Polarization of HIV-1- and CMV-Specific IL-17-Producing T Cells among People with HIV under Antiretroviral Therapy with Cannabis and/or Cocaine Usage
Abstract
1. Introduction
2. Results
2.1. Clinical and Demographic Characteristics of Participants
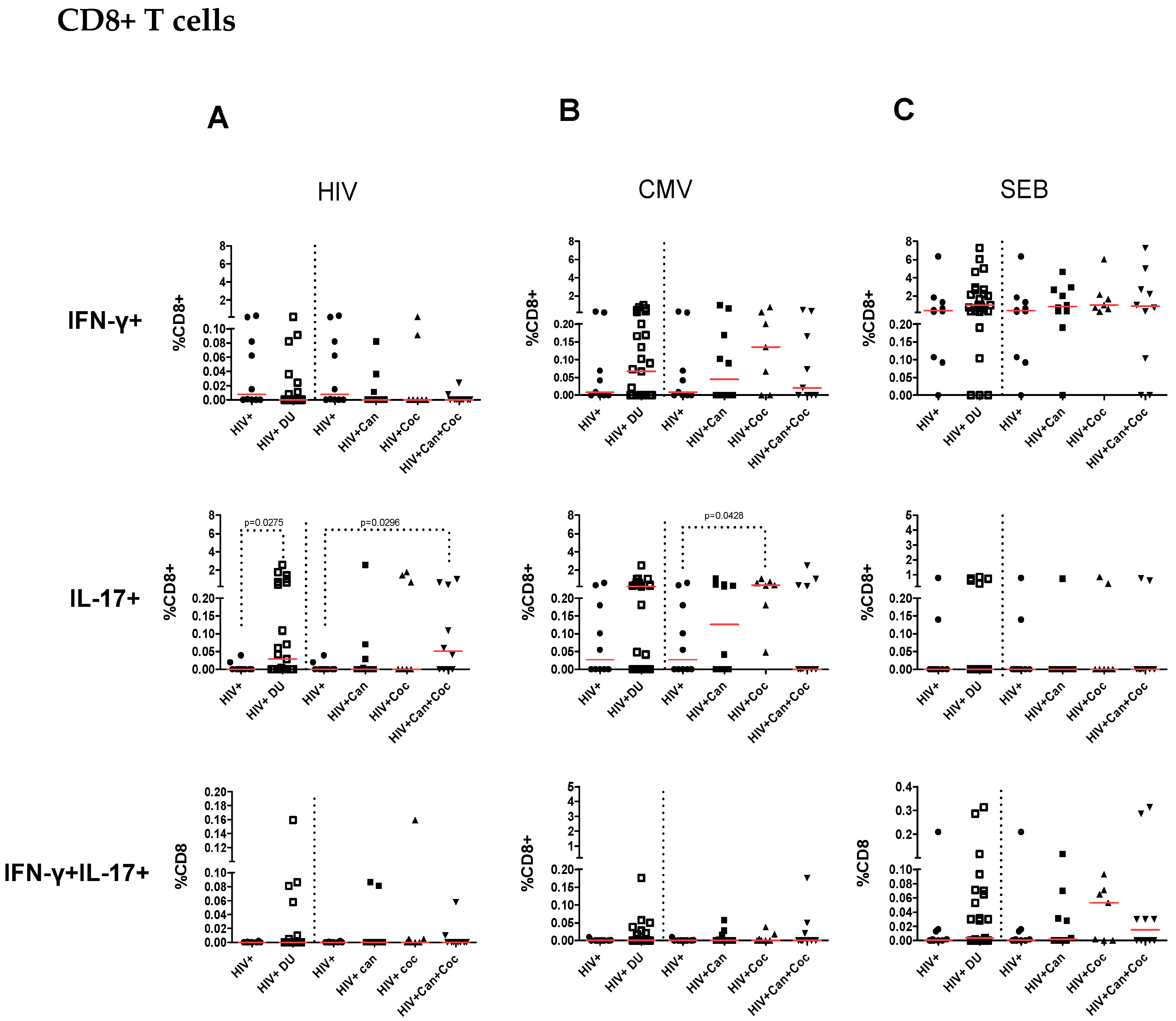
2.2. Increased IL-17-Producing HIV-Specific T-Cell Responses in PWH Drug Users
2.3. Increased CMV-Specific CD8+ T-Cell Response in PWH Cocaine Users
2.4. T-Cell Responses from HIV-Infected Drug Users after SEB Stimulation
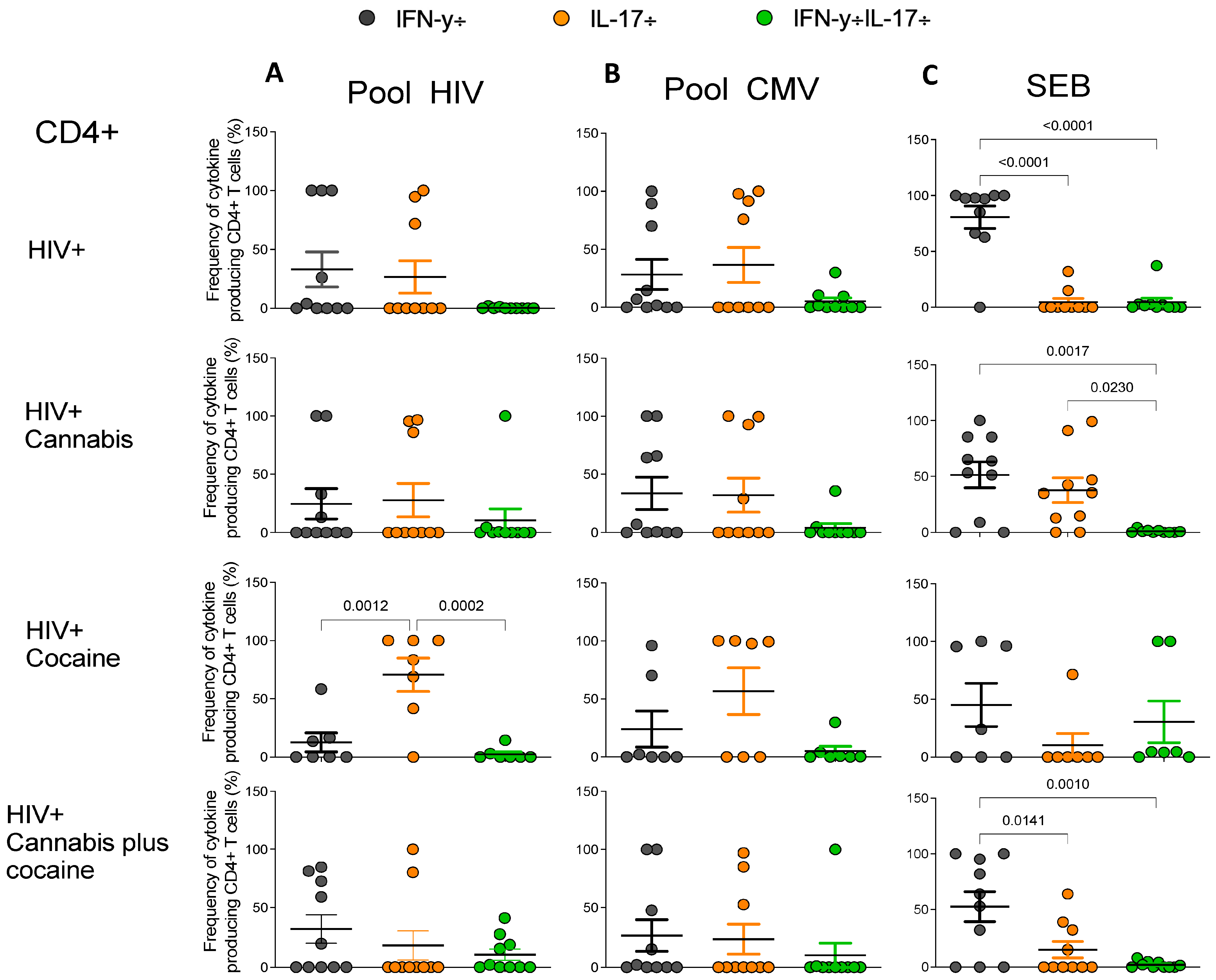
2.5. Frequencies of Antigen-Specific Cytokine-Producing CD4+ and CD8+ T Cells in PWH Drug Users
3. Discussion
4. Material and Methods
4.1. Study Participants and Sample Collection
4.2. Toxicological Testing
4.3. Immunoenzymatic Assay for Anti-CMV IgG
4.4. Cell Preparation
4.5. PBMC Stimulation with HIV-1, CMV Peptide Pools, or SEB
4.6. Cell Surface and Intracellular Cytokine Staining by Flow Cytometry
4.7. Data Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Friedman, H.; Newton, C.; Klein, T.W. Microbial Infections, Immunomodulation, and Drugs of Abuse. Clin. Microbiol. Rev. 2003, 16, 209–219. [Google Scholar] [CrossRef] [PubMed]
- Dash, S.; Muthukumar, B.; Villalta, F.; Dash, C.; Pandhare, J. Impact of cocaine abuse on HIV pathogenesis. Front. Microbiol. 2015, 6, 1111. [Google Scholar] [CrossRef] [PubMed]
- Addai, A.B.; Pandhare, J.; Paromov, V.; Mantri, C.K.; Pratap, S.; Dash, C. Cocaine modulates HIV-1 integration in primary CD4+ T cells: Implications in HIV-1 pathogenesis in drug-abusing patients. J. Leukoc. Biol. 2014, 97, 779–790. [Google Scholar] [CrossRef]
- Kim, S.G.; Jung, J.B.; Dixit, D.; Rovner, R., Jr.; Zack, J.A.; Baldwin, G.C.; Vatakis, D.N. Cocaine exposure enhances permissiveness of quiescent T cells to HIV infection. J. Leukoc. Biol. 2013, 94, 835–843. [Google Scholar] [CrossRef]
- Napuri, J.; Pilakka-kanthikeel, S.; Raymond, A.; Agudelo, M.; Yndart-Arias, A.; Saxena, S.K.; Nair, M. Cocaine enhances HIV-1 infectivity in monocyte derived dendritic cells by suppressing microRNA-155. PLoS ONE 2013, 8, e83682. [Google Scholar] [CrossRef]
- Macmadu, A.; Reddon, H.; Marshall, B.D.L.; Fairbairn, N.; Nolan, S.; Socías, M.E.; Milloy, M.J. Crack cocaine use frequency is associated with HIV disease severity independent of antiretroviral therapy exposure: A prospective cohort study. AIDS Behav. 2022, 26, 3356–3364. [Google Scholar] [CrossRef] [PubMed]
