The Current and Potential Therapeutic Use of Metformin—The Good Old Drug
Abstract
1. Introduction
2. A Brief History of Metformin
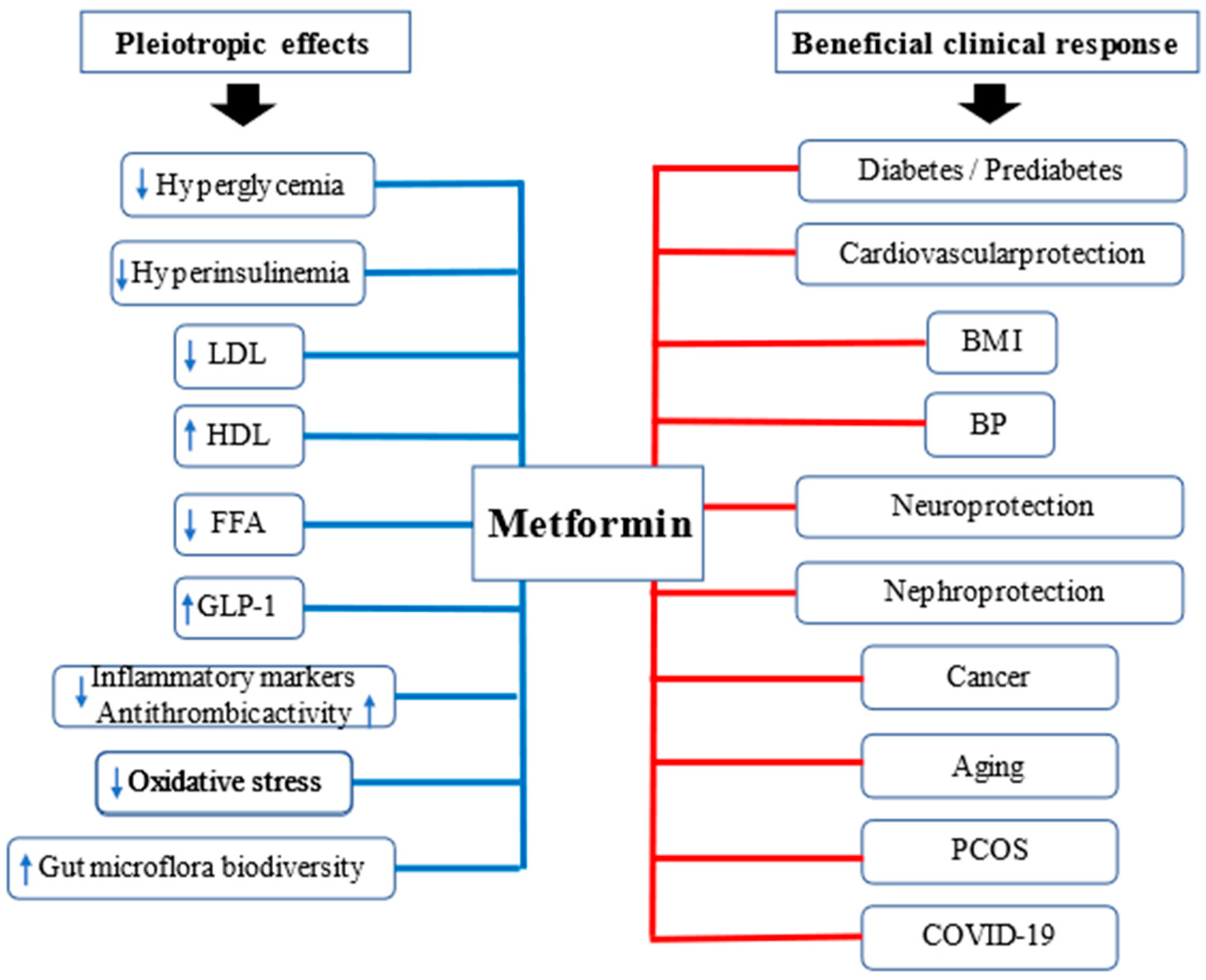
3. Pleiotropic Effects of Metformin
3.1. Effects of Metformin on Glucose Metabolism
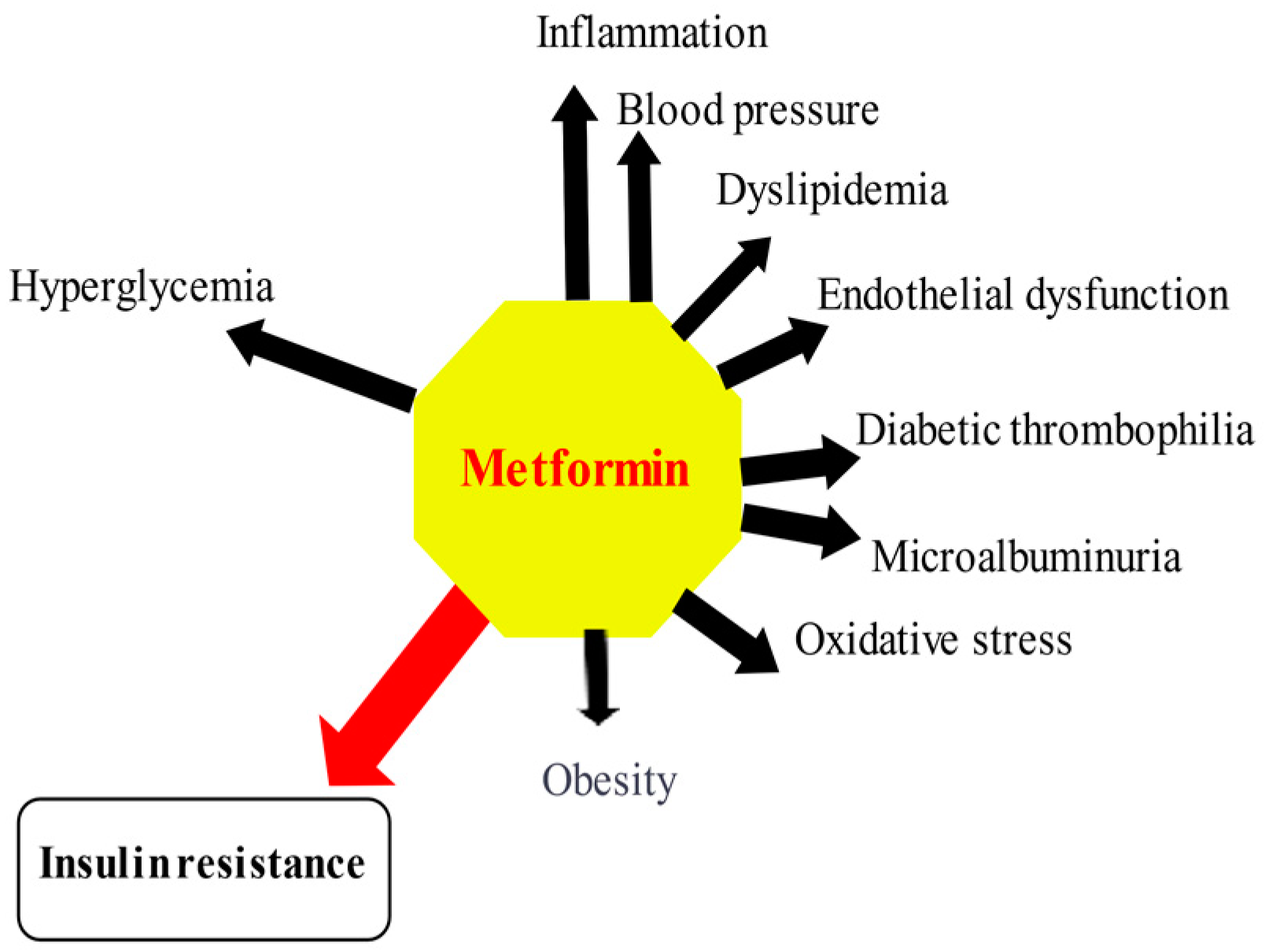
3.2. Pleiotropic Effects of Metformin beyond Glucose Control
3.2.1. Metformin and Inflammation
3.2.2. Metformin and Oxidative Stress
4. Clinical Aspects of Metformin
4.1. Type 2 Diabetes
4.2. Prediabetes
4.3. Type 1 Diabetes
4.4. Gestational Diabetes (GDM)
4.5. Polycystic Ovary Syndrome (PCOS)
4.6. Non-Alcoholic Fatty Liver Disease (NAFLD)
4.7. Cardiovascular Protection
4.8. Nephroprotection
4.9. Cancer
4.10. Longevity
4.11. COVID-19
5. Pharmacogenetics of Metformin
6. Safety of Metformin
7. Conclusions
Funding
Conflicts of Interest
References
- UK Prospective Diabetes Study (UKPDS) Group. Effect of Intensive Blood-Glucose Control with Metformin on Complications in Overweight Patients with Type 2 Diabetes (UKPDS 34). Lancet 1998, 352, 854–865. [Google Scholar] [CrossRef]
- Holman, R.R.; Paul, S.K.; Bethel, M.A.; Matthews, D.R.; Neil, H.A.W. 10-Year Follow-up of Intensive Glucose Control in Type 2 Diabetes. N. Engl. J. Med. 2008, 359, 1577–1589. [Google Scholar] [CrossRef]
- Roussel, R.; Travert, F.; Pasquet, B.; Wilson, P.W.F.; Smith, S.C.; Goto, S.; Ravaud, P.; Marre, M.; Porath, A.; Bhatt, D.L.; et al. Metformin Use and Mortality among Patients with Diabetes and Atherothrombosis. Arch. Intern. Med. 2010, 170, 1892–1899. [Google Scholar] [CrossRef]
- Hong, J.; Zhang, Y.; Lai, S.; Lv, A.; Su, Q.; Dong, Y.; Zhou, Z.; Tang, W.; Zhao, J.; Cui, L.; et al. Effects of Metformin versus Glipizide on Cardiovascular Outcomes in Patients with Type 2 Diabetes and Coronary Artery Disease. Diabetes Care 2013, 36, 1304–1311. [Google Scholar] [CrossRef] [PubMed]
- Solymár, M.; Ivic, I.; Pótó, L.; Hegyi, P.; Garami, A.; Hartmann, P.; Pétervári, E.; Czopf, L.; Hussain, A.; Gyöngyi, Z.; et al. Metformin Induces Significant Reduction of Body Weight, Total Cholesterol and LDL Levels in the Elderly—A Meta-Analysis. PLoS ONE 2018, 13, e0207947. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Xie, H.; Liu, Y.; Gao, P.; Yang, X.; Shen, Z. Effect of Metformin on All-Cause and Cardiovascular Mortality in Patients with Coronary Artery Diseases: A Systematic Review and an Updated Meta-Analysis. Cardiovasc. Diabetol. 2019, 18, 96. [Google Scholar] [CrossRef] [PubMed]
- Halabi, A.; Sen, J.; Huynh, Q.; Marwick, T.H. Metformin Treatment in Heart Failure with Preserved Ejection Fraction: A Systematic Review and Meta-Regression Analysis. Cardiovasc. Diabetol. 2020, 19, 124. [Google Scholar] [CrossRef] [PubMed]
- Selvin, E.; Bolen, S.; Yeh, H.-C.; Wiley, C.; Wilson, L.M.; Marinopoulos, S.S.; Feldman, L.; Vassy, J.; Wilson, R.; Bass, E.B.; et al. Cardiovascular Outcomes in Trials of Oral Diabetes Medications: A Systematic Review. Arch. Intern. Med. 2008, 168, 2070–2080. [Google Scholar] [CrossRef]
- Griffin, S.J.; Leaver, J.K.; Irving, G.J. Impact of Metformin on Cardiovascular Disease: A Meta-Analysis of Randomised Trials among People with Type 2 Diabetes. Diabetologia 2017, 60, 1620–1629. [Google Scholar] [CrossRef] [PubMed]
- Boussageon, R.; Supper, I.; Bejan-Angoulvant, T.; Kellou, N.; Cucherat, M.; Boissel, J.-P.; Kassai, B.; Moreau, A.; Gueyffier, F.; Cornu, C. Reappraisal of Metformin Efficacy in the Treatment of Type 2 Diabetes: A Meta-Analysis of Randomised Controlled Trials. PLoS Med. 2012, 9, e1001204. [Google Scholar] [CrossRef] [PubMed]
- Lamanna, C.; Monami, M.; Marchionni, N.; Mannucci, E. Effect of Metformin on Cardiovascular Events and Mortality: A Meta-Analysis of Randomized Clinical Trials. Diabetes Obes. Metab. 2011, 13, 221–228. [Google Scholar] [CrossRef] [PubMed]
- Nathan, D.M.; Buse, J.B.; Davidson, M.B.; Heine, R.J.; Holman, R.R.; Sherwin, R.; Zinman, B.; Professional Practice Committee, American Diabetes Association. European Association for the Study of Diabetes Management of Hyperglycaemia in Type 2 Diabetes: A Consensus Algorithm for the Initiation and Adjustment of Therapy. A Consensus Statement from the American Diabetes Association and the European Association for the Study of Diabetes. Diabetologia 2006, 49, 1711–1721. [Google Scholar] [CrossRef]
- American Diabetes Association 9. Pharmacologic Approaches to Glycemic Treatment: Standards of Medical Care in Diabetes-2020. Diabetes Care 2020, 43, S98–S110. [Google Scholar] [CrossRef] [PubMed]
- Kato, E.T.; Silverman, M.G.; Mosenzon, O.; Zelniker, T.A.; Cahn, A.; Furtado, R.H.M.; Kuder, J.; Murphy, S.A.; Bhatt, D.L.; Leiter, L.A.; et al. Effect of Dapagliflozin on Heart Failure and Mortality in Type 2 Diabetes Mellitus. Circulation 2019, 139, 2528–2536. [Google Scholar] [CrossRef] [PubMed]
- Cavallari, I.; Maddaloni, E. Cardiovascular Effects of SGLT-2 Inhibitors: What We Have Learned from Cardiovascular Outcome Trials and What We Still Need to Understand. Diabetes Metab. Res. Rev. 2019, 35, e3124. [Google Scholar] [CrossRef] [PubMed]
- Jia, X.; Alam, M.; Ye, Y.; Bajaj, M.; Birnbaum, Y. GLP-1 Receptor Agonists and Cardiovascular Disease: A Meta-Analysis of Recent Cardiac Outcome Trials. Cardiovasc. Drugs Ther. 2018, 32, 65–72. [Google Scholar] [CrossRef] [PubMed]
- Scheen, A.J. Cardiovascular Effects of New Oral Glucose-Lowering Agents: DPP-4 and SGLT-2 Inhibitors. Circ. Res. 2018, 122, 1439–1459. [Google Scholar] [CrossRef] [PubMed]
- Kluger, A.Y.; Tecson, K.M.; Barbin, C.M.; Lee, A.Y.; Lerma, E.V.; Rosol, Z.P.; Rangaswami, J.; Lepor, N.E.; Cobble, M.E.; McCullough, P.A. Cardiorenal Outcomes in the CANVAS, DECLARE-TIMI 58, and EMPA-REG OUTCOME Trials: A Systematic Review. Rev. Cardiovasc. Med. 2018, 19, 41–49. [Google Scholar] [CrossRef] [PubMed]
- Verma, S.; Bain, S.C.; Monk Fries, T.; Mazer, C.D.; Nauck, M.A.; Pratley, R.E.; Rasmussen, S.; Saevereid, H.A.; Zinman, B.; Buse, J.B. Duration of Diabetes and Cardiorenal Efficacy of Liraglutide and Semaglutide: A Post Hoc Analysis of the LEADER and SUSTAIN 6 Clinical Trials. Diabetes Obes. Metab. 2019, 21, 1745–1751. [Google Scholar] [CrossRef] [PubMed]
- Bin Hussain, A.K.; Abdelgadir, E.; Rashid, F.; Al Haj, A.; Thadani, P.; Bashier, A.M.K. Should Metformin Still Be the First-Line of Treatment in Type 2 Diabetes Mellitus? A Comprehensive Review and Suggested Algorithm. Diabetes Metab. Syndr. 2019, 13, 1935–1942. [Google Scholar] [CrossRef] [PubMed]
- Buse, J.B.; Wexler, D.J.; Tsapas, A.; Rossing, P.; Mingrone, G.; Mathieu, C.; D’Alessio, D.A.; Davies, M.J. 2019 Update to: Management of Hyperglycemia in Type 2 Diabetes, 2018. A Consensus Report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care 2020, 43, 487–493. [Google Scholar] [CrossRef] [PubMed]
- Bailey, C.J.; Day, C. Metformin: Its Botanical Background. Pract. Diabetes Int. 2004, 21, 115–117. [Google Scholar] [CrossRef]
- Thomas, I.; Gregg, B. Metformin; a Review of Its History and Future: From Lilac to Longevity. Pediatr. Diabetes 2017, 18, 10–16. [Google Scholar] [CrossRef]
- Watanabe, C.K. Studies in the metabolism changes induced by administration of guanidine bases: I. Influence of injected guanidine hydrochloride upon blood sugar content. J. Biol. Chem. 1918, 33, 253–265. [Google Scholar] [CrossRef]
- Werner, E.A.; Bell, J. CCXIV.—The Preparation of Methylguanidine, and of Ββ-Dimethylguanidine by the Interaction of Dicyanodiamide, and Methylammonium and Dimethylammonium Chlorides Respectively. J. Chem. Soc. Trans. 1922, 121, 1790–1794. [Google Scholar] [CrossRef]
- Barger, G.; White, F.D. The Constitution of Galegine. Biochem. J. 1923, 17, 827–835. [Google Scholar] [CrossRef] [PubMed]
- Holick, M.F. Vitamin D Deficiency. N. Engl. J. Med. 2007, 357, 266–281. [Google Scholar] [CrossRef] [PubMed]
- Müller, H.; Reinwein, H. Zur Pharmakologie des Galegins. Naunyn-Schmiedebergs Arch. Für Exp. Pathol. Pharmakol. 1927, 125, 212–228. [Google Scholar] [CrossRef]
- Lai, Y.-H.; Fang, T.-C. The Pleiotropic Effect of Vitamin D. Available online: https://www.hindawi.com/journals/isrn/2013/898125/ (accessed on 11 September 2018).
