Nitro-Containing Self-Immolative Systems for Biological Applications
Abstract
1. Introduction
2. NO2 Group on the Trigger
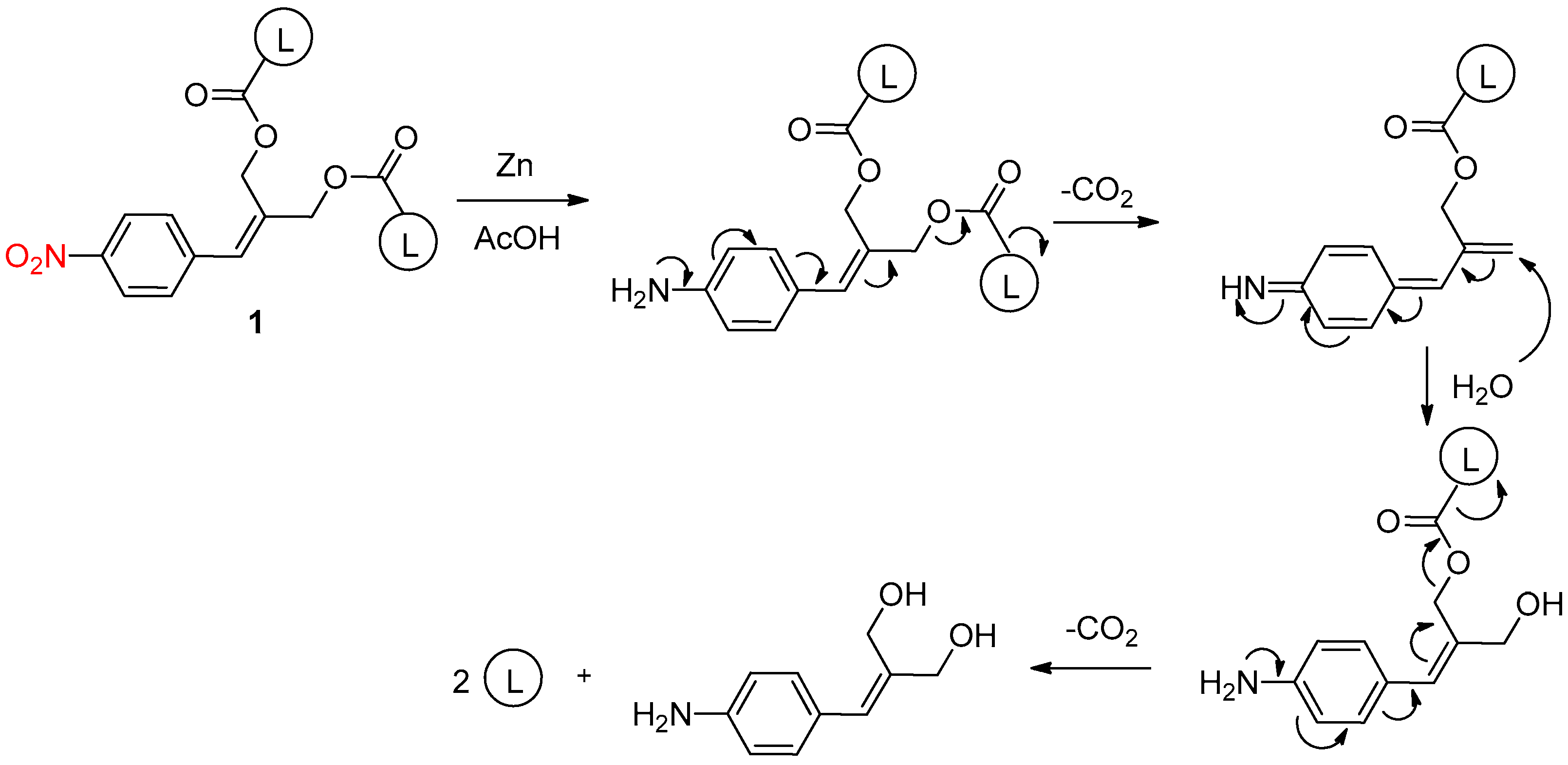
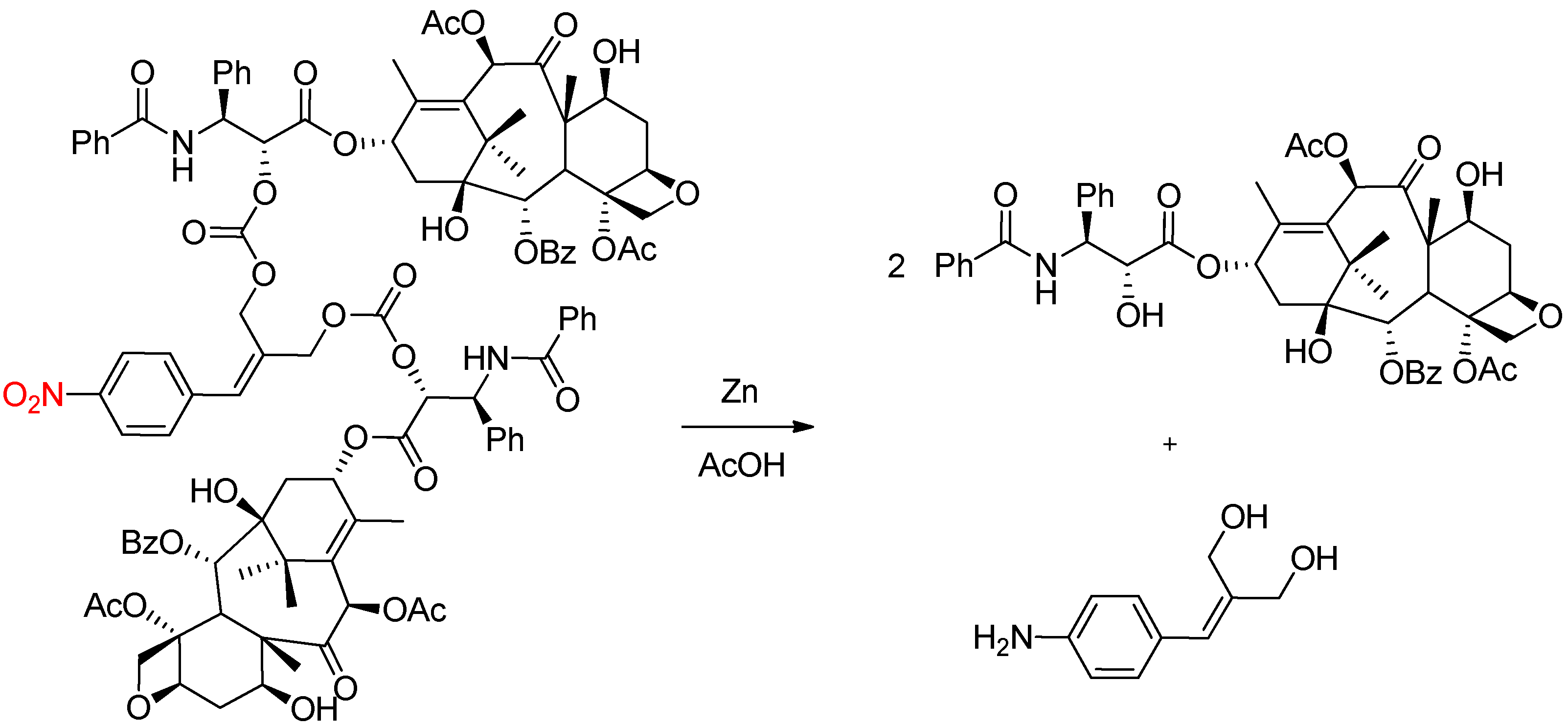
2.1. Chemical Reduction of the Nitro Group
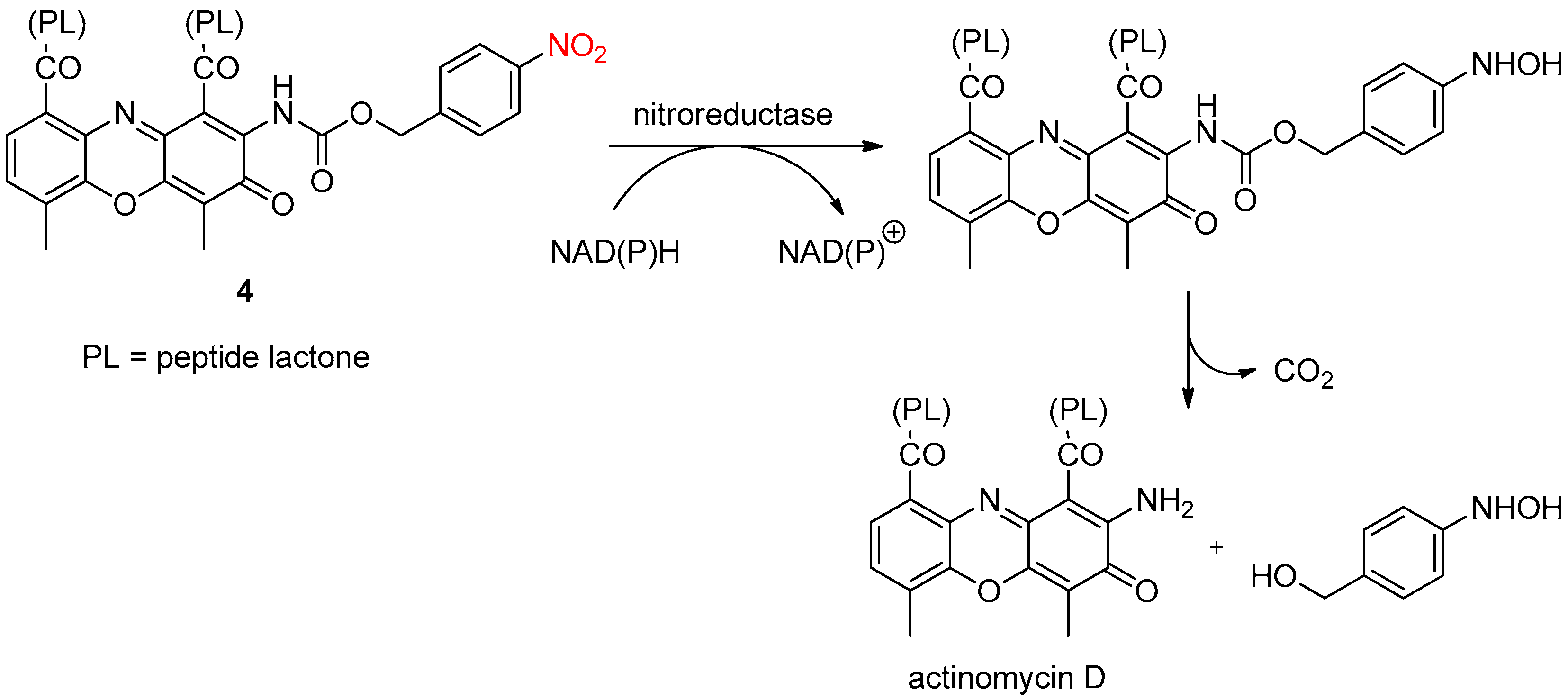
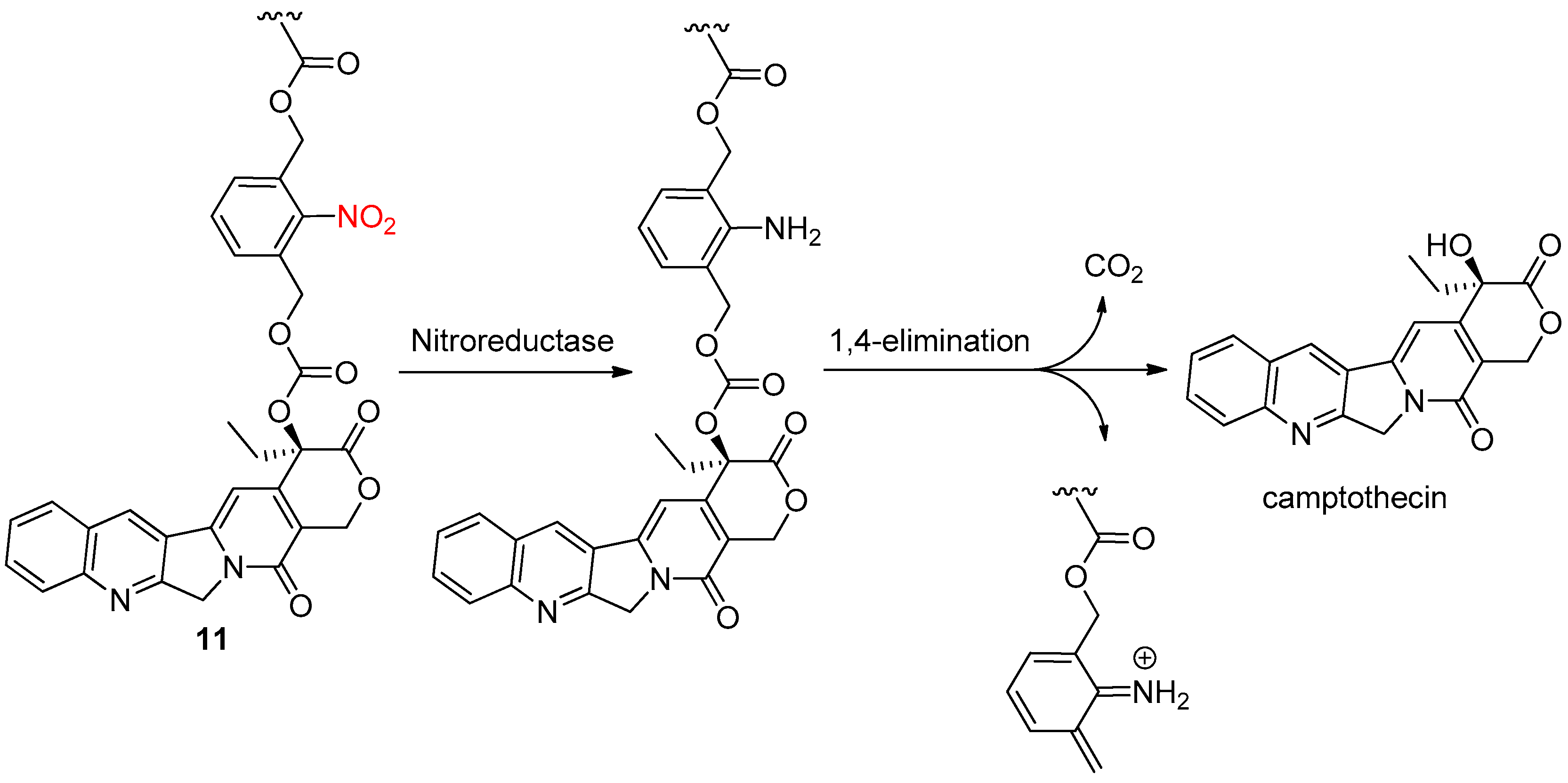
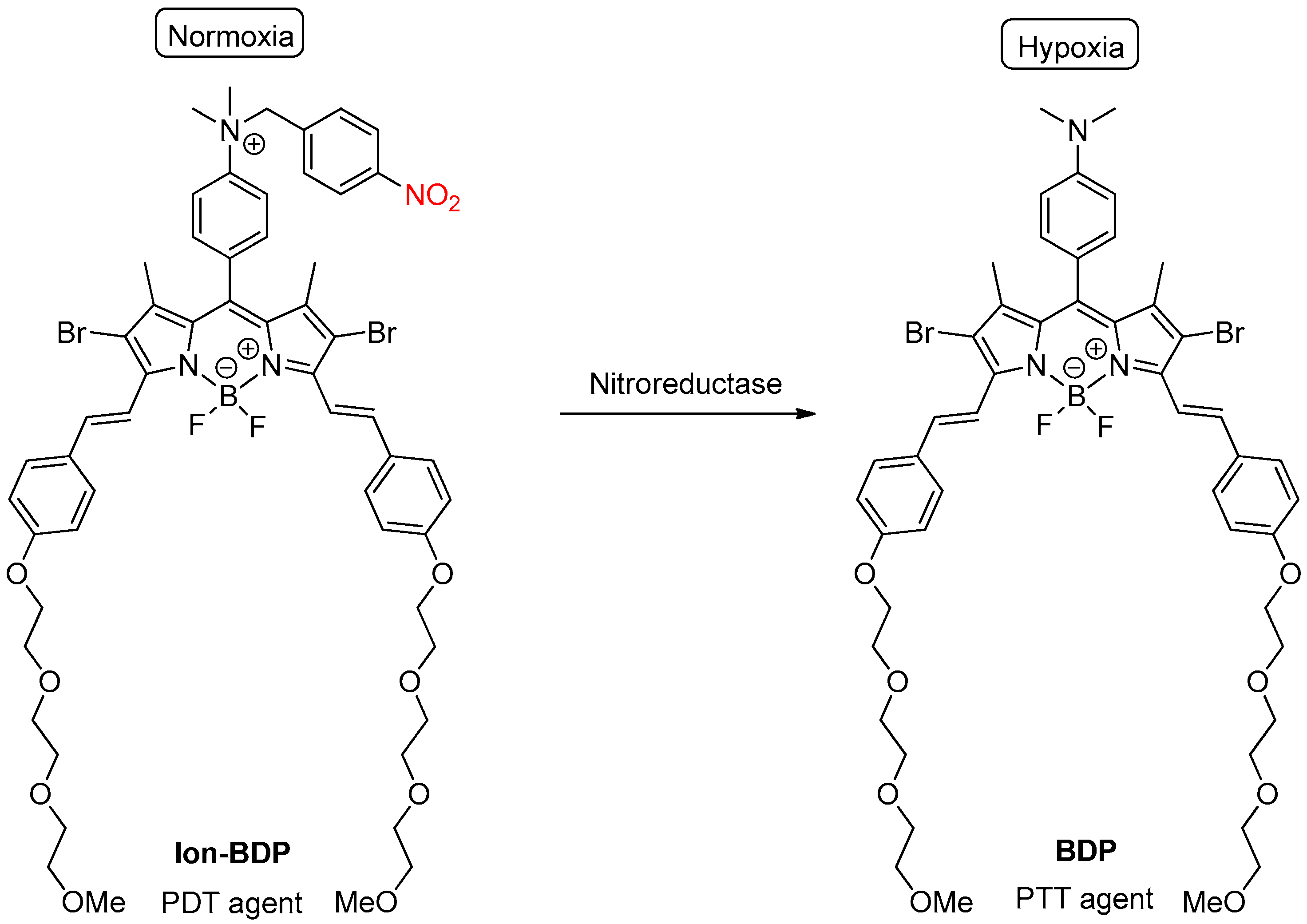
2.2. Enzymatic Reduction of the Nitro Group
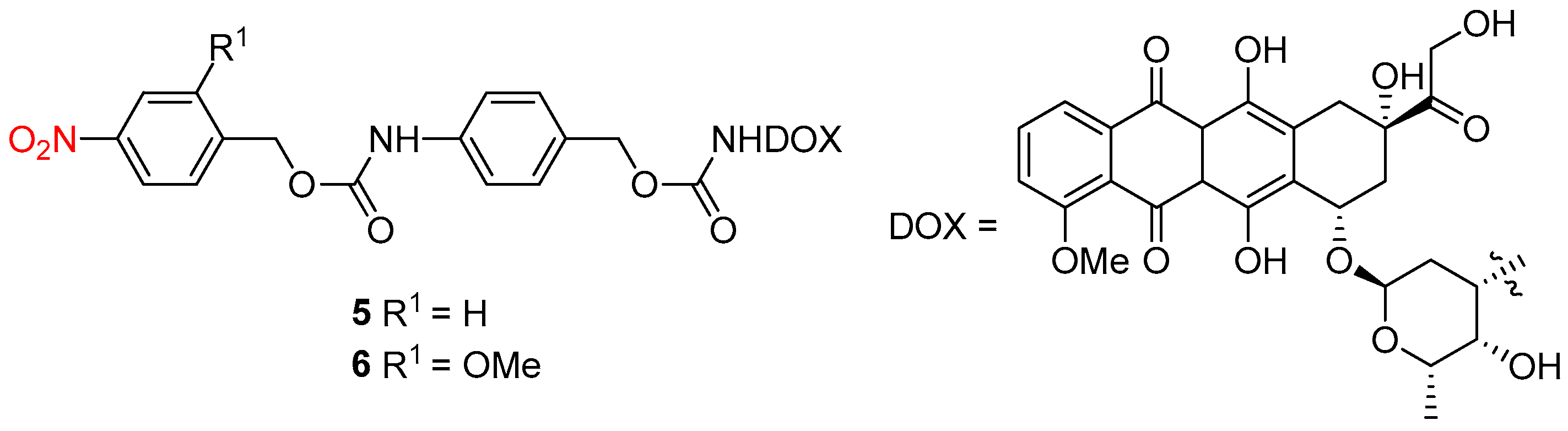
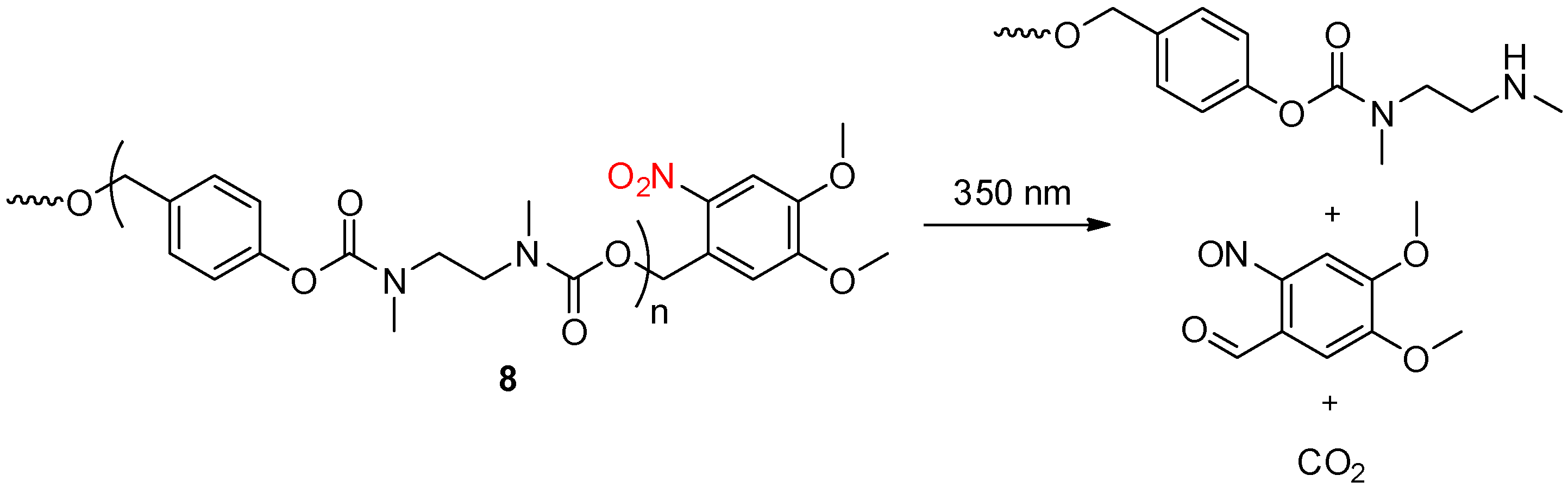
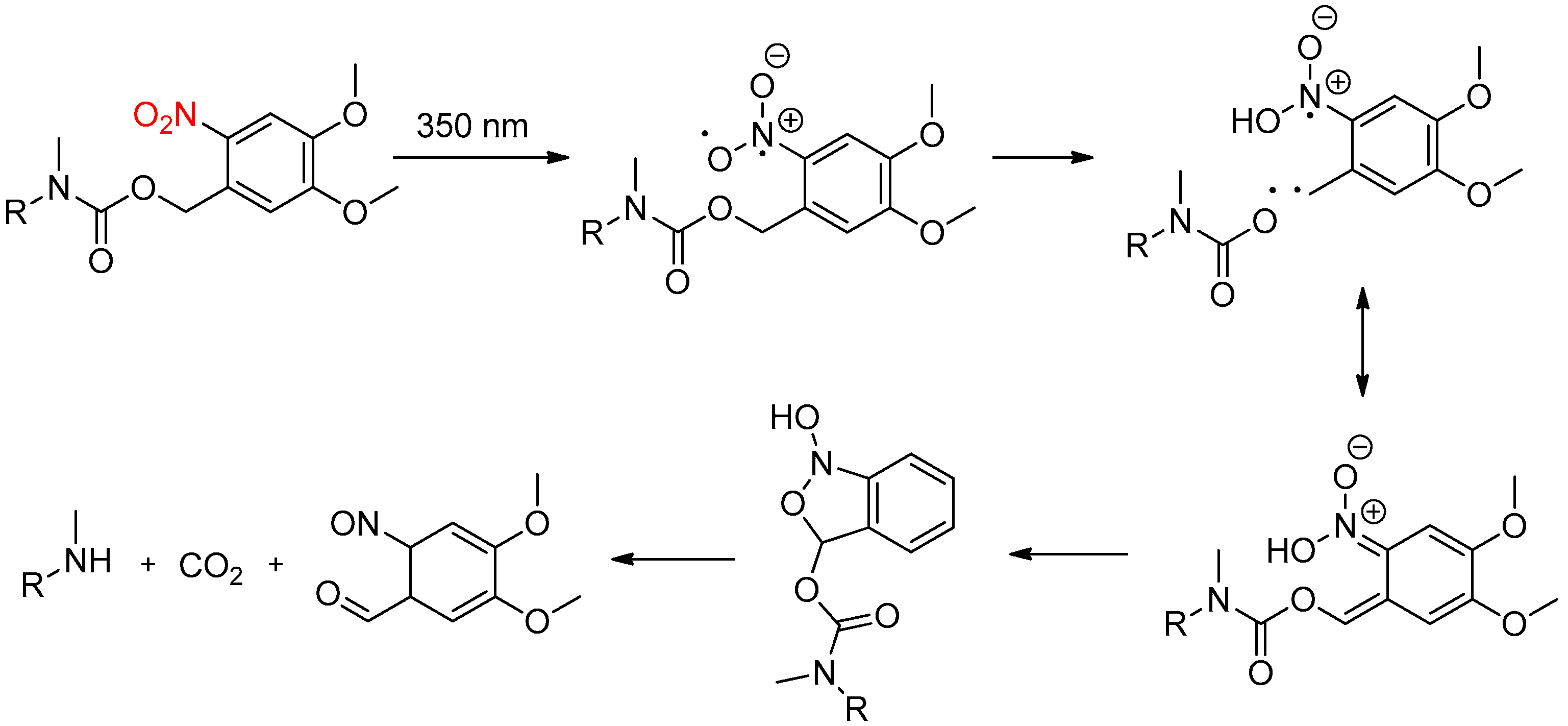
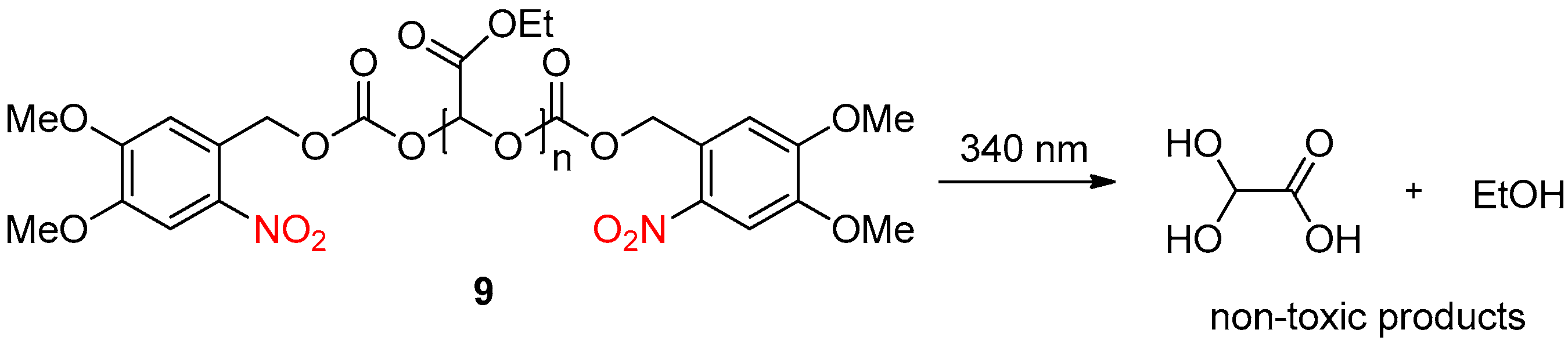
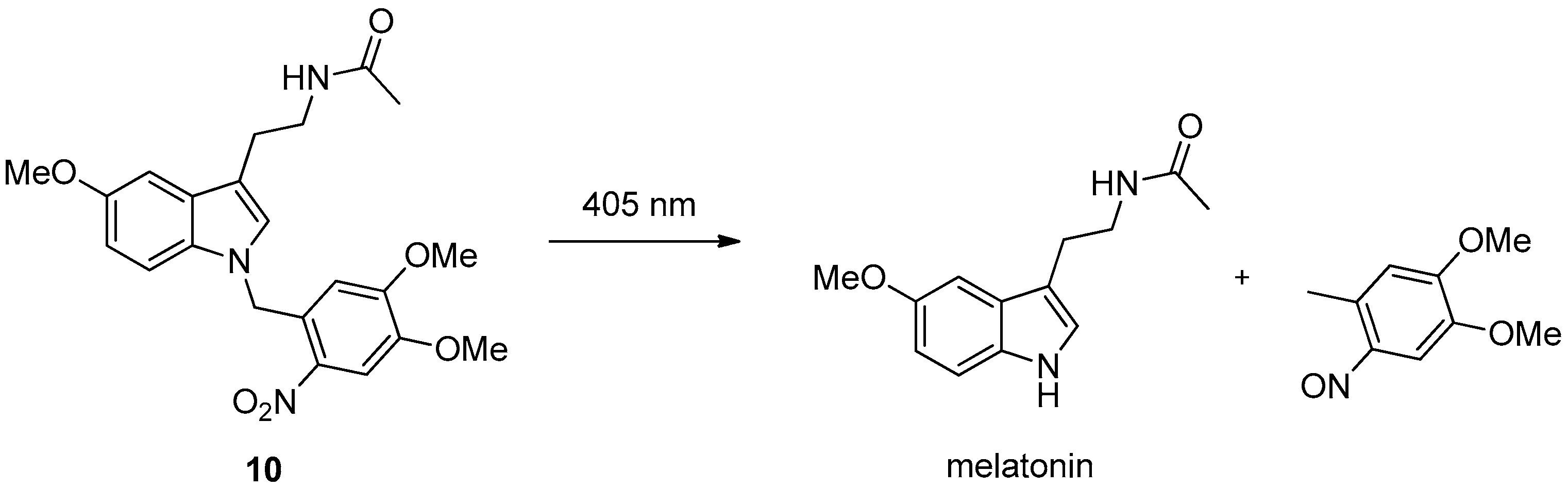
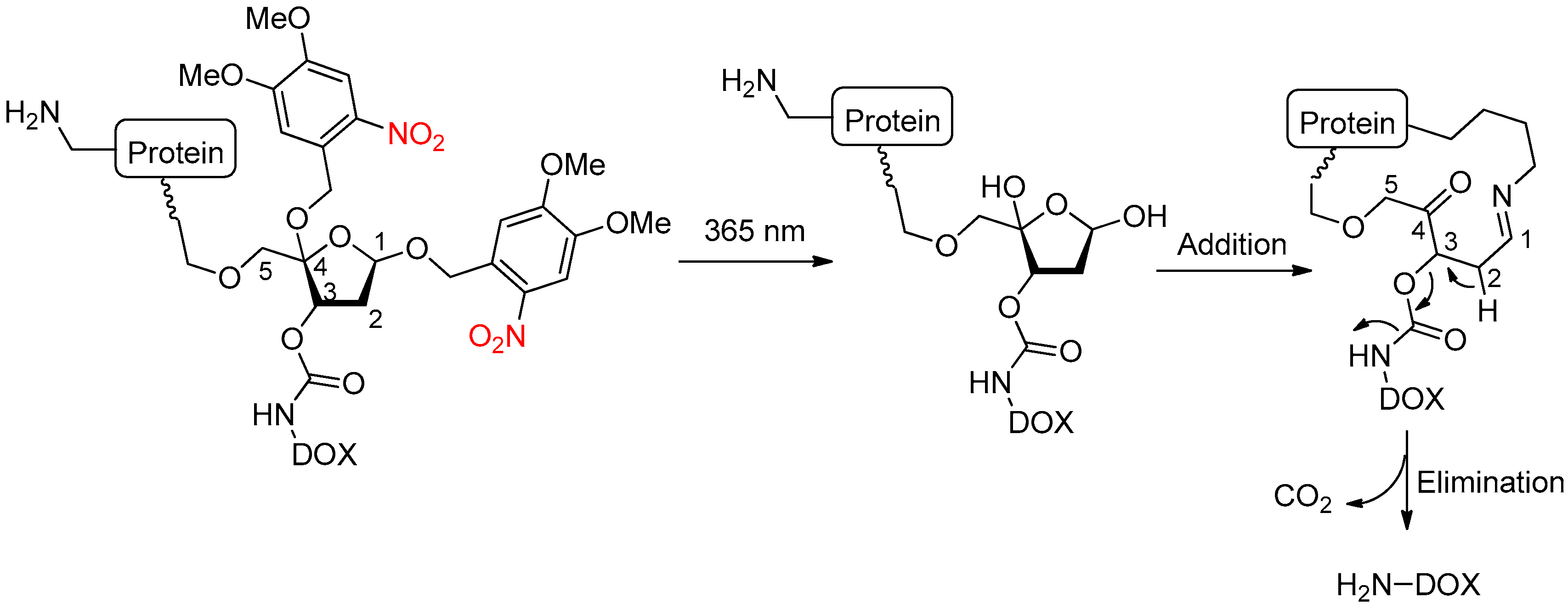
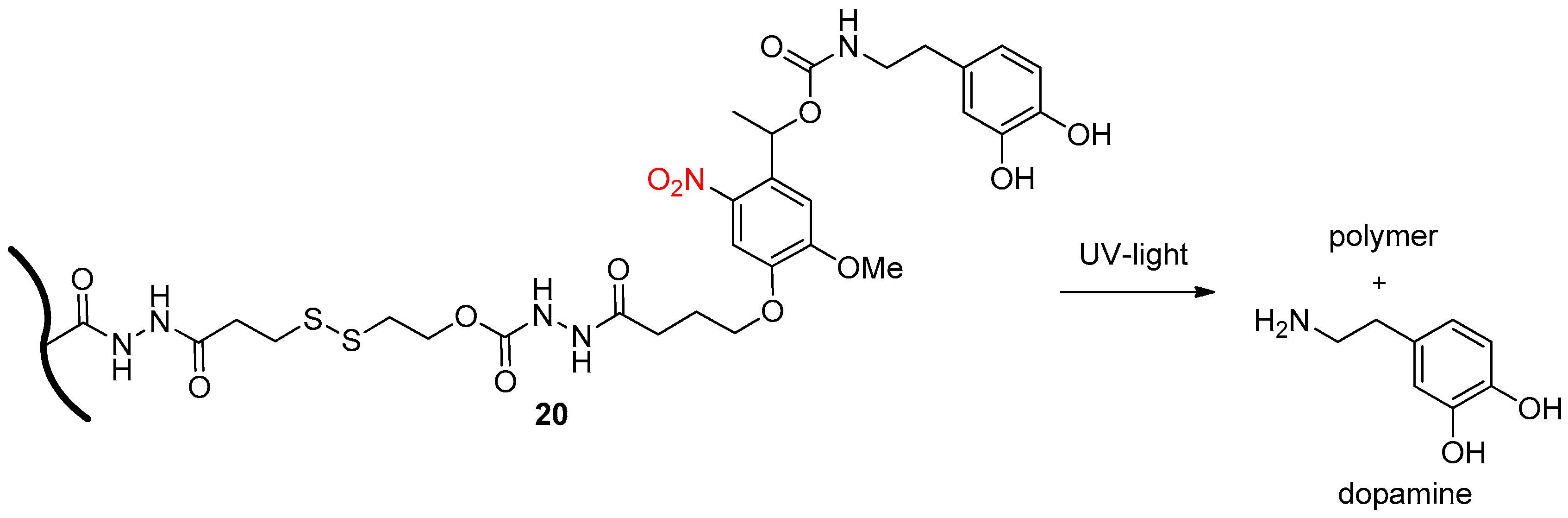
2.3. Light Activation
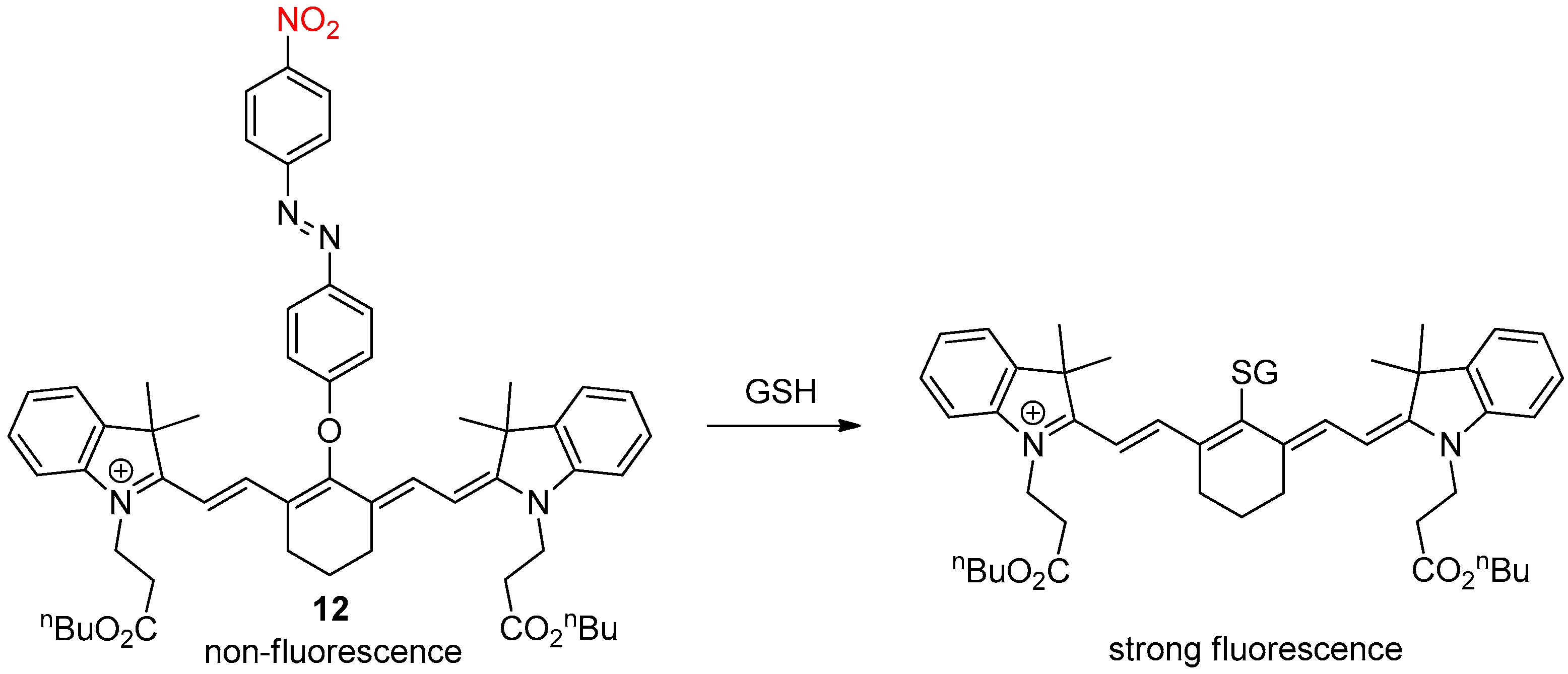
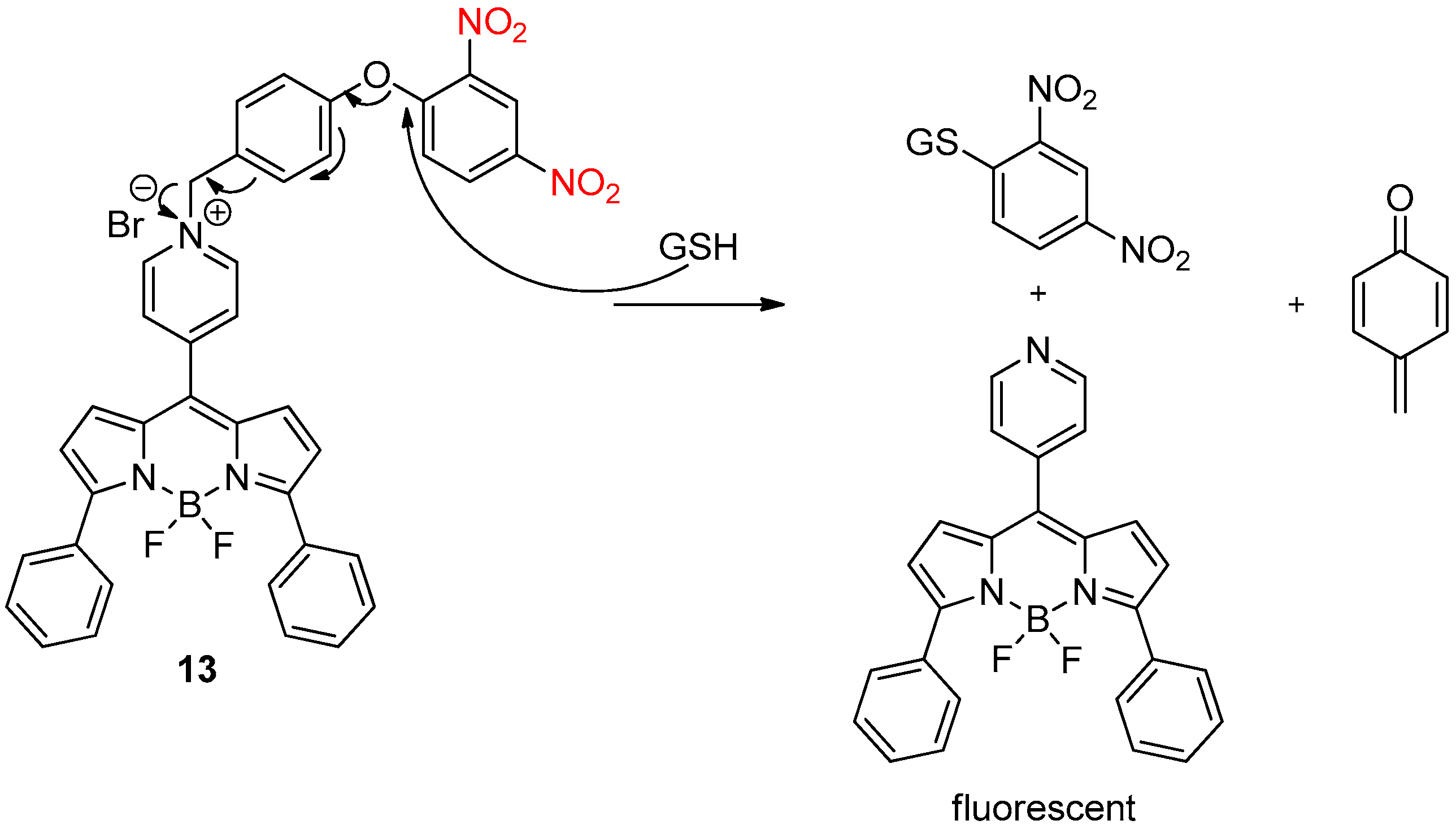
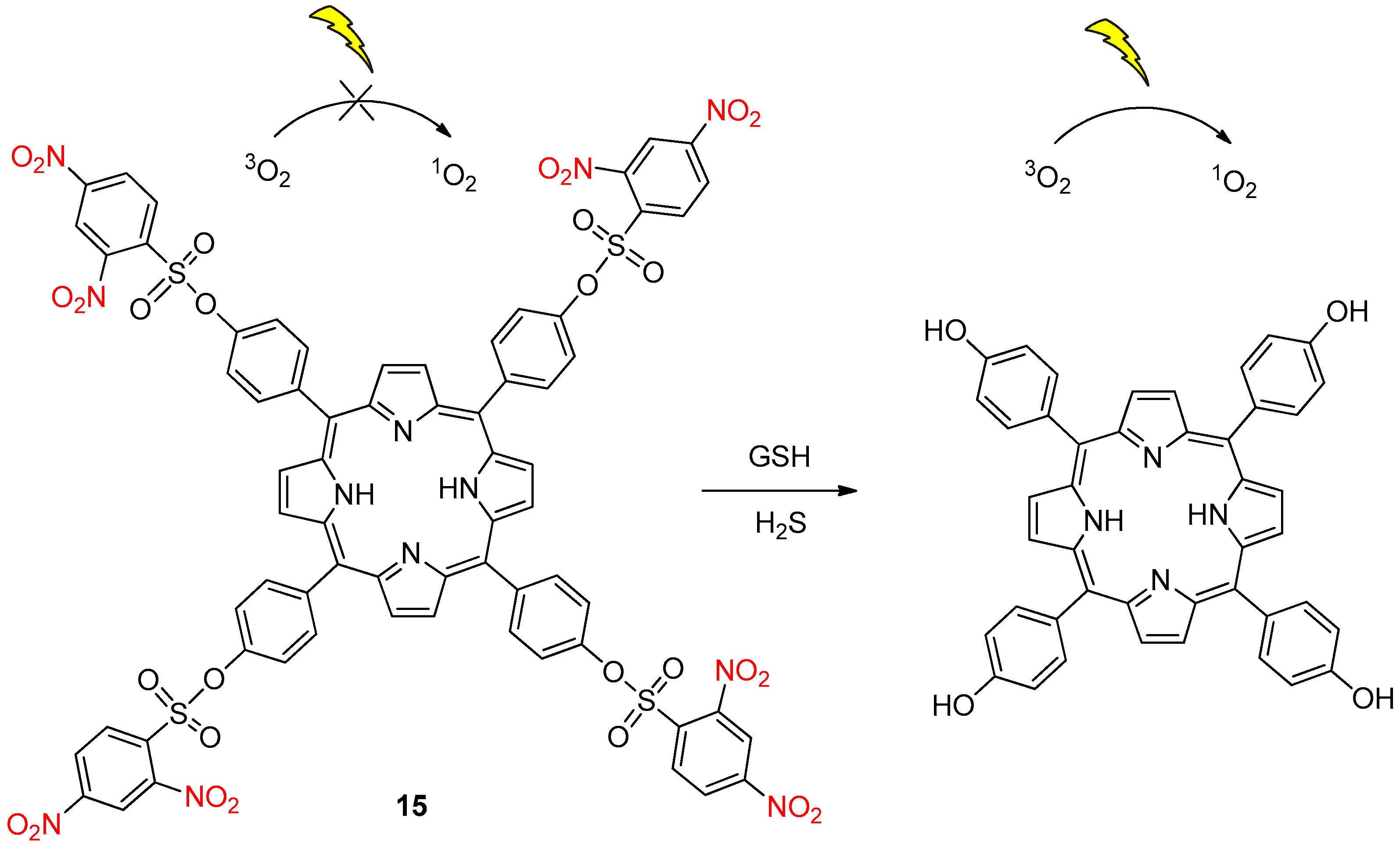
2.4. Glutathione Activation
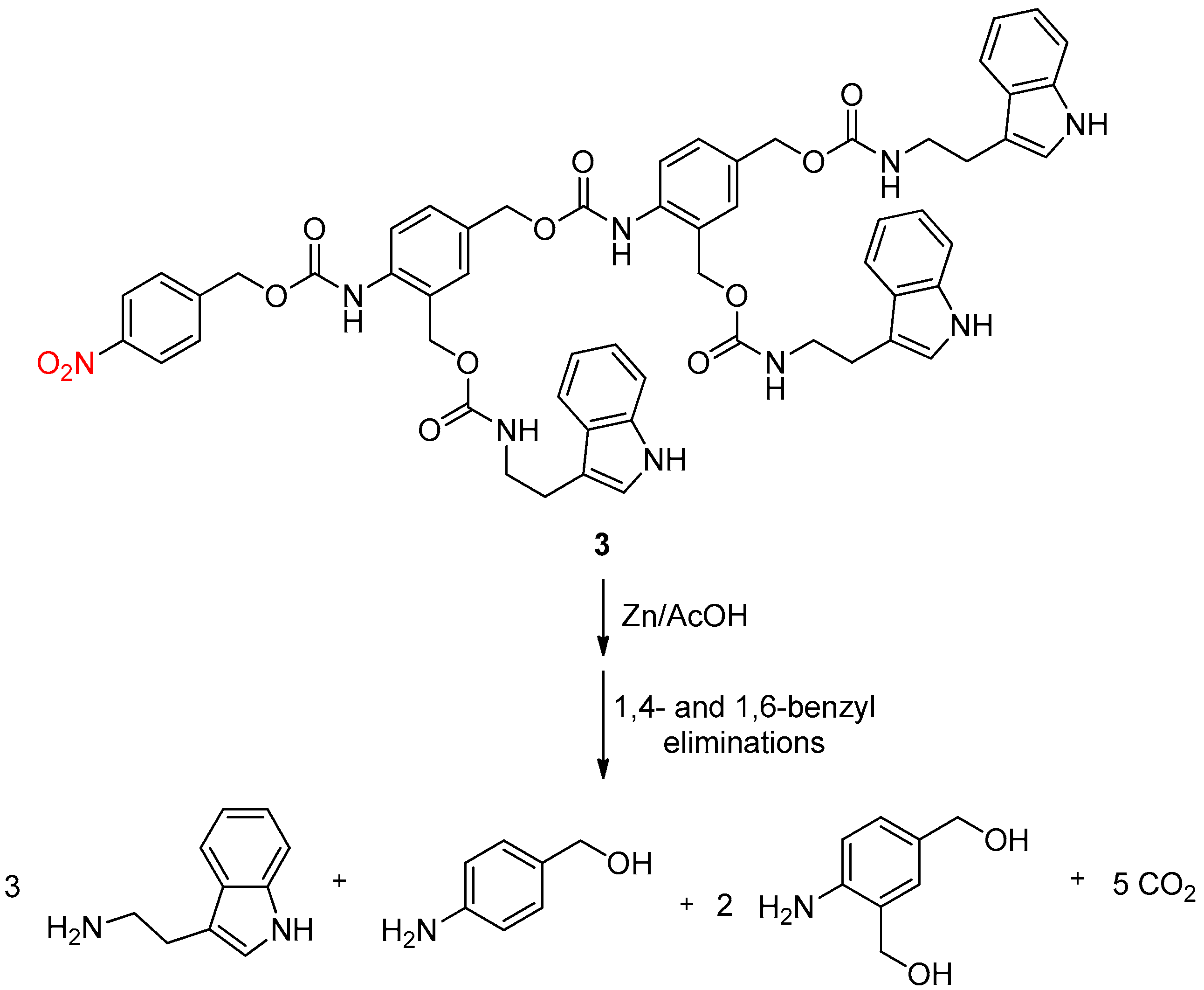
3. NO2 Group on the Linker
4. NO2 Group on the Reporter
4.1. Chemical Activation
4.1.1. Para-Nitrophenoxide Ion as a Reporter Group
4.1.2. Nitroanilines as Reporter Group
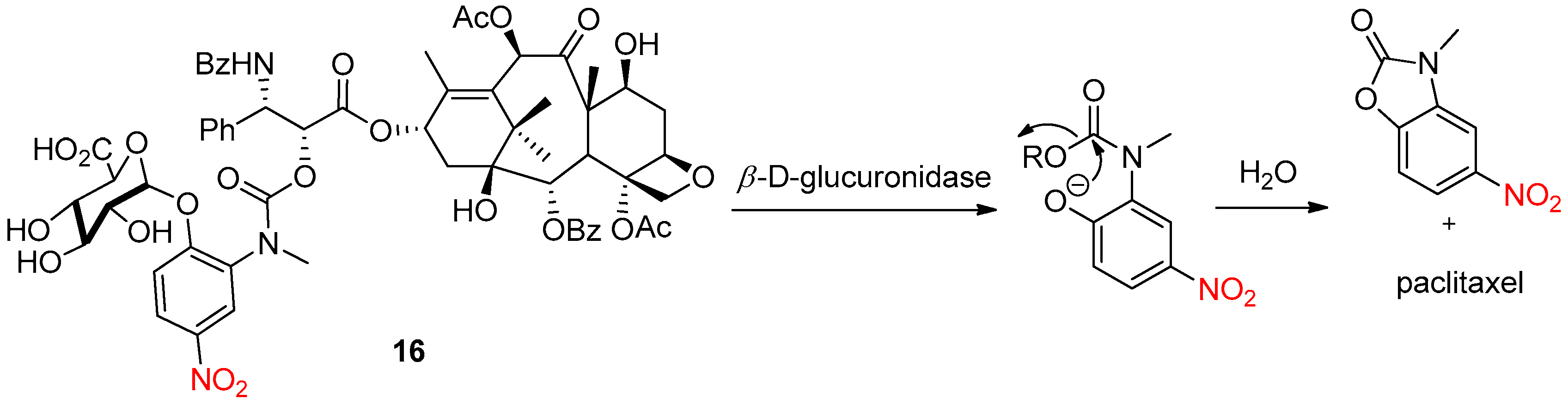
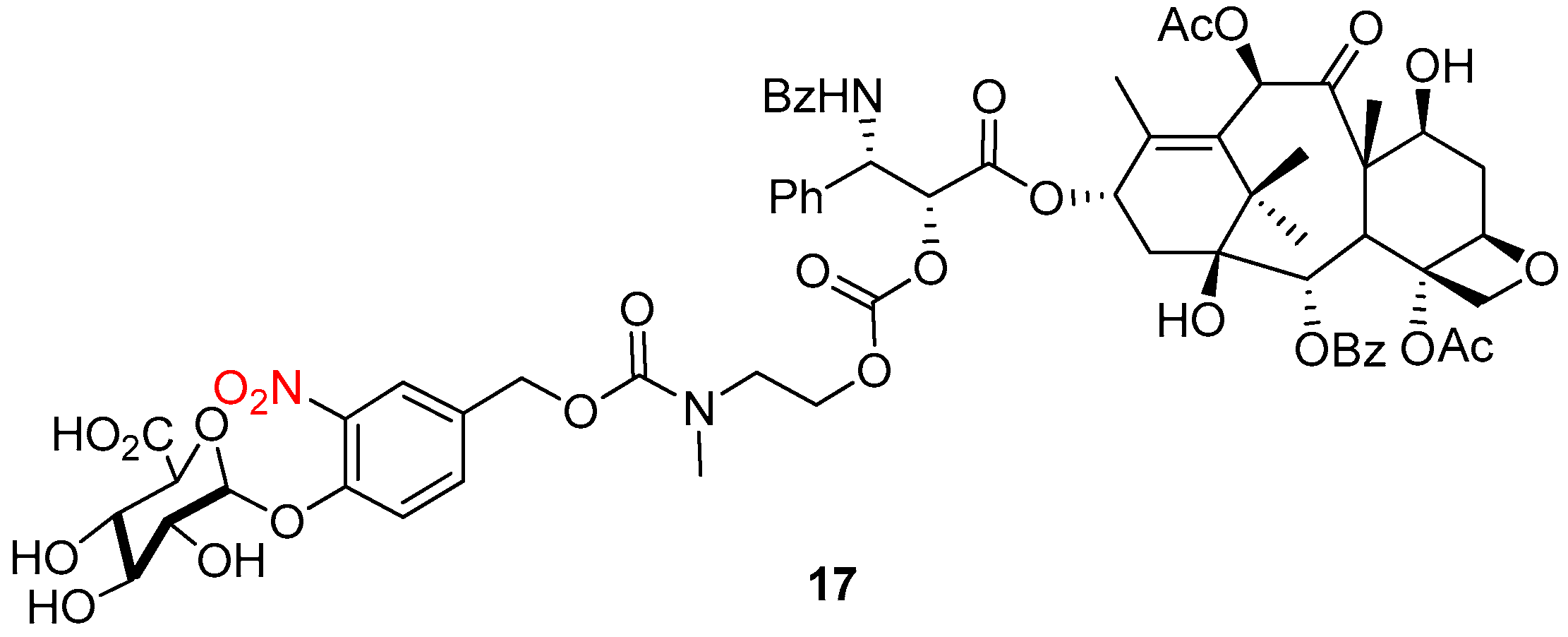
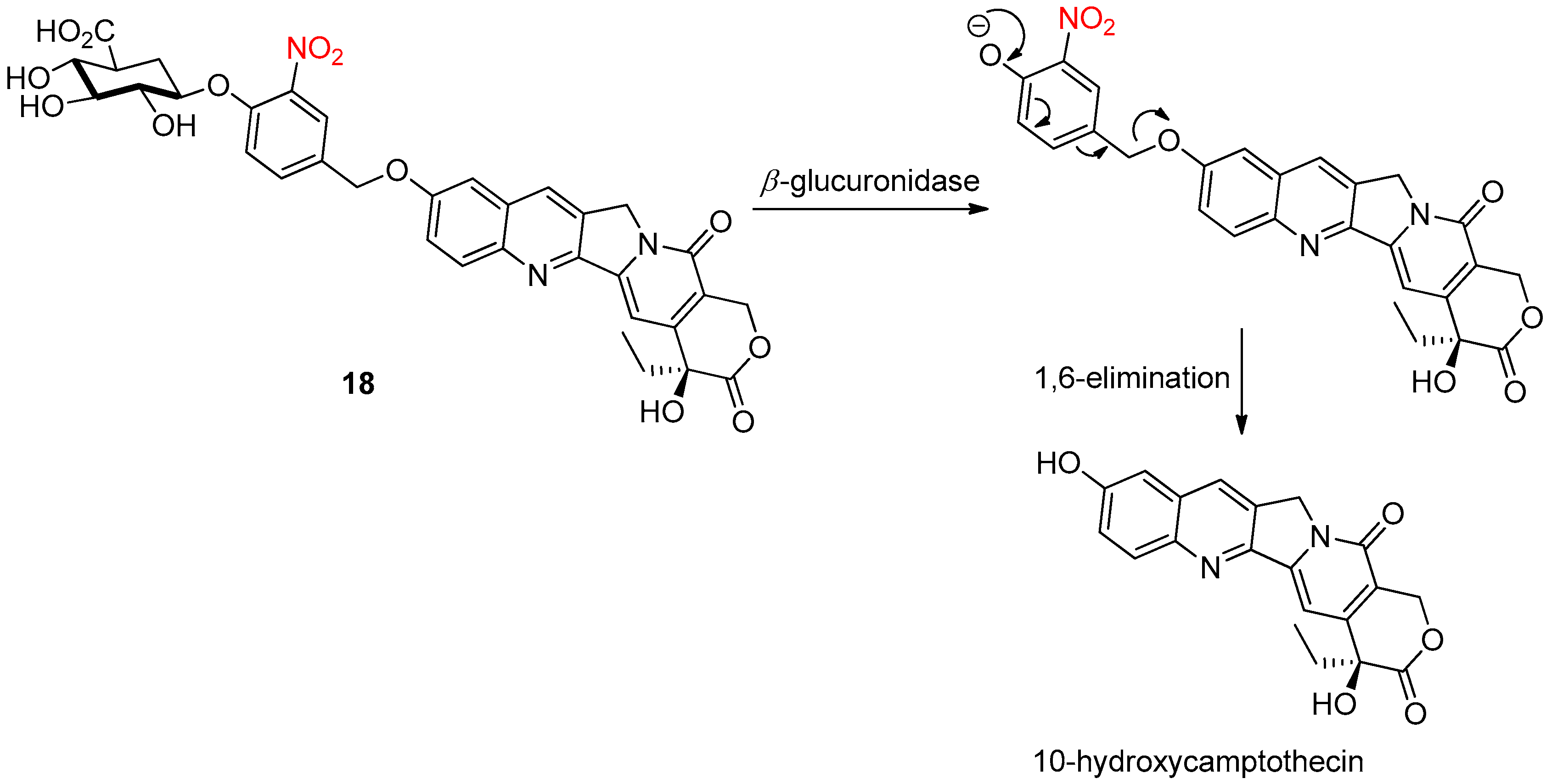
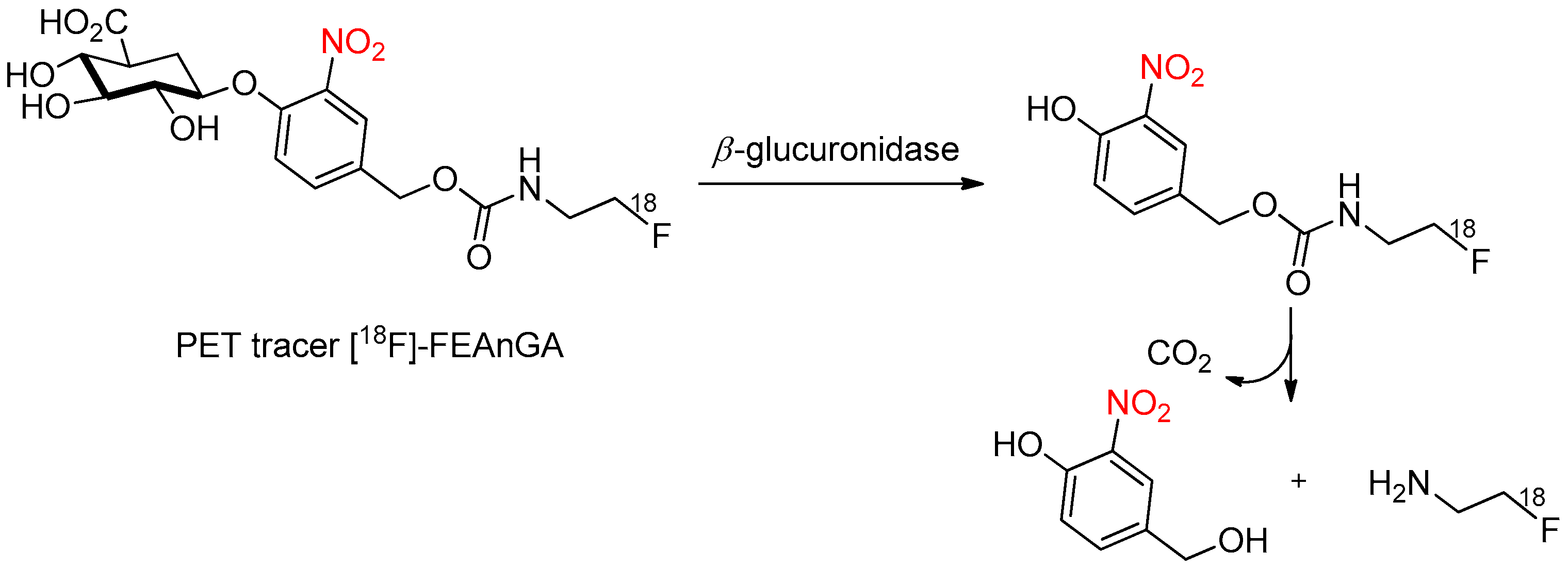
4.2. Enzymatic Activation
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Carl, P.L.; Chakravarty, P.K.; Katzenellenbogen, J.A. Novel Connector Linkage Applicable in Prodrug Design. J. Med. Chem. 1981, 24, 479–480. [Google Scholar] [CrossRef] [PubMed]
