Electrospun Biomimetic Multifunctional Nanofibers Loaded with Ferulic Acid for Enhanced Antimicrobial and Wound-Healing Activities in STZ-Induced Diabetic Rats
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Extraction of Silk Sericin from Silk Cocoons
2.3. Fabrication of Silk Sericin Hybrid Nanofibers by Electrospinning
2.4. Morphology of Nanofibers
2.5. Tensile Strength of Nanofibers
2.6. Swelling Index
2.7. Biodegradability Assay
2.8. In Vitro Drug-Release Studies
2.9. Antimicrobial Activity
2.9.1. Agar Disc Diffusion Method
2.9.2. Time-Kill Assay
2.9.3. Biofilm Formation Assay
2.10. In Vitro Cell-Line Study
2.10.1. Cell Culture and Treatment
2.10.2. In Vitro Cytotoxicity (MTT Assay)
2.10.3. In Vitro Scratch Wound Assay
2.11. In Vivo Wound Healing Activity
2.12. Histopathology
2.13. Statistical Analysis
3. Results and Discussion
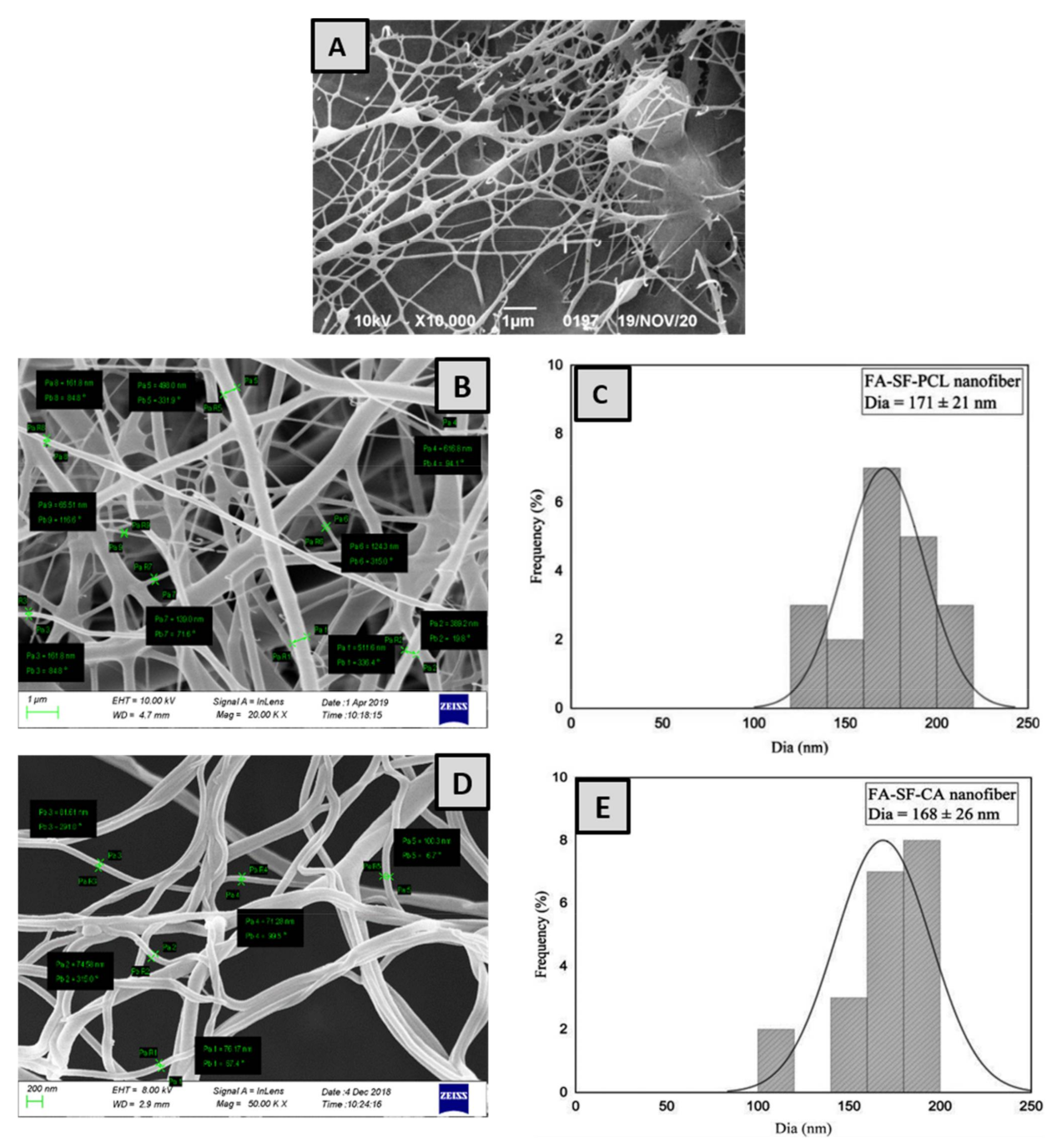
3.1. Surface Morphology
3.2. Mechanical Properties
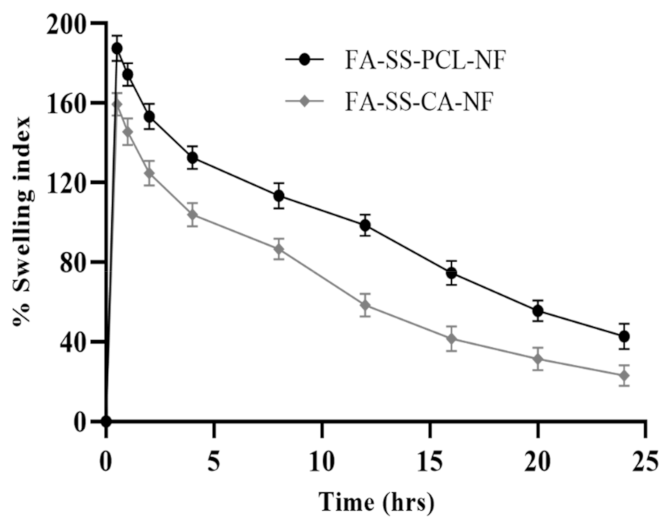
3.3. Swelling Index
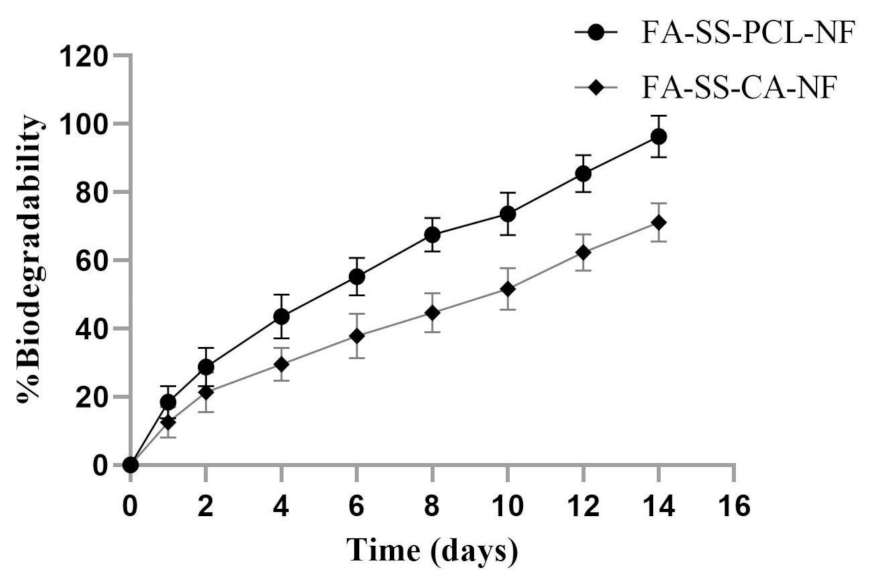
3.4. Biodegradability Assay
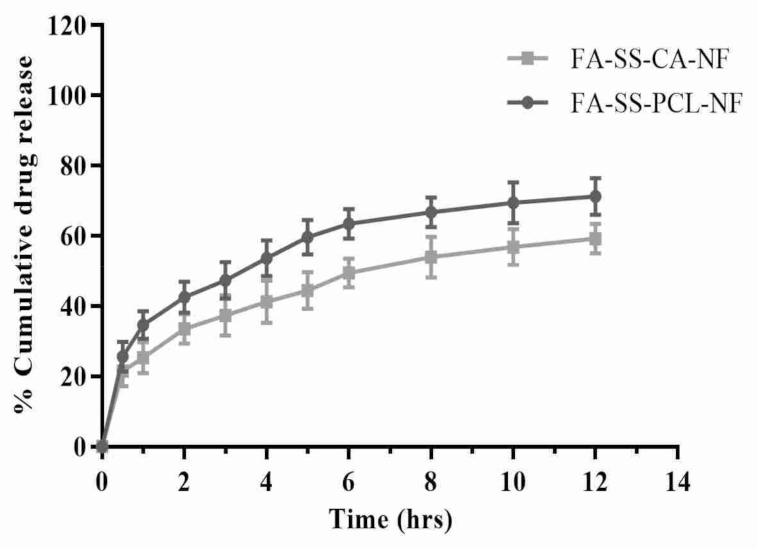
3.5. In Vitro Drug Release Studies
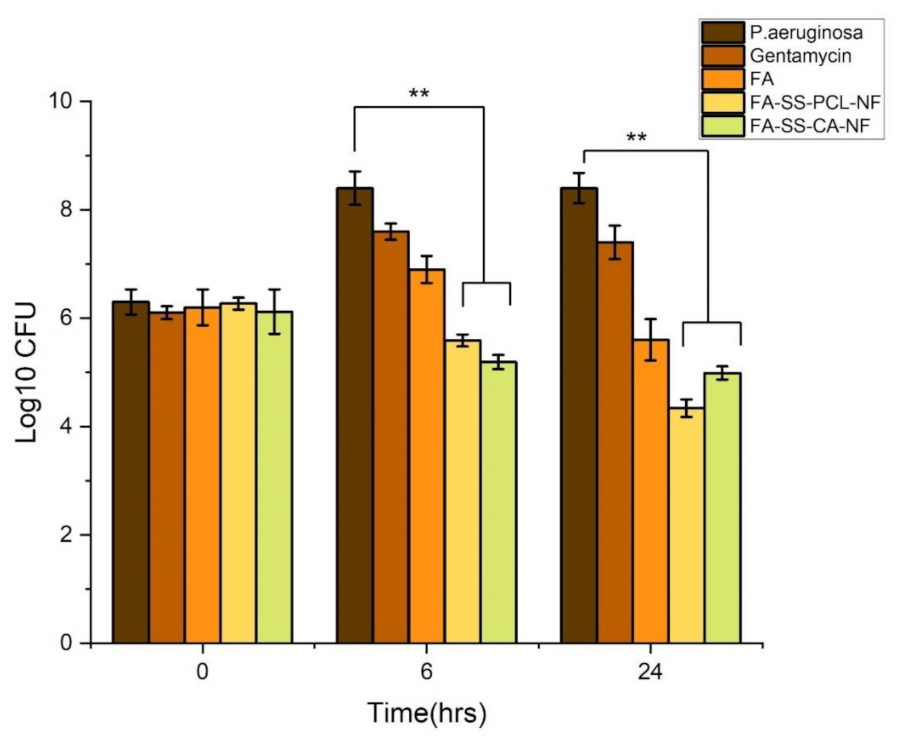
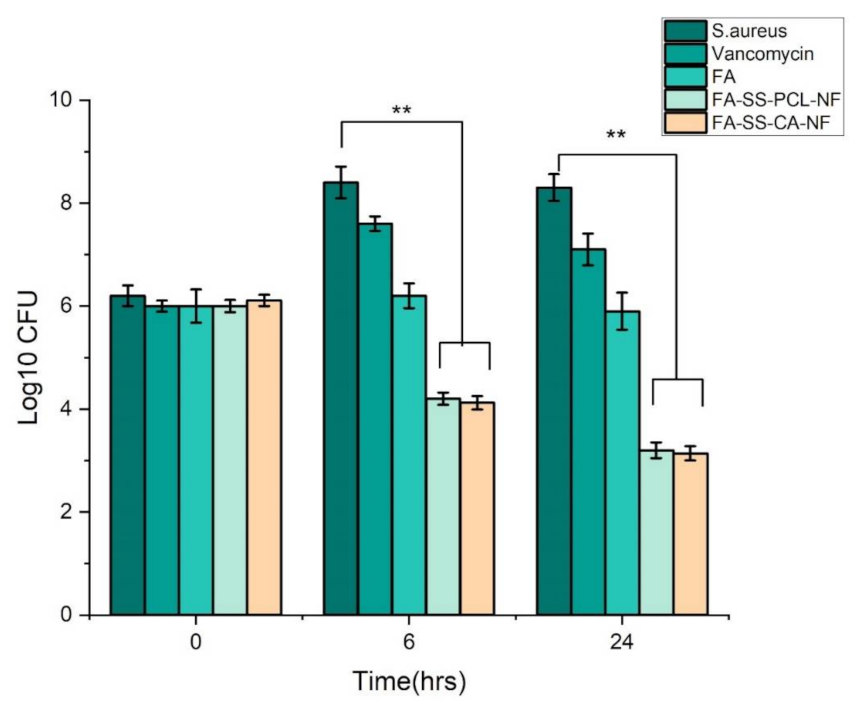
3.6. Antimicrobial Study
3.7. Time-Kill Assay Results
3.8. Biofilm Assay
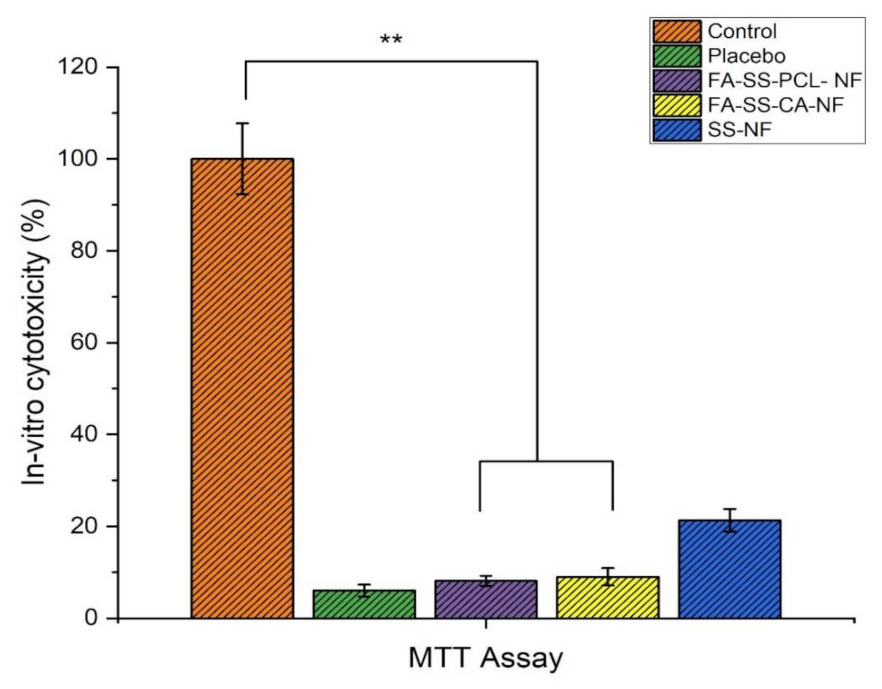
3.9. In Vitro Cytotoxicity (MTT Assay)
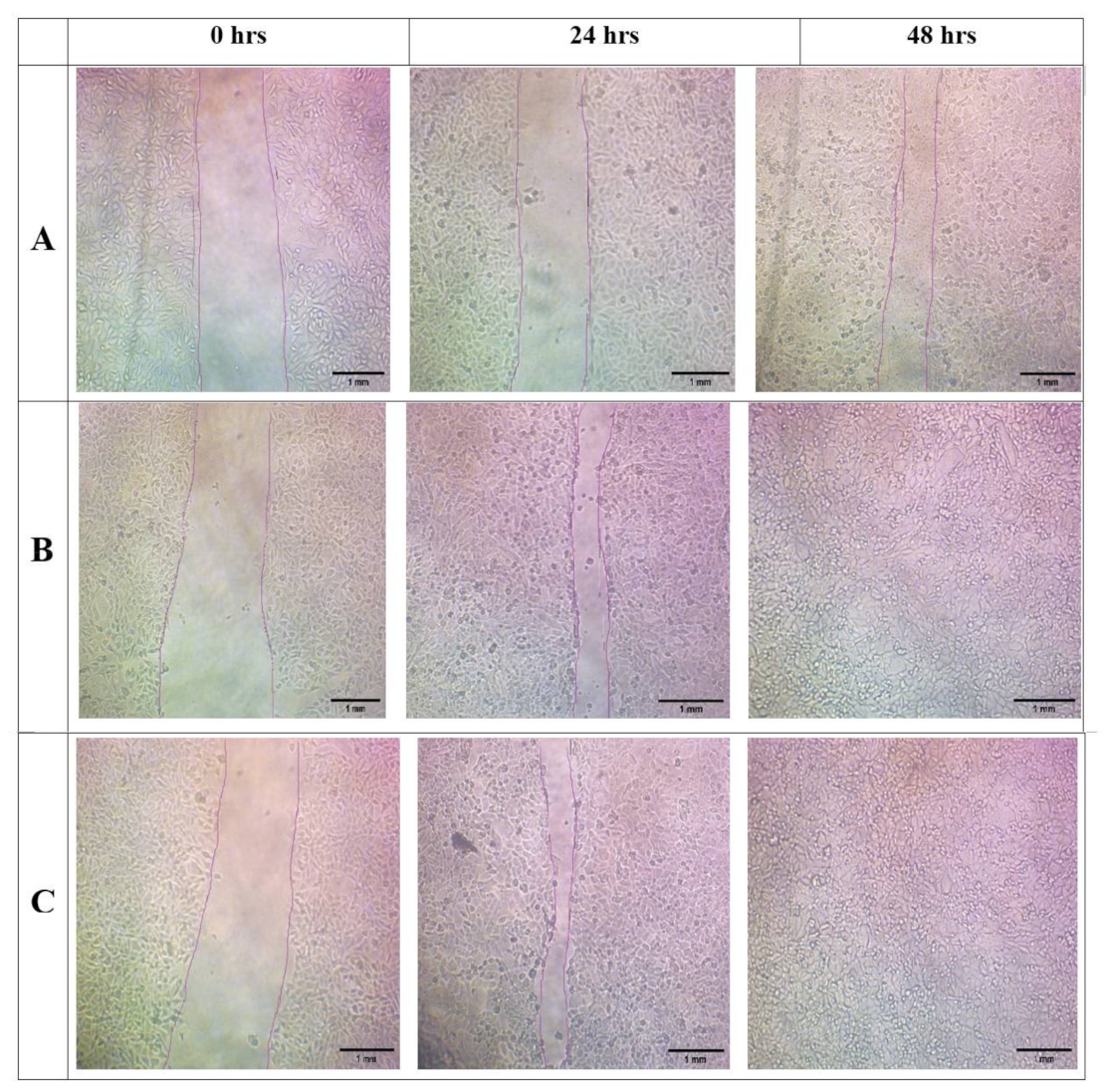
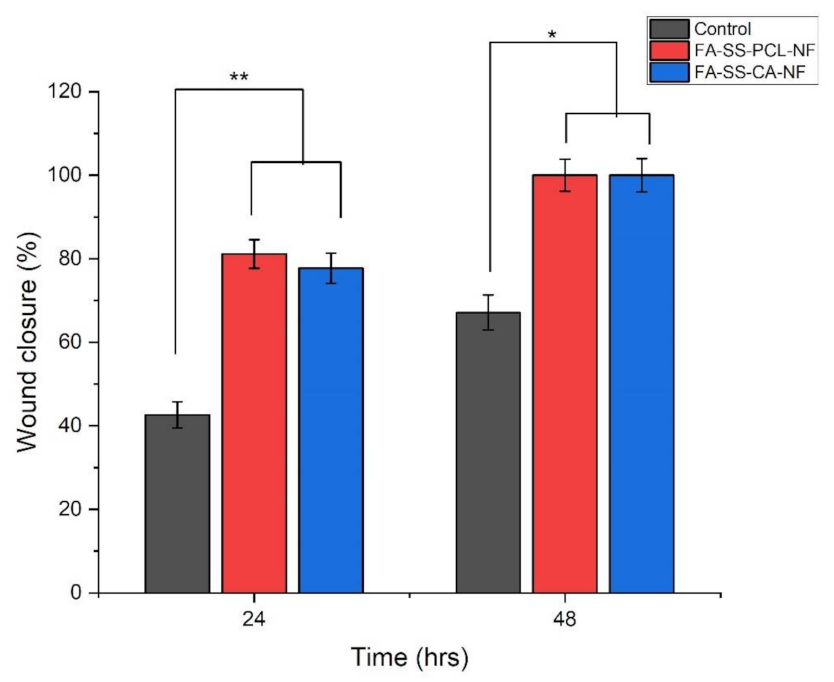
3.10. In Vitro Scratch Wound Assay
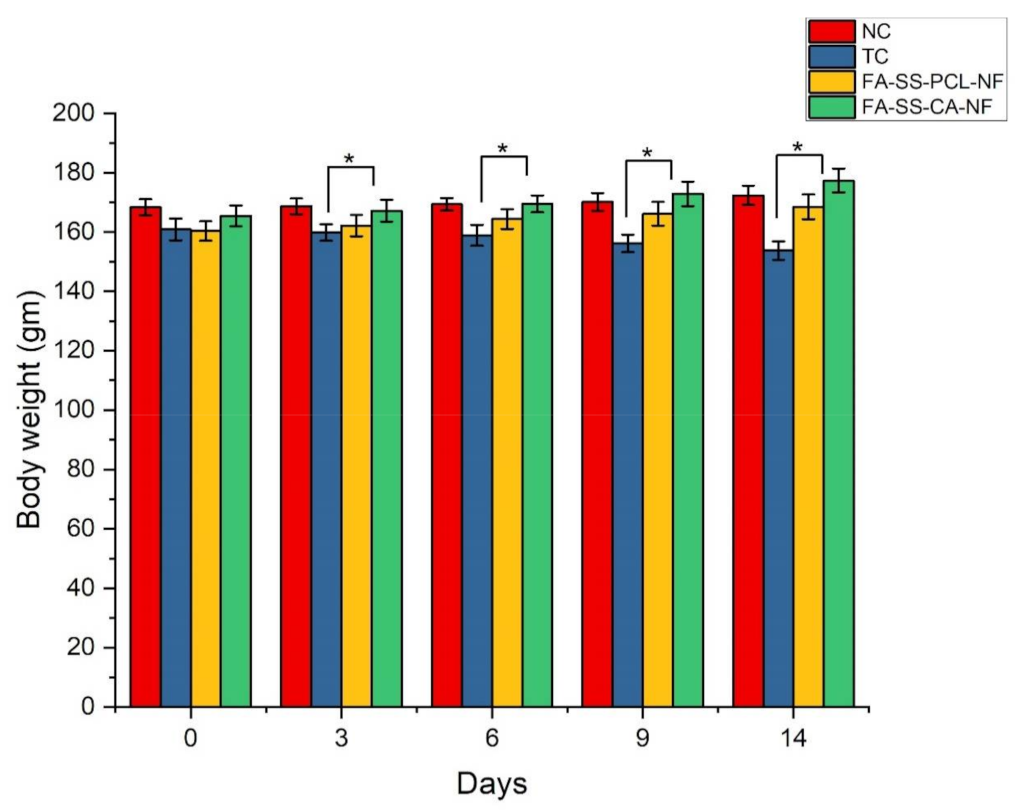
3.11. Weight Variation in Diabetes-Induced Animals
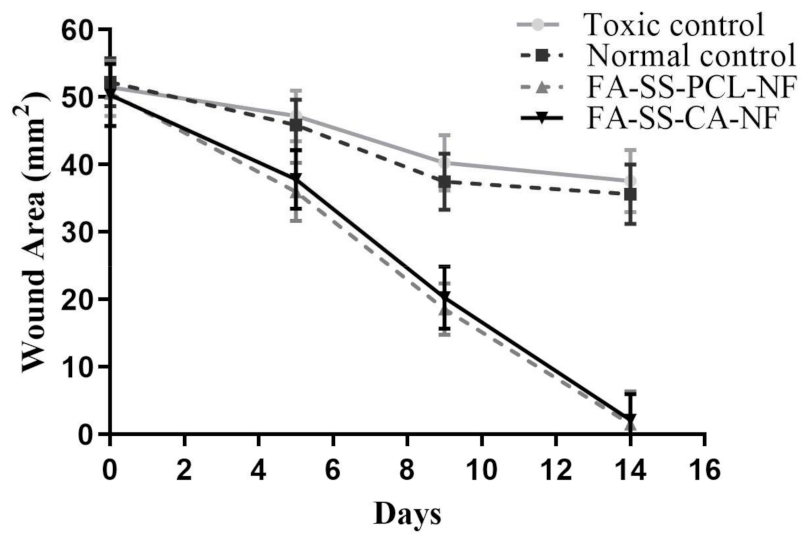
3.12. In Vivo Wound-Healing Study
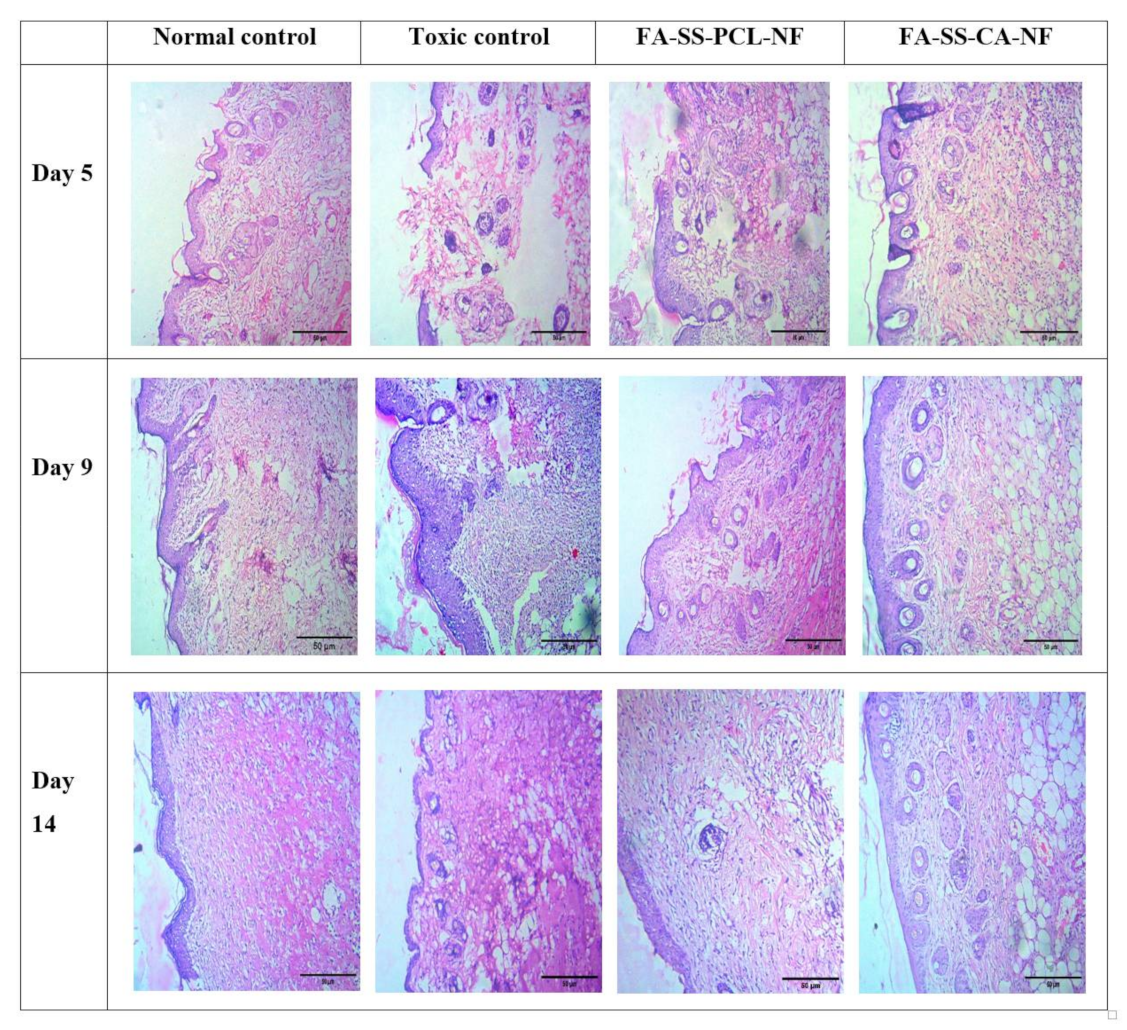
3.13. Histopathology
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yazdanpanah, L.; Nasiri, M.; Adarvishi, S. Literature review on the management of diabetic foot ulcer. World J. Diabetes 2015, 6, 37. [Google Scholar] [CrossRef]
