Phospholipid-Based Microemulsions for Cutaneous Imiquimod Delivery
Abstract
:1. Introduction
2. Results and Discussion
2.1. Pseudo-Ternary Phase Diagram
2.2. Conductivity Studies
2.3. Microemulsion Characterization, Rheological Behaviour, and Stability Studies
2.4. IMQ Permeation through and into Human Skin
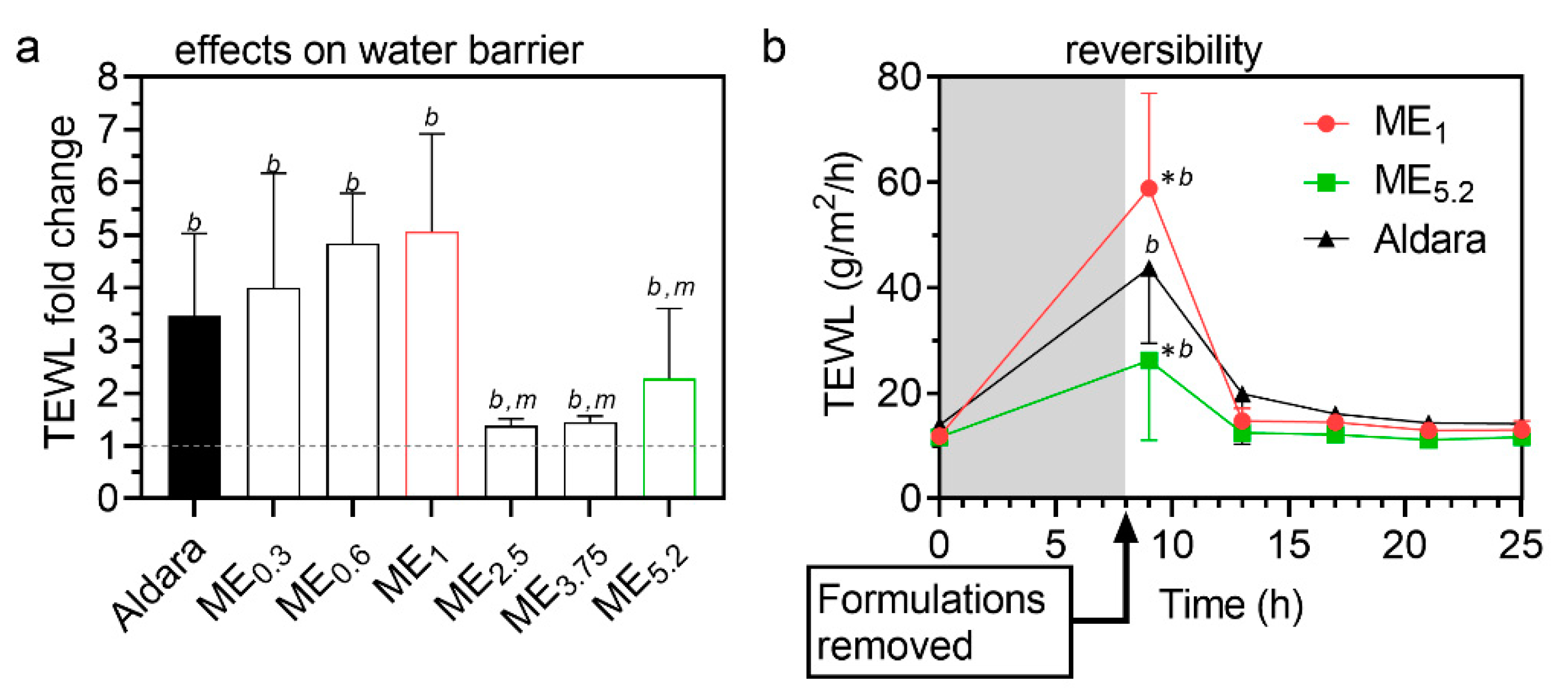
2.5. Reversibility of MEs Effects on Transepidermal Water Loss (TEWL)
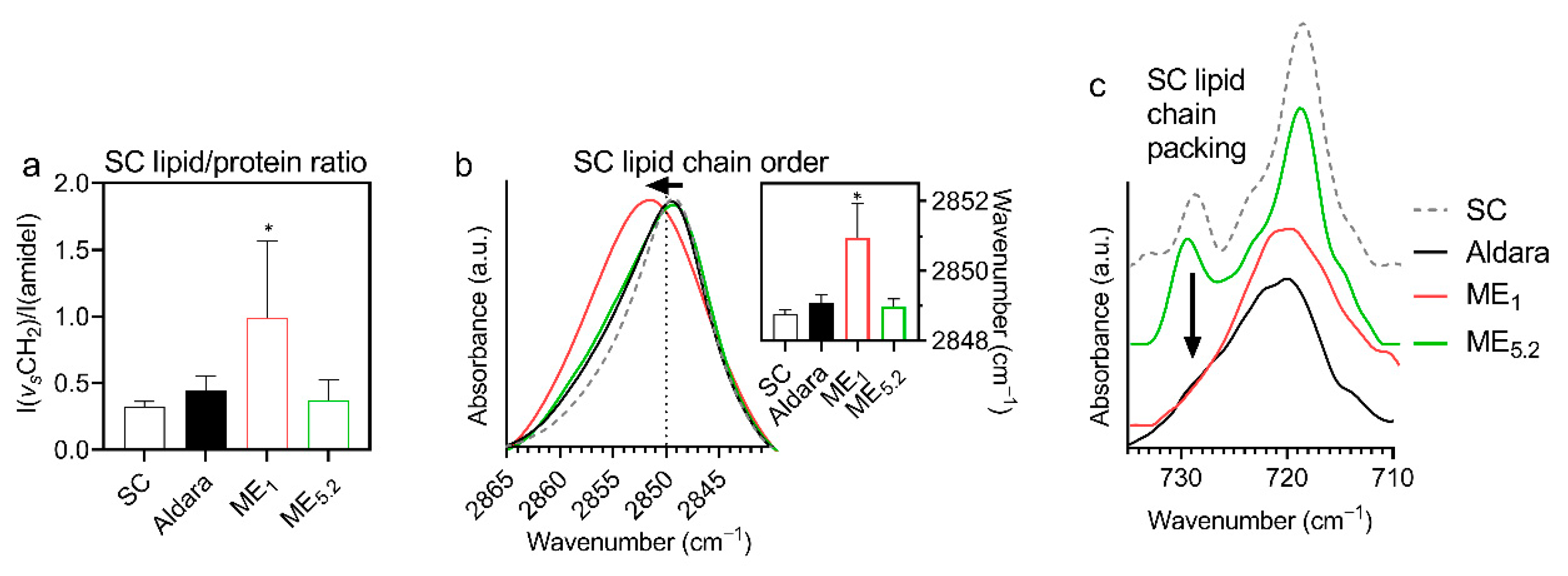
2.6. Interactions of Selected Formulations with the Human SC Studied by FTIR Spectroscopy
3. Materials and Methods
3.1. Materials
3.2. Skin
3.3. Imiquimod HPLC Quantification Method
3.4. Construction of Pseudo-Ternary Diagram
3.5. Conductivity Studies
3.6. Preparation of Selected MEs with IMQ
3.7. ME Characterization, Rheology and Stability
3.8. Skin Permeation and Deposition Experiments
3.9. ME Effects on Transepidermal Water Loss (TEWL)
3.10. Fourier Transform Infrared (FTIR) Spectroscopy
3.11. Data Treatment
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Telò, I.; Pescina, S.; Padula, C.; Santi, P.; Nicoli, S. Mechanisms of imiquimod skin penetration. Int. J. Pharm. 2016, 511, 516–523. [Google Scholar] [CrossRef] [PubMed]
- Horváth, S.; Komlódi, R.; Perkecz, A.; Pintér, E.; Gyulai, R.; Kemény, Á. Methodological refinement of Aldara-induced psoriasiform dermatitis model in mice. Sci. Rep. 2019, 9, 3685. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rossbach, K.; Wahle, K.; Bruer, G.; Brehm, R.; Langeheine, M.; Rode, K.; Schaper-Gerhardt, K.; Gutzmer, R.; Werfel, T.; Kietzmann, M.; et al. Histamine 2 Receptor Agonism and Histamine 4 Receptor Antagonism Ameliorate Inflammation in a Model of Psoriasis. Acta Derm. Venereol. 2020, 100. [Google Scholar] [CrossRef] [PubMed]
- Nerurkar, L.; McColl, A.; Graham, G.; Cavanagh, J. The Systemic Response to Topical Aldara Treatment is Mediated Through Direct TLR7 Stimulation as Imiquimod Enters the Circulation. Sci. Rep. 2017, 7, 16570. [Google Scholar] [CrossRef] [PubMed]
