Malva parviflora Leaves and Fruits Mucilage as Natural Sources of Anti-Inflammatory, Antitussive and Gastro-Protective Agents: A Comparative Study Using Rat Models and Gas Chromatography
Abstract
1. Introduction
2. Results
2.1. In Vitro Hemolytic Activity
2.2. Acute Toxicity Study
2.3. Acute Anti-Inflammatory Activity
2.4. Acute Antitussive Activity
2.5. Antiulcer Activity
2.5.1. Macroscopic Analysis of Stomach Mucosa
2.5.2. Determination of Ulcer Score, Ulcer Index and Percentage Inhibition
2.5.3. Histology of Stomach Wall
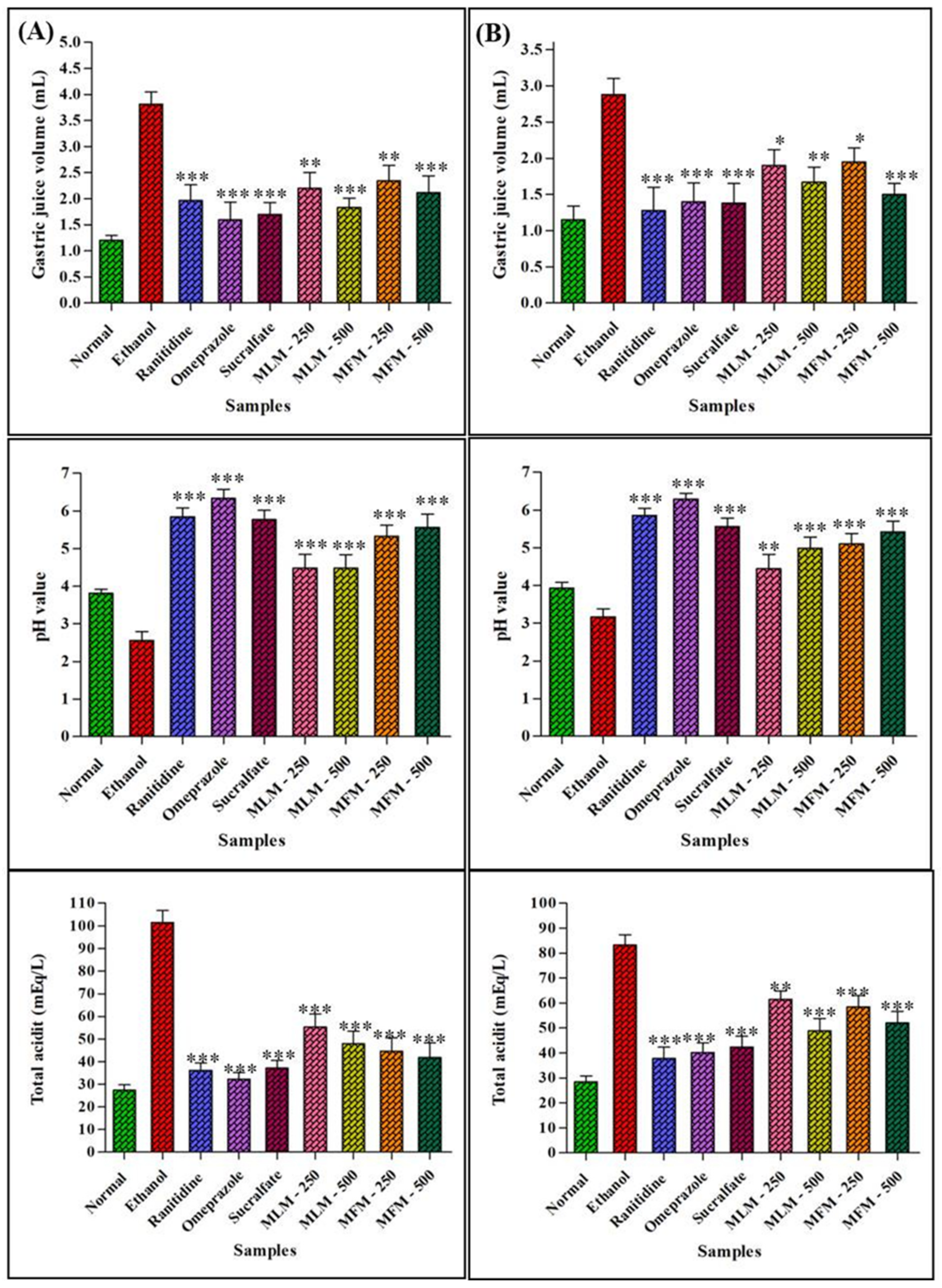
2.5.4. Mechanism of Action
Effect on Gastric Juice Parameters
Effect on Total Protein and Total Mucus Content
2.6. Gas Chromatography Analyses of MLM and MFM
3. Discussion
4. Materials and Methods
4.1. Plant Material
4.2. Drugs and Chemicals
4.3. Animals
4.4. Preparation of the Mucilage
4.5. Evaluation of the Biological Activities of the Mucilage Extracts
4.5.1. In Vitro Hemolytic Activity
4.5.2. Acute Toxicity Study
4.5.3. Anti-Inflammatory Activity
4.5.4. Antitussive Activity
4.5.5. Antiulcer Activity
Study Design
Ethanol Induced Acute Gastric Ulcer
Ethanol Induced Chronic Gastric Ulcer
Parameters of Gastric Ulcer Evaluation
- Macroscopic and Microscopic Evaluation
Ulcer Scoring
Ulcer Index
Ulcer Protection (%)
Histological Analysis
Mode of Gastro-Protective Activity
- Estimation of the Gastric Volume
Determination of the pH Value and Total Acidity
Determination of Gastric Mucin Content
Determination of the Total Protein Content
4.6. Gas Chromatography Coupled with Mass Spectrometry Analysis
4.7. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Eccles, R.; Mallefet, P. Soothing properties of glycerol in cough syrups for acute cough due to common cold. Pharmacy 2017, 5, 4. [Google Scholar] [CrossRef] [PubMed]
- Romm, A.; Ganora, L.; Hoffmann, D.; Yarnell, E.; Abascal, K.; Coven, M. Chapter 3—Fundamental Principles of Herbal Medicine. In Botanical Medicine for Women’s Health; Room, A., Hardy, M.L., Mills, S., Eds.; Churchill Livingstone: Saint Louis, France, 2010; pp. 24–74. [Google Scholar]
- Czibulka, A. Probiotics and Herbal Therapies. In Laryngopharyngeal Reflux Disease; Springer: Berlin/Heidelberg, Germany, 2019; pp. 103–113. [Google Scholar]
- Schmidgall, J.; Schnetz, E.; Hensel, A. Evidence for bioadhesive effects of polysaccharides and polysaccharide-containing herbs in an ex vivo bioadhesion assay on buccal membranes. Planta Med. 2000, 66, 48–53. [Google Scholar] [CrossRef] [PubMed]
- Tosif, M.M.; Najda, A.; Bains, A.; Kaushik, R.; Dhull, S.B.; Chawla, P.; Walasek-Janusz, M. A Comprehensive review on plant-derived mucilage: Characterization, functional properties, applications, and its utilization for nanocarrier fabrication. Polymers 2021, 13, 1066. [Google Scholar] [CrossRef] [PubMed]
- Nazari, M.; Riebeling, S.; Banfield, C.C.; Akale, A.; Crosta, M.; Mason-Jones, K.; Dippold, M.A.; Ahmed, M.A. Mucilage polysaccharide composition and exudation in maize from contrasting climatic regions. Front. Plant Sci. 2020, 11, 1968. [Google Scholar] [CrossRef] [PubMed]