- Alberti, K.G.M.M.; Zimmet, P.Z. Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: Diagnosis and classification of diabetes mellitus. Provisional report of a WHO consultation. Diabet. Med. 1998, 15, 539–553. [Google Scholar] [CrossRef]
- Greenhalgh, D.G. Wound healing and diabetes mellitus. Clin. Plast. Surg. 2003, 30, 37–45. [Google Scholar] [CrossRef]
- Monaco, J.L.; Lawrence, W.T. Acute wound healing: An overview. Clin. Plast. Surg. 2003, 30, 1–12. [Google Scholar] [CrossRef]
- Iacob, A.-T.; Drăgan, M.; Ionescu, O.-M.; Profire, L.; Ficai, A.; Andronescu, E.; Confederat, L.G.; Lupașcu, D. An overview of biopolymeric electrospun nanofibers based on polysaccharides for wound healing management. Pharmaceutics 2020, 12, 983. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Xu, H.; Zhang, M.; Yu, D.-G. Electrospun medicated nanofibers for wound healing. Membranes 2021, 11, 770. [Google Scholar] [CrossRef] [PubMed]
- Sylvester, M.A.; Amini, F.; Tan, C.K. Electrospun nanofibers in wound healing. Mater. Today Proc. 2020, 29, 1–6. [Google Scholar] [CrossRef]
- Agarwal, Y.; Rajinikanth, P.; Ranjan, S.; Tiwari, U.; Balasubramnaiam, J.; Pandey, P.; Arya, D.K.; Anand, S.; Deepak, P. Curcumin loaded polycaprolactone-/polyvinyl alcohol-silk fibroin based electrospun nanofibrous mat for rapid healing of diabetic wound: An in-vitro and in-vivo studies. Int. J. Biol. Macromol. 2021, 176, 376–386. [Google Scholar] [CrossRef] [PubMed]
- Zduńska, K.; Dana, A.; Kolodziejczak, A.; Rotsztejn, H. Antioxidant properties of ferulic acid and its possible application. Ski. Pharmacol. Physiol. 2018, 31, 332–336. [Google Scholar] [CrossRef]
- Balasubashini, M.S.; Rukkumani, R.; Viswanathan, P.; Menon, V.P. Ferulic acid alleviates lipid peroxidation in diabetic rats. Phytother. Res. 2004, 18, 310–314. [Google Scholar] [CrossRef] [PubMed]
