The Role of the Adenosine System on Emotional and Cognitive Disturbances Induced by Ethanol Binge Drinking in the Immature Brain and the Beneficial Effects of Caffeine
Abstract
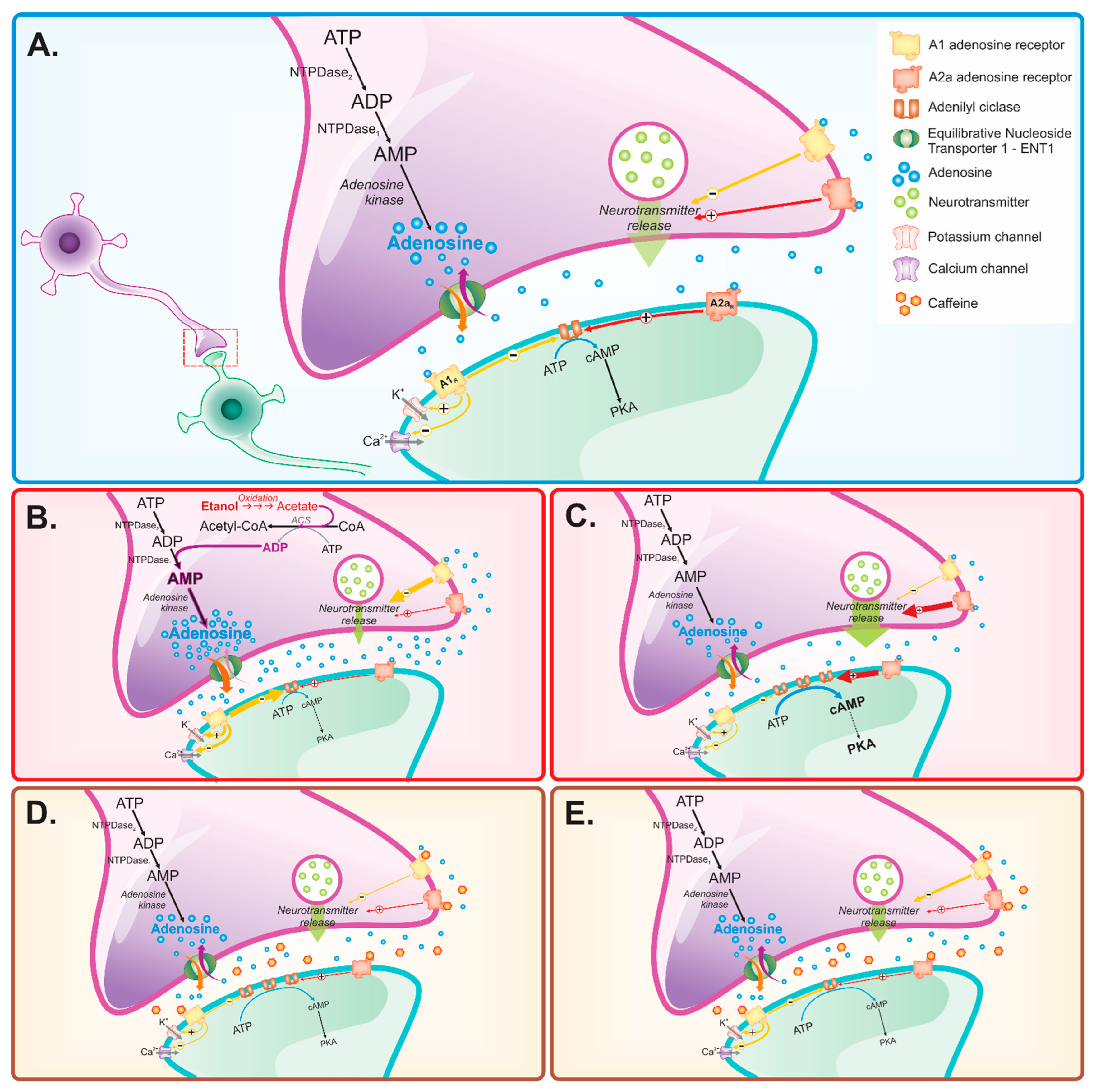
1. Introduction
2. Ethanol versus Adenosine Effects on Anxiety
3. Ethanol versus Adenosine Effects on Depression
4. Ethanol versus Adenosine Effects on Cognition
5. Caffeine as a Therapeutic Tool in Ethanol-Induced Anxiety, Depression, and Cognitive Disorders
5.1. Anxiety
5.2. Depression
5.3. Cognitive Deficits
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- National Institute on Alcohol Abuse and Alcoholism (NIAAA). Council Approves Definition of Binge Drinking. NIAAA Newsletter, Winter 2004; p. 3. Available online: https://pubs.niaaa.nih.gov/publications/Newsletter/winter2004/Newsletter_Number3.pdf (accessed on 1 September 2022).
- Spear, L.P. Effects of Adolescent Alcohol Consumption on the Brain and Behaviour. Nat. Rev. Neurosci. 2018, 19, 197–214. [Google Scholar] [CrossRef] [PubMed]
- Crews, F.; He, J.; Hodge, C. Adolescent Cortical Development: A Critical Period of Vulnerability for Addiction. Pharmacol. Biochem. Behav. 2007, 86, 189–199. [Google Scholar] [CrossRef]
- Izumi, Y.; Nagashima, K.; Murayama, K.; Zorumski, C.F. Acute Effects of Ethanol on Hippocampal Long-Term Potentiation and Long-Term Depression Are Mediated by Different Mechanisms. Neuroscience 2005, 136, 509–517. [Google Scholar] [CrossRef]
- Slawecki, C.J.; Betancourt, M. Effects of Adolescent Ethanol Exposure on Ethanol Consumption in Adult Rats. Alcohol 2002, 26, 23–30. [Google Scholar] [CrossRef]
- Giedd, J.N. The Teen Brain: Insights from Neuroimaging. J. Adolesc. Health 2008, 42, 335–343. [Google Scholar] [CrossRef]
- Engel, J.A.; Jerlhag, E. Alcohol. Prog. Brain Res. 2014, 211, 201–233. [Google Scholar] [CrossRef]
- Pascual, M.; Boix, J.; Felipo, V.; Guerri, C. Repeated Alcohol Administration during Adolescence Causes Changes in the Mesolimbic Dopaminergic and Glutamatergic Systems and Promotes Alcohol Intake in the Adult Rat. J. Neurochem. 2009, 108, 920–931. [Google Scholar] [CrossRef]
- Belém-Filho, I.J.A.; Ribera, P.C.; Nascimento, A.L.; Gomes, A.R.Q.; Lima, R.R.; Crespo-Lopez, M.E.; Monteiro, M.C.; Fontes-Júnior, E.A.; Lima, M.O.; Maia, C.S.F. Low Doses of Methylmercury Intoxication Solely or Associated to Ethanol Binge Drinking Induce Psychiatric-like Disorders in Adolescent Female Rats. Environ. Toxicol. Pharmacol. 2018, 60, 184–194. [Google Scholar] [CrossRef]
- Fernandes, L.M.P.; Cartágenes, S.C.; Barros, M.A.; Carvalheiro, T.C.V.S.; Castro, N.C.F.; Schamne, M.G.; Lima, R.R.; Prediger, R.D.; Monteiro, M.C.; Fontes-Júnior, E.A.; et al. Repeated Cycles of Binge-like Ethanol Exposure Induce Immediate and Delayed Neurobehavioral Changes and Hippocampal Dysfunction in Adolescent Female Rats. Behav. Brain Res. 2018, 350, 99–108. [Google Scholar] [CrossRef]
- Fernandes, L.M.P.; Lopes, K.S.; Santana, L.N.S.; Fontes-Júnior, E.A.; Ribeiro, C.H.M.A.; Silva, M.C.F.; de Oliveira Paraense, R.S.; Crespo-López, M.E.; Gomes, A.R.Q.; Lima, R.R.; et al. Repeated Cycles of Binge-like Ethanol Intake in Adolescent Female Rats Induce Motor Function Impairment and Oxidative Damage in Motor Cortex and Liver, but Not in Blood. Oxidative Med. Cell. Longev. 2018, 2018, 1–14. [Google Scholar] [CrossRef]
- Maia, C.D.S.F.; Queiroz, L.Y.; Oliveira, I.G.; Silva, C.C.S.; Cunha, R.A.; Souza-Monteiro, D.; Ferreira, M.K.M.; Silveira, F.M.; Silva, J.C.; Balbinot, G.d.S.; et al. Binge-like Exposure during Adolescence Induces Detrimental Effects in Alveolar Bone That Persist in Adulthood. Alcohol. Clin. Exp. Res. 2020, 45, 56–63. [Google Scholar] [CrossRef] [PubMed]
- Lamarão-Vieira, K.; Pamplona-Santos, D.; Nascimento, P.C.; Corrêa, M.G.; Bittencourt, L.O.; dos Santos, S.M.; Cartágenes, S.C.; Fernandes, L.M.P.; Monteiro, M.C.; Maia, C.S.F.; et al. Physical Exercise Attenuates Oxidative Stress and Morphofunctional Cerebellar Damages Induced by the Ethanol Binge Drinking Paradigm from Adolescence to Adulthood in Rats. Oxidative Med. Cell. Longev. 2019, 2019, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Maia, C.; Pinheiro, B.G.; Soares da Silva, C.C.; Cunha, R.A.; Souza-Monteiro, D.; Martins Ferreira, M.K.; Schmidt, T.R.; de Souza Balbinot, G.; Collares, F.M.; Martins, M.D.; et al. Prolonged Caffeine Intake Decreases Alveolar Bone Damage Induced by Binge-like Ethanol Consumption in Adolescent Female Rats. Biomed. Pharmacother. 2020, 130, 110608. [Google Scholar] [CrossRef] [PubMed]
- Paiva, I.; Cellai, L.; Meriaux, C.; Poncelet, L.; Nebie, O.; Saliou, J.-M.; Lacoste, A.-S.; Papegaey, A.; Drobecq, H.; Le Gras, S.; et al. Caffeine Intake Exerts Dual Genome-Wide Effects on Hippocampal Metabolism and Learning-Dependent Transcription. J. Clin. Investig. 2022, 132, e149371. [Google Scholar] [CrossRef] [PubMed]
- Cunha, R.A.; Constantino, M.D.; Sebastião, A.M.; Ribeiro, J.A. Modification of A1 and A2A Adenosine Receptor Binding in Aged Striatum, Hippocampus and Cortex of the Rat. NeuroReport 1995, 6, 1583. [Google Scholar] [CrossRef]
- Kaster, M.P.; Machado, N.J.; Silva, H.B.; Nunes, A.; Ardais, A.P.; Santana, M.; Baqi, Y.; Müller, C.E.; Rodrigues, A.L.S.; Porciúncula, L.O.; et al. Caffeine Acts through Neuronal Adenosine A2A Receptors to Prevent Mood and Memory Dysfunction Triggered by Chronic Stress. Proc. Natl. Acad. Sci. USA 2015, 112, 7833–7838. [Google Scholar] [CrossRef]
- Fredholm, B.B.; Bättig, K.; Holmén, J.; Nehlig, A.; Zvartau, E.E. Actions of Caffeine in the Brain with Special Reference to Factors That Contribute to Its Widespread Use. Pharmacol. Rev. 1999, 51, 83–133. [Google Scholar]
- Lopes, J.P.; Pliássova, A.; Cunha, R.A. The Physiological Effects of Caffeine on Synaptic Transmission and Plasticity in the Mouse Hippocampus Selectively Depend on Adenosine A1 and A2A Receptors. Biochem. Pharmacol. 2019, 166, 313–321. [Google Scholar] [CrossRef]
- Cunha, R.A. How Does Adenosine Control Neuronal Dysfunction and Neurodegeneration? J. Neurochem. 2016, 139, 1019–1055. [Google Scholar] [CrossRef]
- Fredholm, B.B.; Chen, J.-F.; Masino, S.A.; Vaugeois, J.-M. Actions of adenosine at its receptors in the CNS: Insights from Knockouts and Drugs. Annu. Rev. Pharmacol. Toxicol. 2005, 45, 385–412. [Google Scholar] [CrossRef]
- Wardas, J. Neuroprotective Role of Adenosine in the CNS. Pol. J. Pharmacol. 2002, 54, 313–326. [Google Scholar] [PubMed]
- Dunwiddie, T.V.; Masino, S.A. The Role and Regulation of Adenosine in the Central Nervous System. Annu. Rev. Neurosci. 2001, 24, 31–55. [Google Scholar] [CrossRef] [PubMed]
- Borycz, J.; Pereira, M.F.; Melani, A.; Rodrigues, R.J.; Köfalvi, A.; Panlilio, L.; Pedata, F.; Goldberg, S.R.; Cunha, R.A.; Ferré, S. Differential Glutamate-Dependent and Glutamate-Independent Adenosine a1Receptor-Mediated Modulation of Dopamine Release in Different Striatal Compartments. J. Neurochem. 2007, 101, 355–363. [Google Scholar] [CrossRef] [PubMed]
- Ferré, S.; Bonaventura, J.; Zhu, W.; Hatcher-Solis, C.; Taura, J.; Quiroz, C.; Cai, N.-S.; Moreno, E.; Casadó-Anguera, V.; Kravitz, A.V.; et al. Essential Control of the Function of the Striatopallidal Neuron by Pre-Coupled Complexes of Adenosine A2A-Dopamine D2 Receptor Heterotetramers and Adenylyl Cyclase. Front. Pharmacol. 2018, 9, 243. [Google Scholar] [CrossRef]
