Study of Cytotoxic and Photodynamic Activities of Dyads Composed of a Zinc Phthalocyanine Appended to an Organotin
Abstract
1. Introduction
2. Results and Discussion.
2.1. Synthesis of the Compounds
2.2. UV-Vis Electronic Absorption Properties
2.3. Infra-Red (IR) Spectroscopic Study
2.4. Fluorescence Spectroscopy
2.5. Singlet Oxygen Measurements
2.6. Biological Effect Analysis of ZnPc(EG3)4:Cytotoxicity, PDT and ROS Production
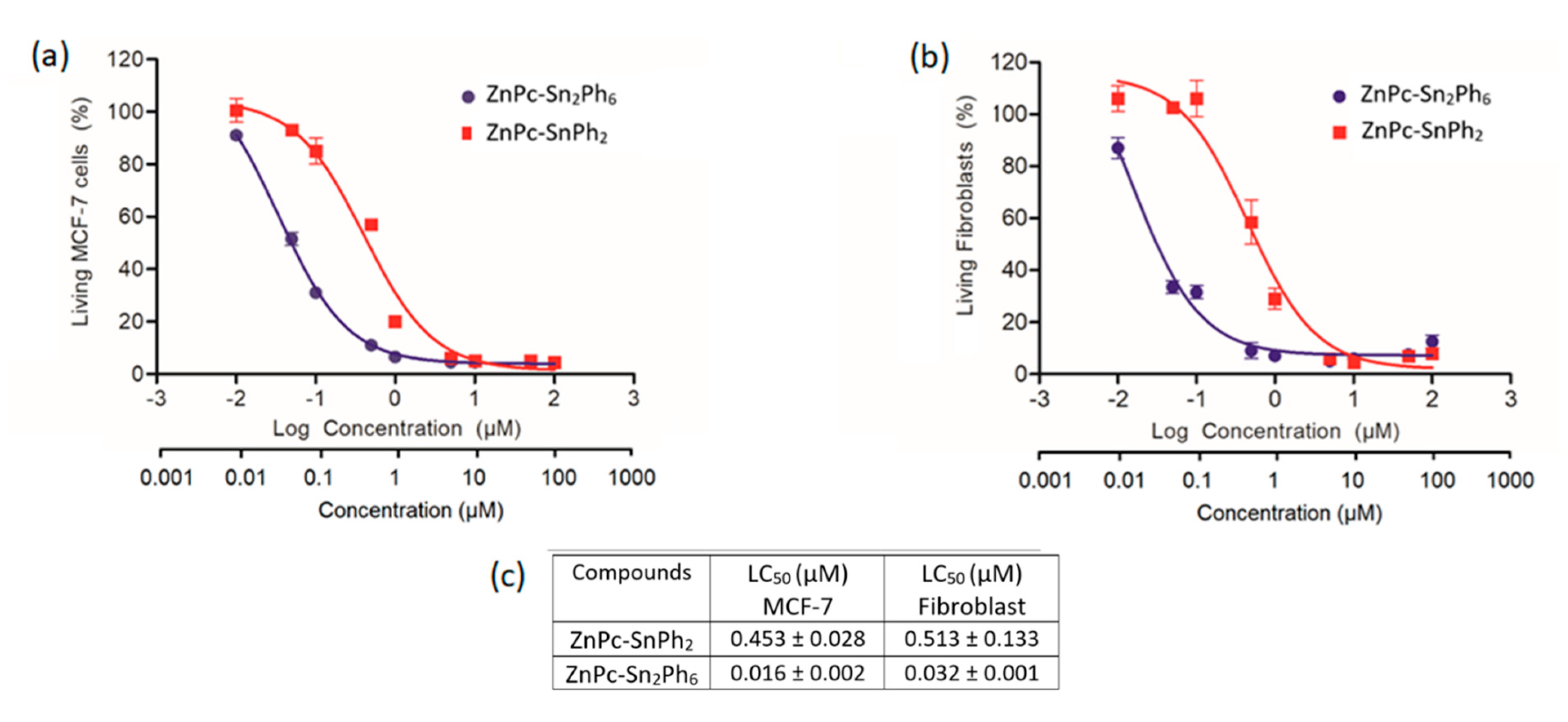
2.7. Cytotoxic Potential Analysis of the Dyads ZnPc-SnPh2 and ZnPc-Sn2Ph6 on Cancer and Healthy Cells
2.8. Photodynamic Therapy Investigations of Dyads
3. Materials and Methods
3.1. Synthesis of the Compounds
Generalities
3.2. Biological Experiments
3.2.1. Cell Culture
3.2.2. Cytotoxicity Study
3.2.3. Phototoxicity Assay at 650 nm
3.3. Statistical Analysis
3.4. Singlet Oxygen Quantum Yield
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Karges, J.; Yempala, T.; Tharaud, M.; Gibson, D.; Gasser, G. A Multi-action and Multi-target RuII–PtIV Conjugate Combining Cancer-Activated Chemotherapy and Photodynamic Therapy to Overcome Drug Resistant Cancers. Angew. Chem. Int. Ed. 2020, 59, 7069–7075. [Google Scholar] [CrossRef]
- Chen, Y.; Gao, Y.; Li, Y.; Wang, K.; Zhu, J. Synergistic chemo-photodynamic therapy mediated by light-activated ROS-degradable nanocarriers. J. Mater. Chem. B 2019, 7, 460–468. [Google Scholar] [CrossRef]
- Zuluaga, F.M.; Lange, N. Combination of Photodynamic Therapy With Anti-Cancer Agents. Curr. Med. Chem. 2008, 15, 1655–1673. [Google Scholar] [CrossRef]
- Li, X.; Lovell, J.F.; Yoon, J.; Chen, X. Clinical development and potential of photothermal and photodynamic therapies for cancer. Nat. Rev. Clin. Oncol. 2020, 17, 657–674. [Google Scholar] [CrossRef]
