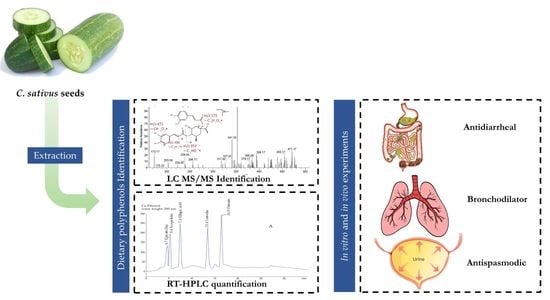
Possible Mechanisms Underlying the Antispasmodic, Bronchodilator, and Antidiarrheal Activities of Polarity–Based Extracts of Cucumis sativus L. Seeds in In Silico, In Vitro, and In Vivo Studies
Abstract
1. Introduction
2. Results
2.1. Identification of Bioactive Compounds by LC–ESI–MS/MS Analysis
2.2. Method Validation and Optimization of HPLC Conditions
2.3. Quantification Analysis of Bioactive Compounds by HPLC
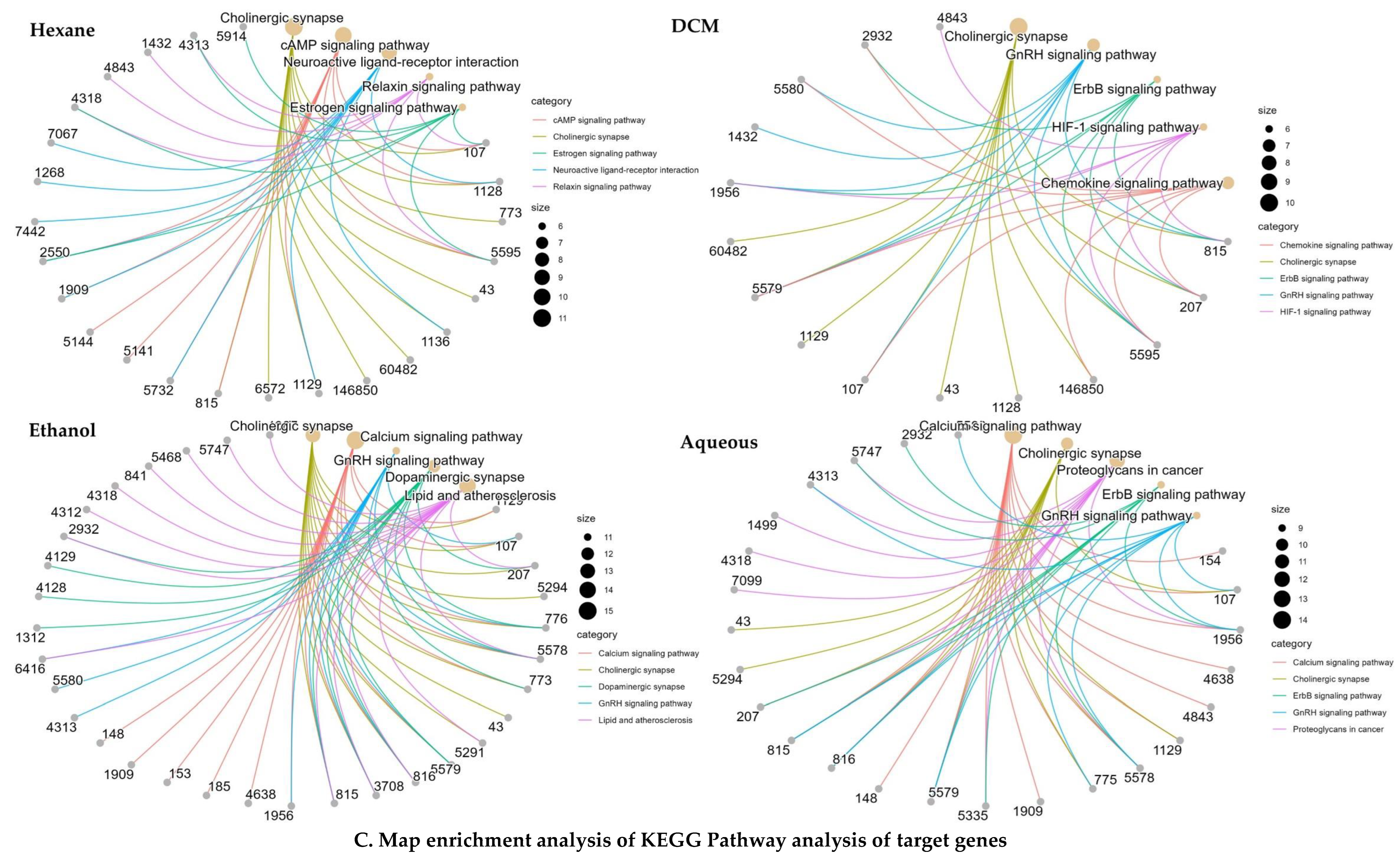
2.4. Network Pharmacology Analysis
2.4.1. Potential Protein Targets Screening
2.4.2. KEGG and GO Analysis
2.4.3. Network Construction
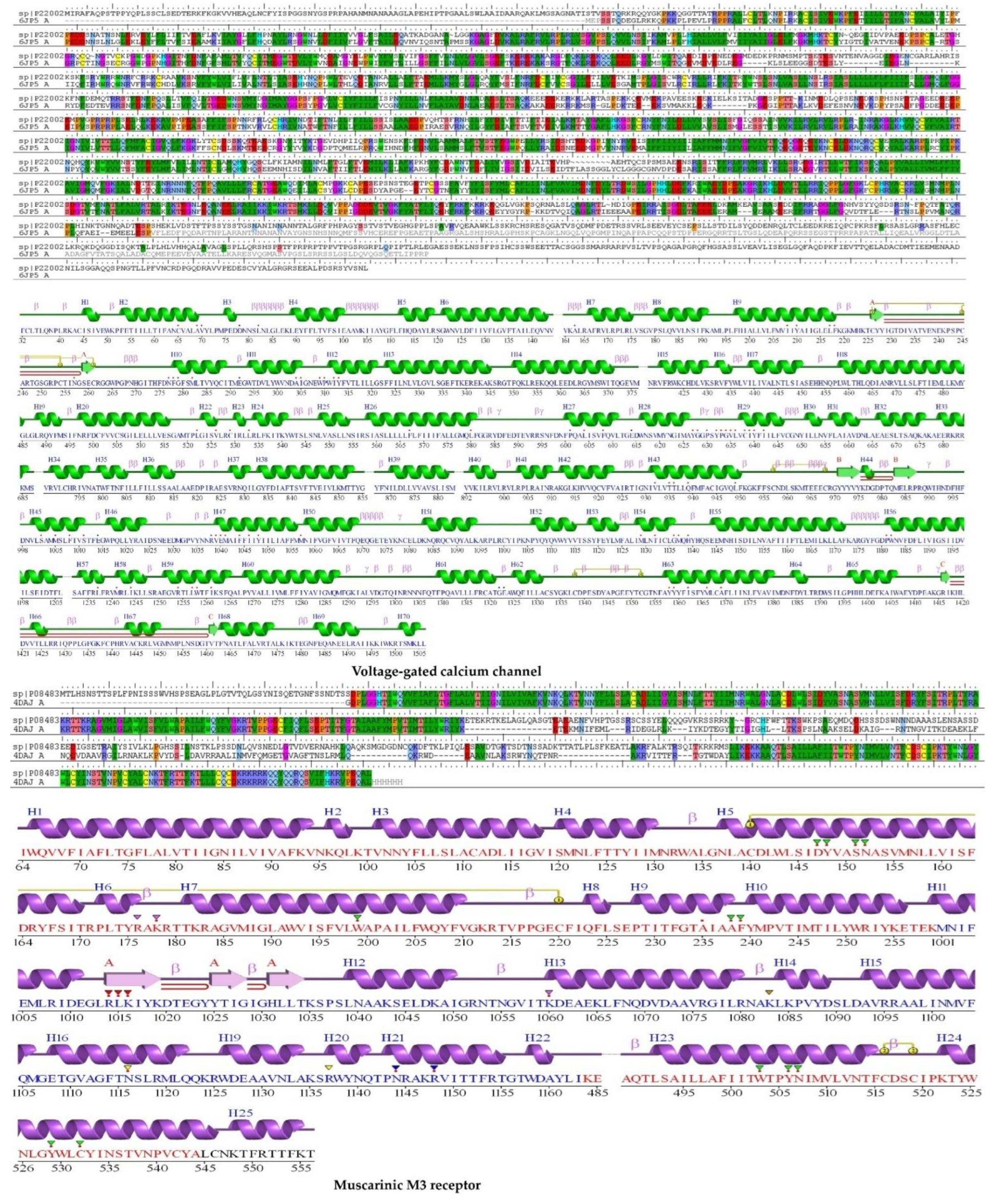
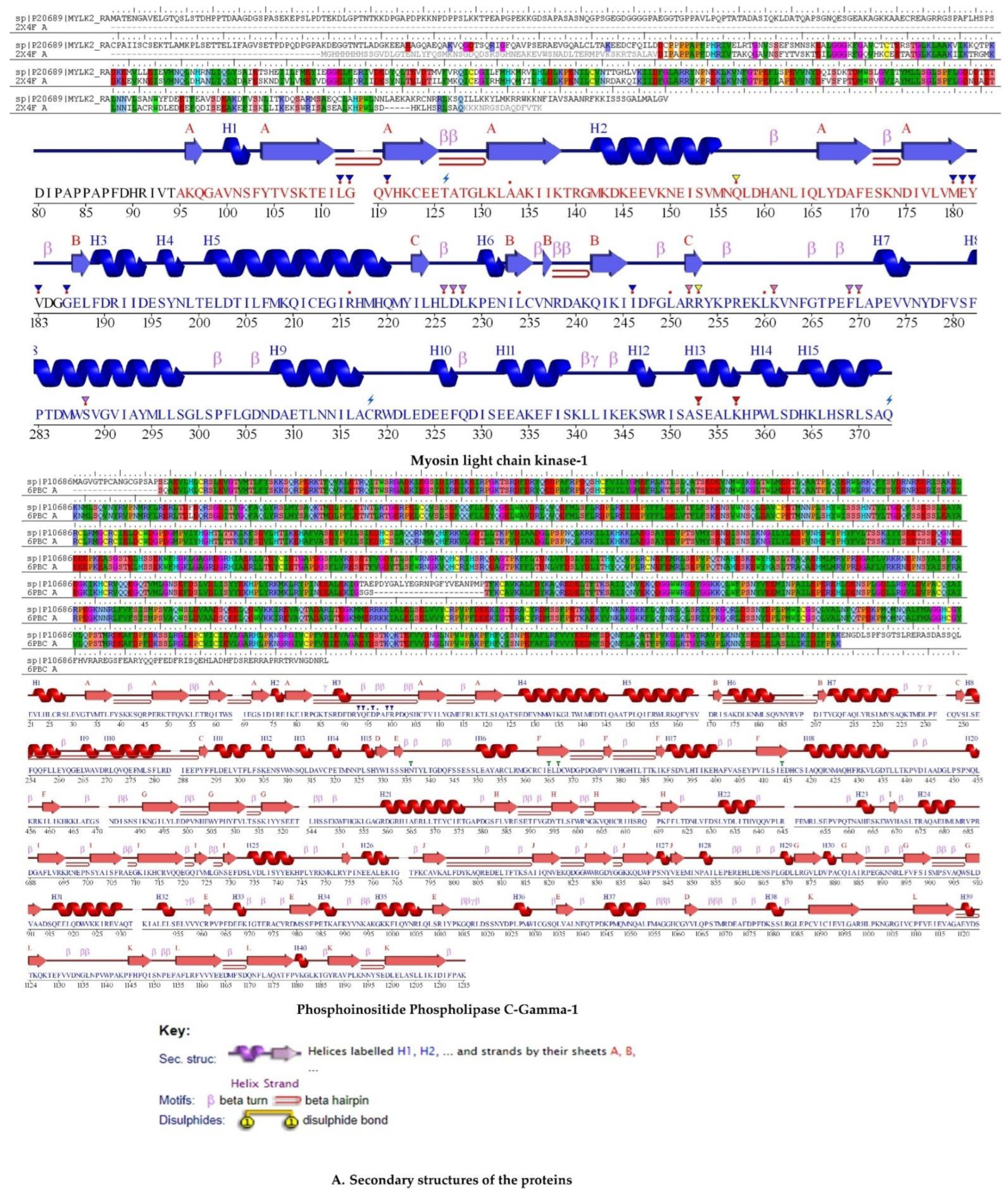
2.5. Protein Homology Modeling
2.5.1. Physicochemical Characteristics
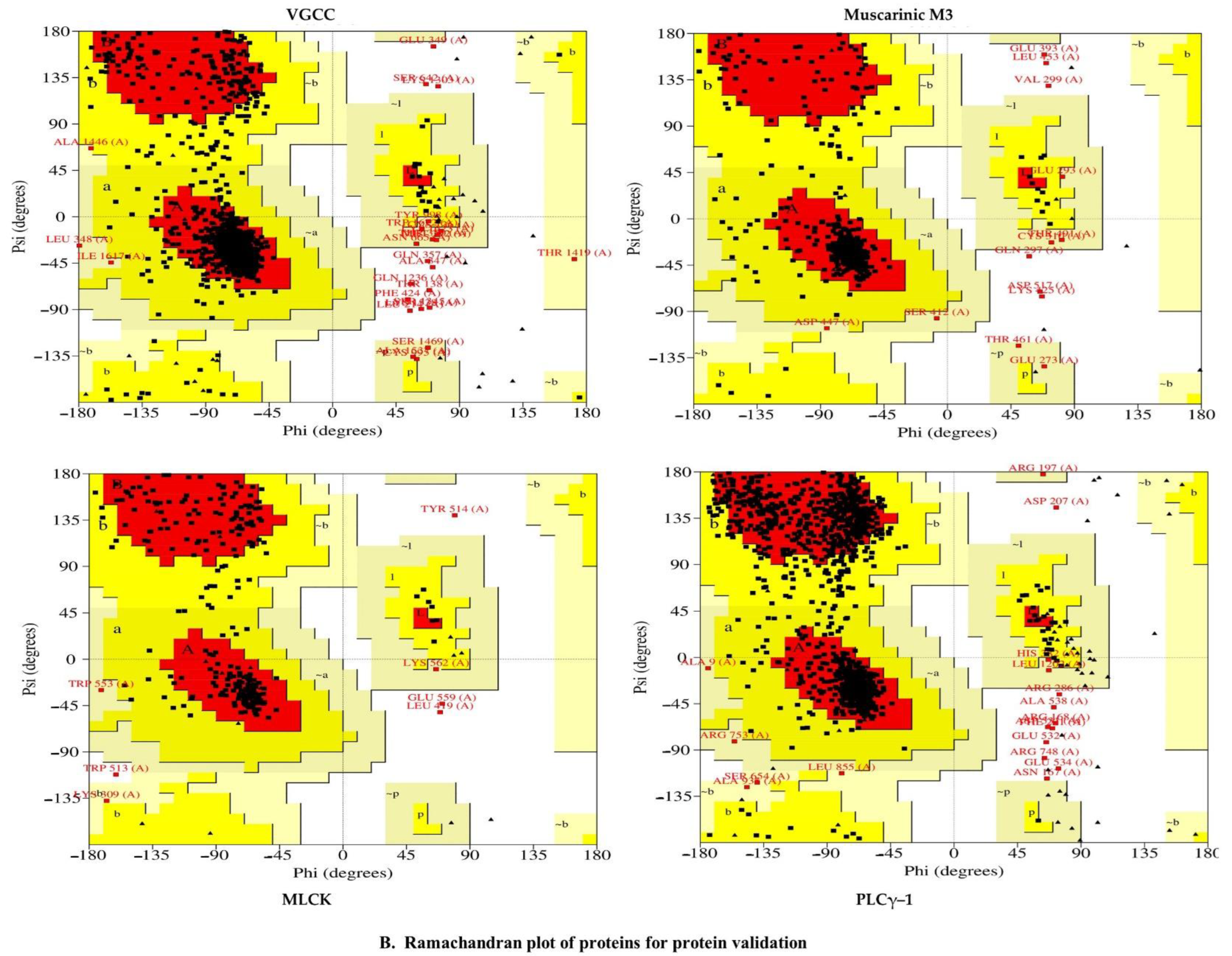
2.5.2. Validation of Homology Modeling
2.6. Molecular Docking
2.6.1. Voltage-Gated Calcium Channel
2.6.2. Muscarinic 3 (M3) Receptor
2.6.3. Myosin Light Chain Kinase
2.6.4. Phosphoinositide Phospholipase C–Gamma–1
2.7. In Vitro Experiments
2.7.1. Effects of C. sativus Seed Extracts on Isolated Rabbit Jejunum Preparation
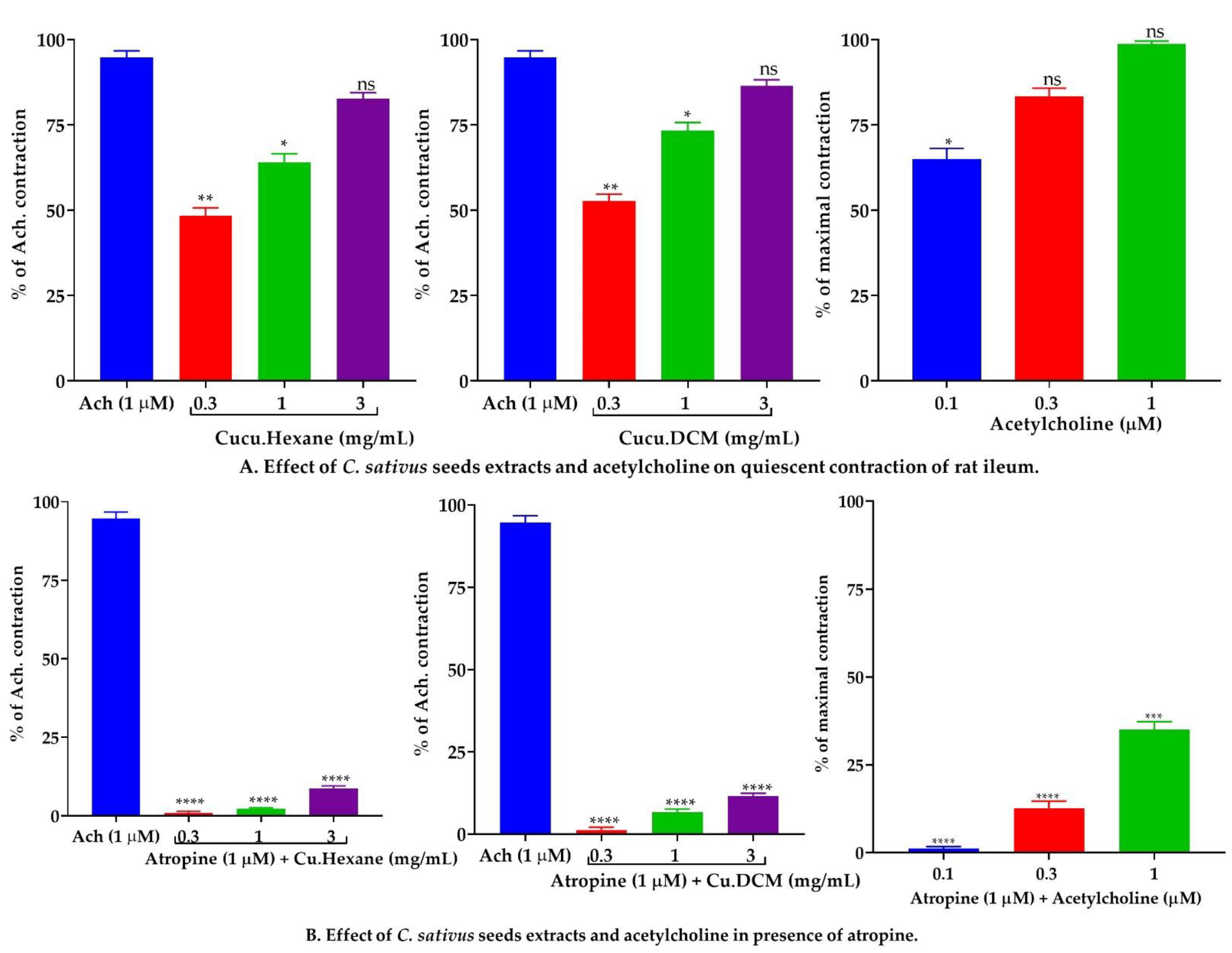
2.7.2. Effect of C. sativus Seed Extracts on Isolated Rat Ileum Preparations
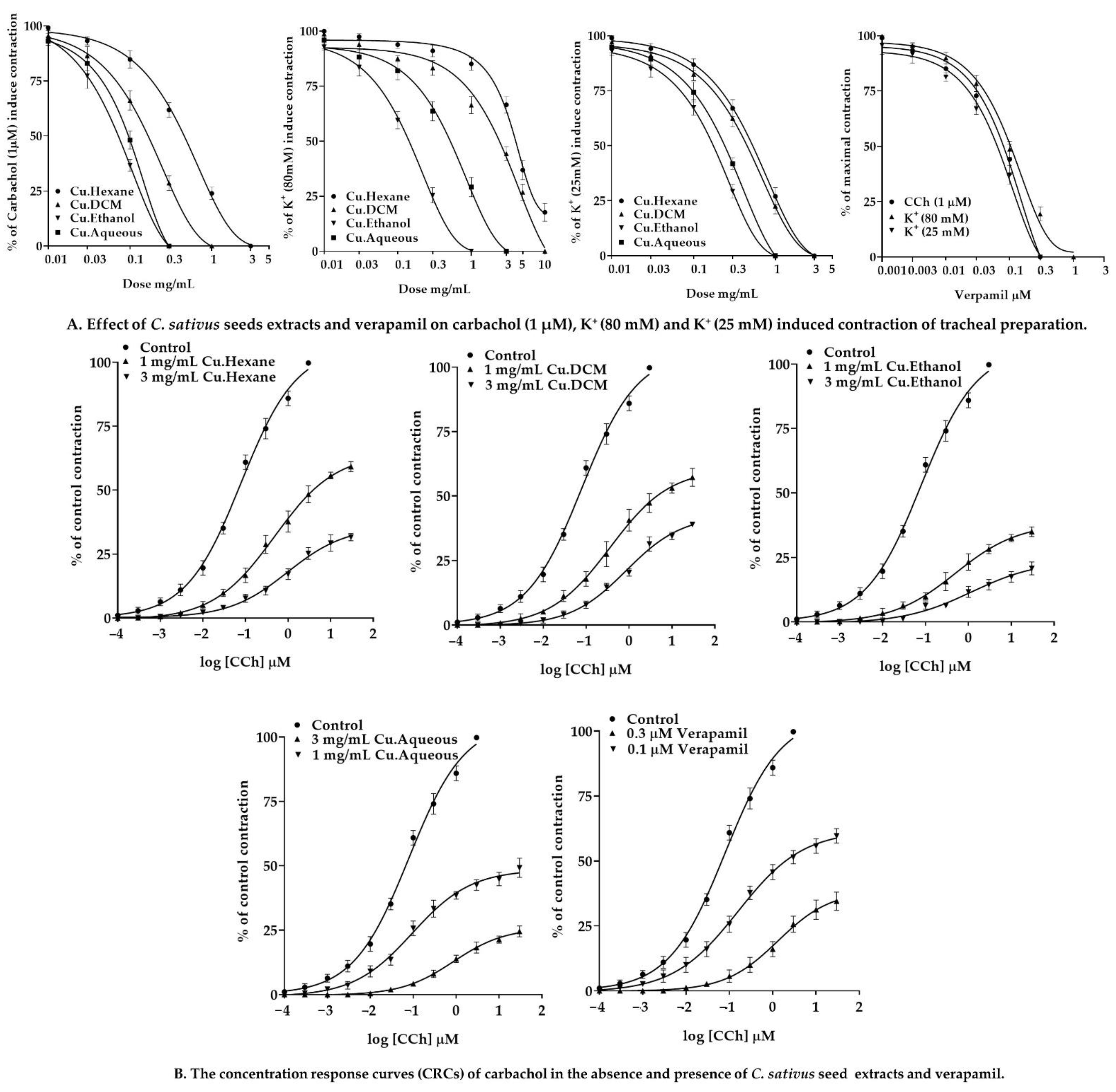
2.7.3. Effect of C. sativus Seed Extracts on Isolated Rabbit Tracheal Preparations
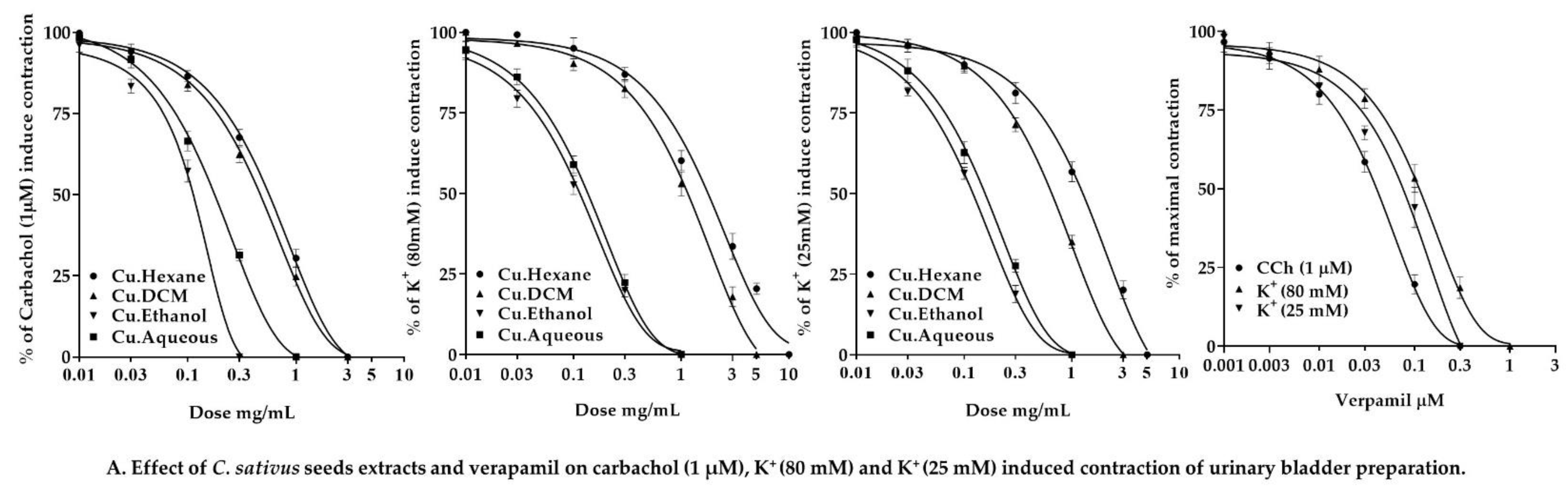
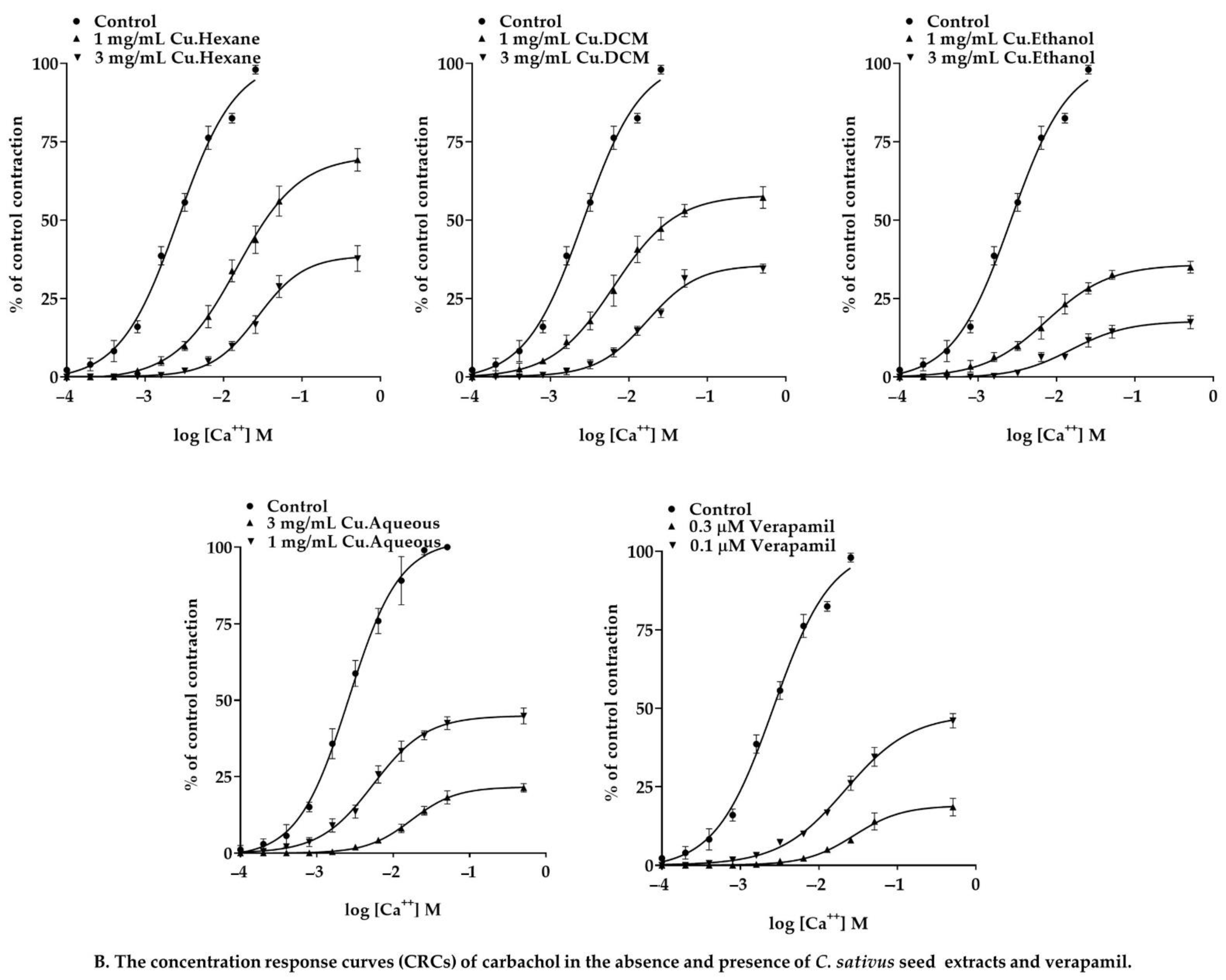
2.7.4. Effect of C. sativus Seed Extracts on Isolated Rabbit Urinary Bladder Preparations
2.8. In Vivo Experiments
2.8.1. Maximum Tolerated Doses for C. sativus Seed Extracts
2.8.2. Effect of C. sativus Seed Extracts on GI Charcoal Meal Intestinal Transit
2.8.3. Effect of C. sativus Seed Extracts on Castor Oil-Induced Diarrhea
2.8.4. Effect of C. sativus Seed Extracts on Intestinal Fluid Accumulation
3. Discussion
4. Materials and Methods
4.1. Preparation of Extracts
4.2. Chemicals
4.3. Sample Preparation for HPLC and LC–ESI–MS/MS
4.4. LC ESI–MS/MS Analysis
4.5. Quantification of Bioactive Compounds by Using Analytical RP-HPLC
4.5.1. RP-HPLC Method Optimization and Validation
4.5.2. Validation of an Analytical Method
4.6. Animals and Housing Conditions
4.7. Network Pharmacology Analysis
4.8. Protein Homology Modeling
4.9. Molecular Docking
4.10. Isolated Tissue Experimentation
4.10.1. Isolated Rabbit Jejunum Preparations
4.10.2. Isolated Rat Ileum Preparation
4.10.3. Isolated Rabbit Tracheal Preparations
4.10.4. Isolated Urinary Bladder Preparations
4.11. In Vivo Experimentation
4.11.1. Evaluation of Maximum Tolerated Dose
4.11.2. Charcoal Meal GI Transit Test
4.11.3. Castor Oil-Induced Diarrhea
4.11.4. Castor Oil-Induced Intestinal Fluid Accumulation
4.12. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CCB | Calcium channel blocker |
| CCh | Carbachol |
| Cu.Aqueous | Aqueous extract of C. sativus seed |
| Cu. DCM | Dichloromethane extract of C. sativus seed |
| Cu.Et | Ethanol extract of C. sativus seed |
| Cu | C. sativus seed |
| Cu.Hexane | n-hexane extract of C. sativus seed |
| CRC | Concentration–response curves |
| FA | Formic acid |
| GO | Gene Ontology |
| KEGG | Kyoto Encyclopedia of Genes and Genomes |
| pKi | Predicted Logarithmic of Inhibition Constant (Ki) |
| MM–GBSA | Molecular mechanics energies combined generalized Born and surface area |
| MLCK–1 | Myosin light chain kinase–1 |
| PLCγ1 | Phosphoinositide Phospholipase C–Gamma–1 |
| TFA | Trifluoroacetic acid |
| VGCC | Voltage-gated calcium channel β2a |
References
- Mariod, A.A.; Saeed Mirghani, M.E.; Hussein, I. Cucumis Sativus Cucumber. In Unconventional Oilseeds and Oil Sources; Elsevier: Alpharetta, GA, USA, 2017; pp. 89–94. ISBN 978-0-12-809435-8. [Google Scholar]
- Renner, S.; Pandey, A. The Cucurbitaceae of India: Accepted Names, Synonyms, Geographic Distribution, and Information on Images and DNA Sequences. PhytoKeys 2013, 20, 53–118. [Google Scholar] [CrossRef] [PubMed]
- Hameed, S.; Hans, S.; Nandan, S.; Fatima, Z. Mechanistic Insights into the Antimycobacterial Action of Unani Formulation, Qurs Sartan Kafoori. J. Tradit. Complement. Med. 2021, 12, 162–171. [Google Scholar] [CrossRef] [PubMed]
- Mosleh, G.; Badr, P.; Zaeri, M.; Mohagheghzadeh, A. Potentials of Antitussive Traditional Persian Functional Foods for COVID-19 Therapy. Front. Pharmacol. 2021, 12, 624006. [Google Scholar] [CrossRef] [PubMed]
- Afzal, M.; Khan, N.A.; Ghufran, A.; Iqbal, A.; Inamuddin, M. Diuretic and Nephroprotective Effect of Jawarish Zarooni Sada—A Polyherbal Unani Formulation. J. Ethnopharmacol. 2004, 91, 219–223. [Google Scholar] [CrossRef]
- Khare, C.P. (Ed.) Indian Medicinal Plants; Springer: New York, NY, USA, 2007; ISBN 978-0-387-70637-5. [Google Scholar]