- Simões, A.P.; Machado, N.J.; Gonçalves, N.; Kaster, M.P.; Simões, A.T.; Nunes, A.; Pereira de Almeida, L.; Goosens, K.A.; Rial, D.; Cunha, R.A. Adenosine A2A Receptors in the Amygdala Control Synaptic Plasticity and Contextual Fear Memory. Neuropsychopharmacology 2016, 41, 2862–2871. [Google Scholar] [CrossRef] [PubMed]
- Delle Donne, K.T.; Sonsalla, P.K. Protection against Methamphetamine-Induced Neurotoxicity to Neostriatal Dopaminergic Neurons by Adenosine Receptor Activation. J. Pharmacol. Exp. Ther. 1994, 271, 1320–1326. [Google Scholar]
- Ferré, S.; Borycz, J.; Goldberg, S.R.; Hope, B.T.; Morales, M.; Lluis, C.; Franco, R.; Ciruela, F.; Cunha, R. Role of Adenosine in the control of Homosynaptic plasticity in striatal excitatory synapses. J. Integr. Neurosci. 2005, 4, 445–464. [Google Scholar] [CrossRef]
- Impagnatiello, F.; Bastia, E.; Ongini, E.; Monopoli, A. Adenosine Receptors in Neurological Disorders. Emerg. Ther. Targets 2000, 4, 635–664. [Google Scholar] [CrossRef]
- Ferré, S.; Quiroz, C.; Woods, A.; Cunha, R.; Popoli, P.; Ciruela, F.; Lluis, C.; Franco, R.; Azdad, K.; Schiffmann, S. An Update on Adenosine A2A-Dopamine D2 Receptor Interactions: Implications for the Function of G Protein-Coupled Receptors. Curr. Pharm. Des. 2008, 14, 1468–1474. [Google Scholar] [CrossRef]
- Ferré, S.; Fuxe, K.; BFredholm, B.B.; Morelli, M.; Popoli, P. Adenosine–Dopamine Receptor–Receptor Interactions as an Integrative Mechanism in the Basal Ganglia. Trends Neurosci. 1997, 20, 482–487. [Google Scholar] [CrossRef]
- Ballesteros-Yáñez, I.; Castillo, C.A.; Merighi, S.; Gessi, S. The Role of Adenosine Receptors in Psychostimulant Addiction. Front. Pharmacol. 2018, 8, 985. [Google Scholar] [CrossRef] [PubMed]
- Diao, L.; Dunwiddie, T.V. Interactions between Ethanol, Endogenous Adenosine and Adenosine Uptake in Hippocampal Brain Slices. J. Pharmacol. Exp. Ther. 1996, 278, 542–546. [Google Scholar] [PubMed]
- Ferré, S.; O’Brien, M.C. Alcohol and Caffeine: The Perfect Storm. J. Caffeine Res. 2011, 1, 153–162. [Google Scholar] [CrossRef]
- Ruby, C.L.; Adams, C.A.; Knight, E.J.; Wook Nam, H.; Choi, D.-S. An Essential Role for Adenosine Signaling in Alcohol Abuse. Curr. Drug Abus. Rev. 2010, 3, 163–174. [Google Scholar] [CrossRef][Green Version]
- Choi, D.-S.; Cascini, M.-G.; Mailliard, W.; Young, H.; Paredes, P.; McMahon, T.; Diamond, I.; Bonci, A.; Messing, R.O. The Type 1 Equilibrative Nucleoside Transporter Regulates Ethanol Intoxication and Preference. Nat. Neurosci. 2004, 7, 855–861. [Google Scholar] [CrossRef] [PubMed]
- Butler, T.R.; Prendergast, M.A. Neuroadaptations in Adenosine Receptor Signaling Following Long-Term Ethanol Exposure and Withdrawal. Alcohol. Clin. Exp. Res. 2011, 36, 4–13. [Google Scholar] [CrossRef]
- Pardo, M.; Betz, A.J.; San Miguel, N.; López-Cruz, L.; Salamone, J.D.; Correa, M. Acetate as an Active Metabolite of Ethanol: Studies of Locomotion, Loss of Righting Reflex, and Anxiety in Rodents. Front. Behav. Neurosci. 2013, 7, 81. [Google Scholar] [CrossRef]
- Bolewska, P.; Martin, B.I.; Orlando, K.A.; Rhoads, D.E. Sequential Changes in Brain Glutamate and Adenosine A1 Receptors May Explain Severity of Adolescent Alcohol Withdrawal after Consumption of High Levels of Alcohol. Neurosci. J. 2019, 2019, 1–7. [Google Scholar] [CrossRef]
- Endesfelder, S.; Weichelt, U.; Strauß, E.; Schlör, A.; Sifringer, M.; Scheuer, T.; Bührer, C.; Schmitz, T. Neuroprotection by Caffeine in Hyperoxia-Induced Neonatal Brain Injury. Int. J. Mol. Sci. 2017, 18, 187. [Google Scholar] [CrossRef]
- Prediger, R.D.S. Effects of Caffeine in Parkinson’s Disease: From Neuroprotection to the Management of Motor and Non-Motor Symptoms. J. Alzheimer’s Dis. 2010, 20, S205–S220. [Google Scholar] [CrossRef]
- Ferreira, S.E.; de Mello, M.T.; Pompeia, S.; de Souza-Formigoni, M.L.O. Effects of Energy Drink Ingestion on Alcohol Intoxication. Alcohol. Clin. Exp. Res. 2006, 30, 598–605. [Google Scholar] [CrossRef] [PubMed]
- Towner, T.T.; Varlinskaya, E.I. Adolescent Ethanol Exposure: Anxiety-like Behavioral Alterations, Ethanol Intake, and Sensitivity. Front. Behav. Neurosci. 2020, 14, 45. [Google Scholar] [CrossRef]
- Kuntsche, E.; Rossow, I.; Engels, R.; Kuntsche, S. Is “Age at First Drink” a Useful Concept in Alcohol Research and Prevention? We Doubt That. Addiction 2015, 111, 957–965. [Google Scholar] [CrossRef] [PubMed]
- Morean, M.E.; L’Insalata, A.; Butler, E.R.; McKee, A.; Krishnan-Sarin, S. Age at Drinking Onset, Age at First Intoxication, and Delay to First Intoxication: Assessing the Concurrent Validity of Measures of Drinking Initiation with Alcohol Use and Related Problems. Addict. Behav. 2018, 79, 195–200. [Google Scholar] [CrossRef] [PubMed]
- Patrick, M.E.; Schulenberg, J.E.; Martz, M.E.; Maggs, J.L.; O’Malley, P.M.; Johnston, L.D. Extreme Binge Drinking among 12th-Grade Students in the United States. JAMA Pediatr. 2013, 167, 1019. [Google Scholar] [CrossRef]
- Ahlström, S.; Österberg, E. International Perspectives on Adolescent and Young Adult Drinking. Alcohol Res. Health 2004, 28, 258. [Google Scholar]
- Dawson, D.A.; Li, T.-K.; Grant, B.F. A Prospective Study of Risk Drinking: At Risk for What? Drug Alcohol Depend. 2008, 95, 62–72. [Google Scholar] [CrossRef]
- Nagel, B.J.; Schweinsburg, A.D.; Phan, V.; Tapert, S.F. Reduced Hippocampal Volume among Adolescents with Alcohol Use Disorders without Psychiatric Comorbidity. Psychiatry Res. Neuroimaging 2005, 139, 181–190. [Google Scholar] [CrossRef]
- Varlinskaya, E.I.; Truxell, E.; Spear, L.P. Chronic Intermittent Ethanol Exposure during Adolescence: Effects on Social Behavior and Ethanol Sensitivity in Adulthood. Alcohol 2014, 48, 433–444. [Google Scholar] [CrossRef]
- Pandey, S.C.; Sakharkar, A.J.; Tang, L.; Zhang, H. Potential Role of Adolescent Alcohol Exposure-Induced Amygdaloid Histone Modifications in Anxiety and Alcohol Intake during Adulthood. Neurobiol. Dis. 2015, 82, 607–619. [Google Scholar] [CrossRef]
- Torcaso, A.; Asimes, A.; Meagher, M.; Pak, T.R. Adolescent Binge Alcohol Exposure Increases Risk Assessment Behaviors in Male Wistar Rats after Exposure to an Acute Psychological Stressor in Adulthood. Psychoneuroendocrinology 2017, 76, 154–161. [Google Scholar] [CrossRef] [PubMed]
- Vetreno, R.P.; Broadwater, M.; Liu, W.; Spear, L.P.; Crews, F.T. Adolescent, but Not Adult, Binge Ethanol Exposure Leads to Persistent Global Reductions of Choline Acetyltransferase Expressing Neurons in Brain. PLoS ONE 2014, 9, e113421. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, G.B.; Fontes, E.d.A.; de Carvalho, S.; da Silva, J.B.; Fernandes, L.M.P.; Oliveira, M.C.S.P.; Prediger, R.D.; Gomes-Leal, W.; Rodrigues Lima, R.; Maia, C.S.F. Minocycline Mitigates Motor Impairments and Cortical Neuronal Loss Induced by Focal Ischemia in Rats Chronically Exposed to Ethanol during Adolescence. Brain Res. 2014, 1561, 23–34. [Google Scholar] [CrossRef]
- Oliveira, A.C.; Pereira, M.C.; Santana, L.N.d.S.; Fernandes, R.M.; Teixeira, F.B.; Oliveira, G.B.; Fernandes, L.M.; Fontes-Júnior, E.A.; Prediger, R.D.; Crespo-López, M.E.; et al. Chronic Ethanol Exposure during Adolescence through Early Adulthood in Female Rats Induces Emotional and Memory Deficits Associated with Morphological and Molecular Alterations in Hippocampus. J. Psychopharmacol. 2015, 29, 712–724. [Google Scholar] [CrossRef]