- Li, X.; Lee, S.; Yoon, J. Supramolecular photosensitizers rejuvenate photodynamic therapy. Chem. Soc. Rev. 2018, 47, 1174–1188. [Google Scholar] [CrossRef]
- Li, X.; Yu, S.; Lee, D.; Kim, G.; Lee, B.; Cho, Y.; Zheng, B.-Y.; Ke, M.-R.; Huang, J.-D.; Nam, K.T.; et al. Facile Supramolecular Approach to Nucleic-Acid-Driven Activatable Nanotheranostics That Overcome Drawbacks of Photodynamic Therapy. ACS Nano 2018, 12, 681–688. [Google Scholar] [CrossRef] [PubMed]
- Lau, J.T.; Lo, P.C.; Fong, W.P.; Ng, D.K. A zinc(II) phthalocyanine conjugated with an oxaliplatin derivative for dual chemo- and photodynamic therapy. J. Med. Chem. 2012, 55, 5446–5454. [Google Scholar] [CrossRef] [PubMed]
- Ryu, J.H.; Koo, H.; Sun, I.C.; Yuk, S.H.; Choi, K.; Kim, K.; Kwon, I.C. Tumor-targeting multi-functional nanoparticles for theragnosis: New paradigm for cancer therapy. Adv. Drug Deliv. Rev. 2012, 64, 1447–1458. [Google Scholar] [CrossRef] [PubMed]
- Lottner, C.; Bart, K.C.; Bernhardt, G.; Brunner, H. Hematoporphyrin-Derived Soluble Porphyrin-Platinum Conjugates with Combined Cytotoxic and Phototoxic Antitumor Activity. J. Med. Chem. 2002, 45, 2064–2078. [Google Scholar] [CrossRef] [PubMed]
- Lottner, C.; Bart, K.C.; Bernhardt, G.; Brunner, H. Soluble Tetraarylporphyrin-Platinum Conjugates as Cytotoxic and Phototoxic Antitumor Agents. J. Med. Chem. 2002, 45, 2079–2089. [Google Scholar] [CrossRef]
- Chung Enhanced apoptotic effect of combined modality of 9-hydroxypheophorbide α-mediated photodynamic therapy and carboplatin on AMC-HN-3 human head and neck cancer cells. Oncol. Rep. 1994, 21, 329–334.
