Sensitization of Antibiotic-Resistant Gram-Negative Bacteria to Photodynamic Therapy via Perfluorocarbon Nanoemulsion
Abstract
:1. Introduction
2. Results and Discussion
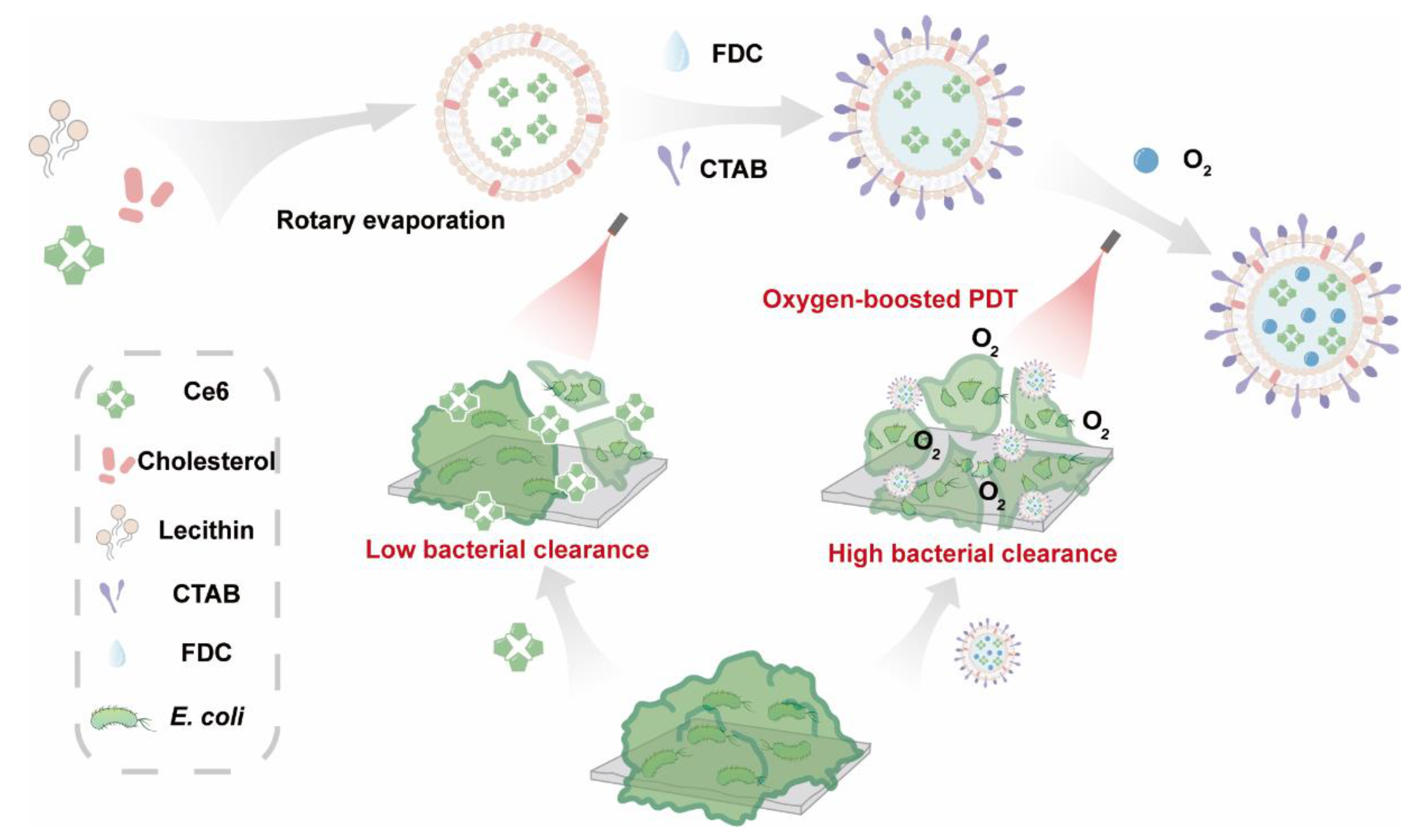
2.1. Preparation of Ce6@FDC


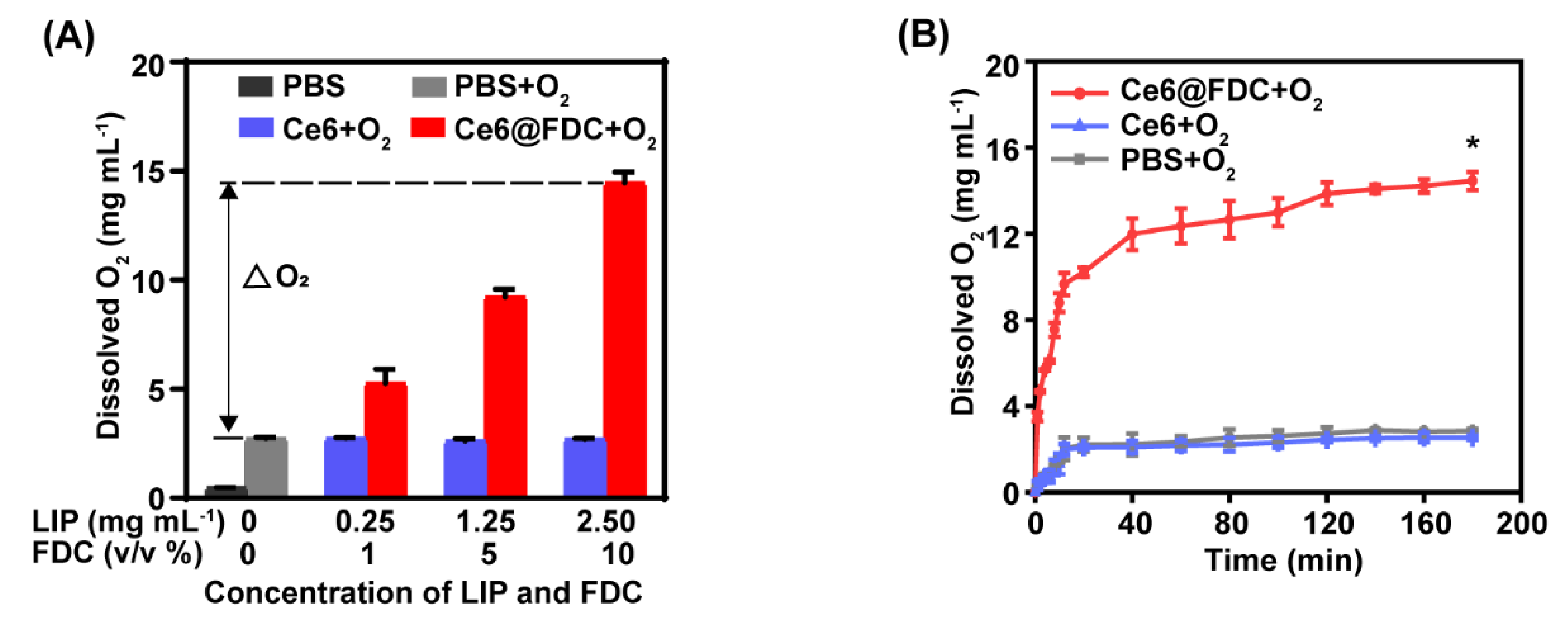
2.2. Oxygen Loading and Releasing Behavior of Ce6@FDC
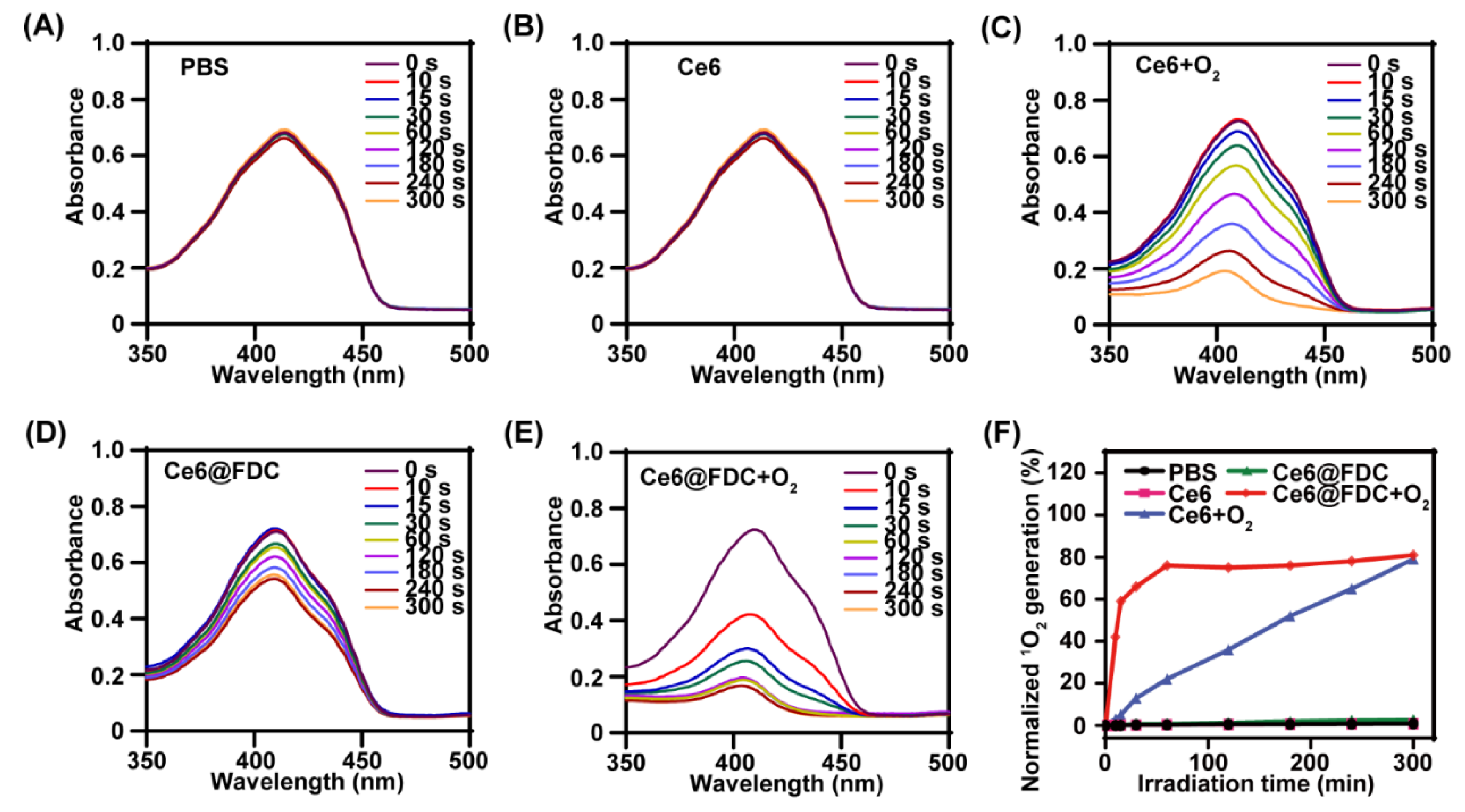
2.3. Enhanced Singlet Oxygen Generation
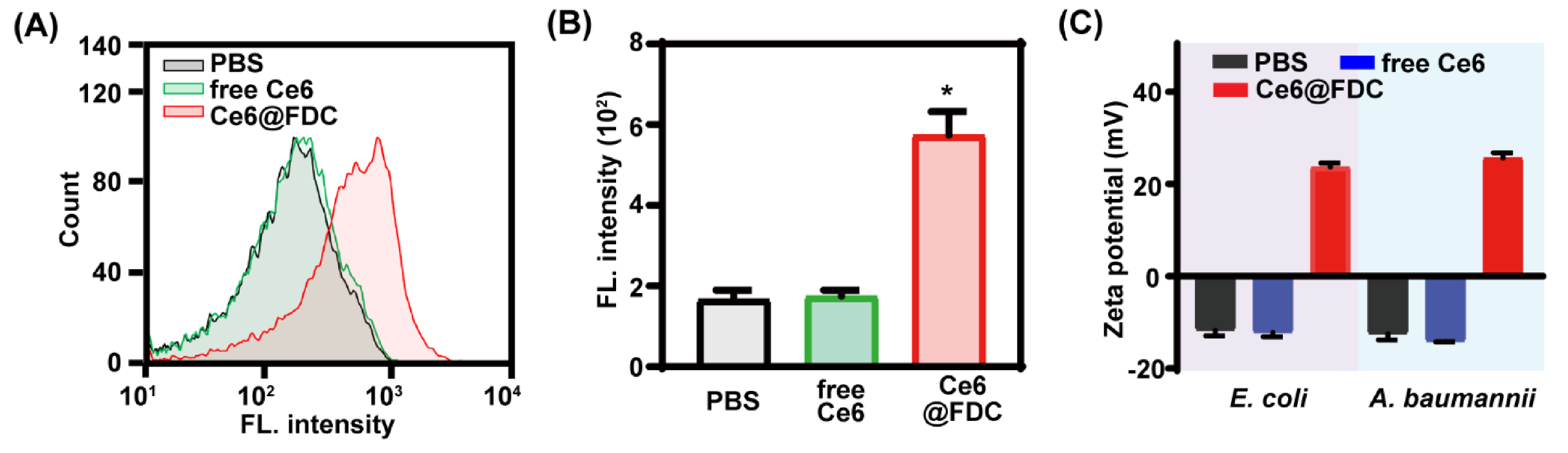
2.4. Facilitated Bacteria Association of Ce6@FDC
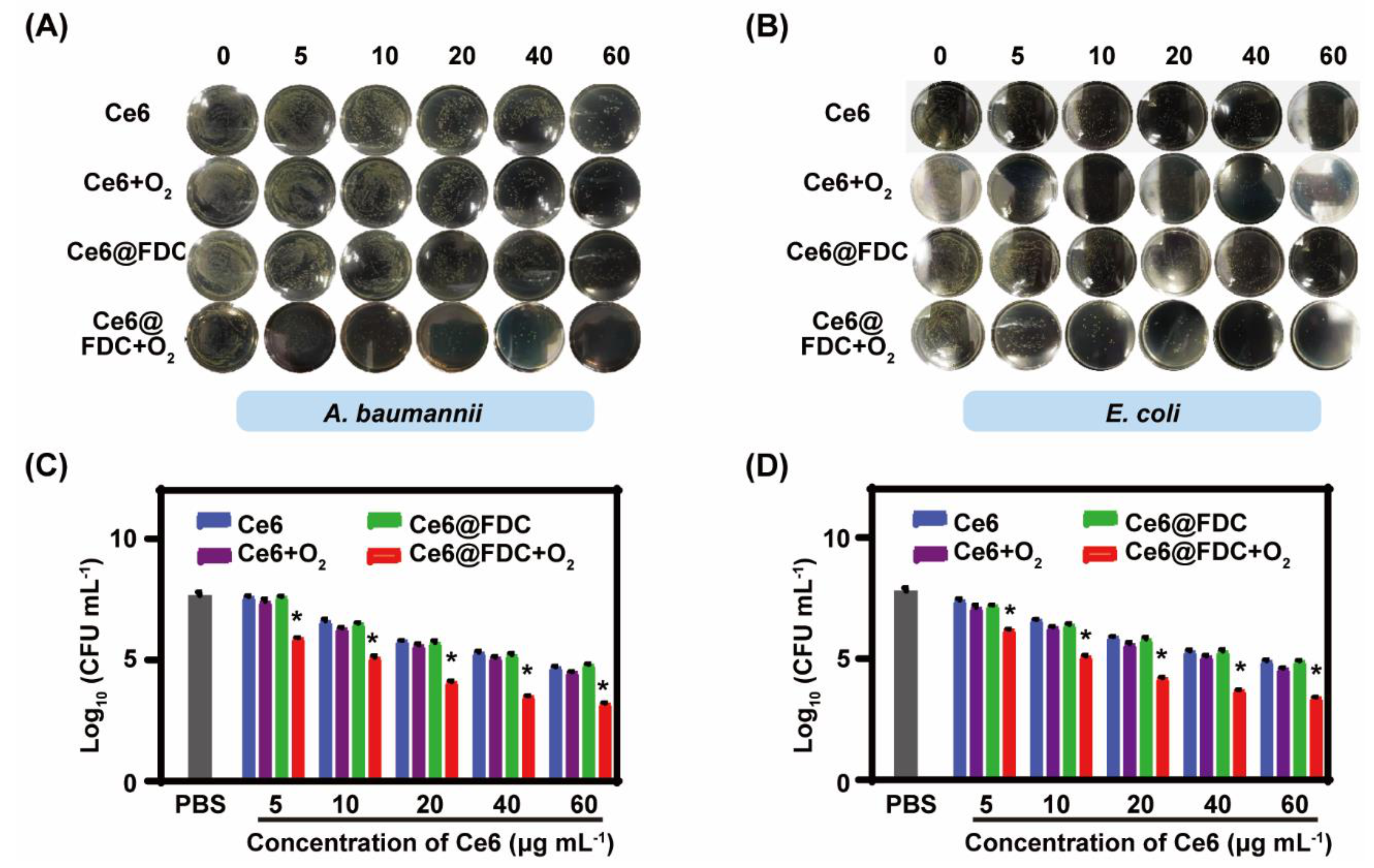
2.5. Photodynamic Bactericidal Performance against Antibiotic-Resistant Gram-Negative Bacteria
2.6. Potent Biofilm Ablation Ability In Vitro
3. Materials and Methods
3.1. Materials
3.2. Preparation of Ce6@FDC Nanoemulsion
3.3. Characterization
3.4. Oxygen Loading and Releasing of Ce6@FDC
3.5. Light-Triggered 1O2-Generation Ability of Ce6@FDC + O2
3.6. Bacterial Association of Ce6@FDC
3.7. In Vitro Photodynamic Antibacterial Activity
3.8. In Vitro Ablation Capacity of Bacterial Biofilm
3.9. Statistics Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Timsit, J.F.; Bassetti, M.; Cremer, O.; Daikos, G.; Waele, J.; Kallil, A.; Kipnis, E.; Kollef, M.; Laupland, K.; Paiva, J.; et al. Rationalizing Antimicrobial Therapy in the ICU: A Narrative Review. Intens. Care Med. 2019, 45, 172–189. [Google Scholar] [CrossRef] [PubMed]
