In Vitro Antibacterial Susceptibility of Different Pathogens to Thirty Nano-Polyoxometalates
Abstract
1. Introduction
2. Results
2.1. Chemistry of Polyoxometalates
2.2. Antimicrobial Activity of Polyoxometalates
3. Discussion
3.1. Chemistry of Polyoxometalates
3.2. POM Pharmacology and Antimicrobial Activity
3.3. POM Structure-Antibacterial Activity Relationship
4. Materials and Methods
4.1. Synthesis and Physico-Chemical Characterizations of Polyoxometalates
4.1.1. Reagents and Chemical Materials
4.1.2. Synthesis of Polyoxometalates
4.1.3. Physico-Chemical Characterizations of Polyoxometalates
4.2. Antimicrobial Activity of Polyoxometalates
4.2.1. Reagents and Materials
4.2.2. Disk Diffusion Method
4.2.3. Minimum Inhibitory Concentration (MIC)
4.2.4. Minimum Bactericidal Concentration (MBC)
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Long, D.L.; Tsunashima, R.; Cronin, L. Polyoxometalates: Building Blocks for Functional Nanoscale Systems. Angew. Chem. Int. Ed. 2010, 49, 1736–1758. [Google Scholar] [CrossRef] [PubMed]
- Pope, M.T. Heteropoly and Isopoly Oxometalates, 1st ed.; Springer: Berlin/Heidelberg, Germany, 1983; pp. 32–65. [Google Scholar]
- Fan, D.; Hao, J. Polyoxometalate-Based Assembly. In Self-Assembled Structures. Properties and Applications in Solution and on Surfaces, 1st ed.; Hao, J., Ed.; CRC Press: Boca Raton, FL, USA; Taylor&Francis Group: Boca Raton, FL, USA, 2011; pp. 141–174. [Google Scholar]
- Hervé, G.; Tézé, A.; Contant, R. General Principles of the Synthesis of Polyoxometalates in Aqueous Solution. In Polyoxometalate Molecular Science NATO Science Series II, 1st ed.; Borrás-Almenar, J.J., Coronado, E., Müller, A., Pope, M.T., Eds.; Kluwer Academic Publishers: Dordrecht, The Netherlands, 2003; Volume 98, pp. 33–54. [Google Scholar] [CrossRef]
- Ivanova, S. Hybrid Organic-Inorganic Materials Based on Polyoxometalates and Ionic Liquids and Their Application in Catalysis. ISRN Chem. Eng. 2014, 2014, 963792. [Google Scholar] [CrossRef]
- Hill, C.L. Progress and Challenges in Polyoxometalate-Based Catalysis and Catalytic Materials Chemistry. J. Mol. Catalysis A Chem. 2007, 262, 2–6. [Google Scholar] [CrossRef]
- Pope, M.T.; Müller, A. Polyoxometalate Chemistry: An Old Field with New Dimensions in Several Disciplines. Angew. Chem. Int. Ed. 1991, 30, 34–48. [Google Scholar] [CrossRef]
- Katsoulis, D.E. A Survey of Applications of Polyoxometalates. Chem. Rev. 1998, 98, 359–387. [Google Scholar] [CrossRef] [PubMed]
- Clemente-Juan, J.; Coronado, E. Magnetic Clusters from Polyoxometalate Complexes. Coord. Chem. Rev. 1999, 193, 361–394. [Google Scholar] [CrossRef]
- Yamase, T.; Abe, H.; Ishikawa, E.; Nojiri, H.; Ohshima, Y. Structure and Magnetism of [n-BuNH3]12[Cu4(GeW9O34)2]·14H2O Sandwiching a Rhomblike Cu48+ Tetragon through α-Keggin Linkage. Inorg. Chem. 2009, 48, 138–148. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Keita, B.; Nadjo, L. Electrochemistry of Isopoly and Heteropoly Oxometalates. In Encyclopedia of Electrochemistry, 1st ed.; Bard, A.J., Stratmann, M., Eds.; Wiley-VCH: Weinheim, Germany, 2006; Volume 7, pp. 607–700. [Google Scholar]
- Rusu, D.; Bâlici, Ș. Polioxometalații. Aplicații Biomedicale, 1st ed.; Casa Cărții de Știință: Cluj-Napoca, România, 2013. [Google Scholar]
- Marcu, G.; Rusu, M. Chimia Polioxometalaților, 1st ed.; Tehnică: București, România, 1997; pp. 22–55. [Google Scholar]
- Long, D.L.; Burkholder, E.; Cronin, L. Polyoxometalate Clusters, Nanostructures and Materials: From Self-Assembly to Designer Materials and Devices. Chem. Soc. Rev. 2007, 36, 105–121. [Google Scholar] [CrossRef]
- Cronin, L. From the Molecular to the Nanoscale: Synthesis, Structure, and Properties. In Comprehensive Coordination Chemistry. II: From Biology to Nanotechnology, 2nd ed.; McCleverty, J.A., Meyer, T.J., Eds.; Elsevier Science: Amsterdam, The Netherlands, 2004; Volume 7, pp. 7–52. [Google Scholar]
- Bernold, H. Polyoxometalates: Introduction to a Class of Inorganic Compounds and Their Biomedical Applications. Front. Biosci. 2005, 10, 275–287. [Google Scholar] [CrossRef]
- Rhule, J.T.; Hill, C.L.; Judd, D.A.; Schinazi, R.F. Polyoxometalate in Medicine. Chem. Rev. 1998, 98, 327–357. [Google Scholar] [CrossRef]
- Yamase, T. Anti-Tumor, -Viral, and -Bacterial Activities of Polyoxometalates for Realizing an Inorganic Drug. J. Mater. Chem. 2005, 15, 4773–4782. [Google Scholar] [CrossRef]
- Nomiya, K.; Torii, H.; Hasegawa, T.; Nemoto, Y.; Nomura, K.; Hashino, K.; Uchida, M.; Kato, Y.; Shimizu, K.; Oda, M. Insulin Mimetic Effect of a Tungstate Cluster. Effect of Oral Administration of Homo-Polyoxotungstates and Vanadium-Substituted Polyoxotungstates on Blood Glucose Level of STZ Mice. J. Inorg. Biochem. 2001, 86, 657–667. [Google Scholar] [CrossRef]
- Ilyas, Z.; Shah, H.S.; Al-Oweini, R.; Kortz, U.; Iqbal, J. Antidiabetic Potential of Polyoxotungstates: In Vitro and In Vivo Studies. Metallomics 2014, 6, 1521–1526. [Google Scholar] [CrossRef] [PubMed]
- Bâlici, Ș.; Wankeu-Nya, M.; Rusu, D.; Nicula, G.Z.; Rusu, M.; Florea, A.; Matei, H. Ultrastructural Analysis of In Vivo Hypoglycemiant Effect of Two Polyoxometalates in Rats with Streptozotocin-Induced Diabetes. Microsc. Microanal. 2015, 21, 1236–1248. [Google Scholar] [CrossRef] [PubMed]
