll-E33288 Complex). US 4970198, 13 November 1990. [Google Scholar]
- Dugger, R.W.; Letendre, L.J.; Patel, V.B.; Prashad, A.S.; Zhang, C. Intermediates and Methods for Synthesizing Calicheamicin Derivatives. WO 2015063680, 7 May 2015. [Google Scholar]
- Heo, Y.A.; Scott, L.J. Deutetrabenazine: A Review in Chorea Associated with Huntington’s Disease. Drugs 2017, 77, 1857–1864. [Google Scholar] [CrossRef]
- Zhang, C. Methods of Manufacturing Benzoquinoline Compounds as Inhibitors of Vesicular Monoamine Transporter 2 (Vmat2). US 20150152099A1, 24 December 2019. [Google Scholar]
- Avery, L.M.; Nicolau, D.P. Investigational Drugs for the Treatment of Infections Caused by Multidrug-Resistant Gram-Negative Bacteria. Expert Opin. Investig. Drugs 2018, 27, 325–338. [Google Scholar] [CrossRef]
- Hecker, S.J.; Reddy, K.R.; Totrov, M.; Hirst, G.C.; Lomovskaya, O.; Griffith, D.C.; King, P.; Tsivkovski, R.; Sun, D.; Sabet, M.; et al. Discovery of a Cyclic Boronic Acid B-Lactamase Inhibitor (Rpx7009) with Utility Vs Class a Serine Carbapenemases. J. Med. Chem. 2015, 58, 3682–3692. [Google Scholar] [CrossRef]
- Markham, A. Telotristat Ethyl: First Global Approval. Drugs 2017, 77, 793–798. [Google Scholar] [CrossRef]
- Bednarz, M.S.; Burgoon, H.A., Jr.; Iimura, S.; Kanamarlapudi, R.C.; Song, Q.; Wu, W.; Yan, J.; Zhang, H. Methods of Preparing 4-Phenyl-6-(2,2,2-trifluoro-1-phenylethoxy)pyrimidineBased Compounds. WO 2009029499A1, 5 March 2009. [Google Scholar]
- Bednarz, M.S.; De Paul, S.; Kanamarlapudi, R.C.; Perlberg, A.; Zhang, H. Preparation of Solids Forms of (S)-Ethyl 2-Amino-3-(4-(2-amino-6-((R)-1-(4-chloro-2-(3-methyl-1H-pyrazol-1-yl)phenyl)-2,2,2-trifluoroethoxy)pyrimidin-4-yl)phenyl)propanoate. WO 2009042733A1, 2 April 2009. [Google Scholar]
- Scott, L.J. Larotrectinib: First Global Approval. Drugs 2019, 79, 201–206. [Google Scholar] [CrossRef]
- Reynolds, M.; Eary, C.T.; Spencer, S.; Juengst, D.; Hache, B.; Jiang, Y.; Haas, J.; Andrews, S.W. Preparation of (S)-N-(5-((R)-2-(2,5-Difluorophenyl)pyrrolidin-1-yl)pyrazolo[1,5-a]pyrimidin-3-yl)-3-hydroxypyrrolidine-1-carboxamide. WO2017201241A1, 23 November 2017. [Google Scholar]
- Hoy, S.M. Glasdegib: First Global Approval. Drugs 2019, 79, 207–213. [Google Scholar] [CrossRef]
- Peng, Z.; Wong, J.W.; Hansen, E.C.; Puchlopek-Dermenci, A.L.A.; Clarke, H.J. Development of a Concise, Asymmetric Synthesis of a Smoothened Receptor (SMO) Inhibitor: Enzymatic Transamination of a 4-Piperidinone with Dynamic Kinetic Resolution. Org. Lett. 2014, 16, 860–863. [Google Scholar] [CrossRef]
- Hoy, S.M. Talazoparib: First Global Approval. Drugs 2018, 78, 1939–1946. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Chu, D.; Liu, Y.; Jiang, Q.; Lu, L. Processes of Synthesizing Dihydropyridophthalazinone Derivatives. WO2011097602A1, 11 August 2011. [Google Scholar]
- Wang, B.; Chu, D.; Liu, Y.; Peng, S. Crystalline (8s,9r)-5-Fluoro-8-(4-fluorophenyl)-9-(1-methyl-1h-1,2,4- triazol-5-yl)-8,9-dihydro-2h-pyrido[4,3,2-de]phthalazin-3(7h)-one Tosylate Salt. WO2012054698A1, 14 August 2014. [Google Scholar]
- Feng, Y.; Gutierrez, A.A.; Shen, Y.; Wang, E.W.; Okhamafe, A.O.; Price, C.P.; Chou, T. Dihydropyridophthalazinone Inhibitors of Poly (Adp-Ribose) Polymerase (Parp) for the Treatment of Multiple Myeloma. WO2013028495A1, 28 February 2013. [Google Scholar]
- Dhillon, S. Ivosidenib: First Global Approval. Drugs 2018, 78, 1509–1516. [Google Scholar] [CrossRef]
