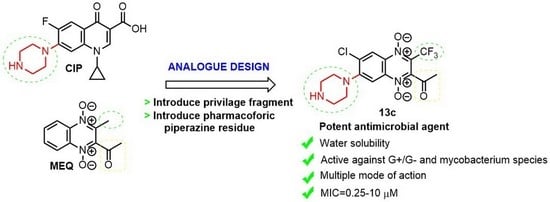
Synthesis and Characterization of Novel 2-Acyl-3-trifluoromethylquinoxaline 1,4-Dioxides as Potential Antimicrobial Agents
Abstract
:1. Introduction
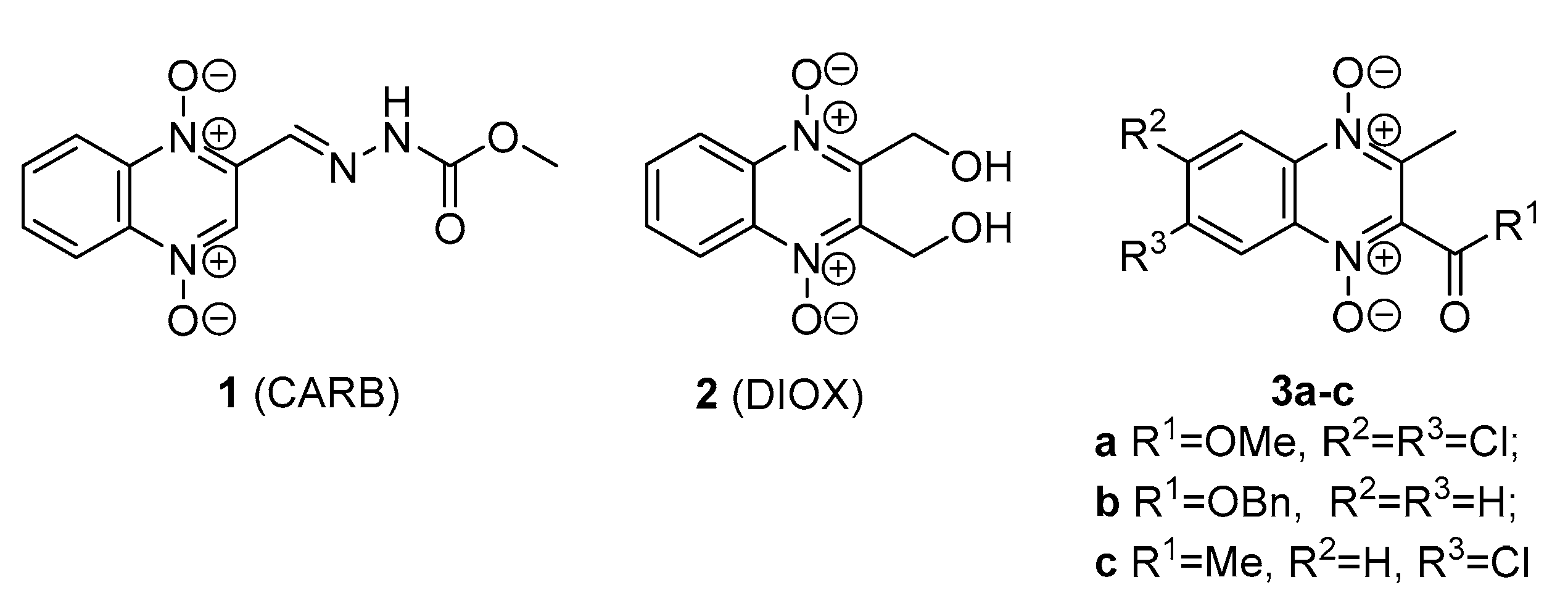
2. Results and Discussion
2.1. Chemistry
2.2. Biological Screening
2.3. Mechanism of Action Determination
2.4. M. smegmatis Drug-Resistant Mutants and Their Analysis
3. Materials and Methods
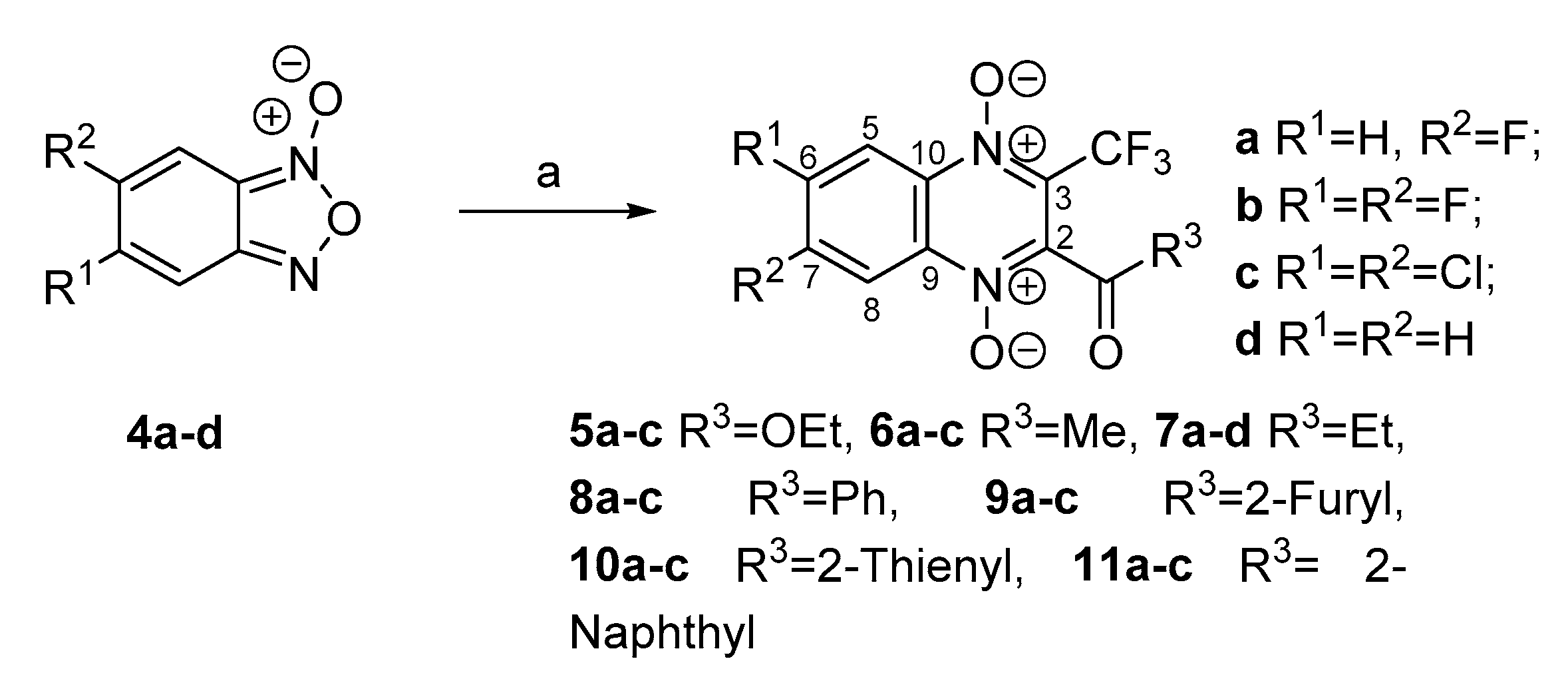
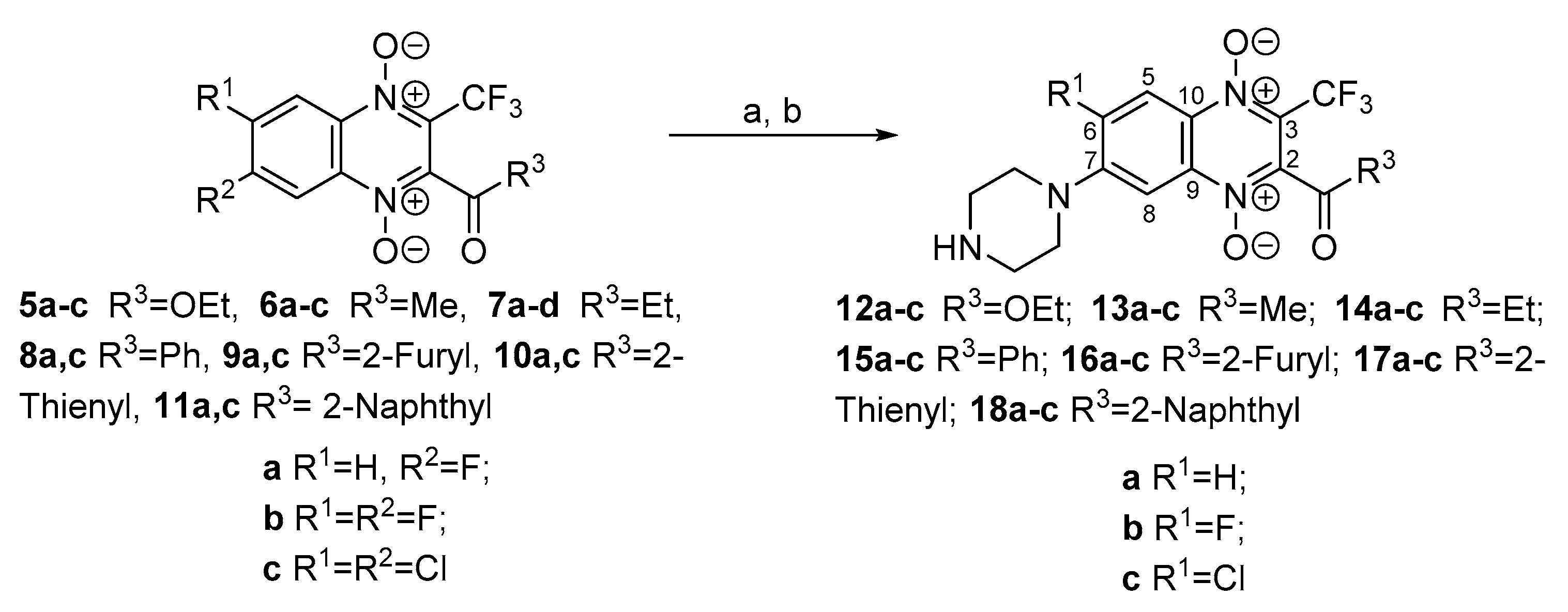
3.1. Synthesis
3.1.1. Materials and General Methods
3.1.2. General Procedure for Synthesis of 5–6a–c, 7a–d and 8–11a–c
3.1.3. 2-Acetyl-7-fluoro-3-trifluoromethylquinoxaline 1,4-Dioxide (6a)
3.1.4. 7-Fluoro-2-propionyl-3-trifluoromethylquinoxaline 1,4-Dioxide (7a)
3.1.5. 2-Propionyl-3-trifluoromethylquinoxaline 1,4-Dioxide (7d)
3.1.6. 2-Ethoxycarbonyl-7-(piperazin-1-yl)-3-trifluoromethylquinoxaline 1,4-Dioxide Hydrochloride (12a)
3.1.7. 2-Ethoxycarbonyl-6-fluoro-7-(piperazin-1-yl)-3-trifluoromethylquinoxaline 1,4-Dioxide Hydrochloride (12b)
3.1.8. 2-Ethoxycarbonyl-6-chloro-7-(piperazin-1-yl)-3-trifluoromethylquinoxaline 1,4-Dioxide Hydrochloride (12c)
3.1.9. 2-Acetyl-7-(piperazin-1-yl)-3-trifluoromethylquinoxaline 1,4-Dioxide Hydrochloride (13a)
3.1.10. 2-Acetyl-6-fluoro-7-(piperazin-1-yl)-3-trifluoromethylquinoxaline 1,4-Dioxide Hydrochloride (13b)
3.1.11. 2-Acetyl-7-chloro-6-(piperazin-1-yl)-3-trifluoromethylquinoxaline 1,4-Dioxide Hydrochloride (13c)
3.1.12. 7-(Piperazin-1-yl)-2-propionyl-3-trifluoromethylquinoxaline 1,4-Dioxide Hydrochloride (14a)
3.1.13. 6-Fluoro-7-(piperazin-1-yl)-2-propionyl-3-trifluoromethylquinoxaline 1,4-Dioxide Hydrochloride (14b)
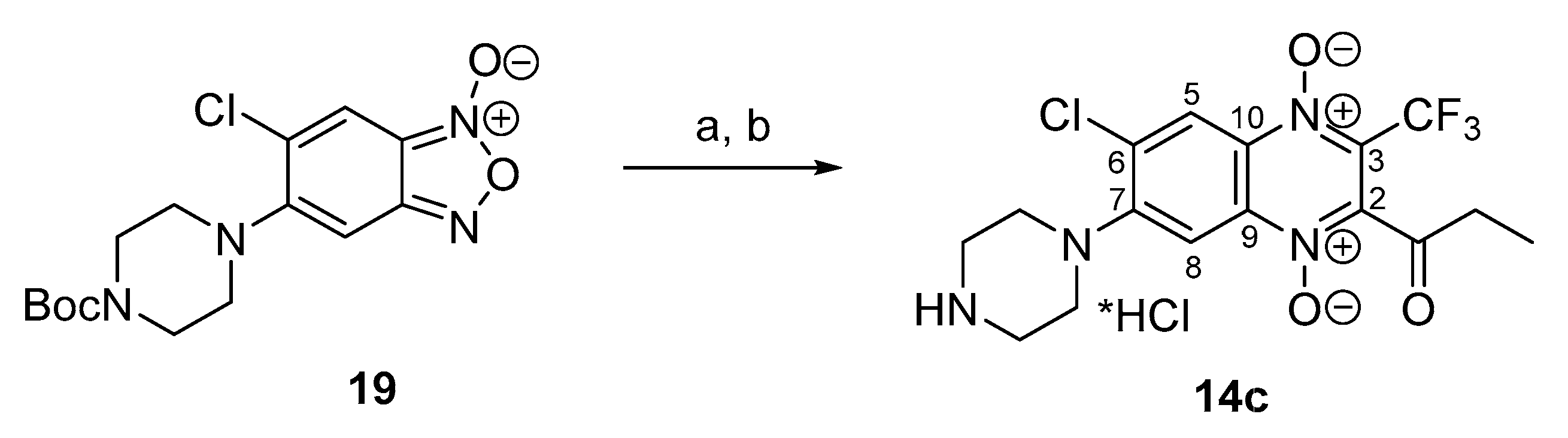
3.1.14. 6-Chloro-7-(piperazin-1-yl)-2-propionyl-3-trifluoromethylquinoxaline 1,4-Dioxide Hydrochloride (14c)
3.1.15. 2-Benzoyl-7-(piperazin-1-yl)-3-trifluoromethylquinoxaline 1,4-Dioxide Hydrochloride (15a)
3.1.16. 2-Benzoyl-6-fluoro-7-(piperazin-1-yl)-3-trifluoromethylquinoxaline 1,4-Dioxide Hydrochloride (15b)
3.1.17. 2-Benzoyl-6-chloro-7-(piperazin-1-yl)-3-trifluoromethylquinoxaline 1,4-Dioxide Hydrochloride (15c)
3.1.18. 2-Benzoyl-6,7-di(piperazin-1-yl)-3-trifluoromethylquinoxaline 1,4-Dioxide Hydrochloride (15d)
3.1.19. 2-(Furan-2-carbonyl)-7-(piperazin-1-yl)-3-trifluoromethylquinoxaline 1,4-Dioxide Hydrochloride (16a)
3.1.20. 6-Fluoro-2-(furan-2-carbonyl)-7-(piperazin-1-yl)-3-trifluoromethylquinoxaline 1,4-Dioxide Hydrochloride (16b)
3.1.21. 6-Chloro-2-(furan-2-carbonyl)-7-(piperazin-1-yl)-3-trifluoromethylquinoxaline 1,4-Dioxide Hydrochloride (16c)
3.1.22. 6,7-(Dipiperazin-1-yl)-2-(furan-2-carbonyl)-3-trifluoromethylquinoxaline 1,4-Dioxide Dihydrochloride (16d)
3.1.23. 7-(Piperazin-1-yl)-2-(thiophene-2-carbonyl)-3-trifluoromethylquinoxaline 1,4-Dioxide Hydrochloride (17a)
3.1.24. 6-Fluoro-7-(piperazin-1-yl)-2-(thiophene-2-carbonyl)-3-trifluoromethylquinoxaline 1,4-Dioxide Hydrochloride (17b)
3.1.25. 6-Chloro-7-(piperazin-1-yl)-2-(thiophene-2-carbonyl)-3-trifluoromethylquinoxaline 1,4-Dioxide Hydrochloride (17c)
3.1.26. 6,7-(Dipiperazin-1-yl)-2-(thiophene-2-carbonyl)-3-trifluoromethylquinoxaline 1,4-Dioxide Hydrochloride (17d)
