Potential Targeted Therapies in Ovarian Cancer
Abstract
1. Introduction
2. Results
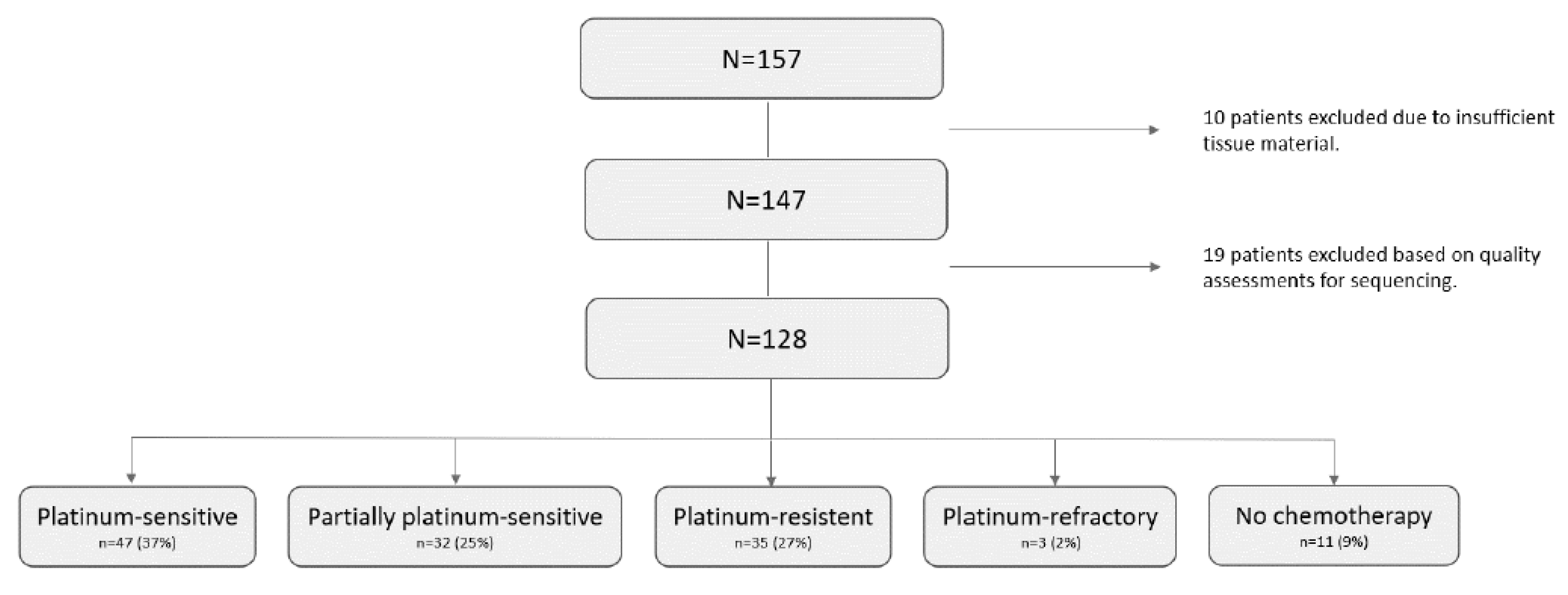
2.1. Clinical Data
2.2. Datamining
2.3. Pathogenic and Likely Pathogenic Mutations
2.4. Druggable Targets
2.5. Potential Targeted Therapies
2.6. Mutations in the Different Platinum Response Groups
3. Discussion
3.1. Mutation Profile in Danish Ovarian Cancer Patients Compared to Other Studies
3.2. Platinum Resistance and PARP Inhibitors
4. Materials and Methods
4.1. Patients and Tissue Samples
4.2. DNA Extraction
4.3. Library Preparation and Sequencing
4.4. Microsatellite Instability (MSI)
4.5. Data Analysis
4.6. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sørensen, S.M.; Bjørn, S.F.; Jochumsen, K.M.; Jensen, P.T.; Thranov, I.R.; Hare-Bruun, H.; Seibæk, L.; Høgdall, C. Danish Gynecological Cancer Database. Clin. Epidemiol. 2016, 8, 485. [Google Scholar] [CrossRef] [PubMed]
- Torre, L.A.; Trabert, B.; DeSantis, C.E.; Miller, K.D.; Samimi, G.; Runowicz, C.D.; Gaudet, M.M.; Jemal, A.; Siegel, R.L. Ovarian cancer statistics, 2018. CA. Cancer J. Clin. 2018, 68, 284–296. [Google Scholar] [CrossRef] [PubMed]
- Lheureux, S.; Gourley, C.; Vergote, I.; Oza, A.M. Epithelial ovarian cancer. Lancet 2019, 393, 1240–1253. [Google Scholar] [CrossRef]
- Kossaï, M.; Leary, A.; Scoazec, J.Y.; Genestie, C. Ovarian Cancer: A Heterogeneous Disease. Pathobiology 2018, 85, 41–49. [Google Scholar] [CrossRef]
- Helleday, T.; Petermann, E.; Lundin, C.; Hodgson, B.; Sharma, R.A. DNA repair pathways as targets for cancer therapy. Nat. Rev. Cancer 2008, 8, 193–204. [Google Scholar] [CrossRef] [PubMed]
- Mittica, G.; Ghisoni, E.; Giannone, G.; Genta, S.; Aglietta, M.; Sapino, A.; Valabrega, G. PARP Inhibitors in Ovarian Cancer. Recent Pat. Anticancer. Drug Discov. 2018, 13, 392–410. [Google Scholar] [CrossRef] [PubMed]
- Konstantinopoulos, P.A.; Ceccaldi, R.; Shapiro, G.I.; D’Andrea, A.D. Homologous recombination deficiency: Exploiting the fundamental vulnerability of ovarian cancer. Cancer Discov. 2015, 5, 1137–1154. [Google Scholar] [CrossRef]
- Myriad Genetics. Available online: https://myriad.com/oncology/mychoice-cdx/ (accessed on 15 April 2022).
- Mirza, M.R.; Pignata, S.; Ledermann, J.A. Latest clinical evidence and further development of PARP inhibitors in ovarian cancer. Ann. Oncol. 2018, 29, 1366–1376. [Google Scholar] [CrossRef]
