Atovaquone Suppresses the Growth of Metastatic Triple-Negative Breast Tumors in Lungs and Brain by Inhibiting Integrin/FAK Signaling Axis
Abstract
1. Introduction
2. Results
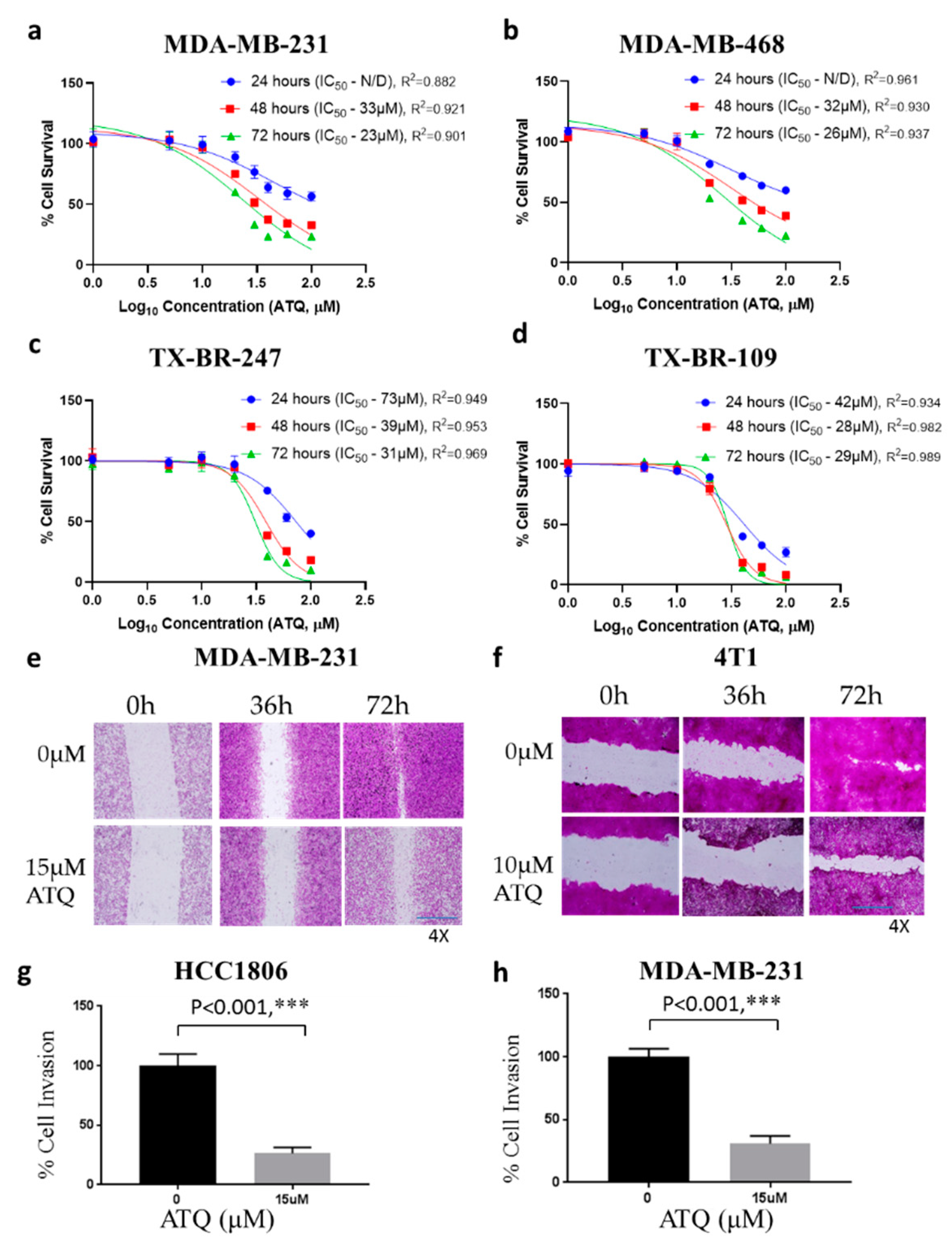
2.1. ATQ Inhibits the Proliferation of Triple-Negative Breast Cancer Cells
2.2. ATQ Reduces the Migration and Invasion of Triple-Negative Breast Cancer Cells
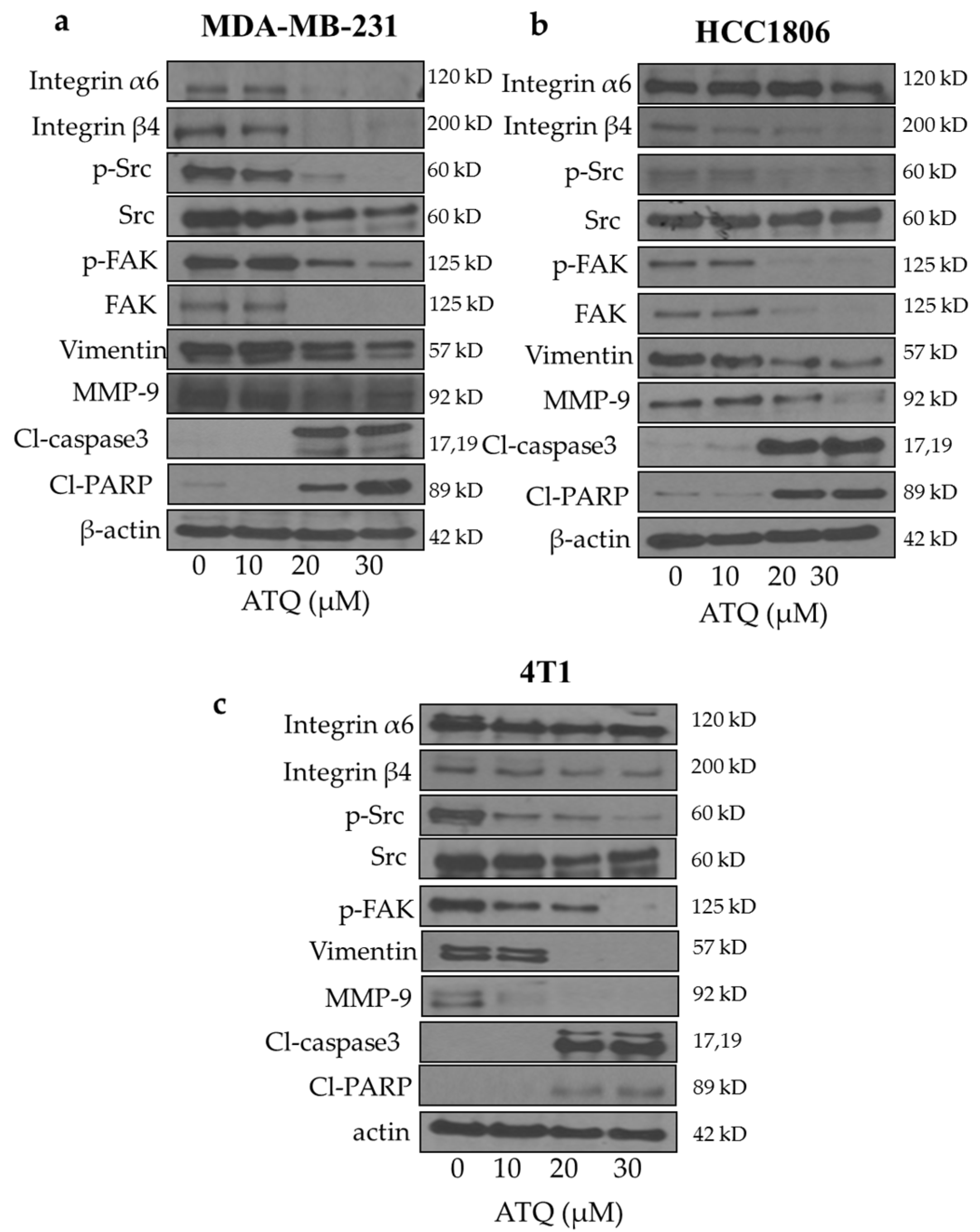
2.3. Inhibition of Integrin/FAK/Src Signaling by ATQ
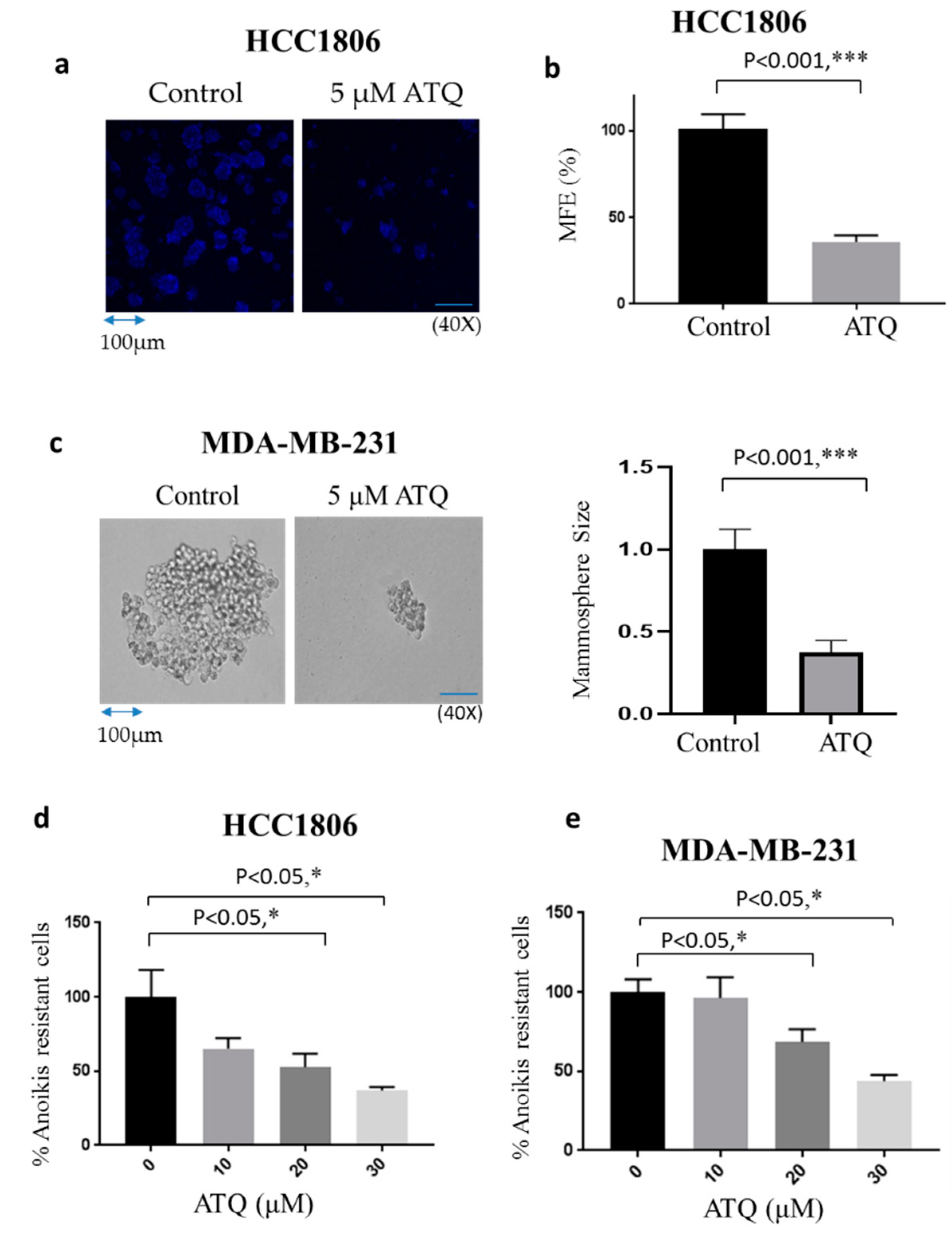
2.4. ATQ Reduces Mammosphere Forming Efficiency of Breast Cancer Cells
2.5. ATQ Reduces Anoikis Resistance of Breast Cancer Cells
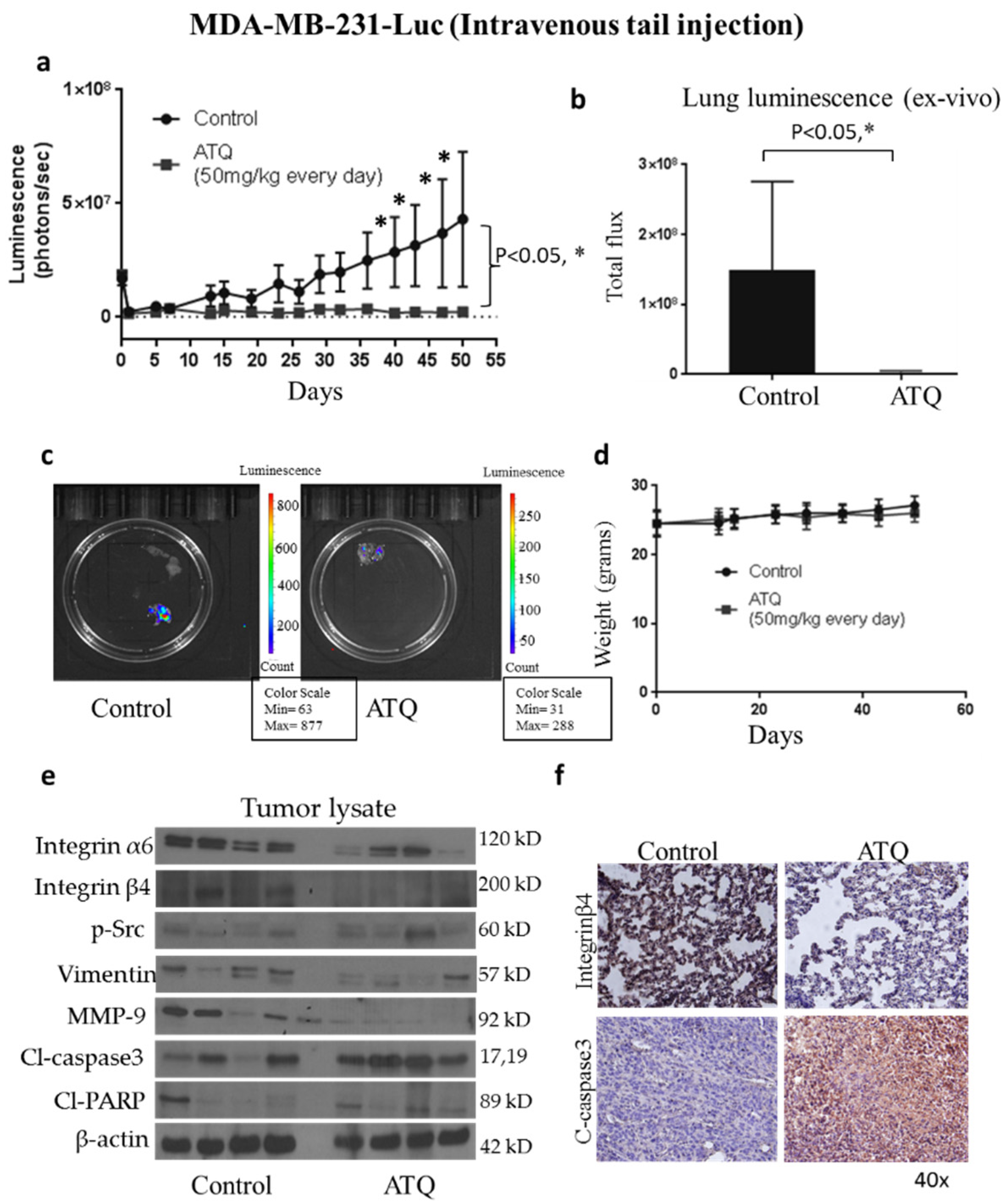
2.6. ATQ Inhibits the Growth of Metastatic Breast Tumors in Lungs
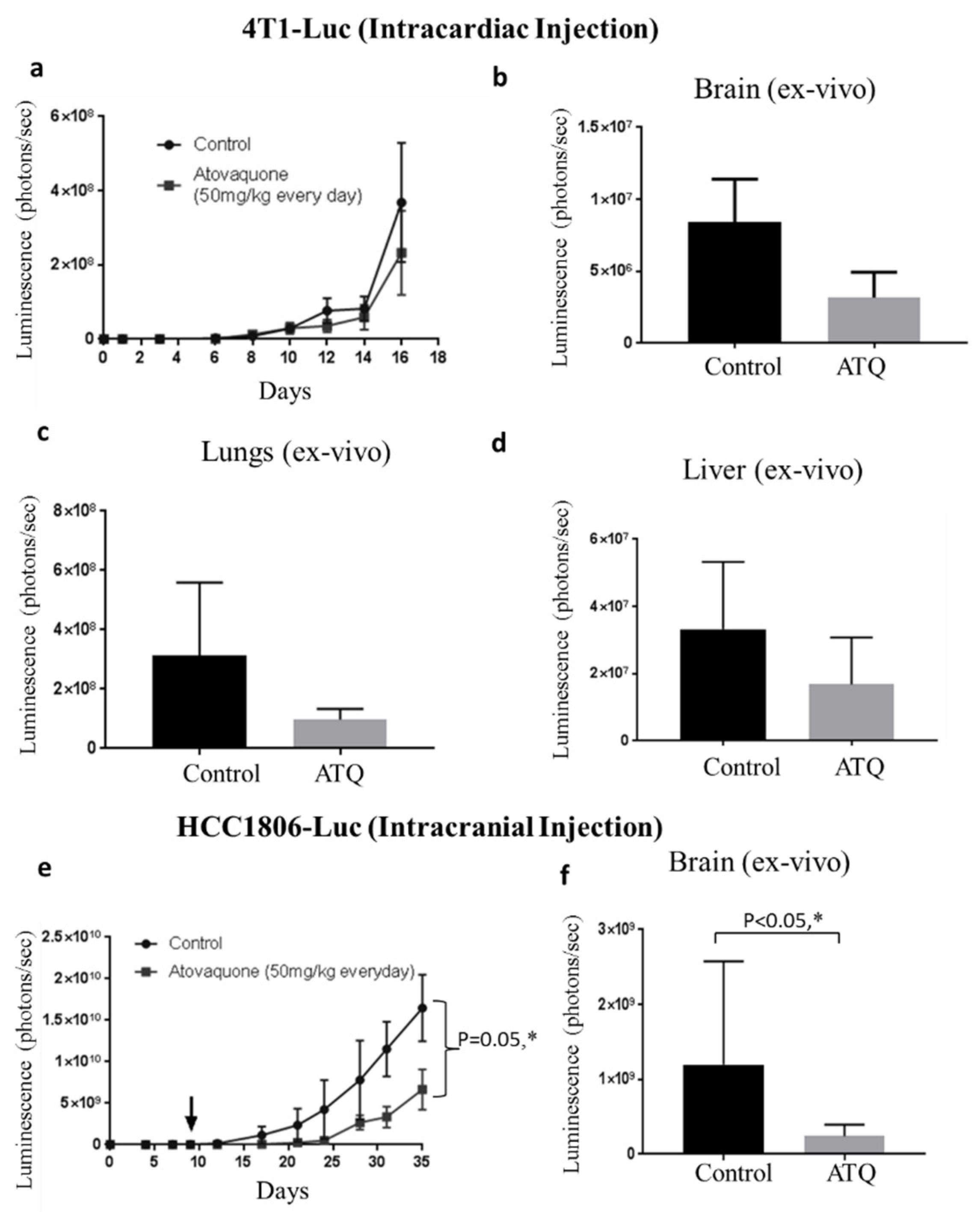
2.7. ATQ Suppresses the Growth of Metastasized Breast Tumors
2.8. ATQ Inhibits the Growth of HCC1806 Tumors in Intracranial Tumor Model
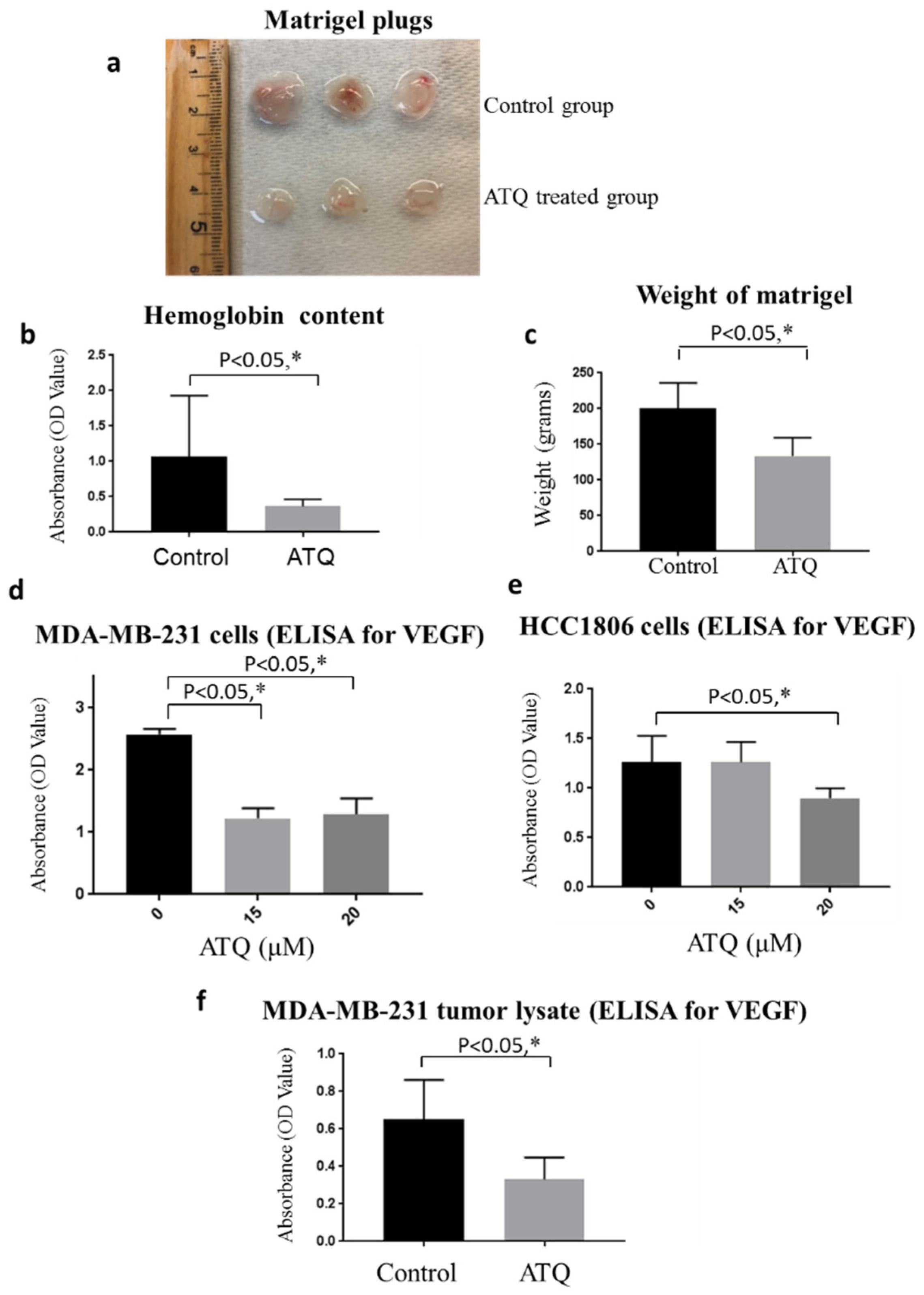
2.9. ATQ Inhibits In Vivo Breast Tumor Angiogenesis
2.10. ATQ Inhibits Secretion of VEGF from Breast Tumor Cells
3. Discussion
4. Materials and Methods
4.1. Cell Culture and Reagents
4.2. Sulphorhodamine Assay
4.3. Wound Healing
4.4. Cell Invasion Assay
4.5. Western Blotting
4.6. Mammospheres Assay
4.7. Anoikis Assay
4.8. Tumor Therapy Model
4.9. In Vivo Metastasis Model (Intravenous Tail Vein Injection)
4.10. Metastasis Model by Intracardiac Injection
4.11. Intracranial Tumor Model
4.12. Matrigel Plug Assay
4.13. Estimation of VEGF Secretion by ELISA
4.14. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sharma, G.N.; Dave, R.; Sanadya, J.; Sharma, P.; Sharma, K. Various types and management of breast cancer: An overview. J. Adv. Pharm. Technol. Res. 2010, 1, 109. [Google Scholar] [PubMed]
- Al-Mahmood, S.; Sapiezynski, J.; Garbuzenko, O.B.; Minko, T. Metastatic and triple-negative breast cancer: Challenges and treatment options. Drug Deliv. Transl. Res. 2018, 8, 1483–1507. [Google Scholar] [PubMed]
- Herold, C.I.; Anders, C.K. New targets for triple-negative breast cancer. Oncology 2013, 27, 846. [Google Scholar]
- Chiu, A.M.; Mitra, M.; Boymoushakian, L.; Coller, H.A. Integrative analysis of the inter-tumoral heterogeneity of triple-negative breast cancer. Sci. Rep. 2018, 8, 11807. [Google Scholar] [CrossRef]
