New Paradigms of Old Psychedelics in Schizophrenia
Abstract
:1. Introduction
1.1. Therapeutic Armamentarium for Schizophrenia
1.2. Psychedelic Drugs in Schizophrenia
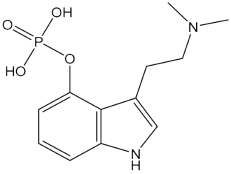
1.3. Psilocybin
1.4. LSD
1.5. MDMA
1.6. DMT
1.7. Mescaline
2. Psychedelic Drug Models in Schizophrenia
Biomarkers of Neuropsychiatry and Their Association with Psychedelics Drugs
3. Neurochemical Mechanisms of Psychedelic Drugs and Their Relationship with APDs
3.1. Psychedelic Pharmacodynamics and Receptor Activation
3.2. Agonist Trafficking of Receptor Signalling and Psychedelics
3.3. Functional Serotoninergic/Glutamatergic Interactions in Psychedelic Responses
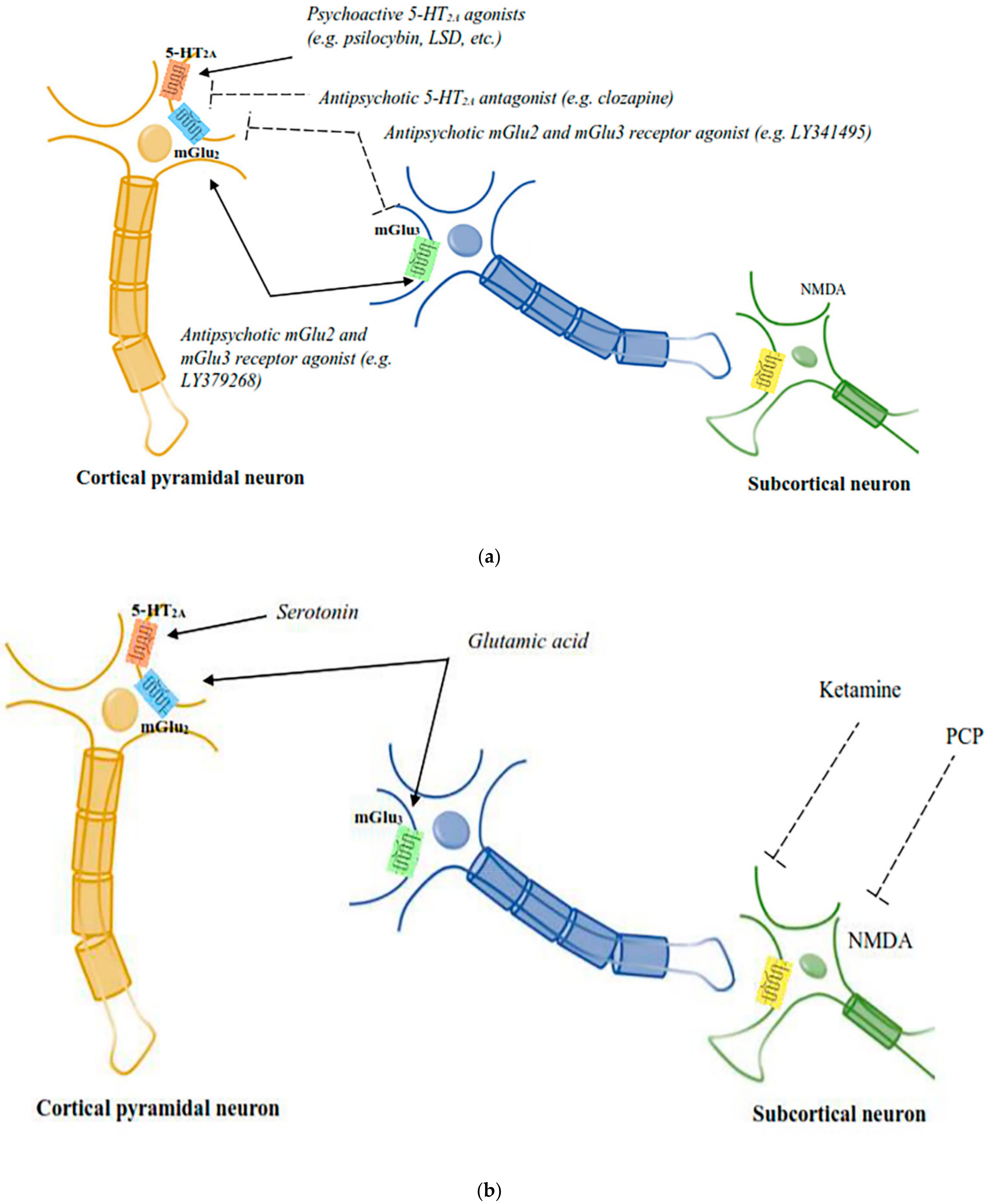
3.4. Role of mGlu2/3 Receptors in Psychedelic Drug Responses
3.5. Role of Central Histamine in Psychedelic Drug Responses
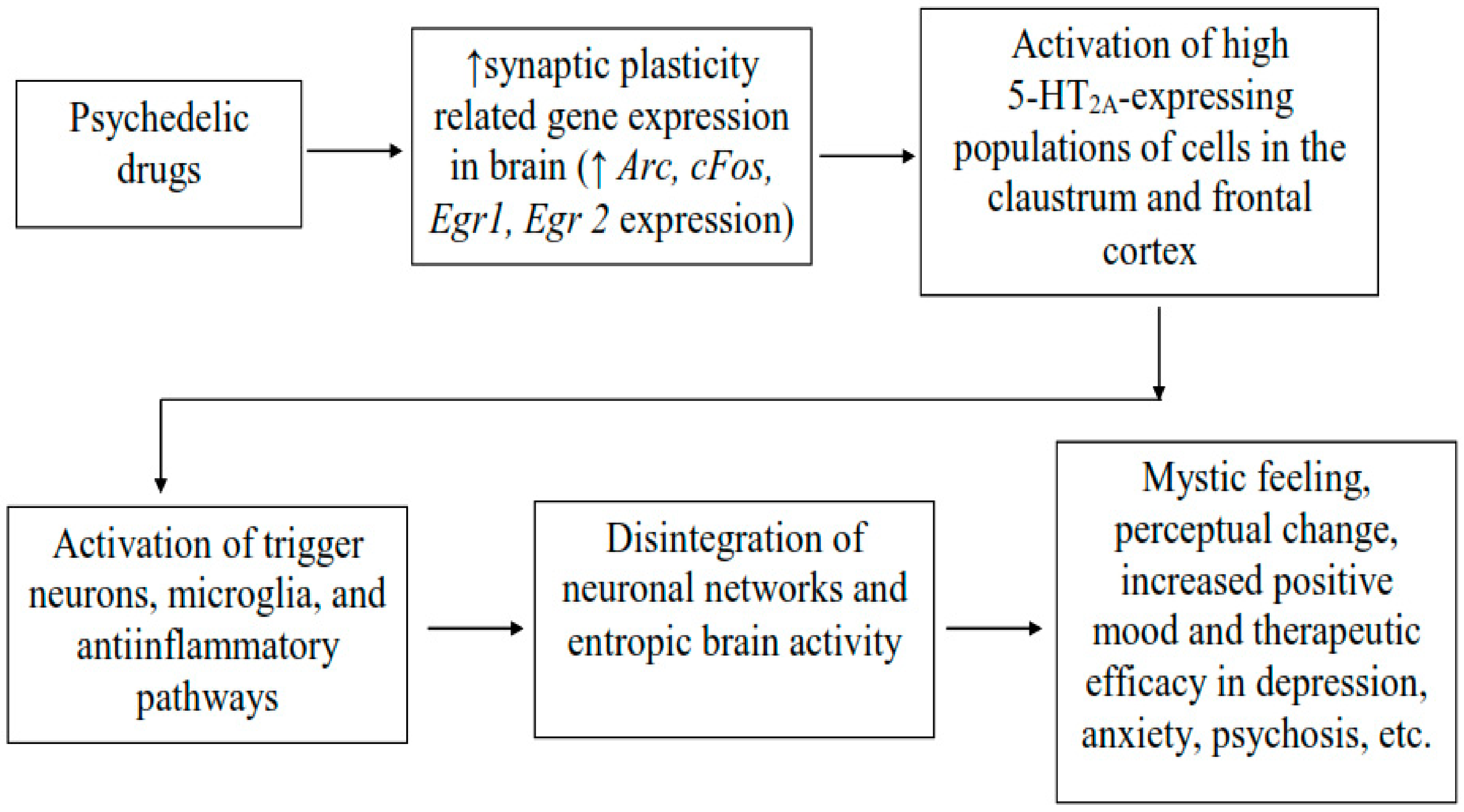
3.6. Psychedelic Effects in Neural Connectivity and Neural Plasticity
3.7. Limitations and Scope of Psychedelic Studies
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Saha, S.; Chant, D.; Welham, J.; McGrath, J. A Systematic Review of the Prevalence of Schizophrenia. PLoS Med. 2005, 2, e141. [Google Scholar] [CrossRef] [PubMed]
- WHO. Schizophrenia. Available online: https://www.who.int/news-room/fact-sheets/detail/schizophrenia (accessed on 7 November 2021).
