Potential Active Constituents from Opophytum forsskalii (Hochst. ex Boiss.) N.E.Br against Experimental Gastric Lesions in Rats
Abstract
:1. Introduction
2. Results and Discussion
2.1. LC-MS Analysis of the Extracts
2.2. Antiulcerogenic Effect of O. forsskalii
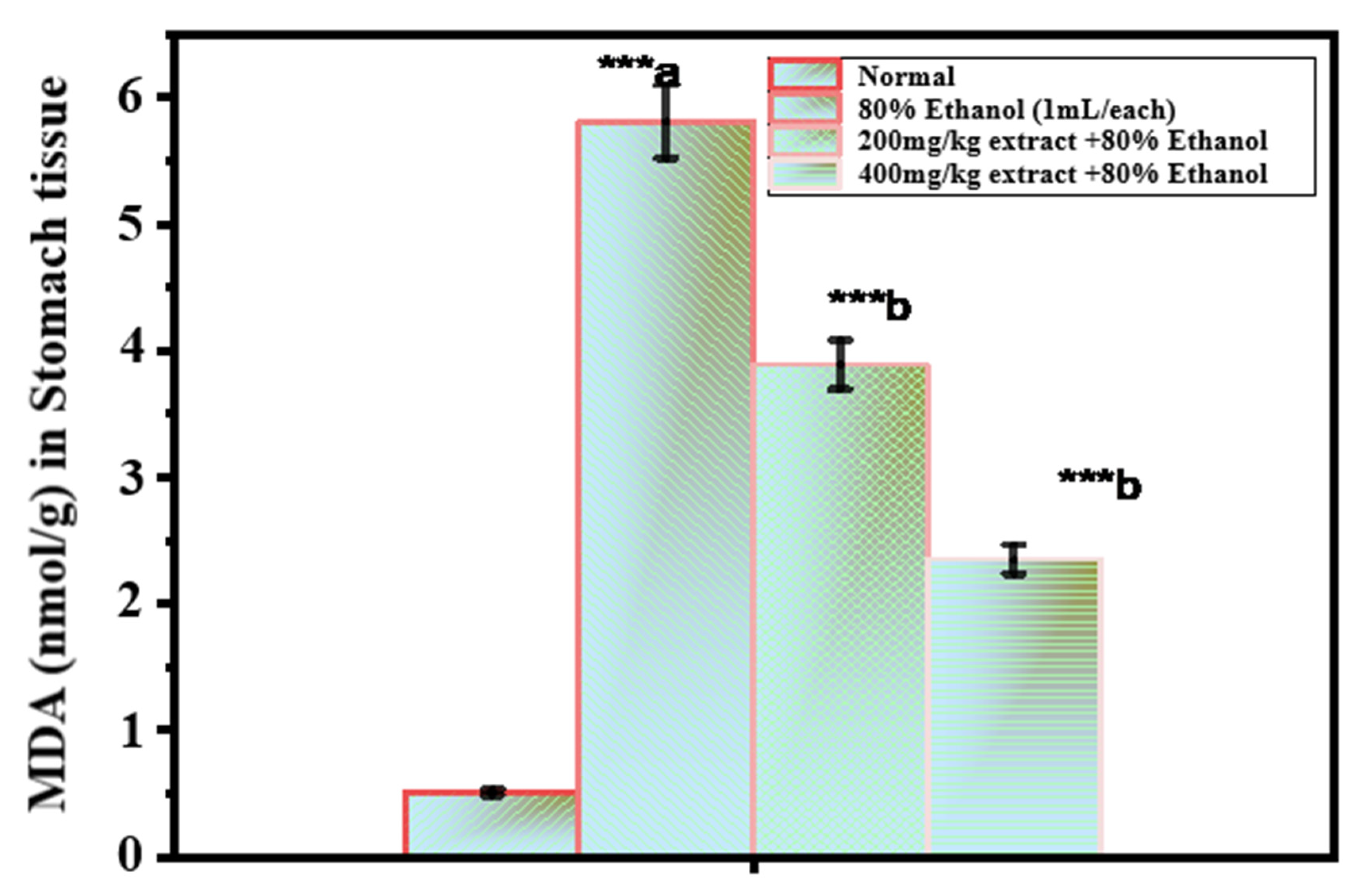
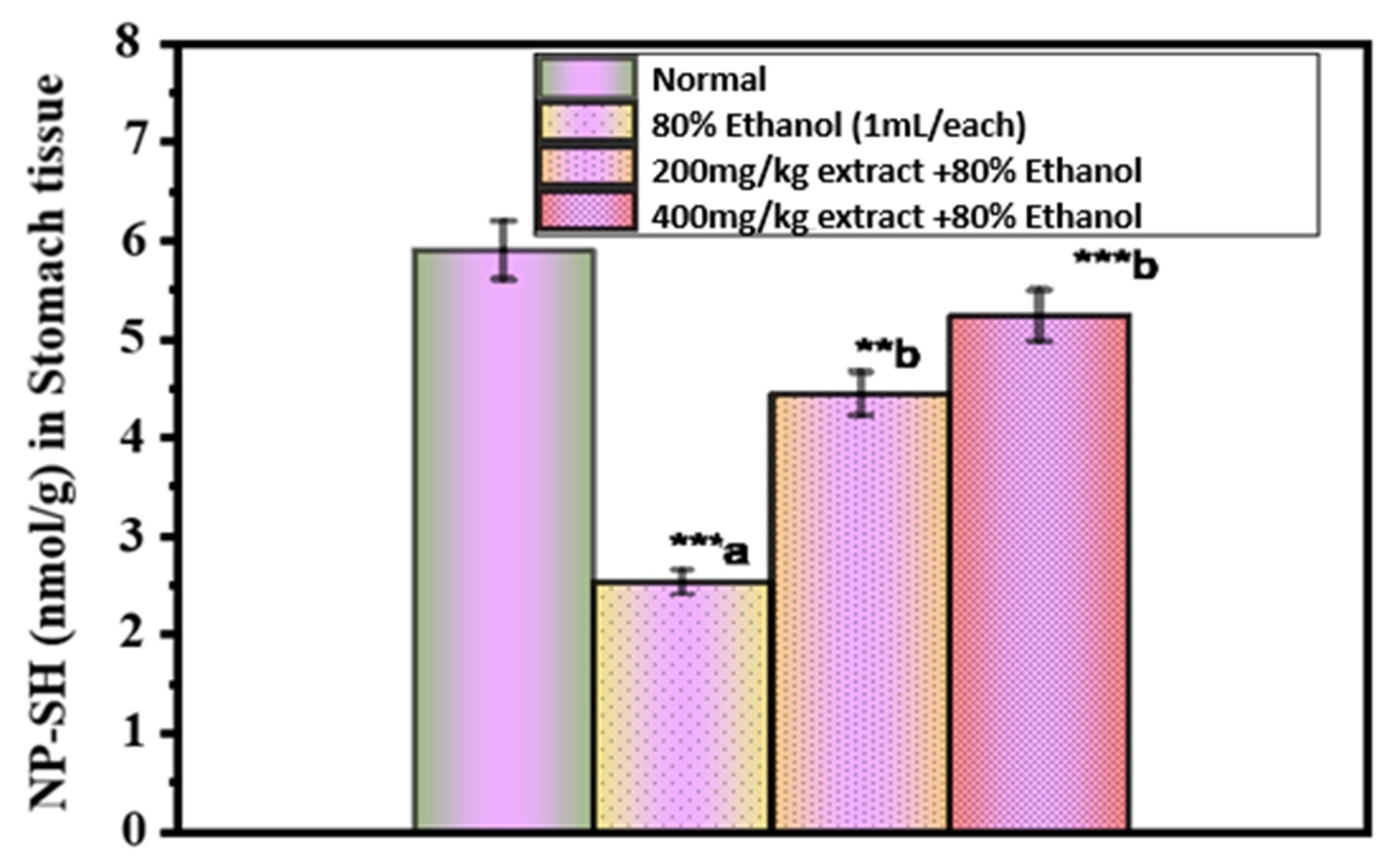
2.3. Assessment of the Oxidative Damage in Ethanol-Induced Ulcer
2.4. Histopathological Studies
3. Materials and Methods
3.1. Materials
3.2. Analysis of Secondary Metabolites Using LC-MS
3.3. In Vivo Antiulcer Assay
3.3.1. Animal Care
3.3.2. Indomethacin-Induced Gastric Lesion
3.3.3. Hypothermic-Restrained Stress-Induced Ulcers
3.3.4. Gastric Lesions Induced by Necrotizing Agents
3.3.5. Assessment of the Oxidative Damage in Ethanol-Induced Ulcer
3.3.6. Histopathological Evaluation
3.4. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Albaqawi, A.; El-Fetoh, N.; Alanazi, R.; Alanazi, N.; Alrayya, S.E.; Alanazi, A.; Alenezi, S.; Alanazi, R.; Alshalan, A.M.; Alenezi, O.T.; et al. Profile of peptic ulcer disease and its risk factors in Arar, Northern Saudi Arabia. Electron. Phys. 2017, 9, 5740–5745. [Google Scholar] [CrossRef]
- Huang, Q.; Jia, X.; Chu, Y.; Zhang, X.; Ye, H. Helicobacter pylori Infection in Geriatric Patients: Current Situation and Treatment Regimens. Front. Med. 2021, 8, 713908. [Google Scholar] [CrossRef]
- Alzahrani, S.; Lina, T.T.; Gonzalez, J.; Pinchuk, I.V.; Beswick, E.J.; Reyes, V.E. Effect of Helicobacter pylori on gastric epithelial cells. World J. Gastroenterol. 2014, 20, 12767–12780. [Google Scholar] [CrossRef]
- Paguigan, N.D.; Castillo, D.H.; Chichioco-Hernandez, C.L. Anti-ulcer activity of leguminosae plants. Arq. Gastroenterol. 2014, 51, 64–67. [Google Scholar] [CrossRef]
- Jarosz, M.; Szkaradek, N.; Marona, H.; Nowak, G.; Młyniec, K.; Librowski, T. Evaluation of anti-inflammatory and ulcerogenic potential of zinc–ibuprofen and zinc–naproxen complexes in rats. Inflammopharmacology 2017, 25, 653–663. [Google Scholar] [CrossRef]
- McEvoy, L.; Carr, D.F.; Pirmohamed, M. Pharmacogenomics of NSAID Induced Upper Gastrointestinal Toxicity. Front. Pharmacol. 2021, 12, 684162. [Google Scholar] [CrossRef]
- Kuna, L.; Jakab, J.; Smolic, R.; Raguz-Lucic, N.; Vcev, A.; Smolic, M. Peptic Ulcer Disease: A Brief Review of Conventional Therapy and Herbal Treatment Options. J. Clin. Med. 2019, 8, 179. [Google Scholar] [CrossRef]
- Stojanović-Radić, Z.; Pejčić, M.; Stojanović, N.; Sharifi-Rad, J.; Stanković, N. Potential of Ocimum basilicum L. and Salvia officinalis L. Essential oils against biofilms of P. aeruginosa clinical isolates. Cell. Mol. Biol. 2016, 62, 27–32. [Google Scholar]
- Salehi, B.; Kumar, N.; Şener, B.; Sharifi-Rad, M.; Kılıç, M.; Mahady, G.; Vlaisavljevic, S.; Iriti, M.; Kobarfard, F.; Setzer, W.N.; et al. Medicinal plants used in the treatment of human immunodeficiency virus. Int. J. Mol. Sci. 2018, 19, 1459. [Google Scholar] [CrossRef]
- Setzer, M.S.; Sharifi-Rad, J.; Setzer, W.N. The search for herbal antibiotics: An in-silico investigation of antibacterial phytochemicals. Antibiotics 2016, 5, 30. [Google Scholar] [CrossRef]
