Accessing Lipophilicity and Biomimetic Chromatography Profile of Biologically Active Ingredients of Botanicals Used in the Treatment of Inflammatory Bowel Disease
Abstract
1. Introduction
2. Results and Discussion
2.1. In Silico Calculation
2.2. Shake-Flask Method
2.3. Chromatographic Methods
2.3.1. Hydrophobicity Evaluation
2.3.2. Lipophilicity Evaluation
2.3.3. Plasma Protein Affinity Evaluation
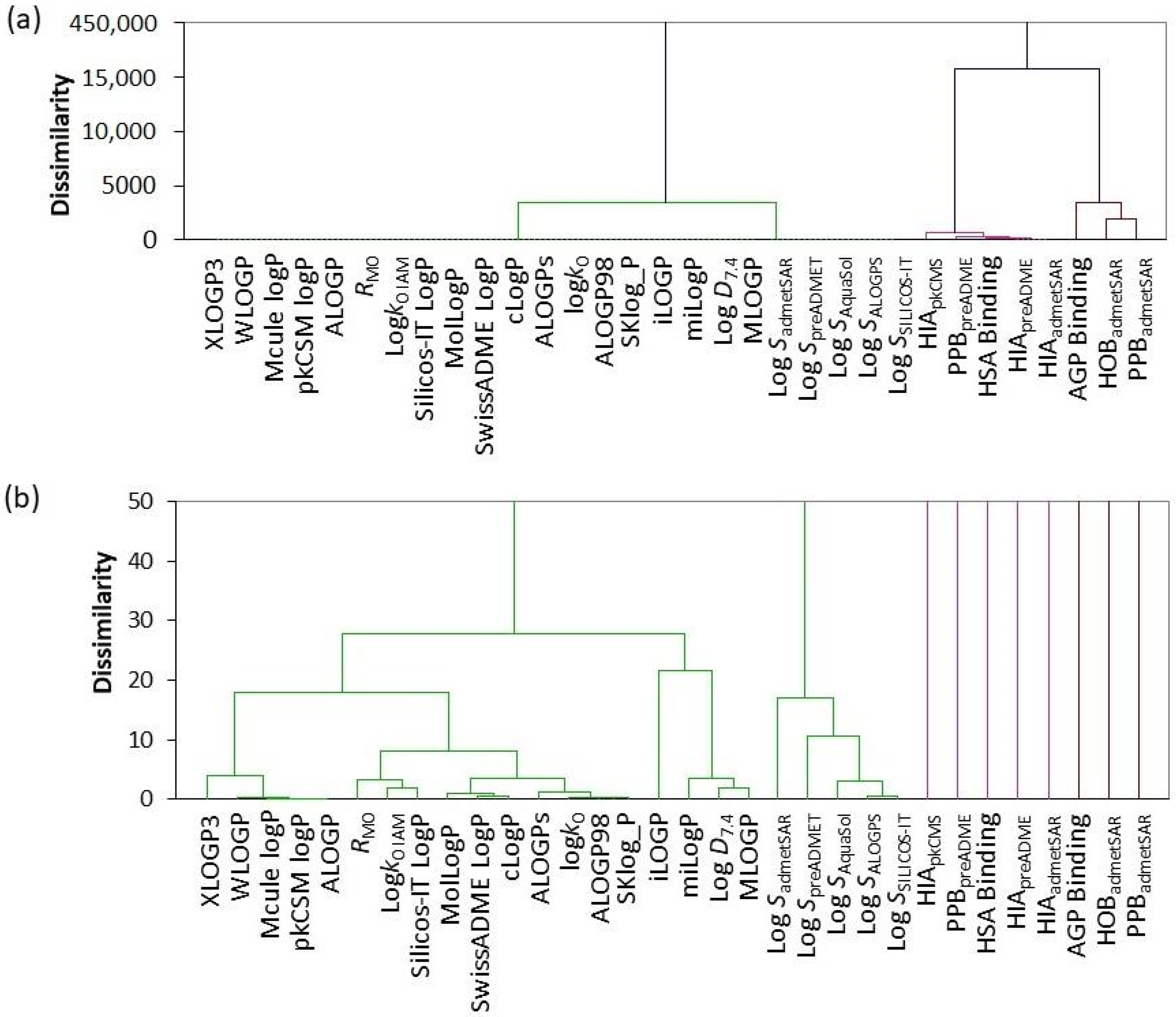
2.4. Comparison of Computational and Experimentally Observed Values Using Statistical Methods
3. Materials and Methods
3.1. Chemicals
3.2. Methods
3.2.1. In Silico Calculation
3.2.2. Shake-Flask Method with Chromatographic Analysis
Sample Preparation
Sample Analysis
3.2.3. Chromatographic Methods
Sample Preparation
C18-TLC Assay
C18- and IAM-HPLC Assays
HSA- and AGP-HPAC Assays
3.2.4. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jairath, V.; Feagan, B.G. Global burden of inflammatory bowel disease. Lancet Gastroenterol. Hepatol. 2020, 5, 2–3. [Google Scholar] [CrossRef]
- Lin, S.C.; Cheifetz, A.S. The use of complementary and alternative medicine in patients with inflammatory bowel disease. Gastroenterol. Hepatol. 2018, 14, 415–425. [Google Scholar]
- Governa, P.; Marchi, M.; Cocetta, V.; De Leo, B.; Saunders, P.T.K.; Catanzaro, D.; Miraldi, E.; Montopoli, M.; Biagi, M. Effects of Boswellia Serrata Roxb. and Curcuma longa L. in an in vitro intestinal inflammation model using immune cells and Caco-2. Pharmaceuticals 2018, 11, 126. [Google Scholar] [CrossRef] [PubMed]
- Mishra, A.; Shaik, H.A.; Sinha, R.K.; Shah, B.R. Andrographolide: A herbal-chemosynthetic approach for enhancing immunity, combating viral infections, and its implication on human health. Molecules 2021, 26, 7036. [Google Scholar] [CrossRef]
- Coelho, M.R.; Romi, M.D.; Ferreira, D.M.T.P.; Zaltman, C.; Soares-Mota, M. The use of curcumin as a complementary therapy in ulcerative colitis: A systematic review of randomized controlled clinical trials. Nutrients 2020, 12, 2296. [Google Scholar] [CrossRef] [PubMed]
- Algieri, F.; Rodriguez-Nogales, A.; Rodriguez-Cabezas, M.E.; Risco, S.; Angeles Ocete, M.; Galvez, J. Botanical drugs as an emerging strategy in inflammatory bowel disease: A review. Mediat. Inflamm. 2015, 2015, 179616. [Google Scholar] [CrossRef]