- Hu, D.; Zou, L.; Gao, Y.; Jin, Q.; Ji, J. Emerging Nanobiomaterials Against Bacterial Infections in Postantibiotic Era. View 2020, 1, 20200014. [Google Scholar] [CrossRef]
- Bassetti, M.; Poulakou, G.; Ruppe, E.; Bouza, E.; Van Hal, S.J.; Brink, A. Antimicrobial Resistance in the Next 30 Years, Humankind, Bugs and Drugs: A Visionary Approach. Intens. Care Med. 2017, 43, 1464–1475. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Yang, Y.; Shi, Y.; Song, H.; Yu, C. Antibiotic-Free Antibacterial Strategies Enabled by Nanomaterials: Progress and Perspectives. Adv. Mater. 2019, 32, 1904106. [Google Scholar] [CrossRef]
- Zhao, Y.; Chen, L.; Wang, Y.; Song, X.; Li, K.; Yan, X.; Yu, L.; He, Z. Nanomaterial-based Strategies in Antimicrobial Applications: Progress and Perspectives. Nano Res. 2021, 14, 4417–4441. [Google Scholar] [CrossRef]
- Tacconelli, E.; Carrara, E.; Savoldi, A.; Harbarth, S.; Mendelson, M.; Monnet, D.L.; Celine, P.; Gunnar, K.; Jan, K.; Yehuda, C.; et al. Discovery, Research, and Development of New Antibiotics: The WHO Priority List of Antibiotic-resistant Bacteria and Tuberculosis. Lancet Infect. Dis. 2018, 18, 318–327. [Google Scholar] [CrossRef]
