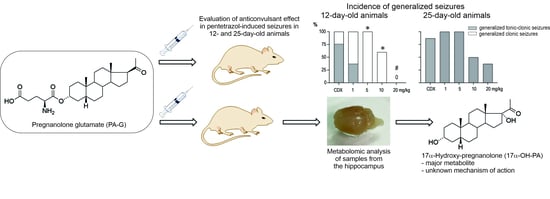
The Neuroactive Steroid Pregnanolone Glutamate: Anticonvulsant Effect, Metabolites and Its Effect on Neurosteroid Levels in Developing Rat Brains
Abstract
1. Introduction
2. Results
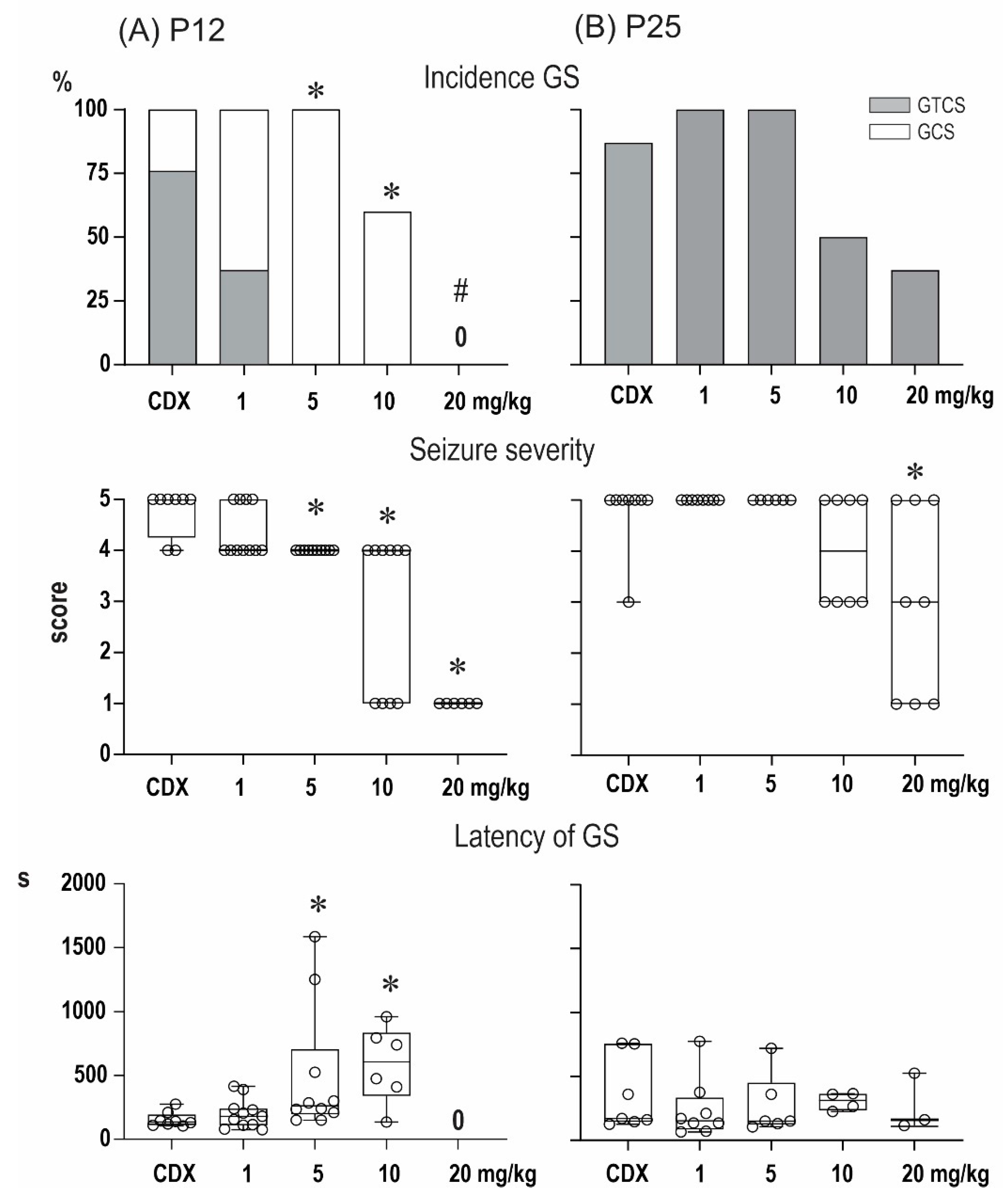
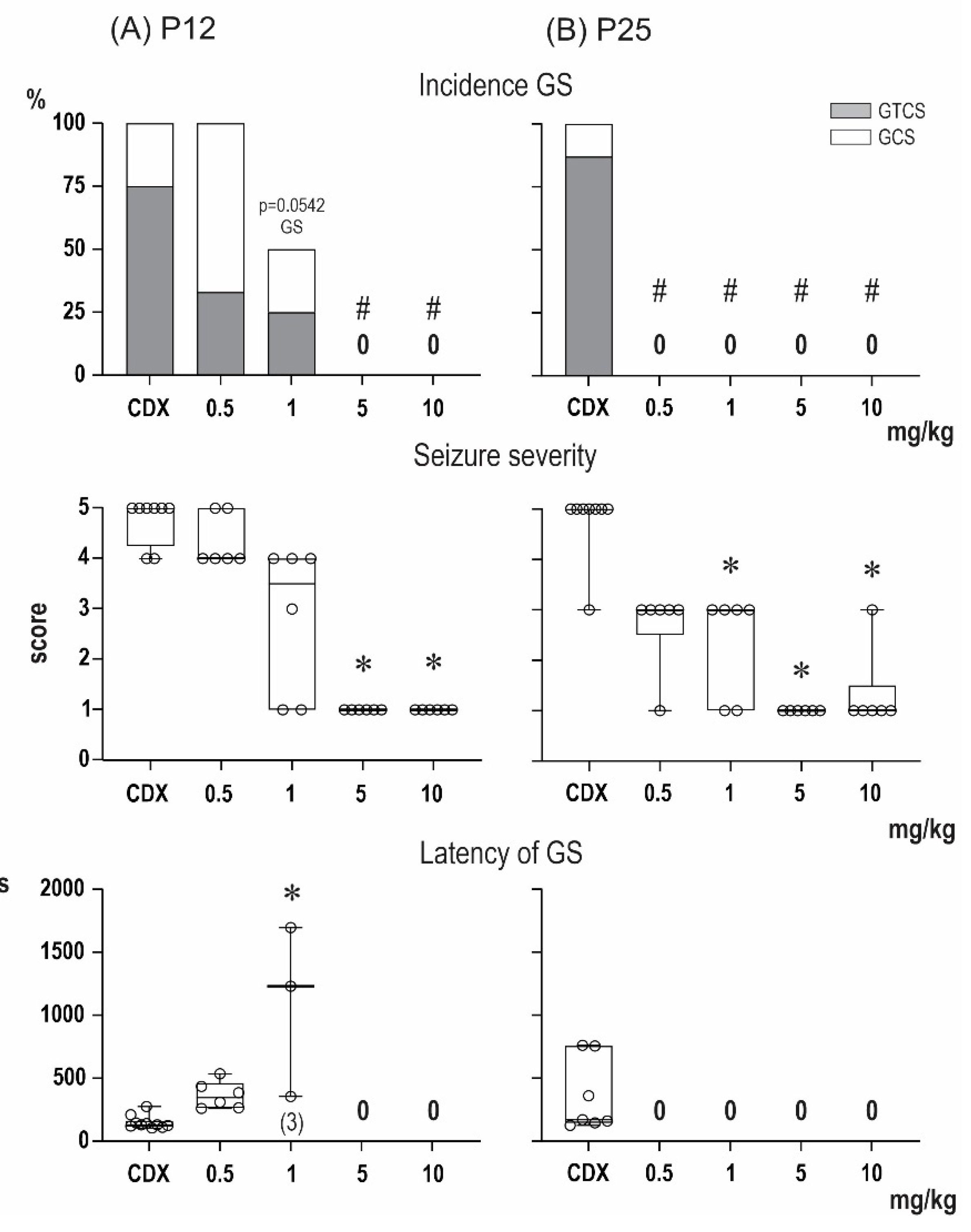
2.1. Anticonvulsant Effects of PA-S and PA-G in the Model of Pentylenetetrazol-Induced Seizures
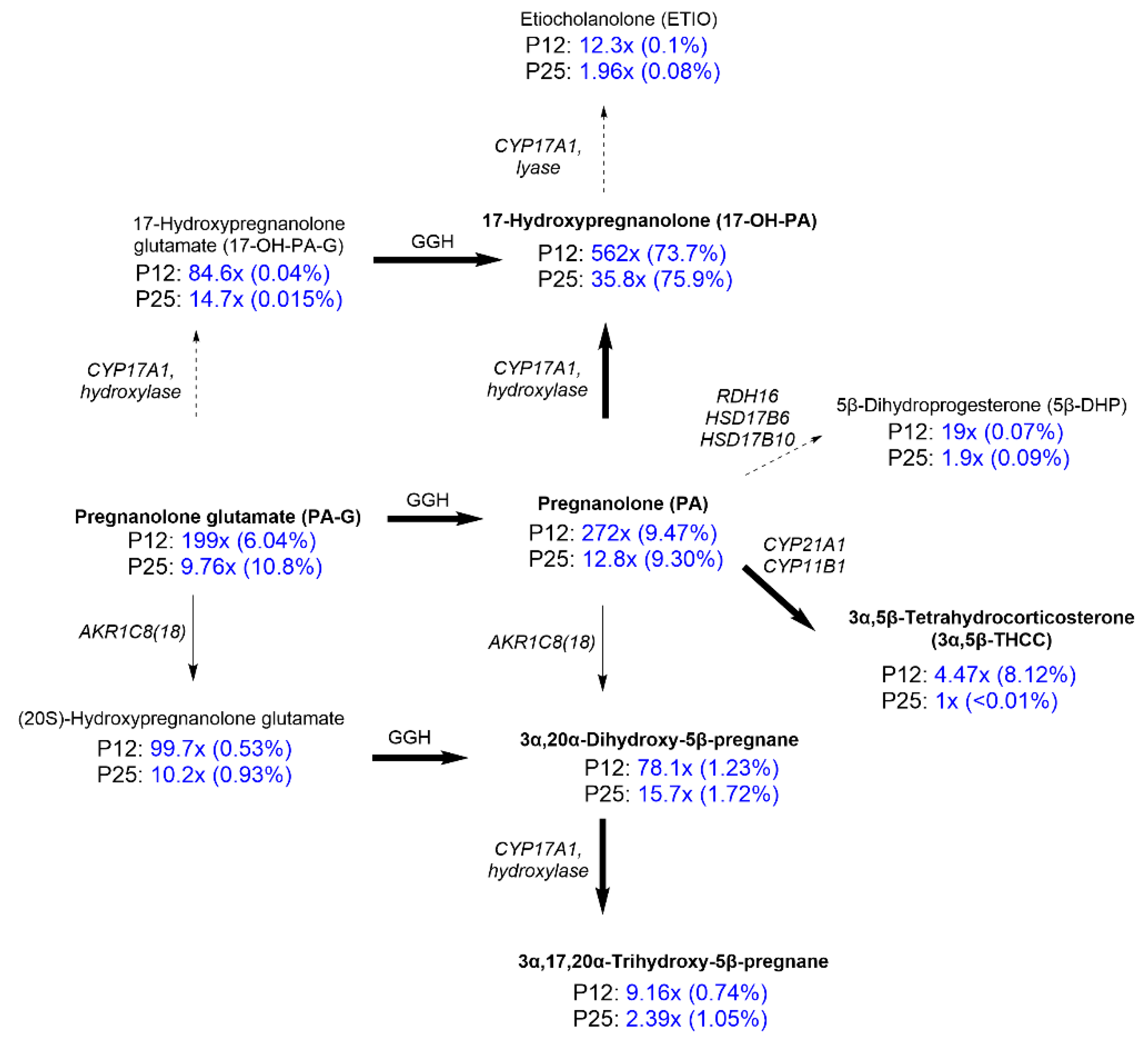
2.2. Steroidomic Analysis
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Pregnanolone Glutamate, Pregnanolone Sulfate, and Zuranolone
4.3. PTZ-Induced Seizures
4.4. Brain Samples for Metabolomic Analysis
4.5. Chemical Analysis
4.6. Sample Preparation
4.7. Nomenclature of Endogenous Steroids and Metabolites of PA-G
4.8. Statistics
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Witt, K.A.; Sandoval, K.E. Steroids and the blood-brain barrier: Therapeutic implications. Adv. Pharmacol. 2014, 71, 361–390. [Google Scholar] [CrossRef] [PubMed]
- Grube, M.; Hagen, P.; Jedlitschky, G. Neurosteroid Transport in the Brain: Role of ABC and SLC Transporters. Front. Pharmacol. 2018, 9, 354. [Google Scholar] [CrossRef] [PubMed]
- Schafer, A.M.; Meyer Zu Schwabedissen, H.E.; Grube, M. Expression and Function of Organic Anion Transporting Polypeptides in the Human Brain: Physiological and Pharmacological Implications. Pharmaceutics 2021, 13, 834. [Google Scholar] [CrossRef] [PubMed]
- Kudova, E. Rapid effects of neurosteroids on neuronal plasticity and their physiological and pathological implications. Neurosci. Lett. 2021, 750, 135771. [Google Scholar] [CrossRef]
- Brown, A.R.; Mitchell, S.J.; Peden, D.R.; Herd, M.B.; Seifi, M.; Swinny, J.D.; Belelli, D.; Lambert, J.J. During postnatal development endogenous neurosteroids influence GABA-ergic neurotransmission of mouse cortical neurons. Neuropharmacology 2016, 103, 163–173. [Google Scholar] [CrossRef]
- Sierra, A.; Lavaque, E.; Perez-Martin, M.; Azcoitia, I.; Hales, D.B.; Garcia-Segura, L.M. Steroidogenic acute regulatory protein in the rat brain: Cellular distribution, developmental regulation and overexpression after injury. Eur. J. Neurosci. 2003, 18, 1458–1467. [Google Scholar] [CrossRef]
- MacKenzie, G.; Maguire, J. Neurosteroids and GABAergic signaling in health and disease. Biomol. Concepts 2013, 4, 29–42. [Google Scholar] [CrossRef]
- Grobin, A.C.; Morrow, A.L. 3α-hydroxy-5α-pregnan-20-one levels and GABAA receptor-mediated 36Cl− flux across development in rat cerebral cortex. Brain Res. Dev. Brain Res. 2001, 131, 31–39. [Google Scholar] [CrossRef]
- Brown, A.R.; Herd, M.B.; Belelli, D.; Lambert, J.J. Developmentally regulated neurosteroid synthesis enhances GABAergic neurotransmission in mouse thalamocortical neurones. J. Physiol. 2015, 593, 267–284. [Google Scholar] [CrossRef]
- Rosenthal, E.S.; Claassen, J.; Wainwright, M.S.; Husain, A.M.; Vaitkevicius, H.; Raines, S.; Hoffmann, E.; Colquhoun, H.; Doherty, J.J.; Kanes, S.J. Brexanolone as adjunctive therapy in super-refractory status epilepticus. Ann. Neurol. 2017, 82, 342–352. [Google Scholar] [CrossRef]
- Levesque, M.; Biagini, G.; Avoli, M. Neurosteroids and Focal Epileptic Disorders. Int. J. Mol. Sci. 2020, 21, 9391. [Google Scholar] [CrossRef] [PubMed]