- Dybka-Stępień, K.; Otlewska, A.; Góźdź, P.; Piotrowska, M. The renaissance of plant mucilage in health promotion and industrial applications: A review. Nutrients 2021, 13, 3354. [Google Scholar] [CrossRef]
- El-Shiekh, R.A.; Salama, A.; Al-Mokaddem, A.K.; Abdel-Sattar, E.A. Gastroprotective effect of mucilage fraction from Solenostemma argel via cytoprotection and attenuation of oxidative stress, inflammation and apoptosis. J. Herb. Pharmacol. 2021, 10, 232–240. [Google Scholar] [CrossRef]
- Wadhwa, J.; Nair, A.; Kumria, R. Potential of plant mucilages in pharmaceuticals and therapy. Curr. Drug Deliv. 2013, 10, 198–207. [Google Scholar] [CrossRef]
- Alam, M.T.; Parvez, N.; Sharma, P.K. FDA-approved natural polymers for fast dissolving tablets. J. Pharm. 2014, 2014, 952970. [Google Scholar] [CrossRef]
- Hou, C.; Chen, L.; Yang, L.; Ji, X. An insight into anti-inflammatory effects of natural polysaccharides. Int. J. Biol. Macromol. 2020, 153, 248–255. [Google Scholar] [CrossRef]
- Aboulwafa, M.M.; Youssef, F.S.; Gad, H.A.; Altyar, A.E.; Al-Azizi, M.M.; Ashour, M.L. A comprehensive insight on the health benefits and phytoconstituents of Camellia sinensis and recent approaches for its quality control. Antioxidants 2019, 8, 455. [Google Scholar] [CrossRef]
- Ashour, M.L.; Youssef, F.S.; Gad, H.A.; El-Readi, M.Z.; Bouzabata, A.; Abuzeid, R.M.; Sobeh, M.; Wink, M. Evidence for the anti-inflammatory activity of Bupleurum marginatum (Apiaceae) extracts using in vitro and in vivo experiments supported by virtual screening. J. Pharm. Pharmacol. 2018, 70, 952–963. [Google Scholar] [CrossRef] [PubMed]
- El Senousy, A. Immunomodulatory and anti-inflammatory activities of the defatted alcoholic extract and mucilage of Hibiscus sabdariffa L. leaves, and their chemical characterization. J. Pharmacog. Phytochem. 2019, 8, 982–990. [Google Scholar]
- Carlotto, J.; Maria-Ferreira, D.; de Souza, L.M.; da Luz, B.B.; Dallazen, J.L.; de Paula Werner, M.F.; Cipriani, T.R. A polysaccharide fraction from “ipê-roxo” (Handroanthus heptaphyllus) leaves with gastroprotective activity. Carbohydr. Polym. 2019, 226, 115239. [Google Scholar] [CrossRef]
- Nascimento, A.M.; Maria-Ferreira, D.; de Souza, E.F.J.; de Souza, L.M.; Sassaki, G.L.; Iacomini, M.; Werner, M.F.d.P.; Cipriani, T.R. Gastroprotective effect and chemical characterization of a polysaccharide fraction from leaves of Croton cajucara. Int. J. Biol. Macromol. 2017, 95, 153–159. [Google Scholar] [CrossRef]
- El-Din, M.I.G.; Youssef, F.S.; Said, R.S.; Ashour, M.L.; Eldahshan, O.A.; Singab, A.N.B. Chemical constituents and gastro-protective potential of Pachira glabra leaves against ethanol-induced gastric ulcer in experimental rat model. Inflammopharmacology 2021, 29, 317–332. [Google Scholar] [CrossRef] [PubMed]
- El-Din, M.I.G.; Youssef, F.S.; Ashour, M.L.; Eldahshan, O.A.; Singab, A.N.B. New γ-pyrone glycoside from Pachira glabra and assessment of its gastroprotective activity using an alcohol-induced gastric ulcer model in rats. Food Funct. 2020, 11, 1958–1965. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.S.A.; Khundmiri, S.U.K.; Khundmiri, S.R.; Al-Sanea, M.M.; Mok, P.L. Fruit-derived polysaccharides and terpenoids: Recent update on the gastroprotective effects and mechanisms. Front. Pharmacol. 2018, 9, 569. [Google Scholar] [CrossRef]