- Mirza, M.R.; Coleman, R.L.; González-Martín, A.; Moore, K.N.; Colombo, N.; Ray-Coquard, I.; Pignata, S. The forefront of ovarian cancer therapy: Update on PARP inhibitors. Ann. Oncol. 2020, 31, 1148–1159. [Google Scholar] [CrossRef]
- Vestergaard, L.K.; Oliveira, D.N.P.; Poulsen, T.S.; Høgdall, C.K.; Høgdall, E.V. Oncomine TM Comprehensive Assay v3 vs. Oncomine TM Com-prehensive Assay Plus. Cancers 2021, 13, 5230. [Google Scholar] [CrossRef]
- Cancer Genome Atlas Research Network. Integrated genomic analyses of ovarian carcinoma. Nature 2011, 474, 609–615. [Google Scholar] [CrossRef]
- Patch, A.M.; Christie, E.L.; Etemadmoghadam, D.; Garsed, D.W.; George, J.; Fereday, S.; Nones, K.; Cowin, P.; Alsop, K.; Bailey, P.J.; et al. Whole-genome characterization of chemoresistant ovarian cancer. Nature 2015, 521, 489–494. [Google Scholar] [CrossRef] [PubMed]
- Richards, S.; Aziz, N.; Bale, S.; Bick, D.; Das, S.; Gastier-Foster, J.; Crody, W.W.; Hegde, M.; Lyon, E.; Spector, E.; et al. Standards and guidelines for the interpretation of sequence variants: A joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet. Med. 2015, 17, 405–424. [Google Scholar] [CrossRef] [PubMed]
- Muzzey, D.; Evans, E.A.; Lieber, C. Understanding the Basics of NGS: From Mechanism to Variant Calling. Curr. Genet. Med. Rep. 2015, 3, 158–165. [Google Scholar] [CrossRef] [PubMed]
- Altmann, A.; Weber, P.; Bader, D.; Preuß, M.; Binder, E.B.; Müller-Myhsok, B. A beginners guide to SNP calling from high-Throughput DNA-Sequencing data. Hum. Genet. 2012, 131, 1541–1554. [Google Scholar] [CrossRef]
- Soegaard, M.; Kjaer, S.K.; Cox, M.; Wozniak, E.; Høgdall, E.; Høgdall, C.; Blaakaer, J.; Jacobs, I.J.; Gayther, S.A.; Ramus, S.J. BRCA1 and BRCA2 mutation prevalence and clinical characteristics of a population-based series of ovarian cancer cases from Denmark. Clin. Cancer Res. 2008, 14, 3761–3767. [Google Scholar] [CrossRef]
- ESMO Guidelines Comittee. Relapsed Epithelial Ovarian Carcinoma Treatment Recommendations. 2020. Available online: https://www.esmo.org/guidelines/guidelines-by-topic/gynaecological-cancers/newly-diagnosed-and-relapsed-epithelial-ovarian-carcinoma/eupdate-ovarian-cancer-treatment-recommendations (accessed on 15 April 2022).
