Functional Connectivity between Task-Positive Networks and the Left Precuneus as a Biomarker of Response to Lamotrigine in Bipolar Depression: A Pilot Study
Abstract
1. Introduction
2. Results
2.1. Clinical and Demographic Data
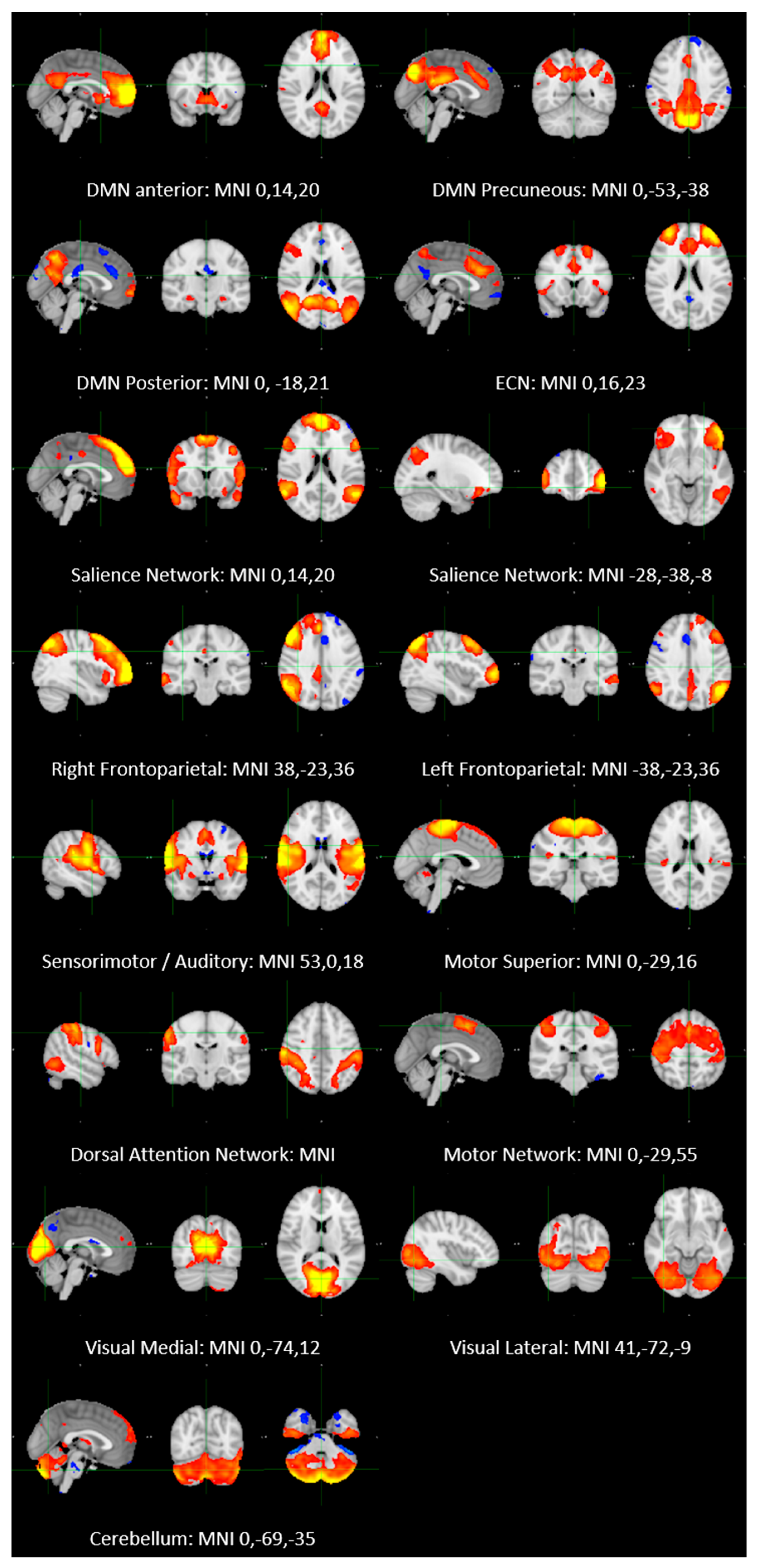
2.2. Resting-State fMRI Imaging Analysis
- (1)
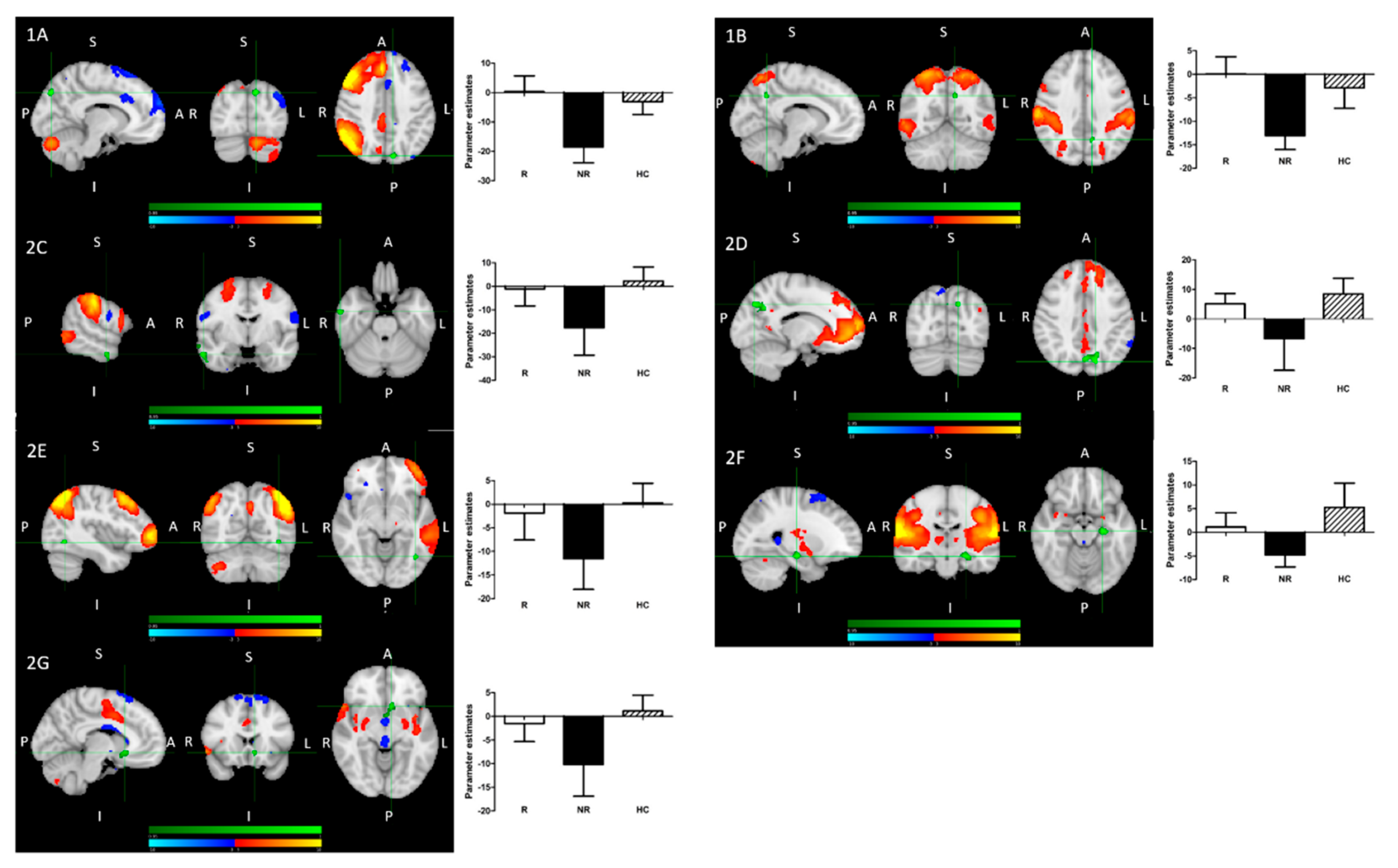
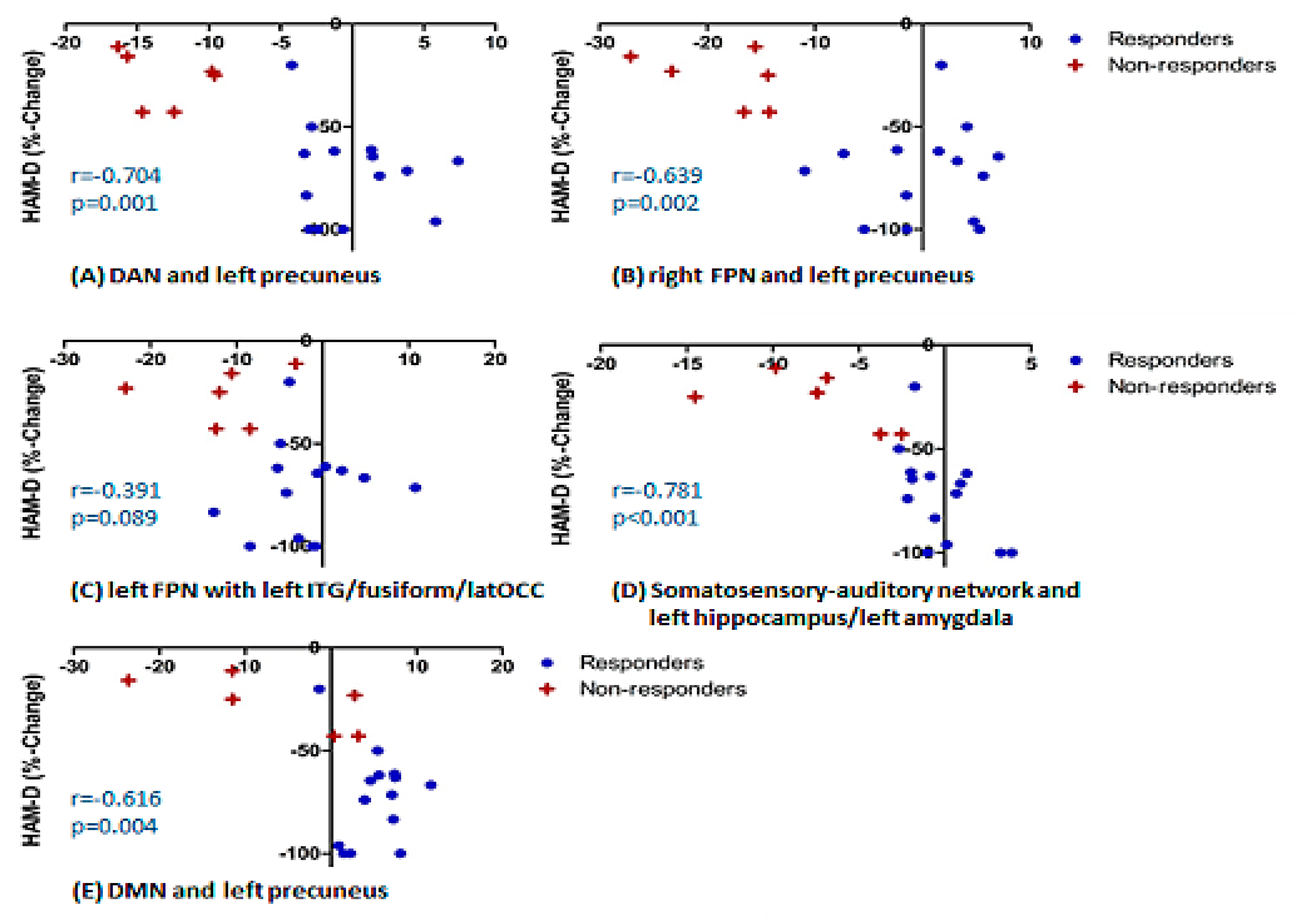
- Treatment R, as compared to NR, showed: greater pre-treatment rsFC between the right FPN and left precuneus; greater pre-treatment FC between the DAN and the left precuneus (Figure 2).