- Rizvi, I.; Celli, J.P.; Evans, C.L.; Abu-Yousif, A.O.; Muzikansky, A.; Pogue, B.W.; Finkelstein, D.; Hasan, T. Synergistic enhancement of carboplatin efficacy with photodynamic therapy in a three-dimensional model for micrometastatic ovarian cancer. Cancer Res. 2010, 70, 9319–9328. [Google Scholar] [CrossRef] [PubMed]
- Kirveliene, V.; Grazeliene, G.; Dabkeviciene, D.; Micke, I.; Kirvelis, D.; Juodka, B.; Didziapetriene, J. Schedule-dependent interaction between Doxorubicin and mTHPC-mediated photodynamic therapy in murine hepatoma in vitro and in vivo. Cancer Chemother. Pharmacol. 2006, 57, 65–72. [Google Scholar] [CrossRef]
- Aniogo, E.C.; George, B.P.A.; Abrahamse, H. In vitro combined effect of Doxorubicin and sulfonated zinc Phthalocyanine-mediated photodynamic therapy on MCF-7 breast cancer cells. Tumour Biol. 2017, 39, 1010428317727278. [Google Scholar] [CrossRef] [PubMed]
- Ali, S.; Muhammad, S.; Khurshid, A.; Ikram, M.; Maqsood, M.; Fisher, C.; Cathcart, J.; Lilge, L. Effective phthalocyanines mediated photodynamic therapy with doxorubicin or methotrexate combination therapy at sub-micromolar concentrations in vitro. Photodiagnosis Photodyn. Ther. 2018, 22, 51–64. [Google Scholar] [CrossRef]
