Abstract
Plectranthus zeylanicus Benth is used in Sri Lankan folk medicine as a remedy for inflammatory conditions and microbial infections. Our previous investigations revealed potent 5-lipoxygenase (5-LO) inhibitory activity in lipophilic extracts of this plant, supporting its anti-inflammatory potential. In-depth studies on the antimicrobial activity have not been conducted and the bioactive ingredients remained elusive. As a continuation of our previous work, the present investigation was undertaken to evaluate the antimicrobial activity of different extracts of P. zeylanicus and to isolate and characterize bioactive secondary metabolites. Different organic extracts of this plant were analyzed for their antibacterial activity, and the most active extract, i.e., dichloromethane extract, was subjected to bioactivity-guided fractionation, which led to the isolation of 7α-acetoxy-6β-hydroxyroyleanone. This compound displayed strong antibacterial activity against methicillin-resistant Staphylococcus aureus with a minimum inhibitory concentration of 62.5 µg/mL, and its disinfectant capacity was comparable to the potency of a commercial disinfectant. Moreover, 7α-acetoxy-6β-hydroxyroyleanone inhibits 5-LO with IC50 values of 1.3 and 5.1 µg/mL in cell-free and cell-based assays, respectively. These findings rationalize the ethnopharmacological use of P. zeylanicus as antimicrobial and anti-inflammatory remedy.
1. Introduction
Plectranthus zeylanicus Benth [synonym: Coleus zeylanicus (Benth)], locally known as Iruveriya [1], is a perennial herb within the family Lamiaceae, which has been widely employed in Ayurvedic and folk medicine in Sri Lanka for hundreds of years. Our previous investigations revealed potent 5-lipoxygenase (5-LO) inhibitory activities in lipophilic extracts of this plant, thus supporting its traditional usage as an anti-inflammatory remedy [2]. Leukotrienes (LT) are bioactive lipid mediators produced from arachidonic acid by 5-LO during the onset of infections with pathogenic bacteria such as Escherichia coli and Staphylococcus aureus [3], and they mediate typical symptoms of inflammation such as swelling, redness and pain [4]. LT also contribute to the development of chronic inflammatory disorders [5].
Apart from its utility for the treatment of inflammatory conditions, P. zeylanicus is also known as a medication for microbial infections, such as dysentery and diarrhea and also against smallpox, the common cold and eye diseases [6]. However, only a limited number of scientific reports are available to reinforce these traditional claims. For example, the essential oils of the plant displayed antimicrobial activity against Proteus vulgaris, Aspergillus parasiticus, Aspergillus niger, Rhizopus oryzae and Colletotrichum musae [7], while very mild antibacterial and antifungal activities with relatively high minimal inhibitory concentration (MIC) values up to 250 mg/mL were observed in the chloroform and ethanol extracts [8].
Previous studies on the phytoconstituents of P. zeylanicus led to the isolation of diterpenoids such as 7β-acetoxy-6β-hydroxyroyleanone, 7β-,6β-dihydroxyroyleanone, and 7α-acetoxy-6β-hydroxyroyleanone from its methanolic extracts [9]. In addition, geraniol, geranyl acetate, caryophyllene, eudesm-7(11)-en-4-ol, p-cymene, fenchyl acetate, fenchyl formate, and bornyl acetate were identified in the essential oils obtained from the aerial and the roots of P. zeylanicus grown in Sri Lanka [10], whereas about 80 compounds were detected in the essential oils of this plant grown in India [11]. Nevertheless, in-depth investigations on the bioactivity of secondary metabolites isolated from P. zeylanicus of Sri Lankan origin are not available to rationalize its therapeutic significance as an anti-inflammatory and antimicrobial remedy. Thus, in continuation of our early attempts of correlating the anti-inflammatory activity with the phytochemicals present in the lipophilic extracts of P. zeylanicus that inhibit 5-LO [2], the present study specifically focused on the determination of the antimicrobial activity of different extracts of this plant and to conduct bioactivity-guided fractionation to obtain the active secondary metabolites with antimicrobial, disinfectant and anti-inflammatory activities.
In the present study, the anti-inflammatory activity of the isolated phytoconstituents was evaluated by analyzing their inhibitory activity on 5-LO in vitro. The antibacterial activity was determined against Gram-positive and Gram-negative bacteria, including methicillin-resistant Staphylococcus aureus (MRSA), while the antifungal activity was evaluated against Candida albicans. In order to determine the suitability of the antimicrobial compounds as potent disinfectants, the disinfectant potency was compared with that of a commercial disinfectant employing a surface disinfectant assay. Together, our data provide valuable insights into the ethnopharmacological significance of P. zeylanicus as a remedy for inflammatory disorders and microbial infections.
2. Results
2.1. Antimicrobial Activity of the Crude Extracts of P. zeylanicus
Our screening approach revealed that different extracts of P. zeylanicus possess antimicrobial activities against S. aureus, S. saprophyticus (Figure 1), E. faecalis, S. typhi and P. aeruginosa to different degrees; however, none of these extracts were active against E. coli and S. flexneri. The dichloromethane (DCM) extract displayed potent and broad-range activity against the tested bacterial species in comparison to the other extracts (Table 1), thus an initial phytochemical screening was performed and, thereafter, it was subjected to bioactivity-guided fractionation.
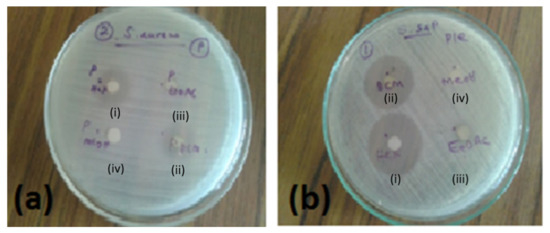
Figure 1.