- (2)
- Treatment NR, as compared to HC, showed reduced baseline rsFC: of the right FPN with the left precuneus; of the DAN with right middle temporal gyrus (MTG) and left precuneus; of the DMN and left precuneus; of the extended somatosensory-motor area (SSMN) including the sensory-motor network (SMN), auditory cortex, posterior insula, central and parietal operculum, midcingulate cortex (MCC), and supplementary motor area (SMA) with the left hippocampus/left amygdala, left subcallosal cortex/nucleus accumbens, right occipital pole and left middle frontal gyrus (MFG); as well as of the left FPN with left inferior temporal gyrus (ITG)/occipital fusiform gyrus/lateral occipital cortex (Figure 2).
- (3)
- All patients, as compared to HC, showed reduced baseline rsFC of the extended sensory-motor area (including the SMN, auditory cortex, posterior insula, central and parietal operculum, MCC, and SMA) with the left hippocampus/left amygdala.
- (1)
- R > NR:
- (A)
- The right fronto-parietal network (FPN) and left precuneus
- (B)
- The dorsal attention network (DAN) and left precuneus
- (2)
- NR < HC:
- (A)
- The right FPN and left precuneus (see 1A);
- (B)
- The DAN and left precuneus (see 1B);
- (C)
- The DAN and right middle temporal gyrus;
- (D)
- The DMN and left precuneus;
- (E)
- The left FPN with left inferior temporal gyrus/occipital fusiform gyrus/lateral occipital cortex cluster;
- (F)
- The extended sensory-motor component with the left hippocampus/left amygdala (also all patients < HC);
- (G)
- The extended sensory-motor component with the left subcallosal cortex/accumbens nucleus cluster.
3. Discussion
4. Materials and Methods
4.1. Participants and Design
4.2. Resting-State fMRI
4.3. Analysis Methods
4.3.1. Pre-Processing
4.3.2. ICA Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bowden, C.L.; Singh, V. The use of antidepressants in bipolar disorder patients with depression. Exp. Opin. Pharmacother. 2016, 17, 7–25. [Google Scholar] [CrossRef]
- Solmi, M.; Veronese, N.; Zaninotto, L.; van der Loos, M.L.; Gao, K.; Schaffer, A.; Reis, C.; Normann, C.; Anghelescu, I.G.; Correll, C.U. Lamotrigine compared to placebo and other agents with antidepressant activity in patients with unipolar and bipolar depression: A comprehensive meta-analysis of efficacy and safety outcomes in short-term trials. CNS Spectr. 2016, 21, 403–418. [Google Scholar] [CrossRef]
- Wang, X.Q.; Xiong, J.; Xu, W.H.; Yu, S.Y.; Huang, X.S.; Zhang, J.T.; Tian, C.L.; Huang, D.H.; Jia, W.Q.; Lang, S.Y. Risk of a lamotrigine-related skin rash: Current meta-analysis and postmarketing cohort analysis. Seizure 2015, 25, 52–61. [Google Scholar] [CrossRef]
- Godlewska, B.R.; Browning, M.; Norbury, R.; Igoumenou, A.; Cowen, P.J.; Harmer, C.J. Predicting treatment response in depression: The role of anterior cingulate cortex. Int. J. Neuropsychopharmacol. 2018, 21, 988–996. [Google Scholar] [CrossRef] [PubMed]
- Ketter, T.A.; Manji, H.K.; Post, R.M. Potential mechanisms of action of lamotrigine in the treatment of bipolar disorders. J. Clin. Psychopharmacol. 2003, 23, 484–495. [Google Scholar] [CrossRef]
- Li, B.J.; Friston, K.; Mody, M.; Wang, H.N.; Lu, H.B.; Hu, D.W. A brain network model for depression: From symptom understanding to disease intervention. CNS Neurosci. Ther. 2018, 24, 1004–1019. [Google Scholar] [CrossRef] [PubMed]
