Evaluation of Novel Inhibitors of Tryptophan Dioxygenases for Enzyme and Species Selectivity Using Engineered Tumour Cell Lines Expressing Either Murine or Human IDO1 or TDO2
Abstract
1. Introduction
2. Results and Discussion
2.1. Establishment of LLTC Lines Constitutively Expressing Either Murine or Human IDO1 for Cell-Based Assays of Tryptophan Dioxygenases
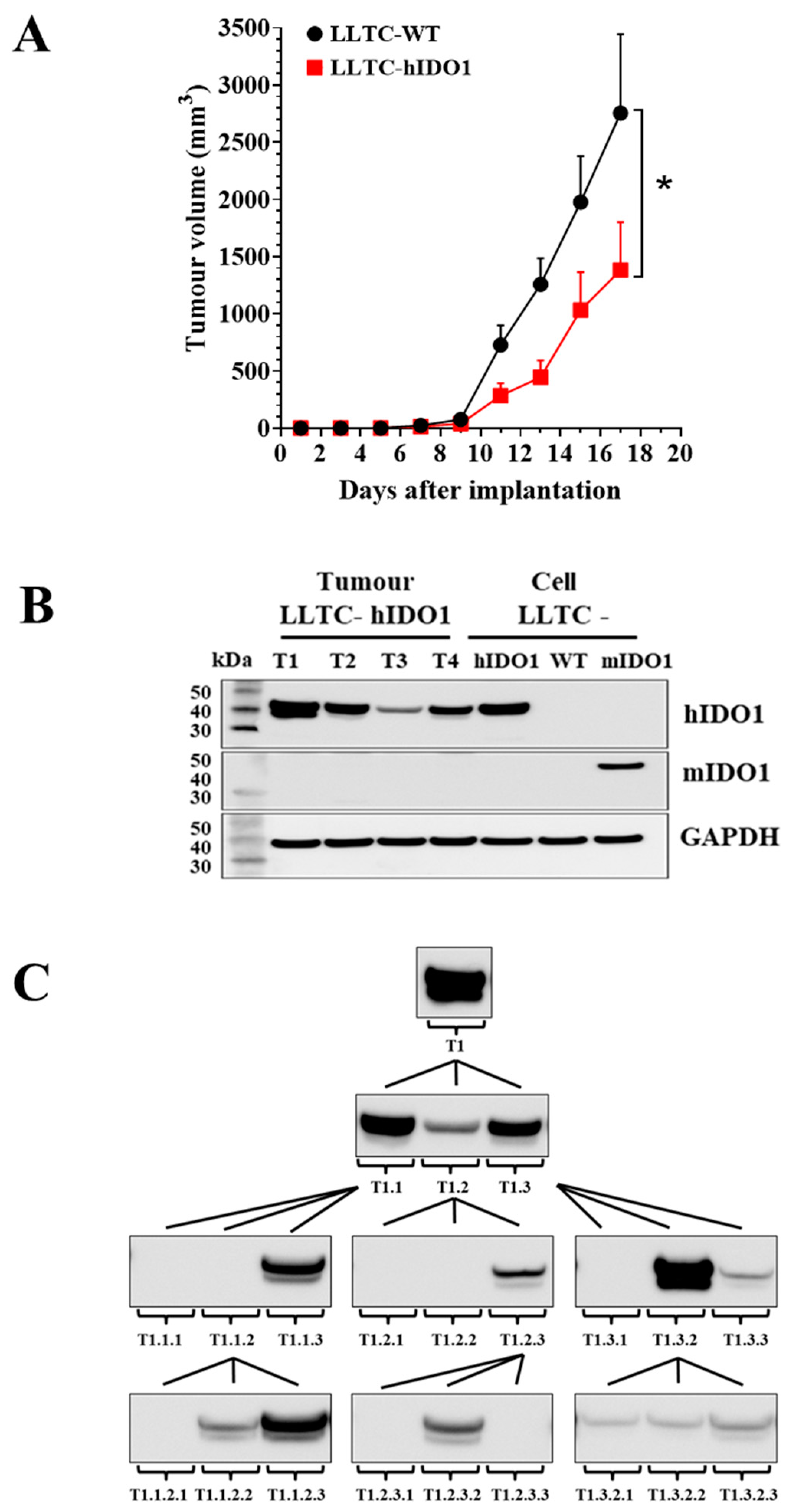
2.2. IDO1 Expression and Growth of LLTC-WT and LLTC-hIDO1 Tumours in Mice
2.3. Growth and IDO1 Expression by GL261-WT and GL261-hIDO1 Tumours in Mice
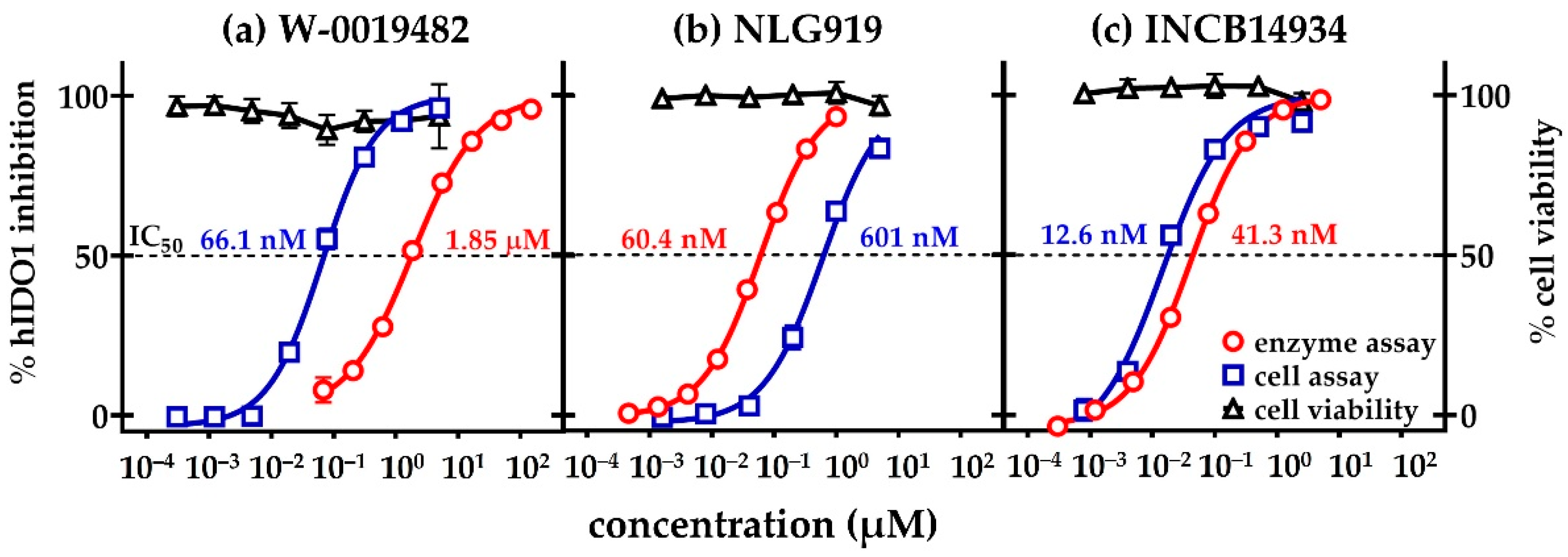
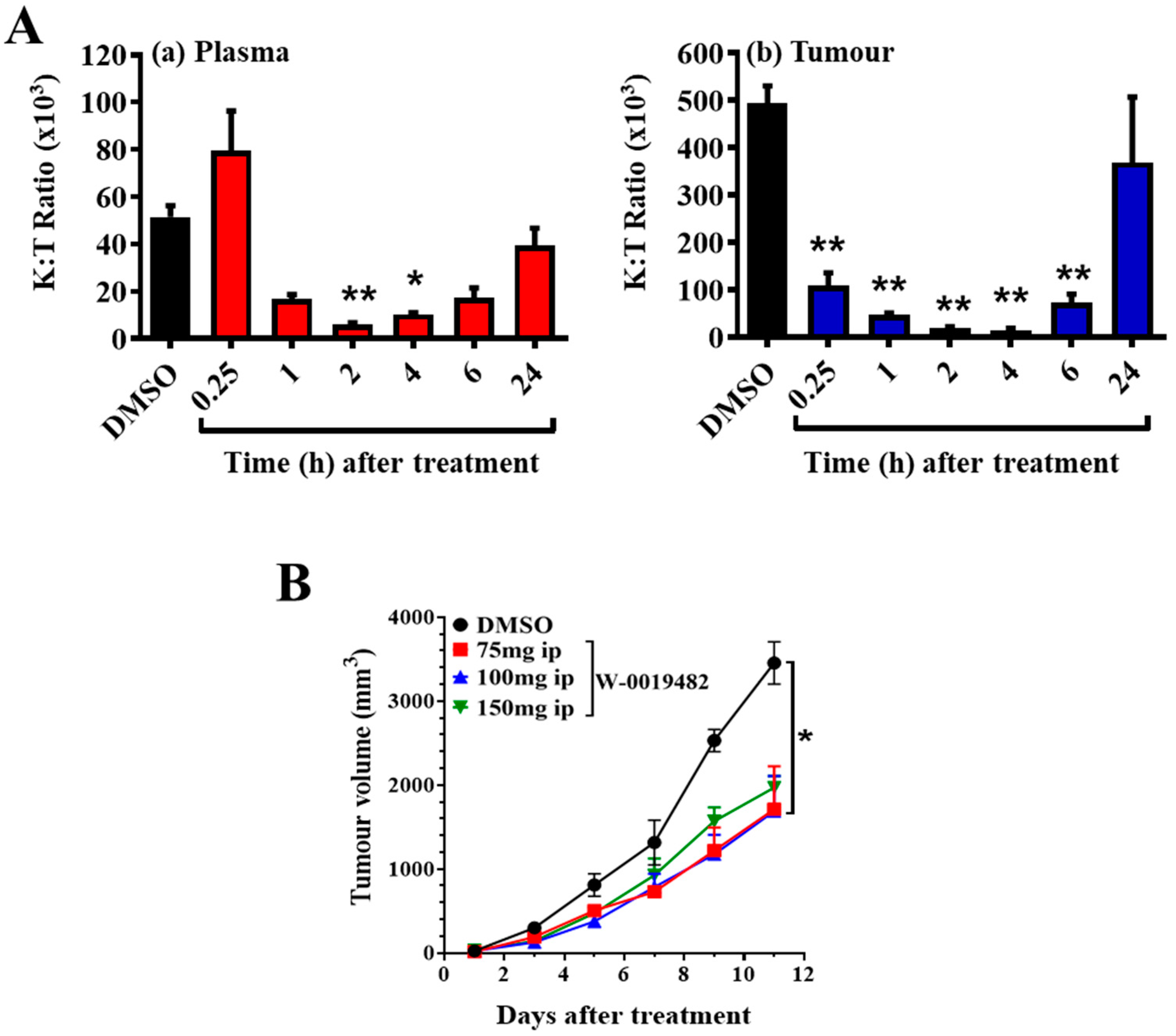
2.4. Evaluation of W-0019482 as a Lead for the Synthesis of New IDO1 Inhibitors for Cancer Therapy
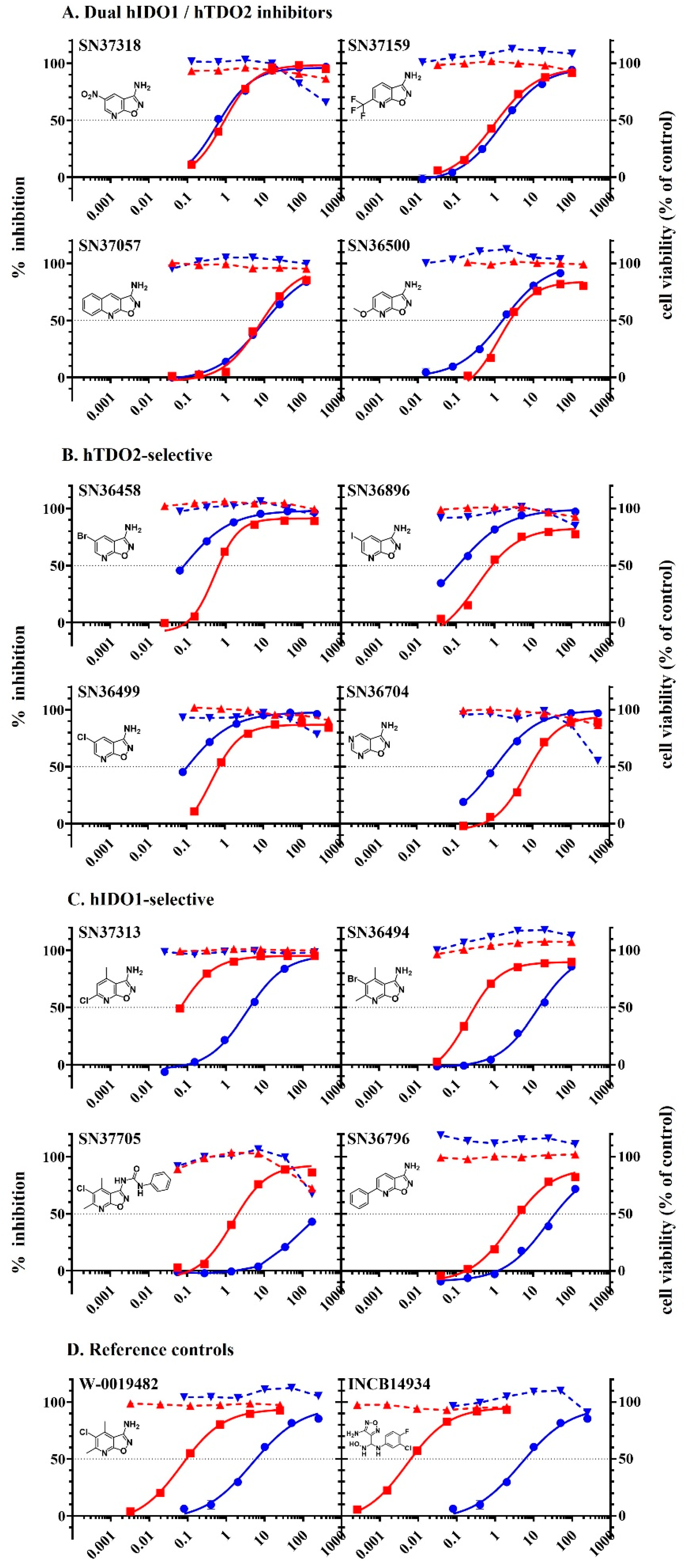
2.5. Inhibition of Tryptophan Dioxygenases by Analogues of W-0019482
3. Materials and Methods
3.1. Drugs and Reagents
3.2. Cell lines and Tissue Culture
3.3. Engineered LLTC and GL261 Cell Lines Constitutively Expressing Tryptophan Dioxygenases
3.4. Western Blots of IDO1 in Cell Lines
3.5. Immunostaining of Cytospots for Dioxygenase Expression
3.6. Cell-Based Assays for Inhibition of IDO1 or TDO2 and Cytotoxicity of Test Compounds
3.7. Mice and Tumour Implantations
3.8. Immune Cell Infiltrates in Subcutaneous GL261-WT or GL261-hIDO1 Tumours
3.9. Determination of Tumour and Plasma Kynurenine and Tryptophan Concentrations
3.10. Inhibition of Tumour Growth by Dioxygenase Inhibitors in Mice
3.11. Statistical Analyses
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef] [PubMed]
- Uyttenhove, C.; Pilotte, L.; Théate, I.; Stroobant, V.; Colau, D.; Parmentier, N.; Boon, T.; Van den Eynde, B.J. Evidence for a tumoral immune resistance mechanism based on tryptophan degradation by indoleamine 2,3-dioxygense. Nat. Med. 2003, 9, 1269–1274. [Google Scholar] [CrossRef] [PubMed]
- Takikawa, O.; Kuroiwa, T.; Yamazaki, F.; Kido, R. Mechanism of interferon-gamma action-characterizarion of indoleamine 2,3-dioxygenase in cultured human cells induced by interferon-gamma and evaluation of the enzyme-mediated tryptophan degradation in its anticellular activity. J. Biol. Chem. 1998, 189, 461–466. [Google Scholar]