- Saha, T.; Aoun, J.; Sarkar, P.; Bourdelais, A.J.; Baden, D.G.; Leblanc, N.; Hamlyn, J.M.; Woodward, O.M.; Hoque, K.M. Cucumis sativus Extract Elicits Chloride Secretion by Stimulation of the Intestinal TMEM16A Ion Channel. Pharm. Biol. 2021, 59, 1008–1015. [Google Scholar] [CrossRef]
- Abbasi, A.M.; Khan, M.A.; Ahmad, M.; Zafar, M.; Jahan, S.; Sultana, S. Ethnopharmacological Application of Medicinal Plants to Cure Skin Diseases and in Folk Cosmetics among the Tribal Communities of North-West Frontier Province, Pakistan. J. Ethnopharmacol. 2010, 128, 322–335. [Google Scholar] [CrossRef]
- Sharma, S.; Dwivedi, J.; Paliwal, S. Evaluation of Antacid and Carminative Properties of Cucumis sativus under Simulated Conditions. Sch. Res. Libr. Der Pharm. Lett. 2012, 4, 234–239. [Google Scholar]
- Sharma, S.; Yadav, S.; Singh, G.; Paliwal, S.; Dwivedi, J. First Report on Laxative Activity of Cucumis sativus. Int. J. Pharm. Sci. Rev. Res. 2012, 12, 129–131. [Google Scholar]
- Rajasree, R.S.; Sibi, P.I.; Francis, F.; William, H. Phytochemicals of Cucurbitaceae Family—A Review. Int. J. Pharmacogn. Phytochem. Res. 2016, 8, 113–123. [Google Scholar]
- Tuama, A.A.; Mohammed, A.A. Phytochemical Screening and in Vitro Antibacterial and Anticancer Activities of the Aqueous Extract of Cucumis sativus. Saudi J. Biol. Sci. 2019, 26, 600–604. [Google Scholar] [CrossRef]
- Wahid, M.; Saqib, F.; Chicea, L.; Ahmedah, H.T.; Sajer, B.H.; Marc (Vlaic), R.A.; Pop, O.L.; Moga, M.; Gavris, C. Metabolomics Analysis Delineates the Therapeutic Effects of Hydroethanolic Extract of Cucumis Sativus L. Seeds on Hypertension and Isoproterenol-Induced Myocardial Infarction. Biomed. Pharmacother. 2022, 148, 112704. [Google Scholar] [CrossRef] [PubMed]
- Agatemor, U.; Nwodo, O.; Anosike, C. Anti-Inflammatory Activity of Cucumis sativus L. Br. J. Pharm. Res. 2015, 8, 1–8. [Google Scholar] [CrossRef]
- Minaiyan, M.; Zolfaghari, B.; Kamal, A. Effect of Hydroalcoholic and Buthanolic Extract of Cucumis sativus Seeds on Blood Glucose Level of Normal and Streptozotocin-Induced Diabetic Rats. Iran. J. Basic Med. Sci. 2011, 14, 436–442. [Google Scholar] [CrossRef] [PubMed]
- Soltani, R.; Hashemi, M.; Farazmand, A.; Asghari, G.; Heshmat-Ghahdarijani, K.; Kharazmkia, A.; Ghanadian, S.M. Evaluation of the Effects of Cucumis sativus Seed Extract on Serum Lipids in Adult Hyperlipidemic Patients: A Randomized Double-Blind Placebo-Controlled Clinical Trial. J. Food Sci. 2017, 82, 214–218. [Google Scholar] [CrossRef]
- Vetriselvan, S.; Subasini, U.; Velmurugan, C.; Muthuramu, T.; Revathy, J. Anti-Inflammatory Activity of Cucumis sativus Seed in Carrageenan and Xylene Induced Edema Model Using Albino Wistar Rats. Int. J. Biopharm. 2013, 4, 34–37. [Google Scholar]
- Wahid, M.; Saqib, F.; Ahmedah, H.T.; Gavris, C.M.; De Feo, V.; Hogea, M.; Moga, M.; Chicea, R. Cucumis sativus L. Seeds Ameliorate Muscular Spasm-Induced Gastrointestinal and Respiratory Disorders by Simultaneously Inhibiting Calcium Mediated Signaling Pathway. Pharmaceuticals 2021, 14, 1197. [Google Scholar] [CrossRef]
- Gill, N.S.; Garg, M.; Bansal, R.; Sood, S.; Muthuraman, A.; Bali, M.; Sharma, P.D. Evaluation of Antioxidant and Antiulcer Potential of Cucumis sativum L. Seed Extract in Rats. Asian J. Clin. Nutr. 2009, 1, 131–138. [Google Scholar] [CrossRef]
- Khan, M.S.; Tariq, S.S.H. Medicinal Treatment of Multiple Renal Calculi (Hisat-E-Kulyah) and Bilateral Ureteric Calculi (Hisat-E-Halib) by Unani Pharmacopoeial Formulations—A Case Study. World J. Adv. Res. Rev. 2021, 11, 183–189. [Google Scholar] [CrossRef]
- Anwar, S.; Khan, N.A.; Amin, K.M.Y.; Ahmad, G. Effects of Banadiq-Al Buzoor in Some Renal Disorders. Hamdard Med. 1999, 42, 31–36. [Google Scholar]
- Alam, S.; Khan, N.A.; Nasiruddin, M. The Effect of Qurs-e-Zarishk Sagheer (A Compound Unani Formulation) on Liver Enzymes in CCl4 Induced Hepatotoxicity in Rats. Hippocrat. J. Unani Med. 2014, 9, 1–12. [Google Scholar]
- Siddika, M.; Hasnat, R.; Bahar, E. Thrombolytic (in Vitro) and Analgesic (in Vivo) Effect of Methanolic Extract of Cucumis sativus. Pharma Innov. 2016, 3, 1–7. [Google Scholar]
- Rehman, S.; Imran, M. An Ethno-Botanical Review of Seeds of Cucumis Sativa (Maghz-e-Tukhme Khiyarain) from Unani Medicine and Its Pharmacological Updates. Int. J. Adv. Pharm. Med. Bioallied Sci. 2021, 9, 31–36. [Google Scholar]
- Mukherjee, P.K.; Nema, N.K.; Maity, N.; Sarkar, B.K. Phytochemical and Therapeutic Potential of Cucumber. Fitoterapia 2013, 84, 227–236. [Google Scholar] [CrossRef] [PubMed]
- Garg, V.K.; Nes, W.R. Occurrence of Δ5-Sterols in Plants Producing Predominantly Δ7-Sterols: Studies on the Sterol Compositions of Six Cucurbitaceae Seeds. Phytochemistry 1986, 25, 2591–2597. [Google Scholar] [CrossRef]
- Abu-Reidah, I.M.; Arráez-Román, D.; Quirantes-Piné, R.; Fernández-Arroyo, S.; Segura-Carretero, A.; Fernández-Gutiérrez, A. HPLC-ESI-Q-TOF-MS for a Comprehensive Characterization of Bioactive Phenolic Compounds in Cucumber Whole Fruit Extract. Food Res. Int. 2012, 46, 108–117. [Google Scholar] [CrossRef]