- Bâlici, Ș.; Șușman, S.; Rusu, D.; Nicula, G.Z.; Sorițău, O.; Rusu, M.; Biris, A.S.; Matei, H. Differentiation of Stem Cells into Insulin-Producing Cells under the Influence of Nanostructural Polyoxometalates. J. Appl. Toxicol. 2016, 36, 373–384. [Google Scholar] [CrossRef]
- Prudent, R.; Moucadel, V.; Laudet, B.; Barette, C.; Lafanechere, L.; Hasenknopf, B.; Li, J.; Bareyt, S.; Lacote, E.; Thorimbert, S.; et al. Identification of Polyoxometalates as Nanomolar Noncompetitive Inhibitors of Protein Kinase CK2. Chem. Biol. 2008, 15, 683–692. [Google Scholar] [CrossRef]
- Ogata, A.; Yanagie, H.; Ishikawa, E.; Mitsui, S.; Yamashita, A.; Hasumi, K.; Takamoto, S.; Yamase, T.; Eriguchi, M. Antitumour Effect of Polyoxomolybdates: Induction of Apoptotic Cell Death and Autophagy in In Vitro and In Vivo Models. Br. J. Cancer 2008, 98, 399–409. [Google Scholar] [CrossRef]
- Wang, J.; Qu, X.; Qi, Y.; Li, J.; Song, X.; Li, L.; Yin, D.; Xu, K.; Li, J. Pharmacokinetics of Anti-HBV Polyoxometalate in Rats. PLoS ONE 2014, 9, e98292. [Google Scholar] [CrossRef]
- Yamase, T. Polyoxometalates for Molecular Devices: Antitumor Activity and Luminescence. In Polyoxometalates: From Platonic Solids to Anti-Retroviral Activity, 1st ed.; Pope, M.T., Müller, A., Eds.; Kluwer Academic: Dordrecht, The Netherlands, 1994; pp. 337–358. [Google Scholar]
- Sarafianos, S.G.; Kortz, U.; Pope, M.T.; Modak, M.J. Mechanism of Polyoxometalate-Mediated Inactivation of DNA Polymerases: An Analysis with HIV-1 Reverse Transcriptase Indicates Specificity for the DNA-Binding Cleft. Biochem. J. 1996, 319, 619–626. [Google Scholar] [CrossRef]
- Inoue, M.; Segawa, K.; Matsunaga, S.; Matsumoto, N.; Oda, M.; Yamase, T. Antibacterial Activity of Highly Negative Charged Polyoxotungstates, K27[KAs4W40O140] and K18[KSb9W21O86], and Keggin-Structural Polyoxotungstates against Helicobacter pylori. J. Inorg. Biochem. 2005, 99, 1023–1031. [Google Scholar] [CrossRef]
- Tajima, Y. The Effects of Tungstophosphate and Tungstosilicate on Various Stress Promoters Transformed in Escherichia Coli. J. Inorg. Biochem. 2003, 94, 155–160. [Google Scholar] [CrossRef]
- Bâlici, Ș.; Niculae, M.; Pall, E.; Rusu, M.; Rusu, D.; Matei, H. Antibiotic-Like Behavior of Polyoxometalates. In Vitro Comparative Study: Seven Polyoxotungstates—Nine Antibiotics against Gram-Positive and Gram-Negative Bacteria. Rev. Chim. 2016, 67, 485–490. [Google Scholar]
- Inoue, M.; Suzuki, T.; Fujita, Y.; Oda, M.; Matsumoto, N.; Yamase, T. Enhancement of Antibacterial Activity of β-Lactam Antibiotics by [P2W18O62]6−, [SiMo12O40]4−, and [PTi2W10O40]7− against Methicillin-Resistant and Vancomycin-Resistant Staphylococcus aureus. J. Inorg. Biochem. 2006, 100, 1225–1233. [Google Scholar] [CrossRef] [PubMed]
- Vică, M.L.; Glevitzky, M.; Tit, D.M.; Behl, T.; Heghedus-Mindru, R.C.; Zaha, D.C.; Ursu, F.; Popa, M.; Glevitzky, I.; Bungau, S. The Antimicrobial Activity of Honey and Propolis Extracts from the Central Region of Romania. Food Biosci. 2021, 41, 101014. [Google Scholar] [CrossRef]
- Vică, M.L.; Glevitzky, I.; Glevitzky, M.; Siserman, C.V.; Matei, H.V.; Teodoru, C.A. Antibacterial Activity of Propolis Extracts from the Central Region of Romania against Neisseria gonorrhoeae. Antibiotics 2021, 10, 689. [Google Scholar] [CrossRef] [PubMed]
- Junie, L.M.; Vică, M.L.; Glevitzky, M.; Matei, H.V. Physico-Chemical Characterisation and Antibacterial Activity of Different Types of Honey Tested on Stains Isolated from Hospitalised Patients. J. Apic. Sci. 2016, 60, 5–17. [Google Scholar] [CrossRef]
- Yan, T.-T.; Xuan, Z.-X.; Wang, S.; Zhang, X.; Luo, F. Facile One-Pot Construction of Polyoxometalate-Based Lanthanide-Amino Acid Coordination Polymers for Proton Conduction. Inorg. Chem. Commun. 2019, 105, 147–150. [Google Scholar] [CrossRef]
- Sablinskas, V.; Steiner, G.; Hof, M. Section II: Methods 1: Optical Spectroscopy, Applications. In Handbook of Spectroscopy, 1st ed.; Gauglitz, G., Vo-Dinh, T., Eds.; WILEY-VCH, Verlag: Weinheim, Germany, 2003; Volume 1, pp. 98–103. [Google Scholar]
- Nakamoto, K. Applications in Inorganic Chemistry. In Infrared and Raman Spectra of Inorganic and Coordination Compounds Part A: Theory and Applications in Inorganic Chemistry, 5th ed.; Nakamoto, K., Ed.; John Wiley & Sons Inc: New York, NY, USA, 1997; pp. 38–52. [Google Scholar]
- Čolović, M.B.; Lacković, M.; Lalatović, J.; Mougharbel, A.S.; Kortz, U.; Krstić, D.Z. Polyoxometalates in Biomedicine: Update and Overview. Curr. Med. Chem. 2020, 27, 362–379. [Google Scholar] [CrossRef]
- Gumerova, N.I.; Rompel, A. Polyoxometalates in Solution: Speciation under Spotlight. Chem. Soc. Rev. 2020, 49, 7568–7601. [Google Scholar] [CrossRef]
- Inoue, M.; Suzuki, T.; Fujita, Y.; Oda, M.; Matsumoto, N.; Iijima, J.; Yamase, T. Synergistic Effect of Polyoxometalates in Combination with Oxacillin against Methicillin-Resistant and Vancomycin-Resistant Staphylococcus Aureus: A High Initial Inoculum of 1 × 108 cfu/ml for In Vivo Test. Biomed. Pharmacother. 2006, 60, 220–226. [Google Scholar] [CrossRef]
- World Health Organization (WHO); International Agency for Research on Cancer (IARC). [Internet]. Cancers Attributable to Infections. Available online: https://gco.iarc.fr/causes/infections/tools-pie?mode=2&sex=0&population=who&continent=0&country=0&population_group=0&cancer=0&key=attr_cases&lock_scale=0&pie_mode=1&nb_results=5 (accessed on 30 September 2021).
