Euphorbia supina Extracts Block NADPH Oxidase-Mediated, Ceramide-Induced Apoptosis Initiated by Diesel Particulate Matter
Abstract
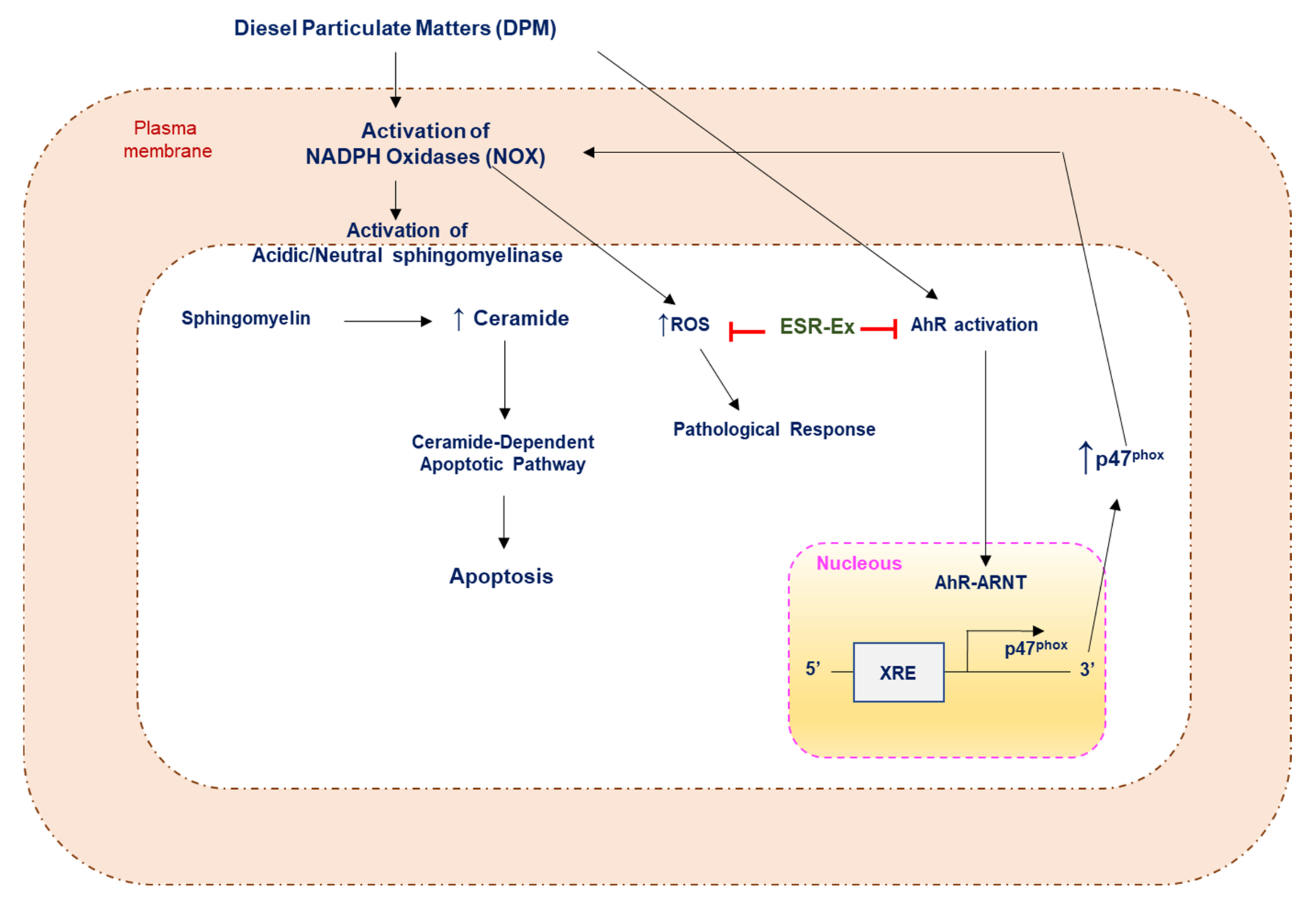
1. Introduction
2. Results
2.1. Component of ESR Extracts
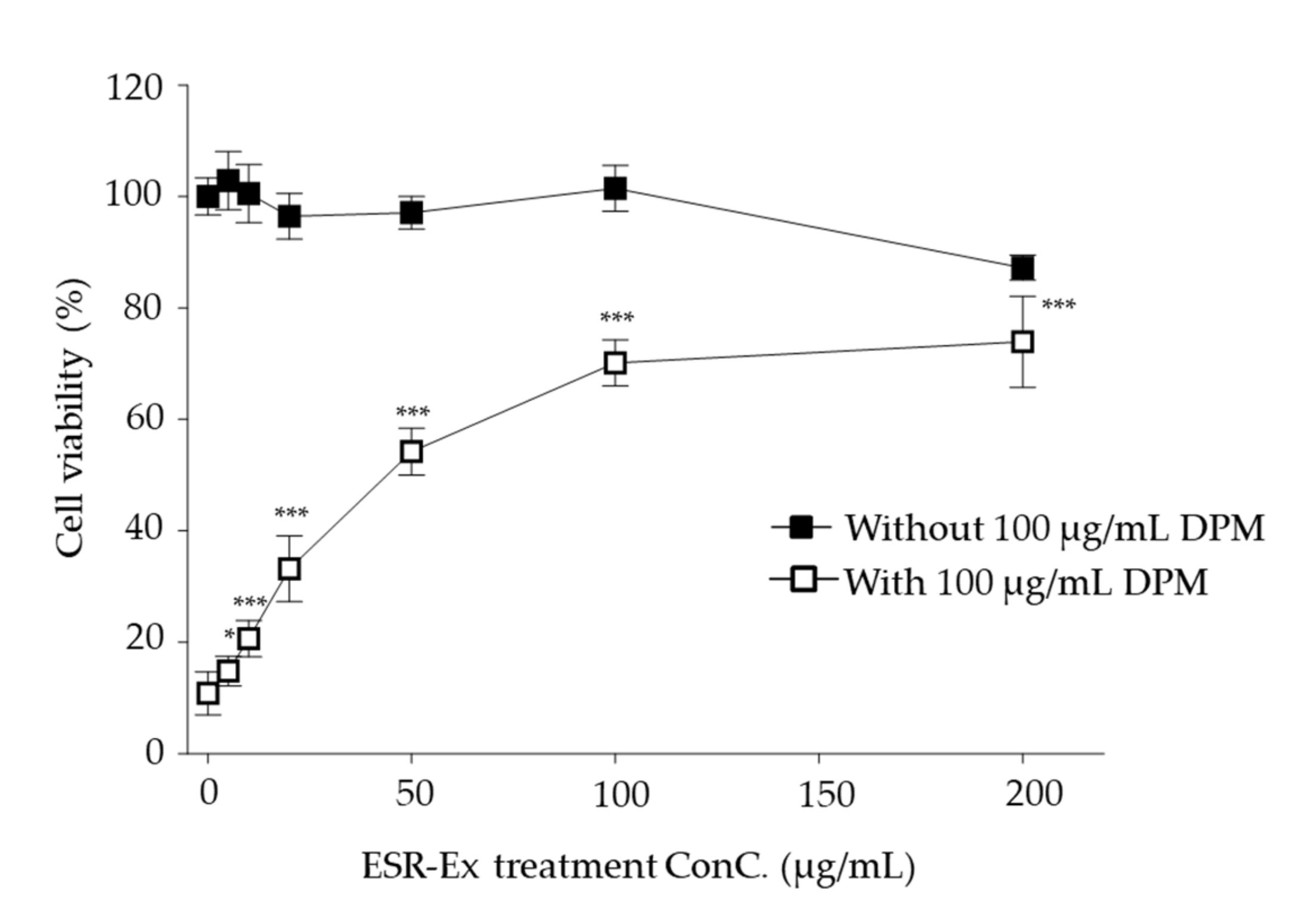
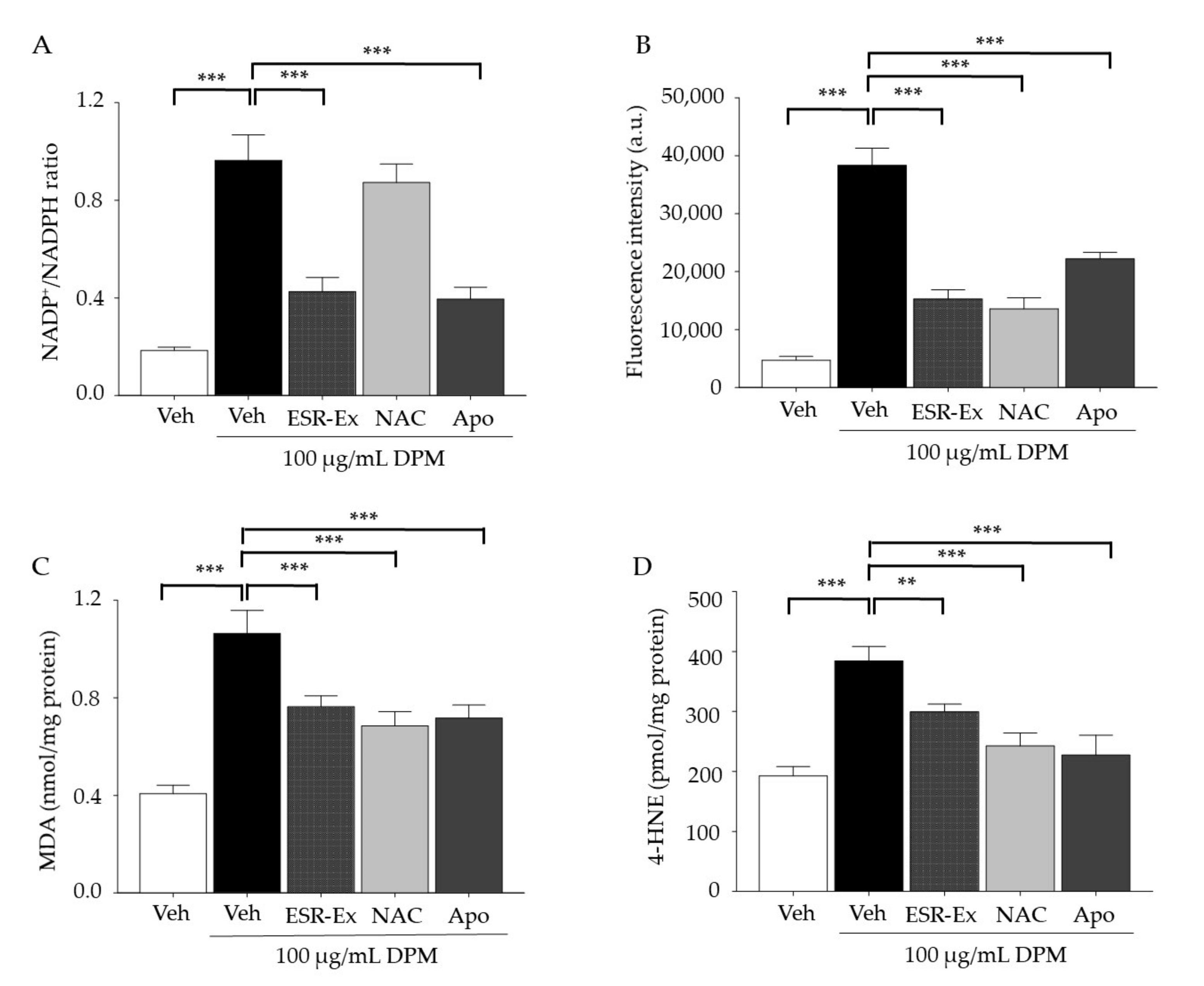
2.2. ESR-Ex Suppresses DPM-Induced Decreases in Cell Viability and NOX Activation
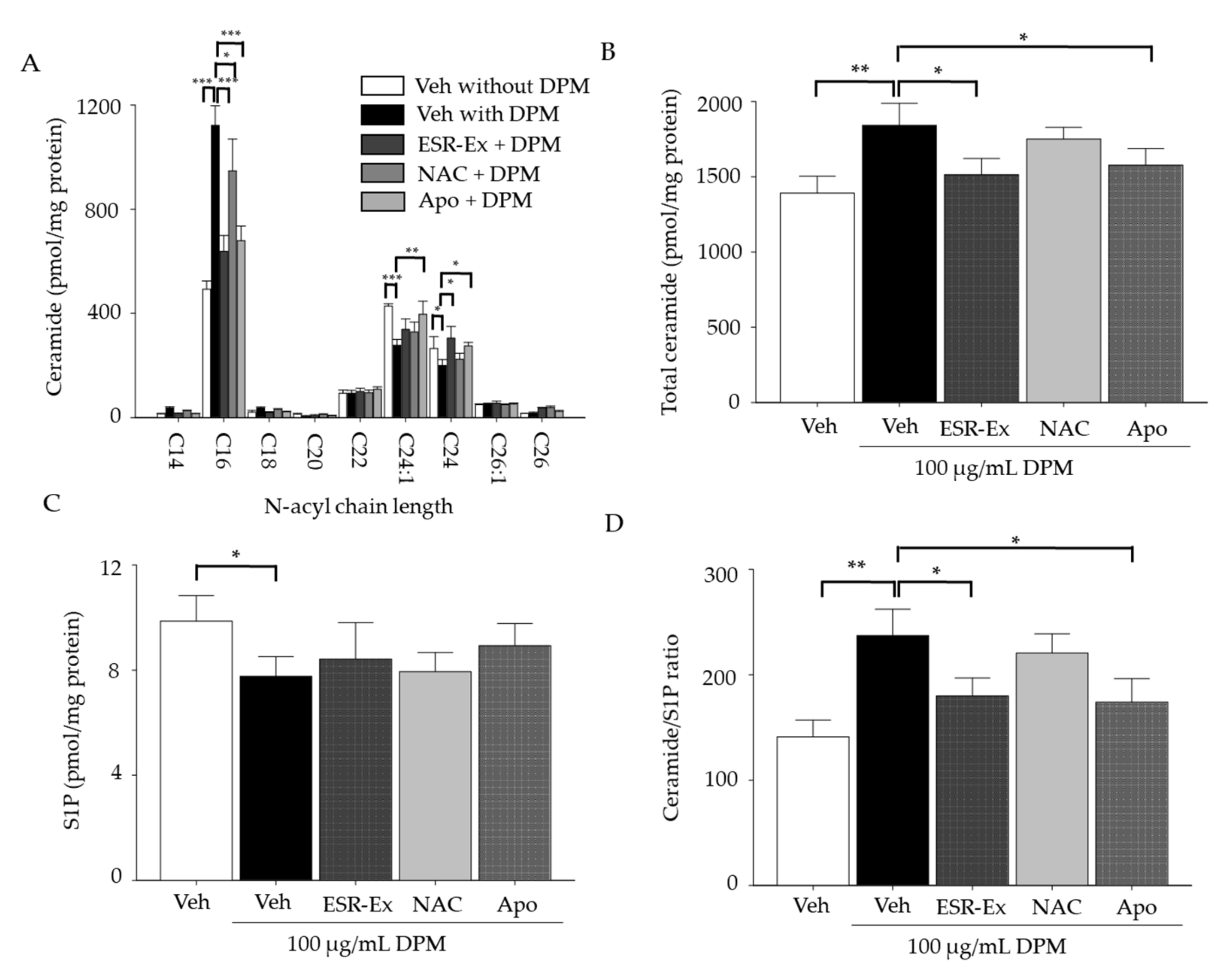
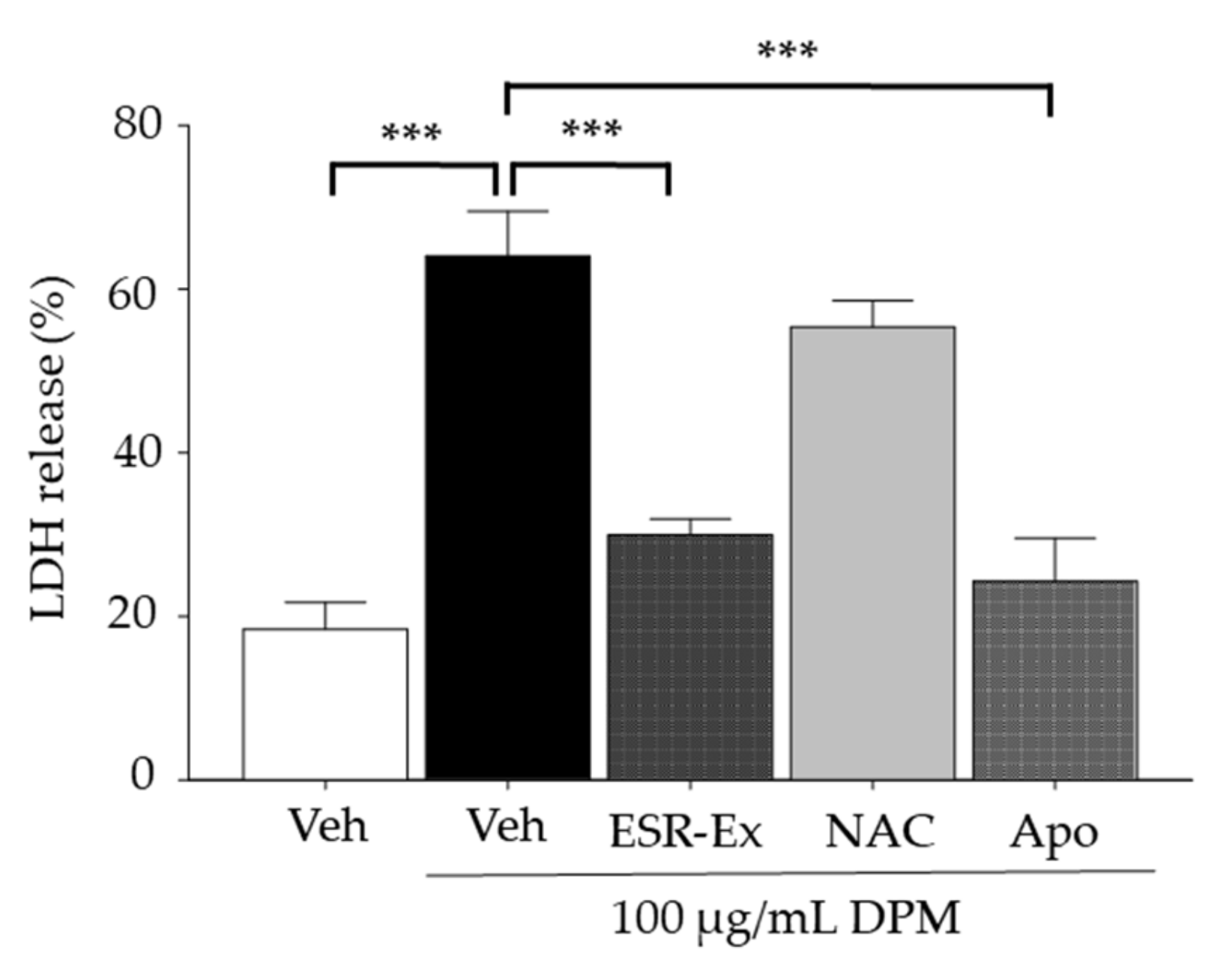
2.3. ESR-Ex Suppresses Changes in Ceramide Profiles and Cell Toxicity in KC Exposed to DPM
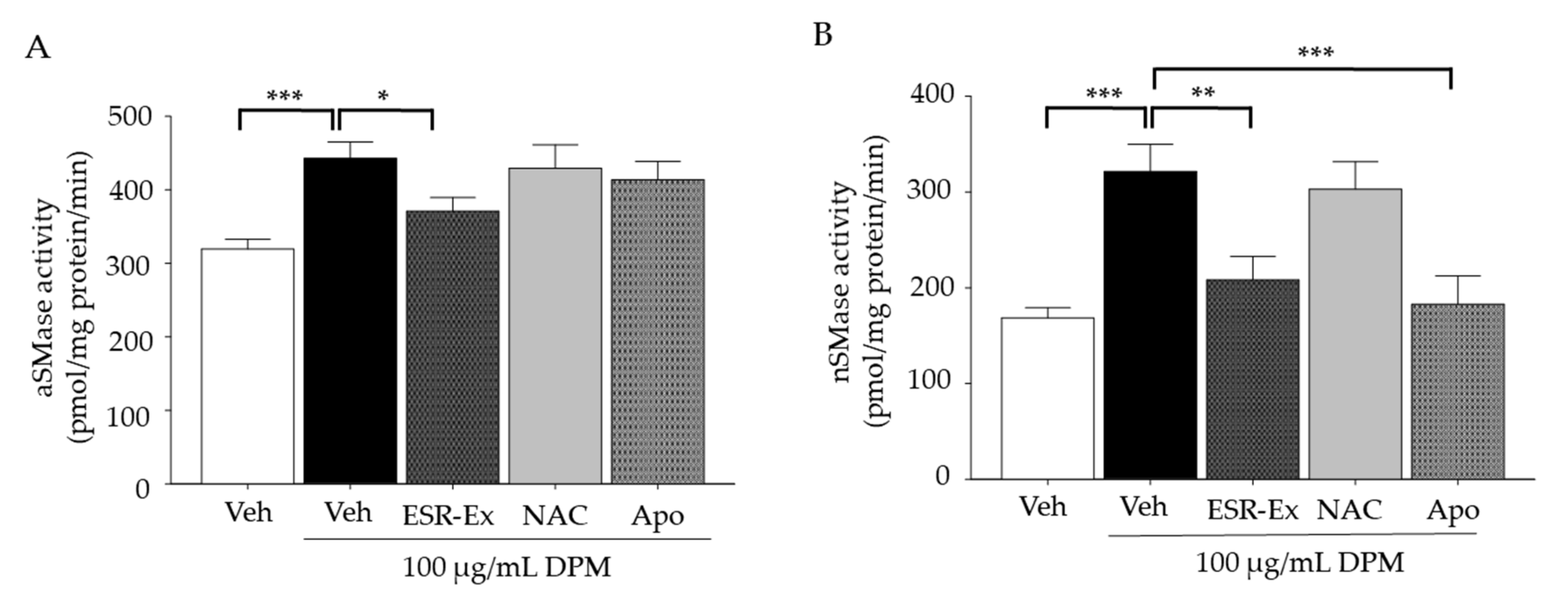
2.4. ESR-Ex Suppresses DPM-Mediated Activation of Sphingomyelinases
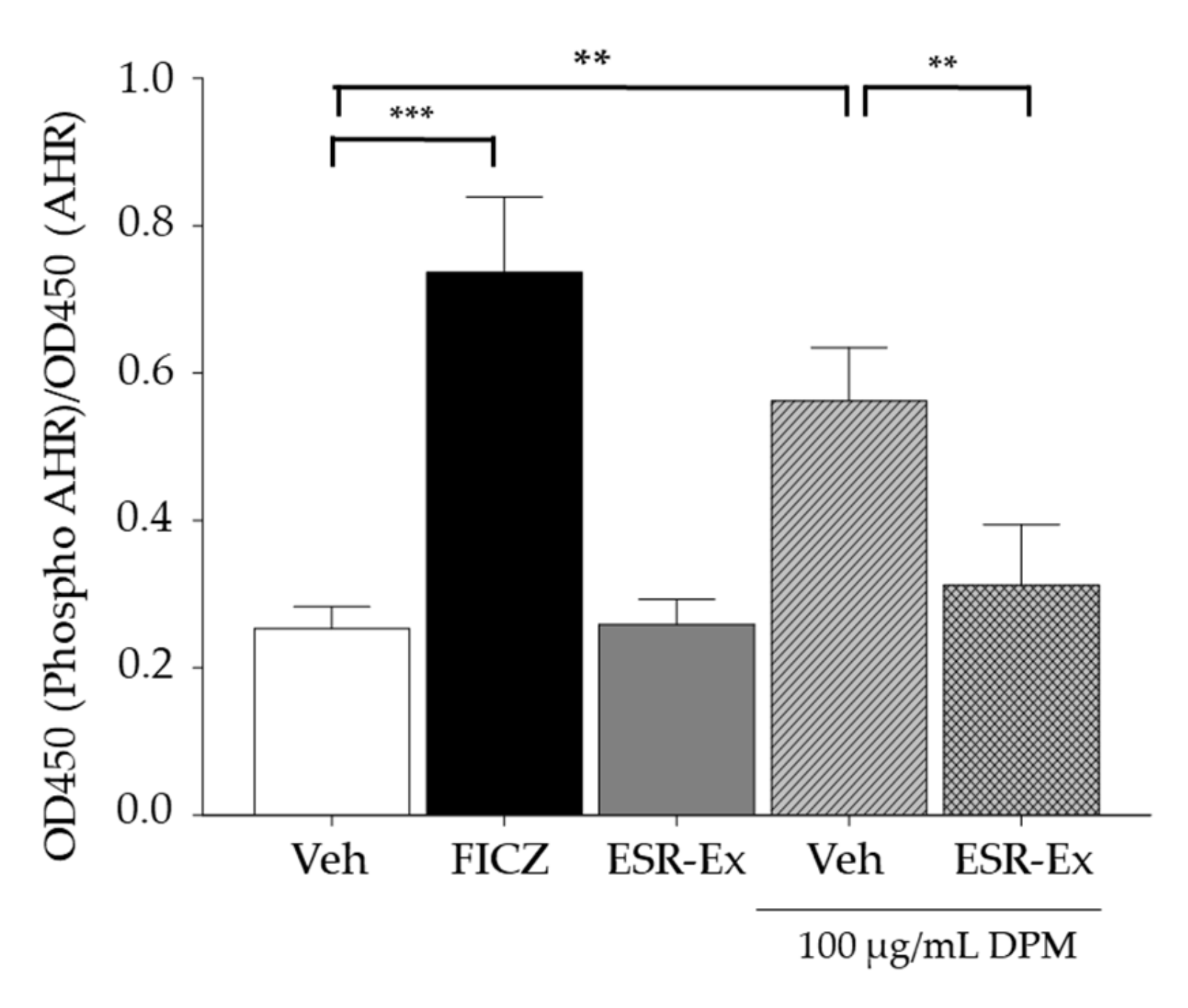
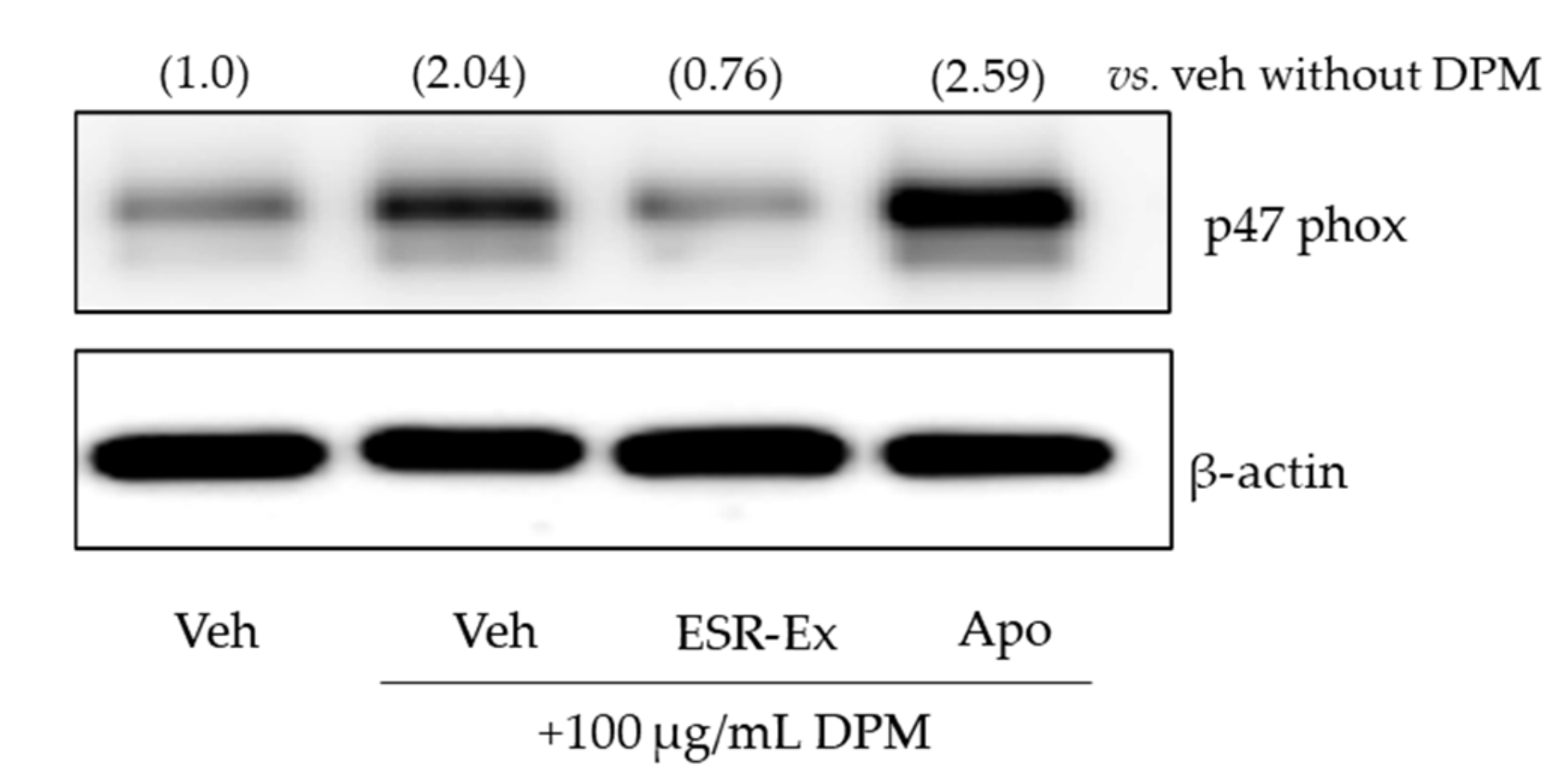
2.5. ESR-Ex Suppresses NOX Activation via Blockage of AhR-Induced p47phox Production
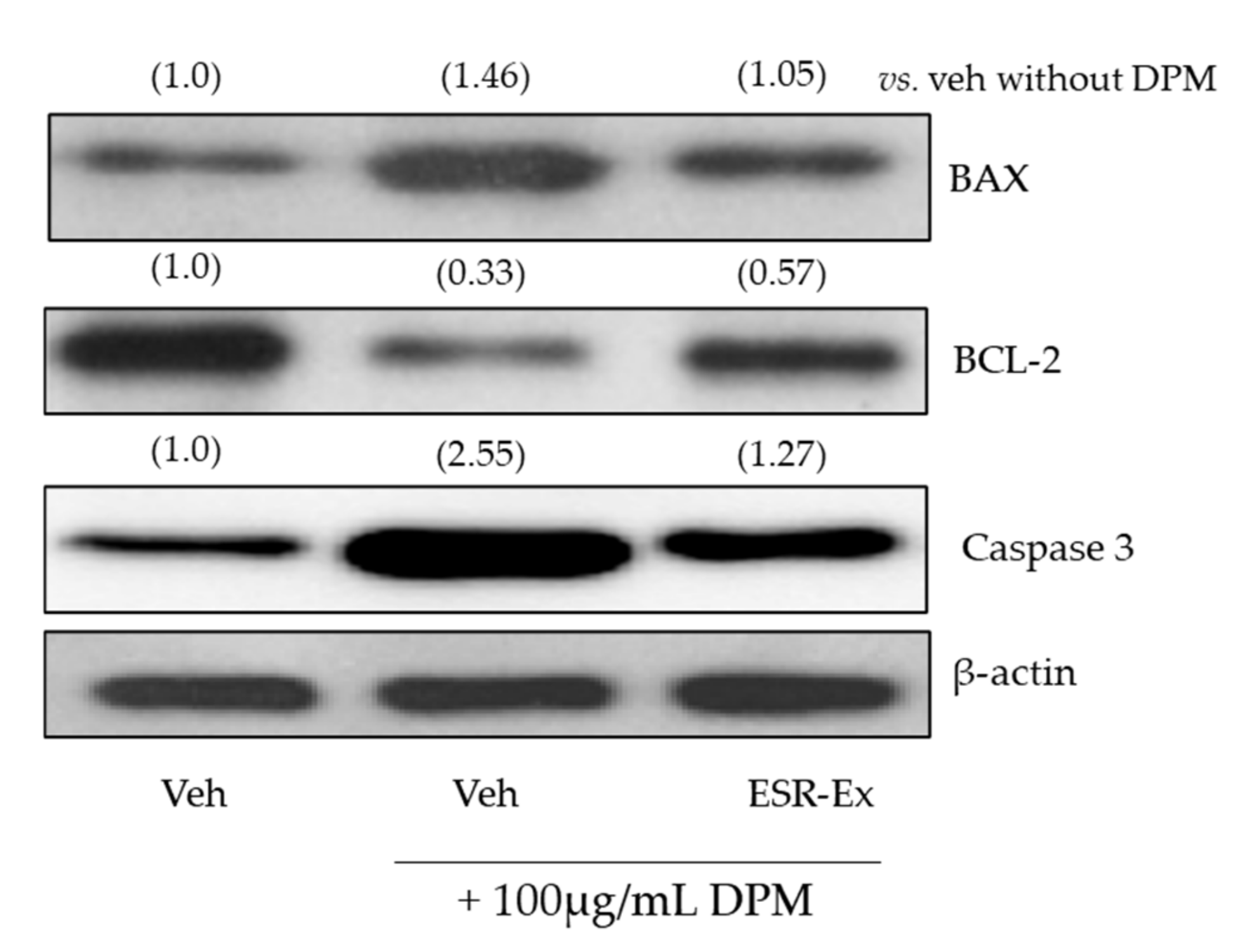
2.6. ESR-Ex Suppresses DPM-Induced Apoptosis
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. ESR-Ex Preparation
4.3. Identification of Phenolic Acid and Flavonoids in ESR-Ex
4.4. Cell Culture
4.5. Cell Viability and Cytotoxicity
4.6. NADPH Oxidases
4.7. Detection of Cellular ROS
4.8. Measurement of Ceramide, and Sphingosine-1-Phosphate (S1P)
4.9. Sphingomyelinase Assay
4.10. Western Blot Analysis
4.11. AhR Phosphorylation Assay
4.12. Statistical Analyses