- Li, Q.; Zhai, W.; Jiang, Q.; Huang, R.; Liu, L.; Dai, J.; Gong, W.; Du, S.; Wu, Q. Curcumin-piperine mixtures in self-microemulsifying drug delivery system for ulcerative colitis therapy. Int. J. Pharm. 2015, 490, 22–31. [Google Scholar] [CrossRef]
- Ermondi, G.; Vallaro, M.; Caron, G. Learning how to use IAM chromatography for predicting permeability. Eur. J. Pharm. Sci. 2018, 114, 385–390. [Google Scholar] [CrossRef]
- Carrasco-Correa, E.J.; Ruiz-Allica, J.; Rodríguez-Fernández, J.F.; Miró, M. Human artificial membranes in (bio)analytical science: Potential for in vitro prediction of intestinal absorption-A review, TrAC-Trend. Anal. Chem. 2021, 145, 116446. [Google Scholar] [CrossRef]
- Obradović, D.; Radan, M.; Đikić, T.; Popović Nikolić, M.; Oljačić, S.; Nikolić, K. The evaluation of drug-plasma protein binding interaction on immobilized human serum albumin stationary phase, aided by different computational approaches. J. Pharmaceut. Biomed. Anal. 2022, 211, 114593. [Google Scholar] [CrossRef]
- Jeličić, M.-L.; Brusač, E.; Klarić, D.A.; Nigović, B.; Turk, N.; Mornar, A. A chromatographic approach to development of 5-aminosalicylate/folic acid fixed-dose combinations for treatment of Crohn’s disease and ulcerative colitis. Sci. Rep. 2020, 10, 20838. [Google Scholar] [CrossRef] [PubMed]
- Brusač, E.; Jeličić, M.-L.; Amidžić Klarić, D.; Nigović, B.; Turk, N.; Klarić, I.; Mornar, A. Pharmacokinetic profiling and simultaneous determination of thiopurine immunosuppressants and folic acid by chromatographic methods. Molecules 2019, 24, 3469. [Google Scholar] [CrossRef] [PubMed]
- Russo, G.; Grumetto, L.; Baert, M.; Lynen, F. Comprehensive two-dimensional liquid chromatography as a biomimetic screening platform for pharmacokinetic profiling of compound libraries in early drug development. Anal. Chim. Acta 2021, 1142, 157–168. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Tawab, M. Considerations to be taken when carrying out medicinal plant research—what we learn from an insight into the IC50 values, bioavailability and clinical efficacy of exemplary anti-inflammatory herbal components. Pharmaceuticals 2021, 14, 437. [Google Scholar] [CrossRef]