- Baum, M.K.; Rafie, C.; Lai, S.; Sales, S.; Page, B.; Campa, A. Crack-cocaine use accelerates HIV disease progression in a cohort of HIV-positive drug users. J. Acquir. Immune Defic. Syndr. 2009, 50, 93–99. [Google Scholar] [CrossRef]
- Cheng, D.; Luo, Z.; Fitting, S.; Stoops, W.; Heath, S.L.; Ndhlovu, L.C.; Jiang, W. The link between chronic cocaine use, B cell perturbations, and blunted immune recovery in HIV-infected individuals on suppressive ART. NeuroImmune Pharmacol. Ther. 2023, 2, 71–79. [Google Scholar] [CrossRef]
- Pandhare, J.; Addai, A.B.; Mantri, C.K.; Hager, C.; Smith, R.M.; Barnett, L.; Villalta, F.; Kalams, S.A.; Dash, C. Cocaine enhances HIV-1-induced CD4+ T-cell apoptosis Implications in disease progression in cocaine-abusing HIV-1 patients. Am. J. Pathol. 2014, 184, 928–936. [Google Scholar] [CrossRef]
- Lorenz, D.R.; Duttla, A.; Mukerji, S.; Holman, A.; Uno, H.; Gabuzda, D. Marijuana use impacts midlife cardiovascular events in HIV-infected men. Clin. Infect. Dis. 2017, 65, 626–635. [Google Scholar] [CrossRef]
- Marcellin, F.; Lions, C.; Rosenthal, E.; Roux, P.; Sogni, P.; Wittkop, L.; Protopopescu, C.; Spire, B.; Salmon-Ceron, D.; Dabis, F.; et al. No significant effect of cannabis use on the count and percentage of circulating CD4 T-cells in HIV-HCV co-infected patients. Drug Alcohol. Rev. 2017, 36, 227–238. [Google Scholar] [CrossRef] [PubMed]
- Sido, J.M.; Jackson, A.R.; Nagarkatti, P.S.; Nagarkatti, M. Marijuana-derived Δ-9-tetrahydrocannabinol suppresses Th1/Th17 cell-mediated delayed-type hypersensitivity through micro RNA regulation. J. Mol. Med. 2016, 94, 1039–1051. [Google Scholar] [CrossRef]
- Rosenberg, E.S.; Billingsley, J.M.; Caliendo, A.M.; Boswell, S.L.; Sax, P.E.; Kalams, S.A.; Walker, B.D. Vigorous HIV+-specific CD4+ T cell responses associated with control of viremia. Science 1997, 278, 1447–1460. [Google Scholar] [CrossRef] [PubMed]
- Meek, S.M.; Williams, M.A. IFN-gamma-dependent and independent mechanism of CD4+ memory T cell-mediated protection from Listeria infection. Pathogens 2018, 7, 22. [Google Scholar] [CrossRef] [PubMed]
- Riou, C.; Berkowitz, N.; Goliath, R.; Burgers, W.A.; Wilkinson, R.J. Analysis of the phenotype of mycobacterium tuberculosis-specific CD4+ T cells to discriminate latent from active tuberculosis in HIV-uninfected and HIV-infected individuals. Front. Immunol. 2017, 8, 968. [Google Scholar] [CrossRef]
- Trautmann, L.; Janbazian, L.; Chomont, N.; Said, E.A.; Gimmig, S.; Bessette, B.; Boulassel, M.-R.; Delwart, E.; Sepulveda, H.; Balderas, R.S.; et al. Upregulation of PD-1 expression on HIV-specific CD8+ T cells leads to reversible immune dysfunction. Nat. Med. 2006, 12, 1198–1202. [Google Scholar] [CrossRef]