- De Groot, F.M.H.; Albrecht, C.; Koekkoek, R.; Beusker, P.H.; Scheeren, H.W. “Cascade-Release Dendrimers” Liberate All End Groups upon a Single Triggering Event in the Dendritic Core. Angew. Chem. Int. Ed. 2003, 42, 4490–4494. [Google Scholar] [CrossRef] [PubMed]
- Warnecke, A.; Kratz, F. 2,4-Bis(hydroxymethyl)aniline as a Building Block for Oligomers with Self-Eliminating and Multiple Release Proporties. J. Org. Chem. 2008, 73, 1546–1552. [Google Scholar] [CrossRef] [PubMed]
- Patterson, S.; Wyllie, S. Nitro drugs for the treatment of trypanosomatid diseases: Past, present, and future prospects. Trends Parasitol. 2014, 30, 289–298. [Google Scholar] [CrossRef]
- Mauger, A.B.; Burke, P.J.; Somani, H.H.; Friedlos, F.; Knox, R.J. Self-Immolative Prodrugs: Candidates for Antibody-Directed Enzyme Prodrug Therapy in Conjunction with a Nitroreductase Enzyme. J. Med. Chem. 1994, 37, 3452–3458. [Google Scholar] [CrossRef]
- Hay, M.P.; Sykes, B.M.; Denny, W.A.; O’Connor, C.J. Substituent effects on the kinetics of reductively-initiated fragmentation of nitrobenzyl carbamates designed as triggers for bioreductive prodrugs. J. Chem. Soc. Perkin Trans. 1 1999, 2759–2770. [Google Scholar] [CrossRef]