- Sharma, T.; Jana, S. Investigation of molecular properties that influence the permeability and oral bioavailability of major β-boswellic acids. Eur. J. Drug Metab. Pharmacokinet. 2020, 45, 243–255. [Google Scholar] [CrossRef]
- Krüger, P.; Daneshfar, R.; Eckert, G.P.; Klein, J.; Volmer, D.A.; Bahr, U.; Müller, W.E.; Karas, M.; Schubert-Zsilavecz, M.; Abdel-Tawab, M. Metabolism of boswellic acids in vitro and in vivo. Drug Metab. Dispos. 2008, 36, 1135–1142. [Google Scholar] [CrossRef]
- Godugu, D.; Rupula, K.; Sashidhar, R.B. Binding studies of andrographolide with human serum albumin: Molecular docking, chromatographic and spectroscopic studies. Protein Pept. Lett. 2018, 25, 330–338. [Google Scholar] [CrossRef]
- Kempińska, D.; Chmiel, T.; Kot-Wasik, A.; Mróz, A.; Mazerska, Z.; Namieśnik, J. State of the art and prospects of methods for determination of lipophilicity of chemical compounds. TrAC-Trend. Anal. Chem. 2019, 113, 54–73. [Google Scholar] [CrossRef]
- Brusač, E.; Jeličić, M.-L.; Amidžić Klarić, D.; Mornar, A. Miniaturized shake-flask HPLC method for determination of distribution coefficient of drugs used in inflammatory bowel diseases. Acta Pharm. 2019, 69, 649–660. [Google Scholar] [CrossRef]
- International Conference on Harmonization of Technical Requirements for Registration of Pharmaceuticals for Human Use, ICH Harmonised Tripartite Guideline. Validation of Analytical Procedures: Text and Methodology Q2(R1), Current Step 4 Version. November 2005. Available online: https://www.ich.org/fileadmin/Public_Web_Site/ICH_Products/Guidelines/Quality/Q2_R1/Step4/Q2_R1__Guideline.pdf (accessed on 20 June 2022).
- Kotagale, N.R.; Charde, P.B.; Helonde, A.; Gupta, K.R.; Umekar, M.J.; Raut, N.S. Studies on bioavailability enhancement of curcumin. Int. J. Pharm. Pharm. Sci. 2020, 12, 20–25. [Google Scholar] [CrossRef][Green Version]
- Jithavech, P.; Suwattananuruk, P.; Muangnoi, C.; Thitikornpong, W.; Towiwat, P.; Vajragupta, O.; Rojsitthisak, P. Physicochemical investigation of a novel curcumin diethyl γ-aminobutyrate, a carbamate ester prodrug of curcumin with enhanced antineuroinflammatory activity. PLoS ONE 2022, 17, e0265689. [Google Scholar] [CrossRef] [PubMed]