3.1.27. 2-(1-Naphthoyl)-7-(piperazin-1-yl)-3-trifluoromethylquinoxaline 1,4-Dioxide Hydrochloride (18a)
3.1.28. 6-Fluoro-2-(1-naphthoyl)-7-(piperazin-1-yl)-3-trifluoromethylquinoxaline 1,4-Dioxide Hydrochloride (18b)
3.1.29. 6-Chloro-2-(1-naphthoyl)-7-(piperazin-1-yl)-3-trifluoromethylquinoxaline 1,4-Dioxide Hydrochloride (18c)
3.1.30. 2-(1-Naphthoyl)-7-(dipiperazin-1-yl)-3-trifluoromethylquinoxaline 1,4-Dioxide Hydrochloride (18d)
3.2. Biology
3.2.1. Microbial Cultures and Growth Conditions
3.2.2. Minimal Inhibitory Concentrations Determination
MIC Determination on M. smegmatis Strains
MIC Determination on M. tuberculosis Strains
MIC Determination on the Rest of the Test Strains
3.2.3. Obtaining Spontaneous Drug-Resistant M. smegmatis Mutants
3.2.4. M. smegmatis Whole-Genomic Sequencing and Analysis
3.2.5. Introduction of the Targeted Mutation in MSMEG_4883 Gene
3.2.6. Mechanism of Action Determination
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cascioferro, S.; Carbone, D.; Parrino, B.; Pecoraro, C.; Giovannetti, E.; Cirrincione, G.; Diana, P. Therapeutic strategies to counteract antibiotic resistance in MRSA biofilm-associated infections. Chem. Med. Chem. 2021, 16, 65–80. [Google Scholar] [CrossRef] [PubMed]
- Karaiskos, I.; Lagou, S.; Pontikis, K.; Rapti, V.; Poulakou, G. The “Old” and the “New” Antibiotics for MDR Gram-Negative Pathogens: For Whom, When, and How. Front. Public Health. 2019, 7, 151. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cascioferro, S.; Parrino, B.; Carbone, D.; Pecoraro, C.; Diana, P. Novel strategies in the war against antibiotic resistance. Future Med. Chem. 2021, 13, 529–531. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. World Health Organization Global Tuberculosis Report 2020; World Health Organization: Geneva, Switzerland, 2020; pp. 1–232. [Google Scholar]
- Carta, A.; Corona, P.; Loriga, M. Quinoxaline 1,4-Dioxide: A Versatile Scaffold Endowed with Manifold Activities. Curr. Med. Chem. 2005, 12, 2259–2272. [Google Scholar] [CrossRef] [PubMed]
- Cheng, G.; Sa, W.; Cao, C.; Guo, L.; Hao, H.; Liu, Z.; Wang, X.; Yuan, Z. Quinoxaline 1,4-Di-N-Oxides: Biological Activities and Mechanisms of Actions. Front. Pharmacol. 2016, 7, 64. [Google Scholar] [CrossRef] [Green Version]
- Maan, M.K.; Weng, Z.; Dai, M.; Liu, Z.; Hao, H.; Cheng, G.; Wang, Y.; Wang, X.; Huang, L. The Spectrum of Antimicrobial Activity of Cyadox against Pathogens Collected from Pigs, Chicken, and Fish in China. Antibiotics 2021, 10, 153. [Google Scholar] [CrossRef]
- Carta, A.; Paglietti, G.; Nikookar, M.E.R.; Sanna, P.; Sechi, L.; Zanetti, S. Novel Substituted Quinoxaline 1,4-Dioxides with In Vitro Antimycobacterial and Anticandida Activity. Eur. J. Med. Chem. 2002, 37, 355–366. [Google Scholar] [CrossRef]
- Constable, P.; Hinchcliff, K.W.; Done, S.; Gruenberg, W. Practical Antimicrobial Therapeutics. Vet. Med. 2017, 1, 153–174. [Google Scholar] [CrossRef]
- Vernaya, O.I.; Shabatin, V.P.; Shabatina, T.I.; Khvatov, D.I.; Semenov, A.M.; Yudina, T.P.; Danilov, V.S. Cryochemical modification, activity, and toxicity of dioxidine. Russ. J. Phys. Chem. 2017, 91, 229–232. [Google Scholar] [CrossRef]