- Fjell, C.D.; Hiss, J.A.; Hancock, R.E.W.; Schneider, G. Designing Antimicrobial Peptides: Form Follows Function. Nat. Rev. Drug Discov. 2012, 11, 37–51. [Google Scholar] [CrossRef]
- Chen, H.; Jin, Y.; Wang, J.; Wang, Y.; Jiang, W.; Dai, H.; Pang, S.; Lei, L.; Ji, J.; Wang, B. Design of Smart Targeted and Responsive Drug Delivery Systems with Enhanced Antibacterial Properties. Nanoscale 2018, 10, 20946–20962. [Google Scholar] [CrossRef]
- Ermini, M.L.; Voliani, V. Antimicrobial Nano-agents: The Copper Age. ACS Nano 2021, 15, 6008–6029. [Google Scholar] [CrossRef]
- Hu, D.; Deng, Y.; Jia, F.; Jin, Q.; Ji, J. Surface Charge Switchable Supramolecular Nanocarriers for Nitric Oxide Synergistic Photodynamic Eradication of Biofilms. ACS Nano 2020, 14, 347–359. [Google Scholar] [CrossRef]
- Zhang, R.; Li, Y.; Zhou, M.; Wang, C.; Feng, P.; Miao, W.; Huang, H. Photodynamic Chitosan Nano-assembly as a Potent Alternative Candidate for Combating Antibiotic-resistant Bacteria. ACS Appl. Mater. Inter. 2019, 11, 26711–26721. [Google Scholar] [CrossRef]
- Zhao, J.; Xu, L.; Zhang, H.; Zhuo, Y.; Weng, Y.; Li, S.; Yu, D.H. Surfactin-methylene Blue Complex Under LED Illumination for Antibacterial Photodynamic Therapy: Enhanced Methylene Blue Transcellular Accumulation Assisted by Surfactin. Colloids Surf. B Biointerfaces 2021, 207, 111974. [Google Scholar] [CrossRef]
