Terbium-Based AGuIX-Design Nanoparticle to Mediate X-ray-Induced Photodynamic Therapy
Abstract
1. Introduction
2. Results
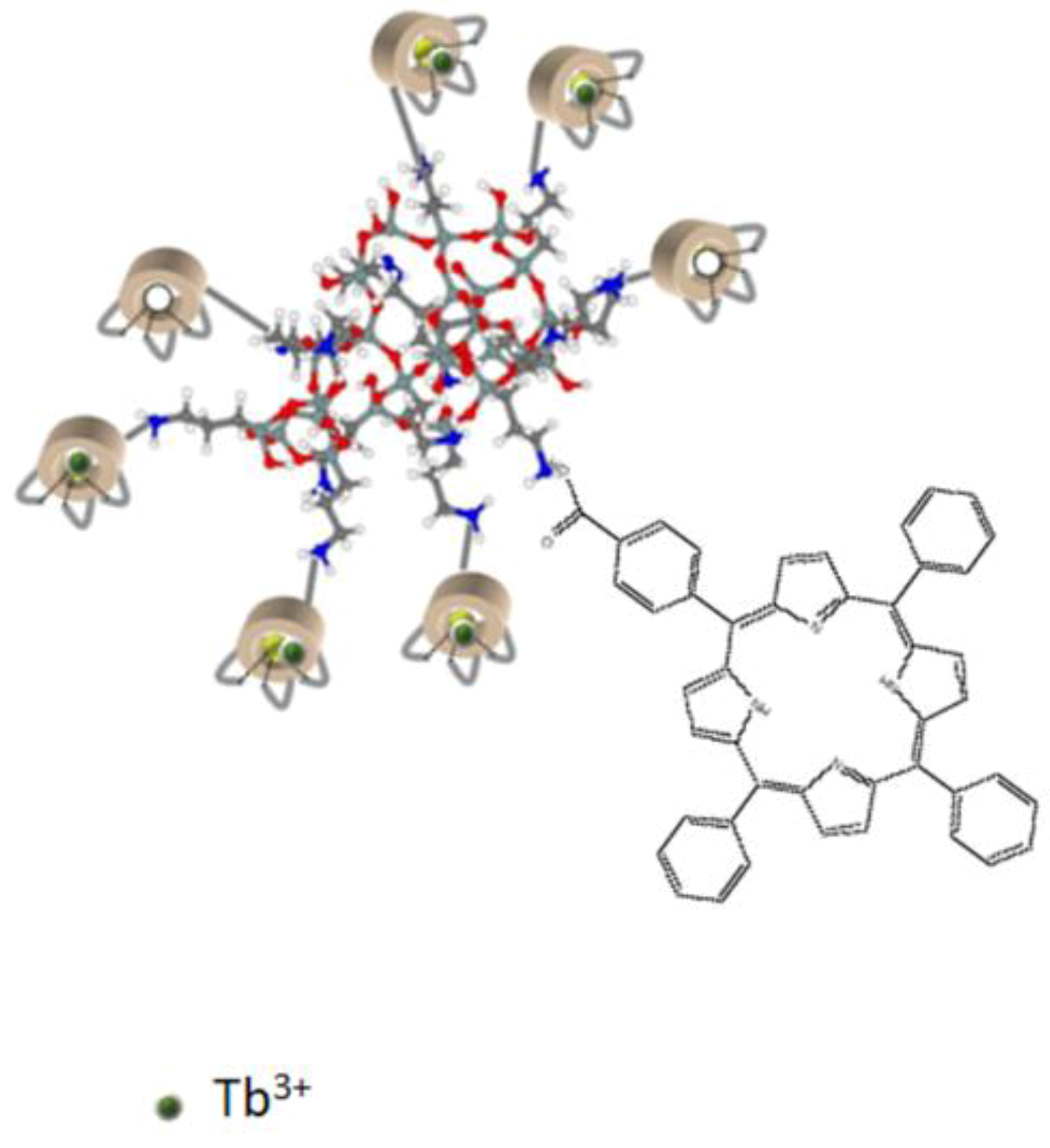
2.1. Characteristics of the AGuIX@Tb-P1
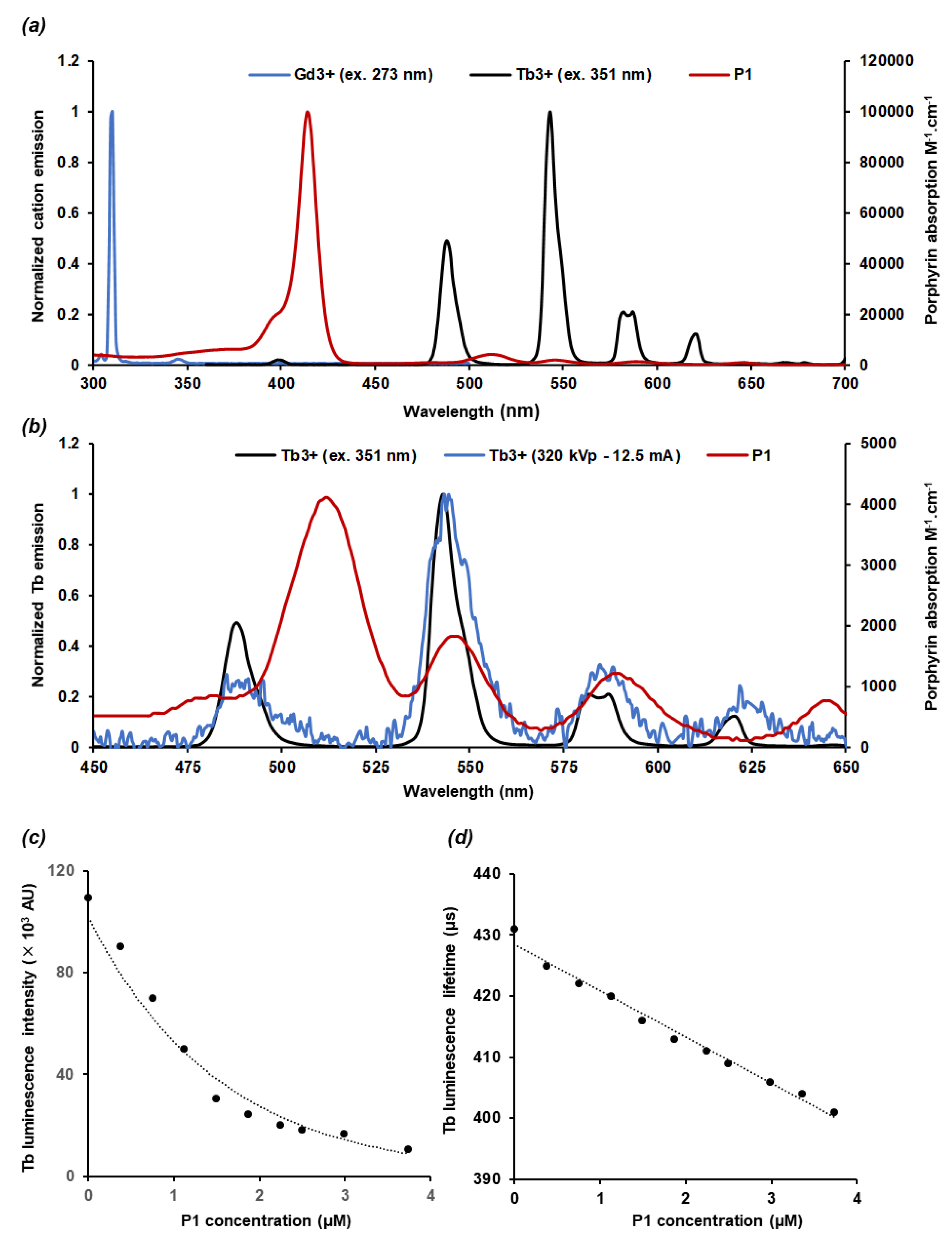
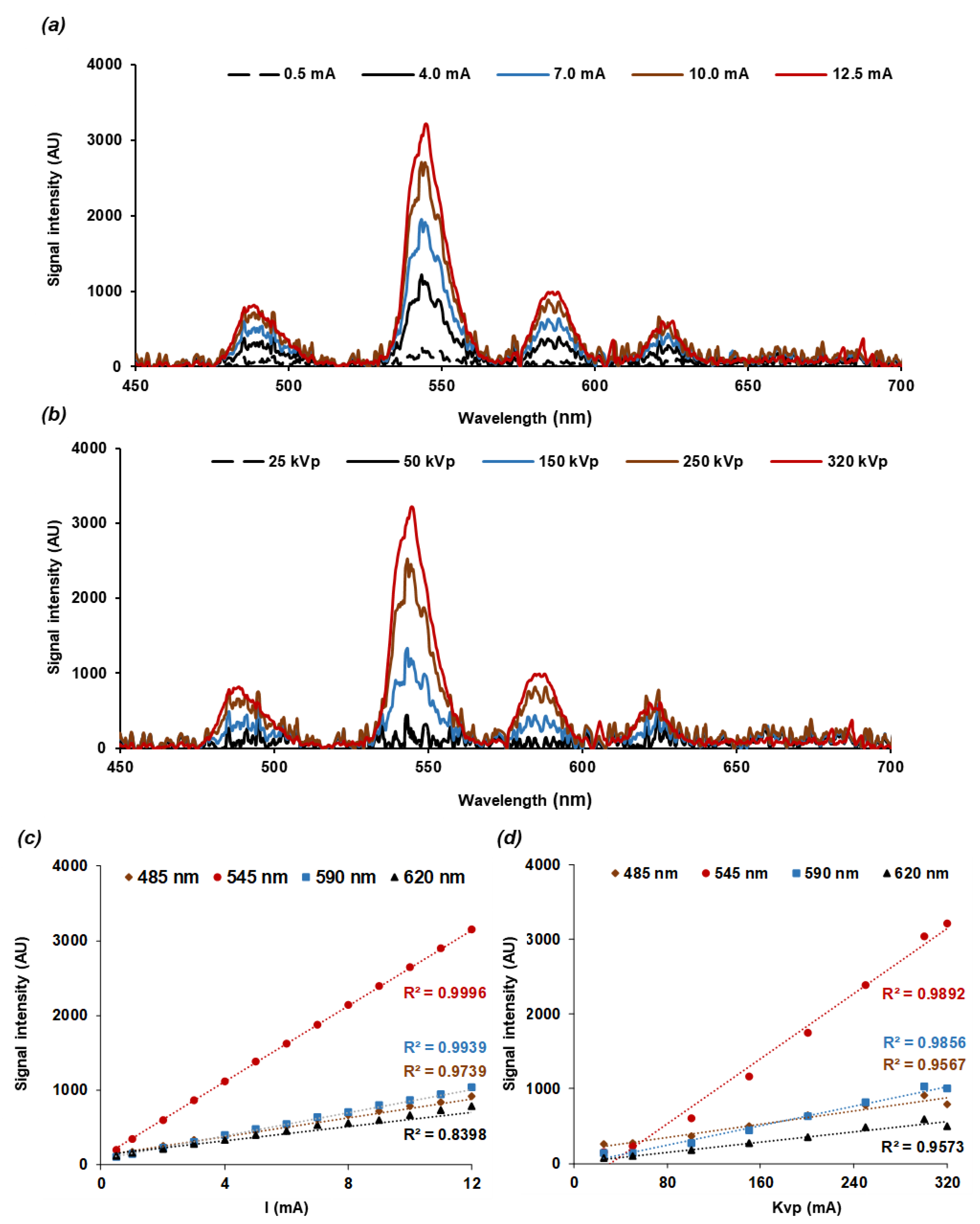
2.2. Nanoscintillator Response to X-ray Excitation
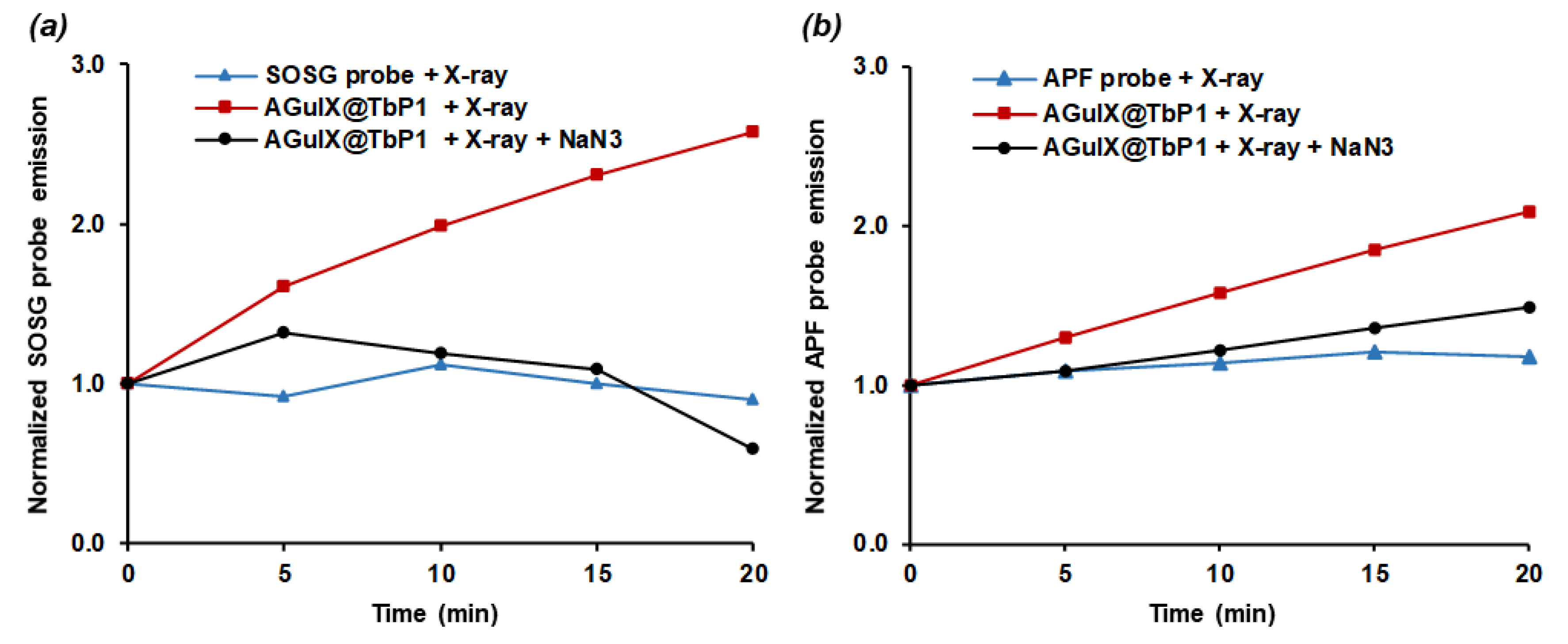
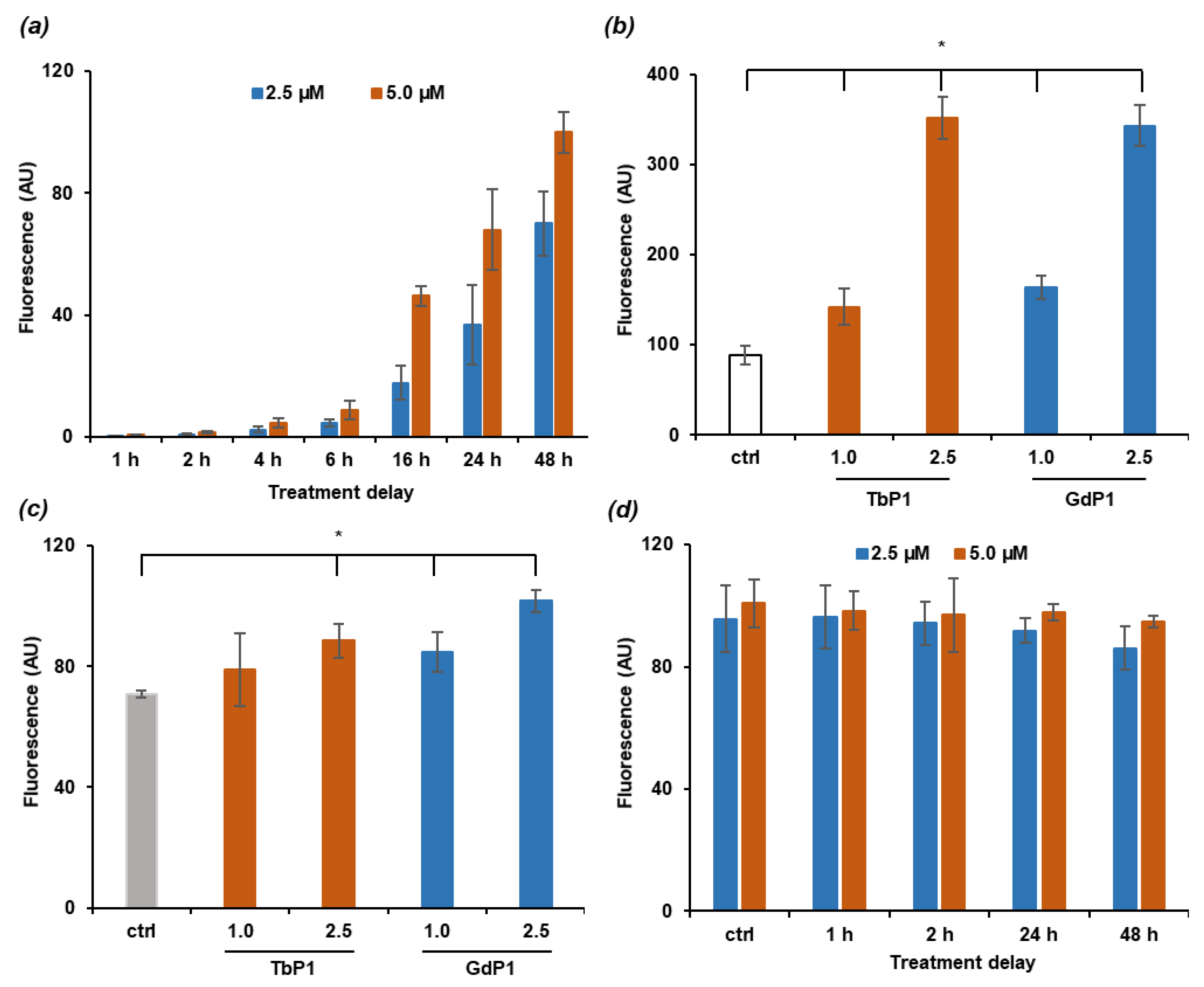
2.3. Energy Transfer and Singlet Oxygen Production
2.4. Cytotoxicity of AGuIX@Tb-P1 on Glioblastoma Cell Growth
2.5. NPs Cell Uptake
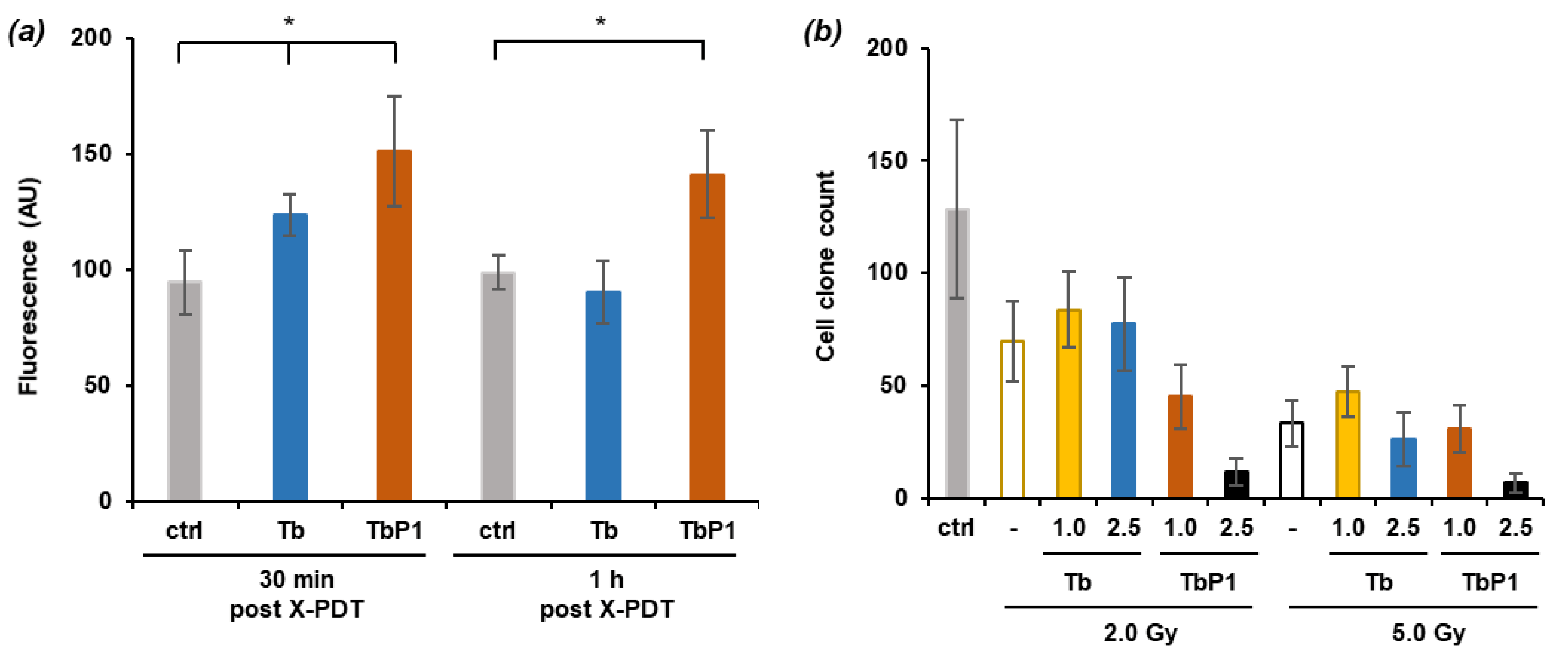
2.6. Photodynamic Effect on U-251 MG Cell Survival
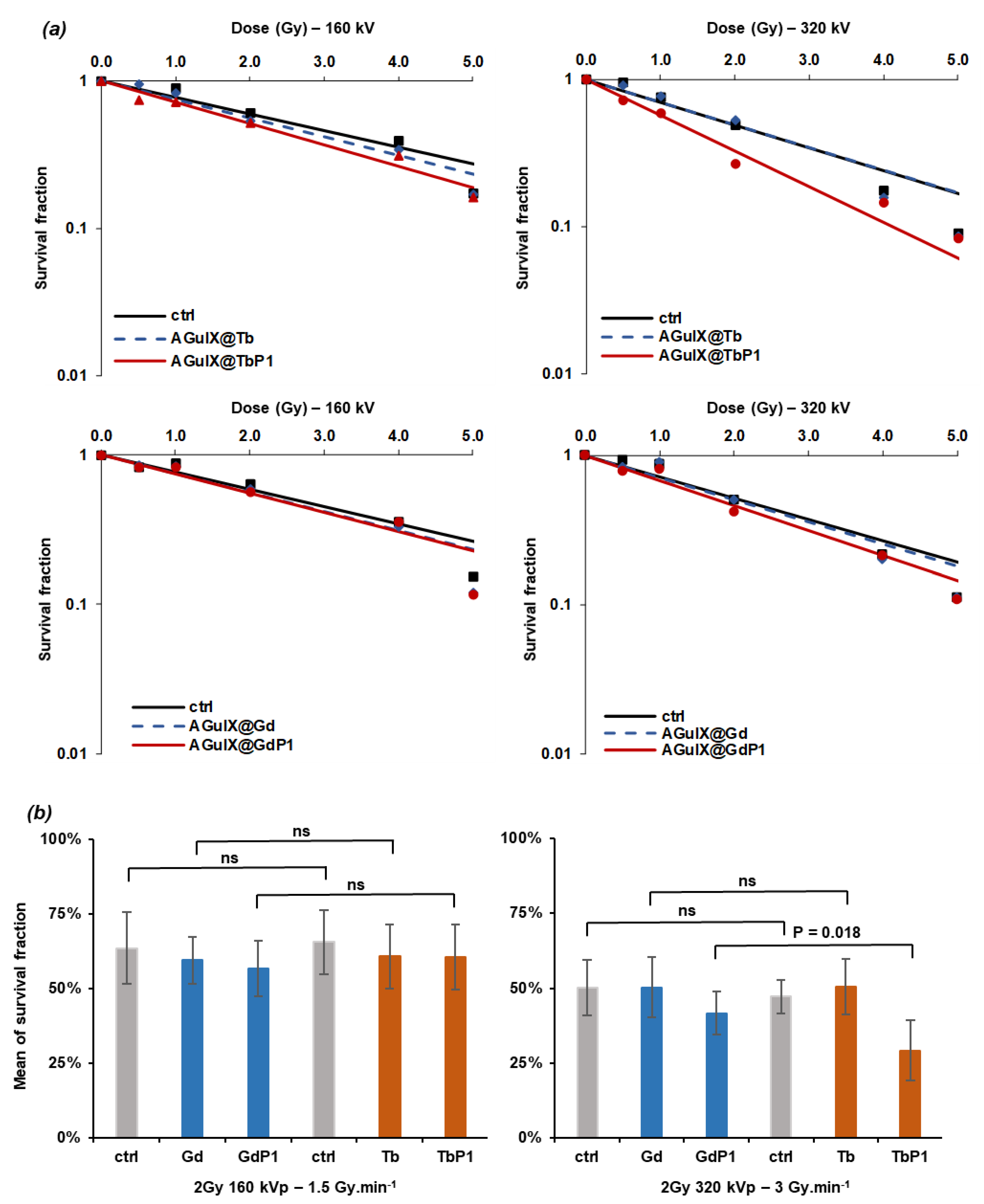
2.7. X-ray-Induced Photodynamic Effect on U-251 MG Cell Survival
3. Discussion
3.1. Gadolinium Substitution by Terbium in the AguIX@ Platform
3.2. Irradiation of AGuIX@Tb-P1 Produces Singlet Oxygen
3.3. Irradiation of AGuIX@Tb-P1 Induces a Photodynamic Effect on U-251 MG Cell Growth
4. Materials and Methods
4.1. Reagents
4.2. Synthesis and Preparation of the AGuIX@-Complexes
4.3. Dynamic Light Scattering Size
4.4. Potential of the AGuIX Conjugates
4.5. Synthesis of AGuIX@Gd and AGuIX@Gd-P1
4.6. Photophysical Properties of AGuIX@Tb-P1
4.7. Tb Scintillation Assessment under X-ray
4.8. Singlet Oxygen Production during Red Light Exposition or X-ray Irradiation
4.9. Biological Experiments
4.9.1. Cell Culture
4.9.2. Cell Growth Assessment
4.9.3. Nanoparticles Cell Uptake
4.9.4. PDT and X-PDT Conditions
4.9.5. Reactive Species Quantification during PDT or X-PDT
4.9.6. Anchorage-Dependent Clonogenic Assay
4.9.7. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Davis, M.E. Glioblastoma: Overview of disease and treatment. Clin. J. Oncol. Nurs. 2016, 20, S2–S8. [Google Scholar] [CrossRef]
- Stupp, R.; Hegi, M.E.; Mason, W.P.; Bent, M.J.V.D.; Taphoorn, M.J.B.; Janzer, R.C.; Ludwin, S.K.; Allgeier, A.; Fisher, B.; Belanger, K.; et al. Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol. 2009, 10, 459–466. [Google Scholar] [CrossRef]
- Calixto, G.M.F.; Bernegossi, J.; De Freitas, L.M.; Fontana, C.R.; Chorilli, M. Nanotechnology-based drug delivery systems for photodynamic therapy of cancer: A review. Molecules 2016, 21, 342. [Google Scholar] [CrossRef]