- Karrar, E.; Sheth, S.; Navicha, W.B.; Wei, W.; Hassanin, H.; Abdalla, M.; Wang, X. A Potential New Source: Nutritional and Antioxidant Properties of Edible Oils from Cucurbit Seeds and Their Impact on Human Health. J. Food Biochem. 2019, 43, e12733. [Google Scholar] [CrossRef]
- Kai, H.; Baba, M.; Okuyama, T. Inhibitory Effect of Cucumis sativus on Melanin Production in Melanoma B16 Cells by Downregulation of Tyrosinase Expression. Planta Med. 2008, 74, 1785–1788. [Google Scholar] [CrossRef]
- Antonia Murcia, M.; Jiménez, A.M.; Martínez-Tomé, M. Vegetables Antioxidant Losses during Industrial Processing and Refrigerated Storage. Food Res. Int. 2009, 42, 1046–1052. [Google Scholar] [CrossRef]
- McNally, D.J.; Wurms, K.V.; Labbé, C.; Quideau, S.; Bélanger, R.R. Complex C-Glycosyl Flavonoid Phytoalexins from Cucumis sativus. J. Nat. Prod. 2003, 66, 1280–1283. [Google Scholar] [CrossRef]
- Saqib, F.; Janbaz, K.H. Ethnopharmacological Basis for Folkloric Claims of Anagallis arvensis Linn. (Scarlet pimpernel) as Prokinetic, Spasmolytic and Hypotensive in Province of Punjab, Pakistan. J. Ethnopharmacol. 2021, 267, 113634. [Google Scholar] [CrossRef]
- Sirous, H.; Chemi, G.; Campiani, G.; Brogi, S. An Integrated in Silico Screening Strategy for Identifying Promising Disruptors of P53-MDM2 Interaction. Comput. Biol. Chem. 2019, 83, 107105. [Google Scholar] [CrossRef] [PubMed]
- Kuhn, B.; Kollman, P.A. Binding of a Diverse Set of Ligands to Avidin and Streptavidin: An Accurate Quantitative Prediction of Their Relative Affinities by a Combination of Molecular Mechanics and Continuum Solvent Models. J. Med. Chem. 2000, 43, 3786–3791. [Google Scholar] [CrossRef] [PubMed]
- Hassan Gilani, A.U.; Aziz, N.; Ahmad, M.; Alam, M.T.; Rizwani, G.H. Spasmogenic and Spasimolytic Constituents in Sida Pakistanica. Pharm. Biol. 1999, 37, 173–180. [Google Scholar] [CrossRef]
- Gilani, A.U.H.; Shah, A.J.; Yaeesh, S. Presence of Cholinergic and Calcium Antagonist Constituents in Saussurea Lappa Explains Its Use in Constipation and Spasm. Phyther. Res. 2007, 21, 541–544. [Google Scholar] [CrossRef]
- Salehi, B.; Capanoglu, E.; Adrar, N.; Catalkaya, G.; Shaheen, S.; Jaffer, M.; Giri, L.; Suyal, R.; Jugran, A.K.; Calina, D.; et al. Cucurbits Plants: A Key Emphasis to Its Pharmacological Potential. Molecules 2019, 24, 1854. [Google Scholar] [CrossRef]
- Patel, S.; Rauf, A. Edible Seeds from Cucurbitaceae Family as Potential Functional Foods: Immense Promises, Few Concerns. Biomed. Pharmacother. 2017, 91, 330–337. [Google Scholar] [CrossRef]
- Dixit, Y.; Kar, A. Protective Role of Three Vegetable Peels in Alloxan Induced Diabetes Mellitus in Male Mice. Plant Foods Hum. Nutr. 2010, 65, 284–289. [Google Scholar] [CrossRef]
- Wahid, M.; Ali, A.; Saqib, F.; Aleem, A.; Bibi, S.; Afzal, K.; Ali, A.; Baig, A.; Khan, S.A.; Bin Asad, M.H.H. Pharmacological Exploration of Traditional Plants for the Treatment of Neurodegenerative Disorders. Phyther. Res. 2020, 34, 3089–3112. [Google Scholar] [CrossRef]
- Mallik, J.; Priyanka, D.; Sourav, D. Pharmacological Activity of Cucumis sativus L.—A Complete Review. Asian J. Pharm. Res. Dev. 2013, 1, 1–6. [Google Scholar]
- Das, J.; Chowdhury, A.; Biswas, S.K.; Karmakar, U.K.; Sharif, S.R.; Raihan, S.Z.; Muhit, M.A. Cytotoxicity and Antifungal Activities of Ethanolic and Chloroform Extracts of Cucumis sativus Linn (Cucurbitaceae) Leaves and Stems. Res. J. Phytochem. 2012, 6, 25–30. [Google Scholar] [CrossRef]
- Gilani, A.H.; Khan, A.U.; Raoof, M.; Ghayur, M.N.; Siddiqui, B.S.; Vohra, W.; Begum, S. Gastrointestinal, Selective Airways and Urinary Bladder Relaxant Effects of Hyoscyamus Niger Are Mediated through Dual Blockade of Muscarinic Receptors and Ca2+ Channels. Fundam. Clin. Pharmacol. 2008, 22, 87–99. [Google Scholar] [CrossRef] [PubMed]
- Gilani, A.H.; Khan, A.U.; Ghayur, M.N.; Ali, S.F.; Herzig, J.W. Antispasmodic Effects of Rooibos Tea (Aspalathus Linearis) Is Mediated Predominantly through K+-Channel Activation. Basic Clin. Pharmacol. Toxicol. 2006, 99, 365–373. [Google Scholar] [CrossRef] [PubMed]
- Wallace, J.L.; Sharkey, K.A. Pharmacotherapy of Gastric Acidity, Peptic Ulcers, and Gastroesophageal Reflux Disease. In Goodman & Gilman’s the Pharmacological Basis of Therapeutics; Brunton, L.L., Hilal-Dandan, R., Knollmann, B.C., Eds.; McGraw-Hill: New York, NY, USA, 2018; pp. 1309–1322. ISBN 978-0-07-176939-6. [Google Scholar]
- Gilani, A.H.; Khan, A.U.; Ghayur, M.N. Ca2+ Antagonist and Cholinergic Activities Explain the Medicinal Use of Olive in Gut Disorders. Nutr. Res. 2006, 26, 277–283. [Google Scholar] [CrossRef]
- Perez-Zoghbi, J.F.; Karner, C.; Ito, S.; Shepherd, M.; Alrashdan, Y.; Sanderson, M.J. Ion Channel Regulation of Intracellular Calcium and Airway Smooth Muscle Function. Pulm. Pharmacol. Ther. 2009, 22, 388–397. [Google Scholar] [CrossRef] [PubMed]