- Iwata, S.; Wakita, M.; Shin, M.C.; Fukuda, A.; Akaike, N. Modulation of allopregnanolone on excitatory transmitters release from single glutamatergic terminal. Brain Res. Bull. 2013, 93, 39–46. [Google Scholar] [CrossRef] [PubMed]
- Park-Chung, M.; Malayev, A.; Purdy, R.H.; Gibbs, T.T.; Farb, D.H. Sulfated and unsulfated steroids modulate gamma-aminobutyric acidA receptor function through distinct sites. Brain Res. 1999, 830, 72–87. [Google Scholar] [CrossRef]
- Park-Chung, M.; Wu, F.S.; Purdy, R.H.; Malayev, A.A.; Gibbs, T.T.; Farb, D.H. Distinct sites for inverse modulation of N-methyl-D-aspartate receptors by sulfated steroids. Mol. Pharmacol. 1997, 52, 1113–1123. [Google Scholar] [CrossRef] [PubMed]
- Weaver, C.E.; Land, M.B.; Purdy, R.H.; Richards, K.G.; Gibbs, T.T.; Farb, D.H. Geometry and charge determine pharmacological effects of steroids on N-methyl-D-aspartate receptor-induced Ca2+ accumulation and cell death. J. Pharmacol. Exp. Ther. 2000, 293, 747–754. [Google Scholar] [PubMed]
- Petrovic, M.; Sedlacek, M.; Horak, M.; Chodounska, H.; Vyklicky, L., Jr. 20-oxo-5beta-pregnan-3alpha-yl sulfate is a use-dependent NMDA receptor inhibitor. J. Neurosci. 2005, 25, 8439–8450. [Google Scholar] [CrossRef] [PubMed]
- Ziolkowski, L.; Mordukhovich, I.; Chen, D.M.; Chisari, M.; Shu, H.J.; Lambert, P.M.; Qian, M.; Zorumski, C.F.; Covey, D.F.; Mennerick, S. A neuroactive steroid with a therapeutically interesting constellation of actions at GABAA and NMDA receptors. Neuropharmacology 2021, 183, 108358. [Google Scholar] [CrossRef]
- Kapur, J. Role of NMDA receptors in the pathophysiology and treatment of status epilepticus. Epilepsia Open 2018, 3, 165–168. [Google Scholar] [CrossRef]
- Hanada, T. Ionotropic Glutamate Receptors in Epilepsy: A Review Focusing on AMPA and NMDA Receptors. Biomolecules 2020, 10, 464. [Google Scholar] [CrossRef]
- Pohl, M.; Mares, P. Effects of flunarizine on Metrazol-induced seizures in developing rats. Epilepsy Res. 1987, 1, 302–305. [Google Scholar] [CrossRef]
- Holubova, K.; Chvojkova, M.; Hrcka Krausova, B.; Vyklicky, V.; Kudova, E.; Chodounska, H.; Vyklicky, L.; Vales, K. Pitfalls of NMDA Receptor Modulation by Neuroactive Steroids. The Effect of Positive and Negative Modulation of NMDA Receptors in an Animal Model of Schizophrenia. Biomolecules 2021, 11, 1026. [Google Scholar] [CrossRef]
- Bukanova, J.V.; Solntseva, E.I.; Kolbaev, S.N.; Kudova, E. Modulation of GABA and glycine receptors in rat pyramidal hippocampal neurones by 3alpha5beta-pregnanolone derivatives. Neurochem. Int. 2018, 118, 145–151. [Google Scholar] [CrossRef]
- Martinez Botella, G.; Salituro, F.G.; Harrison, B.L.; Beresis, R.T.; Bai, Z.; Blanco, M.J.; Belfort, G.M.; Dai, J.; Loya, C.M.; Ackley, M.A.; et al. Neuroactive Steroids. 2. 3alpha-Hydroxy-3beta-methyl-21-(4-cyano-1H-pyrazol-1′-yl)-19-nor-5beta-pregnan-20 -one (SAGE-217): A Clinical Next Generation Neuroactive Steroid Positive Allosteric Modulator of the (gamma-Aminobutyric Acid)A Receptor. J. Med. Chem. 2017, 60, 7810–7819. [Google Scholar] [CrossRef] [PubMed]
- Rambousek, L.; Bubenikova-Valesova, V.; Kacer, P.; Syslova, K.; Kenney, J.; Holubova, K.; Najmanova, V.; Zach, P.; Svoboda, J.; Stuchlik, A.; et al. Cellular and behavioural effects of a new steroidal inhibitor of the N-methyl-D-aspartate receptor 3α5β-pregnanolone glutamate. Neuropharmacology 2011, 61, 61–68. [Google Scholar] [CrossRef]