Antibacterial activity of different extracts of P. zeylanicus at 1000 µg/mL against (a) S. aureus or (b) S. saprophyticus where (i) n-hexane, (ii) DCM, (iii) EtOAc, or (iv) methanol were used for extraction. Photos are representatives out of n = 2 experiments with similar results.

Table 1.
MIC values of different extracts prepared from P. zeylanicus against various bacterial species. Data are given as means, n = 3.
2.2. Phytochemical Screening by Gas Chromatography-Coupled Mass Spectrometric (GC-MS) Analysis
The GC-MS analysis of the DCM extract of P. zeylanicus led to the identification of several components based on the comparison of the obtained experimental mass spectra with those recorded in the NIST MS Search 2.0, Adams mass spectrum library, and also by comparison with those of the respective standards. Some of these compounds (hexadecanoic acid, 9,12,15-octadecatrienoic acid, stigmasterol, sitosterol and amyrin) have also been detected in the bioactive fractions of the n-hexane extract of this plant (Figure 2) as reported in our earlier publication [2]. In addition, the presence of several other diterpenes along with an intense peak representing a compound with the chemical formulae C20–23HxO2–4 was detected in the DCM extract. The compounds that have been tentatively identified are listed in Table 2.
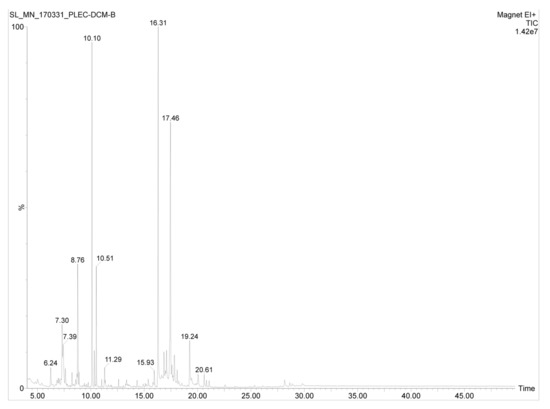
Figure 2.
Total ion chromatogram of the DCM extract of P. zeylanicus.

Table 2.
Tentatively identified compounds in the DCM extract of P. zeylanicus.
2.3. Bioactivity-Guided Fractionation
In order to obtain deeper insights into the identity of the compounds responsible for the potent antimicrobial activity, the DCM extract was fractionated by column chromatography using ethyl acetate, n-hexane, and methanol as solvents into eleven fractions. These fractions were analyzed for antimicrobial activity against S. aureus and S. saprophyticus at 1000 µg/mL by the disc diffusion method. Out of the eleven fractions (F) of the DCM extract, F-5 (15% EtOAc in n-hexane), F-6 (25% EtOAc in n-hexane), F-7 (35% EtOAc in n-hexane) and F-8 (50% EtOAc in n-hexane) displayed antimicrobial activity against both bacterial species, with the highest activity observed in F-5 and F-6 (Table 3). Since analysis by thin-layer chromatography (TLC) indicated identical spots in these two fractions, they were pooled together and further separated by a Sephadex column packed with methanol. This led to the separation of five major sub-fractions (SF), and the most bioactive sub-fraction was further purified by silica gel column chromatography using n-hexane and EtOAc as the solvent system. This resulted in a pure compound, and the NMR and MS data analysis of this isolated compound revealed its identity as 7α-acetoxy-6β-hydroxyroyleanone (AHR) (Figure 3), which is an abietane type diterpenoid with the chemical formula of C22H30O6 (m/z = 391.213).

Table 3.
Antimicrobial activity of the different fractions (F) obtained by silica gel column chromatography of the DCM extract of P. zeylanicus and sub-fractions (SF) obtained from the combined fraction F-5 + F-6. The diameter of the zone of bacterial growth inhibition is given as means ± S.D, n = 2.
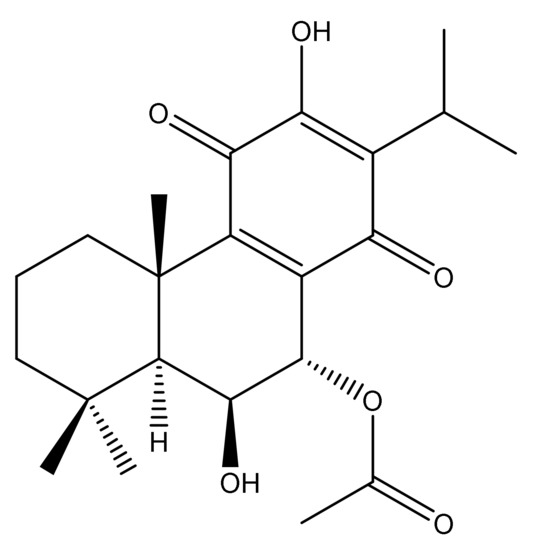
Figure 3.
Chemical structure of 7α-acetoxy-6β-hydroxyroyleanone isolated after bioactivity-guided fractionation from the DCM extract of P. zeylanicus.
The 1H NMR and 13C NMR data of this compound were in agreement with those previously reported for 7α-acetoxy-6β-hydroxyroyleanone [12,13,14,15]. Independently from literature data, chemical shift assignment was achieved by 1D NMR (1H, 13C, APT) and 2D homo- and heterocorrelation experiments (1H-1H COSY, 1H-1H ROESY, HSQC and HMBC). The 1H and 13C NMR data of 7α-acetoxy-6β-hydroxyroyleanone are shown in Appendix A along with most recently reported NMR data of this compound for comparison [15].
2.4. Bioactivity Studies of the Isolated Compound
2.4.1. Antimicrobial Activity and Disinfectant Potency
The isolated compound, 7α-acetoxy-6β-hydroxyroyleanone, displayed potent antibacterial activity against S. saprophyticus with a MIC of 31.5 µg/mL. Moreover, the MIC values of this compound against other Gram-positive bacteria, i.e., S. aureus, E. fecalis and nine clinical isolates of MRSA were found to be 62.5 µg/mL while only a moderate antibacterial activity was observed against the Gram-negative S. typhi and P. aeruginosa with MIC of 500 and 250 µg/mL, respectively. In addition, moderate antifungal activity was observed for this compound against C. albicans, with a MIC of 1000 µg/mL.