- Yang, R.; Xu, J.; Xu, L.; Sun, X.; Chen, Q.; Zhao, Y.; Peng, R.; Liu, Z. Cancer Cell Membrane-Coated Adjuvant Nanoparticles with Mannose Modification for Effective Anticancer Vaccination. ACS Nano 2018, 12, 5121–5129. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Wu, S.; Qin, Y.; Fan, F.; Zhang, Z.; Huang, C.; Ji, W.; Lu, L.; Wang, C.; Sun, H.; et al. Targeted Codelivery of an Antigen and Dual Agonists by Hybrid Nanoparticles for Enhanced Cancer Immunotherapy. Nano Lett. 2019, 19, 4237–4249. [Google Scholar] [CrossRef] [PubMed]
- Jiménez-Sánchez, G.; Pavot, V.; Chane-Haong, C.; Handké, N.; Terrat, C.; Gigmes, D.; Trimaille, T.; Verrier, B. Preparation and in vitro evaluation of imiquimod loaded polylactide-based micelles as potential vaccine adjuvants. Pharm. Res. 2015, 32, 311–320. [Google Scholar] [CrossRef]
- Ma, M.; Wang, J.; Guo, F.; Lei, M.; Tan, F.; Li, N. Development of nanovesicular systems for dermal imiquimod delivery: Physicochemical characterization and in vitro/in vivo evaluation. J. Mater. Sci. Mater. Med. 2015, 26, 191. [Google Scholar] [CrossRef]
- Al-Mayahy, M.H.; Sabri, A.H.; Rutland, C.S.; Holmes, A.; McKenna, J.; Marlow, M.; Scurr, D.J. Insight into imiquimod skin permeation and increased delivery using microneedle pre-treatment. Eur. J. Pharm. Biopharm. 2019, 139, 33–43. [Google Scholar] [CrossRef]
- Myhre, P.E.; Levy, M.L.; Eichenfield, L.F.; Kolb, V.B.; Fielder, S.L.; Meng, T.-C. Pharmacokinetics and Safety of Imiquimod 5% Cream in the Treatment of Molluscum Contagiosum in Children. Pediatric Dermatol. 2008, 25, 88–95. [Google Scholar] [CrossRef]
- Hanna, E.; Abadi, R.; Abbas, O. Imiquimod in dermatology: An overview. Int. J. Dermatol. 2016, 55, 831–844. [Google Scholar] [CrossRef] [PubMed]
- Vicente, S.; Peleteiro, M.; Díaz-Freitas, B.; Sanchez, A.; González-Fernández, Á.; Alonso, M.J. Co-delivery of viral proteins and a TLR7 agonist from polysaccharide nanocapsules: A needle-free vaccination strategy. J. Control. Release 2013, 172, 773–781. [Google Scholar] [CrossRef] [PubMed]
- Telò, I.; Favero, E.D.; Cantù, L.; Frattini, N.; Pescina, S.; Padula, C.; Santi, P.; Sonvico, F.; Nicoli, S. Gel-like TPGS-Based Microemulsions for Imiquimod Dermal Delivery: Role of Mesostructure on the Uptake and Distribution into the Skin. Mol. Pharm. 2017, 14, 3281–3289. [Google Scholar] [CrossRef]