- Lajili, S.; Ammar, H.H.; Mzoughi, Z.; Amor, H.B.H.; Muller, C.D.; Majdoub, H.; Bouraoui, A. Characterization of sulfated polysaccharide from Laurencia obtusa and its apoptotic, gastroprotective and antioxidant activities. Int. J. Biol. Macromol. 2019, 126, 326–336. [Google Scholar] [CrossRef]
- Fathy, S.; Emam, M.; Agwa, S.A.; Zahra, F.A.; Youssef, F.; Sami, R. The antiproliferative effect of Origanum majorana on human hepatocarcinoma cell line: Suppression of NF-kB. Cell. Mol. Biol. 2016, 62, 80–84. [Google Scholar]
- Murgia, V.; Ciprandi, G.; Votto, M.; De Filippo, M.; Tosca, M.A.; Marseglia, G.L. Natural remedies for acute post-viral cough in children. Allergol. Immunopathol. 2021, 49, 173–184. [Google Scholar] [CrossRef]
- Kahramanoğlu, İ.; Wan, C. Determination and improvement of the postharvest storability of Little Mallow (Malva parviflora L.): A novel crop for a sustainable diet. HortScience 2020, 55, 1378–1386. [Google Scholar]
- Ododo, M.M.; Choudhury, M.K.; Dekebo, A.H. Structure elucidation of β-sitosterol with antibacterial activity from the root bark of Malva parviflora. SpringerPlus 2016, 5, 1210. [Google Scholar] [CrossRef] [PubMed]
- Munir, A.; Youssef, F.S.; Ishtiaq, S.; Kamran, S.H.; Sirwi, A.; Ahmed, S.A.; Ashour, M.L.; Elhady, S.S. Malva parviflora leaves mucilage: An eco-friendly and sustainable biopolymer with antioxidant properties. Polymers 2021, 13, 4251. [Google Scholar] [CrossRef] [PubMed]
- Dugani, A.; Dakhil, B.; Treesh, S. Protective effect of the methanolic extract of Malva parviflora L. leaves on acetic acid-induced ulcerative colitis in rats. Saudi J. Gastroenterol. 2016, 22, 226–233. [Google Scholar]
- El-Naggar, M.E.; Hussein, J.; El-sayed, S.M.; Youssef, A.M.; El Bana, M.; Latif, Y.A.; Medhat, D. Protective effect of the functional yogurt based on Malva parviflora leaves extract nanoemulsion on acetic acid-induced ulcerative colitis in rats. J. Mat. Res. Technol. 2020, 9, 14500–14508. [Google Scholar] [CrossRef]
- Afolayan, A.J.; Aboyade, O.M.; Adedapo, A.A.; Sofidiya, M.O. Anti-inflammatory and analgesic activity of the methanol extract of Malva parviflora Linn (Malvaceae) in rats. Afr. J. Biotechnol. 2010, 9, 1225–1229. [Google Scholar]
- Bouriche, H.; Meziti, H.; Senator, A.; Arnhold, J. Anti-inflammatory, free radical-scavenging, and metal-chelating activities of Malva parviflora. Pharm. Biol. 2011, 49, 942–946. [Google Scholar] [CrossRef]
- Jothy, S.L.; Zakaria, Z.; Chen, Y.; Lau, Y.L.; Latha, L.Y.; Sasidharan, S. Acute oral toxicity of methanolic seed extract of Cassia fistula in Mice. Molecules 2011, 16, 5268–5282. [Google Scholar] [CrossRef]
- Afsar, T.; Razak, S.; Khan, M.R.; Mawash, S.; Almajwal, A.; Shabir, M.; Haq, I.U. Evaluation of antioxidant, anti-hemolytic and anticancer activity of various solvent extracts of Acacia hydaspica R. Parker aerial parts. BMC Complement. Altern. Med. 2016, 16, 258. [Google Scholar]
