Assessing the Oxidative State of the Skin by Combining Classical Tape Stripping with ORAC Assay
Abstract
:1. Introduction
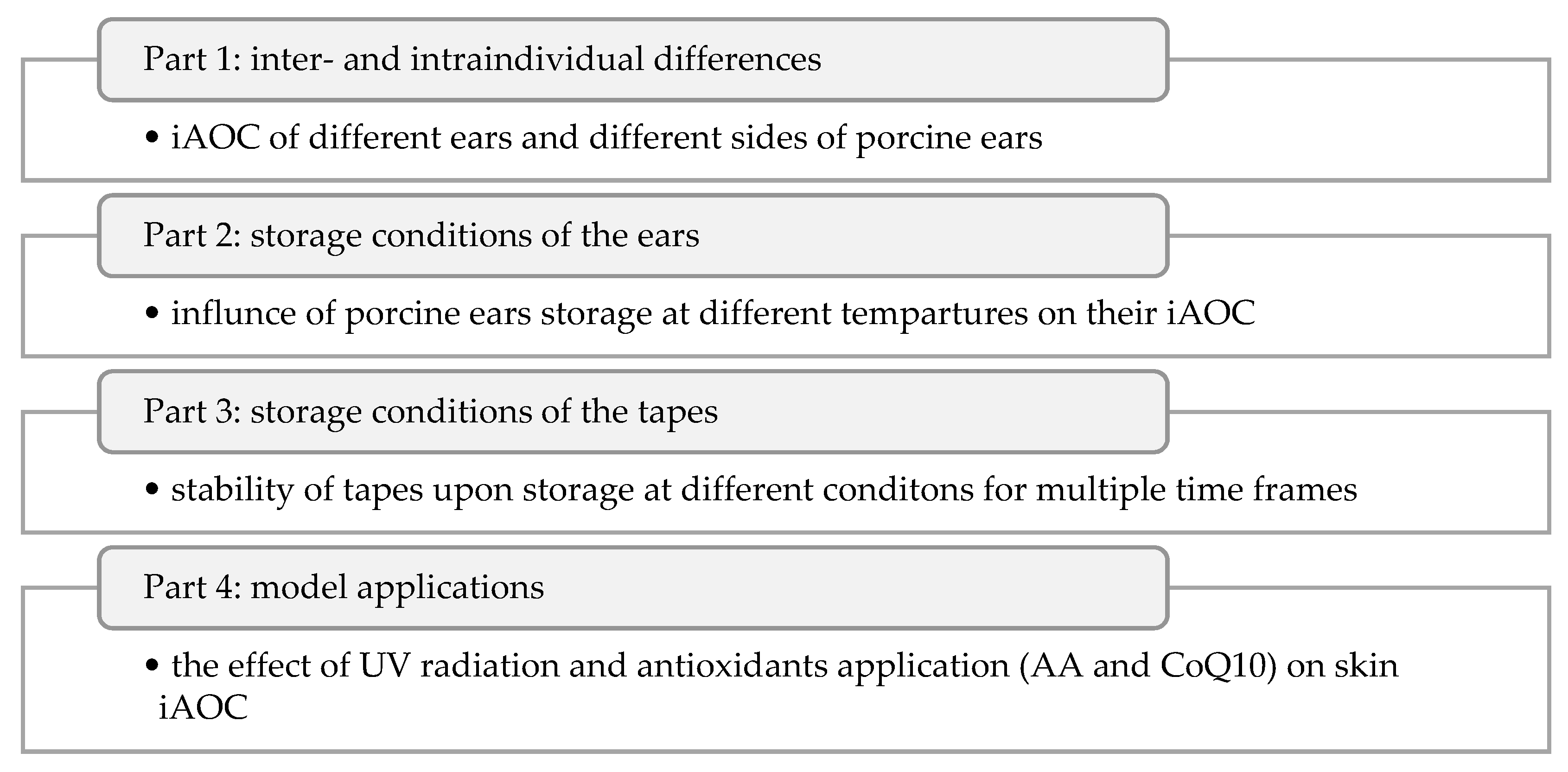
2. Results
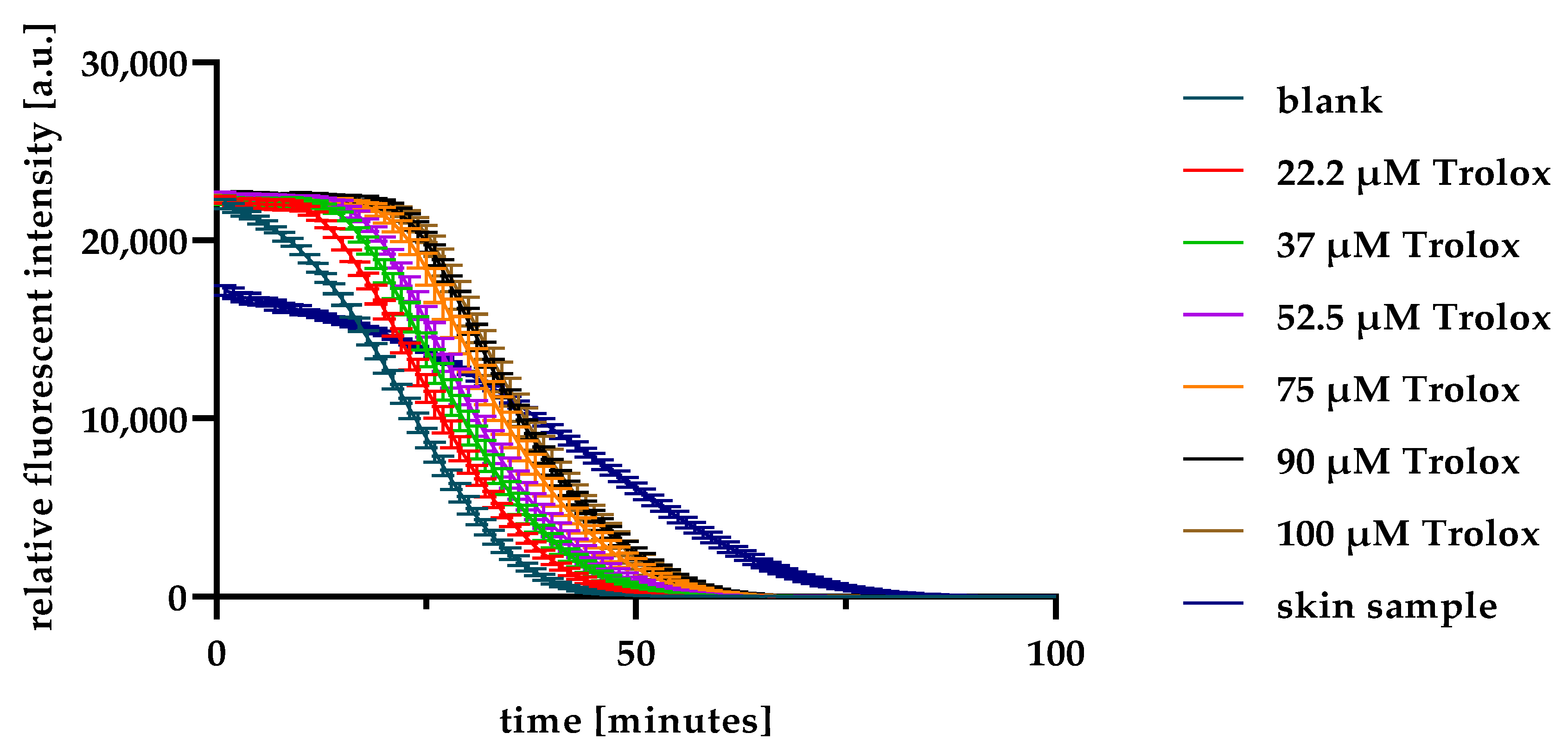
2.1. ORAC Optimisation and Validation
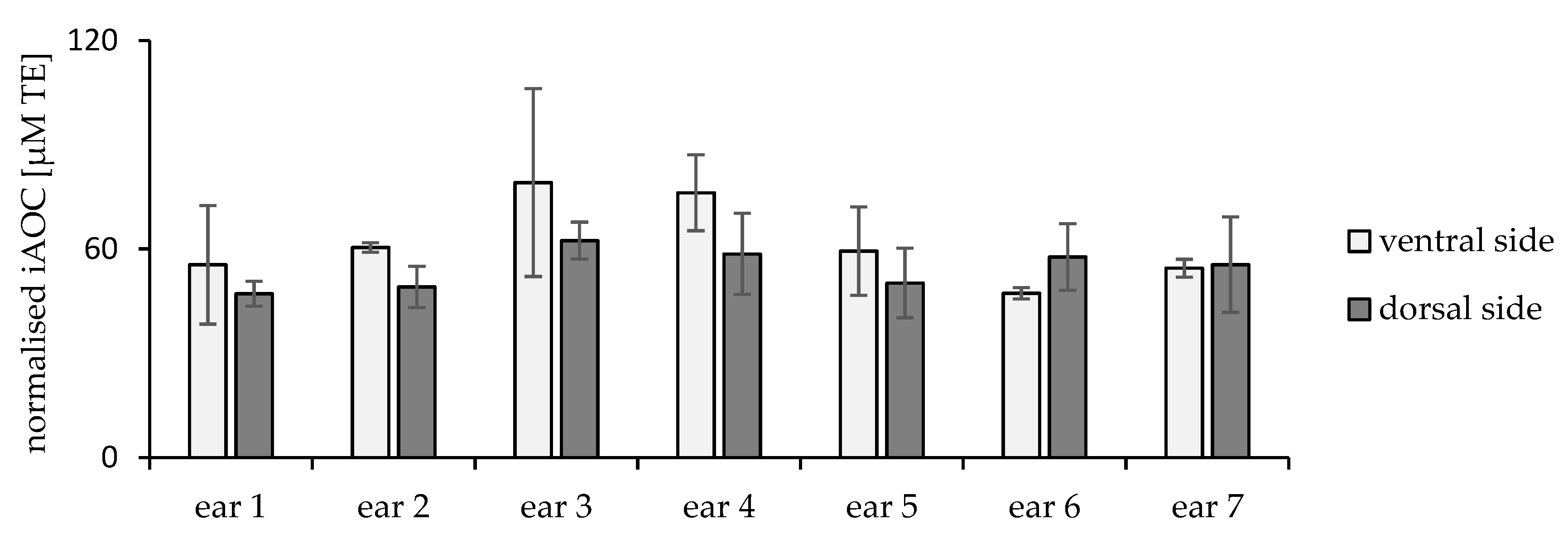
2.2. Inter- and Intraindividual Differences
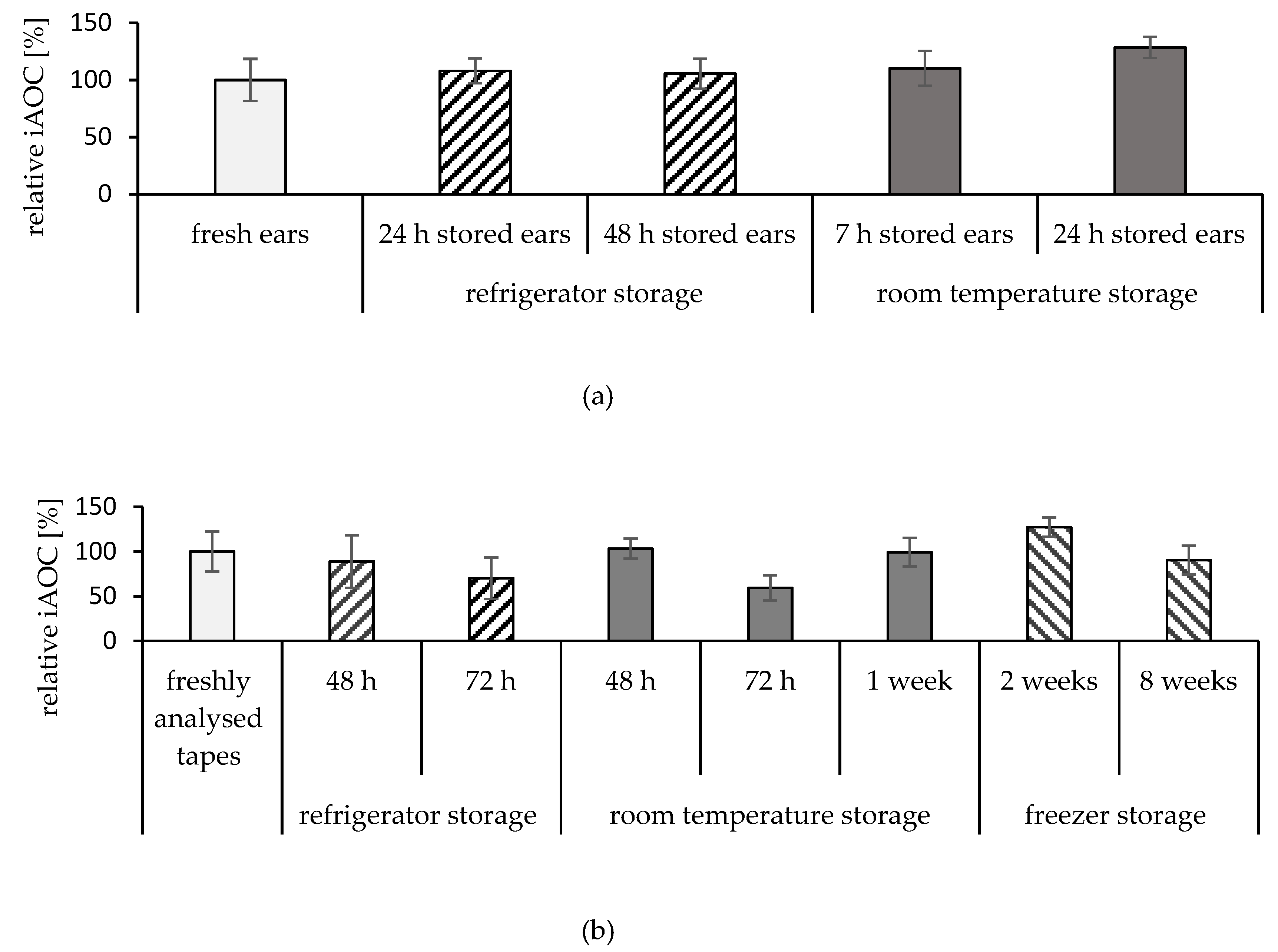
2.3. Storage Conditions of the Ears
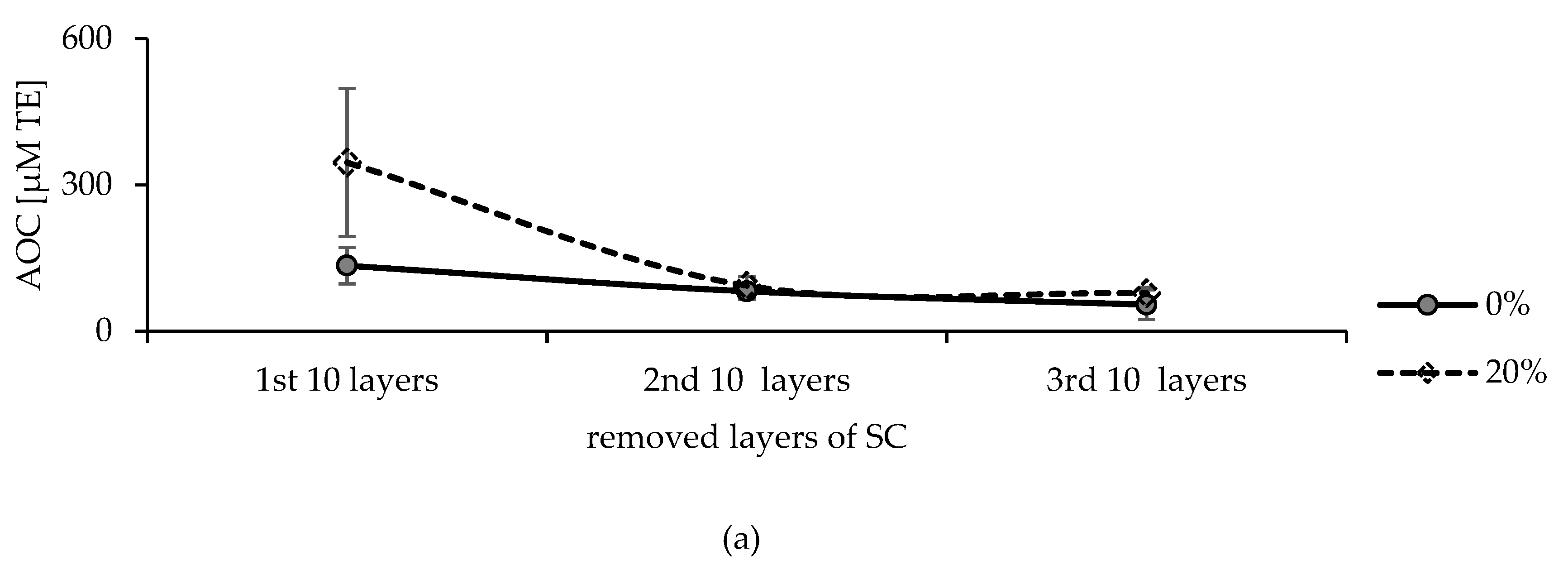
2.4. Storage Conditions of the Tapes
2.5. Model Application
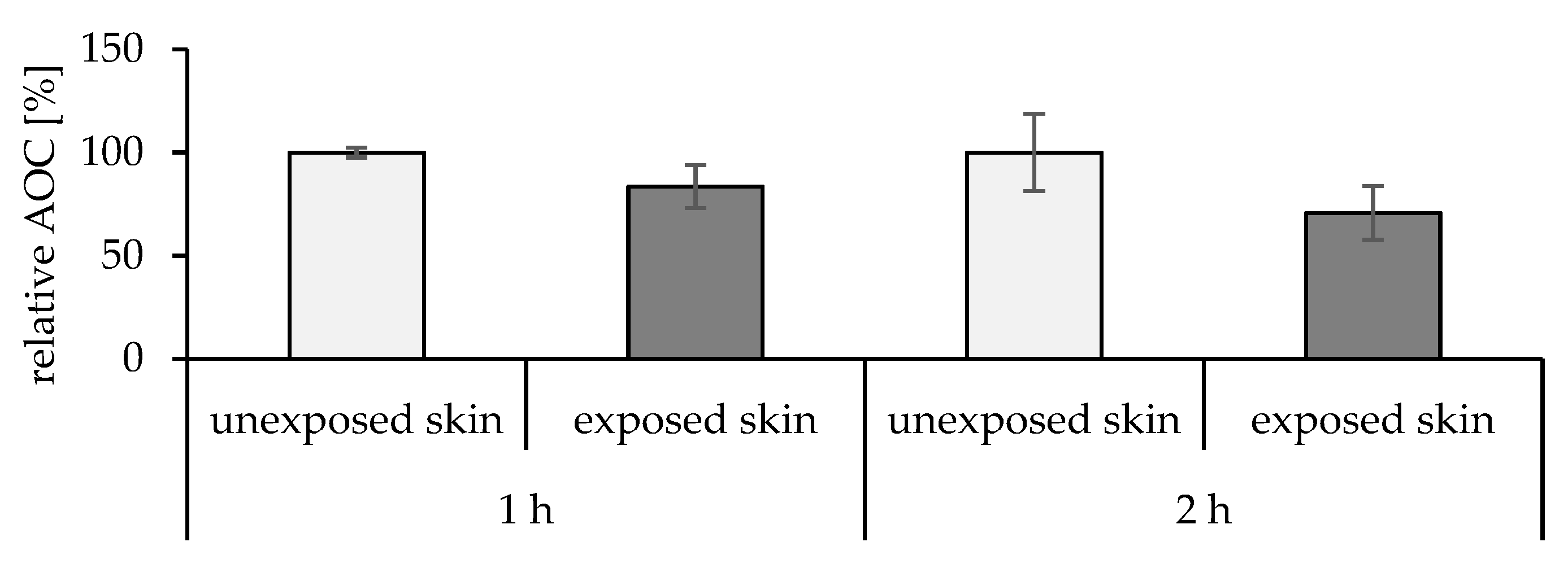
2.5.1. Effect of the UV Radiation on the Skin
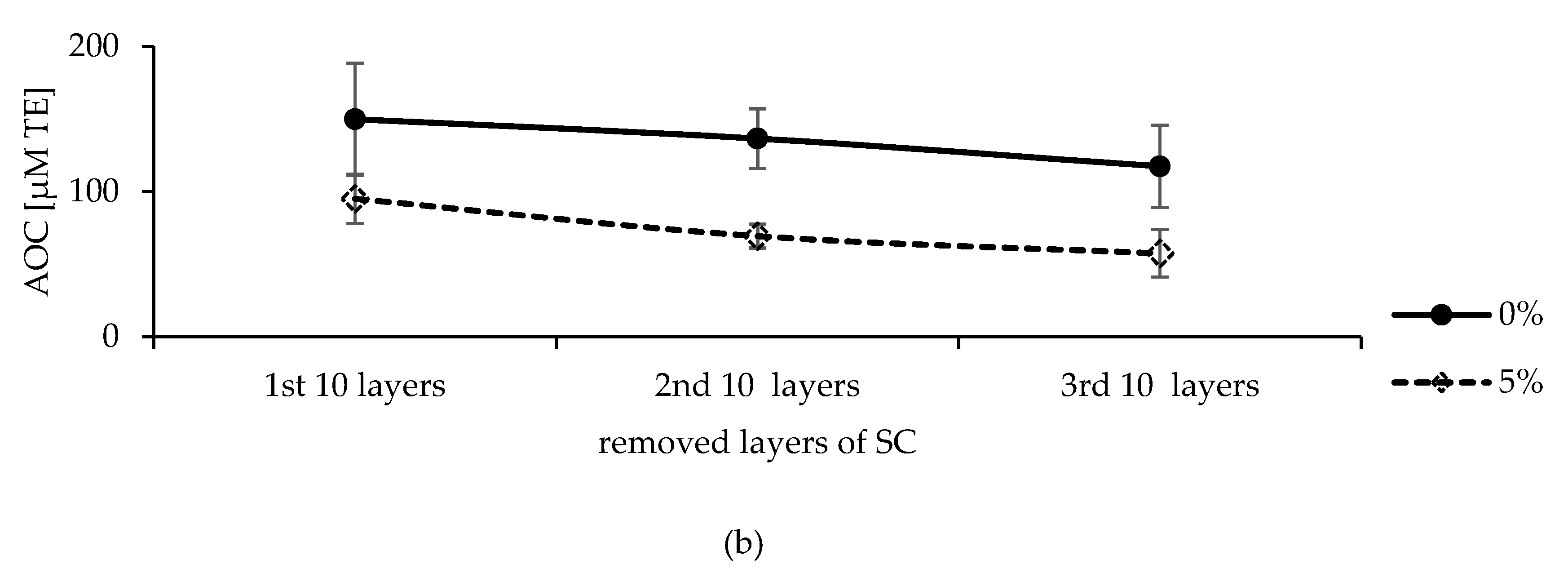
2.5.2. Effect of Cosmeceuticals on Skin
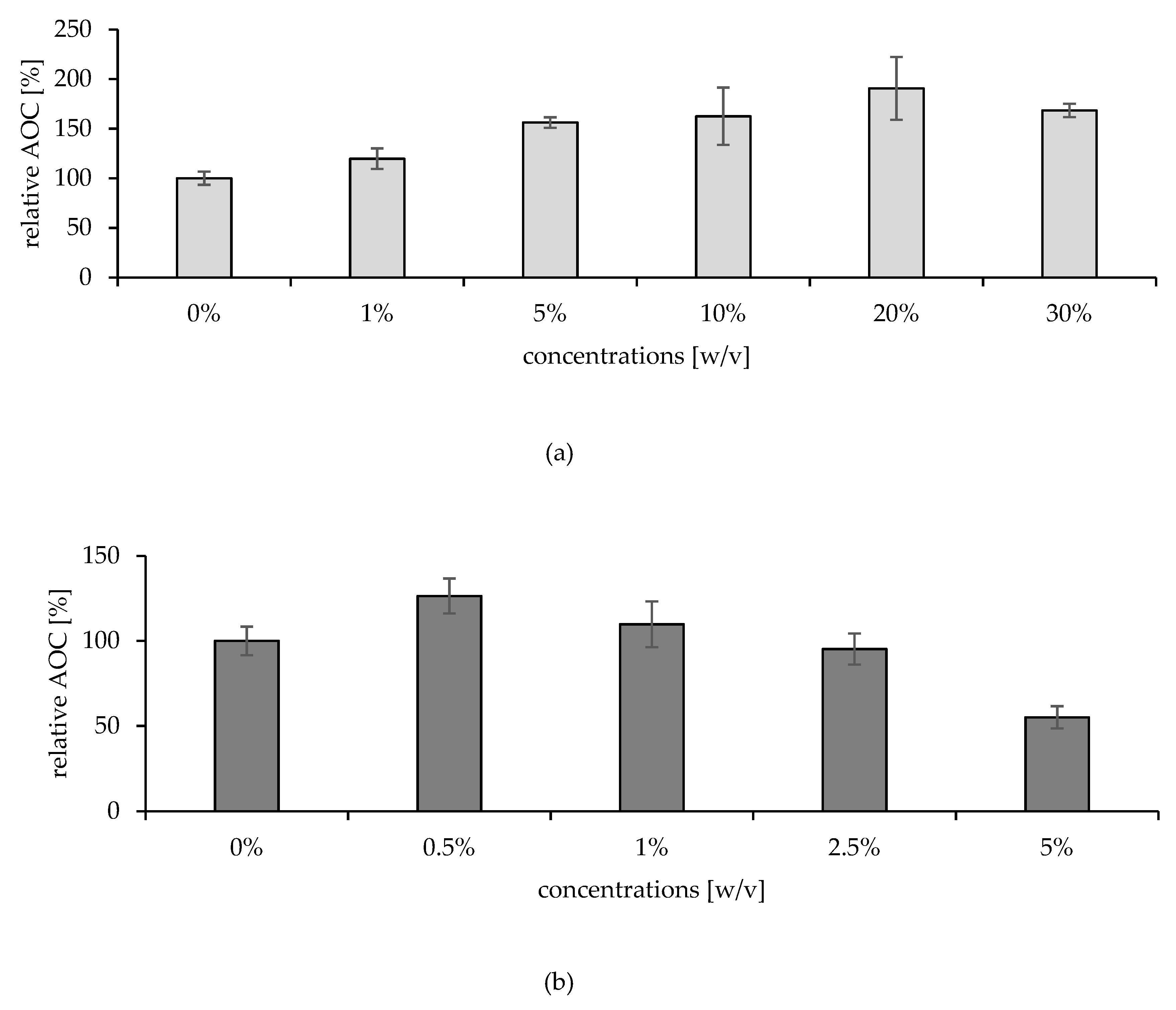
In Vitro Studies
Ex Vivo Studies
3. Discussion
4. Materials and Methods
4.1. Tape Stripping
4.2. Skin Treatment
4.3. Quantification of Protein Content
4.4. Storage and Extraction of Tapes
4.5. ORAC Assay
4.6. Statistics
4.7. Normalisation
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Thiele, J.J.; Schroeter, C.; Hsieh, S.N.; Podda, M.; Packer, L. The antioxidant network of the stratum corneum. Curr. Probl. Dermatol. 2001, 29, 26–42. [Google Scholar] [CrossRef] [PubMed]
- Feingold, K.R.; Elias, P.M. Skin Barrier; Taylor & Francis: New York, NY, USA, 2006; ISBN 0824758153. [Google Scholar]
- Shindo, Y.; Witt, E.; Han, D.; Epstein, W.; Packer, L. Enzymic and non-enzymic antioxidants in epidermis and dermis of human skin. J. Investig. Dermatol. 1994, 102, 122–124. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McMullen, R.L. Antioxidants and the Skin, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2018; ISBN 9781351798822. [Google Scholar]
- D’Aniello, C.; Cermola, F.; Patriarca, E.J.; Minchiotti, G. Vitamin C in stem cell biology: Impact on extracellular matrix homeostasis and epigenetics. Stem Cells Int. 2017, 2017, 8936156. [Google Scholar] [CrossRef] [Green Version]
- Li, R.; Bianchet, M.A.; Talalay, P.; Amzel, L.M. The three-dimensional structure of NAD(P)H:quinone reductase, a flavoprotein involved in cancer chemoprotection and chemotherapy: Mechanism of the two-electron reduction. Proc. Natl. Acad. Sci. USA 1995, 92, 8846–8850. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schallreuter, K.U.; Rokos, H.; Chavan, B.; Gillbro, J.M.; Cemeli, E.; Zothner, C.; Anderson, D.; Wood, J.M. Quinones are reduced by 6-tetrahydrobiopterin in human keratinocytes, melanocytes, and melanoma cells. Free Radic. Biol. Med. 2008, 44, 538–546. [Google Scholar] [CrossRef] [PubMed]