- Aguilar-Barajas, E.; Díaz-Pérez, C.; Ramírez-Díaz, M.I.; Riveros-Rosas, H.; Cervantes, C. Bacterial Transport of Sulfate, Molybdate, and Related Oxyanions. Biometals 2011, 24, 687–707. [Google Scholar] [CrossRef]
- Bijelic, A.; Aureliano, M.; Rompel, A. The Antibacterial Activity of Polyoxometalates: Structures, Antibiotic Effects and Future Perspectives. Chem. Commun. 2018, 54, 1153–1169. [Google Scholar] [CrossRef] [PubMed]
- Fukuda, N.; Yamase, T.; Tajima, Y. Inhibitory Effect of Polyoxotungstates on the Production of Penicillin-binding Proteins and β-Lactamase against Methicillin-resistant Staphylococcus aureus. Biol. Pharm. Bull. 1999, 22, 463–470. [Google Scholar] [CrossRef][Green Version]
- Müller, A.; Kögerler, P.; Bögge, H. Pythagorean Harmony in the World of Metal Oxygen Clusters of the Mo11 Type: Giant Wheels and Spheres both Based on a Pentagonal Type Unit. In Molecular Self-Assembly: Organic versus Inorganic Approaches (Structure and Bonding, Book Series: Structure), 1st ed.; Fujita, M., Ed.; Springer: Berlin/Heidelberg, Germany, 2000; Volume 96, pp. 203–236. [Google Scholar]
- Müller, A.; Kögerler, P.; Dress, A.W.M. Giant Metal-Oxide-Based Spheres and Their Topology: From Pentagonal Building Blocks to Keplerates and Unusual Spin Systems. Coord. Chem. Rev. 2001, 222, 193–218. [Google Scholar] [CrossRef]
- Müller, A.; Kögerler, P.; Kuhlmann, C. A Variety of Combinatorially Linkable Units as Disposition:† From a Giant Icosahedral Keplerate to Multi-Functional Metal–Oxide Based Network Structures. Chem. Commun. 1999, 15, 1347–1358. [Google Scholar] [CrossRef]
- Müller, A.; Serain, C. Soluble Molybdenum Blues-des Pudels Kern. Acc. Chem. Res. 2000, 33, 2–10. [Google Scholar] [CrossRef]
- Prescott, D.M. Cells: Principles of Molecular Structure and Function; Jones and Bartlett Publishers: Boston, MA, USA, 1988; pp. 23–58. [Google Scholar]
- Aureliano, M.; Gumerova, N.; Sciortino, G.; Garribba, E.; Rompel, A.; Crans, D. Polyoxovanadates with Emerging Biomedical Activities. Coord. Chem. Rev. 2021, 447, 214143. [Google Scholar] [CrossRef]
- Yamamoto, N.; Schols, D.; de Clercq, E.; Debyser, Z.; Pauwels, R.; Balzarini, J.; Nakashima, H.; Baba, M.; Hosoya, M.; Snoeck, R.; et al. Mechanism of Anti-Human Immunodeficiency Virus Action of Polyoxometalates, A Class of Broad-Spectrum Antiviral Agents. Mol. Pharmacol. 1992, 42, 1109–1117. [Google Scholar] [PubMed]
- Liu, R.; Cao, K.; Clark, A.H.; Lu, P.; Anjass, M.; Biskupek, J.; Kaiser, U.; Zhang, G.; Streb, C. Top-Down Synthesis of Polyoxometalate-Like Subnanometer Molybdenum-Oxo Clusters As High-Performance Electrocatalysts. Chem. Sci. 2020, 11, 1043–1051. [Google Scholar] [CrossRef]
- Itul, D.; Marcu, G. Co (II) Complexes with Lacunary Heteropolywolfram Ligands and Mixed Addendum Atoms—A Study on the Optimum Formation pH Range. Rev. Chim. 1995, 46, 143–146. [Google Scholar]
- Sadowska, J.M.; Genoud, K.J.; Kelly, D.J.; O’Brien, F.J. Bone Biomaterials for Overcoming Antimicrobial Resistance: Advances in Non-Antibiotic Antimicrobial Approaches for Regeneration of Infected Osseous Tissue. Mater. Today 2021, 46, 136–154. [Google Scholar] [CrossRef]
- Clinical and Laboratory Standards Institute (CLSI). Performance Standards for Antimicrobial Disk Susceptibility Tests, 12th ed.; Approved Standard-Twelfth Edition; CLSI Document M02-A12; Clinical and Laboratory Standards Institute: Wayne, NJ, USA, 2015; Volume 35, pp. 10–15. [Google Scholar]
- Clinical and Laboratory Standards Institute (CLSI). Performance Standards for Antimicrobial Disk Susceptibility Tests, 13th ed.; Approved Standard-Thirteenth Edition; CLSI Document M02; Clinical and Laboratory Standards Institute: Wayne, NJ, USA, 2018; Volume 36, pp. 21–65. [Google Scholar]
- Markey, B.K.; Leonard, F.C.; Archambault, M.; Cullinane, A.; Maguire, D. Clinical Veterinary Microbiology., 2nd ed.; Elsevier: Edinburgh, UK, 2013; pp. 3–48, 105–288. [Google Scholar]
- Bauer, A.W.; Kirby, W.M.M.; Sherris, J.C.; Turck, M. Antibiotic Susceptibility Testing by A Standardized Single Disk Method. Am. J. Clin. Pathol. 1966, 45, 493–496. [Google Scholar] [CrossRef] [PubMed]
- Kronvall, G.; Giske, C.G.; Kahlmeter, G. Setting Interpretive Breakpoints for Antimicrobial Susceptibility Testing Using Disk Diffusion. Int. J. Antimicrob. Agents. 2011, 38, 281–290. [Google Scholar] [CrossRef] [PubMed]