- Lakshmi, G.; Smitha, N.; Ammu, S.; Priya, C.; Bhaskara Rao, K. Phytochemical profile, in vitro antioxidant and hemolytic activities of various leaf extract of Nymphaea Nouchali Linn: An in vitro study. Int. J. Pharm. Pharm. Sci. 2014, 6, 548–552. [Google Scholar]
- Vidhya, R.; Udayakumar, R. Phytochemical screening and evaluation of in vitro haemolytic, thrombolytic and antiinflammatory activities of Aerva lanata (L.). IAJPS 2016, 6, 6–7. [Google Scholar]
- Elmajdoub, A.A.; Awidat, S.K.; El-Mahmoudy, A.M. Anti-inflammatory potential of Agaricus in carrageenan-induced model of local inflammation in rats. Int. J. Basic Clin. Pharmacol. 2015, 4, 497. [Google Scholar] [CrossRef][Green Version]
- Galati, E.; Cavallaro, A.; Ainis, T.; Tripodo, M.; Bonaccorsi, I.; Contartese, G.; Taviano, M.F.; Fimiani, V. Anti-Inflammatory effect of lemon mucilage: In vivo and in vitro studies. Immunopharmacol. Immunotoxicol. 2005, 27, 661–670. [Google Scholar] [CrossRef] [PubMed]
- Alba, K.; Nguyen, P.T.; Kontogiorgos, V. Sustainable polysaccharides from Malvaceae family: Structure and functionality. Food Hydrocolloid. 2021, 118, 106749. [Google Scholar] [CrossRef]
- Kaur, H.B.; Ruknuddin, G.; Nariya, M.; Patgiri, B.; Bedarkar, P.; Prajapati, P.K. Anti-tussive activity of Ashtangavaleha prepared by two different methods against sulphur dioxide induced cough in mice. Med. J. 2018, 11, 471–475. [Google Scholar] [CrossRef]
- Ridh, D.A.A.M.; Sarheed, N.M.; Kokaz, O. Activity of ethanolic extraction of Malva parviflora and liqourice as antifungal and antioxidant in male rats. J. Pharm. Sci. Res. 2018, 10, 777–781. [Google Scholar]
- Sutovska, M.; Capek, P.; Franova, S.; Joskova, M.; Sutovsky, J.; Marcinek, J.; Kalman, M. Antitussive activity of Althaea officinalis L. polysaccharide rhamnogalacturonan and its changes in guinea pigs with ovalbumine-induced airways inflammation. Bratisl. Lek. Listy 2011, 112, 670–675. [Google Scholar]
- Büechi, S.; Vögelin, R.; Eiff, M.; Ramos, M.; Melzer, J. Open trial to assess aspects of safety and efficacy of a combined herbal cough syrup with Ivy and Thyme. Res. Complement. Nat. Class. Med. 2006, 12, 328–332. [Google Scholar] [CrossRef]
- Adefisayo, M.A.; Akomolafe, R.O.; Akinsomisoye, S.O.; Alabi, Q.K.; Ogundipe, O.L.; Omole, J.G.; Olamilosoye, K.P. Gastro-protective effect of methanol extract of Vernonia amygdalina (del.) leaf on aspirin-induced gastric ulcer in Wistar rats. Toxicol. Rep. 2017, 4, 625–633. [Google Scholar] [CrossRef]
- Chen, S.H.; Liang, Y.C.; Chao, J.C.; Tsai, L.H.; Chang, C.C.; Wang, C.C.; Pan, S. Protective effects of Ginkgo biloba extract on the ethanol-induced gastric ulcer in rats. World J. Gastroenterol. 2005, 11, 3746–3750. [Google Scholar] [CrossRef]
- Rahim, N.A.; Hassandarvish, P.; Golbabapour, S.; Ismail, S.; Tayyab, S.; Abdulla, M.A. Gastroprotective effect of ethanolic extract of Curcuma xanthorrhiza leaf against ethanol-induced gastric mucosal lesions in Sprague-Dawley rats. BioMed Res. Int. 2014, 2014, 416409. [Google Scholar] [CrossRef] [PubMed]