- Munn, D.H.; Zhou, M.; Attwood, J.T.; Bondarev, I.; Pashine, A.; Mellor, A.L. Inhibition of T cell proliferation by macrophage tryptophan catabolism. J. Exp. Med. 1999, 189, 1363–1372. [Google Scholar] [CrossRef] [PubMed]
- Mellor, A.L.; Keskin, D.B.; Johnson, T.; Chandler, P.; Munn, D. Cells expressing indoleamine 2,3-dioxygenase inhibit T cell responses. J. Immunol. 2002, 168, 3771–3776. [Google Scholar] [CrossRef]
- Bauer, T.M.; Jiga, L.P.; Chuang, J.-J.; Randazzo, M.; Opelz, G.; Terness, P. Studying the immunosuppressive role of indoleamine 2,3-dioxygenase: Tryptophan metabolites suppress rat allogeneic T-cell responses in vitro and in vivo. Transpl. Int. 2005, 18, 95–100. [Google Scholar] [CrossRef]
- Frumento, G.; Rotondo, R.; Tonetti, M.; Damonte, G.; Benatti, U.; Ferrara, G.B. Tryptophan-derived catabolites are responsible for inhibition of T and natural killer cell proliferation induced by indoleamine 2,3-dioxygenase. J. Exp. Med. 2002, 196, 459–468. [Google Scholar] [CrossRef]
- Munn, D.H.; Zhou, M.; Attwood, J.T.; Bonarev, I.; Conway, S.J.; Marshalll, B.; Brown, C.; Mellor, A.L. Prevention of allogeneic fetal rejection by tryptophan catabolism. Science 1998, 281, 1191–1193. [Google Scholar] [CrossRef]
- Godin-Ethier, J.; Hanafi, L.-A.; Piccirillo, C.A.; Lapointe, R. Indoleamine 2,3-dioxygenase expression in human cancers: Clinical and immunologic perspectives. Clin. Can. Res. 2012, 17, 6985–6991. [Google Scholar] [CrossRef]
- Curti, A.; Trabanelli, S.; Salvestrini, V.; Baccarani, M.; Lemoli, R.M. The role of indoleamine 2,3-dioxygenase in the induction of immune tolerance: Focus on hematology. Blood 2009, 113, 2394–2401. [Google Scholar] [CrossRef]
- Wainwright, D.A.; Balyasnikova, I.V.; Chang, A.L.; Ahmed, A.U.; Moon, K.-S.; Auffinger, B.; Tobias, A.L.; Han, Y.; Lesniak, M.S. IDO expression in brain tumors increase the recruitment of regulatory T cells and negatively impacts survival. Clin. Can. Res. 2012, 18, 6110–6121. [Google Scholar] [CrossRef] [PubMed]