- Tee-Ngam, P.; Nunant, N.; Rattanarat, P.; Siangproh, W.; Chailapakul, O. Simple and rapid determination of ferulic acid levels in food and cosmetic samples using paper-based platforms. Sensors 2013, 13, 13039–13053. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Locarno, S.; Eleta-Lopez, A.; Lupo, M.G.; Gelmi, M.L.; Clerici, F.; Bittner, A.M. Electrospinning of pyrazole-isothiazole derivatives: Nanofibers from small molecules. R. Soc. Chem. 2019, 9, 20565–20572. [Google Scholar] [CrossRef] [Green Version]
- Sell, S.A.; Wolfe, P.S.; Garg, K.; McCool, J.M.; Rodriguez, I.A.; Bowlin, G.L. The use of natural polymers in tissue engineering: A focus on electrospun extracellular matrix analogues. Polymers 2010, 2, 522–553. [Google Scholar] [CrossRef]
- Kasoju, N.; Bora, U. Silk fibroin in tissue engineering. Adv. Healthc. Mater. 2012, 1, 393–412. [Google Scholar] [CrossRef] [PubMed]
- Cao, T.-T.; Zhang, Y.-Q. Processing and characterization of silk sericin from Bombyx mori and its application in biomaterials and biomedicines. Mater. Sci. Eng. C 2016, 61, 940–952. [Google Scholar] [CrossRef] [PubMed]
- Padamwar, M.; Pawar, A. Silk Sericin and Its Applications: A Review; CSIR: New Delhi, India, 2004. [Google Scholar]
- Bakhsheshi-Rad, H.R.; Ismail, A.F.; Aziz, M.; Akbari, M.; Hadisi, Z.; Omidi, M.; Chen, X. Development of the PVA/CS nanofibers containing silk protein sericin as a wound dressing: In vitro and in vivo assessment. Int. J. Biol. Macromol. 2020, 149, 513–521. [Google Scholar] [CrossRef] [PubMed]
- Khoshnevisan, K.; Maleki, H.; Samadian, H.; Shahsavari, S.; Sarrafzadeh, M.H.; Larijani, B.; Dorkoosh, F.A.; Haghpanah, V.; Khorramizadeh, M.R. Cellulose acetate electrospun nanofibers for drug delivery systems: Applications and recent advances. Carbohydr. Polym. 2018, 198, 131–141. [Google Scholar] [CrossRef] [PubMed]
- Negut, I.; Dorcioman, G.; Grumezescu, V. Scaffolds for Wound Healing Applications. Polymers 2020, 12, 2010. [Google Scholar] [CrossRef]
- Yang, Y.; Lee, S.M.; Lee, H.S.; Lee, K.H. Recovery of silk sericin from soap-alkaline degumming solution. Int. J. Ind. Entomol. 2013, 27, 203–208. [Google Scholar] [CrossRef] [Green Version]