- Pescina, S.; Garrastazu, G.; Del Favero, E.; Rondelli, V.; Cantù, L.; Padula, C.; Santi, P.; Nicoli, S. Microemulsions based on TPGS and isostearic acid for imiquimod formulation and skin delivery. Eur. J. Pharm. Sci. 2018, 125, 223–231. [Google Scholar] [CrossRef]
- Gogoll, K.; Stein, P.; Lee, K.D.; Arnold, P.; Peters, T.; Schild, H.; Radsak, M.; Langguth, P. Solid nanoemulsion as antigen and immunopotentiator carrier for transcutaneous immunization. Cell Immunol. 2016, 308, 35–43. [Google Scholar] [CrossRef] [PubMed]
- Lopez, P.A.; Denny, M.; Hartmann, A.K.; Alflen, A.; Probst, H.C.; von Stebut, E.; Tenzer, S.; Schild, H.; Stassen, M.; Langguth, P.; et al. Transcutaneous immunization with a novel imiquimod nanoemulsion induces superior T cell responses and virus protection. J. Derm. Sci. 2017, 87, 252–259. [Google Scholar] [CrossRef] [Green Version]
- Theochari, I.; Ilic, T.; Nicolic, I.; Dobricic, V.; Tenchiou, A.; Papahatjis, D.; Savic, S.; Xenakis, A.; Papadimitriou, V.; Pletsa, V. Biological Evaluation of Oil-in-Water Microemulsions as Carriers of Benzothiophene Analogues for Dermal Applications. Biomimetics 2021, 6, 10. [Google Scholar] [CrossRef]
- Klier, J.; Tucker, C.J.; Kalantar, T.H.; Green, D.P. Properties and Applications of Microemulsions. Adv. Mater. 2000, 12, 1751–1757. [Google Scholar] [CrossRef]
- Malakar, J.; Sen, S.O.; Nayak, A.K.; Sen, K.K. Development and evaluation of microemulsions for transdermal delivery of insulin. ISRN Pharm. 2011, 2011, 780150. [Google Scholar] [CrossRef] [Green Version]
- Ganggwar, N.; Singh, M.; Parashar, P.; Tripathi, C.B.; Arya, M.; Saraf, S.; Saha, S. Topical Delivery of Fluconazole via Microemulsion Incorporated Hydrogel for the Management of Fungal Dermatophytosis. Curr. Drug Ther. 2016, 11, 129–141. [Google Scholar] [CrossRef]
- Kogan, A.; Garti, N. Microemulsions as transdermal drug delivery vehicles. Adv. Colloid Interface Sci. 2006, 123, 369–385. [Google Scholar] [CrossRef] [PubMed]