- Schmitt, F.; Govindaswamy, P.; Zava, O.; Suss-Fink, G.; Juillerat-Jeanneret, L.; Therrien, B. Combined arene ruthenium porphyrins as chemotherapeutics and photosensitizers for cancer therapy. J. Biol. Inorg. Chem. 2009, 14, 101–109. [Google Scholar] [CrossRef]
- Gianferrara, T.; Bergamo, A.; Bratsos, I.; Milani, B.; Spagnul, C.; Sava, G.; Alessio, E. Ruthenium-porphyrin conjugates with cytotoxic and phototoxic antitumor activity. J. Med. Chem. 2010, 53, 4678–4690. [Google Scholar] [CrossRef]
- Ruiz-Gonzalez, R.; Milan, P.; Bresoli-Obach, R.; Stockert, J.C.; Villanueva, A.; Canete, M.; Nonell, S. Photodynamic Synergistic Effect of Pheophorbide a and Doxorubicin in Combined Treatment against Tumoral Cells. Cancers 2017, 9, 18. [Google Scholar] [CrossRef]
- van Leeuwen, M.; Beeby, A.; Fernandes, I.; Ashworth, S.H. The photochemistry and photophysics of a series of alpha octa(alkyl-substituted) silicon, zinc and palladium phthalocyanines. Photochem. Photobiol. Sci. 2014, 13, 62–69. [Google Scholar] [CrossRef]
- Liu, J.Y.; Lo, P.C.; Fong, W.P.; Ng, D.K. Effects of the number and position of the substituents on the in vitro photodynamic activities of glucosylated zinc(II) phthalocyanines. Org. Biomol. Chem. 2009, 7, 1583–1591. [Google Scholar] [CrossRef] [PubMed]