- Quinn, P.J.; Markey, B.K.; Leonard, F.C.; FitzPatrick, E.S.; Fanning, S.; Hartigan, P.J. Veterinary Microbiology and Microbial Disease; Wiley-BlackWell: Hoboken, NJ, USA, 2011; pp. 149–292. [Google Scholar]
- Clinical and Laboratory Standards Institute (CLSI). Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically, 11th ed.; CLSI Standard M07; Clinical and Laboratory Standards Institute: Wayne, NJ, USA, 2018; pp. 15–53. [Google Scholar]
- Clinical and Laboratory Standards Institute (CLSI). Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically, 9th ed.; Approved Standard-Ninth Edition; CLSI Standard M07-A9; Clinical and Laboratory Standards Institute: Wayne, NJ, USA, 2012; Volume 32, pp. 12–58. [Google Scholar]
| POM No. | Chemical Formula of POMs | Structure Types of POMs |
|---|---|---|
| 1. | Na4[FeIII(H2O)PMo11O39]·18H2O | mono-lacunary Keggin |
| 2. | Na9[Fe3(H2O)3(PMo9O34)2] | tri-lacunary Keggin/sandwich type |
| 3. | Na8[SiW11O39]·12H2O | mono-lacunary Keggin |
| 4. | Na11[Fe3(H2O)3(SiW9O34)2]·25H2O | tri-lacunary Keggin/sandwich type |
| 5. | K3[(VO)3PMo9O34]·14H2O | tri-lacunary Keggin |
| 6. | Na6[PMo9VIV3VO40]·16H2O | Keggin with mixed addenda atoms |
| 7. | K6[(VO)SiMo2W9O39]·11H2O | mono-lacunary Keggin with mixed addenda atoms |
| 8. | K10[(VO)4(PW9O34)2]·26H2O | tri-lacunary Keggin/sandwich type |
| 9. | K10[(VO)4(AsW9O34)2]·21H2O | tri-lacunary pseudo-Keggin/sandwich type |
| 10. | K11H[(VO)3(SbIIIW9O33)2]·27H2O | tri-lacunary pseudo-Keggin/sandwich type |
| 11. | Na12[Sb2W22O74(OH)2]⋅38H2O | cluster |
| 12. | H4[SiW12O40]·14H2O | saturated Keggin |
| 13. | H3[PW12O40]·12H2O | saturated Keggin |
| 14. | H3[PMo12O40]·13H2O | saturated Keggin |
| 15. | Na9[SbW9O33]·19,5H2O | tri-lacunary pseudo-Keggin |
| 16a. | Na10[SiW9O34]·24H2O | tri-lacunary Keggin |
| 16b. | Na10[SiW9O34]·24H2O–recryst. | tri-lacunary Keggin |
| 17. | Na27[NaAs4W40O140]·42H2O | cluster |
| 18. | Na8H[PW9O34]·20H2O | tri-lacunary Keggin |
| 19. | (NBu4)27[NaAs4Mo40O140] ·12H2O | cluster |
| 20. | (Bu3Sn)18[NaSb9W21O86] | cluster |
| 21. | K6[Co(H2O)SiMo2W9O39]·14H2O | mono-lacunary Keggin with mixed addenda atoms |
| 22. | K10[Co(H2O)Si2MoW16O61]·18H2O | mono-lacunary Wells-Dawson with mixed addenda atoms |
| 23a. | Na5[FeIII(H2O)SiW11O39]·24H2O | mono-lacunary Keggin |
| 23b. | Na5[FeIII(H2O)SiW11O39]·24H2O–recryst. | mono-lacunary Keggin |
| 24a. | Na5[FeIII(H2O)GeW11O39]·26H2O | mono-lacunary Keggin |
| 24b. | Na5[FeIII(H2O)GeW11O39]·26H2O–recryst. | mono-lacunary Keggin |
| 25. | Na10[Mn4(H2O)2(AsW9O34)2]·27H2O | tri-lacunary pseudo-Keggin/sandwich type |
| 26. | Na12[Co3(H2O)3(BiW9O33)2]·37H2O | tri-lacunary pseudo-Keggin/sandwich type |
| 27. | Na14[Mn3(H2O)3(SiW9O34)2]·28H2O | tri-lacunary Keggin/sandwich type |
| 28. | (NH4)4(NBu4)5[Na(BuSn)3Sb9W21O86]·17H2O | cluster |
| 29. | K27[NaAs4W40O140]·52H2O | cluster |
| 30. | K6[SiVIVW11O40]·12H2O | mono-lacunary Keggin |
| POM No. | Elemental Analysis and TG Data (Found (Calcd.)) | UV (H2O) Data (nm/cm−1): ν2(M=Ot) and ν1(M-Oc,e-M) | FTIR Spectral Data (νmax (cm−1) and Their Contribution in the POMs’ Structure) |
|---|---|---|---|
| 1. | M = 2200.38; Na (4.20 (4.18)); Fe (2.58 (2.54)); P (1.39 (1.41)); Mo (47.98 (47.96)); H2O (15.62 (15.55)). | ν2 = 210/47,619 and ν1 = 228/43,859. | 1128 (w, νas(P-Oi)); 1049 (sh, νas(P-Oi)); 924 (vs, sh, νas(Mo=Ot)); 887 (vs, νas(Mo-Oc-Mo)); 847 (s, sp νas(Mo-Oe-Mo)); 658 (s, br, νas(Mo-Oe-Mo)); 621 (s, br, δ(P-Oi)); 577 (s, br, δ(P-Oi)); 546 (m, sh, δ(Mo-O-Mo)); 486 (m, ν(Fe-O)). |
| 2. | M = 3737.68; Na (5.57 (5.54)); Fe (4.50 (4.48)); P (1.63 (1.66)); Mo (46.24 (46.20)); H2O (13.10 (13.01)). | ν2 = 219/45,662 and ν1 = 271/36,900. | 1180–1044 (s, sp, νas(P-Oi)); 997 (vs, sp, νas(Mo=Ot)); 978 (vs, sp, νas(Mo-Oc-Mo)); 775 (m, b νas(Mo-Oe-Mo)); 667 (w, νas(Mo-Ob-Mo)/sandwich); 514 (m, sp, δ(Mo-O-Mo)). |
| 3. | M = 3074.40; Na (6.04 (5.98)); Si (0.88 (0.91)); W (63.58 (65.78)); H2O (7.10 (7.03)). | ν2 = 206/48,544 and ν1 = 258/38,759. | 3446 (vs, br, νas(O-H)); 1635 (w, δ(O-H)); 1005 (vw, sh, νas(Si-Oi)); 962 (s, sp, νas(W=Ot)); 910 (vs, sp, νas(W-Oc-W)); 798 (vs, br, νas(W-Oe-W)); 517 (vs, br, δ(W-Oc,e-W)). |
