Neutrophil Immunomodulatory Activity of Farnesene, a Component of Artemisia dracunculus Essential Oils
Abstract
1. Introduction
2. Results and Discussion
2.1. Essential Oil Composition
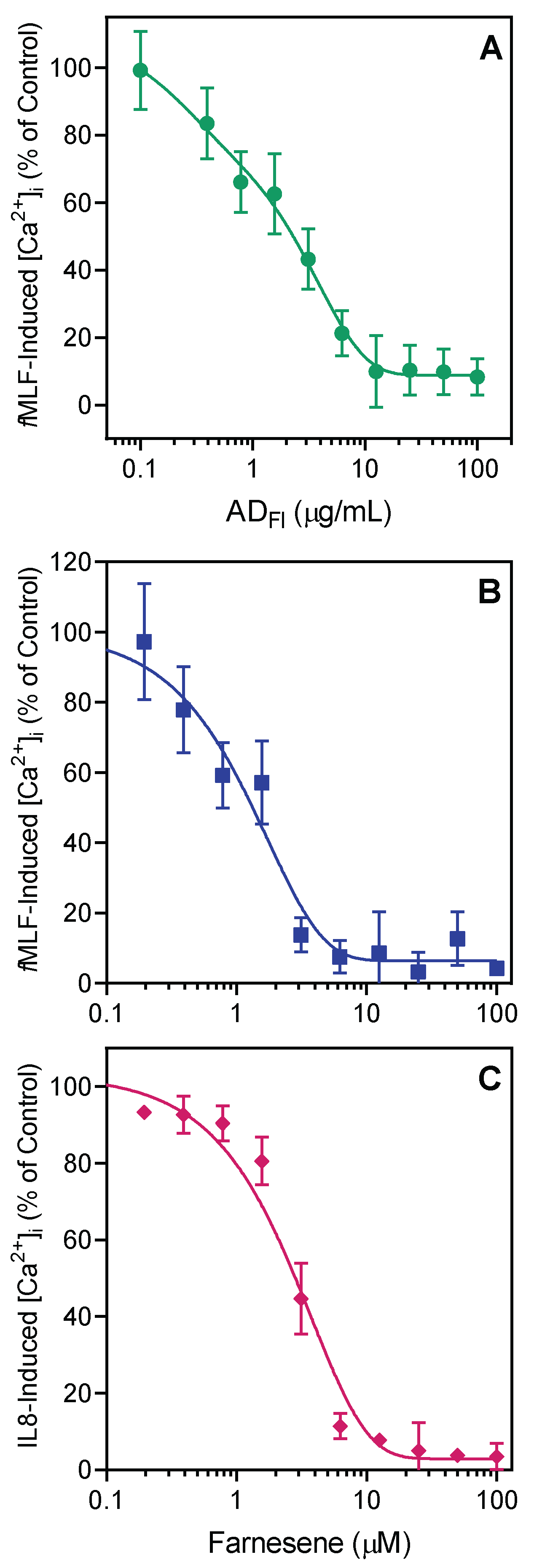
2.2. Effect of Artemisia Essential Oils and Pure Compounds on Neutrophil Ca2+ Influx
2.3. Effect of A. dracunculus Essential Oils and Pure Compounds on Neutrophil Chemotaxis
2.4. Identification of Potential Protein Targets for Selected Compounds
3. Materials and Methods
3.1. Materials
3.2. Essential Oil Extraction
3.3. GC-Flame Ionization Detector (GC-FID) and GC-Mass Spectrometry (GC-MS) Analysis
3.4. Isolation of Human Neutrophils
3.5. Cell Culture
3.6. Ca2+ Mobilization Assay
3.7. Chemotaxis Assay
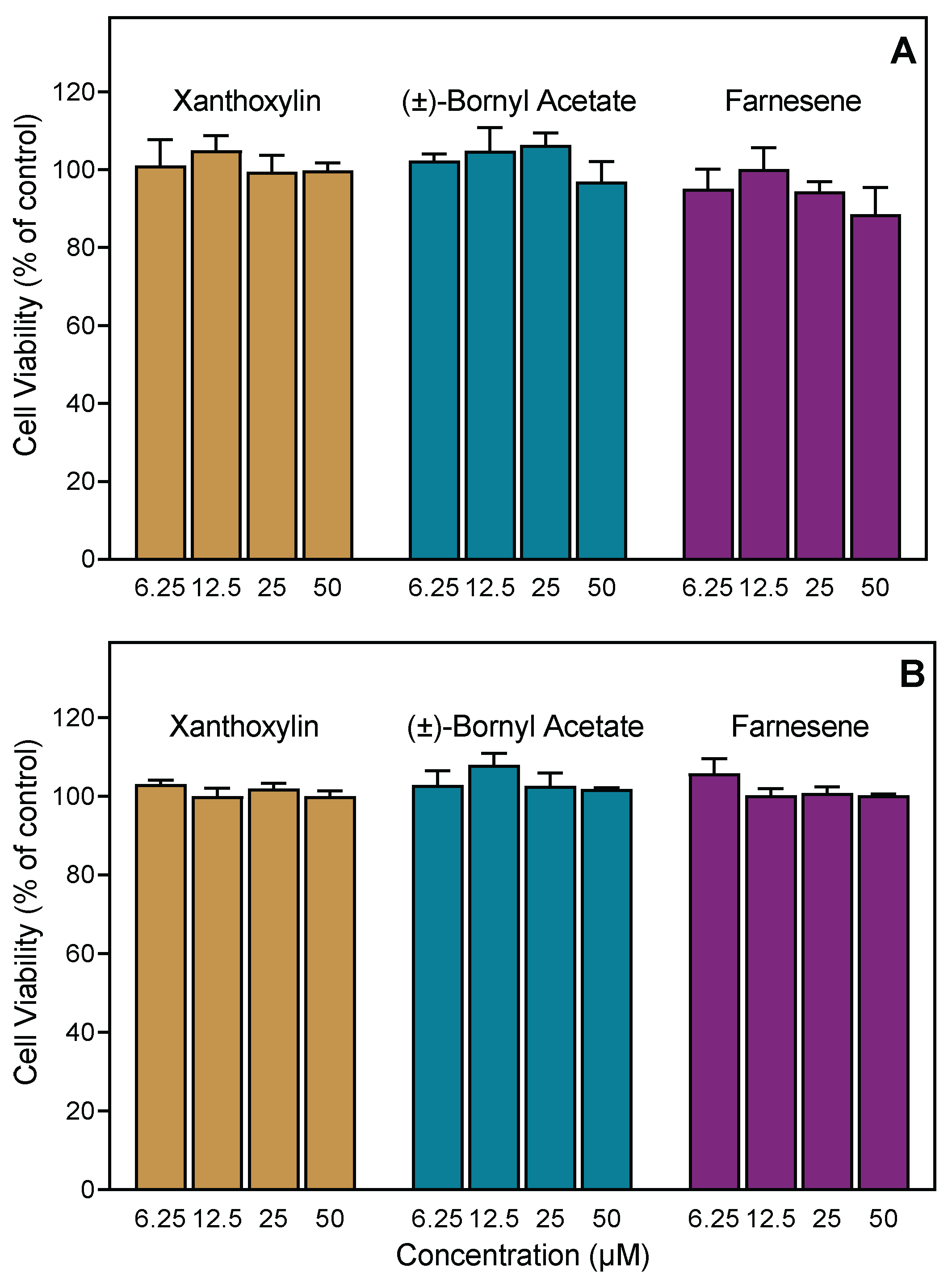
3.8. Cytotoxicity Assay
3.9. Molecular Modeling
3.10. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wright, C.W. Artemisia; CRC Press: London, UK, 2001. [Google Scholar]
- Abad, M.J.; Bedoya, L.M.; Apaza, L.; Bermejo, P. The Artemisia L. genus: A review of bioactive essential oils. Molecules 2012, 17, 2542–2566. [Google Scholar] [CrossRef] [PubMed]
- Garcia, S.; McArthur, E.D.; Pellicer, J.; Sanderson, S.C.; Valles, J.; Garnatje, T. A molecular phylogenetic approach to western North America endemic Artemisia and allies (Asteraceae): Untangling the sagebrushes. Am. J. Bot. 2011, 98, 638–653. [Google Scholar] [CrossRef] [PubMed]
- Katial, R.K.; Lin, F.L.; Stafford, W.W.; Ledoux, R.A.; Westly, C.R.; Weber, R.W. Mugwort and sage (Artemisia) pollen cross-reactivity: ELISA inhibition and immunoblot evaluation. Ann. Allerg. Asthma Immunol. 1997, 79, 340–346. [Google Scholar] [CrossRef]
- Francis, J.K. Wildland Shrubs of the United States and its Territories: Thamnic Descriptions; Gen.Techn. Rep. IITF-GTR-26; International Institute of Tropical Forestry: Fort Collins, CO, USA; San Juan, Puerto Rico, 2004; Volume 1, pp. 47–90. [Google Scholar]
- Liu, Y.; He, Y.; Wang, F.; Xu, R.; Yang, M.; Ci, Z.; Wu, Z.; Zhang, D.; Lin, J. From longevity grass to contemporary soft gold: Explore the chemical constituents, pharmacology, and toxicology of Artemisia argyi H. Lév. & vaniot essential oil. J. Ethnopharmacol. 2021, 279, 114404. [Google Scholar]
- Ding, J.; Wang, L.; He, C.; Zhao, J.; Si, L.; Huang, H. Artemisia scoparia: Traditional uses, active constituents and pharmacological effects. J. Ethnopharmacol. 2021, 273, 113960. [Google Scholar] [CrossRef]
- Du Toit, A.; Van der Kooy, F. Artemisia afra, a controversial herbal remedy or a treasure trove of new drugs? J. Ethnopharmacol. 2019, 244, 112127. [Google Scholar] [CrossRef]
- Kachura, A.; Harris, C.S. An ethnobotanical meta-analysis of North American medicinal Asteraceae. Botany 2021, 100, 207–217. [Google Scholar] [CrossRef]
- Nagy, J.G.; Tengerdy, R.P. Antibacterial action of essential oils of Artemisia as an ecological factor: I. Antibacterial action of the volatile oils of Artemisia tridentata and Artemisia nova on aerobic bacteria. Appl. Microbiol. 1967, 15, 819–821. [Google Scholar] [CrossRef]
- Nagy, J.G.; Tengerdy, R.P. Antibacterial action of essential oils of Artemisia as an ecological factor: II. Antibacterial action of the volatile oils of Artemisia tridentata (big sagebrush) on bacteria from the rumen of mule deer. Appl. Microbiol. 1968, 16, 441–444. [Google Scholar] [CrossRef]
- Natvig, D.O. The effects of volatile and other secondary metabolites from Artemisia tridentata on soil microfungi: An ecological approach. Master’s Thesis, University of Montana, Missoula, MT, USA, 1976. [Google Scholar]
- Eichelbaum, S.R. Screening of Plants for Antibacterial Properties: Growth Inhibition of Staphylococcus aureus by Artemisia tridentata; Florida International University: Miami, FL, USA, 2016. [Google Scholar]
- Baldemir, A.; Karaman, Ü.; İllgün, S.; Kaçmaz, G.; Demirci, B. Antiparasitic efficacy of Artemisia ludoviciana Nutt. (Asteraceae) essential oil for Acanthamoeba castellanii, Leishmania infantum and Trichomonas vaginalis. Indian J. Pharm. Educ. Res. 2018, 52, 416–425. [Google Scholar] [CrossRef]