- Borovska, J.; Vyklicky, V.; Stastna, E.; Kapras, V.; Slavikova, B.; Horak, M.; Chodounska, H.; Vyklicky, L., Jr. Access of inhibitory neurosteroids to the NMDA receptor. Br. J. Pharmacol. 2012, 166, 1069–1083. [Google Scholar] [CrossRef] [PubMed]
- Kudova, E.; Chodounska, H.; Slavikova, B.; Budesinsky, M.; Nekardova, M.; Vyklicky, V.; Krausova, B.; Svehla, P.; Vyklicky, L. A New Class of Potent N-Methyl-D-Aspartate Receptor Inhibitors: Sulfated Neuroactive Steroids with Lipophilic D-Ring Modifications. J. Med. Chem. 2015, 58, 5950–5966. [Google Scholar] [CrossRef]
- Holubova, K.; Nekovarova, T.; Pistovcakova, J.; Sulcova, A.; Stuchlik, A.; Vales, K. Pregnanolone Glutamate, a Novel Use-Dependent NMDA Receptor Inhibitor, Exerts Antidepressant-Like Properties in Animal Models. Front. Behav. Neurosci. 2014, 8, 130. [Google Scholar] [CrossRef] [PubMed]
- Kleteckova, L.; Tsenov, G.; Kubova, H.; Stuchlik, A.; Vales, K. Neuroprotective effect of the 3alpha5beta-pregnanolone glutamate treatment in the model of focal cerebral ischemia in immature rats. Neurosci. Lett. 2014, 564, 11–15. [Google Scholar] [CrossRef]
- Soldin, O.P.; Guo, T.; Weiderpass, E.; Tractenberg, R.E.; Hilakivi-Clarke, L.; Soldin, S.J. Steroid hormone levels in pregnancy and 1 year postpartum using isotope dilution tandem mass spectrometry. Fertil. Steril. 2005, 84, 701–710. [Google Scholar] [CrossRef]
- Hill, M.; Cibula, D.; Havlikova, H.; Kancheva, L.; Fait, T.; Kancheva, R.; Parizek, A.; Starka, L. Circulating levels of pregnanolone isomers during the third trimester of human pregnancy. J. Steroid Biochem. Mol. Biol. 2007, 105, 166–175. [Google Scholar] [CrossRef]
- Hirst, J.J.; Yawno, T.; Nguyen, P.; Walker, D.W. Stress in pregnancy activates neurosteroid production in the fetal brain. Neuroendocrinology 2006, 84, 264–274. [Google Scholar] [CrossRef] [PubMed]
- Hirst, J.J.; Palliser, H.K.; Yates, D.M.; Yawno, T.; Walker, D.W. Neurosteroids in the fetus and neonate: Potential protective role in compromised pregnancies. Neurochem. Int. 2008, 52, 602–610. [Google Scholar] [CrossRef]
- Liang, L.; Rasmussen, M.H.; Piening, B.; Shen, X.; Chen, S.; Rost, H.; Snyder, J.K.; Tibshirani, R.; Skotte, L.; Lee, N.C.; et al. Metabolic Dynamics and Prediction of Gestational Age and Time to Delivery in Pregnant Women. Cell 2020, 181, 1680–1692.e15. [Google Scholar] [CrossRef] [PubMed]
- Hirst, J.J.; Cumberland, A.L.; Shaw, J.C.; Bennett, G.A.; Kelleher, M.A.; Walker, D.W.; Palliser, H.K. Loss of neurosteroid-mediated protection following stress during fetal life. J. Steroid Biochem. Mol. Biol. 2016, 160, 181–188. [Google Scholar] [CrossRef] [PubMed]
- Westcott, K.T.; Hirst, J.J.; Ciurej, I.; Walker, D.W.; Wlodek, M.E. Brain allopregnanolone in the fetal and postnatal rat in response to uteroplacental insufficiency. Neuroendocrinology 2008, 88, 287–292. [Google Scholar] [CrossRef]
- Brunton, P.J.; Russell, J.A. Neuroendocrine control of maternal stress responses and fetal programming by stress in pregnancy. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2011, 35, 1178–1191. [Google Scholar] [CrossRef]
- Howell, K.B.; Harvey, A.S.; Archer, J.S. Epileptic encephalopathy: Use and misuse of a clinically and conceptually important concept. Epilepsia 2016, 57, 343–347. [Google Scholar] [CrossRef]