Furthermore, this compound exhibited a significant disinfectant activity against S. aureus, MRSA isolates and P. aeruginosa. The statistical analysis revealed that the compound was highly effective in reducing the mean colony counts of all the tested bacteria on rough and smooth surfaces when compared to the untreated surface (p < 0.01). The mean colony counts of all the organisms were more or less equal to that of the commercial disinfectant (used as the reference) on both types of surfaces (Table 4).

Table 4.
Comparison of mean colony counts of different microorganisms on different surfaces by one-way ANOVA with post hoc multiple comparisons.
2.4.2. Anti-Inflammatory Activity
Since a significant anti-inflammatory activity has been reported for the DCM extract of P. zeylanicus, that is, inhibition of 5-LO activity with IC50 of 1.2 and 12 µg/mL in cell-free and cell-based activity assays, respectively [2], 7α-acetoxy-6β-hydroxyroyleanone was evaluated for its activity against 5-LO. 7α-Acetoxy-6β-hydroxyroyleanone suppressed 5-LO activity in a cell-free assay using isolated human recombinant 5-LO with IC50 of 1.3 μg/mL and also inhibited 5-LO product formation in human neutrophils challenged with A23187 plus exogenous arachidonic acid with IC50 = 5.1 μg/mL (Figure 4). The reference 5-LO inhibitor zileuton blocked 5-LO activity with IC50 = 0.55 µM, as expected (not shown).
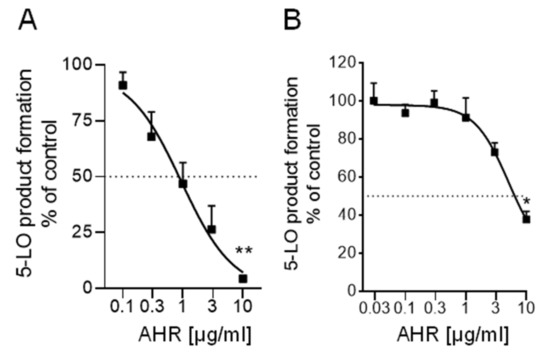
Figure 4.
Inhibition of 5-LO by 7α-acetoxy-6β-hydroxyroyleanone (AHR) in (A) a cell-free assay and (B) in intact human neutrophils. Data are given as mean ± S.E.M, n = 4. Statistical evaluation of the data was performed by one-way ANOVA, * p < 0.05, ** p < 0.01.
3. Discussion
In this study, attempts were made to identify bioactive secondary metabolites from the medicinal plant P. zeylanicus to rationalize and validate its traditional use as an anti-inflammatory and antimicrobial remedy. Our previous investigations revealed potent 5-LO inhibitory activity in the n-hexane and DCM extracts of this plant, thus supporting the traditional claims on its anti-inflammatory potential [2]. In the present study, a potent antibacterial activity was observed in the DCM extract of P. zeylanicus, and the bioactivity-guided fractionation revealed 7α-acetoxy-6β-hydroxyroyleanone as an active ingredient that moreover, efficiently inhibited 5-LO. Therefore, our results disclose potent antibacterial, disinfectant and anti-inflammatory activity for 7α-acetoxy-6β-hydroxyroyleanone and, thus, this rationalizes the ethnopharmacological use of P. zeylanicus.
With the increasing prevalence of multi-drug resistant microbial strains, which is considered as one of the leading causes for mortality and morbidity in hospitalized patients [16], the development of novel antimicrobial agents with diverse structures and mechanisms of action are highly demanding [17]. Since the therapeutic use of medicinal plants for the treatment of infectious diseases is as old as human civilization and has evolved along with it, the secondary metabolites present in the medicinal plants warrant exploration as promising candidates of novel antimicrobial agents [18]. In fact, a vast number of medicinal plants and the secondary metabolites thereof have been investigated for their antimicrobial potency [18,19,20]. The present investigation revealed that 7α-acetoxy-6β-hydroxyroyleanone displays strong antibacterial activity against various clinical isolates of MRSA with MIC of 62.5 µg/mL. Although the antimicrobial activity of 7α-acetoxy-6β-hydroxyroyleanone and its synthetic derivatives have been previously documented [15,21,22], none of these studies accomplished a potential application of this property. Since poor infection control practices in the hospital settings facilitate the transmission of drug-resistant bacteria among crowded hospital populations, appropriate environmental hygienic practices are extremely useful for hospital infection control. Hence, routine cleaning of high-touch surfaces or objects with disinfectants is adopted as one of the important preventive measures against the transmission of hospital-acquired infections [23]. Thus, special emphasis was given in our study to evaluate the disinfectant potency of 7α-acetoxy-6β-hydroxyroyleanone. It is noteworthy that the disinfectant capacity of 7α-acetoxy-6β-hydroxyroyleanone was comparable to the commercial disinfectant used as the positive control. To the best of our knowledge, this is the first report that describes the disinfectant potency of a plant secondary metabolite being equally effective as the commercial disinfectant, which stimulates the prospective development of eco-friendly biodegradable disinfectants. The recently developed protocol by Fonseka et al. [24] for in vitro mass propagation of P. zeylanicus might be helpful for a sustainable supply of plant material to isolate this bioactive compound and to develop a commercial product.