- Anaya-Eugenio, G.D.; Rivero-Cruz, I.; Rivera-Chávez, J.; Mata, R. Hypoglycemic properties of some preparations and compounds from Artemisia ludoviciana Nutt. J. Ethnopharmacol. 2014, 155, 416–425. [Google Scholar] [CrossRef] [PubMed]
- Pelarti, M.S.; Zarehshuran, L.K.; Babaeekhou, L.; Ghane, M. Antibacterial, anti-biofilm and anti-quorum sensing activities of Artemisia dracunculus essential oil (EO): A study against Salmonella enterica serovar Typhimurium and Staphylococcus aureus. Arch. Microbiol. 2021, 203, 1529–1537. [Google Scholar] [CrossRef] [PubMed]
- Anaya-Eugenio, G.D.; Rivero-Cruz, I.; Bye, R.; Linares, E.; Mata, R. Antinociceptive activity of the essential oil from Artemisia ludoviciana. J. Ethnopharmacol. 2016, 179, 403–411. [Google Scholar] [CrossRef] [PubMed]
- Dos Santos, E.; Leitao, M.M.; Aguero Ito, C.N.; Silva-Filho, S.E.; Arena, A.C.; Silva-Comar, F.M.S.; Nakamura Cuman, R.K.; Oliveira, R.J.; Nazari Formagio, A.S.; Leite Kassuya, C.A. Analgesic and anti-inflammatory articular effects of essential oil and camphor isolated from Ocimum kilimandscharicum Gurke leaves. J. Ethnopharmacol. 2021, 269, 113697. [Google Scholar] [CrossRef] [PubMed]
- Darwish, R.S.; Hammoda, H.M.; Ghareeb, D.A.; Abdelhamid, A.S.A.; Bellah El Naggar, E.M.; Harraz, F.M.; Shawky, E. Efficacy-directed discrimination of the essential oils of three Juniperus species based on their in-vitro antimicrobial and anti-inflammatory activities. J. Ethnopharmacol. 2020, 259, 112971. [Google Scholar] [CrossRef] [PubMed]
- Ozek, G.; Schepetkin, I.A.; Yermagambetova, M.; Ozek, T.; Kirpotina, L.N.; Almerekova, S.S.; Abugalieva, S.I.; Khlebnikov, A.I.; Quinn, M.T. Innate immunomodulatory activity of cedrol, a component of essential oils isolated from Juniperus species. Molecules 2021, 26, 7644. [Google Scholar] [CrossRef]
- Schepetkin, I.A.; Ozek, G.; Ozek, T.; Kirpotina, L.N.; Khlebnikov, A.I.; Quinn, M.T. Chemical composition and immunomodulatory activity of essential oils from Rhododendron albiflorum. Molecules 2021, 26, 3652. [Google Scholar] [CrossRef]
- Schepetkin, I.A.; Ozek, G.; Ozek, T.; Kirpotina, L.N.; Khlebnikov, A.I.; Quinn, M.T. Chemical composition and immunomodulatory activity of Hypericum perforatum essential oils. Biomolecules 2020, 10, 916. [Google Scholar] [CrossRef]
- Ozek, G.; Schepetkin, I.A.; Utegenova, G.A.; Kirpotina, L.N.; Andrei, S.R.; Ozek, T.; Baser, K.H.C.; Abidkulova, K.T.; Kushnarenko, S.V.; Khlebnikov, A.I.; et al. Chemical composition and phagocyte immunomodulatory activity of Ferula iliensis essential oils. J. Leukoc. Biol. 2017, 101, 1361–1371. [Google Scholar] [CrossRef]
- Schepetkin, I.A.; Kushnarenko, S.V.; Ozek, G.; Kirpotina, L.N.; Sinharoy, P.; Utegenova, G.A.; Abidkulova, K.T.; Ozek, T.; Baser, K.H.; Kovrizhina, A.R.; et al. Modulation of human neutrophil responses by the essential oils from Ferula akitschkensis and their constituents. J. Agric. Food Chem. 2016, 64, 7156–7170. [Google Scholar] [CrossRef]
- Schepetkin, I.A.; Kushnarenko, S.V.; Ozek, G.; Kirpotina, L.N.; Utegenova, G.A.; Kotukhov, Y.A.; Danilova, A.N.; Ozek, T.; Baser, K.H.; Quinn, M.T. Inhibition of human neutrophil responses by the essential oil of Artemisia kotuchovii and its constituents. J. Agric. Food Chem. 2015, 63, 4999–5007. [Google Scholar] [CrossRef] [PubMed]
- Lopes-Lutz, D.; Alviano, D.S.; Alviano, C.S.; Kolodziejczyk, P.P. Screening of chemical composition, antimicrobial and antioxidant activities of Artemisia essential oils. Phytochemistry 2008, 69, 1732–1738. [Google Scholar] [CrossRef] [PubMed]
- Hosseini, S.F.; Amraie, M.; Salehi, M.; Mohseni, M.; Aloui, H. Effect of chitosan-based coatings enriched with savory and/or tarragon essential oils on postharvest maintenance of kumquat (Fortunella sp.) fruit. Food Sci. Nutr. 2019, 7, 155–162. [Google Scholar] [CrossRef] [PubMed]
- Babushok, V.I.; Linstrom, P.J.; Zenkevich, I.G. Retention indices for frequently reported compounds of plant essential oils. J. Phys. Chem. Ref. Data 2011, 40, 043101. [Google Scholar] [CrossRef]