- Khaitan, A.; Unutmaz, D. Revisiting immune exhaustion during HIV infection. Curr HIV/AIDS Rep. 2011, 8, 4–11. [Google Scholar] [CrossRef]
- Teigler, J.E.; Zelinskyy, G.; Eller, M.A.; Bolton, D.L.; Marovich, M.; Gordon, A.D.; Alrubayyi, A.; Alter, G.; Robb, M.L.; Martin, J.N.; et al. Differential inhibitory receptor expression on T cells delineates functional capacities in chronic viral infection. J. Virol. 2017, 91, e01263-17. [Google Scholar] [CrossRef]
- Szabo, S.J.; Kim, S.T.; Costa, G.L.; Zhang, X.; Fathman, C.G.; Glimcher, L.H. A novel transcription factor, T-bet, directs Th1 lineage commitment. Cell 2000, 100, 655–669. [Google Scholar] [CrossRef]
- Liu, S.Y.; Sanchez, D.J.; Aliyari, R.; Lu, S.; Cheng, G. Systematic identification of type I and type II interferon-induced antiviral factors. Proc. Natl. Acad. Sci. USA 2012, 109, 4239–4244. [Google Scholar] [CrossRef]
- Kane, M.; Zang, T.M.; Rihn, S.J.; Zhang, F.; Kueck, T.; Alim, M.; Schoggins, J.; Rice, C.M.; Wilson, S.J.; Bieniasz, P.D. Identification of Interferon-Stimulated Genes with Antiretroviral Activity. Cell Host Microbe 2016, 20, 392–405. [Google Scholar] [CrossRef]
- Bettelli, E.; Korn, T.; Oukka, M.; Kuchroo, V.K. Induction and effector functions of T(H)17 cells. Nature 2008, 453, 1051–1057. [Google Scholar] [CrossRef]
- d’ettorre, G.; Ceccarelli, G.; Andreotti, M.; Selvaggi, C.; Giustini, N.; Serafino, S.; Schietroma, I.; Nunnari, G.; Antonelli, G.; Vullo, V.; et al. Analysis of Th17 and Tc17 frequencies and antiviral defenses in gut-associated lymphoid tissue of chronic HIV-1 positive patients. Mediat. Inflamm. 2015, 2015, 395484. [Google Scholar] [CrossRef] [PubMed]
- Huber, M.; Heink, S.; Pagenstecher, A.; Reinhard, K.; Lohoff, M. IL-17A secretion by CD8+ T cells supports Th17-mediated autoimmune encephalomyelitis. J. Clin. Investig. 2013, 123, 247–260. [Google Scholar] [CrossRef] [PubMed]
- Henriques, A.; Inês, L.; Couto, M.; Pedreiro, S.; Santos, C.; Magalhães, M.; Santos, P.; Velada, I.; Almeida, A.; Carvalheiro, T.; et al. Frequency and functional activity of Th17, TC17 and other T-cell subsets in systemic lupus erythematosus. Cell. Immunol. 2010, 264, 97–103. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Seongwook, K.; Kim, J.; Kwon, G.; Koo, S. Elevated Levels of T helper 17 cells are associated with disease activity in patients with rheumatoid arthritis. Ann. Lab. Med. 2013, 33, 52–59. [Google Scholar] [CrossRef] [PubMed]