- Demidova, T.N.; Hamblin, M.R. Effect of Cell-photosensitizer Binding and Cell Density on Microbial Photoinactivation. Antimicrob. Agents Chemother. 2005, 49, 2329–2335. [Google Scholar] [CrossRef] [Green Version]
- Bourre, L.; Giuntini, F.; Eggleston, I.M.; Mosse, C.A.; Macrobert, A.J.; Wilson, M. Effective Photoinactivation of Gram-positive and Gram-negative bacterial Strains Using An HIV-1 Tat Peptide-porphyrin Conjugate. Photochem. Photobiol. Sci. 2010, 9, 1613–1620. [Google Scholar] [CrossRef]
- Sun, Y.D.; Zhu, Y.X.; Zhang, X.; Jia, H.R.; Xia, Y.; Wu, F.G. Role of Cholesterol Conjugation in the Antibacterial Photodynamic Therapy of Branched Polyethylenimine-containing Nanoagents. Langmuir 2019, 35, 14324–14331. [Google Scholar] [CrossRef]
- Hu, D.; Zou, L.; Yu, W.; Jia, F.; Han, H.; Yao, K.; Jin, Q.; Ji, J. Relief of Biofilm Hypoxia Using An Oxygen Nanocarrier: A New Paradigm for Enhanced Antibiotic Therapy. Adv. Sci. 2020, 7, 2000398. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, D.; Yang, K.; Sheng, D.; Tan, B.; Wang, Z.; Ran, H.; Yi, H.; Zhong, Y.; Lin, H. Mitochondria-targeted Artificial “Nano-RBCs” for Amplified Synergistic Cancer Phototherapy by A Single NIR Irradiation. Adv. Sci. 2018, 5, 1800049. [Google Scholar] [CrossRef]