- Bechet, D.; Mordon, S.R.; Guillemin, F.; Barberi-Heyob, M. Photodynamic therapy of malignant brain tumours: A complementary approach to conventional therapies. Cancer Treat. Rev. 2014, 40, 229–241. [Google Scholar] [CrossRef] [PubMed]
- Larue, L.; Ben Mihoub, A.; Youssef, Z.; Colombeau, L.; Acherar, S.; André, J.-C.; Arnoux, P.; Baros, F.; Vermandel, M.; Frochot, C. Using X-rays in photodynamic therapy: An overview. Photochem. Photobiol. Sci. 2018, 17, 1612–1650. [Google Scholar] [CrossRef] [PubMed]
- Pinel, S.; Thomas, N.; Boura, C.; Barberi-Heyob, M. Approaches to physical stimulation of metallic nanoparticles for glioblastoma treatment. Adv. Drug Deliv. Rev. 2019, 138, 344–357. [Google Scholar] [CrossRef] [PubMed]
- Maeda, H.; Sano, Y.; Takeshita, J.; Iwai, Z.; Kosaka, H.; Marubayashi, T.; Matsukado, Y. A pharmacokinetic simulation model for chemotherapy of brain tumor with an antitumor protein antibiotic, neocarzinostatin: Theoretical considerations behind a two-compartment model for continuous infusion via an internal carotid artery. Cancer Chemother. Pharmacol. 1981, 5, 243–249. [Google Scholar] [CrossRef]
- Maeda, H. Toward a full understanding of the EPR effect in primary and metastatic tumors as well as issues related to its heterogeneity. Adv. Drug Deliv. Rev. 2015, 91, 3–6. [Google Scholar] [CrossRef] [PubMed]
- Beck, T.J.; Kreth, F.W.; Beyer, W.; Mehrkens, J.H.; Obermeier, A.; Stepp, H.; Stummer, W.; Baumgartner, R. Interstitial photodynamic therapy of nonresectable malignant glioma recurrences using 5-aminolevulinic acid induced protoporphyrin IX. Lasers Surg. Med. 2007, 39, 386–393. [Google Scholar] [CrossRef]
- Krishnamurthy, S.; Powers, S.K.; Witmer, P.; Brown, T. Optimal light dose for interstitial photodynamic therapy in treatment for malignant brain tumors. Lasers Surg. Med. 2000, 27, 224–234. [Google Scholar] [CrossRef]
- Stummer, W.; Beck, T.; Beyer, W.; Mehrkens, J.H.; Obermeier, A.; Etminan, N.; Stepp, H.; Tonn, J.-C.; Baumgartner, R.; Herms, J.; et al. Long-sustaining response in a patient with non-resectable, distant recurrence of glioblastoma multiforme treated by interstitial photodynamic therapy using 5-ALA: Case report. J. Neuro-Oncol. 2007, 87, 103–109. [Google Scholar] [CrossRef]
- Kostron, H.; Fiegele, T.; Akatuna, E. Combination of FOSCAN® mediated fluorescence guided resection and photodynamic treatment as new therapeutic concept for malignant brain tumors. Med. Laser Appl. 2006, 21, 285–290. [Google Scholar] [CrossRef]
