Probiotic Bacteria with High Alpha-Gal Content Protect Zebrafish against Mycobacteriosis
Abstract
:1. Introduction
2. Results and Discussion
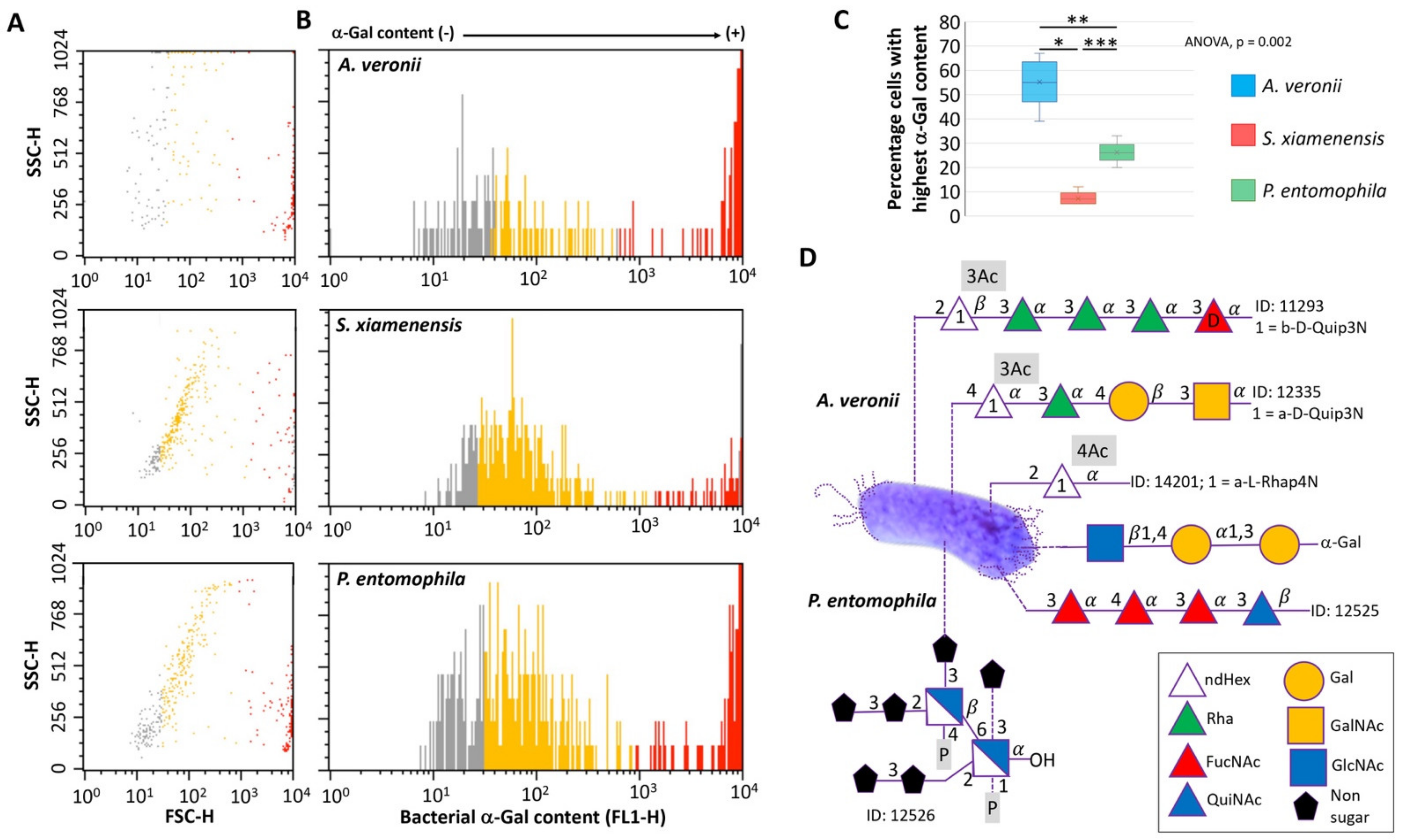
2.1. Zebrafish Gut Microbiota Contains Potential Probiotic Bacteria with High α-Gal Content
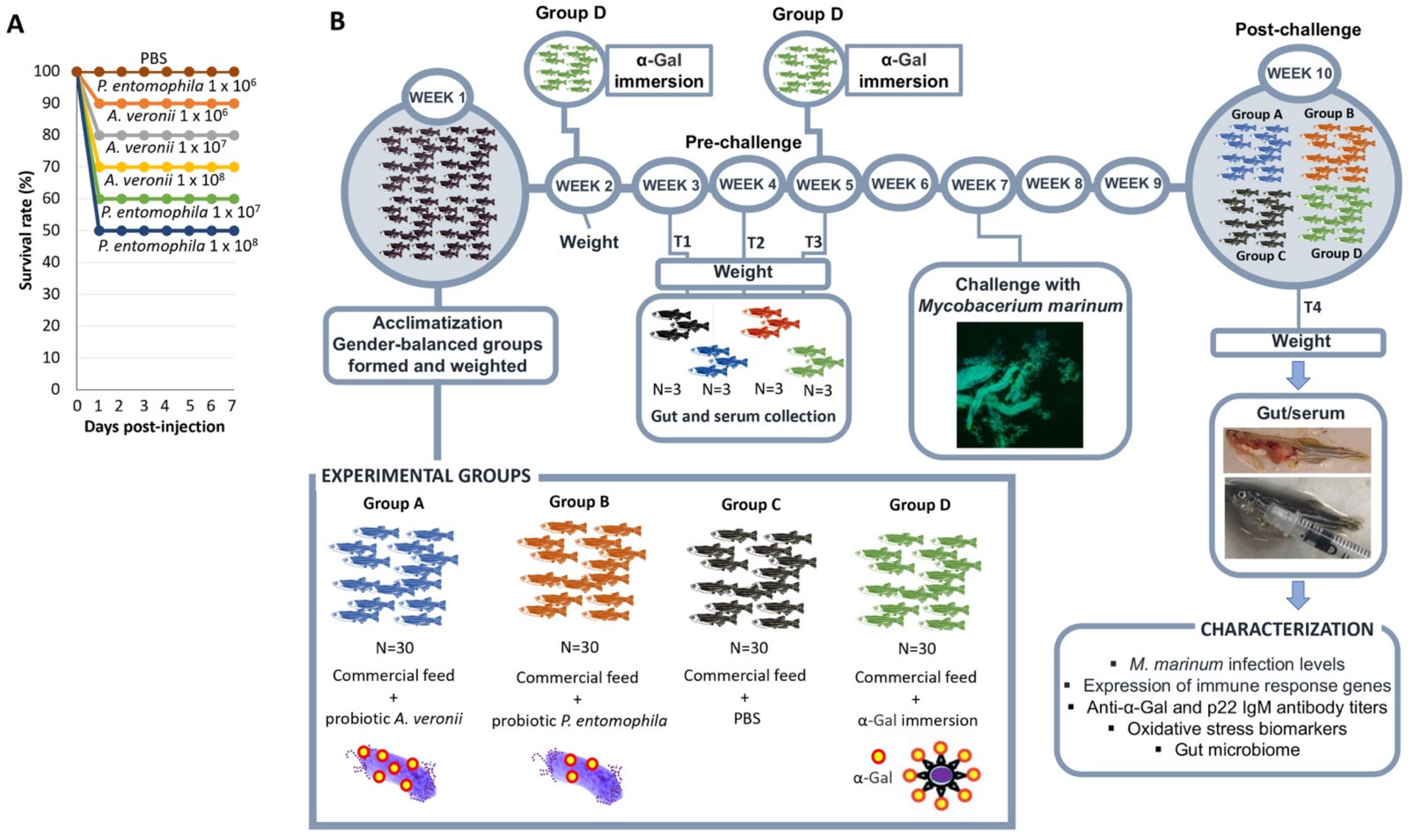
2.2. Bacteria from Zebrafish Gut Microbiota with High Alpha-Gal Content Are Not Toxic
2.3. Bacteria from Zebrafish Gut Microbiota with High Alpha-Gal Content Protect Fish against Mycobacteriosis
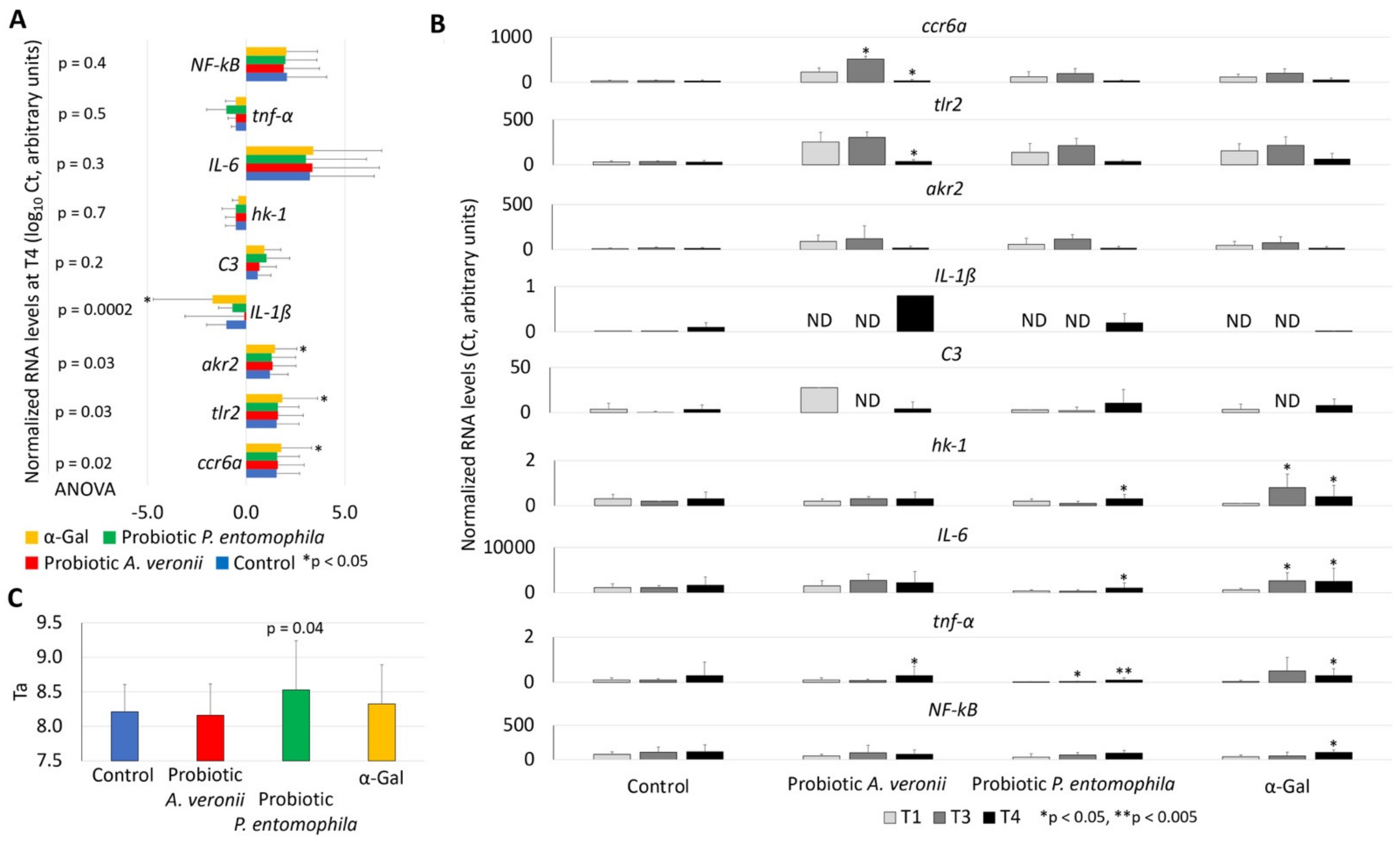
2.4. Treatment with Probiotic Bacteria with High α-Gal Content Induce the Expression of Immune Response and Nutrient Metabolism Genes
2.5. Treatment with Probiotic Bacteria with High α-Gal Content Reduces Oxidative Stress in Fish
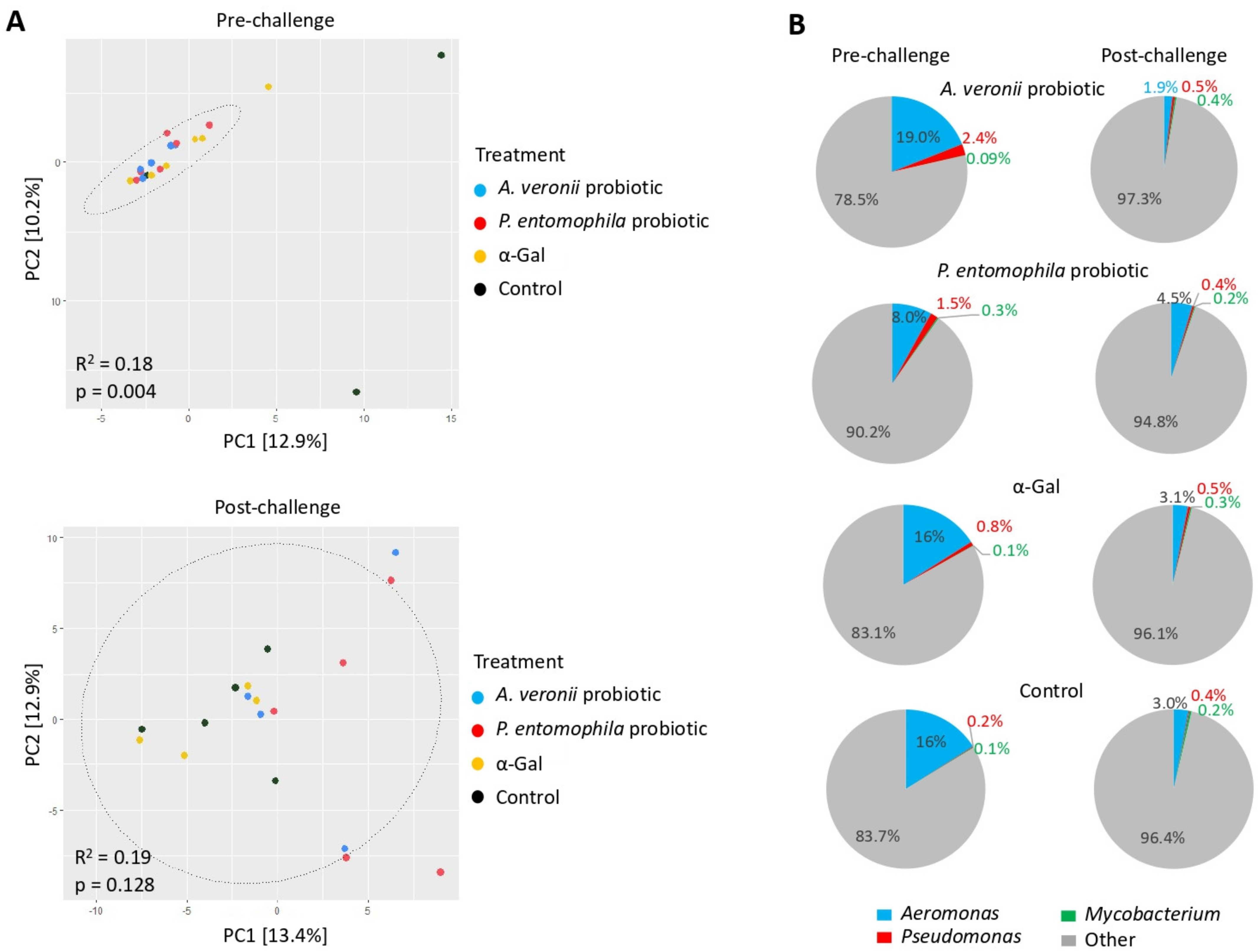
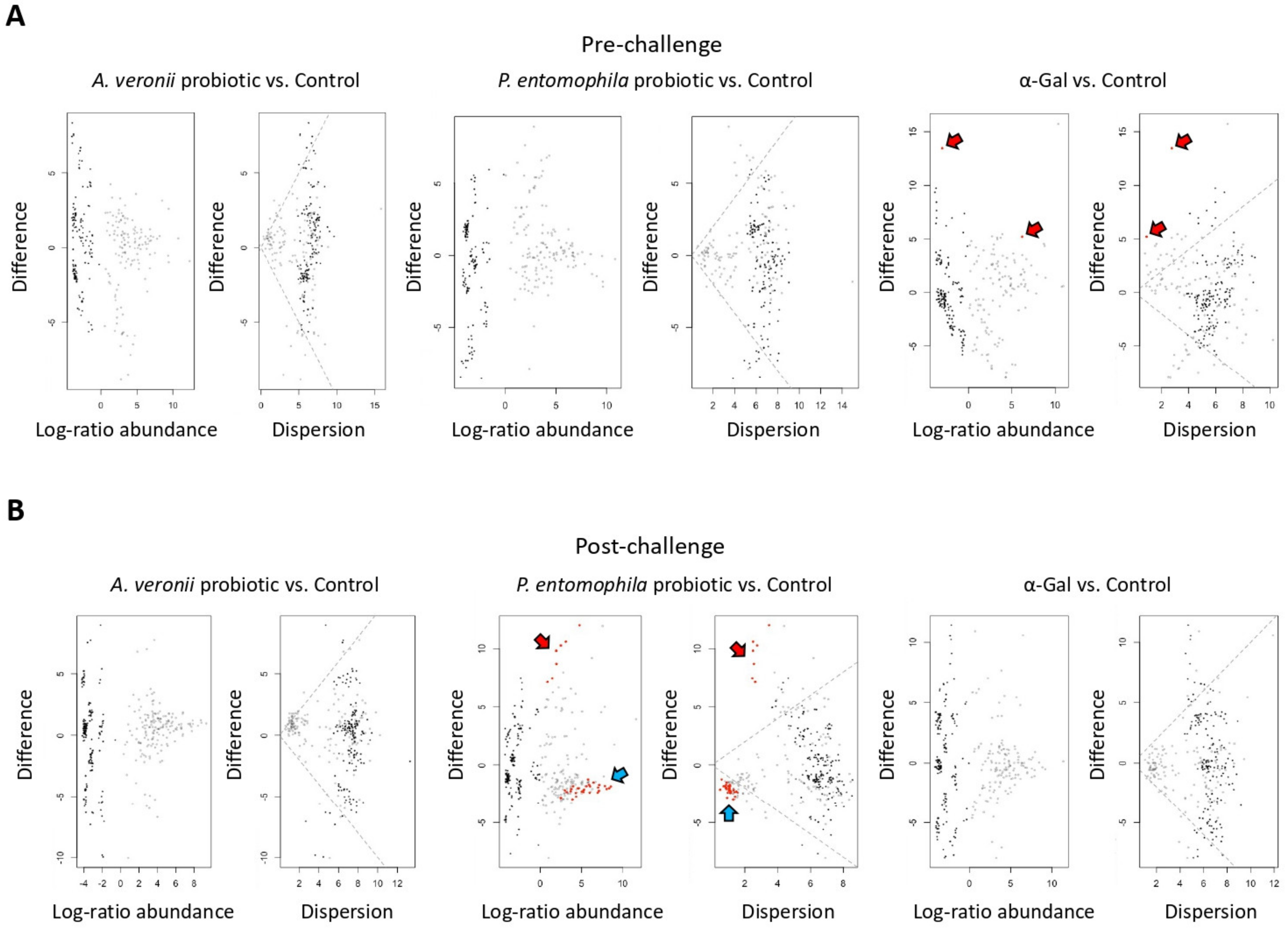
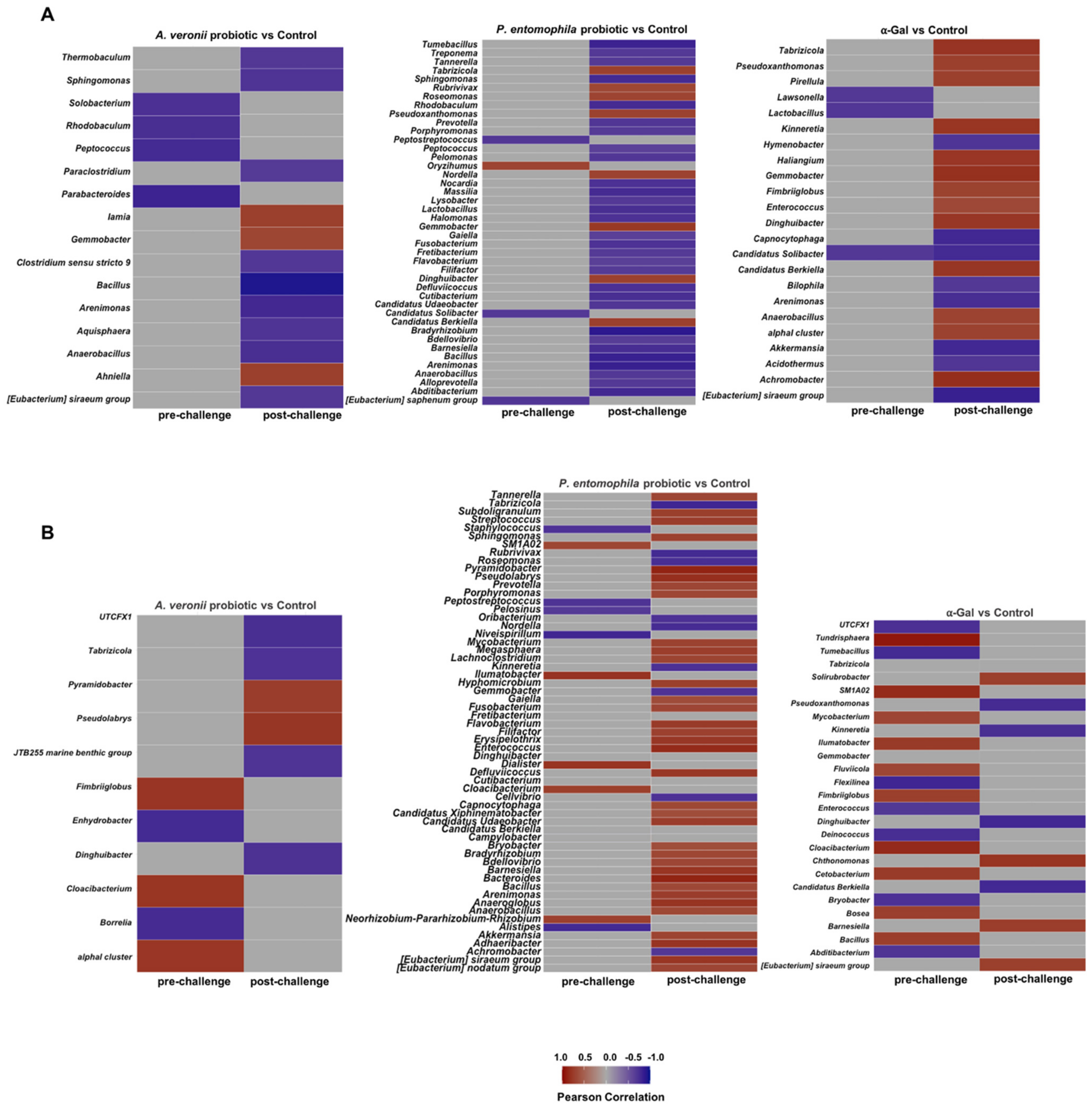
2.6. Microbiota Composition Varies in Response to Treatment with Probiotic Bacteria and α-Gal
2.7. Mechanisms Mediating Protection against Mycobacteriosis by Probiotics with High α-Gal Content
3. Materials and Methods
3.1. Zebrafish
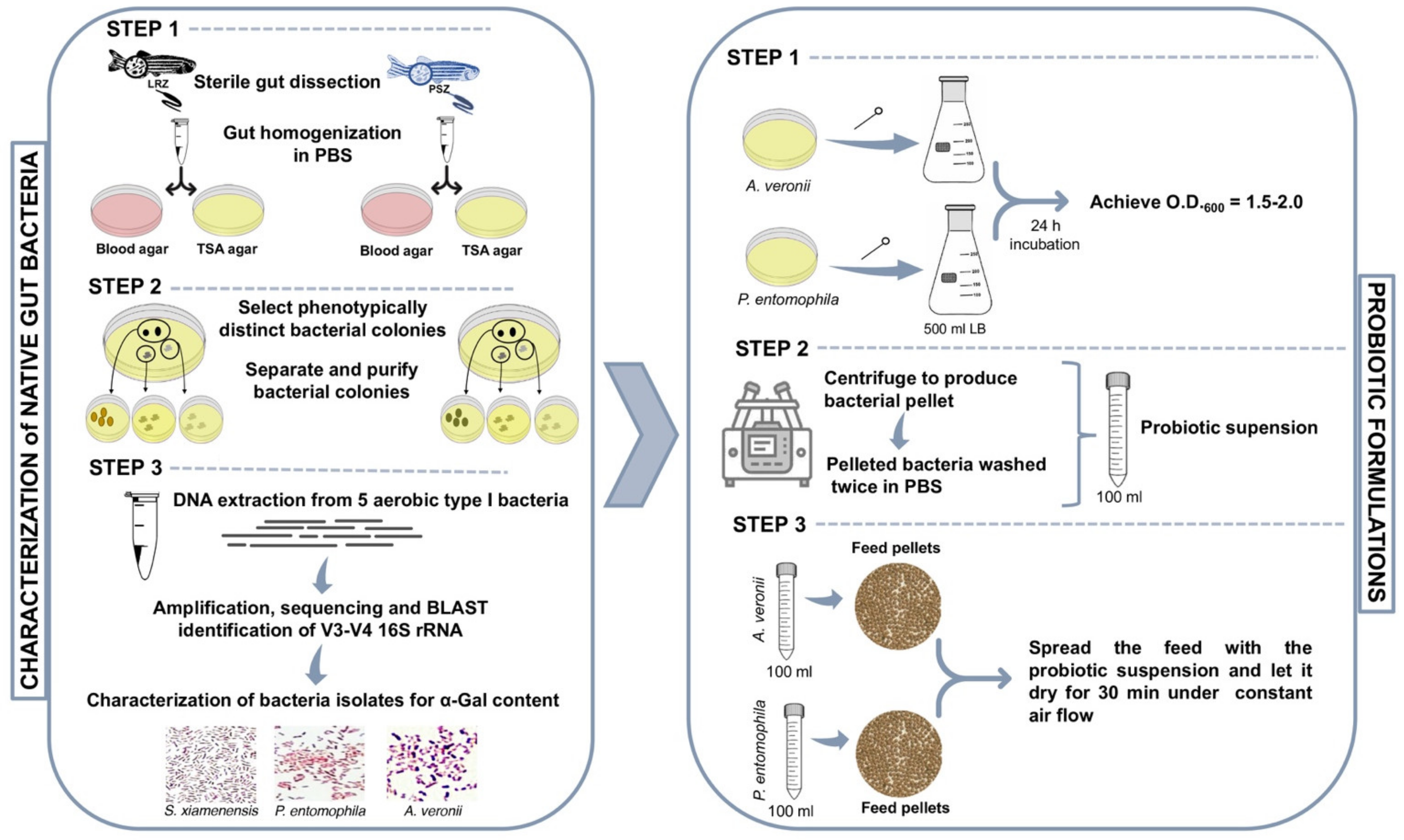
3.2. Sampling and Bacterial Culture from Zebrafish Gut Microbiota
3.3. Bacterial DNA Extraction and 16S rRNA Gene Amplification and Sequencing
3.4. Analysis of Bacterial α-Gal Content
3.5. Bacterial Carbohydrate Structure
3.6. Probiotic Bacteria
3.7. Toxicity Assessment of A. veronii and P. entomophila
3.8. Probiotic Formulation and Feed Administration
3.9. Zebrafish Treatment with Probiotics and Challenge with M. marinum
3.10. Characterization of M. marinum RNA Levels by RT-qPCR
3.11. Characterization of Anti-α-Gal and P22 IgM Antibody Titers in Zebrafish
3.12. Characterization of mRNA Levels of Selected Zebrafish Immune Response and Nutrient Metabolism Genes by RT-qPCR
3.13. Characterization of Serum Total Antioxidant Capacity
3.14. Characterization of The Zebrafish Gut Microbiome
3.14.1. DNA Extraction, Amplicon Preparation, and Sequencing
3.14.2. Downstream Data Analysis for 16S rRNA Sequencing Processing and ASVs Workflow
3.14.3. Statistical Analysis of Gut Zebrafish Microbial Communities
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Duremdez, R.; Al-Marzouk, A.; Qasem, J.A.; Al-Harbi, A.; Gharabally, H. Isolation of Streptococcus agalactiae from cultured silver pomfret, Pampus argenteus (Euphrasen), in Kuwait. J. Fish. Dis. 2004, 27, 307–310. [Google Scholar] [CrossRef]
- Bebak, J.; Wagner, B.; Burnes, B.; Hanson, T. Farm size, seining practices, and salt use: Risk factors for Aeromonas hydrophila outbreaks in farm-raised catfish, Alabama, USA. Prev. Vet. Med. 2015, 118, 161–168. [Google Scholar] [CrossRef] [PubMed]
- Decostere, A.; Hermans, K.; Haesebrouck, F. Piscine mycobacteriosis: A literature review covering the agent and the disease it causes in fish and humans. Vet. Microbiol. 2004, 99, 159–166. [Google Scholar] [CrossRef]
- Jacobs, J.M.; Stine, C.B.; Baya, A.M.; Kent, M.L. A review of mycobacteriosis in marine fish. J. Fish. Dis. 2009, 32, 119–130. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhou, Z.; Wu, N.; Tao, Y.; Xu, L.; Cao, Y.; Zhang, Y.; Yao, B. Gibel carp Carassius auratus gut microbiota after oral administration of trimethoprim/ sulfamethoxazole. Dis. Aquat. Organ. 2012, 99, 207–213. [Google Scholar] [CrossRef] [Green Version]
- Romero, J.; Feijoo, C.G.; Navarrete, P. Antibiotics in Aquaculture—Use, Abuse and Alternatives; INTECH Open Access Publisher: London, UK, 2012. [Google Scholar] [CrossRef] [Green Version]
- Skwor, T.; Shinko, J.; Augustyniak, A.; Gee, C.; Andraso, G. Aeromonas hydrophila and Aeromonas veronii predominate among potentially pathogenic ciprofloxacin- and tetracycline-resistant aeromonas isolates from Lake Erie. Appl. Environ. Microbiol. 2014, 80, 841–848. [Google Scholar] [CrossRef] [Green Version]