- McGrath, J.; Saha, S.; Chant, D.; Welham, J. Schizophrenia: A Concise Overview of Incidence, Prevalence, and Mortality. Epidemiol. Rev. 2008, 30, 67–76. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- González-Maeso, J.; Ang, R.L.; Yuen, T.; Chan, P.; Weisstaub, N.V.; López-Giménez, J.F.; Zhou, M.; Okawa, Y.; Callado, L.F.; Milligan, G.; et al. Identification of a serotonin/glutamate receptor complex implicated in psychosis. Nature 2008, 452, 93–97. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tandon, R.; Gaebel, W.; Barch, D.M.; Bustillo, J.; Gur, R.E.; Heckers, S.; Malaspina, D.; Owen, M.J.; Schultz, S.; Tsuang, M.; et al. Definition and description of schizophrenia in the DSM-5. Schizophr. Res. 2013, 150, 3–10. [Google Scholar] [CrossRef]
- Rahman, T.; Lauriello, J. Schizophrenia: An Overview. Focus 2016, 14, 300–307. [Google Scholar] [CrossRef] [PubMed]
- Bola, J.R. Medication-Free Research in Early Episode Schizophrenia: Evidence of Long-Term Harm? Schizophr. Bull. 2006, 32, 288–296. [Google Scholar] [CrossRef]
- Kapur, S.; Mamo, D. Half a century of antipsychotics and still a central role for dopamine D2 receptors. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2003, 27, 1081–1090. [Google Scholar] [CrossRef]
- Seeman, P. Atypical Antipsychotics: Mechanism of Action. Can. J. Psychiatry 2002, 47, 29–40. [Google Scholar] [CrossRef] [Green Version]
- Ban, T.A. Fifty years chlorpromazine: A historical perspective. Neuropsychiatr. Dis. Treat. 2007, 3, 495–500. [Google Scholar]
- Shen, W.W. A history of antipsychotic drug development. Compr. Psychiatry 1999, 40, 407–414. [Google Scholar] [CrossRef]
- Davies, M.A.; Sheffler, D.J.; Roth, B.L. Aripiprazole: A novel atypical antipsychotic drug with a uniquely robust pharmacology. CNS Drug Rev. 2004, 10, 317–336. [Google Scholar] [CrossRef] [PubMed]
- Conley, R.R.; Kelly, D.L. Current status of antipsychotic treatment. Curr. Drug Targets CNS Neurol. Disord. 2002, 1, 123–128. [Google Scholar] [CrossRef] [PubMed]
- Awad, A.G.; Voruganti, L.N.P. New antipsychotics, compliance, quality of life, and subjective tolerability—Are patients better off? Can. J. Psychiatr. 2004, 49, 297–302. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weickert, T.W.; Goldberg, T.E. First- and second-generation antipsychotic medication and cognitive processing in schizophrenia. Curr. Psychiatry Rep. 2005, 7, 304–310. [Google Scholar] [CrossRef] [PubMed]
- Green, M.F. Impact of cognitive and social cognitive impairment on functional outcomes in patients with schizophrenia. J. Clin. Psychiatry 2016, 77, 8–11. [Google Scholar] [CrossRef]
- Kirschner, M.; Aleman, A.; Kaiser, S. Secondary negative symptomsA review of mechanisms, assessment and treatment. Schizophr. Res. 2017, 186, 29–38. [Google Scholar] [CrossRef]
- Hennekens, C.H.; Hennekens, A.R.; Hollar, D.; Casey, D.E. Schizophrenia and increased risks of cardiovascular disease. Am. Heart J. 2005, 150, 1115–1121. [Google Scholar] [CrossRef]
- Auquier, P.; Lançon, C.; Rouillon, F.; Lader, M.; Holmes, C. Mortality in schizophrenia. Pharmacoepidemiol. Drug Saf. 2006, 15, 873–879. [Google Scholar] [CrossRef]
- Seeman, P. Dopamine and schizophrenia. Scholarpedia 2007, 10, 3634. [Google Scholar] [CrossRef]
- Colton, C.W.M.R. Congruencies in Increased Mortality Rates, Years of Potential Life Lost, and Causes of Death Among Public Mental Health Clients in Eight States. Prev. Chronic Dis. 2006, 3, A42. [Google Scholar]
- Newcomer, J.W.; Hennekens, C.H. Severe mental illness and risk of cardiovascular disease. JAMA 2007, 298, 1794–1796. [Google Scholar] [CrossRef] [PubMed]
- Malhotra, N.; Grover, S.; Chakrabarti, S.; Kulhara, P. Metabolic syndrome in schizophrenia. Indian J. Psychol. Med. 2013, 35, 227–240. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tharoor, H.; Kaliappan, A.; Gopal, S. Sexual dysfunctions in schizophrenia: Professionals and patients perspectives. Indian J. Psychiatry 2015, 57, 85–87. [Google Scholar] [CrossRef] [PubMed]
- Demjaha, A.; Murray, R.M.; McGuire, P.K.; Kapur, S.; Howes, O.D. Dopamine synthesis capacity in patients with treatment-resistant schizophrenia. Am. J. Psychiatry 2012, 169, 1203–1210. [Google Scholar] [CrossRef] [Green Version]
- Tamminga, C.A.; Carlsson, A. Partial dopamine agonists and dopaminergic stabilizers, in the treatment of psychosis. Curr. Drug Targets 2002, 1, 141–147. [Google Scholar] [CrossRef]
- Kane, J.; Honigfeld, G.; Singer, J.; Meltzer, H. Clozapine for the treatment-resistant schizophrenic. A double-blind comparison with chlorpromazine. Arch. Gen. Psychiatry 1988, 45, 789–796. [Google Scholar] [CrossRef]
- Conley, R.R. Optimizing treatment with clozapine. J. Clin. Psychiatry 1998, 59, 44–48. [Google Scholar]
- Wahlbeck, K.; Cheine, M.; Essali, A.; Adams, C. Evidence of clozapine’s effectiveness in schizophrenia: A systematic review and meta-analysis of randomized trials. Am. J. Psychiatry 1999, 156, 990–999. [Google Scholar] [CrossRef]
- Beaumont, G. Antipsychotics—The Future of Schizophrenia Treatment. Curr. Med. Res. Opin. 2000, 16, 37–42. [Google Scholar] [CrossRef]
- Miyamoto, S.; Duncan, G.E.; Marx, C.E.; Lieberman, J.A. Treatments for schizophrenia: A critical review of pharmacology and mechanisms of action of antipsychotic drugs. Mol. Psychiatry 2005, 10, 79–104. [Google Scholar] [CrossRef]
- Jones, P.B.; Barnes, T.R.E.; Davies, L.; Dunn, G.; Lloyd, H.; Hayhurst, K.P.; Murray, R.M.; Markwick, A.; Lewis, S.W. Randomized controlled trial of the effect on Quality of Life of second- vs. first-generation antipsychotic drugs in schizophrenia: Cost Utility of the Latest Antipsychotic Drugs in Schizophrenia Study (CUtLASS 1). Arch. Gen. Psychiatry 2006, 63, 1079–1087. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lieberman, J.A.; Tollefson, G.; Tohen, M.; Green, A.I.; Gur, R.E.; Kahn, R.; McEvoy, J.; Perkins, D.; Sharma, T.; Zipursky, R.; et al. Comparative efficacy and safety of atypical and conventional antipsychotic drugs in first-episode psychosis: A randomized, double-blind trial of olanzapine versus haloperidol. Am. J. Psychiatry 2003, 160, 1396–1404. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lieberman, J.A.; Phillips, M.; Gu, H.; Stroup, S.; Zhang, P.; Kong, L.; Ji, Z.; Koch, G.; Hamer, R.M. Atypical and conventional antipsychotic drugs in treatment-naive first-episode schizophrenia: A 52-week randomized trial of clozapine vs. chlorpromazine. Neuropsychopharmacology 2003, 28, 995–1003. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lieberman, J.A.; Stroup, T.S.; McEvoy, J.P.; Swartz, M.S.; Rosenheck, R.A.; Perkins, D.O.; Keefe, R.S.E.; Davis, S.M.; Davis, C.E.; Lebowitz, B.D.; et al. Effectiveness of antipsychotic drugs in patients with chronic schizophrenia. N. Engl. J. Med. 2005, 353, 1209–1223. [Google Scholar] [CrossRef] [Green Version]
- Geddes, J.; Freemantle, N.; Harrison, P.; Bebbington, P. Atypical antipsychotics in the treatment of schizophrenia: Systematic overview and meta-regression analysis. BMJ 2000, 321, 1371–1376. [Google Scholar] [CrossRef] [Green Version]
- Lieberman, J.A. Effectiveness of antipsychotic drugs in patients with chronic schizophrenia: Efficacy, safety and cost outcomes of CATIE and other trials. J. Clin. Psychiatry 2007, 68, e04. [Google Scholar] [CrossRef]
- Schooler, N.; Rabinowitz, J.; Davidson, M.; Emsley, R.; Harvey, P.D.; Kopala, L.; McGorry, P.D.; Van Hove, I.; Eerdekens, M.; Swyzen, W.; et al. Risperidone and haloperidol in first-episode psychosis: A long-term randomized trial. Am. J. Psychiatry 2005, 162, 947–953. [Google Scholar] [CrossRef]
- Stroup, T.S.; Gray, N. Management of common adverse effects of antipsychotic medications. World Psychiatry 2018, 17, 341–356. [Google Scholar] [CrossRef]
- British Association for Psychopharmacology of Schizophrenia. Evidence-Based Guidelines for the Pharmacological Treatment of Schizophrenia: Recommendations from the British Association for Psychopharmacology. Available online: https://www.bap.org.uk (accessed on 7 November 2021).