- Carta, A.; Loriga, M.; Paglietti, G.; Mattana, A.; Fiori, P.L.; Mollicotti, P.; Sechi, L.; Zanetti, S. Synthesis, anti-mycobacterial, anti-trichomonas and anti-candida in vitro activities of 2-substituted-6,7-difluoro-3-methylquinoxaline 1,4-dioxides. Eur. J. Med. Chem. 2004, 39, 195–203. [Google Scholar] [CrossRef]
- Jaso, A.; Zarranz, B.; Aldana, I.; Monge, A. Synthesis of New Quinoxaline-2-Carboxylate 1,4-Dioxide Derivatives as Anti-Mycobacterium Tuberculosis Agents. J. Med. Chem. 2005, 48, 2019–2025. [Google Scholar] [CrossRef] [PubMed]
- Ortega, M.; Sainz, Y.; Montoya, M.; Jaso, A.; Zarranz, B.; Aldana, I.; Monge, A. Anti-Mycobacterium Tuberculosis Agents Derived from Quinoxaline-2-Carbonitrile and Quinoxaline-2-Carbonitrile 1,4-Di-N-Oxide. Arzneimittelforschung 2002, 52, 113–119. [Google Scholar] [CrossRef] [PubMed]
- Ortega, M.A.; Sainz, Y.; Montoya, M.E.; Ceráin, A.L.D.; Monge, A. Synthesis and Antituberculosis Activity of New 2-Quinoxalinecarbonitrile 1,4-Di-N-Oxides. Die Pharm. 1999, 54, 24–25. [Google Scholar] [CrossRef]
- Montoya, M.E.; Sainz, Y.; Ortega, M.A.; Cerain, A.L.D.; Monge, A. Synthesis and Antituberculosis Activity of Some New 2-Quinoxalinecarbonitriles. Il Farm. 1998, 53, 570–573. [Google Scholar] [CrossRef]
- Vicente, E.; Villar, R.; Pérez-Silanes, S.; Aldana, I.; Goldman, R.C.; Mong, A. Quinoxaline 1,4-Di-N-Oxide and the Potential for Treating Tuberculosis. Infect. Disord. Drug Targets 2011, 11, 196–204. [Google Scholar] [CrossRef] [Green Version]
- Zarranz, B.; Jaso, A.; Aldana, I.; Monge, A. Synthesis and Antimycobacterial Activity of New Quinoxaline-2-Carboxamide 1,4-Di-N-Oxide Derivatives. Bioorg. Med. Chem. 2003, 11, 2149–2156. [Google Scholar] [CrossRef] [Green Version]
- Landelle, G.; Panossian, A.; Leroux, F.R. Trifluoromethyl ethers and -thioethers as tools for medicinal chemistry and drug discovery. Curr. Top. Med. Chem. 2014, 14, 941–951. [Google Scholar] [CrossRef]
- Zanda, M. Trifluoromethyl Group: An Effective Xenobiotic Function for Peptide Backbone Modification. New J. Chem. 2004, 28, 1401–1411. [Google Scholar] [CrossRef]
- Santivañez-Veliz, M.; Pérez-Silanes, S.; Torres, E.; Moreno-Viguri, E. Design and Synthesis of Novel Quinoxaline Derivatives as Potential Candidates for Treatment of Multidrug-Resistant and Latent Tuberculosis. Bioorg. Med. Chem. Lett. 2016, 26, 2188–2193. [Google Scholar] [CrossRef] [Green Version]
- Buravchenko, G.I.; Scherbakov, A.M.; Dezhenkova, L.G.; Bykov, E.E.; Solovieva, S.E.; Korlukov, A.A.; Sorokin, D.V.; Fidalgo, L.M.; Shchekotikhin, A.E. Discovery of Derivatives of 6(7)-Amino-3-Phenylquinoxaline-2-Carbonitrile 1,4-Dioxides: Novel, Hypoxia-Selective HIF-1α Inhibitors with Strong Antiestrogenic Potency. Bioorg. Chem. 2020, 104, 104324. [Google Scholar] [CrossRef]
- Benitez, D.; Cabrera, M.; Hernández, P.; Boiani, L.; Lavaggi, M.L.; Maio, R.D.; Yaluff, G.; Serna, E.; Torres, S.; Ferreira, M.E.; et al. 3-Trifluoromethylquinoxaline N,N′-Dioxides as Anti-Trypanosomatid Agents. Identification of Optimal Anti-T. Cruzi Agents and Mechanism of Action Studies. J. Med. Chem. 2011, 54, 3624–3636. [Google Scholar] [CrossRef] [PubMed]