- Stepp, H.; Beck, T.; Pongratz, T.; Meinel, T.; Kreth, F.-W.; Tonn, J.C.; Stummer, W. ALA and malignant glioma: Fluorescence-guided resection and photodynamic treatment. J. Environ. Pathol. Toxicol. Oncol. 2007, 26, 157–164. [Google Scholar] [CrossRef]
- Eljamel, M.S.; Goodman, C.; Moseley, H. ALA and Photofrin® Fluorescence-guided resection and repetitive PDT in glioblastoma multiforme: A single centre Phase III randomised controlled trial. Lasers Med. Sci. 2007, 23, 361–367. [Google Scholar] [CrossRef] [PubMed]
- Zilidis, G.; Aziz, F.; Telara, S.; Eljamel, M.S. Fluorescence image-guided surgery and repetitive Photodynamic Therapy in brain metastatic malignant melanoma. Photodiagn. Photodyn. Ther. 2008, 5, 264–266. [Google Scholar] [CrossRef] [PubMed]
- Johansson, A.; Palte, G.; Schnell, O.; Tonn, J.-C.; Herms, J.; Stepp, H. 5-aminolevulinic acid-induced protoporphyrin IX levels in tissue of human malignant brain tumors. Photochem. Photobiol. 2010, 86, 1373–1378. [Google Scholar] [CrossRef]
- Aziz, F.; Telara, S.; Moseley, H.; Goodman, C.; Manthri, P.; Eljamel, M.S. Photodynamic therapy adjuvant to surgery in metastatic carcinoma in brain. Photodiagn. Photodyn. Ther. 2009, 6, 227–230. [Google Scholar] [CrossRef]
- Jiang, F.; Robin, A.M.; Katakowski, M.; Tong, L.; Espiritu, M.; Singh, G.; Chopp, M. Photodynamic therapy with photofrin in combination with Buthionine Sulfoximine (BSO) of human glioma in the nude rat. Lasers Med. Sci. 2003, 18, 128–133. [Google Scholar] [CrossRef] [PubMed]
- Zimmermann, A.; Ritsch-Marte, M.; Kostron, H. mTHPC-mediated photodynamic diagnosis of malignant brain tumors. Photochem. Photobiol. 2001, 74, 611–616. [Google Scholar] [CrossRef]
- Stummer, W.; Pichlmeier, U.; Meinel, T.; Wiestler, O.D.; Zanella, F.; Reulen, H.-J. Fluorescence-guided surgery with 5-aminolevulinic acid for resection of malignant glioma: A randomised controlled multicentre phase III trial. Lancet Oncol. 2006, 7, 392–401. [Google Scholar] [CrossRef]
- Van Eijk, C.W. Inorganic scintillators in medical imaging. Phys. Med. Biol. 2002, 47, R85–R106. [Google Scholar] [CrossRef] [PubMed]
- Ren, X.-D.; Hao, X.-Y.; Li, H.-C.; Ke, M.-R.; Zheng, B.-Y.; Huang, J.-D. Progress in the development of nanosensitizers for X-ray-induced photodynamic therapy. Drug Discov. Today 2018, 23, 1791–1800. [Google Scholar] [CrossRef]