- Sykes, B.M.; Hay, M.P.; Bohinc-Herceg, D.; Helsby, N.A.; O’Connor, C.J.; Denny, W.A. Leaving group effects in reductively triggered fragmentation of 4-nitrobenzyl carbamates. J. Chem. Soc. Perkin Trans. 1 2000, 1601–1608. [Google Scholar] [CrossRef]
- Hay, M.P.; Wilson, W.R.; Denny, W.A. Nitroarylmethylcarbamate prodrugs of doxorubicin for use with nitroreductase gene-directed enzyme prodrug therapy. Bioorg. Med. Chem. 2005, 13, 4043–4055. [Google Scholar] [CrossRef]
- Zhou, W.; Wang, C.; Liu, Z.; Gou, S. Hypoxia-Activated Prodrugs with Dual COX-2/CA Inhibitory Effects on Attenuating Cardiac Inflammation under Hypoxia. J. Med. Chem. 2022, 65, 13436–13451. [Google Scholar] [CrossRef]
- De Gracia Lux, C.; McFearin, C.L.; Joshi-Barr, S.; Sankaranarayanan, J.; Fomina, N.; Almutairi, A. Single UV or Near IR Triggering Event Leads to Polymer Degradation into Small Molecules. ACS Macro Lett. 2012, 1, 922–926. [Google Scholar] [CrossRef]
- Romano, A.; Roppolo, I.; Rossegger, E.; Schlögl, S.; Sangermano, M. Recent Trends in Applying Ortho-Nitrobenzyl Esters for the Design of Photo-Responsive Polymer Networks. Materials 2020, 13, 2777. [Google Scholar] [CrossRef]
- Fan, B.; Trant, J.F.; Wong, A.D.; Gillies, E.R. Polyglyoxylates: A Versatile Class of Triggerable Self-Immolative Polymers from Readily Accessible Monomers. J. Am. Chem. Soc. 2014, 136, 10116–10123. [Google Scholar] [CrossRef] [PubMed]
- Somalo-Barranco, G.; Serra, C.; Lyons, D.; Piggins, H.D.; Jockers, R.; Llebaria, A. Design and Validation of the First Family of Photo-Activatable Ligands for Melatonin Receptors. J. Med. Chem. 2022, 65, 11229–11240. [Google Scholar] [CrossRef] [PubMed]