- Gioacchini, G.; Rossi, G.; Carnevali, O. Host-probiotic interaction: New insights into the role of the endocannabinoid system by in vivo and ex vivo approaches. Sci. Rep. 2017, 7, 1261. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Daliri, E.B.; Ofosu, F.K.; Xiuqin, C.; Chelliah, R.; Oh, D.H. Probiotic effector compounds: Current knowledge and future perspectives. Front. Microbiol. 2021, 12, 655705. [Google Scholar] [CrossRef]
- Akhter, N.; Wu, B.; Memon, A.M.; Mohsin, M. Probiotics and prebiotics associated with aquaculture: A review. Fish. Shellfish Immunol. 2015, 45, 733–741. [Google Scholar] [CrossRef]
- Borges, N.; Keller-Costa, T.; Sanches-Fernandes, G.; Louvado, A.; Gomes, N.; Costa, R. Bacteriome structure, function, and probiotics in fish larviculture: The good, the bad, and the gaps. Annu. Rev. Anim. Biosci. 2021, 9, 423–452. [Google Scholar] [CrossRef]
- Hao, K.; Wu, Z.Q.; Li, D.L.; Yu, X.B.; Wang, G.X.; Ling, F. Effects of dietary administration of Shewanella xiamenensis A-1, Aeromonas veronii A-7, and Bacillus subtilis, single or combined, on the Grass Carp (Ctenopharyngodon idella) intestinal microbiota. Probiotics Antimicrob. Proteins. 2017, 9, 386–396. [Google Scholar] [CrossRef]
- Ringø, E.; Hoseinifar, S.H.; Ghosh, K.; Doan, H.V.; Beck, B.R.; Song, S.K. Lactic acid bacteria in Finfish—An update. Front. Microbiol. 2018, 9, 1818. [Google Scholar] [CrossRef] [PubMed]
- Kuebutornye, F.; Abarike, E.D.; Lu, Y. A review on the application of Bacillus as probiotics in aquaculture. Fish. Shellfish Immunol. 2019, 87, 820–828. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, G.; Ray, A.K. The advancement of probiotics research and its application in fish farming industries. Res. Vet. Sci. 2017, 115, 66–77. [Google Scholar] [CrossRef] [PubMed]
- Yi, C.C.; Liu, C.H.; Chuang, K.P.; Chang, Y.T.; Hu, S.Y. A potential probiotic Chromobacterium aquaticum with bacteriocin-like activity enhances the expression of indicator genes associated with nutrient metabolism, growth performance and innate immunity against pathogen infections in zebrafish (Danio rerio). Fish. Shellfish Immunol. 2019, 93, 124–134. [Google Scholar] [CrossRef]
- Huang, H.; Zhou, P.; Chen, P.; Xia, L.; Hu, S.; Yi, G.; Lu, J.; Yang, S.; Xie, J.; Peng, J.; et al. Alteration of the gut microbiome and immune factors of grass carp infected with Aeromonas veronii and screening of an antagonistic bacterial strain (Streptomyces flavotricini). Microb. Pathog. 2020, 143, 104092. [Google Scholar] [CrossRef] [PubMed]
- Arani, M.M.; Salati, A.P.; Keyvanshokooh, S.; Safari, O. The effect of Pediococcus acidilactici on mucosal immune responses, growth, and reproductive performance in zebrafish (Danio rerio). Fish Physiol. Biochem. 2021, 47, 153–162. [Google Scholar] [CrossRef] [PubMed]
- Standen, B.T.; Rodiles, A.; Peggs, D.L.; Davies, S.J.; Santos, G.A.; Merrifield, D.L. Modulation of the intestinal microbiota and morphology of tilapia, Oreochromis niloticus, following the application of a multi-species probiotic. Appl. Microbiol. Biotechnol. 2015, 99, 8403–8417. [Google Scholar] [CrossRef]
- Ljubobratovic, U.; Kosanovic, D.; Vukotic, G.; Molnar, Z.; Stanisavljevic, N.; Ristovic, T.; Peter, G.; Lukic, J.; Jeney, G. Supplementation of lactobacilli improves growth, regulates microbiota composition and suppresses skeletal anomalies in juvenile pike-perch (Sander lucioperca) reared in recirculating aquaculture system (RAS): A pilot study. Res. Vet. Sci. 2017, 115, 451–462. [Google Scholar] [CrossRef]
- Pyclik, M.; Srutkova, D.; Schwarzer, M.; Górska, S. Bifidobacteria cell wall-derived exo-polysaccharides, lipoteichoic acids, peptidoglycans, polar lipids and proteins—Their chemical structure and biological attributes. Int. J. Biol. Macromol. 2020, 147, 333–349. [Google Scholar] [CrossRef] [PubMed]