- Chen, W.; Zhang, J. Using nanoparticles to enable simultaneous radiation and photodynamic therapies for cancer treatment. J. Nanosci. Nanotechnol. 2006, 6, 1159–1166. [Google Scholar] [CrossRef]
- Kamkaew, A.; Chen, F.; Zhan, Y.; Majewski, R.L.; Cai, W. Scintillating nanoparticles as energy mediators for enhanced photodynamic therapy. ACS Nano 2016, 10, 3918–3935. [Google Scholar] [CrossRef]
- Retif, P.; Pinel, S.; Toussaint, M.; Frochot, C.; Chouikrat, R.; Bastogne, T.; Barberi-Heyob, M. Nanoparticles for radiation therapy enhancement: The key parameters. Theranostics 2015, 5, 1030–1044. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Hu, J.; Elmenoufy, A.H.; Yang, X. Highly efficient FRET system capable of deep photodynamic therapy established on X-ray excited mesoporous LAF3:TB scintillating nanoparticles. ACS Appl. Mater. Interfaces 2015, 7, 12261–12269. [Google Scholar] [CrossRef] [PubMed]
- Bulin, A.-L.; Vasil’Ev, A.; Belsky, A.; Amans, D.; LeDoux, G.; Dujardin, C. Modelling energy deposition in nanoscintillators to predict the efficiency of the X-ray-induced photodynamic effect. Nanoscale 2015, 7, 5744–5751. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.-C.; Wang, W.-Y.; Lin, F.-H.; Hou, C.-H. Rare-earth-doped calcium carbonate exposed to X-ray irradiation to induce reactive oxygen species for tumor treatment. Int. J. Mol. Sci. 2019, 20, 1148. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Xue, Z.; Jiang, M.; Li, Y.; Zeng, S.; Liu, H. Soft X-ray activated NaYF4:Gd/Tb scintillating nanorods for in vivo dual-modal X-ray/X-ray-induced optical bioimaging. Nanoscale 2017, 10, 342–350. [Google Scholar] [CrossRef]
- Abliz, E.; Collins, J.E.; Bell, H.; Tata, D.B. Novel applications of diagnostic X-rays in activating a clinical photodynamic drug: Photofrin II through X-ray induced visible luminescence from “rare-earth” formulated particles. J. X-ray Sci. Technol. 2011, 19, 521–530. [Google Scholar] [CrossRef] [PubMed]
- Elmenoufy, A.H.; Tang, Y.; Hu, J.; Xu, H.; Yang, X. A novel deep photodynamic therapy modality combined with CT imaging established via X-ray stimulated silica-modified lanthanide scintillating nanoparticles. Chem. Commun. 2015, 51, 12247–12250. [Google Scholar] [CrossRef] [PubMed]
