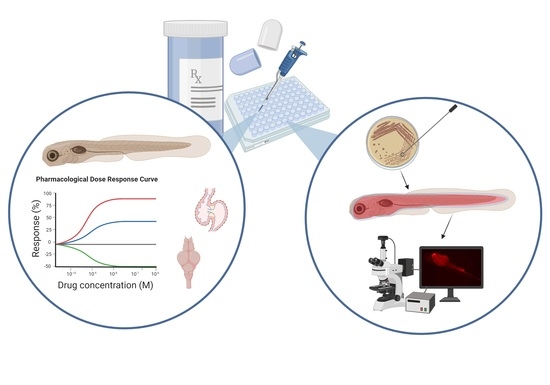
Zebrafish: An Attractive Model to Study Staphylococcus aureus Infection and Its Use as a Drug Discovery Tool
Abstract
:1. Introduction
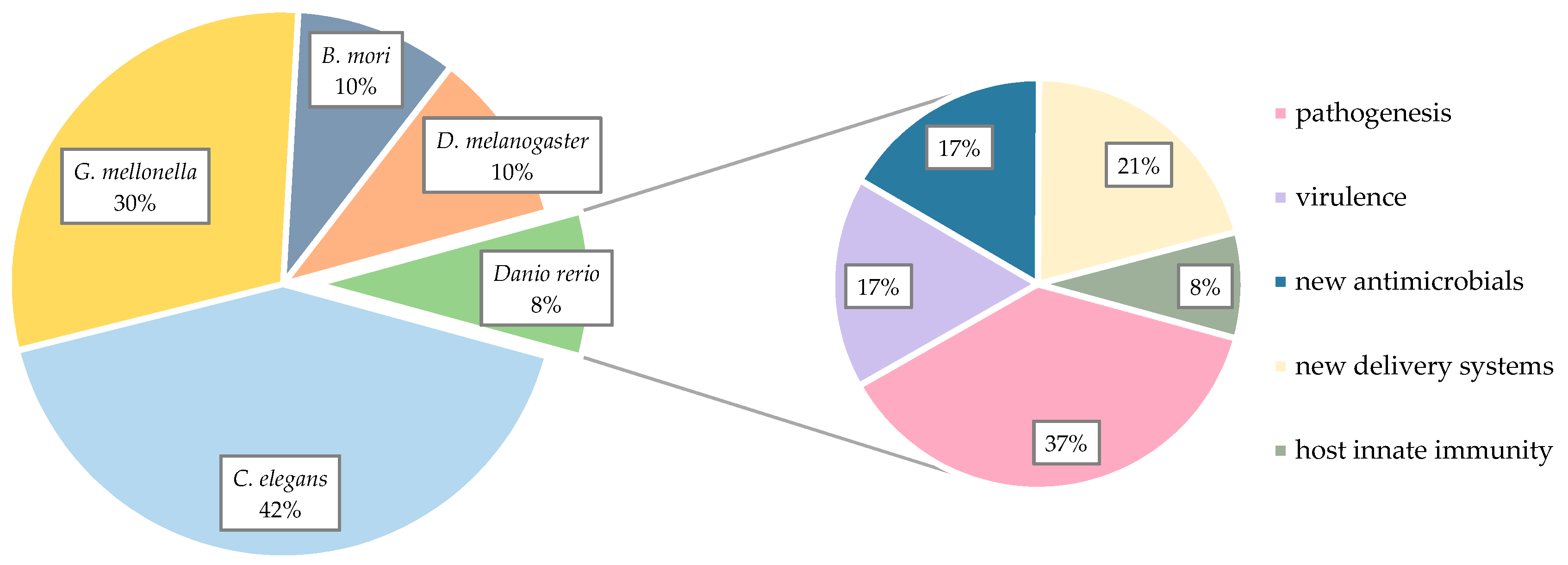
2. Non-Mammalian Models in Pharmaceutical Research
3. Zebrafish Larvae Models of S. aureus Infections
4. Zebrafish Larvae in Drug Discovery
5. Conclusion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Al-Mebairik, N.F.; El-Kersh, T.A.; Al-Sheikh, Y.A.; Marie, M.A.M. A Review of Virulence Factors, Pathogenesis, and Antibiotic Resistance in Staphylococcus Aureus. Rev. Med Microbiol. 2016, 27, 50–56. [Google Scholar] [CrossRef]
- Tong, S.Y.C.; Davis, J.S.; Eichenberger, E.; Holland, T.L.; Fowler, V.G. Staphylococcus Aureus Infections: Epidemiology, Pathophysiology, Clinical Manifestations, and Management. Clin. Microbiol. Rev. 2015, 28, 603–661. [Google Scholar] [CrossRef] [Green Version]
- Enright, M.C.; Robinson, D.A.; Randle, G.; Feil, E.J.; Grundmann, H.; Spratt, B.G. The Evolutionary History of Methicillin-Resistant Staphylococcus Aureus (MRSA). Proc. Natl. Acad. Sci. USA 2002, 99, 7687–7692. [Google Scholar] [CrossRef] [Green Version]
- Hiramatsu, K.; Katayama, Y.; Matsuo, M.; Sasaki, T.; Morimoto, Y.; Sekiguchi, A.; Baba, T. Multi-Drug-Resistant Staphylococcus Aureus and Future Chemotherapy. J. Infect. Chemother. 2014, 20, 593–601. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cong, Y.; Yang, S.; Rao, X. Vancomycin Resistant Staphylococcus aureus Infections: A Review of Case Updating and Clinical Features. J. Adv. Res. 2020, 21, 169–176. [Google Scholar] [CrossRef]
- Hassoun, A.; Linden, P.K.; Friedman, B. Incidence, Prevalence, and Management of MRSA Bacteremia across Patient Populations—A Review of Recent Developments in MRSA Management and Treatment. Crit. Care 2017, 21, 211. [Google Scholar] [CrossRef] [Green Version]
- Tacconelli, E.; Carrara, E.; Savoldi, A.; Harbarth, S.; Mendelson, M.; Monnet, D.L.; Pulcini, C.; Kahlmeter, G.; Kluytmans, J.; Carmeli, Y.; et al. Discovery, Research, and Development of New Antibiotics: The WHO Priority List of Antibiotic-Resistant Bacteria and Tuberculosis. Lancet Infect. Dis. 2018, 18, 318–327. [Google Scholar] [CrossRef]
- Shariati, A.; Dadashi, M.; Chegini, Z.; van Belkum, A.; Mirzaii, M.; Khoramrooz, S.S.; Darban-Sarokhalil, D. The Global Prevalence of Daptomycin, Tigecycline, Quinupristin/Dalfopristin, and Linezolid-Resistant Staphylococcus Aureus and Coagulase–Negative Staphylococci Strains: A Systematic Review and Meta-Analysis. Antimicrob. Resist. Infect. Control. 2020, 9, 56. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sarkar, S.; Heise, M.T. Mouse Models as Resources for Studying Infectious Diseases. Clin. Ther. 2019, 41, 1912–1922. [Google Scholar] [CrossRef] [Green Version]
- Kugelberg, E.; Norström, T.; Petersen, T.K.; Duvold, T.; Andersson, D.I.; Hughes, D. Establishment of a Superficial Skin Infection Model in Mice by Using Staphylococcus Aureus and Streptococcus Pyogenes. AAC 2005, 49, 3435–3441. [Google Scholar] [CrossRef] [Green Version]