- Nandi, A.; Yan, L.-J.; Jana, C.K.; Das, N. Role of catalase in oxidative stress- and age-associated degenerative diseases. Oxid. Med. Cell. Longev. 2019, 2019, 9613090. [Google Scholar] [CrossRef] [Green Version]
- Mugesh, G.; Panda, A.; Singh, H.B.; Punekar, N.S.; Butcher, R.J. Glutathione peroxidase-like antioxidant activity of diaryl diselenides: A mechanistic study. J. Am. Chem. Soc. 2001, 123, 839–850. [Google Scholar] [CrossRef] [PubMed]
- Johnson, F.; Giulivi, C. Superoxide dismutases and their impact upon human health. Mol. Aspects Med. 2005, 26, 340–352. [Google Scholar] [CrossRef]
- Schallreuter, K.U.; Wood, J.M. Thioredoxin reductase—Its role in epidermal redox status. J. Photochem. Photobiol. B Biol. 2001, 64, 179–184. [Google Scholar] [CrossRef]
- Podda, M.; Zollner, T.M.; Grundmann-Kollmann, M.; Thiele, J.J.; Packer, L.; Kaufmann, R. Activity of alpha-lipoic acid in the protection against oxidative stress in skin. Curr. Probl. Dermatol. 2001, 29, 43–51. [Google Scholar] [CrossRef]
- Gülçin, I. Antioxidant and antiradical activities of L-carnitine. Life Sci. 2006, 78, 803–811. [Google Scholar] [CrossRef] [PubMed]
- Turkmen, D. Serum bilirubin and uric acid antioxidant levels in rosacea patients. J. Cosmet. Dermatol. 2020, 19, 2717–2720. [Google Scholar] [CrossRef] [PubMed]
- Hoogduijn, M.J.; Cemeli, E.; Ross, K.; Anderson, D.; Thody, A.J.; Wood, J.M. Melanin protects melanocytes and keratinocytes against H2O2-induced DNA strand breaks through its ability to bind Ca2+. Exp. Cell. Res. 2004, 294, 60–67. [Google Scholar] [CrossRef] [PubMed]
- Shah, A.A.; Sinha, A.A. Oxidative stress and autoimmune skin disease. Eur. J. Dermatol. 2013, 23, 5–13. [Google Scholar] [CrossRef] [PubMed]
- Briganti, S.; Picardo, M. Antioxidant activity, lipid peroxidation and skin diseases. What’s new. J. Eur. Acad. Dermatol. Venereol. 2003, 17, 663–669. [Google Scholar] [CrossRef] [PubMed]
- Rokos, H.; Beazley, W.D.; Schallreuter, K.U. Oxidative stress in vitiligo: Photo-oxidation of pterins produces H(2)O(2) and pterin-6-carboxylic acid. Biochem. Biophys. Res. Commun. 2002, 292, 805–811. [Google Scholar] [CrossRef]