- Gilani, A.H.; Ghayur, M.N.; Saify, Z.S.; Ahmed, S.P.; Choudhary, M.I.; Khalid, A. Presence of Cholinomimetic and Acetylcholinesterase Inhibitory Constituents in Betel Nut. Life Sci. 2004, 75, 2377–2389. [Google Scholar] [CrossRef]
- Wahid, M.; Saqib, F. Scientific Basis for Medicinal Use of Citrullus Lanatus (Thunb.) in Diarrhea and Asthma: In Vitro, in Vivo and in Silico Studies. Phytomedicine 2022, 98, 153978. [Google Scholar] [CrossRef]
- Saqib, F.; Janbaz, K.H. Rationalizing Ethnopharmacological Uses of Alternanthera Sessilis: A Folk Medicinal Plant of Pakistan to Manage Diarrhea, Asthma and Hypertension. J. Ethnopharmacol. 2016, 182, 110–121. [Google Scholar] [CrossRef]
- Hall, J.E.; Hall, M.E. Excitation and Contraction of Smooth Muscle. In Guyton and Hall Textbook of Medical Physiology; Elsevier Health Sciences: Alpharetta, GA, USA, 2020; pp. 101–109. ISBN 9780323597128. [Google Scholar]
- Gilani, A.H.; Jabeen, Q.; Ghayur, M.N.; Janbaz, K.H.; Akhtar, M.S. Studies on the Antihypertensive, Antispasmodic, Bronchodilator and Hepatoprotective Activities of the Carum Copticum Seed Extract. J. Ethnopharmacol. 2005, 98, 127–135. [Google Scholar] [CrossRef]
- Mehmood, M.H.; Anila, N.; Begum, S.; Syed, S.A.; Siddiqui, B.S.; Gilani, A.H. Pharmacological Basis for the Medicinal Use of Carissa Carandas in Constipation and Diarrhea. J. Ethnopharmacol. 2014, 153, 359–367. [Google Scholar] [CrossRef]
- Janbaz, K.H.; Nisa, M.; Saqib, F.; Imran, I.; Zia-Ul-Haq, M.; De Feo, V. Bronchodilator, Vasodilator and Spasmolytic Activities of Methanolic Extract of Myrtus communis L. J. Physiol. Pharmacol. 2013, 64, 479–484. [Google Scholar]
- Janbaz, K.H.; Zaeem Ahsan, M.; Saqib, F.; Imran, I.; Zia-Ul-Haq, M.; Abid Rashid, M.; Jaafar, H.Z.E.; Moga, M. Scientific Basis for Use of Pyrus Pashia Buch.-Ham. Ex D. Don. Fruit in Gastrointestinal, Respiratory and Cardiovascular Ailments. PLoS ONE 2015, 10, e0118605. [Google Scholar] [CrossRef] [PubMed]
- Miyazaki, E.; Yabu, H.; Sunano, S. Excitation and Contraction of the Smooth Muscle. Jpn. J. Smooth Muscle Res. 1971, 7, 83–97. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Iwao, I.; Terada, Y. On the Mechanism of Diarrhea Due to Castor Oil. Jpn. J. Pharmacol. 1962, 12, 137–145. [Google Scholar] [CrossRef] [PubMed]
- Atta, A.H.; Mouneir, S.M. Antidiarrhoeal Activity of Some Egyptian Medicinal Plant Extracts. J. Ethnopharmacol. 2004, 92, 303–309. [Google Scholar] [CrossRef]
- Reynolds, I.J.; Gould, R.J.; Snyder, S.H. Loperamide: Blockade of Calcium Channels as a Mechanism for Antidiarrheal Effects. J. Pharmacol. Exp. Ther. 1984, 231, 628–632. [Google Scholar]
- Crowe, A.; Wong, P. Potential Roles of P-Gp and Calcium Channels in Loperamide and Diphenoxylate Transport. Toxicol. Appl. Pharmacol. 2003, 193, 127–137. [Google Scholar] [CrossRef]
- Seraglio, S.K.T.; Valese, A.C.; Daguer, H.; Bergamo, G.; Azevedo, M.S.; Gonzaga, L.V.; Fett, R.; Costa, A.C.O. Development and Validation of a LC-ESI-MS/MS Method for the Determination of Phenolic Compounds in Honeydew Honeys with the Diluted-and-Shoot Approach. Food Res. Int. 2016, 87, 60–67. [Google Scholar] [CrossRef]
- Rowan, A.N. Guide for the Care and Use of Laboratory Animals; National Academies Press: Washington, DC, USA, 1979; Volume 8, ISBN 0309186633. [Google Scholar]
- Chauhan, V.; Singh, M.P. Immuno-Informatics Approach to Design a Multi-Epitope Vaccine to Combat Cytomegalovirus Infection. Eur. J. Pharm. Sci. 2020, 147, 105279. [Google Scholar] [CrossRef]
- Yu, Z.; Kang, L.; Zhao, W.; Wu, S.; Ding, L.; Zheng, F.; Liu, J.; Li, J. Identification of Novel Umami Peptides from Myosin via Homology Modeling and Molecular Docking. Food Chem. 2021, 344, 128728. [Google Scholar] [CrossRef]
- Subhani, S.; Jayaraman, A.; Jamil, K. Homology Modelling and Molecular Docking of MDR1 with Chemotherapeutic Agents in Non-Small Cell Lung Cancer. Biomed. Pharmacother. 2015, 71, 37–45. [Google Scholar] [CrossRef]
- Elasoru, S.E.; Rhana, P.; de Oliveira Barreto, T.; Naves de Souza, D.L.; Menezes-Filho, J.E.R.; Souza, D.S.; Loes Moreira, M.V.; Gomes Campos, M.T.; Adedosu, O.T.; Roman-Campos, D.; et al. Andrographolide Protects against Isoproterenol-Induced Myocardial Infarction in Rats through Inhibition of L-Type Ca2+ and Increase of Cardiac Transient Outward K+ Currents. Eur. J. Pharmacol. 2021, 906, 174194. [Google Scholar] [CrossRef] [PubMed]









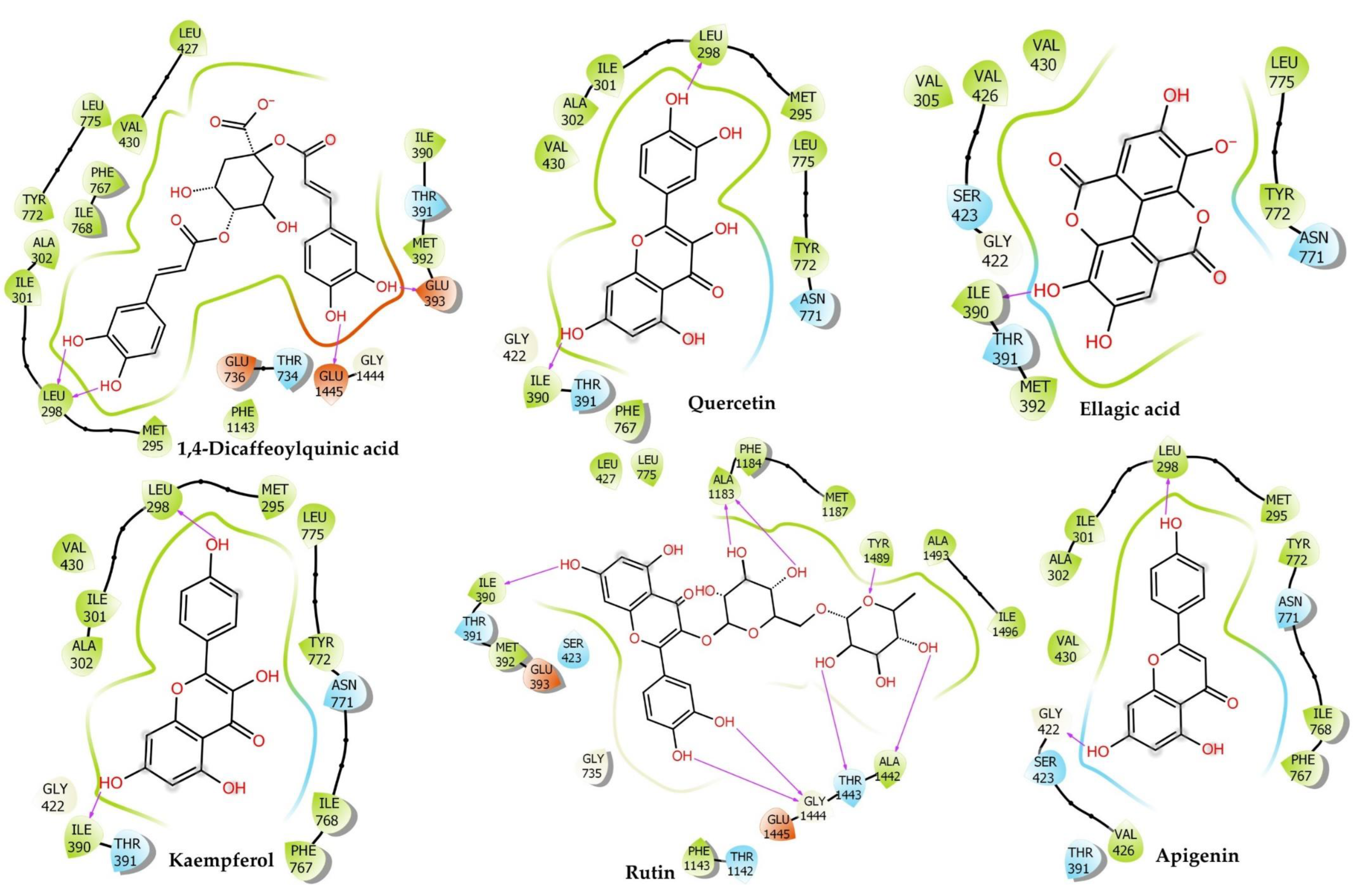

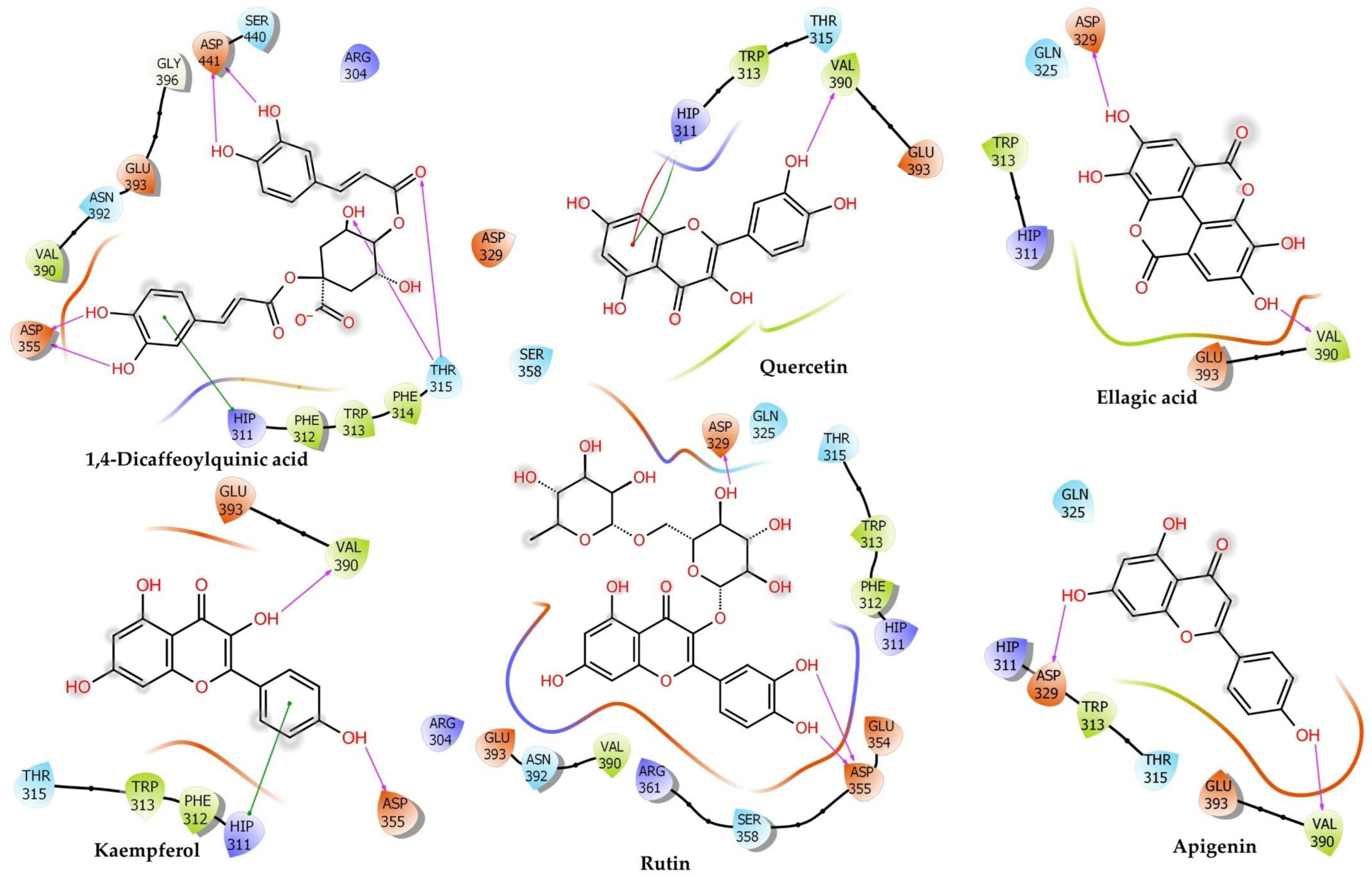

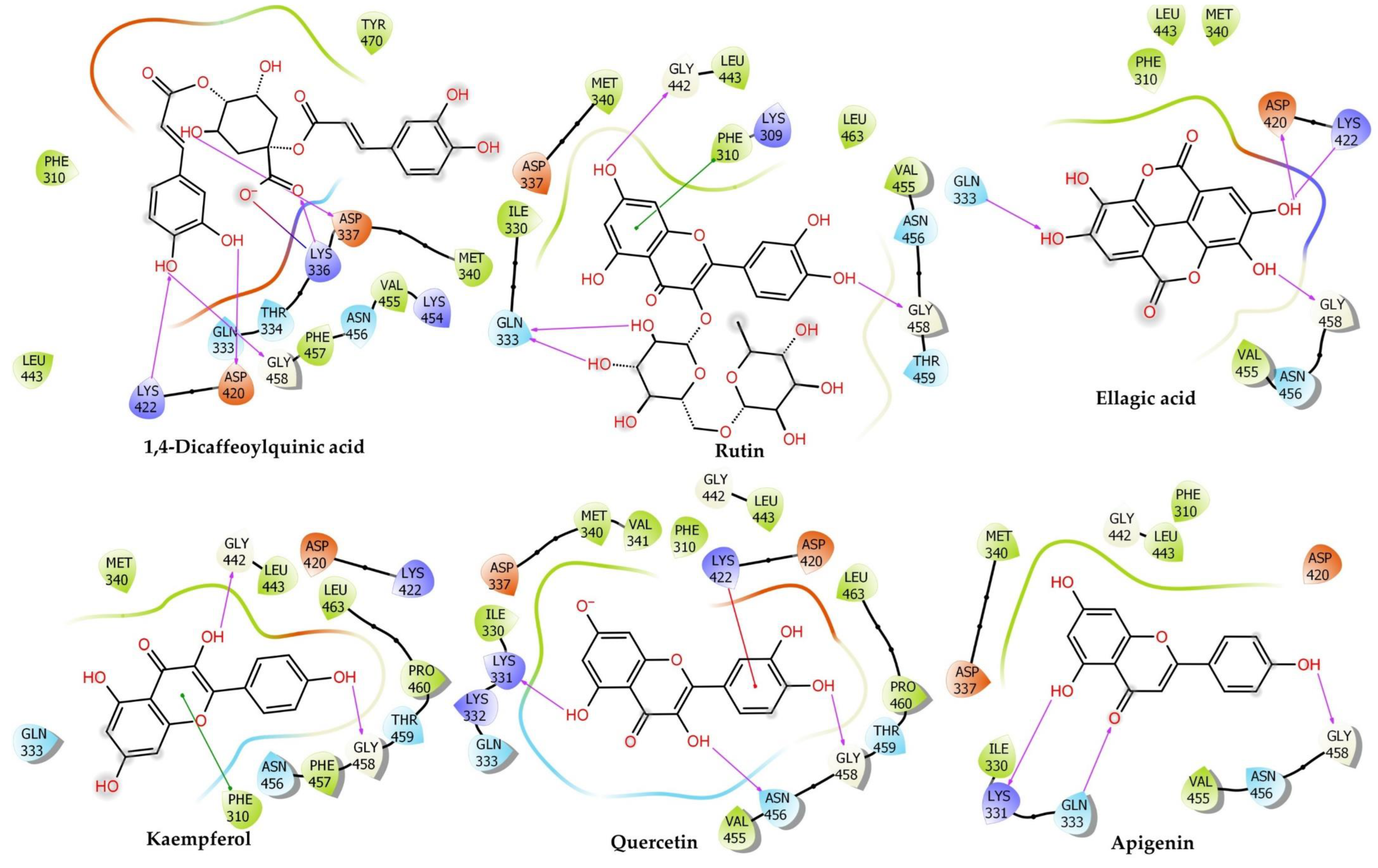
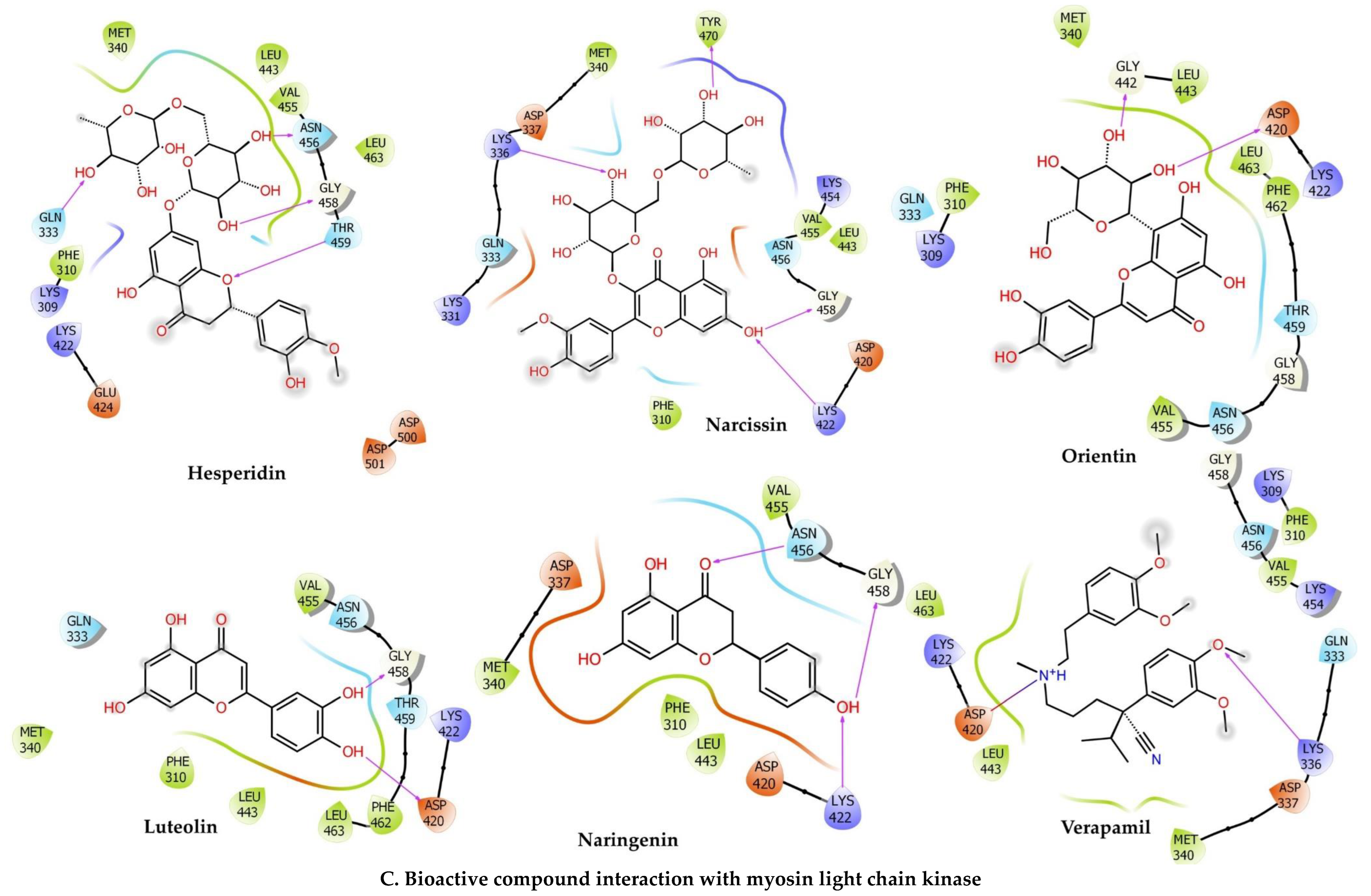
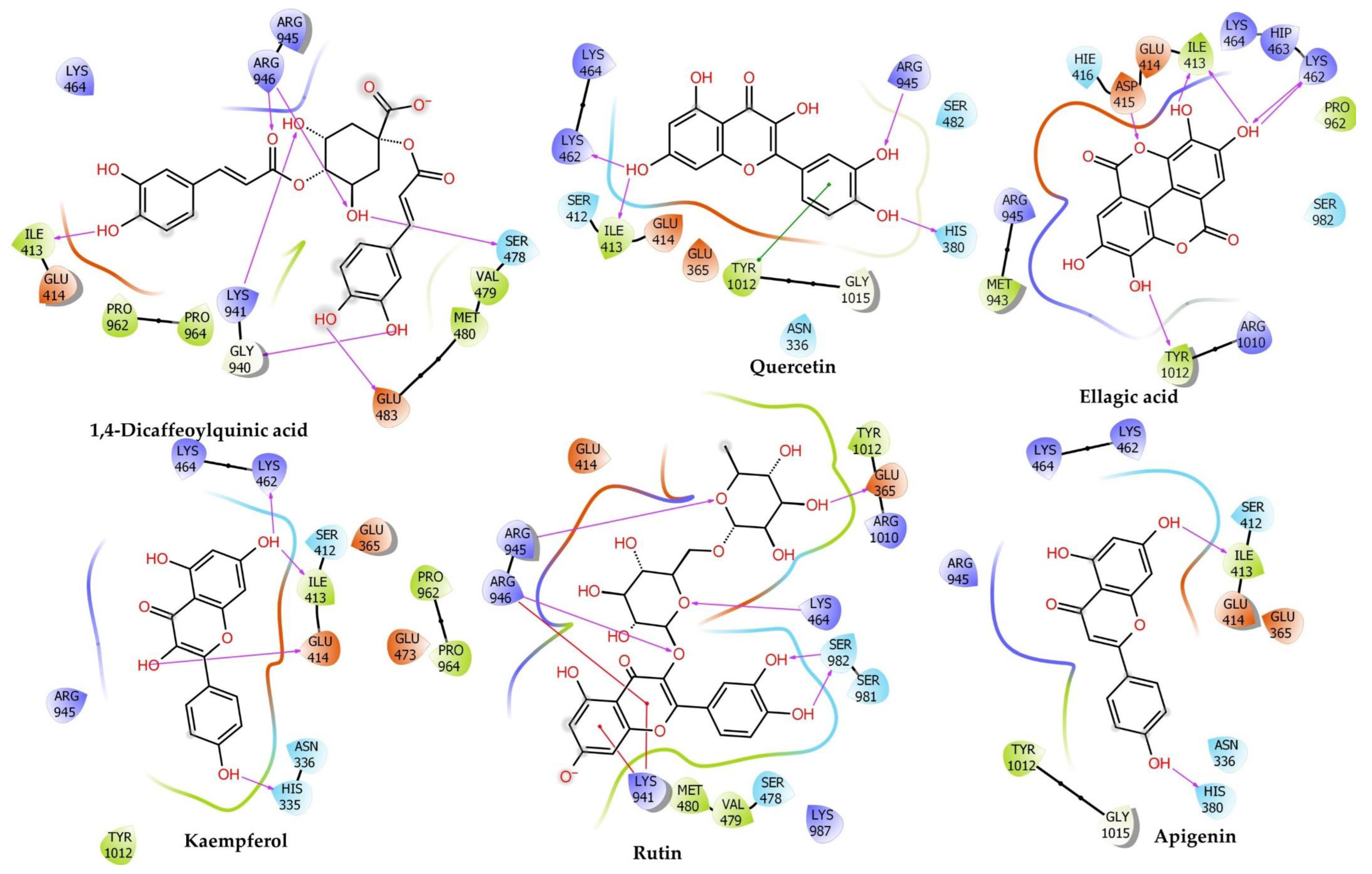
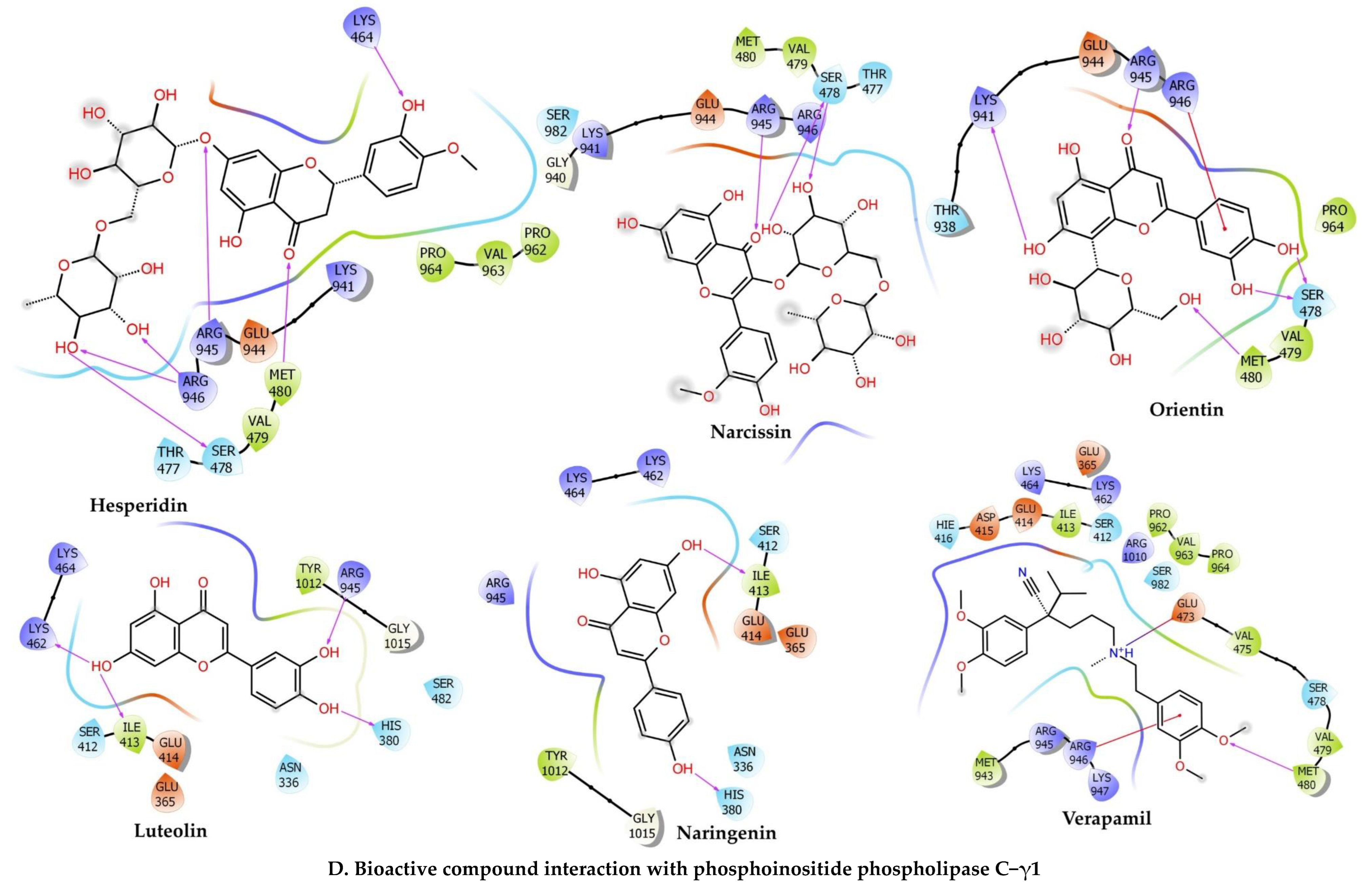


| Compounds | Docking Score | Glide Energy | ∆G Binding | pKi (µM) | ∆G Coulomb | ∆G Covalent | ∆G Hbond | ∆G Lipo | ∆G Packing | ∆G Solv GB | ∆G vdW | Residue–Ligand Interactions with Distance (Å) | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Hydrogen Bonds | Electrostatic/Hydrophobic Bonds | ||||||||||||