- Reizner, W.; Hunter, J.G.; O’Malley, N.T.; Southgate, R.D.; Schwarz, E.M.; Kates, S.L. A Systematic Review of Animal Models for Staphylococcus Aureus Osteomyelitis. Eur. Cell Mater. 2014, 27, 196–212. [Google Scholar] [CrossRef]
- Bremell, T.; Lange, S.; Yacoub, A.; Rydén, C.; Tarkowski, A. Experimental Staphylococcus Aureus Arthritis in Mice. Infect. Immun. 1991, 59, 2615–2623. [Google Scholar] [CrossRef] [Green Version]
- Parsonnet, J.; Gillis, Z.A.; Richter, A.G.; Pier, G.B. A Rabbit Model of Toxic Shock Syndrome That Uses a Constant, Subcutaneous Infusion of Toxic Shock Syndrome Toxin 1. Infect. Immun. 1987, 55, 1070–1076. [Google Scholar] [CrossRef] [Green Version]
- Brouillette, E.; Grondin, G.; Lefebvre, C.; Talbot, B.G.; Malouin, F. Mouse Mastitis Model of Infection for Antimicrobial Compound Efficacy Studies against Intracellular and Extracellular Forms of Staphylococcus Aureus. Vet. Microbiol. 2004, 101, 253–262. [Google Scholar] [CrossRef]
- Buchmann, K. Evolution of Innate Immunity: Clues from Invertebrates via Fish to Mammals. Front. Immunol. 2014, 5, 459. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hoffmann, J.A.; Kafatos, F.C.; Janeway, C.A.; Ezekowitz, R.A. Phylogenetic Perspectives in Innate Immunity. Science 1999, 284, 1313–1318. [Google Scholar] [CrossRef] [Green Version]
- Strähle, U.; Scholz, S.; Geisler, R.; Greiner, P.; Hollert, H.; Rastegar, S.; Schumacher, A.; Selderslaghs, I.; Weiss, C.; Witters, H.; et al. Zebrafish Embryos as an Alternative to Animal Experiments—A Commentary on the Definition of the Onset of Protected Life Stages in Animal Welfare Regulations. Reprod. Toxicol. 2012, 33, 128–132. [Google Scholar] [CrossRef] [PubMed]
- Kaito, C.; Murakami, K.; Imai, L.; Furuta, K. Animal Infection Models Using Non-Mammals. Microbiol. Immunol. 2020, 64, 585–592. [Google Scholar] [CrossRef] [PubMed]
- Gravato-Nobre, M.J.; Hodgkin, J. Caenorhabditis Elegans as a Model for Innate Immunity to Pathogens. Cell Microbiol. 2005, 7, 741–751. [Google Scholar] [CrossRef] [PubMed]
- Kamath, R. Genome-Wide RNAi Screening in Caenorhabditis Elegans. Methods 2003, 30, 313–321. [Google Scholar] [CrossRef]
- Champion, O.L.; Wagley, S.; Titball, R.W. Galleria Mellonella as a Model Host for Microbiological and Toxin Research. Virulence 2016, 7, 840–845. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cook, S.M.; McArthur, J.D. Developing Galleria Mellonella as a Model Host for Human Pathogens. Virulence 2013, 4, 350–353. [Google Scholar] [CrossRef] [Green Version]
- Abolaji, A.; Kamdem, J.P.; Farombi, O.; Rocha, J.B. Drosophila Melanogaster as a Promising Model Organism in Toxicological Studies: A Mini Review. Arch. Bas. App. Med. 2013, 1, 33–38. [Google Scholar]
- Roberts, D.B. Drosophila Melanogaster: The Model Organism. Entomol. Exp. Appl. 2006, 121, 93–103. [Google Scholar] [CrossRef]
- Ugur, B.; Chen, K.; Bellen, H.J. Drosophila Tools and Assays for the Study of Human Diseases. Dis. Models Mech. 2016, 9, 235–244. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prüßing, K.; Voigt, A.; Schulz, J.B. Drosophila Melanogaster as a Model Organism for Alzheimer’s Disease. Mol. Neurodegener. 2013, 8, 35. [Google Scholar] [CrossRef] [Green Version]
