Efficacy of Quetiapine Monotherapy and Combination Therapy for Patients with Bipolar Depression with Mixed Features: A Randomized Controlled Pilot Study
Abstract
1. Introduction
2. Results
2.1. Participants
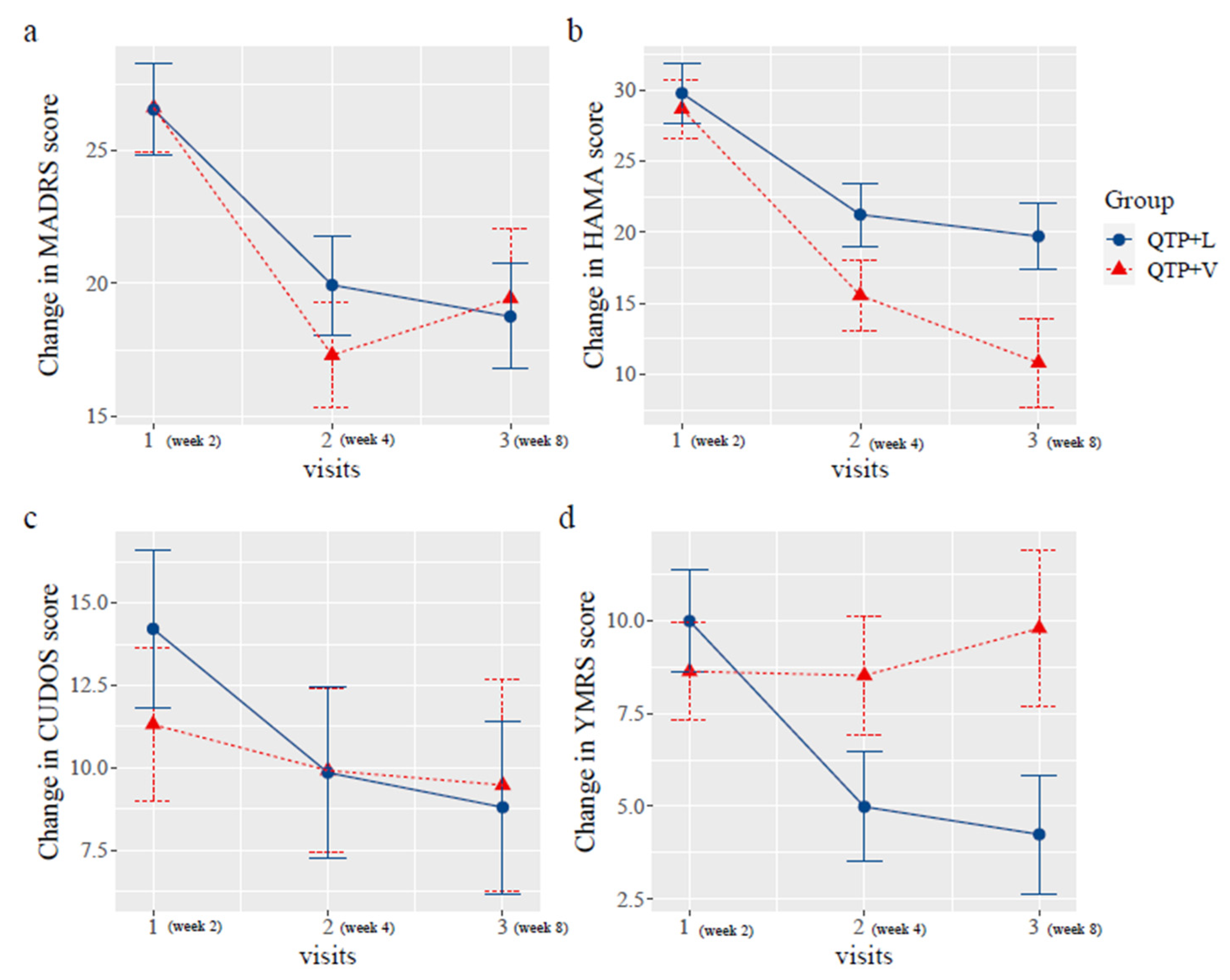
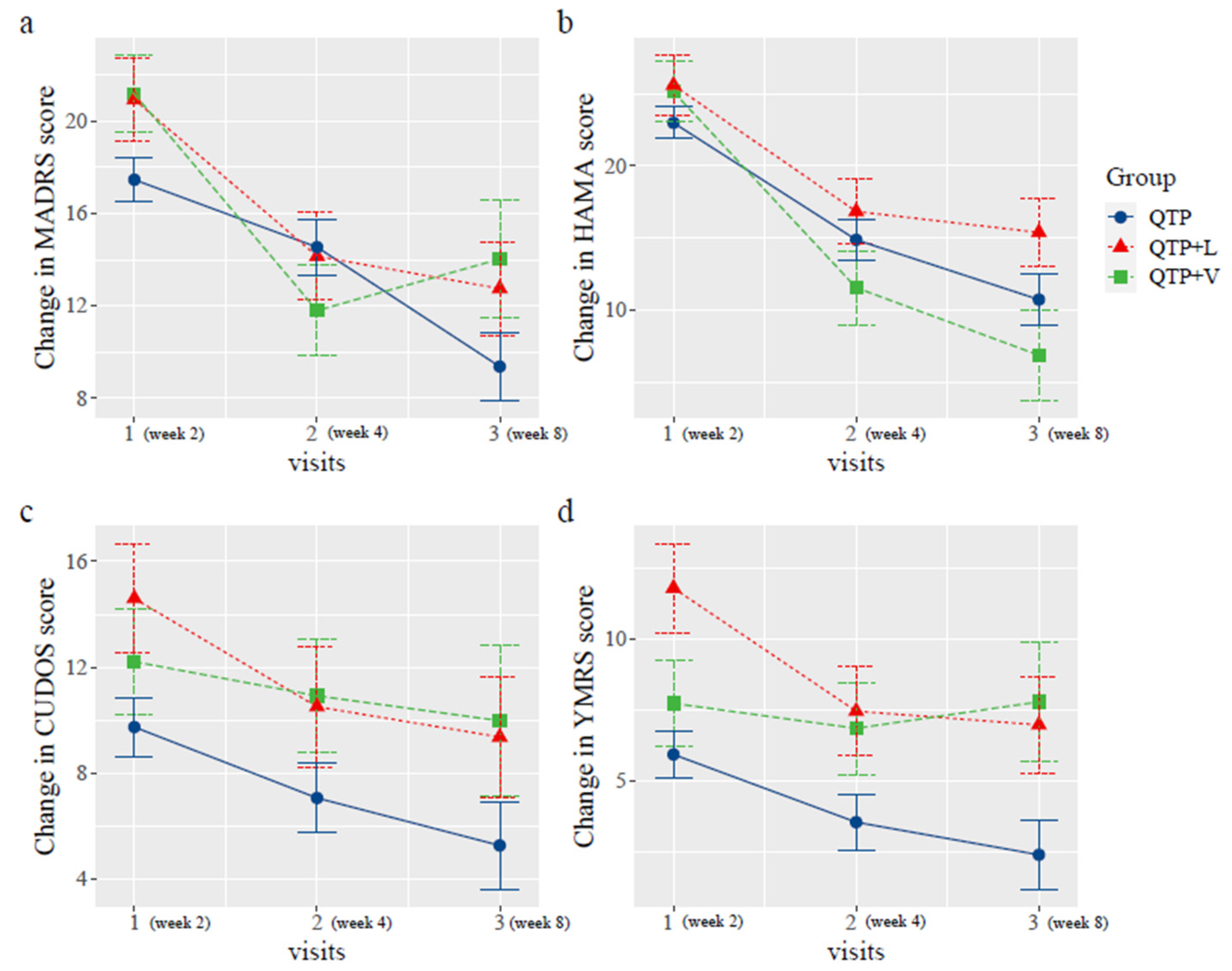
2.2. Change of the Scores
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Patients
5.2. Study Design
5.3. Statistical Analyses
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Craddock, N.; Sklar, P. Genetics of bipolar disorder. Lancet 2013, 381, 1654–1662. [Google Scholar] [CrossRef]
- Niu, Z.; Wu, X.; Zhu, Y.; Yang, L.; Shi, Y.; Wang, Y.; Qiu, H.; Gu, W.; Wu, Y.; Long, X.; et al. Early Diagnosis of Bipolar Disorder Coming Soon: Application of an Oxidative Stress Injury Biomarker (BIOS) Model. Neurosci. Bull. 2022, 38, 979–991. [Google Scholar] [CrossRef]
- Zandi, P.P.; Jaffe, A.E.; Goes, F.S.; Burke, E.E.; Collado-Torres, L.; Huuki-Myers, L.; Seyedian, A.; Lin, Y.; Seifuddin, F.; Pirooznia, M. Amygdala and anterior cingulate transcriptomes from individuals with bipolar disorder reveal downregulated neuroimmune and synaptic pathways. Nat. Neurosci. 2022, 25, 381–389. [Google Scholar] [CrossRef]
- Sublette, M.E.; Cheung, S.; Lieberman, E.; Hu, S.; Mann, J.J.; Uhlemann, A.C.; Miller, J.M. Bipolar disorder and the gut microbiome: A systematic review. Bipolar Disord. 2021, 23, 544–564. [Google Scholar] [CrossRef]
- Swann, A.C.; Steinberg, J.L.; Lijffijt, M.; Moeller, G.F. Continuum of depressive and manic mixed states in patients with bipolar disorder: Quantitative measurement and clinical features. World Psychiatry 2009, 8, 166–172. [Google Scholar] [CrossRef] [PubMed]
- Shim, I.H.; Woo, Y.S.; Bahk, W.M. Prevalence rates and clinical implications of bipolar disorder “with mixed features” as defined by DSM-5. J. Affect. Disord. 2015, 173, 120–125. [Google Scholar] [CrossRef] [PubMed]