- Muthusamy, A.R.; Singh, A.; Yazali, V.S.; Luthra, P.K.; Vasoya, S.L.; Patil, B.T.; Taneja, A.K.; Srivastav, N.C.; Singh, R.; Rengasamy, V.; et al. Solid State Forms of Ivosidenib. WO2019104318A1, 31 May 2019. [Google Scholar]
- Miyazaki, H.; Ikeda, Y.; Sakurai, O.; Miyake, T.; Tsubota, R.; Okabe, J.; Kuroda, M.; Hisada, Y.; Yanagida, T.; Yoneda, H.; et al. Discovery of Evocalcet, a Next-Generation Calcium-Sensing Receptor Agonist for the Treatment of Hyperparathyroidism. Bioorg. Med. Chem. Lett. 2018, 28, 2055–2060. [Google Scholar] [CrossRef]
- Heo, Y.-A. Baloxavir: First Global Approval. Drugs 2018, 78, 693–697. [Google Scholar] [CrossRef]
- Shibahara, S.; Fukui, N.; Maki, T.; Anan, K. Method for Producing Substituted Polycyclic Pyridone Derivative and Crystal of Same. WO2017221869A1, 28 December 2017. [Google Scholar]
- Hata, K.; Kimura, J.; Miki, H.; Toyosawa, T.; Nakamura, T.; Katsu, K. In Vitro and in Vivo Antifungal Activities of ER-30346, a Novel Oral Triazole with a Broad Antifungal Spectrum. Antimicrob. Agents Chemother. 1996, 40, 2237–2242. [Google Scholar] [CrossRef]
- Pesti, J.; Chen, C.-K.; Spangler, L.; DelMonte, A.J.; Benoit, S.; Berglund, D.; Bien, J.; Brodfuehrer, P.; Chan, Y.; Corbett, E.; et al. The Process Development of Ravuconazole: An Efficient Multikilogram Scale Preparation of an Antifungal Agent. Org. Process Res. Dev. 2009, 13, 716–728. [Google Scholar] [CrossRef]
- Gao, Q.; Chen, C.-P.H.; Fakes, M.G.; Pendri, Y.R.; Kiau, S.; Vakkalagadda, B. Preparation of Mono-Lysine Salts of Azole Compounds as Fungicides. WO2006118351A1, 9 November 2006. [Google Scholar]
- Vanover, K.E.; Davis, R.E.; Zhou, Y.; Ye, W.; Brasic, J.R.; Gapasin, L.; Saillard, J.; Weingart, M.; Litman, R.E.; Mates, S.; et al. Dopamine D2 Receptor Occupancy of Lumateperone (ITI-007): A Positron Emission Tomography Study in Patients with Schizophrenia. Neuropsychopharmacology 2019, 44, 598–605. [Google Scholar] [CrossRef]
- Li, P.; Zhang, Q.; Robichaud, A.J.; Lee, T.; Tomesch, J.; Yao, W.; Beard, J.D.; Snyder, G.L.; Zhu, H.; Peng, Y.; et al. Discovery of a Tetracyclic Quinoxaline Derivative as a Potent and orally active multifunctional drug candidate for the treatment of neuropsychiatric and neurological disorders. J. Med. Chem. 2014, 57, 2670–2682. [Google Scholar] [CrossRef]
- Markham, A. Tenapanor: First Approval. Drugs 2019, 79, 1897–1903. [Google Scholar] [CrossRef] [PubMed]
- Richter, J.; Dammer, O.; Krejcik, L.; Hejtmankova, L.; Lustig, P.; Dousa, M. Solid Forms of Tenapanor and Method of Preparation of Tenapanor. WO2019091503, 16 May 2019. [Google Scholar]
- Beuckmann, C.T.; Suzuki, M.; Ueno, T.; Nagaoka, K.; Arai, T.; Higashiyama, H. In vitro and in Silico Characterization of Lemborexant (E2006), A Novel Dual Orexin Receptor Antagonist. J. Pharmacol. Exp. Ther. 2017, 362, 287–295. [Google Scholar] [CrossRef]
- Yoshida, Y.; Naoe, Y.; Terauchi, T.; Ozaki, F.; Doko, T.; Takemura, A.; Tanaka, T.; Sorimachi, K.; Beuckmann, C.T.; Suzuki, M.; et al. Discovery of (1R,2S)-2-{[(2,4-dimethylpyrimidin-5-yl)oxy]methyl}-2-(3- fluorophenyl)-N-(5-fluoropyridin-2-yl)cyclopropanecarboxamide (E2006): A Potent and Efficacious Oral Orexin Receptor Antagonist. J. Med. Chem. 2015, 58, 4648–4664. [Google Scholar] [CrossRef]
- Sato, T.; Yamawaki, K. Cefiderocol: Discovery, Chemistry, and in vivo Profiles of a Novel Siderophore Cephalosporin. Clin. Infect. Dis. 2019, 69, 538–543. [Google Scholar] [CrossRef]