- Kumadoh, D.; Archer, M.A.; Yeboah, G.N.; Kyene, M.O.; Boakye-Yiadom, M.; Adi-Dako, O.; Osei-Asare, C.; Adase, E.; Appiah, A.A.; Mintah, S.O. A review on anti-peptic ulcer activities of medicinal plants used in the formulation of Enterica Dyspepsia and NPK 500 capsules. Heliyon. 2021, 7, e08465. [Google Scholar] [CrossRef]
- Alam, A.; Rehman, N.U.; Ansari, M.N.; Palla, A.H. Effects of Essential Oils of Elettaria cardamomum Grown in India and Guatemala on Gram-Negative Bacteria and Gastrointestinal Disorders. Molecules 2021, 26, 2546. [Google Scholar] [CrossRef]
- Junior, I.F.S.; Balogun, S.O.; Oliveira, R.G.; Damazo, A.S.; Martins, D.T.O. Piper umbellatum l.: A medical plant with gastric-ulcer protective and ulcer healing effects in experimental rodent models. J. Ethnopharmacol. 2016, 192, 123–131. [Google Scholar] [CrossRef]
- de Lacerda Neto, L.J.; Ramos, A.G.; Santos Sales, V.; de Souza, S.D.; Dos Santos, A.T.; de Oliveira, L.R.; Kerntopf, M.R.; de Albuquerque, T.R.; Coutinho, H.D.; Quintans-Júnior, L.J.; et al. Gastroprotective and ulcer healing effects of hydroethanolic extract of leaves of Caryocar coriaceum: Mechanisms involved in the gastroprotective activity. Chem. Biol. Interact. 2017, 261, 56–62. [Google Scholar] [CrossRef]
- Nguelefack, T.B.; Feumebo, C.B.; Ateufack, G.; Watcho, P.; Tatsimo, S.; Atsamo, A.D.; Tane, P.; Kamanyi, A. Anti-ulcerogenic properties of the aqueous and methanol extracts from the leaves of Solanum torvum Swartz (Solanaceae) in rats. J. Ethnopharmacol. 2008, 119, 135–140. [Google Scholar] [CrossRef]
- Alqasoumi, S.; Al-Sohaibani, M.; Al-Howiriny, T.; Al-Yahya, M.; Rafatullah, S. Rocket “Eruca sativa”: A salad herb with potential gastric anti-ulcer activity. World J. Gastroenterol. 2009, 15, 1958–1965. [Google Scholar] [CrossRef]
- Youssif, K.; Elshamy, A.; Rabeh, M.; Gabr, N.; Haggag, E.A. Phytochemical and Biological Review on Plants of the family Aizoaceae. J. Adv. Pharm. Res. 2019, 3, 158–181. [Google Scholar] [CrossRef]
- Dhasan, P.B.; Jegadeesan, M.; Kavimani, S. Antiulcer activity of aqueous extract of fruits of Momordica cymbalaria Hook f. in Wistar rats. Pharmacogn. Res. 2010, 2, 58–61. [Google Scholar] [CrossRef]
- Prakash, R. Gastroprotective and antisecretory properties of methanolic extract of Trianthema portulacastrum. L in aspirin & pyloric ligature induced gastric ulcer in rats. PTB Rep. 2015, 1, 87–91. [Google Scholar]
- Abdel-Farid, I.B.; Mahalel, U.A.; Jahangir, M.J.; Elgebaly, H.A.; El-Naggar, S.A. Metabolomic profiling and antioxidant activity of Opophytum forsskalii. JUSEJ 2016, 3, 19–24. [Google Scholar] [CrossRef]
- Adedamola, A.K.; Eko, E.O.; Omoniyi, O.O. Ethnopharmacology, Therapeutic Properties and Nutritional Potentials of Carpobrotus edulis: A Comprehensive Review. Sci. Pharm. 2020, 88, 39. [Google Scholar] [CrossRef]
- El-Amier, Y.A.; Alghanem, S.M.; Al-hadithy, O.N.; Fahmy, A.A.; El-Zayat, M.M. Phytochemical analysis and biological activities of three wild Mesembryanthemum species growing in heterogeneous habitats. J. Phytol. 2021, 13, 01–08. [Google Scholar] [CrossRef]
- Aabed, K.; Mohammed, A.E. Phytoproduct, Arabic Gum and Opophytum forsskalii Seeds for Bio-Fabrication of Silver Nanoparticles: Antimicrobial and Cytotoxic Capabilities. Nanomaterials 2021, 11, 2573. [Google Scholar] [CrossRef]