- Hamamoto, H.; Tonoike, A.; Narushima, K.; Horie, R.; Sekimizu, K. Silkworm as a Model Animal to Evaluate Drug Candidate Toxicity and Metabolism. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2009, 149, 334–339. [Google Scholar] [CrossRef]
- Nwibo, D.D.; Hamamoto, H.; Matsumoto, Y.; Kaito, C.; Sekimizu, K. Current Use of Silkworm Larvae (Bombyx Mori) as an Animal Model in Pharmaco-Medical Research. Drug Discov. Ther. 2015, 9, 133–135. [Google Scholar] [CrossRef] [Green Version]
- Driever, W.; Solnica-Krezel, L.; Schier, A.F.; Neuhauss, S.C.; Malicki, J.; Stemple, D.L.; Stainier, D.Y.; Zwartkruis, F.; Abdelilah, S.; Rangini, Z.; et al. A Genetic Screen for Mutations Affecting Embryogenesis in Zebrafish. Development 1996, 123, 37–46. [Google Scholar] [CrossRef] [PubMed]
- Veneman, W.J.; Stockhammer, O.W.; de Boer, L.; Zaat, S.A.J.; Meijer, A.H.; Spaink, H.P. A Zebrafish High Throughput Screening System Used for Staphylococcus Epidermidis Infection Marker Discovery. BMC Genom. 2013, 14, 255. [Google Scholar] [CrossRef] [Green Version]
- Lieschke, G.J.; Currie, P.D. Animal Models of Human Disease: Zebrafish Swim into View. Nat. Rev. Genet. 2007, 8, 353–367. [Google Scholar] [CrossRef] [PubMed]
- Prajsnar, T.K.; Cunliffe, V.T.; Foster, S.J.; Renshaw, S.A. A Novel Vertebrate Model of Staphylococcus Aureus Infection Reveals Phagocyte-Dependent Resistance of Zebrafish to Non-Host Specialized Pathogens. Cell. Microbiol. 2008, 10, 2312–2325. [Google Scholar] [CrossRef] [PubMed]
- Prajsnar, T.K.; Serba, J.J.; Dekker, B.M.; Gibson, J.F.; Masud, S.; Fleming, A.; Johnston, S.A.; Renshaw, S.A.; Meijer, A.H. The Autophagic Response to Staphylococcus Aureus Provides an Intracellular Niche in Neutrophils. Autophagy 2020, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Ulhuq, F.R.; Gomes, M.C.; Duggan, G.M.; Guo, M.; Mendonca, C.; Buchanan, G.; Chalmers, J.D.; Cao, Z.; Kneuper, H.; Murdoch, S.; et al. A Membrane-Depolarizing Toxin Substrate of the Staphylococcus Aureus Type VII Secretion System Mediates Intraspecies Competition. Proc. Natl. Acad. Sci. USA 2020, 117, 20836–20847. [Google Scholar] [CrossRef]
- Gibson, J.F.; Prajsnar, T.K.; Hill, C.J.; Tooke, A.K.; Serba, J.J.; Tonge, R.D.; Foster, S.J.; Grierson, A.J.; Ingham, P.W.; Renshaw, S.A.; et al. Neutrophils Use Selective Autophagy Receptor Sqstm1/P62 to Target Staphylococcus Aureus for Degradation in Vivo in Zebrafish. Autophagy 2020, 1–10. [Google Scholar] [CrossRef]
- Kasahara, M.; Suzuki, T.; Pasquier, L.D. On the Origins of the Adaptive Immune System: Novel Insights from Invertebrates and Cold-Blooded Vertebrates. Trends Immunol. 2004, 25, 105–111. [Google Scholar] [CrossRef]
- Lam, S.H.; Chua, H.L.; Gong, Z.; Lam, T.J.; Sin, Y.M. Development and Maturation of the Immune System in Zebrafish, Danio Rerio: A Gene Expression Profiling, in Situ Hybridization and Immunological Study. Dev. Comp. Immunol. 2004, 28, 9–28. [Google Scholar] [CrossRef]