- Godic, A.; Poljšak, B.; Adamic, M.; Dahmane, R. The role of antioxidants in skin cancer prevention and treatment. Oxid. Med. Cell. Longev. 2014, 2014, 860479. [Google Scholar] [CrossRef]
- Schneider, S.L.; Lim, H.W. A review of inorganic UV filters zinc oxide and titanium dioxide. Photodermatol. Photoimmunol. Photomed. 2019, 35, 442–446. [Google Scholar] [CrossRef] [Green Version]
- Ravetti, S.; Clemente, C.; Brignone, S.; Hergert, L.; Allemandi, D.; Palma, S. Ascorbic Acid in Skin Health. Cosmetics 2019, 6, 58. [Google Scholar] [CrossRef] [Green Version]
- Delinasios, G.J.; Karbaschi, M.; Cooke, M.S.; Young, A.R. Vitamin E inhibits the UVAI induction of “light” and “dark” cyclobutane pyrimidine dimers, and oxidatively generated DNA damage, in keratinocytes. Sci. Rep. 2018, 8, 423. [Google Scholar] [CrossRef] [Green Version]
- Marcheggiani, F.; Kordes, S.; Cirilli, I.; Orlando, P.; Silvestri, S.; Vogelsang, A.; Möller, N.; Blatt, T.; Weise, J.M.; Damiani, E.; et al. Anti-ageing effects of ubiquinone and ubiquinol in a senescence model of human dermal fibroblasts. Free Radic. Biol. Med. 2021, 165, 282–288. [Google Scholar] [CrossRef] [PubMed]
- Masaki, H. Role of antioxidants in the skin: Anti-aging effects. J. Dermatol. Sci. 2010, 58, 85–90. [Google Scholar] [CrossRef] [PubMed]
- Lademann, J.; Jacobi, U.; Surber, C.; Weigmann, H.-J.; Fluhr, J.W. The tape stripping procedure—Evaluation of some critical parameters. Eur. J. Pharm. Biopharm. 2009, 72, 317–323. [Google Scholar] [CrossRef] [PubMed]
- Pelikh, O.; Stahr, P.-L.; Huang, J.; Gerst, M.; Scholz, P.; Dietrich, H.; Geisel, N.; Keck, C.M. Nanocrystals for improved dermal drug delivery. Eur. J. Pharm. Biopharm. 2018, 128, 170–178. [Google Scholar] [CrossRef]
- Fujita, H.; Hirao, T.; Takahashi, M. A simple and non-invasive visualization for assessment of carbonylated protein in the stratum corneum. Skin Res. Technol. 2007, 13, 84–90. [Google Scholar] [CrossRef]
- Herrling, T. UV-induced free radicals in the skin detected by ESR spectroscopy and imaging using nitroxides. Free Radic. Biol. Med. 2003, 35, 59–67. [Google Scholar] [CrossRef]