- Zheng, B.-Y.; Zhang, H.-P.; Ke, M.-R.; Huang, J.-D. Synthesis and antifungal photodynamic activities of a series of novel zinc(II) phthalocyanines substituted with piperazinyl moieties. Dyes Pigment. 2013, 99, 185–191. [Google Scholar] [CrossRef]
- Durmus, M.; Nyokong, T. Synthesis, photophysical and photochemical studies of new water-soluble indium(III) phthalocyanines. Photochem. Photobiol. Sci. 2007, 6, 659–668. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zheng, B.-D.; Peng, X.-H.; Li, S.-Z.; Ying, J.-W.; Zhao, Y.; Huang, J.-D.; Yoon, J. Phthalocyanines as medicinal photosensitizers: Developments in the last five years. Coord. Chem. Rev. 2019, 379, 147–160. [Google Scholar] [CrossRef]
- Gómez-Ruiz, S.; Žižak, Ž.; Kaluđerović, G.N. Structural studies and cytotoxic activity against human cancer cell lines of mono and dinuclear tin(IV) complexes with the α,α′-dimercapto-o-xylene ligand. Inorg. Chim. Acta 2014, 423, 117–122. [Google Scholar] [CrossRef]
- Anjorin, C.; Song, X.; Kumar, D.; Pike, R.; De Dios, A.; Eng, G. Crystal structures of two polymeric bis(triphenyltin) malonates. J. Coord. Chem. 2010, 63, 4031–4040. [Google Scholar] [CrossRef]