- Hulley, I.M.; Sadgrove, N.J.; Tilney, P.M.; Ozek, G.; Yur, S.; Ozek, T.; Baser, K.H.C.; van Wyk, B.E. Essential oil composition of Pentzia incana (Asteraceae), an important natural pasture plant in the Karoo region of South Africa. Afr. J. Range Sci. 2018, 35, 137–145. [Google Scholar] [CrossRef]
- Darriet, F. Caractérisation de Nouvelles Molécules et Variabilité Chimique de Trois Plantes du Contin-uum Corse-Sardaigne: Chamaemelum Mixtum, Anthemis Maritima et Eryngium Maritimum; University of Corsica Pasquale: Paoli, France, 2011. [Google Scholar]
- Turkmenoglu, F.P.; Agar, O.T.; Akaydin, G.; Hayran, M.; Demirci, B. Characterization of volatile compounds of eleven Achillea species from Turkey and biological activities of essential oil and methanol extract of A. hamzaoglui Arabaci & Budak. Molecules 2015, 20, 11432–11458. [Google Scholar]
- Demirci, B.; Baser, K.H.C. The essential oil composition of Tanacetum macrophyllum (Waldst. et Kit.) Schultz. Bip. J. Essent. Oil Res. 2007, 19, 255–257. [Google Scholar] [CrossRef]
- Brophy, J.J.; Goldsack, R.J.; Forster, P.I.; Southwell, I.A. Leaf oils of the genus Acradenia (Rutaceae). J. Essent. Oil Res. 2001, 13, 136–139. [Google Scholar] [CrossRef]
- Buttkus, H.A.; Bose, R.J.; Shearer, D.A. Terpenes in the essential oil of sagebrush (Artemisia tridentata). J. Agric. Food Chem. 1977, 25, 288–291. [Google Scholar] [CrossRef]
- Borek, T.T.; Hochrein, J.M.; Irwin, A.N. Composition of the essential oils from Rocky Mountain juniper (Juniperus scopulorum), Big sagebrush (Artemisia tridentata), and White Sage (Salvia apiana); Sandia National Laboratories (SNL): Albuquerque, NM, USA; Livermore, CA, USA, 2003. [Google Scholar]
- Powell, J. Site factor relationships with volatile oils in big sagebrush. Rangel. Ecol. Manag. J. Range Manag. Arch. 1970, 23, 42–46. [Google Scholar] [CrossRef][Green Version]
- Welch, B.L.; McArthur, E.D. Variation of monoterpenoid content among subspecies and accessions of Artemisia tridentata grown in a uniform garden. Rangeland Ecol. Manag. 1981, 34, 380–384. [Google Scholar] [CrossRef]
- Epstein, W.W.; Gaudioso, L.A. Santolinolide B [(2R, 3S, 4S)-4-hydroxy-2, 5-dimethyl-3-vinyl-5-hexenoic acid lactone]. A new irregular monoterpene from Artemisia tridentata tridentata. J. Org. Chem. 1979, 44, 3113–3117. [Google Scholar] [CrossRef]
- Epstein, W.W.; Gaudioso, L.A.; Brewster, G.B. Essential oil constituents of Artemisia tridentata rothrockii. The isolation and characterization of two new irregular monoterpenes. J. Org. Chem. 1984, 49, 2748–2754. [Google Scholar] [CrossRef]
- Gunawardena, K.; Rivera, S.; Epstein, W. The monoterpenes of Artemisia tridentata ssp. vaseyana, Artemisia cana ssp. viscidula and Artemisia tridentata ssp. spiciformis. Phytochemistry 2002, 59, 197–203. [Google Scholar] [CrossRef]
- Collin, G.; St-Gelais, A.; Turcotte, M.; Gagnon, H. Composition of the essential oil and of some extracts of the aerial parts of Artemisia ludoviciana var. latiloba Nutt. Am. J. Essential Oils Nat. Prod. 2016, 4, 28–38. [Google Scholar]
- Ruiz-Cancino, A.; Cano, A.E.; Delgado, G. Sesquiterpene lactones and flavonoids from Artemisia ludoviciana ssp. mexicana. Phytochemistry 1993, 33, 1113–1115. [Google Scholar] [CrossRef]
- Ohno, N.; Gershenzon, J.; Roane, C.; Mabry, T.J. 11, 13-dehydrodesacetylmatricarin and other sesquiterpene lactones from Artemisia ludoviciana var. Ludoviciana and the identity of artecanin and chyrsartemin B. Phytochemistry 1980, 19, 103–106. [Google Scholar] [CrossRef]
- Geissman, T.; Saitoh, T. Ludalbin, a new lactone from Artemisia ludoviciana. Phytochemistry 1972, 11, 1157–1160. [Google Scholar] [CrossRef]
- Mumivand, H.; Ebrahimi, A.; Morshedloo, M.R.; Shayganfar, A. Water deficit stress changes in drug yield, antioxidant enzymes activity and essential oil quality and quantity of Tarragon (Artemisia dracunculus L.). Ind. Crop. Prod. 2021, 164, 113381. [Google Scholar] [CrossRef]