- Brody, J.R.; Costantino, C.L.; Berger, A.C.; Sato, T.; Lisanti, M.P.; Yeo, C.J.; Emmons, R.V.; Witkiewicz, A.K. Expression of indoleamine 2,3-dioxygense in metastatic malignant melanoma recruits regulatory T cell to avoid immune detection and affects survival. Cell Cycle 2009, 8, 1930–1934. [Google Scholar] [CrossRef]
- Feder-Mengus, C.; Wyler, S.; Hudolin, T.; Ruszat, R.; Bubendorf, L.; Chiarugi, A.; Pittelli, M.; Weber, W.P.; Bachmann, A.; Gasser, T.C.; et al. High expression of indoleamine 2,3-dioxygenase gene in prostate cancer. Eur. J. Cancer 2008, 44, 2266–2275. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Inaba, T.; Ino, K.; Kajiyama, H.; Kajiyama, H.; Yamamoto, E.; Shibata, K.; Nawa, A.; Nagasaka, T.; Takikawa, O.; Takahashi, N.; et al. Indoleamine 2,3 dioxygenase is a novel prognostic indicator for endometrial cancer. Br. J. Cancer 2006, 95, 1555–1561. [Google Scholar]
- Ino, K.; Yoshida, N.; Kajiyama, H.; Shibata, K.; Yamamoto, E.; Kidokoro, K.; Terauchi, M.; Nawa, A.; Nomura, S.; Nagasaka, T.; et al. Role of immunosuppressive enzyme indoleamine 2,3-dioxygenase in the progression of ovarian carcinoma. Gynecol. Oncol. 2009, 115, 185–192. [Google Scholar]
- Yu, J.; Sun, J.; Wang, S.E.; Li, H.; Cao, S.; Cong, Y.; Liu, J.; Ren, X. Upregulated expression of indoleamine 2,3-dioxygenase in primary breast cancer correlates with increase of infiltrated regulatory T cells in situ and lymph node metastasis. Clin. Dev. Immunol. 2011, 2011, 469135. [Google Scholar] [CrossRef]
- Witkiewicz, A.; Williams, T.K.; Cozzitorto, J.; Durkan, B.; Showalter, S.L.; Yeo, C.J.; Brody, J.R. Expression of indoleamine 2,3-dioxygenase in metastatic pancreatic ductal adenocarcinoma recruits regulatory T cells to avoid immune detection. J. Am. Coll. Surg. 2008, 206, 849–854. [Google Scholar] [CrossRef]
- Suzuki, Y.; Suda, T.; Furuhashi, K.; Suzuki, M.; Fujie, M.; Hahimoto, D.; Nakamura, Y.; Inui, N.; Nakamura, H.; Chida, K. Increased serum kynurenine/tryptophan ratio correlates with disease progression in lung cancer. Lung Cancer 2010, 67, 361–365. [Google Scholar] [CrossRef]
- Astigiano, S.; Morandi, B.; Costa, R.; Mastracci, L.; D’Agostino, A.; Ratto, G.B.; Melioli, G.; Frumento, G. Eosinophil granulocytes account for indoleamine 2,3-dioxygenase-mediated immune escape in human non-small cell lung cancer. Neoplasia 2005, 7, 390–396. [Google Scholar] [CrossRef]