- Capovilla, G.; Moshe, S.L.; Wolf, P.; Avanzini, G. Epileptic encephalopathy as models of system epilepsy. Epilepsia 2013, 54 (Suppl. 8), 34–37. [Google Scholar] [CrossRef]
- Katsnelson, A.; Buzsaki, G.; Swann, J.W. Catastrophic childhood epilepsy: A recent convergence of basic and clinical neuroscience. Sci. Transl. Med. 2014, 6, 262ps13. [Google Scholar] [CrossRef]
- Deligiannidis, K.M.; Meltzer-Brody, S.; Gunduz-Bruce, H.; Doherty, J.; Jonas, J.; Li, S.; Sankoh, A.J.; Silber, C.; Campbell, A.D.; Werneburg, B.; et al. Effect of Zuranolone vs Placebo in Postpartum Depression: A Randomized Clinical Trial. JAMA Psychiatry 2021, 78, 951–959. [Google Scholar] [CrossRef]
- Blanco, M.J.; La, D.; Coughlin, Q.; Newman, C.A.; Griffin, A.M.; Harrison, B.L.; Salituro, F.G. Breakthroughs in neuroactive steroid drug discovery. Bioorg. Med. Chem. Lett. 2018, 28, 61–70. [Google Scholar] [CrossRef]
- Claahsen-van der Grinten, H.L.; Stikkelbroeck, N.; Falhammar, H.; Reisch, N. Management of endocrine disease: Gonadal dysfunction in congenital adrenal hyperplasia. Eur. J. Endocrinol. 2021, 184, R85–R97. [Google Scholar] [CrossRef]
- Holsboer, F.; Knorr, D. Determination of urinary 17-hydroxypregnanolone by gas chromatography-mass spectrometry in patients with congenital adrenal hyperplasia. J. Steroid Biochem. 1977, 8, 1197–1199. [Google Scholar] [CrossRef]
- Bacchus, H. A method for measurement of total 17-OH, 21-methyl steroids in the urine and its application to clinical problems. Metabolism 1967, 16, 153–161. [Google Scholar] [CrossRef]
- Bell, M.; Varley, H. The estimation of pregnanetriol and 17-hydroxypregnanolone in urine in congenital adrenal hyperplasia. Clin. Chim. Acta 1960, 5, 396–405. [Google Scholar] [CrossRef]
- Pillai, G.V.; Smith, A.J.; Hunt, P.A.; Simpson, P.B. Multiple structural features of steroids mediate subtype-selective effects on human alpha4beta3delta GABAA receptors. Biochem. Pharmacol. 2004, 68, 819–831. [Google Scholar] [CrossRef]
- Meletti, S.; Lucchi, C.; Monti, G.; Giovannini, G.; Bedin, R.; Trenti, T.; Rustichelli, C.; Biagini, G. Low levels of progesterone and derivatives in cerebrospinal fluid of patients affected by status epilepticus. J. Neurochem. 2018, 147, 275–284. [Google Scholar] [CrossRef]
- Lucchi, C.; Costa, A.M.; Rustichelli, C.; Biagini, G. Allopregnanolone and Pregnanolone Are Reduced in the Hippocampus of Epileptic Rats, but Only Allopregnanolone Correlates with Seizure Frequency. Neuroendocrinology 2021, 111, 536–541. [Google Scholar] [CrossRef]
- Lucchi, C.; Costa, A.M.; Senn, L.; Messina, S.; Rustichelli, C.; Biagini, G. Augmentation of endogenous neurosteroid synthesis alters experimental status epilepticus dynamics. Epilepsia 2020, 61, e129–e134. [Google Scholar] [CrossRef]
- Jankovic, S.M.; Djesevic, M.; Jankovic, S.V. Experimental GABA A Receptor Agonists and Allosteric Modulators for the Treatment of Focal Epilepsy. J. Exp. Pharmacol. 2021, 13, 235–244. [Google Scholar] [CrossRef]
- Shafizadeh, T.B.; Halsted, C.H. gamma-Glutamyl hydrolase, not glutamate carboxypeptidase II, hydrolyzes dietary folate in rat small intestine. J. Nutr. 2007, 137, 1149–1153. [Google Scholar] [CrossRef]