- Fraternale, D.; Flamini, G.; Ricci, D. Essential oil composition and antigermination activity of Artemisia dracunculus (Tarragon). Nat. Prod. Commun. 2015, 10, 1469–1472. [Google Scholar] [CrossRef]
- Sahakyan, N.; Andreoletti, P.; Cherkaoui-Malki, M.; Petrosyan, M.; Trchounian, A. Artemisia dracunculus L. essential oil phytochemical components trigger the activity of cellular antioxidant enzymes. J. Food Biochem. 2021, 45, e13691. [Google Scholar] [CrossRef] [PubMed]
- Gilemeister, E.; Hoffmann, F. Die Aetherischen Ole; Academie Verlag.: Berlin, Germany, 1961; Volume 7, pp. 714–715. [Google Scholar]
- Pushkareva, E.S.; Efremov, A.A. Component composition of the essential oil of Artemisia frigida from Krasnoyarsk region and of its individual fractions. Sorption Chromatog. Proc. 2012, 12, 619–623. [Google Scholar]
- Atazhanova, G.A.; Dembitskii, A.D.; Yakovleva, T.D.; Ishmuratova, M.Y.; Mikhailov, V.G.; Adekenov, S.M. Composition of the essential oils of Artemisia radicans and A. frigida. Chem. Nat. Compd. 1999, 35, 427–429. [Google Scholar] [CrossRef]
- Korolyuk, E.; Tkachev, A. Chemical composition of the essential oil from two wormwood species Artemisia frigida and Artemisia argyrophylla. Russ. J. Bioorg. Chem. 2010, 36, 884–893. [Google Scholar] [CrossRef]
- Zhigzhitzhapova, S.V.; Randalova, T.E.; Radnaeva, L.D.; Dylenova, E.P.; Chen, S.; Zhang, F. Chemical composition of essentials oils of Artemisia frigida Willd. (Asteraceae) grown in the North and Central Asia. J. Essent. Oil Bear. Plants 2017, 20, 915–926. [Google Scholar] [CrossRef]
- Liu, X.C.; Li, Y.; Wang, T.; Wang, Q.; Liu, Z.L. Chemical composition and insecticidal activity of essential oil of Artemisia frigida Willd (Compositae) against two grain storage insects. Trop. J. Pharm. Res. 2014, 13, 587–592. [Google Scholar] [CrossRef]
- Borchuluun, S.; Wang, Q.; Hao, J. Extraction of essential oil from the aerial parts of Artemisia frigida Willd by way of hydrodistillation. Proc. Mongolian Acad. Sci. 2020, 60, 25–30. [Google Scholar] [CrossRef]
- Borchuluun, S.; Wang, Q.; Xu, Y.; He, X.; Bao, W.; Pa, B. Structure elucidation and NMR assignments of a new sesquiterpene of volatile oil from Artemisia frigida Willd. Nat. Prod. Res. 2021, 35, 2376–2380. [Google Scholar] [CrossRef]
- Kelsey, R.G.; Shafizadeh, F.; Campbell, J.A.; Craig, A.C.; Campana, C.F.; Craig, R.E. Canin from Artemisia cana Pursh ssp. cana. Crystal structure and identification of chrysartemin A. J. Org. Chem. 1983, 48, 125–127. [Google Scholar] [CrossRef]
- Lee, K.; Simpson, R.; Geissman, T. Sesquiterpenoid lactones of Artemisia. Constituents of Artemisia cana ssp. cana. The structure of canin. Phytochemistry 1969, 8, 1515–1521. [Google Scholar] [CrossRef]
- Shafizadeh, F.; Bhadane, N. Chemical constituents of sagebrush. V. Sesquiterpene lactones of sagebrush. New guaianolides from Artemisia cana subspecies viscidula. J. Org. Chem. 1972, 37, 3168–3173. [Google Scholar] [CrossRef]
- Malech, H.L.; DeLeo, F.R.; Quinn, M.T. The role of neutrophils in the immune system: An overview. Methods Mol. Biol. 2020, 2087, 3–10. [Google Scholar] [PubMed]
- Keir, H.R.; Chalmers, J.D. Neutrophil extracellular traps in chronic lung disease: Implications for pathogenesis and therapy. Eur. Respir. Rev. 2022, 31, 210241. [Google Scholar] [CrossRef] [PubMed]
- Stojkov, D.; Gigon, L.; Peng, S.; Lukowski, R.; Ruth, P.; Karaulov, A.; Rizvanov, A.; Barlev, N.A.; Yousefi, S.; Simon, H.U. Physiological and pathophysiological roles of metabolic pathways for NET formation and other neutrophil functions. Front. Immunol. 2022, 13, 826515. [Google Scholar] [CrossRef]
- Dixit, N.; Kim, M.H.; Rossaint, J.; Yamayoshi, I.; Zarbock, A.; Simon, S.I. Leukocyte function antigen-1, kindlin-3, and calcium flux orchestrate neutrophil recruitment during inflammation. J. Immunol. 2012, 189, 5954–5964. [Google Scholar] [CrossRef]
- Ali, H.; Richardson, R.M.; Haribabu, B.; Snyderman, R. Chemoattractant receptor cross-desensitization. J. Biol. Chem. 1999, 274, 6027–6030. [Google Scholar] [CrossRef]