| Muscarinic Acetylcholine Receptor | |||||||||||||
| Orintnin | −6.88 ± 0.03 | −49.81 | −39.22 ± 0.18 | −13.80 | −23.26 | 2.62 | −2.93 | −7.46 | −2.39 | 28.66 | −34.45 | Conventional H-Bond: Phe312 (1.78), Val390 (2.09), Glu393 (2.95), Asp355 (1.82), Asp355 (1.80), Carbon H-Bond: Phe312 (2.66), Glu393 (2.67), Glu393 (2.28), Glu393 (2.63) | Pi–Cation: His311 (3.89) |
| Rutin | −6.74 ± 0.43 | −56.72 | −36.47 ± 0.08 | −12.61 | −13.87 | 3.49 | −3.33 | −9.58 | −1.07 | 34.7 | −46.81 | Conventional H-Bond: Phe312 (2.94), Asp329 (1.95), Asp355 (2.04), Asp355 (1.96), Carbon H-Bond: Trp313 (2.81), Trp313 (2.74), Trp313 (2.49) | Pi–Anion: Glu393 (3.00), Glu393 (3.61), Pi–Sigma: Glu393 (2.81), Pi–Alkyl: Val390 (5.02) |
| Narcissin | −6.64 ± 0.26 | −52.54 | −20.36 ± 0.32 | −5.61 | −20.5 | 6.8 | −4.03 | −4.71 | −2.55 | 41.91 | −37.28 | Conventional H-Bond: Gln389 (2.52), Glu393 (1.90), Gln389 (2.81), Asp394 (1.91), Asp329 (1.90), Carbon H-Bond: Val390 (2.48), Glu393 (2.37), Gln389 (2.90), Glu393 (2.43), Asp394 (2.97), Glu393 (3.07), Glu393 (2.64), Phe312 (2.51), Glu393 (2.84) | Pi–Cation: His311 (4.67), Pi–Anion: Asp329 (4.78), Pi–Lone Pair: Trp313 (2.94), Pi–Pi T–Shaped: His311 (4.73) |
| Hesperidin | −6.04 ± 0.07 | −53.59 | −49.25 ± 0.16 | −18.16 | −33.84 | 2.88 | −4.5 | −10.82 | −0.86 | 45 | −47.11 | Conventional H-Bond: Asp329 (1.81), Glu393 (1.82), Gln389 (1.85), Glu393 (3.04), Phe312 (1.66), Carbon H-Bond: Arg304 (3.05), Arg304 (2.66), Arg304 (2.74), His311 (2.50), Val390 (2.63), Glu393 (2.70), Asn392 (2.75) | Pi–Anion: Glu393 (3.87), Alkyl: Ala362 (3.86) |
| Quercetin | −5.89 ± 0.11 | −37.98 | −36.36 ± 0.11 | −12.56 | −32.16 | −3.64 | −1.73 | −6.29 | −1.39 | 38.09 | −29.25 | Conventional H-Bond: Val390 (1.68) | Pi–Cation: His311 (4.68), Pi–Anion: Asp355 (5.00), Pi–Anion: Glu393 (3.39) |
| Kaempferol | −4.35 ± 0.08 | −33.19 | −30.24 ± 0.01 | −9.90 | −20.19 | 1.07 | −1.41 | −5.74 | −1.4 | 23.97 | −26.53 | Conventional H-Bond: Val390 (1.86), Asp355 (1.75), Carbon H-Bond: His311 (2.91) | Pi–Anion: Glu393 (3.54), Pi–Anion: Glu393 (4.04), Pi–Lone Pair: Phe312 (3.00), Pi–Alkyl: Val390 (5.49) |
| Ellagic Acid | −4.34 ± 0.16 | −36.18 | −31.54 ± 0.09 | −10.47 | −19.21 | 2.61 | −1.89 | −8.1 | −1.21 | 22.87 | −26.59 | Conventional H-Bond: Asp329 (2.98), Val390 (1.69), Asp329 (1.96), Carbon H-Bond: His311 (2.49) | Pi–Anion: Glu393 (3.74), Pi–Sigma: Trp313 (2.68), Pi–Lone Pair: Phe312 (2.90), Pi–Pi T-Shaped: His311 (5.59) |
| Luteolin | −4.21 ± 0.13 | −32.01 | −26.01 ± 0.16 | −8.07 | −16.92 | 0.63 | −1.92 | −5.01 | −1.44 | 24.95 | −26.31 | Conventional H-Bond: Asp355 (1.74), Asp355 (1.82), Carbon H-Bond: His311 (2.88) | Pi–Anion: Glu393 (3.52) |
| Naringenin | −4.20 ± 0.28 | −28.44 | −28 ± 0.41 | −8.93 | −22.4 | 2.21 | −2.23 | −3.96 | −0.82 | 23.66 | −24.46 | Conventional H-Bond: Gln389 (1.83), Glu393 (2.73), Phe312 (1.80), Asp329 (2.07), Carbon H-Bond: Ser358 (2.92), Ser358 (2.37) | Pi–Cation: His311 (4.35), Pi–Anion: Glu393 (3.84), Pi–Pi T-Shaped: His311 (4.54), Pi–Alkyl: Val390 (4.55) |
| 1,4–Dicaffeoylquinic Acid | −4.17 ± 0.34 | −47.08 | −43.76 ± 0.39 | −15.78 | −21.99 | 3.51 | −5.15 | −12.51 | −2.74 | 33.49 | −38.35 | Conventional H-Bond: Thr315 (2.33), Thr315 (2.11), Asp355 (1.76), Asp441 (2.11), Asp355 (1.73), Asp441 (2.03) | Pi–Cation: Arg304 (4.18), Pi–Anion: Phe314 (4.19), Pi–Pi T-Shaped: His311 (4.60) |
| Verapamil | −3.34 ± 0.17 | −39.26 | −22.93 ± 0.06 | −6.73 | −4.24 | 11.75 | −1.66 | −13.49 | −1.6 | 23.6 | −37.29 | Conventional H-Bond: Arg361 (2.04), Carbon H-Bond: Ser358 (2.96), Phe312 (2.79), Phe312 (2.72), Gln389 (2.85), Glu393 (2.83), Asn392 (2.66) | Salt Bridge;Attractive Charge: Glu393 (1.93), Pi–Sigma: Glu393 (2.39), Alkyl: Ala362 (4.18), Arg361 (4.40) |
| Apigenin | −3.29 ± 0.06 | −31.89 | −23.92 ± 0.14 | −7.16 | −12.62 | 3.65 | −1.56 | −5.99 | −1.04 | 20.44 | −26.79 | Conventional H-Bond: Asp329 (1.97), Val390 (1.73), Carbon H-Bond: His311 (2.96) | Pi–Anion: Glu393 (3.56), Pi–Lone Pair: Phe312 (2.97), Trp313 (2.93) |
| Ferulic Acid | −2.02 ± 0.11 | −16.31 | −2.48 ± 0.43 | 2.15 | 7.96 | 1.21 | −0.54 | −7.96 | −0.03 | 16.1 | −19.22 | Conventional H-Bond: Val390 (1.79), Carbon H-Bond: Glu393 (2.53) | Pi–Lone Pair: Phe312 (2.89) |
| Phosphoinositide Phospholipase C-Gamma-1 | |||||||||||||
| Rutin | −6.81 ± 0.15 | −32.87 | −40.42 ± 0.07 | −14.33 | −26.72 | 3.97 | −2.97 | −8.13 | 0 | 26.09 | −32.66 | Attractive Charge: Lys941 (5.01), Carbon H-Bond: Ser981 (2.85), Ser981 (2.29), Ser982 (2.73), Glu473 (2.63), Glu414 (2.28), Conventional H-Bond: Lys464 (2.44), Arg945 (2.59), Arg946 (1.77), Ser982 (2.06), Tyr1012 (2.52), Glu365 (1.77), Ser982 (1.72), Pi–Alkyl: Tyr1012 (5.03), Arg945 (5.14), Pro964 (4.98), Met480 (5.06), Arg945 (5.31), Met480 (4.13), Pro964 (3.89) | Pi–Cation: Lys941 (4.61), Pi–Cation; Pi–Donor Hydrogen Bond: Arg946 (3.03), Pi–Sulfur: Met480 (4.46) |