- Mignot, A.; Truillet, C.; Lux, F.; Sancey, L.; Louis, C.; Denat, F.; Boschetti, F.; Bocher, L.; Gloter, A.; Stephan, O.; et al. A top-down synthesis route to ultrasmall multifunctional Gd-based silica nanoparticles for theranostic applications. Chemistry 2013, 19, 6122–6136. [Google Scholar] [CrossRef]
- Le Duc, G.; Roux, S.; Paruta-Tuarez, A.; Dufort, S.; Brauer, E.; Marais, A.; Truillet, C.; Sancey, L.; Perriat, P.; Lux, F.; et al. Advantages of gadolinium based ultra-small nanoparticles vs molecular gadolinium chelates for radiotherapy guided by MRI for glioma treatment. Cancer Nanotechnol. 2014, 5, 4. [Google Scholar] [CrossRef] [PubMed]
- Sancey, L.; Lux, F.; Kotb, S.; Roux, S.; Dufort, S.; Bianchi, A.; Cremillieux, Y.; Fries, P.; Coll, J.-L.; Rodriguez-Lafrasse, C.; et al. The use of theranostic gadolinium-based nanoprobes to improve radiotherapy efficacy. Br. J. Radiol. 2014, 87, 20140134. [Google Scholar] [CrossRef]
- McMahon, S.J.; Hyland, W.B.; Muir, M.F.; Coulter, J.A.; Jain, S.; Butterworth, K.T.; Schettino, G.; Dickson, G.R.; Hounsell, A.R.; O’Sullivan, J.M.; et al. Biological consequences of nanoscale energy deposition near irradiated heavy atom nanoparticles. Sci. Rep. 2011, 1, 18. [Google Scholar] [CrossRef]
- Sicard-Roselli, C.; Brun, E.; Gilles, M.; Baldacchino, G.; Kelsey, C.; McQuaid, H.; Polin, C.; Wardlow, N.; Currell, F. A new mechanism for hydroxyl radical production in irradiated nanoparticle solutions. Small 2014, 10, 3338–3346. [Google Scholar] [CrossRef]
- Du, Y.; Sun, H.; LeDuc, G.; Doussineau, T.; Ji, K.; Wang, Q.; Lin, Z.; Wang, Y.; Liu, Q.; Tillement, O.; et al. Radiosensitization effect of AGuIX, a Gadolinium-based nanoparticle, in nonsmall cell lung cancer. ACS Appl. Mater. Interfaces 2020, 12, 56874–56885. [Google Scholar] [CrossRef] [PubMed]
- Gries, M.; Thomas, N.; Acherar, S.; Frochot, C.; Lux, F.; Tillement, O.; Barberi-Heyob, M.; Daouk, J.; Rocchi, P.; Choulier, L.; et al. Multiscale selectivity and in vivo biodistribution of NRP-1-targeted theranostic aguix nanoparticles for PDT of glioblastoma. Int. J. Nanomed. 2020, 15, 8739–8758. [Google Scholar] [CrossRef] [PubMed]
- Franken, N.A.; Oei, A.L.; Kok, H.P.; Rodermond, H.M.; Sminia, P.; Crezee, J.; Stalpers, L.J.; Barendsen, G.W. Cell survival and radiosensitisation: Modulation of the linear and quadratic parameters of the LQ model (Review). Int. J. Oncol. 2013, 42, 1501–1515. [Google Scholar] [CrossRef]