- Mohammadzadehmoghadam, S.; Dong, Y. Fabrication and characterization of electrospun silk fibroin/gelatin scaffolds crosslinked with glutaraldehyde vapor. Front. Mater. 2019, 6, 91. [Google Scholar] [CrossRef]
- Wadke, P.; Chhabra, R.; Jain, R.; Dandekar, P. Silver-embedded starch-based nanofibrous mats for soft tissue engineering. Surf. Interfaces 2017, 8, 137–146. [Google Scholar] [CrossRef]
- Zhang, X.; Chen, Z.; Bao, H.; Liang, J.; Xu, S.; Cheng, G.; Zhu, Y. Fabrication and characterization of silk fibroin/curcumin sustained-release film. Materials 2019, 12, 3340. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Samadian, H.; Salehi, M.; Farzamfar, S.; Vaez, A.; Ehterami, A.; Sahrapeyma, H.; Goodarzi, A.; Ghorbani, S. In vitro and in vivo evaluation of electrospun cellulose acetate/gelatin/hydroxyapatite nanocomposite mats for wound dressing applications. Artif. Cells Nanomed. Biotechnol. 2018, 46, 964–974. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mac Faddin, J.F. Media for Isolation-Cultivation-Identification-Maintenance of Medical Bacteria; Williams & Wilkins: Baltimore, MD, USA, 1985; Volume 1. [Google Scholar]
- Balouiri, M.; Sadiki, M.; Ibnsouda, S.K. Methods for in vitro evaluating antimicrobial activity: A review. J. Pharm. Anal. 2016, 6, 71–79. [Google Scholar] [CrossRef] [Green Version]
- Fguira, L.F.-B.; Fotso, S.; Ameur-Mehdi, R.B.; Mellouli, L.; Laatsch, H. Purification and structure elucidation of antifungal and antibacterial activities of newly isolated Streptomyces sp. strain US80. Res. Microbiol. 2005, 156, 341–347. [Google Scholar] [CrossRef] [PubMed]
- Avila-Novoa, M.-G.; Iñíguez-Moreno, M.; Solís-Velázquez, O.-A.; González-Gomez, J.-P.; Guerrero-Medina, P.-J.; Gutiérrez-Lomelí, M. Biofilm formation by Staphylococcus aureus isolated from food contact surfaces in the dairy industry of Jalisco, Mexico. J. Food Qual. 2018, 2018, 1746139. [Google Scholar] [CrossRef] [Green Version]