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bedard, K.; Krause, K.H. The NOX family of ROS-generating NADPH oxidases: Physiology and pathophysiology. Physiol. Rev. 2007, 87, 245–313. [Google Scholar] [CrossRef] [PubMed]
- Elbatreek, M.H.; Pachado, M.P.; Cuadrado, A.; Jandeleit-Dahm, K.; Schmidt, H. Reactive Oxygen Comes of Age: Mechanism-Based Therapy of Diabetic End-Organ Damage. Trends Endocrinol. Metab. 2019, 30, 312–327. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Wang, A.; Wu, Y.; Gulbins, E.; Grassme, H.; Zhao, Z. Acid Sphingomyelinase-Ceramide System in Bacterial Infections. Cell Physiol. Biochem. 2019, 52, 280–301. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.H.; Chou, F.P.; Lin, H.H.; Wang, C.J. Gaseous nitrogen oxide repressed benzo[a]pyrene-induced human lung fibroblast cell apoptosis via inhibiting JNK1 signals. Arch. Toxicol. 2005, 79, 694–704. [Google Scholar] [CrossRef] [PubMed]
- Tarafdar, A.; Pula, G. The Role of NADPH Oxidases and Oxidative Stress in Neurodegenerative Disorders. Int. J. Mol. Sci. 2018, 19, 3824. [Google Scholar] [CrossRef]
- Lee, H.S.; Park, H.Y.; Kwon, S.P.; Kim, B.; Lee, Y.; Kim, S.; Shin, K.O.; Park, K. NADPH Oxidase-Mediated Activation of Neutral Sphingomyelinase Is Responsible for Diesel Particulate Extract-Induced Keratinocyte Apoptosis. Int. J. Mol. Sci. 2020, 21, 1001. [Google Scholar] [CrossRef]