- Pareja, F.; Geyer, F.C.; Marchiò, C.; Burke, K.A.; Weigelt, B.; Reis-Filho, J.S. Triple-negative breast cancer: The importance of molecular and histologic subtyping, and recognition of low-grade variants. NPJ Breast Cancer 2016, 2, 16036. [Google Scholar] [CrossRef] [PubMed]
- Blows, F.M.; Driver, K.E.; Schmidt, M.K.; Broeks, A.; Van Leeuwen, F.E.; Wesseling, J.; Cheang, M.C.; Gelmon, K.; Nielsen, T.O.; Blomqvist, C.; et al. Subtyping of breast cancer by immunohistochemistry to investigate a relationship between subtype and short and long term survival: A collaborative analysis of data for 10,159 cases from 12 studies. PLoS Med. 2010, 7, e1000279. [Google Scholar] [CrossRef] [PubMed]
- Dent, R.; Hanna, W.M.; Trudeau, M.; Rawlinson, E.; Sun, P.; Narod, S.A. Pattern of metastatic spread in triple-negative breast cancer. Breast Cancer Res. Treat. 2009, 115, 423–428. [Google Scholar] [CrossRef]
- Tseng, L.; Hsu, N.; Chen, S.; Lu, Y.; Lin, C.; Chang, D.; Li, H.; Lin, Y.; Chang, H.; Chao, T.; et al. Distant metastasis in triple-negative breast cancer. Neoplasma 2013, 60, 290–294. [Google Scholar] [CrossRef]
- Kozlowski, J.; Kozlowska, A.; Kocki, J. Breast cancer metastasis-insight into selected molecular mechanisms of the phenomenon. Postepy Hig. I Med. Dosw. 2015, 69, 447–451. [Google Scholar] [CrossRef]
- Wei, S.C.; Fattet, L.; Yang, J. The forces behind EMT and tumor metastasis. Cell Cycle 2015, 14, 2387. [Google Scholar] [CrossRef]
- Hunter, K.W.; Crawford, N.P.; Alsarraj, J. Mechanisms of metastasis. Breast Cancer Res. 2008, 10, S2. [Google Scholar] [CrossRef]
- Talmadge, J.E.; Fidler, I.J. AACR centennial series: The biology of cancer metastasis: Historical perspective. Cancer Res. 2010, 70, 5649–5669. [Google Scholar] [CrossRef]
- Weigelt, B.; Peterse, J.L.; Van’t Veer, L.J. Breast cancer metastasis: Markers and models. Nat. Rev. Cancer 2005, 5, 591. [Google Scholar] [CrossRef] [PubMed]
- Fokstuen, T.; Wilking, N.; Rutqvist, L.E.; Wolke, J.; Liedberg, A.; Signomklao, T.; Fernberg, J.-O. Radiation therapy in the management of brain metastases from breast cancer. Breast Cancer Res. Treat. 2000, 62, 211–216. [Google Scholar] [CrossRef] [PubMed]