- Zang, C.; Wang, H.; Li, T.; Zhang, Y.; Li, J.; Shang, M.; Du, J.; Xi, Z.; Zhou, C. A light-responsive, self-immolative linker for controlled drug delivery via peptide- and protein-drug conjugates. Chem. Sci. 2019, 10, 8973–8980. [Google Scholar] [CrossRef] [PubMed]
- Dutta, D.; Zhou, Q.; Mukerabigwi, J.F.; Lu, N.; Ge, Z. Hypoxia-Responsive Polydrug Nanocarriers for Near-Infrared Light-Boosted Photodynamic Chemotherapy. Biomacromolecules 2021, 22, 4857–4870. [Google Scholar] [CrossRef]
- Lin, H.-H.; Yang, X.-Z.; Yu, M.-Y.; Liu, J.-Y. A switchable phototherapeutic strategy based on a hypoxia-responsive photosensitizer for heterogeneous tumor ablation. Dye. Pigment. 2022, 201, 110217. [Google Scholar] [CrossRef]
- Ossipov, D.A.; Romero, A.B.; Ossipova, E. Light-activable prodrugs based on hyaluronic acid biomaterials. Carbohydr. Polym. 2018, 180, 145–155. [Google Scholar] [CrossRef]
- Lim, S.-Y.; Hong, K.-H.; Kim, D.I.; Kwon, H.; Kim, H.-J. Tunable Heptamethine-Azo Dye Conjugate as an NIR Fluorescent Probe for the Selective Detection of Mitochondrial Glutathione over Cysteine and Homocysteine. J. Am. Chem. Soc. 2014, 136, 7018–7025. [Google Scholar] [CrossRef]
