Immunomodulatory Effects of (R)-Sulforaphane on LPS-Activated Murine Immune Cells: Molecular Signaling Pathways and Epigenetic Changes in Histone Markers
Abstract
:1. Introduction
2. Results
2.1. Effect of (R)-SFN on the Viability of Murine Peritoneal Macrophages
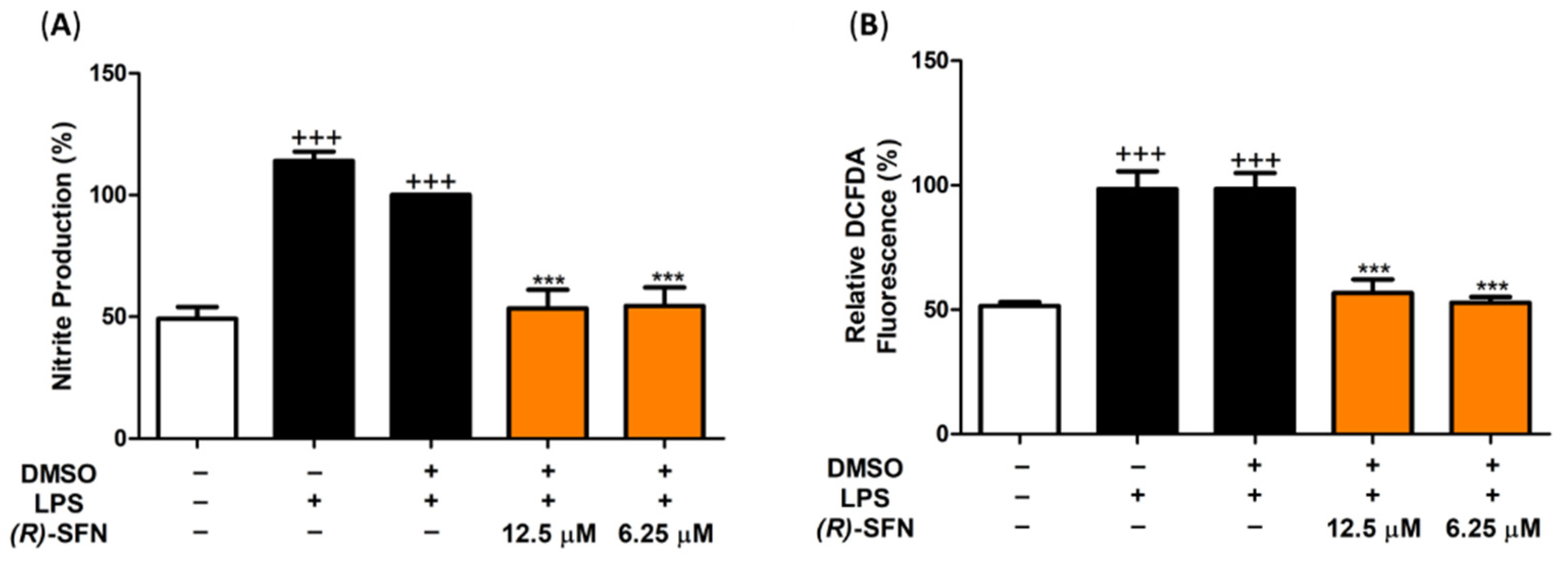
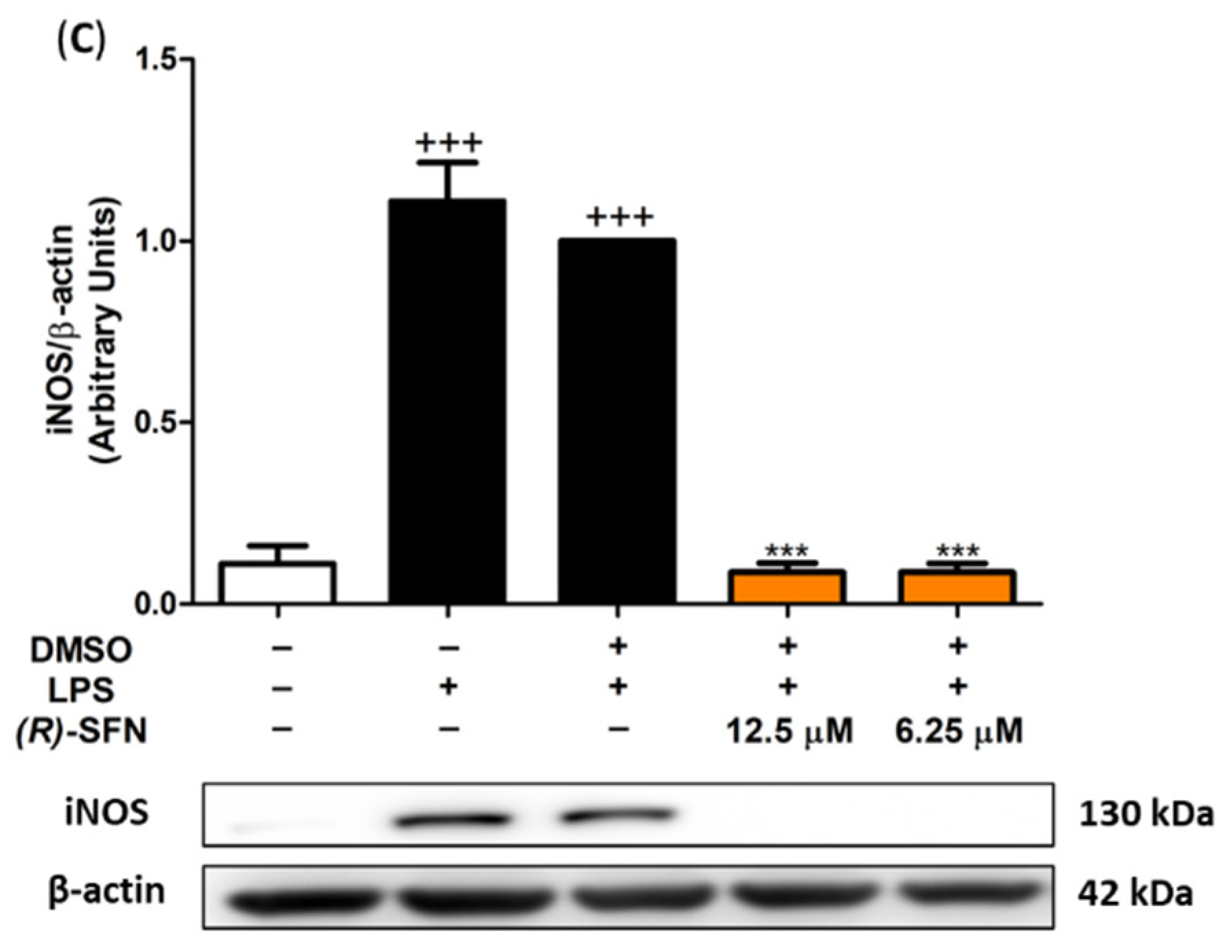
2.2. (R)-SFN Down-Regulated iNOS, Nitrite Production and Intracellular ROS in LPS-Stimulated Murine Peritoneal Macrophages
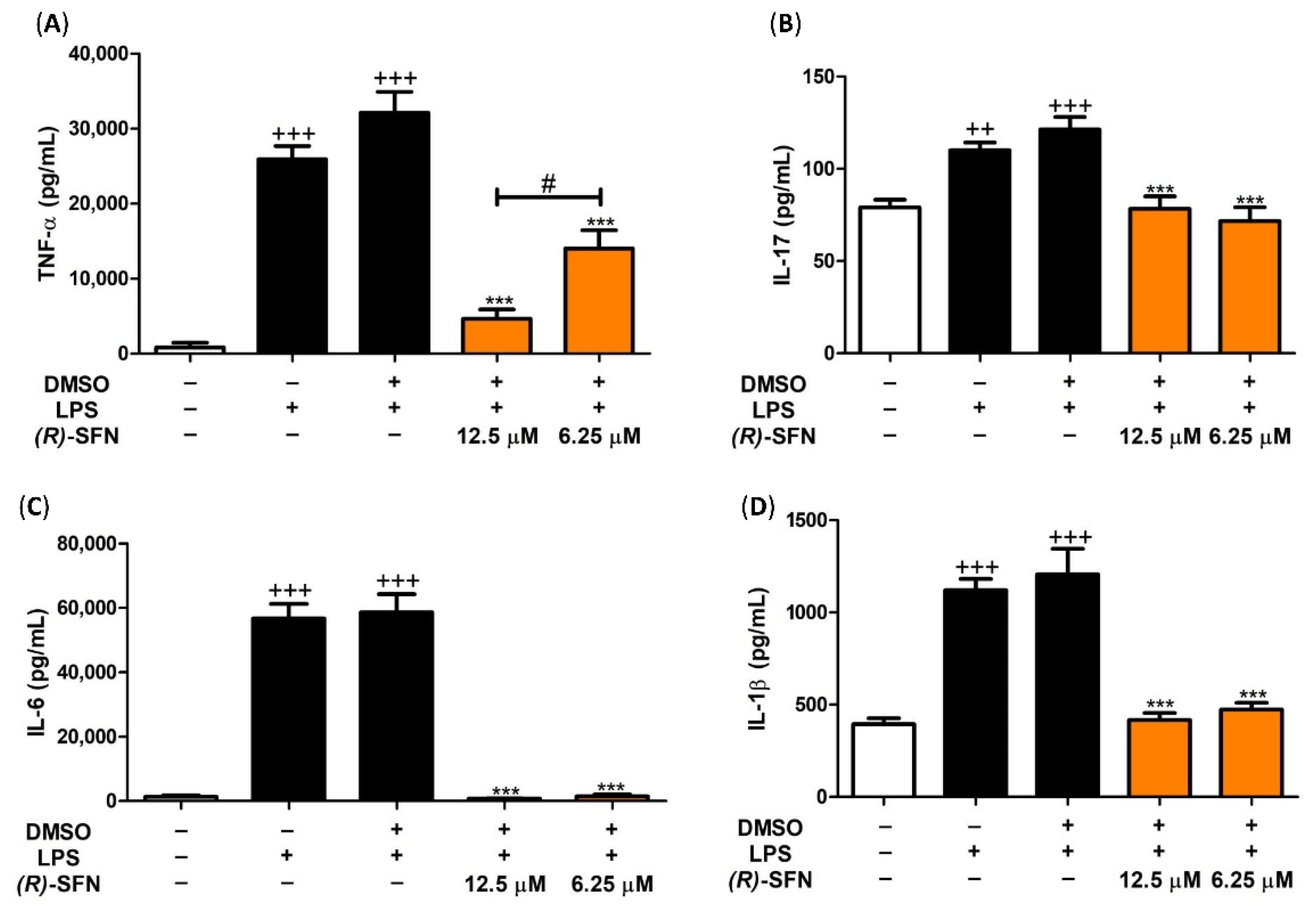
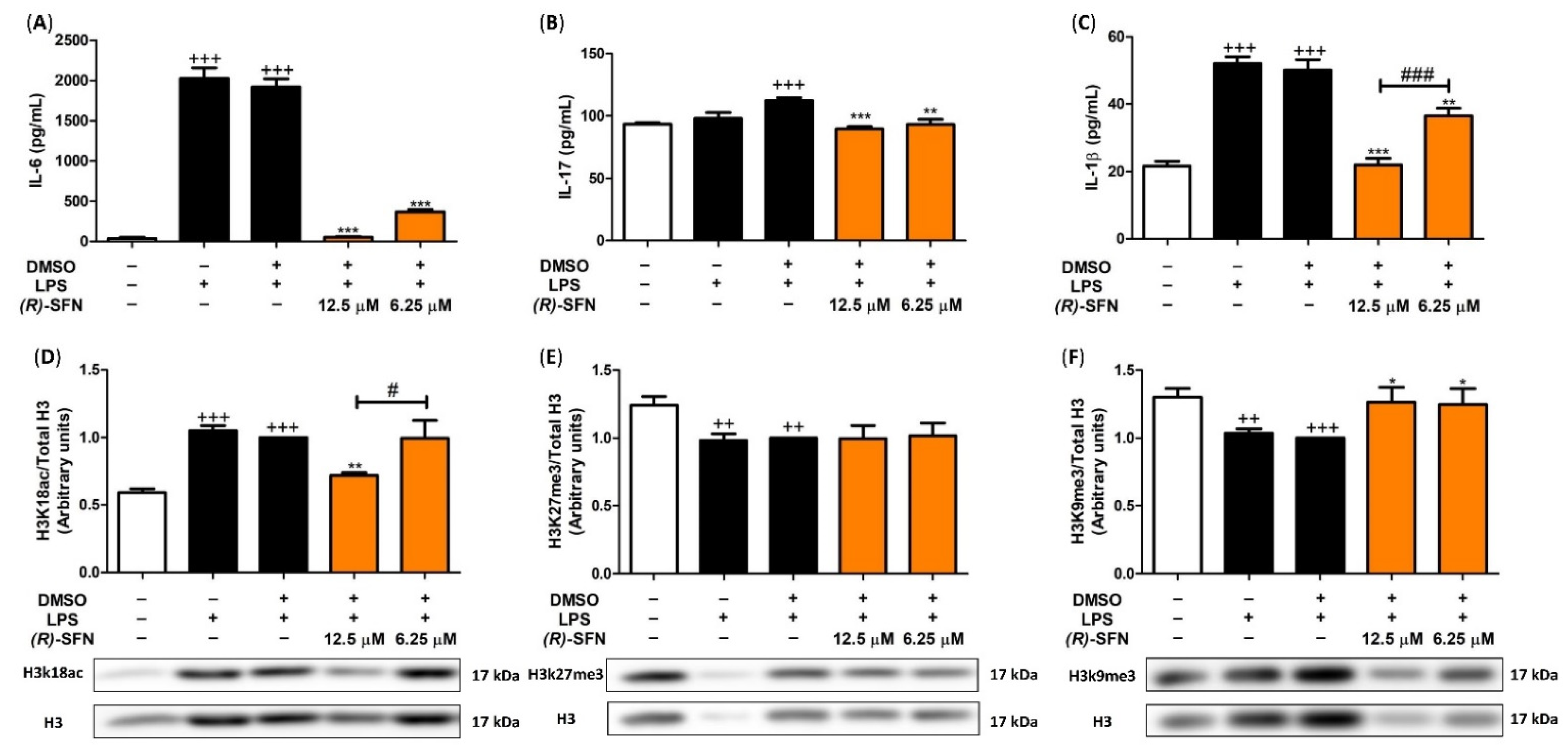
2.3. Effects of (R)-SFN on TNF-α, IL-1β, IL-6 and IL-17 Production
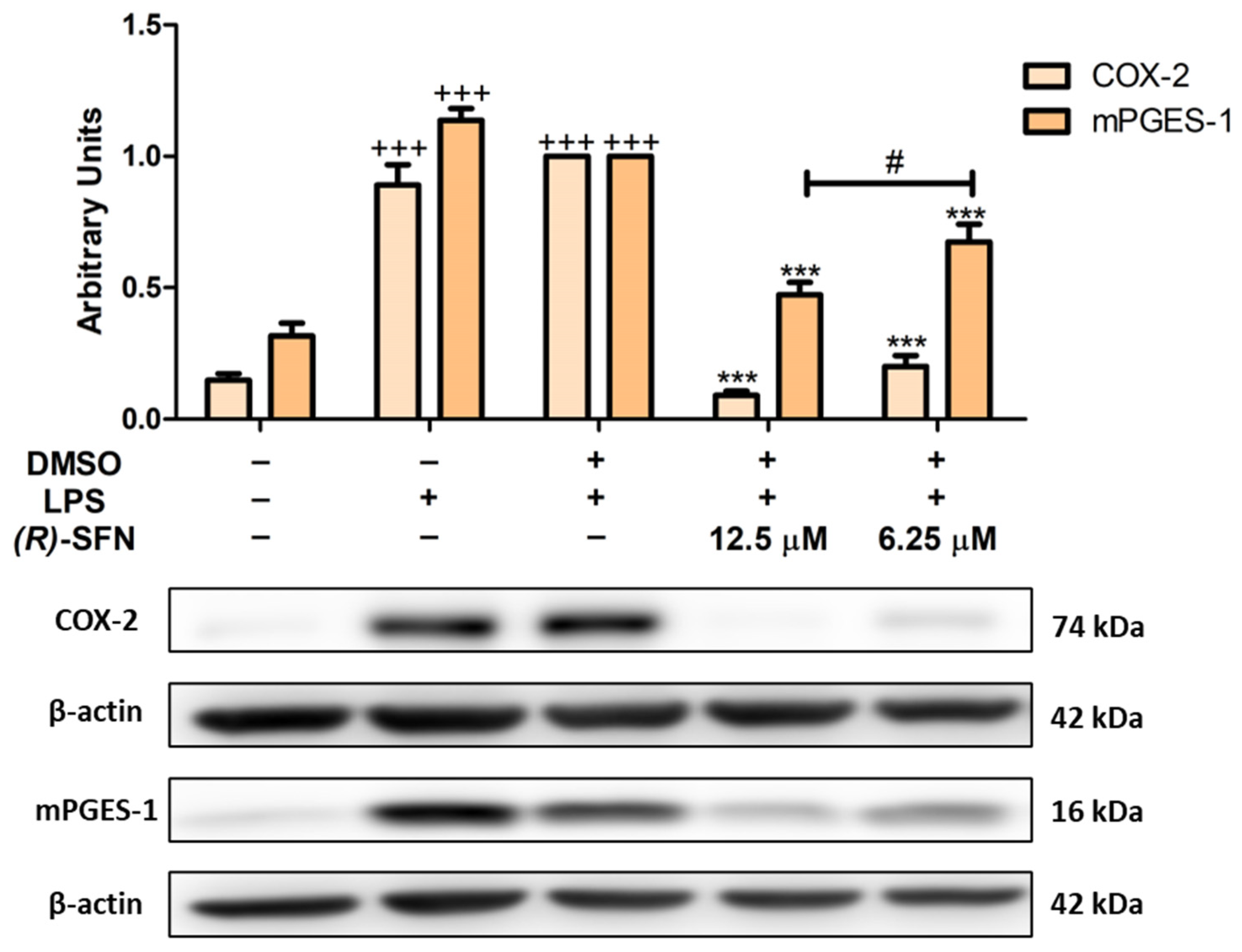
2.4. (R)-SFN Decreased LPS-Induced COX-2 and mPGES-1 Overexpression
2.5. (R)-SFN Up-Regulated Nrf-2/HO-1 Axis Protein Expression in LPS-Stimulated Murine Peritoneal Macrophages
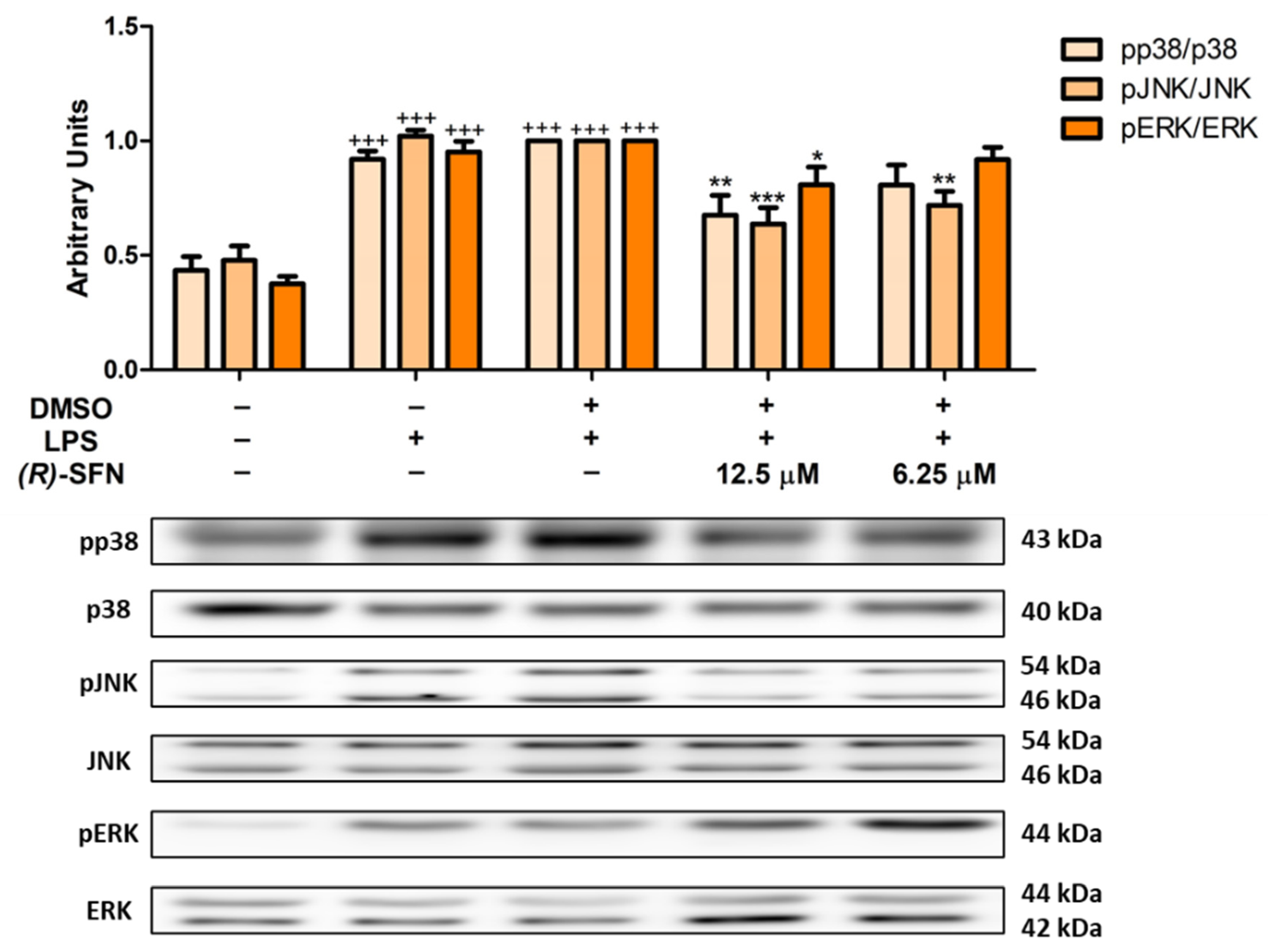
2.6. Effects of (R)-SFN on MAPKs Activation in LPS-Activated Peritoneal Macrophages
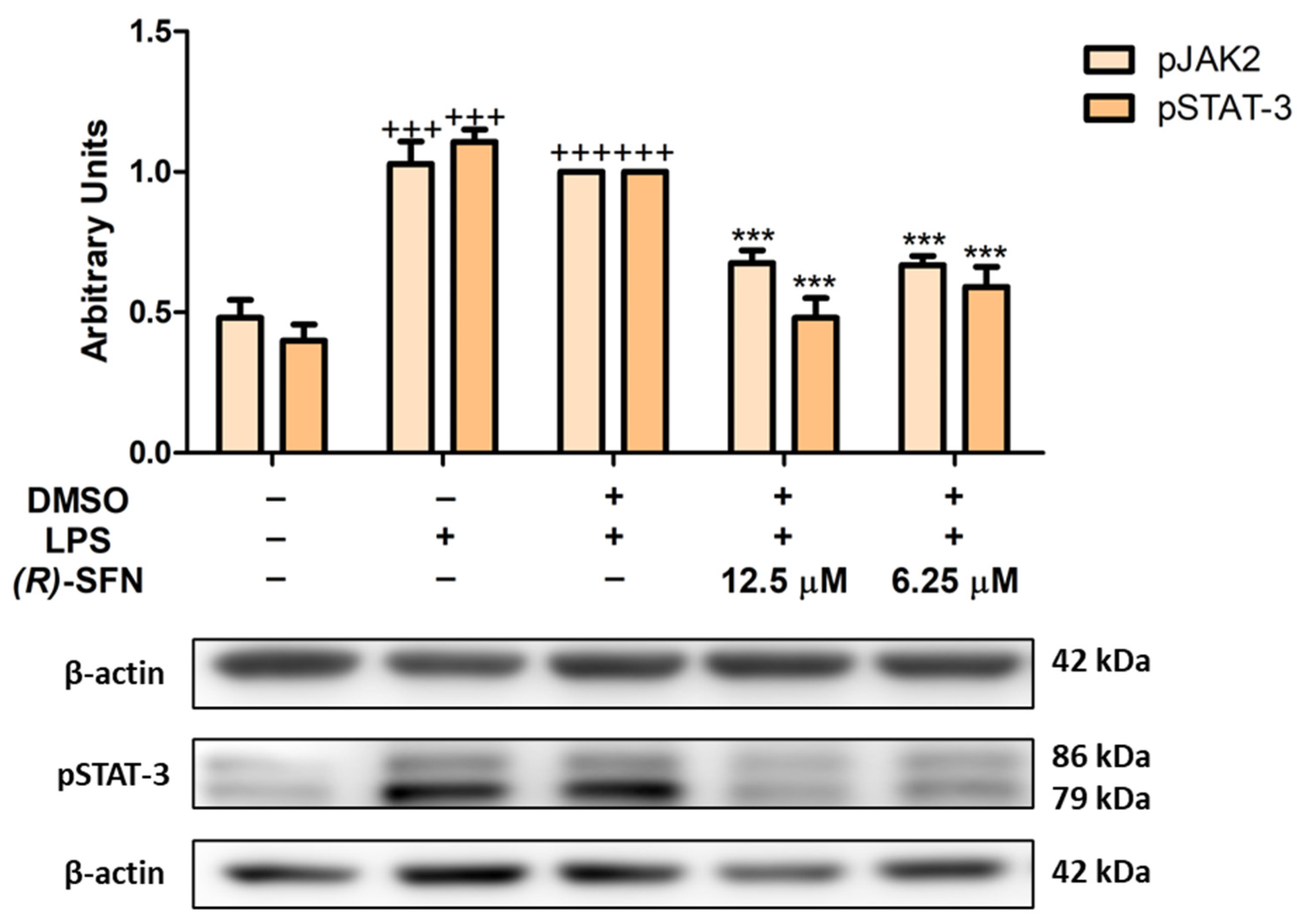
2.7. Effects of (R)-SFN on the JAK2/STAT-3 Signaling Pathway in LPS-Activated Peritoneal Macrophages
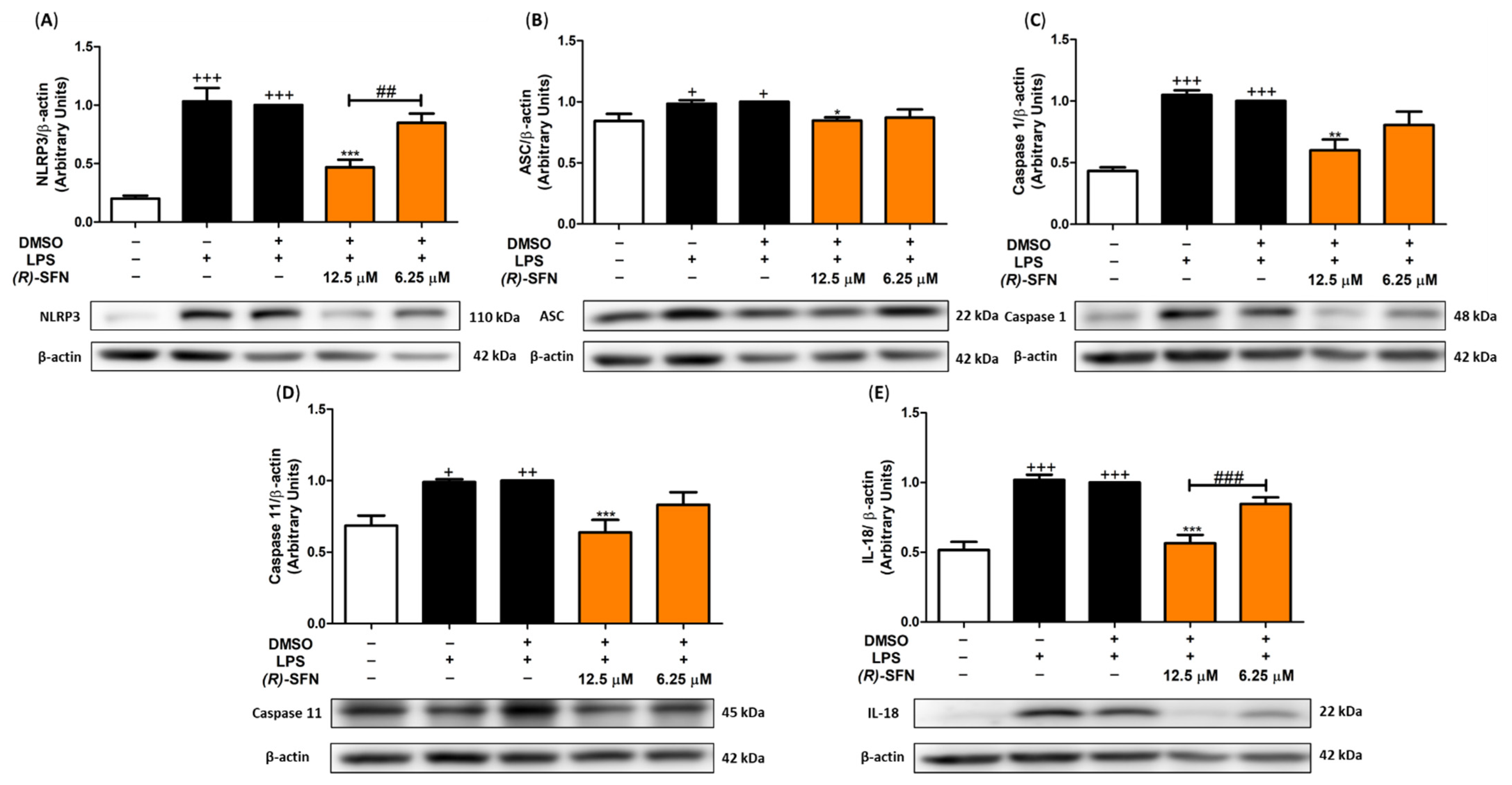
2.8. (R)-SFN Inhibited the Canonical and Non-Canonical Inflammasome in LPS-Activated Murine Peritoneal Macrophages
2.9. (R)-SFN Produced Epigenetic Histone Modifications in Spleen Cells
3. Discussion
4. Materials and Methods
4.1. Chemicals
4.1.1. Reagents and Instruments
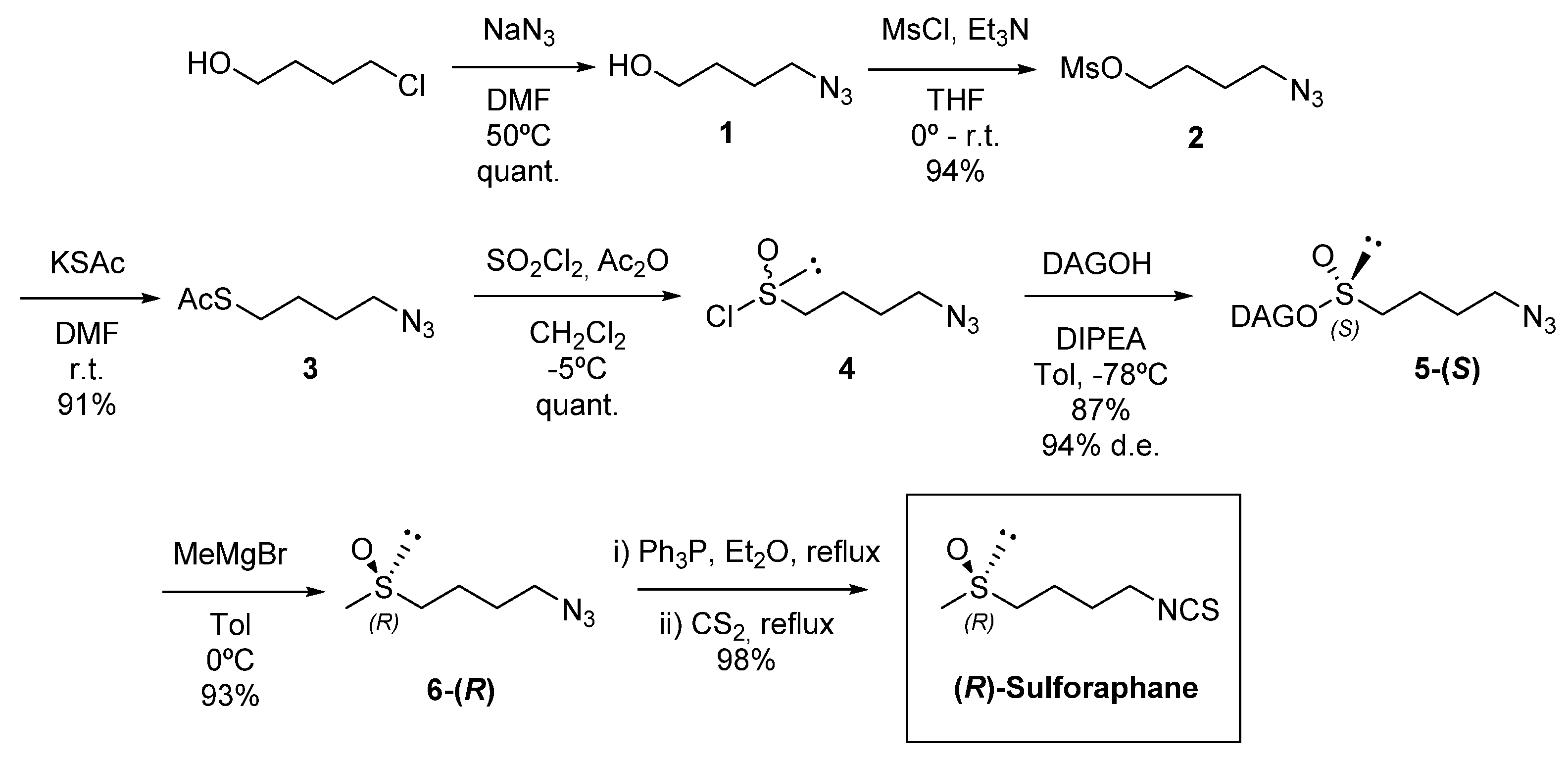
4.1.2. Synthesis of 4-Azidobutan-1-ol (1)
4.1.3. Synthesis of 4-Azidobutyl Methanesulfonate (2)
4.1.4. Synthesis of 4-Azidobutyl-1-thioacetate (3)
4.1.5. Synthesis of 4-Azidobutane-1-sulfinyl chloride (4)
4.1.6. Synthesis of (S)-(1,2:5,6-Di-O-Isopropylidene-α-d-glucofuranosyl) 4-Azidobutanesulfinate (5-(S))