- Yasuhiro, N.; Kenji, Y.; Yusuke, T.; Hideki, S.; Shinya, H.; Toshiaki, A. Cephalosporin Having Catechol Group. AU2009310959, 7 May 2015. [Google Scholar]
- Duggan, S.; Keam, S.J. Upadacitinib: First Approval. Drugs 2019, 79, 1819–1828. [Google Scholar] [CrossRef]
- Wishart, N.; Argiriadi, M.A.; Calderwood, D.J.; Ericsson, A.M.; Fiamengo, B.R.; Frank, K.E.; Friedman, M.; George, D.M.; Goedken, E.R.; Josephsohn, N.S.; et al. Novel Tricyclic Compounds. US20110311474, 23 April 2013. [Google Scholar]
- Scott, L.J.; Chan, H.L.Y. Tenofovir Alafenamide: A Review in Chronic Hepatitis B. Drugs 2017, 77, 1017–1028. [Google Scholar] [CrossRef]
- Indukuri, V.S.K.; Joga, S.R.; Gorantla, S.R.; Chava, S. Process for the Preparation of Tenofovir. US 20140303368, 16-06-2015. US 20140303368, 16 June 2015. [Google Scholar]
- Tsiang, M.; Jones, G.S.; Goldsmith, J.; Mulato, A.; Hansen, D.; Kan, E.; Tsai, L.; Bam, R.A.; Stepan, G.; Stray, K.M.; et al. Antiviral Activity of Bictegravir (GS-9883), a Novel Potent HIV-1 Integrase Strand Transfer Inhibitor with an Improved Resistance Profile. Antimicrob. Agents Chemother. 2016, 60, 7086–7097. [Google Scholar] [CrossRef]
- Chiu, A.; Enquist, J.; Griggs, N.; Hale, C.; Ikemoto, N.; Keaton, K.A.; Kraft, M.; Lazerwith, S.E.; Leeman, M.; Peng, Z.; et al. Synthesis of Polycyclic-Carbamoylpyridone Compounds. US20150368264A1, 24 December 2015. [Google Scholar]
- Hughes, D.L. Review of Synthetic Routes and Final Forms of Integrase Inhibitors Dolutegravir, Cabotegravir, and Bictegravir. Org. Process Res. Dev. 2019, 23, 716–729. [Google Scholar] [CrossRef]
- Doi, Y. Treatment Options for Carbapenem-Resistant Gram-Negative Bacterial Infections. Clin. Infect. Dis. 2019, 69, 565–575. [Google Scholar] [CrossRef]
- Voelker, R. New Antibacterial Should be Used With Caution. J. Am. Med. Assoc. 2019, 322, 807. [Google Scholar] [CrossRef]
- Miller, S.P.; Limanto, J.; Zhong, Y.L.; Yasuda, N.; Liu, Z. Preparation of Tert-Butyl 4-((1R,2S,5R)-6-(benzyloxy)-7-oxo-1,6-diazabicyclo[3.2.1]octane-2- Carboxamido Piperidine-1-Carboxylate. WO2014200786, 18 December 2014. [Google Scholar]
- Terasawa, H.; Ejima, A.; Ohsuki, S.; Uoto, K. Hexa-cyclic Compound. US5834476, 10 November 1998. [Google Scholar]
- Markham, A. Pralsetinib: First Approval. Drugs 2020, 80, 1865–1870. [Google Scholar] [CrossRef] [PubMed]
- Brubaker, J.D.; Kim, J.L.; Wilson, K.J.; Wilson, D.; DiPietro, L.V. Inhibitors of RET. US20170121312, 24 July 2018. [Google Scholar]
- Scott, L.J. Rimegepant: First Approval. Drugs 2020, 80, 741–746. [Google Scholar] [CrossRef]
- Luo, G.; Chen, L.; Conway, C.M.; Denton, R.; Keavy, D.; Signor, L.; Kostich, W.; Lentz, K.A.; Santone, K.S.; Schartman, R.; et al. Discovery of (5S,6S,9R)-5-amino-6-(2,3- difluorophenyl)-6,7,8,9-tetrahydro-5H-cyclohepta[b]pyri din-9-yl-4-(2-oxo2,3-dihydro-1H-imidazo[4,5-b]pyridin-1-yl)piperidine-1-carboxylate (BMS927711): An Oral Calcitonin Gene-Related Peptide (CGRP) Antagonist in Clinical Trials for Treating Migraine. J. Med. Chem. 2012, 55, 10644–10651. [Google Scholar] [PubMed]
- Markham, A. Migalastat: First Global Approval. Drugs 2016, 76, 1147–1152. [Google Scholar] [CrossRef]
- Stuetz, A.; Steiner, A.; Wrodnigg, T. Preparation of IminoSugar Glycopeptide Conjugates via Catalytic Intramolecular Reductive Amination Reaction. EP 1,903,034, 26 March 2008. [Google Scholar]
