Lurasidone versus Quetiapine for Cognitive Impairments in Young Patients with Bipolar Depression: A Randomized, Controlled Study
Abstract
1. Introduction
2. Results
2.1. Subject Characteristics
2.2. Primary Outcome
Cognitive Function
2.3. Secondary Outcome
2.3.1. Depression Severity
2.3.2. Clinical Response Rate
2.3.3. Clinical Remission Rate
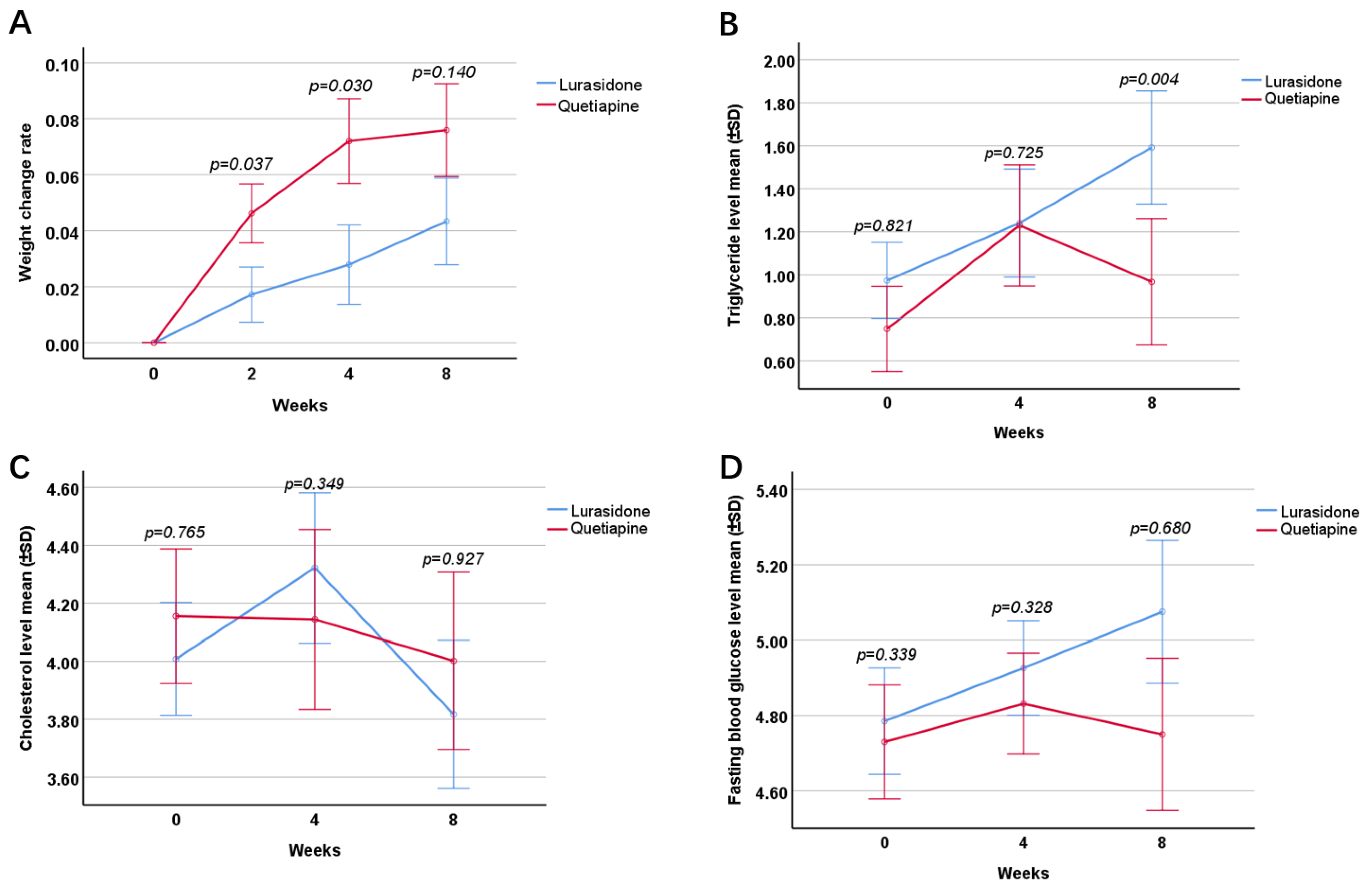
2.3.4. Rate of Change in Body Weight
2.3.5. Triglyceride Level
2.3.6. Cholesterol Level
2.3.7. Fasting Blood Glucose Level
3. Discussion
4. Methods and Materials
4.1. Participants
4.2. Study Design
4.3. Concomitant Medications
4.4. Assessment of Cognitive Function
4.5. Assessment of Depressive Levels
4.6. Assessment of Weight and Serum Metabolic Profiles
4.7. Safety and Tolerability
4.8. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| LSM | least squares mean |
| CI | confidence interval |
| SD | standard deviation |
| PDQ-5-D | 5-item Perceived Deficits Questionnaire for Depression |
References
- Baldessarini, R.J.; Bolzani, L.; Cruz, N.; Jones, P.; Lai, M.; Lepri, B.; Perez, J.; Salvatore, P.; Tohen, M.; Tondo, L. Onset-age of bipolar disorders at six international sites. J. Affect. Disord. 2010, 121, 143–146. [Google Scholar] [CrossRef] [PubMed]
- Chengappa, K.N.R.; Kupfer, D.J.; Frank, E.; Houck, P.R.; Grochocinski, V.J.; Cluss, P.A.; Stapf, D.A. Relationship of Birth Cohort and Early Age at Onset of Illness in a Bipolar Disorder Case Registry. Am. J. Psychiatry 2003, 160, 1636–1642. [Google Scholar] [CrossRef] [PubMed]
- Perlis, R.H.; Miyahara, S.; Marangell, L.B.; Wisniewski, S.R.; Ostacher, M.; DelBello, M.P.; Bowden, C.L.; Sachs, G.S.; Nierenberg, A.A. STEP-BD Investigators: Long-term implications of early onset in bipolar disorder: Data from the fifirst 1000 participants in the systematic treatment enhancement program for bipolar disorder (STEP-BD). Biol. Psychiatry 2004, 55, 875–881. [Google Scholar] [CrossRef]
- Algorta, G.P.; Youngstrom, E.A.; Frazier, T.W.; Freeman, A.J.; Youngstrom, J.K.; Findling, R.L. Suicidality in pediatric bipolar disorder: Predictor or outcome of family processes and mixed mood presentation? Bipolar Disord. 2011, 13, 76–86. [Google Scholar] [CrossRef] [PubMed]
- Joslyn, C.; Hawes, D.J.; Hunt, C.; Mitchell, P.B. Is age of onset associated with severity, prognosis, and clinical features in bipolar disorder? A meta-analytic review. Bipolar Disord. 2016, 18, 389–403. [Google Scholar] [CrossRef]
- Bonnin, C.M.; Torrent, C.; Goikolea, J.M.; Reinares, M.; Solé, B.; Valenti, M.; Sanchez-Moreno, J.; Hidalgo, D.; Tabarés-Seisdedos, R.; Martinez-Aran, A.; et al. The impact of repeated manic episodes and executive dysfunction on work adjustment in bipolar disorder. Eur. Arch. Psychiatry Clin. Neurosci. 2014, 264, 247–254. [Google Scholar] [CrossRef]