4.1.7. Synthesis of (R)-(-)-1-Azido-4-(methylsulfinyl)-butane (6-(R))
4.1.8. Synthesis of (R)-(-)-1-Isothiocyanato-4-(methylsulfinyl)-butane (R-Sulforaphane)
4.2. Animals
4.3. Isolation and In Vitro Culture of Murine Peritoneal Macrophages and Spleen Cells
4.4. Cell Viability Assay
4.5. Measurement of Nitrite Production
4.6. Detection of DCFDA Cell-Reactive Oxygen Species
4.7. Determination of Pro-Inflammatory Cytokines
4.8. Histone Extraction
4.9. Immunoblotting Detection
4.10. Statistical Evaluation
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mangla, B.; Javed, S.; Sultan, M.H.; Kumar, P.; Kohli, K.; Najmi, A.; Alhazmi, H.A.; Al Bratty, M.; Ahsan, W. Sulforaphane: A review of its therapeutic potentials, advances in its nanodelivery, recent patents, and clinical trials. Phytother. Res. 2021, 35, 5440–5458. [Google Scholar] [CrossRef] [PubMed]
- Rose, P. 7-Methylsulfinylheptyl and 8-methylsulfinyloctyl isothiocyanates from watercress are potent inducers of phase II enzymes. Carcinogenesis 2000, 21, 1983–1988. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Y.; Talalay, P.; Cho, C.G.; Posner, G.H. A major inducer of anticarcinogenic protective enzymes from broccoli: Isolation and elucidation of structure. Proc. Natl. Acad. Sci. USA 1992, 89, 2399–2403. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Juge, N.; Mithen, R.F.; Traka, M. Molecular basis for chemoprevention by sulforaphane: A comprehensive review. Cell. Mol. Life Sci. 2007, 64, 1105–1127. [Google Scholar] [CrossRef] [PubMed]
- Vergara, F.; Wenzler, M.; Hansen, B.G.; Kliebenstein, D.J.; Halkier, B.A.; Gershenzon, J.; Schneider, B. Determination of the absolute configuration of the glucosinolate methyl sulfoxide group reveals a stereospecific biosynthesis of the side chain. Phytochemistry 2008, 69, 2737–2742. [Google Scholar] [CrossRef]