- Kim, E.S. Midostaurin: First Global Approval. Drugs 2017, 77, 1251–1259. [Google Scholar] [CrossRef]
- Hoehn, P.; Koch, B.; Mutz, M. Process for Purifying Staurosporine. EP 2272850 B1, 10 May 2017. [Google Scholar]
- Markham, A. Naldemedine: First Global Approval. Drugs 2017, 77, 923–927. [Google Scholar] [CrossRef]
- Tamura, Y.; Noguchi, K.; Inagaki, M.; Morimoto, K.; Haga, N.; Oda, S.; Omura, S. Crystal of 6,7-Unsaturated-7-carbamoyl Morphinan Derivative and Method for Producing the Same. US 9108975B2, 19 April 2016. [Google Scholar]
- Citrome, L. Valbenazine for Tardive Dyskinesia: A Systematic Review of the Efficacy and Safety Profile for This Newly Approved Novel Medication—What Is the Number Needed to Treat, Number Needed to Harm and Likelihood to Be Helped or Harmed? Int. J. Clin. Pract. 2017, 71, e12964. [Google Scholar] [CrossRef]
- Boldt, K.G.; Biggers, M.S.; Phifer, S.S.; Brine, G.A.; Rehder, K.S. Synthesis of (+)- and (−)-Tetrabenazine from the Resolution of A-Dihydrotetrabenazine. Synth. Commun. 2009, 39, 3574–3585. [Google Scholar] [CrossRef]
- Xiao, X.-Y.; Hunt, D.K.; Zhou, J.; Clark, R.B.; Dunwoody, N.; Fyfe, C.; Grossman, T.H.; O’Brien, W.J.; Plamondon, L.; Ronn, M.; et al. Fluorocyclines. 1. 7-Fluoro-9-Pyrrolidinoacetamido-6-Demethyl-6-Deoxytetracycline: A Potent, Broad Spectrum Antibacterial Agent. J. Med. Chem. 2012, 55, 597–605. [Google Scholar] [CrossRef]
- Zhang, W.-Y.; Che, Q.; Crawford, S.; Ronn, M.; Dunwoody, N. A Divergent Route to Eravacycline. J. Org. Chem. 2017, 82, 936–943. [Google Scholar] [CrossRef]
- Zhou, J.; Xiao, X.-Y.; Plamondon, L.; Hunt, D.K.; Clark, R.B.; Zahler, R.B. Preparation of C7-Fluoro Substituted Tetracycline Compounds as Antibacterial Agents. WO 2010017470A1, 5 August 2014. [Google Scholar]
- Zhang, W.-Y.; Hogan, P.C.; Chen, C.-L.; Niu, J.; Wang, Z.; Lafrance, D.; Gilicky, O.; Dunwoody, N.; Ronn, M. Process Research and Development of an Enantiomerically Enriched Allylic Amine, One of the Key Intermediates for the Manufacture of Synthetic Tetracyclines. Org. Process Res. Dev. 2015, 19, 1784–1795. [Google Scholar] [CrossRef]
- Deeks, E.D. Sarecycline: First Global Approval. Drugs 2019, 79, 325–329. [Google Scholar] [CrossRef]
- Abato, P.; Assefa, H.; Berniac, J.; Bhatia, B.; Bowser, T.; Grier, M.; Honeyman, L.; Ismail, M.; Kim, O.K.; Nelson, M.; et al. Substituted Tetracycline Compounds for Treatment of Bacterial Infections and Neoplasms. WO2008079339A1, 3 July 2008. [Google Scholar]
- Nelson, M.L.; McIntyre, L. Preparation of 7-Substituted Fused Ring Tetracycline Compounds as Antibiotics. WO2001087824A1, 22 November 2001. [Google Scholar]
- Tanaka, S.K.; Steenbergen, J.; Villano, S. Discovery, Pharmacology, and Clinical Profile of Omadacycline, a Novel Aminomethylcycline Antibiotic. Bioorg. Med. Chem. 2016, 24, 6409–6419. [Google Scholar] [CrossRef]
- Markham, A.; Keam, S.J. Omadacycline: First Global Approval. Drugs 2018, 78, 1931–1937. [Google Scholar] [CrossRef]
- Johnston, S.; Warchol, T. Methods for Synthesizing and Purifying Aminoalkyl Tetracycline Compounds. WO2008134048A1, 6 November 2008. [Google Scholar]
- Keam, S.J. Vibegron: First Global Approval. Drugs 2018, 78, 1835–1839. [Google Scholar] [CrossRef]
- Xu, F.; Kosjek, B.; Cabirol, F.L.; Chen, H.; Desmond, R.; Park, J.; Gohel, A.P.; Collier, S.J.; Smith, D.J.; Liu, Z.; et al. Synthesis of Vibegron Enabled by a Ketoreductase Rationally Designed for High Ph Dynamic Kinetic Reduction. Angew. Chem. Int. Ed. 2018, 57, 6863–6867. [Google Scholar] [CrossRef]