- Lewis, S.W.; Barnes, T.R.E.; Davies, L.; Murray, R.M.; Dunn, G.; Hayhurst, K.P.; Markwick, A.; Lloyd, H.; Jones, P.B. Randomized controlled trial of effect of prescription of clozapine versus other second-generation antipsychotic drugs in resistant schizophrenia. Schizophr. Bull. 2006, 32, 715–723. [Google Scholar] [CrossRef] [Green Version]
- McEvoy, J.P.; Lieberman, J.A.; Stroup, T.S.; Davis, S.M.; Meltzer, H.Y.; Rosenheck, R.A.; Swartz, M.S.; Perkins, D.O.; Keefe, R.S.E.; Davis, C.E.; et al. Effectiveness of clozapine versus olanzapine, quetiapine, and risperidone in patients with chronic schizophrenia who did not respond to prior atypical antipsychotic treatment. Am. J. Psychiatry 2006, 163, 600–610. [Google Scholar] [CrossRef]
- Howes, O.D.; McCutcheon, R.; Agid, O.; de Bartolomeis, A.; van Beveren, N.J.M.; Birnbaum, M.L.; Bloomfield, M.A.P.; Bressan, R.A.; Buchanan, R.W.; Carpenter, W.T.; et al. Treatment-Resistant Schizophrenia: Treatment Response and Resistance in Psychosis (TRRIP) Working Group Consensus Guidelines on Diagnosis and Terminology. Am. J. Psychiatry 2017, 174, 216–229. [Google Scholar] [CrossRef] [PubMed]
- Lally, J.; Ajnakina, O.; Di Forti, M.; Trotta, A.; Demjaha, A.; Kolliakou, A.; Mondelli, V.; Reis Marques, T.; Pariante, C.; Dazzan, P.; et al. Two distinct patterns of treatment resistance: Clinical predictors of treatment resistance in first-episode schizophrenia spectrum psychoses. Psychol. Med. 2016, 46, 3231–3240. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Howes, O.H.; Kaar, S.J. Antipsychotic drugs: Challenges and future directions. World Psychiatry 2018, 17, 170–171. [Google Scholar] [CrossRef] [PubMed]
- Goff, D.C. The Pharmacologic Treatment of Schizophrenia—2021. JAMA 2021, 325, 175–176. [Google Scholar] [CrossRef]
- Dedic, N.; Jones, P.G.; Hopkins, S.C.; Lew, R.; Shao, L.; Campbell, J.E.; Spear, K.L.; Large, T.H.; Campbell, U.C.; Hanania, T.; et al. SEP-363856, a Novel Psychotropic Agent with a Unique, Non-D(2) Receptor Mechanism of Action. J. Pharmacol. Exp. Ther. 2019, 371, 1–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koblan, K.S.; Kent, J.; Hopkins, S.C.; Krystal, J.H.; Cheng, H.; Goldman, R.; Loebel, A. A Non–D2-Receptor-Binding Drug for the Treatment of Schizophrenia. N. Engl. J. Med. 2020, 382, 1497–1506. [Google Scholar] [CrossRef]
- Andersen, K.A.A.; Carhart-Harris, R.; Nutt, D.J.; Erritzoe, D. Therapeutic effects of classic serotonergic psychedelics: A systematic review of modern-era clinical studies. Acta Psychiatr. Scand. 2021, 143, 101–118. [Google Scholar] [CrossRef]
- Dos Santos, R.G.; Bouso, J.C.; Rocha, J.M.; Rossi, G.N.; Hallak, J.E. The use of classic hallucinogens/psychedelics in a therapeutic context: Healthcare policy opportunities and challenges. Risk Manag. Healthc. Policy 2021, 14, 901. [Google Scholar] [CrossRef]
- González-Maeso, J.; Sealfon, S.C. Psychedelics and schizophrenia. Trends Neurosci. 2009, 32, 225–232. [Google Scholar] [CrossRef]
- Curran, H.V.; Nutt, D.; de Wit, H. Psychedelics and related drugs: Therapeutic possibilities, mechanisms and regulation. Psychopharmacology 2018, 235, 373–375. [Google Scholar] [CrossRef] [Green Version]
- Vollenweider, F.X.; Smallridge, J.W. Classic Psychedelic Drugs: Update on Biological Mechanisms. Pharmacopsychiatry 2022, 55, 121–138. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, C.P. Drug addiction and drug abuse. In Goodman and Gilman’s the Pharmacological Basis of Therapeutics; Goodman, A.G., Rall, T.W., Nies, A.S.T.P., Eds.; McGraw Hill: New York, NY, USA, 1990; pp. 522–573. [Google Scholar]
- Nichols, D.E. Psychedelics. Pharmacol. Rev. 2016, 68, 264–355. [Google Scholar] [CrossRef] [Green Version]
- Kyzar, E.J.; Nichols, C.D.; Gainetdinov, R.R.; Nichols, D.E.; Kalueff, A.V. Psychedelic Drugs in Biomedicine. Trends Pharmacol. Sci. 2017, 38, 992–1005. [Google Scholar] [CrossRef] [PubMed]
- Cameron, L.P.; Nazarian, A.; Olson, D.E. Psychedelic Microdosing: Prevalence and Subjective Effects. J. Psychoact. Drugs 2020, 52, 113–122. [Google Scholar] [CrossRef]
- Cameron, L.P.; Benson, C.J.; DeFelice, B.C.; Fiehn, O.; Olson, D.E. Chronic, Intermittent Microdoses of the Psychedelic N,N-Dimethyltryptamine (DMT) Produce Positive Effects on Mood and Anxiety in Rodents. ACS Chem. Neurosci. 2019, 10, 3261–3270. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cameron, L.P. Asking questions of psychedelic microdosing. eLife 2021, 10, e66920. [Google Scholar] [CrossRef]
- Majić, T.; Jungaberle, H.; Schmidt, T.T.; Zeuch, A.; Hermle, L.; Gallinat, J. Psychotherapy with Adjuvant use of Serotonergic Psychoactive Substances: Possibilities and Challenges. Fortschr. Neurol. Psychiatr. 2017, 85, 383–392. [Google Scholar] [CrossRef]
- Byock, I. Taking Psychedelics Seriously. J. Palliat. Med. 2018, 21, 417–421. [Google Scholar] [CrossRef] [Green Version]
- Nichols, D.E.; Johnson, M.W.; Nichols, C.D. Psychedelics as Medicines: An Emerging New Paradigm. Clin. Pharmacol. Ther. 2017, 101, 209–219. [Google Scholar] [CrossRef]
- Hendricks, P.S.; Thorne, C.B.; Clark, C.B.; Coombs, D.W.; Johnson, M.W. Classic psychedelic use is associated with reduced psychological distress and suicidality in the United States adult population. J. Psychopharmacol. 2015, 29, 280–288. [Google Scholar] [CrossRef]
- Hibicke, M.; Landry, A.N.; Kramer, H.M.; Talman, Z.K.; Nichols, C.D. Psychedelics, but Not Ketamine, Produce Persistent Antidepressant-like Effects in a Rodent Experimental System for the Study of Depression. ACS Chem. Neurosci. 2020, 11, 864–871. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bershad, A.K.; Preller, K.H.; Lee, R.; Keedy, S.; Wren-Jarvis, J.; Bremmer, M.P.; de Wit, H. Preliminary Report on the Effects of a Low Dose of LSD on Resting-State Amygdala Functional Connectivity. Biol. Psychiatry 2020, 5, 461–467. [Google Scholar] [CrossRef] [PubMed]
- Raval, N.R.; Johansen, A.; Donovan, L.L.; Ros, N.F.; Ozenne, B.; Hansen, H.D.; Knudsen, G.M. A Single Dose of Psilocybin Increases Synaptic Density and Decreases 5-HT(2A) Receptor Density in the Pig Brain. Int. J. Mol. Sci. 2021, 22, 835. [Google Scholar] [CrossRef] [PubMed]
- Carhart-Harris, R.; Giribaldi, B.; Watts, R.; Baker-Jones, M.; Murphy-Beiner, A.; Murphy, R.; Martell, J.; Blemings, A.; Erritzoe, D.; Nutt, D.J. Trial of Psilocybin versus Escitalopram for Depression. N. Engl. J. Med. 2021, 384, 1402–1411. [Google Scholar] [CrossRef]