- Evans, A.; James, J.; Cornford, E.; Chan, S.; Burrell, H.; Pinder, S.; Gutteridge, E.; Robertson, J.; Hornbuckle, J.; Cheung, K. Brain metastases from breast cancer: Identification of a high-risk group. Clin. Oncol. 2004, 16, 345–349. [Google Scholar] [CrossRef] [PubMed]
- Gounder, M.M.; Spriggs, D.R. Inclusion of patients with brain metastases in phase I trials: An unmet need. Clin. Cancer Res. 2011, 17, 3855–3857. [Google Scholar] [CrossRef]
- Boogerd, W.; Vos, V.; Hart, A.; Baris, G. Brain metastases in breast cancer; natural history, prognostic factors and outcome. J. Neuro Oncol. 1993, 15, 165–174. [Google Scholar] [CrossRef]
- Clark, E.A.; Brugge, J.S. Integrins and signal transduction pathways: The road taken. Science 1995, 268, 233–239. [Google Scholar] [CrossRef] [PubMed]
- Diaz, L.K.; Cristofanilli, M.; Zhou, X.; Welch, K.L.; Smith, T.L.; Yang, Y.; Sneige, N.; Sahin, A.A.; Gilcrease, M.Z. β4 integrin subunit gene expression correlates with tumor size and nuclear grade in early breast cancer. Mod. Pathol. 2005, 18, 1165. [Google Scholar] [CrossRef] [PubMed]
- Friedrichs, K.; Ruiz, P.; Franke, F.; Gille, I.; Terpe, H.-J.; Imhof, B.A. High expression level of α6 integrin in human breast carcinoma is correlated with reduced survival. Cancer Res. 1995, 55, 901–906. [Google Scholar]
- Jones, J.; Royall, J.; Critchley, D.; Walker, R. Modulation of myoepithelial-associated α6β4 integrin in a breast cancer cell line alters invasive potential. Exp. Cell Res. 1997, 235, 325–333. [Google Scholar] [CrossRef]
- Bolós, V.; Gasent, J.M.; López-Tarruella, S.; Grande, E. The dual kinase complex FAK-Src as a promising therapeutic target in cancer. OncoTargets Ther. 2010, 3, 83. [Google Scholar] [CrossRef]
- Cary, L.A.; Guan, J.-L. Focal adhesion kinase in integrin-mediated signaling. Front. Biosci. 1999, 4, D102–D113. [Google Scholar] [CrossRef]
- Zhao, X.; Guan, J.-L. Focal adhesion kinase and its signaling pathways in cell migration and angiogenesis. Adv. Drug Deliv. Rev. 2011, 63, 610–615. [Google Scholar] [CrossRef]
- Cary, L.A.; Chang, J.F.; Guan, J.-L. Stimulation of cell migration by overexpression of focal adhesion kinase and its association with Src and Fyn. J. Cell Sci. 1996, 109, 1787–1794. [Google Scholar] [CrossRef] [PubMed]