- El-Amier, Y.A.; Haroun, S.; El-Shehaby, O.A.; Al-Hadith, O.N. Antioxidant and Antimicrobial Properties of Some Wild Aizoaceae species Growing in Egyptian Desert. J. Environ. Sci. 2016, 45, 1–10. [Google Scholar]
- Munyai, R.; Raletsena, M.V.; Modise, D.M. LC-MS Based Metabolomics Analysis of Potato (Solanum tuberosum L.) Cultivars Irrigated with Quicklime Treated Acid Mine Drainage Water. Metabolites 2022, 12, 221. [Google Scholar] [CrossRef]
- Shirahata, T.; Ishikawa, H.; Kudo, T.; Takada, Y.; Hoshino, A.; Taga, Y.; Minakuchi, Y.; Hasegawa, T.; Horiguchi, R.; Hirayama, T.; et al. Metabolic fingerprinting for discrimination of DNA-authenticated Atractylodes plants using 1H NMR spectroscopy. J. Nat. Med. 2021, 75, 475–488. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Kim, J.; Kim, N.; Lee, D.; Lee, H.; Lee, D.Y.; Kim, K.H. Metabolomic Elucidation of the Effect of Sucrose on the Secondary Metabolite Profiles in Melissa officinalis by Ultraperformance Liquid Chromatography-Mass Spectrometry. ACS Omega 2020, 5, 33186–33195. [Google Scholar] [CrossRef]
- Hamed, A.I.; Said, R.B.; Kontek, B.; Al-Ayed, A.S.; Kowalczyk, M.; Moldoch, J.; Stochmal, A.; Olas, B. LC-ESI-MS/MS profile of phenolic and glucosinolate compounds in samh flour (Mesembryanthemum forsskalei Hochst. ex Boiss) and the inhibition of oxidative stress by these compounds in human plasma. Food Res. Int. 2016, 85, 282–290. [Google Scholar] [CrossRef]
- Moawad, A.; Mohamed, R. Secondary metabolites from Mesembryanthemum forsskaolii Hochst. ex. Boiss. Planta Medica 2014, 80, 1382558142. [Google Scholar] [CrossRef]
- Moawad, A.; Amin, E.; Mohammed, R. Diffusion-ordered Spectroscopy of Flavonol Mixture from Mesembryanthemum forsskaolii (Aizoaceae). Eur. J. Med. Plants 2016, 16, 1–8. [Google Scholar] [CrossRef]
- Ahmed, S.R.; Rabbee, M.F.; Roy, A.; Chowdhury, R.; Banik, A.; Kubra, K.; Hassan Chowdhury, M.M.; Baek, K.H. Therapeutic Promises of Medicinal Plants in Bangladesh and Their Bioactive Compounds against Ulcers and Inflammatory Diseases. Plants 2021, 10, 1348. [Google Scholar] [CrossRef]
- Shimada, H.; Eto, M.; Ohtaguro, M.; Ohtsubo, M.; Mizukami, Y.; Ide, T.; Imamura, Y. Differential mechanisms for the inhibition of human cytochrome P450 1A2 by apigenin and genistein. J. Biochem. Mol. Toxicol. 2010, 24, 230–234. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Wang, S.; Zhu, M.; Chen, J.; Zhu, X. Effects of genistein, apigenin, quercetin, rutin and astilbin on serum uric acid levels and xanthine oxidase activities in normal and hyperuricemic mice. Food Chem. Toxicol. 2011, 49, 1943–1947. [Google Scholar] [CrossRef]
- Narkhede, K.P.; Satapathy, T.; Bibhas, P. Protective effect of Cod Liver Oil in Experimentally Induced Gastric Ulceration in Rats. Res. J. Pharm. Technol. 2019, 12, 5–10. [Google Scholar] [CrossRef]
- Salomone, F.; Galvano, F.; Li Volti, G. Molecular Bases Underlying the Hepatoprotective Effects of Coffee. Nutrients 2017, 9, 85. [Google Scholar] [CrossRef]
- Huang, C.C.; Lin, K.J.; Cheng, Y.W.; Hsu, C.A.; Yang, S.S.; Shyur, L.F. Hepatoprotective effect and mechanistic insights of deoxyelephantopin, a phyto-sesquiterpene lactone, against fulminant hepatitis. J. Nutr. Biochem. 2013, 24, 516–530. [Google Scholar] [CrossRef]
- Dembitsky, V.M. Antitumor and hepatoprotective activity of natural and synthetic neo steroids. Prog. Lipid Res. 2020, 79, 101048. [Google Scholar] [CrossRef]