- Brandacher, G.; Perathoner, A.; Ladurner, R.; Schneeberger, S.; Obrist, P.; Winkler, C.; Werner, E.R.; Werner-Felmayer, G.; Weiss, H.G.; Gobel, G.; et al. Prognostic value of indoleamine 2,3-dioxygenase expression in colorectal cancer: Effect on tumor-infiltrating T cells. Clin. Cancer Res. 2006, 12, 1114–1151. [Google Scholar] [CrossRef]
- Ferdinande, L.; Decaestecker, C.; Verset, L.; Mathieu, A.; Lopez, X.M.; Negulescu, A.-M.; Van Maerken, T.; Salmon, I.; Cuvelier, C.A.; Demetter, P. Clinicopathological significance of indoleamine 2,3-dioxygenase 1 expression in colorectal cancer. Br. J. Cancer 2011, 106, 141–147. [Google Scholar] [CrossRef] [PubMed]
- Palmer, B.D.; Ching, L.-M. Inhibitors of Indoleamine 2,3-dioxygenase 1 (IDO1) and their Use in Cancer Therapy. IPONZ NZ Patent 628688, 13 August 2014. [Google Scholar]
- Tomek, P.; Palmer, B.D.; Flanagan, J.U.; Fung, S.-P.S.; Bridewell, D.J.A.; Jamie, J.F.; Ching, L.-M. Discovery and characterization of hydrazine derivatives as inhibitors of the immune inhibitory enzyme, indoleamine 2,3-dioxygenase 1 (IDO1). Bioorg. Med. Chem. 2013, 21, 7595–7603. [Google Scholar]
- Tomek, P.; Palmer, B.D.; Flanagan, J.U.; Fung, S.-P.S.; Bridewell, D.J.A.; Jamie, J.F.; Ching, L.-M. Formation of an N-formylkynurenine-derived fluorophore and its use for measuring indoleamine 2,3-dioxygenase 1 activity. Anal. Bioanal. Chem. 2013, 405, 2515–2524. [Google Scholar] [CrossRef] [PubMed]
- Ching, L.-M.; Palmer, B.D.; Tomek, P.; Flanagan, J.U.; Henare, K. A Novel Class of Inhibitors of the Immunosuppressive Enzyme indoleamine 2,3-dioxygenase 1 (IDO1) for the Treatment of Cancer; Abstract 4469; Proc of American Association for Cancer Research: Philadelphia, PA, USA, 2015. [Google Scholar]
- Yue, E.W.; Douty, B.; Wayland, B.; Bower, M.; Liu, X.; Leffet, L.; Wang, Q.; Bowman, K.J.; Hansbury, M.J.; Liu, C.; et al. Discovery of potent competitive inhibitors of indoleamine 2,3-dioxygenase with in vivo pharmacodynamic activity and efficacy in a mouse melanoma model. J. Med. Chem. 2009, 52, 7364–7367. [Google Scholar] [CrossRef] [PubMed]