- Kouidhi, B.; Zmantar, T.; Hentati, H.; Bakhrouf, A. Cell surface hydrophobicity, biofilm formation, adhesives properties and molecular detection of adhesins genes in Staphylococcus aureus associated to dental caries. Microb. Pathog. 2010, 49, 14–22. [Google Scholar] [CrossRef] [PubMed]
- Wsoo, M.A.; Abd Razak, S.I.; Bohari, S.P.M.; Shahir, S.; Salihu, R.; Kadir, M.R.A.; Nayan, N.H.M. Vitamin D3-loaded electrospun cellulose acetate/polycaprolactone nanofibers: Characterization, in-vitro drug release and cytotoxicity studies. Int. J. Biol. Macromol. 2021, 181, 82–98. [Google Scholar] [CrossRef] [PubMed]
- Muniandy, K.; Gothai, S.; Tan, W.S.; Kumar, S.S.; Mohd Esa, N.; Chandramohan, G.; Al-Numair, K.S.; Arulselvan, P. In vitro wound healing potential of stem extract of Alternanthera sessilis. Int. J. Biol. Macromol. 2018, 2018, 3142073. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hadisi, Z.; Farokhi, M.; Bakhsheshi-Rad, H.R.; Jahanshahi, M.; Hasanpour, S.; Pagan, E.; Dolatshahi-Pirouz, A.; Zhang, Y.S.; Kundu, S.C.; Akbari, M. Hyaluronic acid (HA)-based silk fibroin/zinc oxide core–shell electrospun dressing for burn wound management. Macromol. Biosci. 2020, 20, 1900328. [Google Scholar] [CrossRef]
- Cheng, K.-Y.; Lin, Z.-H.; Cheng, Y.-P.; Chiu, H.-Y.; Yeh, N.-L.; Wu, T.-K.; Wu, J.-S. Wound healing in streptozotocin-induced diabetic rats using atmospheric-pressure argon plasma jet. Sci. Rep. 2018, 8, 12214. [Google Scholar] [CrossRef]
- Akbarzadeh, A.; Norouzian, D.; Mehrabi, M.; Jamshidi, S.; Farhangi, A.; Verdi, A.A.; Mofidian, S.; Rad, B.L. Induction of diabetes by streptozotocin in rats. Indian J. Clin. Biochem. 2007, 22, 60–64. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carvalho, M.T.; Araújo-Filho, H.G.; Barreto, A.S.; Quintans-Júnior, L.J.; Quintans, J.S.; Barreto, R.S. Wound healing properties of flavonoids: A systematic review highlighting the mechanisms of action. Phytomedicine 2021, 90, 153636. [Google Scholar] [CrossRef] [PubMed]
- Ghaisas, M.M.; Kshirsagar, S.B.; Sahane, R.S. Evaluation of wound healing activity of ferulic acid in diabetic rats. Int. Wound J. 2014, 11, 523–532. [Google Scholar] [CrossRef] [PubMed]
