A Skin Cancer Prophylaxis Study in Hairless Mice Using Methylene Blue, Riboflavin, and Methyl Aminolevulinate as Photosensitizing Agents in Photodynamic Therapy
Abstract
1. Introduction
2. Results
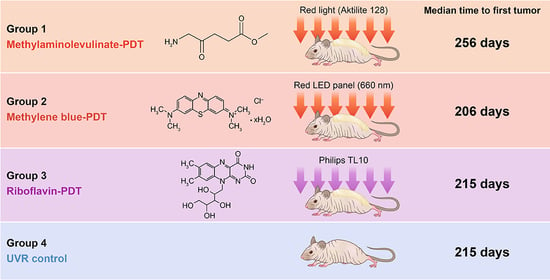
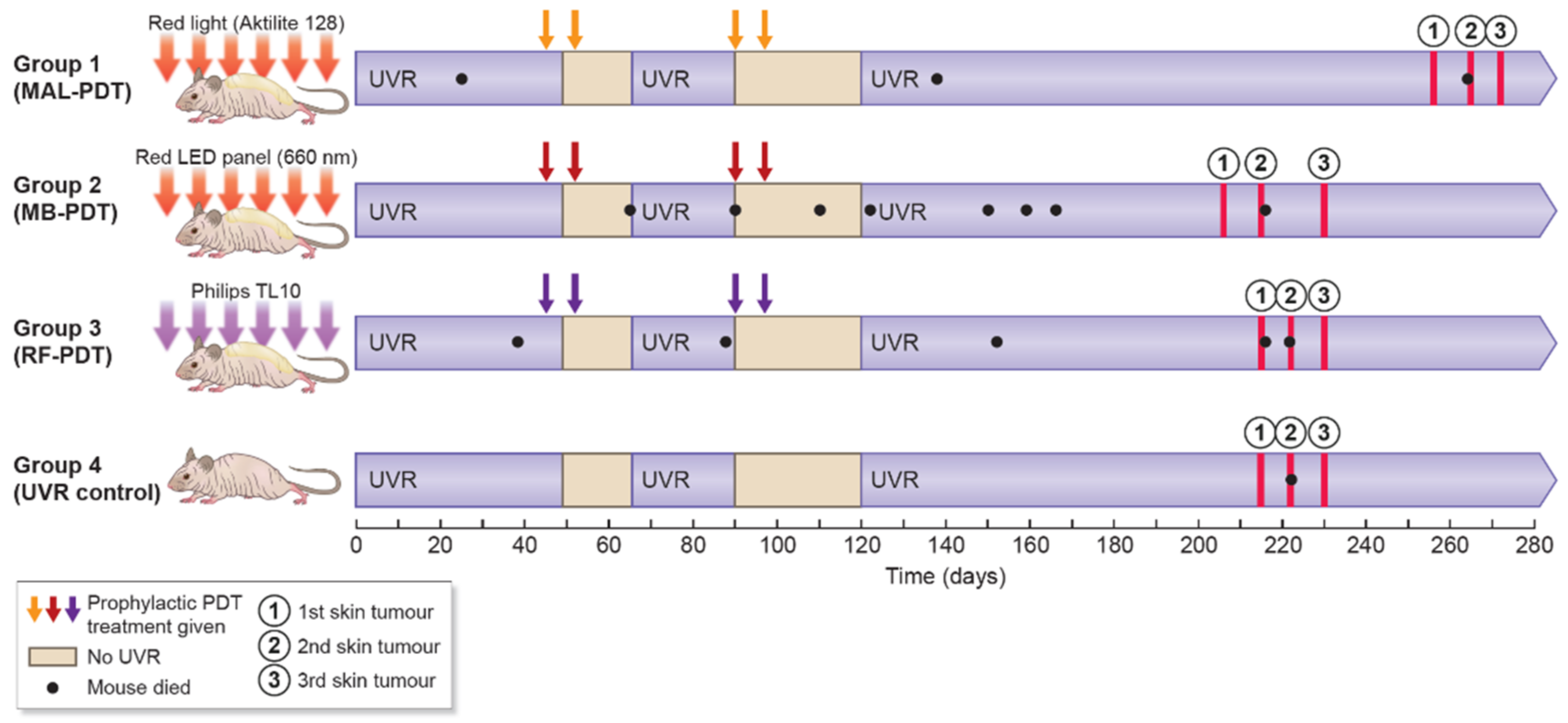
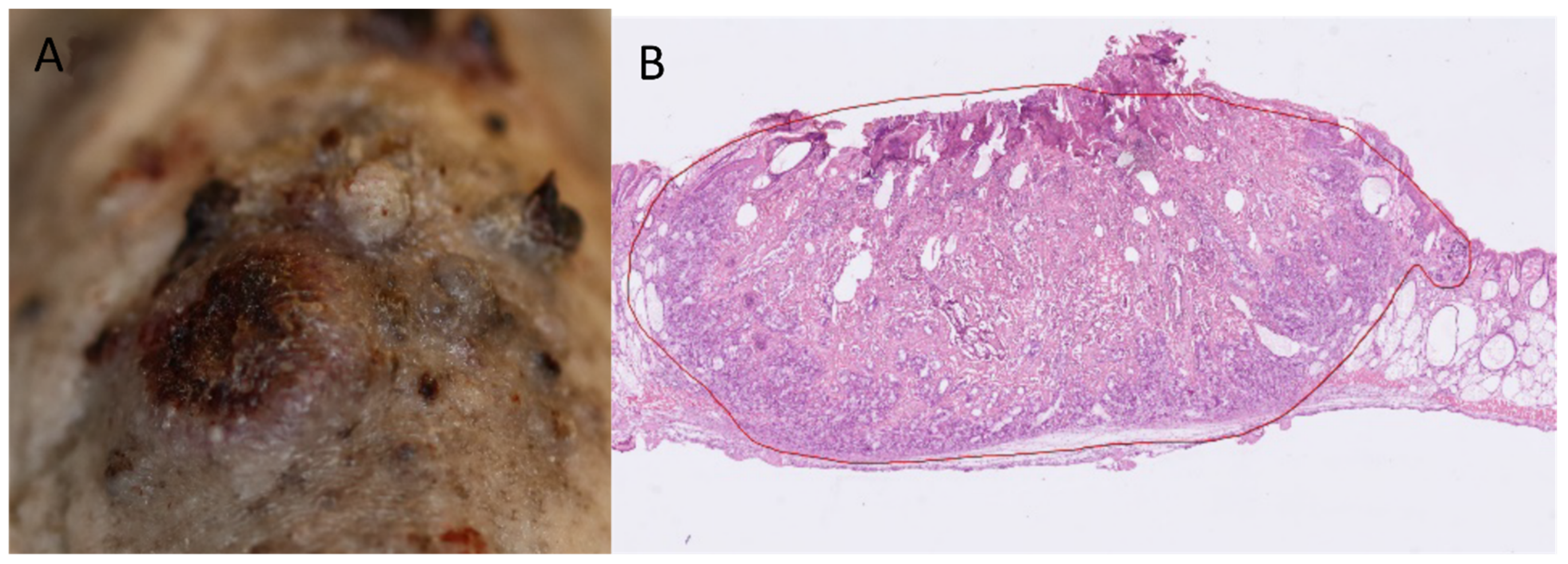
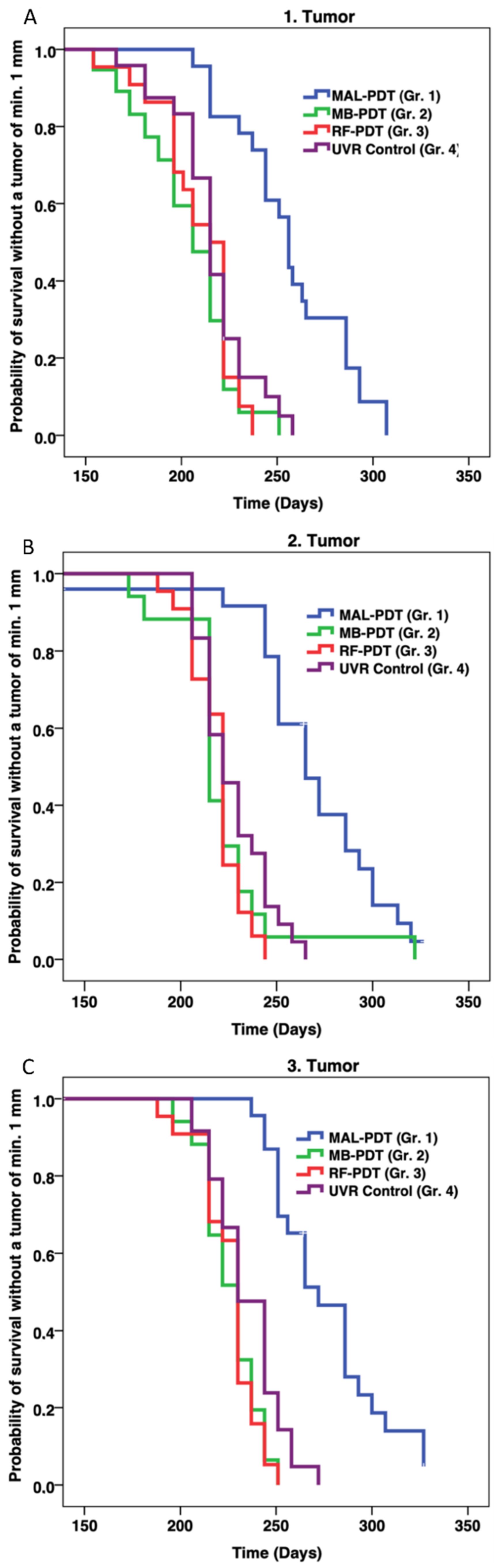
2.1. Efficacy of Prophylactic PDT
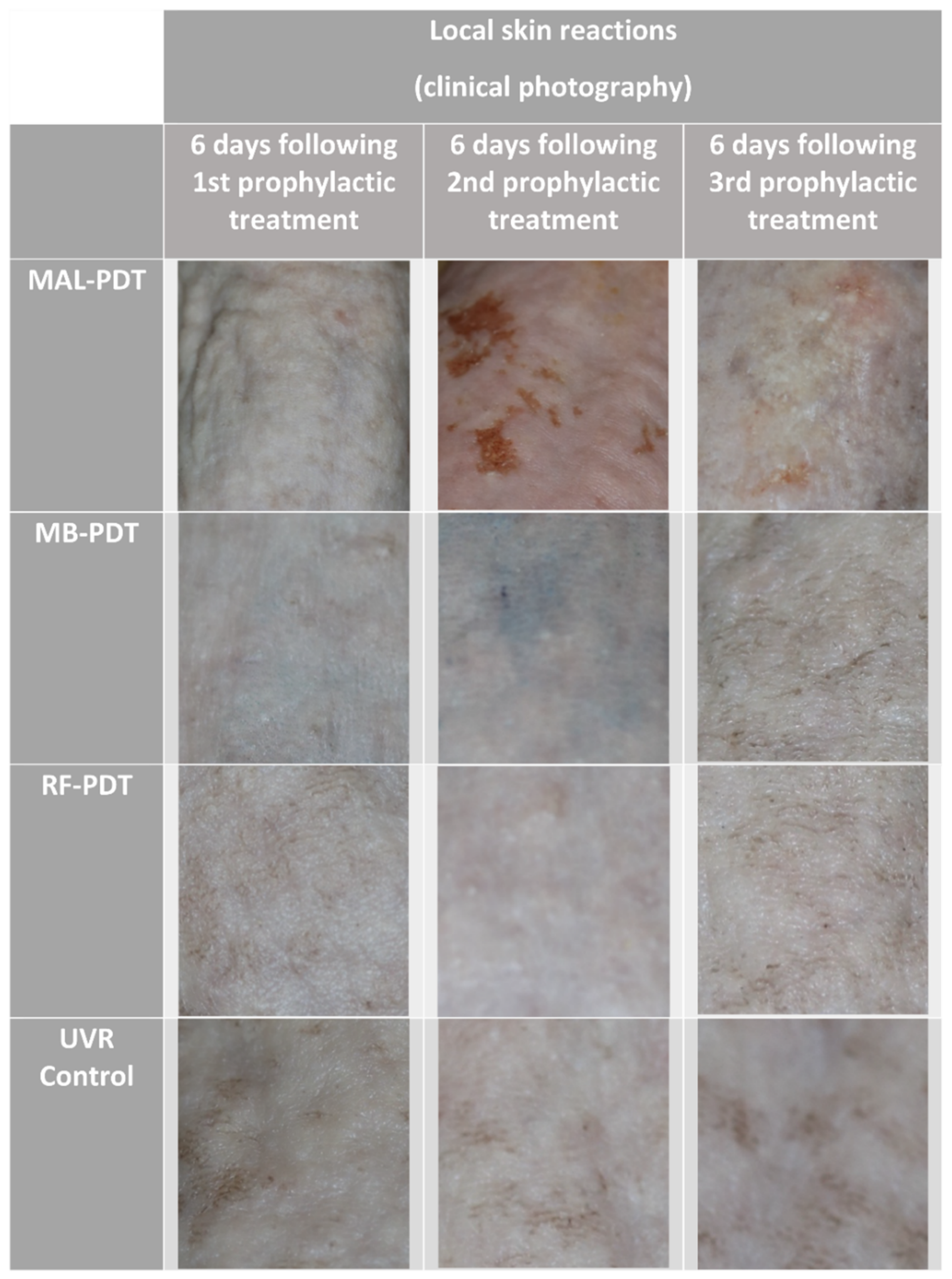
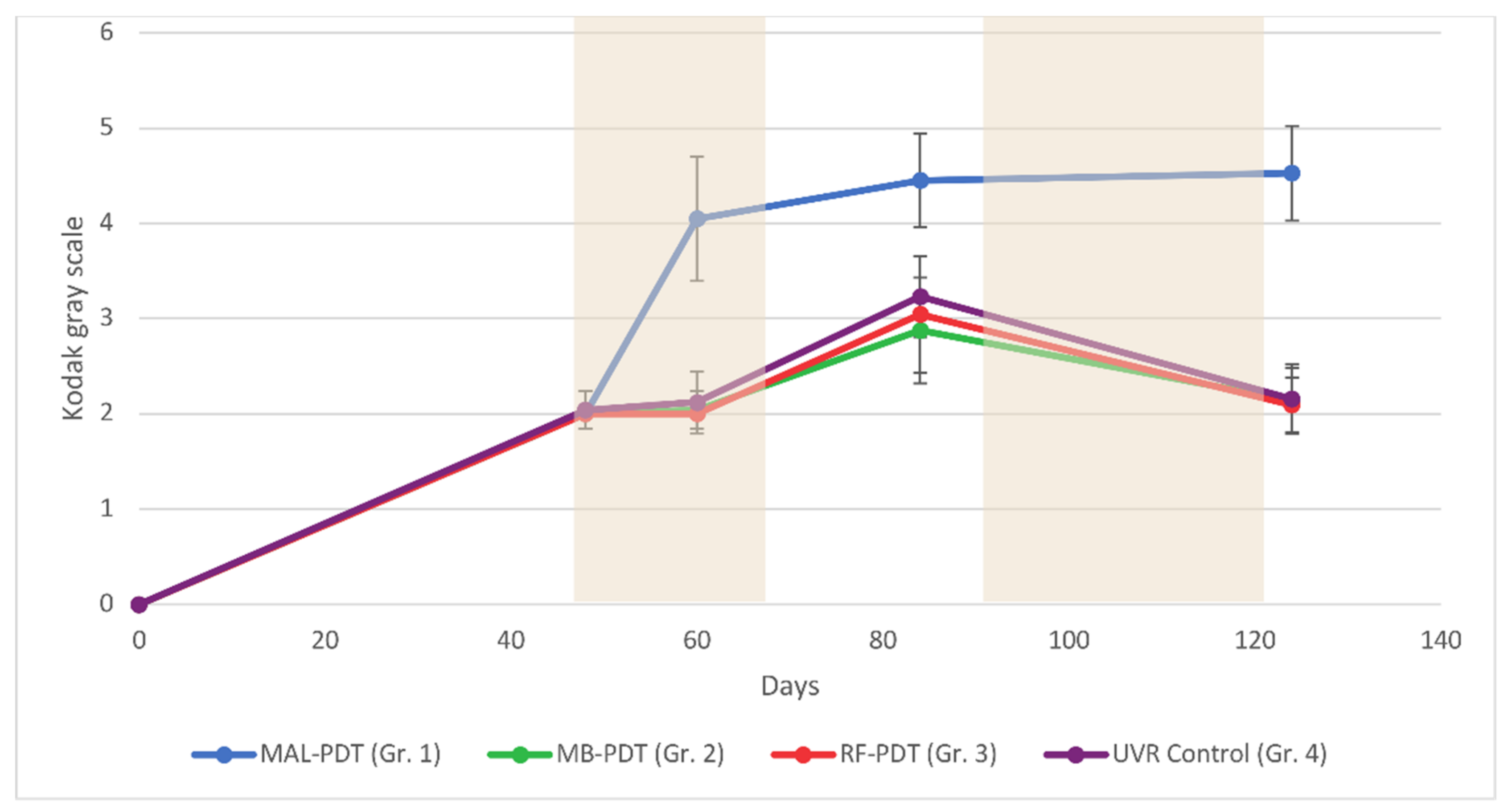
2.2. Pigmentation
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Study Design
4.3. UVR Exposure
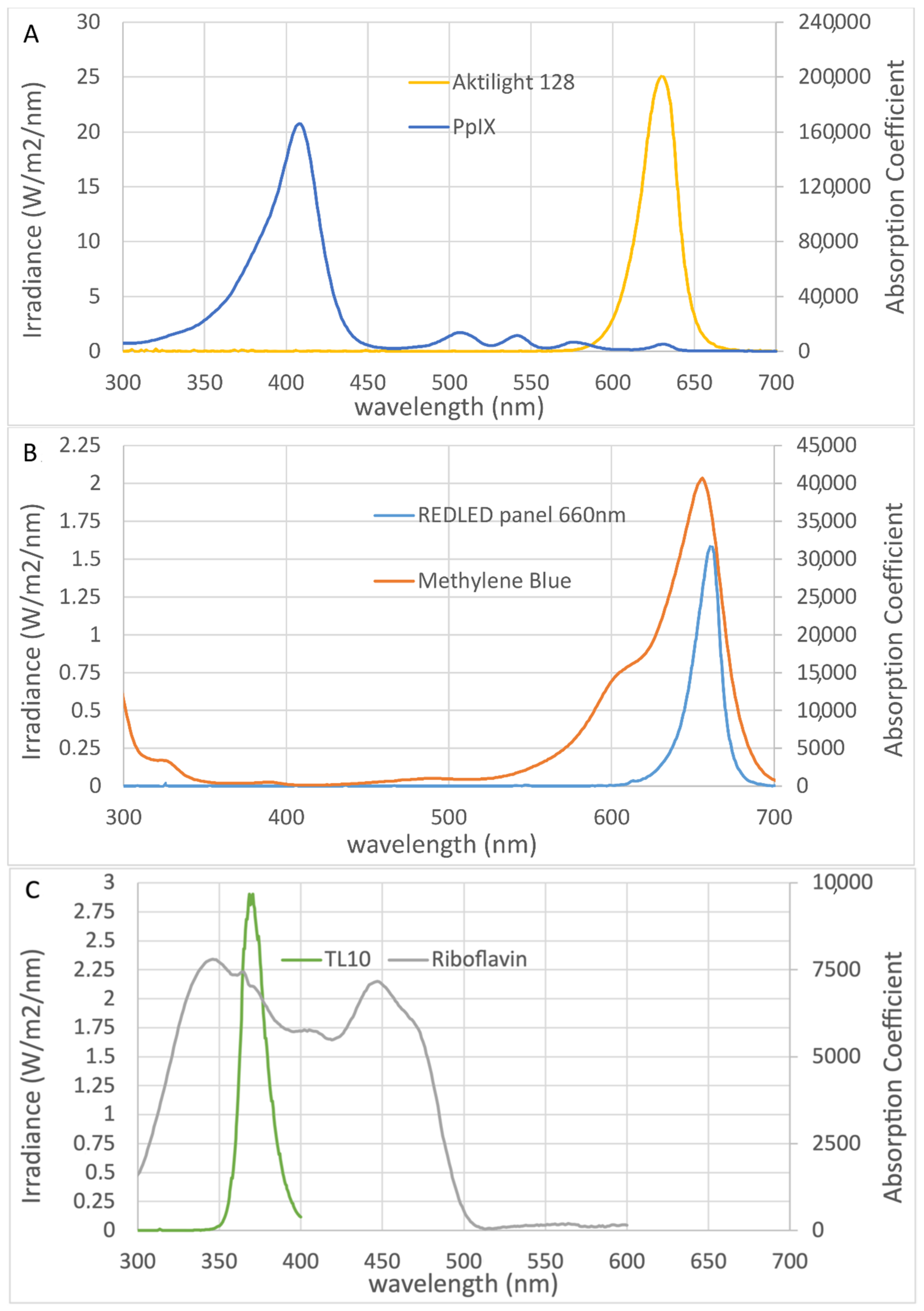
4.4. Photosensitizers
4.5. Statistics
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Abrahamse, H.; Hamblin, M.R. New photossensitizers for photodynamic therapy. Biochem. J. 2017, 473, 347–364. [Google Scholar] [CrossRef]
- Ozog, D.M.; Rkein, A.M.; Fabi, S.G.; Gold, M.H.; Goldman, M.P.; Lowe, N.J.; Martin, G.M.; Munavalli, G.S. Photodynamic Therapy: A Clinical Consensus Guide. Dermatol. Surg. 2016, 42, 804–827. [Google Scholar] [CrossRef] [PubMed]
- Akasov, R.A.; Sholina, N.V.; Khochenkov, D.A.; Alova, A.V.; Gorelkin, P.V.; Erofeev, A.S.; Generalova, A.N.; Khaydukov, E.V. Photodynamic therapy of melanoma by blue-light photoactivation of flavin mononucleotide. Sci. Rep. 2019, 9, 9679. [Google Scholar] [CrossRef] [PubMed]