- Santos, J.L.; Aparicio, A.; Bagney, A.; Sánchez-Morla, E.M.; Rodríguez-Jiménez, R.; Mateo, J.; Jiménez-Arriero, M. A five-year follow-up study of neurocognitive functioning in bipolar disorder. Bipolar Disord. 2014, 16, 722–731. [Google Scholar] [CrossRef]
- Gong, J.; Wang, J.; Qiu, S.; Chen, P.; Luo, Z.; Wang, J.; Huang, L.; Wang, Y. Common and distinct patterns of intrinsic brain activity alterations in major depression and bipolar disorder: Voxel-based meta-analysis. Transl. Psychiatry 2020, 10, 353. [Google Scholar] [CrossRef]
- Martinez-Aran, A.; Vieta, E.; Reinares, M.; Colom, F.; Torrent, C.; Sanchez-Moreno, J.; Benabarre, A.; Goikolea, J.M.; Comes, M.; Salamero, M. Cognitive Function Across Manic or Hypomanic, Depressed, and Euthymic States in Bipolar Disorder. Am. J. Psychiatry 2004, 161, 262–270. [Google Scholar] [CrossRef]
- Wingo, A.P.; Harvey, P.D.; Baldessarini, R.J. Neurocognitive impairment in bipolar disorder patients: Functional implications. Bipolar Disord. 2009, 11, 113–125. [Google Scholar] [CrossRef]
- Goldstein, B.I.; Carnethon, M.R.; Matthews, K.A.; McIntyre, R.S.; Miller, G.E.; Raghuveer, G.; Stoney, C.M.; Wasiak, H.; McCrindle, B.W. American Heart Association Atherosclerosis; Hypertension and Obesity in Youth Committee of the Council on Cardiovascular Disease in the Young: Major depressive disorder and bipolar disorder predispose youth to accelerated atherosclerosis and early cardiovascular disease. A scientifific statement from the American Heart Association. Circulation 2015, 132, 965–986. [Google Scholar] [PubMed]
- Goldstein, B.I.; Baune, B.T.; Bond, D.J.; Chen, P.; Eyler, L.; Fagiolini, A.; Gomes, F.; Hajek, T.; Hatch, J.; McElroy, S.L.; et al. Call to action regarding the vascular-bipolar link: A report from the Vascular Task Force of the International Society for Bipolar Disorders. Bipolar Disord. 2020, 22, 440–460. [Google Scholar] [CrossRef]
- Willett, W.C.; Manson, J.E.; Stampfer, M.J.; Colditz, G.A.; Rosner, B.; Speizer, F.E.; Hennekens, C.H. Weight, weight change, and coronary heart disease in women. Risk within the ‘normal’ weight range. JAMA 1995, 273, 461–465. [Google Scholar] [CrossRef] [PubMed]
- Kenchaiah, S.; Evans, J.C.; Levy, D.; Wilson, P.W.; Benjamin, E.J.; Larson, M.G.; Kannel, W.B.; Vasan, R.S. Obesity and the Risk of Heart Failure. N. Engl. J. Med. 2002, 347, 305–313. [Google Scholar] [CrossRef] [PubMed]
- Cooper, S.J.; Reynolds, G.; Barnes, T.R.E.; England, E.; Haddad, P.; Heald, A.; Holt, R.; Lingford-Hughes, A.; Osborn, D.; McGowan, O.; et al. BAP guidelines on the management of weight gain, metabolic disturbances and cardiovascular risk associated with psychosis and antipsychotic drug treatment. J. Psychopharmacol. 2016, 30, 717–748. [Google Scholar] [CrossRef]
- Carvalho, A.F.; Firth, J.; Vieta, E. Bipolar Disorder. N. Engl. J. Med. 2020, 383, 58–66. [Google Scholar] [CrossRef]