- Adinortey, M.; Ansah, C.; Galyuon, I.; Nyarko, A. In vivo models used for evaluation of potential antigastroduodenal Ulcer Agents. Ulcers 2013, 2013, 489–512. [Google Scholar] [CrossRef]
- Sharifi-Rad, M.; Fokou, P.V.T.; Sharopov, F.; Martorell, M.; Ademiluyi, A.O.; Rajkovic, J.; Salehi, B.; Martins, N.; Iriti, M.; Sharifi-Rad, J. Antiulcer agents: From plant extracts to phytochemicals in healing promotion. Molecules 2018, 23, 1751. [Google Scholar] [CrossRef] [PubMed]
- Balogun, M.E.; Besong, E.E.; Obimma, J.N.; Djobissie, S.F.; Mbamalu, O.S. Gastroprotective effect of ethanolic extract of Vigna subterranea in ethanol-induced gastric mucosal ulceration in Rats. Ind. J. Physiol. Pharmacol. 2018, 62, 347–358. [Google Scholar]
- Zakaria, Z.A.; Balan, T.; Azemi, A.K.; Omar, M.H.; Mohtarrudin, N.; Ahmad, Z.; Abdullah, M.N.H.; Desa, M.N.M.; Teh, L.K.; Salleh, M.Z. Mechanism (s) of action underlying the gastroprotective effect of ethyl acetate fraction obtained from the crude methanolic leaves extract of Muntingia calabura. BMC Complement. Altern. Med. 2016, 16, 78. [Google Scholar] [CrossRef] [PubMed]
- Arawwawala, L.; Arambewela, L.; Ratnasooriya, W. Gastroprotective effect of Piper betle Linn. leaves grown in Sri Lanka. J. Ayurveda Integ. Med. 2014, 5, 38. [Google Scholar] [CrossRef]
- Onoja, S.O.; Chinagorom, O.; Ikpa, C.B.; Madubuike, K.G.; Ezeigbo, I.I.; Ijioma, S.N.; Anaga, A.O.; Ezeja, M.I. Gastroprotective effects of methanol extract of Eremomastax speciosa leaf harvested in Southern part of Nigeria in rat. EuroBiotech J. 2018, 2, 200–208. [Google Scholar] [CrossRef]
- Monrroy, M.; García, E.; Ríos, K.; García, J.R. Extraction and physicochemical characterization of mucilage from Opuntia cochenillifera (L.) Miller. J. Chem. 2017, 2017, 4301901. [Google Scholar] [CrossRef]
- Patel, D.; Prajapati, D.; Patel, N. Seed mucilage from Ocimum americanum linn. as disintegrant in tablets: Separation and evaluation. Ind. J. Pharm. Sci. 2007, 69, 431. [Google Scholar] [CrossRef]
- Kamble, M.S. Studies on isolation and evaluation of Ocimum tenuiflorum linn seed mucilage. J. Drug Del. Therap. 2012, 2, 6. [Google Scholar] [CrossRef][Green Version]
- Zubair, M.; Rizwan, K.; Rashid, U.; Saeed, R.; Saeed, A.A.; Rasool, N.; Riaz, M. GC/MS profiling, in vitro antioxidant, antimicrobial and haemolytic activities of Smilax macrophylla leaves. Arab. J. Chem. 2017, 10, S1460–S1468. [Google Scholar] [CrossRef]
- OECD. Test No. 425: Acute oral toxicity—Up-and-down procedure. In The OECD Guidelines for the Testing of Chemicals; OECD: Paris, France, 2001; ISBN 9789264071049. [Google Scholar]
- Igbe, I.; Ching, F.P.; Eromon, A. Anti-inflammatory activity of aqueous fruit pulp extract of Hunteria umbellata K. schum in acute and chronic inflammation. Acta Pol. Pharm. Drug Res. 2010, 67, 81–85. [Google Scholar]
- Singh, M.; Kumar, V.; Singh, I.; Gauttam, V.; Kalia, A.N. Anti-inflammatory activity of aqueous extract of Mirabilis jalapa Linn. leaves. Pharmacog. Res. 2010, 2, 364. [Google Scholar] [CrossRef] [PubMed]