- Song, Y.; Jeong, S.W.; Lee, W.S.; Park, S.; Kim, Y.H.; Kim, G.S.; Lee, S.J.; Jin, J.S.; Kim, C.Y.; Lee, J.E.; et al. Determination of Polyphenol Components of Korean Prostrate Spurge (Euphorbia supina) by Using Liquid Chromatography-Tandem Mass Spectrometry: Overall Contribution to Antioxidant Activity. J. Anal. Methods Chem. 2014, 2014, 418690. [Google Scholar] [CrossRef]
- Bahar, E.; Lee, G.H.; Bhattarai, K.R.; Lee, H.Y.; Choi, M.K.; Rashid, H.O.; Kim, J.Y.; Chae, H.J.; Yoon, H. Polyphenolic Extract of Euphorbia supina Attenuates Manganese-Induced Neurotoxicity by Enhancing Antioxidant Activity through Regulation of ER Stress and ER Stress-Mediated Apoptosis. Int. J. Mol. Sci. 2017, 18, 300. [Google Scholar] [CrossRef]
- Kang, S.H.; Jeon, Y.D.; Cha, J.Y.; Hwang, S.W.; Lee, H.Y.; Park, M.; Lee, B.R.; Shin, M.K.; Kim, S.J.; Shin, S.M.; et al. Antioxidant and skin-whitening effects of aerial part of Euphorbia supina Raf. Extract. BMC Complement. Altern. Med. 2018, 18, 256. [Google Scholar] [CrossRef]
- Han, M.H.; Lee, W.S.; Nagappan, A.; Kim, H.J.; Park, C.; Kim, G.Y.; Hong, S.H.; Kim, N.D.; Kim, G.; Ryu, C.H.; et al. Polyphenols from Korean prostrate spurge Euphorbia supina induce apoptosis through the Fas-associated extrinsic pathway and activation of ERK in human leukemic U937 cells. Oncol. Rep. 2016, 36, 99–107. [Google Scholar] [CrossRef][Green Version]