- Mukai, H.; Takata, N.; Ishii, H.T.; Tanabe, N.; Hojo, Y.; Furukawa, A.; Kimoto, T.; Kawato, S. Hippocampal synthesis of estrogens and androgens which are paracrine modulators of synaptic plasticity: Synaptocrinology. Neuroscience 2006, 138, 757–764. [Google Scholar] [CrossRef] [PubMed]
- Dalla Valle, L.; Belvedere, P.; Simontacchi, C.; Colombo, L. Extraglandular hormonal steroidogenesis in aged rats. J. Steroid Biochem. Mol. Biol. 1992, 43, 1095–1098. [Google Scholar] [CrossRef]
- Dalla Valle, L.; Vianello, S.; Belvedere, P.; Colombo, L. Rat cytochrome P450c17 gene transcription is initiated at different start sites in extraglandular and glandular tissues. J. Steroid Biochem. Mol. Biol. 2002, 82, 377–384. [Google Scholar] [CrossRef]
- Shin, H.C.; Kim, H.R.; Cho, H.J.; Yi, H.; Cho, S.M.; Lee, D.G.; Abd El-Aty, A.M.; Kim, J.S.; Sun, D.; Amidon, G.L. Comparative gene expression of intestinal metabolizing enzymes. Biopharm. Drug Dispos. 2009, 30, 411–421. [Google Scholar] [CrossRef]
- Vianello, S.; Waterman, M.R.; Dalla Valle, L.; Colombo, L. Developmentally regulated expression and activity of 17alpha-hydroxylase/C-17,20-lyase cytochrome P450 in rat liver. Endocrinology 1997, 138, 3166–3174. [Google Scholar] [CrossRef][Green Version]
- Emanuelsson, I.; Almokhtar, M.; Wikvall, K.; Gronbladh, A.; Nylander, E.; Svensson, A.L.; Fex Svenningsen, A.; Norlin, M. Expression and regulation of CYP17A1 and 3beta-hydroxysteroid dehydrogenase in cells of the nervous system: Potential effects of vitamin D on brain steroidogenesis. Neurochem. Int. 2018, 113, 46–55. [Google Scholar] [CrossRef]
- Missaghian, E.; Kempna, P.; Dick, B.; Hirsch, A.; Alikhani-Koupaei, R.; Jegou, B.; Mullis, P.E.; Frey, B.M.; Fluck, C.E. Role of DNA methylation in the tissue-specific expression of the CYP17A1 gene for steroidogenesis in rodents. J. Endocrinol. 2009, 202, 99–109. [Google Scholar] [CrossRef]
- Le Goascogne, C.; Sananes, N.; Gouezou, M.; Takemori, S.; Kominami, S.; Baulieu, E.E.; Robel, P. Immunoreactive cytochrome P-450(17 alpha) in rat and guinea-pig gonads, adrenal glands and brain. J. Reprod. Fertil. 1991, 93, 609–622. [Google Scholar] [CrossRef][Green Version]
- Miller, W.L.; Auchus, R.J. The “backdoor pathway” of androgen synthesis in human male sexual development. PLoS Biol. 2019, 17, e3000198. [Google Scholar] [CrossRef]
- Sze, Y.; Gill, A.C.; Brunton, P.J. Sex-dependent changes in neuroactive steroid concentrations in the rat brain following acute swim stress. J. Neuroendocrinol. 2018, 30, e12644. [Google Scholar] [CrossRef] [PubMed]
- Kancheva, R.; Hill, M.; Novak, Z.; Chrastina, J.; Kancheva, L.; Starka, L. Neuroactive steroids in periphery and cerebrospinal fluid. Neuroscience 2011, 191, 22–27. [Google Scholar] [CrossRef]
- Qaiser, M.Z.; Dolman, D.E.M.; Begley, D.J.; Abbott, N.J.; Cazacu-Davidescu, M.; Corol, D.I.; Fry, J.P. Uptake and metabolism of sulphated steroids by the blood-brain barrier in the adult male rat. J. Neurochem. 2017, 142, 672–685. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.D.; Wahlstrom, G.; Backstrom, T. The regional brain distribution of the neurosteroids pregnenolone and pregnenolone sulfate following intravenous infusion. J. Steroid Biochem. Mol. Biol. 1997, 62, 299–306. [Google Scholar] [CrossRef]