- Tew, W.P.; Lacchetti, C.; Ellis, A.; Maxian, K.; Banerjee, S.; Bookman, M.; Jones, M.B.; Lee, J.M.; Lheureux, S.; Liu, J.F.; et al. PARP Inhibitors in the Management of Ovarian Cancer: ASCO Guideline. ASCO Guidel. 2020, 38, 3468. [Google Scholar] [CrossRef]
- Medicinrådet. Medicinrådets Behandlingsvejledning Vedrørende Lægemidler til BRCA-Muteret Kræft I Æggestokkene, Ægge-lederne Eller Primær Kræft I Bughinden; Medicinrådet: Copenhagen, Denmark, 2020; pp. 1–10. [Google Scholar]
- Oronsky, B.; Ray, C.M.; Spira, A.I.; Trepel, J.B.; Carter, C.A.; Cottrill, H.M. A brief review of the management of platinum-resistant–platinum-refractory ovarian cancer. Med. Oncol. 2017, 34, 1–7. [Google Scholar] [CrossRef]
- Fong, P.C.; Yap, T.A.; Boss, D.S.; Carden, C.P.; Mergui-Roelvink, M.; Gourley, C.; De Greve, J.; Lubinski, J.; Shanley, S.; Messiou, C.; et al. Poly(ADP)-ribose polymerase inhibition: Frequent durable responses in BRCA carrier ovarian cancer correlating with platinum-free interval. J. Clin. Oncol. 2010, 28, 2512–2519. [Google Scholar] [CrossRef]
- Kaufman, B.; Shapira-Frommer, R.; Schmutzler, R.K.; Audeh, M.W.; Friedlander, M.; Balmaña, J.; Mitchell, G.; Fried, G.; Stemmer, S.M.; Hubert, A.; et al. Olaparib monotherapy in patients with advanced cancer and a germline BRCA1/2 mutation. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2015, 33, 244–250. [Google Scholar] [CrossRef]
- Moore, K.N.; Secord, A.A.; Geller, M.A.; Miller, D.S.; Cloven, N.G.; Fleming, G.F.; Hendrickson, A.E.W.; Azodi, M.; DiSilverstro, P.; Oza, A.M.; et al. QUADRA: A phase 2, open-label, single-arm study to evaluate niraparib in patients (pts) with relapsed ovarian cancer (ROC) in 4th or later line of therapy: Results from the tBRCAmut subset. Ann. Oncol. 2018, 29, viii332–viii358. [Google Scholar] [CrossRef]
- Colombo, N.; Nicoletto, M.O.; Panici, P.B.; Tognon, G.; Bologna, A.; Lissoni, A.A.; DeCensi, A.; Tomao, F.; Fossati, R.; Tettamanzi, F.; et al. BAROCCO: A randomized phase II study of weekly paclitaxel vs cediranib-olaparib combination given with continuous or intermittent schedule in patients with recurrent platinum resistant ovarian cancer (PROC). Ann. Oncol. 2019, 30, v896. [Google Scholar] [CrossRef]
- Kristeleit, R.; Lisyanskaya, A.; Fedenko, A.; Dvorkin, M.; de Melo, A.C.; Shparyk, Y.; Rakhmatullina, I.; Bondarenko, I.; Colombo, N.; Svintsitskiy, V.; et al. Rucaparib versus chemotherapy in patients with advanced, relapsed ovarian cancer and a deleterious BRCA mutation: Efficacy and safety from ARIEL4, a randomized phase III study. Presented Soc. Gynecol. Oncol. 2021, 162, S3–S4. [Google Scholar] [CrossRef]
- ESMO Guidelines. Available online: https://www.esmo.org/guidelines (accessed on 15 April 2022).