- Pal, L.C.; Agrawal, S.; Gautam, A.; Chauhan, J.K.; Rao, C.V. Hepatoprotective and Antioxidant Potential of Phenolics-Enriched Fraction of Anogeissus acuminata Leaf against Alcohol-Induced Hepatotoxicity in Rats. Med. Sci. 2022, 10, 17. [Google Scholar] [CrossRef]
- Swanson, H.I.; Choi, E.Y.; Helton, W.B.; Gairola, C.G.; Valentino, J. Impact of apigenin and kaempferol on human head and neck squamous cell carcinoma. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2014, 117, 214–220. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, D.; Yang, L.; Zhou, D.; Zhang, J. Purification and Characterization of Flavonoids from the Leaves of Zanthoxylum bungeanum and Correlation between Their Structure and Antioxidant Activity. PLoS ONE 2014, 9, e105725. [Google Scholar] [CrossRef]
- Nagy, N.; Kuipers, H.F.; Frymoyer, A.R.; Ishak, H.D.; Bollyky, J.B.; Wight, T.N.; Bollyky, P.L. 4-methylumbelliferone treatment and hyaluronan inhibition as a therapeutic strategy in inflammation, autoimmunity, and cancer. Front. Immunol. 2015, 6, 123. [Google Scholar] [CrossRef]
- Rashid, M.I.; Fareed, M.I.; Rashid, H.; Aziz, H.; Ehsan, N.; Khalid, S.; Ghaffar, I.; Ali, R.; Gul, A.; Hakeem, K.R. Flavonoids and Their Biological Secrets. Plant Hum. Health 2019, 2, 579–605. [Google Scholar] [CrossRef]
- Al-Faris, N.A.; Al-Sawadi, A.D.; Alokail, M.S. Effect of samh seeds supplementation (Mesembryanthemum forsskalei Hochst) on liver enzymes and lipid profiles of streptozotocin (STZ)-induced diabetic Wistar rats. Saudi J. Biol. Sci. 2010, 17, 23–28. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rosaria, A.; Manuguerra, S.; Collins, E.; Mahdhi, A.; Renda, G.; Messina, C.M.; Santulli, A. Antioxidant Properties of a Supercritical Fluid Extract of the Halophyte Mesembryanthemum nodiflorum L. from Sicilian Coasts: Nutraceutical and Cosmeceutical Applications. Appl. Sci. 2020, 10, 2374. [Google Scholar] [CrossRef]
- Serafim, C.; Araruna, M.E.; Júnior, E.A.; Diniz, M.; Hiruma-Lima, C.; Batista, L. A Review of the Role of Flavonoids in Peptic Ulcer (2010-2020). Molecules 2020, 25, 5431. [Google Scholar] [CrossRef]
- Hamdi, A.; Majouli, K.; Abdelhamid, A.; Marzouk, B.; Belghith, H.; Chraief, I.; Bouraoui, A.; Marzouk, Z.; Heyden, Y.V. Pharmacological activities of the organic extracts and fatty acid composition of the petroleum ether extract from Haplophyllum tuberculatum leaves. J. Ethnopharmacol. 2018, 216, 97–103. [Google Scholar] [CrossRef]
- Shahin, N.N.; Abdelkader, N.F.; Safar, M.M. A Novel Role of Irbesartan in Gastroprotection against Indomethacin-Induced Gastric Injury in Rats: Targeting DDAH/ADMA and EGFR/ERK Signaling. Sci. Rep. 2018, 8, 4280. [Google Scholar] [CrossRef]
- Simões, S.; Lopes, R.; Campos, M.C.D.; Marruz, M.J.; da Cruz, M.E.M.; Corvo, L. Animal models of acute gastric mucosal injury: Macroscopic and microscopic evaluation. Anim. Model Exp. Med. 2019, 2, 121–126. [Google Scholar] [CrossRef]
- Di Cerbo, A.; Carnevale, G.; Avallone, R.; Zavatti, M.; Corsi, L. Protective Effects of Borago officinalis(Borago) on Cold Restraint Stress-Induced Gastric Ulcers in Rats: A Pilot Study. Front. Vet. Sci. 2020, 7, 427. [Google Scholar] [CrossRef]