- Thorpe, D.; Callejas, A.; Royzman, D.; Pike, R.D.; Eng, G.; Song, X. Synthesis and crystal structures of ionic triphenyltin complexes with oxalic and malonic acid. J. Coord. Chem. 2013, 66, 3647–3659. [Google Scholar] [CrossRef]
- Song, X.; Zapata, A.; Hoerner, J.; de Dios, A.C.; Casabianca, L.; Eng, G. Synthesis, larvicidal, QSAR and structural studies of some triorganotin 2,2,3,3-tetramethylcyclopropanecarboxylates. Appl. Organomet. Chem. 2007, 21, 545–550. [Google Scholar] [CrossRef]
- Davies, A.G. 6-Tin. In Comprehensive Organometallic Chemistry II; Abel, E.W., Stone, F.G.A., Wilkinson, G., Eds.; Elsevier: Oxford, UK, 1995; pp. 217–303. [Google Scholar]
- Foote, C.S.; Krasnovsky, A.A.; Fu, Y.; Selke, M.; Karney, W.L. Singlet Oxygen Dimol-Sensitized Luminescence and Reactions of Singlet Oxygen with Organometallics. In The Activation of Dioxygen and Homogeneous Catalytic Oxidation; Barton, D.H.R., Martell, A.E., Sawyer, D.T., Eds.; Springer: Boston, MA, USA, 1993; pp. 411–422. [Google Scholar]
- Li, Y.; Wang, J.; Zhang, X.; Guo, W.; Li, F.; Yu, M.; Kong, X.; Wu, W.; Hong, Z. Highly water-soluble and tumor-targeted photosensitizers for photodynamic therapy. Org. Biomol. Chem. 2015, 13, 7681–7694. [Google Scholar] [CrossRef]
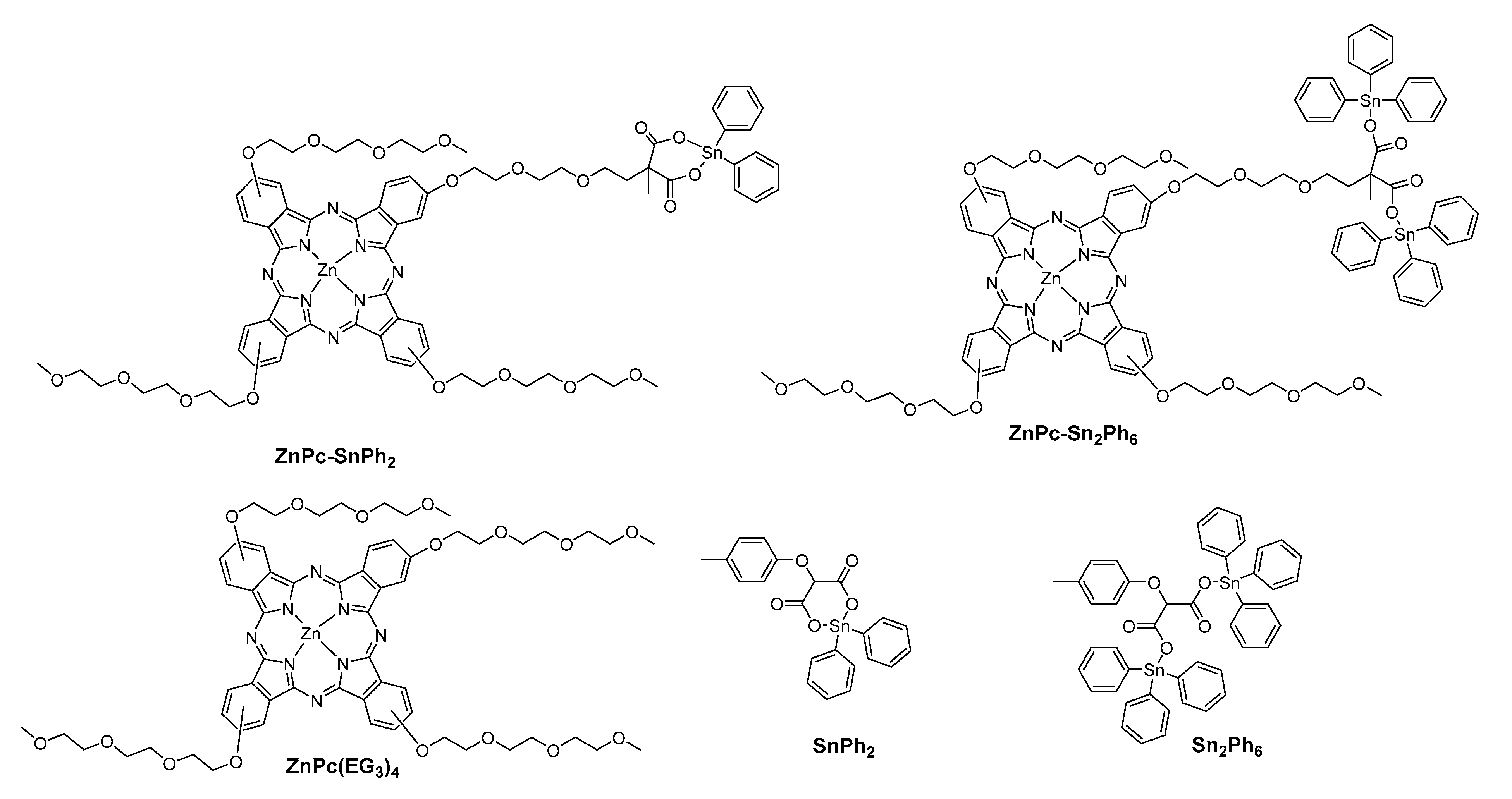
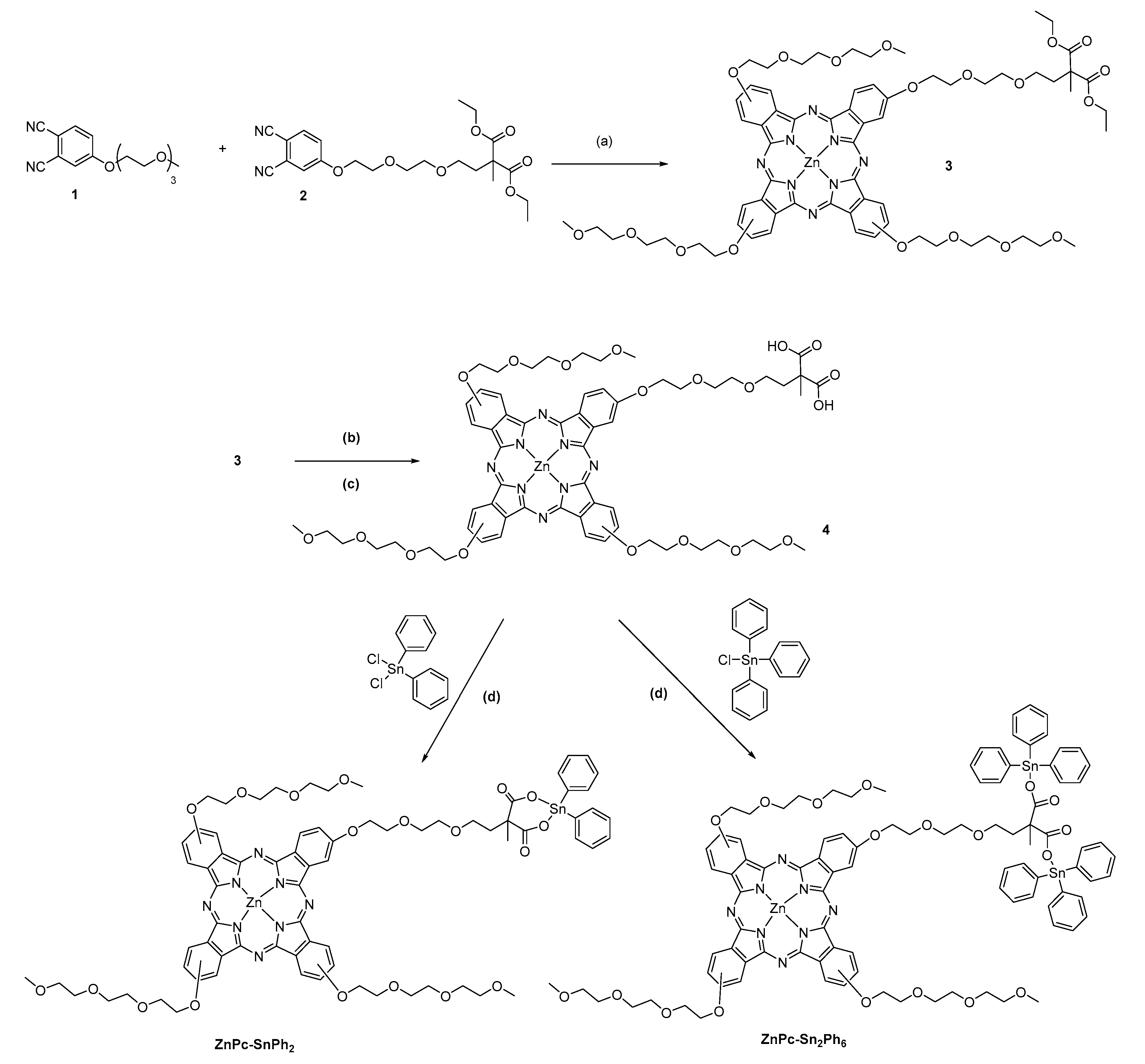
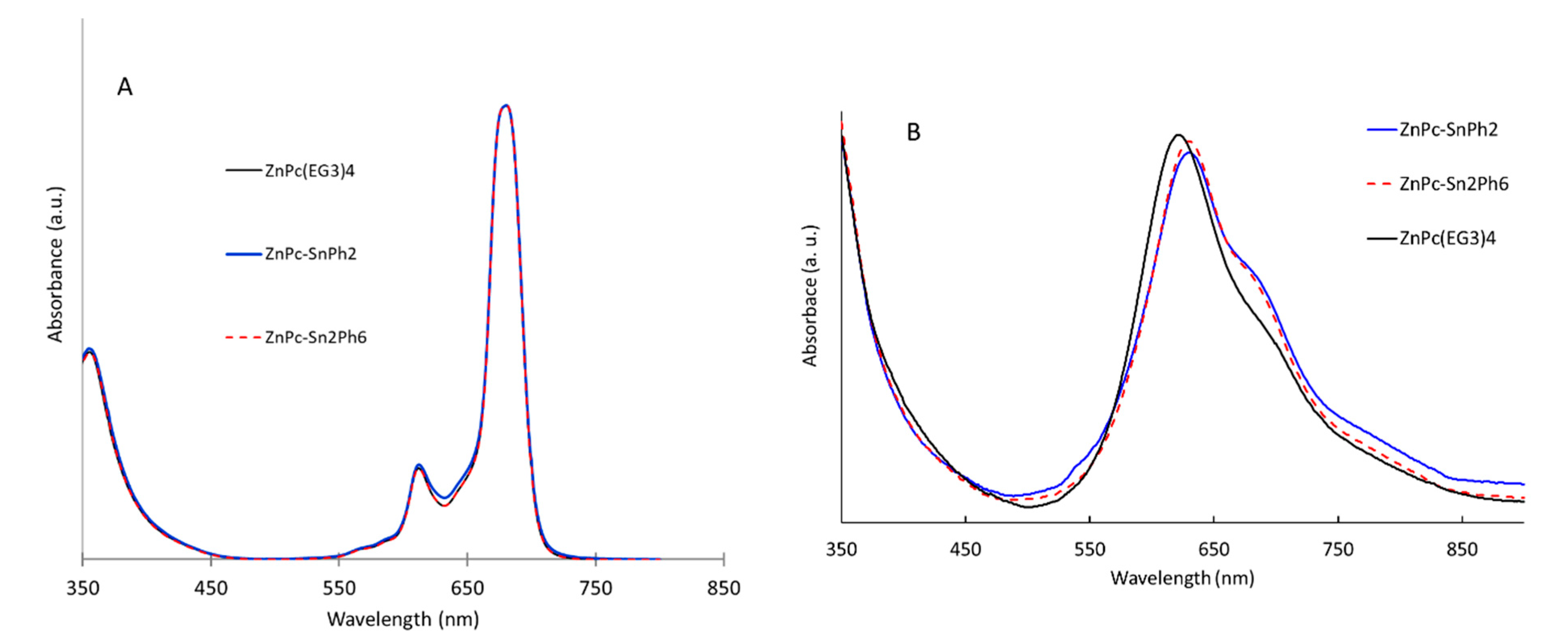