- Astronomo, R.D.; Burton, D.R. Carbohydrate vaccines: Developing sweet solutions to sticky situations? Nat. Rev. Drug Discov. 2010, 9, 308–324. [Google Scholar] [CrossRef] [Green Version]
- Hodžić, A.; Mateos-Hernández, L.; de la Fuente, J.; Cabezas-Cruz, A. α-Gal-based vaccines: Advances, opportunities, and perspectives. Trends Parasitol. 2020, 36, 992–1001. [Google Scholar] [CrossRef] [PubMed]
- Pacheco, I.; Contreras, M.; Villar, M.; Risalde, M.A.; Alberdi, P.; Cabezas-Cruz, A.; Gortázar, C.; de la Fuente, J. Vaccination with alpha-Gal protects against mycobacterial infection in the zebrafish model of tuberculosis. Vaccines 2020, 8, 195. [Google Scholar] [CrossRef]
- Urra, J.M.; Ferreras-Colino, E.; Contreras, M.; Cabrera, C.M.; Fernández de Mera, I.G.; Villar, M.; Cabezas-Cruz, A.; Gortázar, C.; de la Fuente, J. The antibody response to the glycan α-Gal correlates with COVID-19 disease symptoms. J. Med. Virol. 2021, 93, 2065–2075. [Google Scholar] [CrossRef] [PubMed]
- De la Fuente, J.; Gortázar, C.; Cabezas-Cruz, A. Immunity to glycan α-Gal and possibilities for the control of COVID-19. Immunotherapy 2021, 13, 185–188. [Google Scholar] [CrossRef] [PubMed]
- Cosma, C.L.; Swaim, L.E.; Volkman, H.; Ramakrishnan, L.; Davis, J.M. Zebrafish and frog models of Mycobacterium marinum infection. Curr. Protoc. Microbiol. 2006, 3, unit 10B.2, chapter 10. [Google Scholar] [CrossRef]
- Cronan, M.R.; Tobin, D.M. Fit for consumption: Zebrafish as a model for tuberculosis. Dis. Model Mech. 2014, 7, 777–784. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Leeuwen, L.M.; van der Sar, A.M.; Bitter, W. Animal models of tuberculosis: Zebrafish. Cold Spring Harb. Perspect. Med. 2014, 5, a018580. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Myllymäki, H.; Bäuerlein, C.A.; Rämet, M. The zebrafish breathes new life into the study of tuberculosis. Front. Immunol. 2016, 7, 196. [Google Scholar] [CrossRef]
- Masud, S.; Torraca, V.; Meijer, A.H. Modeling infectious diseases in the context of a developing immune system. Curr. Top. Dev. Biol. 2017, 124, 277–329. [Google Scholar] [CrossRef] [Green Version]
- Risalde, M.A.; López, V.; Contreras, M.; Mateos-Hernández, L.; Gortázar, C.; de la Fuente, J. Control of mycobacteriosis in zebrafish (Danio rerio) mucosally vaccinated with heat-inactivated Mycobacterium bovis. Vaccine 2018, 36, 4447–4453. [Google Scholar] [CrossRef]
- López, V.; Risalde, M.A.; Contreras, M.; Mateos-Hernández, L.; Vicente, J.; Gortázar, C.; de la Fuente, J. Heat-inactivated Mycobacterium bovis protects zebrafish against mycobacteriosis. J. Fish Dis. 2018, 41, 1515–1528. [Google Scholar] [CrossRef]
- Lin, Y.S.; Saputra, F.; Chen, Y.C.; Hu, S.Y. Dietary administration of Bacillus amyloliquefaciens R8 reduces hepatic oxidative stress and enhances nutrient metabolism and immunity against Aeromonas hydrophila and Streptococcus agalactiae in zebrafish (Danio rerio). Fish Shellfish Immunol. 2019, 86, 410–419. [Google Scholar] [CrossRef] [PubMed]
- Contreras, M.; Pacheco, I.; Alberdi, P.; Díaz-Sánchez, S.; Artigas-Jerónimo, S.; Mateos-Hernández, L.; Villar, M.; Cabezas-Cruz, A.; de la Fuente, J. Allergic reactions and immunity in response to tick salivary biogenic substances and red meat consumption in the zebrafish model. Front. Cell. Infect. Microbiol. 2020, 10, 78. [Google Scholar] [CrossRef] [PubMed]
- Benard, E.L.; Rougeot, J.; Racz, P.I.; Spaink, H.P.; Meijer, A.H. Transcriptomic approaches in the zebrafish model for tuberculosis-insights into host- and pathogen-specific determinants of the innate immune response. Adv. Genet. 2016, 95, 217–251. [Google Scholar] [CrossRef]
- Roeselers, G.; Mittge, E.K.; Stephens, W.Z.; Parichy, D.M.; Cavanaugh, C.M.; Guillemin, K.; Rawls, J.F. Evidence for a core gut microbiota in the zebrafish. ISME J. 2011, 5, 1595–1608. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cantas, L.; Sørby, J.R.; Aleström, P.; Sørum, H. Culturable gut microbiota diversity in zebrafish. Zebrafish 2012, 9, 26–37. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dahan, D.; Jude, B.A.; Lamendella, R.; Keesing, F.; Perron, G.G. Exposure to arsenic alters the microbiome of larval zebrafish. Front. Microbiol. 2018, 9, 1323. [Google Scholar] [CrossRef] [PubMed]