- Morton, C.; Campbell, S.; Gupta, G.; Keohane, S.; Lear, J.; Zaki, I.; Walton, S.; Kerrouche, N.; Thomas, G.; Soto, P. Intraindividual, right-left comparison of topical methyl aminolaevulinate-photodynamic therapy and cryotherapy in subjects with actinic keratoses: A multicentre, randomized controlled study. Br. J. Dermatol. 2006, 155, 1029–1036. [Google Scholar] [CrossRef]
- Hauschild, A.; Popp, G.; Stockfleth, E.; Meyer, K.G.; Imberger, D.; Mohr, P.; Itschert, G.; Kaufmann, R.; Neuber, K.; Frambach, Y.; et al. Effective photodynamic therapy of actinic keratoses on the head and face with a novel, self-adhesive 5-aminolaevulinic acid patch. Exp. Dermatol. 2009, 18, 116–121. [Google Scholar] [CrossRef]
- Horn, M.; Wolf, P.; Wulf, H.C.; Warloe, T.; Fritsch, C.; Rhodes, L.E.; Kaufmann, R.; De Rie, M.; Legat, F.J.; Stender, I.M.; et al. Topical methyl aminolaevulinate photodynamic therapy in patients with basal cell carcinoma prone to complications and poor cosmetic outcome with conventional treatment. Br. J. Dermatol. 2003, 149, 1242–1249. [Google Scholar] [CrossRef] [PubMed]