- Roy, N.K.; Parama, D.; Banik, K.; Bordoloi, D.; Devi, A.K.; Thakur, K.K.; Padmavathi, G.; Shakibaei, M.; Fan, L.; Sethi, G.; et al. An Update on pharmacological potential of boswellic acids against chronic diseases. Int. J. Mol. Sci. 2019, 20, 4101. [Google Scholar] [CrossRef] [PubMed]
- Dei Cas, M.; Ghidoni, R. Dietary curcumin: Correlation between bioavailability and health potential. Nutrients 2019, 11, 2147. [Google Scholar] [CrossRef] [PubMed]
- Boyle, C.A.; Coatney, R.W.; Wickham, A.; Mukherjee, S.K.; Meunier, L.D. Alpha-1 acid glycoprotein as a biomarker for subclinical illness and altered drug binding in rats. Comp. Med. 2021, 71, 123–132. [Google Scholar] [CrossRef]
- Dezhampanah, H.; Shabanzade, Z. Investigation of binding interaction between human serum albumin with zirconium complex of curcumin and curcumin. J. Biomol. Struct. Dyn. 2022, 40, 722–732. [Google Scholar] [CrossRef]
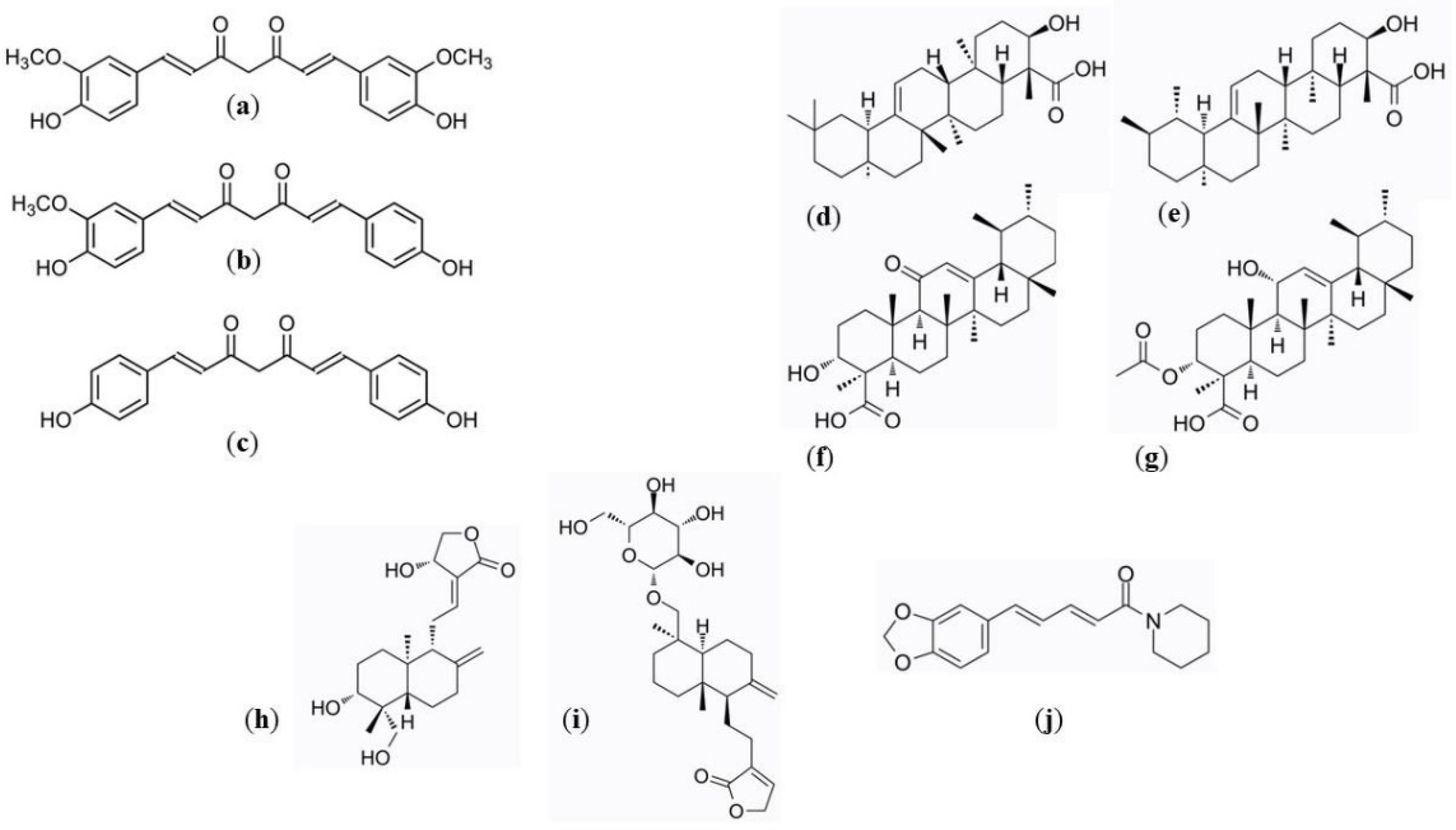

| Parameter | Curcumin | Demetoxycurcumin | Bisdemetoxycurcumin | α-Boswellic Acid | β-Boswellic Acid | 11-Keto-β-boswellic Acid | 3-Acetyl-11-keto-β-boswellic Acid | Andrographolide | Neoandrographolide | Piperine |
|---|---|---|---|---|---|---|---|---|---|---|
| Lipophilicity | ||||||||||
| iLOGP | 3.27 | 2.78 | 1.75 | 3.99 | 3.96 | 3.32 | 3.80 | 2.61 | 3.37 | 3.38 |
| XLOGP3 | 3.20 | 3.32 | 3.26 | 8.41 | 8.26 | 7.20 | 7.22 | 2.16 | 2.63 | 3.46 |
| WLOGP | 3.15 | 3.14 | 3.13 | 7.23 | 7.09 | 6.27 | 6.84 | 1.96 | 1.85 | 2.51 |
| MLOGP | 1.47 | 1.80 | 2.13 | 5.82 | 5.82 | 4.87 | 5.14 | 1.98 | 1.26 | 2.39 |
| Silicos-IT LogP | 4.04 | 3.95 | 3.87 | 5.85 | 5.46 | 5.15 | 5.67 | 2.94 | 2.55 | 3.41 |
| SwissADME LogP | 3.03 | 3.00 | 2.83 | 6.26 | 6.12 | 5.36 | 5.74 | 2.33 | 2.33 | 3.03 |
| ALOGPs | 3.62 | 3.54 | 3.46 | 7.42 | 6.46 | 5.50 | 5.90 | 1.57 | 1.88 | 3.38 |
| miLogP | 2.30 | 2.48 | 2.67 | 6.72 | 6.79 | 5.69 | 6.39 | 1.05 | 1.17 | 3.33 |
| Mcule logP | 3.37 | 3.36 | 3.35 | 7.23 | 7.09 | 6.27 | 6.84 | 1.96 | 1.85 | 2.94 |
| cLogP | 2.95 | 3.02 | 3.09 | 6.06 | 6.00 | 5.29 | 5.78 | 1.88 | 1.74 | 3.60 |
| MolLogP | 2.83 | 2.88 | 2.93 | 5.86 | 5.68 | 4.66 | 5.17 | 2.32 | 2.51 | 3.47 |