- Zhong, X.; Wu, H.; Zhang, W.; Gwack, Y.; Shang, W.; Lee, K.O.; Isakov, N.; He, Z.; Sun, Z. Decoupling the role of RORγt in the differentiation and effector function of TH17 cells. Sci. Adv. 2022, 8, eadc9221. [Google Scholar] [CrossRef]
- Mansouri, M.; Raze, A.A. The potential role of Th17 lymphocytes in patients with psoriasis. Ann. Bras. Dermatol. 2018, 93, 63–66. [Google Scholar] [CrossRef] [PubMed]
- Sokoya, T.; Steel, H.C.; Nieuwoudt, M.; Rossouw, T.M. HIV as a cause of immune activation and immunosenescence. Mediat. Inflamm. 2017, 2017, 6825493. [Google Scholar] [CrossRef]
- El-Far, M.; Halwani, R.; Said, E.; Trautmann, L.; Doroudchi, M.; Janbazian, L.; Fonseca, S.; van Grevenynghe, J.; Yassine-Diab, B.; Sékaly, R.-P.; et al. T-cell exhaustion in HIV infection. Curr HIV/AIDS Rep. 2008, 5, 13–19. [Google Scholar] [CrossRef]
- Deeks, S.G.; Kitchen, C.M.R.; Liu, L.; Guo, H.; Gascon, R.; Narváez, A.B.; Hunt, P.; Martin, J.N.; Kahn, J.O.; Levy, J.; et al. Immune activation set point during early HIV infection predicts subsequent CD4+ T-cell changes independent of viral load. Clin. Obs. Interv. Ther. Trials 2004, 104, 942–947. [Google Scholar] [CrossRef] [PubMed]
- Day, C.L.; Kaufmann, D.E.; Kiepiela, P.; Brown, J.A.; Moodley, E.S.; Reddy, S.; Mackey, E.W.; Miller, J.D.; Leslie, A.J.; DePierres, C.; et al. PD-1 expression on HIV-specific T cells is associated with T-cell exhaustion and disease progression. Nature 2006, 443, 350–354. [Google Scholar] [CrossRef]
- Kahan, S.M.; Wherry, E.J.; Zajac, A.J. T cell exhaustion during persistent viral infections. Virology 2015, 479–480, 180–193. [Google Scholar] [CrossRef] [PubMed]
- Rallón, N.; García, M.; García-Samaniego, J.; Rodríguez, N.; Cabello, A.; Restrepo, C.; Álvarez, B.; García, R.; Górgolas, M.; Benito, J.M. HCV coinfection contributes to HIV pathogenesis by increasing immune exhaustion in CD8 T-cells. PLoS ONE 2017, 12, e017394335. [Google Scholar] [CrossRef][Green Version]
- Li, J.Z.; Segal, F.P.; Bosch, R.J.; Lalama, C.M.; Roberts-Toler, C.; Delagreverie, H.; Getz, R.; Garcia-Broncano, P.; Kinslow, J.; Tressler, R.; et al. ART reduces T cell activation and immune exhaustion markers in HIV controllers. Clin. Infect. Dis. 2019, 70, ciz442. [Google Scholar]
- Lee, J.S.; Tato, C.M.; Joyce-Shaikh, B.; Gulen, M.F.; Cayatte, C.; Chen, Y.; Blumenschein, W.M.; Judo, M.; Ayanoglu, G.; McClanahan, T.K.; et al. Interleukin-23-independent IL-17 production regulates intestinal epithelial permeability. Immunity 2015, 43, 727–738. [Google Scholar] [CrossRef] [PubMed]