- Anand, S.; Rajinikanth, P.S.; Arya, D.K.; Pandey, P.; Gupta, R.K.; Sankhwar, R.; Chidambaram, K. Multifunctional Biomimetic Nanofibrous Scaffold Loaded with Asiaticoside for Rapid Diabetic Wound Healing. Pharmaceutics 2022, 14, 273. [Google Scholar] [CrossRef] [PubMed]
- Selvaraj, S.; Fathima, N.N. Fenugreek Incorporated Silk Fibroin Nanofibers—A Potential Antioxidant Scaffold for Enhanced Wound Healing. ACS Appl. Mater. Interfaces 2017, 9, 5916–5926. [Google Scholar] [CrossRef]
- Stovall, M.; Smith, S.A.; Langholz, B.M.; Boice, J.D., Jr.; Shore, R.E.; Andersson, M.; Buchholz, T.A.; Capanu, M.; Bernstein, L.; Lynch, C.F. Dose to the contralateral breast from radiotherapy and risk of second primary breast cancer in the WECARE study. Int. J. Radiat. Oncol. Biol. Phys. 2008, 72, 1021–1030. [Google Scholar] [CrossRef] [Green Version]
- Mistry, P.; Chhabra, R.; Muke, S.; Narvekar, A.; Sathaye, S.; Jain, R.; Dandekar, P. Fabrication and characterization of starch-TPU based nanofibers for wound healing applications. Mater. Sci. Eng. C 2021, 119, 111316. [Google Scholar] [CrossRef]
- Samadian, H.; Zamiri, S.; Ehterami, A.; Farzamfar, S.; Vaez, A.; Khastar, H.; Alam, M.; Ai, A.; Derakhshankhah, H.; Allahyari, Z. Electrospun cellulose acetate/gelatin nanofibrous wound dressing containing berberine for diabetic foot ulcer healing: In vitro and in vivo studies. Sci. Rep. 2020, 10, 8312. [Google Scholar] [CrossRef]
- Ou, S.; Kwok, K.C. Ferulic acid: Pharmaceutical functions, preparation and applications in foods. J. Sci. Food. Agric. 2004, 84, 1261–1269. [Google Scholar] [CrossRef]
- Santoro, M.M.; Gaudino, G. Cellular and molecular facets of keratinocyte reepithelization during wound healing. Exp. Cell Res. 2005, 304, 274–286. [Google Scholar] [CrossRef] [PubMed]
- Zafar, M.; Naqvi, S.N.H. Effects of STZ-Induced diabetes on the relative weights of kidney, liver and pancreas in albino rats: A comparative study. J. Morphol. 2010, 28, 135–142. [Google Scholar] [CrossRef] [Green Version]