- Smith, S.M.; Fox, P.T.; Miller, K.L.; Glahn, D.C.; Fox, P.M.; Mackay, C.E.; Filippini, N.; Watkins, K.E.; Toro, R.; Laird, A.R.; et al. Correspondence of the brain’s functional architecture during activation and rest. Proc. Natl. Acad. Sci. USA 2009, 106, 13040–13045. [Google Scholar] [CrossRef]
- Hohenfeld, C.; Werner, C.J.; Reetz, K. Resting-state connectivity in neurodegenerative disorders: Is there potential for an imaging biomarker? NeuroImage Clin. 2018, 18, 849–870. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Gao, Y.; Tang, S.; Lu, L.; Zhang, L.; Bu, X.; Li, H.; Hu, X.; Hu, X.; Jiang, P.; et al. Large-scale network dysfunction in the acute state compared to the remitted state of bipolar disorder: A meta-analysis of resting-state functional connectivity. EBioMedicine 2020, 54, 102742. [Google Scholar] [CrossRef] [PubMed]
- Zovetti, N.; Rossetti, M.G.; Perlini, C. Default mode network activity in bipolar disorder. Epidemiol. Psychiatry Sci. 2020, 29, e166. [Google Scholar] [CrossRef]
- Gong, J.; Wang, J.; Qiu, S.; Chen, P.; Luo, Z.; Wang, J.; Huang, L.; Wang, Y. Common and distinct patterns of intrinsic brain activity alterations in major depression and bipolar disorder: Voxel-based meta-analysis. Transl. Psychiatry 2020, 10, 353. [Google Scholar] [CrossRef]
- Spielberg, J.M.; Matyi, M.A.; Karne, H.; Anand, A. Lithium monotherapy associated longitudinal effects on resting state brain networks in clinical treatment of bipolar disorder. Bipolar Disord. 2019, 21, 361–371. [Google Scholar] [CrossRef]
- Zhou, J.; Ma, X.; Li, C.; Liao, A.; Yang, Z.; Ren, H.; Tang, J.; Li, J.; Li, Z.; He, Y.; et al. Frequency-specific changes in the fractional amplitude of the low-frequency fluctuations in the default mode network in medication-free patients with bipolar II depression: A longitudinal functional MRI study. Front. Psychiatry 2021, 11, 574819. [Google Scholar] [CrossRef]
- Altinay, M.; Karne, H.; Anand, A. Lithium monotherapy associated clinical improvement effects on amygdala-ventromedial prefrontal cortex resting state connectivity in bipolar disorder. J. Affect. Disord. 2018, 225, 4–12. [Google Scholar] [CrossRef]
- Dandash, O.; Yücel, M.; Daglas, R.; Pantelis, C.; McGorry, P.; Berk, M.; Fornito, A. Differential effect of quetiapine and lithium on functional connectivity of the striatum in first episode mania. Transl. Psychiatry 2018, 6, 59. [Google Scholar] [CrossRef]
- Beckmann, C.; Mackay, C.; Filippini, N.; Smith, S. Group comparison of resting-state FMRI data using multi-subject ICA and dual regression. NeuroImage 2009, 47, S148. [Google Scholar] [CrossRef]
- Yeo, B.T.; Krienen, F.M.; Sepulcre, J.; Sabuncu, M.R.; Lashkari, D.; Hollinshead, M.; Roffman, J.L.; Smoller, J.W.; Zöllei, L.; Polimeni, J.R.; et al. The organization of the human cerebral cortex estimated by intrinsic functional connectivity. Neurophysiology 2011, 106, 1125–1165. [Google Scholar]
- Fox, M.D.; Snyder, A.Z.; Vincent, J.L.; Corbetta, M.; Van Essen, D.C.; Raichle, M.E. The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proc. Natl. Acad. Sci. USA 2005, 102, 9673–9678. [Google Scholar] [CrossRef] [PubMed]
- Marek, S.; Dosenbach, N.U.F. The frontoparietal network: Function, electrophysiology, and importance of individual precision mapping. Dialog. Clin. Neurosci. 2018, 20, 133–140. [Google Scholar]