- Houghton, C.A.; Fassett, R.G.; Coombes, J.S. Sulforaphane and Other Nutrigenomic Nrf2 Activators: Can the Clinician’s Expectation Be Matched by the Reality? Oxidative Med. Cell. Longev. 2016, 2016, 7857186. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, J.H.; Moon, M.H.; Jeong, J.K.; Park, Y.G.; Lee, Y.J.; Seol, J.W.; Park, S.Y. Sulforaphane induced adipolysis via hormone sensitive lipase activation, regulated by AMPK signaling pathway. Biochem. Biophys. Res. Commun. 2012, 426, 492–497. [Google Scholar] [CrossRef] [PubMed]
- Johansson, N.L.; Pavia, C.S.; Jen, W.C. Growth inhibition of a spectrum of bacterial and fungal pathogens by sulforaphane, an isothiocyanate product found in broccoli and other cruciferous vegetables. Planta Med. 2008, 74, 747–750. [Google Scholar] [CrossRef]
- Amjad, A.I.; Parikh, R.A.; Appleman, L.J.; Hahm, E.-R.; Singh, K.; Singh, S.V. Broccoli-Derived Sulforaphane and Chemoprevention of Prostate Cancer: From Bench to Bedside. Curr. Pharmacol. Rep. 2015, 1, 382–390. [Google Scholar] [CrossRef]
- Giacoppo, S.; Galuppo, M.; Montaut, S.; Iori, R.; Rollin, P.; Bramanti, P.; Mazzon, E. An overview on neuroprotective effects of isothiocyanates for the treatment of neurodegenerative diseases. Fitoterapia 2015, 106, 12–21. [Google Scholar] [CrossRef] [PubMed]
- Bai, Y.; Wang, X.; Zhao, S.; Ma, C.; Cui, J.; Zheng, Y. Sulforaphane Protects against Cardiovascular Disease via Nrf2 Activation. Oxidative Med. Cell. Longev. 2015, 2015, 407580. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lauterbach, M.A.; Hanke, J.E.; Serefidou, M.; Mangan, M.S.J.; Kolbe, C.C.; Hess, T.; Rothe, M.; Kaiser, R.; Hoss, F.; Gehlen, J.; et al. Toll-like Receptor Signaling Rewires Macrophage Metabolism and Promotes Histone Acetylation via ATP-Citrate Lyase. Immunity 2019, 51, 997–1011.e7. [Google Scholar] [CrossRef]