- Mitra, S.K.; Schlaepfer, D.D. Integrin-regulated FAK–Src signaling in normal and cancer cells. Curr. Opin. Cell Biol. 2006, 18, 516–523. [Google Scholar] [CrossRef]
- Wu, Y.; He, L.; Zhang, L.; Chen, J.; Yi, Z.; Zhang, J.; Liu, M.; Pang, X. Anacardic acid (6-pentadecylsalicylic acid) inhibits tumor angiogenesis by targeting Src/FAK/Rho GTPases signaling pathway. J. Pharmacol. Exp. Ther. 2011, 339, 403–411. [Google Scholar] [CrossRef] [PubMed]
- Trimmer, C.; Whitaker-Menezes, D.; Bonuccelli, G.; Milliman, J.N.; Daumer, K.M.; Aplin, A.E.; Pestell, R.G.; Sotgia, F.; Lisanti, M.P.; Capozza, F. CAV1 inhibits metastatic potential in melanomas through suppression of the integrin/Src/FAK signaling pathway. Cancer Res. 2010, 70, 7489–7499. [Google Scholar] [CrossRef] [PubMed]
- Gupta, P.; Gupta, N.; Fofaria, N.M.; Ranjan, A.; Srivastava, S.K. HER2-mediated GLI2 stabilization promotes anoikis resistance and metastasis of breast cancer cells. Cancer Lett. 2019, 442, 68–81. [Google Scholar] [CrossRef] [PubMed]
- Gilmore, A.P. Anoikis. Cell Death Differ. 2005, 12, 1473. [Google Scholar] [CrossRef] [PubMed]
- Simpson, C.D.; Anyiwe, K.; Schimmer, A.D. Anoikis resistance and tumor metastasis. Cancer Lett. 2008, 272, 177–185. [Google Scholar] [CrossRef]
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef]
- Fantozzi, A.; Gruber, D.C.; Pisarsky, L.; Heck, C.; Kunita, A.; Yilmaz, M.; Meyer-Schaller, N.; Cornille, K.; Hopfer, U.; Bentires-Alj, M.; et al. VEGF-mediated angiogenesis links EMT-induced cancer stemness to tumor initiation. Cancer Res. 2014, 74, 1566–1575. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Adjei, A.A. Targeting angiogenesis in cancer therapy: Moving beyond vascular endothelial growth factor. Oncologist 2015, 20, 660. [Google Scholar] [CrossRef]
- Hicklin, D.J.; Ellis, L.M. Role of the vascular endothelial growth factor pathway in tumor growth and angiogenesis. J. Clin. Oncol. 2005, 23, 1011–1027. [Google Scholar] [CrossRef]
- Ashton, T.M.; Fokas, E.; Kunz-Schughart, L.A.; Folkes, L.K.; Anbalagan, S.; Huether, M.; Kelly, C.J.; Pirovano, G.; Buffa, F.M.; Hammond, E.M.; et al. The anti-malarial atovaquone increases radiosensitivity by alleviating tumour hypoxia. Nat. Commun. 2016, 7, 12308. [Google Scholar] [CrossRef]