In addition, the 5-LO inhibitory action of 7α-acetoxy-6β-hydroxyroyleanone was disclosed for the first time by this study. Although several compounds such as β-caryophyllene [25], α-caryophyllene [26], α-tocopherol [27] and amyrin [28], with already proven anti-inflammatory activities, were detected in the DCM extract of P. zeylanicus, a correlation between the 5-LO inhibitory potency of the DCM extract and the presence of 7α-acetoxy-6β-hydroxyroyleanone can be proposed. The IC50 values in the cell-free and cell-based assays obtained for the DCM extract (IC50 = 1.2 and 12 µg/mL, respectively [2] were comparable to those of the 7α-acetoxy-6β-hydroxyroyleanone (IC50 = 1.3 and 5.1 µg/mL). Moreover, the potency of 7α-acetoxy-6β-hydroxyroyleanone against 5-LO in cell-free assays (IC50 of 1.3 µg/mL that corresponds to 3.3 µM) is even slightly superior over that of other well-known 5-LO inhibitory triterpenes like 3-O-acetyl-11-keto-β-boswellic acid (IC50 = 8 μM) and abietic acid (IC50 = 29.5 μM) or sesquiterpenes such as chamazulene (IC50 = 10 μM) [29]. Thus, 7α-acetoxy-6β-hydroxyroyleanone can be added to the list of plant-derived 5-LO inhibitors with high potency against this pro-inflammatory enzyme.
4. Materials and Methods
4.1. Plant Material
P. zeylanicus plants were collected in Nittambuwa (Western Province of Sri Lanka) in 2015/2016 and were identified by one of the authors (MN), who is a botanist. The morphological descriptions given in the books “A Revised Handbook to the Flora of Ceylon: volume-III, M.D. Dassanayake & F.R. Fosberg” and “Medicinal plants (indigenous and exotic) used in Ceylon: Volume 2 by D.M.A. Jayaweera” were used to confirm the identity of the plant. The plant was authenticated by comparison with the herbarium specimens at the National herbarium, Royal Botanical Garden, Peradeniya, Sri Lanka. A voucher specimen (Plec-WP-1603) was deposited at the Department of Biochemistry, Faculty of Medicine, University of Ruhuna, Sri Lanka for future reference.
4.2. Preparation of Crude Extracts
The plant material (whole plant) was thoroughly washed and dried in shade (30 ± 2 °C) for one week. Dried plants were powdered using a domestic grinder (Singer model KA-MIXEE). Three hundred and eighty grams of powdered material was successively extracted with 1800 mL of n-hexane, dichloromethane (DCM), ethyl acetate (EtOAc), and methanol (Roth, Karlsruhe, Germany) at room temperature using a linear shaker for 40 min. The extracts were evaporated to dryness with the use of a rotary evaporator (BÜCHI, R-114, Essen, Germany).
4.3. Evaluation of Antimicrobial Activity for Bioactivity-Guided Fractionation
The crude extracts from above were subjected to a preliminary screening for antimicrobial activity against standard isolates of Staphylococcus aureus (ATCC 25923) and Escherichia coli (ATCC 25922), as well as clinical isolates of Staphylococcus saprophyticus, Enterococcus faecalis, Salmonella typhi, Pseudomonas aeruginosa and Shigella flexneri available at the Department of Microbiology, Faculty of Medicine, University of Ruhuna, Sri Lanka. The disc diffusion method was employed for the pre-screening of extracts for antimicrobial activity and the MIC of the active extracts was determined by the broth-microdilution method.
4.3.1. Pre-Screening of Crude Extracts for Antibacterial Activity
The crude extracts were initially screened for the antimicrobial activity at a concentration of 1000 µg/mL by the disc diffusion method. A loop full from an isolated colony of one-day-old cultures of each organism grown on blood agar was dissolved in normal saline and the turbidity of the solution was adjusted to the McFarland 0.5 standard. Mueller Hinton Agar (MHA) plates were inoculated with a suspension of each organism and thereafter the plates were allowed to dry. Then, filter paper discs (6 mm in diameter), containing the test extract/reference drugs were placed on the agar surface. The plates were incubated at 37 °C overnight and the zone of inhibition around each paper disc was measured. The zones ≥6 mm were considered as inhibition, resulting from significant antibacterial activity. The assay was conducted in triplicate. Gentamicin was used as the reference (positive control), while DCM was used as the negative control. The experiment was performed in duplicate.
4.3.2. Determination of Minimum Inhibitory Concentration
Based on the preliminary observations, the active extracts were further subjected to the broth microdilution assay following the method described by Napagoda et al. [29] for the determination of the MIC values.
Briefly, 100 µL of solvent controls and test samples were added to the first wells of the microplate starting with a concentration of 2 mg/mL and then two-fold serially diluted down the wells. A part of the diluted culture (100 µL) with a turbidity standard equal to 0.5 McFarland and 50 µL of Muller Hinton Broth (MHB) were added to all wells. The microtiter plates were incubated for 24 h at 37 °C. After incubation, the absorbance was measured by a microplate reader (Biotek, ELx800, Minooski, VT, USA). The MIC was determined as the lowest concentration of test agent that prevents the visible growth of a bacterium. MBC (minimum bactericidal concentration) is the lowest concentration of an antibacterial agent required to kill a particular bacterium and was determined by sub-culturing the content of the above microtiter plate wells in agar plates. Gentamicin was used as the reference drug (positive control) for the assay. The assay was conducted in triplicate.
4.4. Phytochemical Profiling and Bioactivity-Guided Fractionation of Most Active Extract
Based on the results of the antimicrobial screening assays, the DCM extract was selected for the initial phytochemical screening and was further subjected to the bioactivity-guided fractionation.
4.4.1. Phytochemical Profiling by GC-MS Analysis
GC-MS analysis was carried out by the method described by Napagoda et al. [30]. Dried crude DCM extract was dissolved in ethyl acetate (1 mg/mL) and analyzed on a gas chromatograph HP6890 (Agilent, Santa Clara, CA, USA) connected to an MS02 mass spectrometer from Micromass (Waters, Manchester, UK) with EI 70 eV using ZB5ms column (30 m × 0.25 mm, 0.25-μm film thickness; Phenomenex). The carrier gas was helium at a flow rate of 1 mL/min. The injector temperature was kept at 250 °C and the temperature program was set as 100 °C (2 min), 15 °C/min to 200 °C, 5 °C/min to 305 °C (20 min).