- Judd, L.L.; Schettler, P.J.; Akiskal, H.; Coryell, W.; Fawcett, J.; Fiedorowicz, J.G.; Solomon, D.A.; Keller, M.B. Prevalence and clinical significance of subsyndromal manic symptoms, including irritability and psychomotor agitation, during bipolar major depressive episodes. J. Affect. Disord. 2012, 138, 440–448. [Google Scholar] [CrossRef] [PubMed]
- Goldberg, J.F.; Perlis, R.H.; Bowden, C.L.; Thase, M.E.; Miklowitz, D.J.; Marangell, L.B.; Calabrese, J.R.; Nierenberg, A.A.; Sachs, G.S. Manic symptoms during depressive episodes in 1,380 patients with bipolar disorder: Findings from the STEP-BD. Am. J. Psychiatry 2009, 166, 173–181. [Google Scholar] [CrossRef] [PubMed]
- Perugi, G.; Angst, J.; Azorin, J.M.; Bowden, C.L.; Mosolov, S.; Reis, J.; Vieta, E.; Young, A.H.; Group, B.R.-I.-M.S. Mixed features in patients with a major depressive episode: The BRIDGE-II-MIX study. J. Clin. Psychiatry 2015, 76, e351–e358. [Google Scholar] [CrossRef] [PubMed]
- Frankland, A.; Cerrillo, E.; Hadzi-Pavlovic, D.; Roberts, G.; Wright, A.; Loo, C.K.; Breakspear, M.; Mitchell, P.B. Comparing the phenomenology of depressive episodes in bipolar I and II disorder and major depressive disorder within bipolar disorder pedigrees. J. Clin. Psychiatry 2015, 76, 32–38, quiz 39. [Google Scholar] [CrossRef] [PubMed]
- Persons, J.E.; Coryell, W.H.; Solomon, D.A.; Keller, M.B.; Endicott, J.; Fiedorowicz, J.G. Mixed state and suicide: Is the effect of mixed state on suicidal behavior more than the sum of its parts? Bipolar Disord. 2018, 20, 35–41. [Google Scholar] [CrossRef]
- Balazs, J.; Benazzi, F.; Rihmer, Z.; Rihmer, A.; Akiskal, K.K.; Akiskal, H.S. The close link between suicide attempts and mixed (bipolar) depression: Implications for suicide prevention. J. Affect. Disord. 2006, 91, 133–138. [Google Scholar] [CrossRef] [PubMed]
- Nusslock, R.; Frank, E. Subthreshold bipolarity: Diagnostic issues and challenges. Bipolar Disord. 2011, 13, 587–603. [Google Scholar] [CrossRef] [PubMed]
- Pacchiarotti, I.; Bond, D.J.; Baldessarini, R.J.; Nolen, W.A.; Grunze, H.; Licht, R.W.; Post, R.M.; Berk, M.; Goodwin, G.M.; Sachs, G.S.; et al. The International Society for Bipolar Disorders (ISBD) task force report on antidepressant use in bipolar disorders. Am. J. Psychiatry 2013, 170, 1249–1262. [Google Scholar] [CrossRef]