- Sterne, J. [Treatment of diabetes mellitus with N,N-dimethylguanylguanidine (LA. 6023, glucophage)]. Therapie 1959, 14, 625–630. [Google Scholar] [PubMed]
- International Diabetes Federation. Type 2 Diabetes. Available online: https://www.idf.org/our-activities/care-prevention/type-2-diabetes.html (accessed on 22 January 2021).
- He, L.; Wondisford, F.E. Metformin Action: Concentrations Matter. Cell Metab. 2015, 21, 159–162. [Google Scholar] [CrossRef] [PubMed]
- Lunger, L.; Melmer, A.; Oberaigner, W.; Leo, M.; Juchum, M.; Pölzl, K.; Gänzer, J.; Innerebner, M.; Eisendle, E.; Beck, G.; et al. Prescription of Oral Antidiabetic Drugs in Tyrol—Data from the Tyrol Diabetes Registry 2012–2015. Wien. Klin. Wochenschr. 2017, 129, 46–51. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Model List of Essential Medicines. Available online: https://www.who.int/publications-detail-redirect/WHOMVPEMPIAU2019.06 (accessed on 22 January 2021).
- Markowicz-Piasecka, M.; Huttunen, K.M.; Mateusiak, L.; Mikiciuk-Olasik, E.; Sikora, J. Is Metformin a Perfect Drug? Updates in Pharmacokinetics and Pharmacodynamics. Curr. Pharm. Des. 2017, 23, 2532–2550. [Google Scholar] [CrossRef]
- Pernicova, I.; Korbonits, M. Metformin—Mode of Action and Clinical Implications for Diabetes and Cancer. Nat. Rev. Endocrinol. 2014, 10, 143–156. [Google Scholar] [CrossRef] [PubMed]
- Piskovatska, V.; Stefanyshyn, N.; Storey, K.B.; Vaiserman, A.M.; Lushchak, O. Metformin as a Geroprotector: Experimental and Clinical Evidence. Biogerontology 2019, 20, 33–48. [Google Scholar] [CrossRef] [PubMed]
- Nasri, H.; Rafieian-Kopaei, M. Metformin: Current Knowledge. J. Res. Med. Sci. Off. J. Isfahan Univ. Med. Sci. 2014, 19, 658–664. [Google Scholar]
- Rena, G.; Hardie, D.G.; Pearson, E.R. The Mechanisms of Action of Metformin. Diabetologia 2017, 60, 1577–1585. [Google Scholar] [CrossRef]
- Sliwinska, A.; Drzewoski, J. Molecular Action of Metformin in Hepatocytes: An Updated Insight. Curr. Diabetes Rev. 2015, 11, 175–181. [Google Scholar] [CrossRef]
- Ruderman, N.B.; Carling, D.; Prentki, M.; Cacicedo, J.M. AMPK, Insulin Resistance, and the Metabolic Syndrome. J. Clin. Investig. 2013, 123, 2764–2772. [Google Scholar] [CrossRef]
- Natali, A.; Ferrannini, E. Effects of Metformin and Thiazolidinediones on Suppression of Hepatic Glucose Production and Stimulation of Glucose Uptake in Type 2 Diabetes: A Systematic Review. Diabetologia 2006, 49, 434–441. [Google Scholar] [CrossRef] [PubMed]
- Cusi, K.; Consoli, A.; DeFronzo, R.A. Metabolic Effects of Metformin on Glucose and Lactate Metabolism in Noninsulin-Dependent Diabetes Mellitus. J. Clin. Endocrinol. Metab. 1996, 81, 4059–4067. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hundal, R.S.; Krssak, M.; Dufour, S.; Laurent, D.; Lebon, V.; Chandramouli, V.; Inzucchi, S.E.; Schumann, W.C.; Petersen, K.F.; Landau, B.R.; et al. Mechanism by Which Metformin Reduces Glucose Production in Type 2 Diabetes. Diabetes 2000, 49, 2063–2069. [Google Scholar] [CrossRef] [PubMed]
- Inzucchi, S.E.; Maggs, D.G.; Spollett, G.R.; Page, S.L.; Rife, F.S.; Walton, V.; Shulman, G.I. Efficacy and Metabolic Effects of Metformin and Troglitazone in Type II Diabetes Mellitus. N. Engl. J. Med. 1998, 338, 867–872. [Google Scholar] [CrossRef] [PubMed]
- Petersen, M.C.; Vatner, D.F.; Shulman, G.I. Regulation of Hepatic Glucose Metabolism in Health and Disease. Nat. Rev. Endocrinol. 2017, 13, 572–587. [Google Scholar] [CrossRef] [PubMed]
- Song, S.; Andrikopoulos, S.; Filippis, C.; Thorburn, A.W.; Khan, D.; Proietto, J. Mechanism of Fat-Induced Hepatic Gluconeogenesis: Effect of Metformin. Am. J. Physiol. Endocrinol. Metab. 2001, 281, E275–E282. [Google Scholar] [CrossRef] [PubMed]
- Stumvoll, M.; Nurjhan, N.; Perriello, G.; Dailey, G.; Gerich, J.E. Metabolic Effects of Metformin in Non-Insulin-Dependent Diabetes Mellitus. N. Engl. J. Med. 1995, 333, 550–554. [Google Scholar] [CrossRef] [PubMed]
- Lage, R.; Diéguez, C.; Vidal-Puig, A.; López, M. AMPK: A Metabolic Gauge Regulating Whole-Body Energy Homeostasis. Trends Mol. Med. 2008, 14, 539–549. [Google Scholar] [CrossRef]
- Xue, B.; Kahn, B.B. AMPK Integrates Nutrient and Hormonal Signals to Regulate Food Intake and Energy Balance through Effects in the Hypothalamus and Peripheral Tissues. J. Physiol. 2006, 574, 73–83. [Google Scholar] [CrossRef]
- Foretz, M.; Hébrard, S.; Leclerc, J.; Zarrinpashneh, E.; Soty, M.; Mithieux, G.; Sakamoto, K.; Andreelli, F.; Viollet, B. Metformin Inhibits Hepatic Gluconeogenesis in Mice Independently of the LKB1/AMPK Pathway via a Decrease in Hepatic Energy State. J. Clin. Investig. 2010, 120, 2355–2369. [Google Scholar] [CrossRef]
- Foretz, M.; Guigas, B.; Bertrand, L.; Pollak, M.; Viollet, B. Metformin: From Mechanisms of Action to Therapies. Cell Metab. 2014, 20, 953–966. [Google Scholar] [CrossRef]
- An, H.; He, L. Current Understanding of Metformin Effect on the Control of Hyperglycemia in Diabetes. J. Endocrinol. 2016, 228, R97–R106. [Google Scholar] [CrossRef] [PubMed]
- Zhou, G.; Myers, R.; Li, Y.; Chen, Y.; Shen, X.; Fenyk-Melody, J.; Wu, M.; Ventre, J.; Doebber, T.; Fujii, N.; et al. Role of AMP-Activated Protein Kinase in Mechanism of Metformin Action. J. Clin. Investig. 2001, 108, 1167–1174. [Google Scholar] [CrossRef]
- Shaw, R.J.; Lamia, K.A.; Vasquez, D.; Koo, S.-H.; Bardeesy, N.; Depinho, R.A.; Montminy, M.; Cantley, L.C. The Kinase LKB1 Mediates Glucose Homeostasis in Liver and Therapeutic Effects of Metformin. Science 2005, 310, 1642–1646. [Google Scholar] [CrossRef]
- Owen, M.R.; Doran, E.; Halestrap, A.P. Evidence That Metformin Exerts Its Anti-Diabetic Effects through Inhibition of Complex 1 of the Mitochondrial Respiratory Chain. Biochem. J. 2000, 348, 607–614. [Google Scholar] [CrossRef]
- El-Mir, M.Y.; Nogueira, V.; Fontaine, E.; Avéret, N.; Rigoulet, M.; Leverve, X. Dimethylbiguanide Inhibits Cell Respiration via an Indirect Effect Targeted on the Respiratory Chain Complex I. J. Biol. Chem. 2000, 275, 223–228. [Google Scholar] [CrossRef]
- Fontaine, E. Metformin-Induced Mitochondrial Complex I Inhibition: Facts, Uncertainties, and Consequences. Front. Endocrinol. 2018, 9, 753. [Google Scholar] [CrossRef] [PubMed]
- Hou, W.-L.; Yin, J.; Alimujiang, M.; Yu, X.-Y.; Ai, L.-G.; Bao, Y.-Q.; Liu, F.; Jia, W.-P. Inhibition of Mitochondrial Complex I Improves Glucose Metabolism Independently of AMPK Activation. J. Cell. Mol. Med. 2018, 22, 1316–1328. [Google Scholar] [CrossRef] [PubMed]
- Matsuzaki, S.; Humphries, K.M. Selective Inhibition of Deactivated Mitochondrial Complex I by Biguanides. Biochemistry 2015, 54, 2011–2021. [Google Scholar] [CrossRef]
- Viollet, B.; Foretz, M.; Guigas, B.; Horman, S.; Dentin, R.; Bertrand, L.; Hue, L.; Andreelli, F. Activation of AMP-Activated Protein Kinase in the Liver: A New Strategy for the Management of Metabolic Hepatic Disorders. J. Physiol. 2006, 574, 41–53. [Google Scholar] [CrossRef]
- Kulkarni, A.S.; Brutsaert, E.F.; Anghel, V.; Zhang, K.; Bloomgarden, N.; Pollak, M.; Mar, J.C.; Hawkins, M.; Crandall, J.P.; Barzilai, N. Metformin Regulates Metabolic and Nonmetabolic Pathways in Skeletal Muscle and Subcutaneous Adipose Tissues of Older Adults. Aging Cell 2018, 17. [Google Scholar] [CrossRef] [PubMed]
- Musi, N.; Hirshman, M.F.; Nygren, J.; Svanfeldt, M.; Bavenholm, P.; Rooyackers, O.; Zhou, G.; Williamson, J.M.; Ljunqvist, O.; Efendic, S.; et al. Metformin Increases AMP-Activated Protein Kinase Activity in Skeletal Muscle of Subjects with Type 2 Diabetes. Diabetes 2002, 51, 2074–2081. [Google Scholar] [CrossRef] [PubMed]
- Kristensen, J.M.; Treebak, J.T.; Schjerling, P.; Goodyear, L.; Wojtaszewski, J.F.P. Two Weeks of Metformin Treatment Induces AMPK-Dependent Enhancement of Insulin-Stimulated Glucose Uptake in Mouse Soleus Muscle. Am. J. Physiol. Endocrinol. Metab. 2014, 306, E1099–E1109. [Google Scholar] [CrossRef] [PubMed]
- Zang, M.; Zuccollo, A.; Hou, X.; Nagata, D.; Walsh, K.; Herscovitz, H.; Brecher, P.; Ruderman, N.B.; Cohen, R.A. AMP-Activated Protein Kinase Is Required for the Lipid-Lowering Effect of Metformin in Insulin-Resistant Human HepG2 Cells. J. Biol. Chem. 2004, 279, 47898–47905. [Google Scholar] [CrossRef]
- Cool, B.; Zinker, B.; Chiou, W.; Kifle, L.; Cao, N.; Perham, M.; Dickinson, R.; Adler, A.; Gagne, G.; Iyengar, R.; et al. Identification and Characterization of a Small Molecule AMPK Activator That Treats Key Components of Type 2 Diabetes and the Metabolic Syndrome. Cell Metab. 2006, 3, 403–416. [Google Scholar] [CrossRef] [PubMed]
- Bourron, O.; Daval, M.; Hainault, I.; Hajduch, E.; Servant, J.M.; Gautier, J.F.; Ferré, P.; Foufelle, F. Biguanides and Thiazolidinediones Inhibit Stimulated Lipolysis in Human Adipocytes through Activation of AMP-Activated Protein Kinase. Diabetologia 2010, 53, 768–778. [Google Scholar] [CrossRef] [PubMed]
- Cao, J.; Meng, S.; Chang, E.; Beckwith-Fickas, K.; Xiong, L.; Cole, R.N.; Radovick, S.; Wondisford, F.E.; He, L. Low Concentrations of Metformin Suppress Glucose Production in Hepatocytes through AMP-Activated Protein Kinase (AMPK). J. Biol. Chem. 2014, 289, 20435–20446. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.-S.; Jiang, B.; Li, M.; Zhu, M.; Peng, Y.; Zhang, Y.-L.; Wu, Y.-Q.; Li, T.Y.; Liang, Y.; Lu, Z.; et al. The Lysosomal V-ATPase-Ragulator Complex Is a Common Activator for AMPK and MTORC1, Acting as a Switch between Catabolism and Anabolism. Cell Metab. 2014, 20, 526–540. [Google Scholar] [CrossRef] [PubMed]
- Miller, R.A.; Chu, Q.; Xie, J.; Foretz, M.; Viollet, B.; Birnbaum, M.J. Biguanides Suppress Hepatic Glucagon Signalling by Decreasing Production of Cyclic AMP. Nature 2013, 494, 256–260. [Google Scholar] [CrossRef]
- McCreight, L.J.; Bailey, C.J.; Pearson, E.R. Metformin and the Gastrointestinal Tract. Diabetologia 2016, 59, 426–435. [Google Scholar] [CrossRef] [PubMed]
- Bailey, C.J.; Wilcock, C.; Scarpello, J.H.B. Metformin and the Intestine. Diabetologia 2008, 51, 1552–1553. [Google Scholar] [CrossRef]
- Minamii, T.; Nogami, M.; Ogawa, W. Mechanisms of Metformin Action: In and out of the Gut. J. Diabetes Investig. 2018, 9, 701–703. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Rangel, E.; Inzucchi, S.E. Metformin: Clinical Use in Type 2 Diabetes. Diabetologia 2017, 60, 1586–1593. [Google Scholar] [CrossRef] [PubMed]
- Bonnet, F.; Scheen, A. Understanding and Overcoming Metformin Gastrointestinal Intolerance. Diabetes Obes. Metab. 2017, 19, 473–481. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, J.; Hiel, S.; Delzenne, N.M. Metformin: Old Friend, New Ways of Action-Implication of the Gut Microbiome? Curr. Opin. Clin. Nutr. Metab. Care 2018, 21, 294–301. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.; Thazhath, S.S.; Bound, M.J.; Jones, K.L.; Horowitz, M.; Rayner, C.K. Mechanism of Increase in Plasma Intact GLP-1 by Metformin in Type 2 Diabetes: Stimulation of GLP-1 Secretion or Reduction in Plasma DPP-4 Activity? Diabetes Res. Clin. Pract. 2014, 106, e3–e6. [Google Scholar] [CrossRef] [PubMed]