4.4.2. Bioactivity-Guided Fractionation
DCM extract (430 mg) was subjected to silica gel column chromatography (Roth Kieselgel 60, 0.04–0.063 mm, 230–400 mesh). The sample was eluted with n-hexane, different mixtures of EtOAc in n-hexane (3%, 5%, 10%, 15%, 25%, 35%, 50%, 75%, 100%) and methanol sequentially to obtain eleven fractions.
The antimicrobial activity of the resulting fractions was determined against S. aureus and S. saprophyticus by the disc diffusion method. Based on the diameter of the zone of inhibition, the most active fraction was selected, and further fractionation was carried out by Sephadex LH-20 (Sigma Aldrich, Steinheim am Albuch, Germany) and silica gel column chromatography to isolate a pure compound. The antimicrobial, disinfectant and anti-inflammatory activities of this compound was determined.
The structure elucidation was achieved by 1H and 13C NMR spectroscopy and mass spectrometry. NMR spectra (1H NMR, 13C NMR, 13C APT, 1H-1H COSY, 1H-1H ROESY, 1H-13C HSQC and 1H-13C HMBC) were recorded on a Bruker Avance III HD 500 NMR spectrometer (Bruker Biospin, Karlsruhe, Germany) operating at 500.13 MHz for 1H and 125.75 MHz for 13C. The spectrometer was equipped with a 5 mm TCI cryoprobe. Standard Bruker pulse programs as implemented in Bruker TopSpin 3.5 were used for acquisition. The residual solvent signals of acetone-d6 (1H-δ 2.04, 13C-δ 29.8) were used for referencing spectra in the 1H and 13C dimensions.
Mass spectrometric data were obtained by QExactive-HF-X and an Ultimate 3000 series RSLC (Dionex, Sunnyvale, CA, USA) chromatography system using electrospray ionization.
4.5. Evaluation of the Bioactivity of Pure Compound
4.5.1. Antimicrobial Activity
Antibacterial Activity
The antibacterial activity and the MIC of the purified compound was determined by the broth microdilution method against S. aureus, S. saprophyticus, E. faecalis, S. typhi, P. aeruginosa and nine clinical isolates of methicillin-resistant S. aureus (MRSA).
Anti-Candida Activity
The in vitro anti-Candida activity of the isolated compound was assessed by the agar well diffusion method in Sabouraud Dextrose Agar (SDA). Candida albicans (ATCC 10231) was obtained from the Department of Microbiology, Faculty of Medicine, University of Ruhuna, Sri Lanka, and a fungal suspension matching with the McFarland 0.5 turbidity was prepared using isolated colonies of 48 h old cultures. Wells (diameter-6 mm, depth-5 mm) were prepared on SDA plates using a sterilized cork borer and the suspensions of the organisms were inoculated on these plates using a sterile cotton swab. The wells were filled with 50 µL of each of the test solutions (at 1000, 500, 250 and 125 µg/mL of the isolated compound, positive and negative controls) separately. Thereafter, the plates were incubated at 37 °C for 24 h and the zone of inhibition around each well was measured. The zones ≥6 mm were considered as inhibition resulting from significant antifungal activity. Fluconazole was used as the reference antifungal drug and DMSO was used as the negative control. The experiments were performed in triplicates and the diameter of the zone of inhibition was expressed as mean ± SD.
Disinfectant Potency
The disinfectant potency was evaluated against S. aureus, P. aeruginosa and three clinical isolates of MRSA following the method described by Napagoda et al. [31]. Pre-autoclaved rough (floor tile) and smooth (stainless steel) surfaces (50 cm2) were treated evenly with 1 mL of the bacterial suspension (equal to McFarland 0.5 turbidity) and allowed to dry for 1 h. On each surface, two squares (25 cm2) were labeled and the test solution (2.5 mL at its MBC concentration dissolved in 1% DMSO in water) was applied by a sterile cotton gauge in one square, while the other (labeled as non-disinfected area) was left without any treatment. After a contact period of 10 min, both areas were swabbed and each swab was vortexed in a tube containing 5 mL of MHB and a dilution was prepared as 1:10. Five drops of the dilution were inoculated on MHA plates and incubated for 48 h. A commercial disinfectant (Lifebuoy® soap solution; Unilever, London, UK containing silver as the active ingredient) was used as the positive control. The experiment was performed in duplicates. Mean colony counts of different microorganisms on different surfaces were obtained, and the data were analyzed by one-way ANOVA with post hoc multiple comparisons. A p-value < 0.05 (*) was considered significant.
4.5.2. Anti-Inflammatory Activity
Cell-Free 5-LO Activity Assay
E. coli (BL21) was transformed with pT3-5-LO plasmid, and recombinant 5-LO protein was expressed at 30 °C as described [31]. Cells were lysed in 50 mM triethanolamine/HCl pH 8.0, 5 mM EDTA, 1 mM phenylmethanesulphonyl fluoride, soybean trypsin inhibitor (60 µg/mL), and lysozyme (1 mg/mL), homogenized by sonication (3 × 15 s), and centrifuged at 40,000× g for 20 min at 4 °C. The 40,000× g supernatant (S40) was applied to an ATP-agarose column to partially purify 5-LO as described [32]. Aliquots of semi-purified 5-LO (0.5 µg) were diluted with 1 mL ice-cold PBS containing 1 mM EDTA. Samples were pre-incubated with the test compound or vehicle (0.1% DMSO). After 10 min at 4 °C, samples were pre-warmed for 30 s at 37 °C, and 2 mM CaCl2 plus 20 µM arachidonic acid were added to start the formation of 5-LO products. After 10 min, the reaction was stopped by the addition of one volume of ice-cold methanol, and the formed 5-LO products were analyzed by RP-HPLC as described [33]. 5-LO products include the all-trans isomers of LTB4 (tr-LTB4 isomers) as well as 5(S)-hydroperoxy-6-trans-8,11,14-cis-eicosatetraenoic acid (5-HPETE) and its corresponding alcohol 5(S)-hydroxy-6-trans-8,11,14-cis-eicosatetraenoic acid (5-HETE).