- Chen, Z.; Wang, Z.; Ren, J.; Qu, X. Enzyme Mimicry for Combating Bacteria and Biofilms. Accounts Chem. Res. 2018, 51, 789–799. [Google Scholar] [CrossRef]
- Yin, Z.; Chen, D.; Zou, J.; Shao, J.; Tang, H.; Xu, H.; Si, W.; Dong, X. Tumor Microenvironment Responsive Oxygen-self-generating Nanoplatform for Dual-imaging Guided Photodynamic and Photothermal Therapy. ChemistrySelect 2018, 3, 4366–4373. [Google Scholar] [CrossRef]
- Chen, Q.; Feng, L.; Liu, J.; Zhu, W.; Dong, Z.; Wu, Y.; Liu, Z. Intelligent Albumin-MnO2 Nanoparticles as pH-/H2O2-responsive Dissociable Nnanocarriers to Modulate Tumor Hypoxia for Effective Combination Therapy. Adv. Mater. 2016, 28, 7129–7136. [Google Scholar] [CrossRef]
- Sheng, D.; Liu, T.; Deng, L.; Zhang, L.; Li, X.; Xu, J.; Hao, L.; Li, P.; Ran, H.; Chen, H.; et al. Perfluorooctyl Bromide & Indocyanine Ggreen co-loaded Nanoliposomes for Enhanced Multimodal Imaging-guided Phototherapy. Biomaterials 2018, 165, 1–13. [Google Scholar] [PubMed]
- Gao, M.; Liang, C.; Song, X.; Chen, Q.; Jin, Q.; Wang, C.; Liu, Z. Erythrocyte-membrane-enveloped Perfluorocarbon as Nanoscale Artificial Red Blood Cells to Relieve Tumor Hypoxia and Enhance Cancer Radiotherapy. Adv. Mater. 2017, 29, 1701429. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Cheng, H.; Jiang, C.; Qiu, X.; Wang, K.; Huan, W.; Yuan, A.; Wu, J.; Hu, Y. Perfluorocarbon Nanoparticles Enhance Eeactive Oxygen Levels and Tumor Growth Inhibition in Photodynamic Therapy. Nat. Commun. 2015, 6, 8785. [Google Scholar] [CrossRef] [PubMed]