- Wattenberg, B.W. The long and the short of ceramides. J. Biol. Chem. 2018, 293, 9922–9923. [Google Scholar] [CrossRef] [PubMed]
- Uchida, Y.; Houben, E.; Park, K.; Douangpanya, S.; Lee, Y.M.; Wu, B.X.; Hannun, Y.A.; Radin, N.S.; Elias, P.M.; Holleran, W.M. Hydrolytic pathway protects against ceramide-induced apoptosis in keratinocytes exposed to UVB. J. Investig. Dermatol. 2010, 130, 2472–2480. [Google Scholar] [CrossRef] [PubMed]
- Brinchmann, B.C.; Skuland, T.; Rambol, M.H.; Szoke, K.; Brinchmann, J.E.; Gutleb, A.C.; Moschini, E.; Kubatova, A.; Kukowski, K.; Le Ferrec, E.; et al. Lipophilic components of diesel exhaust particles induce pro-inflammatory responses in human endothelial cells through AhR dependent pathway(s). Part Fibre Toxicol. 2018, 15, 21. [Google Scholar] [CrossRef] [PubMed]
- Palkova, L.; Vondracek, J.; Trilecova, L.; Ciganek, M.; Pencikova, K.; Neca, J.; Milcova, A.; Topinka, J.; Machala, M. The aryl hydrocarbon receptor-mediated and genotoxic effects of fractionated extract of standard reference diesel exhaust particle material in pulmonary, liver and prostate cells. Toxicol. In Vitro 2015, 29, 438–448. [Google Scholar] [CrossRef] [PubMed]
- Koizumi, M.; Tatebe, J.; Watanabe, I.; Yamazaki, J.; Ikeda, T.; Morita, T. Aryl hydrocarbon receptor mediates indoxyl sulfate-induced cellular senescence in human umbilical vein endothelial cells. J. Atheroscler. Thromb. 2014, 21, 904–916. [Google Scholar] [CrossRef]