- Xiang, M.; Kim, H.; Ho, V.T.; Walker, S.R.; Bar-Natan, M.; Anahtar, M.; Liu, S.; Toniolo, P.A.; Kroll, Y.; Jones, N.; et al. Gene expression-based discovery of atovaquone as a STAT3 inhibitor and anticancer agent. Blood 2016, 128, 1845–1853. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Liu, X.; Shan, W.; Liu, Q.; Wang, C.; Zheng, J.; Yao, H.; Tang, R.; Zheng, J. Anti-malarial atovaquone exhibits anti-tumor effects by inducing DNA damage in hepatocellular carcinoma. Am. J. Cancer Res. 2018, 8, 1697–1711. [Google Scholar]
- Chen, D.; Sun, X.; Zhang, X.; Cao, J. Targeting mitochondria by anthelmintic drug atovaquone sensitizes renal cell carcinoma to chemotherapy and immunotherapy. J. Biochem. Mol. Toxicol. 2018, 32, e22195. [Google Scholar] [CrossRef] [PubMed]
- Gupta, N.; Srivastava, S.K. Atovaquone: An Antiprotozoal Drug Suppresses Primary and Resistant Breast Tumor Growth by Inhibiting HER2/β-Catenin Signaling. Mol. Cancer Ther. 2019, 18, 1708–1720. [Google Scholar] [CrossRef] [PubMed]
- Tester, A.M.; Ruangpanit, N.; Anderson, R.L.; Thompson, E.W. MMP-9 secretion and MMP-2 activation distinguish invasive and metastatic sublines of a mouse mammary carcinoma system showing epithelial-mesenchymal transition traits. Clin. Exp. Metastasis 2000, 18, 553–560. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.-Y.; Lin, H.-H.; Tang, M.-J.; Wang, Y.-K. Vimentin contributes to epithelial-mesenchymal transition cancer cell mechanics by mediating cytoskeletal organization and focal adhesion maturation. Oncotarget 2015, 6, 15966. [Google Scholar] [CrossRef] [PubMed]
- Smart, C.E.; Morrison, B.J.; Saunus, J.M.; Vargas, A.C.; Keith, P.; Reid, L.; Wockner, L.; Amiri, M.A.; Sarkar, D.; Simpson, P.T.; et al. In vitro analysis of breast cancer cell line tumourspheres and primary human breast epithelia mammospheres demonstrates inter-and intrasphere heterogeneity. PLoS ONE 2013, 8, e64388. [Google Scholar] [CrossRef]
- Prasad, S.; Ramachandran, S.; Gupta, N.; Kaushik, I.; Srivastava, S.K. Cancer cells stemness: A doorstep to targeted therapy. Biochim. Biophys. Acta Mol. Basis Dis. 2019, 1866, 165424. [Google Scholar] [CrossRef]
- Wang, R.; Lv, Q.; Meng, W.; Tan, Q.; Zhang, S.; Mo, X.; Yang, X. Comparison of mammosphere formation from breast cancer cell lines and primary breast tumors. J. Thorac. Dis. 2014, 6, 829. [Google Scholar] [PubMed]