- Thondam, S.K.; Cross, A.; Cuthbertson, D.J.; Wilding, J.P.; Daousi, C. Effects of Chronic Treatment with Metformin on Dipeptidyl Peptidase-4 Activity, Glucagon-like Peptide 1 and Ghrelin in Obese Patients with Type 2 Diabetes Mellitus. Diabet. Med. J. Br. Diabet. Assoc. 2012, 29, e205–e210. [Google Scholar] [CrossRef] [PubMed]
- Pollak, M. The Effects of Metformin on Gut Microbiota and the Immune System as Research Frontiers. Diabetologia 2017, 60, 1662–1667. [Google Scholar] [CrossRef]
- Duca, F.A.; Côté, C.D.; Rasmussen, B.A.; Zadeh-Tahmasebi, M.; Rutter, G.A.; Filippi, B.M.; Lam, T.K.T. Metformin Activates a Duodenal Ampk-Dependent Pathway to Lower Hepatic Glucose Production in Rats. Nat. Med. 2015, 21, 506–511. [Google Scholar] [CrossRef] [PubMed]
- Wiernsperger, N.F.; Bailey, C.J. The Antihyperglycaemic Effect of Metformin: Therapeutic and Cellular Mechanisms. Drugs 1999, 58, 31–39. [Google Scholar] [CrossRef]
- DeFronzo, R.A. Pathogenesis of Type 2 Diabetes Mellitus. Med. Clin. North. Am. 2004, 88, 787–835. [Google Scholar] [CrossRef]
- Goldstein, B.J. Insulin Resistance as the Core Defect in Type 2 Diabetes Mellitus. Am. J. Cardiol. 2002, 90, 3G–10G. [Google Scholar] [CrossRef]
- Gunton, J.E.; Delhanty, P.J.D.; Takahashi, S.-I.; Baxter, R.C. Metformin Rapidly Increases Insulin Receptor Activation in Human Liver and Signals Preferentially through Insulin-Receptor Substrate-2. J. Clin. Endocrinol. Metab. 2003, 88, 1323–1332. [Google Scholar] [CrossRef] [PubMed]
- Zimmet, P.; Collier, G. Clinical Efficacy of Metformin against Insulin Resistance Parameters: Sinking the Iceberg. Drugs 1999, 58, 21–28. [Google Scholar] [CrossRef] [PubMed]
- Giannarelli, R.; Aragona, M.; Coppelli, A.; Del Prato, S. Reducing Insulin Resistance with Metformin: The Evidence Today. Diabetes Metab. 2003, 29, 6S28–6S35. [Google Scholar] [CrossRef]
- Hundal, H.S.; Ramlal, T.; Reyes, R.; Leiter, L.A.; Klip, A. Cellular Mechanism of Metformin Action Involves Glucose Transporter Translocation from an Intracellular Pool to the Plasma Membrane in L6 Muscle Cells. Endocrinology 1992, 131, 1165–1173. [Google Scholar] [CrossRef]
- Matthaei, S.; Greten, H. Evidence That Metformin Ameliorates Cellular Insulin-Resistance by Potentiating Insulin-Induced Translocation of Glucose Transporters to the Plasma Membrane. Diabete Metab. 1991, 17, 150–158. [Google Scholar] [PubMed]
- Matthaei, S.; Reibold, J.P.; Hamann, A.; Benecke, H.; Häring, H.U.; Greten, H.; Klein, H.H. In Vivo Metformin Treatment Ameliorates Insulin Resistance: Evidence for Potentiation of Insulin-Induced Translocation and Increased Functional Activity of Glucose Transporters in Obese (Fa/Fa) Zucker Rat Adipocytes. Endocrinology 1993, 133, 304–311. [Google Scholar] [CrossRef]
- Yazıcı, D.; Sezer, H. Insulin Resistance, Obesity and Lipotoxicity. Adv. Exp. Med. Biol. 2017, 960, 277–304. [Google Scholar] [CrossRef] [PubMed]
- Bergman, R.N.; Mittelman, S.D. Central Role of the Adipocyte in Insulin Resistance. J. Basic Clin. Physiol. Pharmacol. 1998, 9, 205–221. [Google Scholar] [CrossRef] [PubMed]
- Engin, A.B. What Is Lipotoxicity? Adv. Exp. Med. Biol. 2017, 960, 197–220. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Li, X.W.; Chen, D.Z.; Hao, F.; Tao, S.X.; Yu, H.Y.; Cheng, R.; Liu, H. Neuro-Protective Role of Metformin in Patients with Acute Stroke and Type 2 Diabetes Mellitus via AMPK/Mammalian Target of Rapamycin (MTOR) Signaling Pathway and Oxidative Stress. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 2019, 25, 2186–2194. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Massey, S.; Story, D.; Li, L. Metformin: An Old Drug with New Applications. Int. J. Mol. Sci. 2018, 19, 2863. [Google Scholar] [CrossRef] [PubMed]
- Moon, J.S.; Karunakaran, U.; Elumalai, S.; Lee, I.-K.; Lee, H.W.; Kim, Y.-W.; Won, K.C. Metformin Prevents Glucotoxicity by Alleviating Oxidative and ER Stress-Induced CD36 Expression in Pancreatic Beta Cells. J. Diabetes Complicat. 2017, 31, 21–30. [Google Scholar] [CrossRef] [PubMed]
- Papachristoforou, E.; Lambadiari, V.; Maratou, E.; Makrilakis, K. Association of Glycemic Indices (Hyperglycemia, Glucose Variability, and Hypoglycemia) with Oxidative Stress and Diabetic Complications. J. Diabetes Res. 2020, 2020, 7489795. [Google Scholar] [CrossRef] [PubMed]
- Podhorecka, M.; Ibanez, B.; Dmoszyńska, A. Metformin—Its Potential Anti-Cancer and Anti-Aging Effects. Postepy Hig. Med. Dosw. Online 2017, 71, 170–175. [Google Scholar] [CrossRef]
- Mallik, R.; Chowdhury, T.A. Metformin in Cancer. Diabetes Res. Clin. Pract. 2018, 143, 409–419. [Google Scholar] [CrossRef] [PubMed]
- Fitch, K.; Abbara, S.; Lee, H.; Stavrou, E.; Sacks, R.; Michel, T.; Hemphill, L.; Torriani, M.; Grinspoon, S. Effects of Lifestyle Modification and Metformin on Atherosclerotic Indices among HIV-Infected Patients with the Metabolic Syndrome. AIDS 2012, 26, 587–597. [Google Scholar] [CrossRef] [PubMed]
- Van Wagner, L.B.; Rinella, M.E. The Role of Insulin-Sensitizing Agents in the Treatment of Nonalcoholic Steatohepatitis. Ther. Adv. Gastroenterol. 2011, 4, 249–263. [Google Scholar] [CrossRef]
- Daniele, G.; Abdul-Ghani, M.; DeFronzo, R.A. What Are the Pharmacotherapy Options for Treating Prediabetes? Expert Opin. Pharmacother. 2014, 15, 2003–2018. [Google Scholar] [CrossRef] [PubMed]
- Romualdi, D.; Versace, V.; Lanzone, A. What Is New in the Landscape of Insulin-Sensitizing Agents for Polycystic Ovary Syndrome Treatment. Ther. Adv. Reprod. Health 2020, 14, 2633494120908709. [Google Scholar] [CrossRef] [PubMed]
- Ludvigsson, J. The Latest Pharmacotherapy Options for Type 1 Diabetes. Expert Opin. Pharmacother. 2014, 15, 37–49. [Google Scholar] [CrossRef] [PubMed]
- Livingstone, R.; Boyle, J.G.; Petrie, J.R. REMOVAL Study Team A New Perspective on Metformin Therapy in Type 1 Diabetes. Diabetologia 2017, 60, 1594–1600. [Google Scholar] [CrossRef] [PubMed]
- Saisho, Y. Metformin and Inflammation: Its Potential Beyond Glucose-Lowering Effect. Endocr. Metab. Immune Disord. Drug Targets 2015, 15, 196–205. [Google Scholar] [CrossRef]
- Kuryłowicz, A.; Koźniewski, K. Anti-Inflammatory Strategies Targeting Metaflammation in Type 2 Diabetes. Molecules 2020, 25, 2224. [Google Scholar] [CrossRef]
- Pu, R.; Shi, D.; Gan, T.; Ren, X.; Ba, Y.; Huo, Y.; Bai, Y.; Zheng, T.; Cheng, N. Effects of Metformin in Obesity Treatment in Different Populations: A Meta-Analysis. Ther. Adv. Endocrinol. Metab. 2020, 11, 2042018820926000. [Google Scholar] [CrossRef] [PubMed]
- Hamidi Shishavan, M.; Henning, R.H.; van Buiten, A.; Goris, M.; Deelman, L.E.; Buikema, H. Metformin Improves Endothelial Function and Reduces Blood Pressure in Diabetic Spontaneously Hypertensive Rats Independent from Glycemia Control: Comparison to Vildagliptin. Sci. Rep. 2017, 7, 10975. [Google Scholar] [CrossRef] [PubMed]
- Landin-Wilhelmsen, K. Metformin and Blood Pressure. J. Clin. Pharm. Ther. 1992, 17, 75–79. [Google Scholar] [CrossRef] [PubMed]
- Randriamboavonjy, V.; Mann, W.A.; Elgheznawy, A.; Popp, R.; Rogowski, P.; Dornauf, I.; Dröse, S.; Fleming, I. Metformin Reduces Hyper-Reactivity of Platelets from Patients with Polycystic Ovary Syndrome by Improving Mitochondrial Integrity. Thromb. Haemost. 2015, 114, 569–578. [Google Scholar] [CrossRef] [PubMed]
- Coyle, C.; Cafferty, F.H.; Vale, C.; Langley, R.E. Metformin as an Adjuvant Treatment for Cancer: A Systematic Review and Meta-Analysis. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2016, 27, 2184–2195. [Google Scholar] [CrossRef]
- Vancura, A.; Bu, P.; Bhagwat, M.; Zeng, J.; Vancurova, I. Metformin as an Anticancer Agent. Trends Pharmacol. Sci. 2018, 39, 867–878. [Google Scholar] [CrossRef] [PubMed]
- Kothari, V.; Galdo, J.A.; Mathews, S.T. Hypoglycemic Agents and Potential Anti-Inflammatory Activity. J. Inflamm. Res. 2016, 9, 27–38. [Google Scholar] [CrossRef]
- Sobel, B.E.; Hardison, R.M.; Genuth, S.; Brooks, M.M.; McBane, R.D.; Schneider, D.J.; Pratley, R.E.; Huber, K.; Wolk, R.; Krishnaswami, A.; et al. Profibrinolytic, Antithrombotic, and Antiinflammatory Effects of an Insulin-Sensitizing Strategy in Patients in the Bypass Angioplasty Revascularization Investigation 2 Diabetes (BARI 2D) Trial. Circulation 2011, 124, 695–703. [Google Scholar] [CrossRef] [PubMed]
- Krysiak, R.; Okopien, B. The Effect of Metformin on Monocyte Secretory Function in Simvastatin-Treated Patients with Impaired Fasting Glucose. Metabolism 2013, 62, 39–43. [Google Scholar] [CrossRef] [PubMed]
- Krysiak, R.; Okopien, B. Lymphocyte-Suppressing and Systemic Anti-Inflammatory Effects of High-Dose Metformin in Simvastatin-Treated Patients with Impaired Fasting Glucose. Atherosclerosis 2012, 225, 403–407. [Google Scholar] [CrossRef] [PubMed]
- Brownlee, M. The Pathobiology of Diabetic Complications: A Unifying Mechanism. Diabetes 2005, 54, 1615–1625. [Google Scholar] [CrossRef]
- Signorini, A.M.; Fondelli, C.; Renzoni, E.; Puccetti, C.; Gragnoli, G.; Giorgi, G. Antioxidant Effects of Gliclazide, Glibenclamide, and Metformin in Patients with Type 2 Diabetes Mellitus. Curr. Ther. Res. 2002, 63, 411–420. [Google Scholar] [CrossRef]
- Esteghamati, A.; Eskandari, D.; Mirmiranpour, H.; Noshad, S.; Mousavizadeh, M.; Hedayati, M.; Nakhjavani, M. Effects of Metformin on Markers of Oxidative Stress and Antioxidant Reserve in Patients with Newly Diagnosed Type 2 Diabetes: A Randomized Clinical Trial. Clin. Nutr. 2013, 32, 179–185. [Google Scholar] [CrossRef]
- Araújo, A.A.D.; Pereira, A.D.S.B.F.; Medeiros, C.A.C.X.D.; Brito, G.A.D.C.; Leitão, R.F.D.C.; Araújo, L.D.S.; Araújo Júnior, R.F.D. Effects of Metformin on Inflammation, Oxidative Stress, and Bone Loss in a Rat Model of Periodontitis. PLoS ONE 2017, 12, e0183506. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Thompson, J.; Hu, Y.; Das, A.; Lesnefsky, E.J. Metformin Attenuates ER Stress-Induced Mitochondrial Dysfunction. Transl. Res. J. Lab. Clin. Med. 2017, 190, 40–50. [Google Scholar] [CrossRef]
- Singh, R.K.; Gupta, B.; Tripathi, K.; Singh, S.K. Anti Oxidant Potential of Metformin and Pioglitazone in Type 2 Diabetes Mellitus: Beyond Their Anti Glycemic Effect. Diabetes Metab. Syndr. 2016, 10, 102–104. [Google Scholar] [CrossRef] [PubMed]
- International Diabetes Federation. Diabetes Atlas, 9th ed.; International Diabetes Federation: Brussels, Belgium, 2019; Available online: https://www.diabetesatlas.org/en/ (accessed on 22 January 2021).