- Davis, A.K.; Barrett, F.S.; May, D.G.; Cosimano, M.P.; Sepeda, N.D.; Johnson, M.W.; Finan, P.H.; Griffiths, R.R. Effects of Psilocybin-Assisted Therapy on Major Depressive Disorder: A Randomized Clinical Trial. JAMA Psychiatry 2021, 78, 481–489. [Google Scholar] [CrossRef] [PubMed]
- Vargas, M.V.; Meyer, R.; Avanes, A.A.; Rus, M.; Olson, D.E. Psychedelics and Other Psychoplastogens for Treating Mental Illness. Front. Psychiatry 2021, 12, 727117. [Google Scholar] [CrossRef]
- Schenberg, E.E. Psychedelic-Assisted Psychotherapy: A Paradigm Shift in Psychiatric Research and Development. Front. Pharmacol. 2018, 9, 733. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reiff, C.M.; Richman, E.E.; Nemeroff, C.B.; Carpenter, L.L.; Widge, A.S.; Rodriguez, C.I.; Kalin, N.H.; McDonald, W.M. Psychedelics and Psychedelic-Assisted Psychotherapy. Am. J. Psychiatry 2020, 177, 391–410. [Google Scholar] [CrossRef]
- Noorani, T. Making psychedelics into medicines: The politics and paradoxes of medicalization. J. Psychedelic Stud. 2020, 4, 34–39. [Google Scholar] [CrossRef] [Green Version]
- Nichols, D.E. Hallucinogens. Pharmacol. Ther. 2004, 101, 131–181. [Google Scholar] [CrossRef]
- Mithoefer, M.C.; Grob, C.S.; Brewerton, T.D. Novel psychopharmacological therapies for psychiatric disorders: Psilocybin and MDMA. Lancet Psychiatry 2016, 3, 481–488. [Google Scholar] [CrossRef]
- Moreno, F.A.; Wiegand, C.B.; Taitano, E.K.; Delgado, P.L. Safety, tolerability, and efficacy of psilocybin in 9 patients with obsessive-compulsive disorder. J. Clin. Psychiatry 2006, 67, 1735–1740. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carhart-Harris, R.L.; Bolstridge, M.; Rucker, J.; Day, C.M.J.; Erritzoe, D.; Kaelen, M.; Bloomfield, M.; Rickard, J.A.; Forbes, B.; Feilding, A.; et al. Psilocybin with psychological support for treatment-resistant depression: An open-label feasibility study. Lancet Psychiatry 2016, 3, 619–627. [Google Scholar] [CrossRef] [Green Version]
- Strumila, R.; Nobile, B.; Korsakova, L.; Lengvenyte, A.; Olie, E.; Lopez-Castroman, J.; Guillaume, S.; Courtet, P. Psilocybin, a naturally occurring indoleamine compound, could be useful to prevent suicidal behaviors. Pharmaceuticals 2021, 14, 1213. [Google Scholar] [CrossRef] [PubMed]
- Slocum, S.T.; DiBerto, J.F.; Roth, B.L. Molecular insights into psychedelic drug action. J. Neurochem. 2021. [Google Scholar] [CrossRef]
- Hwang, K.A.J.; Saadabadi, A. Lysergic Acid Diethylamide (LSD); StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- Lee, M.A.; Shlain, B. Acid Dreams: The Complete Social History of LSD: The CIA, the Sixties, and Beyond; Grove Weidenfeld: New York, NY, USA, 1992. [Google Scholar]
- Mashour, G.A. From LSD to the IRB: Henry Beecher’s psychedelic research and the foundation of clinical ethics. Int. Anesthesiol. Clin. 2007, 45, 105–111. [Google Scholar] [CrossRef]
- Nutt, D. Mind-altering drugs and research: From presumptive prejudice to a Neuroscientific Enlightenment?: Science & Society series on “Drugs and Science”. EMBO Rep. 2014, 15, 208–211. [Google Scholar] [CrossRef] [Green Version]
- Dyck, E. Flashback: Psychiatric experimentation with LSD in historical perspective. Can. J. Psychiatry 2005, 50, 381–388. [Google Scholar] [CrossRef]
- Kvam, T.-M.; Stewart, L.H.; Andreassen, O.A. Psychedelic drugs in the treatment of anxiety, depression and addiction. Tidsskr. Den Nor. Laegeforening 2018, 138. [Google Scholar] [CrossRef] [Green Version]
- Fuentes, J.J.; Fonseca, F.; Elices, M.; Farré, M.; Torrens, M. Therapeutic Use of LSD in Psychiatry: A Systematic Review of Randomized-Controlled Clinical Trials. Front. Psychiatry 2020, 10, 943. [Google Scholar] [CrossRef] [Green Version]
- Ramaekers, J.G.; Hutten, N.; Mason, N.L.; Dolder, P.; Theunissen, E.L.; Holze, F.; Liechti, M.E.; Feilding, A.; Kuypers, K.P. A low dose of lysergic acid diethylamide decreases pain perception in healthy volunteers. J. Psychopharmacol. 2021, 35, 398–405. [Google Scholar] [CrossRef] [PubMed]
- Holze, F.; Avedisian, I.; Varghese, N.; Eckert, A.; Liechti, M.E. Role of the 5-HT(2A) Receptor in Acute Effects of LSD on Empathy and Circulating Oxytocin. Front. Pharmacol. 2021, 12, 711255. [Google Scholar] [CrossRef] [PubMed]
- Gasser, P.; Holstein, D.; Michel, Y.; Doblin, R.; Yazar-Klosinski, B.; Passie, T.; Brenneisen, R. Safety and efficacy of lysergic acid diethylamide-assisted psychotherapy for anxiety associated with life-threatening diseases. J. Nerv. Ment. Dis. 2014, 202, 513–520. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gasser, P.; Kirchner, K.; Passie, T. LSD-assisted psychotherapy for anxiety associated with a life-threatening disease: A qualitative study of acute and sustained subjective effects. J. Psychopharmacol. 2015, 29, 57–68. [Google Scholar] [CrossRef] [PubMed]
- Family, N.; Maillet, E.L.; Williams, L.T.J.; Krediet, E.; Carhart-Harris, R.L.; Williams, T.M.; Nichols, C.D.; Goble, D.J.; Raz, S. Safety, tolerability, pharmacokinetics, and pharmacodynamics of low dose lysergic acid diethylamide (LSD) in healthy older volunteers. Psychopharmacology 2020, 237, 841–853. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carhart-Harris, R.L.; Kaelen, M.; Whalley, M.G.; Bolstridge, M.; Feilding, A.; Nutt, D.J. LSD enhances suggestibility in healthy volunteers. Psychopharmacology 2015, 232, 785–794. [Google Scholar] [CrossRef]
- Kaelen, M.; Barrett, F.S.; Roseman, L.; Lorenz, R.; Family, N.; Bolstridge, M.; Curran, H.V.; Feilding, A.; Nutt, D.J.; Carhart-Harris, R.L. LSD enhances the emotional response to music. Psychopharmacology 2015, 232, 3607–3614. [Google Scholar] [CrossRef]
- Hwang, K.A.J.; Saadabadi, A. Lysergic Acid Diethylamide (LSD); StatPearls Publishing: Treasure Island, FL, USA, 2018. [Google Scholar]
- Kraehenmann, R.; Pokorny, D.; Aicher, H.; Preller, K.H.; Pokorny, T.; Bosch, O.G.; Seifritz, E.; Vollenweider, F.X. LSD Increases Primary Process Thinking via Serotonin 2A Receptor Activation. Front. Pharmacol. 2017, 8, 814. [Google Scholar]
- Preller, K.H.; Burt, J.B.; Ji, J.L.; Schleifer, C.H.; Adkinson, B.D.; Stämpfli, P.; Seifritz, E.; Repovs, G.; Krystal, J.H.; Murray, J.D. Changes in global and thalamic brain connectivity in LSD-induced altered states of consciousness are attributable to the 5-HT2A receptor. eLife 2018, 7, e35082. [Google Scholar] [CrossRef]