- Yi, Y.S. Caspase-11 non-canonical inflammasome: A critical sensor of intracellular lipopolysaccharide in macrophage-mediated inflammatory responses. Immunology 2017, 152, 207–217. [Google Scholar] [CrossRef] [Green Version]
- Jeong, Y.H.; Oh, Y.C.; Cho, W.K.; Yim, N.H.; Ma, J.Y. Hoveniae semen seu fructus ethanol extract exhibits anti-inflammatory activity via MAPK, AP-1, and STAT signaling pathways in LPS-stimulated RAW 264.7 and mouse peritoneal macrophages. Mediat. Inflamm. 2019, 2019, 9184769. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, R.; Hong, P.; Zheng, X. β-carotene attenuates lipopolysaccharide-induced inflammation via inhibition of the NF-κB, JAK2/STAT3 and JNK/p38 MAPK signaling pathways in macrophages. Anim. Sci. J. 2019, 90, 140–148. [Google Scholar] [CrossRef]
- Huang, Y.-P.; Chen, D.-R.; Lin, W.-J.; Lin, Y.-H.; Chen, J.-Y.; Kuo, Y.-H.; Chung, J.-G.; Hsia, T.-C.; Hsieh, W.-T. Ergosta-7,9(11),22-trien-3β-ol Attenuates Inflammatory Responses via Inhibiting MAPK/AP-1 Induced IL-6/JAK/STAT Pathways and Activating Nrf2/HO-1 Signaling in LPS-Stimulated Macrophage-like Cells. Antioxidants 2021, 10, 1430. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Tang, L. Discovery and development of sulforaphane as a cancer chemopreventive phytochemical. Acta Pharmacol. Sin. 2007, 28, 1343–1354. [Google Scholar] [CrossRef] [PubMed]
- Abdull Razis, A.F.; Iori, R.; Ioannides, C. The natural chemopreventive phytochemical R-sulforaphane is a far more potent inducer of the carcinogen-detoxifying enzyme systems in rat liver and lung than the S-isomer. Int. J. Cancer 2011, 128, 2775–2782. [Google Scholar] [CrossRef] [PubMed]