- Penning, T.M.; Wangtrakuldee, P.; Auchus, R.J. Structural and Functional Biology of Aldo-Keto Reductase Steroid-Transforming Enzymes. Endocr. Rev. 2019, 40, 447–475. [Google Scholar] [CrossRef]
- Eechaute, W.P.; Dhooge, W.S.; Gao, C.Q.; Calders, P.; Rubens, R.; Weyne, J.; Kaufman, J.M. Progesterone-transforming enzyme activity in the hypothalamus of the male rat. J. Steroid Biochem. Mol. Biol. 1999, 70, 159–167. [Google Scholar] [CrossRef]
- Shimada, K.; Yago, K. Studies on neurosteroids X. Determination of pregnenolone and dehydroepiandrosterone in rat brains using gas chromatography-mass spectrometry-mass spectrometry. J. Chromatogr. Sci. 2000, 38, 6–10. [Google Scholar] [CrossRef][Green Version]
- Liere, P.; Akwa, Y.; Weill-Engerer, S.; Eychenne, B.; Pianos, A.; Robel, P.; Sjovall, J.; Schumacher, M.; Baulieu, E.E. Validation of an analytical procedure to measure trace amounts of neurosteroids in brain tissue by gas chromatography-mass spectrometry. J. Chromatogr. B Biomed. Sci. Appl. 2000, 739, 301–312. [Google Scholar] [CrossRef]
- Baulieu, E.E.; Robel, P.; Schumacher, M. Neurosteroids: Beginning of the story. Int. Rev. Neurobiol. 2001, 46, 1–32. [Google Scholar] [CrossRef]
- Liu, S.; Sjovall, J.; Griffiths, W.J. Neurosteroids in rat brain: Extraction, isolation, and analysis by nanoscale liquid chromatography-electrospray mass spectrometry. Anal. Chem. 2003, 75, 5835–5846. [Google Scholar] [CrossRef]
- Verleye, M.; Akwa, Y.; Liere, P.; Ladurelle, N.; Pianos, A.; Eychenne, B.; Schumacher, M.; Gillardin, J.M. The anxiolytic etifoxine activates the peripheral benzodiazepine receptor and increases the neurosteroid levels in rat brain. Pharmacol. Biochem. Behav. 2005, 82, 712–720. [Google Scholar] [CrossRef] [PubMed]
- Ebner, M.J.; Corol, D.I.; Havlikova, H.; Honour, J.W.; Fry, J.P. Identification of neuroactive steroids and their precursors and metabolites in adult male rat brain. Endocrinology 2006, 147, 179–190. [Google Scholar] [CrossRef] [PubMed]
- Mathur, C.; Prasad, V.V.; Raju, V.S.; Welch, M.; Lieberman, S. Steroids and their conjugates in the mammalian brain. Proc. Natl. Acad. Sci. USA 1993, 90, 85–88. [Google Scholar] [CrossRef] [PubMed]
- Reddy, D.S. Catamenial Epilepsy: Discovery of an Extrasynaptic Molecular Mechanism for Targeted Therapy. Front. Cell Neurosci. 2016, 10, 101. [Google Scholar] [CrossRef]
- Conklin, P.; Heggeness, F.W. Maturation of tempeature homeostasis in the rat. Am. J. Physiol. 1971, 220, 333–336. [Google Scholar] [CrossRef]
- Hill, M.; Hana, V., Jr.; Velikova, M.; Parizek, A.; Kolatorova, L.; Vitku, J.; Skodova, T.; Simkova, M.; Simjak, P.; Kancheva, R.; et al. A method for determination of one hundred endogenous steroids in human serum by gas chromatography-tandem mass spectrometry. Physiol. Res. B 2019, 68, 179–207. [Google Scholar] [CrossRef] [PubMed]

| Steroid | Age (Days) | Control (C) 1 | Intact (I) 2 | PA-G-Treated (P) 3 |
|---|---|---|---|---|
| Pregnenolone (PE) (ng/g) | 12 | 11.3 (9.4, 13.5) | 9.93 (8.21, 11.9) | 4.69 (3.74, 5.8) |
| 25 | 5.68 (4.59, 6.97) | 4.56 (3.39, 6) | 3.44 (2.68, 4.34) | |
| Age: F = 24.8, p < 0.001, Group: F = 12.8, p < 0.001, Age × Group: F = 1.9, p = 0.176; C25 < C12, I25 < I12, P < I, P < C, P12 < I12, P12 < C12 | ||||
| Pregnenolone sulfate (PE-S) (ng/g) | 12 | 16.9 (12.6, 22.6) | 24 (17.3, 33.4) | 29.5 (22, 39.9) |
| 25 | 8.81 (6.41, 11.9) | 9.86 (7.22, 13.3) | 10.5 (7.74, 14.2) | |