- McIntyre, R.S.; Cha, D.S.; Kim, R.D.; Mansur, R.B. A review of FDA-approved treatment options in bipolar depression. CNS Spectr. 2013, 18 (Suppl. 1), 1–21. [Google Scholar] [CrossRef]
- DelBello, M.P.; Goldman, R.; Phillips, D.; Deng, L.; Cucchiaro, J.; Loebel, A. Efficacy and Safety of Lurasidone in Children and Adolescents With Bipolar I Depression: A Double-Blind, Placebo-Controlled Study. J. Am. Acad. Child Adolesc. Psychiatry 2017, 56, 1015–1025. [Google Scholar] [CrossRef]
- Cifariello, A.; Pompili, A.; Gasbarri, A. 5-HT7 receptors in the modulation of cognitive processes. Behav. Brain Res. 2008, 195, 171–179. [Google Scholar] [CrossRef]
- Enomoto, T.; Ishibashi, T.; Tokuda, K.; Ishiyama, T.; Toma, S.; Ito, A. Lurasidone reverses MK-801-induced impairment of learning and memory in the Morris water maze and radial-arm maze tests in rats. Behav. Brain Res. 2008, 186, 197–207. [Google Scholar] [CrossRef]
- Ishiyama, T.; Tokuda, K.; Ishibashi, T.; Ito, A.; Toma, S.; Ohno, Y. Lurasidone (SM-13496), a novel atypical antipsychotic drug, reverses MK-801-induced impairment of learning and memory in the rat passive-avoidance test. Eur. J. Pharmacol. 2007, 572, 160–170. [Google Scholar] [CrossRef] [PubMed]
- Harvey, P.D.; Siu, C.O.; Hsu, J.; Cucchiaro, J.; Maruff, P.; Loebel, A. Effect of lurasidone on neurocognitive performance in patients with schizophrenia: A short-term placebo- and active-controlled study followed by a 6-month double-blind extension. Eur. Neuropsychopharmacol. 2013, 23, 1373–1382. [Google Scholar] [CrossRef] [PubMed]
- Meltzer, H.Y.; Share, D.B.; Jayathilake, K.; Salomon, R.M.; Lee, M.A. Lurasidone Improves Psychopathology and Cognition in Treatment-Resistant Schizophrenia. J. Clin. Psychopharmacol. 2020, 40, 240–249. [Google Scholar] [CrossRef]
- Dickstein, D.P.; Treland, J.E.; Snow, J.; McClure, E.B.; Mehta, M.S.; Towbin, K.E.; Pine, D.S.; Leibenluft, E. Neuropsychological performance in pediatric bipolar disorder. Biol. Psychiatry 2004, 55, 32–39. [Google Scholar] [CrossRef]
- Doyle, A.E.; Wilens, T.E.; Kwon, A.; Seidman, L.J.; Faraone, S.V.; Fried, R.; Swezey, A.; Snyder, L.; Biederman, J. Neuropsychological Functioning in Youth with Bipolar Disorder. Biol. Psychiatry 2005, 58, 540–548. [Google Scholar] [CrossRef] [PubMed]
- Glahn, D.C.; Bearden, C.E.; Caetano, S.; Fonseca, M.; Najt, P.; Hunter, K.; Pliszka, S.R.; Olvera, R.L.; Soares, J.C. Declarative memory impairment in pediatric bipolar disorder. Bipolar Disord. 2005, 7, 546–554. [Google Scholar] [CrossRef] [PubMed]
- Frías, Á.; Palma, C.; Farriols, N. Neurocognitive impairments among youth with pediatric bipolar disorder: A systematic review of neuropsychological research. J. Affect. Disord. 2014, 166, 297–306. [Google Scholar] [CrossRef]