- Rydén, L.; Mellbin, L. Glucose Perturbations and Cardiovascular Risk: Challenges and Opportunities. Diab. Vasc. Dis. Res. 2012, 9, 170–176. [Google Scholar] [CrossRef]
- Hostalek, U.; Gwilt, M.; Hildemann, S. Therapeutic Use of Metformin in Prediabetes and Diabetes Prevention. Drugs 2015, 75, 1071–1094. [Google Scholar] [CrossRef] [PubMed]
- Knowler, W.C.; Barrett-Connor, E.; Fowler, S.E.; Hamman, R.F.; Lachin, J.M.; Walker, E.A.; Nathan, D.M. Diabetes Prevention Program Research Group Reduction in the Incidence of Type 2 Diabetes with Lifestyle Intervention or Metformin. N. Engl. J. Med. 2002, 346, 393–403. [Google Scholar] [CrossRef] [PubMed]
- Moin, T.; Schmittdiel, J.A.; Flory, J.H.; Yeh, J.; Karter, A.J.; Kruge, L.E.; Schillinger, D.; Mangione, C.M.; Herman, W.H.; Walker, E.A. Review of Metformin Use for Type 2 Diabetes Prevention. Am. J. Prev. Med. 2018, 55, 565–574. [Google Scholar] [CrossRef] [PubMed]
- American Diabetes Association 3. Prevention or Delay of Type 2 Diabetes: Standards of Medical Care in Diabetes—2020. Diabetes Care 2020, 43, S32–S36. [Google Scholar] [CrossRef] [PubMed]
- Cree-Green, M.; Bergman, B.C.; Cengiz, E.; Fox, L.A.; Hannon, T.S.; Miller, K.; Nathan, B.; Pyle, L.; Kahn, D.; Tansey, M.; et al. Metformin Improves Peripheral Insulin Sensitivity in Youth with Type 1 Diabetes. J. Clin. Endocrinol. Metab. 2019, 104, 3265–3278. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, J.; Cummings, E.; Zdravkovic, V.; Finegood, D.; Daneman, D. Metformin as an Adjunct Therapy in Adolescents with Type 1 Diabetes and Insulin Resistance: A Randomized Controlled Trial. Diabetes Care 2003, 26, 138–143. [Google Scholar] [CrossRef] [PubMed]
- Gin, H.; Messerchmitt, C.; Brottier, E.; Aubertin, J. Metformin Improved Insulin Resistance in Type I, Insulin-Dependent, Diabetic Patients. Metabolism 1985, 34, 923–925. [Google Scholar] [CrossRef]
- Nadeau, K.J.; Chow, K.; Alam, S.; Lindquist, K.; Campbell, S.; McFann, K.; Klingensmith, G.; Walravens, P. Effects of Low Dose Metformin in Adolescents with Type I Diabetes Mellitus: A Randomized, Double-Blinded Placebo-Controlled Study. Pediatr. Diabetes 2015, 16, 196–203. [Google Scholar] [CrossRef]
- Meyer, L.; Bohme, P.; Delbachian, I.; Lehert, P.; Cugnardey, N.; Drouin, P.; Guerci, B. The Benefits of Metformin Therapy during Continuous Subcutaneous Insulin Infusion Treatment of Type 1 Diabetic Patients. Diabetes Care 2002, 25, 2153–2158. [Google Scholar] [CrossRef]
- Lund, S.S.; Tarnow, L.; Astrup, A.S.; Hovind, P.; Jacobsen, P.K.; Alibegovic, A.C.; Parving, I.; Pietraszek, L.; Frandsen, M.; Rossing, P.; et al. Effect of Adjunct Metformin Treatment in Patients with Type-1 Diabetes and Persistent Inadequate Glycaemic Control. A Randomized Study. PLoS ONE 2008, 3, e3363. [Google Scholar] [CrossRef]
- Vella, S.; Buetow, L.; Royle, P.; Livingstone, S.; Colhoun, H.M.; Petrie, J.R. The Use of Metformin in Type 1 Diabetes: A Systematic Review of Efficacy. Diabetologia 2010, 53, 809–820. [Google Scholar] [CrossRef] [PubMed]
- Libman, I.M.; Miller, K.M.; DiMeglio, L.A.; Bethin, K.E.; Katz, M.L.; Shah, A.; Simmons, J.H.; Haller, M.J.; Raman, S.; Tamborlane, W.V.; et al. Effect of Metformin Added to Insulin on Glycemic Control Among Overweight/Obese Adolescents with Type 1 Diabetes: A Randomized Clinical Trial. JAMA 2015, 314, 2241–2250. [Google Scholar] [CrossRef] [PubMed]
- Dandona, P.; Mathieu, C.; Phillip, M.; Hansen, L.; Tschöpe, D.; Thorén, F.; Xu, J.; Langkilde, A.M. DEPICT-1 Investigators Efficacy and Safety of Dapagliflozin in Patients with Inadequately Controlled Type 1 Diabetes: The DEPICT-1 52-Week Study. Diabetes Care 2018, 41, 2552–2559. [Google Scholar] [CrossRef]
- Rosenstock, J.; Marquard, J.; Laffel, L.M.; Neubacher, D.; Kaspers, S.; Cherney, D.Z.; Zinman, B.; Skyler, J.S.; George, J.; Soleymanlou, N.; et al. Empagliflozin as Adjunctive to Insulin Therapy in Type 1 Diabetes: The EASE Trials. Diabetes Care 2018, 41, 2560–2569. [Google Scholar] [CrossRef] [PubMed]
- Riddle, M.C.; Cefalu, W.T. SGLT Inhibitors for Type 1 Diabetes: An Obvious Choice or Too Good to Be True? Diabetes Care 2018, 41, 2444–2447. [Google Scholar] [CrossRef]
- Pfützner, A.; Klonoff, D.; Heinemann, L.; Ejskjaer, N.; Pickup, J. Euglycemic Ketosis in Patients with Type 2 Diabetes on SGLT2-Inhibitor Therapy-an Emerging Problem and Solutions Offered by Diabetes Technology. Endocrine 2017, 56, 212–216. [Google Scholar] [CrossRef] [PubMed]
- Ueda, P.; Svanström, H.; Melbye, M.; Eliasson, B.; Svensson, A.-M.; Franzén, S.; Gudbjörnsdottir, S.; Hveem, K.; Jonasson, C.; Pasternak, B. Sodium Glucose Cotransporter 2 Inhibitors and Risk of Serious Adverse Events: Nationwide Register Based Cohort Study. BMJ 2018, 363, k4365. [Google Scholar] [CrossRef]
- Cesta, C.E.; Cohen, J.M.; Pazzagli, L.; Bateman, B.T.; Bröms, G.; Einarsdóttir, K.; Furu, K.; Havard, A.; Heino, A.; Hernandez-Diaz, S.; et al. Antidiabetic Medication Use during Pregnancy: An International Utilization Study. BMJ Open Diabetes Res. Care 2019, 7, e000759. [Google Scholar] [CrossRef]
- Ainuddin, J.; Karim, N.; Hasan, A.A.; Naqvi, S.A. Metformin versus Insulin Treatment in Gestational Diabetes in Pregnancy in a Developing Country: A Randomized Control Trial. Diabetes Res. Clin. Pract. 2015, 107, 290–299. [Google Scholar] [CrossRef] [PubMed]
- Lindsay, R.S.; Loeken, M.R. Metformin Use in Pregnancy: Promises and Uncertainties. Diabetologia 2017, 60, 1612–1619. [Google Scholar] [CrossRef] [PubMed]
- Ainuddin, J.A.; Karim, N.; Zaheer, S.; Ali, S.S.; Hasan, A.A. Metformin Treatment in Type 2 Diabetes in Pregnancy: An Active Controlled, Parallel-Group, Randomized, Open Label Study in Patients with Type 2 Diabetes in Pregnancy. J. Diabetes Res. 2015, 2015, 325851. [Google Scholar] [CrossRef] [PubMed]
- Rowan, J.A.; Hague, W.M.; Gao, W.; Battin, M.R.; Moore, M.P. MiG Trial Investigators Metformin versus Insulin for the Treatment of Gestational Diabetes. N. Engl. J. Med. 2008, 358, 2003–2015. [Google Scholar] [CrossRef]
- Rowan, J.A.; Rush, E.C.; Plank, L.D.; Lu, J.; Obolonkin, V.; Coat, S.; Hague, W.M. Metformin in Gestational Diabetes: The Offspring Follow-up (MiG TOFU): Body Composition and Metabolic Outcomes at 7–9 Years of Age. BMJ Open Diabetes Res. Care 2018, 6, e000456. [Google Scholar] [CrossRef] [PubMed]
- Dandona, P.; Mathieu, C.; Phillip, M.; Hansen, L.; Griffen, S.C.; Tschöpe, D.; Thorén, F.; Xu, J.; Langkilde, A.M. DEPICT-1 Investigators Efficacy and Safety of Dapagliflozin in Patients with Inadequately Controlled Type 1 Diabetes (DEPICT-1): 24 Week Results from a Multicentre, Double-Blind, Phase 3, Randomised Controlled Trial. Lancet Diabetes Endocrinol. 2017, 5, 864–876. [Google Scholar] [CrossRef]
- Henry, R.R.; Thakkar, P.; Tong, C.; Polidori, D.; Alba, M. Efficacy and Safety of Canagliflozin, a Sodium-Glucose Cotransporter 2 Inhibitor, as Add-on to Insulin in Patients with Type 1 Diabetes. Diabetes Care 2015, 38, 2258–2265. [Google Scholar] [CrossRef]
- Garg, S.K.; Henry, R.R.; Banks, P.; Buse, J.B.; Davies, M.J.; Fulcher, G.R.; Pozzilli, P.; Gesty-Palmer, D.; Lapuerta, P.; Simó, R.; et al. Effects of Sotagliflozin Added to Insulin in Patients with Type 1 Diabetes. N. Engl. J. Med. 2017, 377, 2337–2348. [Google Scholar] [CrossRef] [PubMed]
- Sam, S.; Ehrmann, D.A. Metformin Therapy for the Reproductive and Metabolic Consequences of Polycystic Ovary Syndrome. Diabetologia 2017, 60, 1656–1661. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Liu, X.; Zhang, W. The Effect of Metformin Therapy for Preventing Gestational Diabetes Mellitus in Women with Polycystic Ovary Syndrome: A Meta-Analysis. Exp. Clin. Endocrinol. Diabetes Off. J. Ger. Soc. Endocrinol. Ger. Diabetes Assoc. 2020, 128, 199–205. [Google Scholar] [CrossRef] [PubMed]
- Balen, A.H.; Morley, L.C.; Misso, M.; Franks, S.; Legro, R.S.; Wijeyaratne, C.N.; Stener-Victorin, E.; Fauser, B.C.J.M.; Norman, R.J.; Teede, H. The Management of Anovulatory Infertility in Women with Polycystic Ovary Syndrome: An Analysis of the Evidence to Support the Development of Global WHO Guidance. Hum. Reprod. Update 2016, 22, 687–708. [Google Scholar] [CrossRef] [PubMed]
- Zeng, X.-L.; Zhang, Y.-F.; Tian, Q.; Xue, Y.; An, R.-F. Effects of Metformin on Pregnancy Outcomes in Women with Polycystic Ovary Syndrome: A Meta-Analysis. Medicine 2016, 95, e4526. [Google Scholar] [CrossRef]
- Palomba, S.; Falbo, A.; Zullo, F.; Orio, F. Evidence-Based and Potential Benefits of Metformin in the Polycystic Ovary Syndrome: A Comprehensive Review. Endocr. Rev. 2009, 30, 1–50. [Google Scholar] [CrossRef] [PubMed]
- Doi, S.A.R.; Furuya-Kanamori, L.; Toft, E.; Musa, O.A.H.; Islam, N.; Clark, J.; Thalib, L. Metformin in Pregnancy to Avert Gestational Diabetes in Women at High Risk: Meta-Analysis of Randomized Controlled Trials. Obes. Rev. Off. J. Int. Assoc. Study Obes. 2020, 21, e12964. [Google Scholar] [CrossRef]
- Tanbo, T.; Mellembakken, J.; Bjercke, S.; Ring, E.; Åbyholm, T.; Fedorcsak, P. Ovulation Induction in Polycystic Ovary Syndrome. Acta Obstet. Gynecol. Scand. 2018, 97, 1162–1167. [Google Scholar] [CrossRef] [PubMed]
- Javed, Z.; Papageorgiou, M.; Deshmukh, H.; Rigby, A.S.; Qamar, U.; Abbas, J.; Khan, A.Y.; Kilpatrick, E.S.; Atkin, S.L.; Sathyapalan, T. Effects of Empagliflozin on Metabolic Parameters in Polycystic Ovary Syndrome: A Randomized Controlled Study. Clin. Endocrinol. 2019, 90, 805–813. [Google Scholar] [CrossRef] [PubMed]
- Jensterle, M.; Kocjan, T.; Kravos, N.A.; Pfeifer, M.; Janez, A. Short-Term Intervention with Liraglutide Improved Eating Behavior in Obese Women with Polycystic Ovary Syndrome. Endocr. Res. 2015, 40, 133–138. [Google Scholar] [CrossRef]
- Han, Y.; Li, Y.; He, B. GLP-1 Receptor Agonists versus Metformin in PCOS: A Systematic Review and Meta-Analysis. Reprod. Biomed. Online 2019, 39, 332–342. [Google Scholar] [CrossRef] [PubMed]