- Lambert, J.M.R.; Gorzov, P.; Veprintsev, D.B.; Söderqvist, M.; Segerbäck, D.; Bergman, J.; Fersht, A.R.; Hainaut, P.; Wiman, K.G.; Bykow, V.J.N.; et al. PRIMA-1 Reactivates Mutant p53 by Covalent Binding to the Core Domain. Cancer Cell 2009, 15, 376–388. [Google Scholar] [CrossRef] [PubMed]
- Mohell, N.; Alfredsson, J.; Fransson, Å.; Uustalu, M.; Byström, S.; Gullbo, J.; Hallberg, A.; Bykow, V.J.N.; Björklund, U.; Wiman, K.G.; et al. APR-246 overcomes resistance to cisplatin and doxorubicin in ovarian cancer cells. Cell Death Dis. 2015, 6, e1794. [Google Scholar] [CrossRef]
- Hentze, J.L.; Høgdall, C.; Kjær, S.K.; Blaakær, J.; Høgdall, E. Searching for new biomarkers in ovarian cancer patients: Rationale and design of a retrospective study under the Mermaid III project. Contemp. Clin. Trials Commun. 2017, 8, 167–174. [Google Scholar] [CrossRef]

| Median age in year | 65 (41–89) |
| Median CA125 | 792 (9–17.160) |
| Median RMI | 4632 (63–153.432) |
| Median BMI | 24 (15–48) |
| Median follow up time in months | 92 (61–123) |
| Performance score | |
| 0 | 54 (42%) |
| 1 | 52 (41%) |
| 2 | 18 (14%) |
| 3 | 4 (3%) |
| FIGO stage | |
| I | 9 (7%) |
| II | 10 (8%) |
| III | 89 (70%) |
| IV | 20 (15%) |
| Residual tumor after surgery | |
| 0 | 50 (39%) |
| <1 cm | 24 (19%) |
| >1 cm ≤ 2 cm | 17 (13%) |
| >2 cm | 37 (29%) |
| Chemotherapy (n = 117) | |
| Carboplatin and taxol | 113 (97%) |
| Carboplatin | 4 (3%) |
| Chemotherapy response | |
| >12 months (sensitive) | 47 (40%) |
| 6 - ≤ 12 months (partially platinum-sensitive) | 32 (27%) |
| ≤6 months (resistant) | 35 (30%) |
| Refractory | 3 (3%) |
| OS in months | |
| OS (median) | 43 (0.7–190) |
| OS FIGO stage I (median) | 138 (45–184) |
| OS FIGO stage II (median) | 54 (3–140) |
| OS FIGO stage III (median) | 38 (0.7–190) |
| OS FIGO stage IV (median) | 33 (0.8–180) |
| PFS in months | |
| PFS (median) | 15 (0–118) |
| PFS FIGO stage I (median) | 74 (23–118) |
| PFS FIGO stage II (median) | 19 (0–74) |
| PFS FIGO stage III (median) | 15 (0–107) |
| PFS FIGO stage IV (median) | 10 (0–95) |
| All patients (n = 128) | ARID1A ATM * BAP1 BRCA1 ** BRCA2 ** CDK12 * CHEK1 * CREBBP CTNNB1 DDR2 EGFR * FANCA FANCI | FBXW7 FGFR3 * FGFR4 FLT3 FOXL2 H3F3A KRAS MAP2K1 MAX MLH1 * MRE11 MSH2 * MTOR | MYC NF1 NF2 NOTCH1 NOTCH2 NOTCH3 NTRK2 NTRK3 PDGFRB PIK3CA * PIK3CB POLE PTCH1 | PTPN11 RAD51 RB1 SETD2 SLX4 SMARCA4 SMARCB1 SMO TERT TP53 (86%) TSC1 TSC2 |
| Platinum-sensitive (n = 47) | ARID1A ATM * BRCA1 ** BRCA2 ** CDK12 * CREBBP EGFR * | FANCA FGFR4 FLT3 H3F3A KRAS MAP2K1 MSH2 * | NF1 NOTCH1 NOTCH3 PDGFRB PIK3CB PTCH1 RAD51 | RB1 SMARCA4 SMARCB1 TP53 (85%) TSC1 TSC2 |