- Monteiro, P.; Gosseling, A.; Wacleche, V.S.; El-Far, M.; Said, E.A.; Karred, H.; Grandvaux, N.; Boulassel, M.-R.; Routy, J.-P.; Ancuta, P. Memory CCR6+CD4+ T cells are preferential targets from productive HIV type 1 infection regardless of their expression of integrin B7. J. Immunol. 2011, 186, 4618–4630. [Google Scholar] [CrossRef]
- Castro, F.O.F.; Silva, J.M.; Dorneles, G.P.; Barros, J.B.S.; Ribeiro, C.B.; Noronha, I.; Barbosa, G.R.; Souza, L.C.S.; Guilarde, A.O.; Pereira, A.J.C.S.; et al. Distinct inflammatory profiles in HIV-infected individuals under antiretroviral therapy using cannabis, cocaine or cannabis plus cocaine. AIDS 2019, 33, 1831–1842. [Google Scholar] [CrossRef]
- Myers, J.M.; Cooper, L.T.; Kem, D.C.; Stavrakis, S.; Kosanke, S.D.; Shevach, E.M.; Fairweather, D.; Stoner, J.A.; Cox, C.J.; Cunningham, M.W. Cardiac myosin-Th17 responses promote heart failure in human myocarditis. JCI Insight 2016, 1, 85851. [Google Scholar] [CrossRef]
- Lock, C.; Hermans, G.; Pedotti, R.; Brendolan, A.; Schadt, E.; Garren, H.; Langer-Gould, A.; Strober, S.; Cannella, B.; Allard, J.; et al. Gene-microarray analysis of multiple sclerosis lesions yields new targets validated in autoimmune encephalomyelitis. Nat. Med. 2002, 8, 500–508. [Google Scholar] [CrossRef]
- Pellicori, P.; Zhang, J.; Cuthbert, J.; Urbinati, A.; Shah, P.; Kazmi, S.; Cleland, J.G.F. High sensitivity C-Reactive Protein in Chronic Heart Failure: Patient Characteristics, Phenotypes and Mode of Death. Cardiovasc. Res. 2019, pii, cvz198. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.; Awasthi, A.; Yosef, N.; Quintana, F.J.; Xiao, S.; Peters, A.; Wu, C.; Kleinewietfeld, M.; Kunder, S.; Hafler, D.A.; et al. Induction and molecular signature of pathogenic TH17 cells. Nat. Immunol. 2012, 13, 991–999. [Google Scholar] [CrossRef]
- Hu, D.; Notarbartolo, S.; Croonenborghs, T.; Patel, B.; Cialic, R.; Yang, T.H.; Aschenbrenner, D.; Andersson, K.M.; Gattorno, M.; Pham, M.; et al. Transcriptional signature of human pro-inflammatory TH17 cells identifies reduced IL10 gene expression in multiple sclerosis. Nat. Commun. 2017, 8, 1600. [Google Scholar] [CrossRef] [PubMed]
- Stockinger, B.; Omenetti, S. The dichotomous nature of T helper 17 cells. Nat. Rev. Immunol 2017, 17, 535–544. [Google Scholar] [CrossRef] [PubMed]