- Haag, S.F.; Taskoparan, B.; Bittl, R.; Teutloff, C.; Wenzel, R.; Fahr, A.; Chen, M.; Lademann, J.; Schäfer-Korting, M.; Meinke, M.C. Stabilization of reactive nitroxides using invasomes to allow prolonged electron paramagnetic resonance measurements. Skin Pharmacol. Physiol. 2011, 24, 312–321. [Google Scholar] [CrossRef]
- Tsuchida, K.; Kobayashi, M. Oxidative stress in human facial skin observed by ultraweak photon emission imaging and its correlation with biophysical properties of skin. Sci. Rep. 2020, 10, 9626. [Google Scholar] [CrossRef]
- Hagens, R.; Khabiri, F.; Schreiner, V.; Wenck, H.; Wittern, K.-P.; Duchstein, H.-J.; Mei, W. Non-invasive monitoring of oxidative skin stress by ultraweak photon emission measurement. II: Biological validation on ultraviolet A-stressed skin. Skin Pharmacol. Physiol. 2008, 14, 112–120. [Google Scholar] [CrossRef]
- Georgescu, S.R.; Mitran, C.I.; Mitran, M.I.; Nicolae, I.; Matei, C.; Ene, C.D.; Popa, G.L.; Tampa, M. Oxidative Stress in Cutaneous Lichen Planus-A Narrative Review. J. Clin. Med. 2021, 10, 2692. [Google Scholar] [CrossRef]
- Szczepanczyk, M.; Ruzgas, T.; Gullfot, F.; Gustafsson, A.; Björklund, S. Catalase Activity in Keratinocytes, Stratum Corneum, and Defatted Algae Biomass as a Potential Skin Care Ingredient. Biomedicines 2021, 9, 1868. [Google Scholar] [CrossRef] [PubMed]
- Said, E.R.; Nagui, N.A.E.R.; Rashed, L.A.; Mostafa, W.Z. Oxidative stress and the cholinergic system in non-segmental vitiligo: Effect of narrow band ultraviolet b. Photodermatol. Photoimmunol. Photomed. 2021, 37, 306–312. [Google Scholar] [CrossRef] [PubMed]
- Prior, R.L.; Wu, X.; Schaich, K. Standardized methods for the determination of antioxidant capacity and phenolics in foods and dietary supplements. J. Agric. Food Chem. 2005, 53, 4290–4302. [Google Scholar] [CrossRef] [PubMed]
- Summerfield, A.; Meurens, F.; Ricklin, M.E. The immunology of the porcine skin and its value as a model for human skin. Mol. Immunol. 2015, 66, 14–21. [Google Scholar] [CrossRef] [PubMed]
- Jacobi, U.; Kaiser, M.; Toll, R.; Mangelsdorf, S.; Audring, H.; Otberg, N.; Sterry, W.; Lademann, J. Porcine ear skin: An in vitro model for human skin. Skin Res. Technol. 2007, 13, 19–24. [Google Scholar] [CrossRef]
- Klang, V.; Schwarz, J.C.; Lenobel, B.; Nadj, M.; Auböck, J.; Wolzt, M.; Valenta, C. In vitro vs. in vivo tape stripping: Validation of the porcine ear model and penetration assessment of novel sucrose stearate emulsions. Eur. J. Pharm. Biopharm. 2012, 80, 604–614. [Google Scholar] [CrossRef]