| ALOGP98 | 3.22 | 3.24 | 3.26 | 6.42 | 6.47 | 5.54 | 5.92 | 2.06 | 2.38 | 2.86 |
| pkCSM logP | 3.37 | 3.36 | 3.35 | 7.23 | 7.09 | 6.27 | 6.84 | 1.96 | 1.85 | 3.00 |
| SKlog_P | 3.72 | 3.49 | 3.25 | 6.78 | 6.61 | 5.72 | 6.02 | 2.10 | 2.42 | 3.03 |
| ALOGP | 3.37 | 3.36 | 3.35 | 7.23 | 7.09 | 6.27 | 6.84 | 1.96 | 1.85 | 3.00 |
| Solubility | ||||||||||
| log SSILICOS-IT | −4.45 | −4.34 | −4.23 | −6.12 | −5.67 | −5.55 | −6.15 | −2.69 | −2.70 | −3.00 |
| log SALOGPS | −4.81 | −4.70 | −4.46 | −5.86 | −5.97 | −5.51 | −6.06 | −3.09 | −3.37 | −3.28 |
| log SAquaSol | −3.92 | −4.01 | −4.06 | −5.13 | −5.20 | −4.71 | −4.87 | −2.78 | −3.42 | −3.55 |
| log SpreADMET | −4.53 | −4.05 | −3.56 | −7.35 | −7.49 | −6.92 | −7.28 | −3.12 | −4.44 | −3.71 |
| log SadmetSAR | −3.36 | −3.09 | −2.88 | −3.85 | −3.79 | −3.45 | −4.67 | −2.85 | −3.31 | −3.40 |
| Absorption | ||||||||||
| HIApkCMS (%) | 82.190 | 91.393 | 91.159 | 94.363 | 97.466 | 98.826 | 99.510 | 95.357 | 62.263 | 94.444 |
| HIApreADMET (%) | 94.403 | 94.029 | 93.750 | 95.996 | 95.996 | 96.714 | 99.202 | 87.688 | 81.330 | 98.180 |
| HIAadmetSAR (%) | 97.70 | 97.70 | 97.37 | 98.53 | 98.53 | 99.01 | 99.19 | 98.22 | 81.24 | 96.39 |
| HOBadmetSAR (%) | 60.00 | 64.29 | 64.29 | 50.00 | 52.86 | 51.43 | 51.43 | 61.43 | 65.71 | 57.14 |
| Plasma protein binding | ||||||||||
| PPBpreADMET (%) | 88.030 | 89.913 | 93.826 | 100.000 | 100.000 | 100.000 | 94.061 | 94.873 | 93.373 | 90.449 |
| PPBadmetSAR (%) | 64.0 | 68.9 | 42.1 | 51.4 | 65.8 | 62.9 | 79.4 | 49.4 | 52.5 | 100.0 |
| Analyte | Linearity Range (µg/mL) | Regression Equation * | r | LOD (µg/mL) ** | LOQ (µg/mL) *** |
|---|---|---|---|---|---|
| Curcumin | 1–375 | y = 987.3 x + 0.935 | 0.9999 | 0.3 | 1 |
| Demetoxycurcumin | 1–375 | y = 421.0 x + 0.610 | 0.9998 | 0.3 | 1 |
| Bisdemetoxycurcumin | 10–375 | y = 134.4 x + 0.200 | 0.9999 | 3 | 10 |
| Andrographolide | 5–300 | y = 15,039 x + 35.28 | 0.9996 | 2 | 5 |
| Neoandrographolide | 5–250 | y = 1723 x + 3.188 | 0.9995 | 2 | 5 |
| Piperine | 5–300 | y = 10,601 x + 10.15 | 0.9999 | 2 | 5 |
| Analyte | Precision (RSD, %) **** | Accuracy (Recovery and RSD, %) ***** | |||