- Turska-Szewczuk, A.; Duda, K.A.; Schwudke, D.; Pekala, A.; Kozinska, A.; Holst, O. Structural studies of the lipopolysaccharide from the fish pathogen Aeromonas veronii strain Bs19, serotype O16. Mar. Drugs 2014, 12, 1298–1316. [Google Scholar] [CrossRef] [Green Version]
- Molinari, L.M.; Scoaris, D.O.; Pedroso, R.B.; Bittencourt, N.L.R.; Nakamura, C.V.; Nakamura, T.U.; Abreu Filho, B.A.; Dias Filho, B.P. Bacterial microflora in the gastrointestinal tract of Nile tilapia, Oreochromis niloticus, cultured in a semi-intensive system, Acta Sci. Biol. Sci. 2003, 25, 267–271. [Google Scholar] [CrossRef]
- Silver, A.C.; Williams, D.; Faucher, J.; Horneman, A.J.; Gogarten, J.P.; Graf, J. Complex evolutionary history of the Aeromonas veronii group revealed by host interaction and DNA sequence data. PLoS ONE 2011, 6, e16751. [Google Scholar] [CrossRef] [Green Version]
- Song, M.F.; Kang, Y.H.; Zhang, D.X.; Chen, L.; Bi, J.F.; Zhang, H.P.; Zhang, L.; Qian, A.D.; Shan, X.F. Immunogenicity of extracellular products from an inactivated vaccine against Aeromonas veronii TH0426 in koi, Cyprinus carpio. Fish Shellfish Immunol. 2018, 81, 176–181. [Google Scholar] [CrossRef]
- Yang, B.; Zhang, D.; Wu, T.; Zhang, Z.; Raza, S.; Schreurs, N.; Zhang, L.; Yang, G.; Wang, C.; Qian, A.; et al. Maltoporin (LamB protein) contributes to the virulence and adhesion of Aeromonas veronii TH0426. J. Fish. Dis. 2019, 42, 379–389. [Google Scholar] [CrossRef]
- Yang, B.; Song, H.; An, D.; Zhang, D.; Raza, S.; Wang, G.; Shan, X.; Qian, A.; Kang, Y.; Wang, C. Functional analysis of preA in Aeromonas veronii TH0426 reveals a key role in the regulation of virulence and resistance to oxidative stress. Int. J. Mol. Sci. 2019, 21, 98. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, B.; Chen, C.; Sun, Y.; Cao, L.; Zhang, D.; Sun, W.; Zhang, L.; Wang, G.; Shan, X.; Kang, Y.; et al. Comparative genomic analysis of different virulence strains reveals reasons for the increased virulence of Aeromonas veronii. J. Fish Dis. 2021, 44, 11–24. [Google Scholar] [CrossRef]
- Volpe, E.; Mandrioli, L.; Errani, F.; Serratore, P.; Zavatta, E.; Rigillo, A.; Ciulli, S. Evidence of fish and human pathogens associated with doctor fish (Garra rufa, Heckel, 1843) used for cosmetic treatment. J. Fish Dis. 2019, 42, 1637–1644. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Raza, S.; Yang, B.; Sun, Y.; Wang, G.; Sun, W.; Qian, A.; Wang, C.; Kang, Y.; Shan, X. Aeromonas veronii infection in commercial freshwater fish: A potential threat to public health. Animals 2020, 10, 608. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vodovar, N.; Vallenet, D.; Cruveiller, S.; Rouy, Z.; Barbe, V.; Acosta, C.; Cattolico, L.; Jubin, C.; Lajus, A.; Segurens, B.; et al. Complete genome sequence of the entomopathogenic and metabolically versatile soil bacterium Pseudomonas entomophila. Nat. Biotechnol. 2006, 24, 673–679. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thune, R.; Stanley, L.A.; Cooper, R.K. Pathogenesis of gram—Negative bacteria infections in warm-water fish. Fish Annu. Rev. Fish. Dis. 1993, 3, 37–68. [Google Scholar] [CrossRef]
- Preena, P.G.; Dharmaratnam, A.; Swaminathan, T.R. Antimicrobial resistance analysis of pathogenic bacteria isolated from freshwater Nile Tilapia (Oreochromis niloticus) cultured in Kerala, India. Curr. Microbiol. 2020, 77, 3278–3287. [Google Scholar] [CrossRef]
- Hasan, K.N.; Banerjee, G. Recent studies on probiotics as beneficial mediator in aquaculture: A review. J. Basic Appl. Zool. 2020, 81, 53. [Google Scholar] [CrossRef]