- Zarranz, B.; Jaso, A.; Aldana, I.; Monge, A. Synthesis and Anticancer Activity Evaluation of New 2-Alkylcarbonyl and 2-Benzoyl-3-Trifluoromethyl-Quinoxaline 1,4-Di-N-Oxide Derivatives. Bioorg. Med. Chem. 2004, 12, 3711–3721. [Google Scholar] [CrossRef] [PubMed]
- Torres, E.; Moreno-Viguri, E.; Galiano, S.; Devarapally, G.; Crawford, P.W.; Azqueta, A.; Arbillaga, L.; Varela, J.; Birriel, E.; Maio, R.D.; et al. Novel Quinoxaline 1,4-Di-N-Oxide Derivatives as New Potential Antichagasic Agents. Eur. J. Med. Chem. 2013, 66, 324–334. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Solano, B.; Junnotula, V.; Marín, A.; Villar, R.; Burguete, A.; Vicente, E.; Pérez-Silanes, S.; Aldana, I.; Monge, A.; Dutta, S.; et al. Synthesis and Biological Evaluation of New 2-Arylcarbonyl-3-Trifluoromethylquinoxaline 1,4-Di-N-Oxide Derivatives and Their Reduced Analogues. J. Med. Chem. 2007, 50, 5485–5492. [Google Scholar] [CrossRef]
- Kotovskaya, S.K.; Romanova, S.A.; Charushin, V.N.; Kodess, M.I.; Chupakhin, O.N. 5(6)-Fluoro-6(5)-R-Benzofuroxans: Synthesis and NMR 1H, 13C and 19F Studies. J. Fluor. Chem. 2004, 125, 421–428. [Google Scholar] [CrossRef]
- Jovené, C.; Jacquet, M.; Marrot, J.; Bourdreux, F.; Kletsky, M.E.; Burov, O.N.; Gonçalves, A.-M.; Goumont, R. Revisiting the Synthesis of 4,6-Difluorobenzofuroxan: A Study of Its Reactivity and Access to Fluorinated Quinoxaline Oxides: Revisiting the Synthesis of 4,6-Difluorobenzofuroxan. Eur. J. Org. Chem. 2014, 2014, 6451–6466. [Google Scholar] [CrossRef]
- Buravchenko, G.I.; Scherbakov, A.M.; Korlukov, A.A.; Dorovatovskii, P.V.; Shchekotikhin, A.E. Revision of the Regioselectivity of the Beirut Reaction of Monosubstituted Benzofuroxans with Benzoylacetonitrile. 6-Substituted Quinoxaline-2-Carbonitrile 1,4-Dioxides: Structural Characterization and Estimation of Anticancer Activity and Hypoxia Selectivity. Curr. Org. Synth. 2020, 17, 29–39. [Google Scholar] [CrossRef]
- Hansen, P.E. 13C-NMR of Polycyclic Aromatic Compounds. A Review. Org. Magn. Reson. 1979, 12, 109–142. [Google Scholar] [CrossRef]
- Imming, P.; Sinning, C.; Meyer, A. Drugs, Their Targets and the Nature and Number of Drug Targets. Nat. Rev. Drug Discov. 2006, 5, 821–834. [Google Scholar] [CrossRef]
- Buravchenko, G.I.; Scherbakov, A.M.; Dezhenkova, L.G.; Fidalgo, L.; Shchekotikhin, A.E. Synthesis of 7-Amino-6-Halogeno-3-Phenylquinoxaline-2-Carbonitrile 1,4-Dioxides: A Way Forward for Targeting Hypoxia and Drug Resistance of Cancer Cells. RSC Adv. 2021, 11, 38782–38795. [Google Scholar] [CrossRef]
- Pérez-Silanes, S.; Devarapally, G.; Torres, E.; Moreno-Viguri, E.; Aldana, I.; Monge, A.; Crawford, P.W. Cyclic Voltammetric Study of Some Anti-Chagas-Active 1,4-Dioxidoquinoxalin-2-yl Ketone Derivatives. Helv. Chim. Acta 2013, 96, 217–227. [Google Scholar] [CrossRef]
- Pérez-Silanes, S.; Torres, E.; Arbillaga, L.; Varela, J.; Cerecetto, H.; González, M.; Azqueta, A.; Moreno-Viguri, E. Synthesis and Biological Evaluation of Quinoxaline Di-N-Oxide Derivatives with in vitro Trypanocidal Activity. Bioorg. Med. Chem. Lett. 2016, 26, 903–906. [Google Scholar] [CrossRef] [PubMed]
- Pretsch, E.; Bühlmann, P.; Affolter, C. Structure Determination of Organic Compounds, 4th ed.; Springer: Berlin/Heideberg, Germany, 2009; pp. 154–196. [Google Scholar]