- Jyotshna; Srivastava, N.; Singh, B.; Chanda, D.; Shanker, K. Chemical composition and acetylcholinesterase inhibitory activity of Artemisia maderaspatana essential oil. Pharm. Biol. 2015, 53, 1677–1683. [Google Scholar]
- Zhang, H.; Zhou, D.; Luo, Y.; Wang, J.; Zong, S. Identification of volatile compounds emitted by Artemisia ordosica (Artemisia, Asteraceae) and changes due to mechanical damage and weevil infestation. Z. Naturforsch. C. 2013, 68, 313–317. [Google Scholar] [CrossRef]
- Zhang, H.; Zong, S.; Luo, Y.; Wang, T.; Wang, J.; Cao, C. Comparative study of the volatiles’ composition of healthy and larvae-infested Artemisia ordosica. Z. Naturforsch. C. 2013, 68, 8–12. [Google Scholar]
- Padalia, R.C.; Verma, R.S.; Chauhan, A.; Chanotiya, C.S.; Yadav, A. Variation in the volatile constituents of Artemisia annua var. CIM-Arogya during plant ontogeny. Nat. Prod. Commun. 2011, 6, 239–242. [Google Scholar] [CrossRef]
- Gonzalez, S.B.; Gastaldi, B.; Catalan, C.; Di Leo Lira, P.; Retta, D.; van Baren, C.M.; Bandoni, A.L. Artemisia magellanica. Chemical composition of the essential oil from an unexplored endemic species of Patagonia. Chem. Biodivers. 2019, 16, e1900125. [Google Scholar] [CrossRef] [PubMed]
- Govindarajan, M.; Benelli, G. Artemisia absinthium-borne compounds as novel larvicides: Effectiveness against six mosquito vectors and acute toxicity on non-target aquatic organisms. Parasitol. Res. 2016, 115, 4649–4661. [Google Scholar] [CrossRef] [PubMed]
- Cha, J.D.; Jeong, M.R.; Choi, H.J.; Jeong, S.I.; Moon, S.E.; Yun, S.I.; Kim, Y.H.; Kil, B.S.; Song, Y.H. Chemical composition and antimicrobial activity of the essential oil of Artemisia lavandulaefolia. Planta Med. 2005, 71, 575–577. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.; Wang, B.; Yang, F.; Sun, Q.; Yang, Z.; Zhu, L. Chemical compositionand anti-acetyl cholinesterase activity of flower essential oils of Artemisia annua at different flowering stages. Iran J. Pharm. Res. 2011, 10, 265–271. [Google Scholar]
- Qadir, M.; Maurya, A.K.; Agnihotri, V.K.; Shah, W.A. Volatile composition, antibacterial and antioxidant activities of Artemisia tournefortiana Reichb. from Kashmir, India. Nat. Prod. Res. 2021, 35, 152–156. [Google Scholar] [CrossRef]
- Xu, T.; Xu, M.; Lu, Y.Y.; Zhang, W.Q.; Sun, J.H.; Zeng, R.S.; Turlings, T.C.J.; Chen, L. A trail pheromone mediates the mutualism between ants and aphids. Curr. Biol. 2021, 31, 4738–4747. [Google Scholar] [CrossRef]
- Wang, B.; Dong, W.; Li, H.; D’Onofrio, C.; Bai, P.; Chen, R.; Yang, L.; Wu, J.; Wang, X.; Wang, B.; et al. Molecular basis of (E)-β-farnesene-mediated aphid location in the predator Eupeodes corollae. Curr. Biol. 2022, 32, 951–962.e957. [Google Scholar] [CrossRef]
- Orhan, I.E. A Review focused on molecular mechanisms of anxiolytic effect of Valerina officinalis L. in connection with its phytochemistry throughin vitro/in vivo studies. Curr. Pharm. Design 2021, 27, 3084–3090. [Google Scholar] [CrossRef]
- Yang, L.; Liu, J.; Li, Y.; Qi, G. Bornyl acetate suppresses ox-LDL-induced attachment of THP-1 monocytes to endothelial cells. Biomed. Pharmacother. 2018, 103, 234–239. [Google Scholar] [CrossRef]
- Chen, N.; Sun, G.; Yuan, X.; Hou, J.; Wu, Q.; Soromou, L.W.; Feng, H. Inhibition of lung inflammatory responses by bornyl acetate is correlated with regulation of myeloperoxidase activity. J. Surg. Res. 2014, 186, 436–445. [Google Scholar] [CrossRef]
- Wu, X.; Xiao, F.; Zhang, Z.; Li, X.; Xu, Z. Research on the analgesic effect and mechanism of bornyl acetate in volatile oil from amomum villosum. Zhong Yao Cai 2005, 28, 505–507. [Google Scholar] [PubMed]
- Daina, A.; Michielin, O.; Zoete, V. SwissADME: A free web tool to evaluate pharmacokinetics, drug-likeness and medicinal chemistry friendliness of small molecules. Sci. Rep. UK 2017, 7, 42717. [Google Scholar] [CrossRef] [PubMed]
- Tetko, I.V.; Tanchuk, V.Y. Application of associative neural networks for prediction of lipophilicity in ALOGPS 2.1 program. J. Chem. Inf. Comp. Sci. 2002, 42, 1136–1145. [Google Scholar] [CrossRef] [PubMed]