- Fert, A.; Raymond Marchand, L.; Wiche Salinas, T.R.; Ancuta, P. Targeting Th17 cells in HIV-1 remission/cure interventions. Trends Immunol. 2022, 43, 580–594. [Google Scholar] [CrossRef] [PubMed]
- Langrish, C.L.; Chen, Y.; Blumenschein, W.M.; Mattson, J.; Basham, B.; Sedgwick, J.D.; McClanahan, T.; Kastelein, R.A.; Cua, D.J. IL-23 drives a pathogenic T cell population that induces autoimmune inflammation. J. Exp. Med. 2005, 201, 233–240. [Google Scholar] [CrossRef] [PubMed]
- Isailovic, N.; Daigo, K.; Mantovani, A.; Selmi, C. Interleukin-17 and innate immunity in infections and chronic inflammation. J. Autoimmun. 2015, 60, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Renault, C.; Veyrenche, N.; Mennechet, F.; Bedin, A.S.; Routy, J.P.; Van de Perre, P.; Reynes, J.; Tuaillon, E. Th17 CD4+ T-Cell as a Preferential Target for HIV Reservoirs. Front. Immunol. 2022, 13, 822576. [Google Scholar] [CrossRef] [PubMed]
- Manuzak, J.A.; Gott, T.M.; Kirkwood, J.S.; Coronado, E.; Hensley-McBain, T.; Miller, C.; Cheu, R.K.; Collier, A.C.; Funderburg, N.T.; Martin, J.N.; et al. Heavy cannabis use associated with reduction in activated and inflammatory immune cell frequencies in antiretroviral therapy-treated human immunodeficiency virus-infected individuals. Clin. Infect. Dis. 2018, 66, 1872–1882. [Google Scholar] [CrossRef]
- Kozela, E.; Juknat, A.; Kaushansky, N.; Rimmerman, N.; Ben-Nun, A.; Vogel, Z. Cannabinoids decrease the Th17 inflammatory autoimmune phenotype. J. Neuroimmune Pharmacol. 2013, 8, 1265–1276. [Google Scholar] [CrossRef]
- Falcinelli, S.D.; Cooper-Volkheimer, A.D.; Semenova, L.; Wu, E.; Richardson, A.; Ashokkumar, M.; Margolis, D.M.; Archin, N.M.; Rudin, C.D.; Murdoch, D.; et al. Impact of cannabis use on immune cell populations and the viral reservoir in people with HIV on suppressive antiretroviral therapy. J. Infect. Dis. 2023, 228, jiad364. [Google Scholar] [CrossRef] [PubMed]
- Klenerman, P.; Oxenius, A. T cell responses to cytomegalovirus. Nat. Rev. Immunol. 2016, 16, 367–377. [Google Scholar] [CrossRef] [PubMed]
- Lichtner, M.; Cicconi, P.; Vita, S.; Cozzi-Lepri, A.; Galli, M.; Caputo, S.L.; Saracino, A.; De Luca, A.; Moioli, M.; Maggiolo, F.; et al. Cytomegalovirus coinfection is associated with an increased risk of severe non-AIDS-defining events in a large cohort of HIV-infected patients. J. Infect. Dis. 2015, 211, 178–186. [Google Scholar] [CrossRef] [PubMed]
- Ballegaard, V.; Brændstrup, P.; Pedersen, K.K.; Kirkby, N.; Stryhn, A.; Ryder, L.P.; Gerstoft, J.; Nielsen, S.D. Cytomegalovirus-specific T-cells are associated with immune senescence, but not with systemic inflammation, in people living with HIV. Sci. Rep. 2018, 8, 3778. [Google Scholar] [CrossRef] [PubMed]