- Zhu, B.; Wang, L.; Huang, J.; Xiang, X.; Tang, Y.; Cheng, C.; Feng, Y.; Lang, M.; Li, Q. Ultrasound-triggered Perfluorocarbon-derived Nanobombs for Targeted Therapies of Rheumatoid Arthritis. J. Mater. Chem. B 2019, 7, 4581–4591. [Google Scholar] [CrossRef]
- Zou, L.Y.; Hu, D.F.; Wang, F.J.; Jin, Q.; Ji, J. The Relief of Hypoxic Microenvironment Using An O2 Self-sufficient Fluorinated Nanoplatform for Enhanced Photodynamic Eradication of Bacterial Biofilms. Nano Res. 2022, 15, 1636–1644. [Google Scholar] [CrossRef]
- Zhao, C.; Tong, Y.; Li, X.; Shao, L.; Chen, L.; Lü, J.; Deng, X.; Wang, X.; Wu, Y. Photosensitive Nanoparticles Combining Vascular-independent Intratumor Distribution and On-demand Oxygen-depot Delivery for Enhanced Cancer Photodynamic Therapy. Small 2018, 14, e1703045. [Google Scholar] [CrossRef]
- Yu, M.; Xu, X.; Cai, Y.; Zou, L.; Shuai, X. Perfluorohexane-cored Nanodroplets for Stimulations-responsive Ultrasonography and O2-potentiated Photodynamic Therapy. Biomaterials 2018, 175, 61–71. [Google Scholar] [CrossRef]