- Suenderhauf, C.; Hammann, F.; Huwyler, J. Computational prediction of blood-brain barrier permeability using decision tree induction. Molecules 2012, 17, 10429–10445. [Google Scholar] [CrossRef]
- Ferrari, C.; Macchiarulo, A.; Costantino, G.; Pellicciari, R. Pharmacophore model for bile acids recognition by the FPR receptor. J. Comput. Aid. Mol. Des. 2006, 20, 295–303. [Google Scholar] [CrossRef]
- Chen, X.; Yang, D.; Shen, W.; Dong, H.F.; Wang, J.M.; Oppenheim, J.J.; Howard, M.Z. Characterization of chenodeoxycholic acid as an endogenous antagonist of the G-coupled formyl peptide receptors. Inflamm. Res. 2000, 49, 744–755. [Google Scholar] [CrossRef]
- Chen, X.; Mellon, R.D.; Yang, L.; Dong, H.; Oppenheim, J.J.; Howard, O.M. Regulatory effects of deoxycholic acid, a component of the anti-inflammatory traditional Chinese medicine Niuhuang, on human leukocyte response to chemoattractants. Biochem. Pharmacol. 2002, 63, 533–541. [Google Scholar] [CrossRef][Green Version]
- Pamplona, F.A.; Ferreira, J.; de Lima, O.M., Jr.; Duarte, F.S.; Bento, A.F.; Forner, S.; Villarinho, J.G.; Bellocchio, L.; Wotjak, C.T.; Lerner, R.; et al. Anti-inflammatory lipoxin A4 is an endogenous allosteric enhancer of CB1 cannabinoid receptor. Proc. Natl. Acad. Sci. USA 2012, 109, 21134–21139. [Google Scholar] [CrossRef]
- Ge, Y.; Zhang, S.; Wang, J.; Xia, F.; Wan, J.B.; Lu, J.; Ye, R.D. Dual modulation of formyl peptide receptor 2 by aspirin-triggered lipoxin contributes to its anti-inflammatory activity. FASEB J. 2020, 34, 6920–6933. [Google Scholar] [CrossRef]
- Das, U.N. Essential fatty acids and their metabolites in the pathobiology of inflammation and its resolution. Biomolecules 2021, 11, 1873. [Google Scholar] [CrossRef]
- Park, J.; Langmead, C.J.; Riddy, D.M. New advances in targeting the resolution of inflammation: Implications for specialized pro-resolving mediator GPCR drug discovery. ACS Pharmacol. Transl. 2020, 3, 88–106. [Google Scholar] [CrossRef] [PubMed]
- Lu, D.J.; Furuya, W.; Grinstein, S. Involvement of multiple kinases in neutrophil activation. Blood Cells 1993, 19, 343–351. [Google Scholar] [PubMed]
- Bokoch, G.M. Chemoattractant signaling and leukocyte activation. Blood 1995, 86, 1649–1660. [Google Scholar] [CrossRef] [PubMed]
- Schepetkin, I.A.; Khlebnikov, A.I.; Giovannoni, M.P.; Kirpotina, L.N.; Cilibrizzi, A.; Quinn, M.T. Development of small molecule non-peptide formyl peptide receptor (FPR) ligands and molecular modeling of their recognition. Curr. Med. Chem. 2014, 21, 1478–1504. [Google Scholar] [CrossRef]
- He, H.Q.; Ye, R.D. The formyl peptide receptors: Diversity of ligands and mechanism for recognition. Molecules 2017, 22, 455. [Google Scholar] [CrossRef]
- Knall, C.; Young, S.; Nick, J.A.; Buhl, A.M.; Worthen, G.S.; Johnson, G.L. Interleukin-8 regulation of the Ras/Raf/mitogen-activated protein kinase pathway in human neutrophils. J. Biol. Chem. 1996, 271, 2832–2838. [Google Scholar] [CrossRef]
- Ozek, G.; Ishmuratova, M.; Tabanca, N.; Radwan, M.M.; Goger, F.; Ozek, T.; Wedge, D.E.; Becnel, J.J.; Cutler, S.J.; Can Baser, K.H. One-step multiple component isolation from the oil of Crinitaria tatarica (Less.) Sojak by preparative capillary gas chromatography with characterization by spectroscopic and spectrometric techniques and evaluation of biological activity. J. Sep. Sci. 2012, 35, 650–660. [Google Scholar] [CrossRef]
- Schepetkin, I.A.; Kirpotina, L.N.; Khlebnikov, A.I.; Quinn, M.T. High-throughput screening for small-molecule activators of neutrophils: Identification of novel N-formyl peptide receptor agonists. Mol. Pharmacol. 2007, 71, 1061–1074. [Google Scholar] [CrossRef]
- Liu, X.; Ouyang, S.; Yu, B.; Liu, Y.; Huang, K.; Gong, J.; Zheng, S.; Li, Z.; Li, H.; Jiang, H. PharmMapper server: A web server for potential drug target identification using pharmacophore mapping approach. Nucleic Acid. Res. 2010, 38, W609–W614. [Google Scholar] [CrossRef]


| Artemisia spp. | Location | Latitude (N) | Longitude (E) | Altitude (m) | Plant Material | Date of Collection; Specimen No. | Yield (%) |