- Vieta, E.; Valenti, M. Pharmacological management of bipolar depression: Acute treatment, maintenance, and prophylaxis. CNS Drugs 2013, 27, 515–529. [Google Scholar] [CrossRef]
- Faedda, G.L.; Marangoni, C.; Reginaldi, D. Depressive mixed states: A reappraisal of Koukopoulos’ criteria. J. Affect. Disord. 2015, 176, 18–23. [Google Scholar] [CrossRef]
- Yatham, L.N.; Chakrabarty, T.; Bond, D.J.; Schaffer, A.; Beaulieu, S.; Parikh, S.V.; McIntyre, R.S.; Milev, R.V.; Alda, M.; Vazquez, G.; et al. Canadian Network for Mood and Anxiety Treatments (CANMAT) and International Society for Bipolar Disorders (ISBD) recommendations for the management of patients with bipolar disorder with mixed presentations. Bipolar Disord. 2021, 23, 767–788. [Google Scholar] [CrossRef] [PubMed]
- Fornaro, M.; Stubbs, B.; De Berardis, D.; Perna, G.; Valchera, A.; Veronese, N.; Solmi, M.; Gananca, L. Atypical Antipsychotics in the Treatment of Acute Bipolar Depression with Mixed Features: A Systematic Review and Exploratory Meta-Analysis of Placebo-Controlled Clinical Trials. Int. J. Mol. Sci. 2016, 17, 241. [Google Scholar] [CrossRef] [PubMed]
- Suttajit, S.; Srisurapanont, M.; Maneeton, N.; Maneeton, B. Quetiapine for acute bipolar depression: A systematic review and meta-analysis. Drug Des. Devel Ther. 2014, 8, 827–838. [Google Scholar] [CrossRef] [PubMed]
- Scherk, H.; Pajonk, F.G.; Leucht, S. Second-generation antipsychotic agents in the treatment of acute mania: A systematic review and meta-analysis of randomized controlled trials. Arch. Gen. Psychiatry 2007, 64, 442–455. [Google Scholar] [CrossRef]
- Weisler, R.H.; Nolen, W.A.; Neijber, A.; Hellqvist, A.; Paulsson, B.; Trial 144 Study, I. Continuation of quetiapine versus switching to placebo or lithium for maintenance treatment of bipolar I disorder (Trial 144: A randomized controlled study). J. Clin. Psychiatry 2011, 72, 1452–1464. [Google Scholar] [CrossRef]