- Gioacchini, G.; Giorgini, E.; Olivotto, I.; Maradonna, F.; Merrifield, D.L.; Carnevali, O. The influence of probiotics on zebrafish Danio rerio innate immunity and hepatic stress. Zebrafish 2014, 11, 98–106. [Google Scholar] [CrossRef]
- Liu, X.; Wu, H.; Liu, Q.; Wang, Q.; Xiao, J.; Chang, X.; Zhang, Y. Profiling immune response in zebrafish intestine, skin, spleen and kidney bath-vaccinated with a live attenuated Vibrio anguillarum vaccine. Fish Shellfish Immunol. 2015, 45, 342–345. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Liu, N.; Liu, H. Determination of the total mass of antioxidant substances and antioxidant capacity per unit mass in serum using redox titration. Bioinorg. Chem. Appl. 2014, 2014, 928595. [Google Scholar] [CrossRef] [PubMed]
- Slaninova, A.; Smutna, M.; Modra, H.; Svobodova, Z. A review: Oxidative stress in fish induced by pesticides. Neuro Endocrinol. Lett. 2009, 30 (Suppl. S1), 2–12. [Google Scholar]
- Yadav, S.S.; Kumar, R.; Khare, P.; Tripathi, M. Oxidative stress biomarkers in the freshwater fish, Heteropneustes fossilis (Bloch) exposed to sodium fluoride: Antioxidant defense and role of ascorbic acid. Toxicol. Int. 2015, 22, 71–76. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, J.W.; Choi, H.; Hwang, U.K.; Kang, J.C.; Kang, Y.J.; Kim, K.I.; Kim, J.H. Toxic effects of lead exposure on bioaccumulation, oxidative stress, neurotoxicity, and immune responses in fish: A review. Environ. Toxicol. Pharmacol. 2019, 68, 101–108. [Google Scholar] [CrossRef] [PubMed]
- Zamani, B.; Sheikhi, A.; Namazi, N.; Larijani, B.; Azadbakht, L. The effects of supplementation with probiotic on biomarkers of oxidative stress in adult subjects: A systematic review and meta-analysis of randomized trials. Probiotics Antimicrob. Proteins. 2020, 12, 102–111. [Google Scholar] [CrossRef]
- Bernut, A.; Dupont, C.; Ogryzko, N.V.; Neyret, A.; Herrmann, J.L.; Floto, R.A.; Renshaw, S.A.; Kremer, L. CFTR protects against Mycobacterium abscessus infection by fine-tuning host oxidative defenses. Cell Rep. 2019, 26, 1828–1840.e4. [Google Scholar] [CrossRef] [Green Version]
- Black, H.D.; Xu, W.; Hortle, E.; Robertson, S.I.; Britton, W.J.; Kaur, A.; New, E.J.; Witting, P.K.; Chami, B.; Oehlers, S.H. The cyclic nitroxide antioxidant 4-methoxy-TEMPO decreases mycobacterial burden in vivo through host and bacterial targets. Free Radic. Biol. Med. 2019, 135, 157–166. [Google Scholar] [CrossRef]
- Wipperman, M.F.; Bhattarai, S.K.; Vorkas, C.K.; Maringati, V.S.; Taur, Y.; Mathurin, L.; McAulay, K.; Vilbrun, S.C.; Francois, D.; Bean, J.; et al. Gastrointestinal microbiota composition predicts peripheral inflammatory state during treatment of human tuberculosis. Nat. Commun. 2021, 12, 1141. [Google Scholar] [CrossRef] [PubMed]
- Nadal, A.P.; Ikeda-Ohtsubo, W.; Sipkema, D.; Peggs, D.; McGurk, C.; Forlenza, M.; Wiegertjes, G.F.; Brugman, S. Feed, microbiota, and gut immnunity: Using the zebrafish model to understand fish health. Front. Immunol. 2020, 11, 114. [Google Scholar] [CrossRef] [PubMed]
- Robinson, C.D.; Klein, H.S.; Murphy, K.D.; Parthasarathy, R.; Guillemin, K.; Bohannan, B.J.M. Experimental bacterial adaptation to the zebrafish gut reveals a primary role for immigration. PLoS Biol. 2018, 16, e2006893. [Google Scholar] [CrossRef] [Green Version]
- Mateos-Hernández, L.; Obregón, D.; Maye, J.; Borneres, J.; Versille, N.; de la Fuente, J.; Estrada-Peña, A.; Hodžić, A.; Šimo, L.; Cabezas-Cruz, A. Anti-tick microbiota vaccine impacts Ixodes ricinus performance during feeding. Vaccines 2020, 8, 702. [Google Scholar] [CrossRef]
- Bamgbose, T.; Anvikar, A.R.; Alberdi, P.; Abdullahi, I.O.; Inabo, H.I.; Bello, M.; Cabezas-Cruz, A.; de la Fuente, J. Functional food for the stimulation of the immune system against malaria. Probiotics Antimicrob. Proteins. 2021, 1–13. [Google Scholar] [CrossRef]
- Galili, U.; Rachmilewitz, E.A.; Peleg, A.; Flechner, I. A unique natural human IgG antibody with anti-α-galactosyl specificity. J. Exp. Med. 1984, 160, 1519–1531. [Google Scholar] [CrossRef]