- Gupta, R.; Gupta, M.; Bhandari, A.; Gupta, J. Evaluation of antitussive activity of polyherbomineral formulation on cough reflex induced by different cough induced models in mice. Int. J. Drug Dev. Res. 2014, 6, 280–289. [Google Scholar]
- Ateufack, G.; Domgnim Mokam, E.C.; Mbiantcha, M.; Dongmo Feudjio, R.B.; David, N.; Kamanyi, A. Gastroprotective and ulcer healing effects of piptadeniastrum Africanum on experimentally induced gastric ulcers in rats. BMC Complement. Altern. Med. 2015, 15, 214. [Google Scholar] [CrossRef] [PubMed]
- Katary, M.; Salahuddin, A. Gastroprotective Effect of punicalagin against ethanol-induced gastric Ulcer: The possible underlying Mechanisms. Biomark. J. 2017, 3, 1. [Google Scholar] [CrossRef]
- Rafiei, M.; Namazi, F.; Rajaian, H.; Nazifi, S. Gastroprotective effects of various Scrophularia striata extracts on ethanol-induced gastric ulcer in Rats. Turk. J. Pharm. Sci. 2016, 13, 328–334. [Google Scholar] [CrossRef]
- Arab, H.H.; Salama, S.A.; Omar, H.A.; Arafa, E.-S.A.; Maghrabi, I.A. Diosmin protects against ethanol-induced gastric injury in rats: Novel anti-ulcer actions. PLoS ONE 2015, 10, e0122417. [Google Scholar] [CrossRef]
- Tamaddonfard, E.; Erfanparast, A.; Farshid, A.A.; Imani, M.; Mirzakhani, N.; Salighedar, R.; Tamaddonfard, S. Safranal, a constituent of saffron, exerts gastro-protective effects against indomethacin-induced gastric ulcer. Life Sci. 2019, 224, 88–94. [Google Scholar] [CrossRef]
- Sharma, A.L.; Bhot, M.A.; Chandra, N. Gastroprotective effect of aqueous extract and mucilage from Bryophyllum pinnatum (Lam.) Kurz. Anci. Sci. Life. 2014, 33, 252–258. [Google Scholar] [CrossRef]
- Das, A.K.; Bigoniya, P.; Verma, N.K.; Rana, A. Gastroprotective effect of Achyranthes aspera Linn. leaf on rats. Asian Pac. J. Trop. Med. 2012, 5, 197–201. [Google Scholar] [CrossRef]
- Dashputre, N.; Naikwade, N. Evaluation of anti-ulcer activity of methanolic extract of Abutilon indicum Linn. leaves in experimental rats. Int. J. Pharm. Sci. Drug Res. 2011, 3, 97–100. [Google Scholar]
- Gupta, J.; Kumar, D.; Gupta, A. Evaluation of gastric anti–ulcer activity of methanolic extract of Cayratia trifolia in experimental animals. Asian Pac. J. Trop. Dis. 2012, 2, 99–102. [Google Scholar] [CrossRef]
- Gul, H.; Abbas, K.; Qadir, M.I. Gastro-protective effect of ethanolic extract of Mentha longifolia in alcohol-and aspirin-induced gastric ulcer models. Bang. J. Pharmacol. 2015, 10, 241–245. [Google Scholar] [CrossRef]
- Sen, S.; Asokkumar, K.; Umamaheswari, M.; Sivashanmugam, A.; Subhadradevi, V. Antiulcerogenic effect of gallic acid in rats and its effect on oxidant and antioxidant parameters in stomach tissue. Ind. J. Pharm. Sci. 2013, 75, 149. [Google Scholar]
- Kore Kakasaheb, J.; Shete Rajkumar, V.; Patel Apsari, J.; Kulkarni Jitendra, B. Antiulcer activity of aqueous extract of Spinacia oleracia in rats. IJRPC 2011, 13, 29–31. [Google Scholar]
- Shukla, P.; Porwal, A.; Roy, S.; Chaturvedi, S.; Tripathi, S.; Arya, N. Preliminary study on antiulcer effect of agomelatine and its potentiation with pyridoxine. Int. J. Basic Clin. Pharmacol. 2017, 6, 2566–2570. [Google Scholar] [CrossRef]