- Patterson, D.R.; Jensen, M.P. Hypnosis and clinical pain. Psychol. Bull. 2003, 129, 495–521. [Google Scholar] [CrossRef]
- Kirsch, I.; Low, C.B. Suggestion in the treatment of depression. Am. J. Clin. Hypn. 2013, 55, 221–229. [Google Scholar] [CrossRef] [PubMed]
- Geyer, M.A. Lysergic Acid Diethylamide and Psilocybin Revisited. Biol. Psychiatry 2015, 78, 516–518. [Google Scholar] [CrossRef] [PubMed]
- Karch, S.B. A historical review of MDMA. Open Forensic Sci. J. 2011, 4, 20–24. [Google Scholar] [CrossRef]
- Garcia-Romeu, A.; Kersgaard, B.; Addy, P.H. Clinical applications of hallucinogens: A review. Exp. Clin. Psychopharmacol. 2016, 24, 229–268. [Google Scholar] [CrossRef] [PubMed]
- Jardim, A.V.; Jardim, D.V.; Chaves, B.R.; Steglich, M.; Ot’alora, G.M.; Mithoefer, M.C.; da Silveira, D.X.; Tófoli, L.F.; Ribeiro, S.; Matthews, R. 3, 4-methylenedioxymethamphetamine (MDMA)-assisted psychotherapy for victims of sexual abuse with severe post-traumatic stress disorder: An open label pilot study in Brazil. Braz. J. Psychiatry 2020, 43, 181–185. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, J.M.; Bogenschutz, M.; Lilienstein, A.; Harrison, C.; Kleiman, S.; Parker-Guilbert, K.; Ot’alora, G.M.; Garas, W.; Paleos, C.; Gorman, I. MDMA-assisted therapy for severe PTSD: A randomized, double-blind, placebo-controlled phase 3 study. Nat. Med. 2021, 27, 1025–1033. [Google Scholar] [CrossRef]
- Passie, T.; Halpern, J.H.; Stichtenoth, D.O.; Emrich, H.M.; Hintzen, A. The pharmacology of lysergic acid diethylamide: A review. CNS Neurosci. Ther. 2008, 14, 295–314. [Google Scholar] [CrossRef]
- Passie, T.; Seifert, J.; Schneider, U.; Emrich, H.M. The pharmacology of psilocybin. Addict. Biol. 2002, 7, 357–364. [Google Scholar] [CrossRef]
- Bunzow, J.R.; Sonders, M.S.; Arttamangkul, S.; Harrison, L.M.; Zhang, G.; Quigley, D.I.; Darland, T.; Suchland, K.L.; Pasumamula, S.; Kennedy, J.L.; et al. Amphetamine, 3,4-methylenedioxymethamphetamine, lysergic acid diethylamide, and metabolites of the catecholamine neurotransmitters are agonists of a rat trace amine receptor. Mol. Pharmacol. 2001, 60, 1181–1188. [Google Scholar] [CrossRef]
- Fontanilla, D.; Johannessen, M.; Hajipour, A.R.; Cozzi, N.V.; Jackson, M.B.; Ruoho, A.E. The hallucinogen N,N-dimethyltryptamine (DMT) is an endogenous sigma-1 receptor regulator. Science 2009, 323, 934–937. [Google Scholar] [CrossRef] [Green Version]
- Franzen, F.R.; Gross, H. Tryptamine, N,N-Dimethyltryptamine, N,N-Dimethyl-5-hydroxytryptamine and 5-Methoxytryptamine in Human Blood and Urine. Nature 1965, 206, 1052. [Google Scholar] [CrossRef] [PubMed]
- Barker, S.A.; McIlhenny, E.H.; Strassman, R. A critical review of reports of endogenous psychedelic N, N-dimethyltryptamines in humans: 1955–2010. Drug Test. Anal. 2012, 4, 617–635. [Google Scholar] [CrossRef] [PubMed]
- Barker, S.A.; Monti, J.A.; Christian, S.T. Metabolism of the hallucinogen N,N-dimethyltryptamine in rat brain homogenates. Biochem. Pharmacol. 1980, 29, 1049–1057. [Google Scholar] [CrossRef]
- Barker, S.A.; Borjigin, J.; Lomnicka, I.; Strassman, R. LC/MS/MS analysis of the endogenous dimethyltryptamine hallucinogens, their precursors, and major metabolites in rat pineal gland microdialysate. Biomed. Chromatogr. BMC 2013, 27, 1690–1700. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Christian, S.T.; Harrison, R.; Quayle, E.; Pagel, J.; Monti, J. The in vitro identification of dimethyltryptamine (DMT) in mammalian brain and its characterization as a possible endogenous neuroregulatory agent. Biochem. Med. 1977, 18, 164–183. [Google Scholar] [CrossRef]
- Thompson, M.A.; Moon, E.; Kim, U.J.; Xu, J.; Siciliano, M.J.; Weinshilboum, R.M. Human indolethylamine N-methyltransferase: cDNA cloning and expression, gene cloning, and chromosomal localization. Genomics 1999, 61, 285–297. [Google Scholar] [CrossRef]
- Wallach, J.V. Endogenous hallucinogens as ligands of the trace amine receptors: A possible role in sensory perception. Med. Hypotheses 2009, 72, 91–94. [Google Scholar] [CrossRef]
- Grof, S.; Soskin, R.A.; Richards, W.A.; Kurland, A.A. DPT as an adjunct in psychotherapy of alcoholics. Int. Pharm. 1973, 8, 104–115. [Google Scholar] [CrossRef]
- Rhead, J.; Turek, I.; Richards, W.; Yensen, R.; Kurland, A.; Ota, K. Psychedelic Drug (DPT)-Assisted Psychotherapy with Alcoholics: A Controlled Study. J. Psychedelic Drugs 1977, 9, 287–300. [Google Scholar] [CrossRef]
- Soskin, R.A.; Grof, S.; Richards, W.A. Low doses of dipropyltryptamine in psychotherapy. Arch. Gen. Psychiatry 1973, 28, 817–821. [Google Scholar] [CrossRef]
- Richards, W.A. Mystical and archetypal experiences of terminal patients in DPT-assisted psychotherapy. J. Relig. Health 1978, 17, 117–126. [Google Scholar] [CrossRef] [PubMed]
- Richards, W.A.; Rhead, J.C.; Dileo, F.B.; Yensen, R.; Kurland, A.A. The Peak Experience Variable in DPT-Assisted Psychotherapy with Cancer Patients. J. Psychedelic Drugs 1977, 9, 1–10. [Google Scholar] [CrossRef]
- Richards, W.A.; Rhead, J.C.; Grof, S.; Goodman, L.E.; Di Leo, F.; Rush, L. DPT as an Adjunct in Brief Psychotherapy with Cancer Patients. OMEGA—J. Death Dying 1980, 10, 9–26. [Google Scholar] [CrossRef]
- Thompson, C.; Szabo, A. Psychedelics as a novel approach to treating autoimmune conditions. Immunol. Lett. 2020, 228, 45–54. [Google Scholar] [CrossRef] [PubMed]
- Heekeren, K.; Neukirch, A.; Daumann, J.; Stoll, M.; Obradovic, M.; Kovar, K.-A.; Geyer, M.A.; Gouzoulis-Mayfrank, E. Prepulse inhibition of the startle reflex and its attentional modulation in the human S-ketamine and N,N-dimethyltryptamine (DMT) models of psychosis. J. Psychopharmacol. 2007, 21, 312–320. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Romeu, A.; Griffiths, R.R.; Johnson, M.W. Psilocybin-occasioned mystical experiences in the treatment of tobacco addiction. Curr. Drug Abus. Rev. 2014, 7, 157–164. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barker, S.A. Administration of N,N-dimethyltryptamine (DMT) in psychedelic therapeutics and research and the study of endogenous DMT. Psychopharmacology 2022, 1–15. [Google Scholar] [CrossRef]
- Heffter, A. Ueber cacteenalkaloide. Ber. Dtsch. Chem. Ges. 1896, 29, 216–227. [Google Scholar] [CrossRef]