- Galili, U.; Mandrell, R.E.; Hamadeh, R.M.; Shohet, S.B.; Griffiss, J.M. Interaction between human natural anti-α-galactosyl immunoglobulin G and bacteria of the human flora. Infect. Immun. 1988, 56, 1730–1737. [Google Scholar] [CrossRef] [Green Version]
- Koch, B.E.V.; Yang, S.; Lamers, G.; Stougaard, J.; Spaink, H.P. Intestinal microbiome adjusts the innate immune setpoint during colonization through negative regulation of MyD88. Nat. Commun. 2018, 9, 4099. [Google Scholar] [CrossRef] [PubMed]
- Kanther, M.; Sun, X.; Mhlbauer, M.; MacKey, L.C.; Flynn, E.J.; Bagnat, M.; Jobin, C.; Rawls, J.F. Microbial colonization induces dynamic temporal and spatial patterns of NF-κB activation in the zebrafish digestive tract. Gastroenterology 2011, 141, 197–207. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheesman, S.E.; Neal, J.T.; Mittge, E.; Seredick, B.M.; Guillemin, K. Epithelial cell proliferation in the developing zebrafish intestine is regulated by the Wnt pathway and microbial signaling via Myd88. Proc. Natl. Acad. Sci. USA 2011, 108 (Suppl. S1), 4570–4577. [Google Scholar] [CrossRef] [Green Version]
- Jacobs, A.J.; Mongkolsapaya, J.; Screaton, G.R.; McShane, H.; Wilkinson, R.J. Antibodies and tuberculosis. Tuberculosis 2016, 101, 102–113. [Google Scholar] [CrossRef] [Green Version]
- Lee, A.Y.S.; Körner, H. The CCR6-CCL20 axis in humoral immunity and T-B cell immunobiology. Immunobiology 2019, 224, 449–454. [Google Scholar] [CrossRef]
- Haeggström, J.Z.; Tholander, F.; Wetterholm, A. Structure and catalytic mechanisms of leukotriene A4 hydrolase. Prostaglandins Other Lipid Mediat. 2007, 83, 198–202. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, A.R.; Yu, J.J.; Guentzel, M.N.; Navara, C.S.; Klose, K.E.; Forsthuber, T.G.; Chambers, J.P.; Berton, M.T.; Arulanandam, B.P. Mast cell TLR2 signaling is crucial for effective killing of Francisella tularensis. J. Immunol. 2012, 188, 5604–5611. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maji, A.; Misra, R.; Kumar Mondal, A.; Kumar, D.; Bajaj, D.; Singhal, A.; Arora, G.; Bhaduri, A.; Sajid, A.; Bhatia, S.; et al. Expression profiling of lymph nodes in tuberculosis patients reveal inflammatory milieu at site of infection. Sci. Rep. 2015, 5, 15214. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blanc, L.; Gilleron, M.; Prandi, J.; Song, O.R.; Jang, M.S.; Gicquel, B.; Drocourt, D.; Neyrolles, O.; Brodin, P.; Tiraby, G.; et al. Mycobacterium tuberculosis inhibits human innate immune responses via the production of TLR2 antagonist glycolipids. Proc. Natl. Acad. Sci. USA 2017, 114, 11205–11210. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, P.L.; Plessner, H.L.; Voitenok, N.N.; Flynn, J.L. Tumor necrosis factor and tuberculosis. J. Investig. Dermatol. Symp. Proc. 2007, 12, 22–25. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Enes, P.; Panserat, S.; Kaushik, S.; Oliva-Teles, A. Nutritional regulation of hepatic glucose metabolism in fish. Fish. Physiol. Biochem. 2009, 35, 519–539. [Google Scholar] [CrossRef] [PubMed]
- Saputra, F.; Shiu, Y.L.; Chen, Y.C.; Puspitasari, A.W.; Danata, R.H.; Liu, C.H.; Hu, S.Y. Dietary supplementation with xylanase-expressing B. amyloliquefaciens R8 improves growth performance and enhances immunity against Aeromonas hydrophila in Nile tilapia (Oreochromis niloticus). Fish Shellfish Immunol. 2016, 58, 397–405. [Google Scholar] [CrossRef]
- Taneja, N.; Coy, P.E.; Lee, I.; Bryson, J.M.; Robey, R.B. Proinflammatory interleukin-1 cytokines increase mesangial cell hexokinase activity and hexokinase II isoform abundance. Am. J. Physiol. Cell Physiol. 2004, 287, C548–C557. [Google Scholar] [CrossRef]
- Peng, X.; Yu, K.-Q.; Deng, G.-H.; Jiang, Y.-X.; Wang, Y.; Zhang, G.-X.; Zhou, H.-W. Comparison of boiling with commercial kits for extracting fecal microbiome DNA by Illumina sequencing of 16S RNA tags. J. Microbiol. Methods 2013, 9, 455–462. [Google Scholar] [CrossRef]