- Abdull Razis, A.F.; Bagatta, M.; De Nicola, G.R.; Iori, R.; Ioannides, C. Induction of epoxide hydrolase and glucuronosyl transferase by isothiocyanates and intact glucosinolates in precision-cut rat liver slices: Importance of side-chain substituent and chirality. Arch. Toxicol. 2011, 85, 919–927. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.; Jahraus, B.; Balta, E.; Ziegler, J.D.; Hübner, K.; Blank, N.; Niesler, B.; Wabnitz, G.H.; Samstag, Y. Sulforaphane inhibits inflammatory responses of primary human T-cells by increasing ROS and depleting glutathione. Front. Immunol. 2018, 9, 1–17. [Google Scholar] [CrossRef]
- Rakariyatham, K.; Wu, X.; Tang, Z.; Han, Y.; Wang, Q.; Xiao, H. Synergism between luteolin and sulforaphane in anti-inflammation. Food Funct. 2018, 9, 5115–5123. [Google Scholar] [CrossRef]
- Ruhee, R.T.; Ma, S.; Suzuki, K. Sulforaphane protects cells against lipopolysaccharide-stimulated inflammation in murine macrophages. Antioxidants 2019, 8, 577. [Google Scholar] [CrossRef] [Green Version]
- Ranaweera, S.S.; Dissanayake, C.Y.; Natraj, P.; Lee, Y.J.; Han, C.H. Anti-inflammatory effect of sulforaphane on LPS-stimulated RAW 264.7 cells and ob/ob mice. J. Vet. Sci. 2020, 21, e91. [Google Scholar] [CrossRef] [PubMed]
- Qi, T.; Xu, F.; Yan, X.; Li, S.; Li, H. Sulforaphane exerts anti-inflammatory effects against lipopolysaccharide-induced acute lung injury in mice through the Nrf2/ARE pathway. Int. J. Mol. Med. 2016, 37, 182–188. [Google Scholar] [CrossRef] [Green Version]
- Mohanty, S.; Pal, A.; Konkimalla, V.B.; Sudam Chandra, S.I. Anti-inflammatory and anti-granuloma activity of sulforaphane, a naturally occurring isothiocyanate from broccoli (Brassica oleracea). Asian J. Pharm. Clin. Res. 2018, 11, 411–416. [Google Scholar] [CrossRef]