| 4. | M = 5378.10; Na (4.72 (4.70)); Fe (3.15 (3.12)); Si (1.02 (1.04)); W (61.58 (61.53)); H2O (9.41 (9.38)). | ν2 = 200/50,000 and ν1 = 257/38,911. | 1190–1063 (w, sp, νas(Si-Oi)); 964 (vs, sp, νas(W=Ot)); 910 (vs, sp, νas(W-Oc-W)); 879 (m, sh, νas(W-Oc-W)); 787 (vs, vbr, νas(W-Oe-W)); 708 (m, sh, ν(W-Ob-W)/sandwich); 542 (vw, br, δ( W-Oc,e-W)); 499 (m, sp, ν(Fe-O)); 403 (m, sp, ν(Fe-O)). |
| 5. | M = 2008.74; K (5.87 (5.84)); V (7.64 (7.61)); P (1.51 (1.54)); Mo (43.05 (42.99)); H2O (12.62 (12.56)). | ν2 = 218/45,871 and ν1 = 305/32,787. | 1180–1088 (s, sp, νas(P-Oi)); 989 (vs, sp, νas(V=Ot)); 941 (vs, sp, νas(Mo=Ot)); 879 (s, br, νas(Mo-Oc-Mo)); 796 (m, br, νas(Mo-Oe-Mo)); 726 (m, νas(Mo-Oe-Mo)); 625 (s, sp, δ(M-O-M)); 513 (w, sp, δ(Mo-Oc,e-Mo)). |
| 6. | M = 2113.42; Na (6.56 (6.53)); P (1.45 (1.47)); Mo (40.92 (40.86)); V (7.26 (7.23)); H2O (13.70 (13.64)). | ν2 = 221/45,249 and ν1 = 305/32,786. | 1190–1063 (vs, sp, νas(P-Oi)); 989 (m, sh, νas(V=Ot)); 962 (vs, sp, νas(Mo=Ot)); 866 (vs, br, νas(V-Oc-V)+ νas(Mo-Oc-Mo)); 785 (vs, vbr, νas(V-Oe-V)+ νas(Mo-Oe-Mo)); 619 (vs, sp, δ(P-Oi); 519 (vw, br, δ(V-Oc,e-V) + δ(Mo-Oc,e-Mo). |
| 7. | M = 2998.20; K (7.84 (7.82)); V (1.73 (1.70)); Si (0.91 (0.94)); Mo (6.44 (6.40)); W (55.26 (55.19)); H2O (6.62 (6.61)). | ν2 = 199/50,251 and ν1 = 258/38,759. | 1109 (w, νas(Si-Oi)); 968 (s, νas(W=Ot) + νas(Mo=Ot)); 906 (vs, νas(W-Oc-W) + νas(Mo-Oc-Mo)); 783 (vs, νas(W-Oe-W) + (Mo-Oe-Mo)); 669 (m, νas(W-Oe-W)+ νas(Mo-Oe-Mo)). |
| 8. | M = 5586.17; K (7.03 (6.99)); V (3.68 (3.65)); P (1.08 (1.11)); W (59.28 (59.24)); H2O (8.45 (8.38)). | ν2 = 201/49,751 and ν1 = 248/40,323. | 3437 (vs, br, νas(O-H)); 1624 (m, sp, δ(O-H)); 1186 (vs, sp, νas(P-Oi)); 1103 (vs, sp, νas(P-Oi)); 987 (sh, νas(W=Ot)); 968 (vs, br, νas(W=Ot)); 891 (s, br, νas(W-Oc-W)); 850 (sh, νas(W-Oc-W)); 791 (vs, vbr, νas(W-Oe-W)); 719 (s, sp, ν(V-Ob-W)); 619 (vs, sp, νs(P-Oi)); 514 (m, br, δ(W-O-W)); 463 (m, br, δ(W-O-W)). |
| 9. | M = 5583.99; K (7.05 (7.00)); V (3.66 (3.65)); As (2.65 (2.68)); W (59.29 (59.26)); H2O (6.81 (6.78)). | ν2 = 201/49,751 and ν1 = 256/39,062. | 3419 (vs, br, νas(O-H)); 1626 (m, sp, δ(O-H)); 1045 (sh, νas(As-Oi)); 931 (vs, br, νas(W=Ot)); 874 (s, sp, νas(W-Oc-W)); 831 (m, νas(W-Oc-W)); 796 (m, sp, νas(W-Oe-W)); 712 (s, sh, ν(V-Ob-W)/sandwich); 621 (m, br, δ(W-O-W)); 553 (m, br, δ(W-O-W)). |
| 10. | M = 5726.92; K (7.55 (7.51)); V (2.70 (2.67)); Sb (4.22 (4.25)); W (57.83 (57.78)); H2O (8.55 (8.49)). | ν2 = 202/49,505 and ν1 = 251/39,841. | 3423 (vs, br, νas(O-H)); 1697 (m, δ(H-O-H)); 1667 (m, br, δ(H-O-H)); 995 (m, sp, νas(V=Ot)); 930 (m, sp, νas(W=Ot)); 857 (s, sp, νas(W-Oc-W)); 833 (vs, νas(W-Oc-W)); 743 (m, sp, νas(Sb-Oi)); 697 (s, νas(W-Oe-W)); 553 (m, br, δ(W-Oc,e-W)). |
| 11. | M = 6466.43; Na (4.31 (4.27)); Sb (3.75 (3.77)); W (62.59 (62.55)); H2O (10.65 (10.59)). | ν2 = 200/50,000 and ν1 = 255/39,216. | 3332 (vs, br, νas(O-H)); 1619 (m, sp, δ(H-O-H)); 1385 (s, sp νas(NO3−)); 943 (vs, sp, νas(W=Ot)); 887 (vs, νas(W-Oc-W)); 864 (s, sh νas(Sb-Oi)); 837 (vs, νas(W-Oc-W)); 800 (s, sh νas(W-Oe-W)); 771 (vs, br, νas(W-Oe-W)); 673 (s, br, νas(W-Ob-W) +δ(O-Sb-O)); 507 (w, br, δ(W-Oc,e-W)). |
| 12. | M = 3130.39; Si (0.88 (0.90)); W (70.52 (70.47)); H2O (8.11 (8.06)). | ν2 = 207/48,309 and ν1 = 263/38,023. | 1020 (m, sh, νas(Si-Oi)); 982 (s, νas(W=Ot)); 926 (vs, sp, νs(Si-Oi)); 883 (m, sp, νas(W-Oc-W)); 787 (vs, br, νas(W-Oe-W)); 538 (m, δ(W-O-W)). |
| 13. | M = 3096.24; P (0.98 (1.00)); W (71.28 (71.25)); H2O (7.00 (6.98)). | ν2 = 201/49,751 and ν1 = 248/40,323. | 1080 (vs, sp, νas(P-Oi); 984 (vs, νas(W=Ot)); 889 (vs, sp, νas(W-Oc-W)); 808 (vs, sp, νas(W-Oe-W)); 596 (w, sp, δ(W-Oc-W)); 525 (m, δ(W-Oe-W)). |
| 14. | M = 2059.45; P (1.48 (1.50)); Mo (55.93 (55.90)); H2O (11.40 (11.37)). | ν2 = 193/51,550 and ν1 = 270/37,000. | 1065 (vs, sp, νas(P-Oi); 962 (vs, sp, νas(Mo=Ot)); 870 (s, vbr, νas(Mo-Oc-Mo)); 787 (vs, br, νas(Mo-Oe-Mo)); 595 (w, δ(Mo-O-Mo)); 509 (vw, δ(Mo-O-Mo)). |
| 15. | M = 2862.51; Na (7.26 (7.23)); Sb (4.22 (4.25)); W (57.87 (57.80)); H2O (12.33 (12.27)). | ν2 = 207/48,309 and ν1 = 238/42,017. | 920 (vs, sp, νas(W=Ot)); 890 (vs, sp, νas(W-Oc-W)); 767 (s, νas(W-Oe-W)); 743 (s, sp, νas(Sb-Oi); 715 (s, νas(W-Oe-W)); 505 (w, br, δ(W-O-W)). |