- Reynolds, D.S.; Tevis, K.M.; Blessing, W.A.; Colson, Y.L.; Zaman, M.H.; Grinstaff, M.W. Breast Cancer Spheroids Reveal a Differential Cancer Stem Cell Response to Chemotherapeutic Treatment. Sci. Rep. 2017, 7, 10382. [Google Scholar] [CrossRef] [PubMed]
- Iglesias, J.M.; Beloqui, I.; Garcia-Garcia, F.; Leis, O.; Vazquez-Martin, A.; Eguiara, A.; Cufi, S.; Pavon, A.; Menendez, J.A.; Dopazo, J.; et al. Mammosphere formation in breast carcinoma cell lines depends upon expression of E-cadherin. PLoS ONE 2013, 8, e77281. [Google Scholar]
- Fofaria, N.M.; Srivastava, S.K. STAT3 induces anoikis resistance, promotes cell invasion and metastatic potential in pancreatic cancer cells. Carcinogenesis 2014, 36, 142–150. [Google Scholar] [CrossRef]
- Tao, K.; Fang, M.; Alroy, J.; Sahagian, G.G. Imagable 4T1 model for the study of late stage breast cancer. BMC Cancer 2008, 8, 228. [Google Scholar] [CrossRef]
- Folkman, J. Role of angiogenesis in tumor growth and metastasis. Semin. Oncol. 2002, 29, 15–18. [Google Scholar] [CrossRef]
- Xu, H.; Eirew, P.; Mullaly, S.C.; Aparicio, S. The omics of triple-negative breast cancers. Clin. Chem. 2014, 60, 122–133. [Google Scholar] [CrossRef]
- Bon, G.; Folgiero, V.; Di Carlo, S.; Sacchi, A.; Falcioni, R. Involvement of α 6 β 4 integrin in the mechanisms that regulate breast cancer progression. Breast Cancer Res. 2007, 9, 203. [Google Scholar] [CrossRef]
- Lu, S.; Simin, K.; Khan, A.; Mercurio, A.M. Analysis of integrin β4 expression in human breast cancer: Association with basal-like tumors and prognostic significance. Clin. Cancer Res. 2008, 14, 1050–1058. [Google Scholar] [CrossRef]
- van Nimwegen, M.J.; van de Water, B. Focal adhesion kinase: A potential target in cancer therapy. Biochem. Pharmacol. 2007, 73, 597–609. [Google Scholar] [CrossRef]
- Finn, R. Targeting Src in breast cancer. Ann. Oncol. 2008, 19, 1379–1386. [Google Scholar] [CrossRef]
- Deryugina, E.I.; Quigley, J.P. Matrix metalloproteinases and tumor metastasis. Cancer Metastasis Rev. 2006, 25, 9–34. [Google Scholar] [CrossRef]