| Repeatability (n = 6) | Intermediate Precision (n = 9) | Low (n = 3) | Medium (n = 3) | High (n = 3) | |
| Curcumin | 0.42 | 1.59 | 102.5 ± 3.89 | 95.0 ± 3.55 | 97.5 ± 2.95 |
| Demetoxycurcumin | 0.38 | 0.80 | 102.9 ± 3.81 | 95.4 ± 3.41 | 98.8 ± 3.01 |
| Bisdemetoxycurcumin | 0.98 | 0.27 | 103.0 ± 3.40 | 96.2 ± 3.31 | 99.4 ± 2.55 |
| Andrographolide | 1.61 | 3.61 | 97.2 ± 3.66 | 99.1 ± 1.98 | 98.4 ± 1.59 |
| Neoandrographolide | 1.73 | 3.39 | 98.1 ± 2.90 | 102.1 ± 2.50 | 96.5 ± 2.34 |
| Piperine | 0.73 | 0.59 | 102.5 ± 0.92 | 104.3 ± 0.94 | 103.0 ± 0.91 |
| Analyte | log D7.4 ± RSD ** (%) (n = 3) |
|---|---|
| Curcumin | 1.94 ± 2.59 |
| Demetoxycurcumin | 1.52 ± 3.78 |
| Bisdemetoxycurcumin | 1.72 ± 4.21 |
| Andrographolide | 1.32 ± 3.25 |
| Neoandrographolide | 1.51 ± 2.55 |
| Piperine | 1.67 ± 3.55 |
| Analyte | Linear Equation * | r | Standard Error |
|---|---|---|---|
| C18-TLC Assay | |||
| Curcumin | y = −0.0540 x + 4.4855 | 0.9916 | 0.0835 |
| Demetoxycurcumin | y = −0.0504 x + 4.1781 | 0.9932 | 0.0699 |
| Bisdemetoxycurcumin | y = −0.0503 x + 4.1751 | 0.9937 | 0.0675 |
| 11-keto-β-boswellic acid | y = −0.0564 x + 5.3756 | 0.9950 | 0.0399 |
| 3-acetyl-11-keto-β-boswellic acid | y = −0.0722 x + 6.9059 | 0.9798 | 0.1041 |
| Andrographolide | y = −0.0302 x + 2.0908 | 0.9970 | 0.0277 |
| Neoandrographolide | y = −0.0334 x + 2.1672 | 0.9964 | 0.0266 |
| Piperine | y = −0.0526 x + 4.4044 | 0.9789 | 0.0913 |
| C18-HPLC Assay | |||
| Curcumin | y = −0.0442 x + 3.4187 | 0.9918 | 0.0368 |
| Demetoxycurcumin | y = −0.0448 x + 3.4349 | 0.9827 | 0.0542 |
| Bisdemetoxycurcumin | y = −0.0447 x + 3.4174 | 0.9818 | 0.0556 |
| Andrographolide | y = −0.0271 x + 1.9065 | 0.9695 | 0.0581 |
| Neoandrographolide | y = −0.0359 x + 2.8595 | 0.9782 | 0.0489 |
| Piperine | y = −0.0394 x + 3.4218 | 0.9796 | 0.0519 |
| IAM-HPLC Assay | |||
| Curcumin | y = −0.0565 x + 4.3463 | 0.9969 | 0.0530 |
| Demetoxycurcumin | y = −0.0555 x + 4.4229 | 0.9941 | 0.0554 |
| Bisdemetoxycurcumin | y = −0.0568 x + 4.6386 | 0.9940 | 0.0569 |
| α-boswellic acid | y = −0.0788 x + 6.3109 | 0.9956 | 0.0526 |
| β-boswellic acid | y = −0.0816 x + 6.5157 | 0.9982 | 0.0346 |
| 11-keto-β-boswellic acid | y = −0.0570 x + 4.9194 | 0.9874 | 0.0645 |