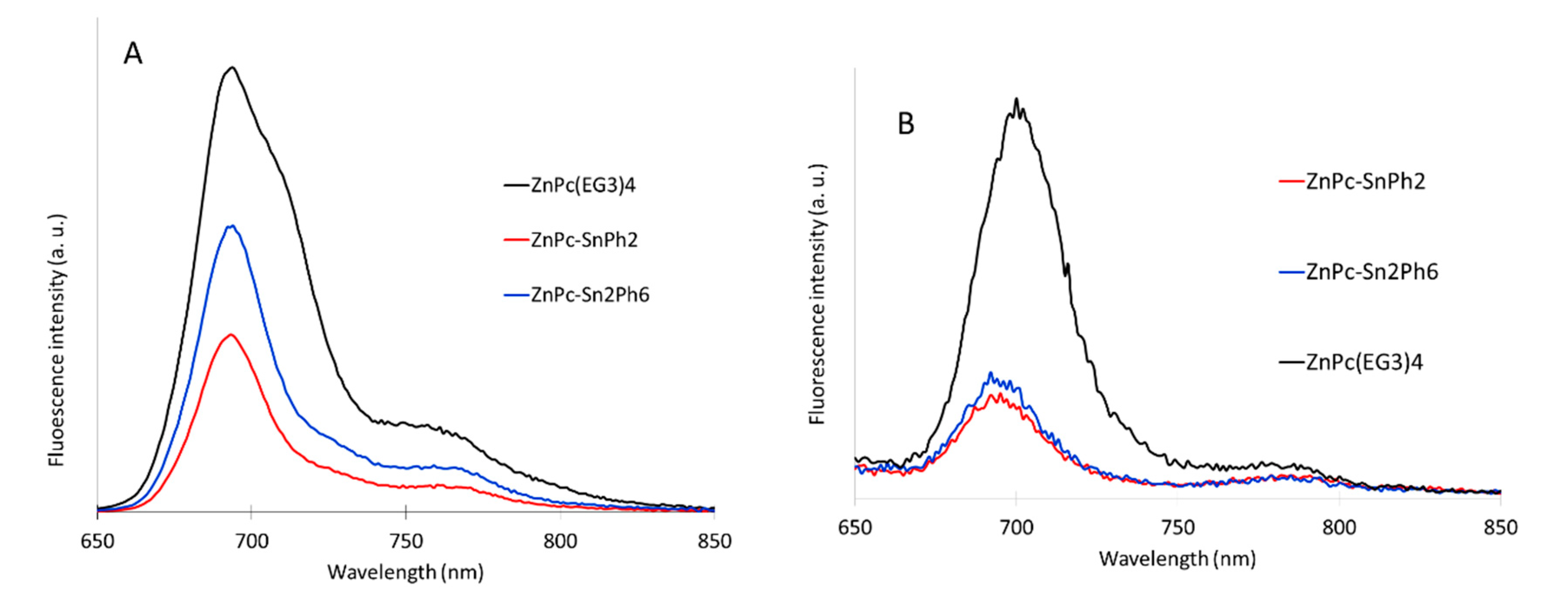


| Compounds | λabs/nm (log Ꜫ/M−1 cm−1) |
|---|---|
| 3 a | 360 (4.83); 616 (4.46); 683 (5.19) |
| 4 a | 360 (4.86); 616 (4.48); 683 (5.21) |
| ZnPc(EG3)4 b | 351 (5.45); 615 (5.04); 681 (5.71) |
| ZnPc-SnPh2 a | 360 (4.82); 616 (4.45); 683 (5.17) |
| ZnPc-Sn2Ph6 a | 360 (4.84); 616 (4.47); 683 (5.2) |
| Compound | ν(Sn-O) cm−1 | νas(OCO) cm−1 | νs (OCO) cm−1 | ν |
|---|---|---|---|---|
| 4 | - | 1725 | 1396 | 329 |
| ZnPc-SnPh2 | 452 | 1557 | 1430sh | 127 |
| ZnPc-Sn2Ph6 | 454 | 1547 | 1429 | 118 |
| Compound | In CHCl3 | In D2O + 1% of DMSO |
|---|---|---|
| ZnPc(EG3)4 | 0.21 ± 0.05 | 0 ± 0.05 |
| ZnPc-SnPh2 | 0.09 ± 0.05 | 0 ± 0.05 |
| ZnPc-Sn2Ph6 | 0.12 ± 0.05 | 0 ± 0.05 |
| MCF-7 with ZnPc-Sn2Ph6 (in µM) | 0 | 0.005 | 0.01 | 0.05 |
|---|---|---|---|---|
| % cell survival, in dark | 100 ± 2 | 99 ± 3 | 77 ± 2 | 38 ± 3 |
| % cell survival, λexc = 650 nm | 98 ± 1 | 98 ± 4 | 75 ± 3 | 41 ± 3 |
| MCF-7 with ZnPc-SnPh2 (in µM) | 0 | 0.05 | 0.1 | 0.5 |
|---|---|---|---|---|
| % cell survival, in dark | 100 ± 5 | 92 ± 3 | 88 ± 4 | 38 ± 5 |