- Kim, C.G.; Lee, H.; Gupta, N.; Ramachandran, S.; Kaushik, I.; Srivastava, S.; Kim, S.-H.; Srivastava, S.K. Role of Forkhead Box Class O proteins in cancer progression and metastasis. Semin. Cancer Biol. 2018, 50, 142–151. [Google Scholar] [CrossRef]
- Messica, Y.; Laser-Azogui, A.; Volberg, T.; Elisha, Y.; Lysakovskaia, K.; Eils, R.; Gladilin, E.; Geiger, B.; Beck, R. The role of vimentin in regulating cell invasive migration in dense cultures of breast carcinoma cells. Nano Lett. 2017, 17, 6941–6948. [Google Scholar] [CrossRef] [PubMed]
- Eccles, S.A.; Welch, D.R. Metastasis: Recent discoveries and novel treatment strategies. Lancet 2007, 369, 1742–1757. [Google Scholar] [CrossRef]
- Frisch, S.M.; Francis, H. Disruption of epithelial cell-matrix interactions induces apoptosis. J. Cell Biol. 1994, 124, 619–626. [Google Scholar] [CrossRef] [PubMed]
- Bielecka, Z.F.; Maliszewska-Olejniczak, K.; Safir, I.J.; Szczylik, C.; Czarnecka, A.M. Three-dimensional cell culture model utilization in cancer stem cell research. Biol. Rev. 2017, 92, 1505–1520. [Google Scholar] [CrossRef]
- Gupta, P.; Srivastava, S.K. Inhibition of HER2-integrin signaling by Cucurbitacin B leads to in vitro and in vivo breast tumor growth suppression. Oncotarget 2014, 5, 1812. [Google Scholar] [CrossRef] [PubMed]
- Lal, S.; Kersch, C.; Beeson, K.A.; Wu, Y.J.; Muldoon, L.L.; Neuwelt, E.A. Interactions between αv-integrin and HER2 and their role in the invasive phenotype of breast cancer cells in vitro and in rat brain. PLoS ONE 2015, 10, e0131842. [Google Scholar] [CrossRef] [PubMed]
- Gupta, N.; Gupta, P.; Srivastava, S.K. Penfluridol overcomes paclitaxel resistance in metastatic breast cancer. Sci. Rep. 2019, 9, 5066. [Google Scholar] [CrossRef] [PubMed]
- Ranjan, A.; Gupta, P.; Srivastava, S.K. Penfluridol: An antipsychotic agent suppresses metastatic tumor growth in triple-negative breast cancer by inhibiting integrin signaling axis. Cancer Res. 2016, 76, 877–890. [Google Scholar] [CrossRef]
- Verma, K.; Gupta, N.; Zang, T.; Wangtrakluldee, P.; Srivastava, S.K.; Penning, T.M.; Trippier, P.C. AKR1C3 Inhibitor KV-37 Exhibits Antineoplastic Effects and Potentiates Enzalutamide in Combination Therapy in Prostate Adenocarcinoma Cells. Mol. Cancer Ther. 2018, 17, 1833–1845. [Google Scholar] [CrossRef] [PubMed]