- AlRashdi, A.S.; Salama, S.M.; Alkiyumi, S.S.; Abdulla, M.A.; Hadi, A.H.A.; Abdelwahab, S.I.; Taha, M.M.; Hussiani, J.; Asykin, N. Mechanisms of gastroprotective effects of ethanolic leaf extract of Jasminum sambac against HCl/ethanol-induced gastric mucosal injury in rats. Evid. Based Complement. Altern. Med. 2012, 2012, 786426. [Google Scholar] [CrossRef]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [CrossRef]
- Xia, Y.G.; Sun, H.M.; Wang, T.L.; Liang, J.; Yang, B.Y.; Kuang, H.X. A modified GC-MS analytical procedure for separation and detection of multiple classes of carbohydrates. Molecules 2018, 23, 1284. [Google Scholar] [CrossRef]

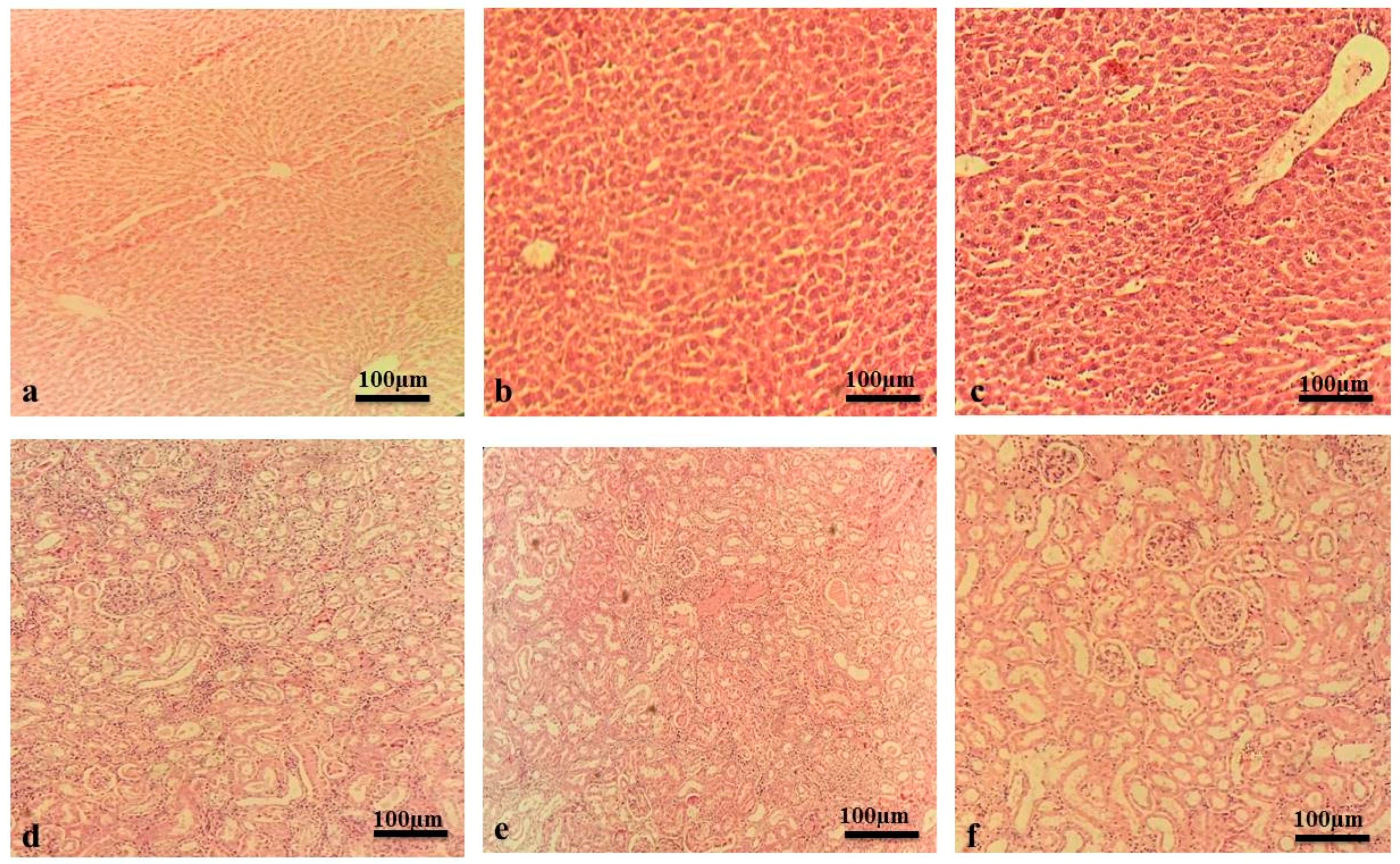


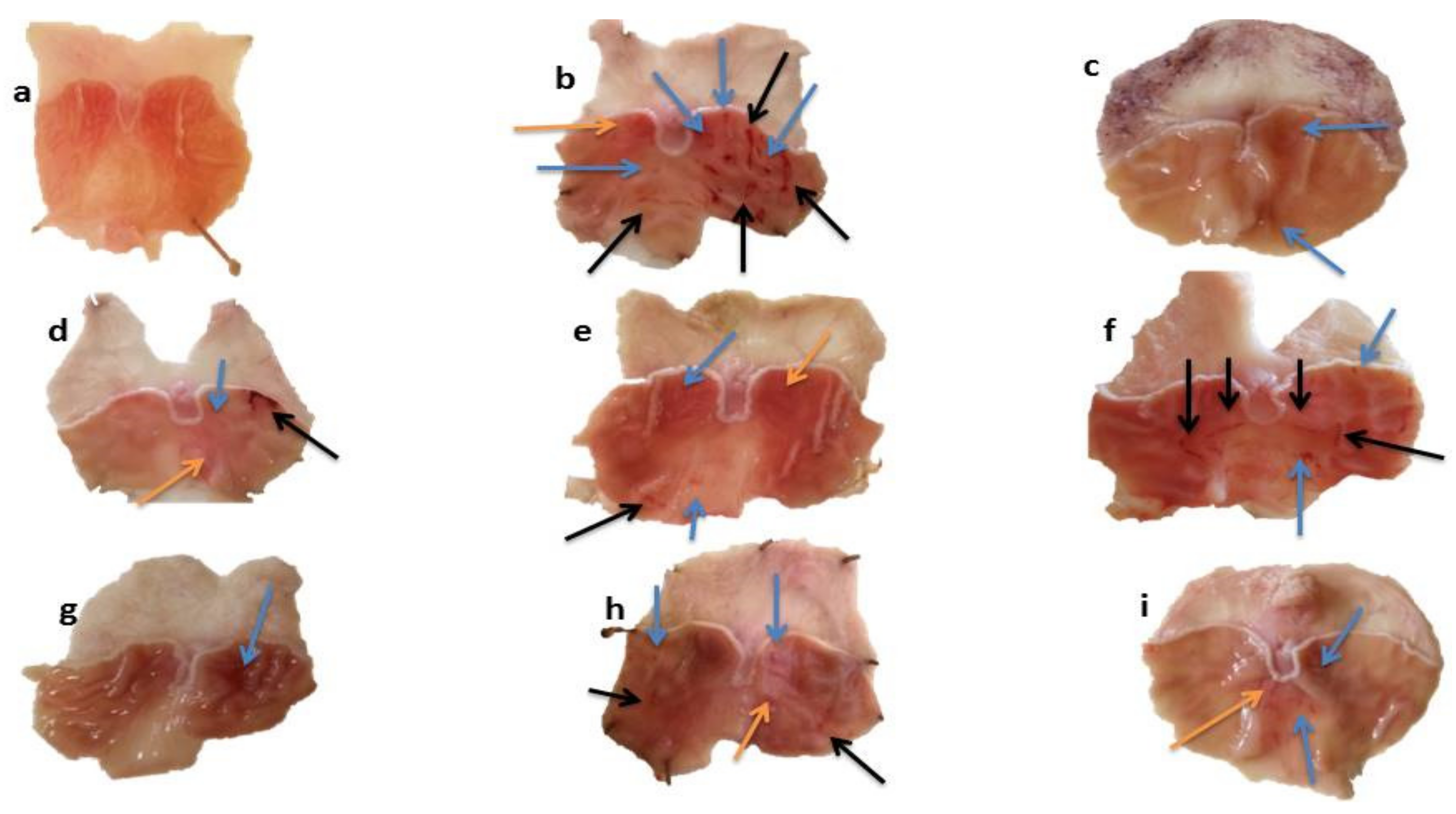
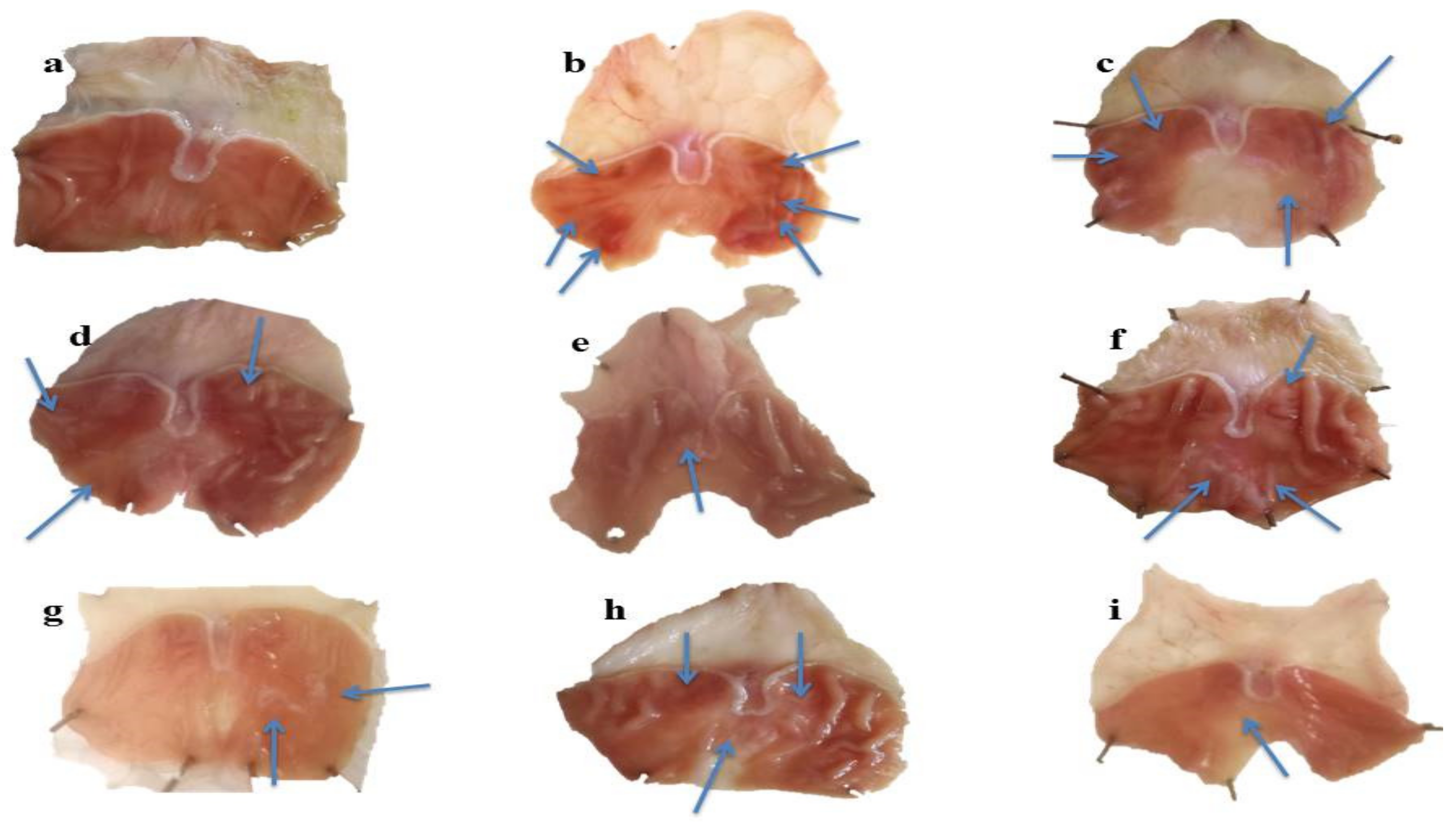
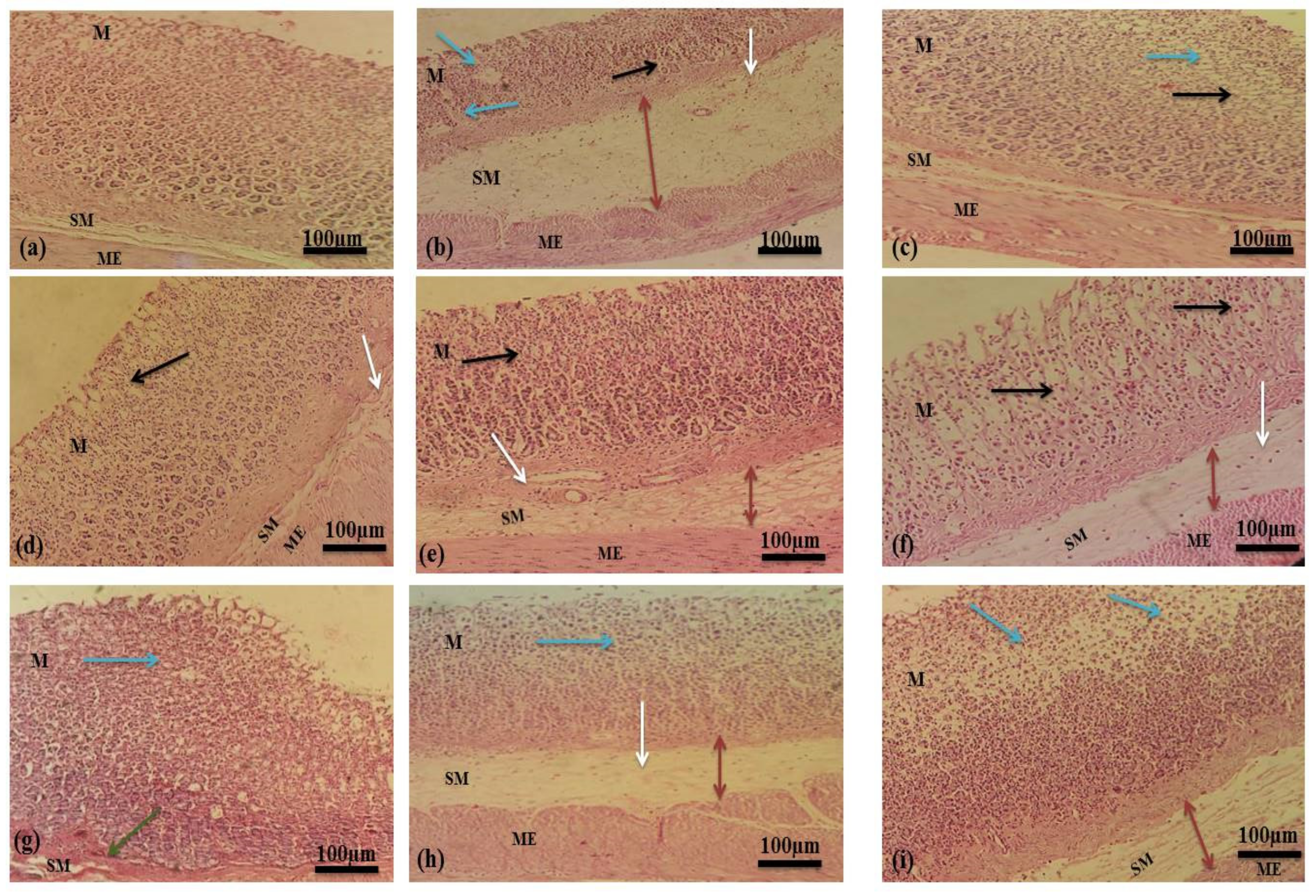

| Groups | Urea (mg/dL) | Creatinine (mg/dL) | Bilirubin (mg/dL) | SGPT (u/L) | SGOT (u/L) | ALP (u/L) |
|---|---|---|---|---|---|---|
| Normal | 42.83 ± 1.05 | 0.9 ± 0.05 | 0.89 ± 0.039 | 107.5 ± 9.98 | 118 ± 8.88 | 139 ± 6.96 |
| MLM | 38.83 ± 1.8 ns | 0.82 ± 0.06 ns | 0.76 ± 0.05 ns | 105.83 ± 11 ns | 114.67 ± 7.8 ns | 128.5 ± 5.5 ns |
| MFM | 40.5 ± 1.57 ns | 0.73 ± 0.05 ns | 0.79 ± 0.04 ns | 86.67 0± 6.66 ns | 114.67 ± 7.8 ns | 133 ± 3.21 ns |
| Group Name | Paw Thickness (mm) | ||||
|---|---|---|---|---|---|
| 0 h | 1 h | 2 h | 3 h | 4 h | |
| Control | 2.09 ± 0.19 | 3.0 ± 0.16 | 3.40 ± 0.16 | 3.52 ± 0.14 | 3.55 ± 0.15 |
| Standard | 2.10 ± 0.26 ns | 2.30 ± 0.25 *** | 2.38 ± 0.17 *** | 2.28 ± 0.20 *** | 2.15 ± 0.25 *** |
| MLM | 2.07 ± 0.25 ns | 2.68 ± 0.18 * | 2.80 ± 0.09 *** | 2.71 ± 0.1 *** | 2.58 ± 0.17 *** |
| MFM | 2.05 ± 0.16 ns | 2.73 ± 0.18 ns | 2.99 ± 0.20 ** | 2.97 ± 0.18 *** | 2.99 ± 0.16 *** |
| Group Name | Ulcer No. | Ulcer Score | Incidence of Ulcer (%) | Ulcer Index | Inhibition of Ulcer (%) |
|---|---|---|---|---|---|
| Normal (10 mL/kg p. o) | - | - | - | - | - |
| Ethanol (10 mL/kg p. o) | 7.83 ± 0.60 | 5.75 ± 0.57 | 100 | 11.36 | - |
| Ranitidine (50 mg/kg p. o) | 1 ± 0.52 *** | 1.0 ± 0.47 *** | 50 | 5.21 | 54.21 |
| Omeprazole (20 mg/kg p. o) | 0.67 ± 0.42 *** | 0.58 ± 0.37 *** | 33.33 | 3.46 | 69.56 |
| Sucralfate (100 mg/kg p. o) | 1.16 ± 0.54 *** | 1.5 ± 0.67 *** | 50 | 5.27 | 53.63 |
| MLM (250 mg/kg p. o) | 2.0 ± 0.73 *** | 2.08 ± 0.69 *** | 83.33 | 8.74 | 23.04 |
| MLM (500 mg/kg p. o) | 1.16 ± 0.60 *** | 1.75 ± 0.70 *** | 66.66 | 6.96 | 38.74 |
| MFM (250 mg/kg p. o) | 2.17 ± 0.48 *** | 2.5 ± 0.56 ** | 100 | 10.47 | 7.85 |
| MFM (500 mg/kg p. o) | 1.5 ± 0.5 *** | 2.17 ± 0.70 *** | 66.66 | 7.03 | 38.08 |
| Group Name | Ulcer No. | Ulcer Score | Incidence of Ulcer (%) | Ulcer Index | Inhibition of Ulcer (%) |
|---|---|---|---|---|---|
| Normal (10 mL/kg p. o) | - | - | - | - | - |
| Ethanol (10 mL/kg p. o) | 3.67 ± 0.71 | 4.33 ± 0.79 | 100 | 10.8 | - |
| Ranitidine (50 mg/kg p. o) | 0.67 ± 0.49 *** | 1 ± 0.74 *** | 33.33 | 3.5 | 67.59 |
| Omeprazole (20 mg/kg p. o) | 0.83 ± 0.40 *** | 1.42 ± 0.70 *** | 50 | 5.22 | 51.66 |