- Peira, E.; Chirio, D.; Carlotti, M.E.; Spagnolo, R.; Trotta, M. Formulation studies of microemulsions for topical applications of acyclovir. J. Drug Deliv. Sci. Technol. 2009, 19, 191–196. [Google Scholar] [CrossRef]
- Asbill, C.S.; El-Kattan, A.F.; Michniak, B. Enhancement of transdermal drug delivery: Chemical and physical approaches. Crit. Rev. Drug Carr. Syst. 2000, 17, 621–658. [Google Scholar] [CrossRef]
- Lopes, L.B. Overcoming the cutaneous barrier with microemulsions. Pharmaceutics 2014, 6, 52–77. [Google Scholar] [CrossRef] [Green Version]
- Theochari, I.; Mitsou, E.; Nikolic, I.; Ilic, T.; Dobricic, V.; Pletsa, V.; Savic, S.; Xenakis, A.; Papadimitriou, V. Colloidal nanodispersions for the topical delivery of Ibuprofen: Structure, dynamics and bioperformances. J. Mol. Liq. 2021, 334, 116021. [Google Scholar] [CrossRef]
- Hoeller, S.; Klang, V.; Valenta, C. Skin-compatible lecithin drug delivery systems for fluconazole: Effect of phosphatidylethanolamine and oleic acid on skin permeation. J. Pharm. Pharm. 2008, 60, 587–591. [Google Scholar] [CrossRef]
- Xu, J.; Yin, A.; Zhao, J.; Li, D.; Hou, W. Surfactant-free microemulsion composed of oleic acid, n-propanol, and H2O. J. Phys. Chem. B 2013, 117, 450–456. [Google Scholar] [CrossRef]
- Cilek, A.; Celebi, N.; Tirnaksiz, F. Lecithin-based microemulsion of a peptide for oral administration: Preparation, characterization, and physical stability of the formulation. Drug Deliv. 2006, 13, 19–24. [Google Scholar] [CrossRef] [Green Version]
- Kato, A.; Ishibashi, Y.; Miyake, Y. Effect of egg yolk lecithin on transdermal delivery of bunazosin hydrochloride. J. Pharm. Pharmacol. 1987, 39, 399–400. [Google Scholar] [CrossRef]
- Raza, K.; Negi, P.; Takyar, S.; Shukla, A.; Amarji, B.; Katare, O.P. Novel dithranol phospholipid microemulsion for topical application: Development, characterization and percutaneous absorption studies. J. Microencapsul. 2011, 28, 190–199. [Google Scholar] [CrossRef]