| Age: F = 25.9, p < 0.001, Group: F = 1.7, p = 0.205, Age × Group: F = 0.5, p = 0.626; C25 < C12, I25 < I12, P25 < P12 | ||||
| Dehydroepiandrosterone (DHEA) (ng/g) | 12 | 1.01 (0.829, 1.26) | 1.37 (1.09, 1.78) | 1.13 (0.916, 1.42) |
| 25 | 0.779 (0.648, 0.946) | 0.668 (0.56, 0.803) | 0.644 (0.54, 0.773) | |
| Age: F = 21.2, p < 0.001, Group: F = 0.3, p = 0.746, Age × Group: F = 1.4, p = 0.277; I25 < I12, P25 < P12 | ||||
| Dehydroepiandrosterone sulfate (DHEA-S) (ng/g) | 12 | 2.5 (2.05, 3.11) | 3.68 (2.83, 5.02) | 4.86 (3.69, 6.81) |
| 25 | 1.42 (1.19, 1.7) | 1.3 (1.09, 1.55) | 1.52 (1.27, 1.82) | |
| Age: F = 63.2, p < 0.001, Group: F = 2.6, p = 0.097, Age × Group: F = 1.8, p = 0.187; C25 < C12, I25 < I12, P25 < P12, P12 > C12 | ||||
| Androstenediol (ng/g) | 12 | 0.57 (0.465, 0.709) | 0.846 (0.67, 1.1) | 0.758 (0.606, 0.968) |
| 25 | 0.39 (0.323, 0.474) | 0.463 (0.373, 0.582) | 0.364 (0.302, 0.441) | |
| Age: F = 23.2, p < 0.001, Group: F = 1.8, p = 0.191, Age × Group: F = 0.7, p = 0.49; I25 < I12, P25 < P12 | ||||
| Androstenediol sulfate (ng/g) | 12 | 5.03 (4.47, 5.69) | 7.18 (6.21, 8.34) | 6.07 (5.36, 6.89) |
| 25 | 1.44 (1.28, 1.62) | 1.39 (1.24, 1.57) | 1.62 (1.44, 1.82) | |
| Age: F = 459, p < 0.001, Group: F = 2.3, p = 0.127, Age × Group: F = 2.8, p = 0.085; C25 < C12, I25 < I12, P25 < P12, I12 > C12 | ||||
| Allopregnanolone (ng/g) | 12 | 0.139 (0.0834, 0.218) | 0.254 (0.165, 0.378) | 0.246 (0.159, 0.367) |
| 25 | 0.151 (0.0919, 0.235) | 0.119 (0.0696, 0.19) | 0.144 (0.0869, 0.224) | |
| Age: F = 2.6, p = 0.118, Group: F = 0.4, p = 0.672, Age × Group: F = 1, p = 0.384 | ||||
| 5β-Dihydroprogesterone (pg/g) | 12 | 25.2 (15.8, 39) | 92.9 (54.8, 173) | 477 (214, 1810) |
| 25 | 49.6 (30.4, 83.2) | 48.8 (31.5, 77.2) | 94.3 (58.6, 164) | |
| Age: F = 1.7, p = 0.21, Group: F = 11.5, p < 0.001, Age × Group: F = 4.2, p = 0.029; P25 < P12 | ||||
| Pregnanolone (ng/g) | 12 | 0.234 (0.162, 0.338) | 0.254 (0.183, 0.353) | 63.6 (26, 200) |
| 25 | 0.393 (0.281, 0.553) | 0.106 (0.0704, 0.156) | 5.02 (3.01, 8.95) | |
| Age: F = 6.9, p = 0.016, Group: F = 131.6, p < 0.001, Age × Group: F = 7.2, p = 0.004; I25 < I12, P25 < P12, P > I, P > C, P12 > I12, P12 > C12, I25 < C25, P25 > I25, P25 > C25 | ||||
| Conjugated pregnanolone (ng/g) | 12 | 0.204 (0.144, 0.285) | 0.381 (0.265, 0.546) | 40.6 (18.7, 110) |
| 25 | 0.615 (0.445, 0.859) | 0.237 (0.162, 0.342) | 6 (3.7, 10.4) | |
| Age: F = 0.3, p = 0.581, Group: F = 115.8, p < 0.001, Age × Group: F = 11.5, p < 0.001; C25 > C12, P25 < P12, P > I, P > C, P12 > I12, P12 > C12, I25 < C25, P25 > I25, P25 > C25 | ||||
| 3α,20α-Dihydroxy-5β-pregnane (ng/g) | 12 | 0.108 (0.0809, 0.144) | 0.339 (0.248, 0.472) | 8.42 (4.58, 17.2) |
| 25 | 0.0583 (0.0431, 0.078) | 0.0284 (0.0186, 0.041) | 0.919 (0.632, 1.38) | |
| Age: F = 75.2, p < 0.001, Group: F = 129.5, p < 0.001, Age × Group: F = 9.5, p = 0.001; C25 < C12, I25 < I12, P25 < P12, P > I, P > C, I12 > C12, P12 > I12, P12 > C12, P25 > I25, P25 > C25 | ||||
| Conjugated 3α,20α-dihydroxy-5β-pregnane (ng/g) | 12 | 35.8 (22.6, 55.8) | 180 (100, 335) | 3570 (1820, 7580) |
| 25 | 50.6 (32.4, 78.5) | 77.8 (50.2, 121) | 518 (311, 899) | |
| Age: F = 5.9, p = 0.024, Group: F = 53.7, p < 0.001, Age × Group: F = 4.3, p = 0.026; P25 < P12, I > C, P > I, P > C, I12 > C12, P12 > I12, P12 > C12, P25 > I25, P25 > C25 | ||||