- Toukach, P.V.; Knirel, Y.A. New database of bacterial carbohydrate structures. Glycoconj. J. 2005, 22, 216–217. [Google Scholar]
- Toukach, P.V.; Egorova, K.S. Carbohydrate structure database merged from bacterial, archaeal, plant and fungal parts. Nucleic Acids Res. 2016, 44, D1229–D1236. [Google Scholar] [CrossRef] [PubMed]
- Toukach, P.V.; Egorova, K.S. New Features of carbohydrate structure database notation (CSDB Linear), as compared to other carbohydrate notations. J. Chem. Inf. Model. 2020, 60, 1276–1289. [Google Scholar] [CrossRef] [PubMed]
- Egorova, K.S.; Toukach, P.V. Glycoinformatics: Bridging isolated islands in the sea of data. Angew. Chem. Int. Ed. Engl. 2018, 57, 14986–14990. [Google Scholar] [CrossRef]
- Wanka, K.M.; Damerau, T.; Costas, B.; Krueger, A.; Schulz, C.; Wuertz, S. Isolation and characterization of native probiotics for fish farming. BMC Microbiol. 2018, 18, 119. [Google Scholar] [CrossRef]
- Meritet, D.M.; Mulrooney, D.M.; Kent, M.L.; Löhr, C.V. Development of quantitative real-time PCR assays for postmortem detection of Mycobacterium spp. common in zebrafish (Danio rerio) research colonies. J. Am. Assoc. Lab. Anim. Sci. 2017, 56, 131–141. [Google Scholar]
- Ririe, K.M.; Rasmussen, R.P.; Wittwer, C.T. Product differentiation by analysis of DNA melting curves during the polymerase chain reaction. Anal. Biochem. 1997, 245, 154–160. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Infantes-Lorenzo, J.A.; Moreno, I.; Risalde, M.; Roy, Á.; Villar, M.; Romero, B.; Ibarrola, N.; de la Fuente, J.; Puentes, E.; de Juan, L.; et al. Proteomic characterisation of bovine and avian purified protein derivatives and identification of specific antigens for serodiagnosis of bovine tuberculosis. Clin. Proteomics 2017, 14, 36. [Google Scholar] [CrossRef] [Green Version]
- Callahan, B.J.; McMurdie, P.J.; Rosen, M.J.; Han, A.W.; Johnson, A.J.A.; Holmes, S.P. DADA2: High-resolution sample inference from Illumina amplicon data. Nat. Methods 2016, 13, 581–583. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yarza, P.; Yilmaz, P.; Pruesse, E.; Glöckner, F.O.; Ludwig, W.; Schleifer, K.H.; Whitman, W.B.; Euzéby, J.; Amann, R.; Roselló-Mora, R. Uniting the classification of cultured and uncultured bacteria and archaea using 16S rRNA gene sequences. Nat. Rev. Microbiol. 2014, 12, 635–645. [Google Scholar] [CrossRef] [PubMed]
- McMurdie, P.J.; Holmes, S. Phyloseq: An R package for reproducible interactive analysis and graphics of microbiome census data. PLoS ONE 2013, 8, e61217. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fernandes, A.D.; Reid, J.N.; Macklaim, J.M.; McMurrough, T.A.; Edgell, D.R.; Gloor, G.B. Unifying the analysis of high-throughput sequencing datasets: Characterizing RNA-seq, 16S rRNA gene sequencing and selective growth experiments by compositional data analysis. Microbiome 2014, 2, 15. [Google Scholar] [CrossRef] [Green Version]



| Organism | Colony Description | Classification |
|---|---|---|
| Aerobic | circular, pink, raised, punctiform bacterial colonies | Type I |
| circular, creamy white, raised, bacterial colonies (≤5 mm) | Type II | |
| irregular, dry white, flat colonies (≤5 mm) | Type III | |
| Anaerobic | circular, creamy white, raised colonies (≤5 mm) | Type Ib |
| circular, white, raised, punctiform colonies | Type IIb |
| ID | BLAST Match to 16S rRNA | Max Score, Total Score, Query Cover, Identity, E-Value | Genebank Accession Number | References in Zebrafish |
|---|---|---|---|---|
| PSZ1 | Aeromonas veronii strain JCM 7375 | 813, 813, 91%, 99.3%, 0.0 | NR_112838.1 NR_118947.1 NR_044845.1 | [37,38,39] |
| PSZ4 | Microbacterium mitrae strain M4-8 | 773, 773, 91%, 99.1%, 0.0 | NR_104520.1 | [37] |
| PSZ9 | Dyadobacter alkalitolerans strain 12116 | 778, 778, 92%, 98.4%, 0.0 | NR_044476.1 | [39] |