| 1,4–Dicaffeoylquinic Acid | −6.80 ± 0.27 | −61.87 | −42.56 ± 0.1 | −15.25 | −14.49 | 11.52 | −5.29 | −15.08 | −0.14 | 26.83 | −45.92 | Conventional H-Bond: Gly940 (2.53), Glu483 (1.85), Ser478 (1.75), Ile413 (1.91), Ile413 (3.00), Arg946 (1.83), Arg946 (2.05), Arg946 (2.33), Lys941 (2.52) | Pi–Alkyl: Val479 (5.36), Lys464 (5.41), Pi–Anion: Glu483 (4.48) |
| Quercetin | −5.56 ± 0.14 | −48.15 | −19.01 ± 0.04 | −5.03 | −47.7 | 6.99 | −3.02 | −6.79 | −1.67 | 70.67 | −37.49 | Conventional H-Bond: Lys462 (2.75), Lys462 (2.44), Lys464 (2.73), Arg945 (2.35), Arg945 (2.62), Glu414 (2.54), Ile413 (2.17), Lys462 (2.67), His380 (1.83), Carbon H-Bond: Lys464 (2.63), Ser482 (2.97), Arg945 (2.43), Gly1015 (2.94) | Pi–Anion: Glu414 (3.56), Pi–Pi T-Shaped: Tyr1012 (4.77) |
| Narcissin | −5.55 ± 0.11 | −41.94 | −29.06 ± 0.23 | −9.39 | −18.1 | 1.3 | −2.19 | −2.79 | 0 | 27.79 | −35.06 | Conventional H-Bond: Ser478 (2.32), Arg945 (2.43), Arg946 (2.74), Ser478 (1.81), Arg946 (2.92), Carbon H-Bond: Thr477 (2.68) | Pi–Sigma: Glu944 (2.85), Pi–Alkyl: Lys941 (4.76), Arg945 (5.34) Val479 (5.33), Lys941 (3.74), |
| Orientin | −5.53 ± 0.26 | −38.33 | −32.07 ± 0.41 | −10.70 | −39.09 | 12.71 | −3.6 | −6.23 | −1.28 | 30.56 | −25.13 | Conventional H-Bond: Met480 (2.53), Arg945 (2.27), Arg946 (2.80), Lys941 (1.66), Ser478 (1.58), Ser478 (1.65), Carbon H-Bond: Glu944 (2.65), Glu944 (2.40), Pi–Cation: Arg946 (3.32) | Pi–Sulfur: Met480 (5.89), Pi–Alkyl: Arg945 (5.38), Lys941 (5.31), Arg945 (5.44) |
| Hesperidin | −5.50 ± 0.06 | −43.48 | −43.21 ± 0.1 | −15.54 | −31.12 | 1.66 | −4.05 | −12.02 | −1.53 | 41.55 | −37.69 | Conventional H-Bond: Lys464 (2.22), Met480 (1.80), Arg945 (2.63), Arg946 (1.91), Arg946 (3.04), Arg946 (2.12), Ser478 (2.32), Carbon H-Bond: Val479 (2.73), Glu944 (2.74), Glu944 (2.77), Pi–Cation; Pi–Donor Hydrogen Bond: Arg946 (3.83) | Pi–Sulfur: Met480 (4.26), Alkyl: Val479 (4.84), Pro962 (4.89), Pi–Alkyl: Lys941 (5.11), Arg945 (4.52), Pro964 (4.43) |
| Ellagic Acid | −4.97 ± 0.12 | −44.95 | −35.17 ± 0.18 | −12.05 | −33.22 | 6.81 | −3.29 | −10.91 | −0.85 | 42.51 | −36.22 | Conventional H-Bond: Asp415 (2.59), Lys462 (2.02), Arg1010 (2.85), Tyr1012 (2.69), Ile413 (1.68), Ile413 (2.56), Lys462 (2.21), Carbon H-Bond: Lys464 (2.49), Lys464 (2.84), Ser982 (2.63) | Pi–Anion: Glu365 (4.85), Glu414 (3.03), Pi–Alkyl: Arg945 (5.46), Arg945 (4.83) |
| Kaempferol | −4.34 ± 0.06 | −48.40 | −17.41 ± 0.23 | −4.33 | −44.99 | 5.48 | −2.54 | −7.15 | −1.63 | 67.64 | −34.21 | Conventional H-Bond: Lys462 (2.32), Lys464 (2.61), Arg945 (2.78), Glu414 (2.35), Ile413 (2.20), Lys462 (2.48), His335 (1.79), Carbon H-Bond: Arg945 (2.51) | Pi–Anion: Glu414 (3.63), Pi–Pi T-Shaped: Tyr1012 (4.54) |
| Luteolin | −4.25 ± 0.15 | −43.17 | −18.33 ± 0.13 | −4.73 | −35.46 | 6.13 | −2.52 | −5.86 | −1.72 | 59.28 | −38.18 | Conventional H-Bond: Lys462 (2.84), Lys462 (2.53), Arg945 (2.28), Ile413 (2.10), Lys462 (2.80), His380 (1.83), Carbon H-Bond: Lys464 (2.74), Ser482 (2.86), Gly1015 (2.86) | Pi–Anion: Glu414 (3.60), Pi–Pi Stacked: Tyr1012 (6.00), Pi–Pi T-Shaped: Tyr1012 (4.79) |
| Ferulic Acid | −3.91 ± 0.19 | −21.31 | −33.17 ± 0.27 | −11.18 | −7.13 | 3.52 | −2.79 | −12.67 | −0.03 | 12.56 | −26.62 | Attractive Charge: Lys941 (5.31), Arg946 (3.16), Conventional H-Bond: Met480 (2.50), Ser982 (1.85), Ser982 (2.90), Val963 (2.09), Met980 (2.85), Carbon H-Bond: Ser981 (2.83), Ser982 (2.66), Ser982 (2.44) | Pi–Alkyl: Met480 (5.05), Pro964 (3.95) |
| Apigenin | −3.85 ± 0.55 | −42.25 | −16.26 ± 0.31 | −3.83 | −32.59 | 6.02 | −1.76 | −5.9 | −1.82 | 56.27 | −36.47 | Conventional H-Bond: Lys462 (2.92), Lys462 (2.59), Ile413 (2.05), Lys462 (2.91), His380 (1.80), Carbon H-Bond: Lys464 (2.73), Arg945 (2.83), Gly1015 (2.83) | Pi–Anion: Glu414 (3.52), Pi–Pi Stacked: Tyr1012 (5.98), Pi–Pi T-Shaped: Tyr1012 (4.78) |
| Naringenin | −3.51 ± 0.17 | −41.80 | −10.88 ± 0.43 | −1.50 | −28.83 | 3.77 | −1.93 | −7.25 | −1.38 | 56.77 | −32.02 | Conventional H-Bond: Lys462 (2.27), Lys464 (2.68), Ile413 (2.17), Lys462 (2.47), His335 (1.82), Carbon H-Bond: Lys464 (2.50), Glu414 (2.36) | |
| Verapamil | −1.89 ± 0.11 | −49.24 | −16.59 ± 0.19 | −3.98 | 22.63 | 7.39 | −0.81 | −18.46 | −1.21 | 20.44 | −46.58 | Salt Bridge;Attractive Charge: Glu473 (3.00), Conventional H-Bond: Met480 (2.53), Arg945 (2.57), Carbon H-Bond: Glu473 (2.41), Asp415 (2.94), Glu414 (2.42), Ser478 (2.76) | Pi–Cation: Arg946 (3.05), Pi–Anion: Glu414 (3.44), Alkyl: Arg945 (3.74), Arg945 (4.43), Arg946 (4.41), Pi–Alkyl: His416 (4.21), Arg945 (5.35), Met480 (4.24), Arg945 (5.12), Pro964 (5.24) |
| Voltage-gated calcium channel | |||||||||||||