- Rinkel, M. Pharmacodynamics of LSD and mescaline. J. Nerv. Ment. Dis. 1957, 125, 424–427. [Google Scholar] [CrossRef]
- Hollister, L.E.; Hartman, A.M. Mescaline, lysergic acid diethylamide and psilocybin comparison of clinical syndromes, effects on color perception and biochemical measures. Compr. Psychiatry 1962, 3, 235–242. [Google Scholar] [CrossRef]
- Prue, B. Indigenous Supports for Recovery from Alcoholism and Drug Abuse: The Native American Church. J. Ethn. Cult. Divers. Soc. Work 2013, 22, 271–287. [Google Scholar] [CrossRef]
- Halpern, J.H.; Sherwood, A.R.; Hudson, J.I.; Yurgelun-Todd, D.; Pope, H.G.J. Psychological and cognitive effects of long-term peyote use among Native Americans. Biol. Psychiatry 2005, 58, 624–631. [Google Scholar] [CrossRef] [PubMed]
- Morris, B.J.; Cochran, S.M.; Pratt, J.A. PCP: From pharmacology to modelling schizophrenia. Curr. Opin. Pharmacol. 2005, 5, 101–106. [Google Scholar] [CrossRef] [PubMed]
- Kristiansen, L.V.; Huerta, I.; Beneyto, M.; Meador-Woodruff, J.H. NMDA receptors and schizophrenia. Curr. Opin. Pharmacol. 2007, 7, 48–55. [Google Scholar] [CrossRef]
- García-Gutiérrez, M.S.; Navarrete, F.; Sala, F.; Gasparyan, A.; Austrich-Olivares, A.; Manzanares, J. Biomarkers in Psychiatry: Concept, Definition, Types and Relevance to the Clinical Reality. Front. Psychiatry 2020, 11, 432. [Google Scholar] [CrossRef]
- George, O.; Koob, G.F. Individual differences in prefrontal cortex function and the transition from drug use to drug dependence. Neurosci. Biobehav. Rev. 2010, 35, 232–247. [Google Scholar] [CrossRef] [Green Version]
- Chase, H.W.; Eickhoff, S.B.; Laird, A.R.; Hogarth, L. The neural basis of drug stimulus processing and craving: An activation likelihood estimation meta-analysis. Biol. Psychiatry 2011, 70, 785–793. [Google Scholar] [CrossRef] [Green Version]
- Hayashi, T.; Ko, J.H.; Strafella, A.P.; Dagher, A. Dorsolateral prefrontal and orbitofrontal cortex interactions during self-control of cigarette craving. Proc. Natl. Acad. Sci. USA 2013, 110, 4422–4427. [Google Scholar] [CrossRef] [Green Version]
- Kühn, S.; Gallinat, J. Common biology of craving across legal and illegal drugs—A quantitative meta-analysis of cue-reactivity brain response. Eur. J. Neurosci. 2011, 33, 1318–1326. [Google Scholar] [CrossRef]
- Habelt, B.; Arvaneh, M.; Bernhardt, N.; Minev, I. Biomarkers and neuromodulation techniques in substance use disorders. Bioelectron. Med. 2020, 6, 4. [Google Scholar] [CrossRef]
- Vollstädt-Klein, S.; Loeber, S.; Richter, A.; Kirsch, M.; Bach, P.; von der Goltz, C.; Hermann, D.; Mann, K.; Kiefer, F. Validating incentive salience with functional magnetic resonance imaging: Association between mesolimbic cue reactivity and attentional bias in alcohol-dependent patients. Addict. Biol. 2012, 17, 807–816. [Google Scholar] [CrossRef] [PubMed]
- Ward, R.J.; Lallemand, F.; de Witte, P. Biochemical and Neurotransmitter Changes Implicated in Alcohol-Induced Brain Damage in Chronic or ‘Binge Drinking’ Alcohol Abuse. Alcohol Alcohol. 2009, 44, 128–135. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Belmer, A.; Patkar, O.L.; Pitman, K.M.; Bartlett, S.E. Serotonergic Neuroplasticity in Alcohol Addiction. Brain Plast. 2016, 1, 177–206. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Müller, C.P.; Pum, M.E.; Schumann, G.; Huston, J.P. The role of serotonin in drug addiction. In Handbook of Behavioral Neuroscience; Elsevier: Amsterdam, The Netherlands, 2010; Volume 21, pp. 507–545. ISBN 1569-7339. [Google Scholar]
- Gladkevich, A.; Kauffman, H.F.; Korf, J. Lymphocytes as a neural probe: Potential for studying psychiatric disorders. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2004, 28, 559–576. [Google Scholar] [CrossRef] [PubMed]
- Quan, N.; Herkenham, M. Connecting cytokines and brain: A review of current issues. Histol. Histopathol. 2002, 17, 273–288. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.-N.; Lee, C.-S.; Wu, B.-J.; Sun, H.-J.; Chang, C.-H.; Chen, C.-Y.; Chen, C.-K.; Wu, L.S.-H.; Cheng, A.T.-A. Immunophenotypes associated with bipolar disorder and lithium treatment. Sci. Rep. 2019, 9, 17453. [Google Scholar] [CrossRef]
- Wang, Z.; Gerstein, M.; Snyder, M. RNA-Seq: A revolutionary tool for transcriptomics. Nat. Rev. Genet. 2009, 10, 57–63. [Google Scholar] [CrossRef]
- Hodgson, K.; Tansey, K.E.; Powell, T.R.; Coppola, G.; Uher, R.; Zvezdana Dernovšek, M.; Mors, O.; Hauser, J.; Souery, D.; Maier, W.; et al. Transcriptomics and the mechanisms of antidepressant efficacy. Eur. Neuropsychopharmacol. 2016, 26, 105–112. [Google Scholar] [CrossRef]
- Viola, T.W.; Heberle, B.A.; Zaparte, A.; Sanvicente-Vieira, B.; Wainer, L.M.; Fries, G.R.; Walss-Bass, C.; Grassi-Oliveira, R. Peripheral blood microRNA levels in females with cocaine use disorder. J. Psychiatr. Res. 2019, 114, 48–54. [Google Scholar] [CrossRef]
- Frantzi, M.; Bhat, A.; Latosinska, A. Clinical proteomic biomarkers: Relevant issues on study design & technical considerations in biomarker development. Clin. Transl. Med. 2014, 3, 7. [Google Scholar] [CrossRef] [Green Version]
- Nascimento, J.M.; Martins-de-Souza, D. The proteome of schizophrenia. NPJ Schizophr. 2015, 1, 14003. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Comes, A.L.; Papiol, S.; Mueller, T.; Geyer, P.E.; Mann, M.; Schulze, T.G. Proteomics for blood biomarker exploration of severe mental illness: Pitfalls of the past and potential for the future. Transl. Psychiatry 2018, 8, 160. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, R.; Liang, J.; Luo, Y.; Wan, X.; Li, K.; Qi, L.; Yuan, W.; Chen, J.; Wu, Z.; Wang, M.; et al. Mass spectrometry identification of potential biomarker proteins in the 150-kD electrophoretic band in patients with schizophrenia. Medicine 2018, 97, e13553. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues-Amorim, D.; Rivera-Baltanás, T.; Vallejo-Curto, M.D.C.; Rodriguez-Jamardo, C.; de Las Heras, E.; Barreiro-Villar, C.; Blanco-Formoso, M.; Fernández-Palleiro, P.; Álvarez-Ariza, M.; López, M.; et al. Proteomics in Schizophrenia: A Gateway to Discover Potential Biomarkers of Psychoneuroimmune Pathways. Front. Psychiatry 2019, 10, 885. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nasca, C.; Bigio, B.; Lee, F.S.; Young, S.P.; Kautz, M.M.; Albright, A.; Beasley, J.; Millington, D.S.; Mathé, A.A.; Kocsis, J.H.; et al. Acetyl-l-carnitine deficiency in patients with major depressive disorder. Proc. Natl. Acad. Sci. USA 2018, 115, 8627–8632. [Google Scholar] [CrossRef] [Green Version]