- Dodick, D.W.; Lipton, R.B.; Ailani, J.; Lu, K.; Finnegan, M.; Trugman, J.M.; Szegedi, A. Ubrogepant for the Treatment of Migraine. N. Engl. J. Med. 2019, 381, 2230–2241. [Google Scholar] [CrossRef]
- Bell, I.M.; Fraley, M.E.; Gallicchio, S.N.; Ginnetti, A.; Mitchell, H.J.; Paone, D.V.; Staas, D.D.; Stevenson, H.E.; Wang, C.; Zartman, C.B. Piperidinone Carboxamide Azaindane CGRP Receptor Antagonists. WO2012064910, 18 May 2012. [Google Scholar]
- Dhillon, S. Decitabine/Cedazuridine: First Approval. Drugs 2020, 80, 1373–1378. [Google Scholar] [CrossRef]
- Ferraris, D.; Duvall, B.; Delahanty, G.; Mistry, B.; Alt, J.; Rojas, C.; Rowbottom, C.; Sanders, K.; Schuck, E.; Huang, K.C.; et al. Design, Synthesis, and Pharmacological Evaluation of Fluorinated Tetrahydrouridine Derivatives as Inhibitors of Cytidine Deaminase. J. Med. Chem. 2014, 57, 2582–2588. [Google Scholar] [CrossRef]
- Edmondson, S.D.; Zhu, C.; Kar, N.F.; Di Salvo, J.; Nagabukuro, H.; Sacre-Salem, B.; Dingley, K.; Berger, R.; Goble, S.D.; Morriello, G.; et al. Discovery of Vibegron: A Potent and Selective Beta3 Adrenergic Receptor Agonist for the Treatment of Overactive Bladder. j. Med. Chem. 2016, 59, 609–623. [Google Scholar] [CrossRef]
- de Bruijne, J.; Bergmann, J.F.; Reesink, H.W.; Weegink, C.J.; Molenkamp, R.; Schinkel, J.; Tong, X.; Li, J.; Treitel, A.; Hughes, E.A.; et al. Antiviral Activity of Narlaprevir Combined with Ritonavir and Pegylated Interferon in Chronic Hepatitis C Patients. Hepatology 2010, 52, 1590–1599. [Google Scholar] [CrossRef] [PubMed]
- Traverse, J.; Leong, W.M.; Miller, S.P.; Albaneze-Walker, J.; Hunter, T.J.; Wang, L.; Liao, H.; Arasappan, A.; Trzaska, S.T.; Smith, R.M.; et al. Enantio- and Stereo Specific Synthesis of β-Amino-αHydroxy Amides. US 8680294B2, 25 March 2014. [Google Scholar]
- Traverse, J.; Leong, W.W.; Miller, S.P.; Albaneze-Walker, J.; Hunter, T.J.; Wang, L.; Liao, H.; Arasappan, A.; Trzaska, S.T.; Smith, R.M.; et al. Processes for Enantioand Stereospecific Syntheses of β-Amino-α-Hydroxy Amides. WO 2011014494 A1, 3 February 2011. [Google Scholar]
- Keating, G.M. Elbasvir/Grazoprevir: First Global Approval. Drugs 2016, 76, 617–624. [Google Scholar] [CrossRef] [PubMed]
- Hayes, A.M.; Morris, D.J.; Clarkson, G.J.; Wills, M. A Class of Ruthenium(II) Catalyst for Asymmetric Transfer Hydrogenations of Ketones. J. Am. Chem. Soc. 2005, 127, 7318–7319. [Google Scholar] [CrossRef]
- Impagnatiello, F.; Bastia, E.; Almirante, N.; Brambilla, S.; Duquesroix, B.; Kothe, A.C.; Bergamini, M.V.W. Prostaglandin Analogues and Nitric Oxide Contribution in the Treatment of Ocular Hypertension and Glaucoma. Br. J. Pharmacol. 2019, 176, 1079–1089. [Google Scholar] [CrossRef]
- Ongini, E.; Benedini, F.; Chiroli, V.; Del Soldato, P. Preparation of Prostaglandin Nitrooxy Derivatives for the Treatment of Glaucoma. WO 2005068421, 9 February 2006. [Google Scholar]
- Vittitow, J.L.; Cavet, M.E. Nitric Oxide Releasing Prostaglandin Derivatives for Treating Normal Tension Glaucoma. WO 2018087092, 17 May 2018. [Google Scholar]
- Markham, A.; Keam, S.J. Danoprevir: First Global Approval. Drugs 2018, 78, 1271–1276. [Google Scholar] [CrossRef]
- Beaulieu, P.L.; Gillard, J.; Bailey, M.D.; Boucher, C.; Duceppe, J.-S.; Simoneau, B.; Wang, X.-J.; Zhang, L.; Grozinger, K.; Houpis, I.; et al. Synthesis of (1r,2s)-1-Amino-2-Vinylcyclopropanecarboxylic Acid Vinyl-ACCA) Derivatives: Key Intermediates for the Preparation of Inhibitors of the Hepatitis C Virus NS3 Protease. J. Org. Chem. 2005, 70, 5869–5879. [Google Scholar] [CrossRef]