Cell-Based 5-LO Activity Assay
Neutrophils were isolated from peripheral blood (University Hospital Jena, Germany) which was taken from healthy adult volunteers with consent by venipuncture in heparinized tubes (16 IE heparin/mL blood). The blood donors had not taken any anti-inflammatory drugs within the prior 10 days. The blood was centrifuged at 4000× g for 20 min at 20 °C for preparation of leukocyte concentrates, which were then subjected to dextran sedimentation and centrifugation on lymphocyte separation medium (LSM 1077, PAA, Colbe, Germany). Contaminating erythrocytes of pelleted neutrophils were removed by hypotonic lysis. Neutrophils were then washed twice in ice-cold PBS pH 7.4 (PBS) and finally resuspended in PBS containing 1 mg/mL glucose or in PBS containing 1 mg/mL glucose plus 1 mM CaCl2 (PGC buffer) (purity > 96–97%). Neutrophils (5 × 106) were resuspended in 1 mL PGC buffer, preincubated for 15 min at 37 °C with test compounds or vehicle (0.1% DMSO), and incubated for 10 min at 37 °C with 2.5 µM Ca2+-ionophore A23187 plus 20 µM arachidonic acid. The reaction was stopped on ice by the addition of 1 mL of methanol, and 30 µL 1 N HCl, 500 µL PBS, and 200 ng prostaglandin B1 were added. The samples were subjected to solid-phase extraction on C18-columns (100 mg, UCT, Bristol, PA, USA) and 5-LO products (LTB4, tr-LTB4 isomers, and 5-HETE) were analyzed by RP-HPLC. Quantities were calculated on the basis of the internal standard PGB1. Cysteinyl-LTs C4, D4 and E4 were not detected (amounts were below detection limit), and oxidation products of LTB4 were not determined.
Statistical Analysis
Data were expressed as mean ± S.E.M. The IC50 values were calculated from averaged measurements at four to five different concentrations of the compounds by nonlinear regression using GraphPad Prism software (San Diego, CA, USA) one site binding competition. Statistical evaluation of the data was performed by one-way ANOVA followed by a Bonferroni or Tukey-Kramer post-hoc test for multiple comparisons, respectively. A p-value < 0.05 (*) was considered significant.
5. Conclusions
Taken together, 7α-acetoxy-6β-hydroxyroyleanone was isolated from a DCM extract of P. zeylanicus and identified as a potent antibacterial and disinfectant agent that also displays efficient 5-LO inhibitory activity. These results further rationalize the traditional use of P. zeylanicus as natural pharmacon in Sri Lanka for the treatment of inflammatory conditions and microbial infections. Particularly, the extremely high disinfectant capacity exhibited by this compound could be considered as a signpost of an effective herbal disinfectant for years to come.
Author Contributions
Conceptualization, M.N. and O.W.; methodology, M.N., J.G., A.N., G.B.W., B.S., A.S., L.J., A.K. and, O.W.; validation, M.N., J.G., A.N., G.B.W., B.S., A.S., L.J., A.K. and O.W.; formal analysis, M.N., J.G., A.N., G.B.W., B.S., A.S., L.J., A.K. and O.W.; investigation, M.N., S.D.S., J.G., H.B., S.L. and M.Q.; resources, M.N., G.B.W., L.J. and O.W.; data curation, J.G., H.B., S.L. and M.Q.; writing—original draft preparation, M.N. and O.W.; writing—review and editing, M.N., O.W., A.K., A.S., A.N., G.B.W., B.S. and L.J.; visualization, M.N.; supervision, M.N. and O.W.; project administration, M.N. and O.W.; funding acquisition, M.N., J.G. and O.W. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Research Grant, RG/BS/2015/01 of National Science Foundation, Sri Lanka received by M.N. J.G. received a Carl-Zeiss-Stipend. The APC was funded by Friedrich Schiller University Jena.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki and approved by the Institutional Review Board of University Hospital Jena (5050-01/17, date: 3 March 2017).
Informed Consent Statement
Written informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data presented in this study are available on reasonable request from the corresponding authors. The data are not publicly available due to privacy.
Acknowledgments
The authors thank Petra Wiecha, Katrin Fischer, Alrun Schumann, and Heidi Traber for expert technical assistance.
Conflicts of Interest
The authors declare that they have no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Appendix A

Table A1.
NMR data (500 MHz for 1H; 125 MHz for 13C) of 7α-acetoxy-6β-hydroxyroyleanone reported in this paper (acetone-d6) and by Bernardes et al. (2018) (CDCl3).
Table A1.