- Kaushik, V.; Ganashalingam, Y.; Schesny, R.; Raab, C.; Sengupta, S.; Keck, C.M. Influence of Massage and Skin Hydration on Dermal Penetration Efficacy of Nile Red from Petroleum Jelly-An Unexpected Outcome. Pharmaceutics 2021, 13, 2190. [Google Scholar] [CrossRef]
- Keck, C.M.; Abdelkader, A.; Pelikh, O.; Wiemann, S.; Kaushik, V.; Specht, D.; Eckert, R.W.; Alnemari, R.M.; Dietrich, H.; Brüßler, J. Assessing the Dermal Penetration Efficacy of Chemical Compounds with the Ex-Vivo Porcine Ear Model. Pharmaceutics 2022, 14, 678. [Google Scholar] [CrossRef]
- Ou, B.; Hampsch-Woodill, M.; Prior, R.L. Development and Validation of an Improved Oxygen Radical Absorbance Capacity Assay Using Fluorescein as the Fluorescent Probe. J. Agric. Food Chem. 2001, 49, 4619–4626. [Google Scholar] [CrossRef]
- Huang, D.; Ou, B.; Prior, R.L. The chemistry behind antioxidant capacity assays. J. Agric. Food Chem. 2005, 53, 1841–1856. [Google Scholar] [CrossRef]
- Ilyasov, I.R.; Beloborodov, V.L.; Selivanova, I.A. Three ABTS•+ radical cation-based approaches for the evaluation of antioxidant activity: Fast- and slow-reacting antioxidant behavior. Chem. Pap. 2018, 72, 1917–1925. [Google Scholar] [CrossRef]
- Klang, V.; Schwarz, J.C.; Hartl, A.; Valenta, C. Facilitating in vitro tape stripping: Application of infrared densitometry for quantification of porcine stratum corneum proteins. Skin Pharmacol. Physiol. 2011, 24, 256–268. [Google Scholar] [CrossRef] [PubMed]
- Voegeli, R.; Heiland, J.; Doppler, S.; Rawlings, A.V.; Schreier, T. Efficient and simple quantification of stratum corneum proteins on tape strippings by infrared densitometry. Skin Res. Technol. 2007, 13, 242–251. [Google Scholar] [CrossRef] [PubMed]
- Hoppel, M.; Baurecht, D.; Holper, E.; Mahrhauser, D.; Valenta, C. Validation of the combined ATR-FTIR/tape stripping technique for monitoring the distribution of surfactants in the stratum corneum. Int. J. Pharm. 2014, 472, 88–93. [Google Scholar] [CrossRef] [PubMed]
- Turner, N.J.; Pezzone, D.; Badylak, S.F. Regional variations in the histology of porcine skin. Tissue Eng. Part C Methods 2015, 21, 373–384. [Google Scholar] [CrossRef]