| Sucralfate (100 mg/kg p. o) | 0.83 ± 0.54 *** | 0.92 ± 0.58 *** | 33.33 | 3.51 | 67.5 |
| MLM (250 mg/kg p. o) | 1.83 ± 0.79 *** | 2.33 ± 1.08 *** | 66.67 | 7.08 | 34.44 |
| MLM (500 mg/kg p. o) | 0.83 ± 0.40 *** | 1.58 ± 0.78 *** | 50 | 5.67 | 47.44 |
| MFM (250 mg/kg p. o) | 1.00 ± 3.7 *** | 1.83 ± 0.69 ** | 66.67 | 6.95 | 35.65 |
| MFM (500 mg/kg p. o) | 0.83 ± 0.54 *** | 1.08 ± 0.76 *** | 33.33 | 3.94 | 63.52 |
| Acute Gastric Ulcer | Chronic Gastric Ulcer | |||
|---|---|---|---|---|
| Group Name | Mucous Content | Total Protein | Mucous Content | Total Protein |
| Normal (10 mL/kg p.o) | 482.5 ± 15.59 | 52.16 ± 3.66 | 504.17 ± 14.52 | 74.64 ± 3.71 |
| Ethanol (10 mL/kg p. o) | 411.17 ± 9.13 | 19.55 ± 3.44 | 295 ± 37.13 | 38.68 ± 3.26 |
| Ranitidine (50 mg/kg p. o) | 452.5 ± 13.14 ns | 44.27 ± 3.66 ** | 445 ± 21.29 ns | 72.72± 4.62 *** |
| Omeprazole (20 mg/kg p. o) | 501.67 ± 13.52 *** | 38.58 ± 6.37 ns | 637.67 ± 32.44 *** | 68.41 ± 3.55 *** |
| Sucralfate (100 mg/kg p. o) | 432.5 ± 12.63 ns | 28.52 ± 5.93 ns | 947.17 ± 65.21 *** | 70.46 ± 4.09 *** |
| MLM (250 mg/kg p. o) | 440.5 ± 12.63 ns | 23.54 ± 4.46 ns | 440.5 ± 12.63 ns | 68.58 ± 4.37 *** |
| MLM (500 mg/kg p. o) | 520 ± 17.89 *** | 47.47 ± 6.50 ** | 960.33 ± 58.24 *** | 69.35 ± 3.40 *** |
| MFM (250 mg/kg p. o) | 430 ± 12.11 ns | 26.73 ± 5.03 ns | 840.17 ± 26.24 *** | 48.64 ± 3.31 ns |
| MFM (500 mg/kg p. o) | 488.16 ± 17.09 ** | 30.28 ± 3.95 ns | 1239.33 ± 54.29 *** | 70.05 ± 4.02 *** |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Altyar, A.E.; Munir, A.; Ishtiaq, S.; Rizwan, M.; Abbas, K.; Kensara, O.; Elhady, S.S.; Rizg, W.Y.; Youssef, F.S.; Ashour, M.L. Malva parviflora Leaves and Fruits Mucilage as Natural Sources of Anti-Inflammatory, Antitussive and Gastro-Protective Agents: A Comparative Study Using Rat Models and Gas Chromatography. Pharmaceuticals 2022, 15, 427. https://doi.org/10.3390/ph15040427
Altyar AE, Munir A, Ishtiaq S, Rizwan M, Abbas K, Kensara O, Elhady SS, Rizg WY, Youssef FS, Ashour ML. Malva parviflora Leaves and Fruits Mucilage as Natural Sources of Anti-Inflammatory, Antitussive and Gastro-Protective Agents: A Comparative Study Using Rat Models and Gas Chromatography. Pharmaceuticals. 2022; 15(4):427. https://doi.org/10.3390/ph15040427
Chicago/Turabian StyleAltyar, Ahmed E., Ans Munir, Saiqa Ishtiaq, Muhammad Rizwan, Khizar Abbas, Osama Kensara, Sameh S. Elhady, Waleed Y. Rizg, Fadia S. Youssef, and Mohamed L. Ashour. 2022. "Malva parviflora Leaves and Fruits Mucilage as Natural Sources of Anti-Inflammatory, Antitussive and Gastro-Protective Agents: A Comparative Study Using Rat Models and Gas Chromatography" Pharmaceuticals 15, no. 4: 427. https://doi.org/10.3390/ph15040427
APA StyleAltyar, A. E., Munir, A., Ishtiaq, S., Rizwan, M., Abbas, K., Kensara, O., Elhady, S. S., Rizg, W. Y., Youssef, F. S., & Ashour, M. L. (2022). Malva parviflora Leaves and Fruits Mucilage as Natural Sources of Anti-Inflammatory, Antitussive and Gastro-Protective Agents: A Comparative Study Using Rat Models and Gas Chromatography. Pharmaceuticals, 15(4), 427. https://doi.org/10.3390/ph15040427