- Joseph, M.F.; Frazier, T.W.; Youngstrom, E.A.; Soares, J.C. A Quantitative and Qualitative Review of Neurocognitive Performance in Pediatric Bipolar Disorder. J. Child Adolesc. Psychopharmacol. 2008, 18, 595–605. [Google Scholar] [CrossRef]
- Viapiana, V.F.; Rodrigues, A.C.R.B.G.; Peters, R.; Tramontina, S.; Passos, I.C.; Fonseca, R.P. Pediatric bipolar disorder: Executive, linguistic, mnemonic, and cognitive efficiency mapping. Appl. Neuropsychol. Child 2021, 11, 350–363. [Google Scholar] [CrossRef]
- Biederman, J.; Seidman, L.J.; Petty, C.R.; Fried, R.; Doyle, A.E.; Cohen, D.R.; Kenealy, D.C.; Faraone, S.V. Effects of stimulant medication on neuropsychological functioning in young adults with attention-deficit/hyperactiv -ity disorder. J. Clin. Psychiatry 2008, 69, 1150–1156. [Google Scholar] [CrossRef]
- Yuen, E.Y.; Li, X.; Wei, J.; Horiguchi, M.; Meltzer, H.Y.; Yan, Z. The Novel Antipsychotic Drug Lurasidone Enhances N-Methyl-d-aspartate Receptor-Mediated Synaptic Responses. Mol. Pharmacol. 2011, 81, 113–119. [Google Scholar] [CrossRef] [PubMed]
- Horisawa, T.; Nishikawa, H.; Toma, S.; Ikeda, A.; Horiguchi, M.; Ono, M.; Ishiyama, T.; Taiji, M. The role of 5-HT7 receptor antagonism in the amelioration of MK-801-induced learning and memory deficits by the novel atypical antipsychotic drug lurasidone. Behav. Brain Res. 2013, 244, 66–69. [Google Scholar] [CrossRef] [PubMed]
- Huang, M.; Panos, J.J.; Kwon, S.; Oyamada, Y.; Rajagopal, L.; Meltzer, H.Y. Comparative effect of lurasidone and blonanserin on cortical glutamate, dopamine, and acetylcholine efflux: Role of relative serotonin (5-HT)2A and DA D2 antagonism and 5-HT1A partial agonism. J. Neurochem. 2014, 128, 938–949. [Google Scholar] [CrossRef]
- Horiguchi, M.; Huang, M.; Meltzer, H.Y. The role of 5-hydroxytryptamine 7 receptors in the phencyclidine-induced novel object recognition defificit in rats. J. Pharmacol. Exp. Ther. 2011, 338, 605–614. [Google Scholar] [CrossRef] [PubMed]
- Horiguchi, M.; Meltzer, H.Y. The Role of 5-HT1A Receptors in phencyclidine (PCP)-induced novel object recognition (NOR) defificit in rats. Psychopharmacology 2012, 221, 205–215. [Google Scholar] [CrossRef] [PubMed]
- Miyauchi, M.; Neugebauer, N.M.; Meltzer, H.Y. Dopamine D4 receptor stimulation contributes to novel object recognition: Relevance to cognitive impairment in schizophrenia. J. Psychopharmacol. 2017, 31, 442–452. [Google Scholar] [CrossRef]
- Murai, T.; Nakako, T.; Ikeda, K.; Ikejiri, M.; Ishiyama, T.; Taiji, M. Lack of dopamine D4 receptor affinity contributes to the procognitive effect of lurasidone. Behav. Brain Res. 2014, 261, 26–30. [Google Scholar] [CrossRef]
- Calabrese, F.; Luoni, A.; Guidotti, G.; Racagni, G.; Fumagalli, F.; Riva, M.A. Modulation of neuronal plasticity following chronic concomitant administration of the novel antipsychotic lurasidone with the mood stabilizer valproic acid. Psychopharmacology 2012, 226, 101–112. [Google Scholar] [CrossRef]