- Hoffman, P.L.; Tabakoff, B. The Role of the NMDA Receptor in Ethanol Withdrawal. EXS 1994, 71, 61–70. [Google Scholar] [CrossRef]
- Olsen, R.W.; Liang, J. Role of GABAA Receptors in Alcohol Use Disorders Suggested by Chronic Intermittent Ethanol (CIE) Rodent Model. Mol. Brain 2017, 10, 1–20. [Google Scholar] [CrossRef]
- Fritz, B.M.; Companion, M.; Boehm, S.L. “Wired,” yet Intoxicated: Modeling Binge Caffeine and Alcohol Co-Consumption in the Mouse. Alcohol. Clin. Exp. Res. 2014, 38, 2269–2278. [Google Scholar] [CrossRef]
- Fastbom, J.; Pazos, A.; Palacios, J.M. The Distribution of Adenosine A1 Receptors and 5’-Nucleotidase in the Brain of Some Commonly Used Experimental Animals. Neuroscience 1987, 22, 813–826. [Google Scholar] [CrossRef]
- Moreau, J.-L.; Huber, G. Central Adenosine A2A Receptors: An Overview. Brain Res. Rev. 1999, 31, 65–82. [Google Scholar] [CrossRef]
- Rosin, D.L.; Robeva, A.; Guyenet, P.G.; Linden, J. Immunohistochemical Localization of Adenosine A2A Receptors in the Rat Central Nervous System. J. Comp. Neurol. 1998, 401, 163–168. [Google Scholar] [CrossRef]
- Prediger, R.D.S.; da Silva, G.E.; Batista, L.C.; Bittencourt, A.L.; Takahashi, R.N. Activation of Adenosine A1 Receptors Reduces Anxiety-like Behavior during Acute Ethanol Withdrawal (Hangover) in Mice. Neuropsychopharmacology 2006, 31, 2210–2220. [Google Scholar] [CrossRef]
- Kaplan, G.B.; Bharmal, N.H.; Leite-Morris, K.A.; Adams, W.R. Role of Adenosine A1 and A2A Receptors in the Alcohol Withdrawal Syndrome. Alcohol 1999, 19, 157–162. [Google Scholar] [CrossRef]
- Giménez-Llort, L.; Fernández-Teruel, A.; Escorihuela, R.M.; Fredholm, B.B.; Tobeña, A.; Pekny, M.; Johansson, B. Mice Lacking the Adenosine a1Receptor Are Anxious and Aggressive, but Are Normal Learners with Reduced Muscle Strength and Survival Rate. Eur. J. Neurosci. 2002, 16, 547–550. [Google Scholar] [CrossRef]
- Johansson, B.; Halldner, L.; Dunwiddie, T.V.; Masino, S.A.; Poelchen, W.; Gimenez-Llort, L.; Escorihuela, R.M.; Fernandez-Teruel, A.; Wiesenfeld-Hallin, Z.; Xu, X.-J.; et al. Hyperalgesia, Anxiety, and Decreased Hypoxic Neuroprotection in Mice Lacking the Adenosine A1 Receptor. Proc. Natl. Acad. Sci. USA 2001, 98, 9407–9412. [Google Scholar] [CrossRef]
- van Calker, D.; Biber, K. The Role of Glial Adenosine Receptors in Neural Resilience and the Neurobiology of Mood Disorders. Neurochem. Res. 2005, 30, 1205–1217. [Google Scholar] [CrossRef] [PubMed]
- Deckert, J. The Adenosine A2A Receptor Knockout Mouse: A Model for Anxiety? Int. J. Neuropsychopharmacol. 1998, 1, 187–190. [Google Scholar] [CrossRef][Green Version]
- Freitag, C.M.; Agelopoulos, K.; Huy, E.; Rothermundt, M.; Krakowitzky, P.; Meyer, J.; Deckert, J.; von Gontard, A.; Hohoff, C. Adenosine A2A Receptor Gene (ADORA2A) Variants May Increase Autistic Symptoms and Anxiety in Autism Spectrum Disorder. Eur. Child Adolesc. Psychiatry 2009, 19, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Ledent, C.; Vaugeois, J.-M.; Schiffmann, S.N.; Pedrazzini, T.; Yacoubi, M.E.; Vanderhaeghen, J.-J.; Costentin, J.; Heath, J.K.; Vassart, G.; Parmentier, M. Aggressiveness, Hypoalgesia and High Blood Pressure in Mice Lacking the Adenosine a 2a Receptor. Nature 1997, 388, 674–678. [Google Scholar] [CrossRef] [PubMed]
- López-Cruz, L.; Carbó-Gas, M.; Pardo, M.; Bayarri, P.; Valverde, O.; Ledent, C.; Salamone, J.D.; Correa, M. Adenosine a 2A Receptor Deletion Affects Social Behaviors and Anxiety in Mice: Involvement of Anterior Cingulate Cortex and Amygdala. Behav. Brain Res. 2017, 321, 8–17. [Google Scholar] [CrossRef]
- Coelho, J.E.; Alves, P.; Canas, P.M.; Valadas, J.S.; Shmidt, T.; Batalha, V.L.; Ferreira, D.G.; Ribeiro, J.A.; Bader, M.; Cunha, R.A.; et al. Overexpression of Adenosine A2A Receptors in Rats: Effects on Depression, Locomotion, and Anxiety. Front. Psychiatry 2014, 5, 67. [Google Scholar] [CrossRef]
- Nam, H.W.; Hinton, D.J.; Kang, N.Y.; Kim, T.; Lee, M.R.; Oliveros, A.; Adams, C.; Ruby, C.L.; Choi, D.-S. Adenosine Transporter ENT1 Regulates the Acquisition of Goal-Directed Behavior and Ethanol Drinking through A2A Receptor in the Dorsomedial Striatum. J. Neurosci. 2013, 33, 4329–4338. [Google Scholar] [CrossRef] [PubMed]
- Yamada, K.; Kobayashi, M.; Shiozaki, S.; Ohta, T.; Mori, A.; Jenner, P.; Kanda, T. Antidepressant Activity of the Adenosine A2A Receptor Antagonist, Istradefylline (KW-6002) on Learned Helplessness in Rats. Psychopharmacology 2014, 231, 2839–2849. [Google Scholar] [CrossRef] [PubMed]
- Jarvis, M.F.; Becker, H.C. Single and Repeated Episodes of Ethanol Withdrawal Increase Adenosine A1, but Not A2A, Receptor Density in Mouse Brain. Brain Res. 1998, 786, 80–88. [Google Scholar] [CrossRef]
- Othman, T.; Legare, D.; Sadri, P.; Lautt, W.W.; Parkinson, F.E. A Preliminary Investigation of the Effects of Maternal Ethanol Intake during Gestation and Lactation on Brain Adenosine A1 Receptor Expression in Rat Offspring. Neurotoxicology Teratol. 2002, 24, 275–279. [Google Scholar] [CrossRef]
- Dar, M.S. Functional Correlation between Subclasses of Brain Adenosine Receptor Affinities and Ethanol-Induced Motor Incoordination in Mice. Pharmacol. Biochem. Behav. 1990, 37, 747–753. [Google Scholar] [CrossRef]
- Proctor, W.R.; Dunwiddie, T.V. Pre- and Postsynaptic Actions of Adenosine in the in Vitro Rat Hippocampus. Brain Res. 1987, 426, 187–190. [Google Scholar] [CrossRef]
- Thompson, S.M.; Haas, H.L.; Gähwiler, B.H. Comparison of the Actions of Adenosine at Pre- and Postsynaptic Receptors in the Rat Hippocampus in Vitro. J. Physiol. 1992, 451, 347–363. [Google Scholar] [CrossRef]
- Deckert, J.; Abel, F.; Künig, G.; Hartmann, J.; Senitz, D.; Maier, H.; Ransmayr, G.; Riederer, P. Loss of Human Hippocampal Adenosine A1 Receptors in Dementia: Evidence for Lack of Specificity. Neurosci. Lett. 1998, 244, 1–4. [Google Scholar] [CrossRef]
- Lewin, E.; Bleck, V. Electroshock Seizures in Mice: Effect on Brain Adenosine and Its Metabolites. Epilepsia 1981, 22, 577–581. [Google Scholar] [CrossRef]
- de Mendonça, A.; Sebastião, A.M.; Ribeiro, A.J. Inhibition of NMDA Receptor-Mediated Currents in Isolated Rat Hippocampal Neurones by Adenosine A1 Receptor Activation. NeuroReport 1995, 6, 1097–1100. [Google Scholar] [CrossRef]
- Scholz, K.P.; Miller, R.J. Analysis of Adenosine Actions on Ca2+ Currents and Synaptic Transmission in Cultured Rat Hippocampal Pyramidal Neurones. J. Physiol. 1991, 435, 373–393. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, M.L.; Cunha, R.A.; Ribeiro, J.A. Adenosine A2A Receptors Facilitate 45Ca2+ Uptake through Class a Calcium Channels in Rat Hippocampal CA3 but Not CA1 Synaptosomes. Neurosci. Lett. 1997, 238, 73–77. [Google Scholar] [CrossRef]
- McCool, B.A. Ethanol Modulation of Synaptic Plasticity. Neuropharmacology 2011, 61, 1097–1108. [Google Scholar] [CrossRef] [PubMed]
- Wydra, K.; Gawliński, D.; Gawlińska, K.; Frankowska, M.; Borroto-Escuela, D.O.; Fuxe, K.; Filip, M. Adenosine A2AReceptors in Substance Use Disorders: A Focus on Cocaine. Cells 2020, 9, 1372. [Google Scholar] [CrossRef]