- Nilsson, I.A.K.; Millischer, V.; Karrenbauer, V.D.; Juréus, A.; Salehi, A.M.; Norring, C.; von Hausswolff-Juhlin, Y.; Schalling, M.; Blennow, K.; Bulik, C.M.; et al. Plasma neurofilament light chain concentration is increased in anorexia nervosa. Transl. Psychiatry 2019, 9, 180. [Google Scholar] [CrossRef] [Green Version]
- Katisko, K.; Cajanus, A.; Jääskeläinen, O.; Kontkanen, A.; Hartikainen, P.; Korhonen, V.E.; Helisalmi, S.; Haapasalo, A.; Koivumaa-Honkanen, H.; Herukka, S.-K.; et al. Serum neurofilament light chain is a discriminative biomarker between frontotemporal lobar degeneration and primary psychiatric disorders. J. Neurol. 2020, 267, 162–167. [Google Scholar] [CrossRef] [Green Version]
- Quintero, M.; Stanisic, D.; Cruz, G.; Pontes, J.G.M.; Costa, T.B.B.C.; Tasic, L. Metabolomic Biomarkers in Mental Disorders: Bipolar Disorder and Schizophrenia. Adv. Exp. Med. Biol. 2019, 1118, 271–293. [Google Scholar] [CrossRef]
- Shih, P.-A.B. Metabolomics Biomarkers for Precision Psychiatry. Adv. Exp. Med. Biol. 2019, 1161, 101–113. [Google Scholar] [CrossRef]
- García-Giménez, J.L. Epigenetic Biomarkers and Diagnostics; Academic Press: Cambridge, MA, USA, 2015; ISBN 0128019212. [Google Scholar]
- García-Giménez, J.L.; Seco-Cervera, M.; Tollefsbol, T.O.; Romá-Mateo, C.; Peiró-Chova, L.; Lapunzina, P.; Pallardó, F.V. Epigenetic biomarkers: Current strategies and future challenges for their use in the clinical laboratory. Crit. Rev. Clin. Lab. Sci. 2017, 54, 529–550. [Google Scholar] [CrossRef]
- Smigielski, L.; Jagannath, V.; Rössler, W.; Walitza, S.; Grünblatt, E. Epigenetic mechanisms in schizophrenia and other psychotic disorders: A systematic review of empirical human findings. Mol. Psychiatry 2020, 25, 1718–1748. [Google Scholar] [CrossRef] [PubMed]
- Ikegame, T.; Bundo, M.; Murata, Y.; Kasai, K.; Kato, T.; Iwamoto, K. DNA methylation of the BDNF gene and its relevance to psychiatric disorders. J. Hum. Genet. 2013, 58, 434–438. [Google Scholar] [CrossRef] [PubMed]
- Angelucci, F.; Brenè, S.; Mathé, A.A. BDNF in schizophrenia, depression and corresponding animal models. Mol. Psychiatry 2005, 10, 345–352. [Google Scholar] [CrossRef] [Green Version]
- Kang, J.I.; Kim, T.Y.; Choi, J.H.; So, H.S.; Kim, S.J. Allele-specific DNA methylation level of FKBP5 is associated with post-traumatic stress disorder. Psychoneuroendocrinology 2019, 103, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.J.; Kim, Y.-K. Panic disorders: The role of genetics and epigenetics. AIMS Genet. 2018, 5, 177–190. [Google Scholar] [CrossRef]
- Cheung, S.; Woo, J.; Maes, M.S.; Zai, C.C. Suicide epigenetics, a review of recent progress. J. Affect. Disord. 2020, 265, 423–438. [Google Scholar] [CrossRef]
- Park, H.-S.; Kim, J.; Ahn, S.H.; Ryu, H.-Y. Epigenetic Targeting of Histone Deacetylases in Diagnostics and Treatment of Depression. Int. J. Mol. Sci. 2021, 22, 5398. [Google Scholar] [CrossRef]
- Schroeder, M.; Hillemacher, T.; Bleich, S.; Frieling, H. The epigenetic code in depression: Implications for treatment. Clin. Pharmacol. Ther. 2012, 91, 310–314. [Google Scholar] [CrossRef]
- Machado-Vieira, R.; Ibrahim, L.; Zarate, C.A.J. Histone deacetylases and mood disorders: Epigenetic programming in gene-environment interactions. CNS Neurosci. Ther. 2011, 17, 699–704. [Google Scholar] [CrossRef]
- Toda, M.; Abi-Dargham, A. Dopamine hypothesis of schizophrenia: Making sense of it all. Curr. Psychiatry Rep. 2007, 9, 329–336. [Google Scholar] [CrossRef]
- Pletnikov, M.; Waddington, J. Modeling the Psychopathological Dimensions of Schizophrenia: From Molecules to Behavior; Academic Press: Cambridge, MA, USA, 2015. [Google Scholar]
- Halberstadt, A.L. Recent advances in the neuropsychopharmacology of serotonergic hallucinogens. Behav. Brain Res. 2015, 277, 99–120. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vollenweider, F.X.; Vollenweider-Scherpenhuyzen, M.F.; Bäbler, A.; Vogel, H.; Hell, D. Psilocybin induces schizophrenia-like psychosis in humans via a serotonin-2 agonist action. Neuroreport 1998, 9, 3897–3902. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Preller, K.H.; Herdener, M.; Pokorny, T.; Planzer, A.; Kraehenmann, R.; Stämpfli, P.; Liechti, M.E.; Seifritz, E.; Vollenweider, F.X. The Fabric of Meaning and Subjective Effects in LSD-Induced States Depend on Serotonin 2A Receptor Activation. Curr. Biol. 2017, 27, 451–457. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Halberstadt, A.L.; Chatha, M.; Klein, A.K.; Wallach, J.; Brandt, S.D. Correlation between the potency of hallucinogens in the mouse head-twitch response assay and their behavioral and subjective effects in other species. Neuropharmacology 2020, 167, 107933. [Google Scholar] [CrossRef]
- Pokorny, T.; Preller, K.H.; Kraehenmann, R.; Vollenweider, F.X. Modulatory effect of the 5-HT1A agonist buspirone and the mixed non-hallucinogenic 5-HT1A/2A agonist ergotamine on psilocybin-induced psychedelic experience. Eur. Neuropsychopharmacol. 2016, 26, 756–766. [Google Scholar] [CrossRef]
- Strassman, R.J. Human psychopharmacology of N,N-dimethyltryptamine. Behav. Brain Res. 1996, 73, 121–124. [Google Scholar] [CrossRef]
- Vollenweider, F.X.; Vontobel, P.; Hell, D.; Leenders, K.L. 5-HT modulation of dopamine release in basal ganglia in psilocybin-induced psychosis in man—A PET study with [11C]raclopride. Neuropsychopharmacology 1999, 20, 424–433. [Google Scholar] [CrossRef] [Green Version]
- De Gregorio, D.; Comai, S.; Posa, L.; Gobbi, G. d-Lysergic Acid Diethylamide (LSD) as a Model of Psychosis: Mechanism of Action and Pharmacology. Int. J. Mol. Sci. 2016, 17, 1953. [Google Scholar] [CrossRef] [Green Version]
- Halberstadt, A.L.; Geyer, M.A. Characterization of the head-twitch response induced by hallucinogens in mice: Detection of the behavior based on the dynamics of head movement. Psychopharmacology 2013, 227, 727–739. [Google Scholar] [CrossRef]
- González-Maeso, J.; Weisstaub, N.V.; Zhou, M.; Chan, P.; Ivic, L.; Ang, R.; Lira, A.; Bradley-Moore, M.; Ge, Y.; Zhou, Q.; et al. Hallucinogens recruit specific cortical 5-HT(2A) receptor-mediated signaling pathways to affect behavior. Neuron 2007, 53, 439–452. [Google Scholar] [CrossRef] [Green Version]