- Öztürk, B.; Gürbüz, A.S.; Durak, Z.E.; Öztürk, H.S. Dipeptidyl Peptidase-4 and Adenosine Deaminase Enzyme Levels in Polycystic Ovary Syndrome. Gynecol. Endocrinol. Off. J. Int. Soc. Gynecol. Endocrinol. 2019, 35, 138–141. [Google Scholar] [CrossRef] [PubMed]
- Ferjan, S.; Janez, A.; Jensterle, M. Dpp4 Inhibitor Sitagliptin as a Potential Treatment Option in Metformin-Intolerant Obese Women with Polycystic Ovary Syndrome: A Pilot Randomized Study. Endocr. Pract. Off. J. Am. Coll. Endocrinol. Am. Assoc. Clin. Endocrinol. 2018, 24, 69–77. [Google Scholar] [CrossRef]
- Ferjan, S.; Janez, A.; Jensterle, M. Dipeptidyl Peptidase-4 Inhibitor Sitagliptin Prevented Weight Regain in Obese Women with Polycystic Ovary Syndrome Previously Treated with Liraglutide: A Pilot Randomized Study. Metab. Syndr. Relat. Disord. 2017, 15, 515–520. [Google Scholar] [CrossRef]
- Zhu, X.; Yan, H.; Xia, M.; Chang, X.; Xu, X.; Wang, L.; Sun, X.; Lu, Y.; Bian, H.; Li, X.; et al. Metformin Attenuates Triglyceride Accumulation in HepG2 Cells through Decreasing Stearyl-Coenzyme A Desaturase 1 Expression. Lipids Health Dis. 2018, 17, 114. [Google Scholar] [CrossRef] [PubMed]
- Smith, B.K.; Marcinko, K.; Desjardins, E.M.; Lally, J.S.; Ford, R.J.; Steinberg, G.R. Treatment of Nonalcoholic Fatty Liver Disease: Role of AMPK. Am. J. Physiol. Endocrinol. Metab. 2016, 311, E730–E740. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, M.J.; Gaunt, P.; Aithal, G.P.; Barton, D.; Hull, D.; Parker, R.; Hazlehurst, J.M.; Guo, K.; LEAN Trial Team; Abouda, G.; et al. Liraglutide Safety and Efficacy in Patients with Non-Alcoholic Steatohepatitis (LEAN): A Multicentre, Double-Blind, Randomised, Placebo-Controlled Phase 2 Study. Lancet 2016, 387, 679–690. [Google Scholar] [CrossRef]
- Takase, T.; Nakamura, A.; Miyoshi, H.; Yamamoto, C.; Atsumi, T. Amelioration of Fatty Liver Index in Patients with Type 2 Diabetes on Ipragliflozin: An Association with Glucose-Lowering Effects. Endocr. J. 2017, 64, 363–367. [Google Scholar] [CrossRef] [PubMed]
- Turner, R.C.; Millns, H.; Neil, H.A.; Stratton, I.M.; Manley, S.E.; Matthews, D.R.; Holman, R.R. Risk Factors for Coronary Artery Disease in Non-Insulin Dependent Diabetes Mellitus: United Kingdom Prospective Diabetes Study (UKPDS: 23). BMJ 1998, 316, 823–828. [Google Scholar] [CrossRef] [PubMed]
- DECODE Study Group; European Diabetes Epidemiology Group. Is the Current Definition for Diabetes Relevant to Mortality Risk from All Causes and Cardiovascular and Noncardiovascular Diseases? Diabetes Care 2003, 26, 688–696. [Google Scholar] [CrossRef]
- Booth, G.L.; Kapral, M.K.; Fung, K.; Tu, J.V. Relation between Age and Cardiovascular Disease in Men and Women with Diabetes Compared with Non-Diabetic People: A Population-Based Retrospective Cohort Study. Lancet London England 2006, 368, 29–36. [Google Scholar] [CrossRef]
- Eeg-Olofsson, K.; Cederholm, J.; Nilsson, P.M.; Zethelius, B.; Svensson, A.-M.; Gudbjörnsdóttir, S.; Eliasson, B. New Aspects of HbA1c as a Risk Factor for Cardiovascular Diseases in Type 2 Diabetes: An Observational Study from the Swedish National Diabetes Register (NDR). J. Intern. Med. 2010, 268, 471–482. [Google Scholar] [CrossRef]
- Sakurai, M.; Saitoh, S.; Miura, K.; Nakagawa, H.; Ohnishi, H.; Akasaka, H.; Kadota, A.; Kita, Y.; Hayakawa, T.; Ohkubo, T.; et al. HbA1c and the Risks for All-Cause and Cardiovascular Mortality in the General Japanese Population: NIPPON DATA90. Diabetes Care 2013, 36, 3759–3765. [Google Scholar] [CrossRef]
- Hesen, N.A.; Riksen, N.P.; Aalders, B.; Brouwer, M.A.; Ritskes-Hoitinga, M.; El Messaoudi, S.; Wever, K.E. A Systematic Review and Meta-Analysis of the Protective Effects of Metformin in Experimental Myocardial Infarction. PLoS ONE 2017, 12, e0183664. [Google Scholar] [CrossRef] [PubMed]
- Zilov, A.V.; Abdelaziz, S.I.; AlShammary, A.; Al Zahrani, A.; Amir, A.; Assaad Khalil, S.H.; Brand, K.; Elkafrawy, N.; Hassoun, A.A.K.; Jahed, A.; et al. Mechanisms of Action of Metformin with Special Reference to Cardiovascular Protection. Diabetes Metab. Res. Rev. 2019, 35, e3173. [Google Scholar] [CrossRef] [PubMed]
- Pryor, R.; Cabreiro, F. Repurposing Metformin: An Old Drug with New Tricks in Its Binding Pockets. Biochem. J. 2015, 471, 307–322. [Google Scholar] [CrossRef]
- Nesti, L.; Natali, A. Metformin Effects on the Heart and the Cardiovascular System: A Review of Experimental and Clinical Data. Nutr. Metab. Cardiovasc. Dis. NMCD 2017, 27, 657–669. [Google Scholar] [CrossRef] [PubMed]
- Paneni, F.; Costantino, S.; Cosentino, F. [Metformin and left ventricular remodeling after acute myocardial infarction: Molecular mechanisms and clinical implications]. G. Ital. Cardiol. 2006 2015, 16, 225–231. [Google Scholar] [CrossRef]
- Dziubak, A.; Wójcicka, G.; Wojtak, A.; Bełtowski, J. Metabolic Effects of Metformin in the Failing Heart. Int. J. Mol. Sci. 2018, 19, 2869. [Google Scholar] [CrossRef] [PubMed]
- Gundewar, S.; Calvert, J.W.; Jha, S.; Toedt-Pingel, I.; Ji, S.Y.; Nunez, D.; Ramachandran, A.; Anaya-Cisneros, M.; Tian, R.; Lefer, D.J. Activation of AMP-Activated Protein Kinase by Metformin Improves Left Ventricular Function and Survival in Heart Failure. Circ. Res. 2009, 104, 403–411. [Google Scholar] [CrossRef]
- Batchuluun, B.; Inoguchi, T.; Sonoda, N.; Sasaki, S.; Inoue, T.; Fujimura, Y.; Miura, D.; Takayanagi, R. Metformin and Liraglutide Ameliorate High Glucose-Induced Oxidative Stress via Inhibition of PKC-NAD(P)H Oxidase Pathway in Human Aortic Endothelial Cells. Atherosclerosis 2014, 232, 156–164. [Google Scholar] [CrossRef] [PubMed]
- Mamputu, J.-C.; Wiernsperger, N.; Renier, G. Metformin Inhibits Monocyte Adhesion to Endothelial Cells and Foam Cell Formation. Br. J. Diabetes Vasc. Dis. 2003, 3, 302–310. [Google Scholar] [CrossRef]
- Yang, Q.; Yuan, H.; Chen, M.; Qu, J.; Wang, H.; Yu, B.; Chen, J.; Sun, S.; Tang, X.; Ren, W. Metformin Ameliorates the Progression of Atherosclerosis via Suppressing Macrophage Infiltration and Inflammatory Responses in Rabbits. Life Sci. 2018, 198, 56–64. [Google Scholar] [CrossRef] [PubMed]
- De Jager, J.; Kooy, A.; Lehert, P.; Bets, D.; Wulffelé, M.G.; Teerlink, T.; Scheffer, P.G.; Schalkwijk, C.G.; Donker, A.J.M.; Stehouwer, C.D.A. Effects of Short-Term Treatment with Metformin on Markers of Endothelial Function and Inflammatory Activity in Type 2 Diabetes Mellitus: A Randomized, Placebo-Controlled Trial. J. Intern. Med. 2005, 257, 100–109. [Google Scholar] [CrossRef] [PubMed]
- Sun, D.; Yang, F. Metformin Improves Cardiac Function in Mice with Heart Failure after Myocardial Infarction by Regulating Mitochondrial Energy Metabolism. Biochem. Biophys. Res. Commun. 2017, 486, 329–335. [Google Scholar] [CrossRef]
- Gin, H.; Freyburger, G.; Boisseau, M.; Aubertin, J. Study of the Effect of Metformin on Platelet Aggregation in Insulin-Dependent Diabetics. Diabetes Res. Clin. Pract. 1989, 6, 61–67. [Google Scholar] [CrossRef]
- Paiva, M.A.; Rutter-Locher, Z.; Gonçalves, L.M.; Providência, L.A.; Davidson, S.M.; Yellon, D.M.; Mocanu, M.M. Enhancing AMPK Activation during Ischemia Protects the Diabetic Heart against Reperfusion Injury. Am. J. Physiol. Heart Circ. Physiol. 2011, 300, H2123–H2134. [Google Scholar] [CrossRef] [PubMed]
- Nafisa, A.; Gray, S.G.; Cao, Y.; Wang, T.; Xu, S.; Wattoo, F.H.; Barras, M.; Cohen, N.; Kamato, D.; Little, P.J. Endothelial Function and Dysfunction: Impact of Metformin. Pharmacol. Ther. 2018, 192, 150–162. [Google Scholar] [CrossRef]
- Sardu, C.; Paolisso, P.; Sacra, C.; Mauro, C.; Minicucci, F.; Portoghese, M.; Rizzo, M.R.; Barbieri, M.; Sasso, F.C.; D’Onofrio, N.; et al. Effects of Metformin Therapy on Coronary Endothelial Dysfunction in Patients with Prediabetes with Stable Angina and Nonobstructive Coronary Artery Stenosis: The CODYCE Multicenter Prospective Study. Diabetes Care 2019, 42, 1946–1955. [Google Scholar] [CrossRef] [PubMed]
- Sardu, C.; D’Onofrio, N.; Torella, M.; Portoghese, M.; Loreni, F.; Mureddu, S.; Signoriello, G.; Scisciola, L.; Barbieri, M.; Rizzo, M.R.; et al. Pericoronary Fat Inflammation and Major Adverse Cardiac Events (MACE) in Prediabetic Patients with Acute Myocardial Infarction: Effects of Metformin. Cardiovasc. Diabetol. 2019, 18, 126. [Google Scholar] [CrossRef]
- Packer, M. Potentiation of Insulin Signaling Contributes to Heart Failure in Type 2 Diabetes: A Hypothesis Supported by Both Mechanistic Studies and Clinical Trials. JACC Basic Transl. Sci. 2018, 3, 415–419. [Google Scholar] [CrossRef]
- Kooy, A.; de Jager, J.; Lehert, P.; Bets, D.; Wulffelé, M.G.; Donker, A.J.M.; Stehouwer, C.D.A. Long-Term Effects of Metformin on Metabolism and Microvascular and Macrovascular Disease in Patients with Type 2 Diabetes Mellitus. Arch. Intern. Med. 2009, 169, 616–625. [Google Scholar] [CrossRef]
- Petrie, J.R.; Chaturvedi, N.; Ford, I.; Brouwers, M.C.G.J.; Greenlaw, N.; Tillin, T.; Hramiak, I.; Hughes, A.D.; Jenkins, A.J.; Klein, B.E.K.; et al. Cardiovascular and Metabolic Effects of Metformin in Patients with Type 1 Diabetes (REMOVAL): A Double-Blind, Randomised, Placebo-Controlled Trial. Lancet Diabetes Endocrinol. 2017, 5, 597–609. [Google Scholar] [CrossRef]
- Wulffelé, M.G.; Kooy, A.; de Zeeuw, D.; Stehouwer, C.D.A.; Gansevoort, R.T. The Effect of Metformin on Blood Pressure, Plasma Cholesterol and Triglycerides in Type 2 Diabetes Mellitus: A Systematic Review. J. Intern. Med. 2004, 256, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Bassols, J.; Martínez-Calcerrada, J.-M.; Osiniri, I.; Díaz-Roldán, F.; Xargay-Torrent, S.; Mas-Parés, B.; Dorado-Ceballos, E.; Prats-Puig, A.; Carreras-Badosa, G.; de Zegher, F.; et al. Effects of Metformin Administration on Endocrine-Metabolic Parameters, Visceral Adiposity and Cardiovascular Risk Factors in Children with Obesity and Risk Markers for Metabolic Syndrome: A Pilot Study. PLoS ONE 2019, 14, e0226303. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, F.; Doi, Y.; Ninomiya, T.; Hirakawa, Y.; Mukai, N.; Hata, J.; Shikata, K.; Yoshida, D.; Matsumoto, T.; Kitazono, T.; et al. Haemoglobin A1c Even within Non-Diabetic Level Is a Predictor of Cardiovascular Disease in a General Japanese Population: The Hisayama Study. Cardiovasc. Diabetol. 2013, 12, 164. [Google Scholar] [CrossRef] [PubMed]