- Ferré, S.; Karcz-Kubicha, M.; Hope, B.T.; Popoli, P.; Burgueño, J.; Gutiérrez, M.A.; Casadó, V.; Fuxe, K.; Goldberg, S.R.; Lluis, C.; et al. Synergistic Interaction between Adenosine A2A and Glutamate MGlu5 Receptors: Implications for Striatal Neuronal Function. Proc. Natl. Acad. Sci. USA 2002, 99, 11940–11945. [Google Scholar] [CrossRef]
- Fuxe, K.; Marcellino, D.; Borroto-Escuela, D.O.; Guescini, M.; Fernández-Dueñas, V.; Tanganelli, S.; Rivera, A.; Ciruela, F.; Agnati, L.F. Adenosine-Dopamine Interactions in the Pathophysiology and Treatment of CNS Disorders. CNS Neurosci. Ther. 2010, 16, e18–e42. [Google Scholar] [CrossRef]
- Batalha, V.L.; Ferreira, D.G.; Coelho, J.E.; Valadas, J.S.; Gomes, R.; Temido-Ferreira, M.; Shmidt, T.; Baqi, Y.; Buée, L.; Müller, C.E.; et al. The Caffeine-Binding Adenosine A2A Receptor Induces Age-like HPA-Axis Dysfunction by Targeting Glucocorticoid Receptor Function. Sci. Rep. 2016, 6, 31493. [Google Scholar] [CrossRef]
- Rees, D.; Scanlon, M.; Ham, J. Adenosine Signalling Pathways in the Pituitary Gland: One Ligand, Multiple Receptors. J. Endocrinol. 2003, 177, 357–364. [Google Scholar] [CrossRef][Green Version]
- Jegou, S.; Yacoubi, M.E.; Mounien, L.; Ledent, C.; Parmentier, M.; Costentin, J.; Vaugeois, J.-M.; Vaudry, H. Adenosine A2A Receptor Gene Disruption Provokes Marked Changes in Melanocortin Content and Pro-Opiomelanocortin Gene Expression. J. Neuroendocrinol. 2003, 15, 1171–1177. [Google Scholar] [CrossRef]
- Fried, E.I.; Nesse, R.M. The Impact of Individual Depressive Symptoms on Impairment of Psychosocial Functioning. PLoS ONE 2014, 9, e90311. [Google Scholar] [CrossRef]
- Maina, G.; Mauri, M.; Rossi, A. Anxiety and Depression. J. Psychopathol. 2016, 22, 236–250. [Google Scholar]
- Tavares, D.F.; Suen, P.; Rodrigues dos Santos, C.G.; Moreno, D.H.; Lane Valiengo, L.D.C.; Klein, I.; Borrione, L.; Marques Forte, P.; Brunoni, A.R.; Alberto Moreno, R. Treatment of Mixed Depression with Theta-Burst Stimulation (TBS): Results from a Double-Blind, Randomized, Sham-Controlled Clinical Trial. Neuropsychopharmacology 2021, 46, 2257–2265. [Google Scholar] [CrossRef] [PubMed]
- Castro, R.L.d.; Zanin, L.; Moraes, L.A.; Ramacciato, J.C.; Bergamaschi, C.d.C.; Flório, F.M. Perfil de Dispensação de Opioides No Brasil Entre Os Anos de 2014 E 2018. Res. Soc. Dev. 2022, 11, e9911326240. [Google Scholar] [CrossRef]
- Thomas, K.H.; Martin, R.M.; Potokar, J.; Pirmohamed, M.; Gunnell, D. Reporting of Drug Induced Depression and Fatal and Non-Fatal Suicidal Behaviour in the UK from 1998 to 2011. BMC Pharmacol. Toxicol. 2014, 15, 54. [Google Scholar] [CrossRef]
- Ziedonis, D.M.; Farren, C.K.; George, T. Depression and Substance Abuse. In Comorbidity in Affective Disorders; Tohen, M., Ed.; Marcel Dekker: New York, NY, USA, 1999; pp. 27–56. [Google Scholar]
- Powers, J.; Duffy, L.; Burns, L.; Loxton, D. Binge Drinking and Subsequent Depressive Symptoms in Young Women in Australia. Drug Alcohol Depend. 2016, 161, 86–94. [Google Scholar] [CrossRef]
- Ju, Y.J.; Kim, W.; Oh, S.S.; Park, E.-C. Solitary Drinking and the Risk of Depressive Symptoms and Suicidal Ideation in College Students: Findings from a Nationwide Survey in Korea. J. Affect. Disord. 2019, 257, 710–715. [Google Scholar] [CrossRef]
- Alasmari, F.; Goodwani, S.; McCullumsmith, R.E.; Sari, Y. Role of Glutamatergic System and Mesocorticolimbic Circuits in Alcohol Dependence. Prog. Neurobiol. 2018, 171, 32–49. [Google Scholar] [CrossRef]
- Hillemacher, T.; Bachmann, O.; Kahl, K.G.; Frieling, H. Alcohol, Microbiome, and Their Effect on Psychiatric Disorders. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2018, 85, 105–115. [Google Scholar] [CrossRef]
- Koob, G.F. Negative Reinforcement in Drug Addiction: The Darkness Within. Curr. Opin. Neurobiol. 2013, 23, 559–563. [Google Scholar] [CrossRef]
- Koob, G.F.; Volkow, N.D. Neurobiology of Addiction: A Neurocircuitry Analysis. Lancet Psychiatry 2016, 3, 760–773. [Google Scholar] [CrossRef]
- Caldwell, K.; Sheema, S.; Paz, R.; Samudioruiz, S.; Laughlin, M.; Spence, N.; Roehlk, M.; Alcon, S.; Allan, A. Fetal Alcohol Spectrum Disorder-Associated Depression: Evidence for Reductions in the Levels of Brain-Derived Neurotrophic Factor in a Mouse Model. Pharmacol. Biochem. Behav. 2008, 90, 614–624. [Google Scholar] [CrossRef] [PubMed]
- Hauser, S.R.; Getachew, B.; Taylor, R.E.; Tizabi, Y. Alcohol Induced Depressive-like Behavior Is Associated with a Reduction in Hippocampal BDNF. Pharmacol. Biochem. Behav. 2011, 100, 253–258. [Google Scholar] [CrossRef] [PubMed]
- Tapia-Arancibia, L.; Rage, F.; Givalois, L.; Dingeon, P.; Arancibia, S.; Beaugé, F. Effects of Alcohol on Brain-Derived Neurotrophic Factor mRNA Expression in Discrete Regions of the Rat Hippocampus and Hypothalamus. J. Neurosci. Res. 2001, 63, 200–208. [Google Scholar] [CrossRef]
- Carbia, C.; Lannoy, S.; Maurage, P.; López-Caneda, E.; O’Riordan, K.J.; Dinan, T.G.; Cryan, J.F. A Biological Framework for Emotional Dysregulation in Alcohol Misuse: From Gut to Brain. Mol. Psychiatry 2020, 26, 1098–1118. [Google Scholar] [CrossRef] [PubMed]
- Crone, E.A.; Dahl, R.E. Understanding Adolescence as a Period of Social–Affective Engagement and Goal Flexibility. Nat. Rev. Neurosci. 2012, 13, 636–650. [Google Scholar] [CrossRef]
- Ganella, D.E.; Barendse, M.E.A.; Kim, J.H.; Whittle, S. Prefrontal-Amygdala Connectivity and State Anxiety during Fear Extinction Recall in Adolescents. Front. Hum. Neurosci. 2017, 11, 587. [Google Scholar] [CrossRef]
- Casey, B.J.; Heller, A.S.; Gee, D.G.; Cohen, A.O. Development of the Emotional Brain. Neurosci. Lett. 2019, 693, 29–34. [Google Scholar] [CrossRef]
- Maia, C.D.S.F.; de Souza Lucena, G.M.R.; Corrêa, P.B.F.; Serra, R.B.; de Melo Matos, R.W.; da Cunha Menezes, F.; dos Santos, S.N.; de Sousa, J.B.; da Costa, E.T.; Ferreira, V.M.M. Interference of Ethanol and Methylmercury in the Developing Central Nervous System. NeuroToxicology 2009, 30, 23–30. [Google Scholar] [CrossRef]
- Hellemans, K.G.C.; Sliwowska, J.H.; Verma, P.; Weinberg, J. Prenatal Alcohol Exposure: Fetal Programming and Later Life Vulnerability to Stress, Depression and Anxiety Disorders. Neurosci. Biobehav. Rev. 2010, 34, 791–807. [Google Scholar] [CrossRef]
- Ruby, C.L.; Walker, D.L. Sex-Specific Regulation of Depression, Anxiety-like Behaviors and Alcohol Drinking in Mice Lacking ENT1. J. Addict. Res. Ther. 2012, 1, S4. [Google Scholar] [CrossRef]
- Asatryan, L.; Nam, H.W.; Lee, M.R.; Thakkar, M.M.; Saeed Dar, M.; Davies, D.L.; Choi, D.-S. Implication of the Purinergic System in Alcohol Use Disorders. Alcohol. Clin. Exp. Res. 2011, 35, 584–594. [Google Scholar] [CrossRef] [PubMed]
- Gass, N.; Ollila, H.M.; Utge, S.; Partonen, T.; Kronholm, E.; Pirkola, S.; Suhonen, J.; Silander, K.; Porkka-Heiskanen, T.; Paunio, T. Contribution of Adenosine Related Genes to the Risk of Depression with Disturbed Sleep. J. Affect. Disord. 2010, 126, 134–139. [Google Scholar] [CrossRef] [PubMed]
- Nagy, L.E.; Diamond, I.; Casso, D.J.; Franklin, C.; Gordon, A.S. Ethanol Increases Extracellular Adenosine by Inhibiting Adenosine Uptake via the Nucleoside Transporter. J. Biol. Chem. 1990, 265, 1946–1951. [Google Scholar] [CrossRef]