- Marek, S.; Dosenbach, N.U.F. Control networks of the frontal lobes. Handb. Clin. Neurol. 2019, 163, 333–347. [Google Scholar]
- Corbetta, M.; Shulman, G.L. Control of goal-directed and stimulus-driven attention in the brain. Nat. Rev. Neurosci. 2002, 3, 201–215. [Google Scholar] [CrossRef]
- Buckner, R.L.; Andrews-Hanna, J.R.; Schacter, D.L. The brain’s default network: Anatomy, function, and relevance to disease. Ann. N. Y. Acad. Sci. 2008, 1124, 1–38. [Google Scholar] [CrossRef]
- Greicius, M.D.; Krasnow, B.; Reiss, A.L.; Menon, V. Functional connectivity in the resting brain: A network analysis of the default mode hypothesis. Proc. Natl. Acad. Sci. USA 2003, 100, 253–258. [Google Scholar] [CrossRef]
- Cavanna, A.E.; Trimble, M.R. The precuneus: A review of its functional anatomy and behavioural correlates. Brain 2006, 129, 564–583. [Google Scholar] [CrossRef]
- Chen, A.C.; Oathes, D.J.; Chang, C.; Bradley, T.; Zhou, Z.W.; Williams, L.M.; Glover, G.H.; Deisseroth, K.; Etkin, A. Causal interactions between fronto-parietal central executive and default-mode networks in humans. Proc. Natl. Acad. Sci. USA 2013, 3, 19944–19949. [Google Scholar] [CrossRef] [PubMed]
- Knyazev, G.G.; Savostyanov, A.N.; Bocharov, A.V.; Tamozhnikov, S.S.; Saprigyn, A.E. Task-positive and task-negative networks and their relation to depression: EEG beamformer analysis. Behav. Brain Res. 2016, 306, 160–169. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Liu, L.; Friston, K.J.; Shen, H.; Wang, L.; Zeng, L.L.; Hu, D. A treatment-resistant default mode subnetwork in major depression. Biol. Psychiatry 2013, 74, 48–54. [Google Scholar] [CrossRef]
- Posner, J.; Hellerstein, D.J.; Gat, I.; Mechling, A.; Klahr, K.; Wang, Z.; McGrath, P.J.; Stewart, J.W.; Peterson, B.S. Antidepressants normalize the default mode network in patients with dysthymia. JAMA Psychiatry 2013, 70, 373–382. [Google Scholar] [CrossRef]
- Shen, Y.; Yao, J.; Jiang, X.; Zhang, L.; Xu, L.; Feng, R.; Cai, L.; Liu, J.; Wang, J.; Chen, W. Sub-hubs of baseline functional, brain networks are related to early improvement following two-week pharmacological, therapy for major depressive disorder. Hum. Brain Mapp. 2015, 36, 2915–2927. [Google Scholar] [CrossRef] [PubMed]
- Kropf, E.; Syan, S.K.; Minuzzi, L.; Frey, B.N. From anatomy to function: The role of the somatosensory cortex in emotional regulation. Braz. J. Psychiatry 2019, 41, 261–269. [Google Scholar] [CrossRef]
- Levenson, R.W. The intrapersonal functions of emotion. Cognit. Emot. 1999, 13, 481–504. [Google Scholar] [CrossRef]
- Wiens, S. Interoception in emotional experience. Curr. Opin. Neurol. 2005, 18, 442–447. [Google Scholar] [CrossRef]
- Pace-Schott, E.F.; Amole, M.C.; Aue, T.; Balconi, M.; Bylsma, L.M.; Critchley, H.; Demaree, H.A.; Friedman, B.H.; Kotynski Gooding, A.E.; Gosseries, O.; et al. Physiological feelings. Neurosci. Biobehav. 2019, 103, 267–304. [Google Scholar] [CrossRef]
- Weiner, K.S.; Zilles, K. The anatomical and functional specialization of the fusiform gyrus. Neuropsychologia 2016, 83, 48–62. [Google Scholar] [CrossRef]
- Haldane, M.; Jogia, J.; Cobb, A.; Kozuch, E.; Kumari, V.; Frangou, S. Changes in brain activation during working memory and facial recognition tasks in patients with bipolar disorder with Lamotrigine monotherapy. Eur. Neuropsychopharmacol. 2008, 18, 48–54. [Google Scholar] [CrossRef]