| Hesperidin | −14.10 ± 0.14 | −78.41 | −45.95 ± 0.29 | −16.73 | −38.15 | 5.37 | −2.88 | −25.96 | −0.94 | 81.9 | −65.28 | Conventional H-Bond:Thr734 (1.96), Leu733 (1.73), Thr1443 (2.20), Thr1142 (2.60), Gly1444 (2.90), Gly1444 (1.75), Leu298 (2.26), Carbon H-Bond:Leu733 (2.44), Leu733 (2.63), Gly1444 (2.34), Thr391 (2.77) | Pi–Pi T-Shaped:Phe767 (5.17), Alkyl:Ala302 (4.28), Met392 (4.91), Met295 (3.90), Pi–Alkyl:Ala302 (5.23), Val430 (5.00), Ile768 (5.25) |
| Rutin | −13.71 ± 0.26 | −76.67 | −30.2 ± 0.13 | −9.89 | −50.79 | 8.82 | −4.12 | −16.79 | −0.11 | 85.82 | −53.04 | Conventional H-Bond:Tyr1489 (2.19), Ala1183 (1.89), Ala1183 (1.88), Thr1443 (1.85), Ala1442 (2.44), Ile390 (1.97), Thr1142 (2.86), Gly1444 (2.21), Gly1444 (1.76), Carbon H-Bond:Thr1443 (2.56) | Amide–Pi Stacked:Gly735; C, O; Glu736 (5.21), Alkyl:Ala1493 (3.37), Pi–Alkyl:Tyr1489 (3.97), Met392 (5.35), Met392 (5.36), Leu427 (5.26), Met392 (5.45) |
| Narcissin | −13.67 ± 0.13 | −67.23 | −37.05 ± 0.04 | −12.86 | −27.08 | 11.31 | −2.16 | −19.82 | −2.54 | 61.68 | −58.45 | Conventional H-Bond:Tyr1489 (2.03), Ala1442 (1.75), Thr1142 (2.10), Ser1141 (2.52), Ala1183 (1.70), Carbon H-Bond:Ala1442 (3.05), Thr1142 (2.66), Thr1443 (2.39), Ala1442 (3.01), Thr1443 (2.46) | Pi–Sulfur:Met1187 (5.56), Pi–Pi T-Shaped:Phe1190 (5.18), Tyr1489 (5.92), Alkyl:Met1187 (3.81), Pi–Alkyl:Phe1190 (4.04), Val1191 (4.78), Ala1493 (4.19) |
| Quercetin | −10.02 ± 0.14 | −40.89 | −36.41 ± 0.31 | −12.58 | −9.91 | 1.18 | −1.15 | −11.72 | −0.49 | 22.42 | −36.74 | Conventional H-Bond: Ile390 (2.07), Leu298 (1.94), Pi–Donor Hydrogen Bond: Tyr772 (2.52) | Pi–Alkyl:Val430 (4.70), Ile768 (5.00), Leu298 (5.02), Ala302 (4.93), Val430 (5.16) |
| Orientin | −9.909 | −56.19 | −28.41 | −9.11 | −54.99 | 12.8 | −3.91 | −10.51 | −1.73 | 69.76 | −39.82 | Conventional H-Bond: Tyr1489 (2.15), Tyr1489 (2.71), Leu733 (2.09), Leu733 (1.93), Asn771 (2.42), Thr391 (1.86), Thr1142 (1.81), Asn1188 (1.72), Asn1188 (1.78), Carbon H-Bond: Leu733 (2.72), Thr734 (2.94) | Pi–Pi T-Shaped: Phe1143 (4.75), Phe1143 (4.87) |
| Kaempferol | −9.442 | −39.31 | −30.9 | −10.19 | −7.73 | 0.4 | −1.15 | −11.67 | −0.51 | 25.56 | −35.81 | Conventional H-Bond: Ile390 (2.08), Leu298 (1.96) | Pi–Alkyl: Val430 (4.74), Ile768 (4.95), Leu298 (5.05), Ala302 (4.90), Val430 (5.16), |
| 1,4–Dicaffeoylquinic Acid | −9.25 ± 0.35 | −63.75 | −3.4 ± 0.33 | 1.75 | 76.32 | 7.05 | −2.42 | −28.74 | −1.23 | 6.2 | −60.59 | Conventional H-Bond: Glu393 (2.14), Leu298 (1.88), Gly1444 (2.93), Glu1445 (2.09), Leu298 (2.05), Carbon H-Bond: Glu736 (3.10) | Pi–Sulfur: Met295 (5.53), Pi–Alkyl: Phe767 (5.40), Leu298 (5.12), Ala302 (4.78) |
| Luteolin | −9.24 ± 0.12 | −40.28 | −33.01 ± 0.37 | −11.11 | −10.47 | 2.16 | −1.01 | −10.39 | −0.36 | 22.63 | −35.57 | Conventional H-Bond: Gly422 (2.02), Leu298 (1.91), Pi–Donor Hydrogen Bond: Tyr772 (2.49) | Pi–Alkyl:Val430 (4.62), Ile768 (5.04), Val430 (5.09), Leu298 (5.02), Ala302 (4.83), Val430 (5.18) |
| Apigenin | −8.76 ± 0.03 | −38.15 | −35.28 ± 0.19 | −12.09 | −1.83 | 0.48 | −1.01 | −10.37 | −0.34 | 12.1 | −34.31 | Conventional H-Bond: Gly422 (1.99), Leu298 (1.93) | Pi–Alkyl: Val430 (4.60), Ile768 (5.06), Val430 (5.13), Leu298 (5.04), Ala302 (4.81), Val430 (5.17) |
| Naringenin | −8.68 ± 0.1 | −38.03 | −34.92 ± 0.17 | −11.94 | −3.32 | 1.01 | −1.02 | −11.6 | −0.42 | 14.58 | −34.16 | Conventional H-Bond: Gly422 (2.02), Leu298 (1.94) | Pi–Alkyl: Leu298 (5.03), Ala302 (4.73), Val430 (5.27), Val430 (5.07) |
| Ellagic Acid | −6.30 ± 0.13 | −33.43 | −16.69 ± 0.21 | −4.02 | −64.45 | 1.7 | −0.96 | −13.41 | −0.82 | 93.13 | −31.88 | Conventional H-Bond:Ile390 (1.64), Ile390 (3.06), Carbon H-Bond:Ser423 (2.47), Tyr772 (2.60) | Pi–Alkyl:Leu775 (5.27), Val430 (4.73), Val430 (4.49), Ile768 (5.01) |
| Ferulic Acid | −5.83 ± 0.2 | −18.71 | −20.9 ± 0.06 | −5.85 | 46.47 | 5.78 | −0.44 | −15.83 | −1.2 | −26.23 | −29.45 | Conventional H-Bond: Met1490 (2.34), Pi–Sulfur:Met1187 (5.74), Alkyl:Met1187 (4.50), Met1490 (5.29) | Pi–Alkyl:Phe1190 (3.58), Ala1493 (4.43) |
| Verapamil | −3.44 ± 0.15 | −45.58 | −32.53 ± 0.1 | −10.90 | −40.4 | 2.16 | 0 | −21.84 | −1 | 76.82 | −48.25 | Carbon H-Bond: Leu733 (2.65), Leu733 (2.49), Asn771 (2.92), Leu733 (3.08), Thr734 (2.78), Thr391 (2.69), Ile390 (2.51) | Pi–Pi T-Shaped: Phe767 (5.41), Alkyl: Leu427 (5.06), Ile1497 (5.33), Leu431 (5.22), Val1191 (3.69), Met392 (3.89), Pi–Alkyl:Val1191 (5.46) |
| Myosin light chain kinase | |||||||||||||
| Rutin | −9.96 ± 0.18 | −60.64 | −40.51 ± 0.21 | −14.36 | −31.74 | 5.46 | −3.4 | −9.77 | −4.28 | 47.25 | −44.03 | Conventional H-Bond:Gln333 (2.43), Lys422 (3.09), Gln333 (1.96), Gln333 (2.01), Gly458 (3.00), Asp337 (2.53), Gly442 (2.67), Gly458 (1.77), Carbon H-Bond:Lys309 (2.58), Gly458 (2.92) | Pi–Sulfur:Met340 (4.28), Pi–Pi Stacked:Phe310 (4.35), Phe310 (3.84), Phe310 (5.74), Pi–Alkyl:Val455 (5.07) |