- Kenakin, T. Ligand-selective receptor conformations revisited: The promise and the problem. Trends Pharmacol. Sci. 2003, 24, 346–354. [Google Scholar] [CrossRef]
- Colpaert, F.C. Discovering risperidone: The LSD model of psychopathology. Nat. Rev. 2003, 2, 315–320. [Google Scholar] [CrossRef] [PubMed]
- Chaki, S.; Yoshida, S.; Okuyama, S. Targeting metabotropic glutamate receptors to develop novel antipsychotics. Jpn. J. Psychopharmacol. 2010, 30, 207–213. [Google Scholar]
- Patil, S.T.; Zhang, L.; Martenyi, F.; Lowe, S.L.; Jackson, K.A.; Andreev, B.V.; Avedisova, A.S.; Bardenstein, L.M.; Gurovich, I.Y.; Morozova, M.A.; et al. Activation of mGlu2/3 receptors as a new approach to treat schizophrenia: A randomized Phase 2 clinical trial. Nat. Med. 2007, 13, 1102–1107. [Google Scholar] [CrossRef] [PubMed]
- Nicoletti, F.; Orlando, R.; Di Menna, L.; Cannella, M.; Notartomaso, S.; Mascio, G.; Iacovelli, L.; Matrisciano, F.; Fazio, F.; Caraci, F.; et al. Targeting mGlu Receptors for Optimization of Antipsychotic Activity and Disease-Modifying Effect in Schizophrenia. Front. Psychiatry 2019, 10, 49. [Google Scholar] [CrossRef]
- Nabeshima, T.; Ishikawa, K.; Yamaguchi, K.; Furukawa, H.; Kameyama, T. Phencyclidine-induced head-twitch responses as 5-HT2 receptor-mediated behavior in rats. Neurosci. Lett. 1987, 76, 335–338. [Google Scholar] [CrossRef]
- Kim, H.S.; Park, I.S.; Park, W.K. NMDA receptor antagonists enhance 5-HT2 receptor-mediated behavior, head-twitch response, in mice. Life Sci. 1998, 63, 2305–2311. [Google Scholar] [CrossRef]
- Carlsson, M.L.; Martin, P.; Nilsson, M.; Sorensen, S.M.; Carlsson, A.; Waters, S.; Waters, N. The 5-HT2A receptor antagonist M100907 is more effective in counteracting NMDA antagonist- than dopamine agonist-induced hyperactivity in mice. J. Neural Transm. 1999, 106, 123–129. [Google Scholar] [CrossRef]
- Kargieman, L.; Santana, N.; Mengod, G.; Celada, P.; Artigas, F. Antipsychotic drugs reverse the disruption in prefrontal cortex function produced by NMDA receptor blockade with phencyclidine. Proc. Natl. Acad. Sci. USA 2007, 104, 14843–14848. [Google Scholar] [CrossRef] [Green Version]
- Schmid, C.L.; Raehal, K.M.; Bohn, L.M. Agonist-directed signaling of the serotonin 2A receptor depends on β-arrestin-2 interactions in vivo. Proc. Natl. Acad. Sci. USA 2008, 105, 1079–1084. [Google Scholar] [CrossRef] [Green Version]
- Mahmood, D.; Khanam, R.; Pillai, K.K.; Akhtar, M. Protective effects of histamine H3-receptor ligands in schizophrenic behaviors in experimental models. Pharmacol. Rep. 2012, 64, 191–204. [Google Scholar] [CrossRef] [PubMed]
- Mahmood, D. Histamine H(3) receptors and its antagonism as a novel mechanism for antipsychotic effect: A current preclinical & clinical perspective. Int. J. Health Sci. 2016, 10, 564–575. [Google Scholar]
- Olson, D.E. Psychoplastogens: A Promising Class of Plasticity-Promoting Neurotherapeutics. J. Exp. Neurosci. 2018, 12, 1179069518800508. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marek, G.J. Interactions of Hallucinogens with the Glutamatergic System: Permissive Network Effects Mediated Through Cortical Layer V Pyramidal Neurons. Curr. Top. Behav. Neurosci. 2018, 36, 107–135. [Google Scholar] [CrossRef] [PubMed]
- Vaidya, V.A.; Marek, G.J.; Aghajanian, G.K.; Duman, R.S. 5-HT2A receptor-mediated regulation of brain-derived neurotrophic factor mRNA in the hippocampus and the neocortex. J. Neurosci. 1997, 17, 2785–2795. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De la Fuente Revenga, M.; Zhu, B.; Guevara, C.A.; Naler, L.B.; Saunders, J.M.; Zhou, Z.; Toneatti, R.; Sierra, S.; Wolstenholme, J.T.; Beardsley, P.M.; et al. Prolonged epigenomic and synaptic plasticity alterations following single exposure to a psychedelic in mice. Cell Rep. 2021, 37, 109836. [Google Scholar] [CrossRef]
- Ly, C.; Greb, A.C.; Cameron, L.P.; Wong, J.M.; Barragan, E.V.; Wilson, P.C.; Burbach, K.F.; Soltanzadeh Zarandi, S.; Sood, A.; Paddy, M.R.; et al. Psychedelics Promote Structural and Functional Neural Plasticity. Cell Rep. 2018, 23, 3170–3182. [Google Scholar] [CrossRef]
- Hutten, N.R.P.W.; Mason, N.L.; Dolder, P.C.; Theunissen, E.L.; Holze, F.; Liechti, M.E.; Varghese, N.; Eckert, A.; Feilding, A.; Ramaekers, J.G.; et al. Low Doses of LSD Acutely Increase BDNF Blood Plasma Levels in Healthy Volunteers. ACS Pharmacol. Transl. Sci. 2021, 4, 461–466. [Google Scholar] [CrossRef]
- Barrett, F.S.; Doss, M.K.; Sepeda, N.D.; Pekar, J.J.; Griffiths, R.R. Emotions and brain function are altered up to one month after a single high dose of psilocybin. Sci. Rep. 2020, 10, 2214. [Google Scholar] [CrossRef]
- Hall, W. The need for publicly funded research on therapeutic use of psychedelic drugs. World Psychiatry 2021, 20, 197–198. [Google Scholar] [CrossRef]
- Hall, W.; Lynskey, M. Assessing the public health impacts of legalizing recreational cannabis use: The US experience. World Psychiatry 2020, 19, 179–186. [Google Scholar] [CrossRef] [PubMed]
- Pilecki, B.; Luoma, J.B.; Bathje, G.J.; Rhea, J.; Narloch, V.F. Ethical and legal issues in psychedelic harm reduction and integration therapy. Harm Reduct. J. 2021, 18, 40. [Google Scholar] [CrossRef] [PubMed]
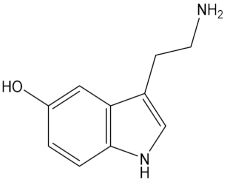 | Serotonin |
 | LSD (Lysergic acid diethylamide) |
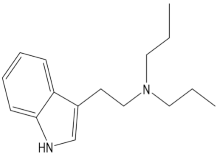 | DPT (N,N-dipropyltryptamine) |
 | Psilocybin |
 | Mescaline |
 | DMT (N,N-dimethyltryptamine) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mahmood, D.; Alenezi, S.K.; Anwar, M.J.; Azam, F.; Qureshi, K.A.; Jaremko, M. New Paradigms of Old Psychedelics in Schizophrenia. Pharmaceuticals 2022, 15, 640. https://doi.org/10.3390/ph15050640
Mahmood D, Alenezi SK, Anwar MJ, Azam F, Qureshi KA, Jaremko M. New Paradigms of Old Psychedelics in Schizophrenia. Pharmaceuticals. 2022; 15(5):640. https://doi.org/10.3390/ph15050640
Chicago/Turabian StyleMahmood, Danish, Sattam K. Alenezi, Md. Jamir Anwar, Faizul Azam, Kamal A. Qureshi, and Mariusz Jaremko. 2022. "New Paradigms of Old Psychedelics in Schizophrenia" Pharmaceuticals 15, no. 5: 640. https://doi.org/10.3390/ph15050640
APA StyleMahmood, D., Alenezi, S. K., Anwar, M. J., Azam, F., Qureshi, K. A., & Jaremko, M. (2022). New Paradigms of Old Psychedelics in Schizophrenia. Pharmaceuticals, 15(5), 640. https://doi.org/10.3390/ph15050640