| LRZ3 | Shewanella xiamenensis strain S4 | 826, 826, 91%, 99.8%, 0.0 | NR_116732.1 | [37,39] |
| LRZ9 | Pseudomonas entomophila L48 | 826, 826, 92%, 99.8%, 0.0 | NR_102854.1 | [37,38,39] |
| α-Gal vs. Control at Pre-Challenge | ||||||
|---|---|---|---|---|---|---|
| Taxon | Diff.btw | Diff.win | Effect | Overlap | We.ep | We.eBH |
| Roseomonas | 13.542346 | 2.9935668 | 4.663723 | 0.000233886 | 3.212340e-04 | 0.02402259 |
| Tabrizicola | 5.322295 | 0.9551066 | 5.490361 | 0.000233886 | 1.855949e-05 | 0.00295325 |
| P. entomophila Probiotic Treatment vs. Control at Post-Challenge | ||||||
| Taxon | Diff.btw | Diff.win | Effect | Overlap | We.ep | We.eBH |
| Barnesiella | −2.124928 | 0.4827484 | −3.883899 | 0.000140345 | 0.0003810454 | 0.02501155 |
| Defluviicoccus | −2.790908 | 0.9249851 | −3.087608 | 0.000140345 | 0.0003750165 | 0.02514513 |
| Arenimonas | −1.797804 | 0.6606933 | −2.640389 | 0.000140345 | 0.0006246535 | 0.03367145 |
| Bradyrhizobium | −1.208369 | 0.5903415 | −2.048345 | 0.000140345 | 0.0017745113 | 0.04961546 |
| Gemmobacter | 12.002481 | 3.4395137 | 3.290789 | 0.000140345 | 0.0019083535 | 0.04779711 |
| Rubrivivax | 10.355734 | 2.8742810 | 3.437225 | 0.000140345 | 0.0017269486 | 0.03403588 |
| Dinghuibacter | 8.695993 | 2.4873367 | 3.648279 | 0.000140345 | 0.0027296612 | 0.04676323 |
| Candidatus Berkiella | 9.832489 | 2.5879052 | 3.665712 | 0.000140345 | 0.0016078763 | 0.03464549 |
| Tabrizicola | 10.649151 | 2.5152927 | 4.325241 | 0.000140345 | 0.0020747599 | 0.03980276 |
| Genes | Oligonucleotide Forward (F) and Reverse (R) Primers | Annealing Conditions | References |
|---|---|---|---|
| ccr6a | F: 5′-AGCTTCTGCGTGGCATCTAT-3′ R: 5′-CAGACGGCTGCACAAACTAA-3′ | 56 °C, 30 s | [24] |
| tlr2 | F: 5′-TGAATGGGTCGAGGAGATTC-3′ R: 5′-CACAAAGTGCTCCGACAGAA-3′ | 56 °C, 30 s | [24] |
| akr2 | F: 5′-ACTATGGACTTCGATCCGCT-3′ R: 5′-GCTCTGTGGTGAGTGCTGAA-3′ | 56 °C, 30 s | [24] |
| IL-1ß | F: 5′-GCATGTCCACATATGCGTCG-3′ R: 5′-GCTGGTCGTATCCGTTTGGA-3′ | 58 °C, 30 s | [24] |
| C3 | F: 5′-ACGCTCTCTGGATTGAAACA-3′ R: 5′-TGCCTTCTTGCATGGCAATC-3′ | 56 °C, 30 s | [24] |
| IL-6 | F: 5′-TCAACTTCTCCAGCGTGATG-3′ R: 5′-TCTTTCCCTCTTTTCCTCCTG-3′ | 56 °C, 30 s | [16] |
| tnf-α | F: 5′-AAGGAGAGTTGCCTTTACCG-3′ R: 5′-ATTGCCCTGGGTCTTATGC-3′ | 54 °C, 30 s | [16] |
| NF-kB | F: 5′-AAGAGGACCAAAATAAGCACAG-3′ R: 5′-AAGTCCAAGGTACATCGCCATGA-3′ | 58 °C, 30 s | [16] |
| hk-1 | F: 5′-ACTTTGGGTGCAATCCTGAC-3′ R: 5′-AGACGACGCACTGTTTTGTG-3′f | 56 °C, 30 s | [16] |
| Time Point | Treatment | |||
|---|---|---|---|---|
| A. veronii | P. entomophila | α-Gal | PBS Control | |
| Pre-Challenge (weeks 3–5) | n = 6 | n = 6 | n = 6 | n = 3 |
| Post-Challenge (week 10) | n = 4 | n = 5 | n = 4 | n = 5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pacheco, I.; Díaz-Sánchez, S.; Contreras, M.; Villar, M.; Cabezas-Cruz, A.; Gortázar, C.; de la Fuente, J. Probiotic Bacteria with High Alpha-Gal Content Protect Zebrafish against Mycobacteriosis. Pharmaceuticals 2021, 14, 635. https://doi.org/10.3390/ph14070635
Pacheco I, Díaz-Sánchez S, Contreras M, Villar M, Cabezas-Cruz A, Gortázar C, de la Fuente J. Probiotic Bacteria with High Alpha-Gal Content Protect Zebrafish against Mycobacteriosis. Pharmaceuticals. 2021; 14(7):635. https://doi.org/10.3390/ph14070635
Chicago/Turabian StylePacheco, Iván, Sandra Díaz-Sánchez, Marinela Contreras, Margarita Villar, Alejandro Cabezas-Cruz, Christian Gortázar, and José de la Fuente. 2021. "Probiotic Bacteria with High Alpha-Gal Content Protect Zebrafish against Mycobacteriosis" Pharmaceuticals 14, no. 7: 635. https://doi.org/10.3390/ph14070635
APA StylePacheco, I., Díaz-Sánchez, S., Contreras, M., Villar, M., Cabezas-Cruz, A., Gortázar, C., & de la Fuente, J. (2021). Probiotic Bacteria with High Alpha-Gal Content Protect Zebrafish against Mycobacteriosis. Pharmaceuticals, 14(7), 635. https://doi.org/10.3390/ph14070635