- Zhang, J.; Bao, X.; Zhou, J.; Peng, F.; Ren, H.; Dong, X.; Zhao, W. A mitochondria-targeted turn-on fluorescent probe for the detection of glutathione in living cells. Biosens. Bioelectron. 2016, 85, 164–170. [Google Scholar] [CrossRef]
- Wang, Z.; Wu, H.; Liu, P.; Zeng, F.; Wu, S. A self-immolative prodrug nanosystem capable of releasing a drug and a NIR reporter for in vivo imaging and therapy. Biomaterials 2017, 139, 139–150. [Google Scholar] [CrossRef]
- Huang, K.; Xie, S.; Jiang, L.; Li, J.; Chen, J. A glutathione and hydrogen sulfide responsive photosensitizer for enhanced photodynamic therapy. Dye. Pigment. 2022, 205, 110529. [Google Scholar] [CrossRef]
- Schmidt, F.; Ungureanu, I.; Duval, R.; Pompon, A.; Monneret, C. Cancer Chemotherapy: A Paclitaxel Prodrug for ADEPT (Antibody-Directed Enzyme Prodrug Therapy). Eur. J. Org. Chem. 2001, 2001, 2129–2134. [Google Scholar] [CrossRef]
- El Alaoui, A.; Saha, N.; Schmidt, F.; Monneret, C.; Florent, J.-C. New Taxol® (paclitaxel) prodrugs designed for ADEPT and PMT strategies in cancer chemotherapy. Bioorg. Med. Chem. 2006, 14, 5012–5019. [Google Scholar] [CrossRef] [PubMed]
- Leu, Y.-L.; Chen, C.-S.; Wu, Y.-J.; Chern, J.-W. Benzyl Ether-Linked Glucuronide Derivative of 10-Hydroxycamptothecin Designed for Selective Camptothecin-Based Anticancer Therapy. J. Med. Chem. 2008, 51, 1740–1746. [Google Scholar] [CrossRef] [PubMed]
- Antunes, I.F.; Haisma, H.J.; Elsinga, P.H.; Dierckx, R.A.; de Vries, E.F.J. Synthesis and Evaluation of [18F]-FEAnGA as a PET Tracer for β-Glucuronidase Activity. Bioconjugate Chem. 2010, 21, 911–920. [Google Scholar] [CrossRef] [PubMed]