- Moretti, S.; Castelli, M.; Franchi, S.; Raggi, M.A.; Mercolini, L.; Protti, M.; Somaini, L.; Panerai, A.E.; Sacerdote, P. Δ⁹-Tetrahydrocannabinol-induced anti-inflammatory responses in adolescent mice switch to proinflammatory in adulthood. J. Leukoc. Biol. 2014, 96, 523–534. [Google Scholar] [CrossRef] [PubMed]
- Zaparte, A.; Schuch, J.B.; Viola, T.W.; Baptista, T.A.S.; Beidacki, A.S.; do Prado, C.H.; Sanvicente-Vieira, B.; Bauer, M.E.; Grassi-Oliveira, R. Cocaine Use Disorder Is Associated with Changes in Th1/Th2/Th17 Cytokines and Lymphocytes Subsets. Front. Immunol. 2019, 10, 2435. [Google Scholar] [PubMed]
- Ivanov, I.I.; McKenzie, B.S.; Zhou, L.; Tadokoro, C.E.; Lepelley, A.; Lafaille, J.J.; Cua, D.J.; Littman, D.R. The orphan nuclear receptor RORt directs the differentiation program of proinflammatory IL-17+ T helper cells. Cell 2006, 126, 1121–1133. [Google Scholar] [CrossRef] [PubMed]
- Muranski, P.; Restifo, N.P. Essentials of Th17 cell commitment and plasticity. Blood 2013, 121, 2402–2414. [Google Scholar] [CrossRef]
- van Voorhis, M.; Knopp, S.; Julliard, W.; Fechner, J.H.; Zhang, X.; Schauer, J.J.; Mezrich, J.D. Exposure to atmospheric particulate matter enhances Th17 polarization through the aryl hydrocarbon receptor. PLoS ONE 2013, 8, e82545. [Google Scholar] [CrossRef]
- Quintana, F.J.; Basso, A.S.; Iglesias, A.H.; Korn, T.; Farez, M.F.; Bettelli, E.; Caccamo, M.; Oukka, M.; Weiner, H.L. Control of T(reg) and T(H)17 cell differentiation by the aryl hydrocarbon receptor. Nature 2008, 453, 65–71. [Google Scholar] [CrossRef]
- Chatterjee, D.; Zhang, Y.; Ngassaki-Yoka, C.D.; Dutilleul, A.; Khalfi, S.; Hernalsteens, O.; Wiche Salinas, T.R.; Dias, J.; Chen, H.; Smail, Y.; et al. Identification of aryl hydrocarbon receptor as a barrier to HIV-1 infection and outgrowth in CD4+ T cells. Cell Rep. 2023, 42, 112634. [Google Scholar] [CrossRef] [PubMed]
- Said, E.A.; Dupuy, F.P.; Trautmann, L.; Zhang, Y.; Shi, Y.; El-Far, M.; Hill, B.J.; Noto, A.; Ancuta, P.; Peretz, Y.; et al. Programmed death-1-induced interleukin-10 production by monocytes impairs CD4+ T cell activation during HIV infection. Nat. Med. 2010, 16, 452–459. [Google Scholar] [CrossRef] [PubMed]
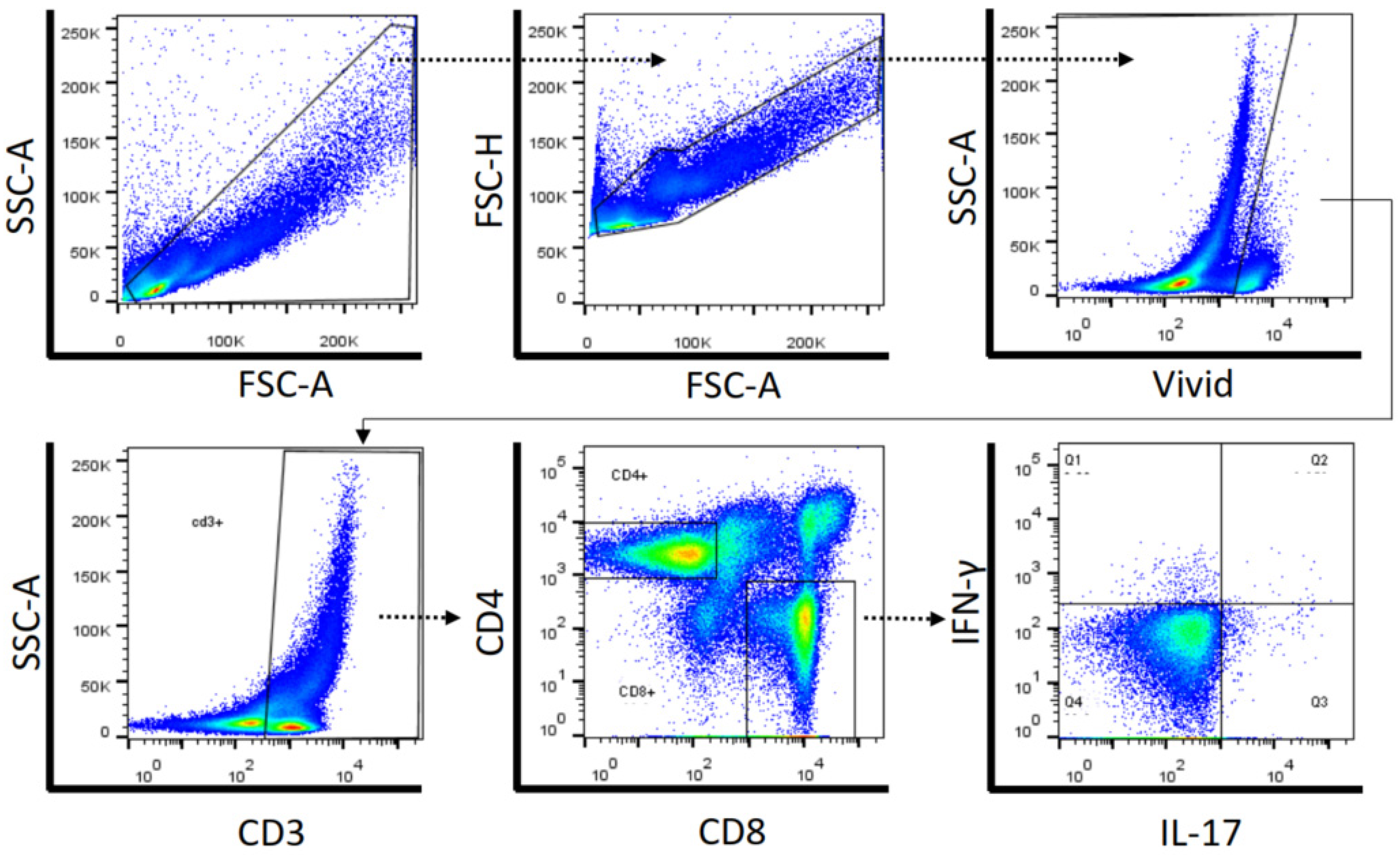


| Characteristics | HIV Non-Drug Users (n = 10) | HIV Cannabis Users (n = 10) | HIV Cocaine Users (n = 7) | HIV Cannabis Plus Cocaine Users (n = 10) | p Value |