| 16a. | M = 2888.89; Na (7.98 (7.96)); Si (0.96 (0.97)); W (57.29 (57.27)); H2O (15.01 (14.97)). | ν2 = 208/48,077 and ν1 = 265/37,736. | 1635 (m, δ(O-H)); 987 (m, sp, νas(W=Ot)); 937 (s, νas(Si-Oi)); 878 (vs, νas(W-Oc-W)); 844 (vs, νas(W-Oc-W)); 810 (vs, νas(W-Oe-W)); 723 (s, νas(W-Oe-W)); 618 (s, νs(Si-Oi)); 528 (m, δ(W-O-W)). |
| 16b. | M = 2888.89; Na (7.98 (7.96)); Si (0.96 (0.97)); W (57.29 (57.27)); H2O (14.91 (14.97)). | ν2 = 208/48,077 and ν1 = 265/37,736. | 1635 (m, δ(O-H)); 987 (m, sp, νas(W=Ot)); 937 (s, νas(Si-Oi)); 878 (vs, νas(W-Oc-W)); 844 (vs, νas(W-Oc-W)); 810 (vs, νas(W-Oe-W)); 723 (s, νas(W-Oe-W)); 618 (s, νs(Si-Oi)); 528 (m, δ(W-O-W)). |
| 17. | M = 11293.56; Na (5.73 (5.70)); As (2.63 (2.65)); W (65.15 (65.11)); H2O (6.75 (6.70)). | ν2 = 200/50,000 and ν1 = 243/41,152. | 951 (vs, sp, νas(W=Ot)); 876 (vs, b νas(As-Oi)+νas(W-Oc-W)); 793 (vs, sp νas(W-Oc-W)); 710 (vs, b νas(W-Oe-W)); 634 (s, b νs(As-Oi)); 577 (m, b, δ(W-O-W)). |
| 18. | M = 2774.75; Na (6.65 (6.63)); P (1.10 (1.12)); W (59.68 (59.63)); H2O (13.05 (12.99)). | ν2 = 208/48,077 and ν1 = 245/40,816. | 1054 (s, sp, νas(P-Oi); 1014 (w, νas(P-Oi); 937 (vs, sp, νas(W=Ot)); 881 (vs, sp, νas(W-Oc-W)); 741 (vs, b, νas(W-Oe-W)); 503 (vw, b, δ(W-O-W)). |
| 19. | M = 13162.90; Na (0.20 (0.17)); C (39.46 (39.42)); H (7.66 (7.63)); N (2.88 (2.87)); As (2.26 (2.28)); Mo (29.21 (29.15)); H2O (1.67 (1.64)). | ν2 = 209/47,847 and ν1 = 228/43,859. | 3446 (vs, br, νas(O-H)); > 2800 (vs, br, νas(C-H)); 1483 (vs, br, νas(C-N)); 1617 (w, b, δ(H-O)); 943 (vs, sh, νas(Mo=Ot)); 924 (vs, sp, νas(Mo=Ot)); 904 (vs, sp, νas(As-Oi)+νas(Mo-Oc-Mo)); 879 (s, sh, νas(Mo-Oc-Mo)); 854 (vs, νas(Mo-Oc-Mo)); 806 (vs, b, νas(Mo-Oe-Mo)); 764 (vs, sh, νas(Mo-Oe-Mo)); 735 (vs, sh, νas(Mo-Oe-Mo)); 706 (vs, b, νas(Mo-Oe-Mo)); 663 (s, νs(As-Oi)); 584 (m, δ(Mo-O-Mo)); 557 (w, b, δ(Mo-O-Mo)); 517 (w, b, δ(Mo-O-Mo)). |
| 20. | M = 11576.37; Na (0.22 (0.20)); C (22.44 (22.41)); H (4.25 (4.23)); Sn (18.48 (18.46)); Sb (9.45 (9.47)); W (33.39 (33.35)). | ν2 = 200/50,000 and ν1 = 254/39,370. | 949 (vs, sp, νas(W=Ot)); 862 (s, b, νas(Sb-Oi) + νas(W-Oc-W)); 796 (s, νas(W-Oe-W)); 739 (vs, νas(W-Oe-W)); 749 (vs, νas(W-Oe-W)); 657 (s, δ(Sb-Oi)); 577 (w, νas(Sb-Oi)); 505 (w, ν(C-Sn-O)); 493 (w, δ(Sb-O)); the presence of bands due to the stretching and deformation vibrations of the C-H and C-C bonds of the butyl groups in the ranges 1000–1300, 1700–1950 and >2800 cm−1 is also observed in the spectrum. |
| 21. | M = 3062.25; K (7.70 (7.66)); Co (1.94 (1.92)); Si (0.90 (0.92)); Mo (6.30 (6.27)); W (54.08 (54.03)); H2O (8.87 (8.82)). | ν2 = 203/49,261 and ν1 = 253/39,526. | 995 (s, sp, νas(Si-Oi); 953 (vs, sp, νas(Mo=Ot)); 901 (vs, sp, νas(W=Ot)); 798 (vs, b, νas(Mo-Oc-Mo)+νas(W-Oc-W)); 739 (vs, b, νas(Mo-Oe-Mo)+νas(W-Oe-W)); 704 (s, vb, νas(Mo-Oe-Mo)+νas(W-Oe-W)); 538 (m, sh, δ(W-O-W)); 524 (m, b, δ(W-O-W)) + δ(Mo-O-Mo)); 482 (m, sh δ(W-O)). |
| 22. | M = 4861.72; K (8.08 (8.04)); Co (1.24 (1.21)); Si (1.14 (1.16)); Mo (1.98 (1.97)); W (60.53 (60.50)); H2O (7.10 (7.04)). | ν2 = 203/49,261 and ν1 = 253/39,526. | 995 (sh, sp, νas(Si-Oi)); 952 (vs, sp, νas(Mo=Ot)); 901 (vs, b νas(W=Ot)); 798 (s, b νas(W-Oc-W) + νas(Mo-Oc-Mo)); 739 (vs, b νas(W-Oc-W)); 704 (s, νas(W-Oe-W)); 525 (s, b, δ(W-O-W) + δ(Mo-O-Mo));); 482 (sh, b νs(W-Oc-Co) + νs(Mo-Oc-Co)). |
| 23a. | M = 3295.48; Na (3.50 (3.49)); Fe (1.70 (1.69)); Si (0.82 (0.85)); W (61.38 (61.36)); H2O (13.68 (13.67)). | ν2 = 200/50,000 and ν1 = 259/38,610. | 1088 (m, νas(Si-Oi); 1005 (sh, νas(Si-Oi); 964 (s, νas(W=Ot)); 910 (vs, b, νs(Si-Oi)+νas(W-Oc-W)); 876 (sh, νas(W-Oc-W)); 787 (vs, b, νas(W-Oe-W)); 704 (sh, νas(W-Oe-W)); 538 (m, δ(W-Oc-W)); 519 (m, b, δ(W-Oe-W)); 418 (m, sh, ν(Fe-O)). |
| 23b. | M = 3295.48; Na (3.50 (3.49)); Fe (1.70 (1.69)); Si (0.82 (0.85)); W (61.38 (61.36)); H2O (13.58 (13.67)). | ν2 = 200/50,000 and ν1 = 259/38,610. | 1088 (m, νas(Si-Oi); 1005 (sh, νas(Si-Oi); 964 (s, νas(W=Ot)); 910 (vs, b, νs(Si-Oi)+νas(W-Oc-W)); 876 (sh, νas(W-Oc-W)); 787 (vs, b, νas(W-Oe-W)); 704 (sh, νas(W-Oe-W)); 538 (m, δ(W-Oc-W)); 519 (m, b, δ(W-Oe-W)); 418 (m, sh, ν(Fe-O)). |