- Azlina, M.F.N.; Qodriyah, H.M.S.; Akmal, M.N.; Ibrahim, I.A.A.; Kamisah, Y. In vivo effect of Piper sarmentosum methanolic extract on stress-induced gastric ulcers in rats. Arch. Med. Sci. 2019, 15, 223–231. [Google Scholar] [CrossRef]
- Sanpinit, S.; Chonsut, P.; Punsawad, C.; Wetchakul, P. Gastroprotective and Antioxidative Effects of the Traditional Thai Polyherbal Formula Phy-Blica-D against Ethanol-Induced Gastric Ulcers in Rats. Nutrients 2021, 14, 172. [Google Scholar] [CrossRef]
- Song, S.H.; Kim, J.E.; Sung, J.E.; Lee, H.A.; Yun, W.B.; Lee, Y.H.; Song, H.; Hwang, D. Anti-ulcer effect of Gallarhois extract with anti-oxidant activity in an ICR model of ethanol/hydrochloride acid-induced gastric injury. J. Tradit. Compl. Med. 2019, 4, 372–382. [Google Scholar] [CrossRef] [PubMed]
- Sharifi-Rad, M.; Fokou, P.V.T.; Sharopov, F.; Martorell, M.; Ademiluyi, A.O.; Rajkovic, J.; Salehi, B.; Martins, N.; Iriti, M.; Sharifi-Rad, J. Antiulcer Agents: From Plant Extracts to Phytochemicals in Healing Promotion. Molecules 2018, 23, 1751. [Google Scholar] [CrossRef] [Green Version]
- Umamaheswari, M.; Asokkumar, K.; Rathidevi, R.; Sivashanmugam, A.T.; Subhadradevi, V.; Ravi, T.K. Antiulcer and in vitro antioxidant activities of Jasminum grandiflorum L. J. Ethnopharmacol. 2007, 110, 464–470. [Google Scholar] [CrossRef] [PubMed]
- Khazaei, M.; Salehi, H. Protective effect of falcaria vulgaris extract on ethanol induced gastric ulcer in rat. Iran J. Pharmacol. Ther. 2006, 5, 43–46. [Google Scholar]
- Michiels, C.; Raes, M.; Toussaint, O.; Remacle, J. Importance of se-glutathione peroxidase, catalase, and Cu/Zn-SOD for cell survival against oxidative stress. Free Radic. Biol. Med. 1994, 17, 235–248. [Google Scholar] [CrossRef]
- Buege, J.A.; Aust, S.D. Microsomal lipid peroxidation. Methods Enzymol. 1978, 52, 302–310. [Google Scholar]
- Kimura, M.; Goto, S.; Ihara, Y.; Wada, A.; Yahiro, K.; Niidome, T.; Aoyagi, H.; Hirayama, T.; Kondo, T. Impairment of glutathione metabolism in human gastric epithelial cells treated with vacuolating cytotoxin from Helicobacter pylori. Microb. Pathog. 2001, 31, 29–36. [Google Scholar] [CrossRef]
- Miller, T.A.; Li, D.; Kuo, Y.J.; Schmidt, K.L.; Shanbour, L.L. Nonprotein sulfhydryl compounds in canine gastric mucosa: Effects of PGE2 and ethanol. Am. J. Physiol. 1985, 249, G137–G144. [Google Scholar] [CrossRef]
- Al Mofleh, I.A.; Alhaider, A.A.; Mossa, J.S.; Al-Soohaibani, M.O.; Rafatullah, S. Aqueous suspension of anise “Pimpinella anisum” protects rats against chemically induced gastric ulcers. World J. Gastroenterol. 2007, 13, 1112–1118. [Google Scholar] [CrossRef]
- Hiraishi, H.; Terano, A.; Ota, S.; Mutoh, H.; Sugimoto, T.; Harada, T.; Razandi, M.; Ivey, K.J. Protection of cultured rat gastric cells against oxidant-induced damage by exogenous glutathione. Gastroenterology 1994, 106, 1199–1207. [Google Scholar] [CrossRef]
- Chiou, S.K.; Moon, W.S.; Jones, M.K.; Tarnawski, A.S. Survivin expression in the stomach: Implications for mucosal integrity and protection. Biochem Biophys Res Commun. 2003, 305, 374–379. [Google Scholar] [CrossRef]
- Zuvarox, T.; Belletieri, C. Malabsorption Syndromes. [Updated 2021 Jul 30]. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, Finland, 2022. Available online: https://www.ncbi.nlm.nih.gov/books/NBK553106 (accessed on 12 July 2022).