- Nam, H.W.; McIver, S.R.; Hinton, D.J.; Thakkar, M.M.; Sari, Y.; Parkinson, F.E.; Haydon, P.G.; Choi, D.-S. Adenosine and Glutamate Signaling in Neuron-Glial Interactions: Implications in Alcoholism and Sleep Disorders. Alcohol. Clin. Exp. Res. 2012, 36, 1117–1125. [Google Scholar] [CrossRef] [PubMed]
- Nagy, L.E.; Diamond, I.; Collier, K.; Lopez, L.; Ullman, B.; Gordon, A.S. Adenosine Is Required for Ethanol-Induced Heterologous Desensitization. Mol. Pharmacol. 1989, 36, 744–748. [Google Scholar]
- Soares-Simi, S.L.; Pastrello, D.M.; Ferreira, Z.S.; Yonamine, M.; Marcourakis, T.; Scavone, C.; Camarini, R. Changes in CREB Activation in the Prefrontal Cortex and Hippocampus Blunt Ethanol-Induced Behavioral Sensitization in Adolescent Mice. Front. Integr. Neurosci. 2013, 7, 94. [Google Scholar] [CrossRef]
- Keedwell, P.A.; Andrew, C.; Williams, S.C.R.; Brammer, M.J.; Phillips, M.L. The Neural Correlates of Anhedonia in Major Depressive Disorder. Biol. Psychiatry 2005, 58, 843–853. [Google Scholar] [CrossRef]
- Girault, J.-A.; Greengard, P. The Neurobiology of Dopamine Signaling. Arch. Neurol. 2004, 61, 641–644. [Google Scholar] [CrossRef]
- Sebastião, A.M.; Ribeiro, J.A. Adenosine Receptors and the Central Nervous System. Adenosine Recept. Health Dis. 2009, 193, 471–534. [Google Scholar] [CrossRef]
- Meyer, J.H.; McNeely, H.E.; Sagrati, S.; Boovariwala, A.; Martin, K.; Verhoeff, N.P.L.G.; Wilson, A.A.; Houle, S. Elevated Putamen D2Receptor Binding Potential in Major Depression with Motor Retardation: An [11C]Raclopride Positron Emission Tomography Study. Am. J. Psychiatry 2006, 163, 1594–1602. [Google Scholar] [CrossRef]
- Cryan, J.F.; Markou, A.; Lucki, I. Assessing Antidepressant Activity in Rodents: Recent Developments and Future Needs. Trends Pharmacol. Sci. 2002, 23, 238–245. [Google Scholar] [CrossRef]
- Cryan, J.F.; Valentino, R.J.; Lucki, I. Assessing Substrates Underlying the Behavioral Effects of Antidepressants Using the Modified Rat Forced Swimming Test. Neurosci. Biobehav. Rev. 2005, 29, 547–569. [Google Scholar] [CrossRef] [PubMed]
- Herszage, J.; Censor, N. Modulation of Learning and Memory: A Shared Framework for Interference and Generalization. Neuroscience 2018, 392, 270–280. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.L.C.; Nader, K.; Schiller, D. An Update on Memory Reconsolidation Updating. Trends Cogn. Sci. 2017, 21, 531–545. [Google Scholar] [CrossRef]
- McClelland, J.L.; McNaughton, B.L.; O’Reilly, R.C. Why There Are Complementary Learning Systems in the Hippocampus and Neocortex: Insights from the Successes and Failures of Connectionist Models of Learning and Memory. Psychol. Rev. 1995, 102, 419–457. [Google Scholar] [CrossRef]
- Miller, G.E.; Cohen, S. Psychological Interventions and the Immune System: A Meta-Analytic Review and Critique. Health Psychol. 2001, 20, 47–63. [Google Scholar] [CrossRef]
- Baudry, M. Synaptic Plasticity and Learning and Memory: 15 Years of Progress. Neurobiol. Learn. Mem. 1998, 70, 113–118. [Google Scholar] [CrossRef]
- Hasselmo, M.E.; Bower, J.M. Acetylcholine and Memory. Trends Neurosci. 1993, 16, 218–222. [Google Scholar] [CrossRef]
- Chen, J.-F.; Lee, C.; Chern, Y. Adenosine Receptor Neurobiology: Overview. Int. Rev. Neurobiol. 2014, 119, 1–49. [Google Scholar] [CrossRef]
- Pasquini, S.; Contri, C.; Merighi, S.; Gessi, S.; Borea, P.A.; Varani, K.; Vincenzi, F. Adenosine Receptors in Neuropsychiatric Disorders: Fine Regulators of Neurotransmission and Potential Therapeutic Targets. Int. J. Mol. Sci. 2022, 23, 1219. [Google Scholar] [CrossRef]
- Viana da Silva, S.; Haberl, M.G.; Zhang, P.; Bethge, P.; Lemos, C.; Gonçalves, N.; Gorlewicz, A.; Malezieux, M.; Gonçalves, F.Q.; Grosjean, N.; et al. Early Synaptic Deficits in the APP/PS1 Mouse Model of Alzheimer’s Disease Involve Neuronal Adenosine A2A Receptors. Nat. Commun. 2016, 7, 11915. [Google Scholar] [CrossRef] [PubMed]
- Pagnussat, N.; Almeida, A.S.; Marques, D.M.; Nunes, F.; Chenet, G.C.; Botton, P.H.S.; Mioranzza, S.; Loss, C.M.; Cunha, R.A.; Porciúncula, L.O. Adenosine a2AReceptors Are Necessary and Sufficient to Trigger Memory Impairment in Adult Mice. Br. J. Pharmacol. 2015, 172, 3831–3845. [Google Scholar] [CrossRef] [PubMed]
- Temido-Ferreira, M.; Ferreira, D.G.; Batalha, V.L.; Marques-Morgado, I.; Coelho, J.E.; Pereira, P.; Gomes, R.; Pinto, A.; Carvalho, S.; Canas, P.M.; et al. Age-Related Shift in LTD Is Dependent on Neuronal Adenosine A2A Receptors Interplay with MGluR5 and NMDA Receptors. Mol. Psychiatry 2018, 25, 1876–1900. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Rial, D.; Canas, P.M.; Yoo, J.-H.; Li, W.; Zhou, X.; Wang, Y.; van Westen, G.J.P.; Payen, M.-P.; Augusto, E.; et al. Optogenetic Activation of Intracellular Adenosine A2A Receptor Signaling in the Hippocampus Is Sufficient to Trigger CREB Phosphorylation and Impair Memory. Mol. Psychiatry 2015, 20, 1339–1349. [Google Scholar] [CrossRef]
- Paul, S.; Elsinga, P.H.; Ishiwata, K.; Dierckx, R.A.J.O.; van Waarde, A. Adenosine A1 Receptors in the Central Nervous System: Their Functions in Health and Disease, and Possible Elucidation by PET Imaging. Curr. Med. Chem. 2011, 18, 4820–4835. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, S.P.; Bittencourt-Hewitt, A.; Sebastian, C.L. Neurocognitive Bases of Emotion Regulation Development in Adolescence. Dev. Cogn. Neurosci. 2015, 15, 11–25. [Google Scholar] [CrossRef]
- Chung, T.; Creswell, K.G.; Bachrach, R.; Clark, D.B.; Martin, C.S. Adolescent Binge Drinking. Alcohol Res. Curr. Rev. 2018, 39, 5–15. [Google Scholar]
- Lees, B.; Meredith, L.R.; Kirkland, A.E.; Bryant, B.E.; Squeglia, L.M. Effect of Alcohol Use on the Adolescent Brain and Behavior. Pharmacol. Biochem. Behav. 2020, 192, 172906. [Google Scholar] [CrossRef]
- Jones, S.A.; Lueras, J.M.; Nagel, B.J. Effects of Binge Drinking on the Developing Brain. Alcohol Res. Curr. Rev. 2018, 39, 87–96. [Google Scholar]
- Steinberg, L. Cognitive and Affective Development in Adolescence. Trends Cogn. Sci. 2005, 9, 69–74. [Google Scholar] [CrossRef]
- Briones, T.L.; Woods, J. Chronic Binge-like Alcohol Consumption in Adolescence Causes Depression-like Symptoms Possibly Mediated by the Effects of BDNF on Neurogenesis. Neuroscience 2013, 254, 324–334. [Google Scholar] [CrossRef] [PubMed]
- Kuzmin, A.; Chefer, V.; Bazov, I.; Meis, J.; Ögren, S.O.; Shippenberg, T.; Bakalkin, G. Upregulated Dynorphin Opioid Peptides Mediate Alcohol-Induced Learning and Memory Impairment. Transl. Psychiatry 2013, 3, e310. [Google Scholar] [CrossRef] [PubMed]
- West, R.K.; Maynard, M.E.; Leasure, J.L. Binge Ethanol Effects on Prefrontal Cortex Neurons, Spatial Working Memory and Task-Induced Neuronal Activation in Male and Female Rats. Physiol. Behav. 2018, 188, 79–85. [Google Scholar] [CrossRef] [PubMed]
- Allen-Gipson, D.S.; Jarrell, J.C.; Bailey, K.L.; Robinson, J.E.; Kharbanda, K.K.; Sisson, J.H.; Wyatt, T.A. Ethanol Blocks Adenosine Uptake via Inhibiting the Nucleoside Transport System in Bronchial Epithelial Cells. Alcohol. Clin. Exp. Res. 2009, 33, 791–798. [Google Scholar] [CrossRef]