- Denat, L.; Kadekaro, A.L.; Marrot, L.; Leachman, S.A.; Abdel-Malek, Z.A. Melanocytes as instigators and victims of oxidative stress. J. Investig. Dermatol. 2014, 134, 1512–1518. [Google Scholar] [CrossRef] [Green Version]
- Silvestre, M.; Saeed, A.; Cervera, R.; Escribá, M.; García-Ximénez, F. Rabbit and pig ear skin sample cryobanking: Effects of storage time and temperature of the whole ear extirpated immediately after death. Theriogenology 2003, 59, 1469–1477. [Google Scholar] [CrossRef]
- Andersson, T.; Ertürk Bergdahl, G.; Saleh, K.; Magnúsdóttir, H.; Stødkilde, K.; Andersen, C.B.F.; Lundqvist, K.; Jensen, A.; Brüggemann, H.; Lood, R. Common skin bacteria protect their host from oxidative stress through secreted antioxidant RoxP. Sci. Rep. 2019, 9, 3596. [Google Scholar] [CrossRef] [Green Version]
- Ketter, P.M.; Kamucheka, R.; Arulanandam, B.; Akers, K.; Cap, A.P. Platelet enhancement of bacterial growth during room temperature storage: Mitigation through refrigeration. Transfusion 2019, 59, 1479–1489. [Google Scholar] [CrossRef] [Green Version]
- Ogai, K.; Nagase, S.; Mukai, K.; Iuchi, T.; Mori, Y.; Matsue, M.; Sugitani, K.; Sugama, J.; Okamoto, S. A Comparison of techniques for collecting skin microbiome samples: Swabbing versus tape-stripping. Front. Microbiol. 2018, 9, 2362. [Google Scholar] [CrossRef]
- Groussard, C.; Morel, I.; Chevanne, M.; Monnier, M.; Cillard, J.; Delamarche, A. Free radical scavenging and antioxidant effects of lactate ion: An in vitro study. J. Appl. Physiol. 2000, 89, 169–175. [Google Scholar] [CrossRef] [PubMed]
- Grazul-Bilska, A.T.; Bilski, J.J.; Redmer, D.A.; Reynolds, L.P.; Abdullah, K.M.; Abdullah, A. Antioxidant capacity of 3D human skin EpiDerm model: Effects of skin moisturizers. Int. J. Cosmet. Sci. 2009, 31, 201–208. [Google Scholar] [CrossRef] [PubMed]
- Maibam, U.; Hooda, O.K.; Sharma, P.S.; Upadhyay, R.C.; Mohanty, A.K. Differential level of oxidative stress markers in skin tissue of zebu and crossbreed cattle during thermal stress. Livest. Sci. 2018, 207, 45–50. [Google Scholar] [CrossRef]
- Abla, M.J.; Banga, A.K. Quantification of skin penetration of antioxidants of varying lipophilicity. Int. J. Cosmet. Sci. 2013, 35, 19–26. [Google Scholar] [CrossRef]