- Pinel-Marie, M.L.; Sparfel, L.; Desmots, S.; Fardel, O. Aryl hydrocarbon receptor-dependent induction of the NADPH oxidase subunit NCF1/p47 phox expression leading to priming of human macrophage oxidative burst. Free Radic. Biol. Med. 2009, 47, 825–834. [Google Scholar] [CrossRef]
- Macpherson, L.; Matthews, J. Inhibition of aryl hydrocarbon receptor-dependent transcription by resveratrol or kaempferol is independent of estrogen receptor alpha expression in human breast cancer cells. Cancer Lett. 2010, 299, 119–129. [Google Scholar] [CrossRef]
- Vrba, J.; Kren, V.; Vacek, J.; Papouskova, B.; Ulrichova, J. Quercetin, quercetin glycosides and taxifolin differ in their ability to induce AhR activation and CYP1A1 expression in HepG2 cells. Phytother. Res. 2012, 26, 1746–1752. [Google Scholar] [CrossRef]
- Tanaka, M.; Fujikawa, M.; Oguro, A.; Itoh, K.; Vogel, C.F.A.; Ishihara, Y. Involvement of the Microglial Aryl Hydrocarbon Receptor in Neuroinflammation and Vasogenic Edema after Ischemic Stroke. Cells 2021, 10, 718. [Google Scholar] [CrossRef]
- Jana, A.; Pahan, K. Fibrillar amyloid-beta peptides kill human primary neurons via NADPH oxidase-mediated activation of neutral sphingomyelinase. Implications for Alzheimer’s disease. J. Biol. Chem. 2004, 279, 51451–51459. [Google Scholar] [CrossRef]