- Li, B.; Cui, W.; Liu, J.; Li, R.; Liu, Q.; Xie, X.H.; Ge, X.L.; Zhang, J.; Song, X.J.; Wang, Y.; et al. Sulforaphane ameliorates the development of experimental autoimmune encephalomyelitis by antagonizing oxidative stress and Th17-related inflammation in mice. Exp. Neurol. 2013, 250, 239–249. [Google Scholar] [CrossRef]
- Zeng, X.; Liu, X.; Bao, H. Sulforaphane suppresses lipopolysaccharide- and Pam3CysSerLys4-mediated inflammation in chronic obstructive pulmonary disease via toll-like receptors. FEBS Openbio 2021, 11, 1313–1321. [Google Scholar] [CrossRef]
- Fernandez, I.; Khiar, N.; Llera, J.M.; Alcudia, F. Asymmetric synthesis of alkane- and arenesulfinates of diacetone-D-glucose (DAG): An improved and general route to both enantiomerically pure sulfoxides. J. Org. Chem. 1992, 57, 6789–6796. [Google Scholar] [CrossRef]
- Fernández, I.; Khiar, N. Recent developments in the synthesis and utilization of chiral sulfoxides. Chem. Rev. 2003, 103, 3651–3705. [Google Scholar] [CrossRef] [PubMed]
- Khiar, N.; Alcudia, F.; Espartero, J.L.; Rodríguez, L.; Fernández, I. Dynamic kinetic resolution of bis-sulfinyl chlorides: A general enantiodivergent synthesis of C2-symmetric bis-sulfinate esters and bis-sulfoxides. J. Am. Chem. Soc. 2000, 122, 7598–7599. [Google Scholar] [CrossRef]
- Balcells, D.; Ujaque, G.; Fernández, I.; Khiar, N.; Maseras, F. How does the achiral base decide the stereochemical outcome in the dynamic kinetic resolution of sulfinyl chlorides? A computational study. Adv. Synth. Catal. 2007, 349, 2103–2110. [Google Scholar] [CrossRef]
- Khiar el Wahabi, N.; Fernández Fernández, I.; Recio Jiménez, R. Sulforaphane-Derived Compounds, Production Method Thereof and the Medical, Food and Cosmetic Use of Same. WO2013132124 A1, 12 September 2013. Available online: http://digital.csic.es/handle/10261/92880 (accessed on 17 June 2022).