- Zhang, H.; Lu, Q.; Zhang, J.; Qu, W.; Xie, S.; Huang, L.; Yuan, Z.; Pan, Y. Discovery of Novel Nitrogenous Heterocyclic-Containing Quinoxaline-1,4-Di-N-Oxides as Potent Activator of Autophagy in M.Tb-Infected Macrophages. Eur. J. Med. Chem. 2021, 223, 113657. [Google Scholar] [CrossRef]
- Soliman, D.H. Synthesis, Characterization, Anti-Bacterial and Anti-Fungal Activities of New Quinoxaline 1,4-Di-N-Oxide Derivatives. Int. J. Org. Chem. 2013, 3, 65–72. [Google Scholar] [CrossRef] [Green Version]
- Mazanko, M.S.; Chistyakov, V.A.; Prazdnova, E.V.; Pokudina, I.O.; Churilov, M.N.; Chmyhalo, V.K.; Batyushin, M.M. Dioxidine induces bacterial resistance to antibiotics. Mol. Gen. Microbiol. Virol. 2016, 31, 227–232. [Google Scholar] [CrossRef]
- Osterman, I.A.; Komarova, E.S.; Shiryaev, D.I.; Korniltsev, I.A.; Khven, I.M.; Lukyanov, D.A.; Tashlitsky, V.N.; Serebryakova, M.V.; Efremenkova, O.V.; Ivanenkov, Y.A.; et al. Sorting Out Antibiotics’ Mechanisms of Action: A Double Fluorescent Protein Reporter for High-Throughput Screening of Ribosome and DNA Biosynthesis Inhibitors. Antimicrob. Agents Chemother. 2016, 60, 7481–7489. [Google Scholar] [CrossRef] [Green Version]
- Cheng, G.; Li, B.; Wang, C.; Zhang, H.; Liang, G.; Weng, Z.; Hao, H.; Wang, X.; Liu, Z.; Dai, M.; et al. Systematic and Molecular Basis of the Antibacterial Action of Quinoxaline 1,4-Di-N-Oxides against Escherichia Coli. PLoS ONE 2015, 10, e0136450. [Google Scholar] [CrossRef] [Green Version]
- Suter, W.; Rosselet, A.; Knüsel, F. Mode of Action of Quindoxin and Substituted Quinoxaline-Di-N-Oxides on Escherichia coli. Antimicrob. Agents Chemother. 1978, 13, 770–783. [Google Scholar] [CrossRef] [Green Version]
- Ganley, B.; Chowdhury, G.; Bhansali, J.; Daniels, J.S.; Gates, K.S. Redox-Activated, Hypoxia-Selective DNA Cleavage by Quinoxaline 1,4-Di-N-Oxide. Bioorg. Med. Chem. 2001, 9, 2395–2401. [Google Scholar] [CrossRef]
- Maslov, D.A.; Shur, K.V.; Vatlin, A.A.; Danilenko, V.N. MmpS5-MmpL5 Transporters Provide Mycobacterium smegmatis Resistance to Imidazo[1,2-b][1,2,4,5]Tetrazines. Pathogens 2020, 9, 166. [Google Scholar] [CrossRef] [Green Version]
- Andries, K.; Villellas, C.; Coeck, N.; Thys, K.; Gevers, T.; Vranckx, L.; Lounis, N.; de Jong, B.C.; Koul, A. Acquired Resistance of Mycobacterium tuberculosis to Bedaquiline. PLoS ONE 2014, 9, e102135. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Milano, A.; Pasca, M.R.; Provvedi, R.; Lucarelli, A.P.; Manina, G.; Ribeiro, A.L.d.J.L.; Manganelli, R.; Riccardi, G. Azole Resistance in Mycobacterium tuberculosis Is Mediated by the MmpS5–MmpL5 Efflux System. Tuberculosis 2009, 89, 84–90. [Google Scholar] [CrossRef] [PubMed]
- Li, X.-Z.; Zhang, L.; Nikaido, H. Efflux Pump-Mediated Intrinsic Drug Resistance in Mycobacterium smegmatis. Antimicrob. Agents Chemother. 2004, 48, 2415–2423. [Google Scholar] [CrossRef] [Green Version]
- Frolova, S.G.; Klimina, K.M.; Kumar, R.; Vatlin, A.A.; Salunke, D.B.; Kendrekar, P.; Danilenko, V.N.; Maslov, D.A. Identification of Mutations Conferring Tryptanthrin Resistance to Mycobacterium smegmatis. Antibiotics 2021, 10, 6. [Google Scholar] [CrossRef] [PubMed]