- Traver, D.; Herbomel, P.; Patton, E.E.; Murphey, R.D.; Yoder, J.A.; Litman, G.W.; Catic, A.; Amemiya, C.T.; Zon, L.I.; Trede, N.S. The zebrafish as a model organism to study development of the immune system. In Advances in Immunology; Elsevier: Amsterdam, The Netherlands, 2003; Volume 81, pp. 254–330. ISBN 978-0-12-022481-4. [Google Scholar]
- Li, Y.; Li, Y.; Cao, X.; Jin, X.; Jin, T. Pattern Recognition Receptors in Zebrafish Provide Functional and Evolutionary Insight into Innate Immune Signaling Pathways. Cell Mol. Immunol. 2017, 14, 80–89. [Google Scholar] [CrossRef] [Green Version]
- Zhang, S.; Cui, P. Complement System in Zebrafish. Dev. Comp. Immunol. 2014, 46, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Howe, K.; Clark, M.D.; Torroja, C.F.; Torrance, J.; Berthelot, C.; Muffato, M.; Collins, J.E.; Humphray, S.; McLaren, K.; Matthews, L.; et al. The Zebrafish Reference Genome Sequence and Its Relationship to the Human Genome. Nature 2013, 496, 498–503. [Google Scholar] [CrossRef] [Green Version]
- Torraca, V.; Mostowy, S. Zebrafish Infection: From Pathogenesis to Cell Biology. Trends Cell Biol. 2018, 28, 143–156. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Y.; Hu, B. Establishment of Multi-Site Infection Model in Zebrafish Larvae for Studying Staphylococcus Aureus Infectious Disease. J. Genet. Genom. 2012, 39, 521–534. [Google Scholar] [CrossRef]
- Prajsnar, T.K.; Hamilton, R.; Garcia-Lara, J.; McVicker, G.; Williams, A.; Boots, M.; Foster, S.J.; Renshaw, S.A. A Privileged Intraphagocyte Niche Is Responsible for Disseminated Infection of Staphylococcus Aureus in a Zebrafish Model. Cell Microbiol. 2012, 14, 1600–1619. [Google Scholar] [CrossRef] [Green Version]
- Boldock, E.; Surewaard, B.G.J.; Shamarina, D.; Na, M.; Fei, Y.; Ali, A.; Williams, A.; Pollitt, E.J.G.; Szkuta, P.; Morris, P.; et al. Human Skin Commensals Augment Staphylococcus Aureus Pathogenesis. Nat. Microbiol. 2018, 3, 881–890. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pollitt, E.J.G.; Szkuta, P.T.; Burns, N.; Foster, S.J. Staphylococcus Aureus Infection Dynamics. PLoS Pathog. 2018, 14, e1007112. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McVicker, G.; Prajsnar, T.K.; Williams, A.; Wagner, N.L.; Boots, M.; Renshaw, S.A.; Foster, S.J. Clonal Expansion during Staphylococcus Aureus Infection Dynamics Reveals the Effect of Antibiotic Intervention. PLoS Pathog. 2014, 10, e1003959. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, D.; Ho, Y.X.; Cowell, L.M.; Jilani, I.; Foster, S.J.; Prince, L.R. A Genome-Wide Screen Identifies Factors Involved in S. Aureus-Induced Human Neutrophil Cell Death and Pathogenesis. Front. Immunol. 2019, 10, 45. [Google Scholar] [CrossRef] [PubMed]
- Connolly, J.; Boldock, E.; Prince, L.R.; Renshaw, S.A.; Whyte, M.K.; Foster, S.J. Identification of Staphylococcus Aureus Factors Required for Pathogenicity and Growth in Human Blood. Infect. Immun. 2017, 85. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ulvila, J.; Vanha-aho, L.-M.; Kleino, A.; Vaha-Makila, M.; Vuoksio, M.; Eskelinen, S.; Hultmark, D.; Kocks, C.; Hallman, M.; Parikka, M.; et al. Cofilin Regulator 14-3-3 Is an Evolutionarily Conserved Protein Required for Phagocytosis and Microbial Resistance. J. Leukoc. Biol. 2011, 89, 649–659. [Google Scholar] [CrossRef] [PubMed]