- Cattaneo, A.; Suderman, M.; Cattane, N.; Mazzelli, M.; Begni, V.; Maj, C.; D’Aprile, I.; Pariante, C.M.; Luoni, A.; Berry, A.; et al. Long-term effects of stress early in life on microRNA-30a and its network: Preventive effects of lurasidone and potential implications for depression vulnerability. Neurobiol. Stress 2020, 13, 100271. [Google Scholar] [CrossRef]
- Fumagalli, F.; Calabrese, F.; Luoni, A.; Bolis, F.; Racagni, G.; Riva, M.A. Modulation of BDNF expression by repeated treatment with the novel antipsychotic lurasidone under basal condition and in response to acute stress. Int. J. Neuropsychopharmacol. 2011, 15, 235–246. [Google Scholar] [CrossRef]
- Luoni, A.; Rocha, F.F.; Riva, M.A. Anatomical specifi city in the modulation of activity-regulated genes after acute or chronic lurasidone treatment. Prog. Neuropsychopharmacol. Biol. Psychiatry 2014, 50, 94–101. [Google Scholar] [CrossRef] [PubMed]
- Detke, H.C.; DelBello, M.P.; Landry, J.; Usher, R.W. Olanzapine/Fluoxetine Combination in Children and Adolescents With Bipolar I Depression: A Randomized, Double-Blind, Placebo-Controlled Trial. J. Am. Acad. Child Adolesc. Psychiatry 2015, 54, 217–224. [Google Scholar] [CrossRef] [PubMed]
- Biederman, J.; Joshi, G.; Mick, E.; Doyle, R.; Georgiopoulos, A.M.; Hammerness, P.; Kotarski, M.; Williams, C.; Wozniak, J. A Prospective Open-Label Trial of Lamotrigine Monotherapy in Children and Adolescents with Bipolar Disorder. CNS Neurosci. Ther. 2010, 16, 91–102. [Google Scholar] [CrossRef] [PubMed]
- Patel, N.C.; Delbello, M.P.; Bryan, H.S.; Adler, C.M.; Kowatch, R.A.; Stanford, K.; Strakowski, S.M. Open-Label Lithium for the Treatment of Adolescents With Bipolar Depression. J. Am. Acad. Child Adolesc. Psychiatry 2006, 45, 289–297. [Google Scholar] [CrossRef] [PubMed]
- Sajatovic, M.; Ng-Mak, D.; Solem, C.; Lin, F.-J.; Rajagopalan, K.; Loebel, A. Dosing patterns and medication adherence in bipolar disorder patients treated with lurasidone: A US retrospective claims database analysis. Ther. Adv. Psychopharmacol. 2016, 6, 355–368. [Google Scholar] [CrossRef] [PubMed]
- Ng-Mak, D.; Halpern, R.; Rajagopalan, K.; Loebel, A. Hospitalization risk in bipolar disorder patients treated with lurasidone versus other atypical antipsychotics. Curr. Med. Res. Opin. 2018, 35, 211–219. [Google Scholar] [CrossRef] [PubMed]
- DelBello, M.P.; Tocco, M.; Pikalov, A.; Deng, L.; Goldman, R. Tolerability, Safety, and Effectiveness of Two Years of Treatment with Lurasidone in Children and Adolescents with Bipolar Depression. J. Child Adolesc. Psychopharmacol. 2021, 31, 494–503. [Google Scholar] [CrossRef] [PubMed]
- Findling, R.L.; Pathak, S.; Earley, W.R.; Liu, S.; DelBello, M.P. Efficacy and Safety of Extended-Release Quetiapine Fumarate in Youth with Bipolar Depression: An 8 Week, Double-Blind, Placebo-Controlled Trial. J. Child Adolesc. Psychopharmacol. 2014, 24, 325–335. [Google Scholar] [CrossRef]