| 17-Hydroxyallopregnanolone (ng/g) | 12 | 7.18 (3.2, 14) | 20 (11.6, 32.4) | 8.36 (4.24, 14.9) |
| 25 | 14 (7.76, 23.5) | 7.57 (3.76, 13.7) | 3.19 (1.2, 6.63) | |
| Age: F = 1.3, p = 0.266, Group: F = 2.2, p = 0.138, Age × Group: F = 2.4, p = 0.117 | ||||
| Conjugated 17-hydroxyallopregnanolone (pg/g) | 12 | 26.5 (13.8, 45.2) | 21.8 (10.7, 38.5) | 18.9 (7.92, 36.4) |
| 25 | 45.3 (24.7, 75.8) | 40.9 (21.8, 69.4) | 27.1 (13, 48.9) | |
| Age: F = 2.3, p = 0.147, Group: F = 0.5, p = 0.597, Age × Group: F = 0.1, p = 0.932 | ||||
| 17-Hydroxypregnanolone (ng/g) | 12 | 0.922 (0.588, 1.43) | 2.54 (1.68, 3.93) | 518 (114, 11,000) |
| 25 | 1.14 (0.767, 1.69) | 0.859 (0.573, 1.27) | 40.8 (17.4, 130) | |
| Age: F = 5.8, p = 0.025, Group: F = 76.6, p < 0.001, Age × Group: F = 2.9, p = 0.077; I25 < I12, P > I, P > C, P12 > I12, P12 > C12, P25 > I25, P25 > C25 | ||||
| Conjugated 17-hydroxypregnanolone [pg/g] | 12 | 3.25 (1.5, 6.77) | 5.04 (2.6, 9.78) | 275 (87.5, 1160) |
| 25 | 5.63 (2.7, 11.9) | 2.8 (1.26, 5.84) | 82.7 (32.1, 257) | |
| Age: F = 0.5, p = 0.506, Group: F = 26.3, p < 0.001, Age × Group: F = 1.1, p = 0.367; P > I, P > C, P12 > I12, P12 > C12, P25 > I25, P25 > C25 | ||||
| 3α,17α,20α-Trihydroxy-5β-pregnane (ng/g) | 12 | 0.64 (0.379, 1.08) | 2.57 (1.59, 4.21) | 5.86 (3.54, 9.88) |
| 25 | 0.399 (0.247, 0.636) | 0.221 (0.132, 0.359) | 0.951 (0.565, 1.61) | |
| Age: F = 32.7, p < 0.001, Group: F = 10.8, p < 0.001, Age × Group: F = 4.5, p = 0.023; I25 < I12, P25 < P12, P > I, P > C, I12 > C12, P12 > C12, P25 > I25 | ||||
| Etiocholanolone (ETIO) (pg/g) | 12 | 53 (45.1, 62.9) | 105 (84.9, 132) | 654 (333, 7570) |
| 25 | 38.9 (33.3, 45.5) | 35.8 (30.1, 42.6) | 76.3 (63.6, 92.9) | |
| Age: F = 69.5, p < 0.001, Group: F = 43.5, p < 0.001, Age × Group: F = 6.3, p = 0.007; I25 < I12, P25 < P12, P > I, P > C, I12 > C12, P12 > I12, P12 > C12, P25 > I25, P25 > C25 | ||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kudova, E.; Mares, P.; Hill, M.; Vondrakova, K.; Tsenov, G.; Chodounska, H.; Kubova, H.; Vales, K. The Neuroactive Steroid Pregnanolone Glutamate: Anticonvulsant Effect, Metabolites and Its Effect on Neurosteroid Levels in Developing Rat Brains. Pharmaceuticals 2022, 15, 49. https://doi.org/10.3390/ph15010049
Kudova E, Mares P, Hill M, Vondrakova K, Tsenov G, Chodounska H, Kubova H, Vales K. The Neuroactive Steroid Pregnanolone Glutamate: Anticonvulsant Effect, Metabolites and Its Effect on Neurosteroid Levels in Developing Rat Brains. Pharmaceuticals. 2022; 15(1):49. https://doi.org/10.3390/ph15010049
Chicago/Turabian StyleKudova, Eva, Pavel Mares, Martin Hill, Katerina Vondrakova, Grygoriy Tsenov, Hana Chodounska, Hana Kubova, and Karel Vales. 2022. "The Neuroactive Steroid Pregnanolone Glutamate: Anticonvulsant Effect, Metabolites and Its Effect on Neurosteroid Levels in Developing Rat Brains" Pharmaceuticals 15, no. 1: 49. https://doi.org/10.3390/ph15010049
APA StyleKudova, E., Mares, P., Hill, M., Vondrakova, K., Tsenov, G., Chodounska, H., Kubova, H., & Vales, K. (2022). The Neuroactive Steroid Pregnanolone Glutamate: Anticonvulsant Effect, Metabolites and Its Effect on Neurosteroid Levels in Developing Rat Brains. Pharmaceuticals, 15(1), 49. https://doi.org/10.3390/ph15010049