- Hepburn, L.; Prajsnar, T.K.; Klapholz, C.; Moreno, P.; Loynes, C.A.; Ogryzko, N.V.; Brown, K.; Schiebler, M.; Hegyi, K.; Antrobus, R.; et al. A Spaetzle-like Role for Nerve Growth Factor in Vertebrate Immunity to Staphylococcus Aureus. Science 2014, 346, 641–646. [Google Scholar] [CrossRef] [PubMed]
- Bao, Y.; Li, Y.; Jiang, Q.; Zhao, L.; Xue, T.; Hu, B.; Sun, B. Methylthioadenosine/S-Adenosylhomocysteine Nucleosidase (Pfs) of Staphylococcus Aureus Is Essential for the Virulence Independent of LuxS/AI-2 System. Int. J. Med. Microbiol. 2013, 303, 190–200. [Google Scholar] [CrossRef]
- Messad, N.; Prajsnar, T.K.; Lina, G.; O’Callaghan, D.; Foster, S.J.; Renshaw, S.A.; Skaar, E.P.; Bes, M.; Dunyach-Remy, C.; Vandenesch, F.; et al. Existence of a Colonizing Staphylococcus Aureus Strain Isolated in Diabetic Foot Ulcers. Diabetes 2015, 64, 2991–2995. [Google Scholar] [CrossRef] [Green Version]
- Bhuiyan, M.S.; Jiang, J.-H.; Kostoulias, X.; Theegala, R.; Lieschke, G.J.; Peleg, A.Y. The Resistance to Host Antimicrobial Peptides in Infections Caused by Daptomycin-Resistant Staphylococcus Aureus. Antibiotics 2021, 10, 96. [Google Scholar] [CrossRef] [PubMed]
- Cassar, S.; Adatto, I.; Freeman, J.L.; Gamse, J.T.; Iturria, I.; Lawrence, C.; Muriana, A.; Peterson, R.T.; Van Cruchten, S.; Zon, L.I. Use of Zebrafish in Drug Discovery Toxicology. Chem. Res. Toxicol. 2020, 33, 95–118. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tannenbaum, J.; Bennett, B.T. Russell and Burch’s 3Rs Then and Now: The Need for Clarity in Definition and Purpose. J. Am. Assoc. Lab. Anim. Sci. 2015, 54, 120–132. [Google Scholar] [PubMed]
- Hill, A.J.; Teraoka, H.; Heideman, W.; Peterson, R.E. Zebrafish as a Model Vertebrate for Investigating Chemical Toxicity. Toxicol. Sci. 2005, 86, 6–19. [Google Scholar] [CrossRef] [Green Version]
- Sovari, S.N.; Vojnovic, S.; Bogojevic, S.S.; Crochet, A.; Pavic, A.; Nikodinovic-Runic, J.; Zobi, F. Design, Synthesis and in Vivo Evaluation of 3-Arylcoumarin Derivatives of Rhenium(i) Tricarbonyl Complexes as Potent Antibacterial Agents against Methicillin-Resistant Staphylococcus Aureus (MRSA). Eur. J. Med. Chem. 2020, 205, 112533. [Google Scholar] [CrossRef]
- Kannan, R.R.; Iniyan, A.M.; Vincent, S.G.P. Production of a Compound against Methicillin Resistant Staphylococcus Aureus (MRSA) from Streptomyces Rubrolavendulae ICN3 & Its Evaluation in Zebrafish Embryos. Indian J. Med. Res. 2014, 139, 913–920. [Google Scholar]
- Jabila Mary, T.R.; Kannan, R.R.; Muthamil Iniyan, A.; Carlton Ranjith, W.A.; Nandhagopal, S.; Vishwakarma, V.; Prakash Vincent, S.G. β-Lactamase Inhibitory Potential of Kalafungin from Marine Streptomyces in Staphylococcus Aureus Infected Zebrafish. Microbiol. Res. 2021, 244, 126666. [Google Scholar] [CrossRef]