- Muneer, A. Pharmacotherapy of bipolar disorder with quetiapine: A recent literature review and an update. Clin. Psychopharmacol. Neurosci. 2015, 13, 25–35. [Google Scholar] [CrossRef] [PubMed]
- Yatham, L.N.; Goldstein, J.M.; Vieta, E.; Bowden, C.L.; Grunze, H.; Post, R.M.; Suppes, T.; Calabrese, J.R. Atypical antipsychotics in bipolar depression: Potential mechanisms of action. J. Clin. Psychiatry 2005, 66, 40–48. [Google Scholar] [PubMed]
- Sokolski, K.N.; Denson, T.F. Adjunctive quetiapine in bipolar patients partially responsive to lithium or valproate. Prog. Neuropsychopharmacol. Biol. Psychiatry 2003, 27, 863–866. [Google Scholar] [CrossRef] [PubMed]
- Goldberg, J.F.; Siu, C.; Mao, Y.; Tsai, J.; Pikalov, A.; Calabrese, J.R.; Loebel, A. Major depressive disorder with mixed features and treatment response to lurasidone: A symptom network model. J. Affect. Disord. 2020, 277, 1045–1054. [Google Scholar] [CrossRef]
- Goldberg, J.F.; Ng-Mak, D.; Siu, C.; Chuang, C.C.; Rajagopalan, K.; Loebel, A. Remission and recovery associated with lurasidone in the treatment of major depressive disorder with subthreshold hypomanic symptoms (mixed features): Post-hoc analysis of a randomized, placebo-controlled study with longer-term extension. CNS Spectr. 2017, 22, 220–227. [Google Scholar] [CrossRef]
- Yatham, L.N.; Kennedy, S.H.; Parikh, S.V.; Schaffer, A.; Bond, D.J.; Frey, B.N.; Sharma, V.; Goldstein, B.I.; Rej, S.; Beaulieu, S.; et al. Canadian Network for Mood and Anxiety Treatments (CANMAT) and International Society for Bipolar Disorders (ISBD) 2018 guidelines for the management of patients with bipolar disorder. Bipolar Disord. 2018, 20, 97–170. [Google Scholar] [CrossRef]
- Vieta, E.; Suppes, T.; Ekholm, B.; Udd, M.; Gustafsson, U. Long-term efficacy of quetiapine in combination with lithium or divalproex on mixed symptoms in bipolar I disorder. J. Affect. Disord. 2012, 142, 36–44. [Google Scholar] [CrossRef]