| Partially platinum-sensitive (n = 32) | ARID1A BRCA1 ** BRCA2 ** CREBBP | FANCA KRAS NF1 | NOTCH1 NOTCH3 PIK3CA * | POLE SMO TP53 (91%) |
| Platinum-resistant (n = 35) | ATM * BAP1 BRCA1 ** BRCA2 ** CDK12 * CHEK1 * CREBBP CTNNB1 DDR2 FANCA | FANCI FBXW7 FGFR3 * FOXL2 KRAS MAX MLH1 * MRE11 MTOR | MYC NF1 NF2 NOTCH1 NOTCH2 NTRK2 NTRK3 PDGFRB POLE | PTPN11 RAD51 RB1 SETD2 SMARCA4 SMARCB1 TERT TP53 (83%) TSC1 |
| Platinum-refractory (n = 3) | ARID1A | CREBBP | TP53 (33%) | |
| No chemotherapy (n = 11) | NF1 | NOTCH1 | SLX4 | TP53 (100%) |
| Gene | Cancer | Therapy |
|---|---|---|
| ATM | Prostata cancer | Olaparib |
| BRCA1 | Prostate cancer | Bevacizumab + olaparib, niraparib, olaparib, rucaparib |
| BRCA2 | Prostate cancer | Bevacizumab + olaparib, niraparib, olaparib, rucaparib |
| CDK12 | Prostate cancer | Olaparib |
| CHEK1 | Prostate cancer | Olaparib |
| EGFR | Non-small cell lung cancer | Afatinib, bevacizumab + erlotinib, bevacizumab + gefitinib, dacominitib, erlotinib + ramucirumab, gefitinib, gefitinib + chemotherapy. |
| FGFR3 | Bladder cancer, bladder urothelial carcinoma | Erdafitinib |
| MLH1 MSH2 | Anaplastic thyroid cancer, bladder cancer, brain metastases from solid tumors, colorectal cancer, cutaneous melanoma, hepatocellular carcinoma, hereditary gastrointestinal cancers, hypopharyngeal cancer, larynx cancer, lung and malignant pleural mesothelioma, non-small cell lung cancer, oral cavity cancer, renal cell carcinoma, small-cell lung cancer | Immunotherapy |
| PIK3CA | Breast cancer | Alpelisib and hormone therapy |
| Platinum-sensitive (N = 47) | Partially platinum-sensitive (N = 32) | Platinum-resistant (N = 35) | |
|---|---|---|---|
| BRCA1/2 | n = 10 BRCA1 p.Leu1216PhefsTer2 BRCA1 p.Asp1305AlafsTer2 BRCA1 p.Arg1699Gln BRCA1 p.Gln494Ter BRCA1 p.Glu1134Ter BRCA1 p.Gln1756PorfsTer74 BRCA1 p.? splice site mutation BRCA1 p.Arg1699Gln BRCA1 p.Gln1756ProfsTer74 BRCA2 p.Trp2626Cys | n = 7 BRCA1 p.? splice site mutation BRCA1 p.Ala1708Glu BRCA1 p.Gly1738Glu BRCA1 p.Asn609IlefsTer3 BRCA2 p.Ser1741ThrfsTer35 BRCA2 p.Ser2219Ter BRCA2 p.Lys2909GlnfsTer16 | n = 2 BRCA1 p.Glu1046Ter BRCA1 p.Glu23ValfsTer17 |
| BRCA1/2 and other druggable targets | n = 1 BRCA1 p.? splice site mutation ATM p.Arg2598Ter EGFR p.Ala289Thr MSH2 p.Gln324Ter MSH2 Trp345Ter MSH2 p.Gln413Ter | n = 1 BRCA2 p.Trp3106Ter FGFR3 p.Trp685Ter | |
| Other druggable targets | n = 1 CDK12 p.Ser301CysfsTer5 | n = 1 PIK3CA p.Glu542Lys | n = 4 ATM p.Glu1751Ter CDK12 p.Gln602Ter CHEK1 p.Gln318Ter MLH1 p.Arg659Ter |