- Elley, C.R.; Kenealy, T.; Robinson, E.; Drury, P.L. Glycated Haemoglobin and Cardiovascular Outcomes in People with Type 2 Diabetes: A Large Prospective Cohort Study. Diabet. Med. J. Br. Diabet. Assoc. 2008, 25, 1295–1301. [Google Scholar] [CrossRef] [PubMed]
- Gerstein, H.C.; Pogue, J.; Mann, J.F.E.; Lonn, E.; Dagenais, G.R.; McQueen, M.; Yusuf, S. HOPE investigators The Relationship between Dysglycaemia and Cardiovascular and Renal Risk in Diabetic and Non-Diabetic Participants in the HOPE Study: A Prospective Epidemiological Analysis. Diabetologia 2005, 48, 1749–1755. [Google Scholar] [CrossRef]
- Khaw, K.T.; Wareham, N.; Luben, R.; Bingham, S.; Oakes, S.; Welch, A.; Day, N. Glycated Haemoglobin, Diabetes, and Mortality in Men in Norfolk Cohort of European Prospective Investigation of Cancer and Nutrition (EPIC-Norfolk). BMJ 2001, 322, 15–18. [Google Scholar] [CrossRef] [PubMed]
- Stratton, I.M.; Adler, A.I.; Neil, H.A.; Matthews, D.R.; Manley, S.E.; Cull, C.A.; Hadden, D.; Turner, R.C.; Holman, R.R. Association of Glycaemia with Macrovascular and Microvascular Complications of Type 2 Diabetes (UKPDS 35): Prospective Observational Study. BMJ 2000, 321, 405–412. [Google Scholar] [CrossRef] [PubMed]
- DeFronzo, R.A.; Goodman, A.M. Efficacy of Metformin in Patients with Non-Insulin-Dependent Diabetes Mellitus. The Multicenter Metformin Study Group. N. Engl. J. Med. 1995, 333, 541–549. [Google Scholar] [CrossRef]
- Bennett, W.L.; Maruthur, N.M.; Singh, S.; Segal, J.B.; Wilson, L.M.; Chatterjee, R.; Marinopoulos, S.S.; Puhan, M.A.; Ranasinghe, P.; Block, L.; et al. Comparative Effectiveness and Safety of Medications for Type 2 Diabetes: An Update Including New Drugs and 2-Drug Combinations. Ann. Intern. Med. 2011, 154, 602–613. [Google Scholar] [CrossRef] [PubMed]
- Ito, H.; Ishida, H.; Takeuchi, Y.; Antoku, S.; Abe, M.; Mifune, M.; Togane, M. Long-Term Effect of Metformin on Blood Glucose Control in Non-Obese Patients with Type 2 Diabetes Mellitus. Nutr. Metab. 2010, 7, 83. [Google Scholar] [CrossRef] [PubMed]
- Gerstein, H.C.; Swedberg, K.; Carlsson, J.; McMurray, J.J.V.; Michelson, E.L.; Olofsson, B.; Pfeffer, M.A.; Yusuf, S. CHARM Program Investigators the Hemoglobin A1c Level as a Progressive Risk Factor for Cardiovascular Death, Hospitalization for Heart Failure, or Death in Patients with Chronic Heart Failure: An Analysis of the Candesartan in Heart Failure: Assessment of Reduction in Mortality and Morbidity (CHARM) Program. Arch. Intern. Med. 2008, 168, 1699–1704. [Google Scholar] [CrossRef] [PubMed]
- Levitan, E.B.; Liu, S.; Stampfer, M.J.; Cook, N.R.; Rexrode, K.M.; Ridker, P.M.; Buring, J.E.; Manson, J.E. HbA1c Measured in Stored Erythrocytes and Mortality Rate among Middle-Aged and Older Women. Diabetologia 2008, 51, 267–275. [Google Scholar] [CrossRef] [PubMed]
- Khaw, K.-T.; Wareham, N.; Bingham, S.; Luben, R.; Welch, A.; Day, N. Association of Hemoglobin A1c with Cardiovascular Disease and Mortality in Adults: The European Prospective Investigation into Cancer in Norfolk. Ann. Intern. Med. 2004, 141, 413–420. [Google Scholar] [CrossRef] [PubMed]
- Stout, R.L.; Fulks, M.; Dolan, V.F.; Magee, M.E.; Suarez, L. Relationship of Hemoglobin A1c to Mortality in Nonsmoking Insurance Applicants. J. Insur. Med. 2007, 39, 174–181. [Google Scholar]
- Eurich, D.T.; Majumdar, S.R.; McAlister, F.A.; Tsuyuki, R.T.; Johnson, J.A. Improved Clinical Outcomes Associated with Metformin in Patients with Diabetes and Heart Failure. Diabetes Care 2005, 28, 2345–2351. [Google Scholar] [CrossRef]
- Mohan, M.; Al-Talabany, S.; McKinnie, A.; Mordi, I.R.; Singh, J.S.S.; Gandy, S.J.; Baig, F.; Hussain, M.S.; Bhalraam, U.; Khan, F.; et al. A Randomized Controlled Trial of Metformin on Left Ventricular Hypertrophy in Patients with Coronary Artery Disease without Diabetes: The MET-REMODEL Trial. Eur. Heart J. 2019, 40, 3409–3417. [Google Scholar] [CrossRef] [PubMed]
- Lexis, C.P.H.; Wieringa, W.G.; Hiemstra, B.; van Deursen, V.M.; Lipsic, E.; van der Harst, P.; van Veldhuisen, D.J.; van der Horst, I.C.C. Chronic Metformin Treatment Is Associated with Reduced Myocardial Infarct Size in Diabetic Patients with ST-Segment Elevation Myocardial Infarction. Cardiovasc. Drugs Ther. 2014, 28, 163–171. [Google Scholar] [CrossRef]
- Eurich, D.T.; McAlister, F.A.; Blackburn, D.F.; Majumdar, S.R.; Tsuyuki, R.T.; Varney, J.; Johnson, J.A. Benefits and Harms of Antidiabetic Agents in Patients with Diabetes and Heart Failure: Systematic Review. BMJ 2007, 335, 497. [Google Scholar] [CrossRef] [PubMed]
- Eurich, D.T.; Weir, D.L.; Majumdar, S.R.; Tsuyuki, R.T.; Johnson, J.A.; Tjosvold, L.; Vanderloo, S.E.; McAlister, F.A. Comparative Safety and Effectiveness of Metformin in Patients with Diabetes Mellitus and Heart Failure: Systematic Review of Observational Studies Involving 34,000 Patients. Circ. Heart Fail. 2013, 6, 395–402. [Google Scholar] [CrossRef]
- Crowley, M.J.; Diamantidis, C.J.; McDuffie, J.R.; Cameron, C.B.; Stanifer, J.W.; Mock, C.K.; Wang, X.; Tang, S.; Nagi, A.; Kosinski, A.S.; et al. Clinical Outcomes of Metformin Use in Populations with Chronic Kidney Disease, Congestive Heart Failure, or Chronic Liver Disease: A Systematic Review. Ann. Intern. Med. 2017, 166, 191–200. [Google Scholar] [CrossRef]
- Romero, S.P.; Andrey, J.L.; Garcia-Egido, A.; Escobar, M.A.; Perez, V.; Corzo, R.; Garcia-Domiguez, G.J.; Gomez, F. Metformin Therapy and Prognosis of Patients with Heart Failure and New-Onset Diabetes Mellitus. A Propensity-Matched Study in the Community. Int. J. Cardiol. 2013, 166, 404–412. [Google Scholar] [CrossRef] [PubMed]
- Tinetti, M.E.; McAvay, G.; Trentalange, M.; Cohen, A.B.; Allore, H.G. Association between Guideline Recommended Drugs and Death in Older Adults with Multiple Chronic Conditions: Population Based Cohort Study. BMJ 2015, 351, h4984. [Google Scholar] [CrossRef] [PubMed]
- Al Ali, L.; Hartman, M.T.; Lexis, C.P.H.; Hummel, Y.M.; Lipsic, E.; van Melle, J.P.; van Veldhuisen, D.J.; Voors, A.A.; van der Horst, I.C.C.; van der Harst, P. The Effect of Metformin on Diastolic Function in Patients Presenting with ST-Elevation Myocardial Infarction. PLoS ONE 2016, 11, e0168340. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Bowe, B.; Mokdad, A.H.; Xian, H.; Yan, Y.; Li, T.; Maddukuri, G.; Tsai, C.-Y.; Floyd, T.; Al-Aly, Z. Analysis of the Global Burden of Disease Study Highlights the Global, Regional, and National Trends of Chronic Kidney Disease Epidemiology from 1990 to 2016. Kidney Int. 2018, 94, 567–581. [Google Scholar] [CrossRef] [PubMed]
- Alicic, R.Z.; Rooney, M.T.; Tuttle, K.R. Diabetic Kidney Disease: Challenges, Progress, and Possibilities. Clin. J. Am. Soc. Nephrol. CJASN 2017, 12, 2032–2045. [Google Scholar] [CrossRef]
- Perkovic, V.; Jardine, M.J.; Neal, B.; Bompoint, S.; Heerspink, H.J.L.; Charytan, D.M.; Edwards, R.; Agarwal, R.; Bakris, G.; Bull, S.; et al. Canagliflozin and Renal Outcomes in Type 2 Diabetes and Nephropathy. N. Engl. J. Med. 2019, 380, 2295–2306. [Google Scholar] [CrossRef] [PubMed]
- Wanner, C.; Inzucchi, S.E.; Lachin, J.M.; Fitchett, D.; von Eynatten, M.; Mattheus, M.; Johansen, O.E.; Woerle, H.J.; Broedl, U.C.; Zinman, B.; et al. Empagliflozin and Progression of Kidney Disease in Type 2 Diabetes. N. Engl. J. Med. 2016, 375, 323–334. [Google Scholar] [CrossRef]
- Rosenstock, J.; Perkovic, V.; Johansen, O.E.; Cooper, M.E.; Kahn, S.E.; Marx, N.; Alexander, J.H.; Pencina, M.; Toto, R.D.; Wanner, C.; et al. Effect of Linagliptin vs Placebo on Major Cardiovascular Events in Adults with Type 2 Diabetes and High Cardiovascular and Renal Risk: The CARMELINA Randomized Clinical Trial. JAMA 2019, 321, 69–79. [Google Scholar] [CrossRef] [PubMed]
- Sloan, L.A. Review of Glucagon-like Peptide-1 Receptor Agonists for the Treatment of Type 2 Diabetes Mellitus in Patients with Chronic Kidney Disease and Their Renal Effects. J. Diabetes 2019, 11, 938–948. [Google Scholar] [CrossRef] [PubMed]
- Garofalo, C.; Borrelli, S.; Liberti, M.E.; Andreucci, M.; Conte, G.; Minutolo, R.; Provenzano, M.; De Nicola, L. SGLT2 Inhibitors: Nephroprotective Efficacy and Side Effects. Med. Kaunas Lith. 2019, 55, 268. [Google Scholar] [CrossRef]
- Williams, D.M.; Nawaz, A.; Evans, M. Renal Outcomes in Type 2 Diabetes: A Review of Cardiovascular and Renal Outcome Trials. Diabetes Ther. Res. Treat. Educ. Diabetes Relat. Disord. 2020, 11, 369–386. [Google Scholar] [CrossRef]
- Fei, Y.; Tsoi, M.-F.; Cheung, B.M.Y. Cardiovascular Outcomes in Trials of New Antidiabetic Drug Classes: A Network Meta-Analysis. Cardiovasc. Diabetol. 2019, 18, 112. [Google Scholar] [CrossRef] [PubMed]
- Davies, M.J.; D’Alessio, D.A.; Fradkin, J.; Kernan, W.N.; Mathieu, C.; Mingrone, G.; Rossing, P.; Tsapas, A.; Wexler, D.J.; Buse, J.B. Management of Hyperglycemia in Type 2 Diabetes, 2018. A Consensus Report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care 2018, 41, 2669–2701. [Google Scholar] [CrossRef] [PubMed]
- Alhaider, A.A.; Korashy, H.M.; Sayed-Ahmed, M.M.; Mobark, M.; Kfoury, H.; Mansour, M.A. Metformin Attenuates Streptozotocin-Induced Diabetic Nephropathy in Rats through Modulation of Oxidative Stress Genes Expression. Chem. Biol. Interact. 2011, 192, 233–242. [Google Scholar] [CrossRef]
- Amini, F.G.; Rafieian-Kopaei, M.; Nematbakhsh, M.; Baradaran, A.; Nasri, H. Ameliorative Effects of Metformin on Renal Histologic and Biochemical Alterations of Gentamicin-Induced Renal Toxicity in Wistar Rats. J. Res. Med. Sci. Off. J. Isfahan Univ. Med. Sci. 2012, 17, 621–625. [Google Scholar]
- De Broe, M.E.; Kajbaf, F.; Lalau, J.-D. Renoprotective Effects of Metformin. Nephron 2018, 138, 261–274. [Google Scholar] [CrossRef]
- Nasri, H.; Baradaran, A.; Ardalan, M.R.; Mardani, S.; Momeni, A.; Rafieian-Kopaei, M. Bright Renoprotective Properties of Metformin: Beyond Blood Glucose Regulatory Effects. Iran. J. Kidney Dis. 2013, 7, 423–428. [Google Scholar]