- Lachenmeier, D.W. Safety evaluation of topical applications of ethanol on the skin and inside the oral cavity. J. Occup. Med. Toxicol. 2008, 3, 26. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chollet, J.L.; Jozwiakowski, M.J.; Phares, K.R.; Reiter, M.J.; Roddy, P.J.; Schultz, H.J.; Ta, Q.V.; Tomai, M.A. Development of a topically active imiquimod formulation. Pharm. Dev. Technol. 1999, 4, 35–43. [Google Scholar] [CrossRef] [PubMed]
- Craig, D.Q.M.; Barker, S.A.; Banning, D.; Booth, S.W. An investigation into the mechanisms of self-emulsification using particle size analysis and low frequency dielectric spectroscopy. Int. J. Pharm. 1995, 114, 103–110. [Google Scholar] [CrossRef]
- Li, J.; Wang, X.; Zhang, T.; Wang, C.; Huang, Z.; Luo, X.; Deng, Y. A review on phospholipids and their main applications in drug delivery systems. Asian J. Pharm. Sci. 2015, 10, 81–98. [Google Scholar] [CrossRef]
- Swanson, N.; Smith, C.C.; Kaur, M.; Goldenberg, G. Imiquimod 2.5% and 3.75% for the treatment of actinic keratoses: Two phase 3, multicenter, randomized, double-blind, placebo-controlled studies. J. Drugs Derm. 2014, 13, 166–169. [Google Scholar]
- Jewell, J.R.; Myers, S.A. Topical Therapy Primer for Nondermatologists. Med. Clin. N. Am. 2015, 99, 1167–1182. [Google Scholar] [CrossRef]
- Zang, J.; Feng, M.; Zhao, J.; Wang, J. Micellar and bicontinuous microemulsion structures show different solute–solvent interactions: A case study using ultrafast nonlinear infrared spectroscopy. Phys. Chem. Chem. Phys. 2018, 20, 19938–19949. [Google Scholar] [CrossRef]
- Szumała, P. Structure of Microemulsion Formulated with Monoacylglycerols in the Presence of Polyols and Ethanol. J. Surfactants Deterg. 2015, 18, 97–106. [Google Scholar] [CrossRef] [Green Version]
- Djordjevic, L.; Primorac, M.; Stupar, M.; Krajisnik, D. Characterization of caprylocaproyl macrogolglycerides based microemulsion drug delivery vehicles for an amphiphilic drug. Int. J. Pharm. 2004, 271, 11–19. [Google Scholar] [CrossRef]
- Yuan, J.S.; Ansari, M.; Samaan, M.; Acosta, E.J. Linker-based lecithin microemulsions for transdermal delivery of lidocaine. Int. J. Pharm. 2008, 349, 130–143. [Google Scholar] [CrossRef]