- Röhrig, U.F.; Awad, L.; Grosdidier, A.; Larrieu, P.; Stroobant, V.; Colau, D.; Cerundolo, V.; Simpson, A.J.G.; Vogel, P.; Eynde, B.J.V.D.; et al. Rational design of indoleamine 2,3-dioxygenase inhibitors. J. Med. Chem. 2010, 53, 1172–1189. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Jaller, D.; Patel, B.; LaLonde, J.M.; DuHadaway, J.B.; Malachowski, W.P.; Prendergast, G.C.; Muller, A.J. Structure based development of phenylimidazole-derived inhibitors of indoleamine 2,3-dioxygenase. J. Med. Chem. 2008, 51, 4968–4977. [Google Scholar] [CrossRef]
- Dolusic, E.; Frederick, R. Indoleamine 2,3-dioxygenase inhibitors: A patent review (2008–2012). Expert Opin. Ther. Pat. 2013, 23, 1367–1381. [Google Scholar] [CrossRef]
- Long, G.V.; Dummer, R.; Hamid, O.; Gajewski, T.F.; Caglevic, C.; Dalle, S.; Arance, A.; Carlino, M.S.; Grob, J.J.; Kim, T.M.; et al. Epacodostat plus pemdrolizumab versus placebo plus pembrolizumab in patients with unresectable or metastatic melanoma (ECHO-301/Keynote-252): A phase 3, randomised, double-blind study. Lancet Oncol. 2019, 20, 1083–1097. [Google Scholar] [CrossRef]
- Batabyal, D.; Yeh, S.R. Human tryptophan dioxygenase: A comparison to indoleamine 2,3-dioxygenase. J. Am. Chem. Soc. 2007, 129, 15690–15701. [Google Scholar] [CrossRef]
- Opitz, C.A.; Litzenburger, U.M.; Sahm, F.; Ott, M.; Tritschier, I.; Trump, S.; Shumacher, T.; Jestaedt, L.; Schrenk, D.; Weller, M.; et al. An endogenous tumour-promoting ligand of the human aryl hydrocarbon receptor. Nature 2011, 478, 197–203. [Google Scholar] [CrossRef]
- Van Baren, N.; van den Eynde, B.J. Tryptophan-degrading enzymes in tumoral immune resistance. Front. Immunol 2015, 6, 34. [Google Scholar] [CrossRef] [PubMed]
- De Iudicibus, R.C.; Tomek, P.; Palmer, B.D.; Tijono, S.M.; Flanagan, J.U.; Ching, L.-M. Parallel discovery of selective and dual inhibitors of tryptophan dioxygenases IDO1 and TDO2 with a newly-modified enzymatic assay. Biorg. Med. Chem. 2021, 39, 116160. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Chen, Y.; He, J.; Njoya, E.M.; Chen, J.; Liu, S.; Xie, C.; Huang, W.; Wang, Z.; Li, Y.; et al. 4,6-Substituted-1H-Indazoles as potent IDO1/TDO dual inhibitors. Bioorg. Med. Chem. 2019, 27, 1087–1098. [Google Scholar] [CrossRef] [PubMed]