- Elhalem, E.; Recio, R.; Werner, S.; Lieder, F.; Calderón-Montaño, J.M.; López-Lázaro, M.; Fernández, I.; Khiar, N. Sulforaphane homologues: Enantiodivergent synthesis of both enantiomers, activation of the Nrf2 transcription factor and selective cytotoxic activity. Eur. J. Med. Chem. 2014, 87, 552–563. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Recio, R.; Elhalem, E.; Benito, J.M.; Fernández, I.; Khiar, N. NMR study on the stabilization and chiral discrimination of sulforaphane enantiomers and analogues by cyclodextrins. Carbohydr. Polym. 2018, 187, 118–125. [Google Scholar] [CrossRef] [PubMed]
- Khiar, N.; Werner, S.; Mallouk, S.; Lieder, F.; Alcudia, A.; Fernández, I. Enantiopure sulforaphane analogues with various substituents at the sulfinyl sulfur: Asymmetric synthesis and biological activities. J. Org. Chem. 2009, 74, 6002–6009. [Google Scholar] [CrossRef] [PubMed]
- Recio, R.; Vengut-Climent, E.; Borrego, L.G.; Khiar, N.; Fernández, I. Biologically Active Isothiocyanates: Protecting Plants and Healing Humans. In Studies in Natural Products Chemistry; Atta-ur, R., Ed.; Elsevier: Amsterdam, The Netherlands, 2017; Volume 53, pp. 167–242. ISBN 9780444639301. [Google Scholar]
- Caradonna, F.; Consiglio, O.; Luparello, C.; Gentile, C. Science and healthy meals in the world: Nutritional epigenomics and nutrigenetics of the mediterranean diet. Nutrients 2020, 12, 1748. [Google Scholar] [CrossRef] [PubMed]
- Yuan, F.; Chen, X.; Liu, J.; Feng, W.; Cai, L.; Wu, X.; Chen, S.Y. Sulforaphane restores acetyl-histone H3 binding to Bcl-2 promoter and prevents apoptosis in ethanol-exposed neural crest cells and mouse embryos. Exp. Neurol. 2018, 300, 60–66. [Google Scholar] [CrossRef] [PubMed]
- Su, X.; Jiang, X.; Meng, L.; Dong, X.; Shen, Y.; Xin, Y. Anticancer activity of sulforaphane: The epigenetic mechanisms and the Nrf2 signaling pathway. Oxidative Med. Cell. Longev. 2018, 2018, 5438179. [Google Scholar] [CrossRef]
- Senanayake, G.V.K.; Banigesh, A.; Wu, L.; Lee, P.; Juurlink, B.H.J. The dietary phase 2 protein inducer sulforaphane can normalize the kidney epigenome and improve blood pressure in hypertensive rats. Am. J. Hypertens. 2012, 25, 229–235. [Google Scholar] [CrossRef] [PubMed]
- Dacosta, C.; Bao, Y. The role of microRNAs in the chemopreventive activity of sulforaphane from cruciferous vegetables. Nutrients 2017, 9, 902. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kou, X.; Qi, S.; Dai, W.; Luo, L.; Yin, Z. Arctigenin inhibits lipopolysaccharide-induced iNOS expression in RAW264.7 cells through suppressing JAK-STAT signal pathway. Int. Immunopharmacol. 2011, 11, 1095–1102. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Wang, H.; Kouadir, M.; Song, H.; Shi, F. Recent advances in the mechanisms of NLRP3 inflammasome activation and its inhibitors. Cell Death Dis. 2019, 10, 128. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mazarakis, N.; Anderson, J.; Toh, Z.Q.; Higgins, R.A.; Do, L.A.H.; Luwor, R.B.; Snibson, K.J.; Karagiannis, T.C.; Licciardi, P.V. Examination of novel immunomodulatory effects of l-sulforaphane. Nutrients 2021, 13, 602. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Maitra, U.; Singh, N.; Gan, L. Molecular mechanism underlying LPS-induced generation of reactive oxygen species in macrophages. FASEB J. 2010, 24, 422.3. [Google Scholar] [CrossRef]
- Cuevas, B.; Arroba, A.I.; de los Reyes, C.; Gómez-Jaramillo, L.; González-Montelongo, M.C.; Zubía, E. Diterpenoids from the brown alga rugulopteryx okamurae and their anti-inflammatory activity. Mar. Drugs 2021, 19, 677. [Google Scholar] [CrossRef] [PubMed]
- Tang, H.; Roy, P.; Di, Q.; Ma, X.; Xiao, Y.; Wu, Z.; Quan, J.; Zhao, J.; Xiao, W.; Chen, W. Synthesis compound XCR-7a ameliorates LPS-induced inflammatory response by inhibiting the phosphorylation of c-Fos. Biomed. Pharmacother. 2022, 145, 112468. [Google Scholar] [CrossRef]
- Mitchell, J.A.; Larkin, S.; Williams, T.J. Cyclooxygenase-2: Regulation and relevance in inflammation. Biochem. Pharmacol. 1995, 50, 1535–1542. [Google Scholar] [CrossRef]
- Montoya, T.; Aparicio-Soto, M.; Castejón, M.L.; Rosillo, M.Á.; Sánchez-Hidalgo, M.; Begines, P.; Fernández-Bolaños, J.G.; Alarcón-de-la-Lastra, C. Peracetylated hydroxytyrosol, a new hydroxytyrosol derivate, attenuates LPS-induced inflammatory response in murine peritoneal macrophages via regulation of non-canonical inflammasome, Nrf2/HO1 and JAK/STAT signaling pathways. J. Nutr. Biochem. 2018, 57, 110–120. [Google Scholar] [CrossRef]
- Montoya, T.; Castejón, M.L.; Sánchez-Hidalgo, M.; González-Benjumea, A.; Fernández-Bolaños, J.G.; Alarcón De-La-Lastra, C. Oleocanthal Modulates LPS-Induced Murine Peritoneal Macrophages Activation via Regulation of Inflammasome, Nrf-2/HO-1, and MAPKs Signaling Pathways. J. Agric. Food Chem. 2019, 67, 5552–5559. [Google Scholar] [CrossRef]
- Vuong, L.D.; Nguyen, Q.N.; Truong, V.L. Anti-inflammatory and anti-oxidant effects of combination between sulforaphane and acetaminophen in LPS-stimulated RAW 264.7 macrophage cells. Immunopharmacol. Immunotoxicol. 2019, 41, 413–441. [Google Scholar] [CrossRef]
- Kubo, E.; Chhunchha, B.; Singh, P.; Sasaki, H.; Singh, D.P. Sulforaphane reactivates cellular antioxidant defense by inducing Nrf2/ARE/Prdx6 activity during aging and oxidative stress. Sci. Rep. 2017, 7, 14130. [Google Scholar] [CrossRef] [Green Version]
- Houghton, C.A. Sulforaphane: Its «Coming of Age» as a Clinically Relevant Nutraceutical in the Prevention and Treatment of Chronic Disease. Oxidative Med. Cell. Longev. 2019, 2019, 2716870. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Subedi, L.; Lee, J.; Yumnam, S.; Ji, E.; Kim, S. Anti-Inflammatory Effect of Sulforaphane on LPS-Activated Microglia Potentially through JNK/AP-1/NF-κB Inhibition and Nrf2/HO-1 Activation. Cells 2019, 8, 194. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Montoya, T.; Alarcón-De-la-lastra, C.; Castejón, M.L.; Ortega-Vidal, J.; Altarejos, J.; Sánchez-Hidalgo, M. (-)-methyl-oleocanthal, a new oleocanthal metabolite reduces LPS-induced inflammatory and oxidative response: Molecular signaling pathways and histones epigenetic modulation. Antioxidants 2022, 11, 56. [Google Scholar] [CrossRef]
- Arthur, J.S.C.; Ley, S.C. Mitogen-activated protein kinases in innate immunity. Nat. Rev. Immunol. 2013, 13, 679–692. [Google Scholar] [CrossRef] [PubMed]
- Deramaudt, T.B.; Ali, M.; Vinit, S.; Bonay, M. Sulforaphane reduces intracellular survival of Staphylococcus aureus in macrophages through inhibition of JNK and p38 MAPK-induced inflammation. Int. J. Mol. Med. 2020, 45, 1927–1941. [Google Scholar] [CrossRef] [Green Version]