- Toussaint, M.; Pinel, S.; Bastogne, T.; Frochot, C.; Tillement, O.; Lux, F.; Barberi-Heyob, M.; Auger, F.; Durieux, N.; Thomassin, M.; et al. Proton MR spectroscopy and diffusion mr imaging monitoring to predict tumor response to interstitial photodynamic therapy for glioblastoma. Theranostics 2017, 7, 436–451. [Google Scholar] [CrossRef] [PubMed]
- Clement, S.; Deng, W.; Camilleri, E.; Wilson, B.C.; Goldys, E.M. X-ray induced singlet oxygen generation by nanoparticle-photosensitizer conjugates for photodynamic therapy: Determination of singlet oxygen quantum yield. Sci. Rep. 2016, 6, 19954. [Google Scholar] [CrossRef]
- Kulmala, S.; Hakanen, A.; Laine, E.; Haapakka, K. X-ray irradiation-induced optical luminescence of terbium(III) chelates in aqueous solutions. J. Alloy. Compd. 1995, 225, 279–283. [Google Scholar] [CrossRef]
- Kulmala, S.; Laine, E.; Hakanen, A.; Raerinne, P.; Haapakka, K. Mechanism of terbium(III)-enhanced lyoluminescence of X-ray irradiated sodium chloride. Anal. Chim. Acta 1994, 294, 1–11. [Google Scholar] [CrossRef]
- Jürgensen, A. XEOL spectroscopy of lanthanides in aqueous solution. Can. J. Chem. 2017, 95, 1198–1204. [Google Scholar] [CrossRef]
- Setsukinai, K.-I.; Urano, Y.; Kakinuma, K.; Majima, H.J.; Nagano, T. Development of novel fluorescence probes that can reliably detect reactive oxygen species and distinguish specific species. J. Biol. Chem. 2003, 278, 3170–3175. [Google Scholar] [CrossRef] [PubMed]
- Price, M.; Reiners, J.J.; Santiago, A.M.; Kessel, D. Monitoring singlet oxygen and hydroxyl radical formation with fluorescent probes during photodynamic therapy. Photochem. Photobiol. 2009, 85, 1177–1181. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, J.; Misawa, M. Characterization of reactive oxygen species generated by protoporphyrin IX under X-ray irradiation. Radiat. Phys. Chem. 2009, 78, 889–898. [Google Scholar] [CrossRef]
- Misawa, M.; Takahashi, J. Generation of reactive oxygen species induced by gold nanoparticles under X-ray and UV Irradiations. Nanomed. Nanotechnol. Biol. Med. 2011, 7, 604–614. [Google Scholar] [CrossRef]