- Kozlova, A.; Thabault, L.; Liberelle, M.; Klaessens, S.; Prevost, J.R.C.; Mathieu, C.; Pilotte, L.; Stroobant, V.; Van den Eynde, B.; Frederick, R. Rational design of original fused-cycle selective inhibitors of tryptophan 2,3-dioxygenase. J. Med. Chem. 2021, 64, 10967–10980. [Google Scholar] [CrossRef] [PubMed]
- Marshall, E.S.; Holdaway, K.M.; Shaw, J.H.; Finlay, G.J.; Matthews, J.H.; Baguley, B.C. Anticancer drug sensitivity profiles of new and established melanoma lines. Oncol. Res. 1993, 5, 301–309. [Google Scholar]
- Wainwright, D.A.; Chang, A.L.; Dey, M.; Balyasnikova, I.V.; Kim, C.K.; Tobias, A.; Cheng, Y.; Kim, J.W.; Qiao, J.; Zhang, L.; et al. Durable therapeutic efficacy utilizing combinatorial blockade against IDO, CTLA-4 and PD-L1 in mice with brain tumors. Clin. Cancer Res. 2014, 20, 5290–5301. [Google Scholar] [CrossRef]
- Tritz, Z.P.; Ayasoufi, K.; Johnson, A.J. Anti-PD-1 checkpoint monotherapy in the orthotopic GL261 glioma model: The devil is in the detail. Neuro-Oncol. Adv. 2021, 3, vdab066. [Google Scholar] [CrossRef]
- Zhai, L.; Ladomersky, E.; Dostal, C.R.; Lauing, K.L.; Swoap, K.; Billingham, G.G.; Wu, M.; McCusker, R.H.; Binder, D.C.; Wainwright, D.A. Non-tumor cell IDO1 predominantly contributes to enzyme activity and response to CTL-4/PD-L1 inhibition in mouse glioblastoma. Brain Behav. Immun. 2017, 62, 24–29. [Google Scholar] [CrossRef]
- Wilkoff, L.J.; Dulmadge, E.A.; Chopra, D.P. Viability of cultured Lewis lung cell populations exposed to α-retinoic acid (40753). Proc. Soc. Exp. Biol. Med. 1980, 163, 233–236. [Google Scholar] [CrossRef]
- Bridewell, D.J.A.; Sperry, J.; Smith, J.R.; Kosim-Satyaputra, P.; Ching, L.-M.; Jamie, J.F.; Brimble, M.A. Natural product-inspired pyranonaphthoquinone inhibitors of indoleamine 2,3-dioxygenase-1 (IDO-1). Aust. J. Chem. 2013, 66, 40–49. [Google Scholar] [CrossRef]
- Widmer, B.; Werner, E.R.; Schennach, H.; Wachter, H.; Fuchs, D. Simultaneous measurement of serum tryptophan and kynurenine by HPLC. Clin. Chem. 1997, 12, 2424–2426. [Google Scholar] [CrossRef]