- Jogia, J.; Haldane, M.; Cobb, A.; Kumari, V.; Frangou, S. Pilot investigation of the changes in cortical activation during facial affect recognition with lamotrigine monotherapy in bipolar disorder. Br. J. Psychiatry 2008, 192, 197–201. [Google Scholar] [CrossRef]
- Pavuluri, M.N.; Passarotti, A.M.; Mohammed, T.; Carbray, J.A.; Sweeney, J.A. Enhanced working and verbal memory after lamotrigine treatment in pediatric bipolar disorder. Bipolar Disord. 2010, 12, 213–220. [Google Scholar] [CrossRef]
- Pavuluri, M.N.; Passarotti, A.M.; Harral, E.M.; Sweeney, J.A. Enhanced prefrontal function with pharmacotherapy on a response inhibition task in adolescent bipolar disorder. J. Clin. Psychiatry 2010, 71, 1526–1534. [Google Scholar] [CrossRef]
- Passarotti, A.M.; Sweeney, J.A.; Pavuluri, M.N. Fronto-limbic dysfunction in mania pre-treatment and persistent amygdala over-activity post-treatment in pediatric bipolar disorder. Psychopharmacology 2011, 216, 485–499. [Google Scholar] [CrossRef] [PubMed]
- Spitzer, R.L.; Williams, J.B.W.; Gibbon, M.; First, M.B. Structured Clinical Interview for DSM–1 V (SCID); Biometrics Research New York State Psychiatric Institute: New York, NY, USA, 1995. [Google Scholar]
- Angst, J.; Delini–Stula, A.; Stabl, M.; Stassen, H.H. Is a cutoff score a suitable measure of treatment outcome in short–term trials in depression? A methodological meta–analysis. Hum. Psychopharmacol. 1993, 8, 311–317. [Google Scholar] [CrossRef]
- Altman, E.G.; Hedeker, D.; Peterson, J.L.; Davis, J.M. The altman self-rating mania scale. Biol. Psychiatry 1997, 42, 948–955. [Google Scholar] [CrossRef]
- Spielberger, C.D. State-Traitmant Anxiety Inventory: Bibliography, 2nd ed.; Consulting Psychologists Press: Palo Alto, CA, USA, 1989. [Google Scholar]
- Beck, A.T.; Ward, C.H.; Mendelson, M.; Mock, J.; Erbaugh, J. An inventory for measuring depression. Arch. Gen. Psychiatry 1961, 4, 561–571. [Google Scholar] [CrossRef]
- Smith, S.M. Fast robust automated brain extraction. Hum. Brain Mapp. 2002, 17, 143–155. [Google Scholar] [CrossRef] [PubMed]
- Jenkinson, M.; Bannister, P.R.; Brady, M.; Smith, S.A.C. Improved optimization for the robust and accurate linear registration and motion correction of brain Images. NeuroImage 2002, 17, 825–841. [Google Scholar] [CrossRef]
- Jenkinson, M.; Smith, S. A global optimisation method for robust affine registration of brain images. Med. Image Anal. 2001, 5, 143–156. [Google Scholar] [CrossRef]
- Andersson, J.L.R.; Jenkinson, M.; Smith, S.M. Non-Linear Optimisation; FMRIB Technical Report TR07JA1; University of Oxford: Oxford, UK, 2007. [Google Scholar]
- Andersson, J.L.R.; Jenkinson, M.; Smith, S.M. Non-linear Registration, aka Spatial Normalisation; FMRIB Technical Report TR07JA2; University of Oxford: Oxford, UK, 2007. [Google Scholar]
- Beckmann, C.F.; Smith, S.M. Probabilistic independent component analysis for functional magnetic resonance imaging. IEEE Trans. Med. Imaging 2004, 23, 137–152. [Google Scholar] [CrossRef]
- Minka, T.P. Automatic choice of dimensionality for PCA. In Advances in Neural Information Processing Systems 13; Leen, T.K., Dietterich, T.G., Tresp, V., Eds.; MIT Press: Cambridge, MA, USA, 2001; pp. 598–604. [Google Scholar]
- Hyvarinen, A. Fast and robust fixed-point algorithms for independent component analysis. IEEE Trans. Neural Netw. 1999, 10, 626–634. [Google Scholar] [CrossRef]