- Greig, S.L. Sofosbuvir/Velpatasvir: A Review in Chronic Hepatitis C. Drugs 2016, 76, 1567–1578. [Google Scholar] [CrossRef]
- Allan, K.M.; Fujimori, S.; Heumann, L.V.; Huynh, G.M.; Keaton, K.A.; Levins, C.M.; Pamulapati, G.R.; Roberts, B.J.; Sarma, K.; Teresk, M.G.; et al. Processes for Preparing Peptide Analog as Antiviral Agent. WO 2015191437A1, 17 December 2015. [Google Scholar]
- Nevens, F.; Andreone, P.; Mazzella, G.; Strasser, S.I.; Bowlus, C.; Invernizzi, P.; Drenth, J.P.H.; Pockros, P.J.; Regula, J.; Beuers, U.; et al. A Placebo-Controlled Trial of Obeticholic Acid in Primary Biliary Cholangitis. N. Engl. J. Med. 2016, 375, 631–643. [Google Scholar] [CrossRef]
- Steiner, A.; Waenerlund Poulsen, H.; Jolibois, E.; Rewolinski, M.; Gross, R.; Sharp, E.; Dubas-Fisher, F.; Eberlin, A. Preparation and Uses of Obeticholic Acid. WO 20130345188, 19 January 2016. [Google Scholar]
- Ferrari, M.; Pellicciari, R. Process for Preparing 3a(β)-7a(β)- Dihydroxy-6a(β)-Alkyl-5β-Cholanic Acid. US 7994352, 9 August 2011. [Google Scholar]
- Bassan, E.M.; Baxter, C.A.; Beutner, G.L.; Emerson, K.M.; Fleitz, F.J.; Johnson, S.; Keen, S.; Kim, M.M.; Kuethe, J.T.; Leonard, W.R.; et al. Multikilogram Scale Synthesis of a Chiral Cyclopropanol and an Investigation of the Safe Use of Lithium Acetylide−Ethylene Diamine Complex. Org. Process Res. Dev. 2012, 16, 87–95. [Google Scholar] [CrossRef]
- Markham, A. Ertugliflozin: First Global Approval. Drugs 2018, 78, 513–519. [Google Scholar] [CrossRef]
- Bowles, P.; Brenek, S.J.; Caron, S.; Do, N.M.; Drexler, M.T.; Duan, S.; Dube, P.; Hansen, E.C.; Jones, B.P.; Jones, K.N.; et al. Commercial Route Research and Development for Sglt2 Inhibitor Candidate Ertugliflozin. Org. Process Res. Dev. 2014, 18, 66–81. [Google Scholar] [CrossRef]
- Heo, Y.-A.; Deeks, E.D. Sofosbuvir/Velpatasvir/Voxilaprevir: A Review in Chronic Hepatitis C. Drugs 2018, 78, 577–587. [Google Scholar] [CrossRef]
- Cagulada, A.; Chan, J.; Chan, L.; Colby, D.A.; Karki, K.K.; Kato, D.; Keaton, K.A.; Kondapally, S.; Levins, C.; Littke, A.; et al. Synthesis of an Antiviral N-(3-Ethyl)prolyl-1- aminocyclopropanecarboxylic Acid Peptide and New Routes to Its Difluoromethylaminocyclopropanecarboxylic Acid Intermediate. US 20150175626A1, 13 September 2016. [Google Scholar]
- Lamb, Y.N. Glecaprevir/Pibrentasvir: First Global Approval. Drugs 2017, 77, 1797–1804. [Google Scholar] [CrossRef]
- Periasamy, M.; Seenivasaperumal, M.; Rao, V.D. Convenient Procedures for the Asymmetric Reduction of 1,4- Diphenylbutane-1,4-Dione and Synthesis of 2,5-Diphenylpyrrolidine Derivatives. Synthesis 2003, 35, 2507–2510. [Google Scholar] [CrossRef]
- Bjornson, K.; Karki, K.K.; Link, J.O.; Pyun, H.-J.; Schrier, A.J.; Stevens, K.L.; Taylor, J.G.; Vivian, R.W.; Zablocki, J.; Zipfel, S. Preparation of Macrocyclic and Bicyclic Derivatives of N-Prolyl-1- aminocyclopropanecarboxylic Acid Peptides as Inhibitors of Hepatitis C Virus. WO 2014145095A1, 18 September 2014. [Google Scholar]
- Mehrhof, W.; Irmscher, K.; Erb, R.; Pohl, L. Synthesewege Zum 17a-Hydroxy-16-Methylen-19-nor-Progesteron Und Seinen Derivaten. Chem. Ber. 1969, 102, 643–658. [Google Scholar] [CrossRef]