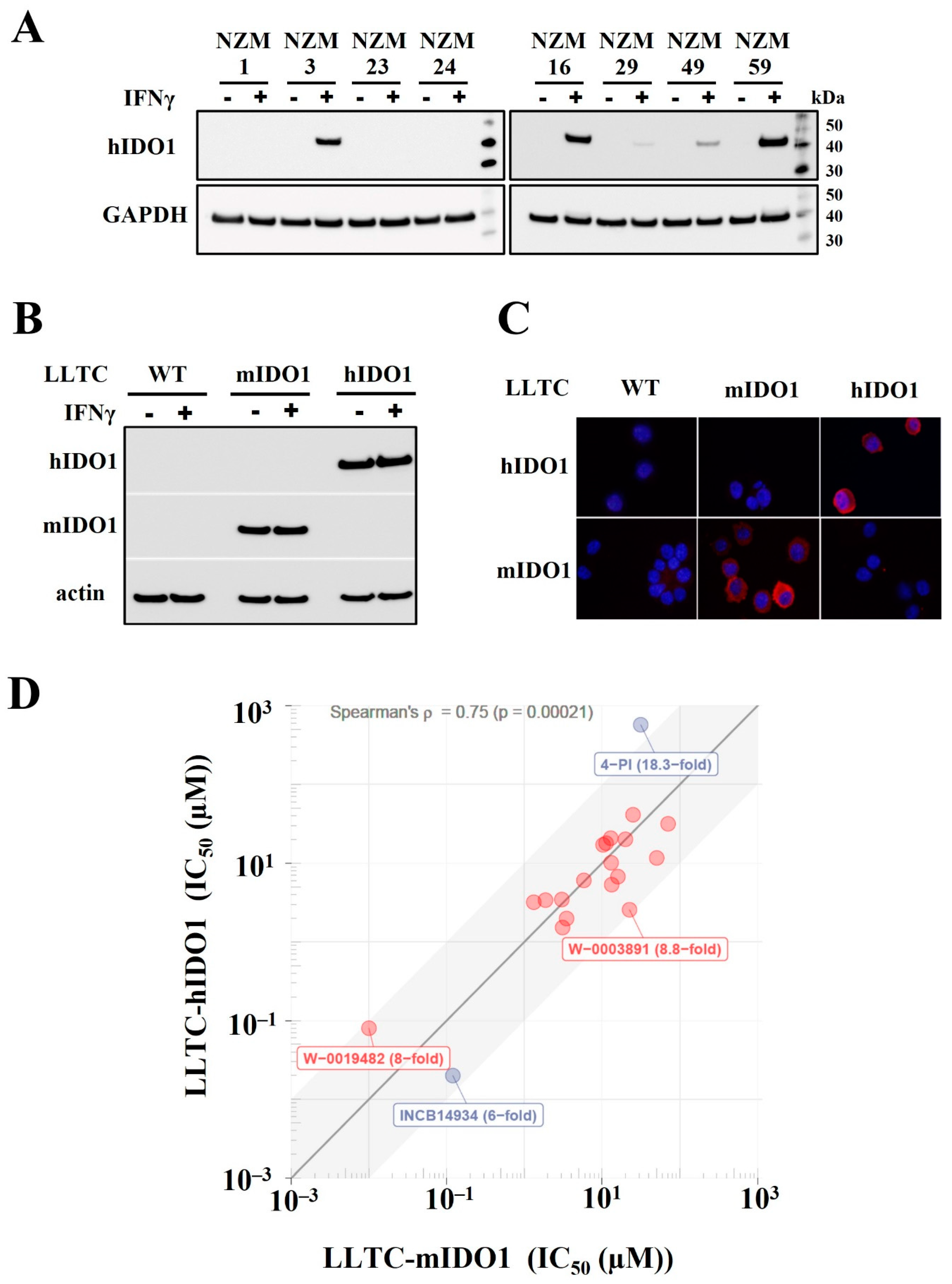

| Compound | Structure | IC50 (µM) | |
|---|---|---|---|
| LLTC-hIDO1 | LLTC-mIDO1 | ||
| W-0019482 | 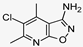 | 0.08 | 0.01 |
| W-0020227 |  | 1.52 | 3.10 |
| W-0137492 | 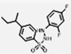 | 1.98 | 3.49 |
| W-0003891 | 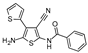 | 2.56 | 22.53 |
| W-0011387 | 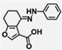 | 3.19 | 1.32 |
| W-0079407 |  | 3.39 | 1.87 |
| W-0079497 |  | 3.45 | 3.03 |
| W-0079433 | 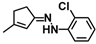 | 5.33 | 13.28 |
| W-0125931 | 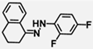 | 6.04 | 5.83 |
| W-0079409 | 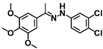 | 6.74 | 15.98 |
| W-0079326 | 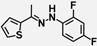 | 10.11 | 13.07 |
| W-0020339 | 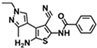 | 11.64 | 50.46 |
| W-0079341 | 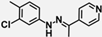 | 17.08 | 10.29 |
| W-0011320 | 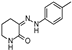 | 17.95 | 11.26 |
| W-0013201 | 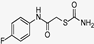 | 20.09 | 19.95 |
| W-0079400 | 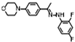 | 20.77 | 12.92 |
| W-0080549 | 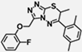 | 31.61 | 70.66 |
| W-0079399 | 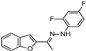 | 41.23 | 25.00 |
| INCB14934 |  | 0.02 | 0.12 |
| 4-PI | 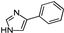 | 573.50 | 31.41 |
| A. Dual hIDO1 and hTDO2 activity. | |||||
| Compound | Structure | IC50 (µM) | IC50 Ratio hTDO2/hIDO1 | ||
| LLTC-hIDO1 | GL261-hTDO2 | ||||
| 1 | SN37318 |  | 0.92 | 0.68 | 1.4−1 |
| 2 | SN37159 |  | 1.14 | 1.81 | 1.6 |
| 3 | SN36500 | 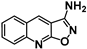 | 2.11 | 1.58 | 1.3−1 |
| 4 | SN37057 |  | 8.88 | 10.68 | 1.2 |
| B. hTDO2 selective. | |||||
| Compound | Structure | IC50 (µM) | IC50 Ratio hTDO2/hIDO1 | ||
| LLTC-hIDO1 | GL261-hTDO2 | ||||
| 5 | SN36458 |  | 0.66 | 0.08 | 7.9−1 |
| 6 | SN36896 | 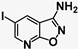 | 0.64 | 0.11 | 5.8−1 |
| 7 | SN36499 | 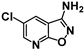 | 0.70 | 0.11 | 6.6−1 |