- Ma, W.; Chen, X.; Fu, L.; Zhu, J.; Fan, M.; Chen, J.; Yang, C.; Yang, G.; Wu, L.; Mao, G.; et al. Ultra-efficient Antibacterial System Based on Photodynamic Therapy and CO Gas Therapy for Synergistic Antibacterial and Ablation Biofilms. ACS Appl. Mater. Inter. 2020, 12, 22479–22491. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Niu, P.; Dai, J.; Wang, Z.; Wang, Y.; Feng, D.; Li, Y.; Miao, W. Sensitization of Antibiotic-Resistant Gram-Negative Bacteria to Photodynamic Therapy via Perfluorocarbon Nanoemulsion. Pharmaceuticals 2022, 15, 156. https://doi.org/10.3390/ph15020156
Niu P, Dai J, Wang Z, Wang Y, Feng D, Li Y, Miao W. Sensitization of Antibiotic-Resistant Gram-Negative Bacteria to Photodynamic Therapy via Perfluorocarbon Nanoemulsion. Pharmaceuticals. 2022; 15(2):156. https://doi.org/10.3390/ph15020156
Chicago/Turabian StyleNiu, Peiyuan, Jialing Dai, Zeyu Wang, Yueying Wang, Duxiang Feng, Yuanyuan Li, and Wenjun Miao. 2022. "Sensitization of Antibiotic-Resistant Gram-Negative Bacteria to Photodynamic Therapy via Perfluorocarbon Nanoemulsion" Pharmaceuticals 15, no. 2: 156. https://doi.org/10.3390/ph15020156
APA StyleNiu, P., Dai, J., Wang, Z., Wang, Y., Feng, D., Li, Y., & Miao, W. (2022). Sensitization of Antibiotic-Resistant Gram-Negative Bacteria to Photodynamic Therapy via Perfluorocarbon Nanoemulsion. Pharmaceuticals, 15(2), 156. https://doi.org/10.3390/ph15020156