- Liu, H.; Carter, P.J.H.; Laan, A.C.; Eelkema, R.; Denkova, A.G. Singlet oxygen sensor green is not a suitable probe for 1O2 in the presence of ionizing radiation. Sci. Rep. 2019, 9, 8393. [Google Scholar] [CrossRef] [PubMed]
- Gollmer, A.; Arnbjerg, J.; Blaikie, F.H.; Pedersen, B.W.; Breitenbach, T.; Daasbjerg, K.; Glasius, M.; Ogilby, P.R. Singlet oxygen sensor green: Photochemical behavior in solution and in a mammalian cell. Photochem. Photobiol. 2011, 87, 671–679. [Google Scholar] [CrossRef] [PubMed]
- Popovich, K.; Tomanová, K.; Čuba, V.; Procházková, L.; Pelikánová, I.T.; Jakubec, I.; Mihóková, E.; Nikl, M. LuAg:Pr3+-porphyrin based nanohybrid system for singlet oxygen production: Toward the next generation of PDTX drugs. J. Photochem. Photobiol. 2018, 179, 149–155. [Google Scholar] [CrossRef]
- Jacobs, V.L.; Valdes, P.A.; Hickey, W.F.; De Leo, J.A. Current review of in vivo GBM rodent models: Emphasis on the CNS-1 tumour model. ASN Neuro 2011, 3, AN20110014. [Google Scholar] [CrossRef]
- Luo, W.; Liu, R.-S.; Zhu, J.-G.; Li, Y.-C.; Liu, H.-C. Subcellular location and photodynamic therapeutic effect of chlorin e6 in the human tongue squamous cell cancer Tca8113 cell line. Oncol. Lett. 2015, 9, 551–556. [Google Scholar] [CrossRef] [PubMed]
- Pavani, C.; Uchoa, A.F.; Oliveira, C.S.; Iamamoto, Y.; Baptista, M.S. Effect of zinc insertion and hydrophobicity on the membrane interactions and PDT activity of porphyrin photosensitizers. Photochem. Photobiol. Sci. 2009, 8, 233–240. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.-C.; Sun, Y.-J.; Chung, P.-H.; Chen, W.-Y.; Swieszkowski, W.; Tian, W.; Lin, F.-H. Development of Ce-doped TiO2 activated by X-ray irradiation for alternative cancer treatment. Ceram. Int. 2017, 43, 12675–12683. [Google Scholar] [CrossRef]
- Kuo, W.-J.; Wang, Y.-C.; Chen, M.-H.; Tung, F.-I.; Liu, T.-Y. Study of a novel vehicle developed for enhancing the efficacy of radiation therapy. Ceram. Int. 2017, 43, S789–S796. [Google Scholar] [CrossRef]
- Chen, M.-H.; Jenh, Y.-J.; Wu, S.-K.; Chen, Y.-S.; Hanagata, N.; Lin, F.-H. Non-invasive photodynamic therapy in brain cancer by use of Tb3+-doped LAF3 nanoparticles in combination with photosensitizer through X-ray irradiation: A proof-of-concept study. Nanoscale Res. Lett. 2017, 12, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.D.; Nguyen, H.T.; Chen, H.; Cox, P.B.; Wang, L.; Nagata, K.; Hao, Z.; Wang, A.; Li, Z.; Xie, J. X-ray induced photodynamic therapy: A combination of radiotherapy and photodynamic therapy. Theranostics 2016, 6, 2295–2305. [Google Scholar] [CrossRef] [PubMed]