|---|---|---|---|---|---|---|---|
| A. ludoviciana | Three Forks, MT | 45.92721 | 111.50106 | 1235 | leaves/flowers | August 2019; 2019-IAS-A1 | 0.3/1.9 |
| A. dracunculus | Bozeman, MT | 45.71475° | 110.97890° | 1646 | leaves/flowers | August 2019; 2019-IAS-A2 | 0.9/0.9 |
| A. frigida | Three Forks, MT | 45.92553° | 111.49730° | 1240 | leaves/flowers | August 2019; 2019-IAS-A3 | 0.3/1.9 |
| A. cana | Three Forks, MT | 45.92274° | 111.49453° | 1238 | leaves/flowers | August 2019; 2019-IAS-A4 | 1.4/1.5 |
| A. tridentata | Bozeman, MT | 45.74118° | 110.98698° | 1415 | leaves/flowers | August 2019; 2019-IAS-A5 | 3.3/2.7 |
| No | RRIa | RRIb | Compound | ATLv | ATFl | ALLv | ALFl | ADLv | ADFl | AFLv | AFFl | ACLv | ACFl |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 1032 | 1008–1039 # | α-Pinene | 2.1 | 1.8 | 2.5 | 3.9 | 0.1 | 0.1 | 2.2 | 3.3 | 0.8 | 2.0 |
| 2 | 1043 | 1011–1063 # | Santolinatriene | 0.7 | 1.7 | 3.5 | 2.2 | 2.4 | |||||
| 3 | 1076 | 1043–1086 # | Camphene | 7.7 | 7.7 | 7.1 | 5.5 | t | t | 4.0 | 6.0 | 1.6 | 4.1 |
| 4 | 1189 | 1179 @ | Artemiseole | 2.1 | 3.1 | 2.5 | 7.5 | 1.2 | |||||
| 5 | 1213 | 1186–1231 # | 1,8-Cineole | 21.8 | 23.8 | 16.3 | 23.1 | 12.5 | 5.7 | 30.0 | 21.9 | ||
| 6 | 1218 | 1188–1233 # | β-Phellandrene | 1.3 | 2.2 | ||||||||
| 7 | 1246 | 1211–1251 # | (Z)-β-Ocimene | 4.6 | 9.4 | ||||||||
| 8 | 1266 | 1232–1267 # | (E)-β-Ocimene | 2.7 | 6.6 | t | |||||||
| 9 | 1280 | 1246–1291 # | p-Cymene | 1.2 | 1.2 | 0.7 | 0.7 | 0.5 | 0.2 | 3.2 | 2.1 | 0.9 | 0.7 |
| 10 | 1290 | 1260–1300 # | Terpinolene | t | t | 0.2 | 0.3 | 8.8 | 5.5 | 0.1 | 0.3 | 0.1 | 0.1 |
| 11 | 1329 | 1312 ^ | Santolina epoxide * | 3.1 | |||||||||
| 12 | 1451 | 1400–1452 # | β-Thujone | 3.3 | 0.1 | ||||||||
| 13 | 1474 | 1453 ** | trans-Chrysanthenol | 2.0 | |||||||||
| 14 | 1532 | 1481–1537 # | Camphor | 51.3 | 41.7 | 41.1 | 26.6 | 23.0 | 37.7 | 32.5 | 35.9 | ||
| 15 | 1538 | 1533–1590 # | trans-Chrysanthenyl acetate | 8.1 | |||||||||
| 16 | 1553 | 1507–1564 # | Linalool | 3.8 | 2.5 | 0.1 | 0.2 | ||||||
| 17 | 1590 | 1549–1597 # | Bornyl acetate | 0.9 | 0.6 | 0.3 | 0.6 | 1.5 | 3.8 | 0.3 | 0.8 | ||
| 18 | 1611 | 1564–1630 # | Terpinen-4-ol | 2.7 | 2.9 | 2.4 | 3.8 | t | 3.3 | 6.9 | 2.0 | 2.3 | |
| 19 | 1687 | 1652–1690 # | Methyl chavicol | 42.9 | 38.8 | ||||||||
| 20 | 1719 | 1653–1728 # | Borneol | 2.4 | 3.3 | 9.6 | 8.1 | t | 6.6 | 14.6 | 2.9 | 2.3 | |
| 21 | 1737 | 1713–1748 # | (Z,E)-α-Farnesene | t | |||||||||
| 22 | 1748 | 1689–1748 # | Piperitone | 0.2 | 1.5 | 1.1 | |||||||
| 23 | 1758 | 1714–1763 # | (E,E)-α-Farnesene | 0.2 | 0.5 | ||||||||
| 24 | 1764 | 1751–1765 # | cis-Chrysanthenol | 0.5 | 0.8 | 1 | 0.5 | 3.9 | 0.5 | 0.2 | |||
| 25 | 1821 | 1807 *** | Fragranol | 3.0 | |||||||||
| 26 | 1827 | 1827 *** | Grandisol | 3.9 | |||||||||
| 27 | 1864 | 1813–1865 # | p-Cymen-8-ol | 0.2 | 0.1 | 0.1 | 2.3 | 0.9 | 0.4 | 0.3 | 0.1 | 0.1 | |
| 28 | 2030 | 1961–2033 # | Methyl eugenol | 26.1 | 26.4 | t | 0.1 | ||||||
| 29 | 2637 | 2608 ## | Xanthoxylin | 2.2 | 1.2 | ||||||||
| Summary of the composition of Artemisia spp. essential oils | |||||||||||||
| Compounds | ATLv | ATFl | ALLv | ALFl | ADLv | ADFl | AFLv | AFFl | ACLv | ACFl | |||
| Monoterpene Hydrocarbons | 13.1 | 14.4 | 13.5 | 18.7 | 20.3 | 25.8 | 11.6 | 17.4 | 6.5 | 11.3 | |||
| Oxygenated Monoterpenes | 84.5 | 79.8 | 82.5 | 77.4 | 4.0 | 1.9 | 79.6 | 79.8 | 84.8 | 81.0 | |||
| Sesquiterpene Hydrocarbons | 2.0 | 0.2 | 0.7 | 1.7 | 2.4 | 0.6 | 0.2 | 0.1 | 0.3 | ||||
| Oxygenated Sesquiterpenes | 0.8 | 1.6 | 1.1 | 1.2 | 1.7 | 1.1 | 2.5 | 0.6 | 0.1 | 0.4 | |||
| Phenylpropanoids | 1.3 | 0.2 | 69.0 | 65.3 | 0.1 | 0.1 | |||||||
| Others | t | t | 0.4 | 0.2 | 2.6 | 2.1 | 0.2 | 0.5 | t | 0.6 | |||
| Total | 98.0 | 97.1 | 98.9 | 97.8 | 98.2 | 97.7 | 94.5 | 98.4 | 91.5 | 93.6 | |||
| Essential Oil or Pure Compound | Activation of [Ca2+]i | Inhibition of [Ca2+]i | Cytotoxicity | |
|---|---|---|---|---|
| fMLF-Induced | WKYMVM-Induced | |||
| EC50 (μg/mL) | IC50 (μg/mL) | IC50 (μg/mL) | ||