- Sintov, A.C. Transdermal delivery of curcumin via microemulsion. Int. J. Pharm. 2015, 481, 97–103. [Google Scholar] [CrossRef] [PubMed]
- Fenner, J.; Clark, R.A.F. Chapter 1—Anatomy, Physiology, Histology, and Immunohistochemistry of Human Skin. In Skin Tissue Engineering and Regenerative Medicine; Albanna, M.Z., Holmes Iv, J.H., Eds.; Academic Press: Boston, MA, USA, 2016; pp. 1–17. [Google Scholar] [CrossRef]
- Jiang, S.J.; Zhou, X.J. Examination of the mechanism of oleic acid-induced percutaneous penetration enhancement: An ultrastructural study. Biol. Pharm. Bull. 2003, 26, 66–68. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Touitou, E.; Godin, B.; Karl, Y.; Bujanover, S.; Becker, Y. Oleic acid, a skin penetration enhancer, affects Langerhans cells and corneocytes. J. Control. Release Off. J. Control. Release Soc. 2002, 80, 1–7. [Google Scholar] [CrossRef]
- Ongpipattanakul, B.; Burnette, R.R.; Potts, R.O.; Francoeur, M.L. Evidence that oleic acid exists in a separate phase within stratum corneum lipids. Pharm. Res. 1991, 8, 350–354. [Google Scholar] [CrossRef] [PubMed]
- Williams, A.C.; Barry, B.W. Penetration enhancers. Adv. Drug Deliv. Rev. 2004, 56, 603–618. [Google Scholar] [CrossRef] [PubMed]
- Naik, A.; Pechtold, L.A.R.M.; Potts, R.O.; Guy, R.H. Mechanism of oleic acid-induced skin penetration enhancement in vivo in humans. J. Control. Release 1995, 37, 299–306. [Google Scholar] [CrossRef]
- Dubey, V.; Mishra, D.; Dutta, T.; Nahar, M.; Saraf, D.K.; Jain, N.K. Dermal and transdermal delivery of an anti-psoriatic agent via ethanolic liposomes. J. Control. Release 2007, 123, 148–154. [Google Scholar] [CrossRef]
- Kim, D.D.; Kim, J.L.; Chien, Y.W. Mutual hairless rat skin permeation-enhancing effect of ethanol/water system and oleic acid. J. Pharm. Sci. 1996, 85, 1191–1195. [Google Scholar] [CrossRef]
- Grissinger, M. Fentanyl Transdermal Patches: More Protection Needed for Patients and Their Families. Pharm. Ther. 2009, 34, 343–390. [Google Scholar]
- Grubauer, G.; Elias, P.M.; Feingold, K.R. Transepidermal water loss: The signal for recovery of barrier structure and function. J. Lipid. Res. 1989, 30, 323–333. [Google Scholar] [CrossRef]