- Reiss, L.K.; Raffetseder, U.; Gibbert, L.; Drescher, H.K.; Streetz, K.L.; Schwarz, A.; Martin, C.; Uhlig, S.; Adam, D. Reevaluation of Lung Injury in TNF-Induced Shock: The Role of the Acid Sphingomyelinase. Mediat. Inflamm. 2020, 2020, 3650508. [Google Scholar] [CrossRef] [PubMed]
- Schikowski, T.; Huls, A. Air Pollution and Skin Aging. Curr. Environ. Health Rep. 2020, 7, 58–64. [Google Scholar] [CrossRef] [PubMed]
- Shin, K.O.; Uchida, Y.; Park, K. Diesel Particulate Extract Accelerates Premature Skin Aging in human Fibroblasts via Ceramide-1-Phosphate-Mediated Signaling Pathway. Int. J. Med. Sci. 2022, 23, 2691. [Google Scholar] [CrossRef] [PubMed]
- Pernomian, L.; Duarte-Silva, M.; de Barros Cardoso, C.R. The Aryl Hydrocarbon Receptor (AHR) as a Potential Target for the Control of Intestinal Inflammation: Insights from an Immune and Bacteria Sensor Receptor. Clin. Rev. Allergy Immunol. 2020, 59, 382–390. [Google Scholar] [CrossRef]
- Leclerc, D.; Staats Pires, A.C.; Guillemin, G.J.; Gilot, D. Detrimental activation of AhR pathway in cancer: An overview of therapeutic strategies. Curr. Opin. Immunol. 2021, 70, 15–26. [Google Scholar] [CrossRef] [PubMed]