- Braathen, L.R.; Szeimies, R.M.; Basset-Seguin, N.; Bissonnette, R.; Foley, P.; Pariser, D.; Roelandts, R.; Wennberg, A.M.; Morton, C.A. Guidelines on the use of photodynamic therapy for nonmelanoma skin cancer: An international consensus. J. Am. Acad. Dermatol. 2007, 56, 125–143. [Google Scholar] [CrossRef] [PubMed]
- Soler, A.M.; Warloe, T.; Berner, A.; Giercksky, K.E. A follow-up study of recurrence and cosmesis in completely responding superficial and nodular basal cell carcinomas treated with methyl 5-aminolaevulinate-based photodynamic therapy alone and with prior curettage. Br. J. Dermatol. 2001, 145, 467–471. [Google Scholar] [CrossRef]
- Szeimies, R.M.; Ibbotson, S.; Murrell, D.F.; Rubel, D.; Frambach, Y.; De Berker, D.; Dummer, R.; Kerrouche, N.; Villemagne, H. A clinical study comparing methyl aminolevulinate photodynamic therapy and surgery in small superficial basal cell carcinoma (8–20 mm), with a 12-month follow-up. J. Eur. Acad. Dermatol. Venereol. 2008, 22, 1302–1311. [Google Scholar] [CrossRef] [PubMed]
- Rhodes, L.E.; De Rie, M.; Enström, Y.; Groves, R.; Morken, T.; Goulden, V.; Wong, G.A.E.; Grob, J.J.; Varma, S.; Wolf, P. Photodynamic Therapy Using Topical Methyl Aminolevulinate vs Surgery for Nodular Basal Cell Carcinoma: Results of a Multicenter Randomized Prospective Trial. Arch. Dermatol. 2004, 140, 17–23. [Google Scholar] [CrossRef] [PubMed]