NMR data (500 MHz for 1H; 125 MHz for 13C) of 7α-acetoxy-6β-hydroxyroyleanone reported in this paper (acetone-d6) and by Bernardes et al. (2018) (CDCl3).
| No. | This Paper | Bernardes et al. [15] | ||
|---|---|---|---|---|
| 1H NMR | 13C NMR | 1H NMR | 13C NMR | |
| δ, mult., J (Hz) | δ | δ | δ | |
| 1β | 2.63, ddd, 12.8/6.6/3.4 | 39.2 | 2.62 | 38.37 |
| 1α | 1.19, overlap | ~1.17 | ||
| 2α | 1.53, ddd, 13.8/7.0/3.6 | 19.8 | 1.55 | 19.70 |
| 2β | 1.86, ddd, 13.8/7.3/3.6 | 1.83 | ||
| 3α | 1.23, m | 43.2 | ~1.20 | 42.28 |
| 3β | 1.44, m | 1.46 | ||
| 4 | 34.4 | 33.67 | ||
| 5α | 1.36, brs, 2.7 fwhm | 50.6 | 1.32 | 49.76 |
| 6α | 4.25, brs, 5.0 fwhm | 67.0 | 4.31 | 67.00 |
| 7β | 5.64, dd, 2.2/0.6 | 69.4 | 5.65 | 68.75 |
| 8 | 137.9 | 137.12 | ||
| 9 | 150.8 | 149.91 | ||
| 10 | 39.6 | 38.63 | ||
| 11 | 184.2 | 183.29 | ||
| 12 | 153.4 | 150.90 | ||
| 13 | 124.9 | 124.69 | ||
| 14 | 186.9 | 185.74 | ||
| 15 | 3.17, sept | 24.9 | 3.14 | 24.17 |
| 16 | 1.17, d, 7.1 | 20.1 | 1.17 * | 19.84 |
| 17 | 1.19, d, 7.1 | 20.3 | 1.20 * | 19.70 |
| 18 | 0.93, s | 34.0 | 0.93 | 33.52 |
| 19 | 1.24, s | 24.1 | 1.21 | 23.81 |
| 20 | 1.64, s | 21.9 | 1.59 | 21.51 |
| 7α-Ac-Me | 1.95, s | 20.9 | 2.02 | 20.93 |
| 7α-Ac-CO | 169.5 | 169.60 | ||
∼ = approximate; fwhm = full width at half maximum; * may be interchanged.
References
- Dassanayake, M.D.; Fosberg, F.R. A Revised Handbook to the Flora of Ceylon, 1st ed.; Amerind Publ. Co., Ltd.: New Delhi, India, 1981; Volume 3, pp. 150–151. [Google Scholar]
- Napagoda, M.; Gerstmeier, J.; Wesely, S.; Popella, S.; Lorenz, S.; Scheubert, K.; Svatoš, A.; Werz, O. Inhibition of 5-lipoxygenase as anti-inflammatory mode of action of Plectranthus zeylanicus Benth and chemical characterization of ingredients by a mass spectrometric approach. J. Ethnopharmacol. 2014, 151, 800–809. [Google Scholar] [CrossRef]
- Chiang, N.; Fredman, G.; Bäckhed, F.; Oh, S.F.; Vickery, T.; Schmidt, B.A.; Serhan, C.N. Infection regulates pro-resolving mediators that lower antibiotic requirements. Nature 2012, 484, 524–528. [Google Scholar] [CrossRef] [Green Version]
- Serhan, C.N. Novel pro-resolving lipid mediators in inflammation are leads for resolution physiology. Nature 2014, 510, 92–101. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koeberle, A.; Werz, O. Natural products as inhibitors of prostaglandin E2 and pro-inflammatory 5-lipoxygenase-derived lipid mediator biosynthesis. Biotechnol. Adv. 2018, 36, 1709–1723. [Google Scholar] [CrossRef] [PubMed]
- Jayaweera, D.M.A. Medicinal Plants (Indigenous and Exotic) Used in Ceylon, Part 2, 1st ed.; National Science Council: Colombo, Sri Lanka, 1982; pp. 108–109. [Google Scholar]
- Deena, M.J.; Sreeranjini, K.; Thoppi, J.E. Antimicrobial screening of essential oils of Coleus aromaticus and Coleus zeylanicus. Int. J. Aromather. 2002, 12, 105–107. [Google Scholar] [CrossRef]
- Kotagiri, D.; Beebi, S.K.; Chaitanya, K.V. Secondary metabolites and the antimicrobial potential of five different Coleus species in response to salinity stress. BioRxiv 2017, 220368. [Google Scholar] [CrossRef] [Green Version]
- Mehrotra, R.; Vishwakarma, R.A.; Thakur, R.S. Abietane diterpenoides from Coleus zeylanicus. Phytochemistry 1989, 28, 3135–3137. [Google Scholar] [CrossRef]
- Arambewela, L.; Wijesinghe, A. Sri Lankan Medicinal Plant Monographs and Analysis—Vol-11 Plectranthus zeylanicus, 1st ed.; National Science Foundation: Colombo, Sri Lanka, 2006. [Google Scholar]
- Jirovetz, L.; Jirovetz, K.; Buchbauer, G.; Fleischhacker, W.; Shafi, P.M.; Saidutty, A. Analyses of the essential oil of the leaves of the medicinal plant Coleus zeylanicus from India. Sci. Pharm. 1998, 66, 223–229. [Google Scholar]
- Hensch, M.; Rüedi, P.; Eugster, C.H. Horminon, Taxochinon und weitere Royleanone aus 2 abessinischen Plectranthus-Spezies (Labiatae). Helv. Chim. Acta 1975, 58, 1921–1934. [Google Scholar] [CrossRef]
- Rüedi, P. 8α,9α-Epoxy-7-oxoroyleanon, ein Diterpen-Epoxychinon aus einer abessinischen Plectranthus-Art (Labiatae). Helv. Chim. Acta 1984, 67, 1116–1120. [Google Scholar] [CrossRef]
- Teixeira, A.P.; Batista, O.; Simoes, M.F.; Nascimento, J.; Duarte, A.; de la Torre, M.C.; Rodriguez, B. Abietane diterpenoids from Plectranthus grandidentatus. Phytochemistry 1997, 44, 325–327. [Google Scholar] [CrossRef] [Green Version]
- Bernardes, C.E.S.; Garcia, C.; Pereira, F.; Mota, J.; Pereira, P.; Cebola, M.J.; Reis, C.P.; Correia, I.; Piedade, M.F.M.; da Piedade, M.E.M.; et al. Extraction optimization and structural and thermal characterization of the antimicrobial abietane 7α-acetoxy-6β-hydroxyroyleanone. Mol. Pharm. 2018, 15, 1412–1419. [Google Scholar] [CrossRef]