- Dwivedi, S.; Singh, D.; Deshmukh, P.T.; Soni, R.; Trivedi, R. Healing potential of ferulic acid on dermal wound in diabetic animals. Asian J. Mol. Model 2015, 1, 1–16. [Google Scholar]
- Zhao, Y.; Qiu, Y.; Wang, H.; Chen, Y.; Jin, S.; Chen, S. Preparation of nanofibers with renewable polymers and their application in wound dressing. Int. J. Polym. Sci. 2016, 4672839. [Google Scholar] [CrossRef] [Green Version]
- Farman, N.; Palacios-Ramirez, R.; Sbeih, M.; Behar-Cohen, F.; Aractingi, S.; Jaisser, F. Cutaneous wound healing in diabetic mice is improved by topical mineralocorticoid receptor blockade. J. Investig. Dermatol. 2020, 140, 223–234.e7. [Google Scholar]
- Martin, P. Wound healing—Aiming for perfect skin regeneration. Science 1997, 276, 75–81. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Xia, T.; Zhi, W.; Wei, L.; Weng, J.; Zhang, C.; Li, X. Promotion of skin regeneration in diabetic rats by electrospun core-sheath fibers loaded with basic fibroblast growth factor. Biomaterials 2011, 32, 4243–4254. [Google Scholar] [CrossRef] [PubMed]




| Formulation | SS | PCL | CA | FA |
|---|---|---|---|---|
| SS-NF | 8% | - | - | - |
| FA-SS-PCL-NF | 8% | 12% | - | 2% |
| FA-SS-CA-NF | 8% | - | 12% | 2% |
| S. No. | Formulations | Strength (MPa) |
|---|---|---|
| 1. | SS-NF | 4.04 ± 0.013 |
| 2. | FA-SS-PCL-NF | 7.73 ± 0.204 |
| 3. | FA-SS-CA-NF | 14.65 ± 0.159 |
| Time (h) | FA-SS-PCL-NF | FA-SS-CA-NF |
|---|---|---|
| 0 | 0 | 0 |
| 0.5 | 25.67 ± 4.21 | 21.56 ± 4.34 |
| 1 | 34.65 ± 3.95 | 25.34 ± 4.45 |
| 2 | 42.56 ± 4.45 | 33.56 ± 4.23 |
| 3 | 47.34 ± 5.21 | 37.34 ± 5.67 |
| 4 | 53.68 ± 5.12 | 41.23 ± 5.98 |
| 5 | 59.65 ± 4.89 | 44.45 ± 5.23 |
| 6 | 63.45 ± 4.21 | 49.46 ± 4.12 |
| 8 | 66.78 ± 4.32 | 53.93 ± 5.85 |
| 10 | 69.45 ± 5.78 | 56.83 ± 5.12 |
| 12 | 71.24 ± 5.23 | 59.23 ± 4.18 |
| Groups | Body Weight (gm) (before Inducing Diabetes) | Body Weight (gm) (after Inducing Diabetes) | ||||
|---|---|---|---|---|---|---|
| 0 Day | 3rd Day | 6th Day | 9th Day | 14th Day | ||