- Vinciullo, C.; Elliott, T.; Francis, D.; Gebauer, K.; Spelman, L.; Nguyen, R.; Weightman, W.; Sheridan, A.; Reid, C.; Czarnecki, D.; et al. Photodynamic therapy with topical methyl aminolaevulinate for “difficult-to-treat” basal cell carcinoma. Br. J. Dermatol. 2005, 152, 765–772. [Google Scholar] [CrossRef] [PubMed]
- Khaydukov, E.V.; Mironova, K.E.; Semchishen, V.A.; Generalova, A.N.; Nechaev, A.V.; Khochenkov, D.A.; Stepanova, E.V.; Lebedev, O.I.; Zvyagin, A.V.; Deyev, S.M.; et al. Riboflavin photoactivation by upconversion nanoparticles for cancer treatment. Sci. Rep. 2016, 6, 35103. [Google Scholar] [CrossRef] [PubMed]
- Silva, A.P.; Neves, C.L.; Silva, E.D.A.; Portela, T.C.L.; Iunes, R.S.; Cogliati, B.; Severino, D.; Baptista, M.D.S.; Dagli, M.L.Z.; Blazquez, F.J.H.; et al. Effects of methylene blue-mediated photodynamic therapy on a mouse model of squamous cell carcinoma and normal skin. Photodiagn. Photodyn. Ther. 2018, 23, 154–164. [Google Scholar] [CrossRef] [PubMed]
- Reis, J.S.; Corrêa, M.A.; Chung, M.C.; Dos Santos, J.L. Synthesis, antioxidant and photoprotection activities of hybrid derivatives useful to prevent skin cancer. Bioorg. Med. Chem. 2014, 22, 2733–2738. [Google Scholar] [CrossRef]