| 8 | SN36704 | 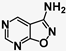 | 8.67 | 1.07 | 8.1−1 |
| C. hIDO1 selective. | |||||
| Compound | Structure | IC50 (µM) | IC50 Ratio hTDO2/hIDO1 | ||
| LLTC-hIDO1 | GL261-hTDO2 | ||||
| 9 | SN37313 | 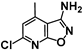 | 0.06 | 4.14 | 67 |
| 10 | SN36494 |  | 0.3 | 14.13 | 47 |
| 11 | SN37705 |  | 2.06 | 175 | 85 |
| 12 | SN36796 | 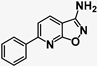 | 4.38 | 36.98 | 8 |
| D. Reference. | |||||
| Compound | Structure | IC50 (µM) | IC50 Ratio hTDO2/hIDO1 | ||
| LLTC-hIDO1 | GL261-hTDO2 | ||||
| Ref 1 | W-0019482 |  | 0.09 | 15.24 | 169 |
| Ref 2 | INCB14934 | 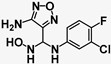 | 0.006 | 6.08 | 1013 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tijono, S.M.; Palmer, B.D.; Tomek, P.; Flanagan, J.U.; Henare, K.; Gamage, S.; Braun, L.; Ching, L.-M. Evaluation of Novel Inhibitors of Tryptophan Dioxygenases for Enzyme and Species Selectivity Using Engineered Tumour Cell Lines Expressing Either Murine or Human IDO1 or TDO2. Pharmaceuticals 2022, 15, 1090. https://doi.org/10.3390/ph15091090
Tijono SM, Palmer BD, Tomek P, Flanagan JU, Henare K, Gamage S, Braun L, Ching L-M. Evaluation of Novel Inhibitors of Tryptophan Dioxygenases for Enzyme and Species Selectivity Using Engineered Tumour Cell Lines Expressing Either Murine or Human IDO1 or TDO2. Pharmaceuticals. 2022; 15(9):1090. https://doi.org/10.3390/ph15091090
Chicago/Turabian StyleTijono, Sofian M, Brian D. Palmer, Petr Tomek, Jack U. Flanagan, Kimiora Henare, Swarna Gamage, Lukas Braun, and Lai-Ming Ching. 2022. "Evaluation of Novel Inhibitors of Tryptophan Dioxygenases for Enzyme and Species Selectivity Using Engineered Tumour Cell Lines Expressing Either Murine or Human IDO1 or TDO2" Pharmaceuticals 15, no. 9: 1090. https://doi.org/10.3390/ph15091090
APA StyleTijono, S. M., Palmer, B. D., Tomek, P., Flanagan, J. U., Henare, K., Gamage, S., Braun, L., & Ching, L.-M. (2022). Evaluation of Novel Inhibitors of Tryptophan Dioxygenases for Enzyme and Species Selectivity Using Engineered Tumour Cell Lines Expressing Either Murine or Human IDO1 or TDO2. Pharmaceuticals, 15(9), 1090. https://doi.org/10.3390/ph15091090