- Sharp, J.D.; Singh, A.K.; Park, S.T.; Lyubetskaya, A.; Peterson, M.W.; Gomes, A.L.C.; Potluri, L.-P.; Raman, S.; Galagan, J.E.; Husson, R.N. Comprehensive Definition of the SigH Regulon of Mycobacterium tuberculosis Reveals Transcriptional Control of Diverse Stress Responses. PLoS ONE 2016, 11, e0152145. [Google Scholar] [CrossRef] [Green Version]
- Rawat, M.; Av-Gay, Y. Mycothiol-dependent Proteins in Actinomycetes. FEMS Microbiol. Rev. 2007, 31, 278–292. [Google Scholar] [CrossRef]
- Gavalda, S.; Bardou, F.; Laval, F.; Bon, C.; Malaga, W.; Chalut, C.; Guilhot, C.; Mourey, L.; Daffé, M.; Quémard, A. The Polyketide Synthase Pks13 Catalyzes a Novel Mechanism of Lipid Transfer in Mycobacteria. Chem. Biol. 2014, 21, 1660–1669. [Google Scholar] [CrossRef] [Green Version]
- Gaughran, R.J.; Picard, J.P.; Kaufman, J.V.R. Contribution to the Chemistry of Benzfuroxan and Benzfurazan Derivatives. J. Am. Chem. Soc. 1954, 76, 2233–2236. [Google Scholar] [CrossRef]
- Liu, Y.; Gao, Y.; Liu, J.; Tan, Y.; Liu, Z.; Chhotaray, C.; Jiang, H.; Lu, Z.; Chiwala, G.; Wang, S.; et al. The Compound TB47 Is Highly Bactericidal against Mycobacterium ulcerans in a Buruli Ulcer Mouse Model. Nat. Commun. 2019, 10, 524. [Google Scholar] [CrossRef] [Green Version]
- Liu, Q.; Liu, Z.; Sun, C.; Shao, M.; Ma, J.; Wei, X.; Zhang, T.; Li, W.; Ju, J. Discovery and Biosynthesis of Atrovimycin, an Antitubercular and Antifungal Cyclodepsipeptide Featuring Vicinal-Dihydroxylated Cinnamic Acyl Chain. Org. Lett. 2019, 21, 2634–2638. [Google Scholar] [CrossRef]
- Maslov, D.A.; Korotina, A.V.; Shur, K.V.; Vatlin, A.A.; Bekker, O.B.; Tolshchina, S.G.; Ishmetova, R.I.; Ignatenko, N.K.; Rusinov, G.L.; Charushin, V.N.; et al. Synthesis and Antimycobacterial Activity of Imidazo[1,2-b][1,2,4,5]Tetrazines. Eur. J. Med. Chem. 2019, 178, 39–47. [Google Scholar] [CrossRef] [PubMed]
- CLSI. Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically, 11th ed.; CLSI Standard M07; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2018. [Google Scholar]
- CLSI. Reference Method for Broth Dilution Antifungal Susceptibility Testing of Yeasts, 4th ed.; CLSI Standard M27; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2017. [Google Scholar]
- CLSI. Reference Method for Broth Dilution Antifungal Susceptibility Testing of Filamentous Fungi, 3rd ed.; CLSI Standard M38; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2017. [Google Scholar]
- Andries, K.; Verhasselt, P.; Guillemont, J.; Göhlmann, H.W.H.; Neefs, J.-M.; Winkler, H.; Gestel, J.V.; Timmerman, P.; Zhu, M.; Lee, E.; et al. A Diarylquinoline Drug Active on the ATP Synthase of Mycobacterium tuberculosis. Science 2005, 307, 223–227. [Google Scholar] [CrossRef] [PubMed]
- O’Malley, T.; Melief, E. Isolation and Characterization of Compound-Resistant Isolates of Mycobacterium tuberculosis. Methods Mol. Biol. 2015, 1285, 317–328. [Google Scholar] [CrossRef]
- Belisle, J.T.; Mahaffey, S.B.; Hill, P.J. Isolation of Mycobacterium Species Genomic DNA. In Mycobacteria Protocols; Humana Press: Totowa, NJ, USA, 2010; Volume 465, pp. 1–12. [Google Scholar] [CrossRef]
- Andrews, S. FastQC: A Quality Control Tool for High Throughput Sequence Data. 2010. Available online: http://www.bioinformatics.babraham.ac.uk/projects/fastqc/ (accessed on 18 April 2020).