- Kim, I.Y.; He, Y.Y. Ultraviolet radiation-induced non-melanoma skin cancer: Regulation of DNA damage repair and inflammation. Genes Dis. 2014, 1, 188–198. [Google Scholar] [CrossRef]
- Lerche, C.M.; Philipsen, P.A.; Poulsen, T.; Wulf, H.C. Topical pimecrolimus and tacrolimus do not accelerate photocarcinogenesis in hairless mice after UVA or simulated solar radiation. Exp. Dermatol. 2009, 18, 246–251. [Google Scholar] [CrossRef]
- Xiong, Z.M.; O’Donovan, M.; Sun, L.; Young Choi, J.; Ren, M.; Cao, K. Anti-Aging Potentials of Methylene Blue for Human Skin Longevity. Sci. Rep. 2017, 7, 2475. [Google Scholar] [CrossRef]
- Damian, D.L.; Matthews, Y.J.; Halliday, G.M. Topical riboflavin attenuates ultraviolet B- and ultraviolet A-induced immunosuppression in humans. Photodermatol. Photoimmunol. Photomed. 2010, 26, 66–69. [Google Scholar] [CrossRef]
- Chen, X.; Shah, D.; Kositratna, G.; Manstein, D.; Anderson, R.R.; Wu, M.X. Facilitation of transcutaneous drug delivery and vaccine immunization by a safe laser technology. J. Control. Release 2012, 159, 43–51. [Google Scholar] [CrossRef]
- Johnson, P.G.; Gallo, S.A.; Hui, S.W.; Oseroff, A.R. A pulsed electric field enhances cutaneous delivery of methylene blue in excised full-thickness porcine skin. J. Investig. Dermatol. 1998, 111, 457–463. [Google Scholar] [CrossRef][Green Version]