| NC | 170 ± 2.11 | 168.4 ± 2.71 | 168.7 ± 2.66 | 169.4 ± 2.11 | 170.2 ± 3.01 | 172.4 ± 3.21 |
| TC | 172 ± 2.63 | 160.9 ± 3.65 | 159.9 ± 2.76 | 158.9 ± 3.46 | 156.2 ± 2.94 | 153.8 ± 3.12 |
| FA-SS-PCL-NF | 171 ± 2.47 | 160.4 ± 3.25 | 162.2 ± 3.66 | 164.4 ± 3.32 | 166.2 ± 4.01 | 169.5 ± 4.21 |
| FA-SS-CA-NF | 175 ± 3.12 | 165.5 ± 3.51 | 167.2 ± 3.71 | 169.5 ± 2.83 | 172.9 ± 4.11 | 177.4 ± 4.03 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Anand, S.; Pandey, P.; Begum, M.Y.; Chidambaram, K.; Arya, D.K.; Gupta, R.K.; Sankhwar, R.; Jaiswal, S.; Thakur, S.; Rajinikanth, P.S. Electrospun Biomimetic Multifunctional Nanofibers Loaded with Ferulic Acid for Enhanced Antimicrobial and Wound-Healing Activities in STZ-Induced Diabetic Rats. Pharmaceuticals 2022, 15, 302. https://doi.org/10.3390/ph15030302
Anand S, Pandey P, Begum MY, Chidambaram K, Arya DK, Gupta RK, Sankhwar R, Jaiswal S, Thakur S, Rajinikanth PS. Electrospun Biomimetic Multifunctional Nanofibers Loaded with Ferulic Acid for Enhanced Antimicrobial and Wound-Healing Activities in STZ-Induced Diabetic Rats. Pharmaceuticals. 2022; 15(3):302. https://doi.org/10.3390/ph15030302
Chicago/Turabian StyleAnand, Sneha, Prashant Pandey, Mohammed Yasmin Begum, Kumarappan Chidambaram, Dilip Kumar Arya, Ravi Kr. Gupta, Ruchi Sankhwar, Shweta Jaiswal, Sunita Thakur, and Paruvathanahalli Siddalingam Rajinikanth. 2022. "Electrospun Biomimetic Multifunctional Nanofibers Loaded with Ferulic Acid for Enhanced Antimicrobial and Wound-Healing Activities in STZ-Induced Diabetic Rats" Pharmaceuticals 15, no. 3: 302. https://doi.org/10.3390/ph15030302
APA StyleAnand, S., Pandey, P., Begum, M. Y., Chidambaram, K., Arya, D. K., Gupta, R. K., Sankhwar, R., Jaiswal, S., Thakur, S., & Rajinikanth, P. S. (2022). Electrospun Biomimetic Multifunctional Nanofibers Loaded with Ferulic Acid for Enhanced Antimicrobial and Wound-Healing Activities in STZ-Induced Diabetic Rats. Pharmaceuticals, 15(3), 302. https://doi.org/10.3390/ph15030302