- Li, H.; Durbin, R. Fast and Accurate Long-Read Alignment with Burrows-Wheeler Transform. Bioinformatics 2010, 26, 589–595. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, H. A Statistical Framework for SNP Calling, Mutation Discovery, Association Mapping and Population Genetical Parameter Estimation from Sequencing Data. Bioinformatics 2011, 27, 2987–2993. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koboldt, D.C.; Zhang, Q.; Larson, D.E.; Shen, D.; McLellan, M.D.; Lin, L.; Miller, C.A.; Mardis, E.R.; Ding, L.; Wilson, R.K. VarScan 2: Somatic Mutation and Copy Number Alteration Discovery in Cancer by Exome Sequencing. Genome Res. 2012, 22, 568–576. [Google Scholar] [CrossRef] [Green Version]
- Parish, T.; Stoker, N.G. Use of a Flexible Cassette Method to Generate a Double Unmarked Mycobacterium Tuberculosis TlyA PlcABC Mutant by Gene Replacement. Microbiology 2000, 146, 1969–1975. [Google Scholar] [CrossRef] [Green Version]
- Goude, R.; Parish, T. Electroporation of Mycobacteria. Methods Mol. Biol. 2010, 465, 203–215. [Google Scholar] [CrossRef]
- Ye, J.; Coulouris, G.; Zaretskaya, I.; Cutcutache, I.; Rozen, S.; Madden, T.L. Primer-BLAST: A Tool to Design Target-Specific Primers for Polymerase Chain Reaction. BMC Bioinform. 2012, 13, 134. [Google Scholar] [CrossRef] [Green Version]
- Shchekotikhin, A.E.; Buravchenko, G.I.; Scherbakov, A.M. Method for Inhibiting Tumor Cells with New Derivatives of 3-Trifluoromethylquinoxaline 1,4-Dioxide. Patent RU 2746395, 13 April 2021. [Google Scholar]



| Strain/MIC (Minimum Inhibitory Concentration, μg/mL) * | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cmpnd | R1 | R2 | M. smegmatis mc2 155 | M. tuberculosis AlRa | M. tuberculosis UAlRv | S. aureus ATCC 29213 | S. epidermidis ATCC 14990 | E. faecalis ATCC 29212 | E. coli ATCC 25922 | K. pneumoniae 1951 | P. aeruginosa ATCC 27853 | C. albicans ATCC 10231 | M. canis B-200 |
| 12a | OEt | H | 4 | 20 | 20 | 4 | 2 | 8 | 4 | 16 | >32 | >32 | >32 |
| 12b | OEt | F | 8 | − | − | 8 | 4 | 8 | 32 | >32 | >32 | 16 | 32 |
| 12c | OEt | Cl | 4 | >20 | 10 | 2 | 0.5 | 2 | 32 | >32 | >32 | 8 | 16 |
| 13a | Me | H | 4 | 20 | 20 | 4 | 2 | 4 | 2 | 8 | >32 | >32 | >32 |
| 13b | Me | F | 4 | 10 | 5 | 1 | 1 | 1 | 1 | 8 | >32 | 8 | >32 |
| 13c | Me | Cl | 4 | 10 | 5 | 1 | 0.25 | 0.5 | 2 | 8 | 32 | 2 | 32 |
| 14a | Et | H | 2 | 20 | 20 | 2 | 2 | 4 | 8 | 32 | >32 | >32 | >32 |
| 14b | Et | F | 8 | 20 | 20 | 2 | 1 | 2 | 4 | 16 | >32 | 16 | >32 |
| 14c | Et | Cl | 8 | >20 | 10 | 2 | 1 | 2 | 8 | >32 | >32 | 4 | >32 |
| 15a | Ph | H | 8 | − | − | 8 | 4 | 4 | 32 | >32 | >32 | >32 | 32 |
| 15b | Ph | F | 8 | 10 | 5 | 2 | 0.5 | 1 | 16 | >32 | >32 | 4 | 8 |
| 15c | Ph | Cl | 8 | 10 | 5 | 2 | 0.25 | 1 | 32 | >32 | >32 | 32 | 8 |
| 15d | Ph |  | 8 | >20 | >20 | 16 | 8 | 16 | >32 | >32 | 16 | 32 | >32 |
| 16a | 2-Furyl | H | >32 | >20 | >20 | 4 | 2 | 4 | 8 | 32 | >32 | >32 | >32 |
| 16b | 2-Furyl | F | 8 | 20 | 10 | 2 | 0.5 | 1 | 8 | 32 | >32 | 32 | 32 |
| 16c | 2-Furyl | Cl | 4 | 20 | 10 | 1 | 0.5 | 1 | 8 | >32 | >32 | >32 | 16 |
| 16d | 2-Furyl |  | 16 | >20 | >20 | >32 | >32 | >32 | >32 | >32 | 32 | 32 | >32 |
| 17a | 2-Tienyl | H | 8 | − | − | 16 | 4 | 16 | >32 | >32 | >32 | 32 | >32 |
| 17b | 2-Tienyl | F | 16 | − | − | 2 | 1 | 1 | >32 | >32 | >32 | 8 | >32 |
| 17c | 2-Tienyl | Cl | 8 | − | − | 2 | 0.5 | 1 | >32 | >32 | >32 | 2 | 32 |
| 17d | 2-Tienyl |  | 16 | − | − | >32 | 32 | 32 | >32 | >32 | >32 | 16 | >32 |
| 18a | 2-Naphtyl | H | 4 | − | − | 16 | 4 | 8 | >32 | >32 | >32 | 8 | 32 |