| Platinum-sensitive (N = 47) | Partially platinum-sensitive (N = 32) | Platinum-resistant (N = 35) | |
|---|---|---|---|
| PARP inhibitor | n = 12 BRCA1 chr17:41243899 p.Leu1216PhefsTer2 BRCA1 chr17:41243633 p.Asp1305AlafsTer2 BRCA1 chr17:41215947 p.Arg1699Gln BRCA1 chr17:41246068 p.Gln494Ter BRCA1 chr17:41244148 p.Glu1134Ter BRCA1 chr17:41209079 p.Gln175ProfsTer74 BRCA1 chr17:41215969 p.? BRCA1 chr17:41215947 p.Arg1699Gln BRCA1 chr17:41209079 p.Gln1756ProfsTer74 BRCA1 chr17:41209153 p.? BRCA2 chr13:32936732 p.Trp2626Cys ATM chr11:108203492 p.Arg2598Ter CDK12 chr17:37619224 p.Ser301CysfsTer5 | n = 7 BRCA1 chr17:41222944 p.? BRCA1 chr17:41215920 p.Ala1708Glu BRCA1 chr17:41209133 p.Gly1738Glu BRCA1 chr17:41245721 p.Asn609IlefsTer3 BRCA2 chr13:32950896 p.Lys2909GlnfsTer16 BRCA2 chr13:32915148 p.Ser2219Ter BRCA2 chr13:32913710 p.Ser1741ThrsfTer35 | n = 6 BRCA1 chr17:41244412 p.Glu1046Ter BRCA1 chr17:41276044 p.Glu23ValfsTer17 BRCA2 chr13:32968887 p.Trp3106Ter ATM chr11:108172448 p.Glu1751Ter CDK12 chr17:37627889 p.Gln602Ter CHEK1 chr11:125514014 p.Gln318Ter |
| Tyrosine kinase inhibitor | n = 1 EGFR chr7:55221821 p.Ala289Thr | ||
| Immunotherapy | n = 1 MSH2 chr2:47643462 p.Gln324Ter MSH2 chr2:47643526 p.Trp345Ter MSH2 chr2:47657041 p.Gln413Ter | n = 1 MLH1 chr3:37090086 p.Arg659Ter | |
| Alpesilib and hormone therapy | n = 1 PIK3CA chr3:178936082 p.Glu542Lys | ||
| Erdafitinib | n = 1 FGFR3 chr4:1808296 p.Trp685Ter |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sisman, Y.; Vestergaard, L.K.; de Oliveira, D.N.P.; Poulsen, T.S.; Schnack, T.H.; Høgdall, C.; Høgdall, E. Potential Targeted Therapies in Ovarian Cancer. Pharmaceuticals 2022, 15, 1324. https://doi.org/10.3390/ph15111324
Sisman Y, Vestergaard LK, de Oliveira DNP, Poulsen TS, Schnack TH, Høgdall C, Høgdall E. Potential Targeted Therapies in Ovarian Cancer. Pharmaceuticals. 2022; 15(11):1324. https://doi.org/10.3390/ph15111324
Chicago/Turabian StyleSisman, Yagmur, Lau Kræsing Vestergaard, Douglas Nogueira Perez de Oliveira, Tim Svenstrup Poulsen, Tine Henrichsen Schnack, Claus Høgdall, and Estrid Høgdall. 2022. "Potential Targeted Therapies in Ovarian Cancer" Pharmaceuticals 15, no. 11: 1324. https://doi.org/10.3390/ph15111324
APA StyleSisman, Y., Vestergaard, L. K., de Oliveira, D. N. P., Poulsen, T. S., Schnack, T. H., Høgdall, C., & Høgdall, E. (2022). Potential Targeted Therapies in Ovarian Cancer. Pharmaceuticals, 15(11), 1324. https://doi.org/10.3390/ph15111324