- Baldessarini, R.J.; Tondo, L. Suicide risk and treatments for patients with bipolar disorder. Jama 2003, 290, 1517–1519. [Google Scholar] [CrossRef]
- Thibaut, F.; De La Barra, F.; Gordon, H.; Cosyns, P.; Bradford, J.M.; Disorders, W.T.F.o.S. The World Federation of Societies of Biological Psychiatry (WFSBP) guidelines for the biological treatment of paraphilias. World J. Biol. Psychiatry 2010, 11, 604–655. [Google Scholar] [CrossRef]
- Fountoulakis, K.N.; Yatham, L.; Grunze, H.; Vieta, E.; Young, A.; Blier, P.; Kasper, S.; Moeller, H.J. The International College of Neuro-Psychopharmacology (CINP) Treatment Guidelines for Bipolar Disorder in Adults (CINP-BD-2017), Part 2: Review, Grading of the Evidence, and a Precise Algorithm. Int. J. Neuropsychopharmacol. 2017, 20, 121–179. [Google Scholar] [CrossRef]
- Grunze, H.; Vieta, E.; Goodwin, G.M.; Bowden, C.; Licht, R.W.; Azorin, J.M.; Yatham, L.; Mosolov, S.; Moller, H.J.; Kasper, S.; et al. The World Federation of Societies of Biological Psychiatry (WFSBP) Guidelines for the Biological Treatment of Bipolar Disorders: Acute and long-term treatment of mixed states in bipolar disorder. World J. Biol. Psychiatry 2018, 19, 2–58. [Google Scholar] [CrossRef] [PubMed]
- Goodwin, G.M.; Haddad, P.M.; Ferrier, I.N.; Aronson, J.K.; Barnes, T.; Cipriani, A.; Coghill, D.R.; Fazel, S.; Geddes, J.R.; Grunze, H.; et al. Evidence-based guidelines for treating bipolar disorder: Revised third edition recommendations from the British Association for Psychopharmacology. J. Psychopharmacol. 2016, 30, 495–553. [Google Scholar] [CrossRef] [PubMed]
- National Institute for Health and Care Excellence: Clinical Guidelines. Bipolar Disorder: The NICE Guideline on the Assessment and Management of Bipolar Disorder in Adults, Children and Young People in Primary and Secondary Care; The British Psychological Society and The Royal College of Psychiatrists: London, UK, 2014. [Google Scholar]
- Sani, G.; Fiorillo, A. The use of lithium in mixed states. CNS Spectr. 2020, 25, 449–451. [Google Scholar] [CrossRef]