| 24a. | M = 3376.06; Na (3.42 (3.40)); Fe (1.67 (1.65)); Ge (2.12 (2.15)); W (59.92 (59.90)); H2O (14.42 (14.41)). | ν2 = 202/49,505 and ν1 = 255/39,216. | 982 (vs, sp νas(W=Ot)); 903 (vs, sh, νas(W-Oc-W)); 876 (vs, b, νas(W-Oc-W)); 814 (s, sh, νas(Ge-O) + νas(W-Oe-W)); 771 (vs, b, νas(Ge-Oi) + νas(W-Oe-W)); 525 (w, b, δ(W-Oc,e-W)). |
| 24b. | M = 3376.06; Na (3.42 (3.40)); Fe (1.67 (1.65)); Ge (2.12 (2.15)); W (59.92 (59.90)); H2O (14.38 (14.41)). | ν2 = 202/49,505 and ν1 = 255/39,216 | 982 (vs, sp νas(W=Ot)); 903 (vs, sh, νas(W-Oc-W)); 876 (vs, b, νas(W-Oc-W)); 814 (s, sh, νas(Ge-O) + νas(W-Oe-W)); 771 (vs, b, νas(Ge-Oi) + νas(W-Oe-W)); 525 (w, b, δ(W-Oc,e-W)) |
| 25. | M = 5519.02; Na (4.18 (4.17)); Mn (3.99 (3.98)); As (2.68 (2.72)); W (59.98 (59.96)); H2O (9.51 (9.47)). | ν2 = 201/49,751 and ν1 = 248/40,323. | 3421 (vs, b, νas(O-H)); 1624 (vs, sp, δ(H-O-H)); 957 (vs, sp, νas(W=Ot)); 877 (vs, b νas(As-Oi)+νas(W-Oc-W)); 839 (s, sp, νas(W-Oc-W)); 768 (vs, νas(W-Oe-W)); 712 (s, νas(W-Oe-W)+νas(W-Ob-W)/sandwich); <514 (m, b, δ(W-O-W)). |
| 26. | M = 5956.33; Na (4.66 (4.63)); Co (2.98 (2.97)); Bi (7.00 (7.02)); W (55.60 (55.56)); H2O (12.15 (12.10)). | ν2 = 194/51,500 and ν1 = 256/38,991. | 946 (s, νas(W=Ot)); 867 (vs, vb, νas(W-Oc-W)); 839 (s, sp, νas(Bi-Oi)); 795 (vs, νas(W-Oe-W)); 740 (s, b, νas(W-Oe-W)); 740 (s, b, νas(W-Ob-W)); 508 (w, δ(W-O-W)). |
| 27. | M = 5498.39; Na (5.88 (5.85)); Mn (3.05 (3.00)); Si (1.00 (1.02)); W (60.25 (60.18)); H2O (10.12 (10.16)). | ν2 = 213/46,948 and ν1 = 256/39,066. | 1631 (m, δ(H2O)); 1568 (m, δ(H2O)); 987 (m, νas(W=Ot)); 940 (s, νas(Si-Oi)); 893 (vs, νas(W-Oc-W)); 807 (vs, νas(W-Oe-W)); 722 (s, νas(W-Oe-W)); 682 (s, νs(Si-Oi-W)); 519 (vw, δ(W-Oc,e-W)); 350 (s, ν(Mn-Oc,e-W)). |
| 28. | M = 8473.62; C (13.06 (13.04)); H (3.10 (3.06)); N (1.54 (1.49)); Na (0.28 (0.27)); Sb (12.94 (12.93)); Sn (4.35 (4.20)); W (45.61 (45.56)); H2O (3.64 (3.61)). | ν2 = 191/52,356 and ν1 = 275/36,363. | 3485 (s, νas(hydrogen bond from lattice water)); 3373 (vs, νas(hydrogen bond from lattice water)); 3171 (m, b, ν(N-H) from NH4+); 1648 (m, δ(O-H)); 1621 (sh, δ(O-H)); 1404 (s, δ(N-H) from NH4+); 1293 (m, νas(C-N) from NBu4); 958 (s, νas(W=Ot)); 927 (m, νas(W=Ot)); 881 (s, νas(W-Oc-W)); 871 (s, νas(W-Oc-W)); 851 (s, νas(W-Oc-W)); 800 (vs, νas(W-Oe-W)); 766 (vs, νas(W-Oe-W)); 701(sh, νas(C-N) from NBu4); 681 (s, νas(Sb-Oi) + νas(Sn-O) + ν(C-Sn-O)); 613 (m, νas(Sb-Oi) + νas(Sn-O) + ν(C-Sn-O)); 549 (s, νas(Sb-Oi) + νas(Sn-O) + ν(C-Sn-O)); 489 (m, νas(Sn-C) + δ(W-O-W)); 431 (w, δ(Sb-O)); 418 (m, νas(Sn-C)). |
| 29. | M = 11908.64; K (8.90 (8.86)); Na (0.21 (0.19)); As (2.50 (2.52)); W (61.81 (61.75)); H2O (7.92 (7.87)). | ν2 = 201/49,751 and ν1 = 254/39,370. | 966 (vs, sp, νas(W=Ot)); 883 (vs, b, νas(As-Oi)+(W-Oc-W)); 783 (vs, b, νas(W-Oe-W)); 733 (s, sh, νas(W-Oe-W)); 671 (vs, b, νas(As-Oi)); 553 (m, b, δ(W-O-W)). |
| 30. | M = 3192.02; K (7.38 (7.35)); Si (0.86 (0.88)); V (1.62 (1.60)); W (63.39 (63.35)); H2O (6.62 (6.77)). | ν2 = 198/50,505 and ν1 = 257/38,910. | 1054 (w, sp, νas(Si-Oi)); 1000 (w, sp, νs(Si-Oi)); 965 (s, sp, νas (W=Ot)); 989 (m, sp, νas (V=O)); 884 (vs, νas(W-Oc-W)); 805 (vs, νas (W-Oe-W)); 741 (vs, vb, νas (W-Oe-W)); 661 (m, δ(Oi-Si-Oi)); 518 (w, δ(W-Oc,e-W)). |
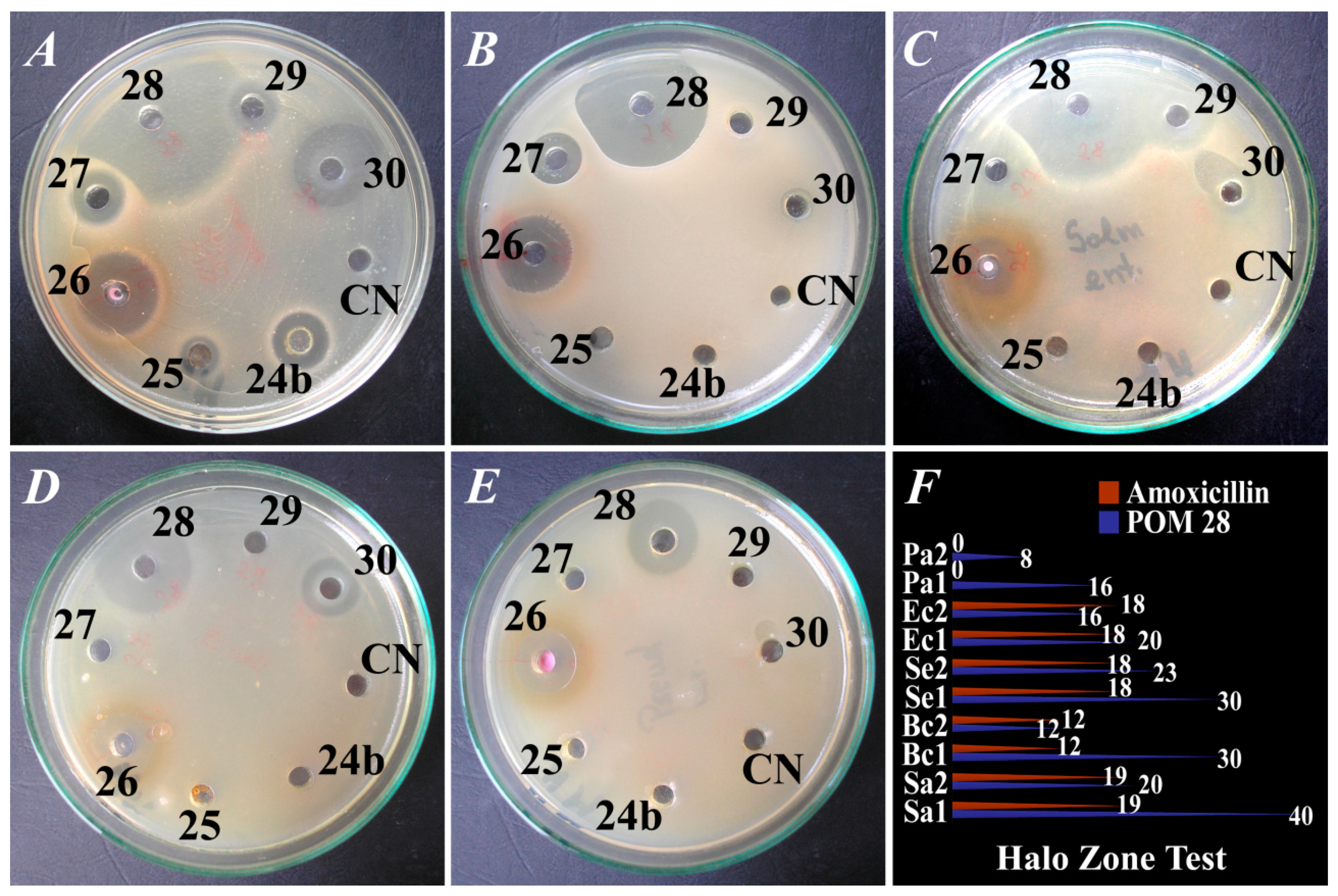
| POM No. | Effect of POMs on Microorganisms (Halo Zone Test/mm) | ||||
|---|---|---|---|---|---|
| S. aureus | B. cereus | S. enteritidis | E. coli | P. aeruginosa | |
| 1. | 12 ± 0.50 R 1 | 7 ± 0.30 7 ± 0.22 2 | 6 ± 0.24 R | R | 9 ± 0.22 R |
| 2. | R | 12 ± 0.30 12 ± 0.44 | R | R | R |
| 3. | R | R | R | R | R |
| 4. | 8 ± 0.23 R | 7 ± 0.45 R | R | R | R |
| 5. | R | R | R | R | R |
| 6. | R | R | R | R | R |
| 7. | 15 ± 0.50 13 ± 0.50 | R | R | R | R |
| 8. | 10 ± 0.50 R | 10 ± 0.20 R | R | R | R |
| 9. | R | R | R | R | R |
| 10. | R | R | R | R | R |
| 11. | 11 ± 0.55 R | R | 10 ± 0.20 R | R | R |
| 12. | R | R | R | R | R |
| 13. | 8 ± 0.12 12 ± 0.5 | 8 ± 0.22 7 ± 0.25 | 10 ± 0.50 10 ± 0.22 | 10 ± 0.50 12 ± 0.25 | R |
| 14. | 8 ± 0.22 7 ± 0.25 | R | 12 ± 0.25 6 ± 0.32 | 12 ± 0.35 8 ± 0.25 | 12 ± 0.50 8 ± 0.42 |
| 15. | 32 ± 0.22 18 ± 0.50 | 23 ± 0.25 12 ± 0.50 | 26 ± 0.25 12 ± 0.50 | R | R |
| 16a. 3 | R | R | R | R | R |
| 16b. 4 | R | R | R | R | R |
| 17. | R | 10 ± 0.25 R | 18 ± 0.25 10 ± 0.50 | R | R |
| 18. | R | R | 8 ± 0.22 R | R | R |