| % cell survival, λexc = 650 nm | 90 ± 9 | 90 ± 4 | 80 ± 2 | 36 ± 2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Toubia, I.; Nguyen, C.; Diring, S.; Pays, M.; Mattana, E.; Arnoux, P.; Frochot, C.; Gary-Bobo, M.; Kobeissi, M.; Odobel, F. Study of Cytotoxic and Photodynamic Activities of Dyads Composed of a Zinc Phthalocyanine Appended to an Organotin. Pharmaceuticals 2021, 14, 413. https://doi.org/10.3390/ph14050413
Toubia I, Nguyen C, Diring S, Pays M, Mattana E, Arnoux P, Frochot C, Gary-Bobo M, Kobeissi M, Odobel F. Study of Cytotoxic and Photodynamic Activities of Dyads Composed of a Zinc Phthalocyanine Appended to an Organotin. Pharmaceuticals. 2021; 14(5):413. https://doi.org/10.3390/ph14050413
Chicago/Turabian StyleToubia, Isabelle, Christophe Nguyen, Stéphane Diring, Marine Pays, Elodie Mattana, Philippe Arnoux, Céline Frochot, Magali Gary-Bobo, Marwan Kobeissi, and Fabrice Odobel. 2021. "Study of Cytotoxic and Photodynamic Activities of Dyads Composed of a Zinc Phthalocyanine Appended to an Organotin" Pharmaceuticals 14, no. 5: 413. https://doi.org/10.3390/ph14050413
APA StyleToubia, I., Nguyen, C., Diring, S., Pays, M., Mattana, E., Arnoux, P., Frochot, C., Gary-Bobo, M., Kobeissi, M., & Odobel, F. (2021). Study of Cytotoxic and Photodynamic Activities of Dyads Composed of a Zinc Phthalocyanine Appended to an Organotin. Pharmaceuticals, 14(5), 413. https://doi.org/10.3390/ph14050413