|---|---|---|---|---|---|
| Gender (male/female) | 9/1 | 10/0 | 5/2 | 8/2 | ns |
| Age, years Media (±SD) | 39 (±9.0) | 37 (±8.2) | 38 (±10.9) | 38 (±7.8) | ns |
| Smokers % (n) | 30% (3) | 30% (3) | 42.8% (3) | 40% (4) | |
| Years of treatment Media (±SD) | 5.4 (±3.0) | 4.8 (±4.3) | 5.7 (±3.6) | 8.7 (±6.7) | |
| Antiretroviral regimen (%) | |||||
| AZT/3TC/EFV | 40.0 | 50.0 | 57.1 | 40.0 | |
| AZT/3TC | 30.0 | - | 14.3 | - | |
| AZT/3TC/LPV/r | 10.0 | 10.0 | 28.6 | - | |
| AZT/RIT | 10.0 | - | - | - | |
| 3TC/TDF/EFV | - | 30.0 | - | 30.0 | |
| Biovir | 10.0 | - | - | - | |
| Biovir/NVP | - | - | - | 20.0 | |
| TDF/3TC/RIT/AZT | - | 10.0 | - | 10.0 | |
| CD4 counts cels/mm3 media (±SD) | 557.4 (±183) | 489.1 (±270) | 627 (±209) | 389 (±288) | ns |
| HIV-1 Viral load | <LD | <LD | <LD | <LD | |
| Nadir CD4 (media ± SD) | 343 (±255) | 252 (±180) | 347 (±179) | 297 (±282) | ns |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Castro, F.d.O.F.d.; Guilarde, A.O.; Souza, L.C.S.; Guimarães, R.F.; Pereira, A.J.C.S.; Romão, P.R.T.; Pfrimer, I.A.H.; Fonseca, S.G. Polarization of HIV-1- and CMV-Specific IL-17-Producing T Cells among People with HIV under Antiretroviral Therapy with Cannabis and/or Cocaine Usage. Pharmaceuticals 2024, 17, 465. https://doi.org/10.3390/ph17040465
Castro FdOFd, Guilarde AO, Souza LCS, Guimarães RF, Pereira AJCS, Romão PRT, Pfrimer IAH, Fonseca SG. Polarization of HIV-1- and CMV-Specific IL-17-Producing T Cells among People with HIV under Antiretroviral Therapy with Cannabis and/or Cocaine Usage. Pharmaceuticals. 2024; 17(4):465. https://doi.org/10.3390/ph17040465
Chicago/Turabian StyleCastro, Fernanda de Oliveira Feitosa de, Adriana Oliveira Guilarde, Luiz Carlos Silva Souza, Regyane Ferreira Guimarães, Ana Joaquina Cohen Serique Pereira, Pedro Roosevelt Torres Romão, Irmtraut Araci Hoffmann Pfrimer, and Simone Gonçalves Fonseca. 2024. "Polarization of HIV-1- and CMV-Specific IL-17-Producing T Cells among People with HIV under Antiretroviral Therapy with Cannabis and/or Cocaine Usage" Pharmaceuticals 17, no. 4: 465. https://doi.org/10.3390/ph17040465
APA StyleCastro, F. d. O. F. d., Guilarde, A. O., Souza, L. C. S., Guimarães, R. F., Pereira, A. J. C. S., Romão, P. R. T., Pfrimer, I. A. H., & Fonseca, S. G. (2024). Polarization of HIV-1- and CMV-Specific IL-17-Producing T Cells among People with HIV under Antiretroviral Therapy with Cannabis and/or Cocaine Usage. Pharmaceuticals, 17(4), 465. https://doi.org/10.3390/ph17040465