- Haab Lutte, A.; Huppes Majolo, J.; Reali Nazario, L.; Da Silva, R.S. Early Exposure to Ethanol Is Able to Affect the Memory of Adult Zebrafish: Possible Role of Adenosine. NeuroToxicology 2018, 69, 17–22. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharya, S.K.; Satyan, K.S.; Chakrabarti, A. Anxiogenic Action of Caffeine: An Experimental Study in Rats. J. Psychopharmacol. 1997, 11, 219–224. [Google Scholar] [CrossRef]
- Bruce, M.S. The Anxiogenic Effects of Caffeine. Postgrad. Med. J. 1990, 66 (Suppl. 2), S18–S24. [Google Scholar]
- Nutt, D.J. The Pharmacology of Human Anxiety. Pharmacol. Ther. 1990, 47, 233–266. [Google Scholar] [CrossRef]
- Fredholm, B.B. Adenosine Actions and Adenosine Receptors after 1 Week Treatment with Caffeine. Acta Physiol. Scand. 1982, 115, 283–286. [Google Scholar] [CrossRef]
- Kulkarni, S.K.; Singh, K.; Bishnoi, M. Involvement of Adenosinergic Receptors in Anxiety Related Behaviours. Indian J. Exp. Biol. 2007, 45, 439–443. [Google Scholar]
- Garcia, A.M.B.; Cardenas, F.P.; Morato, S. The Effects of Pentylenetetrazol, Chlordiazepoxide and Caffeine in Rats Tested in the Elevated Plus-Maze Depend on the Experimental Illumination. Behav. Brain Res. 2011, 217, 171–177. [Google Scholar] [CrossRef]
- Hughes, R.N.; Hancock, N.J.; Henwood, G.A.; Rapley, S.A. Evidence for Anxiolytic Effects of Acute Caffeine on Anxiety-Related Behavior in Male and Female Rats Tested with and without Bright Light. Behav. Brain Res. 2014, 271, 7–15. [Google Scholar] [CrossRef] [PubMed]
- Monopoli, A.; Lozza, G.; Forlani, A.; Mattavelli, A.; Ongini, E. Blockade of Adenosine A2A Receptors by SCH 58261 Results in Neuroprotective Effects in Cerebral Ischaemia in Rats. NeuroReport 1998, 9, 3955–3958. [Google Scholar] [CrossRef] [PubMed]
- Richardson, P.J.; Kase, H.; Jenner, P.G. Adenosine A2A Receptor Antagonists as New Agents for the Treatment of Parkinson’s Disease. Trends Pharmacol. Sci. 1997, 18, 338–344. [Google Scholar] [CrossRef]
- Tebano, M.T.; Domenici, M.R.; Popoli, P. SCH 58261 Differentially Influences Quinolinic Acid-Induced Effects in Striatal and in Hippocampal Slices. Eur. J. Pharmacol. 2002, 450, 253–257. [Google Scholar] [CrossRef]
- Lucas, M. Coffee, Caffeine, and Risk of Depression among Women. Arch. Intern. Med. 2011, 171, 1571. [Google Scholar] [CrossRef]
- Rihs, M.; Müller, C.; Baumann, P. Caffeine Consumption in Hospitalized Psychiatric Patients. Eur. Arch. Psychiatry Clin. Neurosci. 1996, 246, 83–92. [Google Scholar] [CrossRef]
- Wang, L.; Shen, X.; Wu, Y.; Zhang, D. Coffee and Caffeine Consumption and Depression: A Meta-Analysis of Observational Studies. Aust. N. Z. J. Psychiatry 2015, 50, 228–242. [Google Scholar] [CrossRef]
- Espinosa, J.; Rocha, A.; Nunes, F.; Costa, M.S.; Schein, V.; Kazlauckas, V.; Kalinine, E.; Souza, D.O.; Cunha, R.A.; Porciúncula, L.O. Caffeine Consumption Prevents Memory Impairment, Neuronal Damage, and Adenosine A2A Receptors Upregulation in the Hippocampus of a Rat Model of Sporadic Dementia. J. Alzheimer’s Dis. 2013, 34, 509–518. [Google Scholar] [CrossRef]
- Ardais, A.P.; Borges, M.F.; Rocha, A.S.; Sallaberry, C.; Cunha, R.A.; Porciúncula, L.O. Caffeine Triggers Behavioral and Neurochemical Alterations in Adolescent Rats. Neuroscience 2014, 270, 27–39. [Google Scholar] [CrossRef]
- Bevins, R.A.; Besheer, J. Object Recognition in Rats and Mice: A One-Trial Non-Matching-To-Sample Learning Task to Study “Recognition Memory”. Nat. Protoc. 2006, 1, 1306–1311. [Google Scholar] [CrossRef]
- Bora, E.; Harrison, B.J.; Davey, C.G.; Yücel, M.; Pantelis, C. Meta-Analysis of Volumetric Abnormalities in Cortico-Striatal-Pallidal-Thalamic Circuits in Major Depressive Disorder. Psychol. Med. 2011, 42, 671–681. [Google Scholar] [CrossRef] [PubMed]
- Borre, Y.E.; Panagaki, T.; Koelink, P.J.; Morgan, M.E.; Hendriksen, H.; Garssen, J.; Kraneveld, A.D.; Olivier, B.; Oosting, R.S. Neuroprotective and Cognitive Enhancing Effects of a Multi-Targeted Food Intervention in an Animal Model of Neurodegeneration and Depression. Neuropharmacology 2014, 79, 738–749. [Google Scholar] [CrossRef] [PubMed]
- Cacciatore, R.; Helbling, A.; Jost, C.; Hess, B. Episodic Headache, Diminished Performance and Depressive Mood. Praxis 1996, 85, 727–729. [Google Scholar] [PubMed]
- Silverman, K.; Evans, S.M.; Strain, E.C.; Griffiths, R.R. Withdrawal Syndrome after the Double-Blind Cessation of Caffeine Consumption. N. Engl. J. Med. 1992, 327, 1109–1114. [Google Scholar] [CrossRef]
- Khaliq, S.; Haider, S.; Naqvi, F.; Perveen, T.; Saleem, S.; Haleem, D.J. Altered Brain Serotonergic Neurotransmission Following Caffeine Withdrawal Produces Behavioral Deficits in Rats. Pak. J. Pharm. Sci. 2012, 25, 21–25. [Google Scholar]
- Takahashi, R.N. Adenosine Receptor Antagonists for Cognitive Dysfunction: A Review of Animal Studies. Front. Biosci. 2008, 13, 2614. [Google Scholar] [CrossRef]
- Arendash, G.W.; Schleif, W.; Rezai-Zadeh, K.; Jackson, E.K.; Zacharia, L.C.; Cracchiolo, J.R.; Shippy, D.; Tan, J. Caffeine Protects Alzheimer’s Mice against Cognitive Impairment and Reduces Brain β-Amyloid Production. Neuroscience 2006, 142, 941–952. [Google Scholar] [CrossRef]
- Stazi, M.; Lehmann, S.; Sakib, M.S.; Pena-Centeno, T.; Büschgens, L.; Fischer, A.; Weggen, S.; Wirths, O. Long-Term Caffeine Treatment of Alzheimer Mouse Models Ameliorates Behavioural Deficits and Neuron Loss and Promotes Cellular and Molecular Markers of Neurogenesis. Cell. Mol. Life Sci. 2021, 79, 1–18. [Google Scholar] [CrossRef]
- Laurent, C.; Eddarkaoui, S.; Derisbourg, M.; Leboucher, A.; Demeyer, D.; Carrier, S.; Schneider, M.; Hamdane, M.; Müller, C.E.; Buée, L.; et al. Beneficial Effects of Caffeine in a Transgenic Model of Alzheimer’s Disease-like Tau Pathology. Neurobiol. Aging 2014, 35, 2079–2090. [Google Scholar] [CrossRef]
- Canas, P.M.; Porciuncula, L.O.; Cunha, G.M.A.; Silva, C.G.; Machado, N.J.; Oliveira, J.M.A.; Oliveira, C.R.; Cunha, R.A. Adenosine A2A Receptor Blockade Prevents Synaptotoxicity and Memory Dysfunction Caused by -Amyloid Peptides via P38 Mitogen-Activated Protein Kinase Pathway. J. Neurosci. 2009, 29, 14741–14751. [Google Scholar] [CrossRef] [PubMed]
- Faivre, E.; Coelho, J.E.; Zornbach, K.; Malik, E.; Baqi, Y.; Schneider, M.; Cellai, L.; Carvalho, K.; Sebda, S.; Figeac, M.; et al. Beneficial Effect of a Selective Adenosine A2A Receptor Antagonist in the APPswe/PS1dE9 Mouse Model of Alzheimer’s Disease. Front. Mol. Neurosci. 2018, 11, 235. [Google Scholar] [CrossRef]
- Laurent, C.; Burnouf, S.; Ferry, B.; Batalha, V.L.; Coelho, J.E.; Baqi, Y.; Malik, E.; Mariciniak, E.; Parrot, S.; Van der Jeugd, A.; et al. A2A Adenosine Receptor Deletion Is Protective in a Mouse Model of Tauopathy. Mol. Psychiatry 2014, 21, 97–107. [Google Scholar] [CrossRef]
- Alhaider, I.A.; Aleisa, A.M.; Tran, T.T.; Alkadhi, K.A. Caffeine Prevents Sleep Loss-Induced Deficits in Long-Term Potentiation and Related Signaling Molecules in the Dentate Gyrus. Eur. J. Neurosci. 2010, 31, 1368–1376. [Google Scholar] [CrossRef] [PubMed]
- Pandolfo, P.; Machado, N.J.; Köfalvi, A.; Takahashi, R.N.; Cunha, R.A. Caffeine Regulates Frontocorticostriatal Dopamine Transporter Density and Improves Attention and Cognitive Deficits in an Animal Model of Attention Deficit Hyperactivity Disorder. Eur. Neuropsychopharmacol. 2013, 23, 317–328. [Google Scholar] [CrossRef] [PubMed]