- Alexander, H.; Brown, S.; Danby, S.; Flohr, C. Research Techniques Made Simple: Transepidermal Water Loss Measurement as a Research Tool. J. Investig. Dermatol. 2018, 138, 2295–2300.e2291. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kopečná, M.; Macháček, M.; Nováčková, A.; Paraskevopoulos, G.; Roh, J.; Vávrová, K. Esters of terpene alcohols as highly potent, reversible, and low toxic skin penetration enhancers. Sci. Rep. 2019, 9, 14617. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boncheva, M.; Damien, F.; Normand, V. Molecular organization of the lipid matrix in intact Stratum corneum using ATR-FTIR spectroscopy. Biochim. Biophys. Acta 2008, 1778, 1344–1355. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mendelsohn, R.; Flach, C.R.; Moore, D.J. Determination of molecular conformation and permeation in skin via IR spectroscopy, microscopy, and imaging. Biochim. Biophys. Acta BBA—Biomembr. 2006, 1758, 923–933. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schwarz, J.C.; Klang, V.; Hoppel, M.; Mahrhauser, D.; Valenta, C. Natural microemulsions: Formulation design and skin interaction. Eur. J. Pharm. Biopharm. 2012, 81, 557–562. [Google Scholar] [CrossRef] [PubMed]
- Damien, F.; Boncheva, M. The extent of orthorhombic lipid phases in the stratum corneum determines the barrier efficiency of human skin in vivo. J. Invest. Derm. 2010, 130, 611–614. [Google Scholar] [CrossRef]
- Mendelsohn, R.; Moore, D.J. Vibrational spectroscopic studies of lipid domains in biomembranes and model systems. Chem. Phys. Lipids. 1998, 96, 141–157. [Google Scholar] [CrossRef]
- Santos, P.; Watkinson, A.C.; Hadgraft, J.; Lane, M.E. Application of Microemulsions in Dermal and Transdermal Drug Delivery. Ski. Pharmacol. Physiol. 2008, 21, 246–259. [Google Scholar] [CrossRef]
- Hung, W.-H.; Chen, P.-K.; Fang, C.-W.; Lin, Y.-C.; Wu, P.-C. Preparation and Evaluation of Azelaic Acid Topical Microemulsion Formulation: In Vitro and In Vivo Study. Pharmaceutics 2021, 13, 410. [Google Scholar] [CrossRef]