- Rajagopalan, K.; Bacci, E.D.; Wyrwich, K.W.; Pikalov, A.; Loebel, A. The direct and indirect effects of lurasidone monotherapy on functional improvement among patients with bipolar depression: Results from a randomized placebo-controlled trial. Int. J. Bipolar Disord. 2016, 4, 7. [Google Scholar] [CrossRef]
- Loebel, A.; Cucchiaro, J.; Silva, R.; Kroger, H.; Sarma, K.; Xu, J.; Calabrese, J.R. Lurasidone as Adjunctive Therapy With Lithium or Valproate for the Treatment of Bipolar I Depression: A Randomized, Double-Blind, Placebo-Controlled Study. Am. J. Psychiatry 2014, 171, 169–177. [Google Scholar] [CrossRef]
- Forester, B.P.; Sajatovic, M.; Tsai, J.; Pikalov, A.; Cucchiaro, J.; Loebel, A. Safety and Effectiveness of Long-Term Treatment with Lurasidone in Older Adults with Bipolar Depression: Post-Hoc Analysis of a 6-Month, Open-Label Study. Am. J. Geriatr. Psychiatry 2017, 26, 150–159. [Google Scholar] [CrossRef] [PubMed]
- Howland, R.H. Update on Newer Antipsychotic Drugs. J. Psychosoc. Nurs. Ment. Health Serv. 2011, 49, 13–15. [Google Scholar] [CrossRef] [PubMed]
- De Hert, M.; Yu, W.; Detraux, J.; Sweers, K.; van Winkel, R.; Correll, C.U. Body Weight and Metabolic Adverse Effects of Asenapine, Iloperidone, Lurasidone and Paliperidone in the Treatment of Schizophrenia and Bipolar Disorder. CNS Drugs 2012, 26, 733–759. [Google Scholar] [CrossRef] [PubMed]
- Meyer, J.M.; Ng-Mak, D.S.; Chuang, C.-C.; Rajagopalan, K.; Loebel, A. Weight changes before and after lurasidone treatment: A real-world analysis using electronic health records. Ann. Gen. Psychiatry 2017, 16, 36. [Google Scholar] [CrossRef] [PubMed]
- Miller, B.J.; Pikalov, A.; Siu, C.O.; Tocco, M.; Tsai, J.; Harvey, P.D.; Newcomer, J.W.; Loebel, A. Association of C-reactive protein and metabolic risk with cognitive effects of lurasidone in patients with schizophrenia. Compr. Psychiatry 2020, 102, 152195. [Google Scholar] [CrossRef]
- Bellavia, A.; Centorrino, F.; Jackson, J.W.; Fitzmaurice, G.; Valeri, L. The role of weight gain in explaining the effects of antipsychotic drugs on positive and negative symptoms: An analysis of the CATIE schizophrenia trial. Schizophr. Res. 2019, 206, 96–102. [Google Scholar] [CrossRef]
- McIntyre, R.S.; Best, M.W.; Bowie, C.R.; Carmona, N.E.; Cha, D.S.; Lee, Y.; Subramaniapillai, M.; Mansur, R.B.; Barry, H.; Baune, B.T.; et al. The THINC-Integrated Tool (THINC-it) Screening Assessment for Cognitive Dysfunction: Validation in Patients with Major Depressive Disorder. J. Clin. Psychiatry 2017, 78, 873–881. [Google Scholar] [CrossRef]