- Drake, C.L.; Roehrs, T.; Turner, L.; Scofield, H.M.; Roth, T. Caffeine Reversal of Ethanol Effects on the Multiple Sleep Latency Test, Memory, and Psychomotor Performance. Neuropsychopharmacology 2002, 28, 371–378. [Google Scholar] [CrossRef]
- Franks, H.M.; Hagedorn, H.; Hensley, V.R.; Hensley, W.J.; Starmer, G.A. The Effect of Caffeine on Human Performance, Alone and in Combination with Ethanol. Psychopharmacologia 1975, 45, 177–181. [Google Scholar] [CrossRef]
- Santos, L.C.; Ruiz-Oliveira, J.; Oliveira, J.J.; Silva, P.F.; Luchiari, A.C. Irish Coffee: Effects of Alcohol and Caffeine on Object Discrimination in Zebrafish. Pharmacol. Biochem. Behav. 2016, 143, 34–43. [Google Scholar] [CrossRef]
- Spinetta, M.J.; Woodlee, M.T.; Feinberg, L.M.; Stroud, C.; Schallert, K.; Cormack, L.K.; Schallert, T. Alcohol-Induced Retrograde Memory Impairment in Rats: Prevention by Caffeine. Psychopharmacology 2008, 201, 361–371. [Google Scholar] [CrossRef]
| Drug(s) | Evaluation Condition | Study Information | Tests and Analysis | Main Effects | Development Period and Possible Mechanisms | Reference |
|---|---|---|---|---|---|---|
| Ethanol DPCPX-selective adenosine A1 receptor antagonist (CCPA)-selective adenosine A1 receptor agonist | Under drug withdrawal | Pattern of use: acute withdrawal Type: pre-clinical study Dose and use frequency: 0.05 mg/kg i.p. of CCPA 15 min before of 3 mg/kg i.p of DPCPX in ethanol withdrawal of 18 h in the dose of 4 g/kg i.p. | Open field and Elevated Plus Maze test during hangover | The anxiogenic effect of CCPA was reverted within 18h of withdrawal | Adult mice/agonism of A1R and antagonism of A1R supporting the involvement of A1R | [62] |
| Ethanol | Under drug withdrawal | Pattern of use: acute and chronic withdrawal Type: pre-clinical study Dose and use frequency: ethanol 1.6 g/kg (8% w/v) by inhalation in four cycles of 16 h followed by 8 h of abstinence; acutely (single withdrawal in 16 h) and chronically (multiple withdrawal in 64 h) | Effects of single and repeated episodes of ethanol withdrawal on A1R and A2AR in controlling ethanol-induced convulsions | Increase in the convulsion score upon ethanol withdrawal | Adult mice/higher expression of A1R in the cortex | [74] |
| Ethanol | Under drug withdrawal | Pattern of use: chronic withdrawal Type: pre-clinical study Dose and use frequency: administration of ethanol-free liquid diet (3.5% w/v) with discontinuation during 6 h after 18 days | Withdrawal score and relative expression and density of NMDA, AMPA, A1R, and A2AR | Increase in seizures, hyperreflexia, and running episodes | Early adolescence to adulthood/higher expression of NMDA and AMPA, reduction of A1R, and no alterations of A2AR | [39] |
| Ethanol | Under drug effect | Pattern of use: chronic exposition Type: pre-clinical study Dose and use frequency: administration of ethanol in water (15% v/v) during fetal phase in female rats, and after 60 days, the offspring was tested | Body and brain weights, as well A1R expression in cortex, cerebellum, hippocampus and striatum | Reduction in weight and lower expression of A1R in cortex and cerebellum | Fetal development and offspring/ reduction of A1R | [75] |
| Ethanol | Under drug withdrawal | Pattern of use: acute withdrawal Type: pre-clinical study Dose and use frequency: administration of ethanol (6.7% v/v) with discontinuation in 6–7 h | Withdrawal score | Increase of irritability | Late adolescence until adulthood/roleof the adenosine receptors; higher expression acutely of nucleoside transporters | [63] |
| Ethanol CGS21680-selective adenosine A2A receptor agonist | Under drug withdrawal | Pattern of use: acute withdrawal Type: pre-clinical study Dose and use frequency: 0.3 mg/kg i.p, during 6 h (0.5 h withdrawal) to 7 h (1.5 h withdrawal) | Withdrawal score | Reduction of irritability | Late adolescence until adulthood/agonism of A1R and A2AR with high expression of adenosine transporters in striatum | [63] |
| Ethanol | Not informed | Pattern of use: chronic exposition Type: pre-clinical study Dose and use frequency: The concentration of ethanol was raised every fourth day, increasing from 3 to 5 to 10% (v/v) for 10 weeks | Forced swim test, open field and marble-burying test | Anxiogenic and depressive behavior | Adult/ENT1 null mice have lower adenosine levels in the striatum and reduced A1R activation | [112] |
| Ethanol Adenosine | Not informed | Literature review | Not informed | Not informed | Changes of adenosine formation, adenosine uptake, and effects on adenosine receptor coupling | [23] |
| Ethanol Adenosine | Under drug effect | Literature review | Pre-clinical | Ataxia, sleep effects | Not mentioned/relevance of the inhibition of alcohol-sensitive ENT1 in the behavioral effects of ethanol | [113] |
| Ethanol Adenosine | Withdrawal drug effect | Literature review | Pre-clinical | ____________ | Acute ethanol increases extracellular adenosine in cultured cells by selectively inhibiting ENT1 | [72] |
| Ethanol Adenosine | Withdrawal drug effect | Pattern of use: chronic exposure Type: in vitro study Dose and use frequency: ethanol: 100 mM for 2 weeks and adenosine 1.5 units/mL for 48 h | High pressure liquid chromatography | ____________ | Ethanol enhances extracellular adenosine levels in NG108-15 and S49 lymphoma cells, causing increase intracellular cAMP levels mediated by adenosine receptors | [115] |
| Ethanol | Self-administration | Pattern of use: Self-administration Type: pre-clinical study Finality of use: dependence model Dose and use frequency: ethanol: 3–6 to 10% (v/v) for 4 days | Two-bottle choice | Goal-directed behavior, density of A2AR in the Dorsomedial Striatum (DMS) and CREB activity | Adult mice/habitual seeking of ethanol is regulated by ENT1; A2AR in DMS regulate ethanol drinking and CREB levels | [72] |
| Ethanol | Under drug effects | Pattern of use: ethanol acute Type: in vitro study Dose and use frequency: Pretreated with S-(4-nitrobenzyl)-6-thioinosine (100 µM: NBTI); concentrations of ethanol of 0, 25, 50, 100, and 200 mM | Human bronchial epithelial cell line | ------------- | EtOH acutely inhibits adenosine uptake via nucleoside transporters and chronic EtOH exposure desensitizes adenosine transporters | [146] |
| Ethanol AMPCP-inhibitor of ecto-5′-nucleotidase EHNA-inhibitor of adenosine deaminase | Long-lasting effects of ethanol | Pattern of use: chronic withdrawal Type: pre-clinical study Dose and use frequency: Embryos of zebrafish were exposed to 1% (v/v) ethanol; AMPCP at 150 mg/kg or EHNA at 100 mg/kg i.p. using adult fishes | After 30 min of AMPCP, EHNA injections locomotor anxiety, aggressive and social interaction behaviors were evaluated | AMPCP during the adult phase reversed aggressive parameters, and both inhibitors (AMPCP and EHNA) recovered social interaction | Adult/ecto-5′- nucleotidase and adenosine deaminase activities modulate long-lasting ethanol effects | [147] |
| Evaluation Condition | Study Information | Tests and Analysis | Behavioral effects | Development Period and Possible Mechanisms | Reference |
|---|---|---|---|---|---|