| 18b | 2-Naphtyl | F | 8 | − | − | 2 | 0.5 | 2 | >32 | >32 | >32 | 2 | 8 |
| 18c | 2-Naphtyl | Cl | 32 | − | − | 8 | 4 | 4 | >32 | >32 | >32 | 4 | 16 |
| 18d | 2-Naphtyl |  | 8 | − | − | 16 | 4 | 8 | 16 | 32 | 32 | 4 | 16 |
| DIOX | 32 | 10 | 20 | >32 | >32 | >32 | >32 | >32 | >32 | >32 | >32 | ||
| CIP | − | − | − | 0.125 | 0.125 | 0.5 | 0.006 | 0.25 | 0.125 | − | − | ||
| AMPH | − | − | − | − | − | − | − | − | − | 0.75 | 2 | ||
| RIF | 4 | 0.03 | 0.03 | 0.008 | 0.004 | 0.5 | 8 | − | − | − | − | ||
| Cmpnd | M. smegmatis Strains/MIC (μg/mL) | ||||||
|---|---|---|---|---|---|---|---|
| mc2 155 | tfqR1 | tfqR2 | tfqR4 | tfqR5 | tfqR6 | tfqR7 | |
| 12a | 4 | >32 | >32 | >32 | 16 | 8 | 8 |
| 13a | 4 | >32 | 32 | 16 | 16 | 4 | 4 |
| 13b | 4 | >32 | 32 | 16 | 16 | 8 | 8 |
| 13c | 4 | >32 | 32 | 16 | 16 | 16 | 8 |
| 15a | 8 | >32 | >32 | >32 | 8 | 8 | 16 |
| 15b | 8 | >32 | >32 | >32 | 8 | 16 | 16 |
| 15c | 8 | >32 | >32 | >32 | 8 | 16 | 16 |
| 15d | 8 | >32 | >32 | 32 | 8 | 8 | 8 |
| 16b | 8 | >32 | 8 | >32 | 8 | 8 | 16 |
| 16c | 4 | >32 | >32 | >32 | 8 | 8 | 8 |
| 17a | 16 | >32 | 16 | 32 | 8 | 16 | 8 |
| 17b | 16 | >32 | 16 | 32 | 8 | 8 | 16 |
| 17c | 8 | >32 | 16 | >32 | 8 | 16 | 16 |
| 18a | 4 | >32 | 32 | >32 | >32 | 16 | 16 |
| DIOX | 32 | >32 | 32 | >32 | >32 | >32 | 32 |
| Microbial Strains | ||
|---|---|---|
| Name | Comment | Origin |
| M. smegmatis mc2 155 | Wild-type (w.t.) strain | |
| M. smegmatis atR9c | Recombinant strain, mutation: ins C8 (frameshift) in MSMEG_1380. | [42] |
| M. smegmatis qtfR1-2, qtfR4-7 | Spontaneous M. smegmatis mutants, resistant to the compounds 15b and 16b | This study |
| M. smegmatis 4883c | Recombinant M. smegmatis strain, harboring a deletion of CGCTGCTGC176–184 in MSMEG_4883 (ALLP58–61 -> V58). | This study |
| M. tuberculosis UAlRv | Autoluminiscent M. tuberculosis H37Rv strain. | [51] |
| M. tuberculosis AlRa | Autoluminiscent M. tuberculosis H37Ra strain. | [52] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Buravchenko, G.I.; Maslov, D.A.; Alam, M.S.; Grammatikova, N.E.; Frolova, S.G.; Vatlin, A.A.; Tian, X.; Ivanov, I.V.; Bekker, O.B.; Kryakvin, M.A.; et al. Synthesis and Characterization of Novel 2-Acyl-3-trifluoromethylquinoxaline 1,4-Dioxides as Potential Antimicrobial Agents. Pharmaceuticals 2022, 15, 155. https://doi.org/10.3390/ph15020155
Buravchenko GI, Maslov DA, Alam MS, Grammatikova NE, Frolova SG, Vatlin AA, Tian X, Ivanov IV, Bekker OB, Kryakvin MA, et al. Synthesis and Characterization of Novel 2-Acyl-3-trifluoromethylquinoxaline 1,4-Dioxides as Potential Antimicrobial Agents. Pharmaceuticals. 2022; 15(2):155. https://doi.org/10.3390/ph15020155
Chicago/Turabian StyleBuravchenko, Galina I., Dmitry A. Maslov, Md Shah Alam, Natalia E. Grammatikova, Svetlana G. Frolova, Aleksey A. Vatlin, Xirong Tian, Ivan V. Ivanov, Olga B. Bekker, Maxim A. Kryakvin, and et al. 2022. "Synthesis and Characterization of Novel 2-Acyl-3-trifluoromethylquinoxaline 1,4-Dioxides as Potential Antimicrobial Agents" Pharmaceuticals 15, no. 2: 155. https://doi.org/10.3390/ph15020155
APA StyleBuravchenko, G. I., Maslov, D. A., Alam, M. S., Grammatikova, N. E., Frolova, S. G., Vatlin, A. A., Tian, X., Ivanov, I. V., Bekker, O. B., Kryakvin, M. A., Dontsova, O. A., Danilenko, V. N., Zhang, T., & Shchekotikhin, A. E. (2022). Synthesis and Characterization of Novel 2-Acyl-3-trifluoromethylquinoxaline 1,4-Dioxides as Potential Antimicrobial Agents. Pharmaceuticals, 15(2), 155. https://doi.org/10.3390/ph15020155