| ATLv | 23.6 ± 3.6 | 28.9 ± 4.1 | 3.4 ± 0.8 | Nontoxic |
| ATFl | 32.1 ± 4.5 | 19.5 ± 3.3 | 3.7 ± 0.1 | Nontoxic |
| ALLv | 15.8 ± 2.1 | 31.2 ± 3.9 | 8.1 ± 3.4 | Nontoxic |
| ALFl | 16.3 ± 4.2 | 21.4 ± 3.2 | 19.6 ± 6.3 | Nontoxic |
| ADLv | 25.3 ± 7.4 | 4.6 ± 1.3 | 5.3 ± 1.8 | 32.6 ± 4.1 |
| ADFl | 18.8 ± 3.2 | 2.2 ± 0.8 | 2.9 ± 0.8 | 24.7 ± 2.8 |
| AFLv | 44.7 ± 6.2 | 19.3 ± 4.2 | 32.4 ± 6.4 | 42.4 ± 3.2 |
| AFFl | 41.5 ± 3.5 | 13.0 ± 2.6 | 26.6 ± 6.5 | 31.4 ± 4.8 |
| ACLv | 48.7 ± 9.5 | 18.4 ± 7.6 | 36.5 ± 4.8 | Nontoxic |
| ACFl | 41.6 ± 11.7 | 22.6 ± 6.8 | 30.4 ± 7.1 | Nontoxic |
| EC50 (μM) | IC50 (μM) | IC50 (μM) | ||
| 1,8-Cineole | N.A. | N.A. | N.A. | Nontoxic |
| (−)-Camphor | N.A. | N.A. | N.A. | Nontoxic |
| (+)-Camphor | N.A. | N.A. | N.A. | Nontoxic |
| (±)-Bornyl acetate | 50.1 ± 11.5 | 42.6 ± 9.7 | 19.1 ± 0.1 | Nontoxic |
| Farnesene | 28.5 ± 2.6 | 1.1 ± 0.2 | 1.4 ± 0.5 | Nontoxic |
| Piperitone | N.A. | N.A. | N.A. | Nontoxic |
| Xanthoxylin | 53.3 ± 5.0 | 27.2 ± 6.6 | 52.7 ± 11.2 | Nontoxic |
| Property | (E,E)-α-Farnesene | (Z,E)-α-Farnesene | Bornyl Acetate |
|---|---|---|---|
| Formula | C15H24 | C15H24 | C12H20O2 |
| M.W. | 204.35 | 204.35 | 196.29 |
| Heavy Atoms | 15 | 15 | 14 |
| Fraction Csp3 | 0.47 | 0.47 | 0.92 |
| Rotatable Bonds | 6 | 6 | 2 |
| H-Bond Acceptors | 0 | 0 | 2 |
| H-Bond Donors | 0 | 0 | 0 |
| MR | 72.32 | 72.32 | 56.33 |
| tPSA | 0.00 | 0.00 | 26.30 |
| LogP | 5.70 | 5.70 | 3.50 |
| BBB Permeation | Yes | Yes | Yes |
| Rank | PDB ID | Target Name | Fit Score | Rank | PDB ID | Target Name | Fit Score |
|---|---|---|---|---|---|---|---|
| (−)-Bornyl Acetate | (+)-Bornyl Acetate | ||||||
| 1 | 1REU | BMP2 | 1 | 1 | 1J96 | AKR1C2 | 1 |
| 2 | 1J96 | AKR1C2 | 1 | 2 | 1REU | BMP2 | 1 |
| 3 | 1MX1 | LCE1 | 0.996 | 3 | 1OKL | CA2 | 0.9992 |
| 4 | 2AO6 | NR3C4 | 0.9925 | 4 | 2PIQ | NR3C4 | 0.9962 |
| 5 | 2PE0 | PDPK1 | 0.9923 | 5 | 2G01 | JNK1 | 0.9857 |
| 6 | 2G01 | JNK1 | 0.9848 | 6 | 1W8L | PPIase A | 0.9826 |
| 7 | 1IF4 | CA2 | 0.9815 | 7 | 2UZD | Cyclin-A2 | 0.9821 |
| 8 | 1W8L | PPIase A | 0.9811 | 8 | 1MX1 | LCE1 | 0.9784 |
| 9 | 1VJY | TGFBR1 | 0.979 | 9 | 1A28 | PgR | 0.9771 |
| 10 | 1A28 | PgR | 0.9636 | 10 | 2PE0 | PDPK1 | 0.9711 |
| (E,E)-α-Farnesene | (Z,E)-α-Farnesene | ||||||
| 1 | 1J96 | AKR1C2 | 1 | 1 | 1PME | JNK1 | 1 |
| 2 | 3HVC | p38α | 1 | 2 | 3HVC | p38α | 1 |
| 3 | 1E7A | Serum albumin | 1 | 3 | 3BGP | Pim-1 | 0.9998 |
| 4 | 1OJ9 | MAO-B | 1 | 4 | 2PG2 | KIF11 | 0.9993 |
| 5 | 1SHJ | Caspase-7 | 1 | 5 | 1E7A | Serum albumin | 0.999 |
| 6 | 1PME | ERK2 | 1 | 6 | 1OJ9 | MAO-B | 0.9989 |
| 7 | 1P49 | Steryl-sulfatase | 0.9989 | 7 | 1J96 | AKR1C2 | 0.9988 |
| 8 | 2PIN | NR1A2 | 0.9985 | 8 | 1L6L | Apo A-II | 0.9984 |
| 9 | 3BGP | Pim-1 | 0.9982 | 9 | 2PIN | NR1A2 | 0.9978 |
| 10 | 1L6L | Apo A-II | 0.9977 | 10 | 1P49 | Steryl-sulfatase | 0.9975 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schepetkin, I.A.; Özek, G.; Özek, T.; Kirpotina, L.N.; Khlebnikov, A.I.; Klein, R.A.; Quinn, M.T. Neutrophil Immunomodulatory Activity of Farnesene, a Component of Artemisia dracunculus Essential Oils. Pharmaceuticals 2022, 15, 642. https://doi.org/10.3390/ph15050642
Schepetkin IA, Özek G, Özek T, Kirpotina LN, Khlebnikov AI, Klein RA, Quinn MT. Neutrophil Immunomodulatory Activity of Farnesene, a Component of Artemisia dracunculus Essential Oils. Pharmaceuticals. 2022; 15(5):642. https://doi.org/10.3390/ph15050642
Chicago/Turabian StyleSchepetkin, Igor A., Gulmira Özek, Temel Özek, Liliya N. Kirpotina, Andrei I. Khlebnikov, Robyn A. Klein, and Mark T. Quinn. 2022. "Neutrophil Immunomodulatory Activity of Farnesene, a Component of Artemisia dracunculus Essential Oils" Pharmaceuticals 15, no. 5: 642. https://doi.org/10.3390/ph15050642
APA StyleSchepetkin, I. A., Özek, G., Özek, T., Kirpotina, L. N., Khlebnikov, A. I., Klein, R. A., & Quinn, M. T. (2022). Neutrophil Immunomodulatory Activity of Farnesene, a Component of Artemisia dracunculus Essential Oils. Pharmaceuticals, 15(5), 642. https://doi.org/10.3390/ph15050642