| Under drug effect/abstinence | Pattern of use: acute, subchronic, and withdrawal Type: pre-clinical study Dose and use frequency: Under drug effect: 10, 25, 50, and 100 mg/kg i.p.; followed by 50 mg/kg i.p. for 7, 14, and 21 days. Upon abstinence: 50 mg/kg i.p. for 21 days following 2 days of abstinence | Open Field test, Elevated Plus Maze, and Social interaction test | Low doses cause no alterations/moderate to high doses are anxiogenic | Adult/antagonism of adenosine receptors, noradrenaline transmission, and benzodiazepine ligands | [148] |
| Several | Pattern of use: acute, chronic, toxic, and withdrawal Type: clinical studies Dose and use frequency: not found | Clinical data | Anxiogenic | Adult/not investigated | [149] |
| Under drug effect/abstinence | Literature review | Clinical data | Anxiogenic (panic attack at an high dose of 750 mg) under effect/anxiolytic or anxiogenic upon withdrawal | Not mentioned/antagonism of adenosine and noradrenaline overactivity | [150] |
| Under drug Effect | Pattern of use: chronic Type: pre-clinical and in vitro studies Dose and use frequency: 20 mg/kg i.p. after one week | Cortical slice | Increases the binding density of the A1R ligand [3H]L-phenyl-isopropyl-adenosine | Adult/upregulation of adenosine receptors | [151] |
| Under drug effects | Pattern of use: acute and pre-treatment Type: pre-clinical and clinical study Dose and use frequency: 8, 15, 30 and 60 mg/kg | Elevated Plus Maze test | Low doses (not alterations) and high doses (anxiogenic) | Adult/adenosine receptors | [152] |
| Under drug effect | Pattern of use: acute Type: pre-clinical study Dose and use frequency: Under drug effect: 10 and 30 mg/kg i.p. | Elevated Plus Maze | Dose-response curve obtained in a light environment | Adult/participation of the GABAergic pathway | [153] |
| Under drug effect | Pattern of use: acute Type: pre-clinical study; both gender Dose and use frequency: 25 or 50 mg/kg i.p. during one week | Open Field and Elevated Plus Maze | Anxiolytic | Adult/antagonism of A2AR | [154] |
| Under drug effects | Pattern of use: acute Type: pre-clinical study Dose and use frequency: 10 or 30 mg/kg i.p. | Elevated Plus Maze test | High doses (anxiogenic) | Agonism and antagonism of adenosine receptors/involvement of A1R | [62] |
| Withdrawal drug effects | Pattern of use: chronic Type: pre-clinical study Dose and use: 1g/L for 3 weeks frequency: ad libitum | Forced-swimming test, tail-suspension test and elevated plus maze | Depressive, anxiogenic, and anhedonia-like behavior | Adult mice/blockade of A2AR | [17] |
| Withdrawal drug effects | Pattern of use: chronic Type: pre-clinical study Dose and use: 1 g/L for 2 weeks frequency: ad libitum | Open field, novel object recognition task, expression of receptors | Prevented memory impairment and neurodegeneration | Adult/not specified | [161] |
| Withdrawal drug effects | Pattern of use: chronic Type: pre-clinical study Dose and use frequency: 0.1, 0.3 or 1.0 g/L. Frequency: ad libitum | Open field, Novel object recognition task and elevated plus maze | Anxiogenic behavior, negative impacts on non-associative learning | Adolescence to young adult/adenosine antagonism; neuroinflammation and BDNF | [162] |
| Withdrawal drug effects | Pattern of use: chronic exposition Type: clinical study Dose and use frequency: coffee regular consumption (235 and 600 mg/day) twice within one week | Withdrawal symptoms | Headache, increased irritability, decreased performance, and disturbed concentration, as well as depression and anxiety | Adolescents and adults/mood impairments such as depression and anxiety | [166,167] |
| Under drug effects | Pattern of use: acute, subchronic, chronic exposure Type: pre-clinical study Dose and use frequency: several administrations | Alzheimer’s model | Prevention of cognitive decline | Adult mice and cell cultures/neuroprotection by caffeine | [169,170,171] |
| Under drug effect | Pattern of use: chronic Type: clinical study Dose and use: caffeine (300 mg/day) and ethanol (0.5 g/kg) caffeine (150 mg/kg and ethanol 0.5 g/kg) frequency: not specified | Probed-recall memory, sleepiness scale, memory and profile of mood states | Caffeine reversed the effects of ethanol on reaction time in a dose-related manner | Young adult/not investigated | [178] |
| Under drug effect | Pattern of use: chronic Type: clinical study Dose and use frequency: caffeine (300 mg/70 kg) and ethanol (0.75 g/kg) o.r.. for 8 weeks | Standing steadiness, auditory, visual and complex reaction time, manual dexterity, numerical reasoning, perceptual speed, verbal fluency | Body sway in up to 40 min; caffeine reduces simple auditory and complex reaction time | Young adult/caffeine antagonized the ethanol-induced increase in simple auditing, simple visual, and complex reaction time | [179] |
| Withdrawal drug effects | Pattern of use: acute and chronic in combination with ethanol Type: pre-clinical study Dose and use frequency: 0.5% of ethanol and 50 mg/mL of caffeine during 1 day to 15 days | Object discrimination | Learning in the zebrafish model | Adult/combination in withdrawal cause no alterations; low to moderate doses of the combination alter object discrimination | [180] |
| Under drug effects | Pattern of use: acute ethanol Type: pre-clinical study Dose and use frequency: 0.5% of ethanol and 50 mg/mL of caffeine during 1 day to 15 days | Novel odor | Prevention of retrograde amnesia | Adult rats/low doses of caffeine prevent impairments cognitive induced by ethanol | [181] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pinheiro, B.G.; Luz, D.A.; Cartágenes, S.d.C.; Fernandes, L.d.M.P.; Farias, S.V.; Kobayashi, N.H.C.; Fontes-Júnior, E.A.; Ferreira, S.G.; Cunha, R.A.; Prediger, R.D.; et al. The Role of the Adenosine System on Emotional and Cognitive Disturbances Induced by Ethanol Binge Drinking in the Immature Brain and the Beneficial Effects of Caffeine. Pharmaceuticals 2022, 15, 1323. https://doi.org/10.3390/ph15111323
Pinheiro BG, Luz DA, Cartágenes SdC, Fernandes LdMP, Farias SV, Kobayashi NHC, Fontes-Júnior EA, Ferreira SG, Cunha RA, Prediger RD, et al. The Role of the Adenosine System on Emotional and Cognitive Disturbances Induced by Ethanol Binge Drinking in the Immature Brain and the Beneficial Effects of Caffeine. Pharmaceuticals. 2022; 15(11):1323. https://doi.org/10.3390/ph15111323
Chicago/Turabian StylePinheiro, Bruno Gonçalves, Diandra Araújo Luz, Sabrina de Carvalho Cartágenes, Luanna de Melo Pereira Fernandes, Sarah Viana Farias, Natália Harumi Correa Kobayashi, Enéas Andrade Fontes-Júnior, Samira G. Ferreira, Rodrigo A. Cunha, Rui Daniel Prediger, and et al. 2022. "The Role of the Adenosine System on Emotional and Cognitive Disturbances Induced by Ethanol Binge Drinking in the Immature Brain and the Beneficial Effects of Caffeine" Pharmaceuticals 15, no. 11: 1323. https://doi.org/10.3390/ph15111323
APA StylePinheiro, B. G., Luz, D. A., Cartágenes, S. d. C., Fernandes, L. d. M. P., Farias, S. V., Kobayashi, N. H. C., Fontes-Júnior, E. A., Ferreira, S. G., Cunha, R. A., Prediger, R. D., & Maia, C. d. S. F. (2022). The Role of the Adenosine System on Emotional and Cognitive Disturbances Induced by Ethanol Binge Drinking in the Immature Brain and the Beneficial Effects of Caffeine. Pharmaceuticals, 15(11), 1323. https://doi.org/10.3390/ph15111323