- Kawanami, D.; Takashi, Y.; Tanabe, M. Significance of Metformin Use in Diabetic Kidney Disease. Int. J. Mol. Sci. 2020, 21, 4239. [Google Scholar] [CrossRef] [PubMed]
- Cavaglieri, R.C.; Day, R.T.; Feliers, D.; Abboud, H.E. Metformin Prevents Renal Interstitial Fibrosis in Mice with Unilateral Ureteral Obstruction. Mol. Cell. Endocrinol. 2015, 412, 116–122. [Google Scholar] [CrossRef] [PubMed]
- Xue, J.; Wang, L.; Sun, Z.; Xing, C. Basic Research in Diabetic Nephropathy Health Care: A Study of the Renoprotective Mechanism of Metformin. J. Med. Syst. 2019, 43, 266. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Moon, S.Y.; Kim, J.-S.; Baek, C.H.; Kim, M.; Min, J.Y.; Lee, S.K. Activation of AMP-Activated Protein Kinase Inhibits ER Stress and Renal Fibrosis. Am. J. Physiol. Renal Physiol. 2015, 308, F226–F236. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.-C.; Lee, C.-H.; Chang, L.-Y.; Chang, C.-H.; Zhang, J.-F.; Lee, M.-R.; Wang, J.-Y.; Chen, S.-M. Association of Metformin Use with End-Stage Renal Disease in Patients with Type 2 Diabetes Mellitus: A Nationwide Cohort Study Under the Pay-for-Performance Program. J. Clin. Pharmacol. 2019, 59, 1443–1452. [Google Scholar] [CrossRef]
- Kwon, S.; Kim, Y.C.; Park, J.Y.; Lee, J.; An, J.N.; Kim, C.T.; Oh, S.; Park, S.; Kim, D.K.; Oh, Y.K.; et al. The Long-Term Effects of Metformin on Patients with Type 2 Diabetic Kidney Disease. Diabetes Care 2020, 43, 948–955. [Google Scholar] [CrossRef]
- Stephen, J.; Anderson-Haag, T.L.; Gustafson, S.; Snyder, J.J.; Kasiske, B.L.; Israni, A.K. Metformin Use in Kidney Transplant Recipients in the United States: An Observational Study. Am. J. Nephrol. 2014, 40, 546–553. [Google Scholar] [CrossRef] [PubMed]
- Ekström, N.; Schiöler, L.; Svensson, A.-M.; Eeg-Olofsson, K.; Miao Jonasson, J.; Zethelius, B.; Cederholm, J.; Eliasson, B.; Gudbjörnsdottir, S. Effectiveness and Safety of Metformin in 51 675 Patients with Type 2 Diabetes and Different Levels of Renal Function: A Cohort Study from the Swedish National Diabetes Register. BMJ Open 2012, 2. [Google Scholar] [CrossRef] [PubMed]
- Charytan, D.M.; Solomon, S.D.; Ivanovich, P.; Remuzzi, G.; Cooper, M.E.; McGill, J.B.; Parving, H.-H.; Parfrey, P.; Singh, A.K.; Burdmann, E.A.; et al. Metformin Use and Cardiovascular Events in Patients with Type 2 Diabetes and Chronic Kidney Disease. Diabetes Obes. Metab. 2019, 21, 1199–1208. [Google Scholar] [CrossRef] [PubMed]
- Bell, S.; Farran, B.; McGurnaghan, S.; McCrimmon, R.J.; Leese, G.P.; Petrie, J.R.; McKeigue, P.; Sattar, N.; Wild, S.; McKnight, J.; et al. Risk of Acute Kidney Injury and Survival in Patients Treated with Metformin: An Observational Cohort Study. BMC Nephrol. 2017, 18, 163. [Google Scholar] [CrossRef] [PubMed]
- Hippisley-Cox, J.; Coupland, C. Diabetes Treatments and Risk of Amputation, Blindness, Severe Kidney Failure, Hyperglycaemia, and Hypoglycaemia: Open Cohort Study in Primary Care. BMJ 2016, 352, i1450. [Google Scholar] [CrossRef]
- Kosmalski, M.; Drozdowska, A.; Sliwinska, A.; Drzewoski, J. Inappropriate Metformin Prescribing in Elderly Type 2 Diabetes Mellitus (T2DM) Patients. Adv. Med. Sci. 2012, 57, 65–70. [Google Scholar] [CrossRef]
- Bodmer, M.; Meier, C.; Krähenbühl, S.; Jick, S.S.; Meier, C.R. Metformin, Sulfonylureas, or Other Antidiabetes Drugs and the Risk of Lactic Acidosis or Hypoglycemia: A Nested Case-Control Analysis. Diabetes Care 2008, 31, 2086–2091. [Google Scholar] [CrossRef] [PubMed]
- Salpeter, S.R.; Greyber, E.; Pasternak, G.A.; Salpeter, E.E. Risk of Fatal and Nonfatal Lactic Acidosis with Metformin Use in Type 2 Diabetes Mellitus. Cochrane Database Syst. Rev. 2010, CD002967. [Google Scholar] [CrossRef]
- Abudawood, M. Diabetes and Cancer: A Comprehensive Review. J. Res. Med. Sci. Off. J. Isfahan Univ. Med. Sci. 2019, 24, 94. [Google Scholar] [CrossRef]
- Giovannucci, E.; Harlan, D.M.; Archer, M.C.; Bergenstal, R.M.; Gapstur, S.M.; Habel, L.A.; Pollak, M.; Regensteiner, J.G.; Yee, D. Diabetes and Cancer: A Consensus Report. Diabetes Care 2010, 33, 1674–1685. [Google Scholar] [CrossRef] [PubMed]
- Evans, J.M.M.; Donnelly, L.A.; Emslie-Smith, A.M.; Alessi, D.R.; Morris, A.D. Metformin and Reduced Risk of Cancer in Diabetic Patients. BMJ 2005, 330, 1304–1305. [Google Scholar] [CrossRef]
- Sui, X.; Xu, Y.; Wang, X.; Han, W.; Pan, H.; Xiao, M. Metformin: A Novel but Controversial Drug in Cancer Prevention and Treatment. Mol. Pharm. 2015, 12, 3783–3791. [Google Scholar] [CrossRef] [PubMed]
- Chae, Y.K.; Arya, A.; Malecek, M.-K.; Shin, D.S.; Carneiro, B.; Chandra, S.; Kaplan, J.; Kalyan, A.; Altman, J.K.; Platanias, L.; et al. Repurposing Metformin for Cancer Treatment: Current Clinical Studies. Oncotarget 2016, 7, 40767–40780. [Google Scholar] [CrossRef]
- Gonzalez-Angulo, A.M.; Meric-Bernstam, F. Metformin: A Therapeutic Opportunity in Breast Cancer. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2010, 16, 1695–1700. [Google Scholar] [CrossRef] [PubMed]
- Heckman-Stoddard, B.M.; DeCensi, A.; Sahasrabuddhe, V.V.; Ford, L.G. Repurposing Metformin for the Prevention of Cancer and Cancer Recurrence. Diabetologia 2017, 60, 1639–1647. [Google Scholar] [CrossRef] [PubMed]
- Ben Sahra, I.; Regazzetti, C.; Robert, G.; Laurent, K.; Le Marchand-Brustel, Y.; Auberger, P.; Tanti, J.-F.; Giorgetti-Peraldi, S.; Bost, F. Metformin, Independent of AMPK, Induces MTOR Inhibition and Cell-Cycle Arrest through REDD1. Cancer Res. 2011, 71, 4366–4372. [Google Scholar] [CrossRef] [PubMed]
- Lei, Y.; Yi, Y.; Liu, Y.; Liu, X.; Keller, E.T.; Qian, C.-N.; Zhang, J.; Lu, Y. Metformin Targets Multiple Signaling Pathways in Cancer. Chin. J. Cancer 2017, 36, 17. [Google Scholar] [CrossRef]
- Han, G.; Gong, H.; Wang, Y.; Guo, S.; Liu, K. AMPK/MTOR-Mediated Inhibition of Survivin Partly Contributes to Metformin-Induced Apoptosis in Human Gastric Cancer Cell. Cancer Biol. Ther. 2015, 16, 77–87. [Google Scholar] [CrossRef]
- Thakur, S.; Daley, B.; Klubo-Gwiezdzinska, J. The Role of an Anti-Diabetic Drug Metformin in the Treatment of Endocrine Tumors. J. Mol. Endocrinol. 2019, 63, R17–R35. [Google Scholar] [CrossRef]
- Pollak, M.N. Investigating Metformin for Cancer Prevention and Treatment: The End of the Beginning. Cancer Discov. 2012, 2, 778–790. [Google Scholar] [CrossRef] [PubMed]
- Griss, T.; Vincent, E.E.; Egnatchik, R.; Chen, J.; Ma, E.H.; Faubert, B.; Viollet, B.; DeBerardinis, R.J.; Jones, R.G. Metformin Antagonizes Cancer Cell Proliferation by Suppressing Mitochondrial-Dependent Biosynthesis. PLoS Biol. 2015, 13, e1002309. [Google Scholar] [CrossRef] [PubMed]
- Valaee, S.; Yaghoobi, M.M.; Shamsara, M. Metformin Inhibits Gastric Cancer Cells Metastatic Traits through Suppression of Epithelial-Mesenchymal Transition in a Glucose-Independent Manner. PLoS ONE 2017, 12, e0174486. [Google Scholar] [CrossRef]
- Libby, G.; Donnelly, L.A.; Donnan, P.T.; Alessi, D.R.; Morris, A.D.; Evans, J.M.M. New Users of Metformin Are at Low Risk of Incident Cancer. Diabetes Care 2009, 32, 1620–1625. [Google Scholar] [CrossRef]
- Decensi, A.; Puntoni, M.; Goodwin, P.; Cazzaniga, M.; Gennari, A.; Bonanni, B.; Gandini, S. Metformin and Cancer Risk in Diabetic Patients: A Systematic Review and Meta-Analysis. Cancer Prev. Res. 2010, 3, 1451–1461. [Google Scholar] [CrossRef] [PubMed]
- Landman, G.W.D.; Kleefstra, N.; van Hateren, K.J.J.; Groenier, K.H.; Gans, R.O.B.; Bilo, H.J.G. Metformin Associated with Lower Cancer Mortality in Type 2 Diabetes: ZODIAC-16. Diabetes Care 2010, 33, 322–326. [Google Scholar] [CrossRef] [PubMed]
- Park, J.-H.; Kim, Y.-H.; Park, E.H.; Lee, S.-J.; Kim, H.; Kim, A.; Lee, S.B.; Shim, S.; Jang, H.; Myung, J.K.; et al. Effects of Metformin and Phenformin on Apoptosis and Epithelial-Mesenchymal Transition in Chemoresistant Rectal Cancer. Cancer Sci. 2019, 110, 2834–2845. [Google Scholar] [CrossRef]
- Song, C.W.; Lee, H.; Dings, R.P.M.; Williams, B.; Powers, J.; Santos, T.D.; Choi, B.-H.; Park, H.J. Metformin Kills and Radiosensitizes Cancer Cells and Preferentially Kills Cancer Stem Cells. Sci. Rep. 2012, 2, 362. [Google Scholar] [CrossRef]
- Jiralerspong, S.; Palla, S.L.; Giordano, S.H.; Meric-Bernstam, F.; Liedtke, C.; Barnett, C.M.; Hsu, L.; Hung, M.-C.; Hortobagyi, G.N.; Gonzalez-Angulo, A.M. Metformin and Pathologic Complete Responses to Neoadjuvant Chemotherapy in Diabetic Patients with Breast Cancer. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2009, 27, 3297–3302. [Google Scholar] [CrossRef] [PubMed]
- Hirsch, H.A.; Iliopoulos, D.; Tsichlis, P.N.; Struhl, K. Metformin Selectively Targets Cancer Stem Cells, and Acts Together with Chemotherapy to Block Tumor Growth and Prolong Remission. Cancer Res. 2009, 69, 7507–7511. [Google Scholar] [CrossRef]
- Rao, M.; Gao, C.; Guo, M.; Law, B.Y.K.; Xu, Y. Effects of Metformin Treatment on Radiotherapy Efficacy in Patients with Cancer and Diabetes: A Systematic Review and Meta-Analysis. Cancer Manag. Res. 2018, 10, 4881–4890. [Google Scholar] [CrossRef] [PubMed]
- Mekuria, A.N.; Ayele, Y.; Tola, A.; Mishore, K.M. Monotherapy with Metformin versus Sulfonylureas and Risk of Cancer in Type 2 Diabetic Patients: A Systematic Review and Meta-Analysis. J. Diabetes Res. 2019, 2019, 7676909. [Google Scholar] [CrossRef]
- Yin, M.; Zhou, J.; Gorak, E.J.; Quddus, F. Metformin Is Associated with Survival Benefit in Cancer Patients with Concurrent Type 2 Diabetes: A Systematic Review and Meta-Analysis. Oncologist 2013, 18, 1248–1255. [Google Scholar] [CrossRef]
- Dankner, R.; Agay, N.; Olmer, L.; Murad, H.; Keinan Boker, L.; Balicer, R.D.; Freedman, L.S. Metformin Treatment and Cancer Risk: Cox Regression Analysis, with Time-Dependent Covariates, of 320,000 Persons with Incident Diabetes Mellitus. Am. J. Epidemiol. 2019, 188, 1794–1800. [Google Scholar] [CrossRef]