- Urdiales, J.; Morata, P.; De Castro, I.N.; Sánchez-Jiménez, F. Antiproliferative Effect of Dehydrodidemnin B (DDB), a Depsipeptide Isolated from Mediterranean Tunicates. Cancer Lett. 1996, 102, 31–37. [Google Scholar] [CrossRef]
- Rodriguez, I.; Polanco, C.; Cuevas, F.; Mandez, P.; Cuevas, C.; Gallego, P.; Munt, S.; Manzanares, I. Synthetic Methods for Aplidine and New Antitumoral Derivatives, Methods of Making and Using Them. WO2002002596A2, 23 May 2002. [Google Scholar]
- Wilby, K.J.; Eissa, N.A. Clinical Pharmacokinetics and Drug Interactions of Doravirine. Eur. J. Drug. Metab. Pharmacokinet. 2018, 43, 637–644. [Google Scholar] [CrossRef]
- Dai, Y.; Du, Z.; Wang, R. Preparation of Moxidectin. CN104017001B, 13 January 2016. [Google Scholar]
- McCarthy, M.W. Plazomicin for the Treatment of Patients with Complicated Urinary Tract Infection. Drugs Today 2017, 54, 513–518. [Google Scholar] [CrossRef] [PubMed]
- Nagabhushan, T.L. Process for the Manufacture of 6′-NAlkyl Derivatives of Sisomicin and Verdamicin; Novel Intermediate Useful Therein, and Novel 6′-N-Alkylverdamicins Prepared Thereby. US3997524A, 14 December 1976. [Google Scholar]
- Aggen, J.; Goldblum, A.A.; Linsell, M.; Dozzo, P.; Moser, H.E.; Hildebrandt, D.; Gliedt, M. Antibacterial Aminoglycoside Analogs. WO2009067692A1, 28 May 2009. [Google Scholar]
- Aggen, J.B.; Armstrong, E.S.; Goldblum, A.A.; Dozzo, P.; Linsell, M.S.; Gliedt, M.J.; Hildebrandt, D.J.; Feeney, L.A.; Kubo, A.; Matias, R.D.; et al. Synthesis and Spectrum of the Neoglycoside ACHN490. Antimicrob. Agents Chemother. 2010, 54, 4636–4642. [Google Scholar] [CrossRef]
- Bruss, J.B.; Miller, G.H.; Aggen, J.B.; Armstrong, E.S. Treatment of Urinary Tract Infections with Antibacterial Aminoglycoside Compounds. WO2010132777A2, 20 January 2011. [Google Scholar]
- Bruss, J.B.; Miller, G.H.; Aggen, J.B.; Armstrong, E.S. Treatment of Klebsiella Pneumoniae Infections with Antibacterial Aminoglycoside Compounds. WO2010132770A1, 18 November 2010. [Google Scholar]
- Trend, R.; Dappen, M.; Henry, C.E.; Goldblum, A.A.; Aggen, J.B.; Mendonca, R.F.d.J.G.; Sardinha, J.C.F. Synthesis of Antibacterial Aminoglycoside Analogs. WO2019079613A1, 25 April 2019. [Google Scholar]
- Hoy, S.M. Tecovirimat: First Global Approval. Drugs 2018, 78, 1377–1382. [Google Scholar] [CrossRef]
- Hughes, D.L. Review of the Patent Literature: Synthesis and Final Forms of Antiviral Drugs Tecovirimat and Baloxavir Marboxil. Org. Process Res. Dev. 2019, 23, 1298–1307. [Google Scholar] [CrossRef]
- Voelker, R. New Antibiotic for Community-Acquired Pneumonia. J. Am. Med. Assoc. 2019, 322, 1246. [Google Scholar] [CrossRef]
- Riedl, R.; Heilmayer, W.; Spence, L. Process for the Preparation of Pleuromutilins in Crystalline Form. WO201114, 1 December 2011. [Google Scholar]
- Langendonk, J.G.; Balwani, M.; Anderson, K.E.; Bonkovsky, H.L.; Anstey, A.V.; Bissell, D.M.; Bloomer, J.; Edwards, C.; Neumann, N.J.; Parker, C.; et al. Afamelanotide for Erythropoietic Protoporphyria. N. Engl. J. Med. 2015, 373, 48–59. [Google Scholar] [CrossRef]
- Robert, D.; Jung, L. Aromatic Amides Absorbing the UV. WO1987004923, 27 August 1987. [Google Scholar]
- Lensing, C.J.; Freeman, K.T.; Schnell, S.M.; Speth, R.C.; Zarth, A.T.; HaskellLuevano, C. Developing a Biased Unmatched Bivalent Ligand (BUmBL) Design Strategy to Target the GPCR Homodimer Allosteric Signaling (cAMP over barrestin 2 recruitment) Within the Melanocortin Receptors. J. Med. Chem. 2019, 62, 144–158. [Google Scholar] [CrossRef]