- Filippini, N.; MacIntosh, B.J.; Hough, M.G.; Goodwin, G.M.; Frisoni, G.B.; Smith, S.M.; Matthews, P.M.; Beckmann, C.F.; Mackay, C.E. Distinct patterns of brain activity in young carriers of the APOE- 4 allele. Proc. Natl. Acad. Sci. USA 2009, 106, 7209–7214. [Google Scholar] [CrossRef]
| Responders N = 15 | Non-Responders N = 6 | Healthy N = 20 | |
|---|---|---|---|
| Age (years) | 34.8 ± 11.1 | 29.8 ± 11.3 | 30.7 ± 10.0 |
| Gender | 11 F and 4 M | 3 F and 3 M | 13 F and 7 M |
| Age at onset (years) | 20.1 ± 10.1 | 15.5 ± 5.3 | NA |
| Length of illness (years) | 14.1 ± 6.3 | 14.3 ± 10.0 | NA |
| HAMD baseline | 20.8 ± 8.3 | 18.5 ± 4.5 | 0.5 ± 0.9 |
| HAMD at 2nd scan | 5.5 ± 3.9 | 13.8 ± 4.8 | NA |
| BDI baseline | 24.3 ± 12.7 | 26.0 ± 7.1 | 0.9 ± 1.6 |
| BDI at 6 weeks | 6.5 ± 5.2 | 18.8 ± 8.9 | NA |
| Altman baseline | 2.7 ± 2.8 | 3.2 ± 6.3 | 0 ± 0 |
| Altman at 2nd scan | 4.27 ± 4.4 | 5.3 ± 3.5 | NA |
| State anxiety | 34.1 ± 10.7 | 42.8 ± 16.5 | 27.9 ± 8.4 |
| Contrast | Network | Cluster | Cluster Size (Number of Voxels) | Peak Voxel (MNI) | 1-Pmax Value |
|---|---|---|---|---|---|
| R > NR | Right fronto-parietal network | Left precuneus cortex | 43 | −10,−80,40 | >0.999 |
| Dorsal attention network | Left precuneus cortex | 27 | −10,−58,36 | 0.983 | |
| NR < HC | Somatosensory-motor network | Left hippocampus | 112 | −24,−18,−16 | 0.990 |
| Left subcallosal cortex, accumbens | 93 | −8,18,−8 | 0.979 | ||
| Right occipital pole | 36 | 8,−102,4 | 0.971 | ||
| Default Mode Network | Left precuneus cortex | 208 | 14,−74,38 | 0.992 | |
| Right fronto-parietal network | Left precuneus cortex | 39 | −10,−80,40 | 0.997 | |
| Left fronto-parietal network | Left inferior temporal gyrus/occipital fusiform gyrus/lateral occipital cortex | 17 | −40,−62,−8 | 0.967 | |
| Dorsal attention network | Right middle temporal gyrus | 51 | 60,−6,26 | 0.983 | |
| Left precuneus cortex | 31 | −10,−58,34 | 0.986 | ||
| Right middle temporal gyrus | 11 | 64,−6,−16 | 0.962 | ||
| HC > R + NR | Somatosensory-motor network | Left hippocampus | 15 | −24,−18,−16 | 0.966 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martens, M.; Filippini, N.; Masaki, C.; Godlewska, B.R. Functional Connectivity between Task-Positive Networks and the Left Precuneus as a Biomarker of Response to Lamotrigine in Bipolar Depression: A Pilot Study. Pharmaceuticals 2021, 14, 534. https://doi.org/10.3390/ph14060534
Martens M, Filippini N, Masaki C, Godlewska BR. Functional Connectivity between Task-Positive Networks and the Left Precuneus as a Biomarker of Response to Lamotrigine in Bipolar Depression: A Pilot Study. Pharmaceuticals. 2021; 14(6):534. https://doi.org/10.3390/ph14060534
Chicago/Turabian StyleMartens, Marieke, Nicola Filippini, Charles Masaki, and Beata R. Godlewska. 2021. "Functional Connectivity between Task-Positive Networks and the Left Precuneus as a Biomarker of Response to Lamotrigine in Bipolar Depression: A Pilot Study" Pharmaceuticals 14, no. 6: 534. https://doi.org/10.3390/ph14060534
APA StyleMartens, M., Filippini, N., Masaki, C., & Godlewska, B. R. (2021). Functional Connectivity between Task-Positive Networks and the Left Precuneus as a Biomarker of Response to Lamotrigine in Bipolar Depression: A Pilot Study. Pharmaceuticals, 14(6), 534. https://doi.org/10.3390/ph14060534