- Gillman, P.K. Methylene blue implicated in potentially fatal serotonin toxicity. Anaesthesia 2006, 61, 1013–1014. [Google Scholar] [CrossRef]
- Stender, I.M.; Bech-Thomsen, N.; Poulsen, T.; Wulf, H.C. Photodynamic Therapy with Topical δ-aminolevulinic Acid Delays UV Photocarcinogenesis in Hairless Mice. Photochem. Photobiol. 1997, 66, 493–496. [Google Scholar] [CrossRef]
- Togsverd-Bo, K.; Lerche, C.M.; Poulsen, T.; Wulf, H.C.; Hædersdal, M. Photodynamic therapy with topical methyl- and hexylaminolevulinate for prophylaxis and treatment of UV-induced SCC in hairless mice. Exp. Dermatol. 2010, 19, e166–e172. [Google Scholar] [CrossRef]
- Lerche, C.M.; Togsverd-Bo, K.; Philipsen, P.A.; Wulf, H.C. Impact of UVR Exposure Pattern on Squamous Cell Carcinoma-A Dose-Delivery and Dose-Response Study in Pigmented Hairless Mice. Int. J. Mol. Sci. 2017, 18, 2738. [Google Scholar] [CrossRef]
- Del Bino, S.; Sok, J.; Bessac, E.; Bernerd, F. Relationship between skin response to ultraviolet exposure and skin color type. Pigment Cell Res. 2006, 19, 606–614. [Google Scholar] [CrossRef]