- Hashem, F.M.; Shaker, D.S.; Ghorab, M.K.; Nasr, M.; Ismail, A. Formulation, characterization, and clinical evaluation of microemulsion containing clotrimazole for topical delivery. AAPS Pharm. Sci. Tech. 2011, 12, 879–886. [Google Scholar] [CrossRef] [Green Version]
- Kopečná, M.; Macháček, M.; Prchalová, E.; Štěpánek, P.; Drašar, P.; Kotora, M.; Vávrová, K. Dodecyl Amino Glucoside Enhances Transdermal and Topical Drug Delivery via Reversible Interaction with Skin Barrier Lipids. Pharm. Res. 2017, 34, 640–653. [Google Scholar] [CrossRef] [PubMed]
- Kligman, A.M.; Christophers, E. Preparation of Isolated Sheets of Human Stratum Corneum. Arch. Derm. 1963, 88, 702–705. [Google Scholar] [CrossRef] [PubMed]
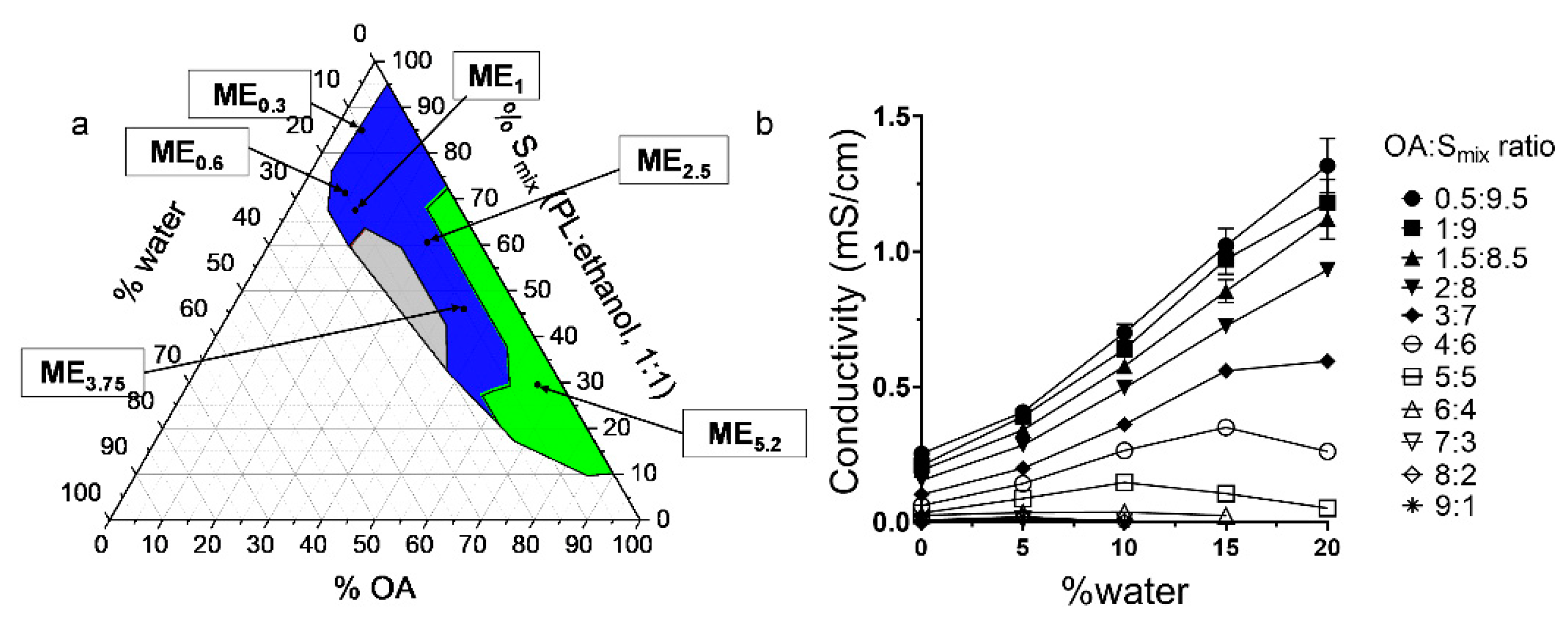
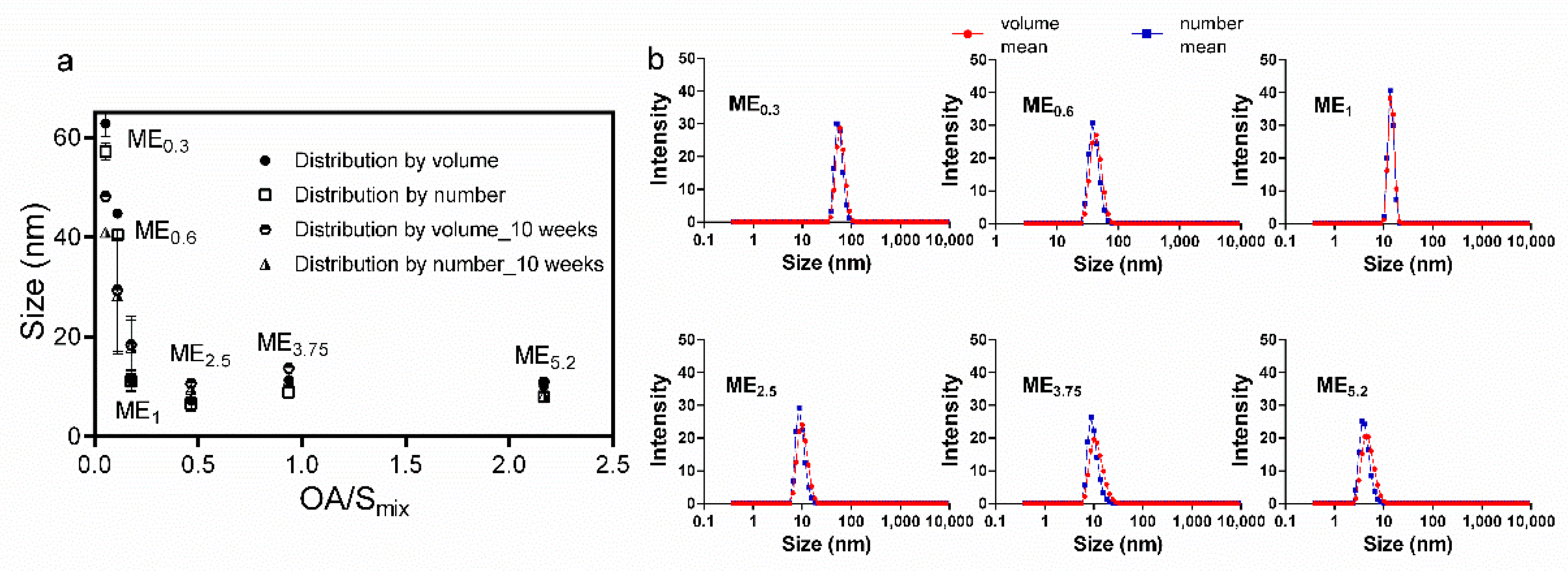
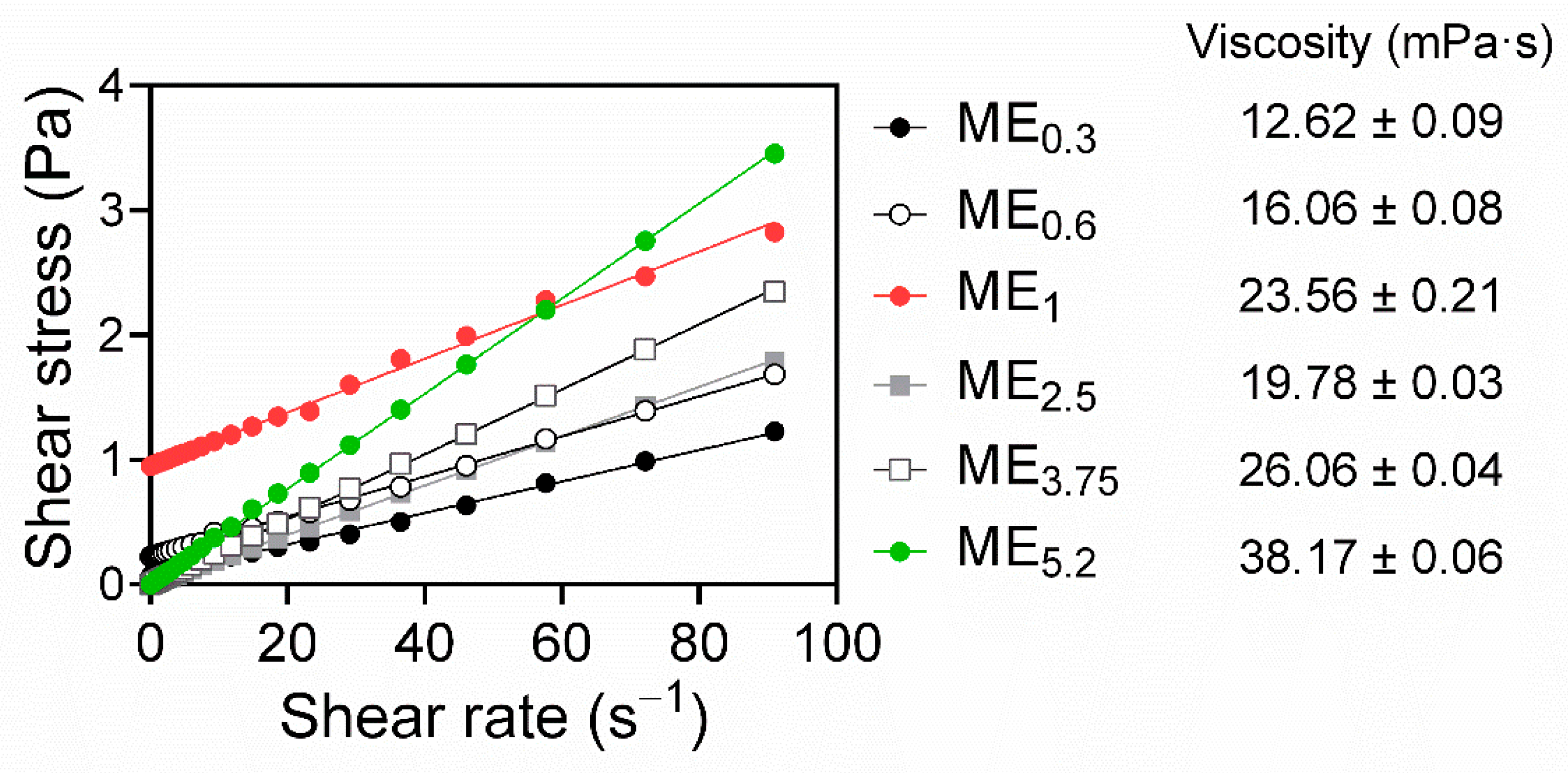
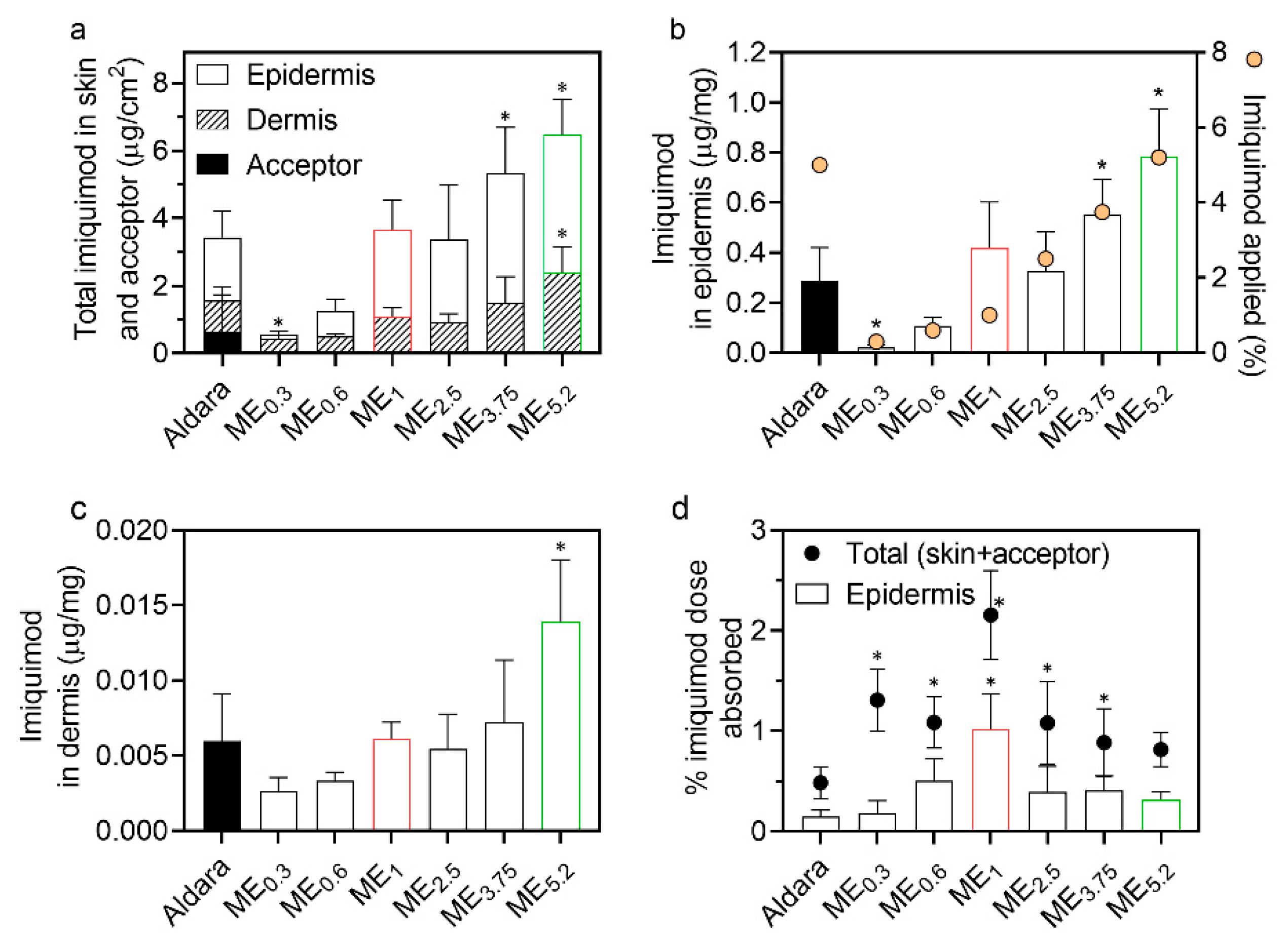
| Formulation | IMQ | OA | Smix (PL/Ethanol 1:1) | Water |
|---|---|---|---|---|
| ME0.3 | 0.30 | 4.50 | 85.20 | 10.00 |
| ME0.6 | 0.60 | 8.00 | 71.50 | 19.90 |
| ME1 | 1.00 | 11.90 | 67.30 | 19.80 |
| ME2.5 | 2.50 | 27.80 | 59.70 | 10.00 |
| ME3.75 | 3.75 | 41.70 | 44.55 | 10.00 |
| ME5.2 | 5.20 | 61.60 | 28.50 | 4.70 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Panoutsopoulou, E.; Zbytovská, J.; Vávrová, K.; Paraskevopoulos, G. Phospholipid-Based Microemulsions for Cutaneous Imiquimod Delivery. Pharmaceuticals 2022, 15, 515. https://doi.org/10.3390/ph15050515
Panoutsopoulou E, Zbytovská J, Vávrová K, Paraskevopoulos G. Phospholipid-Based Microemulsions for Cutaneous Imiquimod Delivery. Pharmaceuticals. 2022; 15(5):515. https://doi.org/10.3390/ph15050515
Chicago/Turabian StylePanoutsopoulou, Eleni, Jarmila Zbytovská, Kateřina Vávrová, and Georgios Paraskevopoulos. 2022. "Phospholipid-Based Microemulsions for Cutaneous Imiquimod Delivery" Pharmaceuticals 15, no. 5: 515. https://doi.org/10.3390/ph15050515
APA StylePanoutsopoulou, E., Zbytovská, J., Vávrová, K., & Paraskevopoulos, G. (2022). Phospholipid-Based Microemulsions for Cutaneous Imiquimod Delivery. Pharmaceuticals, 15(5), 515. https://doi.org/10.3390/ph15050515