- Stevens, C.S.; Rosado, H.; Harvey, R.J.; Taylor, P.W. Epicatechin Gallate, a Naturally Occurring Polyphenol, Alters the Course of Infection with β-Lactam-Resistant Staphylococcus Aureus in the Zebrafish Embryo. Front. Microbiol. 2015, 6. [Google Scholar] [CrossRef] [Green Version]
- Xiong, M.-H.; Li, Y.-J.; Bao, Y.; Yang, X.-Z.; Hu, B.; Wang, J. Bacteria-Responsive Multifunctional Nanogel for Targeted Antibiotic Delivery. Adv. Mater. 2012, 24, 6175–6180. [Google Scholar] [CrossRef]
- Anversa Dimer, F.; de Souza Carvalho-Wodarz, C.; Goes, A.; Cirnski, K.; Herrmann, J.; Schmitt, V.; Pätzold, L.; Abed, N.; De Rossi, C.; Bischoff, M.; et al. PLGA Nanocapsules Improve the Delivery of Clarithromycin to Kill Intracellular Staphylococcus Aureus and Mycobacterium Abscessus. Nanomed. 2020, 24, 102125. [Google Scholar] [CrossRef] [PubMed]
- Fenaroli, F.; Robertson, J.D.; Scarpa, E.; Gouveia, V.M.; Di Guglielmo, C.; De Pace, C.; Elks, P.M.; Poma, A.; Evangelopoulos, D.; Canseco, J.O.; et al. Polymersomes Eradicating Intracellular Bacteria. ACS Nano 2020, 14, 8287–8298. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Song, J.; Klymov, A.; Zhang, Y.; de Boer, L.; Jansen, J.A.; van den Beucken, J.J.; Yang, F.; Zaat, S.A.; Leeuwenburgh, S.C. Monitoring Local Delivery of Vancomycin from Gelatin Nanospheres in Zebrafish Larvae. Int. J. Nanomed. 2018, 13, 5377–5394. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, X.; de Boer, L.; Heiliegers, L.; Man-Bovenkerk, S.; Selbo, P.K.; Drijfhout, J.W.; Høgset, A.; Zaat, S.A.J. Photochemical Internalization Enhances Cytosolic Release of Antibiotic and Increases Its Efficacy against Staphylococcal Infection. J. Control. Release 2018, 283, 214–222. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fraunholz, M.; Sinha, B. Intracellular Staphylococcus Aureus: Live-in and Let Die. Front. Cell. Inf. Microbiol. 2012, 2. [Google Scholar] [CrossRef] [Green Version]
- Cornet, C.; Calzolari, S.; Miñana-Prieto, R.; Dyballa, S.; van Doornmalen, E.; Rutjes, H.; Savy, T.; D’Amico, D.; Terriente, J. ZeGlobalTox: An Innovative Approach to Address Organ Drug Toxicity Using Zebrafish. Int. J. Mol. Sci. 2017, 18, 864. [Google Scholar] [CrossRef] [Green Version]
- Diekmann, H.; Hill, A. ADMETox in Zebrafish. Drug Discov. Today Dis. Models 2013, 10, e31–e35. [Google Scholar] [CrossRef]
- Park, Y.M.; Meyer, M.R.; Müller, R.; Herrmann, J. Drug Administration Routes Impact the Metabolism of a Synthetic Cannabinoid in the Zebrafish Larvae Model. Molecules 2020, 25, 4474. [Google Scholar] [CrossRef]
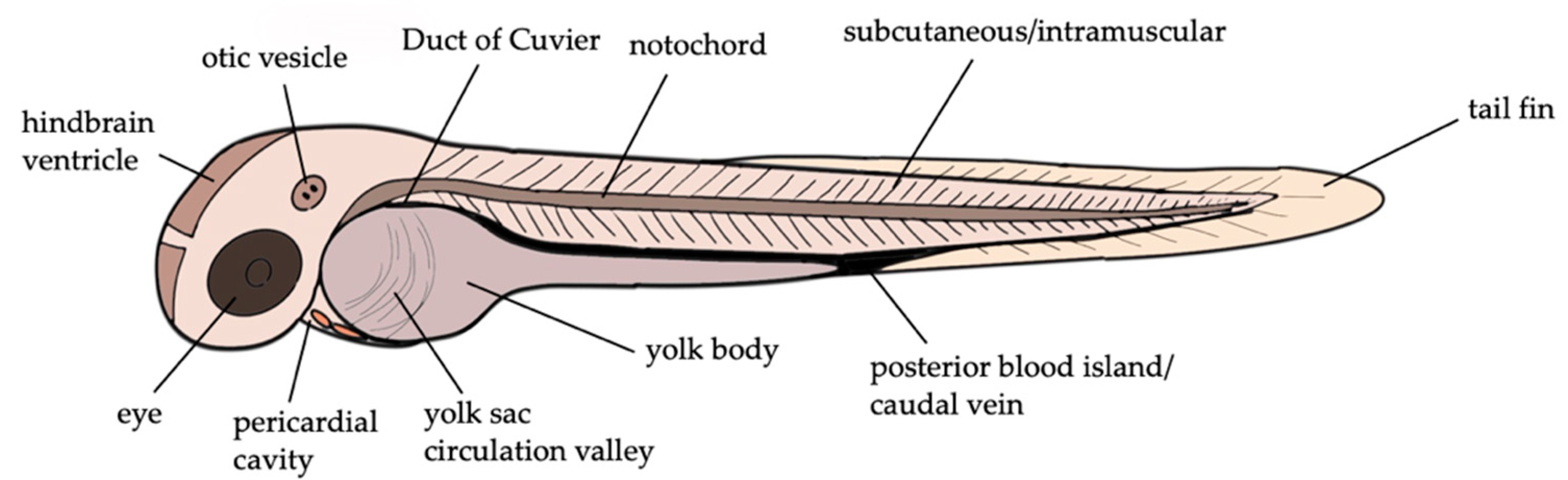
| Aim | Infection Route | Approach | Outcome | Reference |
|---|---|---|---|---|
| Study of pathogenesis | PC, Eye, 4V, YCV, DC, CV, YB | injection of various doses of S. aureus into different sites of zebrafish larvae at 36 hpf | analysis of survival, bacterial proliferation and myeloid cell phagocytosis | [43] |
| YCV | co-infection with two S. aureus strains at 30 or 54 hpf, generation of phagocyte-depleted larvae using the morpholino method | analysis of survival, bacterial strain ratios and myeloid cell phagocytosis | [44] | |