- Taniguchi, M.; Du, H.; Lindsey, J.S. PhotochemCAD 3: Diverse Modules for Photophysical Calculations with Multiple Spectral Databases. Photochem. Photobiol. 2018, 94, 277–289. [Google Scholar] [CrossRef]
- Taniguchi, M.; Lindsey, J.S. Database of Absorption and Fluorescence Spectra of >300 Common Compounds for use in PhotochemCAD. Photochem. Photobiol. 2018, 94, 290–327. [Google Scholar] [CrossRef]
- Diffey, B.L.; Jansen, C.T.; Urbach, F.; Wulf, H.C.; Morison, W.L. The standard erythema dose: A new photobiological concept. Photodermatol. Photoimmunol. Photomed. 1997, 13, 64–66. [Google Scholar] [CrossRef]
- Lerche, C.M.; Philipsen, P.A.; Wulf, H.C. UVR: Sun, lamps, pigmentation and Vitamin D. Photochem. Photobiol. Sci. 2017, 16, 291–301. [Google Scholar] [CrossRef]
| Group No. | 1 | 2 | 3 | 4 |
| No. of Mice (n) | 25 | 25 | 25 | 25 |
| No. of Mice (n) Dy-ing Before End-Point | 3 | 7 | 5 | 1 |
| Treatment | MAL-PDT p-value * | MB-PDT p-value * | RF-PDT p-value * | UVR Control |
| Median Days to 1st Tumor | 256 (237–286) | 206 (188–222) | 215 (196–222) | 215 (206–222) |
| Median Days to 2nd Tumor | 265 (251–293) | 215 (215–230) | 222 (206–222) | 222 (215–244) |