- Bartoli, F. Mixed features and suicidal behavior in bipolar disorder: A clinical relationship that calls for lithium treatment. Bipolar Disord. 2022, 24, 8–9. [Google Scholar] [CrossRef] [PubMed]
- Buoli, M.; Serati, M.; Altamura, A.C. Is the combination of a mood stabilizer plus an antipsychotic more effective than mono-therapies in long-term treatment of bipolar disorder? A systematic review. J. Affect. Disord. 2014, 152–154, 12–18. [Google Scholar] [CrossRef] [PubMed]
- Tom, A.; Bosker, T.A.S.R.J.; Bosker, R.J. Multilevel Analysis: An Introduction to Basic and Advanced Multilevel Modeling; Sage: Los Angeles, CA, USA, 1999. [Google Scholar]
- Ketter, T.A.; Miller, S.; Dell’Osso, B.; Wang, P.W. Treatment of bipolar disorder: Review of evidence regarding quetiapine and lithium. J. Affect. Disord. 2016, 191, 256–273. [Google Scholar] [CrossRef] [PubMed]
- Mixed episodes and suicide risk: A community sample of young adults. J. Affect. Disord. 2020, 266, 252–257. [CrossRef] [PubMed]
- Azorin, J.M.; Kaladjian, A.; Adida, M.; Fakra, E.; Belzeaux, R.; Hantouche, E.; Lancrenon, S. Self-assessment and characteristics of mixed depression in the French national EPIDEP study. J. Affect. Disord. 2012, 143, 109–117. [Google Scholar] [CrossRef] [PubMed]
- Kemp, D.E.; Ganocy, S.J.; Brecher, M.; Carlson, B.X.; Edwards, S.; Eudicone, J.M.; Evoniuk, G.; Jansen, W.; Leon, A.C.; Minkwitz, M.; et al. Clinical value of early partial symptomatic improvement in the prediction of response and remission during short-term treatment trials in 3369 subjects with bipolar I or II depression. J. Affect. Disord. 2011, 130, 171–179. [Google Scholar] [CrossRef]
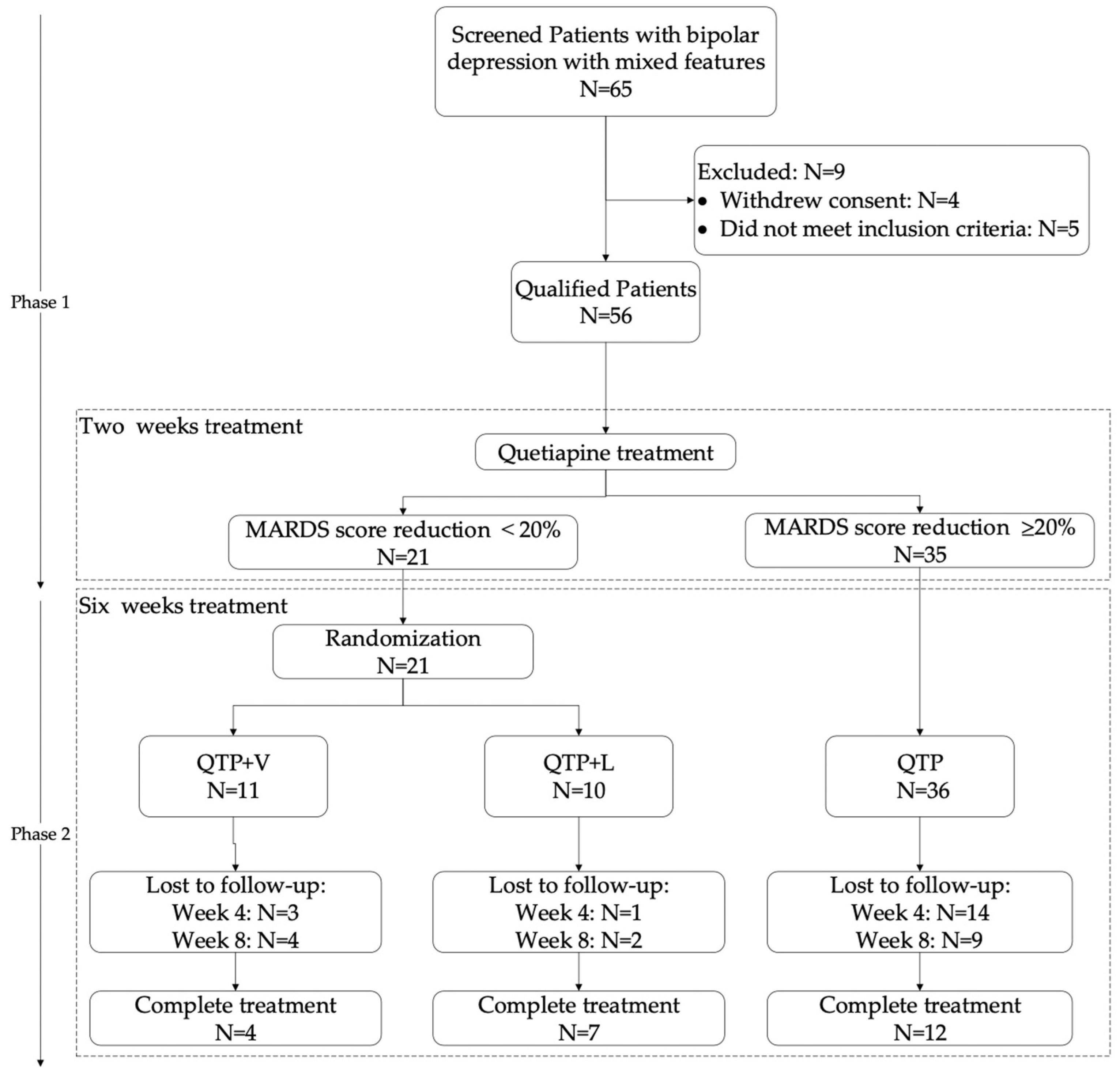
| QTP + V (N = 11) | QTP + L (N = 10) | QTP (N = 35) | Total (N = 56) | |
|---|---|---|---|---|
| Age | 20.18 ± 4.42 | 22.40 ± 8.30 | 22.14 ± 7.44 | 24.80 ± 7.05 |
| Sex (N/%) | ||||
| Male | 3 (27.27) | 4 (40.00) | 18 (51.43) | 25 (44.64) |
| Female | 8 (72.73) | 6 (60.00) | 17 (48.57) | 31 (55.35) |
| Education (year) | 11.50 ± 2.37 | 11.80 ± 2.30 | 12.71 ± 2.32 | 12.33 ± 2.34 |