- Park, K.; Elias, P.M.; Shin, K.O.; Lee, Y.M.; Hupe, M.; Borkowski, A.W.; Gallo, R.L.; Saba, J.; Holleran, W.M.; Uchida, Y. A novel role of a lipid species, sphingosine-1-phosphate, in epithelial innate immunity. Mol. Cell. Biol. 2013, 33, 752–762. [Google Scholar] [CrossRef]
- Park, K.; Ikushiro, H.; Seo, H.S.; Shin, K.O.; Kim, Y.I.; Kim, J.Y.; Lee, Y.M.; Yano, T.; Holleran, W.M.; Elias, P.; et al. ER stress stimulates production of the key antimicrobial peptide, cathelicidin, by forming a previously unidentified intracellular S1P signaling complex. Proc. Natl. Acad. Sci. USA 2016, 113, E1334–E1342. [Google Scholar] [CrossRef]
- Rosenkranz, A.R.; Schmaldienst, S.; Stuhlmeier, K.M.; Chen, W.; Knapp, W.; Zlabinger, G.J. A microplate assay for the detection of oxidative products using 2’,7’-dichlorofluorescin-diacetate. J. Immunol. Methods 1992, 156, 39–45. [Google Scholar] [CrossRef]
| Compound | [M-H]− | Mass Fragmentation |
|---|---|---|
| Quercetin | 301 | 301, 273, 179, 153 |
| Quercetin 3-hexoside | 463 | 463, 301, 300,283, 271, 255, 179, 151 |
| Quercetin-3-O-(2G-α-L-rhamnosyl)-rutinoside | 755 | 755, 300, 283, 271, 255, 179, 151 |
| Kaempferol | 287 | 287, 258, 165, 153, 121 |
| Kaempferol 3-hexoside | 447 | 447, 285, 255 |
| Kaempferol-3-O-rutinoside | 593 | 593, 285, 255, 227, 151 |
| Isorhamnetin | 316 | 316, 301, 272, 256, 164, 151 |
| Isorhamnetin-3-O-neohesperidoside | 623 | 623, 459, 314, 299, 285, 271, 257 |
| Gallic acid | 169 | 169, 125, 97 |