- Raish, M.; Shahid, M.; Bin Jardan, Y.A.; Ansari, M.A.; Alkharfy, K.M.; Ahad, A.; Abdelrahman, I.A.; Ahmad, A.; Al-Jenoobi, F.I. Gastroprotective Effect of Sinapic Acid on Ethanol-Induced Gastric Ulcers in Rats: Involvement of Nrf2/HO-1 and NF-κB Signaling and Antiapoptotic Role. Front. Pharmacol. 2021, 12, 622815. [Google Scholar] [CrossRef]
- Leong, A.S.; Milios, J. Rapid immunoperoxidase staining of lymphocyte antigens using microwave irradiation. J. Pathol. 1986, 148, 183–187. [Google Scholar] [CrossRef] [PubMed]
- Rios, E.; Rocha, N.; Venâncio, E.; Mour, B.; Feitosa, M.; Cerqueira, G.; Soares, P.M.; Woods, D.J.; de Sousa, F.C.; Leal, L.K.; et al. Mechanisms involved in the gastroprotective activity of esculin on acute gastric lesions in mice. Chem. Biol. Interact. 2010, 188, 246–254. [Google Scholar] [CrossRef] [PubMed]
- Mahmood, A.; Fard, A.; Harita, H.; Amin, Z.; Salmah, I. Evaluation of gastroprotective effects of Strobianthes crispus leaf extract on ethanol-induced gastric mucosal injury in rats. Sci. Res. Essays 2011, 6, 2306–2314. [Google Scholar]
- Tarnawski, A.; Tarnawski, A.; Szabo, I.L.; Husain, S.S.; Soreghan, B. Regeneration of gastric mucosa during ulcer healing is triggered by growth factors and signal transduction pathways. J. Physiol. Paris 2001, 95, 337–344. [Google Scholar] [CrossRef]
- Ansari, S.F.; Khan, A.U.; Qazi, N.G.; Shah, F.A.; Naeem, K. In Vivo, Proteomic, and In Silico Investigation of Sapodilla for Therapeutic Potential in Gastrointestinal Disorders. Biomed. Res. Int. 2019, 2019, 4921086. [Google Scholar] [CrossRef]
- Hajrezaie, M.; Salehen, N.; Karimian, H.; Zahedifard, M.; Shams, K.; Al, B.R. Biochanin a gastroprotective effects in ethanol-induced gastric mucosal ulceration in rats. PLoS ONE 2015, 10, e0121529. [Google Scholar]
- Al-Nemi, R.; Makki, A.A.; Sawalha, K.; Hajjar, D.; Jaremko, M. Untargeted Metabolomic Profiling and Antioxidant Capacities of Different Solvent Crude Extracts of Ephedra foeminea. Metabolites 2022, 12, 451. [Google Scholar] [CrossRef]
- Sedlak, J.; Lindsay, R.H. Estimation of total, protein-bound, and nonprotein sulfhydryl groups in tissue with Ellman’s reagent. Anal. Biochem. 1968, 25, 192–205. [Google Scholar] [CrossRef]
- Dursun, H.; Bilici, M.; Albayrak, F.; Ozturk, C.; Saglam, M.B.; Alp, H.H.; Suleyman, H. Antiulcer activity of fluvoxamine in rats and its effect on oxidant and antioxidant parameters in stomach tissue. BMC Gastroenterol. 2009, 9, 36. [Google Scholar] [CrossRef] [PubMed]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [CrossRef]
- Bancroft, J.D.; Layton, C. The Hematoxylins and Eosin in Bancroft’s Theory and Practice of Histological Techniques, 8th ed.; Suvarna, S.K., Layton, C., Bancroft, J.D., Eds.; Elsevier: Amsterdam, The Netherlands, 2018; p. 131. [Google Scholar]
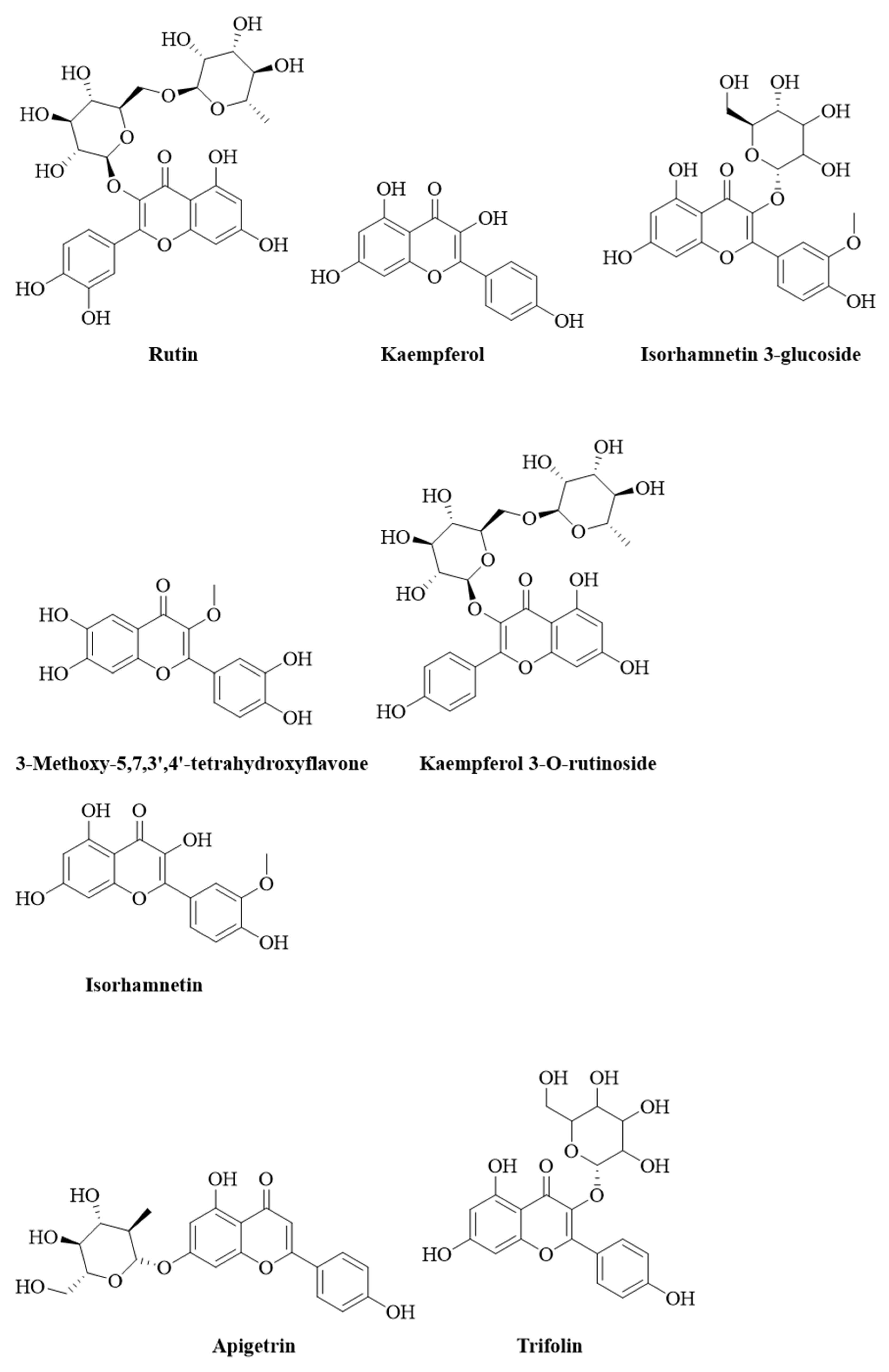
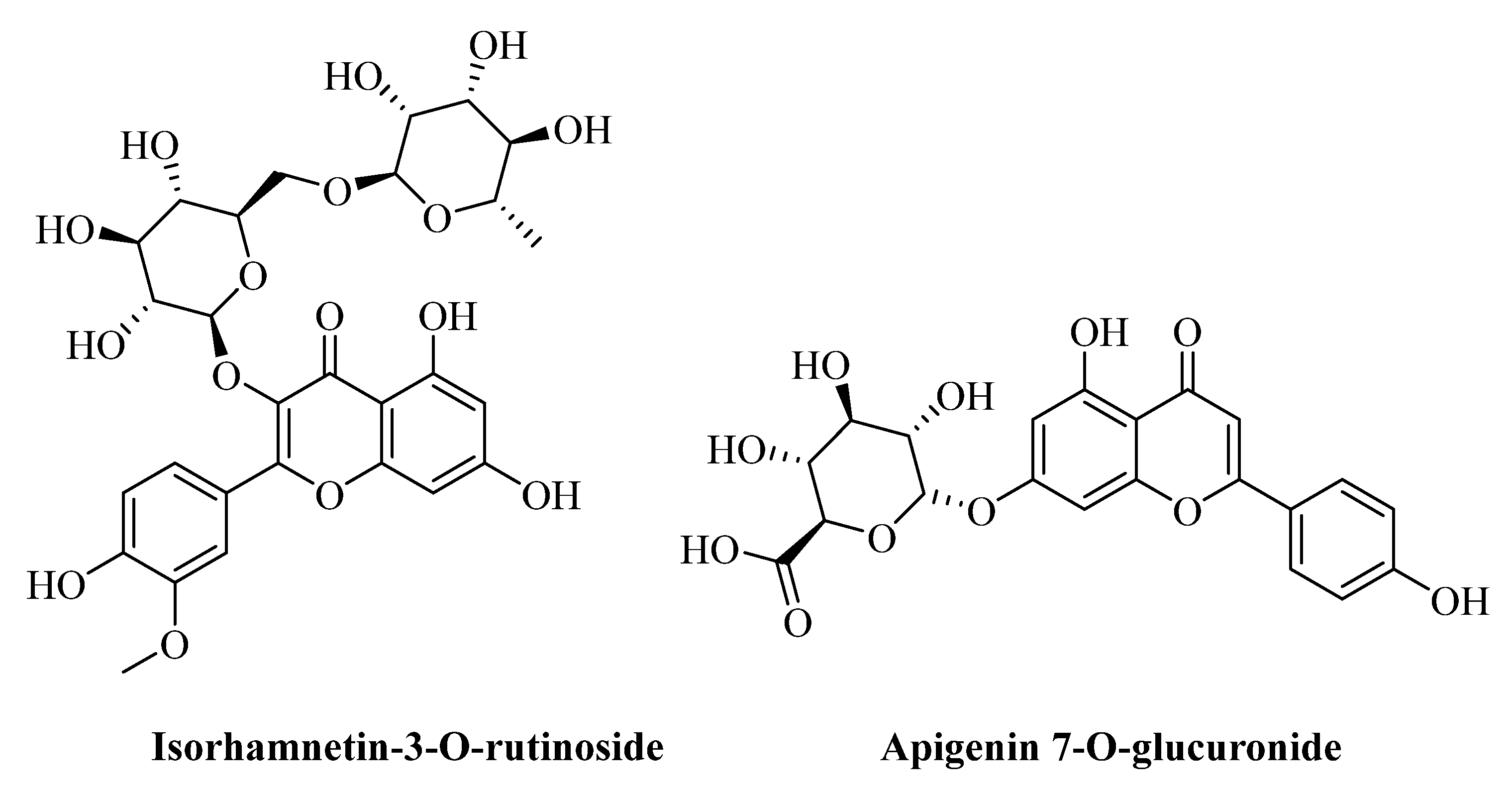