| Illness Duration (month) | 62.40 ± 55.37 | 55.50 ± 36.60 | 66.03 ± 60.85 | 63.41 ± 55.4 |
| QTP + V (N = 11) | QTP + L (N = 10) | QTP (N = 35) | Total (N = 56) | ||
|---|---|---|---|---|---|
| Baseline | MARDS | N = 11 | N = 10 | N = 35 | N = 56 |
| 30.09 ± 8.11 | 33.20 ± 7.77 | 28.66 ± 8.08 | 29.75 ± 8.07 | ||
| HAMA | N = 11 | N = 10 | N = 35 | N = 56 | |
| 25.27 ± 10.55 | 30.50 ± 9.58 | 26.46 ± 9.05 | 26.95 ± 6.43 | ||
| YMRS | N = 11 | N = 10 | N = 35 | N = 56 | |
| 14.55 ± 6.83 | 14.20 ± 6.00 | 13.03 ± 5.62 | 13.54 ± 5.86 | ||
| CUDOS | N = 11 | N = 8 | N = 30 | N = 49 | |
| 16.91 ± 6.47 | 17.88 ± 5.33 | 19.23 ± 8.76 | 18.49 ± 7.76 | ||
| Week 2 | MARDS | N = 10 | N = 9 | N = 34 | N = 53 |
| 26.70 ± 9.81 | 26.78 ± 7.46 | 13.15 ± 6.68 | 18.02 ± 9.84 | ||
| HAMA | N = 9 | N = 9 | N = 33 | N = 51 | |
| 28.89 ± 8.27 | 28.44 ± 9.13 | 19.88 ± 11.43 | 22.98 ± 11.22 | ||
| YMRS | N = 10 | N = 9 | N = 34 | N = 53 | |
| 7.20 ± 5.98 | 11.33 ± 7.63 | 6.06 ± 4.85 | 7.17 ± 5.82 | ||
| CUDOS | N = 10 | N = 9 | N = 32 | N = 51 | |
| 10.50 ± 7.88 | 14.11 ± 9.98 | 9.97 ± 6.67 | 10.08 ± 7.56 | ||
| Week 4 | MARDS | N = 8 | N = 9 | N = 21 | N = 38 |
| 16.25 ± 6.73 | 22.00 ± 10.71 | 10.38 ± 6.86 | 14.37 ± 9.10 | ||
| HAMA | N = 8 | N = 9 | N = 21 | N = 38 | |
| 15.88 ± 8.32 | 21.3 ± 9.70 | 11.81 ± 6.87 | 14.92 ± 8.64 | ||
| YMRS | N = 8 | N = 9 | N = 21 | N = 38 | |
| 5.75 ± 5.97 | 8.00 ± 5.74 | 4.10 ± 2.84 | 5.37 ± 4.57 | ||
| CUDOS | N = 8 | N = 7 | N = 21 | N = 36 | |
| 10.63 ± 8.75 | 12.00 ± 8.14 | 7.38 ± 4.40 | 9.00 ± 6.46 | ||
| Week 8 | MARDS | N = 4 | N = 7 | N = 12 | N = 23 |
| 17.00 ± 7.07 | 19.86 ± 9.84 | 6.92 ± 7.05 | 12.61 ± 9.80 | ||
| HAMA | N = 4 | N = 7 | N = 12 | N = 23 | |
| 13.50 ± 9.54 | 19.57 ± 9.48 | 10.08 ± 9.47 | 13.57 ± 9.99 | ||
| YMRS | N = 4 | N = 7 | N = 12 | N = 23 | |
| 5.00 ± 4.24 | 7.43 ± 7.18 | 3.17 ± 2.52 | 4.78 ± 4.83 | ||
| CUDOS | N = 4 | N = 7 | N = 12 | N = 23 | |
| 6.00 ± 7.35 | 10.29 ± 4.89 | 5.75 ± 5.07 | 7.17 ± 5.58 |
| Week 2 | Week 8 | Difference (95%CI) | p Value | ||