- Pelikh, O.; Eckert, R.W.; Pinnapireddy, S.R.; Keck, C.M. Hair follicle targeting with curcumin nanocrystals: Influence of the formulation properties on the penetration efficacy. J. Control. Release 2020, 10, 598–613. [Google Scholar] [CrossRef]
- Patel, S.R.; Zhong, H.; Sharma, A.; Kalia, Y.N. Controlled non-invasive transdermal iontophoretic delivery of zolmitriptan hydrochloride in vitro and in vivo. Eur. J. Pharm. Biopharm. 2009, 72, 304–309. [Google Scholar] [CrossRef]
- Zhao, X.; Schaffzin, J.K.; Carson, J.; Ankrum, A.; Dobrzykowski, E.; Haslam, D.B.; Dandoy, C.E.; Setchell, K.D.R. Analysis of chlorhexidine gluconate in skin using tape stripping and ultrahigh-performance liquid chromatography-tandem mass spectrometry. J. Pharm. Biomed. Anal. 2020, 183, 113111. [Google Scholar] [CrossRef]
- Rosenquist, M.D.; Cram, A.E.; Kealey, G.P. Skin preservation at 4 °C: A species comparison. Cryobiology 1988, 25, 31–37. [Google Scholar] [CrossRef]
- Zhu, Z.M. Optimal conditions for the storage of split-thickness skin at 4C. Chin. J. Surg. 1989, 27, 169–172. [Google Scholar]
- Lohan, S.B.; Müller, R.; Albrecht, S.; Mink, K.; Tscherch, K.; Ismaeel, F.; Lademann, J.; Rohn, S.; Meinke, M.C. Free radicals induced by sunlight in different spectral regions—in vivo versus ex vivo study. Exp. Dermatol. 2016, 25, 380–385. [Google Scholar] [CrossRef]
- Amaro-Ortiz, A.; Yan, B.; D’Orazio, J.A. Ultraviolet radiation, aging and the skin: Prevention of damage by topical cAMP manipulation. Molecules 2014, 19, 6202–6219. [Google Scholar] [CrossRef] [PubMed]
- Humbert, P.G.; Haftek, M.; Creidi, P.; Lapière, C.; Nusgens, B.; Richard, A.; Schmitt, D.; Rougier, A.; Zahouani, H. Topical ascorbic acid on photoaged skin. Clinical, topographical and ultrastructural evaluation: Double-blind study vs. placebo. Exp. Dermatol. 2003, 12, 237–244. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kishimoto, Y.; Saito, N.; Kurita, K.; Shimokado, K.; Maruyama, N.; Ishigami, A. Ascorbic acid enhances the expression of type 1 and type 4 collagen and SVCT2 in cultured human skin fibroblasts. Biochem. Biophys. Res. Commun. 2013, 430, 579–584. [Google Scholar] [CrossRef] [PubMed]