- Cabreiro, F.; Au, C.; Leung, K.-Y.; Vergara-Irigaray, N.; Cochemé, H.M.; Noori, T.; Weinkove, D.; Schuster, E.; Greene, N.D.E.; Gems, D. Metformin Retards Aging in C. Elegans by Altering Microbial Folate and Methionine Metabolism. Cell 2013, 153, 228–239. [Google Scholar] [CrossRef]
- Martin-Montalvo, A.; Mercken, E.M.; Mitchell, S.J.; Palacios, H.H.; Mote, P.L.; Scheibye-Knudsen, M.; Gomes, A.P.; Ward, T.M.; Minor, R.K.; Blouin, M.-J.; et al. Metformin Improves Healthspan and Lifespan in Mice. Nat. Commun. 2013, 4, 2192. [Google Scholar] [CrossRef] [PubMed]
- Bannister, C.A.; Holden, S.E.; Jenkins-Jones, S.; Morgan, C.L.; Halcox, J.P.; Schernthaner, G.; Mukherjee, J.; Currie, C.J. Can People with Type 2 Diabetes Live Longer than Those without? A Comparison of Mortality in People Initiated with Metformin or Sulphonylurea Monotherapy and Matched, Non-Diabetic Controls. Diabetes Obes. Metab. 2014, 16, 1165–1173. [Google Scholar] [CrossRef]
- Campbell, J.M.; Bellman, S.M.; Stephenson, M.D.; Lisy, K. Metformin Reduces All-Cause Mortality and Diseases of Ageing Independent of Its Effect on Diabetes Control: A Systematic Review and Meta-Analysis. Ageing Res. Rev. 2017, 40, 31–44. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Gong, X.; Wang, L.; Guo, J. Effects of Hypertension, Diabetes and Coronary Heart Disease on COVID-19 Diseases Severity: A Systematic Review and Meta-Analysis. medRxiv 2020. [Google Scholar] [CrossRef]
- Peric, S.; Stulnig, T.M. Diabetes and COVID-19: Disease-Management-People. Wien. Klin. Wochenschr. 2020, 132, 356–361. [Google Scholar] [CrossRef] [PubMed]
- Guan, W.-J.; Liang, W.-H.; Zhao, Y.; Liang, H.-R.; Chen, Z.-S.; Li, Y.-M.; Liu, X.-Q.; Chen, R.-C.; Tang, C.-L.; Wang, T.; et al. Comorbidity and Its Impact on 1590 Patients with COVID-19 in China: A Nationwide Analysis. Eur. Respir. J. 2020, 55. [Google Scholar] [CrossRef] [PubMed]
- Bornstein, S.R.; Rubino, F.; Khunti, K.; Mingrone, G.; Hopkins, D.; Birkenfeld, A.L.; Boehm, B.; Amiel, S.; Holt, R.I.; Skyler, J.S.; et al. Practical Recommendations for the Management of Diabetes in Patients with COVID-19. Lancet Diabetes Endocrinol. 2020, 8, 546–550. [Google Scholar] [CrossRef]
- Bramante, C.T.; Ingraham, N.E.; Murray, T.A.; Marmor, S.; Hovertsen, S.; Gronski, J.; McNeil, C.; Feng, R.; Guzman, G.; Abdelwahab, N.; et al. Metformin and Risk of Mortality in Patients Hospitalised with COVID-19: A Retrospective Cohort Analysis. Alliance Pandemic Prep. 2021, 2, E34–E41. [Google Scholar] [CrossRef]
- Cook, M.N.; Girman, C.J.; Stein, P.P.; Alexander, C.M. Initial Monotherapy with Either Metformin or Sulphonylureas Often Fails to Achieve or Maintain Current Glycaemic Goals in Patients with Type 2 Diabetes in UK Primary Care. Diabet. Med. J. Br. Diabet. Assoc. 2007, 24, 350–358. [Google Scholar] [CrossRef] [PubMed]
- Vallianou, N.G.; Stratigou, T.; Tsagarakis, S. Metformin and Gut Microbiota: Their Interactions and Their Impact on Diabetes. Hormones 2019, 18, 141–144. [Google Scholar] [CrossRef] [PubMed]
- Montastruc, J.-L.; Toutain, P.-L. A New Drug-Drug Interaction Between Hydroxychloroquine and Metformin? A Signal Detection Study. Drug Saf. 2020, 43, 657–660. [Google Scholar] [CrossRef]
- Stocker, S.L.; Morrissey, K.M.; Yee, S.W.; Castro, R.A.; Xu, L.; Dahlin, A.; Ramirez, A.H.; Roden, D.M.; Wilke, R.A.; McCarty, C.A.; et al. The Effect of Novel Promoter Variants in MATE1 and MATE2 on the Pharmacokinetics and Pharmacodynamics of Metformin. Clin. Pharmacol. Ther. 2013, 93, 186–194. [Google Scholar] [CrossRef]
- Shu, Y.; Sheardown, S.A.; Brown, C.; Owen, R.P.; Zhang, S.; Castro, R.A.; Ianculescu, A.G.; Yue, L.; Lo, J.C.; Burchard, E.G.; et al. Effect of Genetic Variation in the Organic Cation Transporter 1 (OCT1) on Metformin Action. J. Clin. Investig. 2007, 117, 1422–1431. [Google Scholar] [CrossRef]
- Shikata, E.; Yamamoto, R.; Takane, H.; Shigemasa, C.; Ikeda, T.; Otsubo, K.; Ieiri, I. Human Organic Cation Transporter (OCT1 and OCT2) Gene Polymorphisms and Therapeutic Effects of Metformin. J. Hum. Genet. 2007, 52, 117–122. [Google Scholar] [CrossRef]
- Becker, M.L.; Visser, L.E.; van Schaik, R.H.N.; Hofman, A.; Uitterlinden, A.G.; Stricker, B.H.C. Genetic Variation in the Organic Cation Transporter 1 Is Associated with Metformin Response in Patients with Diabetes Mellitus. Pharm. J. 2009, 9, 242–247. [Google Scholar] [CrossRef]
- Gong, L.; Goswami, S.; Giacomini, K.M.; Altman, R.B.; Klein, T.E. Metformin Pathways: Pharmacokinetics and Pharmacodynamics. Pharmacogenet. Genom. 2012, 22, 820–827. [Google Scholar] [CrossRef] [PubMed]
- Nasykhova, Y.A.; Tonyan, Z.N.; Mikhailova, A.A.; Danilova, M.M.; Glotov, A.S. Pharmacogenetics of Type 2 Diabetes-Progress and Prospects. Int. J. Mol. Sci. 2020, 21, 6842. [Google Scholar] [CrossRef] [PubMed]
- Florez, J.C. Pharmacogenetics in Type 2 Diabetes: Precision Medicine or Discovery Tool? Diabetologia 2017, 60, 800–807. [Google Scholar] [CrossRef] [PubMed]
- Mofo Mato, E.P.; Guewo-Fokeng, M.; Essop, M.F.; Owira, P.M.O. Genetic Polymorphisms of Organic Cation Transporter 1 (OCT1) and Responses to Metformin Therapy in Individuals with Type 2 Diabetes: A Systematic Review. Medicine 2018, 97, e11349. [Google Scholar] [CrossRef] [PubMed]
- Staud, F.; Cerveny, L.; Ahmadimoghaddam, D.; Ceckova, M. Multidrug and Toxin Extrusion Proteins (MATE/SLC47); Role in Pharmacokinetics. Int. J. Biochem. Cell Biol. 2013, 45, 2007–2011. [Google Scholar] [CrossRef]
- Toyama, K.; Yonezawa, A.; Masuda, S.; Osawa, R.; Hosokawa, M.; Fujimoto, S.; Inagaki, N.; Inui, K.; Katsura, T. Loss of Multidrug and Toxin Extrusion 1 (MATE1) Is Associated with Metformin-Induced Lactic Acidosis. Br. J. Pharmacol. 2012, 166, 1183–1191. [Google Scholar] [CrossRef]
- Zhou, M.; Xia, L.; Wang, J. Metformin Transport by a Newly Cloned Proton-Stimulated Organic Cation Transporter (Plasma Membrane Monoamine Transporter) Expressed in Human Intestine. Drug Metab. Dispos. Biol. Fate Chem. 2007, 35, 1956–1962. [Google Scholar] [CrossRef]
- Dujic, T.; Zhou, K.; Yee, S.W.; van Leeuwen, N.; de Keyser, C.E.; Javorský, M.; Goswami, S.; Zaharenko, L.; Hougaard Christensen, M.M.; Out, M.; et al. Variants in Pharmacokinetic Transporters and Glycemic Response to Metformin: A Metgen Meta-Analysis. Clin. Pharmacol. Ther. 2017, 101, 763–772. [Google Scholar] [CrossRef] [PubMed]
- Tuot, D.S.; Lin, F.; Shlipak, M.G.; Grubbs, V.; Hsu, C.; Yee, J.; Shahinian, V.; Saran, R.; Saydah, S.; Williams, D.E.; et al. Potential Impact of Prescribing Metformin According to EGFR Rather Than Serum Creatinine. Diabetes Care 2015, 38, 2059–2067. [Google Scholar] [CrossRef] [PubMed]
- Fatima, M.; Sadeeqa, S.; Nazir, S.U.R. Metformin and Its Gastrointestinal Problems: A Review. Biomed. Res. 2018, 29. [Google Scholar] [CrossRef]
- Kim, J.; Ahn, C.W.; Fang, S.; Lee, H.S.; Park, J.S. Association between Metformin Dose and Vitamin B12 Deficiency in Patients with Type 2 Diabetes. Medicine 2019, 98, e17918. [Google Scholar] [CrossRef]
- Aroda, V.R.; Edelstein, S.L.; Goldberg, R.B.; Knowler, W.C.; Marcovina, S.M.; Orchard, T.J.; Bray, G.A.; Schade, D.S.; Temprosa, M.G.; White, N.H.; et al. Long-Term Metformin Use and Vitamin B12 Deficiency in the Diabetes Prevention Program Outcomes Study. J. Clin. Endocrinol. Metab. 2016, 101, 1754–1761. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.K.; Kumar, A.; Karmakar, D.; Jha, R.K. Association of B12 Deficiency and Clinical Neuropathy with Metformin Use in Type 2 Diabetes Patients. J. Postgrad. Med. 2013, 59, 253–257. [Google Scholar] [CrossRef] [PubMed]
- Reinstatler, L.; Qi, Y.P.; Williamson, R.S.; Garn, J.V.; Oakley, G.P. Association of Biochemical B12 Deficiency with Metformin Therapy and Vitamin B12 Supplements: The National Health and Nutrition Examination Survey, 1999–2006. Diabetes Care 2012, 35, 327–333. [Google Scholar] [CrossRef] [PubMed]
- Donnelly, L.A.; Dennis, J.M.; Coleman, R.L.; Sattar, N.; Hattersley, A.T.; Holman, R.R.; Pearson, E.R. Risk of Anemia with Metformin Use in Type 2 Diabetes: A MASTERMIND Study. Diabetes Care 2020, 43, 2493–2499. [Google Scholar] [CrossRef] [PubMed]
| Approved to Treat | No Formal Indication (Used Off-Label) | Investigated for New Applications |
|---|---|---|
| T2DM | Prediabetes/obesity | Cardioprotection |
| T1DM | Nephroprotection | |
| GDM | Cancer | |
| PCOS | Anti-aging | |
| NAFLD | COVID-19 |
| Metformin: |
|---|
| Is an effective and safe antihyperglycemic agent in monotherapy as well as in combination with other anti-diabetic medicines. |
| Is indicated as the first -line therapy of newly diagnosed T2DM |
| Inhibits or delays the risk of the transition from prediabetes to T2DM |
| Lowers hyperglycemia mostly via inhibition of hepatic gluconeogenesis along with increasing insulin sensitivity in skeletal muscle |
| Suppresses hepatic glucose production through the inhibition of mitochondrial respiratory chain complex 1 |
| Acts in AMPK—dependent and AMPK-independent manner |
| Shows multiple glucose-independent pleiotropic effects |
| Possesses cardioprotective properties |
| Has possible nephroprotective effect |
| Off-label use is increasing in PCOS, T1DM, NAFLD and obesity |
| Is considered to be promising agent in cancer prevention and treatment as well as an anti-aging agent |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Drzewoski, J.; Hanefeld, M. The Current and Potential Therapeutic Use of Metformin—The Good Old Drug. Pharmaceuticals 2021, 14, 122. https://doi.org/10.3390/ph14020122
Drzewoski J, Hanefeld M. The Current and Potential Therapeutic Use of Metformin—The Good Old Drug. Pharmaceuticals. 2021; 14(2):122. https://doi.org/10.3390/ph14020122
Chicago/Turabian StyleDrzewoski, Józef, and Markolf Hanefeld. 2021. "The Current and Potential Therapeutic Use of Metformin—The Good Old Drug" Pharmaceuticals 14, no. 2: 122. https://doi.org/10.3390/ph14020122
APA StyleDrzewoski, J., & Hanefeld, M. (2021). The Current and Potential Therapeutic Use of Metformin—The Good Old Drug. Pharmaceuticals, 14(2), 122. https://doi.org/10.3390/ph14020122