- Fisher, L.D.; Dixon, D.O.; Herson, J.; Frankowski, R.F.; Hearron, M.S.; Peace, K.E. Intention to treat in clinical trials. In Statistical Issues in Drug Research and Development; Peace, K.E., Ed.; Marcel Dekker: New York, NY, USA, 1990; pp. 331–350. [Google Scholar]

| Characteristic | Lurasidone (n = 29) | Quetiapine (n = 32) | t/χ2/ U | p |
|---|---|---|---|---|
| Age M (P25, P75) (years) | 14 (14, 16) | 15 (14, 16) | 4.45 c | 0.035 * |
| Gender (male/female) | 6/23 | 10/22 | 0.88 b | 0.349 |
| Level of education M (P25, P75) (years) | 8 (7, 10) | 10 (8, 10) | 4.89 c | 0.027 * |
| Duration of illness M (P25, P75) (months) | 16 (12, 30) | 22 (12, 30.75) | 0.01 c | 0.925 |
| Height mean±SD (cm) | 165.07 ± 7.61 | 164.19 ± 6.59 | 0.49 a | 0.630 |
| Weight M (P25, P75) (kg) | 60 (46, 73) | 49.5 (44.88, 57.75) | 4.12 c | 0.042 * |
| Smoking(yes/no) | 2/27 | 0/32 | 0.63 b | 0.429 |
| Drinking(yes/no) | 3/26 | 2/30 | 0.01 b | 0.909 |
| Psychotic symptoms (with/without) | 12/17 | 4/28 | 6.56 b | 0.010 * |
| Family history (positive/recessive) | 5/24 | 4/28 | 0.03 b | 0.873 |
| Project | Lurasidone (Mean ± SD) | Quetiapine (Mean ± SD) | LSM, 95% CI | t | p |
|---|---|---|---|---|---|
| Spotter | 0.07 ± 0.02 | 0.07 ± 0.03 | 0.001, −0.076–0.074 | 0.03 | 0.978 |
| Symbol Check reaction time | 0.08 ± 0.02 | 0.01 ± 0.02 | 0.07, 0.02–0.11 | 2.84 | 0.008 * |
| Symbol Check accuracy | 0.20 ± 0.04 | 0.05 ± 0.04 | 0.16, 0.04–0.28 | 2.68 | 0.012 * |
| Code breaker | 9.19 ± 2.80 | 11.29 ± 3.00 | 2.10, −10.62–6.43 | 0.51 | 0.618 |
| Trails | 6.18 ± 2.01 | 4.35 ± 2.15 | 1.83, −4.20–7.86 | 0.62 | 0.538 |
| PDQ-5-D | 2.35 ± 1.02 | 5.37 ± 1.13 | 3.10, −6.25–0.21 | 1.92 | 0.066 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Diao, X.; Luo, D.; Wang, D.; Lai, J.; Li, Q.; Zhang, P.; Huang, H.; Wu, L.; Lu, S.; Hu, S. Lurasidone versus Quetiapine for Cognitive Impairments in Young Patients with Bipolar Depression: A Randomized, Controlled Study. Pharmaceuticals 2022, 15, 1403. https://doi.org/10.3390/ph15111403
Diao X, Luo D, Wang D, Lai J, Li Q, Zhang P, Huang H, Wu L, Lu S, Hu S. Lurasidone versus Quetiapine for Cognitive Impairments in Young Patients with Bipolar Depression: A Randomized, Controlled Study. Pharmaceuticals. 2022; 15(11):1403. https://doi.org/10.3390/ph15111403
Chicago/Turabian StyleDiao, Xiangyuan, Dan Luo, Dandan Wang, Jianbo Lai, Qunxiao Li, Peifen Zhang, Huimin Huang, Lingling Wu, Shaojia Lu, and Shaohua Hu. 2022. "Lurasidone versus Quetiapine for Cognitive Impairments in Young Patients with Bipolar Depression: A Randomized, Controlled Study" Pharmaceuticals 15, no. 11: 1403. https://doi.org/10.3390/ph15111403
APA StyleDiao, X., Luo, D., Wang, D., Lai, J., Li, Q., Zhang, P., Huang, H., Wu, L., Lu, S., & Hu, S. (2022). Lurasidone versus Quetiapine for Cognitive Impairments in Young Patients with Bipolar Depression: A Randomized, Controlled Study. Pharmaceuticals, 15(11), 1403. https://doi.org/10.3390/ph15111403