| 19. | 20 ± 0.50 12 ± 0.30 | 14 ± 0.50 8 ± 0.65 | 25 ± 0.23 19 ± 0.18 | R R | R R |
| 20. | 30 ± 0.10 13 ± 0.25 | 24 ± 0.15 14 ± 0.22 | 22 ± 0.10 10 ± 0.22 | 12 ± 0.25 8 ± 0.25 | 12 ± 0.22 18 ± 0.25 |
| 21. | R | R | R | R | R |
| 22. | R | R | R | R | R |
| 23a. 3 | 14 ± 0.25 13 ± 0.25 | R | R | R | R |
| 23b. 4 | 14 ± 0.22 13 ± 0.12 | R | R | R | R |
| 24a. 3 | 12 ± 0.15 10 ± 0.25 | R | R | R | R |
| 24b. 4 | 10 ± 0.25 R | R | R | R | R |
| 25. | 13 ± 0.25 16 ± 0.55 | R | R | R | R |
| 26. | 18 ± 0.55 16 ± 0.10 | 20 ± 0.55 12 ± 0.15 | 18 ± 0.55 15 ± 0.15 | 16 ± 0.25 14 ± 0.22 | 15 ± 0.25 22 ± 0.50 |
| 27. | 14 ± 0.50 10 ± 0.35 | 14 ± 0.37 10 ± 0.22 | R | R | R |
| 28. | 40 ± 0.50 20 ± 0.22 | 30 ± 0.50 12 ± 0.55 | 30 ± 0.52 23 ± 0.23 | 20 ± 0.23 16 ± 0.27 | 16 ± 0.45 8 ± 0.56 |
| 29 | 12 ± 0.50 12 ± 0.22 | R | 18 ± 0.50 R | R | R |
| 30 | 18 ± 0.55 11 ± 0.25 | 6 ± 0.51 6 ± 0.45 | 12 ± 0.56 7 ± 0.52 | 14 ± 0.57 8 ± 0.45 | R |
| +ive C 5 | 19 ± 0.52 | 12 ± 0.37 | 18 ± 0.33 | 18 ± 0.26 | R |
| −ive C 6 | R | R | R | R | R |
| POM No. | Minimum Inhibitory Concentration (mg/L) | ||||
|---|---|---|---|---|---|
| S. aureus | B. cereus | S. enteritidis | E. coli | P. aeruginosa | |
| 1. | 0.625 | 1.25 | 1.25 | - | 0.625 |
| 2. | - | 1.25 | - | - | - |
| 4. | 0.625 | 1.25 | - | - | - |
| 7. | 1.25 | - | - | - | - |
| 8. | 1.25 | 1.25 | - | - | - |
| 11. | 0.039 | - | 0.156 | - | - |
| 13. | 1.25 | 2.5 | 0.312 | 0.078 | - |
| 14. | 0.156 | - | 0.312 | 0.156 | 0.312 |
| 15. | 1.25 | 0.312 | 0.625 | - | - |
| 17. | - | 0.625 | 1.25 | - | - |
| 18. | - | - | 1.25 | - | - |
| 19. | 0.156 | 0.625 | 0.312 | - | - |
| 20. | 0.039 | 0.039 | 0.156 | 0.156 | 0.625 |
| 23a. | 0.078 | - | - | - | - |
| 23b. | 0.625 | - | - | - | - |
| 24a. | 0.078 | - | - | - | - |
| 24b. | 0.625 | - | - | - | - |
| 25. | 0.156 | - | - | - | - |
| 26. | 0.312 | 0.078 | 0.156 | 0.312 | 0.625 |
| 27. | 0.156 | 0.156 | - | - | - |
| 28. | 0.625 | 0.0048 | 0.019 | 0.078 | 0.039 |
| 29. | 0.625 | - | 0.625 | - | - |
| 30. | 0.078 | 0.312 | 0.312 | 0.156 | - |
| POM no. | Minimum Bactericidal Concentration (mg/L) | ||||
|---|---|---|---|---|---|
| S. aureus | B. cereus | S. enteritidis | E. coli | P. aeruginosa | |
| 1. | 1.25 | 2.5 | - | - | - |
| 2. | - | - | - | - | - |
| 4. | 2.5 | 2.5 | - | - | - |
| 7. | 1.25 | - | - | - | - |
| 8. | 2.5 | 2.5 | - | - | - |
| 11. | 1.25 | - | 1.25 | - | - |
| 13. | 0.625 | - | 1.25 | 1.25 | - |
| 14. | 0.625 | - | 1.25 | 1.25 | 0.625 |
| 15. | 2.5 | 0.625 | 0.625 | - | - |
| 17. | - | 1.25 | 2.5 | - | - |
| 18. | - | - | 2.5 | - | - |
| 19. | 1.25 | 1.25 | 0.625 | - | - |
| 20. | 1.25 | 0.625 | 2.5 | 1.25 | 2.5 |
| 23a. | 1.25 | - | - | - | - |
| 23b. | 1.25 | - | - | - | - |
| 24a. | 1.25 | - | - | - | - |
| 24b. | 1.25 | - | - | - | - |
| 25. | 2.5 | - | - | - | - |
| 26. | 2.5 | 0.625 | 1.25 | 1.25 | 1.25 |
| 27. | 1.25 | 0.625 | - | - | - |
| 28. | 0.625 | 0.312 | 0.625 | 1.25 | 0.625 |
| 29. | 2.5 | - | 0.625 | - | - |
| 30. | 0.625 | 0.625 | 0.312 | 1.25 | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bâlici, Ș.; Rusu, D.; Páll, E.; Filip, M.; Chirilă, F.; Nicula, G.Z.; Vică, M.L.; Ungur, R.; Matei, H.V.; Fiț, N.I. In Vitro Antibacterial Susceptibility of Different Pathogens to Thirty Nano-Polyoxometalates. Pharmaceuticals 2022, 15, 33. https://doi.org/10.3390/ph15010033
Bâlici Ș, Rusu D, Páll E, Filip M, Chirilă F, Nicula GZ, Vică ML, Ungur R, Matei HV, Fiț NI. In Vitro Antibacterial Susceptibility of Different Pathogens to Thirty Nano-Polyoxometalates. Pharmaceuticals. 2022; 15(1):33. https://doi.org/10.3390/ph15010033
Chicago/Turabian StyleBâlici, Ștefana, Dan Rusu, Emőke Páll, Miuța Filip, Flore Chirilă, Gheorghe Zsolt Nicula, Mihaela Laura Vică, Rodica Ungur, Horea Vladi Matei, and Nicodim Iosif Fiț. 2022. "In Vitro Antibacterial Susceptibility of Different Pathogens to Thirty Nano-Polyoxometalates" Pharmaceuticals 15, no. 1: 33. https://doi.org/10.3390/ph15010033
APA StyleBâlici, Ș., Rusu, D., Páll, E., Filip, M., Chirilă, F., Nicula, G. Z., Vică, M. L., Ungur, R., Matei, H. V., & Fiț, N. I. (2022). In Vitro Antibacterial Susceptibility of Different Pathogens to Thirty Nano-Polyoxometalates. Pharmaceuticals, 15(1), 33. https://doi.org/10.3390/ph15010033