- Verma, K.; Zang, T.; Gupta, N.; Penning, T.M.; Trippier, P.C. Selective AKR1C3 inhibitors potentiate chemotherapeutic activity in multiple acute myeloid leukemia (AML) cell lines. ACS Med. Chem. Lett. 2016, 7, 774–779. [Google Scholar] [CrossRef]
- Lombardo, Y.; de Giorgio, A.; Coombes, C.R.; Stebbing, J.; Castellano, L. Mammosphere formation assay from human breast cancer tissues and cell lines. J. Vis. Exp. JoVE 2015, 97, 52671. [Google Scholar] [CrossRef]
- Kandala, P.K.; Srivastava, S.K. Diindolylmethane-mediated Gli1 protein suppression induces anoikis in ovarian cancer cells in vitro and blocks tumor formation ability in vivo. J. Biol. Chem. 2012, 287, 28745–28754. [Google Scholar] [CrossRef]
- Conley, F.K. Development of a metastatic brain tumor model in mice. Cancer Res. 1979, 39, 1001–1007. [Google Scholar]
- Gupta, P.; Adkins, C.; Lockman, P.; Srivastava, S.K. Metastasis of breast tumor cells to brain is suppressed by phenethyl isothiocyanate in a novel in vivo metastasis model. PLoS ONE 2013, 8, e67278. [Google Scholar] [CrossRef] [PubMed]
- Boreddy, S.R.; Sahu, R.P.; Srivastava, S.K. Benzyl isothiocyanate suppresses pancreatic tumor angiogenesis and invasion by inhibiting HIF-alpha/VEGF/Rho-GTPases: Pivotal role of STAT-3. PLoS ONE 2011, 6, e25799. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gupta, N.; Srivastava, S.K. Atovaquone Suppresses the Growth of Metastatic Triple-Negative Breast Tumors in Lungs and Brain by Inhibiting Integrin/FAK Signaling Axis. Pharmaceuticals 2021, 14, 521. https://doi.org/10.3390/ph14060521
Gupta N, Srivastava SK. Atovaquone Suppresses the Growth of Metastatic Triple-Negative Breast Tumors in Lungs and Brain by Inhibiting Integrin/FAK Signaling Axis. Pharmaceuticals. 2021; 14(6):521. https://doi.org/10.3390/ph14060521
Chicago/Turabian StyleGupta, Nehal, and Sanjay K. Srivastava. 2021. "Atovaquone Suppresses the Growth of Metastatic Triple-Negative Breast Tumors in Lungs and Brain by Inhibiting Integrin/FAK Signaling Axis" Pharmaceuticals 14, no. 6: 521. https://doi.org/10.3390/ph14060521
APA StyleGupta, N., & Srivastava, S. K. (2021). Atovaquone Suppresses the Growth of Metastatic Triple-Negative Breast Tumors in Lungs and Brain by Inhibiting Integrin/FAK Signaling Axis. Pharmaceuticals, 14(6), 521. https://doi.org/10.3390/ph14060521