| 3-acetyl-11-keto-β-boswellic acid | y = −0.0703 x + 5.1157 | 0.9944 | 0.0526 |
| Andrographolide | y = −0.0464 x + 2.2763 | 0.9796 | 0.0753 |
| Neoandrographolide | y = −0.0620 x + 3.4572 | 0.9854 | 0.0755 |
| Piperine | y = −0.0512 x + 3.2026 | 0.9830 | 0.0872 |
| Analyte | HSA Binding ± RSD * (%) (n = 3) | AGP Binding ± RSD (%) (n = 3) |
|---|---|---|
| Curcumin | 97.52 ± 0.20 | 83.07 ± 1.10 |
| Demetoxycurcumin | 97.97 ± 0.35 | 82.92 ± 1.15 |
| Bisdemetoxycurcumin | 98.37 ± 0.10 | 83.14 ± 1.15 |
| α-boswellic acid | 98.94 ± 0.35 | 89.22 ± 0.30 |
| β-boswellic acid | 99.79 ± 0.30 | 89.15 ± 2.04 |
| 11-keto-β-boswellic acid | 98.09 ± 0.02 | 79.01 ± 0.20 |
| 3-acetyl-11-keto-β-boswellic acid | 99.83 ± 0.47 | 83.10 ± 0.37 |
| Andrographolide | 80.07 ± 0.74 | 37.13 ± 1.11 |
| Neoandrographolide | 87.71 ± 0.41 | 64.95 ± 0.41 |
| Piperine | 95.48 ± 0.41 | 71.98 ± 1.33 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jeličić, M.-L.; Amidžić Klarić, D.; Kovačić, J.; Verbanac, D.; Mornar, A. Accessing Lipophilicity and Biomimetic Chromatography Profile of Biologically Active Ingredients of Botanicals Used in the Treatment of Inflammatory Bowel Disease. Pharmaceuticals 2022, 15, 965. https://doi.org/10.3390/ph15080965
Jeličić M-L, Amidžić Klarić D, Kovačić J, Verbanac D, Mornar A. Accessing Lipophilicity and Biomimetic Chromatography Profile of Biologically Active Ingredients of Botanicals Used in the Treatment of Inflammatory Bowel Disease. Pharmaceuticals. 2022; 15(8):965. https://doi.org/10.3390/ph15080965
Chicago/Turabian StyleJeličić, Mario-Livio, Daniela Amidžić Klarić, Jelena Kovačić, Donatella Verbanac, and Ana Mornar. 2022. "Accessing Lipophilicity and Biomimetic Chromatography Profile of Biologically Active Ingredients of Botanicals Used in the Treatment of Inflammatory Bowel Disease" Pharmaceuticals 15, no. 8: 965. https://doi.org/10.3390/ph15080965
APA StyleJeličić, M.-L., Amidžić Klarić, D., Kovačić, J., Verbanac, D., & Mornar, A. (2022). Accessing Lipophilicity and Biomimetic Chromatography Profile of Biologically Active Ingredients of Botanicals Used in the Treatment of Inflammatory Bowel Disease. Pharmaceuticals, 15(8), 965. https://doi.org/10.3390/ph15080965