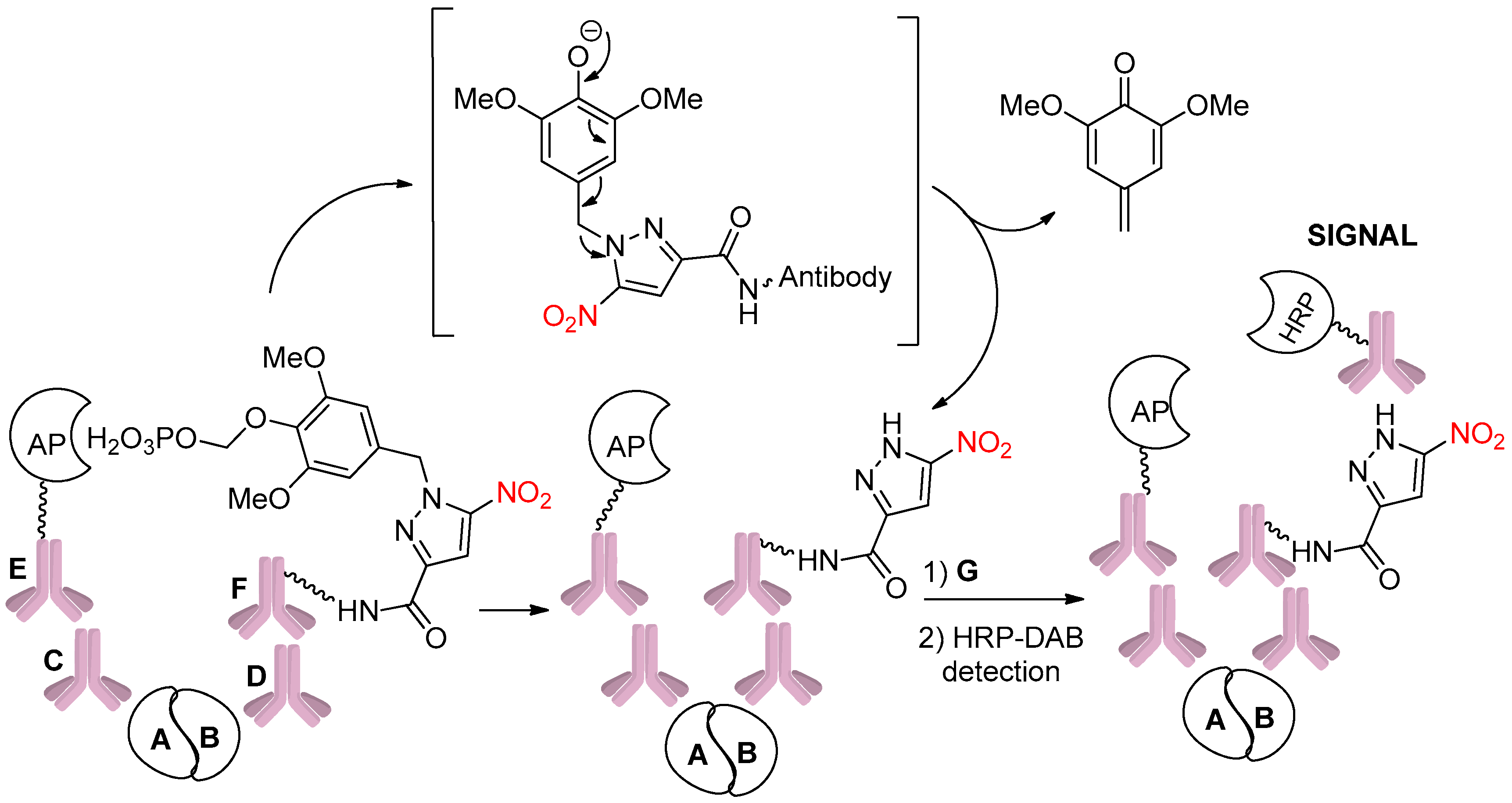
- Polaske, N.W.; Kelly, B.D.; Smith, M.A.; Haura, E.B.; Belosludtsev, Y.Y. Fully Automated Protein Proximity Assay in Formalin-Fixed, Paraffin-Embedded Tissue Using Caged Haptens. Bioconjugate Chem. 2020, 31, 1635–1640. [Google Scholar] [CrossRef]
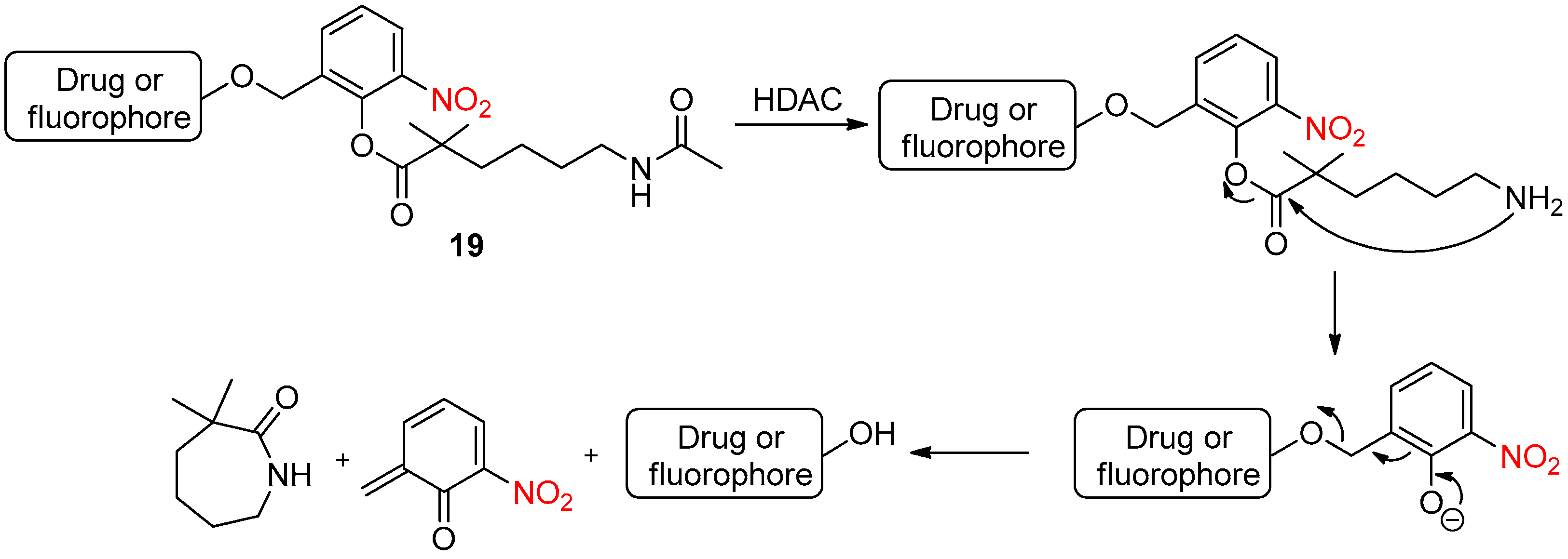
- Liu, F.; Ding, X.; Xu, X.; Wang, F.; Chu, X.; Jiang, J.-H. Reactivity-Tunable Self-Immolative Design Enabling Histone Deacetylase-Targeted Imaging and Prodrug Activation. Angew. Chem. Int. Ed. 2022. [Google Scholar] [CrossRef]
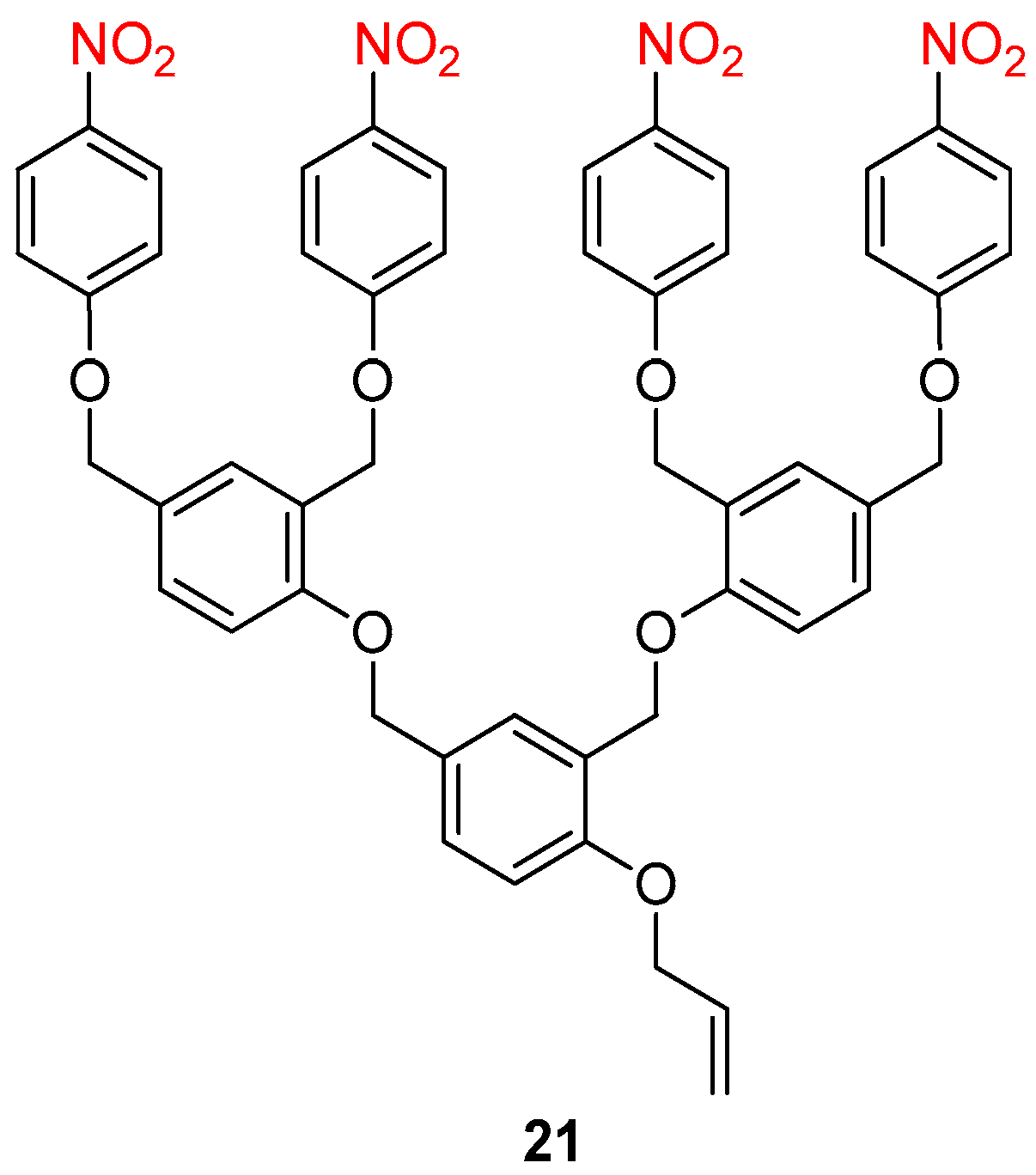
- Szalai, M.L.; Kevwitch, R.M.; McGrath, D.V. Geometric Diassembly of Dendrimers: Dentritic Amplification. J. Am. Chem. Soc. 2003, 125, 15688–15689. [Google Scholar] [CrossRef]
- Ortiz, A.; Shanahan, C.S.; Sisk, D.T.; Perera, S.C.; Rao, P.; McGrath, D.V. Improved Iterative Synthesis of Linearly Diassembling Dendrons. J. Org. Chem. 2010, 75, 6154–6162. [Google Scholar] [CrossRef]
- Li, S.; Szalai, M.L.; Kevwitch, R.M.; McGrath, D.V. Dendrimer Disassembly by Benzyl Ether Depolymerization. J. Am. Chem. Soc. 2003, 125, 10516–10517. [Google Scholar] [CrossRef]