- Pinnell, S.R.; Yang, H.; Omar, M.; Monteiro-Riviere, N.; DeBuys, H.V.; Walker, L.C.; Wang, Y.; Levine, M. Topical L-ascorbic acid: Percutaneous absorption studies. Dermatol. Surg. 2001, 27, 137–142. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Jiang, H.; Li, W.; Qiang, M.; Dong, T.; Li, H. Role of Vitamin C in Skin Diseases. Front. Physiol. 2018, 9, 819. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schallreuter, K.U. Q10-triggered facial vitiligo. Br. J. Dermatol. 2013, 169, 1333–1336. [Google Scholar] [CrossRef]
- Lohan, S.; Lauer, A.-C.; Arndt, S.; Friedrich, A.; Tscherch, K.; Haag, S.; Darvin, M.; Vollert, H.; Kleemann, A.; Gersonde, I.; et al. Determination of the Antioxidant Status of the Skin by In Vivo-Electron Paramagnetic Resonance (EPR) Spectroscopy. Cosmetics 2015, 2, 286–301. [Google Scholar] [CrossRef] [Green Version]
- Albrecht, S.; Elpelt, A.; Kasim, C.; Reble, C.; Mundhenk, L.; Pischon, H.; Hedtrich, S.; Witzel, C.; Lademann, J.; Zastrow, L.; et al. Quantification and characterization of radical production in human, animal and 3D skin models during sun irradiation measured by EPR spectroscopy. Free Radic. Biol. Med. 2019, 131, 299–308. [Google Scholar] [CrossRef]
- Abraham, A.M.; Alnemari, R.M.; Jacob, C.; Keck, C.M. PlantCrystals-Nanosized Plant Material for Improved Bioefficacy of Medical Plants. Materials 2020, 13, 4368. [Google Scholar] [CrossRef]
- Abraham, A.M.; Alnemari, R.M.; Brüßler, J.; Keck, C.M. Improved antioxidant capacity of black tea waste utilizing plantcrystals. Molecules 2021, 26, 592. [Google Scholar] [CrossRef]


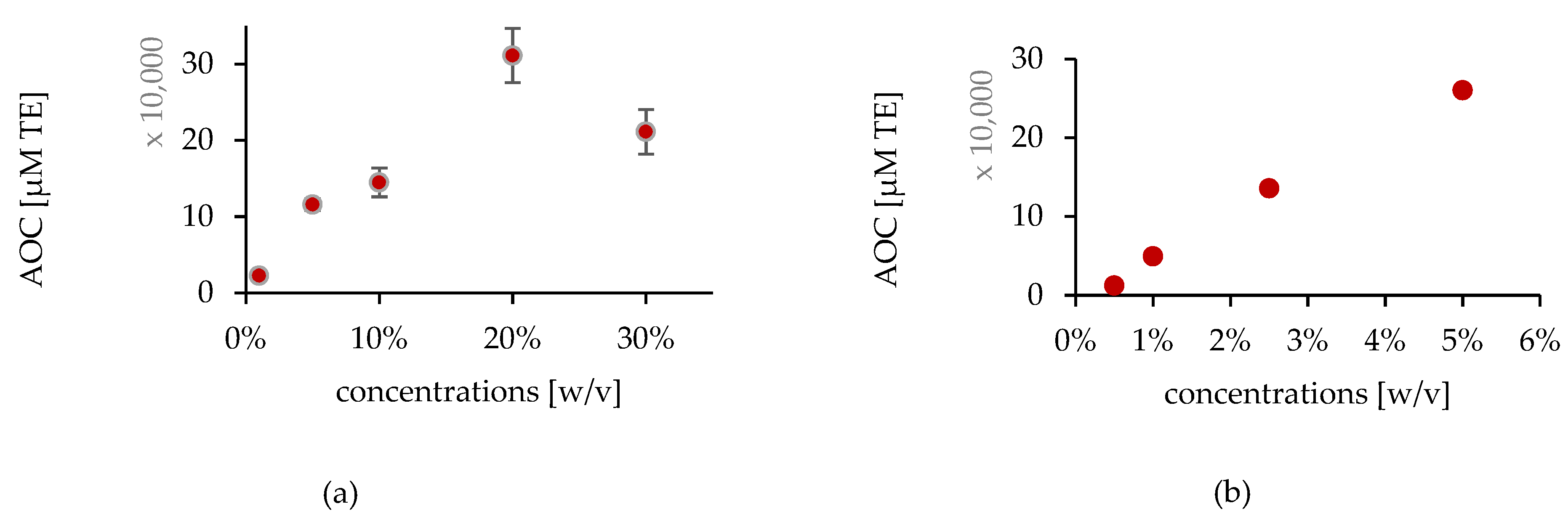

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alnemari, R.M.; Brüßler, J.; Keck, C.M. Assessing the Oxidative State of the Skin by Combining Classical Tape Stripping with ORAC Assay. Pharmaceuticals 2022, 15, 520. https://doi.org/10.3390/ph15050520
Alnemari RM, Brüßler J, Keck CM. Assessing the Oxidative State of the Skin by Combining Classical Tape Stripping with ORAC Assay. Pharmaceuticals. 2022; 15(5):520. https://doi.org/10.3390/ph15050520
Chicago/Turabian StyleAlnemari, Reem M., Jana Brüßler, and Cornelia M. Keck. 2022. "Assessing the Oxidative State of the Skin by Combining Classical Tape Stripping with ORAC Assay" Pharmaceuticals 15, no. 5: 520. https://doi.org/10.3390/ph15050520
APA StyleAlnemari, R. M., Brüßler, J., & Keck, C. M. (2022). Assessing the Oxidative State of the Skin by Combining Classical Tape Stripping with ORAC Assay. Pharmaceuticals, 15(5), 520. https://doi.org/10.3390/ph15050520