| Median Days to 3rd tumor | 272 (251–293) | 230 (215–237) | 230 (215–237) | 230 (222–244) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wulf, H.C.; Al-Chaer, R.N.; Glud, M.; Philipsen, P.A.; Lerche, C.M. A Skin Cancer Prophylaxis Study in Hairless Mice Using Methylene Blue, Riboflavin, and Methyl Aminolevulinate as Photosensitizing Agents in Photodynamic Therapy. Pharmaceuticals 2021, 14, 433. https://doi.org/10.3390/ph14050433
Wulf HC, Al-Chaer RN, Glud M, Philipsen PA, Lerche CM. A Skin Cancer Prophylaxis Study in Hairless Mice Using Methylene Blue, Riboflavin, and Methyl Aminolevulinate as Photosensitizing Agents in Photodynamic Therapy. Pharmaceuticals. 2021; 14(5):433. https://doi.org/10.3390/ph14050433
Chicago/Turabian StyleWulf, Hans Christian, Rami Nabil Al-Chaer, Martin Glud, Peter Alshede Philipsen, and Catharina Margrethe Lerche. 2021. "A Skin Cancer Prophylaxis Study in Hairless Mice Using Methylene Blue, Riboflavin, and Methyl Aminolevulinate as Photosensitizing Agents in Photodynamic Therapy" Pharmaceuticals 14, no. 5: 433. https://doi.org/10.3390/ph14050433
APA StyleWulf, H. C., Al-Chaer, R. N., Glud, M., Philipsen, P. A., & Lerche, C. M. (2021). A Skin Cancer Prophylaxis Study in Hairless Mice Using Methylene Blue, Riboflavin, and Methyl Aminolevulinate as Photosensitizing Agents in Photodynamic Therapy. Pharmaceuticals, 14(5), 433. https://doi.org/10.3390/ph14050433