- Grinda, M.; Legigan, T.; Clarhaut, J.; Peraudeau, E.; Tranoy-Opalinski, I.; Renoux, B.; Thomas, M.; Guilhot, F.; Papot, S. An enzyme-responsive system programmed for the double release of bioactive molecules through an intracellular chemical amplification process. Org. Biomol. Chem. 2013, 11, 7129–7133. [Google Scholar] [CrossRef] [PubMed]
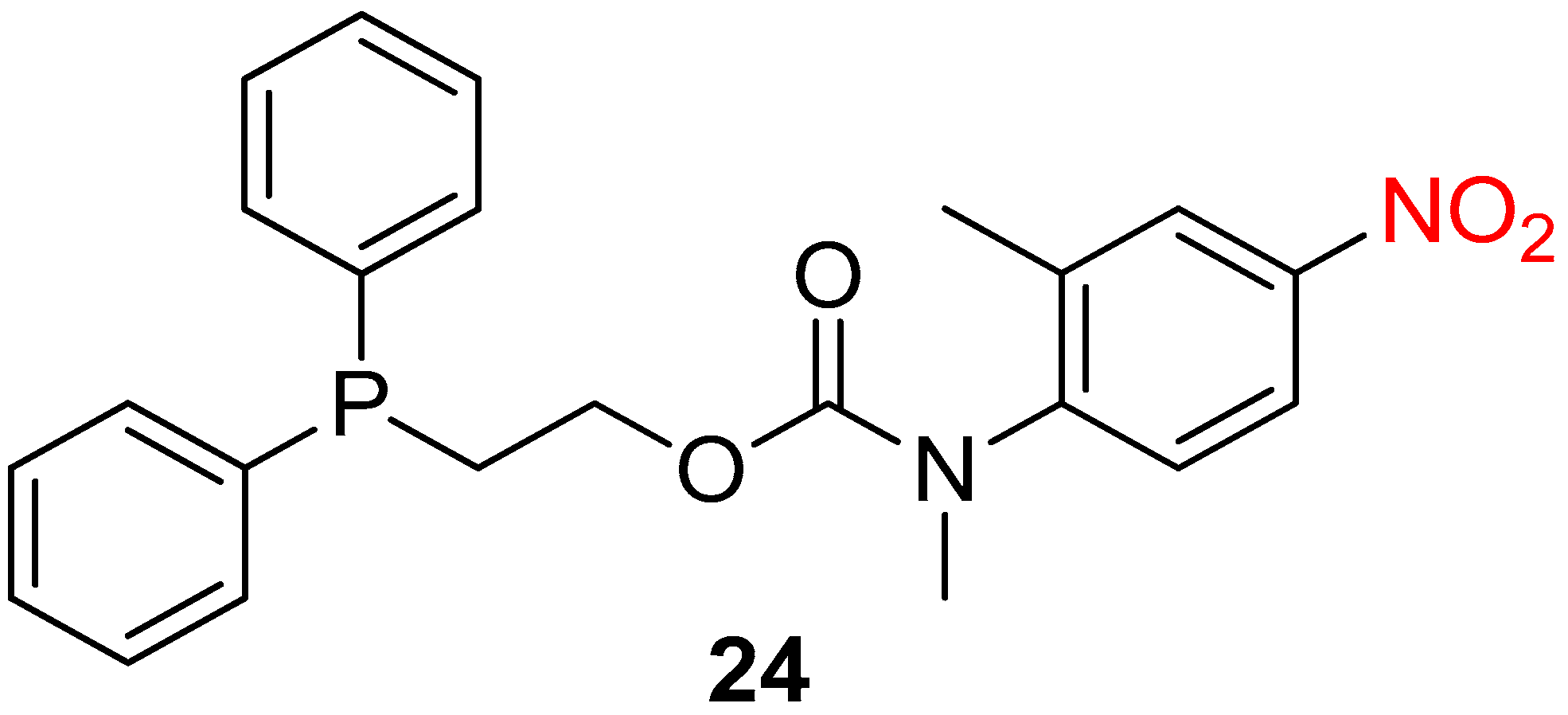
- Acton, A.L.; Leroux, F.; Feula, A.; Melia, K.; Sambrook, M.R.; Hayes, W.; Russell, A.T. Self-immolative systems for the disclosure of reactive electrophilic alkylating agents. Chem. Commun. 2019, 55, 5219–5222. [Google Scholar] [CrossRef] [PubMed]
- Gavriel, A.G.; Leroux, F.; Khurana, G.S.; Lewis, V.G.; Chippindale, A.M.; Sambrook, M.R.; Hayes, W.; Russell, A.T. Self-Immolative System for Disclosure of Reactive Electrophilic Alkylating Agents: Understanding the Role of the Reporter Group. J. Org. Chem. 2021, 86, 10263–10279. [Google Scholar] [CrossRef] [PubMed]
- Russell, A.T.; Hayes, W.; Sambrook, M.R.; Leroux, F.; Acton, A.L.; Feula, A.; Gavriel, A.G. Preparation of Diphenylphosphinoethyl Carbamates as Self-Immolative Systems for Detection of Chemical Warfare Agents. WO2020089571, 5 July 2020. [Google Scholar]
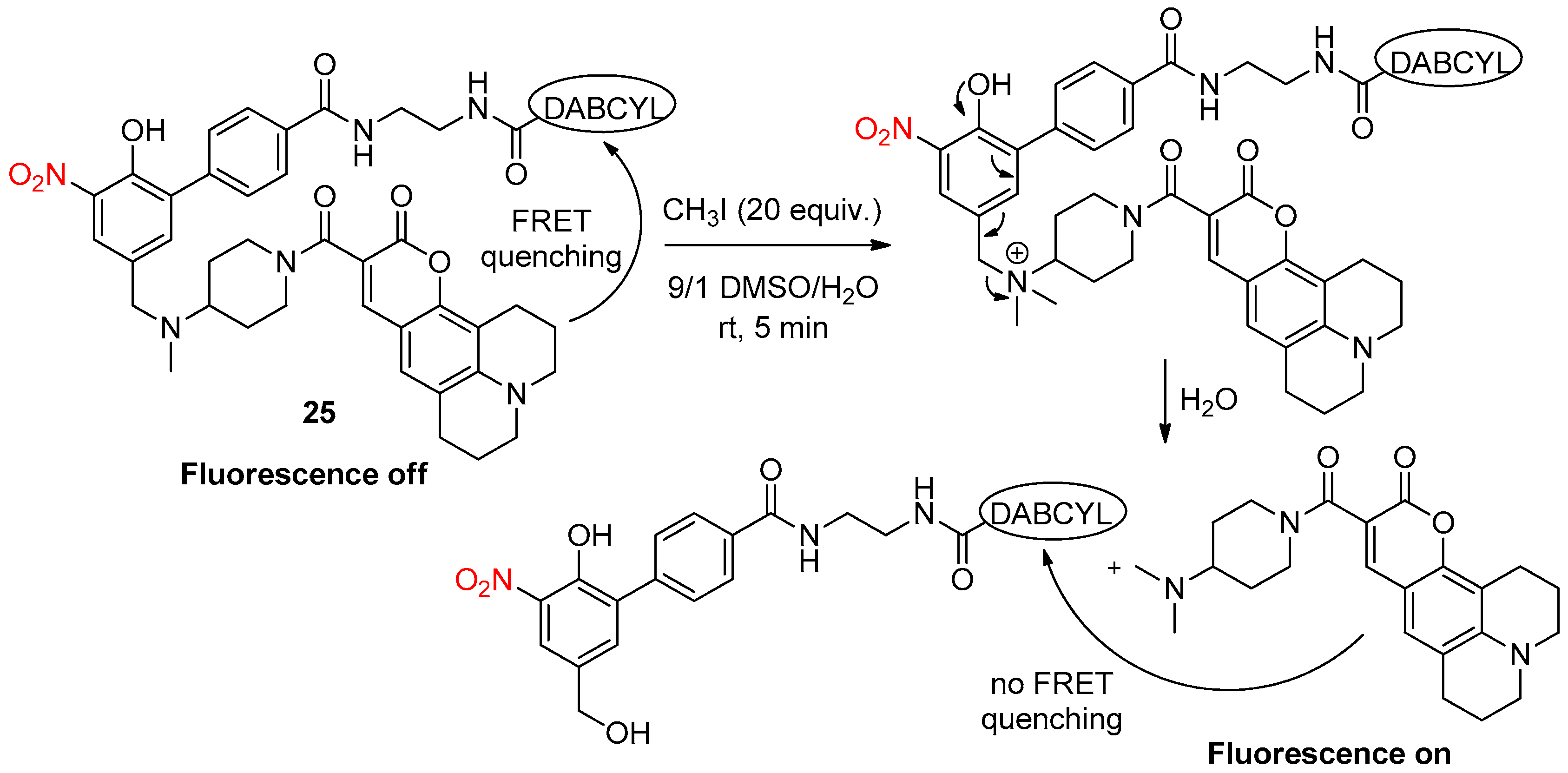
- Tuo, W.; Bouquet, J.; Taran, F.; Le Gall, T. A FRET probe for the detection of alkylating agents. Chem. Commun. 2019, 55, 8655–8658. [Google Scholar] [CrossRef]
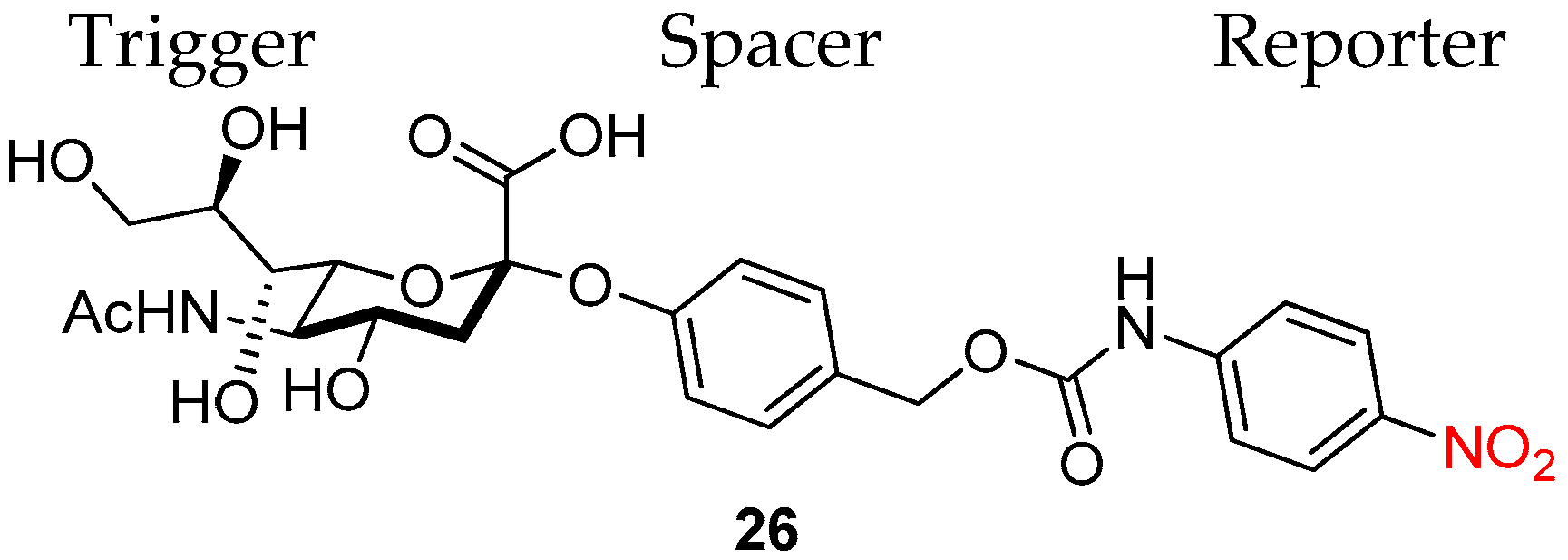
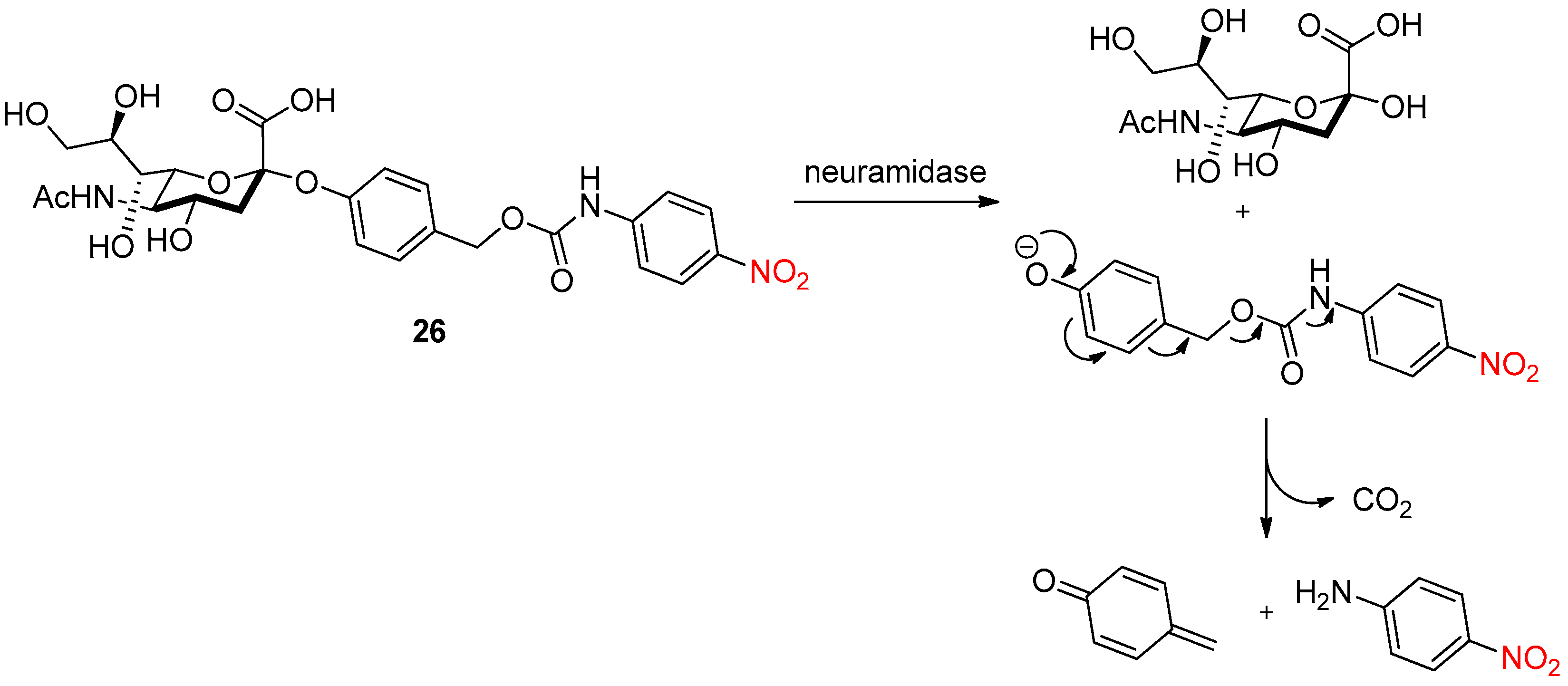