| YCV | injection of S. aureus at 1 or 2 dpf, knockdown of sqstm1 using the morpholino method | analysis of survival, myeloid cell phagocytosis and recruitment of autophagy receptors to S. aureus | [35] | |
| YCV | co-injection of S. aureus combined with a virulence-attenuated mutant as well as S. aureus and M. luteus at 30 hpf | analysis of survival and bacterial proliferation | [45] | |
| YCV | injection of S. aureus at 30 hpf, generation of neutrophil-enriched (irf8 knockdown) as well as NADPH oxidase function deprived embryos using the morpholino method, CRISPR-mediated knockdown of atg5 and atg16l1 | analysis of survival, host–pathogen interactions and recruitment of autophagosomal markers to S. aureus | [33] | |
| YCV | injection of 1:1:1 mixtures of erythromycin-, kanamycin- and teracycline-resistant variants of different S. aureus strains at 30 hpf | analysis of bacterial strain ratios | [46] | |
| Study of antibiotic intervention on staphylococcal infection dynamics | YCV | injection of 1:1:1 mixtures of erythromycin-, kanamycin- and tetracycline-resistant variants of different S. aureus strains at 30 hpf, antibiotic treatment via water immersion, generation of phagocyte-depleted larvae using the morpholino method | analysis of bacterial strain ratios | [47] |
| Study of pathogenesis; identification of virulence factors | YCV | injection of S. aureus wild-type as well as mutants, generation of phagocyte-depleted larvae using the morpholino method | analysis of survival and bacterial proliferation | [48,49] |
| Study of host innate immunity | YCV, PCV | injection of S. aureus at 2 dpf. knockdown of 14-3-3ζ using the morpholino method | analysis of survival and myeloid cell phagocytosis | [50] |
| YCV | injection of S. aureus at 30 hpf, knockdown of trkA using the morpholino method | analysis of survival, bacterial proliferation and neutrophil migration | [51] | |
| Study of virulence | 4V | injection of S. aureus wild-type and mutants as well as co-injection of different strains at 3 dpf | analysis of survival, bacterial proliferation, recruitment of immune cells and cytokine response using qRT-PCR | [34] |
| PC | injection of S. aureus wild-type as well as mutant at 30–32 hpf | analysis of survival and bacterial proliferation | [52] | |
| YCV | injection of different S. aureus strains at 30 hpf | analysis of survival | [53] | |
| Study of virulence and cross-resistance | DC | injection of S. aureus at 48 hpf, knockdown of hepcidin using the morpholino method | analysis of survival | [54] |
| Aim | Infection route | Approach | Outcome | Reference |
|---|---|---|---|---|
| Study of toxicity and efficacy of new antimicrobials | YCV | assessment of lethality, developmental toxicity and cardiotoxicity at 6 hpf, assessment of hepatotoxicity at 72 hpf, injection of MRSA at 30 hpf and treatment with antibacterials at 2 hpi | phenotypic assessment, analysis of survival, fluorescence intensity and bacterial proliferation | [58] |