| Hesperidin | −8.58 ± 0.19 | −59.80 | −41.36 ± 0.34 | −14.73 | −34.46 | 11.78 | −4.51 | −15.67 | 0 | 49.98 | −48.48 | Conventional H-Bond: Gln333 (1.96), Lys422 (2.48), Thr459 (2.09), Asn456 (1.87), Gly458 (1.81), Thr459 (2.90), Carbon H-Bond: Asn456 (3.01), Gly458 (2.56), Gly458 (2.87), Asp501 (2.91) | Pi–Anion:Asp501 (3.60), Alkyl:Met340 (5.38), Val455 (4.85) |
| 1,4–Dicaffeoylquinic Acid | −8.52 ± 0.1 | −54.58 | −37.14 ± 0.07 | −12.90 | 49.74 | 7.27 | −3.34 | −20.29 | −3 | −23.5 | −44.01 | Attractive Charge:Lys336 (4.92), Conventional H-Bond:Gln333 (2.87), Gln333 (2.65), Lys336 (1.78), Lys422 (2.63), Met340 (2.67), Asp337 (1.73), Asp420 (2.30), Gly458 (2.29) | Pi–Alkyl:Val455 (5.34) |
| Narcissin | −7.42 ± 0.11 | −58.25 | −44.61 ± 0.26 | −16.14 | −40.6 | 7.4 | −3.62 | −2.97 | −5.22 | 46.69 | −46.29 | Conventional H-Bond:Lys336 (2.93), Lys336 (2.30), Lys422 (2.30), Met340 (2.52), Tyr470 (1.81), Gly458 (1.71), Carbon H-Bond:Lys331 (2.84) | Pi–Cation:Lys422 (3.61), Pi–Cation;Pi–Donor Hydrogen Bond:Lys422 (2.79), Pi–Anion:Asp420 (4.22), Amide–Pi Stacked:Lys309C, O; Phe310 (3.65) |
| Orientin | −7.41 ± 0.13 | −39.40 | −37.92 ± 0.11 | −13.24 | −25.37 | 0.32 | −2.41 | −10.58 | −1.9 | 33.65 | −31.64 | Conventional H-Bond:Asp420 (2.06), Gly442 (1.98), Gly458 (2.86), Carbon H-Bond:Thr459 (2.69) | |
| Quercetin | −6.68 ± 0.19 | −38.25 | −25.69 ± 0.23 | −7.93 | −11.67 | 5.43 | −2.08 | −8.63 | −3.96 | 26.01 | −30.8 | Conventional H-Bond:Gln333 (2.85), Gly442 (2.88), Gly458 (1.72), Asp420 (2.24) | Pi–Anion:Asp420 (4.09), Pi–Pi Stacked:Phe310 (4.57), Phe310 (5.76), Pi–Alkyl:Leu463 (5.45) |
| Apigenin | −5.89 ± 0.47 | −35.23 | −33.37 ± 0.29 | −11.26 | −27.65 | 2.2 | −1.63 | −6.93 | −4.32 | 34.3 | −29.34 | Conventional H-Bond:Gln333 (2.21), Gln333 (2.47), Lys331 (2.78), Asp337 (2.55), Gly458 (1.78) | Pi–Cation:Lys422 (4.93), Pi–Pi Stacked:Phe310 (4.53), Phe310 (4.14), Phe310 (5.66), Pi–Alkyl:Met340 (4.99), Pi–Alkyl:Val455 (5.34) |
| Naringenin | −5.83 ± 0.31 | −33.27 | −25.15 ± 0.08 | −7.69 | −15.65 | 3.4 | −2.37 | −8.2 | −1.93 | 28.12 | −28.52 | Conventional H-Bond: Lys422 (2.42), Asn456 (2.68), Gly458 (1.82) | Pi–Alkyl: Met340 (4.37) |
| Luteolin | −5.79 ± 0.25 | −35.08 | −28.91 ± 0.34 | −9.33 | −20.37 | 6.65 | −2.17 | −7 | −3.91 | 27.8 | −29.92 | Conventional H-Bond:Gly458 (1.69), Asp420 (1.95), Pi–Cation;Pi–Donor Hydrogen Bond:Lys422 (3.24) | Pi–Anion:Asp420 (4.26), Pi–Pi Stacked:Phe310 (4.86), Pi–Pi Stacked: Phe310 (4.82), Pi–Alkyl:Leu463 (5.47) |
| Kaempferol | −5.78 ± 0.19 | −30.55 | −19.93 ± 0.45 | −5.43 | −13.19 | 3.81 | −1.63 | −8.86 | −3.1 | 30.7 | −27.66 | Conventional H-Bond:Gly442 (2.44), Gly458 (1.90), Thr459 (2.55) | Pi–Pi Stacked:Phe310 (4.29), Pi–Pi Stacked: Phe310 (5.35), Pi–Alkyl:Leu463 (5.36) |
| Ellagic Acid | −5.72 ± 0.11 | −40.57 | −43.15 ± 0.06 | −15.51 | −23.82 | 5.46 | −3.02 | −10.79 | −6.39 | 22.24 | −26.83 | Conventional H-Bond:Gln333 (3.00), Gln333 (2.05), Lys422 (2.11), Gly458 (1.75), Gln333 (2.95), Asp420 (1.79) | Pi–Alkyl:Val455 (5.37) |
| Ferulic Acid | −3.96 ± 0.18 | −23.99 | −12.16 ± 0.1 | −2.05 | 93.74 | 1.53 | −1.77 | −9.27 | −0.88 | −72.31 | −23.2 | Conventional H-Bond:Gln333 (2.32), Lys422 (2.22), Gly458 (1.94), Carbon H-Bond:Asn456 (2.55) | |
| Verapamil | −3.10 ± 0.24 | −41.54 | −49.41 ± 0.45 | −18.23 | −62.32 | 11.1 | −1.12 | −21.03 | −0.81 | 72.28 | −47.51 | Attractive Charge:Asp420 (3.54), Conventional H-Bond:Lys336 (2.74), Carbon H-Bond:Lys309 (3.02), Lys309 (2.61), Asp420 (2.79), Asn456 (2.40), Pi–Donor Hydrogen Bond:Asn456 (2.92) | Alkyl:Met340 (4.71), Pi–Alkyl:Val455 (5.07), Lys309 (5.17) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wahid, M.; Saqib, F.; Akhtar, S.; Ali, A.; Wilairatana, P.; Mubarak, M.S. Possible Mechanisms Underlying the Antispasmodic, Bronchodilator, and Antidiarrheal Activities of Polarity–Based Extracts of Cucumis sativus L. Seeds in In Silico, In Vitro, and In Vivo Studies. Pharmaceuticals 2022, 15, 641. https://doi.org/10.3390/ph15050641
Wahid M, Saqib F, Akhtar S, Ali A, Wilairatana P, Mubarak MS. Possible Mechanisms Underlying the Antispasmodic, Bronchodilator, and Antidiarrheal Activities of Polarity–Based Extracts of Cucumis sativus L. Seeds in In Silico, In Vitro, and In Vivo Studies. Pharmaceuticals. 2022; 15(5):641. https://doi.org/10.3390/ph15050641
Chicago/Turabian StyleWahid, Muqeet, Fatima Saqib, Saeed Akhtar, Anam Ali, Polrat Wilairatana, and Mohammad S. Mubarak. 2022. "Possible Mechanisms Underlying the Antispasmodic, Bronchodilator, and Antidiarrheal Activities of Polarity–Based Extracts of Cucumis sativus L. Seeds in In Silico, In Vitro, and In Vivo Studies" Pharmaceuticals 15, no. 5: 641. https://doi.org/10.3390/ph15050641
APA StyleWahid, M., Saqib, F., Akhtar, S., Ali, A., Wilairatana, P., & Mubarak, M. S. (2022). Possible Mechanisms Underlying the Antispasmodic, Bronchodilator, and Antidiarrheal Activities of Polarity–Based Extracts of Cucumis sativus L. Seeds in In Silico, In Vitro, and In Vivo Studies. Pharmaceuticals, 15(5), 641. https://doi.org/10.3390/ph15050641