- Liu, W.; Gόmez-Durán, C.F.A.; Smith, B.D. Fluorescent Neuraminidase Assay Based on Supramolecular Dye Capture After Enzymatic Cleavage. J. Am. Chem. Soc. 2017, 139, 6390–6395. [Google Scholar] [CrossRef]
- Zhu, R.; Wang, S.; Xue, Z.; Han, J.; Han, S. Senescence-associated sialidase revealed by an activatable fluorescence-on labeling probe. Chem. Commun. 2018, 54, 11566–11569. [Google Scholar] [CrossRef]
- Matsuhita, T.; Danyel, M.N.; Koyama, T.; Hatano, K.; Matsuoka, K. Neuraminidase-triggered activation of prodrug-type substrate of 4-nitroaniline. Bioorg. Med. Chem. Lett. 2020, 30, 126883. [Google Scholar] [CrossRef]







Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Spitz, C.; Primas, N.; Terme, T.; Vanelle, P. Nitro-Containing Self-Immolative Systems for Biological Applications. Pharmaceuticals 2022, 15, 1404. https://doi.org/10.3390/ph15111404
Spitz C, Primas N, Terme T, Vanelle P. Nitro-Containing Self-Immolative Systems for Biological Applications. Pharmaceuticals. 2022; 15(11):1404. https://doi.org/10.3390/ph15111404
Chicago/Turabian StyleSpitz, Cédric, Nicolas Primas, Thierry Terme, and Patrice Vanelle. 2022. "Nitro-Containing Self-Immolative Systems for Biological Applications" Pharmaceuticals 15, no. 11: 1404. https://doi.org/10.3390/ph15111404
APA StyleSpitz, C., Primas, N., Terme, T., & Vanelle, P. (2022). Nitro-Containing Self-Immolative Systems for Biological Applications. Pharmaceuticals, 15(11), 1404. https://doi.org/10.3390/ph15111404