| No. | Name of Compound | RT | Proposed Formula | Theoretical Mwt. | M + H | Observed Mwt. | Mass Error (ppm) | Chemical Class |
|---|---|---|---|---|---|---|---|---|
| 1 | Nylon cyclic dimer | 2.06 | C12H22N2O2 | 226.1681 | 227.1754 | 226.1676 | −2.3434 | Macrocyclic lactam |
| 2 | 4-Amino-3-[(1-carboxyvinyl)oxy]-1,5- cyclohexadiene-1-carboxylic acid | 7.04 | C10H11NO5 | 225.0638 | 226.0711 | 225.0633 | −2.4438 | Carboxylic acid derivative |
| 3 | Hymecromone | 7.93 | C10H8O3 | 176.0473 | 177.0546 | 176.0468 | −2.9538 | Coumarin |
| 4 | Rutin | 9.48 | C27H30O16 | 610.1537 | 611.1610 | 610.1532 | −0.8522 | Flavonoid |
| 5 | Hexyl 2-furoate | 9.98 | C11H16O3 | 196.1100 | 197.1173 | 196.1094 | −2.9575 | Furoic acid esters |
| 6 | (+)-Actinodaphnine | 10.80 | C18H17NO4 | 311.1158 | 312.1231 | 311.1153 | −1.5750 | Alkaloid |
| 7 | 3-Methoxy-5,7,3’,4’-tetrahydroxyflavone | 11.07 | C16H12O7 | 316.0585 | 317.0658 | 316.0580 | −1.6769 | Flavonoid |
| 8 | Apigetrin | 12.05 | C21H20O10 | 432.1057 | 433.1130 | 432.1052 | −1.1340 | Flavonoid |
| 9 | Kaempferol | 12.42 | C15H10O6 | 286.0477 | 287.0550 | 286.0471 | −1.9927 | Flavonoid |
| 10 | kaempferol 3-O-rutinoside | 12.42 | C27H30O15 | 594.1587 | 595.1661 | 594.1583 | −0.7574 | Flavonoid |
| 11 | Trifolin | 12.43 | C21H20O11 | 448.1006 | 449.1077 | 448.0999 | −1.5621 | Flavonoid |
| 12 | Isorhamnetin 3-glucoside | 12.50 | C22H22O12 | 478.1111 | 479.1184 | 478.1106 | −1.1504 | Flavonoid |
| 13 | Isorhamnetin | 12.51 | C16H12O7 | 316.0585 | 317.0657 | 316.0579 | −1.8984 | Flavonoid |
| 14 | Isorhamnetin-3-O-rutinoside | 12.52 | C28H32O16 | 624.1690 | 625.1765 | 624.1687 | −0.5127 | Flavonoid |
| 15 | Apigenin 7-O-glucuronide | 12.58 | C21H18O11 | 446.0852 | 447.0925 | 446.0847 | −1.1881 | Flavonoid |
| 16 | Hexylresorcinol | 14.69 | C12H18O2 | 194.1307 | 195.1380 | 194.1301 | −2.8847 | Phenolic |
| 17 | Genistein | 14.79 | C15H10O5 | 270.0529 | 271.0602 | 270.0523 | −2.1477 | Flavonoid |
| 18 | Hexadecasphinganine | 15.01 | C16H35NO2 | 273.2668 | 274.2742 | 273.2663 | −1.6833 | Sphingoid |
| 19 | Botrydial | 15.85 | C17H26O5 | 310.1782 | 311.1854 | 310.1776 | −1.9666 | Sesquiterpene |
| 20 | Butylparaben | 16.14 | C11H14O3 | 194.0943 | 195.1016 | 194.0937 | −2.9367 | Benzoic acid derivative |
| 21 | (2Z)-3,7-Dimethyl-2,6-octadien-1-yl 3- oxobutanoate | 17.11 | C14H22O3 | 238.1569 | 239.1643 | 238.1565 | −1.8475 | Fatty acids ester |
| 22 | (8E)-2-Amino-8-octadecene-1,3,4-triol | 17.61 | C18H37NO3 | 315.2774 | 316.2846 | 315.2768 | −1.8714 | Fatty acids ester |
| 23 | Phytosphingosine | 18.31 | C18H39NO3 | 317.2930 | 318.3004 | 317.2926 | −1.2922 | Amino alcohol |
| 24 | 2-(14,15-Epoxyeicosatrienoyl) glycerol | 22.36 | C23H38O5 | 376.2590 | 377.2663 | 376.2585 | −1.4086 | Fatty acids ester |
| 25 | 2-Arachidonoyl glycerol | 23.10 | C23H38O4 | 378.2748 | 379.2820 | 378.2742 | −1.5597 | Fatty acids ester |