- Huda, W.; Scalzetti, E.M.; Levin, G. Technique factors and image quality as functions of patient weight at abdominal CT. Radiology 2000, 217, 430–435. [Google Scholar] [CrossRef]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]

| Condition Tested | α Parameter | Dose Modifying Factor (DMF) | Survival Fraction (SF2.0 Gy) | Enhanced Factor (%) | DER |
|---|---|---|---|---|---|
| 160 kVp/1.5 Gy.min−1 | |||||
| Control | 0.263 | 1.000 | 0.592 | ||
| AguIX@Gd | 0.292 | 0.966 | 0.587 | ||
| AguIX@GdP1 | 0.296 | 0.862 | 0.558 | ||
| AguIX@Tb | 0.291 | 0.885 | 0.559 | ||
| AguIX@TbP1 | 0.333 | 0.769 | 0.515 | ||
| 320 kVp/3.0 Gy.min−1 | |||||
| Control | 0.341 | 1.000 | 0.504 | ||
| AguIX@Gd | 0.340 | 1.000 | 0.507 | ||
| AguIX@GdP1 | 0.385 | 0.876 | 0.463 | 8.1 | 1.08 |
| AguIX@Tb | 0.354 | 1.000 | 0.493 | 2.2 | 1.02 |
| AguIX@TbP1 | 0.559 | 0.650 | 0.327 | 35.1 | 1.54 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Daouk, J.; Iltis, M.; Dhaini, B.; Béchet, D.; Arnoux, P.; Rocchi, P.; Delconte, A.; Habermeyer, B.; Lux, F.; Frochot, C.; et al. Terbium-Based AGuIX-Design Nanoparticle to Mediate X-ray-Induced Photodynamic Therapy. Pharmaceuticals 2021, 14, 396. https://doi.org/10.3390/ph14050396
Daouk J, Iltis M, Dhaini B, Béchet D, Arnoux P, Rocchi P, Delconte A, Habermeyer B, Lux F, Frochot C, et al. Terbium-Based AGuIX-Design Nanoparticle to Mediate X-ray-Induced Photodynamic Therapy. Pharmaceuticals. 2021; 14(5):396. https://doi.org/10.3390/ph14050396
Chicago/Turabian StyleDaouk, Joël, Mathilde Iltis, Batoul Dhaini, Denise Béchet, Philippe Arnoux, Paul Rocchi, Alain Delconte, Benoît Habermeyer, François Lux, Céline Frochot, and et al. 2021. "Terbium-Based AGuIX-Design Nanoparticle to Mediate X-ray-Induced Photodynamic Therapy" Pharmaceuticals 14, no. 5: 396. https://doi.org/10.3390/ph14050396
APA StyleDaouk, J., Iltis, M., Dhaini, B., Béchet, D., Arnoux, P., Rocchi, P., Delconte, A., Habermeyer, B., Lux, F., Frochot, C., Tillement, O., Barberi-Heyob, M., & Schohn, H. (2021). Terbium-Based AGuIX-Design Nanoparticle to Mediate X-ray-Induced Photodynamic Therapy. Pharmaceuticals, 14(5), 396. https://doi.org/10.3390/ph14050396