- O’Neill, J. Tackling Drug-Resistant Infections Globally: Final Report and Recommendations—The Review on Antimicrobial Resistance Chaired by Jim O’Neill, Wellcome Trust; HM Government: London, UK, 2016. [Google Scholar]
- Turner, N.A.; Sharma-Kuinkel, B.K.; Maskarinec, S.A.; Eichenberger, E.M.; Shah, P.P.; Carugati, M.; Holland, T.L.; Fowler, V.G., Jr. Methicillin-resistant Staphylococcus aureus: An overview of basic and clinical research. Nat. Rev. Microbiol. 2019, 17, 203–218. [Google Scholar] [CrossRef]
- Anand, U.; Jacobo-Herrera, N.; Altemimi, A.; Lakhssassi, N. A comprehensive review on medicinal plants as antimicrobial therapeutics: Potential avenues of biocompatible drug discovery. Metabolites 2019, 9, 258. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cowan, M.M. Plant products as antimicrobial agents. Clin. Microbiol. Rev. 1999, 12, 564–582. [Google Scholar] [CrossRef] [Green Version]
- Chandra, H.; Bishnoi, P.; Yadav, A.; Patni, B.; Mishra, A.P.; Nautiyal, A.R. Antimicrobial resistance and the alternative resources with special emphasis on plant-based antimicrobials-A review. Plants 2017, 6, 16. [Google Scholar] [CrossRef]
- Rijo, P.; Esteves, M.; Simões, M.F.; Silva, A.; Duarte, A.; Rodriguez, B. Antimicrobial activity of 7α-acetoxy-6β-hydroxyroyleanone 12-O-benzoyl esters. Planta Med. 2008, 74, PB81. [Google Scholar] [CrossRef]
- Pereira, F.; Figueiredo, T.; de Almeida, R.; Antunes, C.; Garcia, C.; Reis, C.P.; Ascensão, L.; Sobral, R.G.; Rijo, P. Unveiling the mechanism of action of 7α-acetoxy-6β-hydroxyroyleanone on an MRSA/VISA strain: Membrane and cell wall interactions. Biomolecules 2020, 10, 983. [Google Scholar] [CrossRef]
- Boswihi, S.S.; Udo, E.E. Methicillin-resistant Staphylococcus aureus: An update on the epidemiology, treatment options and infection control. CMRP 2018, 8, 18–24. [Google Scholar] [CrossRef]
- Fonseka, D.L.C.K.; Wickramaarachchi, W.W.U.I.; Situge, C.D. Mass production of Plectranthus zeylanicus—A valuable medicinal and aromatic plant with a future value. IJMFM & AP 2019, 5, 15–20. [Google Scholar]
- Gertsch, E.J.; Leonti, M.; Raduner, S.; Racz, I.; Chen, J.Z.; Xie, X.Q.; Altmann, K.H.; Karsak, M.; Zimmer, A. α-caryophyllene is a dietary cannabinoid. Proc. Natl. Acad. Sci. USA 2008, 105, 9099–9104. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fernandes, E.S.; Passos, G.F.; Medeiros, R.; da Cunha, F.M.; Ferreira, J.; Campos, M.M.; Pianowski, L.F.; Calixto, J.B. Anti-inflammatory effects of compounds α-humulene and (−)-trans-caryophyllene isolated from the essential oil of Cordia verbenacea. Eur. J. Pharmacol. 2007, 569, 228–236. [Google Scholar] [CrossRef] [PubMed]
- Goetzl, J.E. Vitamin E modulates the lipoxygenation of arachidonic acid in leukocytes. Nature 1980, 288, 183–185. [Google Scholar] [CrossRef] [PubMed]
- Kweifio-Okai, G.; Macrides, T.A. Antilipoxygenase activity of amyrin triterpenes. Res. Commun. Chem. Pathol. Pharmacol. 1992, 78, 367–372. [Google Scholar] [PubMed]
- Werz, O. Inhibition of 5-lipoxygenase product synthesis by natural compounds of plant origin. Planta Med. 2007, 73, 1331–1357. [Google Scholar] [CrossRef] [Green Version]
- Napagoda, M.; Gerstmeier, J.; Butschek, H.; Lorenz, S.; Kanatiwela, D.; De Soyza, S.D.; Qader, M.; Nagahawatte, A.; Wijayaratne, G.B.; Svatoš, A.; et al. Lipophilic extract of Leucas zeylanica, a multi-purpose medicinal plant in the tropics, inhibits key enzymes involved in inflammation and gout. J. Ethnopharmacol. 2018, 224, 474–481. [Google Scholar] [CrossRef]
- Napagoda, M.; Gerstmeier, J.; Butschek, H.; De Soyza, S.; Pace, S.; Lorenz, S.; Qader, M.; Witharana, S.; Nagahawatte, A.; Wijayaratne, G.; et al. The anti-inflammatory and antimicrobial potential of selected ethnomedicinal plants from Sri Lanka. Molecules 2020, 25, 1894. [Google Scholar] [CrossRef]
- Fischer, L.; Szellas, D.; Radmark, O.; Steinhilber, D.; Werz, O. Phosphorylation- and stimulus-dependent inhibition of cellular 5-lipoxygenase activity by nonredox-type inhibitors. FASEB J. 2003, 17, 949–951. [Google Scholar] [CrossRef]
- Steinhilber, D.; Herrmann, T.; Roth, H.J. Separation of lipoxins and leukotrienes from human granulocytes by high-performance liquid chromatography with a Radial-Pak cartridge after extraction with an octadecyl reversed-phase column. J. Chromatogr. 1989, 493, 361–366. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).