| Gallic acid glucoside | 331 | 169, 125 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shin, K.-O.; Kim, S.; Kim, B.; Park, H.-Y.; Jung, E.; Kim, G.; Kim, D.; Cho, H.E.; Uchida, Y.; Park, K. Euphorbia supina Extracts Block NADPH Oxidase-Mediated, Ceramide-Induced Apoptosis Initiated by Diesel Particulate Matter. Pharmaceuticals 2022, 15, 431. https://doi.org/10.3390/ph15040431
Shin K-O, Kim S, Kim B, Park H-Y, Jung E, Kim G, Kim D, Cho HE, Uchida Y, Park K. Euphorbia supina Extracts Block NADPH Oxidase-Mediated, Ceramide-Induced Apoptosis Initiated by Diesel Particulate Matter. Pharmaceuticals. 2022; 15(4):431. https://doi.org/10.3390/ph15040431
Chicago/Turabian StyleShin, Kyong-Oh, Sungeun Kim, Bokyung Kim, Hye-Yoon Park, Eunhee Jung, Garyun Kim, Donghee Kim, Hwang Eui Cho, Yoshikazu Uchida, and Kyungho Park. 2022. "Euphorbia supina Extracts Block NADPH Oxidase-Mediated, Ceramide-Induced Apoptosis Initiated by Diesel Particulate Matter" Pharmaceuticals 15, no. 4: 431. https://doi.org/10.3390/ph15040431
APA StyleShin, K.-O., Kim, S., Kim, B., Park, H.-Y., Jung, E., Kim, G., Kim, D., Cho, H. E., Uchida, Y., & Park, K. (2022). Euphorbia supina Extracts Block NADPH Oxidase-Mediated, Ceramide-Induced Apoptosis Initiated by Diesel Particulate Matter. Pharmaceuticals, 15(4), 431. https://doi.org/10.3390/ph15040431