- Scott, L.J. Brexanolone: First Global Approval. Drugs 2019, 79, 779–783. [Google Scholar] [CrossRef]
- MacNevin, C.J.; Atif, F.; Sayeed, I.; Stein, D.G.; Liotta, D.C. Development and Screening of Water-Soluble Analogues of Progesterone and Allopregnanolone in Models of Brain Injury. J. Med. Chem. 2009, 52, 6012–6023. [Google Scholar] [CrossRef]
- Dhillon, S.; Keam, S.J. Bremelanotide: First Approval. Drugs 2019, 79, 1599–1606. [Google Scholar] [CrossRef]
- Yan, L.; Wang, B.; Li, J.; Jin, Y.; Wang, J.; Yang, Z. A Synthetic Method for Bremelanotide. CN10658911, 29 December 2020. [Google Scholar]
- Markham, A. Lurbinectedin: First Approval. Drugs 2020, 80, 1345–1353. [Google Scholar] [CrossRef]
- He, W.; Zhang, Z.; Ma, D. A scalable Total Synthesis of the Antitumor Agents Et743 and Lurbinectedin. Angew. Chem. Int. Ed. Engl. 2019, 58, 3972–3975. [Google Scholar] [CrossRef]
- Li, X.Q.; Zhang, X.M.; Wu, X.; Lan, Y.; Xu, L.; Meng, X.C.; Li, J.N. Beneficial Effects of Lactitol on the Composition of Gut Microbiota in Constipated Patients. J. Dig. Dis. 2020, 21, 445–453. [Google Scholar] [CrossRef]
- Sun, T.J.; Yang, J.; Lu, H.G.; Zhao, W.J.; Feng, J.P.; Gao, L.H.; Zhu, N.Q.; Zhang, H.Y.; Huo, H.H.; Zhang, Y.B. Preparation of Lactitol. CN101481395, 31 August 2011. [Google Scholar]
- Haws, R.; Brady, S.; Davis, E.; Fletty, K.; Yuan, G.; Gordon, G.; Stewart, M.; Yanovski, J. Effect of Setmelanotide, A Melanocortin-4 Receptor Agonist, on Obesity in Bardet-Biedl Syndrome. Diabetes Obes. Metabol. 2020, 22, 2133–2140. [Google Scholar] [CrossRef]
- Zheng Xin, D. Process for the Synthesis of Ac-Arg-cyclo(Cys-D-Ala-His-DPhe-Arg-Trp-Cys)-NH2. WO2011060355, 19 May 2011. [Google Scholar]
- Dhillon, S. Clascoterone: First Approval. Drugs 2020, 80, 1745–1750. [Google Scholar] [CrossRef]
- Ajani, M.; Moro, L. Enzymatic Process for Obtaining 17 Alpha-Monoesters of Cortexolone And/or its 9,11-dehydroderivatives. WO2009019138, 15 October 2009. [Google Scholar]
- Dong, P.X.; Mei, Z.; Zhao, Z.J.; Yi, Y.Y. Simple Process for Preparing Artesunate by One-Pot Method by Taking Artemisinin as Raw Material. CN 102887908, 23 January 2013. [Google Scholar]
- Lamb, Y.N. Remdesivir: First Approval. Drugs 2020, 80, 1355–1363. [Google Scholar] [CrossRef]
- Clarke, M.O.H.; Jordan, R.; Mackman, R.L.; Ray, A.S.; Siegel, D. Methods for Treating Flaviviridae Virus Infections. WO2017184668, 26 October 2017. [Google Scholar]




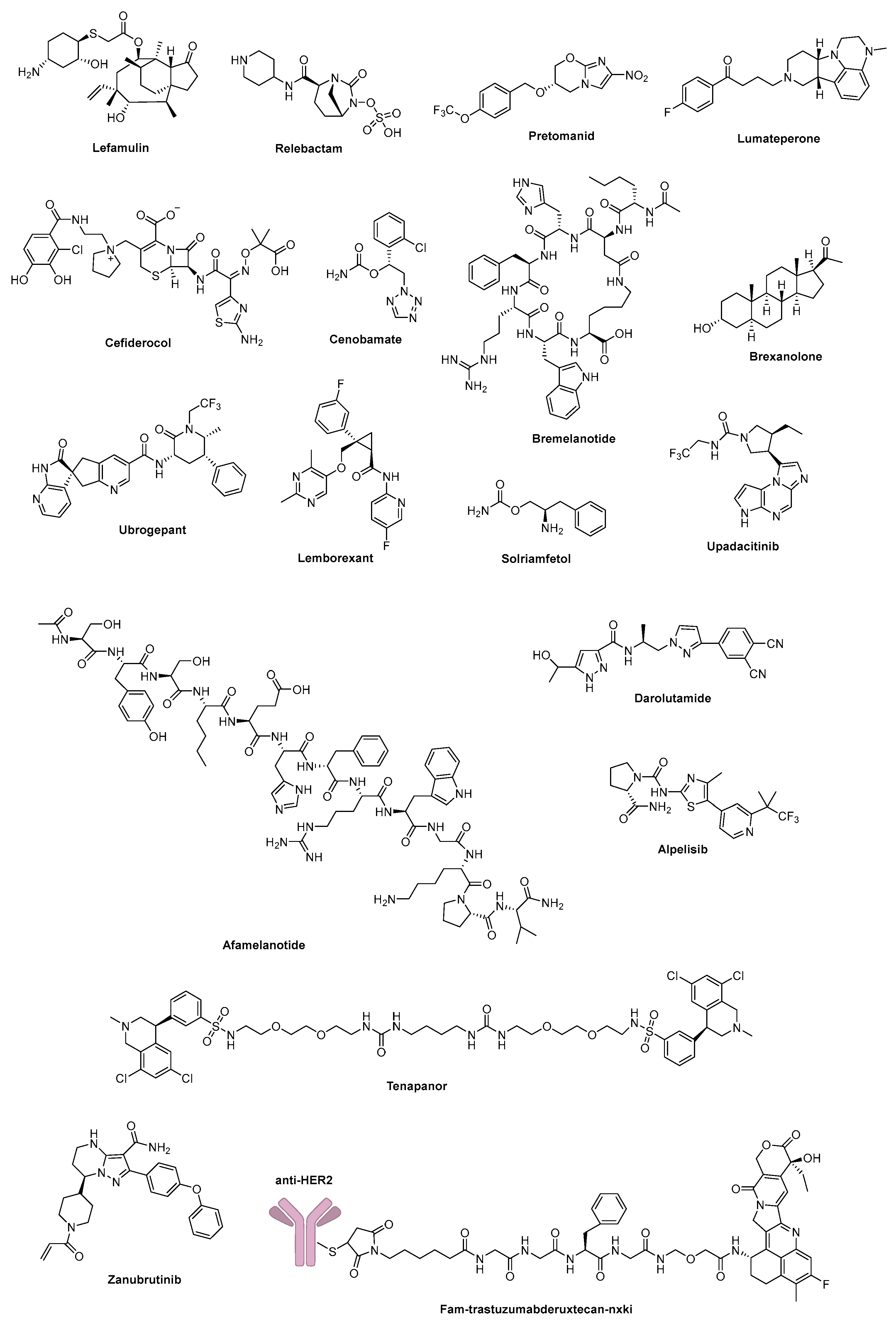






























































Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tamatam, R.; Shin, D. Asymmetric Synthesis of US-FDA Approved Drugs over Five Years (2016–2020): A Recapitulation of Chirality. Pharmaceuticals 2023, 16, 339. https://doi.org/10.3390/ph16030339
Tamatam R, Shin D. Asymmetric Synthesis of US-FDA Approved Drugs over Five Years (2016–2020): A Recapitulation of Chirality. Pharmaceuticals. 2023; 16(3):339. https://doi.org/10.3390/ph16030339
Chicago/Turabian StyleTamatam, Rekha, and Dongyun Shin. 2023. "Asymmetric Synthesis of US-FDA Approved Drugs over Five Years (2016–2020): A Recapitulation of Chirality" Pharmaceuticals 16, no. 3: 339. https://doi.org/10.3390/ph16030339
APA StyleTamatam, R., & Shin, D. (2023). Asymmetric Synthesis of US-FDA Approved Drugs over Five Years (2016–2020): A Recapitulation of Chirality. Pharmaceuticals, 16(3), 339. https://doi.org/10.3390/ph16030339