- Wang, Z.; Zhang, S.; Xiao, Y.; Zhang, W.; Wu, S.; Qin, T.; Yue, Y.; Qian, W.; Li, L. NLRP3 Inflammasome and Inflammatory Diseases. Oxidative Med. Cell. Longev. 2020, 2020, 4063562. [Google Scholar] [CrossRef]
- Matikainen, S.; Nyman, T.A.; Cypryk, W. Function and Regulation of Noncanonical Caspase-4/5/11 Inflammasome. J. Immunol. 2020, 204, 3063–3069. [Google Scholar] [CrossRef]
- Kiser, C.; Gonul, C.P.; Olcum, M.; Genc, S. Inhibitory effects of sulforaphane on NLRP3 inflammasome activation. Mol. Immunol. 2021, 140, 175–185. [Google Scholar] [CrossRef]
- Yu, C.; He, Q.; Zheng, J.; Li, L.Y.; Hou, Y.H.; Song, F.Z. Sulforaphane improves outcomes and slows cerebral ischemic/reperfusion injury via inhibition of NLRP3 inflammasome activation in rats. Int. Immunopharmacol. 2017, 45, 74–78. [Google Scholar] [CrossRef]
- Li, S.; Yang, H.; Chen, X. Protective effects of sulforaphane on diabetic retinopathy: Activation of the nrf2 pathway and inhibition of nlrp3 inflammasome formation. Exp. Anim. 2019, 68, 221–231. [Google Scholar] [CrossRef] [Green Version]
- Yang, G.; Yeon, S.H.; Lee, H.E.; Kang, H.C.; Cho, Y.Y.; Lee, H.S.; Lee, J.Y. Suppression of NLRP3 inflammasome by oral treatment with sulforaphane alleviates acute gouty inflammation. Rheumatology 2018, 57, 727–736. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, J.; Ahn, H.; Hong, E.J.; An, B.S.; Jeung, E.B.; Lee, G.S. Sulforaphane attenuates activation of NLRP3 and NLRC4 inflammasomes but not AIM2 inflammasome. Cell. Immunol. 2016, 306–307, 53–60. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.; Lee, H.E.; Lee, J.Y. A pharmacological inhibitor of NLRP3 inflammasome prevents non-alcoholic fatty liver disease in a mouse model induced by high fat diet. Sci. Rep. 2016, 6, 24399. [Google Scholar] [CrossRef] [PubMed]
- Fu, Q.; Zhai, Z.; Wang, Y.; Xu, L.; Jia, P.; Xia, P.; Liu, C.; Zhang, X.; Qin, T.; Zhang, H. NLRP3 Deficiency Alleviates Severe Acute Pancreatitis and Pancreatitis-Associated Lung Injury in a Mouse Model. Biomed. Res. Int. 2018, 2018, 1294951. [Google Scholar] [CrossRef]
- Deng, Y.; Guo, S.L.; Wei, B.; Gao, X.C.; Zhou, Y.C.; Li, J.Q. Activation of nicotinic acetylcholine α7 receptor attenuates progression of monocrotaline-induced pulmonary hypertension in rats by downregulating the NLRP3 inflammasome. Front. Pharmacol. 2019, 10, 128. [Google Scholar] [CrossRef] [Green Version]
- Greaney, A.J.; Maier, N.K.; Leppla, S.H.; Moayeri, M. Sulforaphane inhibits multiple inflammasomes through an Nrf2-independent mechanism. J. Leukoc. Biol. 2016, 99, 189–199. [Google Scholar] [CrossRef] [Green Version]
- An, Y.W.; Jhang, K.A.; Woo, S.Y.; Kang, J.L.; Chong, Y.H. Sulforaphane exerts its anti-inflammatory effect against amyloid-β peptide via STAT-1 dephosphorylation and activation of Nrf2/HO-1 cascade in human THP-1 macrophages. Neurobiol. Aging 2016, 38, 1–10. [Google Scholar] [CrossRef]
- Tufekci, K.U.; Ercan, I.; Isci, K.B.; Olcum, M.; Tastan, B.; Gonul, C.P.; Genc, K.; Genc, S. Sulforaphane inhibits NLRP3 inflammasome activation in microglia through Nrf2-mediated miRNA alteration. Immunol. Lett. 2021, 233, 20–30. [Google Scholar] [CrossRef]
- Zhao, S.; Zhong, Y.; Fu, X.; Wang, Y.; Ye, P.; Cai, J.; Liu, Y.; Sun, J.; Mei, Z.; Jiang, Y.; et al. H3K4 Methylation Regulates LPS-Induced Proinflammatory Cytokine Expression and Release in Macrophages. Shock 2019, 51, 401–406. [Google Scholar] [CrossRef]
- Liu, K.L.; Zhu, K.; Zhang, H. An overview of the development of EED inhibitors to disable the PRC2 function. RSC Med. Chem. 2022, 13, 39–53. [Google Scholar] [CrossRef]
- Yi, Y.S. Functional interplay between methyltransferases and inflammasomes in inflammatory responses and diseases. Int. J. Mol. Sci. 2021, 22, 7580. [Google Scholar] [CrossRef] [PubMed]
- Aparicio-Soto, M.; Sánchez-Fidalgo, S.; González-Benjumea, A.; Maya, I.; Fernández-Bolaños, J.G.; Alarcón-de-la-Lastra, C. Naturally occurring hydroxytyrosol derivatives: Hydroxytyrosyl acetate and 3,4-dihydroxyphenylglycol modulate inflammatory response in murine peritoneal macrophages. potential utility as new dietary supplements. J. Agric. Food Chem. 2015, 63, 836–846. [Google Scholar] [CrossRef] [PubMed]
- Skehan, P.; Storeng, R.; Scudiero, D.; Monks, A.; McMahon, J.; Vistica, D.; Warren, J.T.; Bokesch, H.; Kenney, S.; Boyd, M.R. New Colorimetric Cytotoxicity Assay for Anticancer-Drug Screening. J. Natl. Cancer Inst. 1990, 82, 1107–1112. [Google Scholar] [CrossRef] [PubMed]
- Hajji, N.; Joseph, B. Epigenetic regulation of cell life and death decisions and deregulation in cancer. Essays Biochem. 2010, 48, 121–146. [Google Scholar] [CrossRef]
- Bradford, M. A Rapid and Sensitive Method for the Quantitation of Microgram Quantities of Protein Utilizing the Principle of Protein-Dye Binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Sánchez-Hidalgo, M.; Martín, A.R.; Villegas, I.; Alarcón De La Lastra, C. Rosiglitazone, an agonist of peroxisome proliferator-activated receptor gamma, reduces chronic colonic inflammation in rats. Biochem. Pharmacol. 2005, 69, 1733–1744. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alcarranza, M.; Villegas, I.; Muñoz-García, R.; Recio, R.; Fernández, I.; Alarcón-de-la-Lastra, C. Immunomodulatory Effects of (R)-Sulforaphane on LPS-Activated Murine Immune Cells: Molecular Signaling Pathways and Epigenetic Changes in Histone Markers. Pharmaceuticals 2022, 15, 966. https://doi.org/10.3390/ph15080966
Alcarranza M, Villegas I, Muñoz-García R, Recio R, Fernández I, Alarcón-de-la-Lastra C. Immunomodulatory Effects of (R)-Sulforaphane on LPS-Activated Murine Immune Cells: Molecular Signaling Pathways and Epigenetic Changes in Histone Markers. Pharmaceuticals. 2022; 15(8):966. https://doi.org/10.3390/ph15080966
Chicago/Turabian StyleAlcarranza, Manuel, Isabel Villegas, Rocío Muñoz-García, Rocío Recio, Inmaculada Fernández, and Catalina Alarcón-de-la-Lastra. 2022. "Immunomodulatory Effects of (R)-Sulforaphane on LPS-Activated Murine Immune Cells: Molecular Signaling Pathways and Epigenetic Changes in Histone Markers" Pharmaceuticals 15, no. 8: 966. https://doi.org/10.3390/ph15080966
APA StyleAlcarranza, M., Villegas, I., Muñoz-García, R., Recio, R., Fernández, I., & Alarcón-de-la-Lastra, C. (2022). Immunomodulatory Effects of (R)-Sulforaphane on LPS-Activated Murine Immune Cells: Molecular Signaling Pathways and Epigenetic Changes in Histone Markers. Pharmaceuticals, 15(8), 966. https://doi.org/10.3390/ph15080966