| 26 | 27-Norcholestane-3,7,12,24,25,26-hexol | 23.36 | C26H46O6 | 454.3272 | 455.3345 | 454.3266 | −1.2326 | Fatty acids ester |
| 27 | Ethyl Linoleate | 23.56 | C20H36O2 | 308.2717 | 309.2790 | 308.2712 | −1.7517 | Fatty acids ester |
| 28 | Bis(2-ethylhexyl) phthalate | 23.79 | C24H38O4 | 390.2772 | 391.2845 | 390.2767 | −1.3580 | Phthalate derivative |
| 29 | 1-α-Linolenoyl-2-arachidonoyl-sn-glycerol | 26.33 | C41H66O5 | 638.4891 | 639.4963 | 638.4885 | −0.9241 | Fatty acids ester |
| 30 | 7alpha,25-Dihydroxycholesterol | 26.82 | C27H46O3 | 418.3447 | 419.3521 | 418.3443 | −1.0757 | Steroid |
| Treatments | Dose mg/kg | Ulcer Index (Mean ± SE) |
|---|---|---|
| Control (Indomethacin Only) | 30 | 37.33 ± 1.25 |
| O. forsskalii Extract + Indomethacin | 200 | 33.33 ± 0.49 * |
| O. forsskalii Extract + Indomethacin | 400 | 28.33 ± 1.68 ** |
| Treatments | Dose mg/kg | Intraluminal Bleeding Score Mean ± SE | Gastric Lesions Ulcer Index Mean ± SE |
|---|---|---|---|
| Control (stress only) | - | 3.50 ± 0.42 | 25.20 ± 3.24 |
| O. forsskalii extract + Stress | 200 | 3.16 ± 0.30 | 23.50 ± 0.42 |
| O. forsskalii extract + Stress | 400 | 1.66 ± 0.21 ** | 18.33 ± 0.84 *** |
| Treatments | Dose mg/kg | Ulcer Index (Mean ± SE) | ||
|---|---|---|---|---|
| 80% EtOH | 0.2 mol/L NaOH | 25% NaCl | ||
| Control (80% Ethanol only) | 1 mL/Rat | 6.83 ± 0.30 | 5.83 ± 0.30 | 6.16 ± 0.30 |
| O. forsskalii extract + 80% Ethanol | 200 | 5.33 ± 0.33 ** | 5.33 ± 0.33 | 5.00 ± 0.36 * |
| O. forsskalii extract + 80% Ethanol | 400 | 4.00 ± 0.36 *** | 4.16 ± 0.30 ** | 4.33 ± 0.42 ** |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Foudah, A.I.; Aloneizi, F.K.; Alqarni, M.H.; Alam, A.; Salkini, M.A.; Abubaker, H.M.; Yusufoglu, H.S. Potential Active Constituents from Opophytum forsskalii (Hochst. ex Boiss.) N.E.Br against Experimental Gastric Lesions in Rats. Pharmaceuticals 2022, 15, 1089. https://doi.org/10.3390/ph15091089
Foudah AI, Aloneizi FK, Alqarni MH, Alam A, Salkini MA, Abubaker HM, Yusufoglu HS. Potential Active Constituents from Opophytum forsskalii (Hochst. ex Boiss.) N.E.Br against Experimental Gastric Lesions in Rats. Pharmaceuticals. 2022; 15(9):1089. https://doi.org/10.3390/ph15091089
Chicago/Turabian StyleFoudah, Ahmed I., Fawwaz Khalaf Aloneizi, Mohammad H. Alqarni, Aftab Alam, Mohammad Ayman Salkini, Hamad M. Abubaker, and Hasan S. Yusufoglu. 2022. "Potential Active Constituents from Opophytum forsskalii (Hochst. ex Boiss.) N.E.Br against Experimental Gastric Lesions in Rats" Pharmaceuticals 15, no. 9: 1089. https://doi.org/10.3390/ph15091089
APA StyleFoudah, A. I., Aloneizi, F. K., Alqarni, M. H., Alam, A., Salkini, M. A., Abubaker, H. M., & Yusufoglu, H. S. (2022). Potential Active Constituents from Opophytum forsskalii (Hochst. ex Boiss.) N.E.Br against Experimental Gastric Lesions in Rats. Pharmaceuticals, 15(9), 1089. https://doi.org/10.3390/ph15091089