|---|---|---|---|---|---|
| MADRS | time * group | 0.545 | |||
| QTP + L | 26.78 | 19.86 | −7.18 (−13.04, −1.32) | 0.025 | |
| QTP + V | 26.70 | 17.00 | −8.6 (−15.75, −1.45) | 0.027 | |
| HAMA | time * group | 0.320 | |||
| QTP + L | 28.44 | 19.57 | −9.25 (−16.29, −2.21) | 0.017 | |
| QTP + V | 28.89 | 13.50 | −16.25 (−24.93, −7.57) | <0.001 | |
| CUDOS | time * group | 0.640 | |||
| QTP + L | 14.11 | 10.29 | −5.41 (−10.98, 0.16) | 0.071 | |
| QTP + V | 10.50 | 6.00 | −1.84 (−8.7, 5.02) | 0.605 | |
| YMRS | time * group | 0.300 | |||
| QTP + L | 11.33 | 7.43 | −4.82 (−9.33, −0.31) | 0.047 | |
| QTP + V | 7.20 | 5.00 | 0.23 (−5.36, 5.82) | 0.936 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Z.; Zhang, D.; Du, Y.; Wang, Y.; Huang, T.; Ng, C.H.; Huang, H.; Pan, Y.; Lai, J.; Hu, S. Efficacy of Quetiapine Monotherapy and Combination Therapy for Patients with Bipolar Depression with Mixed Features: A Randomized Controlled Pilot Study. Pharmaceuticals 2023, 16, 287. https://doi.org/10.3390/ph16020287
Wang Z, Zhang D, Du Y, Wang Y, Huang T, Ng CH, Huang H, Pan Y, Lai J, Hu S. Efficacy of Quetiapine Monotherapy and Combination Therapy for Patients with Bipolar Depression with Mixed Features: A Randomized Controlled Pilot Study. Pharmaceuticals. 2023; 16(2):287. https://doi.org/10.3390/ph16020287
Chicago/Turabian StyleWang, Zheng, Danhua Zhang, Yanli Du, Yin Wang, Tingting Huang, Chee H. Ng, Huimin Huang, Yanmeng Pan, Jianbo Lai, and Shaohua Hu. 2023. "Efficacy of Quetiapine Monotherapy and Combination Therapy for Patients with Bipolar Depression with Mixed Features: A Randomized Controlled Pilot Study" Pharmaceuticals 16, no. 2: 287. https://doi.org/10.3390/ph16020287
APA StyleWang, Z., Zhang, D., Du, Y., Wang, Y., Huang, T., Ng, C. H., Huang, H., Pan, Y., Lai, J., & Hu, S. (2023). Efficacy of Quetiapine Monotherapy and Combination Therapy for Patients with Bipolar Depression with Mixed Features: A Randomized Controlled Pilot Study. Pharmaceuticals, 16(2), 287. https://doi.org/10.3390/ph16020287