| bath water immersion | assessment of cardiotoxicity at 3 dpf, infection of 2 dpf larvae via bath water exposure to different concentrations of MRSA, treatment along with infection via bath water immersion | phenotypic assessment, analysis of survival | [59] | |
| CV, bath water immersion | assessment of acute toxicity at 2 hpf, infection of larvae with S. aureus either via microinjection into the CV or bath water immersion, treatment of larvae along with infection | phenotypic assessment, analysis of survival and bacterial proliferation, histopathological analysis | [60] | |
| Study of efficacy of new antimicrobials | YCV, YB | injection of MRSA or MRSA grown in Epicatechin gallate at 30 hpf, treatment via bath water immersion | analysis of survival and NADPH-oxidase dependent respiratory burst | [61] |
| Study of efficacy using new antibiotic delivery systems | PC, 4V | injection of MRSA at 48 hpf, treatment via injection into the PCV 1 hpi | analysis of survival, fluorescence intensities and delivery of the drug to macrophages | [62] |
| YCV, 4V | injection of S. aureus at 30 hpf, treatment with free Clarithromycin or encapsulated in PLGA nanocapsules via bath water immersion 2 hpi | analysis of survival and bacterial proliferation | [63] | |
| PCV | injection of S. aureus at 2 dpf, treatment with free drugs or drug loaded polymersomes via injection into the PCV at 20 hpi | analysis of bacterial proliferation, biodistribution of polymersomes and delivery of drugs to macrophages | [64] | |
| CV, DC, tail muscle | injection of S. aureus at 3 dpf into the DC and the tail muscle to study biodistribution and internalization of nanospheres, injection of S. aureus at 30 hpf into the CV, treatment with free Vancomycin or Vancomycin loaded gelatin nanospheres via CV injection at 2 hpi | analysis of survival, biodistribution and internalization of the nanospheres into macrophages | [65] | |
| CV, DC | injection of S. aureus at 30 hpf treatment with Gentamicin alone or combined with a photosensitizer at 2 hpi, 10 min illumination at 2 hpt | analysis of survival and interaction of S. aureus with macrophages | [66] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rasheed, S.; Fries, F.; Müller, R.; Herrmann, J. Zebrafish: An Attractive Model to Study Staphylococcus aureus Infection and Its Use as a Drug Discovery Tool. Pharmaceuticals 2021, 14, 594. https://doi.org/10.3390/ph14060594
Rasheed S, Fries F, Müller R, Herrmann J. Zebrafish: An Attractive Model to Study Staphylococcus aureus Infection and Its Use as a Drug Discovery Tool. Pharmaceuticals. 2021; 14(6):594. https://doi.org/10.3390/ph14060594
Chicago/Turabian StyleRasheed, Sari, Franziska Fries, Rolf Müller, and Jennifer Herrmann. 2021. "Zebrafish: An Attractive Model to Study Staphylococcus aureus Infection and Its Use as a Drug Discovery Tool" Pharmaceuticals 14, no. 6: 594. https://doi.org/10.3390/ph14060594
APA StyleRasheed, S., Fries, F., Müller, R., & Herrmann, J. (2021). Zebrafish: An Attractive Model to Study Staphylococcus aureus Infection and Its Use as a Drug Discovery Tool. Pharmaceuticals, 14(6), 594. https://doi.org/10.3390/ph14060594